Highlights
-
•
Alpha-1 antitrypsin deficiency (AATD) may increase the risk of lung cancer.
-
•
The lung cancer histological types most frequently associated with AATD are squamous carcinoma and adenocarcinoma.
-
•
No differences in lung cancer survival have been found acording to the carrier stuatus of alpha 1 antitrypsin deficient alleles.
Keywords: Alpha-1 antitrypsin deficiency, Lung cancer, Histology, Survival
Abstract
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an inherited genetic disorder associated with a risk of developing lung and liver disease. Several studies have examined its possible association with an increased risk of lung cancer.
Materials and Methods
Systematic review of the scientific literature on studies analyzing the risk of LC associated with AATD, as well as its impact on the histological type and survival. The information was located in the Medline (PubMed), Cochrane, and EMBASE databases.
Results
Six studies including a total of 4 038 patients with LC met the inclusion criteria. Most studies included seem to indicate that AATD increases the risk of developing LC, particularly of the squamous and adenocarcinoma types. This risk increases with exposure to tobacco smoke and the diagnosis of chronic obstructive pulmonary disease (COPD). Only one study analyzed the survival of LC patients without finding differences between AATD and non-AATD patients.
Conclusions
These results suggest that AATD may increase the risk of developing LC, particularly of the squamous and adenocarcinoma histological types, but no impact on patient survival has been demonstrated. However, the low quality of the included studies makes it necessary to carry out more studies with a larger sample size and preferably of a prospective nature to confirm these results.
Introduction
Alpha-1 antitrypsin (AAT) is a glycoprotein synthesized primarily by the hepatocytes (80%) and, in smaller amounts, by other types of cells such as monocytes or macrophages [1]. It is encoded by a gene called SERPINA1 that is located on the long arm of chromosome 14 (q31–32.3) and inherited following an autosomal codominant mechanism [2]. Over 125 genetic polymorphisms of this gene associated with a wide variability of alleles are currently known [3]. The normal and most frequent allele is called PI*M (PI: protease inhibitor). It is present in 85–90% of individuals [4]. The most common deficient alleles are PI*S (encodes 40% active protein) and PI*Z (encodes 15% active protein), and they have a heterogeneous incidence worldwide, ranging between 0.8% and 10% according to several studies [5], [6], [7]. The null alleles are characterized by an altered molecular structure and function, with an AAT production <1% and no detectable AAT plasma level [8]. They are much less frequent than PI*S and PI*Z and produced by nucleotid insertion, deletion or substitution mechanisms.
The main function of AAT is the inhibition of serine proteases, being neutrophil elastase the most important one [9]. Other properties of AAT have been described and include an antiinflammatory or antimicrobial action [10,11]. Severe alpha-1 antitrypsin deficiency (AATD) (plasma levels <57 mg/dl) is associated with deficient genotypes such as PI*SZ, PI*ZZ, and other combinations, including rare or null alleles. The protease/antiprotease imbalance associated with AATD is related to the development of lung disease, such as emphysema [12], particularly in relation to exposure to tobacco smoke. Some deficient genotypes are also related with several forms of liver disease according to the age of presentation (from neonatal cholestasis or juvenile hepatitis in the early years of life, to liver cirrhosis or hepatocarcinoma in adulthood) [13]. The main mechanism of liver disease is the intrahepatic accumulation of AAT polymers. The relationship between AATD and lung [14,15], breast [16], liver [17], or colorectal cancer [18], has been studied. Lung cancer (LC) has a high incidence and mortality in developed countries. Given the relationship of AATD with several lung diseases, it is of interest to determine whether this genetic condition might be associated with a greater predisposition to develop LC and whether there might be differences between smokers and non-smokers. Lung is the most affected organ in adults with severe AATD and several studies have been performed to analyze the relationship between AATD and LC, with different results.
The main objective of this systematic review was to analyze the available scientific evidence of the relationship between being a carrier of an AAT deficient genotype and the risk of developing LC. Secondary objectives were to analyze the impact of AATD on the histological type and the survival of patients with LC.
Methods
Literature search
A literature search was performed in the Medline (PubMed), Cochrane Library, and EMBASE databases. To obtain the necessary information, we implemented a predefined search strategy based on a combination of key words (alpha-1 antitrypsin and lung cancer), using both English and Spanish as search languages. The search period covered from 1 January 1975 through 30 April 2020.
