Abstract
Prevention of bronchopulmonary dysplasia (BPD) in premature-birth babies continues to be an unmet medical need. Intramuscular vitamin A is currently employed in preterm neonates to prevent BPD but requires intramuscular injections in fragile neonates. We hypothesized that noninvasive inhaled delivery of vitamin A, targeted to lung, would be a more effective and tolerable strategy. We employed our well-established hyperoxia-injury neonatal rat model, exposing newborn rats to 7 days of constant extreme (95% O2) hyperoxia, comparing vitamin A dosed every 48 h via either aerosol inhalation or intramuscular injection with normoxic untreated healthy animals and vehicle-inhalation hyperoxia groups as positive and negative controls, respectively. Separately, similar vitamin A dosing of normoxia-dwelling animals was performed. Analyses after day 7 included characterization of alveolar histomorphology and protein biomarkers of alveolar maturation [surfactant protein C (SP-C), peroxisome proliferator-activated receptor (PPAR) γ, cholinephosphate cytidylyl transferase, vascular endothelial growth factor and its receptor, FLK-1, and retinoid X receptors (RXR-α, -β, and -γ], apoptosis (Bcl2 and Bax) key injury repair pathway data including protein markers (ALK-5 and β-catenin) and neutrophil infiltration, and serum vitamin A levels. Compared with intramuscular dosing, inhaled vitamin A significantly enhanced biomarkers of alveolar maturation, mitigated hyperoxia-induced lung damage, and enhanced surfactant protein levels, suggesting that it may be more efficacious in preventing BPD in extremely premature infants than the traditionally used IM dosing regimen. We speculate lung-targeted inhaled vitamin A may also be an effective therapy against other lung damaging conditions leading to BPD or, more generally, to acute lung injury.
Keywords: chronic lung disease of prematurity, inhalation dosing, lung injury repair, preterm birth, vitamin a palmitate
INTRODUCTION
Despite continuing improvements in perinatal respiratory support, such as the use of gentler modes of ventilation and the use of lower concentrations of oxygen, the incidence of bronchopulmonary dysplasia (BPD), also known as chronic lung disease (CLD) of prematurity, in extremely premature infants has not decreased (1). In fact, with the increasing survival of extremely premature infants, the prevalence of BPD continues to increase as have associated costs to treat these infants. Children with BPD experience an acute complicated clinical course and too often manifest poor physical growth, neurodevelopmental delays, bronchial hyperreactivity, repeated hospitalizations, and increased mortality (8, 12). The use of postnatal surfactant provides symptomatic relief from respiratory distress syndrome (RDS); however, this has only very modestly translated into a reduction in preventing BPD (13), as surfactants alone do not address the fundamental underlying issue of lung immaturity.
Vitamin A, a potent morphogen, is essential for lung development, particularly for normal alveolarization, and its deficiency has been posited to play a significant role in BPD pathogenesis (2, 7, 26, 27). Vitamin A accumulates primarily in the third trimester of pregnancy, and thus significant vitamin A deficiency and reduced hepatic stores of retinoids are observed with increasing prematurity (11) and are especially prevalent in extremely low birth weight infants, who are at highest risk for BPD (7, 27). Both experimental models and clinical studies have shown that vitamin A supplementation facilitates recovery from lung injury, accelerates alveolar morphogenesis, and reduces the incidence of BPD (6, 30, 35), thus providing compelling evidence to support vitamin A supplementation to prevent and possibly treat BPD and its sequelae. Several clinical studies support vitamin A’s effectiveness in preventing BPD, primarily administered via intramuscular dosing regimens (3, 31). Intramuscular dosing is required because of poor absorption of vitamin A by the immature gastrointestinal tract (22). Nevertheless, despite strong evidence-based clinical data, vitamin A supplementation for preventing BPD has had limited uptake in neonatal clinical practice, in part due to pain and tissue injury associated with repeated invasive intramuscular injections, typically 12 total doses of 5000 IU of vitamin A palmitate delivered over 4 wk as the established clinically effective dose (5, 34).
Direct-to-target organ (lung) delivery of aerosolized vitamin A is a very appealing strategy to prevent and possibly reverse the histopathological changes observed in BPD, a hypothesis we tested in our current study of inhalation dosing of a vitamin A formulation optimized for aerosolization, versus the typical intramuscular delivery. We used a well-established hyperoxia-induced neonatal lung injury rat model known to mimic the histopathologic changes observed in BPD (4) to compare the effects of inhaled vitamin A dosing versus the traditional parenteral intramuscular dosing, analyzing key lung development and injury repair biomarkers and lung morphometry. This approach allows us to establish that inhaled vitamin A delivery offers superior lung injury protective effects, supporting the proposed advantage of direct-to-target organ dosing by aerosol inhalation, while also employing a noninvasive dosing approach that overcomes the concerns of repeated intramuscular dosing in extremely premature infants.
MATERIALS AND METHODS
Animals and conditions.
