Abstract
There is an increased focus on treatments to extend the healthspan. There is solid evidence that exercise extends the healthspan, but other treatments, such as metformin and statins, are also gaining traction. If metformin and statins will be used to prolong healthspan, we must understand their effects in those free of disease and in combination with exercise.
Keywords: aerobic, mitochondria, pharmaceutical, treatment
Introduction
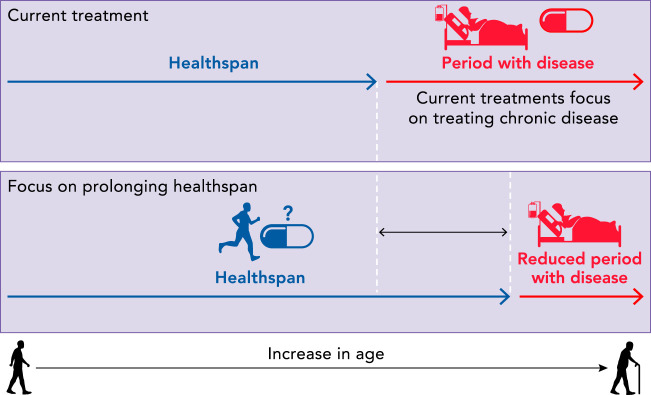
There is an increased appreciation for the goal of increasing healthspan, the period spent free of chronic disease (39). Recently, it was pointed out that with older age 1) chronic diseases become more resistant to treatment and 2) as treatment decreases risk for death from one disease, risk of death from other disease conditions increase (68). The current approach to medicine focuses treatment only after chronic disease has occurred, thus perpetuating competing risks. An alternative strategy is to compress the period of morbidity by preventing the onset of chronic disease. According to one accepted definition of healthspan (39), a treatment to prolong the healthspan begins before chronic disease onset, because healthspan ends once chronic disease starts (FIGURE 1). Although there are some recognized problems with this definition, it has utility in that it frames a period that is spent in a general state of health that is separate from a period spent with accumulating chronic diseases. Strategies to preserve heathspan are therefore preventative in nature.
FIGURE 1.
The period spent free of chronic disease is defined as the healthspan
Once there is a chronic disease, which is often followed by other chronic diseases, the healthspan has ended. Most treatments focus on the period spent with disease, which is in individuals who can have vastly different physiology from those in the healthspan. Therefore, it is crucial to understand the effects of proposed treatments for prolonging the healthspan in healthy individuals and in individuals who may be practicing other health-promoting behaviors.
It is indisputable that exercise is therapeutically beneficial to prevent and treat many chronic diseases (72, 77). Regular exercise prevents the onset of disease, whereas lack of physical activity hastens its development. Aerobic capacity is the maximal capacity of an intact physiological system to deliver and use oxygen during maximal intensity exercise (V̇o2max). Aerobic capacity is likely the most critical health-related outcome measure of healthspan. Low aerobic capacity has been independently linked to increased risk for multiple disease states [cardiovascular disease (CVD), cancer, Type 2 diabetes (T2D), etc.] and increased risk for early mortality (10, 45, 73, 93). In contrast, individuals with high- or moderate-levels of aerobic capacity have significantly lower risk for chronic disease states and much lower rates of early mortality. Importantly, these links between aerobic capacity, health, and mortality have been proven by multiple reports from different laboratories across various populations (races and population demographics) (64).
Despite the clear benefits of exercise, participation in regular exercise is low, with the majority of Americans not meeting Centers for Disease Control and Prevention (CDC) physical activity recommendations. For the 2008 recommendations, the CDC estimated that 24% of people aged 25–64 (39.5 million), and 16.4% (4.8 million) of those aged 65–74 met the physical activity guidelines (CDC statistics). Although there are relatively few side-effects associated with regular physical activity, far more people (~44% of the population) take at least one prescription medicine despite possible side effects. Although physicians may recommend exercise to prevent or treat some chronic diseases, the recommendation is often accompanied by a pharmaceutical prescription. There is also increasing interest in the use of prescription drugs for preventing disease, which could increase use by individuals who do not yet have chronic disease and who are practicing other health-promoting behaviors like exercise. Although the number of regular exercisers are far below what we would hope for the health of the nation, there are still millions of adults who regularly exercise and take a prescribed medication, and this number is expected to increase. In this review, we focus on two highly prescribed drugs, metformin and statins, currently considered for preventative purposes, to highlight what is known, and more importantly not known, about their interactions with exercise.
Metformin and Statins
It is estimated that 150 million people worldwide take metformin. In the U.S., it is the fourth most prescribed medication, with ~78.5 million prescriptions in 2019 (https://clincalc.com/DrugStats/Top300Drugs.aspx). Metformin and lifestyle modifications, including exercise, are the first line therapy for T2D (2). Metformin also has several non-FDA-approved indications, including gestational diabetes, management of antipsychotic-induced weight gain, and both the treatment and prevention of polycystic ovary syndrome (PCOS) (91). There is increasing attention to metformin for treatment of cancer (54), cognitive decline (19), and cardiovascular disease (34). Most of this focus in on treatment of these conditions, but metformin is the only American Diabetes Association (ADA)-recommended antidiabetic for pre-diabetes and is indicated for T2D prevention. This distinction regarding prevention, rather than treatment, is an important one that implies that an individual taking the medication may be free of disease.
Statins are one of the most prescribed drugs in the world, with one specific statin (Atorvastatin) being the second most prescribed medication (>104,000,000) in 2019 (https://clincalc.com/DrugStats/Top300Drugs.aspx). Recent reports indicate that ~40 million U.S. citizens are prescribed statins to lower risk for cardiovascular disease (78). Statins are prescribed as standard of care for individuals who survive a cardiovascular event, for individuals who have T2D, and are increasingly used for primary prevention in individuals with cardiovascular risk factors. In 2013, the American College of Cardiology (ACC) and American Heart Association (AHA) issued a joint statement indicating that more U.S. citizens with elevated cholesterol or other risk factors should be prescribed statins for primary prevention (86, 87). The ACC/AHA statement included an equation of risk factors that was estimated to greatly increase the number of patients taking statins over the coming years. So far, published results suggest only a small shift in statin prescriptions and usage since these guidelines emerged (67). It is, however, still expected that statin use will continue to rise with greater obesity rates and a larger aging population with greater risk for cardiovascular disease. But this continues to highlight a major question in the field: Who should be prescribed statins? There is no doubt that statins are critical for secondary prevention, but their widespread use for primary prevention, especially those with lower than 10 or 20% predicted risk over the next 10 years, remains controversial (1); i.e., the large-scale risk-to-benefit ratios are questioned despite retrospective evidence that lower overall low-density lipoprotein cholesterol (LDL-C) reduced risk for cardiovascular disease (21).
