Abstract
Mucosal surfaces are constantly exposed to a microbiome consisting of microorganisms that heavily influence human immunity and health. In the lung these microorganisms consist of bacteria, viruses, and fungi and exist in a relatively low biomass state. Bacterial communities of the lung modulate local inflammation and correlate with changes in pulmonary physiology and clinical outcomes in patients with lung disease. Instrumental to this progress has been the study of these bacterial communities in the pathogenesis of pulmonary fibrosis, a fatal and progressive disease culminating in respiratory failure. Key pathophysiological mechanisms in pulmonary fibrosis include recurrent idiopathic alveolar epithelial injury, unchecked collagen deposition, mucociliary dysfunction due to muc5b overexpression, hypoxia, and altered host defense. These key mechanisms and their related consequences promote severe progressive architectural lung destruction and loss of local homeostasis. As such, pulmonary fibrosis is an appropriate target disease for the study of the lung microbiome. Herein, we discuss recent advances in our understanding of the role of the lung microbiome in the pathogenesis of pulmonary fibrosis. We highlight fundamental clinical observations and mechanistic insights and identify crucial areas for further discovery science. An improved understanding of how the lung microbiome acts to influence outcomes in patients with pulmonary fibrosis will lead to enhanced therapies for this devastating lung disease.
Keywords: lung microbiome, pulmonary fibrosis
INTRODUCTION
The lungs exist in a constant state of exposure to the outside world. Yet, for centuries biologists purported a hypothesis that the lower respiratory tract was devoid of bacteria, existing in a sterile state (35). This historical perspective was derived from knowledge that long-established bacterial culture-dependent techniques failed to isolate and identify bacteria from clinical respiratory samples or sought only to culture clinically significant pathogens (4). In addition, sampling of the lower airways was technically difficult and associated with risk. This hypothesized “sterile” respiratory tract was therefore only breached in conditions of overwhelming infectious disease (e.g., pneumonia, bronchiectasis). Yet, on reflection, two key observations remained: 1) the surface area of the human respiratory tract is enormous and in continuous contact with the external environment, and 2) the respiratory tract is exposed to microorganisms in inhaled air and through microaspiration from birth. Estimates of the volume of inhaled ambient air are close to 7,000 liters a day and the burden of microorganism therein may be substantial (47). The lower respiratory tract is continuous with the upper respiratory tract and subject to aspiration of oropharyngeal contents, a universal finding in humans (8, 14). The conclusion that the lung was a sterile environment appeared less plausible and required further study. In the last decade, the field of lung microbiome science has expanded through the introduction of culture-independent techniques—an ability to identify bacteria by sequencing of the 16S rRNA gene, a highly conserved gene within the bacterial genome (1). Bacterial communities exist within the human lung, are altered in lung disease, and correlate with physiological measures, alveolar immunity, and clinical outcomes in human lung disease (5, 13, 28, 33, 36, 44). In health, bacterial communities in the respiratory tract can shape local immune tone (40). The sterile lung hypothesis has been debunked. Since the original study by Hilty et al. (11) in 2010, describing bacterial communities in the airways of patients with asthma, our understanding of the interplay between the lung and respiratory tract bacteria has accelerated at a remarkable pace. The microbiome is defined as the “community of commensal, symbiotic and pathogenic organisms that share our body” and the associated complex interactions that occur therein (23). We now know that the microbes existing within the lung are low biomass comparative to other sites in the body, particularly the gut. These microorganisms include fungi, bacteria, and viruses. Complex and for now poorly understood interactions occur between these communities of pulmonary microbiota and the host (6). These field-defining observations have been amalgamated through studies in human patients and biological animal models.
