Abstract
Polyamines regulate a variety of physiological functions and are involved in pathogenesis of diverse human diseases. The epithelium of the mammalian gut mucosa is a rapidly self-renewing tissue in the body, and its homeostasis is preserved through well-controlled mechanisms. Here, we highlight the roles of cellular polyamines in maintaining the integrity of the gut epithelium, focusing on the emerging evidence of polyamines in the regulation of gut epithelial renewal and barrier function. Gut mucosal growth depends on the available supply of polyamines to the dividing cells in the crypts, and polyamines are also essential for normal gut epithelial barrier function. Polyamines modulate expression of various genes encoding growth-associated proteins and intercellular junctions via distinct mechanisms involving RNA-binding proteins and noncoding RNAs. With the rapid advance of polyamine biology, polyamine metabolism and transport are promising therapeutic targets in our efforts to protect the gut epithelium and barrier function in patients with critical illnesses.
Keywords: gut epithelial homeostasis, polyamine biosynthesis, RNA-binding proteins, noncoding RNAs, posttranscriptional regulation
Introduction
The epithelium of mammalian gut mucosa is a rapidly self-renewing tissue in the body and acts as a physical barrier that protects the subepithelial tissues against luminal noxious substances and the microbiome (47). Under physiological conditions, undifferentiated intestinal epithelial cells (IECs) replicate continuously in the proliferating zone within the crypts and differentiate to various mature cell types as they migrate up the villous tips in the small intestine and the luminal surface of the colon to replace exfoliated cells (38, 48, 56). Apoptosis occurs in the crypt area to counterbalance cell division and at the luminal surface of the mucosa, where differentiated cells are lost (47). Differentiated IECs, connected by apical intercellular junctional (IJ) complexes named as tight junctions (TJs) and adherens junctions (AJs), establish a selectively permeable barrier that supports nutrient absorption and prevents intrusion of luminal pathogens and bacteria (61). The gut epithelial renewal and barrier function are tightly regulated by numerous extracellular and intracellular factors at different levels, and their integrity and effectiveness depend on a dynamic balance among IEC proliferation, migration, apoptosis, differentiation, and cell-to-cell interaction (27, 68). Nonetheless, disruption of the gut epithelium homeostasis and barrier function occurs commonly in various human diseases, leading to the translocation of luminal toxic substances and bacteria to the blood stream and, in some instances, resulting in multiple organ dysfunction syndrome and death.
The natural polyamines, including spermidine, spermine, and their precursor putrescine, are ubiquitous organic cations that are found in all eukaryotic cells (53, 59). Although total intracellular polyamine levels are relatively high (in millimolar ranges), free polyamines are considerably less abundant, since they are bound to various cellular anions such as DNA, RNA, proteins, and phospholipids (59). Polyamines are essential for many cell functions and play a crucial role in mammalian development and cell growth (39, 51, 52). Disrupted polyamine metabolism by targeted deletion of the gene encoding ornithine decarboxylase (ODC) or S-adenosylmethionine decarboxylase (SAMDC), two key rate-limiting enzymes in polyamine biosynthesis, is lethal at the early stage of embryonic development (51, 55). Polyamines are absolutely required for normal gut mucosal growth and barrier function (39, 53, 60, 67). Increasing the levels of cellular polyamines, either synthesized endogenously or supplied luminally, stimulates gut mucosal renewal and enhances the barrier function, whereas decreasing polyamines by inhibiting the activity of ODC and SAMDC compromises the gut epithelial integrity and leads to barrier dysfunction (22, 60, 64). Based on emerging evidence that the levels of mucosal tissue polyamines are remarkably altered in patients with colonic cancer (9), inflammatory bowel diseases (IBD) (25), and other mucosal injury-associated disorders (47, 48), the polyamine metabolic pathway has been an attractive target for cancer chemoprevention and for developing new therapeutics for patients with various critical disorders. Since there are already several excellent reviews that focus on polyamine biology and their implication in gastrointestinal physiology in general (9, 44, 53, 60), this review will specifically highlight the roles of polyamines in the regulation of gut epithelial renewal and barrier function, with a particular emphasis on the effects of polyamines on mRNA stability and translation via RNA-binding proteins (RBPs) and noncoding RNAs (ncRNAs).
Polyamine Homeostasis in the Intestinal Epithelium
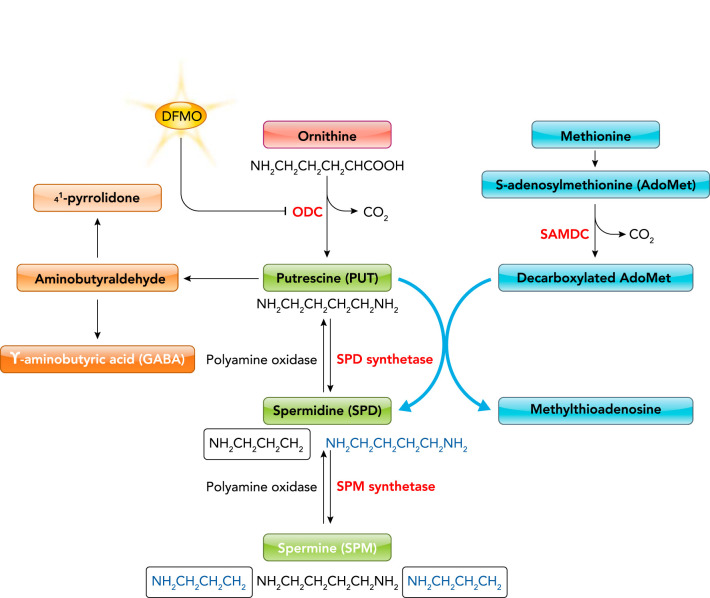
Polyamine homeostasis in the gut epithelium is tightly controlled and depends on endogenous polyamine biosynthesis, degradation, and exogenous polyamine transport (53, 59). Polyamine metabolism consists of both forward and reverse component pathways, although the cellular control of regulatory enzymes and polyamine transporters act in concert with each other to maintain the balance of appropriate levels of individual polyamines. Polyamines are derived from two major amino acids, ornithine and methionine. Ornithine is the specific substrate of ODC for production of putrescine, whereas methionine is the precursor of S-adenosylmethionine (AdoMet). The decarboxylation product of AdoMet is the precursor of the aminopropyl moieties of spermidine and spermine. The de novo synthesis of polyamines begins with the formation of putrescine from ornithine, catalyzed by the enzyme ODC (FIGURE 1). Putrescine is transformed into spermidine by spermidine synthetase via the addition of the first propylamine group derived from AdoMet. Subsequently, spermidine is converted to spermine by spermine synthetase through the addition of the second propylamine group. The interconversion of polyamines within cells is a cyclic process that is initiated from the acetylation of each of the three polyamines and catalyzed by the N-acetyltransferase enzyme with the participation of acetyl coenzyme A. Putrescine, spermidine, and spermine are interconverted frequently in the intestinal epithelium, based on cell physiological needs and in response to changes in environments. On the other hand, the elimination of polyamines from cells is carried out by the oxidative deamination of a primary amino group with the action of diamine oxidase and polyamine oxidase; both catabolic enzymes can act on polyamines and their acetylated derivatives.
