Abstract
Recently, we reported a mutation in γ-adducin (ADD3) was associated with an impaired myogenic response of the afferent arteriole and hypertension-induced chronic kidney disease (CKD) in fawn hooded hypertensive (FHH) rats. However, the mechanisms by which altered renal blood flow (RBF) autoregulation promotes hypertension-induced renal injury remain to be determined. The present study compared the time course of changes in renal hemodynamics and the progression of CKD during the development of DOCA-salt hypertension in FHH 1BN congenic rats [wild-type (WT)] with an intact myogenic response versus FHH 1BN Add3KO (Add3KO) rats, which have impaired myogenic response. RBF was well autoregulated in WT rats but not in Add3KO rats. Glomerular capillary pressure rose by 6 versus 14 mmHg in WT versus Add3KO rats when blood pressure increased from 100 to 150 mmHg. After 1 wk of hypertension, glomerular filtration rate increased by 38% and glomerular nephrin expression decreased by 20% in Add3KO rats. Neither were altered in WT rats. Proteinuria doubled in WT rats versus a sixfold increase in Add3KO rats. The degree of renal injury was greater in Add3KO than WT rats after 3 wk of hypertension. RBF, glomerular filtration rate, and glomerular capillary pressure were lower by 20%, 28%, and 19% in Add3KO rats than in WT rats, which was associated with glomerular matrix expansion and loss of capillary filtration area. The results indicated that impaired RBF autoregulation and eutrophic remodeling of preglomerular arterioles increase the transmission of pressure to glomeruli, which induces podocyte loss and accelerates the progression of CKD in hypertensive Add3KO rats.
Keywords: chronic kidney disease, hemodynamics, hypertension, myogenic response
INTRODUCTION
Patients with diabetes and hypertension are at higher risk for the development of chronic kidney disease (CKD) and together account for two-thirds of the newly diagnosed cases of end-stage renal disease (ESRD) (5). Consequently, the incidence of ESRD continues to escalate as the population ages. Tight control of glycemia and blood pressure slow but do not prevent the progression of renal disease. There is a significant genetic component since less than one-half of patients with diabetes and hypertension develop renal diseases (1, 11). Genome-wide association studies in humans and genetic mapping studies in animal models have identified many quantitative trait loci that are associated with the risk of diabetic and hypertensive nephropathy (27, 29, 30, 36, 39, 46). Knockout (KO) studies in mice and rat models have confirmed that some of these candidate genes alter renal function. However, identification of specific genetic variants that alter the expression and/or function of the proteins involved and cause renal disease in a transgenic model have been difficult (24).
Recently, we identified a recessive mutation in γ-adducin (ADD3), which is shared by the fawn hooded hypertensive (FHH) and Milan normotensive strains of rats, that is associated with increased susceptibility to hypertensive and diabetic nephropathy (7). We further found that the myogenic response of the afferent arteriole and autoregulation of renal blood flow (RBF) following an acute increase in arterial pressure were impaired in both of these strains. Substitution of a 2.4-Mbp region on chromosome 1 from the Brown Norway (BN) rat containing 13 genes including wild-type (WT) Add3 into a congenic model FHH 1BN rat or a transgenic Add3 FHH rat restored the myogenic response of the afferent arteriole and attenuated the development of proteinuria and renal injury (4, 25). However, the mechanism by which impaired autoregulation of RBF promotes the development of proteinuria in FHH rats remains to be determined. A defect in the myogenic response of the afferent arteriole that is not compensated by vascular hypertrophy and remodeling could increase the transmission of pressure to the glomerulus and damage capillaries and podocytes following the development of hypertension (40, 41). On the other hand, hypertension in spontaneously hypertensive rats (12) and angiotensin II-dependent models of hypertension (38) is associated with hypertrophy and narrowing of preglomerular arterioles that protect the glomerulus from elevations in systemic pressure.
Thus, the present followup study characterized the time course of changes in the myogenic response and structure of preglomerular arteries, autoregulation of RBF, glomerular capillary pressure (Pgc), and podocyte loss following the induction of DOCA-salt hypertension in congenic FHH 1BN congenic rats (WT) with the WT Add3 gene and an intact myogenic response versus FHH 1BN Add3KO (Add3KO) rats, in which the myogenic response is impaired.
MATERIALS AND METHODS
Experimental animals.
Experiments were performed on inbred 12- to 15-wk-old male congenic FHH.1BN-(D1Rat09-D1Rat225)/Mcwi rats that express the WT Add3 gene and protein and FHH.1BN Add3KO rats (7). The colonies were maintained in the Laboratory Animal Facility at the University of Mississippi Medical Center and fed a grain-based diet containing a low-Na+ content (0.1% NaCl, Teklad 7034, Envigo, Indianapolis, IN). All protocols were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Time course of the development of hypertension and proteinuria.
Baseline protein excretion was measured overnight in 12-wk-old rats using metabolic cages. Rats were anesthetized with isoflurane, a 200-mg DOCA pellet (90-day release, NM-121, Innovative Research of America, Sarasota, FL) was implanted subcutaneously, and a telemetry transmitter (HD-S10, Data Sciences International, St. Paul, MN) was inserted in the femoral artery for the measurement of mean arterial pressure (MAP). After a 3-day recovery period, baseline MAP was measured on 3 consecutive days, and 1% NaCl was then added to the drinking water. MAP and proteinuria were measured weekly. At the end of the study, rats were anesthetized with isoflurane, and a sample of plasma was collected for the measurement of creatinine concentration. The kidney was also collected for histology, immunohistochemistry, Western blots, or cytokine assays. Urinary protein concentration was quantified with a colorimetric assay (no. 5000006, Bio-Rad, Hercules, CA), and plasma creatinine concentration was measured with a picric acid assay (no. 290-65901, Wako, Richmond, VA).
Myogenic response of renal interlobular arteries.
Interlobular arteries (IAs) were microdissected from the kidneys of WT and Add3KO rats before and after 3 wk of DOCA-salt hypertension. Vessels were cannulated to glass micropipettes in a temperature-controlled chamber and bathed with physiological salt solution (PSS) bubbled with 21% oxygen. Arteries were perfused over a range of intraluminal pressures from 60 to 180 mmHg, and inner and outer diameters were recorded. Pressure diameter curves were repeated in Ca2+-free PSS to study passive mechanical properties of the vessels. Wall thickness and wall-to-lumen ratios were calculated as previously described (48).
Autoregulation of RBF.
Autoregulation of RBF was compared in groups of WT and Add3KO rats under baseline conditions and 1 or 3 wk after the induction of DOCA-salt hypertension, as previously described (35). Briefly, rats were anesthetized using ketamine (30 mg/kg im) and Inactin (50 mg/kg ip). The trachea was cannulated to facilitate breathing, and cannulas were placed in the femoral artery and vein for the measurement of arterial pressure and intravenous infusions. RBF was measured with a ultrasonic flow probe (Transonic System, Ithaca, NY) placed around the left renal artery. Renal perfusion pressure (RPP) was adjusted with clamps on the abdominal aorta above and below the left renal artery. The rat received an intravenous infusion of 0.9% NaCl solution at 6 mL/h throughout the experiment. After a 30-min control period, mesenteric and celiac arteries were tied off to raise arterial pressure, and RBF was measured over the range of 50–180 mmHg in steps of 10 mmHg. The autoregulatory index (AI) was calculated as follows: [(RBF2 − RBF1)/RBF1]/[(RPP2 − RPP1)/RPP1].
