Abstract
Mesothelial cells are arranged as a monolayer on covering membranes that invest surfaces of body cavities like the pleura and peritoneum. Primary human mesothelial cell (HMC) cultures are needed for studying mesothelial cell homeostasis and developing disease models, such as wound healing or cancers. Remarkably, there is a paucity of useable HMC lines that are currently available that faithfully recapitulate normal in vivo phenotypic characteristics. Here, we present a strategy to recover HMC from human pleural tissue and to immortalize them for extended in vitro culturing. Human pleural membrane was harvested by minimally invasive surgical techniques. HMC were isolated using a two-step process combining explant cellular outgrowth from biopsy tissue and flow cytometry based on cell surface expression of cadherin-1 and CD71. Cell cultures were generated after lentiviral transfection with human telomerase. The new HMC cultures retain the same phenotypic traits and physiologic features as their in vivo counterparts, yet they can be adapted for short-term or long-term culture in large-scale in vitro experimentation. In particular, we generated a new HMC line harboring a germline mutation in breast cancer type-1-associated protein-1 (BAP1), a causal tumor suppressor gene, that could be instrumental to malignant mesothelioma research. Patient-specific, normal HMC may serve as novel discovery tools allowing more powerful research models of both normal physiology and disease processes. Our surgically driven approach leads to a limitless resource of novel mesothelial cell cultures.
Keywords: cell culture, flow cytometry, mesothelium, pleura, thoracoscopic biopsy
INTRODUCTION
Human mesothelial cells (HMC) derive from the mesoderm but possess features in common with epithelial cells, namely, apical/ basal polarity, cell-to-cell adhesion, and basement membrane attachment (50). Metabolically active HMC impact many critical biological processes, including: organogenesis, immunity, wound healing, inflammation, or malignancy (both primary and metastatic tumors) (32). Under normal conditions, HMC renew slowly and senesce after few passages so primary cultured cells are not widely available for long-term in vitro experimentation, nor for subsequent verification of reproducibility among research laboratories on a large scale. Consequently, the precise mechanisms governing HMC behavior in these biologic contexts remain to be fully elucidated.
A prime example where improved mechanistic models would provide better understanding of biologic processes is highlighted by malignant pleural mesothelioma (MPM), an aggressive neoplasm arising from mesothelial cells lining the pleural membranes of the thoracic cavity (3). Modern therapeutic strategies have limited efficacy due, in part, to incomplete knowledge of the processes underlying MPM, which continues to increase worldwide unexpectedly. Lack of an accurate model of oncogenesis is a major limitation of in vitro MPM studies (41). Such modeling is needed to explain the step(s) that link asbestos exposure to selection for cytotoxicity resistance as the HMC integrates these molecular perturbations to undergo transformation. Furthermore, such modeling is required to elucidate how genetic alterations accumulate, committing the cell to a fully malignant state. These shortcomings in our current understanding are underpinned by few available normal HMC lines. For over 30 years, the main HMC lines MeT-5A (26), a viral transformed line derived from pleural fluid, and LP-9 (9), an untransformed line obtained from peritoneal ascites fluid, have remained the normal counterpart cells against which most MPM cells have been compared.
Given these limitations, there is a need for new HMC lines that better recapitulate normal mesothelial cells with intact homeostasis machinery. Here, we describe a novel method for isolating and producing HMC lines from patient pleural specimens. We rely on an explant culture system (30) to expand viable HMC from the bulk tissue. The resulting cells maintain their mesothelial identity as evidenced by their structural and functional characteristics as well as any unique patient-specific genetic traits. Our study establishes a perpetual framework for generating normal HMC to be used as preclinical models of benign and oncogenic processes.
MATERIALS AND METHODS
Human tissues and study approval.
All patients whose samples were used for this study were enrolled in Institutional Review Board approved protocols (06-C-0014) under the guidelines of the Center for Cancer Research, National Cancer Institute. Patients, both male and female, were those undergoing video-assisted thoracoscopic surgery (VATS) in the thorax and those who provided written informed consent to be a pleural tissue donor. An area of normal-appearing parietal pleura was harvested from each patient, averaging 2–3 cm2, superficial to the endothoracic fascia, and washed with ice-cold saline before being transferred out of the operating room under sterile conditions. One patient had early stage MPM in the context of germline breast cancer type-1-associated protein-1 (BAP1) mutation where the anatomy permitted harvesting normal-appearing pleura.
Explant culture of cells.
Parietal pleurae (≤200 mg) were minced into 1- to 3-mm pieces on ice and then submerged in mesothelial cell growth (MCG) media (9) in a 10-cm plastic culture dish. The growth surface of this dish was loosely etched with a scalpel to produce parallel lines ~8 mm apart that provided raised anchor points for tissue attachment. The culture dish was incubated at 37°C with 5% CO2 for 10–14 days permitting cells to outgrow from the tissue pieces. Media was exchanged every 4 days.
Flow cytometry (FACS).
Explant-derived cells were detached with TrypLE Express (no. 12605010; Gibco), resuspended in buffer (1× PBS with 2% FBS), and double labeled with anti-CDH1 (rabbit-anti-human, ab223582; Abcam) and anti-CD71 (mouse-anti-human, ab40772; Abcam) diluted at 1:500. Secondary antibodies, AlexaFluor647-conjugated donkey anti-rabbit (ab150075; Abcam) or AlexaFluor488-conjugated goat-anti-mouse (ab150113; Abcam) at 1:2,000 were used for analysis. A Sony SH800 Cell Sorter platform was used for live sorting cells to isolate HMC.
Normal mesothelial cell lines (normal pleura).
After recovering for 48 h from FACS live sorting, HMC were immortalized via lentiviral transduction with human telomerase reverse transcriptase (hTERT) cDNA encoding sequences according to the manufacturer’s protocol (PLV-10081; Cellomics Technology). These newly derived HMC cell lines were designated normal pleura (NP) 1, NP2, NP3, and so forth.
Cell culture.
