Abstract
Tubular changes contribute to the development of renal pathologies in diabetic kidney disease (DKD), including interstitial fibrosis. It is unclear how tubular cells relay signals to interstitial fibroblasts. Recently, exosomes have been recognized as crucial mediators of intercellular communication. We hypothesized that exosomes secreted from tubular cells may stimulate fibroblasts for interstitial fibrosis in DKD. In this study, we isolated and purified exosomes from the renal cortex of DKD mice and high glucose-treated mouse proximal tubular cells. Compared with nondiabetic mice, exosome secretion in kidney tissues decreased in DKD mice. Likewise, high glucose incubation reduced exosome secretion in mouse kidney proximal tubular BUMPT cells. To study the effect of tubular cell exosomes on fibroblasts, exosomes from BUMPT cells were added to renal fibroblast NRK-49F cell cultures. Notably, exosomes from high glucose conditioned BUMPT cells induced higher proliferation, significant morphological change, and substantial production of fibronectin, α-smooth muscle actin, and collagen type Ι in NRK-49F fibroblasts. Proteomics analysis was further performed to profile the proteins within tubular cell exosomes. Interestingly, 22 proteins were found to be differentially expressed between tubular exosomes derived from high glucose conditioned cells and those from normal glucose conditioned cells. Cytoscape analysis suggested the existence of two protein-protein interaction networks in these exosomal differentially expressed proteins. While one of the protein-protein interaction networks comprised enolase 1 (Eno1), heat shock protein family A member 8 (Hspa8), thioredoxin 1 (Txn1), peptidylprolyl isomerase A (Ppia), phosphoglycerate kinase 1 (Pgk1), DNA topoisomerase II-β (Top2b), and β-actin (Actb), the other had the family proteins of human leucocyte antigen F (Ywhag), a component of the ND10 nuclear body (Ywhae), interferon regulatory factor-8 (Ywhaq), and human leucocyte antigen A (Ywhaz). Gene expression analysis via Nephroseq showed a correlation of Eno1 expression with DKD clinical manifestation. In conclusion, DKD is associated with a decrease in exosome secretion in renal tubular cells. Exosomes from high glucose conditioned tubular cells may regulate the proliferation and activation of fibroblasts, contributing to the paracrine signaling mechanism responsible for the pathological onset of renal interstitial fibrosis in DKD.
Keywords: diabetic kidney disease, exosome, renal fibrosis, tubular injury
INTRODUCTION
Diabetic kidney disease (DKD) develops in ~25−40% of patients with diabetes and is the leading cause of end-stage renal disease worldwide (29, 39). With its high prevalence, DKD has become a significant public health problem on a global scale, which imposes a significant economic burden and therefore requires urgent preventive and therapeutic strategies. However, the pathogenesis of DKD remains poorly understood. Despite the involvement of multiple genetic, epigenetic, and metabolic factors, renal interstitial fibrosis is regarded as a shared outcome and predominant pathological feature of DKD that can be characterized by tubular atrophy, extracellular matrix accumulation, and myofibroblast expansion (14, 20).
In renal interstitial fibrosis, pathological changes in kidney tubules may be an initial factor. There is a general consensus that tubulointerstitial communication between tubular cells and fibroblasts is a key to the development of interstitial fibrosis, which functions to regulate both renal repair/regeneration and progressive kidney disease (13, 24). Specifically, when the fibroblast activation is transient and followed by controlled deactivation, renal fibrosis is a protective mechanism that helps repair renal injury and restore renal function. However, when tubular injury and fibroblast activation are sustained over time, fibrosis can lead to irreversible tubulointerstitial damage, manifesting as scar tissues with an abundant extracellular matrix to replace normal kidney tissues (21). It remains unclear how tubulointerstitial communication is established between tubular epithelial cells and neighboring fibroblasts that triggers interstitial fibrosis in kidney diseases, such as DKD.
Notably, emerging evidence reveals that exosomes may play a crucial role in intercellular communication (25, 32, 37). As a type of nanosized (~30–150 nm) membrane vesicles, exosomes can package and deliver cargos containing various cellular constituents, such as proteins, nucleic acid, and lipids. Because of their size and lipophilic membrane constituents, exosomes can efficiently mediate the transfer of their molecular cargos between different cells, thus emphasizing their roles in cell-cell communications and paracrine signaling. Since the transfer of these cargos reflects the physiological state of the originating cells, consequent regulation on the biological functions of the recipient cells can vary between physiological and pathological settings (15, 43, 46). Despite substantial knowledge in the understanding on how intercellular communication is implemented by the exosomes and how exosomes modulate pathological processes in diabetes and diabetic complications (5, 44), it remains unclear whether exosomes play a role in the communication between tubular cells and fibroblasts in renal interstitial fibrosis in DKD. Hence, the primary aim of the present study was to characterize exosome secretion in the pathological DKD setting and to investigate whether exosomes could mediate tubulointerstitial communication and regulate renal fibrosis progression in DKD.
MATERIALS AND METHODS
Antibodies and special reagents.
Antibodies were purchased from the following sources: anti-CD63, anti-fibronectin, anti-α-smooth muscle actin (α-SMA), and anti-cyclophilin B antibodies from Abcam (Cambridge MA), anti-CD9 from Santa Cruz Biotechnology (Dallas, TX), anti-collagen type Ι antibodies from Cell Signaling Technology (Danvers, MA), and all secondary antibodies from Jackson ImmunoResearch (West Grove, PA). Exosome-depleted FBS was from ThermoFisher Scientific (Waltham, MA).
Cell culture.
The Boston University mouse proximal tubular cell line (BUMPT cells) was originally obtained from Dr. Wilfred Lieberthal (Boston University School of Medicine, Boston, MA). Cells were cultured in DMEM with 10% FBS at 37°C in an atmosphere of 5% CO2 as previously described (33). The rat kidney interstitial fibroblast line (NRK-49F cells) was from the American Type Culture Collection (Manassas, VA).
High glucose treatment on renal tubular cell lines.
BUMPT cells were cultured with DMEM containing high (30 mM) or normal (5.5 mM) glucose for 8 days. High glucose medium was prepared by adding d-glucose to DMEM to reach 30 mM glucose. As a control, 24.5 mM d-mannitol was added to the 5.5 mM glucose DMEM. During culture, the medium with 10% FBS was refreshed on the third and fifth day. On the seventh day, cells were changed to serum-free DMEM for 24 h, and the medium was replaced with serum-free DMEM again on the eigth day. The medium was collected at the end of the eigth day for exosome isolation.
Tubular exosome treatment of renal fibroblasts.