Measurement of glomerular filtration rate.
Glomerular filtration rate (GFR) was estimated from the renal clearance of FITC-inulin (F3272, Sigma-Aldrich, St. Louis, MO) from groups of WT and Add3KO rats under baseline conditions and 1 or 3 wk after the induction of DOCA-salt hypertension. The rat was anesthetized using ketamine (30 mg/kg im) and Inactin (50 mg/kg ip). The femoral vein was cannulated to infuse a bolus of FITC-inulin (40 mg/kg). After a 10-min equilibration period for redistribution of FITC-inulin in the extracellular fluid, blood was sampled at 10-min intervals for 1 h. The concentration of FITC-inulin in the plasma was measured using a microplate reader (BioTek, Winooski, VT). A concentration versus time curve was fit using linear regression on a semilog plot. The concentration at time 0 (C0) was estimated by extrapolation to the y-axis, and the volume of distribution (VD) was calculated from C0 divided by the administered dose. The half-life (t1/2) was derived from the plot of FITC-inulin concentration versus time. The clearance of FITC-inulin was then calculated by 0.693 × VD/t1/2. At the end of the study, the kidney was collected and stored for histology and Western blot analysis.
Glomerular capillary pressure.
Pgc was measured in groups of WT and Add3KO rats under baseline conditions and 1 or 3 wk after the induction of hypertension, as previously described (42). Briefly, the rat was prepared as for the measurement of RBF autoregulation, and the kidney was placed in a holder for micropuncture. Pgc was estimated by measuring the sum of oncotic pressure of the plasma, and stop-flow pressures were measured in wax-blocked proximal tubules using a servo-null micropressure device (model 900, World Precision Instruments, Sarasota, FL) at RPPs of 100 and 150 mmHg. At the end of the study, the kidney was collected for histology, immunohistochemistry, Western blot, and cytokine assays.
Histology.
The kidneys were fixed by immersion in 10% formalin, embedded in paraffin, and cut into 3-µm sections. Sections were stained with Masson’s trichrome to quantitate the degree of glomerular injury, tubular protein casts, and renal interstitial fibrosis. Thirty glomeruli per rat were randomly chosen in each section and scored on a scale from 0 to 4 for the percent loss of glomerular capillary area. The degree of renal interstitial fibrosis was determined by measuring the percentage of blue fibrotic tissue per field using NIS-Elements D4.6 software (Nikon, Melville, NY). Protein cast area was quantitated as a percentage of eosin red fluorescence in tubules in outer medullary sections.
Immunohistochemistry.
Paraffin sections were deparaffinized in xylene, rehydrated in steps of 100%, 95%, and 70% ethanol and water, and then permeabilized in 0.1% trypsin in PBS at 37°C. Heat-induced epitope retrieval was performed by heating to 98°C in 0.1% citrate buffer, and sections were permeabilized by rinsing them in −20°C methanol. Sections were blocked using 10% serum matching the host of the secondary antibody and then incubated with primary antibodies targeting ADD3 (1:100, sc-25733, Santa Cruz Biotechnology, Dallas, TX), nephrin (1:50, 20R-NP002, Fitzgerald, Acton, MA), or CD68 (1:200, MCA341R, Bio-Rad) followed by incubation with Alexa Fluor 488-conjugated secondary antibodies at 4°C. Sections were counterstained with 0.005% Evans blue to quench endogenous fluorescence, rinsed, and then coverslipped using an antifade mounting medium with DAPI (H-1800, Vector Laboratories, Burlingame, CA). Images were captured using a Nikon Eclipse 55i microscope equipped with a DS-Fi11 color camera (Nikon). Nephrin expression was quantified as the mean intensity of fluorescence in individual glomeruli and proximal tubules. Ten fields were quantitated for each rat. The numbers of CD68+ macrophages were individually counted in all of the glomeruli in the field. Ten fields were quantitated for each rat.
Western blot analysis.
The renal cortex was homogenized in RIPA buffer (R0278, Sigma-Aldrich) supplemented with protease and phosphatase inhibitors (A32959, Thermo Scientific, Grand Island, NY). Membrane and cytosol fractions were obtained by centrifugation at 9,000 g for 15 min at 4°C. Aliquots were electrophoresed on SDS-polyacrylamide gels, transferred to nitrocellulose membranes using a Trans-Blot Turbo Transfer System (Bio-Rad), blocked, and incubated with primary antibodies targeting ADD3 (sc-365177, Santa Cruz Biotechnology), E-cadherin (no. 610181, BD Biosciences, San Jose, CA), vimentin (no. 5471, Cell Signaling, Danvers, MA), or α-smooth muscle actin (A5228, Sigma-Aldrich) and GAPDH (sc-32233, Santa Cruz Biotechnology) as a loading control. Blots were developed using enhanced chemiluminescence reagent (no. 32106, Thermo Scientific) and analyzed with the Chemidoc XRS+ imaging system (Bio-Rad).
Cytokine assay.
The renal cortex was homogenized in cell lysis buffer (no. 171304011, Bio-Rad) using a tissue homogenizer (FastPrep-24, MP Biomedicals, Irvine, CA). Tissue fragments were removed by centrifugation at 12,000 g for 15 min at 4°C. The supernatant was collected and loaded to a 23-plex cytokine assay (no. 12005641, Bio-Rad) in accordance with the manufacturer’s instructions. The cytokine assay detected IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, IL-18, granulocyte colony-stimulating factor (G-CSG), granulocyte-macrophage colony-stimulating factor (CM-CSF), growth-regulated oncogene/keratinocyte chemoattractant (GRO/KC), interferon (INF)-γ, macrophage colony-stimulating factor (M-CSF), macrophage inflammatory protein (MIP)-1α, MIP-3α, regulated on activation, normal T cell expressed and secreted (RANTES), TNF-α, VEGF, and monocyte chemoattractant protein (MCP)-1. The cytokine assay was analyzed using a Bio-Plex 200 Suspension Assay System (Bio-Rad). Data were compared as fold changes from the corresponding values in the kidneys of nontreated WT rats.
Statistical analysis.
Values are presented as means ± SE. Two-way ANOVA followed by a Holm-Sidak t test for multiple comparisons was used to evaluate the significance of differences in the mean values in continuously measured groups or a Student’s t test for two unpaired groups. P values of <0.05 were considered statistically significant.
RESULTS
Distribution of ADD3 in the kidney.
ADD3 was expressed in renal vasculature (Fig. 1, A and B), podocytes (Fig. 1B), parietal epithelial cells (Fig. 1B), and proximal tubules (Fig. 1, B and C) in FHH 1BN rats (WT rats expressing WT ADD3) but not in Add3KO rats (Fig. 1, D−F). Expression of ADD3 in the renal cortex was also markedly reduced in Add3KO versus WT rats (Fig. 1G).
