Keywords: acute kidney injury, acyl-CoA synthetase, chronic kidney disease, epigenetics, fatty acid oxidation, proximal tubules
Abstract
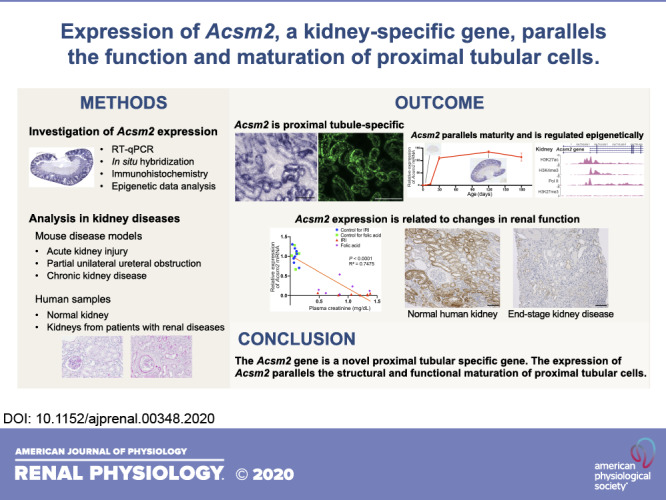
The acyl-CoA synthetase medium-chain family member 2 (Acsm2) gene was first identified and cloned by our group as a kidney-specific “KS” gene. However, its expression pattern and function remain to be clarified. In the present study, we found that the Acsm2 gene was expressed specifically and at a high level in normal adult kidneys. Expression of Acsm2 in kidneys followed a maturational pattern: it was low in newborn mice and increased with kidney development and maturation. In situ hybridization and immunohistochemistry revealed that Acsm2 was expressed specifically in proximal tubular cells of adult kidneys. Data from the Encyclopedia of DNA Elements database revealed that the Acsm2 gene locus in the mouse has specific histone modifications related to the active transcription of the gene exclusively in kidney cells. Following acute kidney injury, partial unilateral ureteral obstruction, and chronic kidney diseases, expression of Acsm2 in the proximal tubules was significantly decreased. In human samples, the expression pattern of ACSM2A, a homolog of mouse Acsm2, was similar to that in mice, and its expression decreased with several types of renal injuries. These results indicate that the expression of Acsm2 parallels the structural and functional maturation of proximal tubular cells. Downregulation of its expression in several models of kidney disease suggests that Acms2 may serve as a novel marker of proximal tubular injury and/or dysfunction.
INTRODUCTION
The acyl-CoA synthetase (ACS) medium-chain family member 2 (Acsm2) gene was first identified and cloned by our group as a kidney-specific “KS” gene (16). However, very little research has been done on the Acsm2 gene. To the best of our knowledge, this gene is not recognized as a kidney-specific gene at this point (42).
ACSs generate acyl-CoA from free fatty acids and CoA in an ATP-dependent manner, as the first step in fatty acid metabolism (11, 36, 43, 47). Acyl-CoA molecules produced by ACS are crucial in the production of energy in the mitochondria via β-oxidation of activated fatty acids (17). There are 25 ACS genes in the mouse, which are classified into 6 subfamilies based on their substrate specificities toward the chain length of fatty acids and on sequence similarity (46): the ACS short-chain (ACSS) subfamily, the ACS medium-chain (ACSM) subfamily, the ACS long-chain (ACSL) subfamily, the ACS very-long-chain (ACSVL) subfamily, the ACS bubblegum (ACSBG) subfamily, and the ACS family (ACSF) subfamily. The majority of the ACS are enriched in one or a few tissues as opposed to being expressed widely, which may reflect the evolutionary diversification of the enzyme families to regulate acyl-CoA metabolism in a tissue-specific manner (11).
The function of Acsm2 is unclear. Some other ACS genes, especially ACSL family genes, have been investigated in several ways and are known to play different roles depending on tissues or cell types (12, 22). However, there are no data regarding the specific role of Acsm2 in the mouse so far. The Acsm2 gene is highly conserved among species (42). In the human genome, two genes share homology with the mouse Acsm2 gene: ACSM2A and ACSM2B (46). The biological function of ACSM2A has not been defined in any species, whereas ACSM2B was partially purified from human liver mitochondria and shown to have ligase activity for fatty acids with high substrate specificity for benzoate (42, 44). With that knowledge, it has been proposed that Acms2 may participate in fatty acid metabolism and glycine conjugation pathways (42). However, the expression pattern and function of the Acsm2 gene in the kidney were not studied well and remain to be clarified.
In the present study, we investigated the expression of the Acsm2 gene in the mouse using multiple approaches, including quantitative RT-PCR, in situ hybridization (ISH), and immunohistochemistry. We found that Acsm2 is expressed specifically in proximal tubules of mouse kidneys in a developmentally regulated fashion. Furthermore, we found that in a variety of kidney diseases, in mice and humans, expression of the Acsm2 gene is intimately related to changes in renal function.
MATERIALS AND METHODS
Animals.
All animals were handled in accordance with National Institutes of Health guidelines for the care and use of experimental animals, and the study was approved by the Institutional Animal Care and Use Committee of the University of Virginia. C57Bl/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME) and bred at the University of Virginia. We used Ren1c gene knockout (Ren1cKO) mice generated by gene targeting, as previously described (37, 41). Mice with conditional deletion of the β1-integrin (Itgb1) gene in renin lineage cells (Itgb1cKO) were generated by crossing the Ren1dCre mouse (39) and Itgb1 floxed mice from Jackson Laboratory (no. 004605) (33).
RNA extraction and quantitative RT-PCR.
RNA was extracted from multiple organs (aorta, adrenal, bladder, brain, colon, heart, kidney, liver, lung, ovary, pancreas, skeletal muscle, small intestine, spleen, stomach, testis, and uterus) of 4-mo-old C57Bl/6 mice and kidneys from mice at multiple ages using TRIzol reagent (ThermoFisher Scientific, Waltham, MA). Reverse transcription was performed using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Quantitative PCR was performed with SYBR Green I (ThermoFisher Scientific) in a CFX Connect system (Bio-Rad Laboratories, Hercules, CA). mRNA expression of Acsm2 and ACSM family member 1 (Acsm1) was normalized to mRNA expression of 40S ribosomal protein S14 (Rps14), and changes in expression were determined by the ∆∆Ct method (where Ct is threshold cycle) and reported as relative expression (29). Primers are listed in Supplemental Table S1 (Supplemental Material is available online at https://doi.org/10.6084/m9.figshare.12609998.v1).
Synthesis of probes for ISH.
