Keywords: cyclooxygenase-2, deoxycorticosterone acetate-salt hypertension, (pro)renin receptor
Abstract
It has been shown that cyclooxygenase (COX)-2-dependent activation of renal (pro)renin receptor (PRR) contributes to angiotensin II (ANG II)-induced hypertension. However, less is known about the involvement of this mechanism in ANG II-independent hypertension. The goal of the present study was to test whether or not COX-2-dependent upregulation of PRR serves as a universal mechanism contributing to ANG II-dependent and -independent hypertension. Here, we examined the association between renal COX-2 and PRR during deoxycorticosterone acetate (DOCA)-salt hypertension in rats. By immunoblot analysis and immunofluorescence, renal protein expression of PRR was remarkably upregulated by DOCA-salt treatment. Surprisingly, this upregulation of renal PRR expression was unaffected by a COX-2 inhibitor, celecoxib. To address the role of renal PRR to the pathogenesis of DOCA-salt hypertension, a decoy PRR inhibitor, PRO20, was infused to the renal medulla of uninephrectomized Sprague-Dawley rats for 14 days. Radiotelemetry demonstrated effective attenuation of DOCA-salt hypertension by intramedullary infusion of a PRR inhibitor, PRO20. In parallel, DOCA-salt-induced hypertrophy in the heart and kidney as well as proteinuria were improved, accompanied with blunted polydipsia and polyuria. In contrast, intravenous infusion of PRO20 was less effective in attenuating DOCA-salt hypertension and cardiorenal injury. Together, these results suggest that COX-2-independent activation of renal PRR contributes to DOCA-salt hypertension.
INTRODUCTION
The incidence of hypertension is increasing, affecting one in three adults in the United States. Hypertension, often labeled as “the silent killer,” is an independent rick factor of cardiovascular disease stroke and renal failure, costing more than $75 billion annually (14). Hypertension in nearly half of the patient population is not controlled (14). Increased salt intake represents the major environmental factor that contributes to the pathogenesis of essential hypertension. Enhanced sensitivity of blood pressure (BP) to salt intake is present in nearly half of Americans who are afflicted with hypertension (26), including ~75% of African American patients with hypertension (2, 28, 39). Yet, the underlying causes of salt sensitivity in essential hypertension remain elusive. Aldosterone (Aldo) is a major effector hormone in the renin-angiotensin-aldosterone system, and its overproduction in primary aldosteronism represents the most common form of secondary hypertension (12, 13). Idiopathic hyperaldosteronism is the most common subtype of primary aldosteronism and accounts for 40–60% of cases (30). Increasing evidence supports the involvement of Aldo in the pathogenesis of essential hypertension. It has been shown that ~10% of patients with essential hypertension have a high ratio of plasma aldosterone to plasma renin activity, suggesting inappropriately increased production of Aldo (5, 25). Mineralocorticoid receptor blockade is reported to have antihypertensive and protective effects on cardiovascular and renal injury in animals and humans (29). Despite the recognized role of Aldo in hypertension and target organ damage, the precise mechanism of action of Aldo remains incompletely understood.
(Pro)renin receptor (PRR) is a specific receptor for prorenin and renin with high affinity in the nanomolar range (17). In vitro evidence shows that renin bound to PRR exhibits a three- to fivefold increased renin activity (17). Furthermore, when PRR binds prorenin, the later undergoes conformational changes to unfold the inhibitory prosegment of prorenin, leading to nonproteolytic activation (17). Our recent study has proven the in vivo renin-regulatory function of PRR in the kidney (18, 32), as previously reported in the central nervous system (9–11) despite some negative studies (31). A soluble form of PRR (sPRR) is generated by intracellular cleavage by furin or a disintegrin and metalloproteinase 19 (ADAM19) and secreted in plasma (3). sPRR binds renin and prorenin and has been reported to activate prorenin (6). Apart from regulation of the activity of prorenin and renin, PRR is an accessory protein, named ATP6ap2, that associates with vacuolar H+-ATPase, a key mediator of final urinary acidification (15).
Within the kidney, PRR mRNA and protein were predominantly expressed in the distal nephron, particularly in the collecting duct (CD) (1), where it is localized almost exclusively to the microvilli at the apical surface of A-type intercalated cells (1, 7). Increasing evidence supports PRR as an important player in the regulation of distal tubular Na+ and water transport as well as intrarenal renin under physiological and pathophysiological conditions (22, 23, 41–43). In particular, a significant number of studies have examined the contribution of renal PRR to angiontensin II (ANG II)-induced hypertension (18, 24, 31, 32). Despite some inconsistent findings, majorities of these reports favor renal PRR as a positive regulator of BP during ANG II infusion. Further evidence suggests that activation of the intrarenal renin-angiotensin system and epithelial Na+ channel (ENaC) contributes to ANG II-induced hypertension (18). Relatively, less is known about the involvement of renal PRR in ANG II-independent hypertension. The goal of the present study was to examine the role of renal PRR in deoxycorticosterone acetate (DOCA)-salt hypertension, which features suppressed circulating renin levels.