Fig. 1.
Immunohistochemistry and Western blot comparing the expression and distribution of γ-adducin (ADD3) in the kidney of control and fawn hooded hypertensive ADD3 knockout (Add3KO) rats. The kidneys of wild-type (WT) and Add3KO rats were immunostained with ADD3 antibody (green) and counterstained with Evans blue (red) and DAPI (blue). A: ADD3 was found in renal interlobular arteries in WT rats. B: ADD3 was also found to be expressed in afferent/efferent arterioles (“a/e”), podocytes (white arrows), and parietal glomerular epithelial cells (yellow arrows). C: ADD3 was found in microvilli of the luminal surface of the proximal tubule (PT; red arrows). D–F: ADD3 staining was not detected in Add3KO rats. G: expression of ADD3 was detected by Western blot in renal cortical homogenates of WT rats but not Add3KO rats. DT, distal tubule.
Comparison of blood pressure and renal injury in WT and Add3KO rats during DOCA-salt hypertension.
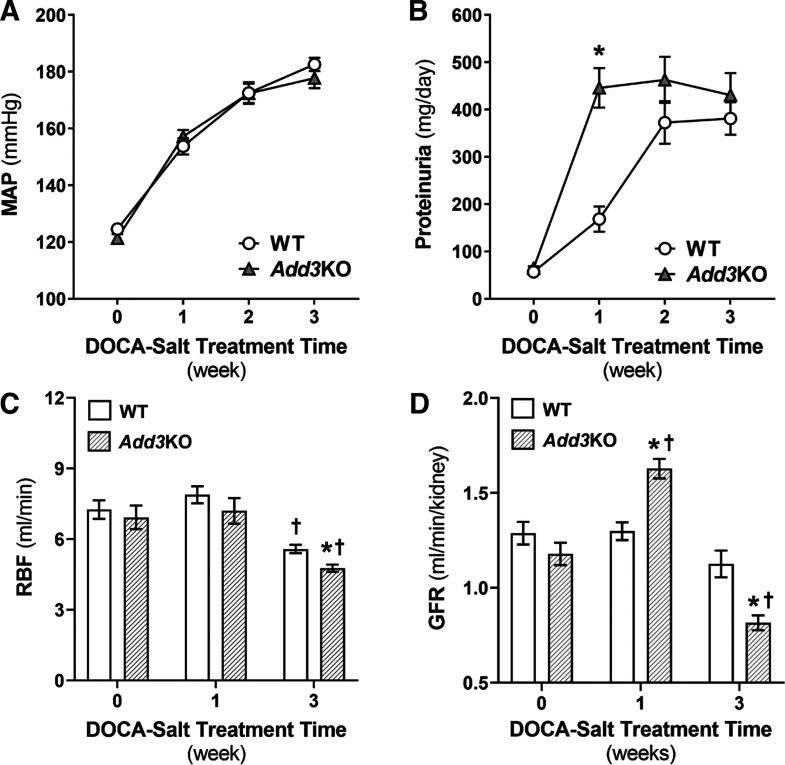
Baseline MAP was similar in 12-wk-old WT and Add3KO rats and increased similarly after the induction of DOCA-salt hypertension (Fig. 2A). Protein excretion increased sixfold in the first week after the induction of hypertension in Add3KO rats versus only a twofold increase in WT rats (Fig. 2B). In the second week, MAP continued to increase to ~170 mmHg in both strains, which exceeds the breakthrough point for autoregulation of RBF. Proteinuria increased in WT rats to the same level as that seen in Add3KO rats. After 3 wk of hypertension, proteinuria did not increase any further in Add3KO rats.
Fig. 2.
Comparison of the time course of hypertension and proteinuria in control and fawn hooded hypertensive γ-adducin knockout (Add3KO) rats treated with DOCA and salt. A: time course of development of DOCA-salt hypertension in wild-type (WT) and Add3KO rats. n = 9–12 rats/group. Statistical analysis was performed using two-way ANOVA with multiple comparisons. B: time course of development of proteinuria during DOCA-salt hypertension in WT and Add3KO rats. n = 9–12 rats/group. Statistical analysis was performed using two-way ANOVA with multiple comparisons. Data are expressed as means ± SE. *P < 0.05 vs. the corresponding value in WT rats. C: baseline renal blood flow (RBF) of WT and Add3KO rats at their physiological blood pressure without DOCA-salt treatment and after 1 or 3 wk of DOCA-salt hypertension. n = 6–9 rats/group. Statistical analysis was performed using an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control. Data are expressed as means ± SE. D: glomerular filtration rate (GFR) of WT and Add3KO rats without DOCA-salt treatment and with 1 or 3 wk of DOCA-salt hypertension. n = 6–7 rats/group. Statistical analysis was performed using an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control. Data are expressed as means ± SE. MAP, mean arterial pressure.
Baseline RBF was not significantly different in WT and Add3KO rats (Fig. 2C) and averaged 7.3 ± 0.4 versus 6.9 ± 0.5 mL/min. Although both strains exhibited a similar increase in blood pressure to ~155 mmHg in the first week of DOCA-salt hypertension, RBF was not altered in either strain. However, after 3 wk of hypertension, RBF decreased significantly in both strains even though their blood pressures rose to ~180 mmHg. The fall in RBF was greater in Add3KO rats (4.8 ± 0.2 mL/min) than in WT control rats (5.6 ± 0.2 mL/min). Baseline GFR was not significantly different in the strains. After 1 wk of hypertension, GFR increased by 38% in Add3KO rats, but it did not increase in WT rats (Fig. 2D). After 3 wk of hypertension, GFR declined in Add3KO rats and was 28% lower than the value seen in WT rats. The degree of glomerular injury was slightly higher in Add3KO than WT rats during the control period (Fig. 3, A and B). After 3 wk of DOCA-salt hypertension, a greater degree of mesangial matrix expansion, occlusion of capillaries, and periglomerular fibrosis were seen in Add3KO rats compared with WT rats. Plasma creatinine concentration rose to a greater extent from 0.5 to 1.2 ± 0.1 mg/dL in WT rats versus 1.6 ± 0.1 mg/dL in Add3KO rats over the course of the study (Fig. 3C).
Fig. 3.
Histology indicating the degree of glomerulosclerosis and renal fibrosis was greater in fawn hooded hypertensive γ-adducin knockout (Add3KO) rats than control rats 3 wk after the development of DOCA-salt hypertension. A: representative images of Masson’s trichrome-stained renal sections from wild-type (WT) and Add3KO rats before and after 3 wk of DOCA-salt hypertension. B: glomerular injury scores in WT and Add3KO rats at baseline and after 3 wk of DOCA-salt hypertension. Thirty glomeruli were randomly chosen and evaluated for each rat. n = 9–12 rats/group. C: baseline plasma creatinine levels were similar in WT and Add3KO rats. n = 7–12 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control.
Changes of the myogenic response and passive mechanical properties of renal arterioles after DOCA-salt hypertension.