Inclusion and exclusion criteria
We applied the following inclusion and exclusion criteria to select the studies to be included in the systematic review: a) type of study design: case-control studies, cohort studies, systematic reviews and meta-analyses; b) patients’ characteristics: age ≥18 years and belonging to the general population; c) sample size: ≥50 cases of LC and total sample size ≥100 patients; d) AAT plasma levels and phenotype/genotype; and e) pathological diagnosis of LC and absence of a history of previous neoplasms; f) quality of the study≥ 5 points.
We obtained the same information from each of the studies (following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] clinical guidelines available at www.prisma-statement.org) using a standardized procedure [19].
Extraction and synthesis of the information
A data extraction table was created to extract the same information from each of the studies included in the review and assess their quality. We were unable to perform a meta-analysis due to the heterogeneity of the included studies.
Study quality assessment
The full text of the included articles was read by two reviewers (RATP and MTD), and any discrepancy in the selection was resolved by consensus. To evaluate the quality of the studies, we developed a quality scale consisting of the following six items for which we set different scores according to the characteristics of each study: sample size, number of patients with LC, adjustment for outcome variables, type of study design, distribution by genotype, and distribution by histological type in patients presenting with both AATD and LC. By setting different scores for each of the characteristics, we were able to create the continuous numerical scale shown in Table 1.
Table 1.
Quality scale used to evaluate the different studies.
| Measured item | Characteristic | |
|---|---|---|
| 100–500 | 0 | |
| 500–1 000 | 1 | |
| >1 000 | 2 | |
| Number of LC patients | 50–100 | 0 |
| 100–500 | 1 | |
| >500 | 2 | |
| Adjustment for variables (number) | 2 (age and sex) | 0 |
| >2 | 2 | |
| Study design | In-hospital case-control | 0 |
| Population-based case-control | 1 | |
| Cohort | 2 | |
| AATD-LC | No | 0 |
| genotype distribution | Yes | 0 |
| Histological distribution in | No | 1 |
| AATD-LC cases | Yes | 1 |
| Total | 10 | |
AATD: alpha-1 antitrypsin deficiency. LC: lung cancer.
Results
Search results
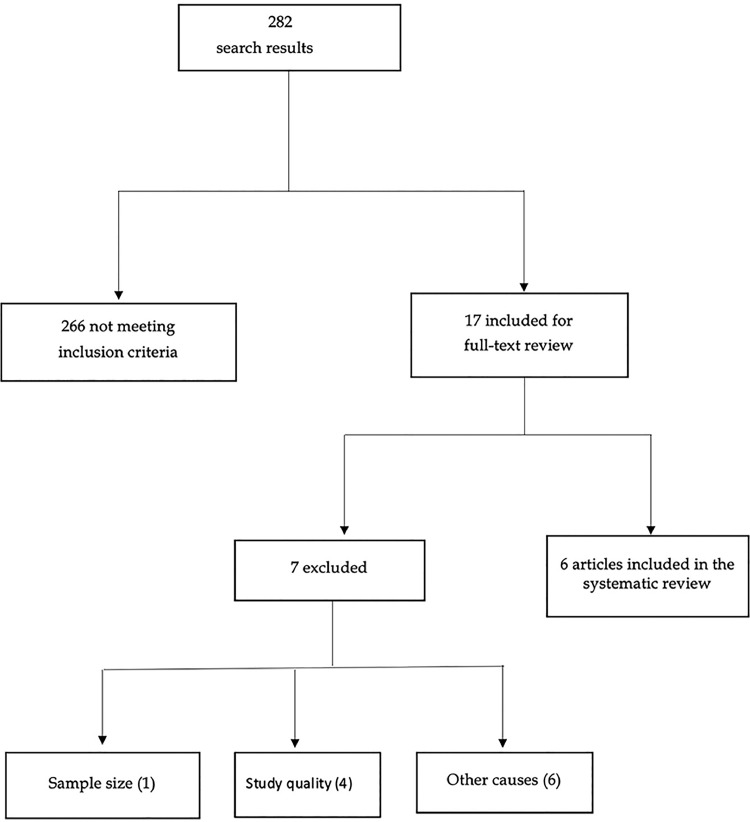
By applying the literature search methods described above, we identified a total of 282 studies of which only six finally met the inclusion criteria. Fig. 1 outlines the detailed description of the study search and inclusion process.