NRK-49F renal fibroblasts were cultured in DMEM with 10% FBS and serum starved for 24 h in serum-free DMEM. Exosomes were collected from high and normal glucose tubular cells and quantified by nanoparticle tracking analysis (NTA). The same particle number (~1 × 1010) of high glucose-treated tubular cell exosomes (HG-Exo) or normal glucose-treated tubular cell exosomes (NG-Exo) were added to NRK-49F cells for 48 h of incubation. After exosome treatment, cell morphology was observed by microscopy. Cell numbers were counted by a TC20 Automated Cell Counter. The cell lysate was harvested for protein analysis.
Mouse model of DKD.
For the Akita diabetic model, C57BL/6J-Ins2 +/Akita mice were originally from Jackson Laboratory. Offspring genotyping performed according to the protocol recommended by Jackson Laboratory. Ins2 +/Akita heterozygous male mice and wild-type (WT) Ins2 +/+ littermates (11 and 20 wk old) were used for experiments. All mice were housed in the animal facility of Charlie Norwood Veterans Affairs Medical Center under a 12:12-h light-dark pattern with free access to food and water. Fasting blood glucose was measured twice a week using a glucometer after the mice were fasted for ∼8 h. Animals with >200 mg/dL fasting blood glucose for two consecutive readings were considered diabetic. All animal experiments were conducted according to a protocol approved by the Institutional Animal Care and Use Committee of Charlie Norwood Veterans Affairs Medical Center.
Isolation and purification of exosomes from conditioned medium.
Exosomes were isolated and purified from the conditioned medium (CM) by a defined series of centrifugation as previously described (43). Briefly, CM was collected and subjected to sequential centrifugation steps as follows: 300 g for 10 min, 2,000 g for 20 min, and 10,000 g for 30 min at 4°C. The supernatant was then subjected to ultracentrifugation (F37L-8 × 100 Fixed- Angle Rotor, Thermo Scientific) at 100,000 g for 90 min at 4°C. The pellet was washed by cold particle-free 1× PBS buffer (Life Technologies) and then subjected to ultracentrifugation (Tft. −80.4 Fixed Angle Rotor, Thermo Scientific) at another 100,000 g for 90 min at 4°C. The resulting pellet was resuspended in PBS for quantification or transmission electron microscopy (TEM) analysis, placed in DMEM for treatment, lysed for protein analysis, or harvested and stored at −80°C for further processing.
Isolation and purification of exosomes from kidney tissue.
The kidney cortex was obtained from Akita or WT mice, cut into small slices, and homogenized in cold Hank’s solution. The homogenate was digested with 0.75 mg/mL collagenase type IV in Hank's solution containing 0.75 mg/mL trypsin inhibitor on a shaker at 120 rpm at 37°C for 15 min and gently inverted in up and down motions for 10 times every 5 min. The digestion was stopped by adding the same volume of cold Hank’s solution with 10% exosome-depleted FBS. The resulting solution was subjected to a series of centrifugations (300 g for 10 min, 2,000 g for 20 min, and 10,000 g for 30 min) at 4°C. The supernatant was then filtered by a 0.22-μm filter, and a pellet was recovered at 120,000-g ultracentrifugation (630 Swing Bucket Rotor, Thermo Scientific) at 4°C for 90 min. The pellet was washed by cold PBS and subjected to ultracentrifugation (Tft. −80.4 Fixed Angle Rotor, Thermo Scientific) again at 120,000 g at 4°C for 70 min. Exosomes were harvested for analysis or stored at −80°C for later processing.
NTA of exosomes.
NTA was performed with ZetaView (Particle Metrix) to measure the size distribution and concentration of exosomes as previously described (46). Isolated fresh exosomes were diluted in particle-free 1× PBS buffer (Life Technologies) and resuspended and scattered by pipette before being injected into the sample cell chamber. The NTA measurement was recorded and analyzed at 11 different positions. The ZetaView system was calibrated using 100-nm polystyrene particles, and the temperature was maintained at 23°C. Size distributions and particle concentrations were assessed with NTA software. Exosome concentration was normalized by tissue weight or the numbers of cells where exosomes were collected. To quantify the cell number, cells were harvested at the end of treatment, digested into suspension using trypsin, and quantified with a TC20 Automated Cell Counter.
Transmission electron microscopy.
TEM was conducted by the Electron Microscopy Core of Augusta University as previously described (12, 16). Briefly, three microliters of exosome pellet solution were applied on Formvar/carbon-coated 200 mesh copper electron microscopy grids, incubated at room temperature for 5 min, and then subjected to standard uranyl acetate staining. The grid was washed with PBS three times and allowed to semi-dry at room temperature before observations in a transmission electron microscope (Hitachi H7500 TEM, Tokyo, Japan).
Immunoblot analysis.
Whole cell or exosomal lysates were extracted in 2% SDS buffer [62.5 mM Tris·HCl (pH 6.8), 2% SDS, and 10% glycerol] with protease inhibitor cocktail (Sigma-Aldrich) and Benzonase nuclease (EMD Millipore). Protein concentration was determined with a Pierce BCA protein assay kit (Thermo Scientific). Equal amounts of whole cell protein or total exosomal protein (normalized by original cell numbers) were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The blot was blocked with 5% milk for 1 h at room temperature and then immunoblotted with primary antibody at 4°C overnight. The blot membrane was then subsequently incubated with primary antibodies and secondary antibodies. The blot signals were revealed with a chemiluminescence kit (Bio-Rad or Thermo Scientific).
Proteomics of tubular exosomal proteins.
Tubular cell CM was collected from four independent experiments and stored at −80°C. Within 1 mo, CMs were pooled together for ultracentrifugation to isolate exosomes. The fresh exosome pellets were resuspended in exosome-depleted 1× PBS buffer and digested for proteomics analysis. Normalization was done using particle numbers by NTA. Proteomics was performed on proteins with liquid chromatography-tandem mass spectrometry (LC-MS/MS) at the Integrated Genomics Core of Augusta University. Details of the procedure can be found online at https://www.augusta.edu/cancer/research/shared-resources/proteomics/index.php. The protein profile was analyzed between NG-Exo and HG-Exo samples.
Histological analysis.
Kidney tissue was harvested, fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned at 4 μm for histological examinations. Sirius red staining was performed according to a standard procedure provided by the manufacturer (HT15, Sigma-Aldrich). To quantitatively estimate the fibrotic area, around 10 fields (×10 and ×20 magnification) were randomly selected from each section and analyzed by ImageJ software.
Quantitative RT-PCR.