One-day-old Sprague-Dawley rat pups were exposed to hyperoxia following our previously described methods (4, 15, 21, 24). Briefly, time-mated pregnant dams delivered spontaneously at term and postnatal day (PD) 1 pups were placed in either 21% or 95% O2 for 7 days in Plexiglas chambers along with lactating mothers. Oxygen concentration was monitored and adjusted based on monitoring via an oxygen analyzer. Pups were separated from dams only to effectuate the vitamin A or vehicle dosing, as outlined below. To avoid the confounding effects of variation in nutritional intake between the experimental groups, litter size was adjusted to 8 pups (4 males and 4 females) and, to avoid O2 toxicity to dams exposed to hyperoxia, dams were switched every 24 h between the 21% O2 and 95% O2 exposure groups. At the end of the experimental period, pups were euthanized for sample collections, as described below. All animal procedures were performed at The Lundquist Institute for Biomedical Innovation at Harbor-ULCA Medical Center following the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at The Lundquist Institute (Approval # 31324–01). In line with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association, animals were euthanized with an excess of pentobarbital sodium (200 mg/kg) administered intraperitoneally and cervical dislocation for the pups as the secondary method of euthanasia.
Vitamin A formulation.
We prepared water-miscible vitamin A palmitate as a solution of 50,000 IU/mL pharmaceutical-grade vitamin A palmitate (DSM Nutritionals, Parsippany, NJ) solubilized with 12% polysorbate 80, with the balance of the volume being water and with citric acid or sodium hydroxide to achieve neutral pH. A vehicle-only 12% polysorbate 80 solution was also prepared, lacking only vitamin A palmitate but otherwise identical to the test article. Solutions were sterile-filtered through 0.22-μm membranes as a final step in preparation.
Vitamin A dose level.
The target vitamin A dose employed for neonatal rats was 5 IU/g body mass, scaled by body mass based on typical neonatal intensive care unit (NICU) dosing: NICU dosing of 5,000 IU delivered intramuscularly every 48 h equates to 5 IU/g based on the average body mass of a 1-kg extremely low birth weight (ELBW) premature human infant. Pups were weighed to determine the exact dose at each time point. Dosing was performed on alternate days (PD 1, 3, 5, and 7), maintaining the every-other-day cadence (3 times/wk) of the typical NICU course (although we acknowledge that the development cycle of newborn rats is accelerated vs. human neonates). The stock vitamin A palmitate formulation was diluted as necessary in sterile saline to facilitate accurate dose delivery by either injection or inhalation routes. Vehicle doses were of identical volume and formulation, only lacking vitamin A and including the same saline dilution.
Intramuscular dosing.
For injection, the dose was 5 IU/g body mass. Intramuscular injections were administered on the anterior aspect of the upper thighs using insulin syringes. Injection volume was 30 μL.
Inhalation dosing.
Whole body aerosol exposure was performed using our previously described methods (15, 28). Matching previous efforts, we nebulized a 5-fold excess of the target vitamin A dose to account for a nominal 20% dose delivery efficiency based on our experience and per previous description (17, 18). As such, 25 IU/g body mass was aerosolized for each animal. Pups were typically dosed communally using whole body exposure chambers. The cup of a vibrating-mesh nebulizer (Aerogen, Galway, Ireland) was loaded with a final volume of 1.5 mL comprised of vitamin A or vehicle stocks diluted into sterile isotonic saline, which was then aerosolized into the exposure chamber for inhalation. Pups (without dams) dwell in the chamber for 30 min to ensure thorough respiratory deposition well beyond the time for complete nebulization of the 1.5 mL (~12 min; data not shown).
Samples.
Animals were euthanized on PD 7, 4–6 h after any PD 7 exposure, to collect lungs and blood. Portions of lung were either snap-frozen, paraformaldehyde-inflation fixed, or cultured as explants, following previously described methods (15, 28). Blood was allowed to clot and centrifuged to yield serum that was quickly aliquoted and frozen. All samples were stored at −80°C and freeze-thaw cycling was minimized to preserve sample integrity.
Lung morphometry.
For lung histomorphometry, radial alveolar count (RAC), mean linear intercept (MLI), and alveolar septal thickness (AST) were determined following previously described methods (4, 15, 28).
Protein extraction and western blot analysis.
Liquid nitrogen flash-frozen lung tissue was homogenized with a tissue grinder in lysis buffer (RIPA buffer) containing 1 mM EDTA and EGTA each (Boston Bioproducts, Ashland, MA) supplemented with 1 mM PMSF and complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). For each sample, 50 μg of total protein was denatured with SDS sample buffer and electrophoresed in 10% SDS polyacrylamide gel. Resolved samples were then transferred onto a 0.45-μm nitrocellulose membrane and, after blocking with TBS-Tween (TBST) + 5% milk, were probed with primary antibodies [Bcl-2 (1:200, cat# sc-492), Bcl-2-associated X protein (Bax; 1:350; cat# sc-493), peroxisome proliferator-activated receptor gamma (PPAR-γ; 1:500; cat # sc-7196), surfactant protein C (SP-C; 1:250; cat # sc-518029), CTP:phosphocholine cytidylyltransferase subunit alpha (CCT-α; 1:200; cat # sc-376107), β-catenin (1:500; cat# sc-7963), activin receptor-like kinase 5 (ALK-5; 1;200; cat# sc-398), fetal liver kinase 1 (FLK-1; 1:200; cat# sc-6251) (all from Santa Cruz Biotechnology, Santa Cruz, CA), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000; cat# MAb374, MilliporeSigma, Burlington, MA), and retinoid X receptor alpha (RXRα; 1:800; cat# NBP2–20130, Novus Biologics, Littleton, CO)] overnight at 4°C, followed by appropriate secondary antibody and Super-Signal Chemiluminescent substrate (Pierce Chemicals, Rockford, IL). The validation and specificity of the antibodies used have been demonstrated in previous publications (4, 9, 19, 23), specifically demonstrating alveolar cell specific localization of SP-C, β-catenin, and PPARγ by immunostaining for the relevant cell-specific markers. ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantitate protein bands, which were measured by densitometry and expressed relative to accompanying GAPDH bands as relative units.