Fig. 1.
Diagram explaining the search and inclusion process.
Five studies had a case-control design, and the other one was a cohort study. These studies were performed in the United States and Europe, and their sample size ranged between 530 and 3 441 patients, of which 186 to 1 856 had a diagnosis of LC. The most frequent causes of exclusion were the study quality (4), inclusion of neoplastic diseases other than LC (2), being conference papers (2), and the text language (2).
AATD and risk of LC
A description of the studies included in the review can be found in Table 2. Yang et al. [20] analyzed the rate of carriers of AAT-deficient phenotypes among 260 LC patients compared with the rate of carriers of this deficiency reported among the Caucasian population of the United States [21]. They found that 12.3% of the patients (11.1% smokers and 20.6% never-smokers) were carriers of deficient phenotypes, and the prevalence of an AAT deficient phenotype rose to 15% among the group of patients with a diagnosis of both chronic obstructive pulmonary disease (COPD) and LC. The same authors carried out a study with a dual case-control design that included a total of 1 856 LC patients, 1 585 community controls, and 902 siblings of the LC patients [14]. In this study they found that the risk of developing LC among the carriers of AAT deficient phenotypes was significantly higher than that of the controls (OR = 1.7; 95% CI = 1.2-2.4) and the siblings of the LC patients (OR = 2; 95% CI = 1.4-2.7). Topic et al. [15] performed a case-control study in Serbia comparing the rate of carriers of AAT-deficient genotypes in a sample of 186 LC patients and in a control group comprised of 1 060 subjects. They found that 5.9% of the LC patients versus 3.7% of the controls had AATD, without finding significant differences between both groups (p = 0.055). In Spain, Torres-Durán et al. [22] performed a multicenter case-control study with never-smoking patients, including 212 cases of LC and 318 controls. They found an increased risk of LC in subjects with a Pi*SS genotype (OR = 4.64; 95% CI = 1.08-19.92), particularly in women (OR = 7.58; 95% CI = 1.40-40.87) and subjects with environmental exposure to tobacco smoke at home for more than 20 years (OR = 12.10; 95% CI = 1.18-123.77).
Table 2.
Characteristics of the studies included in the systematic review.
| Author | Year | Type of study | N | % of smokers with AATD-LC | % of AATD in LC patients | AATD-LC alleles-genotypes (%) | % COPD-LC | AATD-LC OR; 95% CI | AATD-LC histological type (%) | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al. [20] | 1999 | Case-control | LC = 260 Controls = U.S. white population |
78.1 | 12.3 | 9.2% PI*S 2.3% PI*Z |
15 | NS | 23.8% BAC 15.9% Squamous 11.1% ADC |
6 |
| Topic et al. [15] | 2006 | Case-control | LC = 186 Controls =1 060 |
90.9 | 5.9 | 4% PI*MZ 1.6% PI*MS |
NS | 4.51; 1.66–12.29 (Squamous) | 15.1% Squamous 4% ADC |
7 |
| Yang et al. [14] | 2008 | Case-control | LC = 1 856 Controls = 1 585 Siblings-LC = 902 |
NS | 13.4 | NS | 38 | Controls: 1.7; 1.2–2.4 Siblings LC: 2; 1.4–2.7 |
ADC: OR 2; 1.1–3.8 Squamous: OR 2.5; 1.2–2 |
8 |
| Li et al. [24] | 2011 | Cohort | LC = 1 321 | 84.8 | 13.5 | 8% PI*MS 3.3% PI*MZ 2.1% Other (P*MI, PI*SZ, PI*MNull) |
NS | Survival analysis: 0.98; 0.82-1.18 | 55.3% ADC 31.8% Squamous |
10 |
| Enewold et al. [23] | 2012 | Case-control | LC = 203 LC + COPD = 1 18 COPD = 145 Controls = 317 |
NS | 10.8 | COPD-NSCLC African-American: 13.6% PI*S/P*Z Caucasian: 12% PI*S/PI*Z |
NS | NSCLC + COPD (African-Americans): 7.39; 1.03-53.21 | NS | 5 |
| Torres-Duran et al. [22] | 2015 | Case-control | LC = 212 Controls = 318 |
0 | 30.2 | 21.1% PI*MS 3.8% PI*MZ 3.3% PI*SS |
NS | PI*SS: 4.64; 1.08–19.92 Women PI*SS: 7.58; 1.40–40.87 |
77% ADC 9.5% Squamous |
6 |
LC: lung cancer. AATD: alpha-1 antitrypsin deficiency. N: sample size: NS: not specified. ADC: adenocarcinoma. BAC: bronchoalveolar carcinoma.