FIGURE 1.
Polyamine biosynthesis and catabolism
ODC, ornithine decarboxylase; SAMDC, S-adenosylmethionine decarboxylase; DFMO, α-difluoromethylornithine. Putrescine, spermidine, and spermine carry two, three, and four positive-charge free amine groups, respectively, which reflects the strength of their binding ability in cells.
Besides endogenous biosynthesis, polyamines also have an enriched exogenous origin predominantly from the gut lumen, including intestinal and pancreatic secretions, catabolic productions of IECs, and dietary foods (41). The gut microbiome also produces polyamines and has been described as a major source of luminal polyamines for years, although there is limited information available regarding the capability to form polyamines of the gut microbiome and the corresponding synthetic pathways (51). The uptake of polyamines by IECs is mediated via distinct mechanisms, including transcellular transporters, co-transporters, and passive diffusion (48, 53, 59). The levels of intracellular polyamines are critically controlled by feedback mechanisms, mostly by the polyamines themselves via the regulation of de novo synthesis, catabolism, and uptake. In mammalian cells, the degradation of ODC is facilitated by a specific ODC-antizyme (49, 59). Interestingly, this antizyme protein also appears to downregulate polyamine transport, and its activity is tightly regulated by interaction between polyamines and mTOR signaling (50). DL-α-difluoromethylornithine (DFMO) is an irreversible inactivator of the ODC enzyme, and it has been widely used to deplete cellular polyamines in vivo and in vitro for many years (FIGURE 1). DFMO has provided an enormous stimulus to the field of mammalian polyamine biology and led to clinical application in human diseases such as African sleeping sickness and potential chemoprevention for colonic cancer and other tumors.
Polyamines Are Required for Gut Epithelial Renewal
The epithelium of the human small intestine undergoes ~1011 mitoses/day; this rapid, dynamic turnover rate is critical for maintaining gut epithelial homeostasis (47). Disruption of epithelial renewal causes maladaptation and impairs the gut barrier function, which occurs commonly in critically ill patients supported with total parenteral nutrition (TPN). Gut epithelial renewal is tightly regulated by numerous intracellular and extracellular factors. Consistent with most other tissues in the body, the control of gut epithelial renewal is unique and affected by the same hormones, such as insulin, growth hormone, thyroxine, and cortisol, that alter metabolism in general (47, 62). However, the gut epithelium responds to a host of events triggered by the ingestion and presence of food within the digestive tract. Food directly interacts with the gut epithelium and results in the release of several gut hormones that only modulate the rate of gut mucosal growth. Compared with gut hormones, various factors theorized to regulate the gut epithelial renewal within the lumen are less well defined. An increasing body of evidence indicates that the supply of polyamines to the dividing cells in the crypts is essential for normal gut epithelial renewal and repair of damaged mucosa (63, 64). Fasting in mice and food starvation in critically ill patients supported with TPN inhibit ODC activity, decrease tissue polyamine levels, and cause gut mucosal atrophy (23, 47); by contrast, oral administration of polyamines stimulates gut epithelial regeneration and enhances healing after mucosal injury (25, 62, 64). Polyamines enhance gut epithelial renewal by regulating a complex signaling network that controls expression of various genes involved in proliferation, growth arrest, and apoptosis.
Polyamines Stimulate Gut Epithelial Renewal by Altering Expression of Various Genes
The levels of cellular polyamines increase rapidly in cells stimulated to grow and divide, whereas depleting polyamines by inhibiting ODC activity inhibits gut epithelial renewal in vivo and in vitro (21, 37, 60, 65). Polyamines are required for the stimulation of the mucosal growth through their ability to regulate expression of various genes encoding growth-promoting proteins such as Myc, c-Fos, and c-Jun (43, 65, 66), and growth-inhibiting factors including p53, nucleophosmin (NPM), JunD, TGF-β, TGF-β-receptor, and Smads via distinct mechanisms (34, 46, 67, 84, 86). Elevation of polyamine levels activates expression of the growth-promoting genes primarily through activation of their gene transcription (2, 43, 65), but it inhibits the growth-inhibiting gene expression at the posttranscription level (28, 34, 84). In a stress-induced gut mucosal injury model in rats (24, 66), induction in the epithelial regeneration after mucosal injury is accompanied by an increase in the levels of both tissue polyamines and expression of Myc, c-fos, and c-jun genes, whereas polyamine depletion by inhibiting ODC activity with DFMO decreases expression of these proto-oncogenes, inhibits DNA synthesis, and delays mucosal healing. Consistently, in cultured IECs, increased polyamines by ectopically overexpressing ODC also induce the expression levels of these growth-promoting proteins primarily by increasing their gene transcription, enhance G1 to S phase transition during cell cycle, and finally increase cell division (31).
To define the role of negative growth control, including growth arrest and apoptosis, in this process, several studies have revealed that inhibition of gut epithelial renewal following polyamine depletion is not due to a simple decrease in expression of these growth-promoting genes, because polyamine-deficient IECs continuously maintain high basal levels of Myc and c-Jun (31, 43). In other words, inhibition of gut epithelial renewal following polyamine depletion is also an active process that results from the activation of growth-inhibiting gene expression. In fact, expression levels of p53, NPM, JunD, TGF-β, TGF-β-receptor, and Smads increase remarkably following polyamine depletion in the intestinal epithelium (34, 46, 67, 83, 84). Increased levels of cellular polyamines by ectopic ODC overexpression in cultured IECs inhibit the expression of these growth-inhibiting genes, contributing to the stimulation of IEC proliferation, whereas polyamine depletion increases the levels of these inhibitory factors, leading to growth arrest. Unlike the effect on growth-promoting genes, polyamines modulate expression of these growth-inhibiting genes at the posttranscription level. Polyamine depletion increases the stability and translation of mRNAs encoding these growth-inhibiting proteins, but it has no effect on these gene transcriptions (28, 84, 86, 87). In addition, polyamines also regulate the activity of Akt kinase, NF-κB, activating transcription factor-2 (ATF-2), and XIAP, contributing to the control of IEC apoptosis under biological conditions (67, 71, 81).