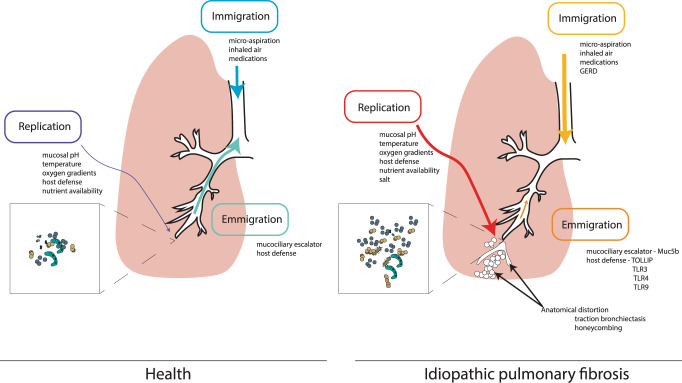
The respiratory tract consists of both an upper segment—nasopharynx, oropharynx, and larynx—and a lower respiratory tract. While the respiratory tract is continuous, the microbiota that reside within the upper respiratory tract are similar yet distinct from those of the lower respiratory tract (4). The bacterial communities of the lower respiratory tract exist in a delicate balance dependent on several ecological pressures. The ecology of the lung microbiome is dependent on microbial immigration related to ubiquitous microaspiration of oropharyngeal communities, which can equally be perturbed by pathology of the upper airway or gastrointestinal tract (4). Emigration of bacterial community members is dependent on local host defense and the mucociliary escalator. The active replication of microbial communities is linked to many factors, including community interactions, host-microbiota interactions, and local environmental conditions (e.g., mucosal pH, anatomy, oxygen gradients, nutrient availability, and temperature) (Fig. 1). In health, the population dynamics of lung bacterial communities are mostly related to a balance of immigration and emigration influences with minimal contributions from changes in replication (4). However, in lung disease, there is considerable disruption to local conditions promoting alterations in microbial immigration, emigration, and replication. This disruption can result in changes in bacterial burden, the composition of bacterial communities between subjects (beta diversity), or changes to the evenness and richness of bacterial communities within subjects (alpha diversity) (Fig. 2). Central to this progress in advancing our knowledge of the role of the lung microbiome has been the dedicated study of lung microbiota in pulmonary fibrosis. As a heterogeneous disorder, pulmonary fibrosis results in the unchecked deposition of collagen within the lung interstitium (34). Incremental thickening of the alveolar capillary membrane progressively impairs gas exchange and distorts pulmonary architecture with subsequent further physiological derangement and organ failure. The most common form of pulmonary fibrosis is idiopathic pulmonary fibrosis (IPF), a progressive and fatal disease (24). IPF has an increasing worldwide incidence and no known cure, and current therapies attenuate disease progression but do not arrest or reverse lung fibrosis (37). The natural history and prognosis are varied and the disease course may be punctuated by acute clinical deterioration, termed acute exacerbations (AE-IPF), that can lead to significant morbidity and mortality (2). A role for lung bacteria in IPF is supported by several clinical observations in recent decades. Richter et al. (38) reported that bacteria were commonly cultured in bronchoalveolar fluid (BAL) from patients with IPF; 36% of patients cultured bacteria including Streptococcus pneumonia and Moraxella catarrhalis among others. The landmark PANTHER trial was a practice-defining study leading to the reversal of employing immunosuppression in IPF disease management (15). Patients with IPF treated with prednisone, azathioprine, and N-aetylcysteine had deleterious outcomes with increased mortality and hospitalizations, suggesting that decreased host defense might result in harm. In addition, patients with IPF treated with broad-spectrum antibiotics have reported improved outcomes in clinical studies (26, 42). Here, we discuss recent research on the lung microbiome in IPF, which has consolidated early findings, supported causal significance, and furthered the role of potential microbiome-based therapies in pulmonary fibrosis. This review will focus solely on the role of bacterial communities in the respiratory tract in pulmonary fibrosis. A detailed discussion of the role of others, including viruses, in the pathogenesis of IPF can be found elsewhere (29, 31). Our review will describe the putative causal links between pulmonary immunity and respiratory tract microbiota. We will examine key features of the respiratory tract microbiome, namely 1) burden, 2) composition, and 3) diversity, as they pertain to pulmonary fibrosis pathogenesis. Within this framework, we discuss the urgent need for further discovery and mechanistic science to address the ongoing unanswered questions in this exciting and revolutionary field of lung microbiome science and pulmonary fibrosis.
Fig. 1.
Ecological pressures on the lung microbiome in idiopathic pulmonary fibrosis. The lung microbiome is determined by relative contributions from microbial immigration, microbial emigration, and microbial replication. In health, immigration and emigration pressures contribute significantly. Factors contributing to immigration include inhaled air and universal microaspiration, and emigration is determined by the mucociliary escalator and host defense. However, in pulmonary fibrosis, significant physiological, immunological, and anatomical disruptions that occur, including changes in immigration (e.g., gastroesophageal reflux disease: GERD) and changes to emigration pressures [increased airway Muc5b expression, altered host defense, modified Toll-like receptor (TLR) signaling], can lead to severe disruption in immigration, emigration pressures, and growth conditions for respiratory tract taxa. Studies have shown that the fibrotic lung represents a salty microenvironment that can favor the growth of “salt-loving” or halophilic bacteria (see Ref. 3). Subsequent fibrotic remodeling with traction bronchiectasis and honeycombing may in turn alter emigration pressures (graphic arrows are weighted to illustrate the plausible “relative” contributions of these selective ecological factors, i.e., immigration, emigration, and replication on the lung microbiome in health and pulmonary fibrosis).
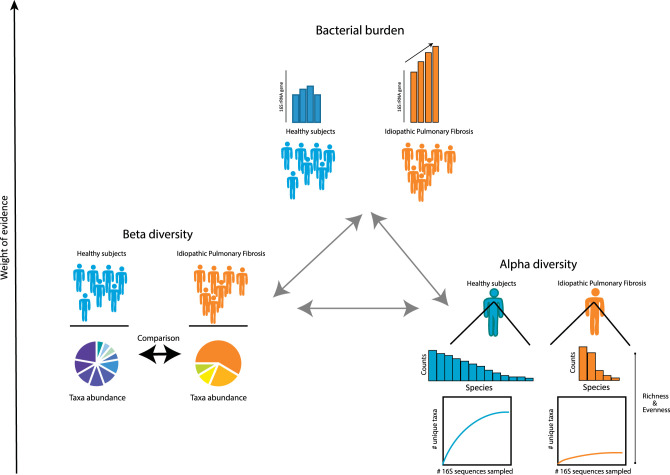
Fig. 2.