Total RNA was extracted from kidney tissues using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA (500 ng) was reverse transcribed using the cDNA transcription kit (Bio-Rad), and quantitative RT-PCR was performed using SYBR Green PCR Master Mix (Bio-Rad). β-Actin (Actb) was used for normalization in all experiments. cDNA was reverse transcribed according to the instructions of the Takara kit. The genes of fibronectin, α-SMA, collagen type I, and collagen type IV were amplified by quantitative RT-PCR. The primer sequences of β-actin, fibronectin, α-SMA, collagen type I, and collagen type IV were as follows: β-actin, primer 1 5′-GATTACTGCTCTGGCTCCTAG-3′ and primer 2 5′-GACTCATCGTACTCCTGCTTG-3′; fibronectin, primer 1 5′-GAGCTATCCATTTCACCTTCAGA-3′ and primer 2 5′-TTGTTCGTAGACACTGGAGAC-3′; α-SMA, primer 1 5′-GAGCTACGAACTGCCTGAC-3′ and primer 2 5′-CTGTTATAGGTGGTTTCGTGG A-3′; collagen type I, primer 1 5′-CGCAAAGAGTCTACATGTCTAGG-3′ and primer 2 5′-CATTGTGTATGCAGCTGACTTC-3′; and collagen type IV, primer 1 5′-TCTGGCTGTGGAAAATGTGA-3′ and primer 2 5′-AATCCAATGACACCTTGCAAC-3′.
Statistics.
Data are expressed as means ± SD. Statistical analysis was conducted using GraphPad Prism software. Statistical differences in multiple groups were determined by multiple comparisons with one-way ANOVA. Statistical differences between two groups were assessed by a Student’s t test. P values of <0.05 were considered statistically different.
RESULTS
Exosome secretion decreases in kidney cortex tissues of DKD mice.
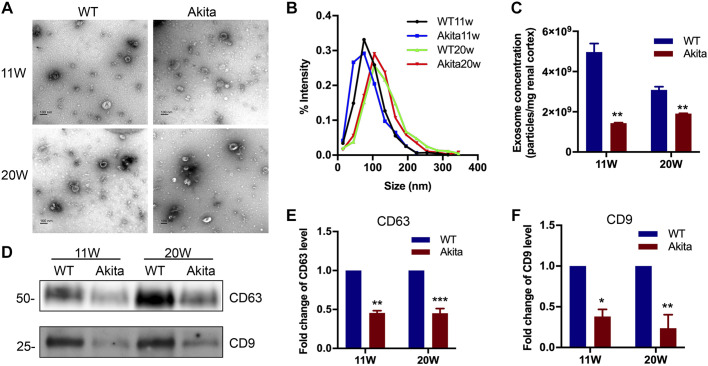
Akita mice have a spontaneous mutation in the insulin 2 gene resulting in incorrect folding of the insulin protein and toxicity in pancreatic β-cells with ensuing type 1 diabetes at 3–4 wk of age (3). To determine exosome production in DKD, we first isolated and purified exosomes from kidney cortical tissues of Akita mice at 11 and 20 wk. As shown in Fig. 1A, isolated exosomes were tiny vesicles with a characteristic bilayer cup-shaped morphology in TEM. No obvious morphological differences were found between exosomes from Akita mice and WT littermate mice at 11 or 20 wk. To further examine exosome size and concentration, NTA was applied. Exosomes from both WT and Akita mice were around 100 nm in diameter in size, and there appeared to be a small size increase from 11 to 20 wk, but the difference was not significant (Fig. 1B). However, the amount or concentration of exosomes in Akita mouse kidneys was markedly decreased compared with WT mouse kidneys (Fig. 1C). We further examined the exosome protein markers CD63 and CD9 in kidney tissues by immunoblot analysis. Compared with WT mice, Akita mice had lower expression of CD63 and CD9 in kidney cortical tissues at both 11 and 20 wk of age (Fig. 1, D–F). Collectively, these results indicated that the kidney cortical tissue of DKD mice had decreased exosome production or secretion.
Fig. 1.
Characterization of exosomes derived from kidney cortex tissues of diabetic kidney disease (DKD) mice. Kidney cortical tissue of 11 wk (11W) or 20 wk (20W) Akita and wild-type (WT) mice were harvested and homogenized for exosome isolation and purification. The exosome pellets were collected for morphological and immunoblot analysis. A: representative morphological images of exosomes observed by transmission electron microscopy. Scale bars = 100 nm. B: size distribution of exosomes analyzed by nanoparticle tracking analysis. C: exosome quantification by nanoparticle tracking analysis after normalization with tissue weight. Values (in particle number/mg renal cortex tissue) are means ± SE of 4 animal groups (n = 4). **P < 0.01 vs. WT mice. D−F: induction of CD63 and CD9 expression in exosomes derived from 11W and 20W DKD mice. D: representative Western blot analysis demonstrating the decreased production of CD63 and CD9 expression in Akita mice. The loading volume of exosome protein lysis was normalized by tissue weight. E and F: semiquantitative analysis of the average optical density of CD63 (E) and CD9 (F). Values are presented as mean ± SD; n = 4. *P < 0.05, **P < 0.01, and *** P < 0.001 vs. the WT group.
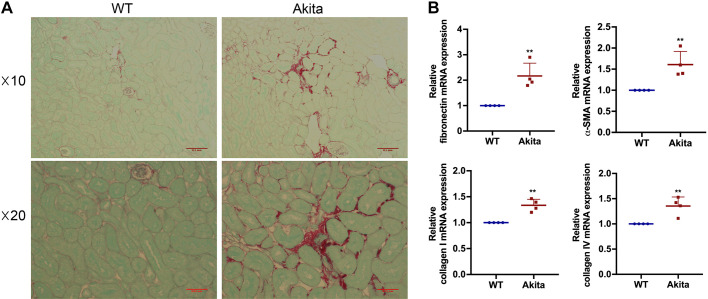
Renal interstitial fibrosis increases in DKD mice.
We next determined the degree of renal interstitial fibrosis, which is commonly known as a late stage feature of DKD. By Sirius red staining, we detected an increased deposition of collagen fibrils in the kidney interstitium in Akita mice at 20 wk compared with WT mice (Fig. 2A). To further confirm the results, we also examined the protein markers of fibrosis by quantitative RT-PCR analysis. Consistently, kidney tissues of Akita mice showed significantly higher expression of fibronectin, α-SMA, collagen type I, and collagen type IV than WT mice (Fig. 2B). These data verify the occurrence of renal interstitial fibrosis in DKD mice.
Fig. 2.
