Abstract
In cultured fetal liver cells, insulin-like growth factor (IGF) binding protein (IGFBP)-1 hyperphosphorylation in response to hypoxia and amino acid deprivation is mediated by inhibition of mechanistic target of rapamycin (mTOR) and activation of amino acid response (AAR) signaling and casein kinase (CK)2. We hypothesized that fetal liver mTOR inhibition, activation of AAR and CK2, and IGFBP-1 hyperphosphorylation occur before development of intrauterine growth restriction (IUGR). Pregnant baboons were fed a control (C) or a maternal nutrient restriction (MNR; 70% calories of control) diet starting at gestational day (GD) 30 (term GD 185). Umbilical blood and fetal liver tissue were obtained at GD 120 (C, n = 7; MNR, n = 10) and 165 (C, n = 7; MNR, n = 8). Fetal weights were unchanged at GD 120 but decreased at GD 165 in the MNR group (−13%, P = 0.03). IGFBP-1 phosphorylation, as determined by parallel reaction monitoring mass spectrometry (PRM-MS), immunohistochemistry, and/or Western blot, was enhanced in MNR fetal liver and umbilical plasma at GD 120 and 165. IGF-I receptor autophosphorylationTyr1135 (−64%, P = 0.05) was reduced in MNR fetal liver at GD 120. Furthermore, fetal liver CK2 (α/α′/β) expression, CK2β colocalization, proximity with IGFBP-1, and CK2 autophosphorylationTyr182 were greater at GD 120 and 165 in MNR vs. C. Additionally, mTOR complex (mTORC)1 (p-P70S6KThr389, −52%, P = 0.05) and mTORC2 (p-AktSer473, −56%, P < 0.001) activity were decreased and AAR was activated (p-GCN2Thr898, +117%, P = 0.02; p-eIF2αSer51, +294%, P = 0.002; p-ERKThr202, +111%, P = 0.03) in MNR liver at GD 120. Our data suggest that fetal liver IGFBP-1 hyperphosphorylation, mediated by mTOR inhibition and both AAR and CK2 activation, is a key link between restricted nutrient and oxygen availability and the development of IUGR.
Keywords: casein kinase II, fetal insulin-like growth factor I, primates, TOR serine-threonine kinases
INTRODUCTION
Intrauterine growth restriction (IUGR) is associated with increased perinatal morbidity and mortality and greater risk for adult-onset diabetes, hypertension, and cardiovascular disease (10, 46). Although IUGR may be the result of multiple in utero challenges, decreased fetal oxygen and nutrient availability as a result of placental insufficiency is a common denominator in the development of restricted fetal growth. The molecular mechanisms linking limited oxygen and nutrient supply to the slowing of fetal growth remain to be fully established but likely involve decreased fetal plasma concentrations of insulin, an important growth-stimulating hormone in utero (26, 27).
In addition to insulin, the fetal and maternal insulin-like growth factor (IGF) systems are among the most important regulators of fetal growth and development (18). IGF-I is a positive regulator of fetal growth (56); fetal circulating concentrations of IGF-I are positively correlated with birth weight (60), and concentrations of cord plasma IGF-I are decreased in IUGR (45). IGF-I bioavailability is determined by IGF binding proteins (IGFBPs), in particular IGFBP-1, which sequesters IGF-I, thereby inhibiting IGF-I action (21). The fetal liver is the major source of synthesis of fetal circulating IGFBP-1 (37). The IGFBP-1 concentration in fetal plasma (85) and expression in fetal liver are reported to be higher in IUGR (82).
Phosphorylation of IGFBP-1 markedly increases its affinity for binding IGF-I (40). Our studies have shown that IGFBP-1 is hyperphosphorylated in both maternal and fetal plasma in human IUGR pregnancies (1, 35). Furthermore, we have established a direct link between hypoxia and/or leucine deprivation and the degree of IGFBP-1 phosphorylation at discrete serine sites in vitro (77). Changes in the secretion and phosphorylation of fetal liver IGFBP-1 due to reduced nutrient availability are therefore likely to have a major impact on IGF-I bioavailability in fetal circulation, resulting in altered fetal growth.
Mechanistic target of rapamycin (mTOR) regulates cell growth in response to the availability of oxygen and nutrients such as amino acids, glucose, and growth factors. mTOR forms two distinct signaling complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 regulates cellular metabolism, mitochondrial function, and protein translation mediated by phosphorylation of downstream targets, in particular eukaryotic translation initiation factor 4E-binding protein (4E-BP1) and P70S6 kinase (P70S6K). mTORC1 activates P70S6K by direct phosphorylation at Thr389, which promotes the initiation of translation through many different mechanisms. mTORC1 is particularly sensitive to the concentrations of specific amino acids, and lack of leucine is known to inhibit mTORC1 activity. On the other hand, mTORC2 regulates lipid and glucose metabolism (73) and phosphorylates Akt at Ser473 (38), which results in promotion of cell survival.
In contrast, the amino acid response (AAR) pathway is activated by limitation or imbalance of essential amino acids (43, 83). In these situations, there is an increase in uncharged tRNA species, which bind to general control nonderepressible 2 (GCN2) kinase. As a result, the eukaryotic translation initiation factor (eIF2α) is phosphorylated, leading to inhibition of global translation but increased translation of activating transcription factor (ATF)4. ATF4 upregulates the expression of a small group of genes involved in transport, metabolism, and oxidative stress in a tissue-specific manner (43). Inhibition of mTOR and activation of AAR signaling are linked to increased IGFBP-1 secretion and phosphorylation in IUGR (34).
Casein kinase CK2 is one of the key kinases phosphorylating IGFBP-1 (1, 5, 29, 50, 80). CK2 is a pleiotropic and constitutively active kinase that can phosphorylate secreted proteins (65). CK2 is composed of two catalytic α-subunits (α and/or α′) and two regulatory β-subunits in an α2β2 configuration, forming stable heterotetramers (47). The catalytic α/α′ subunits of CK2 undergo intermolecular (trans) tyrosine-autophosphorylation, which depends on the catalytic activity of the kinase (24). Because there is a direct correlation between autophosphorylation of CK2 at Tyr182 and subsequent increase in CK2 catalytic activity, Tyr182 phosphorylation of CK2 is considered a functional readout of CK2 activation (24). IGFBP-1 has consensus sequences consistent with the substrate recognition motif of CK2 (50), supporting that CK2 can phosphorylate IGFBP-1 (1, 5, 29, 50, 80). We have reported that that CK2 and IGFBP-1 are colocalized and IGFBP-1 is hyperphosphorylated in human decidua from IUGR pregnancies (35).
Concentrations of circulating amino acids (15–17), glucose, and oxygen (25, 57) are often decreased in utero in human IUGR fetuses. We have also shown decreased amino acid concentrations in baboon IUGR due to maternal nutrient restriction (MNR) (42, 53). The adverse effects of poor maternal nutrition on fetal development (54) have been highlighted in a recent review (28). Low amino acid concentrations inhibit mTOR, which also results in IGFBP-1 hyperphosphorylation in the fetal liver (23, 34, 36, 51), and can contribute to restricted fetal growth (68).
Using cultured HepG2 cells and baboon fetal primary hepatocytes, we have proposed a model that inhibition of mTOR and activation of AAR and CK2 in the fetal liver constitutes a key mechanistic link between decreased oxygen and nutrient availability, increased IGFBP-1 concentration and phosphorylation, and decreased IGF-I bioavailability in IUGR (1, 23, 51). Because our model of regulation of IGFBP-1 hyperphosphorylation in response to amino acid deprivation cannot be tested in pregnant women, we used a well-established MNR model of IUGR in pregnant baboons. MNR (70% global intake of Control diet) in baboons results in an ∼10–15% decrease in fetal weight by gestational day (GD) 165 (term GD 185) (22, 74) but not at GD 120. We hypothesized that inhibition of mTOR, activation of AAR and CK2, hyperphosphorylation of IGFBP-1, and decreased IGF-I bioactivity in the fetal liver occur before the development of IUGR. We determined placental and fetal weight, IGFBP-1 expression/phosphorylation, IGF-I receptor (IGF-IR) phosphorylation, mTOR and AAR signaling, CK2 expression/activity in fetal liver, and IGFBP-1 concentration/phosphorylation in umbilical plasma at GD 120 and studied some of these outcomes also at GD 165.
MATERIALS AND METHODS
Animals
Baboons (Papio cynocephalus hamadryas, P. cynocephalus anubis, and P. hamadryas anubis) from the Southwest National Primate Research Center were studied. All animal procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee (no. 1134 PC) and conducted in compliance with the principles and policies outlined by Grundy (32). Housing and environmental enrichment have been described in detail previously (75). Pregnancy was confirmed by ultrasound at GD 30. In this model of 30% global MNR, mothers were fed 70% of the amount of food (Purina Monkey Diet 5038) eaten by the ad libitum Control females (n = 14). Cesarean section was performed at GD 120 (0.65 of gestation, term = 185 days) and at GD 165 (0.9 of gestation) as previously described (75).
Fetuses were sourced from singleton pregnancies. After morphometric measurements, fetal liver tissue and cord plasma were collected and frozen at −80°C. The number and gestational age (GA) of fetal liver tissue and cord plasma sample used for each experiment are provided in Supplemental Table S1 (see https://doi.org/10.6084/m9.figshare.12609053).
Protein Extraction from Baboon Fetal Liver Tissue
Frozen pieces of the left lobe of baboon fetal liver (∼0.2 g each) from GD 120 and GD 165 were homogenized at 4°C in lysis buffer (Cell Signaling Technologies, Danvers, MA) containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). Subsequently, the homogenate was centrifuged, and the clear supernatant was stored at −80°C.
Sample Preparation for Mass Spectrometry Analysis
Immunoprecipitation of IGFBP-1 from fetal liver.