Features of the lung microbiome in health and idiopathic pulmonary fibrosis (IPF). Graphical representation of the key features of the lung microbiome in healthy subjects and patients with IPF. The weight of evidence to date supports associations between bacterial burden (quantity of 16S rRNA gene counts by PCR) within the lung and clinical outcomes. An increased bacterial burden may result in increased abundance of all bacteria present (without a change in alpha diversity) or increases in certain bacteria and loss of others (with or without changes in alpha diversity). Common measures of alpha diversity include Shannon diversity index, Simpson index, and rarefaction curves. The Shannon diversity index measures the number of local species present and the inequality between species counts/abundance. Rarefaction curves involve the calculation of richness by plotting curves where the number of unique species identified serves as a function of increasing numbers of sequences sampled. In addition, an increase in bacterial burden may or may not result in changes in beta diversity (or the compositional heterogeneity) of bacterial communities present between subjects or samples (i.e., comparison of lung bacterial communities between healthy subjects and patients with IPF). A common measure of beta diversity includes the Bray-Curtis dissimilarity index (comparison of species between two or more samples, where a score of 0 means communities in samples are identical in species presence and abundances and a score of 1 means samples have completely different species abundances). Other measures of beta diversity include the Jaccard distance and UniFrac. In comparisons of lung microbiota in patients with IPF and healthy controls, notable differences are seen in beta diversity and alpha diversity, with lower alpha diversity in patients with IPF (see Ref. 28). Furthermore, in IPF, increasing bacterial burden is associated with a reduction in alpha diversity supporting the gain and/or loss of certain taxa within the lung microbiome (see Ref. 33).
THE LOWER RESPIRATORY TRACT MICROBIOME IN PULMONARY FIBROSIS
In pulmonary fibrosis, progressive distortion and loss of normal lung architecture occurs through unchecked collagen deposition, traction bronchiectasis, reticulation, and honeycomb cyst formation (Fig. 1). Local ecological conditions may be considerably altered, leading to changes in mucosal homeostasis and the loss or gain of bacterial species. The lung microbiome reported in patients with IPF is predominated by several species, including Hemophilus sp., Neisseria sp., Streptococcus sp., and Veillonella sp. (found at higher abundance in IPF lungs compared with healthy volunteers) (30). In this study, a key correlative finding was the association between lung microbiota and gene signatures of host defense in the peripheral blood of patients, suggesting that candidate lung bacteria may be responsible for an alveolar injury pattern that can be detected in blood. While the data do not support causation, this observation was further supported by another study that correlated gene signatures of innate immune function in peripheral blood mononuclear cells (PBMCs) and lung microbiota in patients with IPF (12). The downregulation of several innate immune receptor genes in PBMCs was associated with lung microbial diversity, individual genera, and worse progression-free survival in patients with IPF, strengthening these putative links between host immunity and lung microbiota. Mucin glycoproteins, particularly Muc5B, have established roles in the pathogenesis of IPF. In human patients with IPF, the presence of variants of the Muc5b rs35705950 polymorphism are associated with a several-fold increased risk of IPF development (41). Variant alleles are associated with muc5b overexpression (41). Muc5b is crucial to appropriate airway defense: deficiency is associated with alterations to airway bacteria, whereas overexpression of Muc5b in the lung enhances lung fibrosis and promotes honeycomb cyst formation in animal models (10, 22, 39). Muc5b overexpression in the distal airway and alveolar segment in tandem with honeycomb cyst formation are plausibly associated with a significant loss of local physiological homeostasis (7). In turn, preliminary studies in human patients suggest that the presence of honeycomb cysts may be associated with bacterial community disruption (5). In IPF patients the bacterial communities identified in IPF bronchoalveolar fluid (BAL) to date show features of community dysbiosis (e.g., increased burden, loss of diversity) which correlate with innate immune activation, local alveolar inflammation, and worse clinical outcomes (9, 28, 30, 33). In the next sections, we present a treatise on the evidence supporting the role of the lung microbiome in pulmonary fibrosis and discuss possible future strategies for leveraging host microbiome interactions to develop new therapies.