METHODS
Rat experiments.
Male Sprague-Dawley rats (220–250 g, Charles River Laboratories, Wilmington, MA) were cage housed and maintained in a temperature-controlled room with a 12:12-h light-dark cycle, with free access to tap water and standard rat chow for 14 days. The animal protocols were approved by the Animal Care and Use Committee of the University of Utah. Animals were subjected to the following two protocols. Protocol 1 attempted to examine the regulation of PRR related to cyclooxygenase (COX)-2 in the DOCA-salt model. In this protocol, Sprague-Dawley rats were treated with vehicle or subcutaneous implantation of a 50-mg DOCA pellet (50 mg, 21-day release, Innovative Research of America, Sarasota, FL) plus 0.9% NaCl as drinking fluid with or without celecoxib treatment at 30 mg·kg−1·day−1 from diet for 2 wk. Protocol 2 represented a functional study of the role of renal medullary PRR in DOCA-salt hypertension. Under this protocol, Sprague-Dawley rats underwent nephrectomy and were instrumented with radiotelemetric devices. After 1 wk of recovery from the surgery, a second surgery was performed. DOCA pellets were implanted subcutaneously, and a catheter was placed in the renal medulla for the infusion of vehicle or PRO20 at 120 µg·kg−1·day−1. For this procedure, the kidney was exposed from the flank region, and a catheter was placed ~4.0 mm underneath the surface and secured using vetbond glue; the other end of the catheter was connected to an osmotic minipump containing vehicle or PRO20. Intravenous infusion of PRO20 via a jugular vein was performed to control the spillover. Rats were maintained on a normal-Na+ diet and provided 0.9% saline for drinking water. The radiotelemetric device was turned on 4 h/day from 5:00 PM to 9:00 PM. In a separate experiment, rats were acclimatized to metabolic cages for 7 days before the surgeries. After DOCA pellet implantation, rats were placed in metabolic cages for measurements of food and water intake and 24-h urine collection.
Enzyme immunoassay.
Urinary albumin and neutrophil gelatinase-associated lipocalin (NGAL) were measured with the following commercially available enzyme immunoassay kits according to the manufacturer’s instructions: the kit for albumin (catalog no. ab108789, Abcam, Cambridge, MA) and NGAL (catalog no. 501530, Cayman Chemical).
Immunoblot analysis.
Renal tissues were lysed and subsequently sonicated in PBS that contained 1% Triton X-100, 250 µM PMSF, 2 mM EDTA, and 5 mM dithiothrietol (pH 7.5). Protein concentrations were determined using Coomassie reagent. Forty micrograms of protein for each sample were denatured in boiling water for 10 min, separated by SDS-PAGE, and then transferred onto nitrocellulose membranes. Blots were blocked overnight with 5% nonfat dry milk in Tris-buffered saline followed by incubation for 1 h with anti-PRR antibody (catalog no. ab40790, Abcam). After being washed with Tris-buffered saline, blots were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody and visualized using enhanced chemiluminescence.
Quantitative RT-PCR.
Snap-frozen renal samples were homogenized in TRIzol reagent (catalog no. 15596018, Life Technologies). Total RNA isolation and reverse transcription were performed according to the manufacturer’s recommendations. We used 1 µg of total RNA as a template for reverse transcription using the Transcriptor First Strand cDNA Synthesis Kit (catalog no. 4368813, ThermoFisher) according to the manufacturer’s instructions. Real-time PCR (quantitative PCR) was performed using the ABI Prism StepOnePlus System (Applied Biosystems, Life Technologies) and the SYBR Premix Ex Taq kit (Tli RNaseH Plus, catalog no. 1803132, ThermoFisher). Primers for COX-2 were 5′-AGGACTCTGCTCACGAAGGA-3′ (sense) and 5′-TCAGATGGATTGGAACAGCA-3′ (antisense), primers for renin were 5′- GATCACCATGAAGGGG GTCTCTGT-3′ (sense) and 5′-GTTCCTGAAGGGATTCTTTTGCAC-3′ (antisense), and primers for GAPDH were 5′-GTCTTCACTACCATG GAGAAGG-3′ (sense) and 5′-TCATGGATGACCTTGGCCA G-3′(antisense).
Immunofluorescence staining.