Immunostaining.
Immunofluorescence staining of PPAR-γ; 1:50, β-catenin; 1:100, RXRα; 1:200, RXRβ (Cat: NBP1–33543, Proteintech, Rosemont, IL); 1:100, and RXRγ (Cat: ab15518, Proteintech), 1:100 was performed as previously described (15, 36) and was merged using Photoshop software. Briefly, 5-µm sections were incubated with appropriate primary antibodies at 4°C overnight; Alexa Fluor 594 donkey anti-mouse IgG (1:250 dilution for β-catenin; cat# A 10037, Invitrogen, Carlsbad, CA), and Alexa Flour 488 goat anti-rabbit IgG (1:250 dilution for RXRα and 1:100 dilutions for both RXRβ and RXRγ; cat# A1137, Invitrogen), and Alexa Fluor 594 or 488 goat anti-rabbit IgG (1:250 dilution for PPARγ; cat# A11034 and A11037, Invitrogen) were applied to the sections for 15 min at room temperature. The sections were washed with phosphate-buffered saline and then mounted with Pro-Long Gold antifade reagent with DAPI (Invitrogen) for visualization under a fluorescence microscope by a single blinded investigator. Immunostaining for myeloperoxidase (MPO) [1:50, primary antibody (Cat: 22225–1-AP, Proteintech) and 1: 200 secondary antibody (anti rabbit, Cat: UC282766, Invitrogen)] was performed using a previously described method (4).
Measurement of rate of surfactant phospholipid synthesis.
De novo surfactant phospholipid synthesis was determined by the incorporation of [3H]choline chloride (NEN Dupont, Boston, MA) into DSPC in cultured lung explants, as described previously (20).
Vitamin A level in serum was determined by enzyme-linked immunosorbent assay (ELISA) following manufacturer’s protocol (Aviva Systems Biology, San Diego, CA).
Statistical analyses.
The data were analyzed using ANOVA for pairwise comparisons. The results are based on 5–7 independent experiments, and the values are expressed as means ± SE. A P value of <0.05 is considered to represent statistically significant difference between the experimental groups. Sex was tracked as a variable; however, since no significant differences were observed between males and females in any of the parameters examined, for all statistical comparisons, data from both sexes were combined. Furthermore, since the vehicle-treated group was not different from the untreated group, data only from vehicle-treated controls are included for comparison with the experimental groups.
RESULTS
Study design.
Parallel groups were defined to enable various relevant comparisons, including vitamin A dosing versus vehicle dosing, inhalation-dosing versus intramuscular dosing, and hyperoxia versus normoxia conditions. A separate control group of completely untreated normoxia-exposed animals was also assessed.
Effect of vitamin A on hyperoxia-induced damage to neonatal lung tissue.
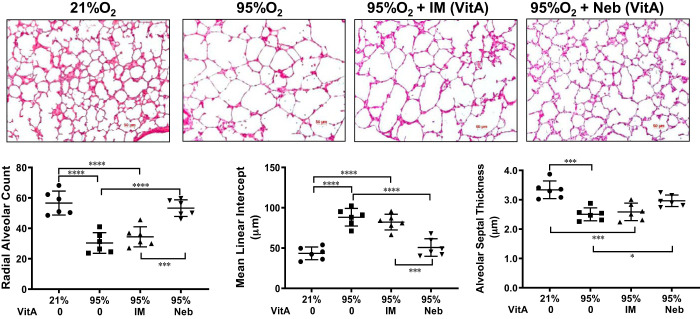
In line with the known effects of hyperoxia on postnatal lung development (4), lungs of hyperoxia-exposed untreated pups demonstrated larger alveoli, i.e., a significant reduction in RAC and an increase in MLI, accompanied by a significant reduction in alveolar septal thickness (Fig. 1). The expected damage observed in untreated (vehicle treated) hyperoxia-exposed animals is evident versus healthy normoxia control (vehicle treated) lungs. These respective conditions also define the boundaries of the range of effects to judge the efficacy of concurrent vitamin A dosing in blocking hyperoxia-induced damage. Hyperoxia-exposed lungs treated with inhaled vitamin A demonstrated near normal RAC and MLI and AST; in contrast, intramuscular vitamin A-administered lungs showed only a modest, statistically insignificant, difference from the vehicle-treated hyperoxia group in all morphometric parameters examined.
Fig. 1.
Histomorphology images (top) show the inherent tissue damage caused to neonatal lung tissue by untreated hyperoxia (95% O2 vehicle) compared with healthy normal (21% O2). The unique ability of nebulized vitamin A (VitA) dosing (right) to substantially mitigate that damage contrasts with weakly effective intramuscular (IM)-dosed results. Vehicle doses were by nebulizer (Neb). A representative image for each group is shown. Histomorphometric measurements (bottom) show quantitation from images of radial alveolar count, mean linear intercept, and alveolar septal thickness; n = 6 animals for each group. The mean (wide horizontal line) ± SE (narrow horizontal lines) is shown for each group. Pairwise P value comparisons where differences are statistically significant are shown: *P < 0.05, ***P < 0.001, or ****P < 0.0001.
Effect of vitamin A on hyperoxia-induced neonatal lung injury.