To perform parallel reaction monitoring mass spectrometry (PRM-MS), equal amounts of total protein (500 µg) from liver tissue lysate obtained from MNR and Control samples were immunoprecipitated (IP) with the well-established highly specific IGFBP-1 mAb 6303 as described previously (80). Before PRM-MS analysis, Western blotting was performed by using a small aliquot of IP to confirm adequate amount of IGFBP-1 present to perform PRM-MS. The remaining IP samples were digested in solution as described below for PRM-MS analysis.
Protein digestion and PRM-MS analysis.
PRM-MS analysis was performed with the IP samples for the quantification of site-specific phosphorylation of IGFBP-1 as well as for determining the expression and autophosphorylation of CK2. PRM-MS technique is thus targeted to the detection of IGFBP-1 and coimmunoprecipitated (co-IP) CK2-specific peptides and peptide modifications (phosphorylation) based on high-resolution and high-precision mass spectrometry. Internal peptides for IGFBP-1 (NH2-ALPGEQQPLHALTR-COOH) and CK2 (NH2-WERFVHSENQHLVSPEAL-COOH) were used to normalize respective phosphopeptide data.
In-solution digestion of the IP samples was performed as described previously (23). In brief, the protein sample was first digested with endoproteinase Asp-N incubated overnight at 37°C (Roche Diagnostics, Laval, QC, Canada), followed by digestion with trypsin (Roche Diagnostics) overnight at 37°C. Peptide digests were desalted with C18-ZipTip and dried in a Thermo SpeedVac. After desalting and drying, samples were loaded onto a Thermo Easy-Spray analytical column (75-µm inner diameter × 500 mm) C18 column with an Easy-nLC 1000 chromatography pump. For each analysis, we reconstituted peptides in 20 µL of 0.1% trifluoroacetic acid (TFA) and loaded 4 µL onto the column. Peptides were separated on a 125-min (5–40% acetonitrile) gradient. Mass spectra were collected on a Q-Exactive hybrid quadrupole-Orbitrap mass spectrometer coupled to an Easy-nLC 1000 system (ThermoFisher). The spectrometer was set in full MS/data-dependent-MS2 TopN mode: mass analyzer over a mass-to-charge ratio (m/z) range of 400–1,600 with a mass resolution of 70,000 (at m/z = 200), 35 NCE (normalized collision energy), 2.0 m/z isolation window, and 15-s dynamic exclusion. The isolation list (not shown) with the mass [m/z] and the sequences of the peptides used to identify IGFBP-1 and CK2 by PRM-MS were recorded. Each trace on the chromatograph represents the detection of each individual transition ion used to monitor phosphorylation of IGFBP-1 or CK2. The retention time provides an indication that the transition ions result from the same parent peptide (correlates with the time that the parental peptide had eluted from the C18 column). Data were generated by PRM-MS using the isolation list that was specific to each phosphorylation state and site corresponding to IGFBP-1 and CK2 in samples of baboon fetal liver from MNR (n = 3) and Control (n = 3) at GD 120. “Doubly phosphorylated” sites (higher m/z) here refers to the presence of two adjacent phosphorylation sites on a single peptide, as opposed to “singly phosphorylated” sites (lower m/z). “Dually phosphorylated” site detection was thus used to confirm phosphorylation being two sites on the same peptide.
SDS-PAGE and Western Blots
Western blotting was performed with fetal liver tissue extracts and cord plasma samples. Equal amounts of total protein (30–100 µg) of liver tissue lysate and equal loading volumes (10 μL) of umbilical cord plasma were run on SDS-PAGE. The proteins were transferred to nitrocellulose membranes by electrophoresis to determine total expression and phosphorylation of IGFBP-1 at Ser101, Ser119, and Ser169 in liver tissue extracts and in cord plasma samples. Liver tissue extracts were further analyzed for total expression and phosphorylation of P70S6K at Thr389 (mTORC1 readout), Akt at Ser473 (mTORC2 readout), GCN2 at Thr898, extracellular signal-regulated kinase (ERK) at Thr202, and elF2α at Ser51 (AAR readouts), IGF-IR autophosphorylation at Tyr1135, and expression of CK2 α-, α′-, and β-subunits. Samples were also probed for β-actin to account for any differences in protein load and transfer.
For immunoblot analysis, either 5% skim milk or 5% BSA was used in Tris-buffered saline (TBS) plus 0.1% Tween 20 (TBST) for blocking. The blots for CK2 α, α′, and β were blocked in Li-Cor blocking buffer (LI-COR Biosciences, Lincoln, NE). All primary antibodies were obtained from Cell Signaling Technologies (Beverly, MA) with the exception of the monoclonal anti-human IGFBP-1 (mAb 6303) (Medix Biochemica, Kauniainen, Finland) and CK2 (α, α′, and β) antibodies (a gift from Dr. David W. Litchfield, University of Western Ontario). Custom IGFBP-1 polyclonal antibodies targeting Ser101, Ser119, and Ser169, generated at YenZyme Antibodies LLC (San Francisco, CA) and previously validated (1, 3), were used for detection of site-specific IGFBP-1 phosphorylation. The sources of all commercial antibodies are shown in Supplemental Table S2 (see https://doi.org/10.6084/m9.figshare.12609071).
Incubation with primary antibodies was overnight at 4°C, and peroxidase-labeled goat anti-mouse and goat anti-rabbit antibodies (1:10,000; Bio-Rad Laboratories Inc.) were used as secondary antibodies. The corresponding bands were detected with the Clarity Western ECL Chemiluminescence substrate reagent, and the images were captured with the Quantity One Molecular Imager VersaDoc (Bio-Rad Laboratories, Burlington, ON, Canada) imaging system; the band intensities were determined with densitometry and Image Laboratory (Beta 3) software (Bio-Rad Laboratories, Burlington, ON, Canada). The Control group was arbitrarily assigned a value of 1 to facilitate comparisons between groups. Full Western blots are included in the Supplemental Figures (all Supplemental Figures are available at https://doi.org/10.6084/m9.figshare.12605279).
Immunohistochemistry
Tissues (GD 120 and GD 165) from the left lobe of Control and MNR fetal baboons were fixed in 4% paraformaldehyde, embedded in paraffin blocks, and sectioned (5 μm). Control and GA-matched MNR liver tissue sections were fixed on the same microscope slides and were then baked overnight at 45°C. The sections were deparaffinized and washed. Subsequently, endogenous peroxidases were blocked by submersion in 3% hydrogen peroxide, rinsed, and incubated with Background Sniper blocking solution (Biocare Medical, LLC, Pacheco, CA). The sections were incubated overnight at 4°C with primary antibody IGFBP-1 mAb 6303 (1:200; Medix Biochemica, Kauniainen, Finland) or rabbit polyclonal CK2β (1:1,000; a gift from Dr. David W. Litchfield, University of Western Ontario) diluted with antibody diluent (Dako, Agilent Technologies, Santa Clara, CA).
The sections were subsequently incubated for 45 min at room temperature with secondary antibody (anti-rabbit or anti-mouse horseradish peroxidase polymer complex, ImmPRESS HRP Reagent Kit; Vector Laboratories, Burlingame, CA) Sections were then treated with 3,3′-diaminobenzidine (DAB) substrate (ImmPACT DAB Peroxidase Substrate Kit; Vector Laboratories, Burlingame, CA) for 2 min and counterstained with CAT Hematoxylin (Biocare Medical, LLC, Pacheco, CA) for 20 s, rinsed, and immersed in Tacha’s Bluing Solution (Biocare Medical, LLC, Pacheco, CA) for 30 s. Images were analyzed under a Zeiss AxioImager Z1 Microscope (Carl Zeiss Canada Ltd., North York, ON, Canada) with bright-field imaging. The immunohistochemistry (IHC) images (n = 3) were analyzed quantitatively to determine percent area of staining of either IGFBP-1 or CK2β for each of the images with Image Pro software. A macro was created using this software to highlight areas with the characteristic brown stain created by DAB to represent the localization of IGFBP-1 or CK2β.
For dual IHC, Control and GA-matched MNR liver tissue sections were similarly prepared on the same slide. A horseradish peroxidase-labeled goat anti-mouse IgG was used as the secondary antibody against IGFBP-1 mAb 6303 (1:200), and an alkaline phosphatase-labeled goat anti-rabbit IgG was subsequently used as the secondary antibody against rabbit polyclonal CK2β (1:1,000). After incubation with alkaline phosphatase, the slides were treated with 3,3′-diaminobenzidine (DAB) substrate for 2 min and then the ImmPACT Vector Red Alkaline Phosphatase Substrate Kit for 5 min (Vector Laboratories, Burlingame, CA) before proceeding to the counterstaining and dehydration methods mentioned above. In dual IHC, DAB staining is for horseradish peroxidase (IGFBP-1) and Vector Red is for alkaline phosphatase (CK2β). Primary antibody was replaced with respective normal preimmune IgG serum and used as negative control. The sections were then dehydrated, cleared with xylene, and mounted with Permount (Fisher Scientific, London, ON, Canada). Glass coverslips were applied, and images were captured under a Zeiss AxioImager Z1 Microscope (Carl Zeiss Canada Ltd., North York, ON, Canada) with bright-field imaging.
Proximity Ligation Assay
Fixed sections of baboon fetal liver tissues from GA-matched Control and MNR on the same slide were utilized. The sections were deparaffinized, washed, and treated with Duolink blocking solution (Sigma-Aldrich, St. Louis, MO) for 1 h at 37°C and then probed with specific combination of primary antibodies overnight at 4°C [mouse IGFBP-1 mAb 6303 (1:200) and rabbit polyclonal CK2β (1:1,000)]. Preimmune sera used as negative controls were Dako mouse IgG and Dako rabbit immunoglobulin fraction (2 μg/mL for each) (Agilent Technologies, Santa Clara, CA). The proximity ligation assay (PLA) reaction was performed with PLA secondary probes diluted 1:5 in Duolink antibody diluent (Sigma-Aldrich, St. Louis, MO) and incubated for 1 h at 37°C. The PLA secondary probes utilized were Duolink PLA Anti-rabbit Plus and Anti-mouse Minus. Subsequent ligation and amplification were performed with reagents and per manufacturer’s instructions and as we described in detail previously (80).