AATD and histological type
Yang et al. [20] found an association between AATD and the risk of two LC histological types: bronchoalveolar carcinoma (adenocarcinoma histological subtype) and squamous cell carcinoma, with a carrier rate of 23.8% (37.5% never-smokers; p = 0.015) and 15.9% (16.1% smokers; p = 0.011), respectively. The increased risk of adenocarcinoma in carriers AAT deficient alleles, particularly bronchoalveolar carcinoma (OR = 2; 95% CI = 1.1-3.8) and squamous cell carcinoma (OR = 2.5; 95% CI = 1.2-5.3), was confirmed in the subsequent study performed by the same research group [14]. Topic et al. [15] also identified an increased risk of squamous cell carcinoma (OR = 4.51; 95% CI = 1.66-12.29) in carriers of PI*MZ and PI*MS genotypes.
AATD and survival
In a prospective cohort study, Li et al. [24] analyzed the survival of patients with non-small cell lung cancer (NSCLC) associated with AATD. This study included 1 321 LC patients (179 AATD carriers, of which 106 [59%] had a PI*MS genotype, 44 [25%] a PI*MZ genotype, and 29 [16%] another genotype) with a mean survival of 2.9 years in the group of AATD carriers (95% CI = 2.3-4) and of 2.7 years in the group of non-carriers (95% CI = 2.4-3). Although they detected no difference between the survival rates of both groups (OR = 0.98; 95% CI = 0.82-1.18), they did observe a higher mortality associated with higher plasma concentrations of AAT ([AHR] for increments of 50 mg/dl = 1.15; 95% CI = 1.11-1.21; p <0.01).
Quality of the included studies
The scores of the different studies ranged between 5 and 10 points, with a mean score of 7. The highest score was obtained by Li et al.’s cohort study [24], whose sample size was greater than 1 000 LC patients.
Discussion
This is the first systematic review analyzing the relationship between AATD and the risk of developing LC. The results of the studies included in this review suggest that AATD may increase the risk of developing LC and be associated with the histological types adenocarcinoma and squamous cell carcinoma. The authors of the only study analyzing the impact of the AATD on survival [24] found no differences based on the carrier state of AAT-deficient alleles, and even observed worse prognosis and survival rates associated with higher plasma levels of AAT. This finding could be related with the AAT role as an acute phase reactant [25].
As defined by the World Health Organization (WHO), AATD is a rare (<5 cases/10,000 people) and clearly under-diagnose genetic condition [26]. This data, along with the known heterogeneity in the geographical distribution of deficient genotypes [27], is a key fact in the interpretation of the results of studies that have analized the association between AATD and risk of LC.
Torres-Duran et al. [22], which study was carried out in the northwest of Spain, where there is a high incidence of carriers of AAT deficient alleles, especially PI*S; found an association of lung cancer risk with the homozygous PI*SS genotype (OR = 4.64, 95% CI = 1.08–19.92). In Serbia, Topic et al. [15] found that 11 patients from sample of 186 lung cancer cases (5.9%) were AATD heterozygotes with moderate deficiencies (PI*MZ and PI*MS phenotypes). When compared with AATD heterozygote frequency in Serbian healthy populations (3.7%), the difference was close to the statistical significance (p = 0.055). In this study, individuals with AATD phenotypes had as an increased risk of developing squamous cell lung cancer than those with non-deficient AAT variants (OR = 4.51; 95% CI = 1.66-12.29); consistent with the data described in previous epidemiological studies [5,6]. However, other studies performed in geographical areas with a low prevalence of AAT deficient alleles failed to demonstrate an association between AATD and the risk of developing LC [28]. Ethnic differences can also have an impact on the risk of developing LC, as demonstrated by Enewold et al., [23] who observed an increased risk of NSCLC in African-American patients with AATD and a simultaneous diagnosis of COPD (OR = 7.39; 95% CI = 1.03-53.21), but not among the caucasian population.