Why Is It Important to Consider Healthspan for Treatment Recommendations?
It is well known that exercise is an ideal preventative medicine (77). Although diet and nutritional supplements are also used as preventative medicine (sometimes with unsubstantiated benefits), the use of prescription medications as preventative medicine is less widespread. However, recent efforts could increase the use of prescription drugs to prolong the healthspan. The Targeting Aging with Metformin (TAME) is a clinical trial that will test whether metformin can delay the onset of age-related diseases and conditions (9). If successful, the FDA will consider biological aging a modifiable condition to be targeted by therapeutic interventions. Moreover, the AHA/ACC statin guidelines also call for an increased utilization of statins to prevent cardiovascular disease. Although a drug to increase healthspan is an exciting prospect, the task of providing evidence for efficacy of such a treatment is daunting. Especially challenging is the prolonged period encompassing the healthspan. A treatment that extends healthspan would start before the onset of chronic disease, which could be a short period in some individuals or decades for others.
Since a treatment that extends healthspan could start when an individual is still healthy, the apparent benefits might not be immediately obvious and may take years to emerge. This issue makes interventions that prolong the healthspan difficult to study in human subjects. A greater concern is that a potential treatment should not do any harm to already healthy individuals—either at the initiation of treatment or in the future. It is a reasonable possibility that the positive or negative results of a pharmaceutical treatment are dictated by the prevailing physiological milieu. It is often that a person interested in a healthy lifestyle will use multiple strategies such as exercise, nutrition, supplements, cognitive exercises, sleep, and other strategies. If a treatment in pill form emerges with the promise of extending the healthspan, it is important to know how that treatment interacts with other bona fide healthspan-extending treatments, such as exercise, because of the high likelihood that these treatments will be used simultaneously.
Metformin and Exercise Interactions
Recently the National Institutes of Health (NIH), more specifically the National Institute on Aging (NIA), issued an RFA seeking proposals on the “Biological Mechanisms of Metformin Effects on Aging and Longevity.” This call for proposals highlights that, even though metformin has been used for nearly 50 years, the mechanisms of action are still unknown. The primary target tissue of metformin is the liver, but metformin also influences metabolic processes in skeletal muscle, adipose tissue, intestine, brain, and cardiovascular tissues (33, 40, 46, 47, 49, 57). For treatment of T2D, it is known that metformin decreases hepatic glucose production by inhibiting gluconeogenesis (46, 57) and increases skeletal muscle glucose uptake (38). It is thought that metformin activates 5′ AMP-activated protein kinase (AMPK) (18, 82), but how metformin activates AMPK is an issue of some debate.
Although it is somewhat clear that metformin impacts mitochondria, the exact mechanisms are unknown (28). There is evidence for direct complex I inhibition (61, 70) or against direct complex I inhibition (29) when used at physiological concentrations. An intriguing hypothesis is that the positive charge of metformin may lead to its accumulation within mitochondria, thus decreasing mitochondrial membrane potential (14, 70). In skeletal muscle specifically, the evidence of the impact of metformin on complex I function is murky with support for (92) and against (40, 51) complex I inhibition. Regarding overall mitochondrial function, 2 weeks of metformin treatment increased mitochondrial function in mice with muscle-specific α2 AMPK kinase dead but not in wild type (48). Permeabilized muscle fibers from obese rats showed a decrease in mitochondrial reactive oxygen species (ROS) emissions when the rats were treated with metformin and when metformin was added ex vivo to the sample chamber, indicating a direct inhibition by metformin (40). Again, most of these studies were done at supraphysiological doses, which leaves many questions of mechanism still unresolved.
At first glance, combining exercise with metformin seems logical for two reasons: 1) the primary target tissues (liver and skeletal muscle) are different but complimentary, and 2) the positive effects of both are thought to be at least partially from the stimulation of AMPK. The first evidence that the combination of metformin and exercise might not be as beneficial as expected came from studies out of the laboratory of Barry Braun. When metformin was provided to subjects who were insulin resistant (but without T2D) before an acute bout of aerobic exercise, the expected increase in insulin sensitivity from the bout of exercise was abolished (81). Subsequent studies showed that metformin blunted the beneficial effects of aerobic exercise training on cardiovascular risk factors in glucose-intolerant individuals (59) and blunted the positive effects of aerobic exercise training on insulin sensitivity in pre-diabetic individuals (58). An important finding of the latter study was that metformin treatment blunted the aerobic exercise-induced improvement in V̇o2max (58). This study extended a previous study from the group that showed that metformin blunts V̇o2max in young, healthy individuals (13).
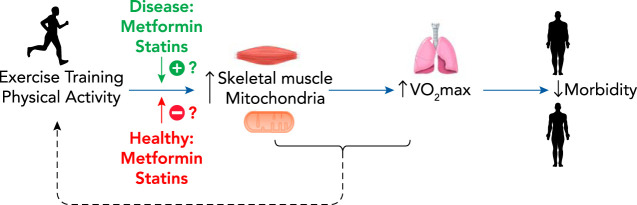
To further address the potential inhibitory effects of metformin on aerobic exercise training, we performed a randomized, double-blind, placebo-controlled study on older healthy human subjects with at least one risk factor for T2D. In this study, subjects completed 12 weeks of aerobic exercise training while taking either a placebo or a clinically relevant dose of metformin (1,500–2,000 mg/day) (47). Our study confirmed previous studies that metformin blunts the expected improvement in V̇o2max from aerobic exercise training (13, 16, 58). Furthermore, we showed that aerobic exercise training with placebo had the expected increase in mitochondrial oxygen consumption with ADP titration in permeabilized muscle fibers, but metformin treatment completely abolished this expected increase in complex I-linked respiration. Related to metabolic outcomes, as expected, there was a significant improvement in whole-body insulin sensitivity in the placebo group, but in the metformin group this improvement was abolished (47). Perhaps most striking in this study was that we observed large variability in responses to metformin treatment (but not in placebo), which included a number of subjects who decreased insulin sensitivity after aerobic exercise training while taking metformin. We then performed follow-up examinations of the data for clues on what subject characteristics may impact the positive or negative response to metformin and exercise. After examining baseline characteristics, we found that subjects who had low fasting insulin, low fasting blood glucose, and high mitochondrial complex I activity had decreases in insulin sensitivity. A likely hypothesis is therefore that metabolic health may dictate the effects of metformin (FIGURE 2).