Tissues were fixed in 10% neutral buffered formalin for 24 h and then embedded in paraffin. After deparaffinization, thin sections (4 µm) were processed for double labeling with immunofluorescence. Slides were blocked in 1% BSA for 1 h and were then incubated with primary antibody at 4°C overnight. After the primary antibody had been washed off, sections were incubated for 1 h at room temperature with donkey anti-goat IgG-FITC (1:75, sc-2024, Santa Cruz Biotechnology, Santa Cruz, CA) or donkey anti-rabbit IgG-tetramethylrhodamine (1:100, A31572, Life Technologies). Goat anti-aquaporin-2 antibody was purchased from Santa Cruz Biotechnology (catalog no. sc-9882). Rabbit anti-PRR antibody was raised against residues 335–350 in the COOH terminus (catalog no. ab40790, Abcam).
Statistical Analyses.
Data are summarized as means ± SE. All data points and animals were included in the statistical analyses. Sample sizes were determined on the basis of similar previous studies or pilot experiments, and no test of normality or power analysis was performed. Statistical analyses for animal and cell culture experiments were performed using ANOVA with the Bonferroni test for multiple comparisons or a paired or unpaired t test for two comparisons. P < 0.05 was considered statistically significant.
RESULTS
Regulation of renal PRR expression during mineralocorticoid excess.
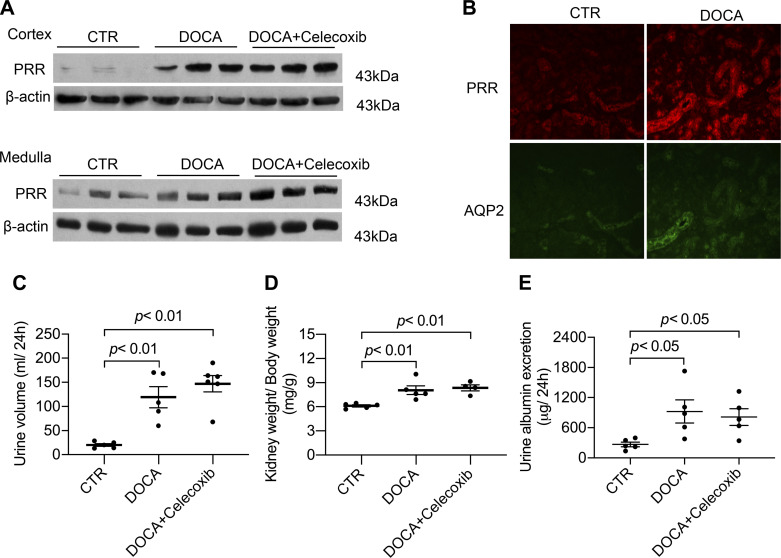
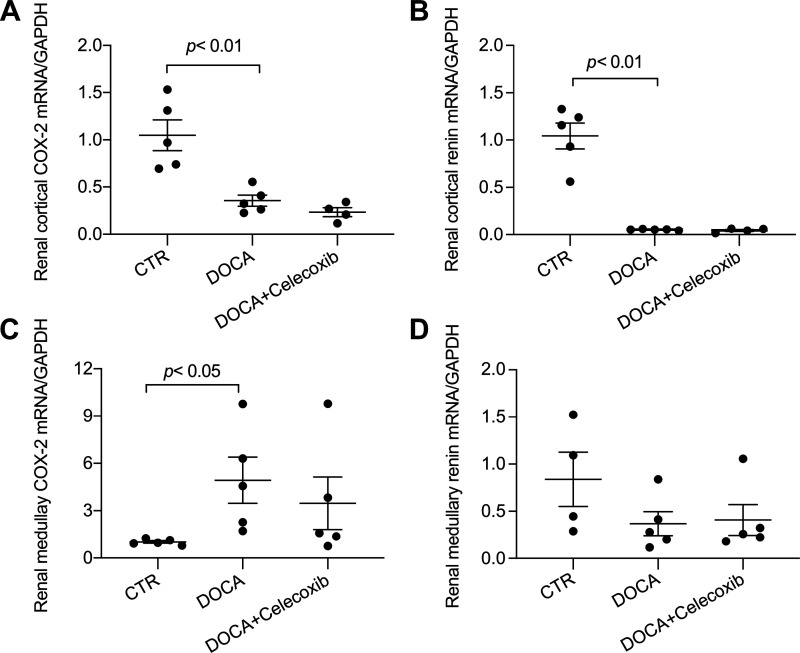
We have previously shown that COX-2 mediates ANG II-induced renal expression of PRR and the intrarenal renal-angiotensin system and thus the development of hypertension (32, 34, 36). Here, we examined the status of renal PRR and its relationship with COX-2 during DOCA-salt treatment. Uninephrectomized Sprague-Dawley rats received vehicle or subcutaneous implantation of DOCA pellet in combination of 0.9% NaCl as drinking fluid for 14 days with or without celecoxib treatment. Immunoblot analysis demonstrated increases in 43-kDa full-length PRR abundance in both the renal cortex and inner medulla in DOCA-salt rats (Fig. 1A). Immunostaining for PRR and AQP2 was performed on consecutive kidney sections from control and DOCA-salt rats. Increased PRR immunoreactivity was found mostly in the distal nephron, including the CD (Fig. 1B). The upregulation of renal PRR in the DOCA-salt model was unaffected by celecoxib. In agreement with this negative observation, celecoxib did not affect DOCA-salt-induced polyuria, renal hypertrophy, and proteinuria (Fig. 1, C–E). In the DOCA-salt model, COX-2 mRNA expression was downregulated in the renal cortex but upregulated in the renal inner medulla, whereas renin mRNA expression was suppressed in both kidney regions, which was unaffected by celecoxib at either location (Fig. 2). The authentication of anti-PRR antibody has already been published in a previous study (35).