Polyamines Regulate the Stability and Translation of mRNAs Through RBPs
In responses to stressful environments, increasing the levels of cellular polyamines potently affects posttranscriptional events (67, 81), besides regulating gene transcription (43, 66). Posttranscriptional processes, such as mRNA transport, turnover, and translation, involve specific mRNA sequences (cis-elements) that interact with trans-acting factors, including RBPs and micro-RNA (miRNAs) (17, 73). U- or AU-rich elements (AREs) are the best-characterized cis-acting sequences located in the 3′-untranslated regions (3′ UTRs) of many labile mRNAs (10, 68). Although AREs often function as decay elements, they also regulate translation and mRNA export. Several RBPs, including AU-binding factor 1 (AUF1), CUG-binding protein 1 (CUGBP1), BRF1, TTP, and KSRP, have been shown to promote ARE-mRNA decay through the recruitment of the ARE-bearing mRNA to sites of mRNA degradation, such as the exosome, the proteasome, or processing bodies (P-bodies) (10, 73). RBPs that stabilize target mRNAs and stimulate translation include the Hu/ELAV proteins, which comprise a family of three primary neuronal members (HuB, HuC, and HuD) and one ubiquitous member HuR (16, 19).
HuR plays an essential role in the posttranscriptional control of mRNAs bearing AREs and is implicated in many aspects of cellular function (16, 17, 36, 73). HuR is predominantly nuclear in unstimulated cells, but it rapidly translocates to the cytoplasm in response to stress, where HuR stabilizes specific mRNAs and affects the translation of several target transcripts (36, 54). HuR is a biological posttranscriptional enhancer in gut epithelium homeostasis, since intestinal epithelial tissue-specific deletion of HuR in mice inhibits small intestinal epithelial renewal (35) and delays mucosal repair after acute injury (68, 78, 88). Polyamine depletion enhances the cytoplasmic accumulation of HuR, although it fails to alter the levels of total cellular HuR (84). Conversely, increased cellular polyamines inhibit HuR cytoplasmic translocation but promote the nuclear accumulation of HuR. Further study has shown that polyamines modulate subcellular distribution of HuR through AMP-activated protein kinase 1 (AMPK1)-regulated phosphorylation and acetylation of importin α1 (85). Increased levels of cellular polyamines stimulate AMPK activity, in turn triggering HuR nuclear import through the activation of both phosphorylation and acetylation of importin α1, whereas polyamine depletion increases the cytoplasmic levels of HuR by inactivating the AMPK-mediated dual modifications of importin α1 (FIGURE 2). A series of studies from our group (70, 71, 84, 86) and others (5, 8) has demonstrated that HuR directly interacts with mRNAs encoding p53, NPM, JunD, ATF-2, XIAP, and MEK-1 via their 3′ UTRs and/or coding regions (CR) and that these HuR/mRNA associations increase dramatically in the cytoplasm of IECs following polyamine depletion, along with a significant increase in the stability and translation of these target mRNAs. HuR silencing abolishes the increase in stability and translation of these mRNAs in polyamine-deficient cells, indicating the importance of increased cytoplasmic HuR in stimulating expression of these growth-inhibiting genes following polyamine depletion (FIGURE 2).
FIGURE 2.

Polyamine depletion inhibits gut epithelial renewal by increasing the expression of growth-inhibiting genes via HuR
Polyamines are essential for the nuclear accumulation of HuR through activation of AMP-activated protein kinase 1 (AMPK1) and acetylation (Ac) of importin α1. Polyamine depletion increases cytoplasmic HuR levels by repressing AMPK1-dependent HuR phosphorylation (P). Cytoplasmic HuR in polyamine-deficient cells directly interacts with and increases the stability and translation of mRNAs encoding growth-inhibiting proteins, leading to an inhibition of gut epithelial renewal.
Induced polyamines in IECs also enhance the degradation of JunD mRNA by altering the competitive binding of its 3′ UTR to HuR and another RBP AUF1 (destabilizer). JunD mRNA is a target of both HuR and AUF1, and polyamine depletion increases HuR association with JunD transcript but decreases the levels of JunD mRNA bound to AUF1, thus stabilizing JunD mRNA (28, 86). HuR silencing enhances AUF1 binding to the JunD mRNA, along with a decrease in the abundance of HuR/JunD mRNA association, renders the JunD mRNA unstable, and prevents the increased stability of JunD mRNA in polyamine-deficient cells. In contrast, increasing the cellular levels of polyamines by ODC overexpression inhibits JunD mRNA association with HuR and increases its interaction with AUF1, leading to an inhibition of JunD expression (86). These results show that polyamines regulate JunD expression via control of HuR/AUF1 interaction and provide new insight into the molecular function of cellular polyamines.
HuR is constitutively expressed in the intestinal epithelium, and its binding motif is widely distributed among numerous ARE- and non-ARE-containing mRNAs (20), suggesting that the ability of HuR to bind a given mRNA and influence its posttranscriptional fate is tightly regulated at the posttranslational level. In support of this possibility, it has been shown that the checkpoint kinase 2 (Chk2) phosphorylates HuR and thereby alters the affinity of HuR for given target transcripts after exposure to oxidative stress and other pathologies (1, 78). We have demonstrated that elevating the levels of cellular polyamines enhances HuR association with Myc mRNA through Chk2-dependent HuR phosphorylation, promotes Myc translation, and contributes to an elevation in Myc steady-state levels and polyamine-induced IEC proliferation (33). In contrast, polyamine depletion decreases HuR/Myc mRNA complexes by repressing HuR phosphorylation, in turn inhibiting Myc translation and cell division.
Polyamines also promote the translation of cyclin-dependent kinase 4 (CDK4) by the action of CUGBP1 and miRNA-222 (miR-222) in the intestinal epithelium (72, 73). Both CUGBP1 and miR-222 directly bind to the Cdk4 mRNA via both CR and 3′ UTR and repress CDK4 translation synergistically. Depletion of cellular polyamines by inhibiting ODC with DFMO increases cytoplasmic CUGBP1 abundance and miR-222 levels, induces their associations with the Cdk4 mRNA, and inhibits CDK4 translation, whereas increasing the levels of cellular polyamines by ODC overexpression decreases Cdk4 mRNA interaction with CUGBP1 and miR-222, in turn inducing CDK4 expression. Polyamine-deficient cells exhibit an increased co-localization of tagged Cdk4 mRNA with P-bodies where mRNAs are degraded; this co-localization is abolished by silencing CUGBP1 and miR-222. Taken together, these observations reveal that polyamines stimulate gut epithelial renewal by modulating expression of various growth-associated genes through different mechanisms and that HuR (FIGURE 2), AUF1, and CUGBP1 play an important role in the posttranscriptional control of growth-inhibiting gene expression by polyamines.