Renal interstitial fibrosis increases in 20-wk diabetic kidney disease mice. Renal interstitial fibrosis was estimated by sirius red staining. A: representative images of sirius red staining under microscopy with both ×10 and ×20 lens. Scale bars = 0.1 and 0.05 mm. B: quantitative analysis of fibronectin, α-smooth muscle actin (α-SMA), collagen type I, and collagen type IV by quantitative RT-PCR (n = 4). **P < 0.01 vs. the wild-type (WT) group.
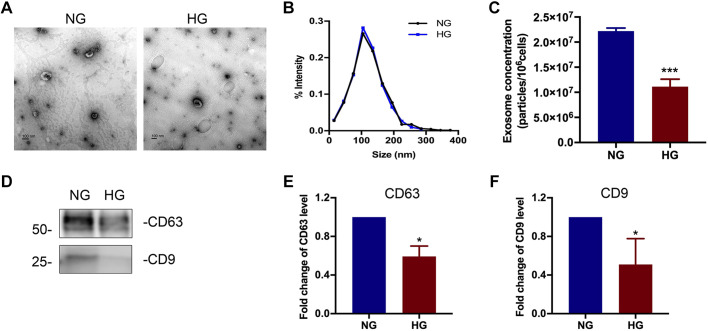
Exosome secretion is decreased in high glucose-treated renal tubular cells.
We next investigated if exosomes from tubule cells play a role in mediating the activation of fibroblasts in renal fibrosis. To mimic the in vivo DKD situation, we cultured renal tubular BUMPT cells in high glucose medium for 8 days to establish an in vitro chronic exposure model. Exosomes were isolated and purified from tubular cell CM harvested after 8 days. We first analyzed the tubular cell exosomes by TEM, NTA, and immunoblot analysis. As shown in Fig. 3, A and B, exosomes from high glucose-treated cells and normal glucose-treated cells had a similar morphology and a similar size distribution of ~100 nm in diameter. Consistent with what we observed in mice (Fig. 1), high glucose-treated cells secreted significantly fewer exosomes than normal glucose-treated cells (Fig. 3C). Expression of exosomal markers CD63 and CD9 was also lower in high glucose-treated cells (Fig. 3D–F).
Fig. 3.
Characterization of exosomes derived from high glucose (HG)-treated renal tubular cells. A: representative images of exosomes observed by transmission electron microscopy. Scale bars = 100 nm. B: size distribution of exosomes analyzed by nanoparticle tracking analysis. C: exosome quantification by nanoparticle tracking analysis after normalization with cell numbers. n = 5. ***P < 0.001 vs. the normal glucose (NG)-treated group. D−F: comparison of CD63 and CD9 expression in exosomes derived from tubular cells in NG- or HG-treated groups. D: representative Western blot analysis demonstrating the decreased production of CD63 and CD9 expression in the HG-treated group. The loading volume of exosome protein lysis was normalized by cell numbers. E and F: semiquantitative analysis of the average optical density of CD63 (E) and CD9 (F). Values are presented as means ± SD; n = 5. *P < 0.05 vs. the NG-treated group.
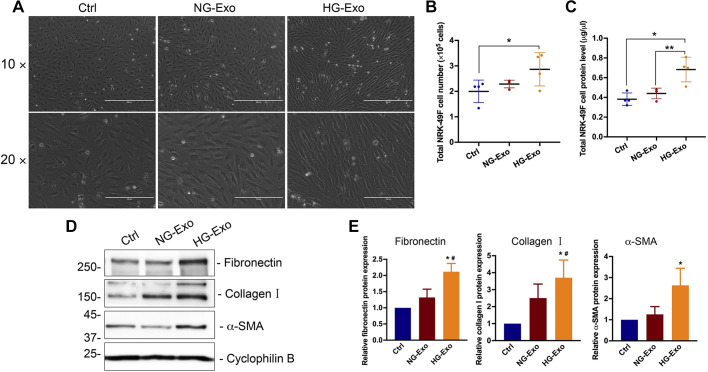
Exosomes from high glucose-treated renal tubular cells activate fibroblasts.
Next, we determined whether renal tubular exosomes can mediate paracrine signaling and establish tubulointerstitial communication. To this end, we treated the recipient NRK-49F renal fibroblasts with tubular cell-derived exosomes for 48 h. As shown in Fig. 4A, exosomes from high glucose-treated BUMPT cells (HG-Exo) induced striking morphological changes of myofibroblasts in NRK-49F cells compared with either the vehicle control group or the group treated with exosomes from normal glucose-treated BUMPT cells (NG-Exo). This suggests that the recipient NRK-49F cells underwent significant differentiation after exposure to the exosomes from high glucose-treated BUMPT cells. We further quantified total cell numbers and protein levels of NRK-49F cells to evaluate the effect on fibroblast cell proliferation. Compared with those treated with vehicle or NG-Exo, there was significant increases in fibroblast cell numbers and proteins in the HG-Exo treatment group (Fig. 4, B and C). Besides, HG-Exo induced protein levels of fibronectin, collagen type I, and α-SMA, commonly used fibrotic markers (Fig. 4, D and E). Collectively, these results demonstrate that high glucose conditioned tubular cells produce fewer exosomes but that their exosomes have a higher capacity of stimulating proliferation and myofibroblast transition in renal fibroblasts.
Fig. 4.
Exosomes from high glucose-treated renal tubular cells activate fibroblasts. High glucose-treated tubular cell exosomes (HG-Exo) or normal glucose-treated tubular cell exosomes (NG-Exo) were added to NRK-49F cells. A: representative images of phase contrast showing the cellular morphology of fibroblasts (n = 4). Scale bars = 100 and 200 μm. B: total cell numbers of NRK-49F fibroblasts. Data are expressed as means ± SD. *P < 0.05, significantly different from the control (Ctrl) group. C: total protein levels of NRK-49F fibroblasts. Data are expressed as means ± SD; n = 4. *P < 0.05, significantly different from the Ctrl group; **P < 0.01 vs. the NG-Exo group. D: representative images of immunoblot analysis of fibronectin, collagen type I, and α-smooth muscle actin (α-SMA). Cyclophilin B was used as a loading control (n = 4). E: densitometric analysis of fibronectin, collagen type I, and α-SMA signals. After being normalized with cyclophilin B, the protein signal of control was arbitrarily set as 1, and the signals of other conditions were normalized with controls to calculate fold changes. Data are expressed as means ± SD; n = 4. *P < 0.05 vs. the Ctrl group; #P < 0.05 vs. the NG-Exo group.
Proteomic analysis of exosomes from high glucose- and normal glucose-treated tubular cells.