To develop the probes specific for Acsm2 and Acsm1 mRNA, DNA fragments were synthesized by PCR using cDNA from wild-type C57Bl/6 mouse kidneys with a 3′ T3 promoter and a 5′ T7 promoter. Primers are provided online in Supplemental Table S2. After purification and confirmation of DNA sequences, digoxigenin (DIG)-labeled RNA sense and antisense probes were generated by in vitro transcription using DIG RNA Labeling Mix and T3 or T7 polymerase (Millipore-Sigma, Burlington, MA).
In situ hybridization.
ISH was performed with 4% paraformaldehyde-fixed, paraffin-embedded kidney and liver tissues. Formalin (10%)-fixed, paraffin-embedded tissues were used from mice subjected to ischemia-reperfusion injury (IRI). Tissues were sectioned, deparaffinized, rehydrated, and post fixed followed by acetylation and permeabilization with proteinase K (10 µg/mL) for 30 min. After preincubation with hybridization buffer (50% formamide, 5× saline sodium citrate, 50 µg/mL yeast transfer RNA, 1% SDS, and 50 µg/mL heparin), slides were incubated with DIG-labeled sense or antisense riboprobes (500 ng/mL) at 55°C overnight. Slides then received stringency washes and blocking and were incubated with anti-DIG-alkaline phosphatase antibody (1:4,000, Millipore-Sigma) overnight at 4°C. After being washed, slides were treated with NTMT solution [100 mM NaCl, 100 mM Tris (pH 9.5), 50 mM MgCl2, 0.1% Tween 20, and 2 mM levamisole]. Sections were incubated with BM purple (Millipore-Sigma) for 1.5 and 7 days for Acsm2 and Acsm1, respectively. Reactions were terminated, and sections were fixed by in 0.2% glutaraldehyde plus 4% paraformaldehyde and mounted with Glycergel Mounting Medium (Agilent Technologies, Santa Clara, CA). To compare the intensity of signals, we placed kidney sections from different mice on the same slides and treated the sections equally during the whole procedure.
Immunohistochemistry.
Tissue sections from paraffin blocks fixed with Bouin’s solution were deparaffinized, rehydrated, and treated with 0.3% hydrogen peroxide in methanol. Slides were microwave treated with 10 mM sodium citrate buffer (pH 6.0) for 10 min. After being blocked with 3% BSA and 2% goat serum, sections were incubated with anti-ACSM2A antibody (ab181865, Abcam, Cambridge, MA, diluted at 1:100) at 4°C overnight. After being washed, sections were incubated with biotinylated secondary antibody, goat anti-rabbit IgG (BA-1000, Vector Laboratories, Burlingame, CA, diluted at 1:200). Staining was amplified using the Vectastain ABC kit (Vector Laboratories) and developed with 3,3′-diaminobenzidine (Millipore-Sigma). Sections were counterstained with hematoxylin (Millipore-Sigma), dehydrated, and mounted with Cytoseal XYL (ThermoFisher Scientific).
Immunofluorescence staining.
Kidney sections from adult wild-type C57Bl/6 mice fixed with Bouin’s solution were deparaffinized, rehydrated, and microwave treated with 10 mM sodium citrate buffer. After being blocked with 3% BSA, 5% donkey serum, 0.04% cold fish skin gelatin, and 0.05% Triton X-100 in PBS, sections were incubated with primary antibodies at 4°C overnight. On the next day, sections were washed, blocked again, and incubated with Alexa Fluor 488-conjugated donkey anti-rabbit antibody (A-21206, ThermoFisher Scientific, diluted at 1:500) and Alexa Fluor 568-conjugated donkey anti-mouse antibody (A10037, ThermoFisher Scientific, diluted at 1:500). After washes, autofluorescence was quenched by Vector TrueVIEW Autofluorescence Quenching Kit (Vector Laboratories). Sections were mounted in VECTASHIELD Vibrance Antifade Mounting Medium (Vector Laboratories). As primary antibodies, anti-ACSM2A antibody (ab181865, Abcam, diluted at 1:100), anti-low-density lipoprotein-related protein 2/megalin antibody (ab184676, Abcam, diluted at 1:50), and anti-calbindin-D-28K antibody (C9848, Millipore-Sigma, diluted at 1:1,000) were used.
Epigenetic data analysis.
Data from the Encyclopedia of DNA Elements (ENCODE) project (9) was analyzed to examine the epigenetic state at the Acsm2 gene locus in each mouse organ. We used data from the ENCODE portal (10) with the following identifiers: ENCSR000CDG, ENCSR000CAN, ENCSR000CFP, ENCSR000CBK, ENCSR000CDF, ENCSR000CDH, ENCSR000CDJ, ENCSR000CCQ, ENCSR000CCL, ENCSR000CDO, and ENCSR000CCU.
Ischemia-reperfusion injury.
The kidney IRI model was generated as previously described (2, 18). Wild-type C57BL/6 male mice at 8−12 wk of age were anesthetized with an intraperitoneal injection of ketamine (120 mg/kg) and xylazine (12 mg/kg). Bilateral or unilateral renal IRI was performed by clamping the renal pedicle for 26 min. The clamps were then removed, and the wound was sutured after the restoration of blood flow was visually observed. Sham-operated mice underwent the same procedure except that the renal pedicles were not clamped. Kidneys with bilateral or unilateral IRI were harvested after 24 h or 14 days of reperfusion, respectively. Plasma was separated by centrifuging heparinized blood at 7,000 g for 5 min, and plasma creatinine was determined using an enzymatic method as previously described (18).
Folic acid treatment.
Wild-type C57BL/6 male mice at 8−12 wk of age were injected intraperitoneally with either folic acid (250 mg/kg) in a vehicle of 0.3 M sodium bicarbonate or solely with vehicle. Kidneys were harvested at 24 h or 14 days after the injection as previously described (2). Plasma creatinine was measured as described above.
Partial unilateral ureteral obstruction.
Partial unilateral ureteral obstruction (pUUO) was performed with C57BL/6 newborn mice at 24−48 h following birth (34). Partial obstruction was created by a ligature tied around the ureter apposed to a 3- to 4-mm length of 0.2-mm-thick stainless steel wire, and the wire was removed after the ligation. Kidneys were harvested 3 wk after obstruction.
Human samples.
Human kidney samples were obtained from biopsy samples previously performed and stored at Niigata University. This study was approved by the Institutional Review Board of Niigata University Graduate School of Medical and Dental Sciences (Niigata, Japan, approval no. G2019-0015) and was carried out according to the principles of the Declaration of Helsinki.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism (version 8.4.0, GraphPad Software, La Jolla, CA). To compare expression levels of Acsm2, two-tailed Student’s t tests were used. Linear regression analysis was used to analyze the relationship between the relative expression of Acsm2 mRNA and plasma creatinine. P values of <0.05 were considered statistically significant.