Image standardization and acquisition.
Image acquisition was performed with fluorescent images acquired on an AxioImager Z1 Epifluorescent Microscope (Carl Zeiss Canada Ltd., Toronto, ON, Canada) as reported previously (80). Regions were randomly selected per slide for each sample. ImagePro Premier software was used to create a macro to process the images for qualitative and quantitative presentation and visual comparison for assessment of the relative PLA signals in Control and MNR baboon fetal liver tissues.
Identification of IGFBP-1 phosphorylation and CK2 autophosphorylation.
PRM-MS allowed us to specifically determine the presence of CK2 coimmunoprecipitated with IGFBP-1, and further CK2 Tyr182 autophosphorylation levels were determined as a readout for CK2 activity. The chromatographs of all transitions were recorded to monitor the relative level of CK2 Tyr182 phosphorylation (NH2-LIDWGLAEFpYHPGQEYNV-COOH; 1194.0465++) that was associated with endogenous IGFBP-1 in fetal liver tissue extracts from MNR and Control samples at GD 120.
Data Presentation and Statistics
Statistics were performed with GraphPad Prism 5 (Graph Pad Software Inc., California). Unpaired t tests were used to compare mean (±SD) differences between the two groups. Differences were considered statistically significant at P < 0.05. For most analyses, samples from the following numbers of animals were used: GD 120 Control, n = 7 and MNR, n = 10 and GD 165 Control, n = 7 and MNR, n = 8. Samples from three animals per group were used in a few analyses because of limited availability. The number of samples for the different analyses are summarized in Supplemental Table S1.
We tested all data sets for heterogeneity of variance with Levene’s test. Levene’s test was significant for a small number of data sets (suggesting some heterogeneity of variance); these data were transformed before analyses.
Because of a limited number of samples in each of the MNR and Control groups at GD 120 and GD 165, sex differences in response to MNR could not be examined with an adequate power. We have shown n values for sex in Supplemental Table S1.
RESULTS
Fetal and Placental Weights and Fetal Plasma Amino Acid Concentrations
Fetal and placental morphometric measure data have been published in our previous reports (42, 63). In brief, placental weights were unchanged in MNR at GD 120 but reduced by 17% (P = 0.06) in MNR at GD 165. Similarly, fetal weights were unaffected by MNR at GD 120 and reduced (−13%, P = 0.03) in the MNR group at GD 165. A decrease in fetal liver weight (−18%, P = 0.043) and relative fetal liver weight (−8.7%, P = 0.047) was observed at GD 165, alongside reduced fetal weight, signifying the onset of IUGR. In contrast, the concentrations of some key essential amino acids, such as leucine, isoleucine, and phenylalanine, were decreased in fetal cord plasma at GD 120 and/or GD 165 (42, 63).
Fetal Liver IGFBP-1 Phosphorylation Is Greater in MNR at GD 120
As shown in Fig. 1, we used a targeted approach to identify novel and or dually phosphorylated sites in fetal liver before IUGR onset. Phosphopeptide detection enabled by PRM-MS analysis allowed the relative quantification of IGFBP-1 phosphorylation events on singly and doubly phosphorylated serine sites, not detectable by other approaches, in fetal liver tissue from MNR and Control at GD 120 (Fig. 1A) and GD 165 (Fig. 1B). Marked increases in phosphorylation in MNR at GD 120 were detected, in particular, at the dually phosphorylated IGFBP-1 sites Ser98+Ser101 (+340%, P = 0.022). Moreover, increases in phosphorylation were observed at three singly phosphorylated serine residues, Ser119 (+154%, P = 0.01), Ser169 (+321%, P = 0.04), and Ser174 (+245%, P = 0.03), in MNR. No statistically significant change in the abundance of phosphorylation of IGFBP-1 at Ser98 (+54%, P = 0.13) or Ser101 (+85%, P = 0.41) or dual phosphorylation at Ser169+Ser174 in MNR (+348%, P = 0.18) was detected.
Fig. 1.
Fetal liver insulin-like growth factor binding protein (IGFBP)-1 phosphorylation is increased in maternal nutrient restriction (MNR) at gestational day (GD) 120: phosphorylation of liver IGFBP-1 at GD 120 (A) and GD 165 (B) determined by parallel reaction monitoring mass spectrometry (PRM-MS) showing phosphorylation (p) singly at Ser98, 101, 119, 169, and 174 and dually at Ser98+101 and Ser169+174 sites. Data were generated by PRM-MS using an isolation list (not shown) that was specific to each phosphorylation state and site in samples of baboon fetal liver from MNR [n = 3; 1 female (F), 2 males (M)] and Control (CTR; n = 3; 1 F, 2 M) at GD 120 and GD 165. The data represent pooled technical triplicates from 3 separate biological liver tissue samples immunoprecipitated with IGFBP-1. Bar graphs summarize data represented as total transition peak intensity relative to control samples (set to a value of 1). Data are normalized to an internal IGFBP-1 (nonphosphorylated) peptide to control for total IGFBP-1. Means + SD, unpaired t test. P < 0.05 is considered significant.
At GD 165, phosphorylation of IGFBP-1 in MNR was markedly increased at singly phosphorylated site Ser119 (+350%, P = 0.04) and dually phosphorylated site Ser169+Ser174 (+1767%, P = 0.04) (Fig. 1B). On the other hand, MNR did not show statistically significant changes compared with Control at sites Ser98 (+277%, P = 0.06), Ser101 (+157%, P = 0.07), Ser169 (+170%, P = 0.3), or Ser174 (+273%, P = 0.07) or at dually phosphorylated site Ser98+Ser101 (+127%, P = 0.1) at GD 165.
Furthermore, using immunoblotting and three phosphosite-specific IGFBP-1 antibodies (pSer101, pSer119, and pSer169) (3, 35), we found increased phosphorylation of IGFBP-1 at Ser101 (+131%, P < 0.001), Ser119 (+153%, P < 0.001), and Ser169 (+113%, P = 0.003) in the MNR fetal liver at GD 120 (Fig. 2, A–C). Although less pronounced, phosphorylation at Ser101 (+81%, P < 0.001), Ser119 (+79%, P = 0.003), and Ser169 (+56%, P = 0.03) remained increased at GD 165 (Fig. 2, A–C).
Fig. 2.

Fetal liver insulin-like growth factor binding protein (IGFBP)-1 expression and phosphorylation are increased in maternal nutrient restriction (MNR) at gestational day (GD) 120: representative Western blots for phosphorylated IGFBP-1 at Ser101 (A), Ser119 (B), and Ser169 (C) and total IGFBP-1 (D) in baboon fetal liver tissue extracts in Control (CTR) and MNR liver at GD 120 and GD 165. Equal protein amounts (50 µg) from cell extracts were loaded. Bar graphs summarize the Western blot data. Means + SD, unpaired t test, Bonferroni corrections. P < 0.05 is considered significant. Control GD 120: n = 7; 2 females (F), 5 males (M); MNR GD 120: n = 10; 5 F, 5 M; Control GD 165: n = 7; 2 F, 5 M; MNR GD 165: n = 8; 3 F, 5 M, except for pSer169: Control: n = 3; 1 F, 2 M and MNR: n = 3; 1 F, 2 M for both GD 120 and GD 165.
We used immunoblotting with IGFBP-1 mAb 6303, which we have extensively validated and used previously (1–4, 23, 35, 51, 79–81), to determine total IGFBP-1 protein expression. As shown in Fig. 2D, total IGFBP-1 expression in fetal liver was higher in MNR (+144%, P = 0.003) compared with the Control group at GD 120. The expression of IGFBP-1 remained elevated in MNR at GD 165 (+48%, P = 0.03) compared with Control.
IGFBP-1 Expression in Fetal Liver Is Higher in MNR at GD 120
Using the same IGFBP-1 mAb 6303, we performed immunohistochemistry (IHC) and compared total IGFBP-1 expression between Control and MNR groups at GD 120 and GD 165 using image analysis (Fig. 3, A–C). Total IGFBP-1 expressed in the parenchymal cells of fetal liver was higher in MNR at GD 120 (Fig. 3b) and at GD 165 (Fig. 3h), compared with Control (Fig. 3, a and g, respectively). Moreover, MNR was also associated with greater IGFBP-1 expression around the central vein as expected for a secretory protein, in the tissues at both GD 120 and GD 165 (Fig. 3, d and j) versus Control (Fig. 3, c and i, respectively). No obvious staining was detected in negative control (Fig. 3, e, f, k, and l). These data suggest that increase in fetal liver IGFBP-1 expression precedes IUGR and the expression remains increased at GD 165. IHC images were quantified. Percent area of IGFBP-1 expression in parenchymal cells was increased in MNR at GD 120 (6-fold increase, P < 0.001) and at GD 165 (25-fold increase, P < 0.001) compared with Control (Fig. 3C). Percent area of IGFBP-1 expression surrounding the central vein was also increased in MNR at GD 120 (11-fold increase, P = 0.03) and GD 165 (18-fold increase, P = 0.03) compared with Control (Fig. 3C).
Fig. 3.