Previous studies in spontaneously hypertensive rats (12) and angiotensin II hypertension models (38) indicated that an enhanced myogenic response, hypertrophy, and narrowing of the preglomerular vasculature shifts the RBF autoregulation curve to higher pressures and protects the kidney from hypertension-induced injury. In the present study, we examined the compensatory changes in the myogenic response and vascular remodeling in IAs exposed to high systemic pressure in DOCA-salt-treated WT and Add3KO rats. The inner diameter of the IA decreased to 81% of control in response to an elevation in transmural pressure from 40 to 100 mmHg before the development of hypertension in WT rats (Fig. 4A). In contrast, the myogenic response of the IA was markedly impaired, and the inner diameter increased in Add3KO rats when pressure was increased over this range. The IA of WT rats exhibited forced dilation at pressure > 100 mmHg and dilated to 111% of control at a pressure of 180 mmHg. The IA of Add3KO rats exhibited forced dilation at pressures > 80 mmHg, and the diameter increased to 135% at a pressure of 180 mmHg. The pressure-diameter curve was shifted to lower pressures by 20 mmHg after 3 wk of hypertension in WT rats, and the myogenic response was impaired to a greater extent in hypertensive Add3KO rats than in WT rats.
Fig. 4.
Pressure-diameter curves of renal interlobular arteries (IAs) indicating that the myogenic response was impaired in fawn hooded hypertensive γ-adducin knockout (Add3KO) rats and that vessels of both control and Add3KO rats exhibit eutrophic outward remodeling after the development of DOCA-salt hypertension. A: inner diameter of the IA over the range of perfusion pressure from 40 to 180 mmHg demonstrating the myogenic response and forced dilation of wild-type (WT) and Add3KO rats from control groups and groups with 3 wk of DOCA-salt hypertension. B: passive pressure-diameter relationships in the IA of Add3KO and WT rats before and after 3 wk of DOCA-salt hypertension. C: wall thickness of the IA was greater in Add3KO rats than in WT rats in Ca2+-free media. D: wall-to-lumen ratios were similar in the IA of Add3KO and WT rats in Ca2+-free media. n = 5–7 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with two-way ANOVA with multiple comparisons. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control.
The passive mechanical properties of the vessels were also studied in Ca2+-free media. No difference was found in the baseline inner diameter of the IA of WT and Add3KO rats. The luminal diameter increased in both strains after 3 wk of DOCA-salt hypertension, and the passive diameter of the IA was 21% larger in Add3KO than in WT rats (Fig. 4B). Add3KO rats had a thicker vascular wall than WT rats, and wall thickness increased in both strains after 3 wk of hypertension (Fig. 4C). However, the wall-to-lumen ratio was not significantly different in control and hypertensive IAs (Fig. 4D) after 3 wk of DOCA-salt hypertension in either strain, indicating that the vessels exhibited outward eutrophic remodeling.
Time course of changes in renal hemodynamics and glomerular injury in WT and Add3KO rats during DOCA-salt hypertension.
Autoregulation of RBF was similar under control conditions and after 1 wk of hypertension. RBF was well autoregulated in control WT rats and only increased by 23% (AI: 0.2) when renal RPP was increased from 80 to 170 mmHg. It increased by 55% (AI: 0.5) over the same range of RPP in control Add3KO rats (Fig. 5A). After the induction of hypertension (1 wk), the percent increase in RBF over the same range of RPP was not significantly altered in either strain [31% (AI: 0.3) in WT rats vs. 52% (AI: 0.5) in Add3KO rats]. After 3 wk of hypertension, RBF increased by 51% (AI: 0.5) in WT rats and by 89% (AI: 0.8) in Add3KO rats in response to this increase in pressure from 80 to 170 mmHg. WT rats were also able to maintain RBF against reductions in pressure from 120 to 80 mmHg better than in Add3KO rats under baseline conditions. No difference in RBF autoregulatory responses to reductions in pressure was found in the strains after 1 or 3 wk of hypertension, which is consistent with some loss of endothelial function. Baseline kidney weight was no different between WT and Add3KO rats at 1.4 versus 1.3 ± 0.1 g. However, 1 wk after DOCA-salt hypertension, kidney weight increased in WT rats to 2.0 ± 0.1 g compared with Add3KO rats with greater renal hypertrophy at 2.9 ± 0.1 g. After 3 wk of hypertension, WT kidneys weighed 2.4 ± 0.2 g versus a higher mass in Add3KO kidneys at 3.2 ± 0.1 g.
Fig. 5.
Autoregulation of renal blood flow (RBF) and glomerular capillary pressure (Pgc) was impaired in fawn hooded hypertensive γ-adducin knockout (Add3KO) rats versus control rats before and after the development of DOCA-salt hypertension. A: autoregulation curves of RBF over renal perfusion pressure (RPP) from 50 to 180 mmHg of wild-type (WT) and Add3KO rats without DOCA-salt treatment and with 1 or 3 wk of DOCA-salt hypertension. n = 6–11 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with two-way ANOVA with multiple comparisons. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control. B: Pgc at 100 and 150 mmHg of RPP of WT and Add3KO rats without DOCA-salt treatment and with 1 or 3 wk of DOCA-salt hypertension. Six to nine measurements per rat were performed. 6–7 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control.
Baseline Pgc at 100 mmHg was similar in WT and Add3KO rats. It increased by 13% in WT rats but by 28% in Add3KO rats when RPP was increased to 150 mmHg (Fig. 5B). Similar responses were seen between the two strains after 1 wk of DOCA-salt hypertension (16% in WT rats vs. 26% in Add3KO rats). However, after 3 wk of DOCA-salt hypertension, baseline Pgc at 100 mmHg decreased by 11% in Add3KO animals, but it remained unchanged in WT animals. In response to an increase in RPP from 100 to 150 mmHg, Pgc rose to a greater extent in WT rats (29%) than in Add3KO rats (13%).
Nephrin was highly expressed along glomerular capillaries in 12-wk-old WT and Add3KO rats (Fig. 6, A vs. D). After 1 wk of hypertension, as Add3KO glomeruli were exposed to higher Pgc (Fig. 5B) and exhibited hyperfiltration (Fig. 2D), glomerular nephrin expression decreased. It did not change in WT rats (Fig. 6, B vs. E). The loss of nephrin was most pronounced at the vascular pole in Add3KO rats (Fig. 6E) and was associated with an accumulation of nephrin in the proximal tubule (Fig. 6E). After 3 wk of hypertension, glomerular nephrin expression also fell in WT rats (Fig. 6C) and was nearly absent in Add3KO rats (Fig. 6F). Quantitation of the intensity of nephrin staining in glomeruli and proximal tubules is shown in Fig. 6G.
Fig. 6.