FIGURE 2.
V̇o2max is one of the best predictors of morbidity and mortality
Regular aerobic exercise improves V̇o2max at least partially through its benefits on mitochondria. The effect of metformin and statins on this mitochondrial adaptation to exercise may be dictated by the health or disease of the individual. There is limited evidence that pharmaceutical treatment, and, conversely, positive exercise adaptations, can change the overall comfort of exercise, thus impacting adherence.
Soon after our study on aerobic exercise training and metformin was published, results from the MASTERS clinical trial were published (90). This study examined the impact of metformin on a second exercise modality, resistance training, in older individuals. Existing literature had suggested that chronic inflammation in skeletal muscle impaired exercise-induced hypertrophy. Thus the authors hypothesized that metformin would blunt the chronic, low-grade inflammation of aging and enhance muscle hypertrophy responsiveness to strength training. Contrary to the hypothesis, the metformin-treated group had a blunted increase in lean body mass and thigh muscle mass as well as a blunted increase in thigh muscle area and density. These results, along with trends toward blunted strength gains, demonstrated that metformin blunted improvements in strength training in older, but healthy, individuals.
The goal of this review is to highlight exercise drug interactions in those free of disease. However, it is also worth noting that we also do not have a clear understanding of how metformin and exercise interact in individuals with T2D. In a rat model of T2D (OLETF rats), combined metformin and aerobic exercise training did not improve multiple markers of non-alcoholic fatty liver disease (NAFLD) and T2D, and blunted exercise-induced increases in hepatic mitochondrial fatty acid oxidation (55). In humans, a previous study examined the acute insulin-sensitizing effect of a bout of exercise in insulin-resistant individuals with or without their normal dose of metformin 24 h before the bout of exercise (69). Although the glucose disappearance rate was higher in metformin plus exercise compared with metformin or exercise alone, the postprandial glucose excursions for the next three meals were not different between groups. The design of this study made it difficult to assess the chronic impact of metformin since there was only a 24-h washout period of metformin. An additional study in T2D subjects compared placebo versus metformin for 28 days before an acute bout of exercise (12). In this study, metformin treatment increased both the heart rate and rating of perceived exertion at a given exercise workload, and did not benefit postprandial glucose regulation compared with exercise alone. Finally, a recent meta-analysis examined data from nine studies with metformin treatment and exercise capacity (23) and concluded that metformin has no impact on exercise adaptations. Unfortunately, the meta-analysis only included two studies with subjects with T2D. Moving forward, it is important to clearly differentiate how health status, free of disease or with T2D, affects the interaction of metformin and exercise.
Statins, Exercise Interactions, Insulin Sensitivity, and Mitochondria
Many patients are advised by their primary care physician to exercise regularly and to take statins to reduce cardiovascular risk. Statins are potent cholesterol-lowering drugs that work through inhibiting an enzyme critical for cholesterol synthesis in the liver [3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase)]. Statins come in different forms (hydrophilic versus lipophilic) resulting in different pharmokinetic and biochemical effects. Hydrophilic statins (pravastatin) primarily target the liver via active transport and have less absorption in other tissues. In contrast, the more commonly used lipophilic statins (simavastatin, atorvastatin, etc.) diffuse into extra-hepatic tissues non-selectively in addition to targeting the liver. We initially started a trial to compare the effects of statin therapy versus statin therapy plus exercise to test the hypothesis that the combined treatment may synergize and have the greatest effect on lowering metabolic syndrome risk factors (63). Individuals who were overweight/obese and had 2/5 metabolic syndrome risk factors plus physical inactivity were randomized to either 12 wk of statin therapy (40 mg/day simvastatin) or 12 wk of statin therapy plus monitored exercise training. Exercise training was moderate-intensity exercise (5 days/wk, 60–75% heart rate reserve, 45 min/day) under direct study staff supervision. Participants were advised to maintain their normal diet and remain weight stable. We also obtained skeletal muscle biopsies and performed V̇o2max measures at baseline and posttreatment. Although statins undoubtedly had a profound effect on lowering LDL-C and triglycerides, we observed that statins also had negative effects. Participants on statin therapy plus exercise did not improve absolute or relative V̇o2max despite 12 wk of exercise training. In contrast, the exercise only group increased V̇o2max by 10%. Because of this impairment, we also probed whether statin therapy prevented exercise-induced increase in mitochondrial content in skeletal muscle (measured by citrate synthase enzyme activity). Again, we found no increase in citrate synthase in the statin therapy plus exercise group compared with a 13% increase in the exercise only group. These results clearly demonstrated that statins impaired classic and important exercise adaptations, adaptations that may be critical for exercise to evoke positive effects on lifespan.
Rodent studies have also revealed that statins can impair exercise adaptations (12, 67), but it is unknown whether the effects of statins on exercise responses, mitochondrial content, and aerobic capacity are uniform or context specific. Cross-sectional studies have revealed that older individuals can be both highly fit and on long-term statin therapy. Kokkinos et al. compared older dyslipidemic men who statin-treated or untreated and stratified by quartiles of fitness (low to high) (47). They reported that the greatest benefit in protection against early mortality occurred in highly fit men taking statins, whereas the most at-risk group was the low-fit men not taking statins. In addition, they found that fitness level significantly impacted risk for early mortality for those taking statins. These results provided additional clues as to why statins may impair exercise adaptations.
