Abstract
Rapidly growing mycobacteria (RGM) are ubiquitous in the environment and can cause a variety of human infections. Catheter-related bloodstream infections (CRBSI) caused by RGM have been reported predominantly among immunocompromised patients. Removal of central lines and antimicrobial therapy with at least 2 active agents are generally recommended for immunocompromised patients. RGM bloodstream infections (BSIs) are rare in immunocompetent patients and clinical data are very limited. Retrospective medical record review was conducted on patients with blood cultures positive for RGM from July 2012 through March 2015 at Lemuel Shattuck Hospital, a public teaching hospital in Jamaica Plain, Massachusetts, United States. RGM was suspected by presence of beaded Gram-positive bacilli on Gram staining of positive conventional blood cultures and it was confirmed as RGM by Massachusetts State Public Health Laboratory. Nineteen episodes of RGM BSI were identified in 17 patients who had no known immunocompromised conditions that predispose them to opportunistic pathogens. They were predominantly young male with history of intravenous drug use. Peripherally inserted central catheter (PICC) was present in all episodes of RGM BSI and 74% of them clinically improved with PICC removal alone without specific antibiotic therapy for RGM. They were followed up for median duration of 45 days (interquartile range 25–385). The patients remained alive and asymptomatic until the end of follow-up periods. In immunocompetent patients, removal of catheters alone without adding specific antibiotics may be sufficient for RGM CRBSI.
Keywords: Rapidly growing mycobacteria, Bacteremia, Catheter-related bloodstream infection, Immunocompetent, Non-tuberculous mycobacteria, Peripherally inserted central catheter
1. Introduction
Rapidly growing mycobacteria (RGM) are ubiquitous environmental organisms associated with a wide variety of human infections [1]. Catheter-related bloodstream infection (CRBSI) caused by RGM has been predominantly reported among those with immunocompromised conditions, especially hematology/oncology patients [2], [3], [4], [5], [6]. In immunocompromised patients with RGM CRBSI, it is generally recommended to remove catheters and treat with at least two active antimicrobial agents [7]. However, antimicrobial susceptibilities are variable among species and strains, which makes selection of empiric antimicrobial therapy challenging. There is no consensus on duration of treatment, but among case reports and retrospective studies at least 4 weeks of therapy was associated with favorable outcomes [6]. In immunocompetent patients, RGM bloodstream infections (BSIs) are rare and available clinical data are extremely limited [8], [9]. We present a case series of RGM CRBSI among immunocompetent patients.
2. Methods
Retrospective medical record review was conducted on patients who had blood cultures positive for RGM from July 2012 through March 2015 at Lemuel Shattuck Hospital (LSH) in Jamaica Plain, Massachusetts, United States. We obtained approval for retrospective medical record review from Massachusetts Department of Public Health Institutional Review Board. The RGM BSI cases were identified by going through the final identification results of the blood cultures obtained during the study period. The initial number of cases considered for chart review was 20. LSH is a 255-bed, public health, teaching hospital which provides acute, sub-acute and outpatient care for an array of patients from all over the state of Massachusetts, primarily who are socially and economically disadvantaged. The inpatient services offer extended care for patients with a variety of medical, surgical and psychiatric illnesses, as well as services addressing complex socioeconomic issues and substance use disorders.
The microbiology laboratory at LSH initially recognized RGM as resembling beaded Gram-positive bacilli on Gram stain (Fig. 1) prepared from positive BacT/ALERT FAN (fastidious antimicrobial neutralization) FA (aerobic) and FN (anaerobic) blood culture bottles (bioMérieux, Durham, NC) detected by the BacT/ALERT system (bioMérieux, Durham, NC, USA). The blood cultures were plated on conventional blood, chocolate and McConkey agars (Remel, Lenexa, KS). When Gram-positive bacilli resembling acid fast bacilli morphologically were seen on Gram stain and presence of mycobacteria was suspected, blood culture specimens were sent to the Massachusetts State Public Health Laboratory (MA SPHL), Boston, Massachusetts for acid fast staining and species identification due to lack of laboratory conditions required for mycobacterial culture and identification at LSH. At the MA SPHL the specimens were inoculated to Mitchison 7H11 agar (Ramel, Lenexa, KS) and Löwenstein-Jensen medium (Becton Dickinson, Franklin Lakes, NJ). Mycobacterial identification was performed using AccuProbe (Gen-Probe, San Diego, CA), a combination of biochemical reaction tests and matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) Biotyper (Bruker Daltonics, Wissembourg, France).