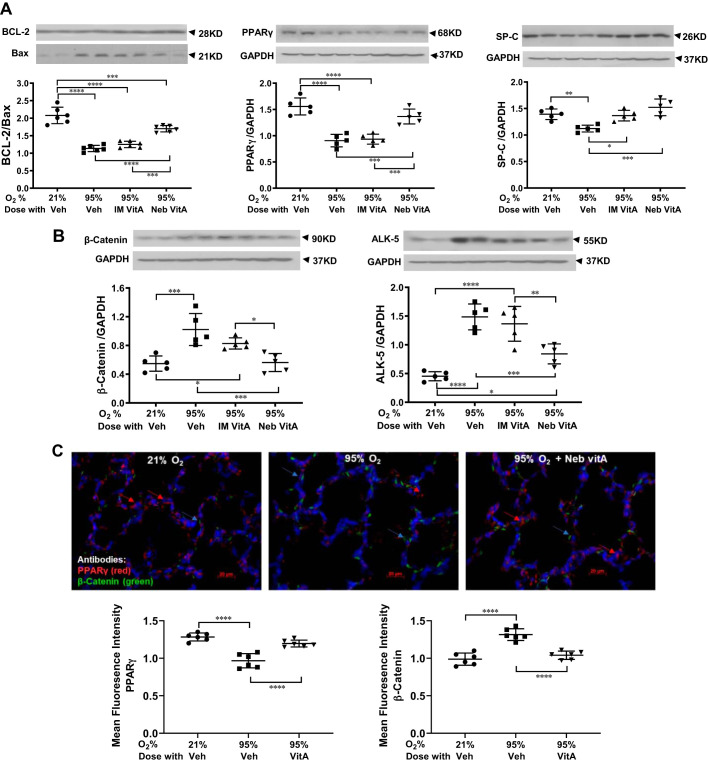
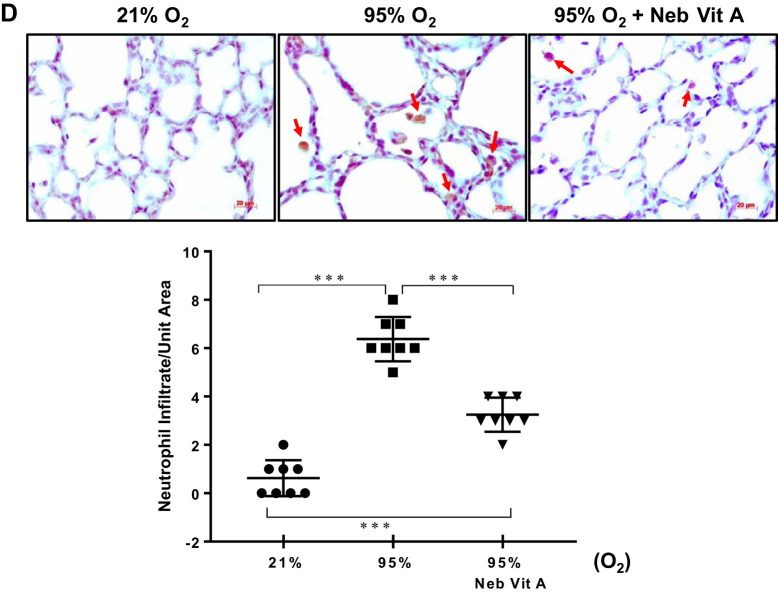
Similar to the lung histomorphometric data, lung maturation biomarkers PPARγ and SP-C, examined by Western analysis, demonstrated a significant reduction in the vehicle-treated hyperoxia-exposed group, an effect that was observed to be mitigated with vitamin A inhalation. In contrast, intramuscular administration improved only the hyperoxia-induced reduction in SP-C protein level, but not the PPARγ protein level (Fig. 2A). For all other injury repair biomarker proteins, BCL-2 and BAX (shown in ratio), ALK-5, and β-catenin, near to normal control levels were maintained in animals receiving inhaled vitamin A, despite continuous exposure to extreme hyperoxia, whereas the intramuscular vitamin A group did not show improvement in any of these parameters (Fig. 2B). Immunofluorescence costaining of lung tissue for PPARγ and β-catenin (Fig. 2C) confirmed the mitigating effects of inhaled vitamin A dosing as evidenced on Western analysis (Fig. 2B). As a further corroboration of vitamin A’s protective effect against hyperoxia-induced neonatal lung injury, alveolar neutrophil infiltration induced by hyperoxia is markedly reduced in the nebulized vitamin A group (Fig. 2D).
Fig. 2.
Nebulized vitamin A (VitA) reduces the extent of hyperoxia-induced damage, as judged by protein biomarkers in the lung quantitated by Western blot (A and B) and immunostaining (C and D). A: proteins involved in lung maturation are significantly diminished upon hyperoxia, and levels are significantly maintained at near normal levels with inhaled vitamin A dosing. Intramuscular (IM) vitamin A dosing shows a range of effects upon these biomarkers ranging from equally effective as inhaled delivery to modest to nearly ineffective. B: proteins representative of repair pathways are elevated upon untreated hyperoxia, with the effect being significantly mitigated by inhaled vitamin A dosing (but not IM dosing). A and B: Western blot images of 2 representative samples from each group aligned above their respective groups in the charts; protein biomarkers are quantitated relative to GAPDH; charts show Western blot data from n = 5 or 6 animals. C: 2-color fluorescence immunostaining of intracellular proteins in fixed lung tissue sections illustrated the simultaneous yet opposite trends (from A and B), using peroxisome proliferator-activated receptor (PPAR) γ and β-catenin as examples. Representative images are shown and protein concentration, quantitated from these images (from n = 6 animals), is shown in the charts. Hyperoxia (95% O2, middle image) lowers PPARγ (red) and raises β-catenin (green) compared with normal control (left), and inhaled VitA during hyperoxia (right) restores more normal-like levels. D: immunostaining for myeloperoxidase illustrates extent of neutrophil infiltration. Representative images are shown, and quantitation from n = 8 for each group is graphed. Veh, vehicle. In each panel, any pairwise P value comparisons where differences are statistically significant are indicated: *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.0001.