RESULTS
Expression of Acsm2 is kidney specific and increases with maturation.
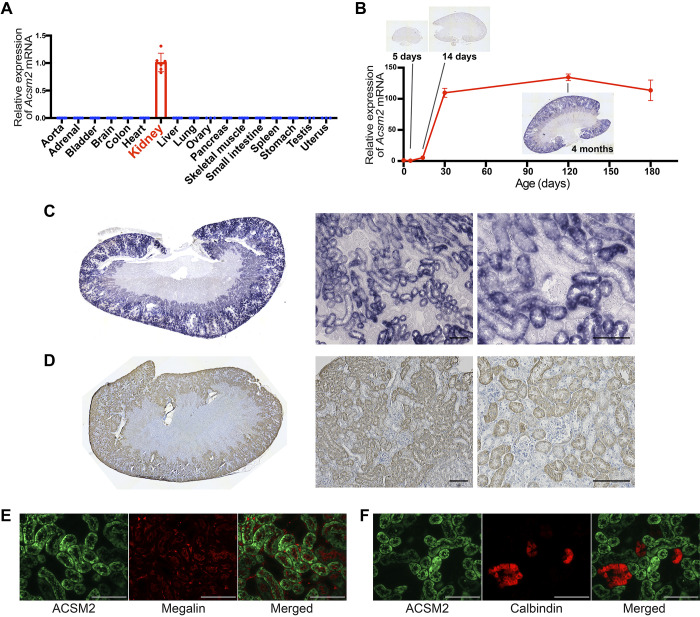
We examined the expression of Acsm2 and Acsm1 genes in multiple organs of adult mice. Acsm2 was expressed in the kidneys at a high level. Acsm2 expression in the liver was less than 1/1,000 of the expression in the kidney. No other organs expressed Acsm2 (Fig. 1A). Acsm1 was expressed in the liver as well as kidneys (Supplemental Fig. S1A), as previously reported (11). Acsm1 mRNA levels in the kidneys were significantly lower than those for Acsm2 (Supplemental Fig. S1B). We then investigated the expression of the Acsm2 gene during kidney development. The expression level of Acsm2 was very low in newborn mice, increased with animal growth, and reached a plateau by 2 mo of age (Fig. 1B).
Fig. 1.
Expression of acyl-CoA synthetase medium-chain family member 2 (Acsm2) is restricted in proximal tubular cells in kidneys. A: quantitative RT-PCR for Acsm2 mRNA with multiple organs from adult wild-type C57/Bl6 mice showed the specific expression of Acsm2 in the kidneys [n = 3 for sex-specific organs, n = 6 for other organs (from 3 male and 3 female mice)]. Data are presented as means ± SD. B: the expression level of Acsm2 in the kidneys was very low at birth, increased with animal growth, and reached a plateau by 2 mo of age, which was shown with quantitative RT-PCR for Acsm2 mRNA at multiple ages in male wild-type C57/Bl6 mice (n = 6 at postnatal day 0, n = 3 per group at other ages) and in situ hybridization. C: in situ hybridization showed the high expression of Acsm2 in the kidneys from adult wild-type mice. Expression of Acsm2 mRNA was observed in proximal tubular cells. Bars = 100 µm. D: immunohistochemistry for ACSM2 protein showed signals restricted in the tubular cells in adult wild-type mice. Bars = 100 µm. E: immunofluorescence staining showed that ACSM2 exists in megalin-positive proximal tubular cells. Bars = 100 µm. F: immunofluorescence staining showed that ACSM2 is not expressed in cells positive for calbindin-D-28K. Bars = 100 µm.
Expression of Acsm2 is restricted to proximal tubular cells.
ISH for Acsm2 mRNA showed high expression of Acsm2 in the kidneys of adult wild-type mice (Fig. 1C). We confirmed the specificity of the method by comparing antisense and sense probes, and there was no signal in liver samples (Supplemental Fig. S2). Expression of Acsm2 was observed, specifically in proximal tubular cells (Fig. 1C). We also performed ISH for Acsm1 mRNA, which may have a similar function to Acsm2. The expression level of Acsm1 in the kidney was much lower than that for Acsm2. This was reflected in the longer incubation time of the chromogenic substrate for alkaline phosphatase to detect ISH signals (7 days compared with 1.5 days for Acsm2), which was also consistent with the higher Ct value (lower mRNA levels) for Acsm1 by quantitative RT-PCR as described above (Supplemental Fig. S1B). The distribution of Acsm1 expression was different from that of Acsm2. Acsm1 expression was relatively higher at the corticomedullary junction, and Acsm2 showed relatively higher expression in the cortex (Supplemental Fig. S3).
Similar to the results of ISH, immunohistochemistry for ACSM2 protein showed the signals restricted to proximal tubular cells in kidneys of adult wild-type mice (Fig. 1D). Double immunofluorescence staining showed that ACSM2 protein localized in cells that expressed megalin protein, a marker of proximal tubular cells (Fig. 1E), whereas ACSM2 was not observed in cells positive for calbindin D-28k, a marker of distal tubules and collecting ducts (Fig. 1F). Thus, Acsm2 mRNA and protein are expressed in proximal tubular cells. Male mice showed higher mRNA levels, by quantitative RT-PCR (Supplemental Fig. S4), than female mice, but the overall distribution pattern of Acsm2 was similar in both sexes.
The Acsm2 gene is epigenetically activated in the kidneys.