Fig. 1.
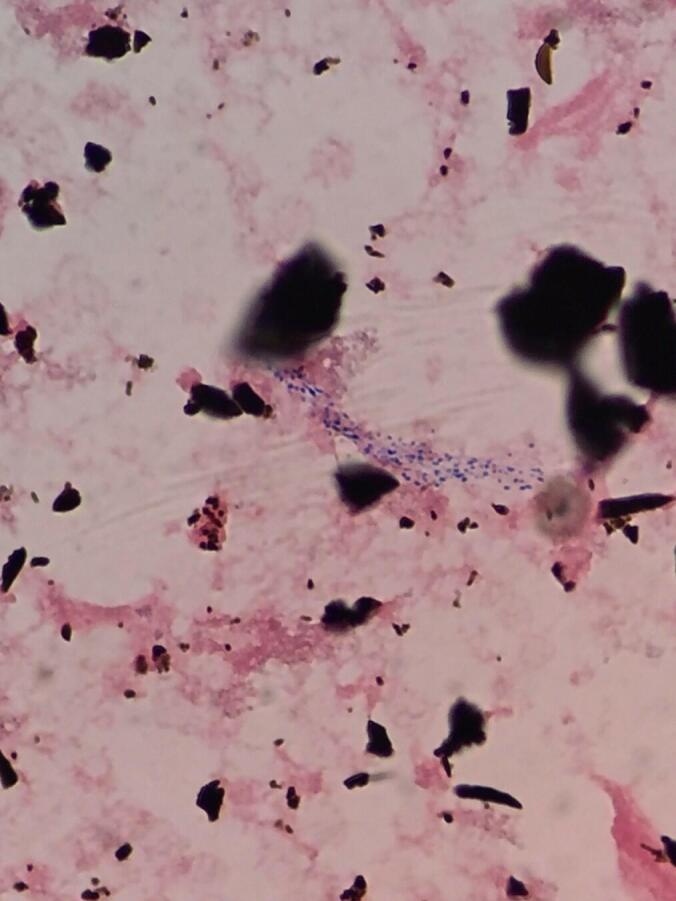
Mycobacterium fortuitum resembling beaded Gram-positive bacilli on Gram stain (×1000) prepared from a positive blood culture bottle (BacT/ALERT FA aerobic bottle).
Fever was defined as temperature ≥38.0 °C and leukocytosis was defined as white blood cell counts ≥10.5 × 103 cells/mm3. RGM CRBSI was defined as either 1) two sets of blood cultures positive for RGM or 2) one set of blood culture positive for RGM with fever or leukocytosis and 3) no potential sources of bacteremia other than indwelling venous catheters based on the chart review.
As RGM BSI cases were increasingly identified, two changes were made by the microbiology laboratory at LSH in December 2013. First, physicians were encouraged to specifically order a send-out mycobacterial blood culture in addition to conventional blood cultures when they suspected BSI. For the mycobacterial blood culture, blood samples were collected in an ethylenediaminetetraacetic acid (EDTA) tube and sent directly to the MA SPHL for direct acid fast staining, inoculation to mycobacterial culture media and identification of mycobacteria if any growth. Second, the routine incubation period for conventional blood culture bottles at LSH was extended from 5 days to 7 days as RGM can take more than 5 days to have growth.