Renal histology indicating that glomerular expression of nephrin decreased to a greater extent in fawn hooded hypertensive γ-adducin knockout (Add3KO) rats than control rats during the development of DOCA-salt hypertension. A−F: representative images of nephrin (green) expression merged with Evans blue counterstaining (red) and DAPI (blue) of wild-type (WT) rats before DOCA-salt treatment (A) and 1 wk (B) or 3 wk (C) after DOCA-salt hypertension and Add3KO rats before DOCA-salt treatment (D) and 1 wk (E) or 3 wk (F) after DOCA-salt hypertension. In E, yellow arrows indicate proximal tubules and the white arrow indicates the vascular pole of the glomerulus. G: quantitation of nephrin mean intensity in glomeruli and proximal tubules in WT and Add3KO rats without and with 1 or 3 wk of DOCA-salt hypertension. Ten fields were quantitated. n = 5–7 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control.
Comparison of the degree of kidney injury.
The degree of renal interstitial fibrosis was greater in hypertensive Add3KO rats than in WT rats after 3 wk of hypertension (Fig. 7A, quantitation in Fig. 7B). Tubular protein casts increased markedly in the kidneys of both WT and Add3KO rats after 3 wk of DOCA-salt hypertension (Fig. 7C, quantitation in Fig. 7D). However, the increase in fibrosis was greater in the kidney of Add3KO rats in than WT rats. Expression of E-cadherin decreased, and expression of epithelial-mesenchymal transition (EMT) markers, vimentin and α-smooth muscle actin (α-SMA), increased to a greater extent in the kidney of Add3KO rats than in WT rats over the course of the study (Fig. 8A, quantitation in Fig. 8, B−D).
Fig. 7.
Renal histology indicating that the degree of renal interstitial fibrosis (top) and tubular protein casts (bottom) was greater in fawn hooded hypertensive γ-adducin knockout (Add3KO) rats than control rats before and after the development of DOCA-salt hypertension. A: comparison of the degree of renal interstitial fibrosis in wild-type (WT) and Add3KO rats before and after 3 wk of DOCA-salt hypertension. Slides were stained with Masson’s trichrome. B: quantitation of interstitial fibrosis area as a percentage of the blue area. Four fields were quantitated for each rat. n = 6 rats/group. C: images of Masson’s trichrome-stained tubular protein casts in WT and Add3KO rats before and after 3 wk of DOCA-salt hypertension. D: quantitation of tubular protein cast as a percentage of the eosin fluorescence area. Four fields were quantitated for each rat. n = 6 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control.
Fig. 8.
Western blots indicating that the expression of markers of epithelial mesenchymal transition were greater in the kidney of fawn hooded hypertensive γ-adducin knockout (Add3KO) than control rats after the development of DOCA-salt hypertension. A: Western blots of E-cadherin, vimentin, and α-smooth muscle actin. B–D: densitometric analyses of E-cadherin, vimentin, and α-smooth muscle actin (α-SMA) relative expression compared with GAPDH. n = 6 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with an unpaired t test. *P < 0.05 vs. the corresponding value in wild-type (WT) rats; †P < 0.05 vs. the same strain control. UNX, uninephrectomized.
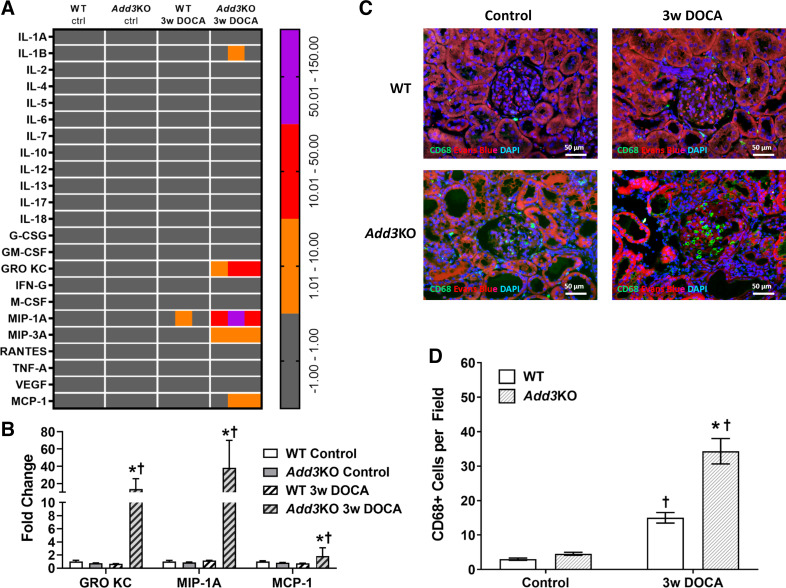
Three macrophage-related proinflammatory cytokines, GRO/KC, MIP-1α, and MCP-1, among the 23 cytokines measured, were significantly upregulated in the kidneys of hypertensive Add3KO rats compared with WT rats (Fig. 9A, quantitation in Fig. 9B). This was associated with an increase in the number of infiltrating CD68+ macrophages in the glomerulus of hypertensive Add3KO rats versus WT rats (Fig. 9C, quantitation in Fig. 9D).
Fig. 9.
Heat map and immunohistochemistry indicating that the expression of cytokines and CD68+ immune cells was greater in the kidney of fawn hooded hypertensive γ-adducin knockout (Add3KO) rats than control rats after the development of DOCA-salt hypertension. A: heat map of a cytokine assay accessing the expression of cytokines in kidney homogenates prepared from wild-type (WT) and Add3KO rats before and after 3 wk of DOCA-salt hypertension. Data are expressed as increased fold changes from control strains. Gray, fold change from −1 to 1; orange, fold change from 1 to 10; red, fold change from 10 to 50; purple, fold change from 50 to 150. n = 3 rats/group. B: quantitation of the three cytokines with significant fold changes from corresponding controls. n = 3 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control. C: representative images of expression of CD68 (green) merged with Evans blue counterstaining (red) and DAPI (blue) in the kidney from WT and Add3KO rats before and after DOCA-salt hypertension. D: quantitation of the number of CD68-positive infiltrating macrophages in glomeruli. Ten fields were quantitated in each rat. n = 4 rats/group. Data are expressed as means ± SE. Statistical analysis was performed with an unpaired t test. *P < 0.05 vs. the corresponding value in WT rats; †P < 0.05 vs. the same strain control. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GRO/KC, growth-regulated oncogene/keratinocyte chemoattractant; IFN-G, interferon-γ; M-CSF, macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T cell expressed and secreted; MCP-1, monocyte chemoattractant protein-1.
DISCUSSION
The present study characterized changes in renal hemodynamics and vascular properties in preglomerular arteries during the development of DOCA-salt hypertension in congenic FHH 1BN rats [WT rats with an intact myogenic response in the afferent arteriole (7)] versus Add3KO rats, in which the myogenic response is impaired.