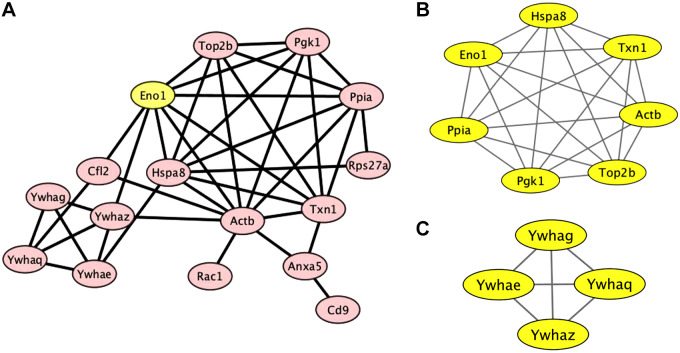
Exosomes mediate intercellular communications by delivering their content molecules to recipient cells, including various proteins and RNAs. To understand how the exosomes from high glucose-treated tubular cells activate fibroblasts, we conducted proteomic analysis to compare the proteins in the exosomes from high glucose- and normal glucose-treated BUMPT cells. Indeed, the HG-Exo group showed quite a few differentially expressed proteins (DEPs). Compared with control exosomes, 22 proteins were upregulated in the HG-Exo group (Table 1). Bioinformatics analysis with online Cytoscape software revealed two protein-protein interaction (PPI) networks in these DEPs (Fig. 5A). One of the PPI networks included enolase 1 (Eno1), heat shock protein family A member 8 (Hspa8), thioredoxin 1 (Txn1), peptidylprolyl isomerase A (Ppia), phosphoglycerate kinase 1 (Pgk1), DNA topoisomerase II-β (Top2b), and β-actin (Actb) (Fig. 5B), while the other comprised the 14-3-3 protein family members of human leucocyte antigen F (Ywhag), a component of the ND10 nuclear body (Ywhae), interferon regulatory factor-8 (Ywhaq), and human leucocyte antigen A (Ywhaz) (Fig. 5C). Interestingly, albeit the existence of two different PPI networks, Eno1 may be the pivotal link between these networks (Fig. 5A). Eleven of the proteins within the identified PPI networks were shown to be “hub proteins,” and some of these proteins have been implicated in fibrosis and DKD (Table 2).
Table 1.
Differentially expressed proteins in exosomes from high glucose-treated renal tubular cells
| Accession Number | Gene Symbol | Fold Change | P Value |
|---|---|---|---|
| P97384 | Anxa11 | 2.55 | 3.60e−2 |
| P51829 | Adcy7 | 2.53 | 1.04e−2 |
| A3KGS3 | Ralgapa2 | 2.45 | 4.97e−2 |
| P45591 | Cfl2 | 2.28 | 2.71e−2 |
| Q9CYL5 | Glipr2 | 2.22 | 4.82e−2 |
| P10639 | Txn | 2.18 | 3.95e−2 |
| P62259 | Ywhae | 2.15 | 3.06e−2 |
| P17742 | Ppia | 2.09 | 2.85e−2 |
| P61982 | Ywhag | 2.07 | 4.21e−2 |
| P63101 | Ywhaz | 2.03 | 3.20e−2 |
| P68254 | Ywhaq | 2 | 3.74e−2 |
| P48036 | Anxa5 | 1.89 | 3.75e−2 |
| P63001 | Rac1 | 1.82 | 3.52e−2 |
| P17182 | Eno1 | 1.8 | 2.10e−2 |
| P62983 | Rps27a | 1.77 | 3.55e−2 |
| P63017 | Hspa8 | 1.74 | 3.00e−2 |
| Q8BHL4 | Gprc5a | 1.71 | 3.48e−2 |
| Q64511 | Top2b | 1.68 | 4.99e−2 |
| P60710 | Actb | 1.66 | 4.77e−2 |
| P40240 | Cd9 | 1.6 | 2.56e−2 |
| P09411 | Pgk1 | 1.58 | 2.59e−2 |
| Q6P5F7 | Ttyh3 | 1.1 | 2.99e−2 |
Exosomes derived from high glucose-treated renal tubular cells and normal glucose-treated cells were isolated for proteomics analysis and compared. A total of 22 proteins were identified as differentially expressed proteins.
Fig. 5.
Correlation network of the differentially expressed proteins displayed by Cytoscape. A: the protein-protein interaction (PPI) network of differentially expressed proteins was constructed using Cytoscape. The most significant modules were obtained from the PPI network. B: one of the PPI networks comprised enolase 1 (Eno1), heat shock protein family A member 8 (Hspa8), thioredoxin 1 (Txn1), peptidylprolyl isomerase A (Ppia), phosphoglycerate kinase 1 (Pgk1), DNA topoisomerase II-β (Top2b), and β-actin (Actb). C: the other network had the family proteins of human leucocyte antigen F (Ywhag), a component of the ND10 nuclear body (Ywhae), interferon regulatory factor-8 (Ywhaq), and human leucocyte antigen A (Ywhaz).
Table 2.
Implications of 11 hub proteins in fibrosis and DKD
| Gene Symbol | Full Name | Implications | |
|---|---|---|---|
| 1 | Eno1 | Enolase 1 | Significant increase of Eno1 expression represents transdifferentiation of murine alveolar epithelial cells, which can be regulated by the Wnt/β-catenin pathway and contributes to pulmonary fibrosis (26). Eno1 inhibition confers antidiabetic effects, which alleviates fibrosis in type 2 diabetes mellitus (6). Eno1 may be a serum biomarker in hepatitis B-induced hepatic fibrosis (42). |
| 2 | Pgk1 | Phosphoglycerate kinase 1 | PGK1 is involved in the cell proliferation of scleroderma. Pgk1, a glycolysis-related protein, is upregulated in uncontrolled diabetes (34). |
| 3 | Ppia | Peptidylprolyl isomerase A | The association between Ppia and fibrosis or DKD has not been reported. |
| 4 | Hspa8 | Heat shock protein family A member 8 | HSP70 overexpression is likely to play an important role in mediating the excessive collagen production by keloid fibroblasts (31) and myocardial fibrosis in streptozotocin-induced diabetic rats. HSP70 increases extracellular matrix production in human vascular smooth muscle by upregulating transforming growth factor-β1 (10). The induction of HSP70 expression is a protective effect against pancreatic fibrosis (17). Intracellular HSP70 deficiency has been found in fulmonary fibrosis (30). The heat shock potein family is involved in the pathogenesis of organ fibrosis (2). Hspa8 is involved in the protein-protein interaction network of diabetic retinopathy (28). |
| 5 | Txn1 | Thioredoxin 1 | Txn1 is a key protein of liver fibrosis in rats. |
| 6 | Actb | β-Actin | Actb variants confer the genetic susceptibility for DKD in a Han Chinese population (18). Actb is involved in the protein-protein interaction network of diabetic retinopathy (28). |
| 7 | Top2b | DNA topoisomerase II-β | The association between Top2b and fibrosis or DKD has not been reported. |
| 8 | Ywhae | A component of the ND10 nuclear body | The association between Ywhae and fibrosis or DKD has not been reported. |
| 9 | Ywhaz | Human leucocyte antigen A | Ywhaz, a negative regulator for insulin signal transduction, can regulate the mesangial hypertrophy in early diabetic nephropathy (45). |
| 10 | Ywhaq | Interferon regulatory factor-8 | The association between Ywhaq and fibrosis or DKD has not been reported. |
| 11 | Ywhag | Human leucocyte antigen F | The association between Ywhag and fibrosis or DKD has not been reported. |
DKD, diabetic kidney disease; Hsp70, heat shock protein 70.