Several mechanisms have been described as the causes of this potential association. The protease/antiprotease imbalance associated with moderate-severe AATD results in an increase in the proteolytic activity of various serine proteases (neutrophil elastase being the most important one), which leads to the generation of a cellular microenvironment that favors the molecular mechanisms of carcinogenesis and tumor progression, as previously described in the case of solid organ tumors such as those of liver, bladder, or lung cancers [29]. In addition, the intrahepatocyte polymerization of the PI*Z allele and, to a lesser extent, the PI*S allele; their structural alteration and reduced production, results in a decrease in the circulating plasma levels of AAT that, added to the molecular oxidation associated with exposure to tobacco smoke, acts as an intermediary mechanism in the onset of pulmonary emphysema [29] and further increase in the risk of developing LC [30].
The studies with the largest sample size were those conducted in the USA. The two studies carried out by Yang et al. [14,20]at the Mayo Clinic (Rochester, Minnesota, USA) included over 2 000 cases of LC in a geographical area with 2.45% and 1.42% of PI*S and PI*Z allele carriers, respectively. These authors consistently observed an increased rate of AAT deficient alleles carriers in the LC patients group compared with the general US white population (12.3%vs. 7%; p = 0.002 [20]) and they found a significantly increased lung cancer risk among carriers of these deficient alleles compared with controls (OR = 1.7, 95% CI = 1.2-2.4 [14]); however, none of the studies describe the distribution by deficient alleles in patients with LC. Not being aware of the distribution of these alleles limits the interpretation of the potential role and mechanisms of AATD in the carcinogenic process in LC.
The role of AAT in the survival of patients with different neoplasms has been analyzed in several studies [31,32]. AAT is an acute phase reactant (APR) and its plasma levels increase in response to the systemic inflammatory states that arise in different types of neoplastic diseases. This factor has been shown to be related to poorer survival rates in neoplasms such as pancreatic and breast cancers [32,33]. The results of the study performed by Li et al.[24]. are similar for LC. These authors found no differences in the survival of carriers and non-carriers of AAT-deficient alleles (OR = 0.98; 95% CI = 0.82-1.18), but did observe worse survival rates associated with higher plasma levels of AAT (AHR for increments of 50 mg/dl = 1.15; 95% CI = 1.11-1.21; p <0.01) and in the blood samples collected before starting treatment (AHR for increments of 50 mg/dl = 1.39; 95% CI = 1.19-1.63). Although we identified no studies analyzing the response of patients with LC and AATD to treatment, we did find evidence of a worse response to chemotherapy among patients with AATD and other types of malignancies, such as prostate cancer or melanoma [34].
Not all of the studies analyzed the histological distribution among the patients with LC and AATD, but those that did [14,15,20,22,24], found an association of AATD with the adenocarcinoma and squamous cell carcinoma histological types, although without being able to establish a different onset mechanism other than the fact that they represent the two most frequent histological types present in the general population [35].
The main advantage of our review is that it includes studies carried out in different geographical areas, thus providing risk information of international nature, including areas with different frequencies and distribution of the deficient AAT alleles.
The main limitation of this review is the low quality and heterogeneity of the study designs, followed by the lack of a description of the deficient alleles distribution and the histological type in the AATD and LC group.
To conclude, AATD may increase the risk of developing LC, particularly of squamous and adenocarcinoma types. This risk increases with exposure to tobacco and previous diagnosis of COPD. The influence of AATD on the survival of patients with LC has not been demonstrated. The findings of this systematic review should be interpreted with caution given the low quality and the small number of included studies, nevertheless it represents the starting point for future research to confirm these results. In this direction, it would be useful to design a study in countries with a relative high prevalence of AATD, such as the countries of Central and Western Europe, which would allow a better quantification of this possible relationship with LC and to delve into the mechanisms responsible for the association of AATD with the histology of squamous carcinoma and adenocarcinoma as well as providing more information on the survival of patients with AATD and LC.