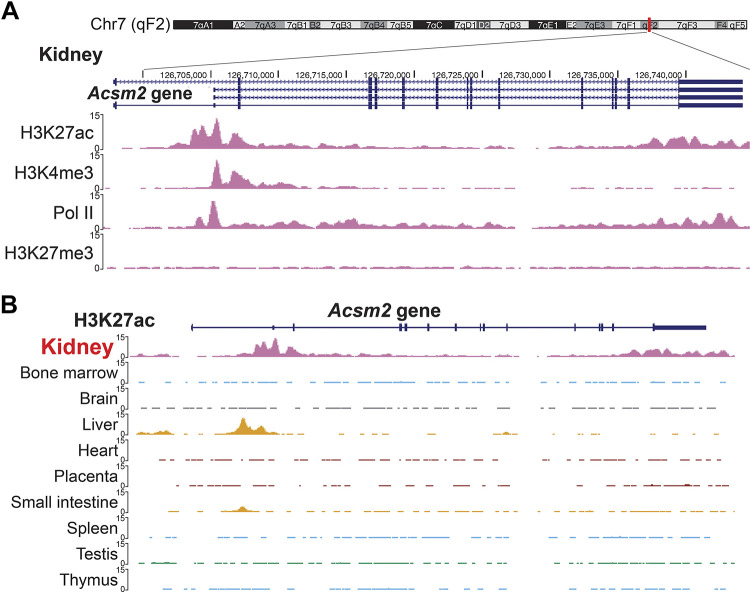
When we explored the data from the ENCODE project database, we found that the Acsm2 gene locus in the cells from adult mouse kidneys has specific histone modifications. Chromatin immunoprecipitation with sequencing (ChIP-seq) data from adult mouse kidneys showed that the Acsm2 gene locus has significant peaks for H3K27ac, H3K4me3, and RNA polymerase II. These epigenomic marks are associated with active enhancers, promoters, and transcriptional activity, respectively. H3K27me3, a silencing mark governed by the polycomb repression complex, was negative at the Acsm2 locus (Fig. 2A). These histone modifications were only seen in kidneys. Cells from other organs did not have the aforementioned activating histone marks at the Acsm2 gene locus (Fig. 2B).
Fig. 2.
The acyl-CoA synthetase medium-chain family member 2 (Acsm2) locus in the mouse has specific histone modifications that are related to active enhancers and promoters and transcription only in kidney cells. A: data from the Encyclopedia of DNA Elements (ENCODE) database showed the epigenetic marks related to the active gene at the Acsm2 gene locus in kidneys from adult mice. B: H3K27ac at the Acsm2 gene locus is specific in the kidneys. Liver cells showed a different pattern, and the other organs did not have H3K27ac at the Acsm2 gene locus.
Acsm2 is downregulated in mouse kidney disease models.
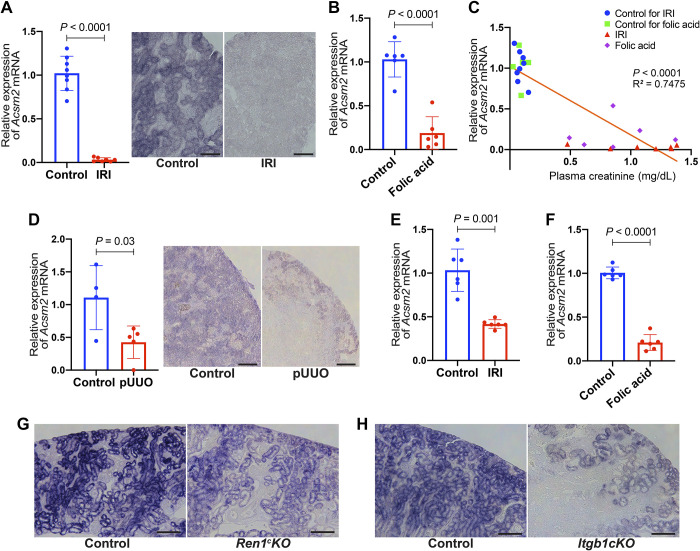
mRNA expression of Acsm2 was significantly decreased in kidneys of mice subjected to acute kidney injury (AKI) by bilateral kidney IRI. Quantitative RT-PCR showed a significant decrease in Acsm2 expression in kidneys with IRI (P < 0.0001; Fig. 3A). In addition, ISH showed lower expression of Acsm2 in proximal tubular cells of kidneys subjected to IRI compared with kidneys subjected to sham surgeries (Fig. 3A). We also examined the kidneys from mice 24 h after the injection of folic acid, another model of AKI. Acsm2 expression was significantly decreased in this model compared with its vehicle control (P < 0.0001; Fig. 3B). These AKI models showed a negative correlation between the relative expression of Acsm2 mRNA and plasma creatinine values (R2 = 0.7475; Fig. 3C).
Fig. 3.
Acyl-CoA synthetase medium-chain family member 2 (Acsm2) expression decreases in multiple types of mouse kidney disease models. A: Acsm2 gene expression was decreased in the kidneys with acute kidney injury (AKI) by bilateral kidney ischemia-reperfusion injury (IRI). Quantitative RT-PCR was performed (n = 8 control; n = 7 IRI). In situ hybridization showed lower expression of Acsm2 in proximal tubular cells of kidneys subjected to bilateral IRI compared with control. Bars = 100 µm. B: quantitative RT-PCR showed a decrease in Acsm2 mRNA expression in kidneys with AKI 24 h after injection of folic acid (n = 6 each). C: there was a negative correlation between Acsm2 expression in the kidney and plasma creatinine. The x-axis shows plasma creatinine levels (in mg/dL), whereas the y-axis shows relative expression of Acsm2 mRNA. Each dot represents the values (creatinine, mRNA) obtained from each individual animal belonging to the AKI models and their controls. The line is the fitted correlation value. D: Acsm2 mRNA expression by quantitative RT-PCR in kidneys was decreased following partial unilateral ureteral obstruction (pUUO; n = 4 control, n = 5 pUUO). In situ hybridization showed lower expression of Acsm2 in tubular cells from kidneys subjected to pUUO. Bars = 200 µm. E: quantitative RT-PCR showed a decrease in Acsm2 mRNA expression in the kidneys with chronic kidney disease, 14 days after unilateral IRI (n = 6 each). F: quantitative RT-PCR showed a decrease in Acsm2 mRNA expression in another chronic kidney disease model at 14 days after injection of folic acid (n = 6 each). G: expression of Acsm2 mRNA in proximal tubular cells of kidneys of Ren1c gene knockout (Ren1cKO) mice was lower than that of control. Bars = 200 µm. H: conditional knockout of the β1-integrin (Itgb1) gene in cells of kidneys from the renin progeny (Itgb1cKO) showed lower expression of Acsm2 in proximal tubular cells compared with control. Bars = 200 µm. All data are reported as means ± SD. Statistical comparison was by Student’s t test.
The neonatal mouse kidneys that underwent pUUO soon after birth showed decreased mRNA expression of Acsm2 at 3 wk postobstruction compared with sham-operated kidneys using quantitative RT-PCR (P = 0.03; Fig. 3D). ISH with pUUO kidneys showed decreased expression of Acsm2 in proximal tubular cells (Fig. 3D).