BACTERIAL BURDEN AND DISEASE PROGRESSION IN PULMONARY FIBROSIS
Studies of the lung microbiome in patients with IPF have evolved since the original field defining paper in 2013. Molyneaux et al. (28) identified an association between the bacterial burden in the lung and mortality in patients with IPF. Bacterial burden was quantified by measuring the 16S rRNA gene by PCR in standardized bronchoalveolar lavage fluid aliquots from patients and reported to be correlated with mortality, the higher the bacterial burden the greater the risk of mortality. Subsequently, we validated this in an independent cohort of patients with IPF using an alternate and more accurate quantification platform (droplet digital PCR of the 16S rRNA gene in human BAL fluid), demonstrating a robust association between bacterial burden and disease progression. Eleanor et al. have shown that in IPF whole lung explants, a proportion of patients with IPF had a higher bacterial burden in explant airways that correlated with clinical outcomes, an increased risk of acute exacerbation (AE), and mortality (6). An AE-IPF has been defined recently as a clinical deterioration that can be classified as triggered or idiopathic (2). These events are poorly understood and carry substantial mortality and morbidity. Life expectancy after an AE-IPF is severely curtailed at 3–4 months (19, 43). Interestingly, a proposed feature of AE-IPF is the presence of an increased bacterial burden in the lower respiratory tract. Molyneaux et al. (27) reported a fourfold increased bacterial burden in the airways of patients with acute exacerbations of IPF compared with patients with stable disease. The increased bacterial burden may lead to direct bacteria-related epithelial injury within the fibrotic lung and heightened inflammatory signaling that we will discuss in the next section. Given our knowledge of IPF pathogenesis and the universal presence of alveolar epithelial injury, the crucial question remains, whether the consistent observation of increased bacterial burden and fibrosis progression is causally related? Recent data attempt to address this key question. Invernizzi et al. (16) quantified bacterial burden in BAL from a large cohort of well-characterized patients with IPF and then examined correlations between bacterial burden and disease extent on computed tomography (CT) scan of the chest. The canonical radiographic features of IPF, e.g., the honeycomb cyst, traction bronchiectasis, and overall extent of fibrosis on CT quantification, have reported associations with mortality and are markers of fibrosis progression (18, 32). However, no correlations between bacterial burden and disease severity, including radiographic honeycombing, were found. Similarly, no association between bacterial burden and honeycombing was reported in a separate cohort of patients, validating this finding (5). Therefore, the increased bacterial burden is not simply the result of distorted pulmonary architecture and tissue fibrosis. More recently, Invernizzi et al. have demonstrated that the association between increased bacterial burden in the lung and mortality in IPF is not a universal finding in interstitial lung disease (ILD). In a study of 110 patients with chronic hypersensitivity pneumonitis (CHP), a fibrotic lung disease often indistinguishable from IPF on clinical, radiographic, and histopathological findings, there was no correlation between bacterial burden and mortality (17). Indeed, the bacterial burden seen in the lungs of patients with CHP was significantly less than in IPF. The increased bacterial burden of IPF may be located in the airway, distal to the actual fibrotic remodeling of the parenchyma, a hypothesis supported by the very low levels of bacteria identified in IPF parenchymal lung tissue (20). There is also a lack of evidence to date supporting an association between key radiographic features (i.e., honeycombing) and BAL bacterial burden in published studies (16, 33). Importantly, bacterial burden remains an independent predictor of mortality even after adjusting for varied physiological and radiographic predictors of mortality in IPF (17).
An increased airway bacterial burden is consistently correlated with progressive disease in IPF, an established and rigorous clinical observation now (16, 28, 33). Furthermore, in the clinical setting of acute exacerbation where disease activity is clearly higher, there is also an increased bacterial burden within the fibrotic lung (27). In biological models, we and others have recently reported that germ-free mice, (experimental mice devoid of microbiota) are protected from mortality in models of pulmonary fibrosis, further supporting a role for bacterial burden in driving disease pathogenesis (33, 49). Key to understanding these observations is the knowledge that an increased bacterial burden or biomass may represent both an increased abundance of all species present (without a change in alpha diversity) or a gain of abundance of certain species and loss of others (with or without a change in alpha diversity) (Fig. 2). An increased burden may or may not be associated with changes in beta diversity. We have previously shown that in IPF the increased bacterial burden correlates with a reduction in alpha diversity (33). Others have shown that, when compared with healthy subjects, patients with IPF have reduced alpha diversity, and differences in numbers of species present and their relative abundance (beta diversity) (17, 28). In that context, we are of the opinion that an increased airway burden in IPF is indicative of dysbiosis, resulting from the growth of certain species with competitive advantage and/or loss of other species as key events. We will discuss some of these putative candidate taxa in detail in the next sections.
Sources of an increased bacterial airway burden in IPF may result from increased immigration of species and/or reduced emigration of species from the respiratory tract (Fig. 1). gastroesophageal reflux disease (GERD) and microaspiration as sources of this increased immigration are plausible, given the previous studied and controversial association between GERD and IPF (25). A reduction in emigration of bacterial species is also plausible in IPF. Alterations in local host defense and increased distal airway mucin glycoproteins (e.g., Muc5b) are likely to lead to altered bacterial clearance. However, the precise mechanisms through which an increased bacterial burden in the distal airway and progressive lung fibrosis are related is not clearly understood and requires future mechanistic studies. Given the improved clinical outcomes observed in trials of antibiotics in patients with IPF, it remains plausible although not yet defined, that the effects seen with antibiotic treatment are attributable to a reduced bacterial burden within the lung. Further studies should be focused on exploring these possibilities.
