Abstract
The data presented in this article are relevant to the research article, “Electrochemistry of Os bipyridyl and phenanthroline complexes, comparison with Ru and Fe” (van der Westhuizen, 2020). Cyclic voltammograms illustrating OsII/III oxidations of eight osmium(II) complexes are presented in this article. The data were obtained under similar experimental conditions, at scan rates with magnitudes ranging from 0.05 V.s−1 to 10.00 V.s−1, in acetonitrile as solvent and tetrabutylammonium hexafluorophosphate as supporting electrolyte. Potentials are reported versus the iron(II) redox couple in ferrocene.
Keywords: Osmium, Bipyridyl, Phenanthroline, Photocatalyst, Dye, Redox prediction
Specifications Table
| Subject | Chemistry |
| Specific subject area | Electrochemistry |
| Type of data | Table, text file, graph, figure |
| How data were acquired | PARTAT 2273, Advanced Electrochemical System |
| Data format | Raw and Analyzed |
| Parameters for data collection | CV measurements were done at 293 K. Synthesized samples were used. Degassed the solvent-electrolyte solution, in this case acetonitrile, in the electrochemical cell with Ar(g) for approximately 10 min. Sample addition to the acetonitrile-electrolyte solution and degassed for approximately 3 min. A blanket of Ar(g) was maintained in the cell for the duration of the electrochemical analysis. |
| Description of data collection | Electrochemical analyses of all the samples were done in an electrochemical cell (2 mL), containing a glassy carbon working electrode, a Pt pseudo reference electrode and a Pt auxiliary electrode. The electrochemical cell was connected to a BAS 100 B/W and electrochemical analyzer, and the obtained data were transferred to Excel for data analysis and diagram preparation. |
| Data source location | Department of Chemistry, University of the Free State: Bloemfontein: South Africa: 29°06′36.1″S 26°11′09.2″E |
| Data accessibility | Primary data available as Supplementary Information |
| Related research article | K.G. von Eschwege, J. Conradie, D. van der Westhuizen, Electrochemistry of Os bipyridyl and phenanthroline complexes, comparison with Ru and Fe, Electroanalysis (2020) in press. https://doi.org/10.1002/elan.202060300 |
Value of the Data
-
•
The electrochemical data illustrate the influence of different polypyridine ligand functional groups on the ease of OsII/III oxidation, at scan rates 0.05 – 10.0 V s−1.
-
•
The data is relevant to research and development of redox indicators, and electro- or photocatalysts in dye-sensitized solar cell or solar liquid fuels manufacturing, etc.
-
•
The range of nine cyclic voltammetry scan rates for each of the eight compounds provide data sets from which best methods and derivatives may be selected in future experiments.
1. Data Description
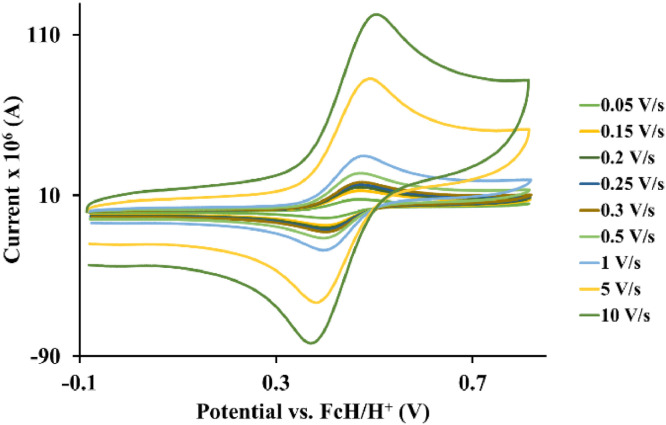
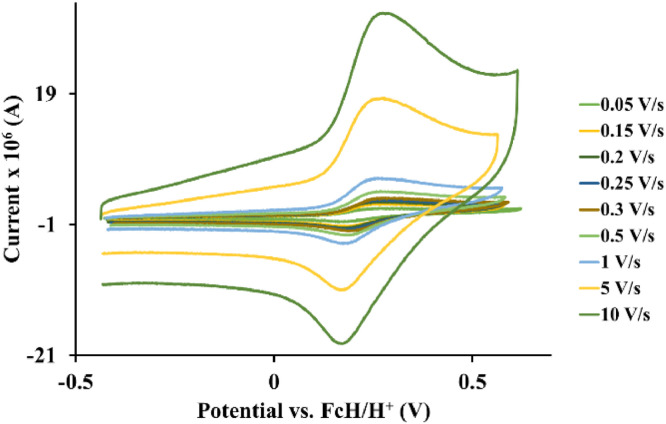