To investigate expression changes in Acsm2 in kidneys with chronic kidney disease (CKD) and renal fibrosis, we examined the effect of AKI on kidneys 14 days following injury. Kidneys 14 days after unilateral IRI, a model of progressive fibrosis, showed a significant decrease in the expression of Acsm2 (P = 0.0001; Fig. 3E). We also found that kidney Acsm2 expression was significantly decreased 14 days after the injection of folic acid (P < 0.0001; Fig. 3F). We examined the expression of Acsm2 in kidney sections of CKD models: Ren1cKO mice (37) and Itgb1cKO mice (33). Both Ren1cKO and Itgb1cKO mice showed low blood pressure and renal failure, although the renal pathology is different: Ren1cKO mice show hypertrophic arteries and arterioles surrounded by cells that retain the program of the renin phenotype (37) and Itgb1cKO mice show thinner afferent arterioles due to apoptosis of renin lineage cells (33). We observed decreased Acsm2 expression in the kidneys from those CKD models with ISH. We found that not only the area of the proximal tubular cell compartment but also the actual Acsm2 expression in each proximal tubular cell was decreased in both CKD models (Fig. 3, G and H). Expression of Acsm1 was also decreased in kidneys exposed to AKI and CKD as described above (Supplemental Fig. S5).
Human and mouse kidneys show similar expression patterns of the ACSM2 gene.
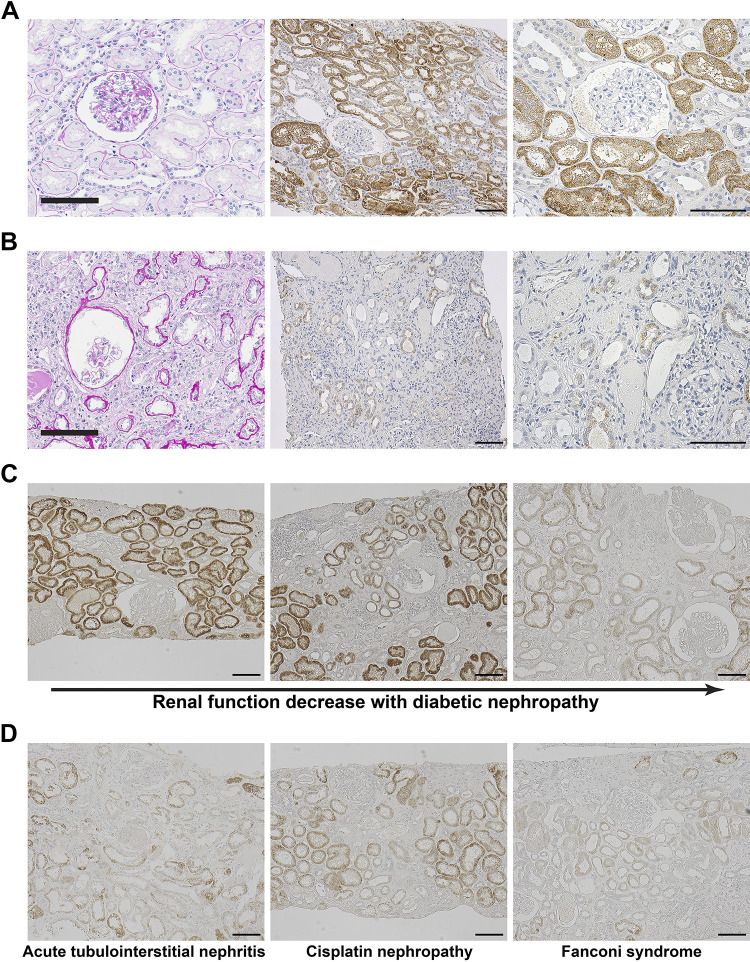
Given that Acsm2 is highly conserved across species, we investigated the expression of ACSM2 in human kidneys (Table 1). ACSM2A protein was observed in adult kidney samples from healthy donors of living renal transplantation (Fig. 4A). ACSM2A was restricted to proximal tubular cells also in human kidneys. We then tested ACSM2A in the kidney from a patient with end-stage kidney disease (creatinine clearance: 4.38 mL/min) who was just initiated on maintenance hemodialysis. ACSM2A expression in the kidney from this patient was significantly decreased compared with expression of ACSM2A in the kidneys from healthy humans (Fig. 4B).
Table 1.
Information on the human kidney samples
| Sample | Figure | Age, yr | Sex | Diagnosis | Creatinine Clearance, mL/min | Urinary Protein, g/day |
|---|---|---|---|---|---|---|
| Case 1 | Figure 4A | 55 | Male | Healthy kidney donor | 97.5 | 0 |
| Case 2 | Figure 4B | 49 | Male | End-stage kidney disease | 4.4 | 1.3 |
| Case 3 | Figure 4C, left | 38 | Female | Diabetic nephropathy | 112 | 0 |
| Case 4 | Figure 4C, middle | 63 | Male | Diabetic nephropathy | 51.8 | 1.8 |
| Case 5 | Figure 4C, right | 70 | Male | Diabetic nephropathy | 29 | 5.6 |
| Case 6 | Figure 4D, left | 66 | Male | Acute tubulointerstitial nephritis | 36 | 1.27 |
| Case 7 | Figure 4D, middle | 63 | Male | Cisplatin nephropathy | 7.98 | 0 |
| Case 8 | Figure 4D, right | 64 | Female | Fanconi syndrome | 36.7 | 0.6 |
Fig. 4.
Acyl-CoA synthetase medium-chain family member 2 (ACSM2) in humans follows similar expression patterns to that in mice. A: normal kidney tissue was obtained from the kidney of a healthy transplant donor. Periodic acid-Schiff staining showed no abnormalities. Immunohistochemistry for ACSM2A protein in this sample showed expression of ACSM2 at high levels in proximal tubules. Bars = 100 µm. B: periodic acid-Schiff staining of kidney tissue from a patient with end-stage kidney disease showed marked interstitial fibrosis, interstitial infiltration, and tubular atrophy. Immunohistochemistry for ACSM2A showed a marked decrease in the kidney from this patient. Bars = 100 µm. C: immunohistochemistry for ACSM2A in kidney samples from patients with diabetic nephropathy at different stages. ACSM2A expression decreased with decreased renal function. Bars = 100 µm. D: ACSM2A expression was lower in patients with acute tubulointerstitial nephritis, cisplatin nephropathy, and Fanconi syndrome with decreased renal function. Bars = 100 µm.
ACSM2A expression in human patients decreased in parallel with decreased renal function.
We examined the expression of ACSM2 in kidneys from patients with several types of renal injuries (Table 1 and Supplemental Fig. S6). We performed immunohistochemistry for ACSM2A in kidney sections from different patients with diabetic nephropathy at different stages of CKD. Expression of ACSM2A was maintained in patients with preserved renal function even with marked pathological findings of diabetic nephropathy. Expression of ACSM2A was decreased in parallel to the decrease in renal function encountered in patients with diabetic nephropathy (Fig. 4C). We also observed decreased expression of ACSM2A in patients with acute tubulointerstitial nephritis, cisplatin nephropathy, and Fanconi syndrome with decreased renal function (Fig. 4D).