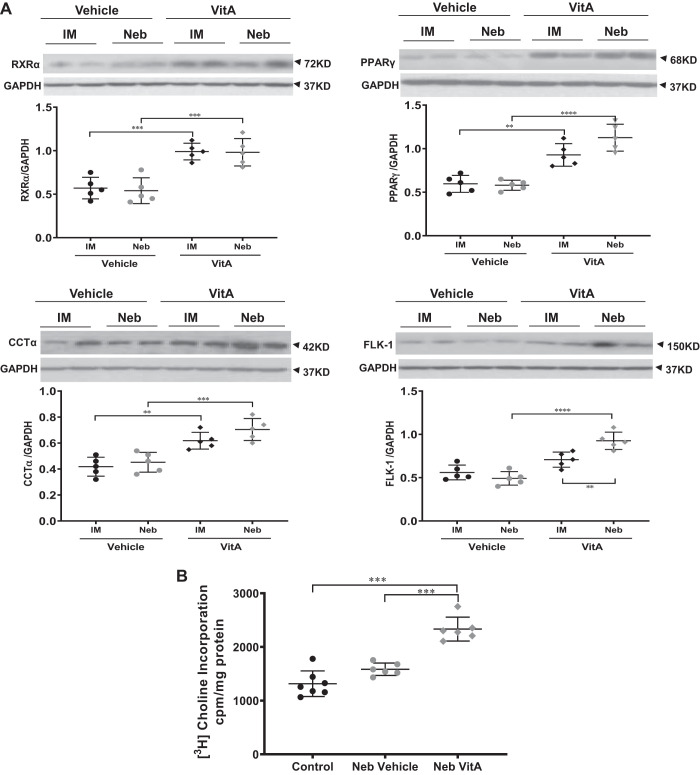
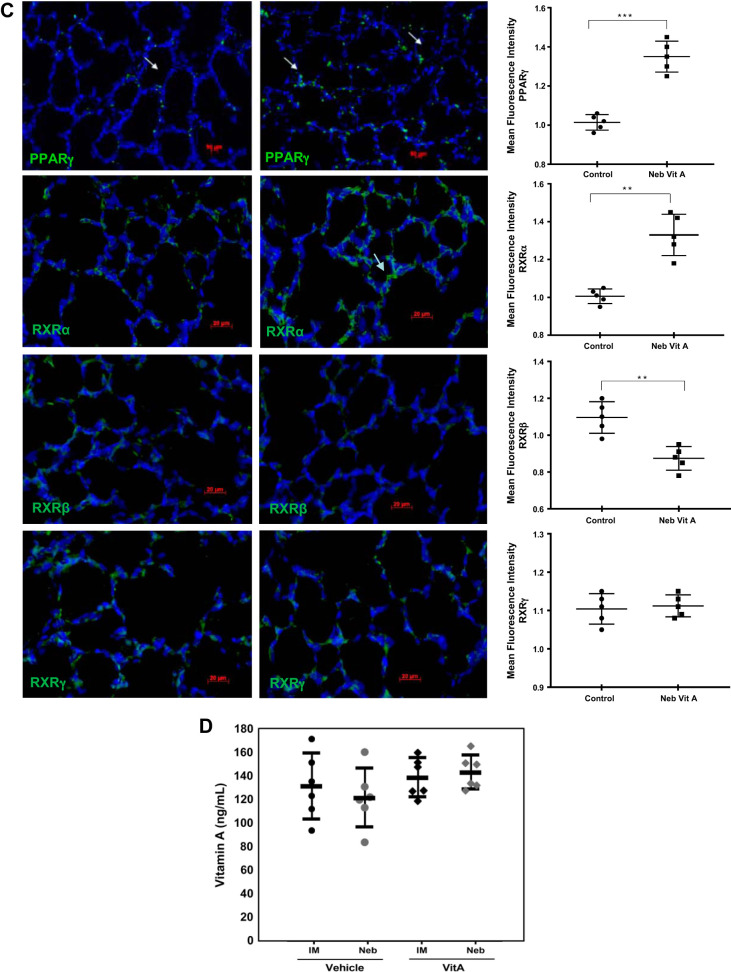
Effect of vitamin A on neonatal lungs and serum under normoxia.