Total insulin-like growth factor (IGF) binding protein (IGFBP)-1 expression in fetal liver is increased and IGF-I receptor (IGF-IR) autophosphorylation is decreased in maternal nutrient restriction (MNR) at gestational day (GD) 120. A: representative immunohistochemistry (IHC) images for IGFBP-1 expression in the parenchyma (brown staining) of left liver lobules from GD 120 in Control (a) and MNR (b) and in the parenchyma with central vein in Control (c) and MNR (d). B: IHC images for IGFBP-1 expression in the parenchyma of left liver lobules from GD 165 in Control (g) and MNR (h) and in the parenchyma with central vein in Control (i) and MNR (j). Liver tissue sections were fixed on the same slide and stained with IGFBP-1 primary antibody. Hematoxylin nuclear counterstain is shown in blue or dark purple. A and B: respective negative control images (e, f, k, and l) with primary antibody replaced with diluent only; ×20 magnification is used (original scale bars, 50 μm). C: IGFBP-1 IHC was quantified (n = 3) with ImagePro software, and data are presented. Bar graph summarizes % area of IGFBP-1 staining in Control (CTR) and MNR parenchyma and central vein images from both gestation days. D: a representative blot of IGF-IR autophosphorylation at Tyr1135 in fetal liver from Control [n = 3; 1 female (F), 2 males (M)] and MNR (n = 3; 1 F, 2 M) fetuses at GD 120. Representative blots for phosphorylated (p-)IGF-IR and IGF-IR are from the same gel but 2 nonadjacent lanes, because control and MNR samples were run on separate sides of the gel. A white space is shown between CTR and MNR lanes for demarcation. Equal loading (100 µg) was performed. Data are presented as means + SD, unpaired t test. P < 0.05 is considered significant.
MNR Is Associated with Decreased Fetal Liver IGF-IR Phosphorylation at GD 120
We assessed the status of IGF-IR autophosphorylation as an indicator for receptor stimulation in fetal liver extracts from MNR at GD 120 potentially due to IGFBP-1 hyperphosphorylation. Western blot data shown in Fig. 3D indicate that IGF-IRβ autophosphorylation at Tyr1135 was reduced in MNR (−64%, P = 0.045) compared with Control at GD 120.
Increased Fetal Liver Protein Kinase CK2 Expression Precedes the Development of IUGR
We performed immunoblot analysis using CK2 α-, α′-, and β-subunit-specific antibodies to determine the expression of CK2 protein in fetal liver (Fig. 4, A–C). Expression of CK2α (+311%, P = 0.002), CK2α′ (+596%, P = 0.04), and CK2β (+264%, P = 0.03) was higher in MNR compared with Control at GD 120, before the onset of IUGR. Fetal liver expression of CK2α (+191%, P = 0.04), CK2α′ (+181%, P = 0.006), and CK2β (+116%, P = 0.007) remained increased in MNR at GD 165 (Fig. 4, A–C).
Fig. 4.

Maternal nutrient restriction (MNR) is associated with increased fetal liver casein kinase (CK)2 expression at gestational day (GD) 120. A–C: representative Western blots showing expression of CK2α (A), CK2α′ (B), and CK2β (C) subunits in Control (CTR) and MNR (n = 3 each; 1 female, 2 males) liver tissue at GD 120 and GD 165 with bar graphs showing means + SD. Equal loading (50 µg) was performed. Analyzed with unpaired t test; P < 0.05 is considered significant. D and E: representative immunohistochemistry (IHC) images for CK2β expression (brown staining) in the parenchyma in left liver lobules at GD 120 (D) in Control (a) and MNR (b) and in the parenchyma with central vein (c and d) and at GD 165 (E) in the parenchyma in Control (g) and MNR (h) and in the parenchyma with central vein (i and j). Liver tissue sections were fixed on the same slide and stained with CK2β primary antibody. Anti-rabbit horseradish peroxidase was used the secondary antibody. Hematoxylin nuclear counterstain is shown in blue or dark purple. D and E: negative control (e, f, k, and l); primary antibody was replaced with normal serum; ×20 magnification is used (original scale bars, 50 µm). F: IHC was quantified (n = 3) with ImagePro software, and data are presented. Bar graph summarizes % area of CK2β staining in CTR and MNR parenchyma and central vein images from both gestation days.
Further using CK2β-specific antibodies, we determined the expression of CK2β subunit in liver tissue by IHC (Fig. 4, D–F). Total CK2 expressed in the parenchymal cells of fetal liver was higher in MNR at GD 120 (Fig. 4b) and at GD 165 (Fig. 4h) compared with Control (Fig. 4, a and g, respectively). MNR also showed greater CK2 expression around the central vein at both GD 120 and GD 165 (Fig. 4, d and j) versus Control (Fig. 4, c and i, respectively). No obvious staining was detected in negative control (Fig. 4, e, f, k, and l). These data demonstrated that, similar to IGFBP-1 (Fig. 3, A–C), percent area of CK2β expression in parenchymal cells was increased in MNR at GD 120 (5-fold increase, P = 0.005) and at GD 165 (12-fold increase, P = 0.003) compared with Control (Fig. 4F). Percent area of CK2β expression surrounding the central vein was also increased in MNR at GD 120 (5-fold increase, P = 0.046) and GD 165 (250-fold increase, P < 0.001) versus Control (Fig. 4F).
Fetal Liver IGFBP-1 and CK2β Colocalization Precedes the Development of IUGR
We then performed dual IHC with fixed liver tissue from GD 120 and GD 165 (Fig. 5, A and B). With the same (mouse) IGFBP-1 mAb 6303 and (rabbit polyclonal) CK2β primary antibodies, the dual IHC images using bright-field microscopy showed an overlap suggesting potential colocalization of CK2β (red-pink stain) and IGFBP-1 (brown stain). The dual pink-brown staining was visually predominant in MNR at GD 120 in the parenchymal cells (Fig. 5, b and d) but much more at GD 165 in MNR (Fig. 5, h and j). High-resolution scans of the general liver parenchyma and central vein regions of the livers showed this colocalization notably greater in MNR compared with Control in zoomed extended focus images (Fig. 5, A and B, insets). Negative control using mouse and rabbit IgG antibody depicted no nonspecific staining.
Fig. 5.

Insulin-like growth factor binding protein (IGFBP)-1 and casein kinase (CK)2β colocalization and proximity at gestational day (GD) 120 precede the development of intrauterine growth restriction (IUGR): representative dual immunohistochemistry (IHC) images for combined IGFBP-1 (brown stain) and CK2β (pink stain) expression appear as overlapping red-pink-brown stain denoted by a black box with dashed line within tissue morphology at GD 120 and GD 165. A, a and b: shown are the parenchyma of left liver lobules from GD 120 in Control (a) and maternal nutrient restriction (MNR; b). Black-outlined regions in a and b are shown in insets as cropped and zoomed extended focus images. c and d: Combined IGFBP-1 and CK2β expression in the parenchyma with central vein in Control (c) and MNR (d). Black-outlined regions in c and d are shown in insets as cropped and zoomed extended focus images. B, g and h: shown are the parenchyma of left liver lobules from GD 165 in Control (g) and MNR (h). Black-outlined regions in g and h are shown in insets as cropped and zoomed extended focus images. i and j: Combined IGFBP-1 and CK2β expression in the parenchyma with central vein in Control (i) and MNR (j). Black-outlined regions in i and j are shown in insets as cropped and zoomed extended focus images. Hematoxylin nuclear counterstain is shown in blue or dark purple. Liver tissue sections were fixed on the same slide and stained with IGFBP-1 and CK2β primary antibody. A and B: negative control (e, f, k, and l); primary antibody was replaced with normal serum; ×63 magnification is used (original scale bars, 10 µm). C and D: proximity ligation assay (PLA) signals demonstrating the potential interaction of CK2β with IGFBP-1 in the left fetal liver lobules from GD 120 (C) in Control (m) and MNR (n) and from GD 165 (D) in Control (q) and MNR (r). Liver tissue sections were fixed on the same slide for PLA. Positive PLA signal appears as red punctate spots denoted by white arrows and DAPI nuclear counterstain (blue) with tissue autofluorescence (pseudogreen) used to show liver tissue morphology. o, p, s, and t: Negative controls. Scale bars, 20 μm (m, n, q, r) and 50 μm (o, p, s, t).
Fetal Liver IGFBP-1 and CK2β Proximity Precedes the Development of IUGR
PLA represents a highly specific approach that allows the detection of protein-protein interactions (at distances <40 nm) at endogenous protein levels in situ with high sensitivity and specificity. PLA images acquired with Z stack and extended depth of focus were compared for IGFBP-1+CK2β in both MNR and Control at GD 120 (Fig. 5C) and GD 165 (Fig. 5D) liver tissues. PLA signals were artificially enhanced with high precision for visibility. Increased numbers of signals were visualized in MNR tissues from GD 120 and 165 (Fig. 5, n and r) compared with Control (Fig. 5, m and q), as can be distinguished from the normal background autofluorescence present in the liver samples. The signals (PLA spots) were highly present in MNR, suggesting increased proximity between CK2β and IGFBP-1 in MNR compared with Control. PLA signals were absent in negative controls. The similarity of outcome between colocalization (IHC) and PLA data suggests the potential for greater IGFBP-1 phosphorylation in MNR at GD 120, due to increased IGFBP-1+CK2 interaction, which remained consistent at GD 165.
Increased Fetal Liver Protein Kinase CK2 Activity Precedes the Development of IUGR
Next, we performed PRM-MS analysis for detection of CK2 autophosphorylation at the Tyr182 site as a readout for CK2 activity, using samples co-IP with IGFBP-1 as shown in Fig. 6A. The chromatograph of all detected PRM-MS transitions was used to monitor the CK2 peptides with autophosphorylation at the Tyr182 site (Fig. 6A). Retention time(s) confirmed the elution time of parental CK2 peptides, containing the Tyr182 site, from the C18 column and demonstrated specificity of our peptide detection protocol. The data in Fig. 6B show relatively higher CK2 autophosphorylation at Tyr182 in MNR corresponding to greater catalytic activity in MNR compared with Control at GD 120 (+105%, P = 0.06) before the onset of IUGR, which remained consistent at GD 165 (+180%, P = 0.08; Fig. 6C).
Fig. 6.