Polyamines Enhance Gut Epithelial Barrier Function
IECs line the mucosa and form a protective barrier to a wide array of noxious substances in the lumen. The epithelial barrier is the specialized structures composing different IJs, including TJs and AJs, that surround the subapical region of epithelial cells (57, 61). The TJ is the apical-most element of the junctional complex and seals epithelial cells together in a way that prevents even small molecules from leaking between cells. Immediately below the TJs are the cadherin-rich AJs that provide strong cell-to-cell adhesion and play functional roles in forming and regulating the barrier function. The AJ E-cadherin integrates cellular signals and is crucial for the control of gut permeability. The assembly of TJ and AJ complexes is highly dynamic and essential for barrier function, and their constituent proteins turnover at a rapid pace. Moreover, IECs also react to noxious stimuli by secreting different antimicrobial peptides and proteins. Paneth cells are specialized IECs residing at the bottom of the crypts that produce high quantities of defensins and other antibiotic proteins when exposed to pathogenic bacteria and bacterial products. Autophagy in IECs sequesters cytoplasmic structures and pathogens targeted for degradation and is also involved in gut epithelial defense and barrier function. Polyamines regulate gut epithelial barrier function by altering IJ expression and epithelial defense via distinct mechanisms involved in RBPs and ncRNAs.
Polyamines Are Essential for Expression of TJs and AJs
Numerous studies have revealed that cellular polyamines are essential for expression of TJs and AJs and are implicated in control of the epithelial barrier function under various pathophysiological conditions (18, 42, 45, 78). The first evidence showing the role of polyamines in maintaining the gut epithelial barrier integrity is from our observations that polyamines are required for expression of AJ E-cadherin (18). Depletion of cellular polyamines by DFMO decreases abundance of cellular E-cadherin and increases paracellular permeability in vitro, which is prevented by exogenous polyamine spermidine given together with DFMO. Polyamines stimulate E-cadherin expression at least partially by altering intracellular free Ca2+ concentration ([Ca2+]cyt), since polyamine depletion reduces [Ca2+]cyt, leading to the degradation of E-cadherin protein, whereas elevation of [Ca2+]cyt by the Ca2+ ionophore ionomycin increases E-cadherin stability in polyamine-deficient cells. Polyamines also enhance E-cadherin gene transcription by activating Myc that directly interacts with the E-Pal box located at the E-cadherin-promoter (32). On the other hand, polyamines stimulate translation of TJ occludin without effect on its mRNA and protein stability (78). The decreased level of occludin translation in polyamine-deficient cells is not due to the reduction of [Ca2+]cyt, because either increased or decreased [Ca2+]cyt does not affect the rate of newly occludin protein synthesis in the presence or absence of polyamines. Polyamines also regulate ZO-1 expression by modulating its gene transcription via JunD, and decreasing cellular polyamines represses ZO-1 transcription through CREB-binding site within the proximal region of the ZO-1-promoter (7, 28). Other TJs such as ZO-2, claudin-2, and claudin-3 are also regulated by polyamines, but their mechanisms remain to be elucidated.
Polyamines regulate occludin translation through Chk2-dependent HuR phosphorylation in the gut epithelium (78, 79). Occludin is a transmembrane TJ protein that plays an important role in TJ assembly and control of the epithelial barrier function (58). The occludin mRNA is a target of HuR, and interaction of HuR with occludin mRNA depends on Chk2-dependent HuR phosphorylation (78). Reduced HuR phosphorylation by silencing Chk2 decreases HuR binding to the occludin mRNA and represses occludin translation without effect on its mRNA stability. Polyamine depletion by inhibiting ODC with DFMO decreases Chk2 levels, reduces HuR phosphorylation, and inhibits occludin translation in IECs. Conversely, increasing the levels of cellular polyamines by ODC overexpression induces Chk2 kinase activity, enhances HuR phosphorylation, and increases HuR binding affinity for occludin mRNA, thereby promoting occludin translation and the barrier function. In mice exposed to septic stress induced by cecal ligation and puncture (CLP), decreasing the Chk2 levels in the intestinal mucosa by treatment with DFMO prevents the restoration of HuR/occludin mRNA association after CLP, thus inhibiting occludin expression and delaying recovery of the gut barrier function. Since CUGBP1 inhibits occludin translation through blocking HuR association with occludin mRNA (79) and polyamine depletion also increases cytoplasmic CUGBP1 levels (72), polyamines might also regulate occludin translation by altering competitive binding of HuR and CUGBP1 to occludin mRNA.
Implication of Long ncRNAs in Polyamine-Regulated IJ Expression
Long ncRNAs (lncRNAs) are defined as transcripts spanning >200 nucleotides in length, capable of regulating a variety of cellular processes (3). Several lncRNAs, including SPRY4-IT1, H19, uc.173, and Gata6, are highly expressed in the gut epithelium and are shown to regulate the barrier function through interaction with RBPs and miRNAs (29, 68, 77). SPRY4-IT1 was the first lncRNA found to regulate gut permeability by altering the TJ expression at the posttranscriptional level (74). Decreasing the levels of cellular SPRY4-IT1 inhibits expression of TJs claudin-1, claudin-3, occludin, and JAM-1, and causes the epithelial barrier dysfunction, whereas increasing the levels of SPRY4-IT1 in the intestinal mucosa protects the gut barrier function in mice exposed to CLP. SPRY4-IT1 interacts with and increases the stability and translation of these TJ mRNAs via HuR, since HuR silencing reduces the SPRY4-IT1 association with the TJ mRNAs without effect on whole-cell SPRY4-IT1 levels. Given the fact that polyamines affect HuR phosphorylation and its binding affinity (33, 78), it is likely that polyamines also regulate TJ barrier function by altering SPRY4-IT1 association with the TJ mRNAs via control of HuR phosphorylation (FIGURE 3).
FIGURE 3.

Polyamines regulate gut barrier function by altering HuR interaction with lncRNAs SPRY4-IT1 and H19
Polyamines increase SPRY4-IT1 association with TJ mRNAs but prevent miR-675 processing from H19 via regulation of HuR binding affinity, thus enhancing IJ expression and promoting gut epithelial barrier function.
Unlike SPRY4-IT1, lncRNA H19 inhibits expression of ZO-1 and E-cadherin, and disrupts the gut epithelial barrier function, whereas targeted deletion of H19 in mice enhances the gut barrier function (80). The levels of H19 increase dramatically in the inflamed human intestinal mucosa from patients with IBD and sepsis, along with increased gut permeability (11, 80). H19 reduces production of ZO-1 and E-cadherin by decreasing stability and translation of the ZO-1 and E-cadherin mRNAs through miR-675-5p and miR-675-3p that are embedded in H19 exon 1. HuR directly interacts with H19 and prevents miR-675 processing from H19, thus rescuing the expression of ZO-1 and E-cadherin, and promoting the barrier dysfunction in the epithelium overexpressing H19 (88). In contrast, intestinal epithelial tissue-specific deletion of HuR or inhibition of HuR phosphorylation by polyamine depletion induces miR-675 production and delays the recovery of the epithelial barrier function in mice after exposure to CLP or mesenteric ischemia/reperfusion. Interestingly, the human gut mucosal tissues from patients with IBD and sepsis exhibit decreased levels of HuR and increased content of H19, associated with the gut barrier dysfunction (30, 75, 80). These findings suggest that increasing the association of HuR with H19 by polyamines also contributes to the regulation of the epithelial barrier function by inhibiting miR-675 production from H19 (FIGURE 3). Recently, it has been reported that lncRNA uc.173 and Gata6 are also intimately involved in the gut epithelial homeostasis and barrier function (69, 75, 77), but their roles in polyamine-modulated IJ expression remain to be investigated.