We first established that ADD3 was expressed in the renal vasculature, podocytes, and proximal tubules in the WT strain. ADD3 was not expressed in the kidney of Add3KO rats (Fig. 1). ADD3, as a capping protein of F-actin filaments, regulates actin-cytoskeleton dynamics and the myogenic response of renal and cerebral arteries (4, 25). A mutation in Add3 in FHH rats or KO of Add3 is associated with a loss of cytoskeleton-dependent intracellular protein trafficking of large-conductance Ca2+-activated K+ channels from the cell membrane, which results in hyperpolarized membrane potential and impaired vascular reactivity (26). In the present study, we demonstrated that the myogenic response of renal IAs was impaired in Add3KO rats, similar to what we previously reported for the afferent arteriole in this strain (7, 8). The loss of myogenic response of the preglomerular arteries in Add3KO rats was associated with impaired autoregulation of RBF and Pgc before the development of hypertension. On the other hand, the tubuloglomerular feedback (TGF) response also participates in renal autoregulation. A previous study by Verseput et al. (43) reported that FHH rats do exhibit a TGF response. However, one would expect that KO of Add3 and elevated K+ channel activity would impair the vasoconstrictor response to adenosine and/or ATP that is the proposed mediator of TGF. Hence, reduced TGF responsiveness might also contribute to elevated Pgc and renal injury in Add3KO rats.
In control animals, Pgc of both strains was similar at a perfusion pressure of 100 mmHg, which is below the forced dilation pressure on the autoregulation curve for both strains. This finding suggests that young normotensive Add3KO rats may not be exposed to glomerular hyperfiltration before the development of hypertension. As perfusion pressure rose, FHH 1BN rats expressing wild-type ADD3 were able to maintain RBF to 160 mmHg. However, Add3KO rats exhibited a breakthrough of RBF autoregulation at pressures above140 mmHg. This difference in RBF autoregulation was also reflected in measurements of Pgc. WT rats maintained Pgc when perfusion pressure was increased to 150 mmHg, whereas Add3KO rats exhibited a significant elevation in Pgc. However, baseline MAP, RBF, and GFR were not significantly different before the induction of DOCA-salt hypertension in WT and Add3KO rats.
MAP increased similarly from 120 to 155 mmHg in WT and Add3KO rats during the first week of DOCA-salt hypertension. This was associated with impaired autoregulation of RBF and Pgc, a 38% increase in GFR, and a sixfold increase in proteinuria in Add3KO rats. In contrast, RBF and Pgc were autoregulated to a much greater extent in WT rats, and the increase in proteinuria was much less than that seen in Add3KO rats.
The mechanisms responsible for the increase in GFR and proteinuria in the first week of hypertension in Add3KO rats are unclear. We hypothesized that the impaired myogenic response in the afferent arteriole and IA would lead to a greater increase in RBF, Pgc, and GFR in Add3KO rats compared with WT rats. However, we found that Pgc and GFR increased, whereas RBF was unaltered, in Add3KO rats due to an increase in both afferent and efferent resistances calculated from the mean values of MAP, RBF, GFR, and Pgc. In this regard, afferent resistance increased similarly by 19% and 27% in WT and Add3KO rats. Efferent resistance, however, increased by 46% in Add3KO rats versus 11% in WT rats. The rise in efferent resistance could be because of a loss in the release of paracrine vasodilators, such as nitric oxide, prostaglandins, and epoxyeicosatrienoic acids, from the glomerulus (28, 45) due to podocyte detachment and more advanced glomerular injury in Add3KO rats (Fig. 6E). In addition, glomerular hypertrophy, which is commonly found early in the development of DOCA-salt hypertension, could contribute to hyperfiltration due to an increase of capillary filtration area and the ultrafiltration coefficient (2, 47). Thus, the rise in GFR was associated with a greater increase in kidney weight in the first week of hypertension in Add3KO rats. The mechanism by which Add3KO enhances renal hypertrophy is unknown and will have to be examined in future studies. Finally, we cannot exclude the possibility that the acute measurements of RBF and GFR in the present study might have underestimated the values in conscious animals because of elevated sympathetic tone, increased vasopressin, and renin-angiotensin release due to anesthesia and surgical stress.
In the second week of DOCA-salt hypertension, MAP increased to ~170 mmHg in both strains, which then exceeded the breakthrough point of RBF autoregulation in the WT strain. Proteinuria now increased in WT rats to the same level as that seen in Add3KO rats in which RBF autoregulation is impaired under baseline conditions. After 3 wk of hypertension, glomerular nephrin staining fell in WT rats, similar to what was seen at 1 wk in Add3KO rats. Proteinuria did not increase further in Add3KO rats even though nephrin staining in glomeruli was nearly absent. The failure to see an increase in proteinuria was likely because of a 50% decrease in GFR (Fig. 2D) that occurred between the first and third week of hypertension in Add3KO rats. The fall in GFR was associated with mesangial matrix expansion, a marked reduction in glomerular capillary filtration area, and a greater increase in glomerular fibrosis (Fig. 3A) in Add3KO rats relative to WT control rats.
Alterations in renal hemodynamics and hypertrophy of the preglomerular vasculature have been reported in patients with essential hypertension (6, 18), spontaneously hypertensive rats (12, 33), and angiotensin II models of hypertension in rats and mice (38). These changes contribute to renoprotection in patients and these strains after the development of hypertension by increasing vascular resistance and shifting the RBF autoregulation curve to higher pressures. With time, inward vascular remodeling eventually leads to ischemic glomerular injury and CKD (3). In the present study, we found that the inner diameter of IAs increased rather than decreased and that the wall-to-lumen ratio was not significantly different after 3 wk of DOCA-salt hypertension in both FHH 1BN and FHH 1BN Add3KO strains. Thus, renal arterioles exhibited outward eutrophic vascular remodeling in our DOCA-salt hypertensive rats rather than inward vascular hypertrophy typically seen in other models of hypertension. The compensatory increase in the diameters of the preglomerular arteries likely contributed to the decrease in RBF autoregulation and increase in Pgc observed in WT rats and the rapid progression of glomerulosclerosis after 3 wk of DOCA-salt hypertension relative to that seen in other hypertension models in which renal vascular resistance is elevated. In contrast, baseline Pgc decreased in Add3KO rats after 3 wk of hypertension because of occlusion of glomerular capillaries despite similar outward eutrophic remodeling of the preglomerular arteries seen in WT rats. Thus, the increase in renal vascular resistance and fall in RBF and GFR in both strains after 3 wk of hypertension may be the result of progressive matrix expansion that occluded glomerular capillaries.
Previous studies have demonstrated that the loss of glomerular barrier function and glomerular hyperfiltration that increases the filtered load of protein in hypertensive, diabetic, and reduced renal mass models is associated with the development of EMT, immune cell infiltration, elevated levels of cytokines, and renal interstitial fibrosis (22, 23, 31). Tubular damage has also been reported to be activated in protein-overload models of renal injury (14, 31). It causes tubular cells to undergo EMT and transdifferentiate into interstitial myofibroblasts, characterized by loss of the epithelial marker E-cadherin and increased expression of vimentin and α-SMA (16, 17). In the present study, we found that loss of the glomerular podocyte marker nephrin was associated with increased protein excretion and the formation of tubular protein casts as well as changes in the expression of EMT markers (Fig. 8) and increased renal interstitial fibrosis (Fig. 7A).