The direct effects of statins on insulin sensitivity and glucose homeostasis have been mixed and controversial. Early evidence suggested statins had beneficial effects on insulin sensitivity (71), whereas subsequent studies showed that the lipophilic statins (simvastatin, atorvastatin, etc.) have minimal effect (3, 26) or impair fasting markers of insulin sensitivity (30, 43, 44, 66). In contrast, hydrophilic statins (pravastatin) are not linked with impairments. A recent small investigation cross-sectionally examined statin (simvastatin) users versus non-users matched for age and weight, and found that those on simvastatin had impaired glucose tolerance and reduced insulin sensitivity calculated by an index measure (52). The authors attributed the difference to possible defects in the capacity to synthesize and store lipids appropriately in skeletal muscle (53) in addition to mitochondrial defects (52). The same group performed a larger cross-sectional analysis that included patients who did or did not experienced statin-induced myalgia or a non-statin control group. The study found no impairments in insulin sensitivity by multiple measures. However, the statin “myalgic” group had reduced insulin secretion during the glucose tolerance test (63). Thus there are inconsistent results of statins on insulin sensitivity that are likely due to statin type (lipophilic versus hydrophilic), dose, length of treatment, and baseline levels of metabolic health (healthy, insulin resistant, or existing T2D).
Multiple lines of evidence (trials and meta-analysis) have shown that statins significantly increase the risk for developing T2D, an effect that is worsened by dosage (74). In addition, statins are associated with increased glycosylated hemoglobin (Hba1c) in patients with T2D (25). Although the risk for developing T2D after initiating statin therapy is small at a population level, it is statistically significant. There are disagreements in the field about the risk-to-benefit ratios for using statins that may potentially cause T2D versus lowering CVD risk through lowering LDL-cholesterol (76). Thus more work needs to be done examining long-term use of statins and metabolic health, including understanding mechanisms of action and determining which patients are most at risk for statin-induced impairments. Prospective randomized control trials in individuals who would normally be prescribed statins with clinically relevant statin doses combined with in-depth physiological analysis of insulin sensitivity are needed.
There is some indication that statins have detrimental effects on skeletal muscle mitochondria. Carefully controlled ex vivo studies of rat muscle show that lipophilic statins impaired mitochondrial respiratory capacity, whereas hydrophilic statin (pravastatin) had fewer negative effects (41). High doses of statins (nonphysiological; ~50 μM) in permeabilized human skeletal muscle fibers were shown to dramatically reduce ADP-stimulated respiration (84, 85). However, intramuscular concentrations (<2–5 µM) are much lower on typical oral doses of statins (20–80 mg) (7). A follow-up study using physiologically relevant concentrations (1–5 µM) in primary human skeletal muscle myotubes reported 30% reductions in ADP-stimulated state 3 respiration within 48 h of statin exposure (50). Importantly, these changes occurred without a measured reduction in mitochondrial content or integrity, despite increased markers of apoptosis and atrophy. These data are supported by previous clinical findings that high-dose simvastatin (80 mg/day) treatment in human patients lowered skeletal muscle mitochondrial DNA (mtDNA), a disputed marker of mitochondrial content, by 50% in 8 weeks (79). The decline correlated strongly with reduced CoQ10 levels in muscle. A more recent cross-sectional study in age- and weight-matched participants with or without regular statin (simvastatin) medication also measured mitochondrial respiratory outcomes in permeabilized muscle fibers obtained by skeletal muscle biopsies (52). The authors reported no change in mitochondrial content, but they did find a significant ~18% decrease in ADP-stimulated mitochondrial respiration when complex I- and II-linked substrates were utilized. A follow up cross-sectional analysis of patients on statins showed significant reductions in skeletal muscle respiratory capacity with complex I- and II-linked substrates, but found no evidence of reduced mitochondrial content measured by citrate synthase (24). Finally, it was shown that 2 weeks of statin therapy does not alter mitochondrial respiratory capacity in skeletal muscle (6), which provides evidence that both dose and duration of treatment impact changes in mitochondrial function. In summary, both older studies in rodent muscle and newer studies in human subjects suggest statins can impair mitochondrial function in skeletal muscle (80). Thus more studies are needed to examine whether the inhibitory effects of statins on skeletal muscle mitochondrial adaptations to exercise are due to a specific type of statin, whether dose matters, and whether timing of the intervention is critical. Because millions of patients are prescribed statins and counseled to exercise to mitigate cardiovascular risk, these clinically relevant scientific questions deserve further study.
Potential Impact on Healthspan
Metformin
There is strong supporting evidence that metformin increases lifespan and health outcomes in lower-order model organisms (15, 36). However, studies in rodent models have been more mixed with either increases in lifespan (4, 60) or no change (88). In human clinical trials, retrospective data and trials in populations with T2D show improved overall survival with metformin (8) as well as decreased risk of cardiovascular disease (89a), cancer incidence (31, 94), and cognitive decline (20, 65). A recent meta-analysis of clinical trials in human subjects demonstrated that people with T2D taking metformin have lower all-cause mortality than those not taking metformin and the general population (17). This study also indicated that people with T2D taking metformin had decreased incidence of cancer compared with the general population and decreased cardiovascular disease compared with people with T2D not on metformin (17). Although these human trials build a strong rationale for using metformin to increase the healthspan, the completed studies were in subjects with T2D and/or other co-morbidities, and none were in subjects absent of disease.
If metformin is to be used to extend healthspan, we need to consider how it impacts those who are already healthy. The landmark Diabetes Prevention Program Study assigned nondiabetic persons with elevated fasting and post-load plasma glucose concentrations to placebo, metformin, or lifestyle-modification (42). Over the 3 years of study, lifestyle modification was most effective at preventing progression to T2D, but metformin treatment also had significantly less progression to T2D compared with controls. However, the effect of metformin was minimal in those with a lower body mass index (BMI) and lower fasting glucose. In addition, metformin was not effective in those over 60 yr of age, a group that had nearly twice the variability of the younger groups, with some older individuals ending up with faster transition to T2D. As discussed above, our clinical trial with aerobic exercise training supported that the subjects with the highest insulin sensitivity, or mitochondrial complex I function, were the ones who ended up worse than when they started. Furthermore, a small study of 20 insulin-resistant individuals found that metformin improved indexes of insulin sensitivity in subjects who had T2D or a family history of T2D but worsened insulin sensitivity in obese subjects without T2D or family history of T2D (37). It is worth noting that, when genome-wide association studies (GWAS) have attempted to predict genetic determinants of metformin treatment efficacy, only 20–34% (depending on physiological outcome) of variability is accounted for (27), further indicating that complex physiological traits likely determine metformin treatment efficacy.