MeT-5A [CRL-9444; American Type Culture Collection (ATCC)] and NP-designated cell lines were maintained in MCG media as follows (9): Medium 199 (11150-059; Gibco) supplemented with 1% antibiotic-antimycotic solution (15140–122; Gibco), 10 ng/mL human epidermal growth factor (E9644; Sigma), 400 nM hydrocortisone (H4001; Sigma), 870 μM insulin (I9278; Sigma), 0.3% Trace Elements B (25–022-CI; Corning), and 10% FBS (FB-11; Omega Scientific). While in MCG media, each NP-designated line could be passaged beyond the normal HMC senescence limit of 45–50 population doublings. Otherwise, NP-designated cell lines were passaged at six to eight doublings in regular Medium 199 media without supplements. The H2052 and H2373 (CRL-5915 and CRL-5943; ATCC) MPM cell line was cultured and maintained according to ATCC instructions. MB52 is a patient-derived MPM cell line obtained from MesobanK (www.mesobank.com) via a Materials Transfer Agreement and maintained according to their instructions.
Tumorsphere formation assay.
NP-designated cell lines were detached and resuspended in the following sphere-forming medium: RPMI 1640 media (11875093; ThermoFisher) with 4 mg/mL insulin, 20 ng/mL human epidermal growth factor, 10 ng/mL fibroblast growth factor-basic (233-FB; R&D Systems), 1× B27 (17504044; ThermoFisher), and1% antibiotic-antimycotic solution (15140–122; Gibco). The H2052 MPM cell line was used as a positive control. All cell lines were seeded at a density of 1.0 × 104 cells/well in six-well ultra-low attachment plates (3471; Corning). Lines were incubated at 37°C for 7 days. Micrographs of resulting spheres were imaged with a light microscope (IX51; Olympus) at ×20 magnification.
Immunocytofluorescence.
Cells were seeded to four-well chamber slides (Nunc Lab-TEK II, 154526; ThermoFisher) at 50,000 cells/well and grown overnight. Cells were fixed in 4% paraformaldehyde and rinsed in 1× PBS. Standard antigen retrieval with heated citrate buffer (H3300; Vector Laboratories) was performed according to the manufacturer’s instructions. The following primary antibodies were used: anti-CDH1 (ab40772; Abcam), anti-CD71 (ab1086; Abcam), and anti-mesothelin (MSLN, sc-33672; Santa Cruz Biotechnology), anti-zonula occludens-1 (ZO-1, 33–9100; Invitrogen), anti-Wilms’ tumor protein 1 (WT1) (NB110-60011; NOVUS), and anti-calretinin (CALB2) (ab16694; Abcam). The following secondary antibodies were used: Alexafluor488-conjugated goat anti-rabbit (111–545–144; Jackson ImmunoResearch) and Alexafluor488-conjugated, goat anti-mouse (ab150113; Abcam). Slide imaging was performed on a Zeiss LSM 710 NLO confocal microscope.
ELISA.
NP-designated cell lines and MeT-5A cells were seeded in a six-well culture plate, stimulated with or without 20 ng/mL human TNF-α (SRP3177; Sigma), and cultured ~16 h. The supernatants were processed using a Human Cytokine ELISA Plate Array I Kit (EA-4001; Signosis) according to the manufacturer’s protocol. Each plate comprised triplicate arrays. One array served as readout for the untreated group (no TNF-α) while the remaining two served as replicate arrays for the experimental group (treated with TNF-α).
Imaging.
Light microscopy was performed on an Olympus IX51 inverted microscope fitted with a Diagnostic SPOT RT camera (Meyer Instruments) and workstation. Scanning electron microscopy was performed at the NIH core facilities.
Karyotyping.
Cells were exposed to colcemid (250 ng/mL) for 3 h, treated in 0.075 M potassium chloride for 20 min, and fixed in methanol-acetic acid (3:1). The cells were then air-dried onto glass slides. Chromosome count (modal value from scoring 20 chromosome spreads) and g-banding analyses were conducted by the Molecular Cytogenetics core facility at NIH.
Baseline cell identification.
Short-tandem repeat DNA profiling of NP cell lines was performed using the FTA Sample Collection Kit from the Human Cell Authentication Service (135-XV; ATCC).
BAP1 mutation analysis.
Genomic DNA was isolated from H2373, NP1, and NP3 cell pellets according to the manufacturer’s protocol (Wizard Genomic DNA Purification Kit, 1120; Promega). DNA template (30 ng) was used for PCR amplification of a 275-bp genomic sequence covering Exon 10 of the BAP1 gene using forward (5′-CCCTGTGAGTGAATGGGTAG-3′) and reverse (5′-CCCAGTACCTGTGTGGTTG-3′) primers. The Expand High Fidelity PCR System was used according to the manufacturer’s instruction (03310256103; Roche). PCR reactions were gel purified and sequenced accordingly (Zymoclean Gel DNA Recovery Kit, D4007). Sequence alignment and mutation identification were performed using Seqman Pro (DNASTAR Lasergene 13 Software).
Statistics.
Means and standard deviation (SD) were calculated from numerical data. Changes indicate the difference between experimental and control samples. Bar graphs depict means ± SD for specific experimental runs of replicate experiments. GraphPad Prism v.8.3.0 software was used for statistical calculations. Two-tailed unpaired Student’s t test assessed the significance between two conditions.
RESULTS
Human pleural biopsy.
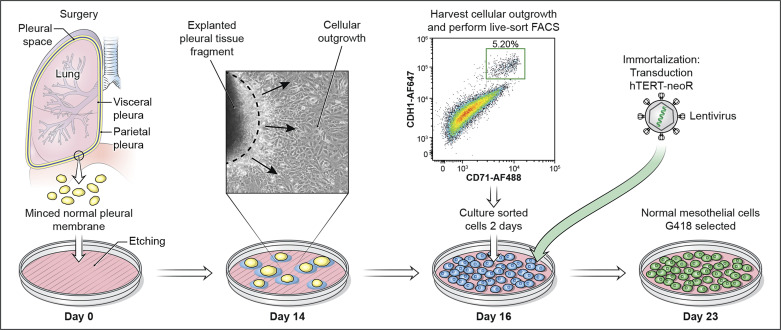
All of the patients in our study underwent elective minimally invasive VATS for various clinical indications, during which pleural biopsies from normal-appearing surfaces were retrieved. These simple yet effective criteria afforded us maximal flexibility to procure pleura in an ongoing manner from a spectrum of patients of both sexes who, occasionally, may harbor specific genetic backgrounds that were previously unavailable as naturally occurring cell lines. In our continuing series of patients, no surgical complications due to pleural biopsy have occurred consistent with this being a feasible and safe procedure. Pleural tissues were subjected to explant outgrowth to allow HMC to adapt to in vitro conditions before isolation for production of immortal cell lines. The major steps of our framework to generate HMC cultures are illustrated (Fig. 1).