BETA DIVERSITY: COMPOSITIONAL HETEROGENEITY AND KEY BACTERIAL SPECIES/GENUS LEVEL EFFECTS
The evidence to date supports an increased bacterial burden as the key observation in the progression of lung fibrosis. This increased bacterial burden, indicative of dysbiosis, has not been rigorously interrogated to date. Knowledge of bacterial communities would strongly suggest that, within the nutrient-limited mucosal surface of the lung, an increase in bacterial burden represents the increased replication of specific taxa with competitive advantages, increased immigration of species, and/or reduced emigration of species. While all microbes within the lung and airway microenvironment are of relevance, it is likely that some are more important than others. We have previously shown correlations between taxa and BAL inflammatory cytokines (33) and Wang et al. (46) have reported significant correlations between lower airway taxa i.e., Porphyromonas and Dialister, abundance and local alveolar levels of Th-17 precursor cytokines in patients with IPF. Han et al. reported an association between an increased abundance of airway Staphylococcus operational taxonomic units (OTU) and Streptococcus OTU and disease progression in patients with IPF (9). However, the mechanisms through which the increased abundance of these taxa promotes disease progression are unknown. In addition, not all patients in the study had evidence of Streptococcus or Staphylococcus OTU within their lavage fluid, suggesting that the signal may be limited to a specific subgroup of patients. Biological models have produced data to support certain species-specific mechanisms. Knippenberg et al. reported that, in murine models of pulmonary fibrosis, Streptococcus pneumonia exposure promoted progressive fibrosis that was alleviated with targeted antibiotic treatment (21). The Streptococcus-derived toxin pneumolysin (Ply) was responsible for progressive fibrosis. Mice treated with Streptococcus species deficient in Ply were protected from progressive fibrosis. In contrast, mice treated with recombinant Ply demonstrated increased alveolar epithelial cell death and lung fibrosis. The mechanism was independent of Toll-like receptor 4 (TLR4). D’Alessandro-Gabazza et al. (3) recently reported an association between a Staphylococcus-derived peptide, corisin, and progressive fibrosis in animal models. Exposure of mice to intratracheal corisin or Staphylococcus nepalensis, a corisin-producing species, exacerbated lung fibrosis through the induction of alveolar epithelial apoptosis. Corisin is a highly conserved peptide common among the Staphylococcus genus. Furthermore, the authors report higher levels of corisin in BAL fluid of patients with IPF experiencing an acute disease exacerbation compared with patients with stable disease, establishing biological plausibility in human patients. In a Japanese study of patients with IPF hospitalized for probable pulmonary infections, the second most common pathogen isolated in clinical respiratory samples was Staphylococcus aureus (4.1% of isolates) (48). It is possible that through alterations in local physiology, Staphylococcus spp. gain a competitive advantage, the increasing airway abundance disrupts homeostasis, and the production of higher levels of Staphylococcus-derived profibrotic peptides promotes further profibrotic cytokine signaling and disease activity. However, Molyneaux et al. (27) did not identify changes in relative abundance of Staphylococcus and Streptococcus spp. in a previous IPF study comparing the lung microbiota of stable patients and patients with AE-IPF. This study was limited by sample size. However, in an individual patient with matched BAL samples captured during stable and exacerbation disease states, there was a marked increased abundance of Streptococcus spp. during the IPF exacerbation. While studies of health in humans and animal models have reported the capacity of lung microbiota to regulate pulmonary immune tone, Yang et al. (49) demonstrated that, in pulmonary fibrosis, commensal bacteria can modulate local immunity and promote fibrosis through outer membrane vesicles (OMVs). OMVs were isolated from three species found at increased abundance in the fibrotic murine lung (Bacteroidetes ovatus, Bacteroidetes stercoris, and Prevotella melaninogenica), and OMVs from two species (B. stercoris and P. melaninogenica), when inoculated into bleomycin-challenged mice, induced IL-17 immunity and profibrotic gene expression. The authors reported that the lung microbiota-induced IL-17B production was TLR and MyD88 signaling dependent with specific roles mentioned for TLR4 and TLR2. The evidence supporting specific species-level effects in animal models supports a causal role for lung microbiota in pulmonary fibrosis. However, this evidence also creates formidable challenges for the future. Therapy that can broadly effect bacterial communities in the lung, e.g., antimicrobials, may have limited efficacy and conceivably alter selective pressures resulting in further dysbiosis and unintended deleterious outcomes. The case for future mechanistic studies to not only understand candidate profibrotic and proinflammatory bacterial mechanisms, but also the local mucosal changes in immunity and physiology that alter the composition of bacterial community networks, is warranted. There may be considerable benefit in directly targeting specific features of microbial physiology or metabolism in the treatment of progressive lung fibrosis or enhancing a specific host response to these candidate bacteria and their profibrotic mechanisms.
ALPHA DIVERSITY: LUNG MICROBIOTA DIVERSITY, CAUSAL EFFECT, AND REGIONAL INFLAMMATION
Microbial diversity is an important component of the lung microbiome. Diversity is a measure of the evenness and richness of a bacterial community. Diversity can be measured within a biological sample (alpha diversity) or between samples (beta diversity). In a comparison of microbial alpha diversity of lung bacterial communities in patients with IPF and healthy subjects, Molyneaux et al. (28) reported reduced alpha diversity (Shannon diversity index) and reduced species numbers in patients with IPF. Takahashi et al. (45) reported in a study of 34 patients with IPF that patients with progressive disease and those with early mortality had reportedly lower alpha diversity (Shannon and Simpson diversity indexes), suggesting that less diverse respiratory tract communities were associated with poor clinical outcomes. In addition, the authors found that Shannon diversity index was positively correlated with forced vital capacity, a physiological measure of lung function known to correlate with clinical outcomes in IPF (greater diversity linked to better lung function). The authors postulated that these observations were likely driven by changes in the abundance of Streptococcaceae and Veillonellaceae at the family taxa level. In our study, we reported robust correlations between lower microbial alpha diversity (Shannon diversity index) and increased alveolar inflammation in patients with IPF (33). Han et al. (9) reported an association between Shannon diversity index and IPF disease progression using Cox regression modeling while accounting for common modifiers of disease progression and the abundance of BAL Staphylococcus OTU and Streptococcus OTU. Clearly, microbial alpha diversity in the respiratory tract of patients with IPF has a complex relationship with the host and may act as an important determinant of local alveolar immune responses. Considerable work remains in deciphering these complex interactions between microbial diversity, bacterial burden, community composition, and how the host responds and/or promotes these changes in pulmonary fibrosis.