The redox data of eight octahedral OsII complexes are presented in this article. These complexes, 1–8, contain different polypyridine ligands, namely, bipyridine-, substituted bipyridine-, phenanthroline- and substituted phenanthroline ligands, see Fig 1. The data presented in this study are related to the research article [1] where cyclic voltammograms (CVs) and peak voltage data obtained at 0.10 Vs−1 scan rates are reported and correlated with DFT computed descriptors. The redox data obtained for the OsII complexes, containing different polypyridine ligands with different electron donating properties [2], are useful in applications of redox indicators, catalysts and photo-active mediators in dye sensitized solar cells (DSSC) [3,[4], [5]. Electrochemical data obtained from CVs at scan rates 0.05 Vs−1 – 10.00 Vs−1, see Figs. 2–9, are tabulated in Tables 1–8. All related primary data are available in the supplementary data file.
Fig. 3.
Cyclic voltammograms of tris(2,2′-bipyridine)osmium bis(tetrafluoroborate), 2, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Fig. 4.
Cyclic voltammograms of tris(1,10-phenanthroline)osmium bis(tetrafluoroborate), 3, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Fig. 5.
Cyclic voltammograms of tris(4-methyl-1,10-phenanthroline)osmium bis(tetrafluoroborate), 4, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Fig. 6.
Cyclic voltammograms of tris(4,4′-di‑tert‑butyl‑2,2′-bipyridine)osmium bis(tetrafluoroborate), 5, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Fig. 7.
Cyclic voltammograms of tris(4,4′-dimethyl-2,2′-bipyridine)osmium bis(tetrafluoroborate), 6, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Fig. 8.
Cyclic voltammograms of tris(3,4,7,8-tetramethyl −1,10-phenanthroline)osmium bis(tetrafluoroborate), 7, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Table 2.
Tris(2,2′-bipyridine)osmium bis(tetrafluoroborate), 2, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.490 | 1.87 | 0.415 | 1.90 | 0.452 | 0.075 | 0.98 |
| 0.15 | 0.486 | 3.53 | 0.418 | 3.60 | 0.452 | 0.068 | 0.98 |
| 0.20 | 0.485 | 4.16 | 0.419 | 4.25 | 0.452 | 0.066 | 0.98 |
| 0.25 | 0.485 | 4.76 | 0.419 | 4.85 | 0.452 | 0.066 | 0.98 |
| 0.30 | 0.485 | 5.27 | 0.419 | 5.40 | 0.452 | 0.066 | 0.98 |
| 0.50 | 0.486 | 6.55 | 0.419 | 6.71 | 0.452 | 0.067 | 0.98 |
| 1.00 | 0.486 | 9.96 | 0.419 | 10.12 | 0.452 | 0.067 | 0.98 |
| 5.00 | 0.494 | 22.16 | 0.410 | 22.58 | 0.452 | 0.084 | 0.98 |
| 10.0 | 0.501 | 32.89 | 0.403 | 33.65 | 0.452 | 0.098 | 0.98 |
Table 3.
Tris(1,10-phenanthroline)osmium bis(tetrafluoroborate), 3, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.479 | 5.15 | 0.409 | 5.25 | 0.444 | 0.070 | 0.98 |
| 0.15 | 0.478 | 10.34 | 0.411 | 10.59 | 0.444 | 0.067 | 0.98 |
| 0.20 | 0.481 | 12.09 | 0.408 | 12.36 | 0.444 | 0.073 | 0.98 |
| 0.25 | 0.480 | 13.16 | 0.408 | 13.47 | 0.444 | 0.072 | 0.98 |
| 0.30 | 0.481 | 14.41 | 0.408 | 14.71 | 0.444 | 0.073 | 0.98 |
| 0.50 | 0.484 | 19.01 | 0.404 | 19.42 | 0.444 | 0.080 | 0.98 |
| 1.00 | 0.486 | 25.64 | 0.402 | 26.26 | 0.444 | 0.084 | 0.98 |
| 5.00 | 0.499 | 54.95 | 0.389 | 56.26 | 0.444 | 0.110 | 0.98 |
| 10.0 | 0.512 | 80.50 | 0.376 | 81.93 | 0.444 | 0.136 | 0.98 |
Table 4.