Fig. 1.
Upregulation of renal (pro)renin receptor (PRR) protein expression in DOCA-salt (DOCA) rats and its independence on cyclooxygenase (COX)-2. Sprague-Dawley rats were treated with vehicle or subcutaneous implantation of a 50-mg DOCA pellet plus 0.9% NaCl as drinking fluid with or without celecoxib treatment at 30 mg·kg−1·day−1 from diet for 2 wk. n = 5 rats/group. A: representative blots of renal full-length PRR protein expression. B: immunolabeling of PRR and aquaporin-2 (AQP2) in the rat renal cortex. The same sections were stained with anti-PRR antibody and anti-AQP2 antibody. The images shown are representative of 6 animals/group. C: urine volume. D: kidney weight. E: urinary albumin excretion. CTR, control. Statistical significance was determined using one-way ANOVA with the Bonferroni test for multiple comparisons, and P values are indicated. Data are means ± SE.
Fig. 2.
Cyclooxygenase (COX)-2 and renin mRNA expression in the kidneys of DOCA-salt (DOCA) rats. After DOCA-salt treatment, the kidney cortex and medulla were subjected to quantitative RT-PCR analysis of COX-2 (A and C) and renin (B and D). n = 5 animals/group. CTR, control. Statistical significance was determined using one-way ANOVA with the Bonferroni test for multiple comparisons, and P values are indicated. Data are means ± SE.
Functional role of renal PRR in DOCA-salt hypertension.
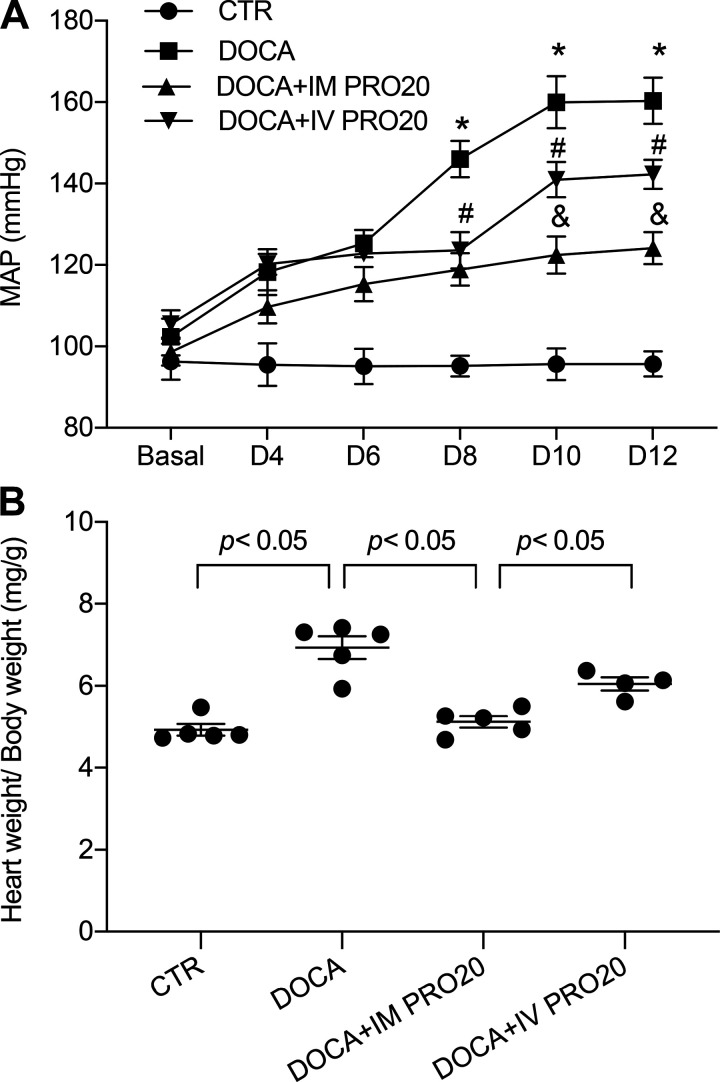