Increased fetal liver casein kinase (CK)2 autophosphorylation in maternal nutrient restriction (MNR) precedes development of intrauterine growth restriction (IUGR). A: parallel reaction monitoring mass spectrometry (PRM-MS) transitions are shown in a representative chromatograph used to monitor phosphorylation of protein kinase CK2 at Tyr182 in fetal liver tissue extracts from gestational day (GD) 120 in immunoprecipitated (IP) sample using insulin-like growth factor binding protein (IGFBP)-1 antibody. Individual traces show individual transition ions used to monitor phosphorylation of CK2 at Tyr182. Peak retention times shown indicate that transition ions result from the same parent peptide. Data were collected with a Q-Exactive hybrid quadrupole-Orbitrap mass spectrometer. B and C: representation of PRM-MS analysis for detection of relative level of phosphorylated CK2 at Tyr182 in Control [n = 3; 1 female (F), 2 males (M)] and MNR (n = 3; 1 F, 2 M) fetal liver at GD 120 (B) and GD 165 (C). The data represent pooled technical triplicates from 3 separate biological liver tissue samples immunoprecipitated with IGFBP-1 antibody. Bar graphs show means + SD, analyzed with unpaired t test. P < 0.05 is considered significant.
Inhibition of Fetal Liver mTORC1 and mTORC2 Signaling in MNR at GD 120 and 165
We determined mTORC1 and mTORC2 signaling activity in baboon fetal liver at GD 120 and GD 165, using phosphorylation of P70S6K at Thr389 (mTORC1) and Akt at Ser473 (mTORC2) as functional readouts. Phosphorylation of (p-)P70S6K (Thr389) (Fig. 7A) was lower in MNR compared with Control, at both GD 120 (−52%, P = 0.05) and GD 165 (−55%, P = 0.03). Similarly, p-Akt (Ser473) (Fig. 7B) was lower in MNR at GD 120 (−56%, P < 0.001) and at GD 165 (−46%, P < 0.001) compared with Control.
Fig. 7.
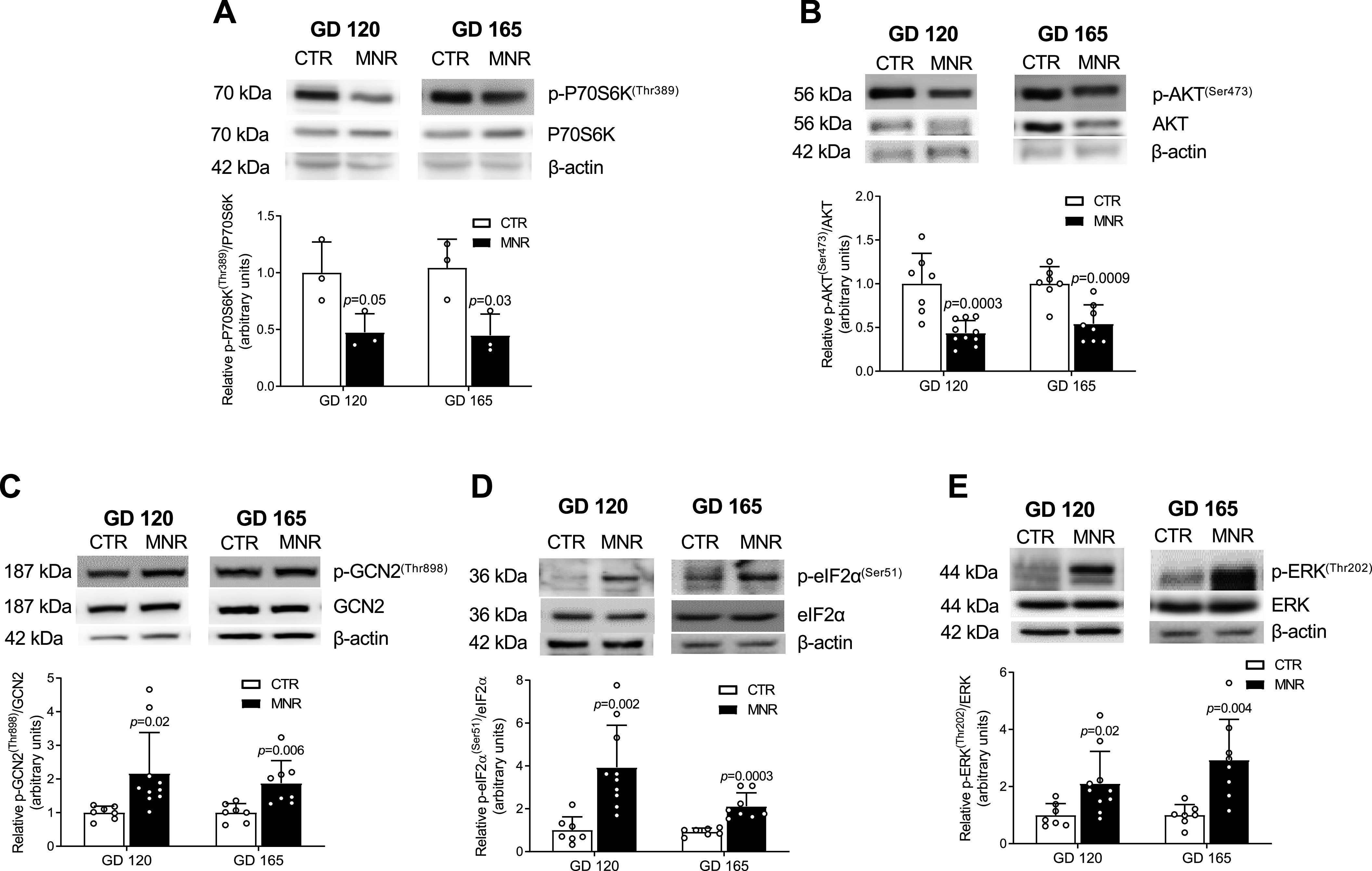
Inhibition of fetal liver mechanistic target of rapamycin complex (mTORC)1 and mTORC2 signaling and activation of amino acid response (AAR) signaling at gestational day (GD) 120 precedes development of intrauterine growth restriction (IUGR). A and B: representative Western blots for total and phosphorylated P70S6 kinase (P70S6K) at Thr389 in Control and maternal nutrient restriction (MNR) fetal liver at GD 120 and GD 165 [n = 3 each; 1 female (F), 2 males (M)] (A) and total and phosphorylated Akt at Ser473 in Control (n = 7; 2 F, 5 M) and MNR (n = 10; 5 F, 5 M) at GD 120 and in Control (n = 7; 2 F, 5 M) and MNR (n = 8; 3 F, 5 M) at GD 165 (B). Equal protein loading (30 µg) was performed. Bar graphs summarize the Western blot data as the ratio of phosphorylated to total protein, with β-actin as the loading control. Means + SD, unpaired t test. P < 0.05 is considered significant. C–E: representative Western blots for the expression of total and phosphorylated general control nonderepressible 2 (GCN2) at Thr898 (C), eukaryotic translation initiation factor (eIF2α) at Ser51 (D), and extracellular signal-regulated kinase (ERK) at Thr202 (E) in Control (n = 7; 2 F, 5 M) and MNR (n = 10; 5 F, 5 M) at GD 120 and in Control (n = 7; 2 F, 5 M) and MNR (n = 8; 3 F, 5 M) at GD 165 fetal liver. Equal protein loading was performed (50 µg). Bar graphs summarize the Western blot data as the ratio of phosphorylated to total protein, with β-actin as the loading control. Means + SD, unpaired t test. P < 0.05 is considered significant.
MNR Is Associated with Activation of Fetal Liver AAR Signaling at GD 120 and 165
Western blot analysis performed with fetal liver tissue extracts to determine the activity of the fetal liver AAR pathway from GD 120 and GD 165 is shown in Fig. 7, C–E. Phosphorylation of GCN2 (Thr898) was greater in MNR at both GD 120 (+117%, P = 0.02) and GD 165 (+88%, P = 0.006) (Fig. 7C) relative to Control. Furthermore, eIF2α (Ser51) phosphorylation was also markedly higher at GD 120 (+294%, P = 0.002) and GD 165 (+112%, P < 0.001) (Fig. 7D) in MNR. Similarly, fetal liver ERK (Thr202) phosphorylation was higher in MNR compared with Control at GD 120 (+111%, P = 0.02) and GD 165 (+194%, P = 0.004) (Fig. 7E).
Total IGFBP-1 Abundance and IGFBP-1 Phosphorylation Are Increased in Cord Plasma in MNR at GD 120 and 165
We determined the site-specific phosphorylation of fetal circulating IGFBP-1 in umbilical plasma from MNR and Control fetuses at GD 120 and GD 165. Western blot analysis showed that total IGFBP-1 was higher at GD 120 (+83%, P = 0.04) and GD 165 (+67%, P = 0.04) in MNR (Fig. 8A). Similarly with validated phosphosite-specific IGFBP-1 antibodies, IGFBP-1 phosphorylation was increased in MNR at Ser101 at both GD 120 (+144%, P = 0.003) and GD 165 (+114%, P = 0.04) (Fig. 8B) and at Ser119 at GD 120 (+78%, P = 0.04) (Fig. 8C). Similarly, IGFBP-1 phosphorylation at Ser169 was also higher in MNR at GD 120 (+131%, P = 0.002) and GD 165 (+138%, P = 0.002) compared with Control (Fig. 8D).
Fig. 8.