Expression of Eno1 in human DKD revealed by analysis via Nephroseq.
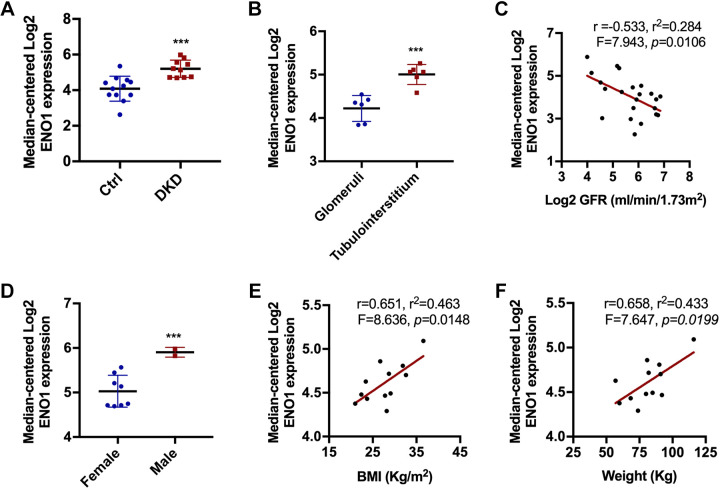
To further understand the potential implications of the above identified DEPs in DKD, we analyzed the proteins using Nephroseq v5 (www.nephroseq.org), an online platform with integrative gene expression data in various kidney diseases. In this database, Eno1 expression was significantly increased in DKD in human patients (Fig. 6A). To further identify if Eno1 is specifically expressed within the tubulointerstitium, we compared the gene expression in the glomeruli and tubulointerstitium in patients with DKD, which showed higher levels of Eno1 expression in the tubulointerstitium than in the glomeruli (Fig. 6B). Notably, Eno1 expression showed a negative correlation with glomerular filtration rate, suggesting that the Eno1 increase in DKD is associated with the decline of renal function (Fig. 6C). We further analyzed the relationship between Eno1 expression and the basic clinical features of patients with DKD. As shown in Fig. 6D, higher Eno1 expression was detected in men compared with women. In male patients, Eno1 expression was positively correlated with the increase in body mass index and weight (Fig. 6, E and F). Collectively, the correlation of Eno1 expression with DKD suggests a role of Eno1 in the pathogenesis of DKD.
Fig. 6.
The association between the differentially expressed protein enolase 1 (Eno1) and diabetic kidney disease (DKD). The 22 differentially expressed proteins in the study of DKD were analyzed on the Nephroseq v5 online platform. Eno1 demonstrated a strong correlation in patients with DKD. A: the expression level of Eno1 increased in patients with DKD. ***P < 0.001 vs. the nondiabetic control (Ctrl). B: Eno1 increased in tubulointerstitium tissue. ***P < 0.001 vs. glomeruli. C: Eno1 was negatively correlated with glomerular filtration rate (GFR). P = 0.0106. D: Eno1 increased in male patients. ***P < 0.001 vs. female patients. E and F: Eno1 was positively correlated with the increase in body mass index (BMI) and weight. P = 0.0148 and 0.0199 respectively.
DISCUSSION
In this study, we demonstrated that exosome secretion decreased in the kidney cortex of Akita diabetic mice and in renal tubular cells cultured with high glucose media. Functionally, exosomes derived from high glucose-treated tubular cells could stimulate the proliferation and activation of fibroblasts, suggesting a role of tubular exosomes in mediating interstitial fibrosis in DKD. Our proteomics analysis identified 22 proteins that are upregulated in exosomes derived from high glucose-treated tubular cells, which form protein interaction networks. Notably, Eno1 in these proteins showed correlations with DKD and related clinical features, suggesting Eno1 as a possible exosomal protein involved in renal interstitial fibrosis and DKD.
DKD was traditionally thought to be a glomerular disease, but recent work has indicated an important role of tubule-interstitial pathologies (14, 38). Tubular injury and dysregulated tubular reabsorption have been regarded as an important cause of albuminuria and proteinuria (22). Lv and colleagues (4, 23) found that exosomes transfer albumin-induced inflammatory signals from tubular epithelial cells to the interstitium. Liu et al. (19) recently reported an interesting finding that exosome production stimulated by transforming growth factor-β1 in renal tubular cells may promote fibroblast activation. Our present study demonstrates that exosomes are the pivotal mediators that can activate fibroblasts and promote fibrogenesis in DKD. This conclusion is supported by our data showing that renal fibroblasts underwent a significant morphological and phenotypic transformation into myofibroblasts after incubation with exosomes secreted by high glucose conditioned tubular cells. These exosomes also increased expression of fibrotic markers such as fibronectin, α-SMA, and collagen type I, indicating substantial fibroblasts differentiation into myofibroblasts. Moreover, they enhanced fibroblast proliferation. Together, these results indicate that tubular exosomes play a vital role in renal fibrosis in DKD via tubulointerstitial communication.
There are very few studies demonstrating exosomes as the underlying mechanism mediating cellular communication in renal fibrosis of DKD. One study particularly had a focus on the cellular communication between glomerular endothelial cells and mesangial cells (41), while another study highlighted the cross-talk between glomerular endothelial cells and podocytes (40). Although the focus of our study was to delineate the communication between tubular cells and fibroblasts, it is not surprising that glomerular endothelial cells, mesangial cells, and podocytes also participate in DKD pathogenesis via exosomes given the nature of these extracellular vesicles. In our current understanding, even without the participation of other cell types, tubular cells can sufficiently initiate and contribute to DKD pathogenesis by mediating the development of diabetic tubulopathy. Consequently, our results can add to the current knowledge and potentially underlie the mechanism of diabetic tubulopathy by driving interstitial fibrosis and induces nephron injuries and dysfunction in DKD.