Separate control experiments were performed to examine the effects of inhaled versus intramuscular vitamin A doses on protein levels of its receptor (RXRα) and select downstream alveolar epithelial (CCT-α), mesenchymal (PPARγ) and endothelial (FLK-1 and VEGF) targets under normoxic conditions (Fig. 3). As determined by Western analysis, under normoxia both dosing routes induced statistically significant responses over vehicle-treated control levels of RXR-α, CCTα, PPARγ, and FLK-1. Although resulting levels from inhaled and intramuscular dosing on the aforementioned biomarkers were not statistically different, the effects suggest that the inhaled dose produced a more robust response. The stimulatory effect of inhaled vitamin A administration on lung maturation was also confirmed by immunofluorescence staining for biomarkers as well as by choline incorporation into disaturated phosphatidylcholine, a marker of surfactant phospholipid synthesis. Nebulized vitamin A increased choline incorporation into disaturated phosphatidylcholine (Fig. 3B), and immunostaining (Fig. 3C) demonstrated increases for PPARγ and diverse effects on the RXR family, increasing RXRα, decreasing RXRβ, and not altering RXRγ staining. The different directionality of effects of vitamin A dosing on RXRs likely reflects the complex cell- and temporal-specific roles of RAR- and RXR-mediated signaling (14) in the developing lung. The fact that intramuscular vitamin A doses elicited a response across all tested biomarkers under normoxia (Fig. 3A) is an important confirmation that intramuscular dosing can indeed stimulate lung response, confirming that this known NICU dosing regimen indeed can elicit a positive response in the lung. This contrasts with the modest to absent response of intramuscular dosing under harsh hyperoxia conditions.
Fig. 3.
Vitamin A (Vit A) doses elevate biomarkers of lung maturation under normoxic conditions. A: representative protein biomarkers stimulated by inhaled or injected vitamin A doses are quantitated by Western blot. Blot results are shown for 2 representative samples from each group; chart has data from n = 5 animals. B: phospholipid synthesis level is quantitated via incorporation of radiolabeled choline. C: confirmatory data are shown by fluorescence immunostaining of intracellular proteins. Representative images are shown; chart has data from n = 5 animals. D: serum vitamin A levels quantitated by ELISA. n = 6–7 each group. In each panel, any pairwise P value comparisons where differences are statistically significant are indicated, with **P < 0.01, ***P < 0.001, or ****P < 0.0001.
Measured 4 to 6 h after administration, serum vitamin A levels (Fig. 3D) following both inhaled and intramuscular vitamin A doses were in the upper half of the apparently normal healthy range, defined by the vehicle-dosed groups, but were not significantly different between the modes of administration. However, comprehensive pharmacokinetic studies are required to determine more thoroughly the impact of inhaled and intramuscular administrations on specific tissue (e.g., lung and liver) and circulating vitamin A levels.
DISCUSSION
Inhaled, aerosolized vitamin A palmitate significantly mitigates hyperoxia-induced lung tissue damage. Compared with intramuscular dosing, inhaled vitamin A dosing resulted in near to normal lung parameters, as judged by assessment of lung histomorphology and morphometry (Fig. 1), biomarkers representative of reduced lung injury including decrease in neutrophil infiltration, and upregulation of maturation and damage-repair pathways (Fig. 2). The extent of the effect of vitamin A dosing can be judged against the extremes defined by untreated (vehicle treated) hyperoxic controls and healthy normoxic controls. Importantly, the extreme hyperoxia condition used in this study (95% O2 for 7 days) represents an aggressive pathobiological challenge, far exceeding the typical NICU oxygen supplementation exposure levels. Our results clearly indicate that inhaled vitamin A helps avoid rampant hyperoxia lung damage across virtually all metrics we tested, and we note that the particular dosing regimen studied to date offers opportunity for improvement, since a subset of the metrics, e.g., the suppressed neutrophil infiltration, are not entirely restored to healthy control levels. Collectively, our data clearly and consistently support that inhaled aerosolized vitamin A dosing mitigates hyperoxic lung damage. Given the commonality of lung injury response pathways leading to BPD (8, 29), we expect potential for inhaled vitamin A also to mitigate acute lung injury resulting from other pulmonary insults, such as inflammation and volutrauma.
As recently reviewed (16), there are few examples in literature of the effect of vitamin A doses upon lung in rodent BPD models. Veness-Meehan et al. (32) reported little to no evidence of intramuscular-dosed vitamin A benefits upon newborn rat lung immediately after 14 days of severe hyperoxia, so our intramuscular results match published precedent in a similar study design. The same group subsequently showed that the same intramuscular dosing ultimately did provide lung benefits after rats were allowed to wean and mature for an additional 4 wk under normoxia (33), although there were still notable differences in certain lung metrics versus healthy controls. Since our novel vitamin A inhalation dosing appears to provide benefits to lung maturation concurrent with the hyperoxia damage, we expect that the longer-term benefits to maturing lung may be improved beyond the dosing seen for the previous intramuscular-dosing study (33); however, this needs confirmation in future studies.
The superior lung responses to inhaled, aerosolized vitamin A doses supports our core hypothesis, namely that inhaled doses offer direct-to-target organ (i.e., lung) exposure, which should improve BPD treatment efficacy versus intramuscular dosing typically used in NICU today. As recently as 2017, there were no aerosol-dosed drugs approved for neonates (10), despite known uses of aerosolized medications for treating various other adult and pediatric lung conditions. Despite several technical hurdles of aerosol delivery to neonates (10), the aerosol dosing we used and the resulting data clearly demonstrate feasibility, since our system relied on passive inhalation by the neonatal rats, with matched intramuscular-dosed groups run in parallel. The specific mechanisms underlying greater efficacy of inhaled versus intramuscular vitamin A dosing in preventing hyperoxia-induced neonatal lung injury are not yet clear, needing deeper evaluation in future studies. It is possible that hyperoxia exposure reduces the uptake or transit ability of vitamin A from the injection site, reduces the uptake ability of lung tissue for blood-borne vitamin A via intramuscular dosing versus the inhaled dosing, and/or intrinsically suppresses vitamin A metabolism systemically, which might potentially include intrinsic hepatic processes like retinol binding protein synthesis and metabolism. The biological and biochemical effects of vitamin A, a potent morphogen, on lung maturation are part of our ongoing studies, including quantitation of the efficiency of delivering vitamin A as an aerosol via the nasal route, which would be more aligned with our vision of leveraging use of noninvasive respiratory support [e.g., nasal continuous positive airway pressure (nCPAP) devices] to deliver nebulized vitamin A. Nose only dosing will help minimize the confounding effects of transdermal absorption that might have occurred with using inhalation following a whole body aerosol exposure approach. Furthermore, beyond the therapeutic or prophylactic superiority of aerosolized vitamin A, efficacious noninvasive delivery via the inhalation route overcomes a variety of known hurdles of intramuscular injection (5, 34), particularly in extremely premature neonates, and our data suggest potential for further development into a viable option to treat other vitamin A deficiency conditions beyond BPD.