Total insulin-like growth factor binding protein (IGFBP)-1 abundance and IGFBP-1 phosphorylation are increased in cord plasma in maternal nutrient restriction (MNR) at gestational day (GD) 120. A: representative Western blots of total IGFBP-1 from GD 120 Control [n = 7; 2 females (F), 5 males (M)] and MNR (n = 10; 5 F, 5 M) and GD 165 Control (n = 7; 2 F, 5 M) and MNR (n = 8; 3 F, 5 M). B–D: representative blots of IGFBP-1 phosphorylation at Ser101 (B) from GD 120 (n = 7 Control; 2 F, 5 M and n = 10 MNR; 5 F, 5 M) and GD 165 (n = 7 Control; 2 F, 5 M and n = 8 MNR; 3 F, 5 M), Ser119 (C) from GD 120 (n = 7 Control; 2 F, 5 M and n = 10 MNR; 5 F, 5 M), and Ser169 (D) from GD 120 (n = 7 Control; 2 F; 5 M and n = 7 MNR; 3 F, 4 M) and GD 165 (n = 7 Control; 2 F, 5 M and n = 8 MNR; 3 F, 5 M) in umbilical cord plasma; equal aliquots of plasma samples were loaded (10 µL). Means + SD, unpaired t test. P < 0.05 is considered significant.
DISCUSSION
We report that inhibition of mTOR, activation of AAR and CK2, and increased IGFBP-1 expression and phosphorylation in the fetal liver and increased concentration and phosphorylation of IGFBP-1 in cord plasma precede the development of IUGR in fetal baboons experiencing nutrient restriction (Fig. 9). Importantly, using primary baboon fetal hepatocytes and HepG2 cells, we have previously demonstrated a mechanistic link between mTOR inhibition, AAR activation, and increased IGFBP-1 expression and phosphorylation (1, 23, 51), which markedly decreases the bioavailability and bioactivity of IGF-I (3, 23, 50). Moreover, mice overexpressing IGFBP-1 are born growth restricted (12, 30, 66, 76). Our data are therefore consistent with the model that increased fetal liver IGFBP-1 phosphorylation, mediated by mTOR inhibition and AAR activation, is a key link between restricted fetal nutrient and oxygen availability and the development of IUGR (Fig. 9).
Fig. 9.

A baboon model of intrauterine growth restriction (IUGR) linking maternal nutrient restriction (MNR) to mechanistic target of rapamycin (mTOR) inhibition, amino acid response (AAR) and casein kinase (CK)2 signaling, and increased insulin-like growth factor (IGF) binding protein (IGFBP)-1 expression and phosphorylation in the fetal liver, preceding restricted fetal growth. Inhibition of mTOR signaling and activation of AAR and CK2 as a result of MNR lead to greater IGFBP-1 expression and phosphorylation and reduced IGF-I bioavailability at gestational day (GD) 120, before IUGR onset (GD 165, full term GD 185).
In the present study we used a well-established nonhuman primate model of MNR, associated with moderate IUGR, reduced fetal concentrations of circulating essential amino acids, and structural and functional changes in many fetal organs (7, 22, 41, 58, 59, 68, 74, 87). Importantly, the IUGR offspring of MNR mothers develop peripheral insulin resistance (19), which provides evidence of the translational significance of this model for human IUGR (39, 61, 62). The striking similarities in placental structure and the close evolutionary relationship between baboons and humans (64, 69, 70) provide additional justification for the use of the baboon as the animal model in this study. Critical for this study, primates have a unique signature of IGFBP-1 phosphorylation (1). This is illustrated by the observation that none of the residues phosphorylated in human IGFBP-1, which enhance binding affinity of IGFBP-1 for IGF-I (2–4), are functionally relevant in rodents (72). It is likely that nonhuman primates may have evolved a distinct mechanism for regulation of IGF-I action that is similar to humans. One limitation of the study is that the number of animals used did not provide sufficient power to systematically explore sex differences in the response to MNR, which should be a focus in future studies.
By studying baboon fetuses at GD 120 and GD 165, we can indirectly address cause-and-effect relationships between key changes in signaling pathways and concentrations of IGFBP-1 and phosphorylation in fetal liver and cord plasma and fetal growth. Fetal growth and fetal liver weight were unaffected by MNR at GD 120, yet there were marked changes in signaling and IGFBP-1 expression and phosphorylation in both fetal liver and cord plasma at this stage of gestation, suggesting that these changes are not a secondary consequence of IUGR.
Our data are consistent with the possibility that there is a mechanistic link (i.e., cause-and-effect relationship) between changes in liver signaling/IGFBP-1 expression and phosphorylation and reduced fetal growth in IUGR. Changes in liver signaling/IGFBP-1 expression and phosphorylation were apparent also after IUGR onset, supporting that despite the slowing of fetal growth at this stage of gestation, nutrient availability remains limited. Decreased fetal liver weight is observed in many models of IUGR, including humans (78) and rats (67). Reduced liver weight in IUGR at GD 165 in this study may perpetuate decreased nutrient availability in the fetus by decreasing the ability to process and store nutrients.
PRM-MS analysis identified significantly increased dual-site Ser98+Ser101 phosphorylation of IGFBP-1 at GD 120 in response to MNR. We also showed concomitantly reduced IGF-IR activation in MNR at GD 120. The affinity of IGFBP-1 for IGF-I is greater than that of IGF-IR for IGF-I, and therefore IGF-I preferentially binds to IGFBP-1 (20). It is thus conceivable that hyperphosphorylation of IGFBP-1 before IUGR onset markedly increases sequestration of IGF-I, thereby decreasing IGF-IRβ autophosphorylation in MNR already at GD 120.
Using phosphorylated P70S6K and Akt as functional readouts, we found that fetal liver mTORC1 and mTORC2 signaling, respectively, were markedly inhibited in MNR baboons at GD 120, before IUGR develops, and remained inhibited at GD 165. mTOR is inhibited in the fetal liver of IUGR animal models, such as in newborn piglets (48) and baboons (1). mTOR signaling functions as a central regulator of cellular metabolism, growth, and survival (44, 73). mTOR is activated by factors that are decreased in the IUGR fetus, such as insulin/IGF-I signaling (42), glucose (84) and amino acid concentrations (8), as well as folate levels (71). Moreover, hypoxemia, common in the IUGR fetus, typically inhibits mTOR signaling (31, 55).
mTOR signaling is also highly responsive to changes in the concentrations of essential amino acids, in particular leucine (9, 52). The decline in amino acid availability may lead to inhibited mTOR signaling, ultimately resulting in IUGR. The lower plasma concentrations of leucine in the MNR fetus at GD 120 and GD 165 in our study may be one important factor promoting mTOR inhibition in the fetal liver.
Several molecular networks are responsive to nutritional stress in fetal liver. A reduction in total dietary protein or an imbalance in amino acid composition, resulting in amino acid deprivation, activates AAR (43) in different organs and cells (49) Moreover, leucine deprivation in HepG2 cells causes activation of AAR signaling (50, 51). In mice in vivo, leucine deprivation results in cell-specific responses that modulate the AAR in the liver and muscle (6, 14). Increased placental eIF2α phosphorylation indicating activation of AAR has also been reported to be higher in human IUGR (86).
We observed activation of AAR signaling in the MNR fetal liver, as evidenced by increased phosphorylation of key functional readouts of the AAR (GCN2, eIF2α, and ERK) at GD 120 that remained activated at GD 165. These findings in MNR fetal liver corroborate human IUGR, where decidua functions as a nutrient sensor linking limited oxygen and nutrient availability to IGFBP-1 hyperphosphorylation mediated by mTOR and AAR (35). MNR in both human and animal models (11) results in reduced maternal-fetal transfer of essential amino acids (13). These observations suggest that the activation of AAR signaling in the fetal liver in response to MNR may represent a key link between limited amino acid supply and IGFBP-1 phosphorylation, and therefore reduced fetal growth.
Higher fetal liver IGFBP-1 phosphorylation at GD 120 relative to Control occurs in conjunction with greater fetal liver CK2 expression in MNR. Furthermore, data obtained with multiple strategies strongly suggest that fetal liver CK2 is activated and the interaction between CK2 and IGFBP-1 is increased before development of IUGR in response to MNR. Using IHC, we observed increased IGFBP-1 and CK2β expression in the parenchyma and surrounding central vein regions of the MNR fetal liver, whereas these data show increased CK2 in close proximity to IGFBP-1 and we have previously reported that CK2 can increase phosphorylation of IGFBP-1 (35, 80). The significance of the two subcellular localizations (parenchyma vs. around the central vein) remains to be fully established. CK2 has been implicated in various disease processes (33); however, studies on the role of CK2 and its regulation in IUGR are limited (1, 35). We propose that CK2 mediates the increased IGFBP-1 phosphorylation and reduced IGF-I bioactivity in the fetal liver in response to MNR before development of IUGR.
In conclusion, this study suggests that increased fetal liver IGFBP-1 phosphorylation, mediated by inhibition of mTOR and activation of AAR and CK2, constitutes a key link between restricted nutrient and oxygen availability and the development of IUGR (Fig. 9). This work will help improve our mechanistic understanding of the molecular links between reduced nutrient availability and restricted fetal growth.
GRANTS
This work was supported by grants from the National Institutes of Health (R03 HD-078313 to M.B.G. and T.J.), by Program Project grants (P01 HD-021350 and R03 HD-093950 to P.W.N.), and in part by a Lawson Research Grant (F0609 to M.B.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.B.G. and T.J. conceived and designed research; J.H.K., B.B.J., K.B., A.S., K.N. and C.L. performed experiments; J.H.K., B.B.J., K.B., A.S., K.N., and M.B.G. analyzed data; J.H.K., B.B.J., K.B., K.N., T.J., and M.B.G. interpreted results of experiments; J.H.K., B.B.J., K.B., A.S., K.N., and M.B.G. prepared figures; J.H.K., B.B.J., K.B., A.S., K.N., and M.B.G. drafted manuscript; J.H.K., B.B.J., K.B., K.N., C.L., P.W.N., T.J., and M.B.G. edited and revised manuscript; J.H.K., B.B.J., K.B., A.S., K.N., C.L., P.W.N., T.J., and M.B.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. David W. Litchfield (Department of Biochemistry, University of Western Ontario) for the antibodies for protein kinase CK2 and Dr. Mike Miller (Department of Paediatrics, University of Western Ontario) for guidance in statistical analyses. We thank Biotron Integrated Microscopy, Western University, London, ON, Canada, for immunohistochemical and image acquisition analyses. J.H.K. acknowledges a Children’s Health Research Institute (Graduate) Trainee Award funded by Children’s Health Foundation, London, ON, Canada.