Fig. 1.
Workflow to generate normal mesothelial cultures. On day 0, patient pleural sample is minced, placed in an etched culture dish, and incubated to allow for attachment of tissue fragments to the plate bottom. By day 14 (explant culturing), cells have migrated out onto the plate surface and are ready for harvest. Black arrows indicate direction of growth leaving the tissue fragment. Dashed line indicates boundary of tissue. Live-cell FACS recovers human mesothelial cell (HMC) according to CDH1 and CD71 surface expression. At day 16 cells are transduced with human telomerase reverse transcriptase (hTERT)-neoR lentivirus. Cells are exposed to neomycin over 5 days to ensure effective selection by resistance. By day 23, normal HMC are ready for propagation in vitro.
Mesothelial cells from pleural tissue.
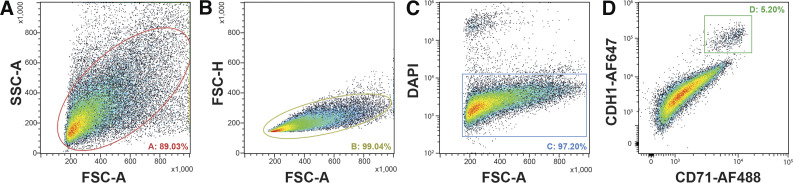
Cells were first gated by size (forward scatter area, FSC-A) and granularity (side scatter area, SSC-A) characteristics to exclude cellular debris (Fig. 2A). Next, cells were subgated for singlets (single cells) based on their FSC-A vs. forward scatter height (FSC-H) properties (Fig. 2B). Live cells were subgated using DAPI substrate as a viability dye (Fig. 2C). Mesothelin (MSLN) (4), the most common HMC-associated membrane protein whose physiologic function remains unknown, is very sensitive to trypsin (used to detach HMC after explant outgrowth), so FACS using anti-MSLN antibody was inconsistent with our objectives. Recently, CD71 (i.e., transferrin receptor) was shown to be efficient at discriminating HMC from contaminating CD45+ cells (22). Cadherin-1 (CDH1) is a cell-cell adhesion glycoprotein expressed on HMC surfaces, specifically concentrated at intercellular junctions (31, 51). Thus, after testing the performance of several HMC surface markers, the final FACS gate used to isolate this subpopulation was set to capture CDH1/CD71 double-positive cells (Fig. 2D). The mean and SD of frequency values determined for the isolated subpopulations of individual cultures (n = 3) were 90.6 ± 2.6% (total cells), 99.4 ± 0.6% (singlets), 96.8 ± 1.2% (live cells), and 4.8 ± 0.8% (HMC). The overall yield of HMC was typically 5.0 × 105 to 1.0 × 106 per tissue specimen.
Fig. 2.
Cell sorting of pleural explants to recover mesothelial cells. A: cells were first gated by size [forward scatter area (FSC-A)] and granularity [side scatter area (SSC-A)] characteristics to exclude cellular debris. B: selected cells were subgated for singlets based on their FSC-A vs. forward scatter height (FSC-H) properties. C: live cells were subgated using DAPI as a viability dye. D: a final gate isolating the mesothelial cell subpopulation was set to capture CDH1/CD71 double-positive cells.
Immortalization of primary mesothelial cells.
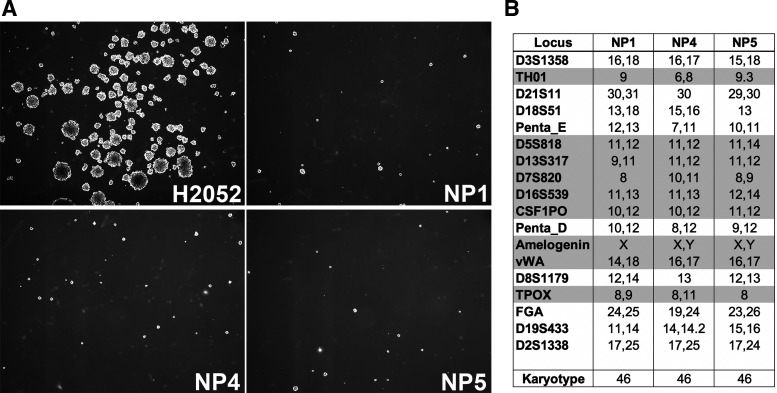
Following purification of HMC, each cell specimen was immortalized for extended passaging via transduction with lentivirus harboring hTERT cDNA (Fig. 1). Of note, use of hTERT does not produce cancer-associated changes (27) while overcoming the inherent problem of senescence-limited short lifespan of primary HMC in vitro (14). Once immortalized, the primary HMC cell lines were designated NP1, NP2, NP3, etc. Before validation experiments, a minimum of 60 population doublings per NP-designated cell line was required. During this period, no change in morphology or doubling time was observed (data not shown). Immortalization of NP-designated cell lines did not predispose those cells to cellular transformation as verified by no growth in tumorsphere assay (19), which measures ability of anchorage-independent growth, a hallmark of cancer cells (Fig. 3A). In the absence of immortalization, HMC typically senesce after 45–50 population doublings (9). Karyotyping of each NP-designated cell line revealed normal chromosome counts and chromosomal stability (no rearrangements) after prolonged culture (Fig. 3B). The typical time to reach this stage from the surgical harvest is about 21 to 24 days.
Fig. 3.
Tumorsphere growth and genetic profiles of normal pleura (NP)-designated cell lines. A: phase-contrast images of NP1, NP4, and NP5 cell lines compared with H2052 malignant pleural mesothelioma cell line (known to be tumorigenic) under conditions of anchorage-independent cell growth. Tumorsphere assay results are shown. B: representative NP cell lines (NP1, NP4, NP5) were characterized by karyotype and short-tandem-repeat analyses.