Statins
Despite the widespread use of statins for primary prevention, there is still disagreements on risk-to-benefit ratio. The cardiology community has indicated that the increased risk for T2D with statin therapy is nonconsequential due to the far larger cardiovascular disease risk reduction (75). There are undoubtedly great benefits of statins on healthspan in specific populations. For example, statins have been successful in preventing cardiovascular disease in patients with familial hypercholesteremia (genetic condition), resulting in far greater benefits than in the parents of these individuals (56). But there is less clear information on statins extending healthspan in individuals who are disease free but prescribed the drug for elevated LDL cholesterol. There is evidence from both primary prevention trials and from meta-analysis that statins used for primary prevention in relatively low-risk individuals provide benefit for atherosclerotic disease, suggesting that statins can aid in extending healthspan (75). Evidence indicates that statins may be most effective at inducing atherosclerotic plaque stabilization early in the course of the disease while also benefiting vascular stiffness (75). Thus the lines between prevention and management of disease onset often become muddled. There has been a recent focus and relative disagreement among worldwide guidelines on the use of statins for primary prevention in older adults (>65 yr) where risks for T2D and musculoskeletal issues may be greater but prevention of cardiovascular disease are also a concern (62). Other questions remain regarding how statins may influence individual physical activity or exercise behavior, as well as aerobic capacity, both of which are factors that profoundly impact healthspan (11). Multiple reports indicate that patients experience muscle pain and discomfort that is sub-clinical in nature, leading them to discontinue statin therapy. This myopathy may be more widespread in people who regularly exercise. Statin intolerance is increased in those who regularly exercise (22, 83), and it is known that statins magnify the increase in creatine kinase, a marker of muscle injury, postexercise (89). Overall, there is strong evidence that statins can synergize with high aerobic capacity to prolong healthspan in dyslipidemic men, but factors related to what healthspan-inducing aid should be initiated first (regular exercise versus statins) requires further evaluation. As indicated by a commentary by Golubic et al. (32), it is likely that physical activity should be regarded as the first step in disease prevention and the addition of statins should be considered only if it does not interfere with exercise.
What Do We Not Know and Recommendations for Moving Forward
It is widely recognized that we do not yet know the exact mechanisms of action of metformin, although we do understand some downstream consequences. This lack of knowledge about mechanisms was acceptable because metformin had clear benefits for its primary indication of treatment of T2D. However, as the potential indications for metformin expand, it becomes increasingly important to understand its mechanisms to understand when it may or may not be effective as preventative medicine. In terms of statins, the direct benefit on disease is well understood since it lowers circulating cholesterol through inhibition of the hepatic enzyme HMGCoA reductase. However, questions do remain as to how statins impose negative effects on skeletal muscle mitochondrial function. Variations in genes that encode proteins important for hepatocellular uptake or biodegradation may lead to higher circulating levels of statins and thus greater skeletal muscle exposure (5). At the level of the skeletal muscle, statins do inhibit mitochondrial respiration, and mixed findings suggest this too may be due depletion of CoQ10, inhibition of complex 3 (80) of the electron transport chain via statin lactones, and other factors that deserve further study (5).
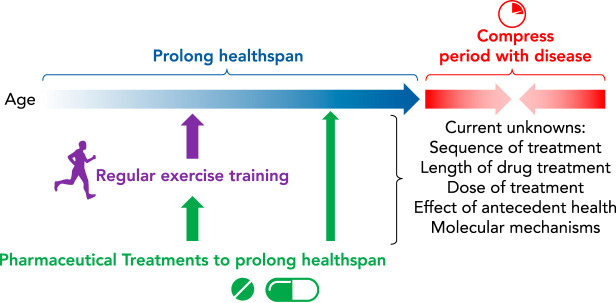
There are many other unanswered questions related to exercise and drug interactions (FIGURE 3). For example, as identified earlier, it is largely unknown how exercise and metformin interact in those who already have T2D. Although many studies have identified the negative impact of metformin on exercise adaptations in healthy individuals, it is unknown how they interact in diseased individuals. This unknown begs the question: Is there something about the metabolic health, aerobic capacity, or skeletal muscle mitochondrial function that impacts the interaction of a medication and exercise, especially a medication that is thought to have mitochondrial effects? In addition, we do not know how the order of initiation impacts outcomes. For example, if someone is already a regular exerciser or is already on metformin or a statin, does initiating the second treatment impact the outcome? Furthermore, does dosing impact the interaction with exercise? In other words, could using metformin or statins at a subclinical dose for preventative purposes lessen the potential negative interactions with exercise?
FIGURE 3.
Current unknowns that need to be understood before widely prescribing metformin and statins to individuals free of disease to prolong healthspan
Answering these questions requires additional studies examining basic mechanisms, as well as clinical trials. In addition, the all too common critique that “we already know that exercise works, so why study it?” has to be reframed and contextualized. We need to clearly understand mechanisms by which both exercise and certain classes of medication evoke positive outcomes if we are to effectively utilize them together to extend healthspan and maximize benefit. For this latter point, the NIH Common Fund Molecular Transducers of Physical Activity Consortium (MoTrPAC) trial designed to study the molecular map that underlies the benefits of physical activity should be particularly useful. Finally, we need to remember the principle of do no harm since, in some cases, there has been unexpected detrimental outcomes with drug and exercise interactions. In conclusion, millions of Americans will be prescribed metformin and/or statins while also being recommended regular daily exercise to extend healthspan. A great deal of work needs to be performed to understand these interactions and the impact on long-term health and disease.
Acknowledgments
B. F. Miller is supported by National Institutes of Health (NIH) Grant R01 AG-064951. J. P. Thyfault is supported by a VA-Merit Grant 1I01BX002567, and NIH Grants R01 DK-121497 and R01 AR-071263.
No conflicts of interest, financial or otherwise, are declared by the author(s).
B.F.M. and J.P.T. conceived and designed research; B.F.M. and J.P.T. prepared figures; B.F.M. and J.P.T. drafted manuscript; B.F.M. and J.P.T. edited and revised manuscript; B.F.M. and J.P.T. approved final version of manuscript.