REFERENCES
- 1.Abu Shehab M, Damerill I, Shen T, Rosario FJ, Nijland M, Nathanielsz PW, Kamat A, Jansson T, Gupta MB. Liver mTOR controls IGF-I bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction. Endocrinology 155: 1327–1339, 2014. doi: 10.1210/en.2013-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Shehab M, Inoue S, Han VK, Gupta MB. Site specific phosphorylation of insulin-like growth factor binding protein-1 (IGFBP-1) for evaluating clinical relevancy in fetal growth restriction. J Proteome Res 8: 5325–5335, 2009. doi: 10.1021/pr900633x. [DOI] [PubMed] [Google Scholar]
- 3.Abu Shehab M, Iosef C, Wildgruber R, Sardana G, Gupta MB. Phosphorylation of IGFBP-1 at discrete sites elicits variable effects on IGF-I receptor autophosphorylation. Endocrinology 154: 1130–1143, 2013. doi: 10.1210/en.2012-1962. [DOI] [PubMed] [Google Scholar]
- 4.Abu Shehab M, Khosravi J, Han VK, Shilton BH, Gupta MB. Site-specific IGFBP-1 hyper-phosphorylation in fetal growth restriction: clinical and functional relevance. J Proteome Res 9: 1873–1881, 2010. doi: 10.1021/pr900987n. [DOI] [PubMed] [Google Scholar]
- 5.Ankrapp DP, Jones JI, Clemmons DR. Characterization of insulin-like growth factor binding protein-1 kinases from human hepatoma cells. J Cell Biochem 60: 387–399, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 279: 36553–36561, 2004. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 7.Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA 108: 3011–3016, 2011. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aplin JD. Hypoxia and human placental development. J Clin Invest 105: 559–560, 2000. doi: 10.1172/JCI9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 24: 400–406, 2014. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298: 564–567, 1989. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beede KA, Limesand SW, Petersen JL, Yates DT. Real supermodels wear wool: summarizing the impact of the pregnant sheep as an animal model for adaptive fetal programming. Anim Front 9: 34–43, 2019. doi: 10.1093/af/vfz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben Lagha N, Seurin D, Le Bouc Y, Binoux M, Berdal A, Menuelle P, Babajko S. Insulin-like growth factor binding protein (IGFBP-1) involvement in intrauterine growth retardation: study on IGFBP-1 overexpressing transgenic mice. Endocrinology 147: 4730–4737, 2006. doi: 10.1210/en.2006-0171. [DOI] [PubMed] [Google Scholar]
- 13.Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 3: 428–444, 2011. doi: 10.2741/s162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carraro V, Maurin AC, Lambert-Langlais S, Averous J, Chaveroux C, Parry L, Jousse C, Ord D, Ord T, Fafournoux P, Bruhat A. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2α/ATF4 pathway. PLoS One 5: e15716, 2010. doi: 10.1371/journal.pone.0015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162: 253–261, 1990. doi: 10.1016/0002-9378(90)90860-A. [DOI] [PubMed] [Google Scholar]
- 16.Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol 158: 120–126, 1988. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- 17.Cetin I, Marconi AM, Corbetta C, Lanfranchi A, Baggiani AM, Battaglia FC, Pardi G. Fetal amino acids in normal pregnancies and in pregnancies complicated by intrauterine growth retardation. Early Hum Dev 29: 183–186, 1992. doi: 10.1016/0378-3782(92)90136-5. [DOI] [PubMed] [Google Scholar]
- 18.Chard T. Insulin-like growth factors and their binding proteins in normal and abnormal human fetal growth. Growth Regul 4: 91–100, 1994. [PubMed] [Google Scholar]
- 19.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol 301: R757–R762, 2011. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemmons DR. Insulin-like growth factor binding proteins: roles in regulating IGF physiology. J Dev Physiol 15: 105–110, 1991. [PubMed] [Google Scholar]
- 21.Clemmons DR. Structural and functional analysis of insulin-like growth factors. Br Med Bull 45: 465–480, 1989. doi: 10.1093/oxfordjournals.bmb.a072335. [DOI] [PubMed] [Google Scholar]
- 22.Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol 572: 67–85, 2006. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damerill I, Biggar KK, Abu Shehab M, Li SS, Jansson T, Gupta MB. Hypoxia increases IGFBP-1 phosphorylation mediated by mTOR inhibition. Mol Endocrinol 30: 201–216, 2016. doi: 10.1210/me.2015-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donella-Deana A, Cesaro L, Sarno S, Brunati AM, Ruzzene M, Pinna LA. Autocatalytic tyrosine-phosphorylation of protein kinase CK2 alpha and alpha′ subunits: implication of Tyr182. Biochem J 357: 563–567, 2001. doi: 10.1042/bj3570563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Economides DL, Nicolaides KH. Blood glucose and oxygen tension levels in small-for-gestational-age fetuses. Am J Obstet Gynecol 160: 385–389, 1989. doi: 10.1016/0002-9378(89)90453-5. [DOI] [PubMed] [Google Scholar]
- 26.Economides DL, Nicolaides KH, Campbell S. Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J Perinat Med 19: 97–105, 1991. doi: 10.1515/jpme.1991.19.1-2.97. [DOI] [PubMed] [Google Scholar]
- 27.Economides DL, Proudler A, Nicolaides KH. Plasma insulin in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 160: 1091–1094, 1989. doi: 10.1016/0002-9378(89)90167-1. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 62: 1789–1801, 2019. doi: 10.1007/s00125-019-4951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frost RA, Tseng L. Insulin-like growth factor-binding protein-1 is phosphorylated by cultured human endometrial stromal cells and multiple protein kinases in vitro. J Biol Chem 266: 18082–18088, 1991. [PubMed] [Google Scholar]
- 30.Gay E, Seurin D, Babajko S, Doublier S, Cazillis M, Binoux M. Liver-specific expression of human insulin-like growth factor binding protein-1 in transgenic mice: repercussions on reproduction, ante- and perinatal mortality and postnatal growth. Endocrinology 138: 2937–2947, 1997. doi: 10.1210/endo.138.7.5282. [DOI] [PubMed] [Google Scholar]
- 31.Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J Clin Endocrinol Metab 77: 1174–1179, 1993. doi: 10.1210/jcem.77.5.8077309. [DOI] [PubMed] [Google Scholar]
- 32.Grundy D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593: 2547–2549, 2015. doi: 10.1113/JP270818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerra B, Issinger OG. Protein kinase CK2 in human diseases. Curr Med Chem 15: 1870–1886, 2008. doi: 10.2174/092986708785132933. [DOI] [PubMed] [Google Scholar]
- 34.Gupta MB. The role and regulation of IGFBP-1 phosphorylation in fetal growth restriction. J Cell Commun Signal 9: 111–123, 2015. doi: 10.1007/s12079-015-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta MB, Abu Shehab M, Nygard K, Biggar K, Singal SS, Santoro N, Powell TL, Jansson T. IUGR is associated with marked hyperphosphorylation of decidual and maternal plasma IGFBP-1. J Clin Endocrinol Metab 104: 408–422, 2019. doi: 10.1210/jc.2018-00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta MB, Jansson T. Novel roles of mechanistic target of rapamycin signaling in regulating fetal growth. Biol Reprod 100: 872–884, 2019. doi: 10.1093/biolre/ioy249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han VK, Matsell DG, Delhanty PJ, Hill DJ, Shimasaki S, Nygard K. IGF-binding protein mRNAs in the human fetus: tissue and cellular distribution of developmental expression. Horm Res 45: 160–166, 1996. doi: 10.1159/000184780. [DOI] [PubMed] [Google Scholar]
- 38.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 39.Jensen CB, Martin-Gronert MS, Storgaard H, Madsbad S, Vaag A, Ozanne SE. Altered PI3-kinase/Akt signalling in skeletal muscle of young men with low birth weight. PLoS One 3: e3738, 2008. doi: 10.1371/journal.pone.0003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones JI, D’Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci USA 88: 7481–7485, 1991. doi: 10.1073/pnas.88.17.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamat A, Nijland MJ, McDonald TJ, Cox LA, Nathanielsz PW, Li C. Moderate global reduction in maternal nutrition has differential stage of gestation specific effects on beta1- and beta2-adrenergic receptors in the fetal baboon liver. Reprod Sci 18: 398–405, 2011. doi: 10.1177/1933719110386496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J 28: 1294–1305, 2014. doi: 10.1096/fj.13-242271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20: 436–443, 2009. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol 21: 63–71, 2019. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 45.Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res 29: 219–225, 1991. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Limesand SW, Thornburg KL, Harding JE. 30th anniversary for the developmental origins of endocrinology. J Endocrinol 242: E1–E4, 2019. doi: 10.1530/JOE-19-0227. [DOI] [PubMed] [Google Scholar]
- 47.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369: 1–15, 2003. doi: 10.1042/bj20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long B, Yin C, Fan Q, Yan G, Wang Z, Li X, Chen C, Yang X, Liu L, Zheng Z, Shi M, Yan X. Global liver proteome analysis using iTRAQ reveals AMPK-mTOR-autophagy signaling is altered by intrauterine growth restriction in newborn piglets. J Proteome Res 15: 1262–1273, 2016. doi: 10.1021/acs.jproteome.6b00001. [DOI] [PubMed] [Google Scholar]
- 49.Malandro MS, Beveridge MJ, Kilberg MS, Novak DA. Effect of low-protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol Cell Physiol 271: C295–C303, 1996. doi: 10.1152/ajpcell.1996.271.1.C295. [DOI] [PubMed] [Google Scholar]