Mesothelial morphology and growth characteristics.
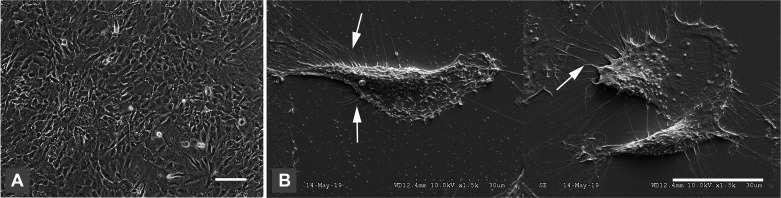
NP-designated cells grown to confluence exhibited cobblestone patterning, a typical appearance of HMC in vitro (37) (Fig. 4A). More specifically, scanning electron microscopy revealed the presence of long thin branching microvilli, a unique and defining feature of HMC (8), on the cell surfaces of all NP-designated cultures (Fig. 4B). Depending on exact culture conditions, the doubling time for NP-designated cell lines produced thus far ranged from 24 to 36 h.
Fig. 4.
Mesothelial cell morphology. A: phase-contrast image of normal pleura (NP)-4 cells at confluence in vitro showing typical cobblestone appearance. Scale bar indicates 50 µm. B: scanning electron microscopy of NP4 cells showing characteristic microvilli (white arrows) on the cell surface. A total of 3 human mesothelial cell are depicted in this composite of 2 images. Scale bar indicates 30 µm.
Normal mesothelial cell lines biomarkers.
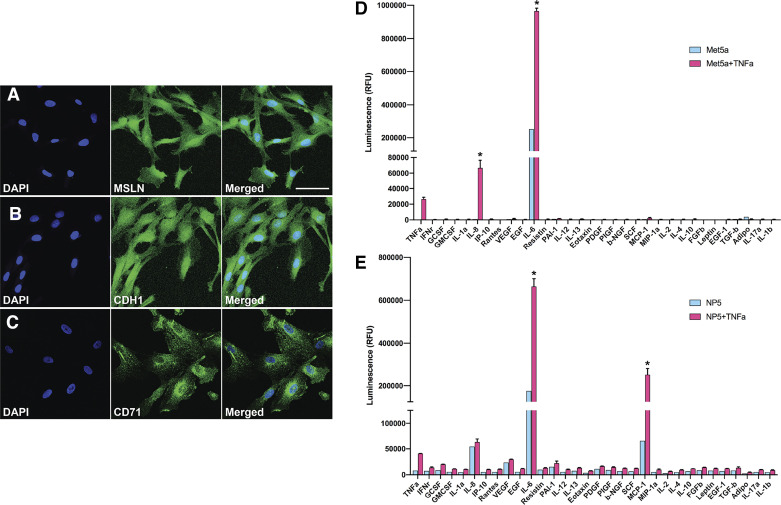
Using immunocytofluorescence, we further characterized the NP-designated lines. Generally, MSLN is expressed on normal HMC (4). Accordingly, diffuse MSLN expression was observed in our NP-designated lines (Fig. 5A). In nonconfluent conditions, CDH1 was expressed diffusely on the cell surface (Fig. 5B). Expression of CD71 was consistently observed on the cell surface of NP-designated lines with punctate perinuclear expression (Fig. 5C). Nonspecific background signal was undetectable for these three HMC-associated biomarkers (data not shown).
Fig. 5.
Mesothelial cell markers and cytokine profile. A–C: immunocytofluorescence analysis of mesothelin (MSLN), CDH1, and CD71 expression (green signals) in the normal pleura (NP)-5 cell line after extended passaging. Scale bar indicates 50 µm. D and E: comparison of cytokine and growth factor expression profiles of MeT-5A and NP5 cell lines with TNF-α stimulation (red) and without (blue). IFN, interferon; GCSF, granulocyte colony-stimulating factor; GMCSF, granulocyte-macrophage colony-stimulating factor; IP-10, interferon-γ-induced protein 10; RANTES, regulated upon activation normal T cell expressed and secreted; VEGF, vascular endothelial growth factor; EGF, endothelial growth factor; PAI-1, plasminogen activator inhibitor-1; PDGF, platelet-derived growth factor; NGF, nerve growth factor; MCP-1, monocyte chemoattractant protein-1; MIP-1, macrophage inflammatory protein-1; FGF, fibroblast growth factor; PIGF, placental growth factor; SCF, stem cell factor. Data are means ± SD; n = 2. *P < 0.05.
Mesothelial cell cytokine profile.
A defining characteristic of the HMC phenotype is their secretory profile in response to stimulation with the proinflammatory cytokine TNF-α. The NP-designated cell lines showed canonical elevated levels of IL-6 (35) and IL-8 (46) upon TNF-α stimulation, similar to MeT-5A cells (Fig. 5D). Additionally, but quite unlike MeT-5A, several other HMC-specific cytokines were secreted by NP-designated lines, including granulocyte colony stimulating factor (25), vascular endothelial growth factor (45), monocyte chemoattractant protein-1 (2), and plasminogen activator inhibitor-1 (49) (Fig. 5E). MeT-5A cells, which fail to secrete any of these other cytokines upon stimulation, are therefore not well representative of in vivo HMC, nor can they be considered an accurate normal-type cell for comparison against disease processes such as MPM.
Expression of tight junction proteins.
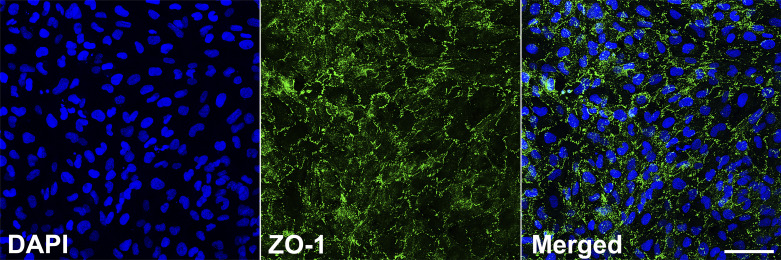
An inherent function of the pleural membrane is its tissue barrier function that results from HMC being interconnected via tight junctions, structures comprised of an intramembrane multiprotein complex, which govern permeability (29). Hence, one of the cardinal features of HMC is their ability to form tight junctions Zonula occludens 1 (ZO-1) is one of the many ubiquitous proteins that contribute to the molecular architecture of tight junctions, acting as scaffolding (43). The NP-designated lines all demonstrated consistent expression of ZO-1 localized to the cell periphery, revealing the typical honeycomb-like pattern on immunostaining (Fig. 6).