3. Results
Twenty-two episodes of RGM BSI among 20 patients were identified during the study period. One patient was excluded because of a known immunocompromised condition (HIV) and two patients were excluded because Mycobacterium abscessus-chelonae complex grew after 10 days and 43 days of incubation, respectively in mycobacterial culture media only which was suspicious for environmental contamination. Nineteen episodes in 17 patients with RGM BSI with no known history of immunocompromised conditions that predispose them to opportunistic pathogens were included for retrospective chart review. Two patients (patient No. 1 and 2 on Table 1) had 2 separate episodes of RGM BSI at different hospitalizations. Negative blood cultures were confirmed prior to the second episodes and they had new peripherally inserted central catheters (PICCs) when they were admitted to LSH for the second time. Total number of culture positive BSI during the study period was 175. Two sets of blood cultures were obtained in 9 episodes and one set of blood culture was obtained in 10 episodes of RGM BSI. All the blood cultures were obtained peripherally. The reasons for obtaining blood cultures were fever (14 episodes, 74%), leukocytosis (1 episode, 5%) and follow-up blood cultures for the previous bacteremia with organisms other than RGM (4 episodes, 21%). The patients were predominantly young male (Table 1) and 94% (n = 16) and 71% (n = 12) of the patients had a history of intravenous drug use and positive anti-hepatitis C antibody, respectively. In all 19 episodes, PICCs were present for long-term intravenous antibiotic therapy for the primary infectious diseases, most commonly Staphylococcus aureus infections (13 episodes, 68%), either at the time of, or several days prior to RGM BSI. The median length of hospitalization at the time of positive RGM blood cultures was 16 days (interquartile range, 10–32). Fever was common (14 episodes, 74%). Leukocytosis was uncommon (3 episodes, 16%). The most common species of RGM identified was M. fortuitum (11 episodes, 58%) (Table 1). RGM was detected by conventional blood cultures in all episodes of RGM BSI and the median time to blood culture positivity by conventional blood cultures was 5 days (interquartile range, 4–6). In 8 episodes (42%) organisms other than RGM grew from the conventional blood cultures within 14 days before or after the blood cultures positive for RGM (Table 1). All the PICCs were removed and 5 episodes (26%) were treated with specific antimicrobial agents for RGM BSI based on the primary providers’ discretion. The median time from collection of blood cultures that grew RGM to removal of PICCs was 5 days (interquartile range, 0–8). In 11 episodes that were treated with specific antimicrobial agents for RGM or other organisms, sterilization of blood cultures was confirmed by repeat blood cultures, but the rest of 8 episodes did not have follow-up blood cultures to confirm clearance of RGM although they all had clinical improvement and stability. The median follow-up period was 45 days (interquartile range, 25–385), and all the patients were alive and asymptomatic at the end of the follow-up periods.
Table 1.
Demographic, microbiological and treatment characteristics of the patients with rapidly growing mycobacterial catheter-related blood stream infections.
| Pt No | Age in years, sex | Identified RGM species | Antibiotics at blood culture collection | Time to positive blood culture (days) | Antibiotic Tx for RGM | Tx duration (days) | Follow-up duration (days) | Other organisms grown in the blood cultures within 14 days before or after RGM positive |
|---|---|---|---|---|---|---|---|---|
| 1a | 31, M | M. fortuitum | Ceftriaxone | 4 | None | – | 21 | None |
| M. fortuitum | Vancomycin | 5 | Doxycycline, Levofloxacin | NAb | 37 | None | ||
| 2a | 41, M | M. fortuitum | Nafcillin | 4 | None | – | 42 | None |
| M. fortuitum | Nafcillin, Rifampin, Fluconazole | 3 | Linezolid, Rifampin | 7 | 1,013 | None | ||
| 3 | 41, F | RGM spp. | Cefepime | 6 | None | – | 119 | Candida glabrata |
| 4 | 21, M | M. fortuitum | Nafcillin | 5 | None | – | 28 | Acinetobacter lwoffii, Staphylococcus haemolyticus |