Polyamines in the Gut Epithelial Defense
Several studies show that polyamines protect the gut epithelium against various pathological stresses through antioxidant activity, epithelial defense, and autophagy (14, 26, 82). Polyamines suppress gut mucosal inflammation by inhibiting inflammatory cytokine synthesis in macrophages (4, 40). Infection with H. pylori (a major pathological factor of gut mucosal injury and inflammation) disrupts gut mucosal immune host defense by altering polyamine metabolism and nitric oxide production (6, 12, 15). Dysregulation of polyamine biosynthesis by depletion of AdoMet or deleting the gene encoding spermine oxidase leads to immunosuppression in the gut epithelium and alters the protection against dextran sulfate sodium-induced colitis (13, 53). Moreover, spermidine was found to induce autophagy via hypusination of EIF5A, thus improving the function of B cells in aging mice (40). Polyamines might also regulate autophagy by altering expression of the autophagy-related gene 16L1 (ATG16L1) via HuR, since target deletion of HuR in IECs specifically decreases the levels of ATG16L1 in the intestinal mucosa (30). We have recently demonstrated that HuR plays an essential role for Paneth cell function by altering membrane localization of TLR2 via posttranscriptional control of the chaperone canopy3 (76). Since HuR phosphorylation depends on Chk2 kinase that requires polyamines for its activation (33, 78), polyamines can regulate autophagy and Paneth cell function by modulating HuR binding affinity via Chk2.
Conclusions and Future Perspectives
Polyamines have a vast spectrum of cell functions in the gut epithelium and are involved in many pathologies. The experimental results summarized here provide strong evidence that polyamines are essential for normal gut epithelial renewal and barrier function through the activation of multiple signaling pathways, especially altering the stability and translation of mRNAs encoding growth-associated proteins and IJs via RBPs and ncRNAs. Polyamines regulate gut epithelium homeostasis and barrier integrity through transcriptional and posttranscriptional control of expression of various genes involved in IEC proliferation, migration, apoptosis, and cell-to-cell interactions. Polyamines upregulate expression of growth-promoting genes primarily by increasing gene transcription but downregulate growth-inhibiting genes through destabilization of mRNAs. Several studies aimed at investigating the mechanism underlying posttranscriptional regulation by polyamines indicate that polyamines modulate the stability and translation of various mRNAs via alterations in HuR subcellular distribution, binding affinity, and its interaction with lncRNAs. In addition, the protective effect on gut barrier and enhancement of epithelial host defense described for polyamines can play an important role in the prevention of acute systemic gut barrier dysfunction in patients with critical illnesses.
However, there are still many gaps in our understanding of polyamine functions in the intestinal epithelium. The molecular processes controlling the gut mucosal tissue levels of polyamines in response to stressful environments remain largely unknown. The development of tissue-specific genetic mouse models should provide crucial information on the polyamine homeostasis in the intestinal epithelium under various pathological conditions. Moreover, it is unclear whether the mutations in RBP functional motifs affect their ability to interact with polyamines and/or mRNAs and thus influence polyamine-dependent gut epithelium homeostasis. Future experiments must also define the mechanism by which IECs uptake polyamine from luminal origin of the intestine. Finally, studies using human mucosal samples from patients with disrupted epithelial renewal/adaptation and gut barrier dysfunction should be essential to establish the impact of polyamines and their regulatory RBPs and ncRNAs on disease pathogenesis and to devise therapeutic venues.
Acknowledgments
This work was supported by Merit Review Awards (to J-Y.W; J.N.R) from U.S. Department of Veterans Affairs; National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-57819, DK-61972, and DK-68491 (to J-Y.W). J-Y.W is a Senior Research Career Scientist, Biomedical Laboratory Research & Development Service, U.S. Department of Veterans Affairs.
No conflicts of interest, financial or otherwise, are declared by the author(s).
J.N.R. and J.-Y.W. conceived and designed research; J.N.R., L.X., and J.-Y.W. interpreted results of experiments; J.N.R. drafted manuscript; J.N.R., L.X., and J.-Y.W. edited and revised manuscript; L.X. analyzed data; L.X. prepared figures; J.-Y.W. approved final version of manuscript.
References
- 1.Akaike Y, Masuda K, Kuwano Y, Nishida K, Kajita K, Kurokawa K, Satake Y, Shoda K, Imoto I, Rokutan K. HuR regulates alternative splicing of the TRA2β gene in human colon cancer cells under oxidative stress. Mol Cell Biol 34: 2857–2873, 2014. doi: 10.1128/MCB.00333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann AS, Geerts D. Polyamine synthesis as a target of MYC oncogenes. J Biol Chem 293: 18757–18769, 2018. doi: 10.1074/jbc.TM118.003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 152: 1298–1307, 2013. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekebrede AF, Keijer J, Gerrits WJJ, Boer VCJ. The molecular and physiological effects of protein-derived polyamines in the intestine. Nutrients 12: 197, 2020. doi: 10.3390/nu12010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergalet J, Fawal M, Lopez C, Desjobert C, Lamant L, Delsol G, Morello D, Espinos E. HuR-mediated control of C/EBPbeta mRNA stability and translation in ALK-positive anaplastic large cell lymphomas. Mol Cancer Res 9: 485–496, 2011. doi: 10.1158/1541-7786.MCR-10-0351. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP, de Sablet T, Piazuelo MB, Sarvaria AR, Cheng Y, Closs EI, Casero RA Jr, Gobert AP, Wilson KT. Polyamines impair Immunity to helicobacter pylori by inhibiting L-arginine uptake required for nitric oxide production. Gastroenterology 139: 1686–1698.e6, 2010. doi: 10.1053/j.gastro.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Xiao L, Rao JN, Zou T, Liu L, Bellavance E, Gorospe M, Wang JY. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell 19: 3701–3712, 2008. doi: 10.1091/mbc.e08-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degese MS, Tanos T, Naipauer J, Gingerich T, Chiappe D, Echeverria P, LaMarre J, Gutkind JS, Coso OA. An interplay between the p38 MAPK pathway and AUBPs regulates c-fos mRNA stability during mitogenic stimulation. Biochem J 467: 77–90, 2015. doi: 10.1042/BJ20141100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao JH, Guo LJ, Huang ZY, Rao JN, Tang CW. Roles of cellular polyamines in mucosal healing in the gastrointestinal tract. J Physiol Pharmacol 64: 681–693, 2013. [PubMed] [Google Scholar]