Tris(4-methyl-1,10-phenanthroline)osmium bis(tetrafluoroborate), 4, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.391 | 4.13 | 0.322 | 4.23 | 0.356 | 0.069 | 0.98 |
| 0.15 | 0.398 | 4.99 | 0.314 | 5.11 | 0.356 | 0.084 | 0.98 |
| 0.20 | 0.403 | 5.36 | 0.310 | 5.48 | 0.356 | 0.093 | 0.98 |
| 0.25 | 0.401 | 5.99 | 0.312 | 6.12 | 0.356 | 0.089 | 0.98 |
| 0.30 | 0.403 | 6.32 | 0.309 | 6.48 | 0.356 | 0.094 | 0.98 |
| 0.50 | 0.414 | 8.49 | 0.299 | 8.67 | 0.356 | 0.115 | 0.98 |
| 1.00 | 0.421 | 10.33 | 0.290 | 10.58 | 0.356 | 0.131 | 0.98 |
| 5.00 | 0.464 | 19.57 | 0.249 | 19.90 | 0.356 | 0.215 | 0.98 |
| 10.0 | 0.496 | 28.17 | 0.217 | 28.65 | 0.356 | 0.279 | 0.98 |
Table 5.
Tris(4,4′-di‑tert‑butyl‑2,2′-bipyridine)osmium bis(tetrafluoroborate), 5, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.338 | 2.35 | 0.259 | 2.40 | 0.298 | 0.079 | 0.98 |
| 0.15 | 0.331 | 4.55 | 0.266 | 4.63 | 0.298 | 0.065 | 0.98 |
| 0.20 | 0.336 | 5.49 | 0.260 | 5.59 | 0.298 | 0.076 | 0.98 |
| 0.25 | 0.335 | 5.87 | 0.261 | 5.97 | 0.298 | 0.074 | 0.98 |
| 0.30 | 0.332 | 6.34 | 0.264 | 6.46 | 0.298 | 0.068 | 0.98 |
| 0.50 | 0.329 | 8.03 | 0.267 | 8.18 | 0.298 | 0.062 | 0.98 |
| 1.00 | 0.333 | 11.83 | 0.262 | 12.06 | 0.298 | 0.071 | 0.98 |
| 5.00 | 0.343 | 25.41 | 0.254 | 26.06 | 0.298 | 0.089 | 0.98 |
| 10.0 | 0.353 | 32.62 | 0.243 | 33.40 | 0.298 | 0.110 | 0.98 |
Table 6.
Tris(4,4′-methyl-2.2′-bipyridine)osmium bis(tetrafluoroborate), 6, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.335 | 2.21 | 0.237 | 2.27 | 0.286 | 0.098 | 0.98 |
| 0.15 | 0.324 | 4.39 | 0.247 | 4.46 | 0.286 | 0.077 | 0.98 |
| 0.20 | 0.333 | 4.56 | 0.239 | 4.68 | 0.286 | 0.094 | 0.98 |
| 0.25 | 0.335 | 5.32 | 0.237 | 5.45 | 0.286 | 0.098 | 0.98 |
| 0.30 | 0.334 | 5.78 | 0.238 | 5.92 | 0.286 | 0.096 | 0.98 |
| 0.50 | 0.335 | 7.49 | 0.236 | 7.62 | 0.286 | 0.099 | 0.98 |
| 1.00 | 0.339 | 11.23 | 0.232 | 11.50 | 0.286 | 0.107 | 0.98 |
| 5.00 | 0.351 | 20.99 | 0.221 | 21.52 | 0.286 | 0.130 | 0.98 |
| 10.0 | 0.365 | 31.24 | 0.206 | 31.90 | 0.286 | 0.159 | 0.98 |
Table 7.