CRediT authorship contribution statement
Ramón A. Tubío-Pérez: Data curation, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. María Torres-Durán: Conceptualization, Data curation, Formal analysis, Supervision, Validation, Writing - review & editing. Alberto Fernández-Villar: Supervision, Validation, Writing - review & editing. Alberto Ruano-Raviña: Conceptualization, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
None
Acknowledgments
Acknowledgments
This study is part of the work aimed of the completion of the PhD Degree of Ramón A. Tubío-Pérez
Funding
This paper has been funding by the Instituto de Salud Carlos III (ISCIII) and FEDER (PI19/00350)
Footnotes
This work is part of the research conducting to the PhD Degree of Ramón A. Tubío-Pérez.
References
- 1.Carlson J.A., Rogers B.B., Sifers R.N. Multiple tissues express alpha 1-antitrypsin in transgenic mice and man. J. Clin. Invest. 1988;82(1):26–36. doi: 10.1172/JCI113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long G.L., Chandra T., Woo S.L. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- 3.Salahuddin P. Genetic variants of alpha1-antitrypsin. Curr. Protein Pept. Sci. 2010;11(2):101–117. doi: 10.2174/138920310790848368. [DOI] [PubMed] [Google Scholar]
- 4.Kueppers F., Christopherson M.J. Alpha1-antitrypsin: further genetic heterogeneity revealed by isoelectric focusing. Am. J. Hum. Genet. 1978;30(4):359–365. [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco I., Bueno P., Diego I. Alpha-1 antitrypsin Pi*SZ genotype: estimated prevalence and number of SZ subjects worldwide. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:1683–1694. doi: 10.2147/COPD.S137852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco I., Bueno P., Diego I. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:561–569. doi: 10.2147/COPD.S125389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Serres F., Blanco I. Role of alpha-1 antitrypsin in human health and disease. J. Intern. Med. 2014;276(4):311–335. doi: 10.1111/joim.12239. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.H., Brantly M. Molecular mechanisms of alpha1-antitrypsin null alleles. Respir. Med. 2000;94(Suppl C):S7–11. doi: 10.1053/rmed.2000.0851. [DOI] [PubMed] [Google Scholar]
- 9.Sinden N.J., Baker M.J., Smith D.J. α-1-antitrypsin variants and the proteinase/antiproteinase imbalance in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(2):L179–L190. doi: 10.1152/ajplung.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pott G.B., Chan E.D., Dinarello C.A. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 2009;85(5):886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaner Z., Ochayon D.E., Shahaf G. Acute phase protein α1-antitrypsin reduces the bacterial burden in mice by selective modulation of innate cell responses. J. Infect. Dis. 2015;211(9):1489–1498. doi: 10.1093/infdis/jiu620. [DOI] [PubMed] [Google Scholar]
- 12.Tobin M.J., Cook P.J., Hutchison D.C. Alpha 1 antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in subjects homozygous for Pi type Z. A survey by the British thoracic association. Br. J. Dis. Chest. 1983;77(1):14–27. doi: 10.1016/0007-0971(83)90002-5. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell E.L., Khan Z. Liver disease in alpha-1 antitrypsin deficiency: current approaches and future directions. Curr. Pathobiol. Rep. 2017;5(3):243–252. doi: 10.1007/s40139-017-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang P., Sun Z., Krowka M.J. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch. Intern. Med. 2008;168(10):1097–1103. doi: 10.1001/archinte.168.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topic A.S., Jelic-Ivanovic Z.D., Spasojevic-Kalimanovska V.V. Association of moderate alpha-1 antitrypsin deficiency with lung cancer in the Serbian population. Arch. Med. Res. 2006;37(7):866–870. doi: 10.1016/j.arcmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 16.El-Akawi Z., Nusier M., Sawalha D. Alpha-1 antitrypsin genotypes in breast cancer patients. Breast Cancer Res. 2005;7(S2) P1.19, bcr1106. [Google Scholar]