CONCLUSIONS
The advent of culture-independent techniques for the identification and study of bacteria in the respiratory tract has revolutionized our understanding of chronic lung disease. The dedicated study of the role of the lung microbiome in pulmonary fibrosis has contributed to these advances. Key features of the lung microbiome correlate with alveolar inflammation, physiological measures, and clinical outcomes in pulmonary fibrosis. The evidence to date supporting continued work in this field has been accrued with clear scientific rigor but has just scratched the surface. The next steps in discovery will involve well-designed mechanistic studies interrogating both the nature of the host response to dysbiosis and the response of bacterial communities and candidate proinflammatory microbiota to changes in alveolar homeostasis and also an improved understanding of how these complex bacterial communities interact with each other within the lung environment. These key studies will lay a foundation for microbiome-based therapies and improved outcomes for patients with pulmonary fibrosis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R00 HL139996 (D.N.O.) and R35 HL144481 (B.B.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.H.L. and D.N.O. drafted manuscript; J.H.L., B.B.M., and D.N.O. edited and revised manuscript; J.H.L., B.B.M., and D.N.O. approved final version of manuscript; D.N.O. prepared figures.
REFERENCES
- 1.Carney SM, Clemente JC, Cox MJ, Dickson RP, Huang YJ, Kitsios GD, Kloepfer KM, Leung JM, LeVan TD, Molyneaux PL, Moore BB, O’Dwyer DN, Segal LN, Garantziotis S. Methods in lung microbiome research. Am J Respir Cell Mol Biol 62: 283–299, 2020. doi: 10.1165/rcmb.2019-0273TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, Behr J, Brown KK, Cottin V, Flaherty KR, Fukuoka J, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kolb M, Lynch DA, Myers JL, Raghu G, Richeldi L, Taniguchi H, Martinez FJ. Acute exacerbation of idiopathic pulmonary fibrosis. an international working group report. Am J Respir Crit Care Med 194: 265–275, 2016. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro-Gabazza CN, Kobayashi T, Yasuma T, Toda M, Kim H, Fujimoto H, Hataji O, Takeshita A, Nishihama K, Okano T, Okano Y, Nishii Y, Tomaru A, Fujiwara K, D’Alessandro VF, Abdel-Hamid AM, Ren Y, Pereira GV, Wright CL, Hernandez A, Fields CJ, Yau PM, Wang S, Mizoguchi A, Fukumura M, Ohtsuka J, Nosaka T, Kataoka K, Kondoh Y, Wu J, Kawagishi H, Yano Y, Mackie RI, Cann I, Gabazza EC. A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis. Nat Commun 11: 1539, 2020. doi: 10.1038/s41467-020-15344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The microbiome and the respiratory tract. Annu Rev Physiol 78: 481–504, 2016. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson RP, Huffnagle GB, Flaherty KR, White ES, Martinez FJ, Erb-Downward JR, Moore BB, O’Dwyer DN. Radiographic honeycombing and altered lung microbiota in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 200: 1544–1547, 2019. doi: 10.1164/rccm.201903-0680LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eleanor B Valenzi HY, Sembrat JC, Yang L, Winters S, Nettles R, Kass DJ, Qin S, Wang X, Myerburg MM, Methe B, Fitch A, Alder JK, Benos PV, McVerry BV, Rojas M, Morris A, Kitsios GD. Topographic heterogeneity of lung microbiota in end-stage idiopathic pulmonary fibrosis: the microbiome in lung explants-2 (MiLEs-2) study (Preprint). medRxiv 2020. doi: 10.1101/2020.03.05.20031021. [DOI] [PMC free article] [PubMed]
- 7.Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, Yang IV, Schwartz DA. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev 96: 1567–1591, 2016. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest 111: 1266–1272, 1997. doi: 10.1378/chest.111.5.1266. [DOI] [PubMed] [Google Scholar]
- 9.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, Moore BB, White ES, Flaherty KR, Huffnagle GB, Martinez FJ, Investigators C; COMET Investigators . Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med 2: 548–556, 2014. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock LA, Hennessy CE, Solomon GM, Dobrinskikh E, Estrella A, Hara N, Hill DB, Kissner WJ, Markovetz MR, Grove Villalon DE, Voss ME, Tearney GJ, Carroll KS, Shi Y, Schwarz MI, Thelin WR, Rowe SM, Yang IV, Evans CM, Schwartz DA. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun 9: 5363, 2018. doi: 10.1038/s41467-018-07768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO. Disordered microbial communities in asthmatic airways. PLoS One 5: e8578, 2010. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Ma SF, Espindola MS, Vij R, Oldham JM, Huffnagle GB, Erb-Downward JR, Flaherty KR, Moore BB, White ES, Zhou T, Li J, Lussier YA, Han MK, Kaminski N, Garcia JGN, Hogaboam CM, Martinez FJ, Noth I; COMET-IPF Investigators . Microbes are associated with host innate immune response in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 196: 208–219, 2017. doi: 10.1164/rccm.201607-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, Calhoun WJ, Castro M, Denlinger LC, Dimango E, Kraft M, Peters SP, Wasserman SI, Wechsler ME, Boushey HA, Lynch SV; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network . Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol 127: 372–381.1-3, 2011. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med 64: 564–568, 1978. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 15.Idiopathic Pulmonary Fibrosis Clinical Research Network Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 366: 1968–1977, 2012. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Invernizzi R, Barnett J, Rawal B, Nair A, Ghai P, Kingston S, Chua F, Wu Z, Wells AU, Renzoni ER, Nicholson AG, Rice A, Lloyd CM, Byrne AJ, Maher TM, Devaraj A, Molyneaux PL. Bacterial burden in the lower airways predicts disease progression in idiopathic pulmonary fibrosis and is independent of radiological disease extent. Eur Respir J 55: 55, 2020. doi: 10.1183/13993003.01519-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Invernizzi R, Wu BG, Barnett J, Ghai P, Kingston S, Hewitt RJ, Feary J, Li Y, Chua F, Wu Z, Wells AU, Renzoni EA, Nicholson AG, Rice A, Devaraj A, Segal LN, Byrne AJ, Maher TM, Lloyd CM, Molyneaux PL. The respiratory microbiome in chronic hypersensitivity pneumonitis is distinct from that of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. In press. 10.1164/rccm.202002-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob J, Aksman L, Mogulkoc N, Procter AJ, Gholipour B, Cross G, Barnett J, Brereton CJ, Jones MG, van Moorsel CH, van Es W, van Beek F, Veltkamp M, Desai SR, Judge E, Burd T, Kokosi M, Savas R, Bayraktaroglu S, Altmann A, Wells AU. Serial CT analysis in idiopathic pulmonary fibrosis: comparison of visual features that determine patient outcome. Thorax 75: 648–654, 2020. doi: 10.1136/thoraxjnl-2019-213865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon K, Chung MP, Lee KS, Chung MJ, Han J, Koh WJ, Suh GY, Kim H, Kwon OJ. Prognostic factors and causes of death in Korean patients with idiopathic pulmonary fibrosis. Respir Med 100: 451–457, 2006. doi: 10.1016/j.rmed.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Kitsios GD, Rojas M, Kass DJ, Fitch A, Sembrat JC, Qin S, Veraldi KL, Gibson KF, Lindell K, Pilewski JM, Methe B, Li K, McDyer J, McVerry BJ, Morris A. Microbiome in lung explants of idiopathic pulmonary fibrosis: a case-control study in patients with end-stage fibrosis. Thorax 73: 481–484, 2018. doi: 10.1136/thoraxjnl-2017-210537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knippenberg S, Ueberberg B, Maus R, Bohling J, Ding N, Tort Tarres M, Hoymann HG, Jonigk D, Izykowski N, Paton JC, Ogunniyi AD, Lindig S, Bauer M, Welte T, Seeger W, Guenther A, Sisson TH, Gauldie J, Kolb M, Maus UA. Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin. Thorax 70: 636–646, 2015. doi: 10.1136/thoraxjnl-2014-206420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurche JS, Dobrinskikh E, Hennessy CE, Huber J, Estrella A, Hancock LA, Schwarz MI, Okamoto T, Cool CD, Yang IV, Evans CM, Schwartz DA. Muc5b enhances murine honeycomb-like cyst formation. Am J Respir Cell Mol Biol 61: 544–546, 2019. doi: 10.1165/rcmb.2019-0138LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lederberg J, McCray AT. ‘Ome sweet ’omics—A genealogical treasury of words. Scientist 15: 8–8, 2001. https://lhncbc.nlm.nih.gov/system/files/pub2001047.pdf. [Google Scholar]
- 24.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 378: 1811–1823, 2018. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, Yow E, Raghu G, Investigators IP; IPFnet Investigators . Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med 1: 369–376, 2013. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macaluso C, Maritano Furcada J, Alzaher O, Chaube R, Chua F, Wells AU, Maher TM, George PM, Renzoni EA, Molyneaux PL. The potential impact of azithromycin in idiopathic pulmonary fibrosis. Eur Respir J 53: 1800628, 2019. doi: 10.1183/13993003.00628-2018. [DOI] [PubMed] [Google Scholar]