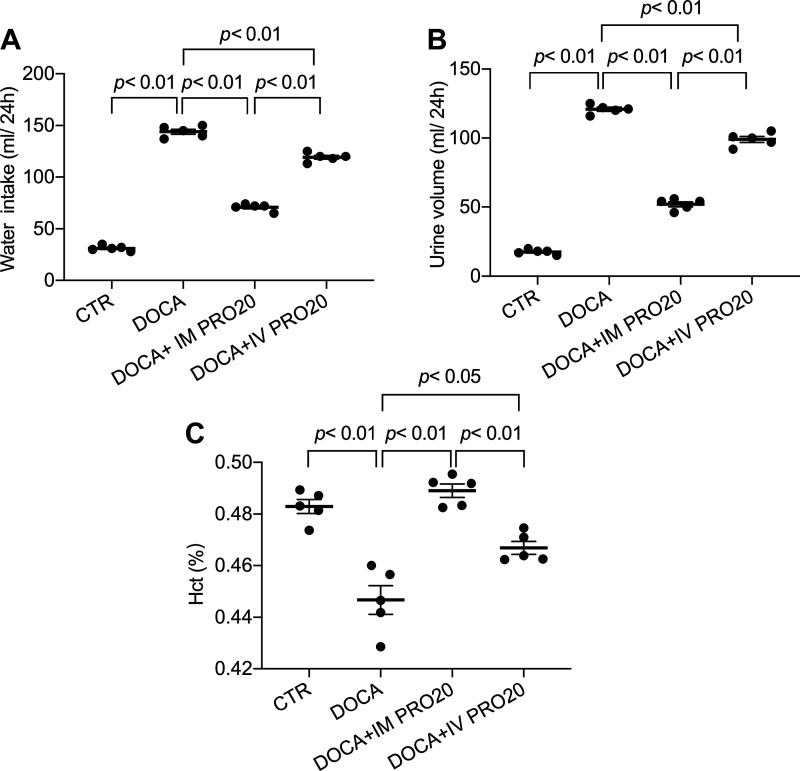
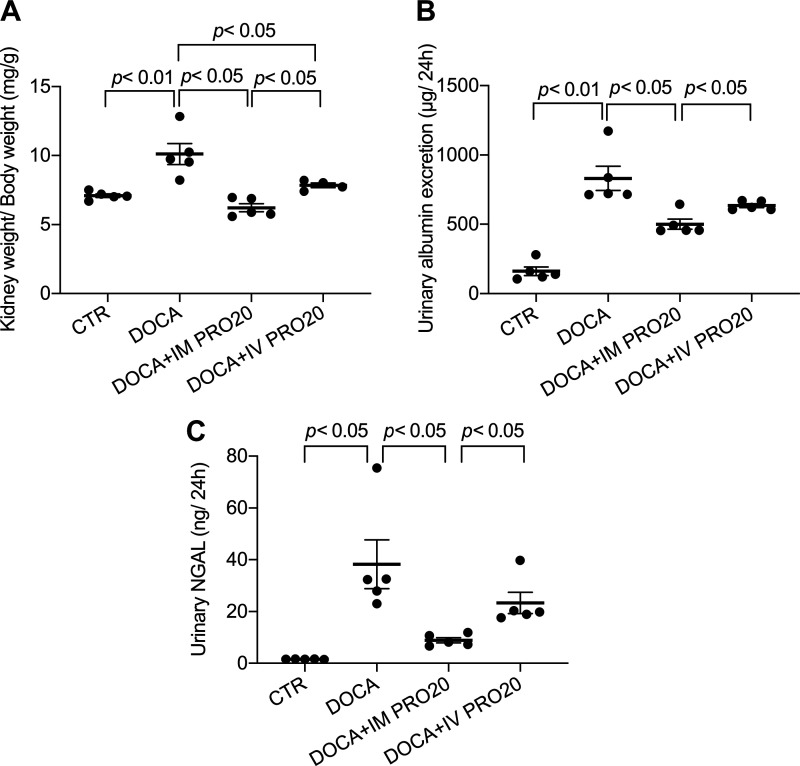
To address the functional role of renal PRR in BP regulation, we examined the effect of intramedullary infusion of PRO20, a 20-amino acid specific decoy inhibitor of PRR (11), on DOCA-salt hypertension in uninephrectomized rats. To control spillover, another group was included to receive intravenous infusion of PRO20. Over 14-day DOCA-salt treatment, mean arterial pressure was gradually and significantly increased in the vehicle-treated group. This mean arterial pressure increase was significantly attenuated by intramedullary infused PRO20, and this attenuation was less with intravenous infusion of PRO20 (Fig. 3A). Cardiac hypertrophy is a well-known consequence of hypertension. Indeed, the heart-to-body weight ratio was increased by DOCA-salt treatment, which was attenuated more effectively with intramedullary infused PRO20 compared with intravenously infused PRO20 (Fig. 3B). Similar patterns of changes were observed for water intake, urine volume, and hematocrit (Fig. 4) as well as for indexes of renal injury including renal hypertrophy, proteinuria, and urinary NGAL levels (Fig. 5). Of note, the change in hematocrit was taken an index of plasma volume assuming that red blood cell count is unaffected by DOCA-salt treatment. These results support an important role of renal PRR in mediating DOCA-salt hypertension and volume expansion.