| 5 | 23, F | RGM spp. | Ceftaroline | 6 | None | – | 383 | Bacillus cereus, Peptostreptococcus spp. |
| 6 | 29, F | M. fortuitum | Cefazolin | 4 | Ciprofloxacin, Imipenem | 38 | 45 | Candida albicans |
| 7 | 48, F | RGM spp. | Cephalexin | 4 | None | – | 878 | None |
| 8 | 24, F | RGM spp. | Vancomycin | 5 | None | – | 11 | None |
| 9 | 20, M | M. fortuitum | Nafcillin | 5 | Azithromycin, Doxycycline | >13c | 45 | None |
| 10 | 29, F | RGM spp. | Vancomycin | 5 | None | – | 9 | Candida zeylanoides |
| 11 | 23, F | M. abscessus-chelonae | Nafcillin | 4 | None | – | 846 | None |
| 12 | 39, M | RGM spp. | Nafcillin | 5 | None | – | 605 | None |
| 13 | 47, M | M. fortuitum | Ceftriaxone, Vancomycin | 4 | None | – | 20 | None |
| 14 | 25, F | M. fortuitum | Ceftriaxone, Gentamicin | 5 | Amikacin, Clarithromycin Imipenem |
31 | 35 | None |
| 15 | 31, M | RGM spp. | Daptomycin | 5 | None | – | 386 | Cryseobacteria meningosepticum |
| 16 | 37, M | M. fortuitum | Cefepime, Daptomycin | 5 | None | – | 20 | Staphylococcus haemolyticus |
| 17 | 40, M | M. fortuitum | Daptomycin, Piperacillin/tazobactam | 5 | None | – | 176 | None |
Abbreviations: Pt No, patient number; M, male; F, female; RGM, rapidly growing mycobacteria; Tx, treatment; RGM spp., rapidly growing mycobacteria species, not further characterized; NA, not available.
The patient had 2 separate episodes of rapidly growing mycobacterial catheter-related blood stream infection at different hospitalization during the study period.
A decision on duration of therapy was deferred to a primary care provider after discharge.
The patient received 13 days of antibiotic therapy while in the hospital and a decision on the final duration of therapy was deferred to a primary care provider.
4. Discussion
To the best of our knowledge, this is the largest case series of RGM BSI in immunocompetent patients. Rodriguez-Coste et al reported 33 RGM BSIs and 85% of them were CRBSI [9]. They included malignancy, chronic gastrointestinal pathology (inflammatory bowel disease and chronic pancreatitis), diabetes, autoimmune disease, IVDU, sickle cell disease and chronic kidney disease on hemodialysis as comorbidities [9]. Each patient had 3–4 comorbidities which suggests that their patients had at least one immunocompromised condition [9]. Chang et al reported 12 patients with venous-catheter associated RGM bacteremia and one of them had noncancer diagnoses (paroxysmal dystonia, hypoxic encephalopathy and chronic diarrhea) [10]. Hawkins et al reported 6 patients with RGM CRBSI and they included a patient with diabetes mellitus and intractable nausea and vomiting as well as another patient who had gut dysmotility as underlying diseases, but the rest of patients had malignancy, immune system disorder or autoimmune disease [8]. Edun et al conducted a case-control study on non-tuberculous mycobacterial blood stream infections with indwelling vascular catheters [3]. For 16 identified cases, sickle cell disease, diabetes mellitus, COPD, end stage renal disease and malignancy were listed as their comorbidities. Based on the number of patients who had those comorbidities, at least 2 patients did not have any underlying comorbidities, but further details are not available [3]. In our case series there were no patients who had leukopenia, lymphopenia or neutropenia. We had high proportion of patients with history of hepatitis C, but none of them had cirrhosis. Patients with cirrhosis do have multiple immune dysfunctions in neutrophils, lymphocytes, monocytes, macrophages, compliment system, bacterial translocation and pattern recognition receptors [11] which contributes to susceptibilities to opportunistic infections [12]. However, those with chronic hepatitis C without cirrhosis often remain generally healthy for many years [13] and they are not known to be susceptible to opportunistic pathogens. The patients with intravenous drug use are at high risks for acquiring blood-borne infections such as hepatitis B, hepatitis C, HIV and bacteremia through the use of contaminated injection drug equipment. However, those risks are not immunological abnormalities and therefore they are not considered as immunocompromised.