Fig. 6.
Tight junction-associated protein expression. Immunocytofluorescence analysis of zonula occludens-1 (ZO-1) protein expression at intercellular junctions of normal pleura (NP)-1 cells (a typical result in a representative NP line) at 24 h after grown to confluency (green signal). Nuclear labeling (DAPI) is indicated by blue signal. Scale bar indicates 50 µm.
BAP1 mutation analysis.
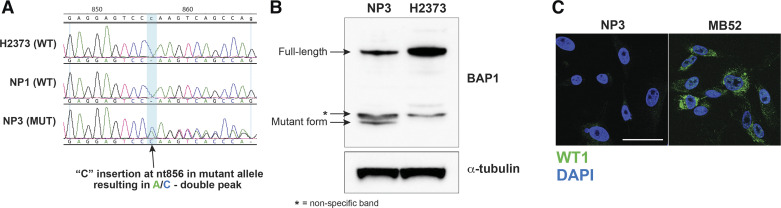
The NP3 cell line, generated from HMC of an MPM patient carrying a germline breast cancer type 1 (BRCA1) associated protein-1 (BAP1) mutation (cytosine insertion mutation at nucleotide 856 determined by Oncomine Comprehensive Assay v3M), harbored this same genetic lesion as verified by PCR profiling (Fig. 7A). This is evidenced by the adenine/cytosine double peak in the NP3 sample of the aligned sequence electropherograms. The BAP1 mutation results in a truncated nonfunctional protein, which can be detected by Western blotting (Fig. 7B). So generally, with our tissue-based technique of generating HMC cultures, specific genetic characteristics are preserved and faithfully represented in subsequent cell passages. In contrast to the surrounding MPM deposits present at the time of pleural biopsy, the NP3 cell line did not overexpress tumor markers (Fig. 7C).
Fig. 7.
Breast cancer type-1-associated protein-1 (BAP1) analysis in mesothelial cell line. A: mutation analysis to detect heterozygous BAP1 germline mutation in normal pleura (NP)-3 cells. Comparison is made among wild-type (WT) H2373 malignant pleural mesothelioma (MPM) cell line and the wild-type NP1 cell line. nt856, nucleotide 856. B: detection of truncated protein produced by BAP1 mutated allele via Western blotting. C: immunocytofluorescence analysis of MPM tumor marker Wilms’ tumor protein 1 (WT1; green signal) in the NP3 cell line compared with the MPM cell line MB52 indicating a lack of signal in NP3 cells. Nuclear labeling (DAPI) is indicated by blue signal. Scale bar indicates 50 µm. MUT, mutant. *Nonspecific band.
Summary of NP cell line traits.
Overall, these new NP-designated cell lines display characteristics matching those of normal primary HMC as delineated from the research literature. The degree of similarity based on the essential physiologic features examined here is striking, especially when contrasted against the traditional, long-established HMC lines currently in use (Table 1). The main difference of NP-designated cell lines from primary HMC is that they are immortal, which makes them more suited to reproducible large-scale experimentation. In any future studies that may require use of these NP-designated cell lines, application-specific baseline characterizations can be added to our table of summary features; for example, the gene expression profiles of these cells with and without exposure to asbestos in oncogenesis modeling.
Table 1.
Comparison of traits among human mesothelial cell lines
| Characteristic | HMC Primary | NP Cells | MeT-5a | LP-9 |
|---|---|---|---|---|
| Nonadherent growth | No (5) | No* | Yes (36) | No (28) |
| Cell surface microvilli | Yes (1) | Yes* | Yes (7) | Yes (39) |
| Mesothelial markers (CDH1, MSLN) | CDH1 (23) | CDH1* MSLN* |
CDH1 (47) MSLN (12) |
MSLN (17) |
| Karyotype | Normal (11) | Normal* | Hyperploidy (41) | Normal (48) |
| Senescence limit | ~14 wk (9) | hTERT immortalized* | SV40 immortalized (20) | ~14 wk (24) |
| Mesothelial-specific cytokine profile | ||||
| IL-6 | Yes (35) | Yes* | Yes* | Yes (15) |
| IL-8 | Yes (46) | Yes* | Yes* | Yes (40) |
| GCSF | Yes (25) | Yes* | No* | Yes (16) |
| VEGF | Yes (45) | Yes* | No* | Yes (16) |
| MCP-1 | Yes (2) | Yes* | No* | Not reported |
| PAI-1 | Yes (49) | Yes* | No* | Not reported |
| Intercellular tight junctions | Yes (18) | Yes* | Yes (42) | Not reported |
HMC, human mesothelial cell; NP, normal pleura; CDH1, cadherin 1; MSLN, mesothelin; hTERT, human telomerase reverse transcriptase; SV40, simian virus 40; GCSF, granulocyte colony-stimulating factor; VEGF, vascular endothelial growth factor; MCP-1, monocyte chemoattractant protein-1; PAI-1, plasminogen activator inhibitor-1. References are in parentheses.
Experimental data from the current study.
DISCUSSION
We have established a straightforward reproducible protocol to isolate HMC from patient-derived pleural biopsies and for generating novel stable cell lines (i.e., NP1, NP3, NP4, NP5, etc.) adaptable to short-term or long-term in vitro experimentation (Fig. 1). Explant culturing (30) with cellular outgrowth is an effective technique well-suited, but underutilized, to adapting normal epithelial-like cells to in vitro propagation. Moreover, we verify the effectiveness of harvesting normal-appearing pleura coupled with dual CDH1/CD71 positivity to highly enrich for normal HMC applicable to diverse research scenarios. This strategy obviates the more difficult circumstance, if not practically infeasible, of only performing pleural biopsy in normal human subjects without any disease. Relying on surgical biopsy to directly obtain HMC is highly advantageous: 1) patients who consent to being tissue donors are a convenient, endless resource; and 2) typical cellular yields are known and consistent. In contrast, previous methods that obtained HMC from effusion fluids have never been easily reproduced because of lack of consensus on standardized technical details (5).