References
- 1.Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin? BMJ 347, oct22 3: f6123, 2013. doi: 10.1136/bmj.f6123. A correction for this article is available at https://doi.org/10.1136/bmj.g3329. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 41, Suppl 1: S73–S85, 2018. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 3.Ando H, Sugimoto K, Yanagihara H, Tsuruoka S, Saito T, Takamura T, Kaneko S, Fujimura A. Effects of atorvastatin and pravastatin on glucose tolerance, adipokine levels and inflammatory markers in hypercholesterolaemic patients. Clin Exp Pharmacol Physiol 35: 1012–1017, 2008. doi: 10.1111/j.1440-1681.2008.04945.x. [DOI] [PubMed] [Google Scholar]
- 4.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 7: 2769–2773, 2008. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 5.Apostolopoulou M, Corsini A, Roden M. The role of mitochondria in statin-induced myopathy. Eur J Clin Invest 45: 745–754, 2015. doi: 10.1111/eci.12461. [DOI] [PubMed] [Google Scholar]
- 6.Asping M, Stride N, Søgaard D, Dohlmann TL, Helge JW, Dela F, Larsen S. The effects of 2 weeks of statin treatment on mitochondrial respiratory capacity in middle-aged males: the LIFESTAT study. Eur J Clin Pharmacol 73: 679–687, 2017. doi: 10.1007/s00228-017-2224-4. [DOI] [PubMed] [Google Scholar]
- 7.Baer AN, Wortmann RL. Myotoxicity associated with lipid-lowering drugs. Curr Opin Rheumatol 19: 67–73, 2007. doi: 10.1097/BOR.0b013e328010c559. [DOI] [PubMed] [Google Scholar]
- 8.Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 16: 1165–1173, 2014. doi: 10.1111/dom.12354. [DOI] [PubMed] [Google Scholar]
- 9.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab 23: 1060–1065, 2016. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- 11.Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1402, 2017. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulé NG, Robert C, Bell GJ, Johnson ST, Bell RC, Lewanczuk RZ, Gabr RQ, Brocks DR. Metformin and exercise in type 2 diabetes: examining treatment modality interactions. Diabetes Care 34: 1469–1474, 2011. doi: 10.2337/dc10-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun B, Eze P, Stephens BR, Hagobian TA, Sharoff CG, Chipkin SR, Goldstein B. Impact of metformin on peak aerobic capacity. Appl Physiol Nutr Metab 33: 61–67, 2008. doi: 10.1139/H07-144. [DOI] [PubMed] [Google Scholar]
- 14.Bridges HR, Jones AJY, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462: 475–487, 2014. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabreiro F, Au C, Leung K-Y, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene NDE, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153: 228–239, 2013. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadeddu C, Nocco S, Cugusi L, Deidda M, Bina A, Fabio O, Bandinu S, Cossu E, Baroni MG, Mercuro G. Effects of metformin and exercise training, alone or in association, on cardio-pulmonary performance and quality of life in insulin resistance patients. Cardiovasc Diabetol 13: 93, 2014. doi: 10.1186/1475-2840-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev 40: 31–44, 2017. doi: 10.1016/j.arr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Meng S, Chang E, Beckwith-Fickas K, Xiong L, Cole RN, Radovick S, Wondisford FE, He L. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J Biol Chem 289: 20435–20446, 2014. doi: 10.1074/jbc.M114.567271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhari K, Reynolds CD, Yang S-H. Metformin and cognition from the perspectives of sex, age, and disease. Geroscience 42: 97–116, 2020. doi: 10.1007/s11357-019-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng C, Lin C-H, Tsai Y-W, Tsai C-J, Chou P-H, Lan T-H. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci 69: 1299–1305, 2014. doi: 10.1093/gerona/glu073. [DOI] [PubMed] [Google Scholar]
- 21.Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388: 2532–2561, 2016. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 22.Corsini A. Statin-related muscle complaints: an underestimated risk. Cardiovasc Drugs Ther 19: 379–381, 2005. doi: 10.1007/s10557-005-6352-1. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Behera SK, Srinivasan A, Xavier AS, Selvarajan S, Kamalanathan S, Sahoo JP, Nair NS. Effect of metformin on exercise capacity: a meta-analysis. Diabetes Res Clin Pract 144: 270–278, 2018. doi: 10.1016/j.diabres.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Dohlmann TL, Morville T, Kuhlman AB, Chrøis KM, Helge JW, Dela F, Larsen S. Statin treatment decreases mitochondrial respiration but muscle coenzyme Q10 levels are unaltered: The LIFESTAT Study. J Clin Endocrinol Metab 104: 2501–2508, 2019. doi: 10.1210/jc.2018-01185. [DOI] [PubMed] [Google Scholar]
- 25.Erqou S, Lee CC, Adler AI. Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia 57: 2444–2452, 2014. doi: 10.1007/s00125-014-3374-x. [DOI] [PubMed] [Google Scholar]
- 26.Farrer M, Winocour PH, Evans K, Neil HAW, Laker MF, Kesteven P, Alberti KGMM. Simvastatin in non-insulin-dependent diabetes mellitus: effect on serum lipids, lipoproteins and haemostatic measures. Diabetes Res Clin Pract 23: 111–119, 1994. doi: 10.1016/0168-8227(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 27.Florez JC. The pharmacogenetics of metformin. Diabetologia 60: 1648–1655, 2017. doi: 10.1007/s00125-017-4335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol 15: 569–589, 2019. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 29.Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369, 2010. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forst T, Pfützner A, Lübben G, Weber M, Marx N, Karagiannis E, Koehler C, Baurecht W, Hohberg C, Hanefeld M. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk–the PIOSTAT Study. Metabolism 56: 491–496, 2007. doi: 10.1016/j.metabol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 7: 867–885, 2014. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golubic R, Ray KK. Statin and exercise prescription. Lancet 381: 1622, 2013. doi: 10.1016/S0140-6736(13)61017-X. [DOI] [PubMed] [Google Scholar]
- 33.Gormsen LC, Søndergaard E, Christensen NL, Brøsen K, Jessen N, Nielsen S. Metformin increases endogenous glucose production in non-diabetic individuals and individuals with recent-onset type 2 diabetes. Diabetologia 62: 1251–1256, 2019. doi: 10.1007/s00125-019-4872-7. [DOI] [PubMed] [Google Scholar]