In summary, our data show significantly superior efficacy of inhaled aerosolized vitamin A versus intramuscular dosing in preventing experimental hyperoxia-induced neonatal lung injury, potentially providing more relevance for use of vitamin A in preventing BPD in extremely premature infants. In clinical practice, intramuscular vitamin A dosing appears to have a modest effect in mitigating BPD; the typical regimen used in NICU is 5,000 IU/dose, 3 doses/wk for 4 wk, and has a well-documented number needed to treat (NNT) = 14 (31). Other doses and regimens, summarized in a thorough meta-analysis (3), have been judged as having “moderate” value against lung conditions or death. With a potential for a significantly more efficacious treatment effect, such as might be achieved with direct-to-target organ delivery of aerosolized vitamin A via a noninvasive inhalation route, the NNT to prevent BPD would be expected to be much lower, achieving the goal of significantly reducing the incidence of BPD in extremely preterm infants.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL142353 (SBIR Phase I); NIH Grants HL127137 and HD071731; Tobacco-Related Disease Research Program Grants 23RT-0018, 27IP-0050, and T29IR0737; and from Ben Franklin Technology Partners of Southeastern Pennsylvania.
DISCLOSURES
Drs. Segal and Gelfand each hold an ownership interest in Advent Therapeutics. Drs. Rehan, Segal, and Gelfand are listed as inventors on intellectual property on the use of aerosolized vitamin A for preventing bronchopulmonary dysplasia. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
C.A.G., R. Segal, and V.K.R. conceived and designed research; C.A.G., R. Sakurai, Y.W., and Y.L. performed experiments; C.A.G., R. Sakurai, Y.W., R. Segal, and V.K.R. interpreted results of experiments; C.A.G., R. Segal, and V.K.R. drafted manuscript; C.A.G., R. Segal, and V.K.R. edited and revised manuscript; C.A.G., R. Sakurai, Y.W., Y.L., R. Segal, and V.K.R. approved final version of manuscript; R. Sakurai and Y.W. analyzed data; R. Sakurai and Y.W. prepared figures.
ACKNOWLEDGMENTS
Pharmaceutical-grade vitamin A palmitate was kindly provided by DSM Nutritionals, Parsippany, NJ.
REFERENCES
- 1.Alvira CM, Morty RE. Can we understand the pathobiology of bronchopulmonary dysplasia? J Pediatr 190: 27–37, 2017. doi: 10.1016/j.jpeds.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chytil F. The lungs and vitamin A. Am J Physiol Lung Cell Mol Physiol 262: L517–L527, 1992. doi: 10.1152/ajplung.1992.262.5.l517. [DOI] [PubMed] [Google Scholar]
- 3.Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst Rev 8:CD000501, 2016. doi: 10.1002/14651858.CD000501.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-beta and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 296: L1031–L1041, 2009. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green J, Darbyshire P, Adams A, Jackson D. It’s agony for us as well: neonatal nurses reflect on iatrogenic pain. Nurs Ethics 23: 176–190, 2016. doi: 10.1177/0969733014558968. [DOI] [PubMed] [Google Scholar]
- 6.Guimarães H, Guedes MB, Rocha G, Tomé T, Albino-Teixeira A. Vitamin A in prevention of bronchopulmonary dysplasia. Curr Pharm Des 18: 3101–3113, 2012. doi: 10.2174/1381612811209023101. [DOI] [PubMed] [Google Scholar]
- 7.Hustead VA, Gutcher GR, Anderson SA, Zachman RD. Relationship of vitamin A (retinol) status to lung disease in the preterm infant. J Pediatr 105: 610–615, 1984. doi: 10.1016/S0022-3476(84)80432-1. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JS, Rehan VK. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung 196: 129–138, 2018. doi: 10.1007/s00408-018-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalmar GB, Kay RJ, LaChance AC, Cornell RB. Primary structure and expression of a human CTP:phosphocholine cytidylyltransferase. Biochim Biophys Acta 1219: 328–334, 1994. doi: 10.1016/0167-4781(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 10.MacLoughlin R, Telfer C, Clark A, Fink J. Aerosol: a novel vehicle for pharmacotherapy in neonates. Curr Pharm Des 23: 5928–5934, 2017. doi: 10.2174/1381612823666170918122136. [DOI] [PubMed] [Google Scholar]
- 11.Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed 90: F103–F108, 2005. doi: 10.1136/adc.2004.057547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, Pryhuber GS; Prematurity and Respiratory Outcomes Program . Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol 35: 313–321, 2015. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin RJ, Fanaroff AA. The preterm lung and airway: past, present, and future. Pediatr Neonatol 54: 228–234, 2013. doi: 10.1016/j.pedneo.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Mey J. RAR/RXR-mediated signaling. In Gene Regulation, Epigenetics and Hormone Signaling, edited by Mandal SS. Weinheim, Germany: Wiley-VCH, 2017, p. 457–510, doi: 10.1002/9783527697274.ch16. [DOI] [Google Scholar]