- 50.Malkani N, Biggar K, Shehab MA, Li SS, Jansson T, Gupta MB. Increased IGFBP-1 phosphorylation in response to leucine deprivation is mediated by CK2 and PKC. Mol Cell Endocrinol 425: 48–60, 2016. doi: 10.1016/j.mce.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malkani N, Jansson T, Gupta MB. IGFBP-1 hyperphosphorylation in response to leucine deprivation is mediated by the AAR pathway. Mol Cell Endocrinol 412: 182–195, 2015. doi: 10.1016/j.mce.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazor KM, Dong L, Mao Y, Swanda RV, Qian SB, Stipanuk MH. Effects of single amino acid deficiency on mRNA translation are markedly different for methionine versus leucine. Sci Rep 8: 8076, 2018. doi: 10.1038/s41598-018-26254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald TJ, Wu G, Nijland MJ, Jenkins SL, Nathanielsz PW, Jansson T. Effect of 30% nutrient restriction in the first half of gestation on maternal and fetal baboon serum amino acid concentrations. Br J Nutr 109: 1382–1388, 2013. doi: 10.1017/S0007114512003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison JL, Regnault TR. Nutrition in pregnancy: optimising maternal diet and fetal adaptations to altered nutrient supply. Nutrients 8: 342, 2016. doi: 10.3390/nu8060342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mparmpakas D, Zachariades E, Goumenou A, Gidron Y, Karteris E. Placental DEPTOR as a stress sensor during pregnancy. Clin Sci (Lond) 122: 349–359, 2012. doi: 10.1042/CS20110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev 27: 141–169, 2006. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 57.Nicolaides KH, Economides DL, Soothill PW. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 161: 996–1001, 1989. doi: 10.1016/0002-9378(89)90770-9. [DOI] [PubMed] [Google Scholar]
- 58.Nijland MJ, Mitsuya K, Li C, Ford S, McDonald TJ, Nathanielsz PW, Cox LA. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol 588: 1349–1359, 2010. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nijland MJ, Schlabritz-Loutsevitch NE, Hubbard GB, Nathanielsz PW, Cox LA. Non-human primate fetal kidney transcriptome analysis indicates mammalian target of rapamycin (mTOR) is a central nutrient-responsive pathway. J Physiol 579: 643–656, 2007. doi: 10.1113/jphysiol.2006.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D; The ALSPAC Study Team; Avon Longitudinal Study of Pregnancy and Childhood . Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. J Clin Endocrinol Metab 85: 4266–4269, 2000. doi: 10.1210/jcem.85.11.6998. [DOI] [PubMed] [Google Scholar]
- 61.Ozanne SE, Jensen CB, Tingey KJ, Martin-Gronert MS, Grunnet L, Brons C, Storgaard H, Vaag AA. Decreased protein levels of key insulin signalling molecules in adipose tissue from young men with a low birthweight: potential link to increased risk of diabetes? Diabetologia 49: 2993–2999, 2006. doi: 10.1007/s00125-006-0466-2. [DOI] [PubMed] [Google Scholar]
- 62.Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48: 547–552, 2005. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- 63.Pantham P, Rosario FJ, Weintraub ST, Nathanielsz PW, Powell TL, Li C, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in maternal nutrient restricted baboons. Biol Reprod 95: 98, 2016. doi: 10.1095/biolreprod.116.141085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perelygin AA, Kammerer CM, Stowell NC, Rogers J. Conservation of human chromosome 18 in baboons (Papio hamadryas): a linkage map of eight human microsatellites. Cytogenet Cell Genet 75: 207–209, 1996. doi: 10.1159/000134484. [DOI] [PubMed] [Google Scholar]
- 65.Pinna LA, Meggio F. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog Cell Cycle Res 3: 77–97, 1997. doi: 10.1007/978-1-4615-5371-7_7. [DOI] [PubMed] [Google Scholar]
- 66.Rajkumar K, Barron D, Lewitt MS, Murphy LJ. Growth retardation and hyperglycemia in insulin-like growth factor binding protein-1 transgenic mice. Endocrinology 136: 4029–4034, 1995. doi: 10.1210/endo.136.9.7544274. [DOI] [PubMed] [Google Scholar]
- 67.Ramadan WS, Alshiraihi I, Al-karim S. Effect of maternal low protein diet during pregnancy on the fetal liver of rats. Ann Anat 195: 68–76, 2013. doi: 10.1016/j.aanat.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Rodríguez-Bernal CL, Rebagliato M, Iñiguez C, Vioque J, Navarrete-Muñoz EM, Murcia M, Bolumar F, Marco A, Ballester F. Diet quality in early pregnancy and its effects on fetal growth outcomes: the Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr 91: 1659–1666, 2010. doi: 10.3945/ajcn.2009.28866. [DOI] [PubMed] [Google Scholar]
- 69.Rogers J, Hixson JE. Baboons as an animal model for genetic studies of common human disease. Am J Hum Genet 61: 489–493, 1997. doi: 10.1086/515527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogers J, Mahaney MC, Witte SM, Nair S, Newman D, Wedel S, Rodriguez LA, Rice KS, Slifer SH, Perelygin A, Slifer M, Palladino-Negro P, Newman T, Chambers K, Joslyn G, Parry P, Morin PA. A genetic linkage map of the baboon (Papio hamadryas) genome based on human microsatellite polymorphisms. Genomics 67: 237–247, 2000. doi: 10.1006/geno.2000.6245. [DOI] [PubMed] [Google Scholar]
- 71.Rosario F, Aye I, Powell T, Jansson T. Folate sensing by placental mtor signaling: a novel mechanism linking maternal folate levels, placental function and fetal development. Placenta 36: 471, 2015. doi: 10.1016/j.placenta.2015.01.386. [DOI] [Google Scholar]
- 72.Sakai K, D’Ercole AJ, Murphy LJ, Clemmons DR. Physiological differences in insulin-like growth factor binding protein-1 (IGFBP-1) phosphorylation in IGFBP-1 transgenic mice. Diabetes 50: 32–38, 2001. doi: 10.2337/diabetes.50.1.32. [DOI] [PubMed] [Google Scholar]
- 73.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. [Erratum in Cell 169: 361–371, 2017.] doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schlabritz-Loutsevitch NE, Dudley CJ, Gomez JJ, Nevill CH, Smith BK, Jenkins SL, McDonald TJ, Bartlett TQ, Nathanielsz PW, Nijland MJ. Metabolic adjustments to moderate maternal nutrient restriction. Br J Nutr 98: 276–284, 2007. doi: 10.1017/S0007114507700727. [DOI] [PubMed] [Google Scholar]
- 75.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol 33: 117–126, 2004. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 76.Schneider MR, Lahm H, Wu M, Hoeflich A, Wolf E. Transgenic mouse models for studying the functions of insulin-like growth factor-binding proteins. FASEB J 14: 629–640, 2000. doi: 10.1096/fasebj.14.5.629. [DOI] [PubMed] [Google Scholar]
- 77.Seferovic MD, Ali R, Kamei H, Liu S, Khosravi JM, Nazarian S, Han VK, Duan C, Gupta MB. Hypoxia and leucine deprivation induce human insulin-like growth factor binding protein-1 hyperphosphorylation and increase its biological activity. Endocrinology 150: 220–231, 2009. doi: 10.1210/en.2008-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah RS, Rajalakshmi R. Studies on human fetal tissues–II. Lipid composition of human fetal tissues in relation to gestational age, fetal size and maternal nutritional status. Indian J Pediatr 55: 272–282, 1988. doi: 10.1007/BF02722197. [DOI] [PubMed] [Google Scholar]
- 79.Shehab MA, Biggar K, Singal SS, Nygard K, Shun-Cheng Li S, Jansson T, Gupta MB. Exposure of decidualized HIESC to low oxygen tension and leucine deprivation results in increased IGFBP-1 phosphorylation and reduced IGF-I bioactivity. Mol Cell Endocrinol 452: 1–14, 2017. doi: 10.1016/j.mce.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singal SS, Nygard K, Dhruv MR, Biggar K, Shehab MA, Li SS, Jansson T, Gupta MB. Co-localization of insulin-like growth factor binding protein-1, casein kinase-2β, and mechanistic target of rapamycin in human hepatocellular carcinoma cells as demonstrated by dual immunofluorescence and in situ proximity ligation assay. Am J Pathol 188: 111–124, 2018. doi: 10.1016/j.ajpath.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singal SS, Nygard K, Gratton R, Jansson T, Gupta MB. Increased insulin-like growth factor binding protein-1 phosphorylation in decidualized stromal mesenchymal cells in human intrauterine growth restriction placentas. J Histochem Cytochem 66: 617–630, 2018. doi: 10.1369/0022155418772574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tapanainen PJ, Bang P, Wilson K, Unterman TG, Vreman HJ, Rosenfeld RG. Maternal hypoxia as a model for intrauterine growth retardation: effects on insulin-like growth factors and their binding proteins. Pediatr Res 36: 152–158, 1994. doi: 10.1203/00006450-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Thiaville MM, Dudenhausen EE, Zhong C, Pan YX, Kilberg MS. Deprivation of protein or amino acid induces C/EBPbeta synthesis and binding to amino acid response elements, but its action is not an absolute requirement for enhanced transcription. Biochem J 410: 473–484, 2008. doi: 10.1042/BJ20071252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C, Zhang R, Zhou L, He J, Huang Q, Siyal FA, Zhang L, Zhong X, Wang T. Intrauterine growth retardation promotes fetal intestinal autophagy in rats via the mechanistic target of rapamycin pathway. J Reprod Dev 63: 547–554, 2017. doi: 10.1262/jrd.2017-050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. J Endocrinol 129: 459–464, 1991. doi: 10.1677/joe.0.1290459. [DOI] [PubMed] [Google Scholar]
- 86.Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 173: 451–462, 2008. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zürcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, Nathanielsz PW, Nijland MJ. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Methods 188: 219–225, 2010. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]