- 34.Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 60: 1620–1629, 2017. doi: 10.1007/s00125-017-4337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, Braeckman BP, Schoofs L, Temmerman L. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci USA 111: E2501–E2509, 2014. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iannello S, Camuto M, Cavaleri A, Milazzo P, Pisano MG, Bellomia D, Belfiore F. Effects of short-term metformin treatment on insulin sensitivity of blood glucose and free fatty acids. Diabetes Obes Metab 6: 8–15, 2004. doi: 10.1111/j.1463-1326.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- 38.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med 338: 867–872, 1998. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 39.Kaeberlein M. How healthy is the healthspan concept? Geroscience 40: 361–364, 2018. doi: 10.1007/s11357-018-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kane DA, Anderson EJ, Price JW III, Woodlief TL, Lin C-T, Bikman BT, Cortright RN, Neufer PD. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med 49: 1082–1087, 2010. doi: 10.1016/j.freeradbiomed.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufmann P, Török M, Zahno A, Waldhauser KM, Brecht K, Krähenbühl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci 63: 2415–2425, 2006. doi: 10.1007/s00018-006-6235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koh KK, Quon MJ, Han SH, Lee Y, Ahn JY, Kim SJ, Koh Y, Shin EK. Simvastatin improves flow-mediated dilation but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Diabetes Care 31: 776–782, 2008. doi: 10.2337/dc07-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Park JB, Shin EK. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis 204: 483–490, 2009. doi: 10.1016/j.atherosclerosis.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation 117: 614–622, 2008. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 46.Konopka AR, Esponda RR, Robinson MM, Johnson ML, Carter RE, Schiavon M, Cobelli C, Wondisford FE, Lanza IR, Nair KS. Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes. Cell Rep 15: 1394–1400, 2016. doi: 10.1016/j.celrep.2016.04.024. A correction for this article is available at https://doi.org/10.1016/j.celrep.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA, Biela LM, Bailey SM, Hamilton KL, Miller BF. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 18: e12880, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen JM, Larsen S, Helge JW, Dela F, Wojtaszewski JFP. Two weeks of metformin treatment enhances mitochondrial respiration in skeletal muscle of AMPK kinase dead but not wild type mice. PLoS One 8: e53533, 2013. doi: 10.1371/journal.pone.0053533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP, Barzilai N. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 17: e12723, 2018. doi: 10.1111/acel.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwak H-B, Thalacker-Mercer A, Anderson EJ, Lin C-T, Kane DA, Lee N-S, Cortright RN, Bamman MM, Neufer PD. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med 52: 198–207, 2012. doi: 10.1016/j.freeradbiomed.2011.10.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen S, Rabøl R, Hansen CN, Madsbad S, Helge JW, Dela F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 55: 443–449, 2012. doi: 10.1007/s00125-011-2340-0. [DOI] [PubMed] [Google Scholar]
- 52.Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Bang LE, Bundgaard H, Nielsen LB, Helge JW, Dela F. Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol 61: 44–53, 2013. doi: 10.1016/j.jacc.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 53.Larsen S, Vigelsø A, Dandanell S, Prats C, Dela F, Helge JW. Simvastatin-induced insulin resistance may be linked to decreased lipid uptake and lipid synthesis in human skeletal muscle: the LIFESTAT Study. J Diabetes Res 2018: 9257874, 2018. doi: 10.1155/2018/9257874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M, Li X, Zhang H, Lu Y. Molecular mechanisms of metformin for diabetes and cancer treatment. Front Physiol 9: 1039, 2018. doi: 10.3389/fphys.2018.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linden MA, Fletcher JA, Morris EM, Meers GM, Kearney ML, Crissey JM, Laughlin MH, Booth FW, Sowers JR, Ibdah JA, Thyfault JP, Rector RS. Combining metformin and aerobic exercise training in the treatment of Type 2 diabetes and NAFLD in OLETF rats. Am J Physiol Endocrinol Metab 306: E300–E310, 2014. doi: 10.1152/ajpendo.00427.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20-Year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med 381: 1547–1556, 2019. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 57.Madiraju AK, Qiu Y, Perry RJ, Rahimi Y, Zhang X-M, Zhang D, Camporez JG, Cline GW, Butrico GM, Kemp BE, Casals G, Steinberg GR, Vatner DF, Petersen KF, Shulman GI. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med 24: 1384–1394, 2018. doi: 10.1038/s41591-018-0125-4. A correction for this article is available at https://doi.org/10.1038/s41591-018-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malin SK, Braun B. Effect of metformin on substrate utilization after exercise training in adults with impaired glucose tolerance. Appl Physiol Nutr Metab 38: 427–430, 2013. doi: 10.1139/apnm-2012-0433. [DOI] [PubMed] [Google Scholar]
- 59.Malin SK, Nightingale J, Choi S-E, Chipkin SR, Braun B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity (Silver Spring), 2012. doi: 10.1038/oby.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin M-J, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun 4: 2192, 2013. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller RA, Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest 120: 2267–2270, 2010. doi: 10.1172/JCI43661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol 71: 85–94, 2018. doi: 10.1016/j.jacc.2017.10.080. [DOI] [PubMed] [Google Scholar]
- 63.Morville T, Dohlmann T, Kuhlman AB, Monberg T, Torp M, Hartmann B, Holst JJ, Larsen S, Helge JW, Dela F. Glucose homeostasis in statin users-The LIFESTAT study. Diabetes Metab Res Rev 35: e3110, 2019. doi: 10.1002/dmrr.3110. [DOI] [PubMed] [Google Scholar]
- 64.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 65.Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41: 61–68, 2014. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- 66.Öhrvall M, Lithell H, Johansson J, Vessby B. A comparison between the effects of gemfibrozil and simvastatin on insulin sensitivity in patients with non-insulin-dependent diabetes mellitus and hyperlipoproteinemia. Metabolism 44: 212–217, 1995. doi: 10.1016/0026-0495(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 67.Okerson T, Patel J, DiMario S, Burton T, Seare J, Harrison DJ. Effect of 2013 ACC/AHA blood cholesterol guidelines on statin treatment patterns and low-density lipoprotein cholesterol in atherosclerotic cardiovascular disease patients. J Am Heart Assoc 6: e004909, 2017. doi: 10.1161/JAHA.116.004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olshansky SJ. From lifespan to healthspan. JAMA 320: 1323–1324, 2018. doi: 10.1001/jama.2018.12621. [DOI] [PubMed] [Google Scholar]