We also found that the expression of macrophage-related proinflammatory cytokines GRO/KC, MIP-1α, and MCP-1 were elevated in the kidneys of hypertensive Add3KO rats (Fig. 9A). This was associated with increased numbers of infiltrating macrophages in the glomerulus (Fig. 9C). Increased numbers of CD68+ macrophages in glomeruli have been reported in patients with diabetic nephropathy (19, 49) and was accompanied by upregulated levels of MIP-1 (34) and MCP-1 (44) cytokines. Increased infiltration of macrophages has also been reported to play an important role in the development of hypertension and renal injury in Dahl salt-sensitive rats (10).
Overall, the results of the present study support the view that altered renal hemodynamics and increased transmission of pressure to the glomerulus play an important role in the rapid development of proteinuria and glomerulosclerosis in hypertensive Add3KO rats, similar to what has been seen in Dahl salt-sensitive (32, 37), reduced renal mass hypertensive rats (11) and in some diabetic models (13, 20). However, there are some limitations to the present study. First, it is important to recognize that the reason why hypertension is a major cause of CKD in patients is because of the high prevalence of hypertension. Indeed, except for certain racial groups and individuals with genetic susceptibility variants, the risk of most patients with hypertension to develop advanced CKD or ESRD is relatively low (15, 21). Most develop benign nephrosclerosis characterized by narrowing of preglomerular arteries and arterioles, which limits damage to glomerular capillaries and preserves the filtration area (11). We have previously reported that ADD3 was expressed in renal vascular smooth muscle cells, which explains why KO of Add3 impairs renal autoregulation (7, 8). However, we now found that ADD3 is expressed in podocytes in the present study. Because ADD3 affects the actin cytoskeleton, which plays a vital role in podocytes (9), we cannot exclude the possibility that KO of Add3 may also facilitate hypertension-induced renal injury by altering podocytes function or make them more susceptible to high pressure-induced effacement and detachment.
In conclusion, the present study indicates that loss of the myogenic response of preglomerular arterioles and autoregulation of RBF in Add3KO rats exposed glomeruli to higher pressures, resulting in hyperfiltration, accelerated podocytes detachment, glomerulosclerosis, EMT, inflammation, renal interstitial fibrosis, and a progressive decline in GFR following the development of hypertension. Eutrophic vascular remodeling, rather than hypertrophy as seen 3 wk after the development of DOCA-salt hypertension, further attenuated RBF autoregulation and contributed to hypertension-induced renal injury. This study provides direct evidence supporting the hypothesis that increased susceptibility to renal injury in genetic and experimental models of hypertension and diabetes are related to differences in the control of renal hemodynamics and the transmission of pressure to the glomerulus (11).
GRANTS
This work was supported by National Institutes of Health Grants DK104184, HL138685, P20GM104357, AG060049, and AG057842.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.F., F.F., and R.J.R. conceived and designed research; L.F., W.G., B.V.N., J.R.J., Y.L., and R.J.R. performed experiments; L.F., W.G., B.V.N., and R.J.R. analyzed data; L.F., W.G., B.V.N., and R.J.R. interpreted results of experiments; L.F. and R.J.R. prepared figures; L.F. and R.J.R. drafted manuscript; L.F., W.G., B.V.N., J.R.J., Y.L., F.F., and R.J.R. edited and revised manuscript; L.F., W.G., B.V.N., J.R.J., Y.L., F.F., and R.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Birdie B. Lamarca and Tarek Ibrahim for helping in the analysis of cytokine assays and Goldie M. Faircloth for maintaining animal colonies.
REFERENCES
- 1.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basting T, Lazartigues E. DOCA-salt hypertension: an update. Curr Hypertens Rep 19: 32, 2017. doi: 10.1007/s11906-017-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 4.Burke M, Pabbidi M, Fan F, Ge Y, Liu R, Williams JM, Sarkis A, Lazar J, Jacob HJ, Roman RJ. Genetic basis of the impaired renal myogenic response in FHH rats. Am J Physiol Renal Physiol 304: F565–F577, 2013. doi: 10.1152/ajprenal.00404.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Chronic Kidney Disease in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. [Google Scholar]
- 6.Elliott WJ. Clinical features in the management of selected hypertensive emergencies. Prog Cardiovasc Dis 48: 316–325, 2006. doi: 10.1016/j.pcad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Fan F, Geurts AM, Pabbidi MR, Ge Y, Zhang C, Wang S, Liu Y, Gao W, Guo Y, Li L, He X, Lv W, Muroya Y, Hirata T, Prokop J, Booz GW, Jacob HJ, Roman RJ. A mutation in γ-adducin impairs autoregulation of renal blood flow and promotes the development of kidney disease. J Am Soc Nephrol 31: 687–700, 2020. doi: 10.1681/ASN.2019080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan F, Pabbidi MR, Ge Y, Li L, Wang S, Mims PN, Roman RJ. Knockdown of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. Am J Physiol Renal Physiol 312: F971–F981, 2017. doi: 10.1152/ajprenal.00529.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Renal Physiol 317: F361–F374, 2019. doi: 10.1152/ajprenal.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin KA. Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension 70: 687–694, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi K, Epstein M, Loutzenhiser R. Enhanced myogenic responsiveness of renal interlobular arteries in spontaneously hypertensive rats. Hypertension 19: 153–160, 1992. doi: 10.1161/01.HYP.19.2.153. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Epstein M, Loutzenhiser R, Forster H. Impaired myogenic responsiveness of the afferent arteriole in streptozotocin-induced diabetic rats: role of eicosanoid derangements. J Am Soc Nephrol 2: 1578–1586, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol 27: 901–909, 2012. doi: 10.1007/s00467-011-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 165: 923–928, 2005. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 16.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RJ, Feehally J, Floege J. Comprehensive Clinical Nephrology E-Book. Amsterdam, The Netherlands: Elsevier Health Sciences, 2014. [Google Scholar]
- 18.Kaplan NM. Kaplan’s Clinical Hypertension. Philadelphia, PA: Lippincott, Williams & Wilkins, 2010. [Google Scholar]
- 19.Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, IJpelaar DHT. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant 32: 1322–1329, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Kojima N, Slaughter TN, Paige A, Kato S, Roman RJ, Williams JM. Comparison of the development diabetic induced renal disease in strains of Goto-Kakizaki rats. J Diabetes Metab Suppl 9: S9-005, 2013. doi: 10.4172/2155-6156.S9-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp JB. Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens 22: 266–272, 2013. doi: 10.1097/MNH.0b013e3283600f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 23.Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med 43: 9–17, 2004. doi: 10.2169/internalmedicine.43.9. [DOI] [PubMed] [Google Scholar]