DISCUSSION
We found that the Acsm2 gene is specifically expressed in proximal tubules of mouse kidneys. Acsm2 was first identified and cloned by our group as a KS gene in rats (16). In that study, we found that Acsm2 expression was also restricted to the kidneys, as we found now in mice. It is also consistent with a previous report using PCR in several organs in mice (11). In our first report of KS, we had found that KS was expressed after renal function became mature in rat kidneys (16). Our present findings in mice support this; we found that the expression of Acsm2 was at very low levels until 2 wk after birth and increased with functional and morphological development. These findings suggest that expression of Acsm2 is likely related to the remarkable growth and maturational changes that occur in the kidney during the first few weeks of life in both mice and rats (15). When KS/Acsm2 was first cloned, we could not determine the precise cellular localization of its expression due to limitations of the methods available at that time. In this study, we show by ISH and immunohistochemistry that Acsm2 is expressed exclusively and at high levels in proximal tubules. Moreover, we tested the localization of ACSM2 protein by comparing it with proteins that distribute in specific tubular segments. Megalin is abundantly expressed in the apical membranes of proximal tubules; more specifically, it is located in the brush border, endocytic vesicles, and dense apical tubules as well as, to some degree, in lysosomes (35). Calbindin-D-28K is a calcium-binding protein located in distal convoluted tubules and connecting tubules (5, 24). Our results indicate that ACSM2 is present in cells that express megalin and is not in cells that express calbindin-D-28K, again indicating that Acsm2 is restricted to proximal tubular cells and is not present in distal tubules. Although previously overlooked, our results indicate that Acsm2 can be used as a proximal tubular cell marker. This is in line with a recent single cell transcriptomic study in mouse kidneys that identified Acsm2 as one of 119 proximal tubule-specific marker genes (38). By ISH, Acsm2 expression in proximal tubules was relatively lower at the corticomedullary junction compared with the cortex, suggesting that Acsm2 is relatively higher in the proximal convoluted tubule than in the proximal straight tubule.
The Acsm2 gene locus in the mouse showed specific histone modifications within an active enhancer and promoter only in kidney cells. We analyzed ChIP-seq data from the total kidney cells that are available at this point. The problem in performing ChIP-seq in the kidney is that unlike other tissues, this organ contains numerous cell types possessing each different and unique epigenetic modifications (32). Fortunately, proximal tubular cells account for >50% of all kidney cells (6, 38); hence, the ChIP-seq data we analyzed plus the restricted cellular expression of Acsm2 indicate that the histone modifications most likely originate in proximal tubular cells. Epigenetic changes regulate the expression of specific genes without altering underlying DNA sequences (30). The results that proximal tubular cells have specific histone modifications at the Acsm2 gene regulatory region suggest that epigenetic regulatory mechanisms, yet to be determined and specified in proximal tubules, may be controlling the expression of Acsm2. It is proposed that the patterns of coordinated regulation within and between these ACS gene families exist in a tissue- and physiologically dependent manner (11). To elucidate how epigenetic modifications for Acsm2 are controlled only in proximal tubules will provide new insights into the regulation of genes in proximal tubules.
Acsm2 is considered to participate in fatty acid metabolism and glycine conjugation pathways (21, 25, 43); however, the specific role of Acsm2 in certain tissues remains to be clarified. Considering other genes in the ACS family may be useful to understand the Acsm2 gene. Transgenic mouse lines that overexpress ACSL in the heart demonstrated marked cardiac myocyte triglyceride accumulation and initial cardiac hypertrophy followed by the development of left ventricular dysfunction and premature death (8). Loss of ACSL family member 1 (Acsl1) severely inhibited fatty acid oxidation in muscle and adipose tissues (13), although loss of Acsl1 showed little effect on fatty acid metabolism in the liver (26). These data suggest tissue-specific coordinated regulation of fatty acid activation by ACS (12). The kidney is one of the most energy-demanding organs in the body, and proximal tubules contain more mitochondria and energy utilization than any other kidney structures (3, 40). Renal tubular epithelial cells depend critically on fatty acid oxidation as their energy source (19). We speculate that Acsm2 in proximal tubular cells may play an essential role in energy generation by using activated acyl-CoA for β-oxidation. Further work is needed to test this hypothesis.
The present study found expression levels of the Acsm2 gene in proximal tubular cells were significantly decreased in multiple kidney disease models in mice and humans: multiple AKI models, a pUUO model, and multiple CKD models. We observed a decrease in Acsm2 expression with quantitative RT-PCR in total kidneys. Also, with ISH, we found that each proximal tubular cell expressed less Acsm2 mRNA in those injury models. The proximal tubules are the primary target of AKI and progression of CKD (6). Some similar gene regulatory mechanisms in injured proximal tubular cells may induce a decrease in Acsm2 expression in those models. Decreased expression of Acsm2 may induce reduced acyl-CoA and reduced activation of fatty acid, leading to reduced fatty acid oxidation in the mitochondria. It has been reported that proximal tubular cells under biological stress, such as transient hypoxia or ischemia, shut down fatty acid oxidation for a period that outlasts injury (40). In the cells, fatty acid oxidation is accomplished mainly in both mitochondria and peroxisomes (14). Of the ACS family genes, only a few have been reported to be peroxisomal, including Acsl4, Slc27a2, and Scl27a4 (45). Peroxisome proliferator-activated receptor-α plays a critical role in regulating fatty acid β-oxidation in kidneys, and this directly correlated with the preservation of kidney morphology and function during AKI (27). A recent study has reported that switching mitochondrial fatty acid oxidation to peroxisomal fatty acid oxidation by sirtuin 5 knockout showed protection against AKI with a reduction in oxygen requirements and oxidative stress, suggesting that the balance of mitochondrial versus peroxisomal fatty acid oxidation in proximal tubular cells is important in kidney injuries (7). Further studies are required to define the factors that induce decreased expression of Acsm2 during kidney injury. Identification of those factors may provide insights into the mechanisms of fatty acid metabolism and cell injury in AKI and CKD.