- 69.Ortega JF, Hamouti N, Fernández-Elías VE, de Prada MVG, Martínez-Vizcaíno V, Mora-Rodríguez R. Metformin does not attenuate the acute insulin-sensitizing effect of a single bout of exercise in individuals with insulin resistance. Acta Diabetol 51: 749–755, 2014. doi: 10.1007/s00592-014-0580-4. [DOI] [PubMed] [Google Scholar]
- 70.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614, 2000. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paolisso G, Barbagallo M, Petrella G, Ragno E, Barbieri M, Giordano M, Varricchio M. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis 150: 121–127, 2000. doi: 10.1016/S0021-9150(99)00352-4. [DOI] [PubMed] [Google Scholar]
- 72.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25, Suppl 3: 1–72, 2015. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 73.Pimentel AE, Gentile CL, Tanaka H, Seals DR, Gates PE. Greater rate of decline in maximal aerobic capacity with age in endurance-trained than in sedentary men. J Appl Physiol (1985) 94: 2406–2413, 2003. doi: 10.1152/japplphysiol.00774.2002. [DOI] [PubMed] [Google Scholar]
- 74.Preiss D, Seshasai SRK, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJP, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 305: 2556–2564, 2011. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 75.Robinson JG. Starting primary prevention earlier with statins. Am J Cardiol 114: 1437–1442, 2014. doi: 10.1016/j.amjcard.2014.07.076. [DOI] [PubMed] [Google Scholar]
- 76.Rochlani Y, Kattoor AJ, Pothineni NV, Palagiri RDR, Romeo F, Mehta JL. Balancing primary prevention and statin-induced diabetes mellitus prevention. Am J Cardiol 120: 1122–1128, 2017. doi: 10.1016/j.amjcard.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 77.Ruegsegger GN, Booth FW. Health benefits of exercise. Cold Spring Harb Perspect Med 8: a029694, 2018. doi: 10.1101/cshperspect.a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salami JA, Warraich H, Valero-Elizondo J, Spatz ES, Desai NR, Rana JS, Virani SS, Blankstein R, Khera A, Blaha MJ, Blumenthal RS, Lloyd-Jones D, Nasir K. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the Medical Expenditure Panel Survey. JAMA Cardiol 2: 56–65, 2017. doi: 10.1001/jamacardio.2016.4700. [DOI] [PubMed] [Google Scholar]
- 79.Schick BA, Laaksonen R, Frohlich JJ, Päivä H, Lehtimäki T, Humphries KH, Côté HCF. Decreased skeletal muscle mitochondrial DNA in patients treated with high-dose simvastatin. Clin Pharmacol Ther 81: 650–653, 2007. doi: 10.1038/sj.clpt.6100124. [DOI] [PubMed] [Google Scholar]
- 80.Schirris TJJ, Renkema GH, Ritschel T, Voermans NC, Bilos A, van Engelen BGM, Brandt U, Koopman WJH, Beyrath JD, Rodenburg RJ, Willems PHGM, Smeitink JAM, Russel FGM. Statin-induced myopathy is associated with mitochondrial complex III inhibition. Cell Metab 22: 399–407, 2015. doi: 10.1016/j.cmet.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H, Hirshman MF, Goodyear LJ, Braun B. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab 298: E815–E823, 2010. doi: 10.1152/ajpendo.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw RJ, Lamia KA, Vasquez D, Koo S-H, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646, 2005. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 57: 525–528, 2004. doi: 10.1111/j.1365-2125.2003.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sirvent P, Bordenave S, Vermaelen M, Roels B, Vassort G, Mercier J, Raynaud E, Lacampagne A. Simvastatin induces impairment in skeletal muscle while heart is protected. Biochem Biophys Res Commun 338: 1426–1434, 2005. doi: 10.1016/j.bbrc.2005.10.108. [DOI] [PubMed] [Google Scholar]
- 85.Sirvent P, Mercier J, Vassort G, Lacampagne A. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem Biophys Res Commun 329: 1067–1075, 2005. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 86.Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W-K, Smith SC, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation 129, 25 Suppl 2: S1–S45, 2014. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 87.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PWF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63, 25 Pt B: 2889–2934, 2014. doi: 10.1016/j.jacc.2013.11.002. Corrections for this article are available at https://doi.org/10.1016/j.jacc.2014.03.007 and https://doi.org/10.1016/j.jacc.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 88.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15: 872–884, 2016. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson PD, Zmuda JM, Domalik LJ, Zimet RJ, Staggers J, Guyton JR. Lovastatin increases exercise-induced skeletal muscle injury. Metabolism 46: 1206–1210, 1997. doi: 10.1016/S0026-0495(97)90218-3. [DOI] [PubMed] [Google Scholar]
- 89a.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with Type 2 diabetes (UKPDS 34). Lancet 352: 854–865, 1998. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 90.Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G, Windham ST, Ovalle F, Bamman MM, Kern PA, Peterson CA. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell 18: e13039, 2019. doi: 10.1111/acel.13039. A corrigendum for this article is available at https://doi.org/10.1111/acel.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y-W, He S-J, Feng X, Cheng J, Luo Y-T, Tian L, Huang Q. Metformin: a review of its potential indications. Drug Des Devel Ther 11: 2421–2429, 2017. doi: 10.2147/DDDT.S141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wessels B, Ciapaite J, van den Broek NMA, Nicolay K, Prompers JJ. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PLoS One 9: e100525, 2014. doi: 10.1371/journal.pone.0100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Willis BL, Gao A, Leonard D, Defina LF, Berry JD. Midlife fitness and the development of chronic conditions in later life. Arch Intern Med 172: 1333–1340, 2012. doi: 10.1001/archinternmed.2012.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu JW, Boudreau DM, Park Y, Simonds NI, Freedman AN. Commonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literature. Expert Opin Drug Saf 13: 1071–1099, 2014. doi: 10.1517/14740338.2014.926887. [DOI] [PubMed] [Google Scholar]