Fig. 3.
Effect of intramedullary (pro)renin receptor inhibition on DOCA-salt hypertension in rats. Uninephrectomized Sprague-Dawley rats were divided into the following four groups: 1) control (CTR), 2) DOCA-salt (DOCA), 3) DOCA + intramedullary PRO20 infusion (IM PRO20), and 4) DOCA + intravenous PRO20 infusion (IV PRO20). The DOCA-salt protocol consisted of subcutaneous implantation of a 50-mg DOCA pellet in combination with 0.9% NaCl as drinking fluid. IM PRO20 (PRO20 at 120 µg·kg−1·day−1) was performed via a catheter chronically implanted in the renal medulla. To control the spillover, IV PRO (PRO20 at 120 µg·kg−1·day−1) was performed via catheterization of the jugular vein. Telemetry was performed to monitor mean arterial pressure (MAP), and it was turned on 4 h/day from 5:00 PM to 9:00 PM. A: radiotelemetry monitoring of MAP. *P < 0.05 vs. CTR; #P < 0.05 vs. DOCA alone; &P < 0.05 vs. IV PRO20. B: heart weight/body weight. n = 5 animals/group. For A, statistical significance was determined using two-way ANOVA with repeated measurements. For B, one-way ANOVA with the Bonferroni test for multiple comparisons were performed. Data are means ± SE.
Fig. 4.
Effect of intramedullary (pro)renin receptor inhibition on fluid homeostasis in DOCA-salt (DOCA) rats. In a separate experiment, following 2-wk treatment with DOCA and PRO20, rats were acclimatized in metabolic cages. Day 14 water intake (A) and urine volume (B) were measured. Plasma samples were harvested and subjected to analysis of hematocrit (Hct; C). n = 5 animals/group. CTR, control; IM PRO20, intramedullary PRO20 infusion; IV PRO20, intravenous PRO20 infusion. Statistical significance was determined using one-way ANOVA with the Bonferroni test for multiple comparisons, and P values are indicated. Data are means ± SE.
Fig. 5.
Effect of intramedullary (pro)renin receptor inhibition on DOCA-salt (DOCA) kidney injury in rats. Following 2-wk treatment with DOCA and PRO20, kidney weight (A) was measured, and day 14 urine was harvested and subjected to ELISA analysis of urinary albumin (B) and urinary neutrophil gelatinase-associated lipocalin (NGAL; C). n = 5 animals/group. CTR, control; IM PRO20, intramedullary PRO20 infusion; IV PRO20, intravenous PRO20 infusion. Statistical significance was determined using one-way ANOVA with the Bonferroni test for multiple comparisons, and P values are indicated. Data are means ± SE.
DISCUSSION
The goal of the present study was to examine the involvement of renal PRR in a rodent model of DOCA-salt hypertension. We, for the first time, report that renal PRR expression was remarkably elevated during DOCA-salt treatment and that this stimulation was largely localized to the distal nephron. Although COX-2 has been shown to mediate upregulation of renal PRR during ANG II infusion, DOCA-salt-induced upregulation of renal PRR expression appears to be COX-2 independent, as suggested by the lack of effect of COX-2 inhibition on PRR upregulation. The functional role of renal PRR in DOCA-salt hypertension was assessed by intramedullary infusion of a decoy PRR inhibitor, PRO20, and radiotelemetry measurement of BP. Site-specific inhibition of renal medullary PRR effectively attenuated DOCA-salt hypertension, accompanied with the improvement of cardiac hypertrophy, volume expansion, renal injury, etc.
PRR is expressed in a wide variety of trusses. Within the kidney, PRR expression was found in various nephron segments with particularly high expression in the CD, where it is restricted to intercalated cells (1, 7). Renal expression of PRR along with indexes of various components of the intrarenal renal-angiotensin system is upregulated by ANG II infusion (34) and water deprivation (35), compatible with its roles in physiological and pathophysiological processes. To our knowledge, the present study is the first to show the upregulation of renal PRR during DOCA-salt treatment. Immunoblot analysis demonstrated an increase in renal PRR protein abundance accompanied with increased urinary sPRR excretion. Immunostaining further mapped the expression upregulation mainly in the CD. This finding suggests a potential role of renal PRR in the regulation of renal function during DOCA-salt treatment.