- 17.Sparos L., Tountas Y., Chapuis-Cellier C. Alpha 1-antitrypsin levels and phenotypes and hepatitis B serology in liver cancer. Br. J. Cancer. 1984;49(5):567–570. doi: 10.1038/bjc.1984.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Holanda S., Blanco I., Menéndez M. Serum concentration of alpha-1 antitrypsin is significantly higher in colorectal cancer patients than in healthy controls. BMC Cancer. 2014;14:355. doi: 10.1186/1471-2407-14-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang P., Wentzlaff K.A., Katzmann J.A. Alpha1-antitrypsin deficiency allele carriers among lung cancer patients. Cancer Epidemiol. Biomark. Prev. 1999;8(5):461–465. [PubMed] [Google Scholar]
- 21.DeCroo S., Kamboh M.I., Ferrell R.E. Population genetics of alpha-1-antitrypsin polymorphism in US whites, US blacks and African blacks. Hum. Hered. 1991;41(4):215–221. doi: 10.1159/000154004. [DOI] [PubMed] [Google Scholar]
- 22.Torres-Durán M., Ruano-Ravina A., Parente-Lamelas I. Alpha-1 antitrypsin deficiency and lung cancer risk: a case-control study in never-smokers. J. Thorac. Oncol. 2015;10(9):1279–1284. doi: 10.1097/JTO.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 23.Enewold L., Mechanic L.E., Bowman E.D. SERPINA1 and ELA2 polymorphisms are not associated with COPD or lung cancer. Anticancer Res. 2012;32(9):3923. -8. [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Krowka M.J., Qi Y., Katzmann J.A. Alpha1-antitrypsin deficiency carriers, serum alpha 1-antitrypsin concentration, and non-small cell lung cancer survival. J. Thorac. Oncol. 2011;6(2):291–295. doi: 10.1097/JTO.0b013e31820213fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouronte-Roibás C., Leiro-Fernández V., Ruano-Raviña A. Predictive value of a series of inflammatory markers in COPD for lung cancer diagnosis: a case-control study. Respir. Res. 2019;20(1):198. doi: 10.1186/s12931-019-1155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpha 1-antitrypsin deficiency: memorandum from a WHO meeting. Bull. World Health Organ. 1997;75(5):397–415. [PMC free article] [PubMed] [Google Scholar]
- 27.de Serres F.J., Blanco I., Fernández-Bustillo E. Genetic epidemiology of alpha-1 antitrypsin deficiency in North America and Australia/New Zealand: australia, Canada, New Zealand and the United States of America. Clin. Genet. 2003;64(5):382–397. doi: 10.1034/j.1399-0004.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 28.El-;Akawi Z.J., Nusier M.K., Zoughool F.E. Relationship between alpha-;1 antitrypsin deficient genotypes S and Z and lung cancer in Jordanian lung cancer patients. Saudi Med. J. 2006;27(2):181. -;4. [PubMed] [Google Scholar]
- 29.Sun Z., Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5(3):182–190. doi: 10.1016/S1470-2045(04)01414-7. [DOI] [PubMed] [Google Scholar]
- 30.Senn O., Russi E.W., Imboden M. alpha1-Antitrypsin deficiency and lung disease: risk modification by occupational and environmental inhalants. Eur. Respir. J. 2005;26(5):909–917. doi: 10.1183/09031936.05.00021605. [DOI] [PubMed] [Google Scholar]
- 31.Mouronte-Roibás C., Leiro-Fernández V., Fernández-Villar A. COPD, emphysema and the onset of lung cancer. Syst. Rev. Cancer Lett. 2016;382(2):240–244. doi: 10.1016/j.canlet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Trichopoulos D., Tzonou A., Kalapothaki V. Alpha 1-antitrypsin and survival in pancreatic cancer. Int. J. Cancer. 1990;45(4):685–686. doi: 10.1002/ijc.2910450419. [DOI] [PubMed] [Google Scholar]
- 33.El-Akawi Z.J., Al-Hindawi F.K., Bashir N.A. Alpha-1 antitrypsin (alpha1-AT) plasma levels in lung, prostate and breast cancer patients. Neuro Endocrinol. Lett. 2008;29(4):482–484. [PubMed] [Google Scholar]
- 34.Ljujic M., Mijatovic S., Bulatovic M.Z. Alpha-1-antitrypsin antagonizes cisplatin-induced cytotoxicity in prostate cancer (PC3) and melanoma cancer (A375) cell lines. Pathol. Oncol. Res. POR. 2017;23(2):335–343. doi: 10.1007/s12253-016-0104-3. [DOI] [PubMed] [Google Scholar]
- 35.Cheng T.-Y.D., Cramb S.M., Baade P.D. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J. Thorac. Oncol. 2016;11(10):1653–1671. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]