Tris(3,4,7,8-tetramethyl-1,10-phenanthroline)osmium bis(tetrafluoroborate), 7, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.263 | 0.79 | 0.164 | 0.82 | 0.213 | 0.099 | 0.97 |
| 0.15 | 0.254 | 1.58 | 0.173 | 1.61 | 0.213 | 0.081 | 0.98 |
| 0.20 | 0.260 | 1.76 | 0.166 | 1.80 | 0.213 | 0.094 | 0.97 |
| 0.25 | 0.258 | 1.97 | 0.169 | 2.06 | 0.213 | 0.089 | 0.96 |
| 0.30 | 0.260 | 2.24 | 0.167 | 2.37 | 0.213 | 0.093 | 0.95 |
| 0.50 | 0.254 | 2.69 | 0.173 | 2.82 | 0.213 | 0.081 | 0.95 |
| 1.00 | 0.262 | 3.10 | 0.164 | 3.25 | 0.213 | 0.098 | 0.96 |
| 5.00 | 0.268 | 10.37 | 0.159 | 10.60 | 0.213 | 0.109 | 0.98 |
| 10.0 | 0.265 | 17.39 | 0.162 | 17.99 | 0.213 | 0.103 | 0.97 |
Fig. 1.
Complex numbering and structure of OsII polypyridine complexes.
Fig. 2.
Cyclic voltammograms of tris(5‑chloro-1,10-phenanthroline)osmium bis(tetrafluoroborate), 1, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Fig. 9.
Cyclic voltammograms of tris(4,4′-dimethoxy-2,2′-bipyridine)osmium bis(tetrafluoroborate), 8, at scan rates 0.05 V/s to 10 V/s in the positive direction.
Table 1.
Tris(5‑chloro-1,10-phenanthroline)osmium bis(perchlorate), 1, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.572 | 3.13 | 0.498 | 3.20 | 0.535 | 0.074 | 0.98 |
| 0.15 | 0.573 | 5.99 | 0.497 | 6.13 | 0.535 | 0.076 | 0.98 |
| 0.20 | 0.571 | 6.63 | 0.499 | 6.75 | 0.535 | 0.072 | 0.98 |
| 0.25 | 0.574 | 7.50 | 0.497 | 7.68 | 0.535 | 0.077 | 0.98 |
| 0.30 | 0.577 | 7.99 | 0.494 | 8.17 | 0.535 | 0.083 | 0.98 |
| 0.50 | 0.574 | 11.46 | 0.496 | 11.72 | 0.535 | 0.078 | 0.98 |
| 1.00 | 0.575 | 14.02 | 0.495 | 14.37 | 0.535 | 0.080 | 0.98 |
| 5.00 | 0.585 | 32.69 | 0.486 | 33.46 | 0.535 | 0.099 | 0.98 |
| 10.0 | 0.592 | 45.56 | 0.479 | 46.44 | 0.535 | 0.113 | 0.98 |
Table 8.
Tris(4,4′-dimethoxy-2,2′bipyridine)osmium bis(tetrafluoroborate), 8, electrochemical data (potential in V vs. FcH/FcH+) of the OsII/III redox couple in acetonitrile (CH3CN) for ca 0.005 mol.dm−3 complex solution at the indicated scan rates.