- 27.Molyneaux PL, Cox MJ, Wells AU, Kim HC, Ji W, Cookson WO, Moffatt MF, Kim DS, Maher TM. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res 18: 29, 2017. doi: 10.1186/s12931-017-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, Cookson WO, Maher TM, Moffatt MF. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 190: 906–913, 2014. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molyneaux PL, Maher TM. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir Rev 22: 376–381, 2013. doi: 10.1183/09059180.00000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molyneaux PL, Willis-Owen SAG, Cox MJ, James P, Cowman S, Loebinger M, Blanchard A, Edwards LM, Stock C, Daccord C, Renzoni EA, Wells AU, Moffatt MF, Cookson WOC, Maher TM. Host-microbial interactions in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 195: 1640–1650, 2017. doi: 10.1164/rccm.201607-1408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore BB, Moore TA. Viruses in Idiopathic Pulmonary Fibrosis. Etiology and Exacerbation. Ann Am Thorac Soc 12, Suppl 2: S186–S192, 2015. doi: 10.1513/AnnalsATS.201502-088AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa H, Ogawa E, Fukunaga K, Kinose D, Yamaguchi M, Nagao T, Tanaka-Mizuno S, Nakano Y. Quantitative CT analysis of honeycombing area predicts mortality in idiopathic pulmonary fibrosis with definite usual interstitial pneumonia pattern: a retrospective cohort study. PLoS One 14: e0214278, 2019. doi: 10.1371/journal.pone.0214278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, Norman KC, Arnold KB, Huffnagle GB, Salisbury ML, Han MK, Flaherty KR, White ES, Martinez FJ, Erb-Downward JR, Murray S, Moore BB, Dickson RP. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med 199: 1127–1138, 2019. doi: 10.1164/rccm.201809-1650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Dwyer DN, Ashley SL, Moore BB. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 311: L590–L601, 2016. doi: 10.1152/ajplung.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 196: 4839–4847, 2016. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Dwyer DN, Zhou X, Wilke CA, Xia M, Falkowski NR, Norman KC, Arnold KB, Huffnagle GB, Murray S, Erb-Downward JR, Yanik GA, Moore BB, Dickson RP. Lung dysbiosis, inflammation, and injury in hematopoietic cell transplantation. Am J Respir Crit Care Med 198: 1312–1321, 2018. doi: 10.1164/rccm.201712-2456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, Flaherty KR, Wells A, Martinez FJ, Azuma A, Bice TJ, Bouros D, Brown KK, Collard HR, Duggal A, Galvin L, Inoue Y, Jenkins RG, Johkoh T, Kazerooni EA, Kitaichi M, Knight SL, Mansour G, Nicholson AG, Pipavath SNJ, Buendía-Roldán I, Selman M, Travis WD, Walsh S, Wilson KC; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society; Latin American Thoracic Society . Diagnosis of idiopathic pulmonary fibrosis. An fficial ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198: e44–e68, 2018. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 38.Richter AG, Stockley RA, Harper L, Thickett DR. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax 64: 692–697, 2009. doi: 10.1136/thx.2008.110445. [DOI] [PubMed] [Google Scholar]
- 39.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature 505: 412–416, 2014. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, Li Y, Shen N, Ghedin E, Morris A, Diaz P, Huang L, Wikoff WR, Ubeda C, Artacho A, Rom WN, Sterman DH, Collman RG, Blaser MJ, Weiden MD. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 1: 16031, 2016. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364: 1503–1512, 2011. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Wilson EC, Twentyman OP, Davison AG, Curtin JJ, Crawford MB, Wilson AM. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax 68: 155–162, 2013. doi: 10.1136/thoraxjnl-2012-202403. [DOI] [PubMed] [Google Scholar]
- 43.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 37: 356–363, 2011. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 44.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 185: 1073–1080, 2012. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi Y, Saito A, Chiba H, Kuronuma K, Ikeda K, Kobayashi T, Ariki S, Takahashi M, Sasaki Y, Takahashi H. Impaired diversity of the lung microbiome predicts progression of idiopathic pulmonary fibrosis. Respir Res 19: 34, 2018. doi: 10.1186/s12931-018-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Lesko M, Badri MH, Kapoor BC, Wu BG, Li Y, Smaldone GC, Bonneau R, Kurtz ZD, Condos R, Segal LN. Lung microbiome and host immune tone in subjects with idiopathic pulmonary fibrosis treated with inhaled interferon-γ. ERJ Open Res 3: 00008-2017, 2017. doi: 10.1183/23120541.00008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winslow CE. A new method of enumerating bacteria in air. Science 28: 28–31, 1908. doi: 10.1126/science.28.705.28. [DOI] [PubMed] [Google Scholar]
- 48.Yamazaki R, Nishiyama O, Sano H, Iwanaga T, Higashimoto Y, Kume H, Tohda Y. Clinical features and outcomes of IPF patients hospitalized for pulmonary infection: a Japanese cohort study. PLoS One 11: e0168164, 2016. doi: 10.1371/journal.pone.0168164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang D, Chen X, Wang J, Lou Q, Lou Y, Li L, Wang H, Chen J, Wu M, Song X, Qian Y. Dysregulated lung commensal bacteria drive interleukin-17B production to promote pulmonary fibrosis through their outer membrane vesicles. Immunity 50: 692–706.e7, 2019. doi: 10.1016/j.immuni.2019.02.001. [DOI] [PubMed] [Google Scholar]