- 10.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol 8: 113–126, 2007. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 11.Geng H, Bu HF, Liu F, Wu L, Pfeifer K, Chou PM, Wang X, Sun J, Lu L, Pandey A, Bartolomei MS, De Plaen IG, Wang P, Yu J, Qian J, Tan XD. In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology 155: 144–155, 2018. doi: 10.1053/j.gastro.2018.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobert AP, Wilson KT. The immune battle against helicobacter pylori infection: NO offense. Trends Microbiol 24: 366–376, 2016. doi: 10.1016/j.tim.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gobert AP, Al-Greene NT, Singh K, Coburn LA, Sierra JC, Verriere TG, Luis PB, Schneider C, Asim M, Allaman MM, Barry DP, Cleveland JL, Destefano Shields CE, Casero RA Jr, Washington MK, Piazuelo MB, Wilson KT. Distinct immunomodulatory effects of spermine oxidase in colitis induced by epithelial injury or infection. Front Immunol 9: 1242, 2018. doi: 10.3389/fimmu.2018.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gobert AP, Latour YL, Asim M, Finley JL, Verriere TG, Barry DP, Milne GL, Luis PB, Schneider C, Rivera ES, Lindsey-Rose K, Schey KL, Delgado AG, Sierra JC, Piazuelo MB, Wilson KT. Bacterial pathogens hijack the innate immune response by activation of the reverse transsulfuration pathway. MBio 10: e02174-e19, 2019. doi: 10.1128/mBio.02174-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gogoi M, Datey A, Wilson KT, Chakravortty D. Dual role of arginine metabolism in establishing pathogenesis. Curr Opin Microbiol 29: 43–48, 2016. doi: 10.1016/j.mib.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorospe M. HuR in the mammalian genotoxic response: post-transcriptional multitasking. Cell Cycle 2: 412–414, 2003. doi: 10.4161/cc.2.5.491. [DOI] [PubMed] [Google Scholar]
- 17.Gorospe M, Tominaga K, Wu X, Fähling M, Ivan M. Post-transcriptional control of the hypoxic response by RNA-binding proteins and microRNAs. Front Mol Neurosci 4: 7, 2011. doi: 10.3389/fnmol.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Rao JN, Liu L, Zou TT, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol 285: C1174–C1187, 2003. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci 65: 3168–3181, 2008. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hitti E, Bakheet T, Al-Souhibani N, Moghrabi W, Al-Yahya S, Al-Ghamdi M, Al-Saif M, Shoukri MM, Lánczky A, Grépin R, Győrffy B, Pagès G, Khabar KS. Systematic analysis of AU-rich element expression in cancer reveals common functional clusters regulated by key RNA-binding proteins. Cancer Res 76: 4068–4080, 2016. doi: 10.1158/0008-5472.CAN-15-3110. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi K, Kashiwagi K. The functional role of polyamines in eukaryotic cells. Int J Biochem Cell Biol 107: 104–115, 2019. doi: 10.1016/j.biocel.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Jainu M, Vijaimohan K, Kannan K. Cissus quadrangularis L. extract attenuates chronic ulcer by possible involvement of polyamines and proliferating cell nuclear antigen. Pharmacogn Mag 6: 225–233, 2010. doi: 10.4103/0973-1296.66941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson LR, McCormack SA. Healing of gastrointestinal mucosa: involvement of polyamines. News Physiol Sci 14: 12–17, 1999. doi: 10.1152/physiologyonline.1999.14.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Konturek PC, Brzozowski T, Konturek SJ, Szlachcic A, Hahn EG. Polyamines and epidermal growth factor in the recovery of gastric mucosa from stress-induced gastric lesions. J Clin Gastroenterol 27, Suppl 1: S97–S104, 1998. doi: 10.1097/00004836-199800001-00016. [DOI] [PubMed] [Google Scholar]
- 25.Lan A, Blachier F, Benamouzig R, Beaumont M, Barrat C, Coelho D, Lancha A Jr, Kong X, Yin Y, Marie JC, Tomé D. Mucosal healing in inflammatory bowel diseases: is there a place for nutritional supplementation? Inflamm Bowel Dis 21: 198–207, 2015. doi: 10.1097/MIB.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 26.Latour YL, Gobert AP, Wilson KT. The role of polyamines in the regulation of macrophage polarization and function. Amino Acids 52: 151–160, 2020. doi: 10.1007/s00726-019-02719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8: 1327–1336, 2006. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Liu L, Rao JN, Esmaili A, Strauch ED, Bass BL, Wang JY. JunD stabilization results in inhibition of normal intestinal epithelial cell growth through P21 after polyamine depletion. Gastroenterology 123: 764–779, 2002. doi: 10.1053/gast.2002.35386. [DOI] [PubMed] [Google Scholar]
- 29.Li XX, Rao JN, Wang JY. Posttranscriptional regulation of gut epithelium homeostasis by RNA-binding proteins and long noncoding RNAs. In: Encyclopedia of Gastroenterology(2nd ed.), edited by Kuipers EJ. Cambridge, MA: Acedemic Press, 2020, p. 247–256. [Google Scholar]
- 30.Li XX, Xiao L, Chung HK, Ma XX, Liu X, Song JL, Jin CZ, Rao JN, Gorospe M, Wang JY. Interaction between HuR and circPABPN1 modulates autophagy in the intestinal epithelium by altering ATG16L1 translation. Mol Cell Biol 40: e00492-e19, 2020. doi: 10.1128/MCB.00492-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Guo X, Rao JN, Zou T, Marasa BS, Chen J, Greenspon J, Casero RA Jr, Wang JY. Polyamine-modulated c-Myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region. Biochem J 398: 257–267, 2006. doi: 10.1042/BJ20060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Guo X, Rao JN, Zou T, Xiao L, Yu T, Timmons JA, Turner DJ, Wang JY. Polyamines regulate E-cadherin transcription through c-Myc modulating intestinal epithelial barrier function. Am J Physiol Cell Physiol 296: C801–C810, 2009. doi: 10.1152/ajpcell.00620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Rao JN, Zou T, Xiao L, Wang PY, Turner DJ, Gorospe M, Wang JY. Polyamines regulate c-Myc translation through Chk2-dependent HuR phosphorylation. Mol Biol Cell 20: 4885–4898, 2009. doi: 10.1091/mbc.e09-07-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Santora R, Rao JN, Guo X, Zou T, Zhang HM, Turner DJ, Wang JY. Activation of TGF-β-Smad signaling pathway following polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 285: G1056–G1067, 2003. doi: 10.1152/ajpgi.00151.2003. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Christodoulou-Vafeiadou E, Rao JN, Zou T, Xiao L, Chung HK, Yang H, Gorospe M, Kontoyiannis D, Wang JY. RNA-binding protein HuR promotes growth of small intestinal mucosa by activating the Wnt signaling pathway. Mol Biol Cell 25: 3308–3318, 2014. doi: 10.1091/mbc.e14-03-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.López de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA 101: 2987–2992, 2004. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mäkitie LT, Kanerva K, Andersson LC. Ornithine decarboxylase regulates the activity and localization of rhoA via polyamination. Exp Cell Res 315: 1008–1014, 2009. doi: 10.1016/j.yexcr.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 38.McCole DF, Barrett KE. Varied role of the gut epithelium in mucosal homeostasis. Curr Opin Gastroenterol 23: 647–654, 2007. doi: 10.1097/MOG.0b013e3282f0153b. [DOI] [PubMed] [Google Scholar]
- 39.McCormack SA, Viar MJ, Johnson LR. Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am J Physiol 264: G367–G374, 1993. doi: 10.1152/ajpgi.1993.264.2.G367. [DOI] [PubMed] [Google Scholar]
- 40.Metur SP, Klionsky DJ. The curious case of polyamines: spermidine drives reversal of B cell senescence. Autophagy 16: 389–390, 2020. doi: 10.1080/15548627.2019.1698210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñoz-Esparza NC, Latorre-Moratalla ML, Comas-Basté O, Toro-Funes N, Veciana-Nogués MT, Vidal-Carou MC. Polyamines in food. Front Nutr 6: 108, 2019. doi: 10.3389/fnut.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlando A, Linsalata M, Bianco G, Notarnicola M, D’Attoma B, Scavo MP, Tafaro A, Russo F. Lactobacillus rhamnosus GG protects the epithelial barrier of Wistar rats from the pepsin-trypsin-digested gliadin (PTG)-induced enteropathy. Nutrients 10: 1698, 2018. doi: 10.3390/nu10111698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel AR, Wang JY. Polyamines modulate transcription but not posttranscription of c-myc and c-jun in IEC-6 cells. Am J Physiol 273: C1020–C1029, 1997. doi: 10.1152/ajpcell.1997.273.3.C1020. [DOI] [PubMed] [Google Scholar]
- 44.Pegg AE. Introduction to the Thematic Minireview Series: Sixty plus years of polyamine research. J Biol Chem 293: 18681–18692, 2018. doi: 10.1074/jbc.TM118.006291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penrose HM, Marchelletta RR, Krishnan M, McCole DF. Spermidine stimulates T cell protein-tyrosine phosphatase-mediated protection of intestinal epithelial barrier function. J Biol Chem 288: 32651–32662, 2013. doi: 10.1074/jbc.M113.475962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao JN, Li L, Bass BL, Wang JY. Expression of the TGF-β receptor gene and sensitivity to growth inhibition following polyamine depletion. Am J Physiol Cell Physiol 279: C1034–C1044, 2000. doi: 10.1152/ajpcell.2000.279.4.C1034. [DOI] [PubMed] [Google Scholar]
- 47.Rao JN, Wang JY. Regulation of Gastrointestinal Mucosal Growth (2nd ed.), edited by Granger ND, Granger J. Williston, VT: Morgan and Claypool Publishers, 2016, p. 1–135. [PubMed] [Google Scholar]
- 48.Ray RM, Johnson LR. Regulation of intestinal mucosal growth by amino acids. Amino Acids 46: 565–573, 2014. doi: 10.1007/s00726-013-1565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ray RM, Bhattacharya S, Bavaria MN, Viar MJ, Johnson LR. Antizyme (AZ) regulates intestinal cell growth independent of polyamines. Amino Acids 46: 2231–2239, 2014. doi: 10.1007/s00726-014-1777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray RM, Bavaria M, Johnson LR. Interaction of polyamines and mTOR signaling in the synthesis of antizyme (AZ). Cell Signal 27: 1850–1859, 2015. doi: 10.1016/j.cellsig.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sánchez-Jiménez F, Medina MÁ, Villalobos-Rueda L, Urdiales JL. Polyamines in mammalian pathophysiology. Cell Mol Life Sci 76: 3987–4008, 2019. doi: 10.1007/s00018-019-03196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seiler N, Raul F. Polyamines and apoptosis. J Cell Mol Med 9: 623–642, 2005. doi: 10.1111/j.1582-4934.2005.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seiler N, Raul F. Polyamines and the intestinal tract. Crit Rev Clin Lab Sci 44: 365–411, 2007. doi: 10.1080/10408360701250016. [DOI] [PubMed] [Google Scholar]
- 54.Seko Y, Azmi H, Fariss R, Ragheb JA. Selective cytoplasmic translocation of HuR and site-specific binding to the interleukin-2 mRNA are not sufficient for CD28-mediated stabilization of the mRNA. J Biol Chem 279: 33359–33367, 2004. doi: 10.1074/jbc.M312306200. [DOI] [PubMed] [Google Scholar]
- 55.Shantz LM, Holm I, Jänne OA, Pegg AE. Regulation of S-adenosylmethionine decarboxylase activity by alterations in the intracellular polyamine content. Biochem J 288: 511–518, 1992. doi: 10.1042/bj2880511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol 47: 217–229, 1985. doi: 10.1146/annurev.ph.47.030185.001245. [DOI] [PubMed] [Google Scholar]
- 57.Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest 120: 1824–1835, 2010. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J 91: e13357, 2020. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tabor CW, Tabor H. Polyamines. Annu Rev Biochem 53: 749–790, 1984. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 60.Timmons J, Chang ET, Wang JY Rao JN. Polyamines and gut mucosal homeostasis. J Gastrointest Digest Sys 2, Suppl 7: 001, 2012. [PMC free article] [PubMed] [Google Scholar]
- 61.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Li GR, Tan BE, Xiong X, Kong XF, Xiao DF, Xu LW, Wu MM, Huang B, Kim SW, Yin YL. Oral administration of putrescine and proline during the suckling period improves epithelial restitution after early weaning in piglets. J Anim Sci 93: 1679–1688, 2015. doi: 10.2527/jas.2014-8230. [DOI] [PubMed] [Google Scholar]
- 63.Wang JY, Johnson LR. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 100: 333–343, 1991. doi: 10.1016/0016-5085(91)90200-5. [DOI] [PubMed] [Google Scholar]
- 64.Wang JY, McCormack SA, Viar MJ, Johnson LR. Stimulation of proximal small intestinal mucosal growth by luminal polyamines. Am J Physiol 261: G504–G511, 1991. doi: 10.1152/ajpgi.1991.261.3.G504. [DOI] [PubMed] [Google Scholar]