We investigated the expression of ACSM2A in human renal biopsy samples and observed similar results to those from mouse disease models. ACSM2A was observed in proximal tubular cells specifically, and it was decreased in renal failure. In the human genome, there are two genes that share homology with mouse Acsm2 gene: ACSM2A and ACSM2B (46). They are both at chromosome 16 and share high sequence homology with an amino acid identity of 97.1%. These genes are expressed not only in the kidneys but also in the liver in humans (4, 42). However, we found that the expression pattern of ACSM2A in human kidneys was the same as that in the mouse. Considering that Acsm2 expression changes with renal injuries were also similar between mice and humans, the functions of Acsm2 in mice and ACSM2A and ACSM2B in humans are likely to be similar. Interestingly, ACSM2A and ACSM2B were detected as one of the CKD-related genes in a study combining genome-wide association data and RNA sequencing of patients with CKD (23). We found that ACSM2A expression was maintained even in patients with pathological abnormalities of diabetic nephropathy if creatinine clearance was preserved. Its expression may parallel renal function closely. We also found a decrease in ACSM2 in patients with several types of renal diseases, including cisplatin nephropathy and Fanconi syndrome. Notably, it has been reported that cisplatin inhibits fatty acid oxidation through a reduction in peroxisome proliferator-activated receptor-α (28, 31). Furthermore, Fanconi syndrome has been reported to be related to reduced fatty acid oxidation (1, 20). Despite the limitations when using human kidney tissue from renal biopsies that may affect the intensity of the stainings (due to kidney tissue damage or differences in tissue fixation or storage), our results suggest that Acsm2 may serve as a novel proximal tubular marker of renal injuries not only in mice but also in human patients. Because ACSM2 protein is expressed in proximal tubular cells at high levels, we expect that it may be detectable in the urine in conditions such as acute tubular necrosis. Further studies are needed to determine the potential of ACSM2 in the urine as a marker of renal injuries.
In summary, the Acsm2 gene is a novel proximal tubule-specific gene. The expression of Acsm2 parallels the structural and functional maturation of proximal tubular cells. Downregulation of its expression in several kidney diseases suggests that Acms2 may serve as a novel marker of proximal tubular injury and renal function.
GRANTS
This work was supported by National Institutes of Health Grants P50DK096373 and R01DK116718 (to R.A.G.), R01DK116196, DK096373, and HL148044 (to M.L.S.S.-L.), and R01DK105133 and 1R01DK085259 (to M.D.O.) as well as the Japan Society for the Promotion of Science Overseas Research Fellowships (to H.W.) and the Japan Society for the Promotion of Science Overseas Research Fellowships and the Uehara Memorial Foundation Research Fellowship (to S.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.W. and R.A.G. conceived and designed research; H.W., R.L.P., M.R.T., V.K.N., and S.T. performed experiments; H.W. analyzed data; H.W., M.L.S.S.-L., and R.A.G. interpreted results of experiments; H.W. prepared figures; H.W. drafted manuscript; H.W., S.W., M.L.S.S.-L., and R.A.G. edited and revised manuscript; H.W., R.L.P., M.R.T., V.K.N., S.T., M.D.O., S.G., I.N., S.W., M.L.S.S.-L., and R.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Xiuyin Liang, Minghong Li, Tiffany J. Southard, and Devon L. Farrar for excellent technical assistance. We thank the ENCODE Consortium and the ENCODE production laboratories for generating the particular data sets. A portion of this study was presented in the American Physiological Society/American Society of Nephrology Control of Renal Function in Health and Disease Conference in Charlottesville, VA and the Annual Meeting of the American Society of Nephrology, Kidney Week 2019, in Washington DC.
REFERENCES
- 1.Assmann N, Dettmer K, Simbuerger JMB, Broeker C, Nuernberger N, Renner K, Courtneidge H, Klootwijk ED, Duerkop A, Hall A, Kleta R, Oefner PJ, Reichold M, Reinders J. Renal Fanconi syndrome is caused by a mistargeting-based mitochondriopathy. Cell Reports 15: 1423–1429, 2016. doi: 10.1016/j.celrep.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 2.Bajwa A, Huang L, Kurmaeva E, Ye H, Dondeti KR, Chroscicki P, Foley LS, Balogun ZA, Alexander KJ, Park H, Lynch KR, Rosin DL, Okusa MD. Sphingosine kinase 2 deficiency attenuates kidney fibrosis via IFN-γ. J Am Soc Nephrol 28: 1145–1161, 2017. doi: 10.1681/ASN.2016030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boomgaarden I, Vock C, Klapper M, Döring F. Comparative analyses of disease risk genes belonging to the acyl-CoA synthetase medium-chain (ACSM) family in human liver and cell lines. Biochem Genet 47: 739–748, 2009. doi: 10.1007/s10528-009-9273-z. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Clark JZ, Nelson JW, Kaissling B, Ellison DH, Knepper MA. Renal-tubule epithelial cell nomenclature for single-cell RNA-sequencing studies. J Am Soc Nephrol 30: 1358–1364, 2019. doi: 10.1681/ASN.2019040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiba T, Peasley KD, Cargill KR, Maringer KV, Bharathi SS, Mukherjee E, Zhang Y, Holtz A, Basisty N, Yagobian SD, Schilling B, Goetzman ES, Sims-Lucas S. Sirtuin 5 regulates proximal tubule fatty acid oxidation to protect against AKI. J Am Soc Nephrol 30: 2384–2398, 2019. doi: 10.1681/ASN.2019020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107: 813–822, 2001. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74, 2012. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis CA, Hitz BC, Sloan CA, Chan ET, Davidson JM, Gabdank I, Hilton JA, Jain K, Baymuradov UK, Narayanan AK, Onate KC, Graham K, Miyasato SR, Dreszer TR, Strattan JS, Jolanki O, Tanaka FY, Cherry JM. The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res 46, D1: D794–D801, 2018. doi: 10.1093/nar/gkx1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis JM, Bowman CE, Wolfgang MJ. Metabolic and tissue-specific regulation of acyl-CoA metabolism. PLoS One 10: e0116587, 2015. doi: 10.1371/journal.pone.0116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol 21: 212–217, 2010. doi: 10.1097/MOL.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab 12: 53–64, 2010. doi: 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fransen M, Lismont C, Walton P. The peroxisome-mitochondria connection: how and why? Int J Mol Sci 18: 1126, 2017. doi: 10.3390/ijms18061126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez RA, Sequeira Lopez ML, Fernandez L, Cherñavvsky DR, Norwood VF. The maturing kidney: development and susceptibility. Ren Fail 21: 283–291, 1999. doi: 10.3109/08860229909085090. [DOI] [PubMed] [Google Scholar]