A large body of experimental evidence has demonstrated that COX-2-derived PGE2 via EP4 receptors plays a pivotal role in mediation of the low salt-induced renin response in juxtaglomerular cells, a key component of the systemic renin-angiotensin system. A recent study has shown that the COX-2/EP4 pathway also mediates upregulation of renal PRR and the intrarenal renin-angiotensin system during ANG II infusion (34). In contrast, in the present study, COX-2 inhibition exerted no effect on DOCA-salt-induced upregulation of renal PRR expression. These results suggest distinct mechanisms of activation of renal PRR during DOCA-salt- and ANG II-induced hypertension depending on the involvement of COX-2. Although the underlying mechanism of this phenomenon remains unknown, the regulation of intrarenal renin clearly differs during the response to the two types of hypertensive stimuli. CD renin is elevated in vivo by ANG II infusion (19) as well as renal artery stenosis (20) and in vitro by exposing CD cells to ANG II (21). In contrast, renal cortical and medullary renin mRNA expression was remarkably suppressed by DOCA-salt treatment, as shown by the present study as well as the study by Ramkumar et al. (27). Based on these observations, a novel mechanism of renal PRR activation during DOCA-salt treatment is conceivable and should be pursued by future studies.
In the present study, the functional role of renal medullary PRR in DOCA-salt hypertension was examined by administrating PRO20 to the renal medulla of unineprectomized rats. The specificity and effectiveness of the compound have been extensively characterized by a large number of previous studies (4, 11, 32, 35, 38, 40). The intramedullary infusion technique used in the present study is a well-established approach capable of achieving site-specific drug delivery to the renal medulla (16). Intravenous infusion is usually included to control the spillover. Based on a series of parameters indicative of hypertension and hypertensive complications in the heart and the kidney, it is evident that intramedullary infusion of PRO20 was more effective than the systemic infusion, suggesting an important role of renal medullary PRR in the pathogenesis of DOCA-salt hypertension. Besides the involvement of renal PRR in this hypertensive model, it has been shown that neuronal PRR contributes to activation of the local renin-angiotensin system and the development of hypertension during DOCA-salt treatment (10). Therefore, it is important to take a tissue-specific approach to distinguish between renal and extrarenal actions of PRR. We believe that this issue has been adequately addressed by the present study.
The present study is limited in that no mechanism is provided to explain how PRR is involved in DOCA-salt hypertension. This issue may be minimized by our recently published study on site-1-protease-derived sPRR meditation of Aldo-induced activation of ENaC expression and activity in cultured mpkCCD cells (37). Interestingly, sPRR acutely stimulates ENaC activity via Nox4-derived ROS and chronically induces α-ENaC transcription via β-catenin signaling. We suspect that PRR/sPRR may contribute to pathogenesis of DOCA-salt hypertension via activation of ENaC in the distal nephron.
In summary, the present study examines the regulation and function of renal PRR in a rat model of DOCA-salt hypertension. We, for the first time, report that renal PRR expression is upregulated in this model via a COX-2-indepdent mechanism. Intramedullary inhibition of PRR attenuates DOCA-salt hypertension and improves hypertensive complications in the heart and kidney. Together, these results suggest an important role of PRR in the renal mechanism of DOCA-salt hypertension.
GRANTS
This work was supported by National Institutes of Health Grants HL139689, DK104072, and HL135851 as well as a Veterans Affairs Merit Review from the Department of Veterans Affairs. T. Yang is a Senior Research Career Scientist in the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.Y. conceived and designed research; F.W., Y.S., R.L., X.L., B.Y., and T.Y. performed experiments; F.W., Y.S., R.L., X.L., B.Y., and T.Y. analyzed data; F.W., Y.S., R.L., X.L., B.Y., and T.Y. interpreted results of experiments; F.W., Y.S., B.Y., and T.Y. prepared figures; F.W., B.Y., and T.Y. drafted manuscript; F.W., B.Y., and T.Y. edited and revised manuscript; B.Y. and T.Y. approved final version of manuscript.