- 65.Wang JY, McCormack SA, Viar MJ, Wang H, Tzen CY, Scott RE, Johnson LR. Decreased expression of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am J Physiol 265: G331–G338, 1993. doi: 10.1152/ajpgi.1993.265.2.G331. [DOI] [PubMed] [Google Scholar]
- 66.Wang JY, Johnson LR. Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am J Physiol 266: G878–G886, 1994. doi: 10.1152/ajpgi.1994.266.5.G878. [DOI] [PubMed] [Google Scholar]
- 67.Wang JY. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 33: 241–252, 2007. doi: 10.1007/s00726-007-0518-z. [DOI] [PubMed] [Google Scholar]
- 68.Wang JY, Xiao L, Wang JY. Posttranscriptional regulation of intestinal epithelial integrity by noncoding RNAs. Wiley Interdiscip Rev RNA 8: e1399, 2017. doi: 10.1002/wrna.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang JY, Cui YH, Xiao L, Chung HK, Zhang Y, Rao JN, Gorospe M, Wang JY. Regulation of intestinal epithelial barrier function by long nRNA uc.173 through interaction with microRNA 29b. Mol Cell Biol 38: e00010–e00018, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang PY, Rao JN, Zou T, Liu L, Xiao L, Yu TX, Turner DJ, Gorospe M, Wang JY. Post-transcriptional regulation of MEK-1 by polyamines through the RNA-binding protein HuR modulating intestinal epithelial apoptosis. Biochem J 426: 293–306, 2010. doi: 10.1042/BJ20091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao L, Rao JN, Zou T, Liu L, Marasa BS, Chen J, Turner DJ, Zhou H, Gorospe M, Wang JY. Polyamines regulate the stability of activating transcription factor-2 mRNA through RNA-binding protein HuR in intestinal epithelial cells. Mol Biol Cell 18: 4579–4590, 2007. doi: 10.1091/mbc.e07-07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao L, Cui YH, Rao JN, Zou T, Liu L, Smith A, Turner DJ, Gorospe M, Wang JY. Regulation of cyclin-dependent kinase 4 translation through CUG-binding protein 1 and microRNA-222 by polyamines. Mol Biol Cell 22: 3055–3069, 2011. doi: 10.1091/mbc.e11-01-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao L, Wang JY. RNA-binding proteins and microRNAs in gastrointestinal epithelial homeostasis and diseases. Curr Opin Pharmacol 19: 46–53, 2014. doi: 10.1016/j.coph.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao L, Rao JN, Cao S, Liu L, Chung HK, Zhang Y, Zhang J, Liu Y, Gorospe M, Wang JY. Long noncoding RNA SPRY4-IT1 regulates intestinal epithelial barrier function by modulating the expression levels of tight junction proteins. Mol Biol Cell 27: 617–626, 2016. doi: 10.1091/mbc.E15-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao L, Wu J, Wang JY, Chung HK, Kalakonda S, Rao JN, Gorospe M, Wang JY. Long noncoding RNA uc.173 promotes renewal of the intestinal mucosa by inducing degradation of microRNA 195. Gastroenterology 154: 599–611, 2018. doi: 10.1053/j.gastro.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao L, Li XX, Chung HK, Kalakonda S, Cai JZ, Cao S, Chen N, Liu Y, Rao JN, Wang HY, Gorospe M, Wang JY. RNA-binding protein HuR regulates Paneth cell function by altering membrane localization of TLR2 via post-transcriptional control of CNPY3. Gastroenterology 157: 731–743, 2019. doi: 10.1053/j.gastro.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao L, Gorospe M, Wang JY. Long noncoding RNAs in intestinal epithelium homeostasis. Am J Physiol Cell Physiol 317: C93–C100, 2019. doi: 10.1152/ajpcell.00092.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu TX, Wang PY, Rao JN, Zou T, Liu L, Xiao L, Gorospe M, Wang JY. Chk2-dependent HuR phosphorylation regulates occludin mRNA translation and epithelial barrier function. Nucleic Acids Res 39: 8472–8487, 2011. doi: 10.1093/nar/gkr567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu TX, Rao JN, Zou T, Liu L, Xiao L, Ouyang M, Cao S, Gorospe M, Wang JY. Competitive binding of CUGBP1 and HuR to occludin mRNA controls its translation and modulates epithelial barrier function. Mol Biol Cell 24: 85–99, 2013. doi: 10.1091/mbc.e12-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu TX, Chung HK, Xiao L, Piao JJ, Lan S, Jaladanki SK, Turner DJ, Raufman JP, Gorospe M, Wang JY. Long noncoding RNA H19 impairs the intestinal barrier by suppressing autophagy and lowering Paneth and Goblet cell function. Cell Mol Gastroenterol Hepatol 9: 611–625, 2020. doi: 10.1016/j.jcmgh.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang X, Zou T, Rao JN, Liu L, Xiao L, Wang PY, Cui YH, Gorospe M, Wang JY. Stabilization of XIAP mRNA through the RNA binding protein HuR regulated by cellular polyamines. Nucleic Acids Res 37: 7623–7637, 2009. doi: 10.1093/nar/gkp755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Simon AK. Polyamines reverse immune senescence via the translational control of autophagy. Autophagy 16: 181–182, 2020. doi: 10.1080/15548627.2019.1687967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zou T, Rao JN, Liu L, Marasa BS, Keledjian KM, Zhang AH, Xiao L, Bass BL, Wang JY. Polyamine depletion induces nucleophosmin modulating stability and transcriptional activity of p53 in intestinal epithelial cells. Am J Physiol Cell Physiol 289: C686–C696, 2005. doi: 10.1152/ajpcell.00085.2005. [DOI] [PubMed] [Google Scholar]
- 84.Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang AH, Xiao L, Pullmann R, Gorospe M, Wang JY. Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem 281: 19387–19394, 2006. doi: 10.1074/jbc.M602344200. [DOI] [PubMed] [Google Scholar]
- 85.Zou T, Liu L, Rao JN, Marasa BS, Chen J, Xiao L, Zhou H, Gorospe M, Wang JY. Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin α1. Biochem J 409: 389–398, 2008. doi: 10.1042/BJ20070860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zou T, Rao JN, Liu L, Xiao L, Yu TX, Jiang P, Gorospe M, Wang JY. Polyamines regulate the stability of JunD mRNA by modulating the competitive binding of its 3′ untranslated region to HuR and AUF1. Mol Cell Biol 30: 5021–5032, 2010. doi: 10.1128/MCB.00807-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou T, Rao JN, Liu L, Xiao L, Cui YH, Jiang Z, Ouyang M, Donahue JM, Wang JY. Polyamines inhibit the assembly of stress granules in normal intestinal epithelial cells regulating apoptosis. Am J Physiol Cell Physiol 303: C102–C111, 2012. doi: 10.1152/ajpcell.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou T, Jaladanki SK, Liu L, Xiao L, Chung HK, Wang JY, Xu Y, Gorospe M, Wang JY. H19 long noncoding RNA regulates intestinal epithelial barrier function via microRNA 675 by interacting with RNA-binding protein HuR. Mol Cell Biol 36: 1332–1341, 2016. doi: 10.1128/MCB.01030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]