Our experience suggests a benign clinical course of RGM CRBSI after PICC removal, without specific antimicrobial therapy. Many RGM species can participate in biofilms which is likely contributing to CRBSI, and removal of PICC would lead to significant decreases in bacterial burden. In immunocompromised patients, due to risks of disseminated infection, RGM CRBSI is typically treated with empiric combination antibiotic therapy while waiting for identification and susceptibility testing results [7], [9], [14]. However, in immunocompetent patients, with short-term bacteremia, resolution of fever, and no suspected metastatic infection, risks associated with long-term multi-drug antibiotic therapy may outweigh the benefit of empiric therapy for RGM CRBSI.
The median time to blood culture positivity by conventional blood cultures was 5 days in our case series and it took more than 5 days to grow in 2 episodes of RGM BSI. The routine incubation period for bacterial blood culture systems is typically 5 days; RGM BSI cases may be under-recognized among immunocompetent patients due to premature termination of conventional blood culture incubation prior to sufficient growth of RGM. When bacterial concentration is high, mycobacteria may be seen on Gram stain as Gram-positive beaded bacilli [15]. However, microorganisms appearing as Gram-positive bacilli in conventional blood cultures may be left without further speciation if, after clinical judgment, they are deemed to be a contaminant. We believe that a small amount of RGM from the environment or skin contamination would not have grown from the conventional bacterial blood culture bottles in a short incubation period. Biofilm formation of RGM on the catheter lumen should have contributed to increased bacterial load and an event was unlikely by chance in a patient with a central venous catheter.
Our case series has some limitations. We were unable to confirm species level identification for RGM in 7 episodes due to insufficient growth of the isolates in sub-culture. Eight episodes had growth of other organisms within 14 days before or after detection of RGM and fever and leukocytosis could have been related to pathogens other than mycobacteria. We do not expect that the antimicrobial agents used for the primary infection at the time of RGM positivity would have treated RGM BSI of those who improved with PICC removal alone as those antimicrobial agents are not active against RGM except for rifampin used in the second episode of the patient number 2, and this patient did receive specific antibiotic therapy for RGM. Adherence to outpatient clinic visits after discharge was poor and long-term follow-up was undertaken only in the limited number of patients. The source of RGM could not be identified because RGM is ubiquitous in the environment with multiple different strains present simultaneously which made environmental investigation very challenging. Sterilization of blood cultures was not confirmed for all the patients although clinical improvement and stability were confirmed in all cases. We were unable to calculate the incidence of RGM CRBSI per 1,000 PICC days due to lack of data and could not compare our CRBSI incidence with other published incidences.
5. Conclusions
Removal of catheters alone without adding specific antibiotics may be sufficient for immunocompetent patients with RGM CRBSI. Awareness and identification of RGM BSI among immunocompetent patients, especially those with history of intravenous drug use, are needed to accumulate more cases, further characterize them, and establish optimal management strategy.
Ethical Statement
This study was approved by the institutional review boards of the Massachusetts Department of Public Health.
CRediT authorship contribution statement
Masako Mizusawa: Conceptualization, Data curation, Writing - original draft. Tine Vindenes: Writing - review & editing. Sarah Buckley: Writing - review & editing. Catharina Armstrong: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors wish to thank Drs. David Stone (Tufts Medical Center), Katherine McGowan (Brigham and Women's Faulkner Hospital), Roger Clark (Brigham and Women's Faulkner Hospital), and Alfred DeMaria (the Massachusetts Department of Public Health) for offering invaluable guidance and insight during the initial outbreak and for review of the manuscript.
Author statement
Masako Mizusawa conceptualized the manuscript structure, curated data and wrote the original draft of the manuscript. The rest of the authors reviewed and edited the original draft. All authors read and approved the final manuscript.