The baseline HMC lines in wide usage have inherent limitations that, in part, have contributed to slow progress into new insights of MPM biology. MeT-5A cells are altered with simian virus-40 early region and Rous sarcoma virus long terminal repeat (oncogenic potential) in addition to being aneuploid due to long-term adaptation in cell culture [originally hypodiploid (20) and more recently hyperploid (41)]. LP-9 cells too easily senesce and typically grow very slowly, making cultivation of sufficient cell numbers for ongoing experimentation inconsistent. Devoid of such encumbrances, our newly derived NP cell lines all exhibit normal ploidy (Fig. 3B) and, significantly, retain in vivo physiologic functions like cytokine response to noxious stimuli or tight junction formation (Table 1). So, these NP lines represent a more meaningful normal counterpart in MPM in vitro studies. Of further importance to cancer research is how these NP lines retain their genetic integrity. Specifically, the NP3 cell line faithfully displays the identical BAP1 mutation harbored in the germline of the patient who originated these cells. BAP1 is a tumor suppressor gene inactivated in familial MPM (44). It is causally linked to tumor predisposition syndrome, which manifests as increased risk for developing MPM along with melanoma and renal carcinoma, among other cancers (38). As a novel cell line, NP3 could uniquely provide insight into the mechanistic role(s) of BAP1 in tumorigenesis. Furthermore, our approach can be adapted, as an example, to obtain peritoneal HMC lines. Mesothelial cells from this location have a unique physiologic response to asbestos exposure (10). Having such patient-specific normal specimens could improve our knowledge of peritoneal MPM, a poorly understood, yet distinct, primary abdominal cancer (6).
Beyond cancer-specific studies and mechanisms of oncogenesis (for MPM), normal HMC lines can additionally, and as equally important, serve as model systems to study other consequential biologic phenomena. A major area of investigation centers on understanding mechanisms of metastasis of any solid tumors, such as ovarian, colorectal, or breast carcinoma to mesothelial surfaces (21). Presumably, the starting point for these endeavors must be centered on the HMC where the process of tumor implantation takes place as metastatic tumor cells interact. Another highly impactful inquiry field is concerned with dissecting the mechanisms of mesothelial wound healing that occurs after surgery, infection, or peritoneal dialysis, all leading to necessary regenerative processes (33). A defect in the regulation of this process can result in diseases such as mesothelioma. To gain insight into the pathophysiology of such conditions, we need to better understand the mechanisms regulating normal mesothelial repair. And lastly, but not an exhaustive listing of examples, there is a large area of research studying mesothelial-to-mesenchymal transition (34), a pathologic cellular transition state contributing to thickening and fibrosis of mesothelial surfaces impairing physiological functions such as fluid transport or barrier protection.
Despite our technical improvements, there are some limitations to our approach. The ideal resource to conduct some of the baseline measurements would be normal primary cells, not immortalized cells. In vivo, HMC are slowly replicating cells so are very unlikely to adapt to in vitro conditions, which mostly explains why they have not been widely available for experimentation. Hence, immortalization was a practical compromise in our method to generate new HMC lines. Insertion of hTERT has minimal impact on the cellular physiology (as shown here in the NP-designated cell line validations, Table 1), yet allow for large-scale expansion of cell numbers necessary for mechanistic studies. Undergoing surgical biopsy to obtain tissue for cell line initiation can expose patients to potential procedural complications. When performed as part of other necessary surgical treatment, pleural biopsy (especially by VATS) is very safe with minimal morbidity. Additionally, the need for a surgical team and associated resources is a relative disadvantage. However, the opportunity to access unique specimens such as germline BAP1 mutants without employing a surgical approach is infeasible. In balance, the many benefits of our methodology outweigh the few risks.
In summary, mesothelial cells comprising the lining of all coelomic compartments are involved in critical biologic processes such as organogenesis, immune interactions, wound healing, or cancer (both primary and metastases). Mechanistic studies to examine these phenomena are less readily performed owing to lack of appropriate human cell lines. Our technique combining minimally invasive tissue biopsy and explant culturing is a novel high-fidelity approach to obtain patient-specific mesothelial cell cultures.
GRANTS
This work was supported by NIH Intramural Research Program with funding from National Cancer Institute Center for Cancer Research Grant ZIA BC 011657 (to C. D. Hoang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.D.H. conceived and designed research; N.P. and A. Singh performed experiments; N.P., A. Singh, A. Shankar, and C.D.H. analyzed data; N.P., A. Singh, D.S.S., and C.D.H. interpreted results of experiments; N.P., A. Shankar, and C.D.H. prepared figures; N.P. and C.D.H. drafted manuscript; A. Singh and C.D.H. edited and revised manuscript; N.P., A. Singh, A. Shankar, D.S.S., and C.D.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ethan Tyler, Medical Illustrator in the Medical Arts Branch of the National Institutes of Health.