| Scan Rate (V/s) |
Epa (V) |
106 Ipa (A) |
Epc (V) |
106Ipc (A) |
E°’ (V) |
ΔE (V) |
Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| 0.05 | 0.113 | 2.10 | 0.036 | 2.14 | 0.074 | 0.077 | 0.98 |
| 0.15 | 0.114 | 3.70 | 0.035 | 3.77 | 0.074 | 0.079 | 0.98 |
| 0.20 | 0.112 | 4.26 | 0.036 | 4.37 | 0.074 | 0.076 | 0.98 |
| 0.25 | 0.110 | 4.79 | 0.039 | 4.90 | 0.074 | 0.071 | 0.98 |
| 0.30 | 0.110 | 5.43 | 0.039 | 5.54 | 0.074 | 0.071 | 0.98 |
| 0.50 | 0.113 | 6.94 | 0.036 | 7.09 | 0.074 | 0.077 | 0.98 |
| 1.00 | 0.113 | 10.57 | 0.035 | 10.79 | 0.074 | 0.078 | 0.98 |
| 5.00 | 0.121 | 19.98 | 0.027 | 20.32 | 0.074 | 0.094 | 0.98 |
| 10.0 | 0.132 | 28.20 | 0.016 | 28.65 | 0.074 | 0.116 | 0.98 |
2. Experimental Design, Materials and Methods
The experimental setup is the same as described in our previous articles [6,7], i.e. electrochemical studies utilizing cyclic voltammetric measurements were done on an Advanced Electrochemical System with personal computer, utilizing PARSTAT 2273 Powersuite software. Measurements were done at 293 K. Consecutive experiments under similar experimental conditions illustrated that all the formal oxidation potentials could be duplicated within 0.005 V and were not influenced by scan rate. CV data presented in this article are obtained from single CVs at different scan rates as indicated in the tables and figures. Cyclic voltammetric measurements were performed on 0.005 mol dm−3 solutions of the complex, dissolved in CH3CN, containing 0.200 mol dm−3 tetrabutylammonium hexafluorophosphate (TBAPF6, [NBu4][PF6]) as supporting electrolyte. Measurements were conducted under a blanket of purified Argon. A three-electrode cell consisting of a Pt auxiliary electrode, a glassy carbon (surface area 3.14 × 10−6 m2) working electrode and a Pt-wire pseudo reference electrode were used. The working electrode was polished on a Buhler polishing mat; first with 1 µm and lastly with 0.25 µm diamond paste. Scan rates were between 0.05 and 10.00 V.s − 1. All experimental potentials were referenced against the redox couple of ferrocene FcH/FcH+ (IUPAC) [8].
CRediT Author Statement
Deidré van der Westhuizen: Synthesis, Experimental measurements, Writing. Ayyavoo Kannan: Synthesis. Jeanet Conradie: Supervision, Editing. Karel von Eschwege: Conceptualization, Supervision, Editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have or could be perceived to have influenced the work reported in this article.
Acknowledgments
Acknowledgments
This work received support from the South African National Research Foundation (Grant numbers 113327 and 96111) and the Central Research Fund of the University of the Free State, Bloemfontein, South Africa.
Supplementary files
Raw data of all cyclic voltammograms are supplied in Excel files.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2020.106454.
Appendix. Supplementary materials
References
- 1.van der Westhuizen D., von Eschwege K.G., Conradie J. Electrochemistry of Os bipyridyl and phenanthroline complexes, comparison with Ru and Fe. Electroanalysis. 2020 doi: 10.1002/elan.202060300. (in press) [DOI] [Google Scholar]
- 2.Kuhn A., von Eschwege K.G., Conradie J. Reduction potentials of parasubstituted nitrobenzenes – a theoretical approach. J. Phys. Org. Chem. 2012;25:58e68. doi: 10.1002/poc.1868. [DOI] [Google Scholar]
- 3.Boschloo G., Hagfeldt A. Characteristics of the iodide/triiodide redox mediat or in dye-sensitized solar cells. Acc. Chem. Res. 2009;42:1819–1826. doi: 10.1021/ar900138m. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira H., von Eschwege K.G., Conradie J. Electronic properties of Fe charge transfer complexes – a combined experimental and theoretical approach. Electrochim. Acta. 2016;216:339–346. doi: 10.1016/j.electacta.2016.09.034. [DOI] [Google Scholar]
- 5.Grätzel M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005;44:6841–6851. doi: 10.1021/ic0508371. [DOI] [PubMed] [Google Scholar]
- 6.van der Westhuizen D., von Eschwege K.G., Conradie J. Electrochemical data of polypyridine complexes of Ru(II) Data Brief. 2019;27 doi: 10.1016/j.dib.2019.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conradie J., von Eschwege K.G. Cyclic voltammograms and electrochemical data of FeII polypyridine complexes. Data Brief. 2020;31 doi: 10.1016/j.dib.2020.105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gritzner G., Kuta J. Recommendations on reporting electrode potentials in nonaqueous solvents. Pure Appl. Chem. 1984;56:461–466. doi: 10.1016/0013-4686(84)80027-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.