References
- 1.Porvaznik I., Solovič I., Mokrý J. Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Adv Exp Med Biol. 2017;944:19–25. doi: 10.1007/978-3-319-44488-8_45. [DOI] [PubMed] [Google Scholar]
- 2.Baird S.F., Taori S.K., Dave J., Willocks L.J., Roddie H., Hanson M. Cluster of non-tuberculous mycobacteraemia associated with water supply in a haemato-oncology unit. J Hosp Infect. 2011;79:339–343. doi: 10.1016/j.jhin.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Edun B., Shah A., Durkin M., Whitmire M., Williams S.P., Albrecht H. Non-tuberculous mycobacterial bloodstream infections in patients with indwelling vascular catheters – the role of sickle cell anaemia. Infect Dis. 2017;49:341–346. doi: 10.1080/23744235.2016.1262058. [DOI] [PubMed] [Google Scholar]
- 4.Ashraf M.S., Swinker M., Augustino K.L., Nobles D., Knupp C., Liles D. Outbreak of Mycobacterium mucogenicum bloodstream infections among patients with sickle cell disease in an outpatient setting. Infect Control Hosp Epidemiol. 2012;33:1132–1136. doi: 10.1086/668021. [DOI] [PubMed] [Google Scholar]
- 5.Kline S., Cameron S., Streifel A., Yakrus M.A., Kairis F., Peacock K. An outbreak of bacteremias associated with Mycobacterium mucogenicum in a hospital water supply. Infect Control Hosp Epidemiol. 2004;25:1042–1049. doi: 10.1086/502341. [DOI] [PubMed] [Google Scholar]
- 6.El Helou G., Hachem R., Viola G.M., El Zakhem A., Chaftari A.-M., Jiang Y. Management of rapidly growing mycobacterial bacteremia in cancer patients. Clin Infect Dis. 2013;56:843–846. doi: 10.1093/cid/cis1032. [DOI] [PubMed] [Google Scholar]
- 7.El Helou G., Viola G.M., Hachem R., Han X.Y., Raad I.I. Rapidly growing mycobacterial bloodstream infections. Lancet Infect Dis. 2013;13:166–174. doi: 10.1016/S1473-3099(12)70316-X. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins C., Qi C., Warren J., Stosor V. Catheter-related bloodstream infections caused by rapidly growing nontuberculous mycobacteria: a case series including rare species. Diagn Microbiol Infect Dis. 2008;61:187–191. doi: 10.1016/j.diagmicrobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Coste M.A., Chirca I., Steed L.L., Salgado C.D. Epidemiology of rapidly growing mycobacteria bloodstream infections. Am J Med Sci. 2016;351:253–258. doi: 10.1016/j.amjms.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Chang C.Y., Tsay R.W., Lin L.C., Liu C.E. Venous catheter-associated bacteremia caused by rapidly growing mycobacteria at a medical center in central Taiwan. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2009;42:343–350. [PubMed] [Google Scholar]
- 11.Noor M.T., Manoria P. Immune dysfunction in cirrhosis. J Clin Transl Hepatol. 2017;5:50–58. doi: 10.14218/JCTH.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartoletti M., Giannella M., Tedeschi S., Viale P. Opportunistic infections in end stage liver disease. Infect Dis Rep. 2018:10. doi: 10.4081/idr.2018.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Convery O., Gargan S., Kickham M., Schroder M., O’Farrelly C., Stevenson N.J. The hepatitis C virus (HCV) protein, p7, suppresses inflammatory responses to tumor necrosis factor (TNF)-α via signal transducer and activator of transcription (STAT)3 and extracellular signal-regulated kinase (ERK)–mediated induction of suppressor of cytokine signaling (SOCS)3. FASEB J. 2019;33:8732–8744. doi: 10.1096/fj.201800629RR. [DOI] [PubMed] [Google Scholar]
- 14.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 15.Holland SM. 333 - The Nontuberculous Mycobacteria. In: Goldman L, Schafer AI, editors. Goldmans Cecil Med. Twenty Fourth Ed., Philadelphia: W.B. Saunders; 2012, p. 1948–50. https://doi.org/10.1016/B978-1-4377-1604-7.00333-X.