REFERENCES
- 1.Andrews PM, Porter KR. The ultrastructural morphology and possible functional significance of mesothelial microvilli. Anat Rec 177: 409–426, 1973. doi: 10.1002/ar.1091770307. [DOI] [PubMed] [Google Scholar]
- 2.Antony VB, Hott JW, Kunkel SL, Godbey SW, Burdick MD, Strieter RM. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am J Respir Cell Mol Biol 12: 581–588, 1995. doi: 10.1165/ajrcmb.12.6.7766422. [DOI] [PubMed] [Google Scholar]
- 3.Beckett P, Edwards J, Fennell D, Hubbard R, Woolhouse I, Peake MD. Demographics, management and survival of patients with malignant pleural mesothelioma in the National Lung Cancer Audit in England and Wales. Lung Cancer 88: 344–348, 2015. doi: 10.1016/j.lungcan.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol 20: 2902–2906, 2000. doi: 10.1128/MCB.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, Pass HI, Rizzo P, Carbone M. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci USA 97: 10214–10219, 2000. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borczuk AC, Cappellini GC, Kim HK, Hesdorffer M, Taub RN, Powell CA. Molecular profiling of malignant peritoneal mesothelioma identifies the ubiquitin-proteasome pathway as a therapeutic target in poor prognosis tumors. Oncogene 26: 610–617, 2007. doi: 10.1038/sj.onc.1209809. [DOI] [PubMed] [Google Scholar]
- 7.Cavallo D, Campopiano A, Cardinali G, Casciardi S, De Simone P, Kovacs D, Perniconi B, Spagnoli G, Ursini CL, Fanizza C. Cytotoxic and oxidative effects induced by man-made vitreous fibers (MMVFs) in a human mesothelial cell line. Toxicology 201: 219–229, 2004. doi: 10.1016/j.tox.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Chernova T, Sun XM, Powley IR, Galavotti S, Grosso S, Murphy FA, Miles GJ, Cresswell L, Antonov AV, Bennett J, Nakas A, Dinsdale D, Cain K, Bushell M, Willis AE, MacFarlane M. Molecular profiling reveals primary mesothelioma cell lines recapitulate human disease. Cell Death Differ 23: 1152–1164, 2016. doi: 10.1038/cdd.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell ND, Rheinwald JG. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell 34: 245–253, 1983. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- 10.Dragon J, Thompson J, MacPherson M, Shukla A. Differential susceptibility of human pleural and peritoneal mesothelial cells to asbestos exposure. J Cell Biochem 116: 1540–1552, 2015. doi: 10.1002/jcb.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Factor RE, Dal Cin P, Fletcher JA, Cibas ES. Cytogenetics and fluorescence in situ hybridization as adjuncts to cytology in the diagnosis of malignant mesothelioma. Cancer 117: 247–253, 2009. doi: 10.1002/cncy.20036. [DOI] [PubMed] [Google Scholar]
- 12.He X, Wang L, Riedel H, Wang K, Yang Y, Dinu CZ, Rojanasakul Y. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Mol Cancer 16: 63, 2017. doi: 10.1186/s12943-017-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog R, Tarantino S, Rudolf A, Aufricht C, Kratochwill K, Witowski J. Senescence-Associated changes in proteome and O-GlcNAcylation pattern in human peritoneal mesothelial cells. BioMed Res Int 2015: 382652, 2015. doi: 10.1155/2015/382652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillegass JM, Miller JM, MacPherson MB, Westbom CM, Sayan M, Thompson JK, Macura SL, Perkins TN, Beuschel SL, Alexeeva V, Pass HI, Steele C, Mossman BT, Shukla A. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part Fibre Toxicol 10: 39, 2013. doi: 10.1186/1743-8977-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillegass JM, Shukla A, MacPherson MB, Bond JP, Steele C, Mossman BT. Utilization of gene profiling and proteomics to determine mineral pathogenicity in a human mesothelial cell line (LP9/TERT-1). J Toxicol Environ Health A 73: 423–436, 2010. doi: 10.1080/15287390903486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilliard TS. The impact of mesothelin in the ovarian cancer tumor microenvironment. Cancers (Basel) 10: 277, 2018. doi: 10.3390/cancers10090277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito T, Yorioka N, Yamamoto M, Kataoka K, Yamakido M. Effect of glucose on intercellular junctions of cultured human peritoneal mesothelial cells. J Am Soc Nephrol 11: 1969–1979, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Johnson S, Chen H, Lo PK. In vitro tumorsphere formation assays. Bio Protoc 3: 3, 2013. doi: 10.21769/BioProtoc.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke Y, Reddel RR, Gerwin BI, Reddel HK, Somers AN, McMenamin MG, LaVeck MA, Stahel RA, Lechner JF, Harris CC. Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos-induced mesothelioma. Am J Pathol 134: 979–991, 1989. [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny HA, Lal-Nag M, White EA, Shen M, Chiang CY, Mitra AK, Zhang Y, Curtis M, Schryver EM, Bettis S, Jadhav A, Boxer MB, Li Z, Ferrer M, Lengyel E. Quantitative high throughput screening using a primary human three-dimensional organotypic culture predicts in vivo efficacy. Nat Commun 6: 6220, 2015. doi: 10.1038/ncomms7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kienzle A, Servais AB, Ysasi AB, Gibney BC, Valenzuela CD, Wagner WL, Ackermann M, Mentzer SJ. Free-floating mesothelial cells in pleural fluid after lung surgery. Front Med (Lausanne) 5: 89, 2018. doi: 10.3389/fmed.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C, Kim DG, Park SH, Hwang YI, Jang SH, Kim CH, Jung KS, Min K, Lee JW, Jang YS. Epithelial to mesenchymal transition of mesothelial cells in tuberculous pleurisy. Yonsei Med J 52: 51–58, 2011. doi: 10.3349/ymj.2011.52.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ksiazek K, Mikula-Pietrasik J, Olijslagers S, Jörres A, von Zglinicki T, Witowski J. Vulnerability to oxidative stress and different patterns of senescence in human peritoneal mesothelial cell strains. Am J Physiol Regul Integr Comp Physiol 296: R374–R382, 2009. doi: 10.1152/ajpregu.90451.2008. [DOI] [PubMed] [Google Scholar]
- 25.Lanfrancone L, Boraschi D, Ghiara P, Falini B, Grignani F, Peri G, Mantovani A, Pelicci PG. Human peritoneal mesothelial cells produce many cytokines (granulocyte colony-stimulating factor [CSF], granulocyte-monocyte-CSF, macrophage-CSF, interleukin-1 [IL-1], and IL-6) and are activated and stimulated to grow by IL-1. Blood 80: 2835–2842, 1992. doi: 10.1182/blood.V80.11.2835.2835. [DOI] [PubMed] [Google Scholar]