REFERENCES
- 1.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 2.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension 43: 707–713, 2004. doi: 10.1161/01.HYP.0000120155.48024.6f. [DOI] [PubMed] [Google Scholar]
- 3.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 4.Fang H, Xu C, Lu A, Zou CJ, Xie S, Chen Y, Zhou L, Liu M, Wang L, Wang W, Yang T. (Pro)renin receptor mediates albumin-induced cellular responses: role of site-1 protease-derived soluble (pro)renin receptor in renal epithelial cells. Am J Physiol Cell Physiol 313: C632–C643, 2017. doi: 10.1152/ajpcell.00006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fardella CE, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, Pinto M, Huete A, Oestreicher E, Foradori A, Montero J. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab 85: 1863–1867, 2000. doi: 10.1210/jc.85.5.1863. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (pro)renin receptor in rat renal inner medullary cells. Hypertension 61: 443–449, 2013. doi: 10.1161/HYPERTENSIONAHA.112.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Peng H, Cao T, Sato R, McDaniels SJ, Kobori H, Navar LG, Feng Y. Brain-targeted (pro)renin receptor knockdown attenuates angiotensin II-dependent hypertension. Hypertension 59: 1188–1194, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension 65: 352–361, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lifton RP. Genetic determinants of human hypertension. Proc Natl Acad Sci USA 92: 8545–8551, 1995. doi: 10.1073/pnas.92.19.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001. doi: 10.1016/S0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: 948–954, 2010. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schägger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 16.Miyata N, Cowley AW Jr. Renal intramedullary infusion of L-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension 33: 446–450, 1999. doi: 10.1161/01.HYP.33.1.446. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF, Yang T. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. Am J Physiol Renal Physiol 312: F245−F253, 2017. doi: 10.1152/ajprenal.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens 3: 96–104, 2009. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutiérrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramkumar N, Kohan DE. The nephron (pro)renin receptor: function and significance. Am J Physiol Renal Physiol 311: F1145–F1148, 2016. doi: 10.1152/ajprenal.00476.2016. [DOI] [PubMed] [Google Scholar]
- 23.Ramkumar N, Kohan DE. The (pro)renin receptor: an emerging player in hypertension and metabolic syndrome. Kidney Int 95: 1041–1052, 2019. doi: 10.1016/j.kint.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramkumar N, Stuart D, Mironova E, Bugay V, Wang S, Abraham N, Ichihara A, Stockand JD, Kohan DE. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiology Renal Physiol 311: F186−F194, 2016. doi: 10.1152/ajprenal.00088.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens 15: 896–902, 2002. doi: 10.1016/S0895-7061(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 26.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Curr Hypertens Rep 13: 55–66, 2011. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song K, Stuart D, Abraham N, Wang F, Wang S, Yang T, Sigmund CD, Kohan DE, Ramkumar N. Collecting duct renin does not mediate DOCA-salt hypertension or renal injury. PLoS One 11: e0159872, 2016. doi: 10.1371/journal.pone.0159872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan JM, Prewitt RL, Ratts TE. Sodium sensitivity in normotensive and borderline hypertensive humans. Am J Med Sci 295: 370–377, 1988. doi: 10.1097/00000441-198804000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Takeda Y. Effects of eplerenone, a selective mineralocorticoid receptor antagonist, on clinical and experimental salt-sensitive hypertension. Hypertens Res 32: 321–324, 2009. doi: 10.1038/hr.2009.29. [DOI] [PubMed] [Google Scholar]
- 30.Takeda Y. Genetic alterations in patients with primary aldosteronism. Hypertens Res 24: 469–474, 2001. doi: 10.1291/hypres.24.469. [DOI] [PubMed] [Google Scholar]
- 31.Trepiccione F, Gerber SD, Grahammer F, López-Cayuqueo KI, Baudrie V, Păunescu TG, Capen DE, Picard N, Alexander RT, Huber TB, Chambrey R, Brown D, Houillier P, Eladari D, Simons M. Renal Atp6ap2/(pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol 27: 3320–3330, 2016. doi: 10.1681/ASN.2015080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T. Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 13: 278, 2015. doi: 10.1186/s12916-015-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 64: 369–377, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Antidiuretic action of collecting duct (pro)renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhu SF, Yang T. COX-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol 307: F25–F32, 2014. doi: 10.1152/ajprenal.00548.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F, Luo R, Peng K, Liu X, Xu C, Lu X, Soodvilai S, Yang T. Soluble (pro)renin receptor regulation of enac involved in aldosterone signaling in cultured collecting duct cells. Am J Physiol Renal Physiol 318: F817−F825, 2020. doi: 10.1152/ajprenal.00436.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F, Xu C, Luo R, Peng K, Ramkumar N, Xie S, Lu X, Zhao L, Zuo CJ, Kohan DE, Yang T. Site-1 protease-derived soluble (pro)renin receptor targets vasopressin receptor 2 to enhance urine concentrating capability. JCI Insight 4: e124174, 2019. doi: 10.1172/jci.insight.124174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996. doi: 10.1161/01.HYP.27.3.481. [DOI] [PubMed] [Google Scholar]
- 40.Xu C, Lu A, Lu X, Zhang L, Fang H, Zhou L, Yang T. Activation of renal (pro)renin receptor contributes to high fructose-induced salt sensitivity. Hypertension 69: 339–348, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang T. Unraveling the physiology of (pro)renin receptor in the distal nephron. Hypertension 69: 564–574, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol 28: 1040–1049, 2017. doi: 10.1681/ASN.2016070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Q, Yang T. Enzymatic sources and physio-pathological functions of soluble (pro)renin receptor. Curr Opin Nephrol Hypertens 27: 77–82, 2018. doi: 10.1097/MNH.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]