- 15.Morales E, Sakurai R, Husain S, Paek D, Gong M, Ibe B, Li Y, Husain M, Torday JS, Rehan VK. Nebulized PPARγ agonists: a novel approach to augment neonatal lung maturation and injury repair in rats. Pediatr Res 75: 631–640, 2014. doi: 10.1038/pr.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morty RE. Using experimental models to identify pathogenic pathways and putative disease management targets in bronchopulmonary dysplasia. Neonatology 117: 233–239, 2020. doi: 10.1159/000506989. [DOI] [PubMed] [Google Scholar]
- 17.O’Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax 52, Suppl 2: S31–S44, 1997. doi: 10.1136/thx.52.2008.S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rau JL. Design principles of liquid nebulization devices currently in use. Respir Care 47: 1257–1275, 2002. [PubMed] [Google Scholar]
- 19.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol 305: L501–L507, 2013. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehan VK, Wang Y, Sugano S, Santos J, Patel S, Sakurai R, Boros LG, Lee WP, Torday JS. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol 292: L323–L333, 2007. [Erratum in Am J Physiol Lung Cell Mol Physiol 293: L820, 2007.] doi: 10.1152/ajplung.00071.2006. [DOI] [PubMed] [Google Scholar]
- 21.Richter J, Toelen J, Vanoirbeek J, Kakigano A, Dekoninck P, Verbeken E, Deprest J. Functional assessment of hyperoxia-induced lung injury after preterm birth in the rabbit. Am J Physiol Lung Cell Mol Physiol 306: L277–L283, 2014. doi: 10.1152/ajplung.00315.2013. [DOI] [PubMed] [Google Scholar]
- 22.Rush MG, Shenai JP, Parker RA, Chytil F. Intramuscular versus enteral vitamin A supplementation in very low birth weight neonates. J Pediatr 125: 458–462, 1994. doi: 10.1016/S0022-3476(05)83295-0. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai R, Liu J, Gong M, Bo J, Rehan VK. Perinatal nicotine exposure induces myogenic differentiation, but not epithelial-mesenchymal transition in rat offspring lung. Pediatr Pulmonol 51: 1142–1150, 2016. doi: 10.1002/ppul.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakurai R, Villarreal P, Husain S, Liu J, Sakurai T, Tou E, Torday JS, Rehan VK. Curcumin protects the developing lung against long-term hyperoxic injury. Am J Physiol Lung Cell Mol Physiol 305: L301–L311, 2013. doi: 10.1152/ajplung.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenai JP, Chytil F, Parker RA, Stahlman MT. Vitamin A status and airway infection in mechanically ventilated very-low-birth-weight neonates. Pediatr Pulmonol 19: 256–261, 1995. doi: 10.1002/ppul.1950190503. [DOI] [PubMed] [Google Scholar]
- 27.Shenai JP, Chytil F, Stahlman MT. Vitamin A status of neonates with bronchopulmonary dysplasia. Pediatr Res 19: 185–188, 1985. doi: 10.1203/00006450-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SK, Sakurai R, Sakurai T, Rehan VK. Inhaled vitamin D: a novel strategy to enhance neonatal lung maturation. Lung 194: 931–943, 2016. doi: 10.1007/s00408-016-9939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torday JS, Rehan VK. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr Res 62: 2–7, 2007. doi: 10.1203/PDR.0b013e31806772a1. [DOI] [PubMed] [Google Scholar]
- 30.Tropea K, Christou H. Current pharmacologic approaches for prevention and treatment of bronchopulmonary dysplasia. Int J Pediatr 2012: 598606, 2012. doi: 10.1155/2012/598606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, Korones SB, Donovan EF, Carlo WA, Shankaran S, Stark AR, Papile L-A, Jobe A, Stacewicz-Sapuntzakis M, Verter J, Fanaroff AA; National Institute of Child Health and Human Development Neonatal Research Network . Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med 340: 1962–1968, 1999. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 32.Veness-Meehan KA, Bottone FG Jr, Stiles AD. Effects of retinoic acid on airspace development and lung collagen in hyperoxia-exposed newborn rats. Pediatr Res 48: 434–444, 2000. doi: 10.1203/00006450-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD. Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol 283: L971–L980, 2002. doi: 10.1152/ajplung.00266.2001. [DOI] [PubMed] [Google Scholar]
- 34.Victorian Agency for Health Information Intramuscular Injections for Neonates (Online). https://www.bettersafercare.vic.gov.au/resources/clinical-guidance/maternity-and-newborn-clinical-network/intramuscular-injections-for-neonates.
- 35.Young TE. Nutritional support and bronchopulmonary dysplasia. J Perinatol 27, S1: S75–S78, 2007. doi: 10.1038/sj.jp.7211725.18034183 [DOI] [Google Scholar]
- 36.Yurt M, Liu J, Sakurai R, Gong M, Husain SM, Siddiqui MA, Husain M, Villarreal P, Akcay F, Torday JS, Rehan VK. Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. Am J Physiol Lung Cell Mol Physiol 307: L859–L867, 2014. doi: 10.1152/ajplung.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]