- 16.Hilgers KF, Nagaraj SK, Karginova EA, Kazakova IG, Chevalier RL, Carey RM, Pentz ES, Gomez RA. Molecular cloning of KS, a novel rat gene expressed exclusively in the kidney. Kidney Int 54: 1444–1454, 1998. doi: 10.1046/j.1523-1755.1998.00143.x. [DOI] [PubMed] [Google Scholar]
- 17.Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis 33: 469–477, 2010. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue T, Abe C, Kohro T, Tanaka S, Huang L, Yao J, Zheng S, Ye H, Inagi R, Stornetta RL, Rosin DL, Nangaku M, Wada Y, Okusa MD. Non-canonical cholinergic anti-inflammatory pathway-mediated activation of peritoneal macrophages induces Hes1 and blocks ischemia/reperfusion injury in the kidney. Kidney Int 95: 563–576, 2019. doi: 10.1016/j.kint.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klootwijk ED, Reichold M, Unwin RJ, Kleta R, Warth R, Bockenhauer D. Renal Fanconi syndrome: taking a proximal look at the nephron. Nephrol Dial Transplant 30: 1456–1460, 2015. doi: 10.1093/ndt/gfu377. [DOI] [PubMed] [Google Scholar]
- 21.Knights KM. Role of hepatic fatty acid:coenzyme A ligases in the metabolism of xenobiotic carboxylic acids. Clin Exp Pharmacol Physiol 25: 776–782, 1998. doi: 10.1111/j.1440-1681.1998.tb02152.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuwata H, Yoshimura M, Sasaki Y, Yoda E, Nakatani Y, Kudo I, Hara S. Role of long-chain acyl-coenzyme A synthetases in the regulation of arachidonic acid metabolism in interleukin 1β-stimulated rat fibroblasts. Biochim Biophys Acta 1841: 44–53, 2014. doi: 10.1016/j.bbalip.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Ledo N, Ko YA, Park AS, Kang HM, Han SY, Choi P, Susztak K. Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD. J Am Soc Nephrol 26: 692–714, 2015. doi: 10.1681/ASN.2014010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CT, Ng HY, Lee YT, Lai LW, Lien YH. The role of calbindin-D28k on renal calcium and magnesium handling during treatment with loop and thiazide diuretics. Am J Physiol Renal Physiol 310: F230–F236, 2016. doi: 10.1152/ajprenal.00057.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemarié F, Beauchamp E, Legrand P, Rioux V. Revisiting the metabolism and physiological functions of caprylic acid (C8:0) with special focus on ghrelin octanoylation. Biochimie 120: 40–48, 2016. doi: 10.1016/j.biochi.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Li LO, Ellis JM, Paich HA, Wang S, Gong N, Altshuller G, Thresher RJ, Koves TR, Watkins SM, Muoio DM, Cline GW, Shulman GI, Coleman RA. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J Biol Chem 284: 27816–27826, 2009. doi: 10.1074/jbc.M109.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int 76: 1049–1062, 2009. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Wu P, Yarlagadda P, Vadjunec NM, Proia AD, Harris RA, Portilla D. PPAR-α ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol 286: F572–F580, 2004. doi: 10.1152/ajprenal.00190.2003. [DOI] [PubMed] [Google Scholar]
- 29.Martinez MF, Medrano S, Brown EA, Tufan T, Shang S, Bertoncello N, Guessoum O, Adli M, Belyea BC, Sequeira-Lopez MLS, Gomez RA. Super-enhancers maintain renin-expressing cell identity and memory to preserve multi-system homeostasis. J Clin Invest 128: 4787–4803, 2018. doi: 10.1172/JCI121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matilainen O, Quirós PM, Auwerx J. Mitochondria and epigenetics−crosstalk in homeostasis and stress. Trends Cell Biol 27: 453–463, 2017. doi: 10.1016/j.tcb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimura I, Kanki Y, Kodama T, Nangaku M. Revolution of nephrology research by deep sequencing: ChIP-seq and RNA-seq. Kidney Int 85: 31–38, 2014. doi: 10.1038/ki.2013.321. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed TH, Watanabe H, Kaur R, Belyea BC, Walker PD, Gomez RA, Sequeira-Lopez MLS. Renin-expressing cells Require β1-integrin for survival and for development and maintenance of the renal vasculature. Hypertension 76: 458–467; Epub ahead of print, 2020. doi: 10.1161/HYPERTENSIONAHA.120.14959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagalakshmi VK, Li M, Shah S, Gigliotti JC, Klibanov AL, Epstein FH, Chevalier RL, Gomez RA, Sequeira-Lopez MLS. Changes in cell fate determine the regenerative and functional capacity of the developing kidney before and after release of obstruction. Clin Sci (Lond) 132: 2519–2545, 2018. doi: 10.1042/CS20180623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int 89: 58–67, 2016. doi: 10.1016/j.kint.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Ohkuni A, Ohno Y, Kihara A. Identification of acyl-CoA synthetases involved in the mammalian sphingosine 1-phosphate metabolic pathway. Biochem Biophys Res Commun 442: 195–201, 2013. doi: 10.1016/j.bbrc.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 37.Oka M, Medrano S, Sequeira-Lόpez MLS, Gómez RA. Chronic stimulation of renin cells leads to vascular pathology. Hypertension 70: 119–128, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Suszták K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004. doi: 10.1016/S1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 40.Simon N, Hertig A. Alteration of fatty acid oxidation in tubular epithelial cells: from acute kidney injury to renal fibrogenesis. Front Med (Lausanne) 2: 52, 2015. doi: 10.3389/fmed.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi N, Lopez ML, Cowhig JE Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 42.van der Sluis R. Analyses of the genetic diversity and protein expression variation of the acyl: CoA medium-chain ligases, ACSM2A and ACSM2B. Mol Genet Genomics 293: 1279–1292, 2018. doi: 10.1007/s00438-018-1460-3. [DOI] [PubMed] [Google Scholar]
- 43.van der Sluis R, Erasmus E. Xenobiotic/medium chain fatty acid: CoA ligase−a critical review on its role in fatty acid metabolism and the detoxification of benzoic acid and aspirin. Expert Opin Drug Metab Toxicol 12: 1169–1179, 2016. doi: 10.1080/17425255.2016.1206888. [DOI] [PubMed] [Google Scholar]
- 44.Vessey DA, Kelley M, Warren RS. Characterization of the CoA ligases of human liver mitochondria catalyzing the activation of short- and medium-chain fatty acids and xenobiotic carboxylic acids. Biochim Biophys Acta 1428: 455–462, 1999. doi: 10.1016/S0304-4165(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 45.Watkins PA, Ellis JM. Peroxisomal acyl-CoA synthetases. Biochim Biophys Acta 1822: 1411–1420, 2012. doi: 10.1016/j.bbadis.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res 48: 2736–2750, 2007. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Watkins PA. Fatty acid activation. Prog Lipid Res 36: 55–83, 1997. doi: 10.1016/S0163-7827(97)00004-0. [DOI] [PubMed] [Google Scholar]