Interestingly, initially proposed as the mechanism to excrete waste and eliminate unnecessary proteins (27), exosomes have been increasingly recognized as the means for cells to eliminate hazardous DNA damage to maintain cellular homeostasis (36). Besides, the contents of exosome cargo and the number of exosomes were also undoubtedly found to be essential for the intended biological function of exosomes on their recipient target cells (7, 9). In alignment with these studies, our present work demonstrates a significant downregulation of exosome secretion in both the diabetic kidney cortex and renal tubular cells conditioned with high glucose. Specifically, a long period of high glucose incubation for tubular cells is a prerequisite to mimic the in vivo chronic DKD model and obtain consistent results. However, our experiments did not investigate the mechanisms of exosome biogenesis and retention, which might be crucial to further understand how diabetes and high glucose regulate exosome secretion. Hence, a more indepth study clarifying the mechanisms of exosome secretion is required to understand the complete exosome regulatory mechanism in DKD.
Another important point to consider is that exosome secretion could be either good or bad depending on the contents of their cargos. However, the exosomal cargoes may vary between different physiological and pathological conditions, which can also be influenced by cell types (1, 8). The highlight of our study is that we have successfully identified 22 exosomal DEPs from the tubular HG-Exo group using proteomic profiling analysis. The presence of these DEPs indicates that distinct cellular processes can be influenced in response to different conditions, which underlie the possible regulatory roles of the DEPs in cellular homeostasis, such as glucose metabolism. For example, Eno1, also known as pyruvate dehydrogenase 1, is a multifunctional glycolytic enzyme that was differently expressed in patients with DKD and promotes organ fibrosis (11). In addition, Tweety family member 3 (Ttyh3) and Ral GTPase activating protein catalytic subunit α2 (Ralgapa2) are also involved in glucose transport pathways. In addition, Ywhae and Ywhaz were previously found to be the regulators of insulin sensitivity. On the other hand, cell structure, growth, differentiation, and intercellular signal transduction may be also implicated since Eno1, CD9, G protein-coupled receptor class C group 5 member A (Gprc5a), Rac1, annexin A5 (Anxa5), and Actb are also a part of the identified DEPs. While only 11 of 22 DEPs were identified as hub proteins involved in the PPI network, genes such as Eno1, Pgk1, Hspa8, Txn1 have significant correlations with the progression of organ fibrosis. Meanwhile, implications on glycolysis or insulin signal transduction were observed from the previous study targeting Pgk1, Actb, and Ywhaz (35). To reiterate the significance of our study, exosomal Eno1 was further identified to be a potential crucial promoter in renal fibrosis of DKD through our Nephroseq v5 online platform analysis using available clinical data. In agreement with our results, a recent study revealed increased expression of Eno1 in DKD conditions and a negative association of Eno1 expression with renal glomerular filtration rate in DKD (11). Hence, a closer look into how exosomal Eno1 regulates DKD warrants further investigation.
In summary, our results demonstrate a significant decrease of exosome secretion from renal tubular cells in DKD. The exosomes produced by tubular cells in DKD have a potent profibrotic activity on renal fibroblasts. The differentially expressed proteins in these exosomes may stimulate fibroblasts. Collectively, these results suggest that tubular exosomes may contribute to tubulointerstitial communication in fibrosis and DKD in a paracrine fashion.
GRANTS
This work was partly supported by grants from the National Institutes of Health and the Department of Veterans Affairs. Z.D. is a recipient of the Senior Research Career Scientist award from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.W. and Z.M. performed experiments; J.W., Z.M., M.J.L., W.Z., C.G., Y.L., and Z.D. analyzed data; J.W., Y.Y., and C.G. interpreted results of experiments; J.W. prepared figures; J.W. drafted manuscript; J.W., Y.Y., and Z.D. edited and revised manuscript; P.F. and Z.D. conceived and designed research; Z.D. approved final version of manuscript.
REFERENCES
- 1.Abbasian N, Herbert KE, Pawluczyk I, Burton JO, Bevington A. Vesicles bearing gifts: the functional importance of micro-RNA transfer in extracellular vesicles in chronic kidney disease. Am J Physiol Renal Physiol 315: F1430–F1443, 2018. doi: 10.1152/ajprenal.00318.2018. [DOI] [PubMed] [Google Scholar]
- 2.Bellaye PS, Burgy O, Causse S, Garrido C, Bonniaud P. Heat shock proteins in fibrosis and wound healing: good or evil? Pharmacol Ther 143: 119–132, 2014. doi: 10.1016/j.pharmthera.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Brosius FC III, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T; Animal Models of Diabetic Complications Consortium . Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carney EF. Chronic kidney disease: key role of exosomes in albumin-induced inflammation. Nat Rev Nephrol 14: 142, 2018. doi: 10.1038/nrneph.2018.6. [DOI] [PubMed] [Google Scholar]
- 5.Chang W, Wang J. Exosomes and their noncoding RNA cargo are emerging as new modulators for diabetes mellitus. Cells 8: 853, 2019. doi: 10.3390/cells8080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho H, Um J, Lee JH, Kim WH, Kang WS, Kim SH, Ha HH, Kim YC, Ahn YK, Jung DW, Williams DR. ENOblock, a unique small molecule inhibitor of the non-glycolytic functions of enolase, alleviates the symptoms of type 2 diabetes. Sci Rep 7: 44186, 2017. doi: 10.1038/srep44186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez JH, Liu Y, Gao H, Dominguez JM II, Xie D, Kelly KJ. Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. J Am Soc Nephrol 28: 3533–3544, 2017. doi: 10.1681/ASN.2016121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, Li Y, Zhang J, Nestor J, Krithivasan P, Lam WY, Mitrotti A, Piva S, Kil BH, Chatterjee D, Reingold R, Bradbury D, DiVecchia M, Snyder H, Mu X, Mehl K, Balderes O, Fasel DA, Weng C, Radhakrishnan J, Canetta P, Appel GB, Bomback AS, Ahn W, Uy NS, Alam S, Cohen DJ, Crew RJ, Dube GK, Rao MK, Kamalakaran S, Copeland B, Ren Z, Bridgers J, Malone CD, Mebane CM, Dagaonkar N, Fellström BC, Haefliger C, Mohan S, Sanna-Cherchi S, Kiryluk K, Fleckner J, March R, Platt A, Goldstein DB, Gharavi AG. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 380: 142–151, 2019. doi: 10.1056/NEJMoa1806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 27: 12–26, 2016. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González-Ramos M, Calleros L, López-Ongil S, Raoch V, Griera M, Rodríguez-Puyol M, de Frutos S, Rodríguez-Puyol D. HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-β1 up-regulation. Int J Biochem Cell Biol 45: 232–242, 2013. doi: 10.1016/j.biocel.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Gordin D, Shah H, Shinjo T, St-Louis R, Qi W, Park K, Paniagua SM, Pober DM, Wu IH, Bahnam V, Brissett MJ, Tinsley LJ, Dreyfuss JM, Pan H, Dong Y, Niewczas MA, Amenta P, Sadowski T, Kannt A, Keenan HA, King GL. Characterization of glycolytic enzymes and pyruvate kinase M2 in type 1 and 2 diabetic nephropathy. Diabetes Care 42: 1263–1273, 2019. doi: 10.2337/dc18-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One 12: e0170628, 2017. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys BD. Mechanism of renal fibrosis. Annu Rev Physiol 80: 309–326, 2018. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6: 395–423, 2011. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpman D, Ståhl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol 13: 545–562, 2017. doi: 10.1038/nrneph.2017.98. [DOI] [PubMed] [Google Scholar]
- 16.Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, Isales CM, Guldberg RE, Hamrick MW, Fulzele S. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep 7: 2029, 2017. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JM, Lee KG, Choi HS, Kim ES, Keum B, Seo YS, Jeen YT, Chun HJ, Lee HS, Um SH, Kim CD. Increased heat shock protein 70 expression attenuates pancreatic fibrosis induced by dibutyltin dichloride. Scand J Gastroenterol 53: 1404–1410, 2018. doi: 10.1080/00365521.2018.1516799. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Wu M, Qin Y, Zhou J, Su J, Pan E, Zhang Q, Zhang N, Sheng H, Dong J, Tong Y, Shen C. ACTB variants confer the genetic susceptibility to diabetic kidney disease in a Han Chinese population. Front Genet 10: 663, 2019. doi: 10.3389/fgene.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Miao J, Wang C, Zhou S, Chen S, Ren Q, Hong X, Wang Y, Hou FF, Zhou L, Liu Y. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int 97: 1181–1195, 2020. doi: 10.1016/j.kint.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12: 976–998, 2016. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long YS, Zheng S, Kralik PM, Benz FW, Epstein PN. Impaired albumin uptake and processing promote albuminuria in OVE26 diabetic mice. J Diabetes Res 2016: 8749417, 2016. doi: 10.1155/2016/8749417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL, Zhou LT, Wang B, Zhang JD, Crowley SD, Liu BC. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol 29: 919–935, 2018. doi: 10.1681/ASN.2017050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 26.Mutze K, Vierkotten S, Milosevic J, Eickelberg O, Königshoff M. Enolase 1 (ENO1) and protein disulfide-isomerase associated 3 (PDIA3) regulate Wnt/β-catenin-driven trans-differentiation of murine alveolar epithelial cells. Dis Model Mech 8: 877–890, 2015. doi: 10.1242/dmm.019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101: 942–948, 1985. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safaei A, Rezaei Tavirani M, Zamanian Azodi M, Lashay A, Mohammadi SF, Ghasemi Broumand M, Peyvandi AA, Okhovatian F, Peyvandi H, Rostami Nejad M. Diabetic retinopathy and laser therapy in rats: a protein-protein interaction network analysis. J Lasers Med Sci 8, Suppl 1: S20–S21, 2017. doi: 10.15171/jlms.2017.s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 75, Suppl 1: A6–A7, 2020. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Sellares J, Veraldi KL, Thiel KJ, Cárdenes N, Alvarez D, Schneider F, Pilewski JM, Rojas M, Feghali-Bostwick CA. Intracellular heat shock protein 70 deficiency in pulmonary fibrosis. Am J Respir Cell Mol Biol 60: 629–636, 2019. doi: 10.1165/rcmb.2017-0268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin JU, Lee WJ, Tran TN, Jung I, Lee JH. Hsp70 knockdown by siRNA decreased collagen production in keloid fibroblasts. Yonsei Med J 56: 1619–1626, 2015. doi: 10.3349/ymj.2015.56.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons M, Raposo G. Exosomes−vesicular carriers for intercellular communication. Curr Opin Cell Biol 21: 575–581, 2009. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W. Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol 284: F488–F497, 2003. doi: 10.1152/ajprenal.00172.2002. [DOI] [PubMed] [Google Scholar]
- 34.Soongsathitanon J, Umsa-Ard W, Thongboonkerd V. Proteomic analysis of peripheral blood polymorphonuclear cells (PBMCs) reveals alteration of neutrophil extracellular trap (NET) components in uncontrolled diabetes. Mol Cell Biochem 461: 1–14, 2019. doi: 10.1007/s11010-019-03583-y. [DOI] [PubMed] [Google Scholar]
- 35.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D. The GeneCards Suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics 54: 1.30.1–1.30.33, 2016. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, Hara E. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8: 15287, 2017. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164: 1226–1232, 2016. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 16: 317–336, 2020. doi: 10.1038/s41581-020-0256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290: 2159–2167, 2003. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Gao Y, Xu L, Dang W, Yan H, Zou D, Zhu Z, Luo L, Tian N, Wang X, Tong Y, Han Z. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep 7: 9371, 2017. doi: 10.1038/s41598-017-09907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu XM, Gao YB, Cui FQ, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open 5: 484–491, 2016. doi: 10.1242/bio.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, Wang Z, Deng B, Wu X, Liu J, Feng X. Identification of Enolase 1 and Thrombospondin-1 as serum biomarkers in HBV hepatic fibrosis by proteomics. Proteome Sci 11: 30, 2013. doi: 10.1186/1477-5956-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Zhou X, Yao Q, Liu Y, Zhang H, Dong Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol 313: F906–F913, 2017. doi: 10.1152/ajprenal.00178.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311: F844–F851, 2016. doi: 10.1152/ajprenal.00429.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Luo X, Ding S, Chen J, Chen T, Chen X, Zha H, Yao L, He X, Peng H. MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS Lett 586: 20–26, 2012. doi: 10.1016/j.febslet.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Zhang W, Yao Q, Zhang H, Dong G, Zhang M, Liu Y, Chen JK, Dong Z. Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am J Physiol Renal Physiol 312: F963–F970, 2017. doi: 10.1152/ajprenal.00078.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]