- 24.O’Meara CC, Lutz MM, Sarkis AB, Xu H, Kothinti RK, Hoffman M, Moreno C, Tabatabai NM, Lazar J, Roman RJ, Jacob HJ. A 4.1-Mb congenic region of Rf-4 contributes to glomerular permeability. J Am Soc Nephrol 23: 825–833, 2012. doi: 10.1681/ASN.2011080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pabbidi MR, Juncos J, Juncos L, Renic M, Tullos HJ, Lazar J, Jacob HJ, Harder DR, Roman RJ. Identification of a region of rat chromosome 1 that impairs the myogenic response and autoregulation of cerebral blood flow in fawn-hooded hypertensive rats. Am J Physiol Heart Circ Physiol 304: H311–H317, 2013. doi: 10.1152/ajpheart.00622.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pabbidi MR, Mazur O, Fan F, Farley JM, Gebremedhin D, Harder DR, Roman RJ. Enhanced large conductance K+ channel activity contributes to the impaired myogenic response in the cerebral vasculature of Fawn Hooded Hypertensive rats. Am J Physiol Heart Circ Physiol 306: H989–H1000, 2014. doi: 10.1152/ajpheart.00636.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer ND, Ng MC, Hicks PJ, Mudgal P, Langefeld CD, Freedman BI, Bowden DW. Evaluation of candidate nephropathy susceptibility genes in a genome-wide association study of African American diabetic kidney disease. PLoS One 9: e88273, 2014. doi: 10.1371/journal.pone.0088273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palygin O, Ilatovskaya DV, Levchenko V, Endres BT, Geurts AM, Staruschenko A. Nitric oxide production by glomerular podocytes. Nitric Oxide 72: 24–31, 2018. doi: 10.1016/j.niox.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattaro C, Köttgen A, Teumer A, Garnaas M, Böger CA, Fuchsberger C, Olden M, Chen M-H, Tin A, Taliun D, et al.; CARDIoGRAM Consortium; ICBP Consortium; CARe Consortium; Wellcome Trust Case Control Consortium 2 (WTCCC2) . Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet 8: e1002584, 2012. doi: 10.1371/journal.pgen.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattaro C, Teumer A, Gorski M, Chu AY, Li M, Mijatovic V, Garnaas M, Tin A, Sorice R, Li Y, et al.; ICBP Consortium; AGEN Consortium; CARDIOGRAM; CHARGe-Heart Failure Group; ECHOGen Consortium . Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun 7: 10023, 2016. doi: 10.1038/ncomms10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remuzzi G. Nephropathic nature of proteinuria. Curr Opin Nephrol Hypertens 8: 655–663, 1999. doi: 10.1097/00041552-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Ren Y, D’Ambrosio MA, Garvin JL, Peterson EL, Carretero OA. Mechanism of impaired afferent arteriole myogenic response in Dahl salt-sensitive rats: role of 20-HETE. Am J Physiol Renal Physiol 307: F533–F538, 2014. doi: 10.1152/ajprenal.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Y, D’Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298: H1769–H1775, 2010. doi: 10.1152/ajpheart.00537.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojewska E, Zychowska M, Piotrowska A, Kreiner G, Nalepa I, Mika J. Involvement of macrophage inflammatory protein-1 family members in the development of diabetic neuropathy and their contribution to effectiveness of morphine. Front Immunol 9: 494, 2018. doi: 10.3389/fimmu.2018.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roman RJ, Cowley AW Jr. Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 248: F190–F198, 1985. doi: 10.1152/ajprenal.1985.248.2.F190. [DOI] [PubMed] [Google Scholar]
- 36.Sandholm N, Salem RM, McKnight AJ, Brennan EP, Forsblom C, Isakova T, McKay GJ, Williams WW, Sadlier DM, Mäkinen V-P, et al.; DCCT/EDIC Research Group New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 8: e1002921, 2012. doi: 10.1371/journal.pgen.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoh M, Haruna Y, Fujimoto S, Sasaki T, Kashihara N. Telmisartan improves endothelial dysfunction and renal autoregulation in Dahl salt-sensitive rats. Hypertens Res 33: 135–142, 2010. doi: 10.1038/hr.2009.190. [DOI] [PubMed] [Google Scholar]
- 38.Schleifenbaum J, Kassmann M, Szijártó IA, Hercule HC, Tano J-Y, Weinert S, Heidenreich M, Pathan AR, Anistan Y-M, Alenina N, Rusch NJ, Bader M, Jentsch TJ, Gollasch M. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ Res 115: 263–272, 2014. doi: 10.1161/CIRCRESAHA.115.302882. [DOI] [PubMed] [Google Scholar]
- 39.Schulz A, Kreutz R. Mapping genetic determinants of kidney damage in rat models. Hypertens Res 35: 675–694, 2012. doi: 10.1038/hr.2012.77. [DOI] [PubMed] [Google Scholar]
- 40.Simons JL, Provoost AP, Anderson S, Rennke HG, Troy JL, Brenner BM. Modulation of glomerular hypertension defines susceptibility to progressive glomerular injury. Kidney Int 46: 396–404, 1994. doi: 10.1038/ki.1994.287. [DOI] [PubMed] [Google Scholar]
- 41.Simons JL, Provoost AP, Anderson S, Troy JL, Rennke HG, Sandstrom DJ, Brenner BM. Pathogenesis of glomerular injury in the fawn-hooded rat: early glomerular capillary hypertension predicts glomerular sclerosis. J Am Soc Nephrol 3: 1775–1782, 1993. [DOI] [PubMed] [Google Scholar]
- 42.van Dokkum RP, Sun C-W, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Renal Physiol 276: R855–R863, 1999. doi: 10.1152/ajpregu.1999.276.3.R855. [DOI] [PubMed] [Google Scholar]
- 43.Verseput GH, Braam B, Provoost AP, Koomans HA. Tubuloglomerular feedback and prolonged ACE-inhibitor treatment in the hypertensive fawn-hooded rat. Nephrol Dial Transplant 13: 893–899, 1998. doi: 10.1093/ndt/13.4.893. [DOI] [PubMed] [Google Scholar]
- 44.Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda S-I, Takasawa K, Yoshimura M, Kida H, Kobayashi KI, Mukaida N, Naito T, Matsushima K, Yokoyama H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58: 1492–1499, 2000. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Garvin JL, Falck JR, Ren Y, Sankey SS, Carretero OA. Glomerular cytochrome P-450 and cyclooxygenase metabolites regulate efferent arteriole resistance. Hypertension 46: 1175–1179, 2005. doi: 10.1161/01.HYP.0000187531.93389.63. [DOI] [PubMed] [Google Scholar]
- 46.Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M, Tin A, Wang L, Chu AY, Hoppmann A, et al.; Lifelines Cohort Study; V. A. Million Veteran Program . A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51: 957–972, 2019. doi: 10.1038/s41588-019-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida Y, Fogo A, Ichikawa I. Glomerular hemodynamic changes vs. hypertrophy in experimental glomerular sclerosis. Kidney Int 35: 654–660, 1989. doi: 10.1038/ki.1989.35. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Zhang C, Liu Y, Gao W, Wang S, Fang X, Guo Y, Li M, Liu R, Roman RJ, Sun P, Fan F. Influence of dual-specificity protein phosphatase 5 on mechanical properties of rat cerebral and renal arterioles. Physiol Rep 8: e14345, 2020. doi: 10.14814/phy2.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Yang Y, Zhao Y. Macrophage phenotype and its relationship with renal function in human diabetic nephropathy. PLoS One 14: e0221991, 2019. doi: 10.1371/journal.pone.0221991. [DOI] [PMC free article] [PubMed] [Google Scholar]