- 26.Lechner JF, Tokiwa T, LaVeck M, Benedict WF, Banks-Schlegel S, Yeager H Jr, Banerjee A, Harris CC. Asbestos-associated chromosomal changes in human mesothelial cells. Proc Natl Acad Sci USA 82: 3884–3888, 1985. doi: 10.1073/pnas.82.11.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology 45: 33–38, 2004. doi: 10.1007/10.1007/s10616-004-5123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohcharoenkal W, Wang L, Stueckle TA, Park J, Tse W, Dinu CZ, Rojanasakul Y. Role of H-Ras/ERK signaling in carbon nanotube-induced neoplastic-like transformation of human mesothelial cells. Front Physiol 5: 222, 2014. doi: 10.3389/fphys.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markov AG, Amasheh S. Tight junction physiology of pleural mesothelium. Front Physiol 5: 221, 2014. doi: 10.3389/fphys.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer TG, Naipal KA, Jager A, van Gent DC. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Sci OA 3: FSO190, 2017. doi: 10.4155/fsoa-2017-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mubarak KK, Montes-Worboys A, Regev D, Nasreen N, Mohammed KA, Faruqi I, Hensel E, Baz MA, Akindipe OA, Fernandez-Bussy S, Nathan SD, Antony VB. Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur Respir J 39: 133–140, 2012. doi: 10.1183/09031936.00141010. [DOI] [PubMed] [Google Scholar]
- 32.Mutsaers SE, Prêle CM, Pengelly S, Herrick SE. Mesothelial cells and peritoneal homeostasis. Fertil Steril 106: 1018–1024, 2016. doi: 10.1016/j.fertnstert.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Mutsaers SE, Whitaker D, Papadimitriou JM. Stimulation of mesothelial cell proliferation by exudate macrophages enhances serosal wound healing in a murine model. Am J Pathol 160: 681–692, 2002. doi: 10.1016/S0002-9440(10)64888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namvar S, Woolf AS, Zeef LA, Wilm T, Wilm B, Herrick SE. Functional molecules in mesothelial-to-mesenchymal transition revealed by transcriptome analyses. J Pathol 245: 491–501, 2018. doi: 10.1002/path.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Offner FA, Obrist P, Stadlmann S, Feichtinger H, Klingler P, Herold M, Zwierzina H, Hittmair A, Mikuz G, Abendstein B, Zeimet A, Marth C. IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine 7: 542–547, 1995. doi: 10.1006/cyto.1995.0073. [DOI] [PubMed] [Google Scholar]
- 36.Papazoglou ED, Jagirdar RM, Kouliou OA, Pitaraki E, Hatzoglou C, Gourgoulianis KI, Zarogiannis SG. In vitro characterization of cisplatin and pemetrexed effects in malignant pleural mesothelioma 3D culture phenotypes. Cancers (Basel) 11: 1446, 2019. doi: 10.3390/cancers11101446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pronk A, Leguit P, Hoynck van Papendrecht AA, Hagelen E, van Vroonhoven TJ, Verbrugh HA. A cobblestone cell isolated from the human omentum: the mesothelial cell; isolation, identification, and growth characteristics. In Vitro Cell Dev Biol 29A: 127–134, 1993. doi: 10.1007/BF02630943. [DOI] [PubMed] [Google Scholar]
- 38.Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet 89: 285–294, 2016. doi: 10.1111/cge.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rilla K, Tiihonen R, Kultti A, Tammi M, Tammi R. Pericellular hyaluronan coat visualized in live cells with a fluorescent probe is scaffolded by plasma membrane protrusions. J Histochem Cytochem 56: 901–910, 2008. doi: 10.1369/jhc.2008.951665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shukla A, MacPherson MB, Hillegass J, Ramos-Nino ME, Alexeeva V, Vacek PM, Bond JP, Pass HI, Steele C, Mossman BT. Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. Am J Respir Cell Mol Biol 41: 114–123, 2009. doi: 10.1165/rcmb.2008-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh A, Pruett N, Hoang CD. In vitro experimental models of mesothelioma revisited. Transl Lung Cancer Res 6: 248–258, 2017. doi: 10.21037/tlcr.2017.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song LJ, Zhou LL, Wang M, Liu F, Xiong L, Xiang F, Yu F, He XL, Xu JJ, Shi HZ, Xin JB, Ye H, Ma WL. Lethal (2) giant larvae regulates pleural mesothelial cell polarity in pleural fibrosis. Biochim Biophys Acta Mol Cell Res 1865: 1201–1210, 2018. doi: 10.1016/j.bbamcr.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103: 755–766, 1986. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, Hesdorffer M, Nasu M, Powers A, Rivera Z, Comertpay S, Tanji M, Gaudino G, Yang H, Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 43: 1022–1025, 2011. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax 54: 707–710, 1999. doi: 10.1136/thx.54.8.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topley N, Brown Z, Jörres A, Westwick J, Davies M, Coles GA, Williams JD. Human peritoneal mesothelial cells synthesize interleukin-8. Synergistic induction by interleukin-1 beta and tumor necrosis factor-alpha. Am J Pathol 142: 1876–1886, 1993. [PMC free article] [PubMed] [Google Scholar]
- 47.Turini S, Bergandi L, Gazzano E, Prato M, Aldieri E. Epithelial to mesenchymal transition in human mesothelial cells exposed to asbestos fibers: role of TGF-β as mediator of malignant mesothelioma development or metastasis via EMT event. Int J Mol Sci 20: 150, 2019. doi: 10.3390/ijms20010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services DHHS Publication No. (NIH). Bethesda, MD: Dept. of Health and Human Services, 1985. [Google Scholar]
- 49.Whawell SA, Scott-Coombes DM, Vipond MN, Tebbutt SJ, Thompson JN. Tumour necrosis factor-mediated release of plasminogen activator inhibitor 1 by human peritoneal mesothelial cells. Br J Surg 81: 214–216, 1994. doi: 10.1002/bjs.1800810218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winters N, Bader D. Development of the serosal mesothelium. J Dev Biol 1: 64–81, 2013. doi: 10.3390/jdb1020064. [DOI] [Google Scholar]
- 51.Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, Aguilera A, Sánchez-Tomero JA, Bajo MA, Alvarez V, Castro MA, del Peso G, Cirujeda A, Gamallo C, Sánchez-Madrid F, López-Cabrera M. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med 348: 403–413, 2003. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]