Abstract
Many mouse models of allergic asthma exhibit eosinophil-predominant cellularity rather than the mixed-granulocytic cytology in steroid-unresponsive severe disease. Therefore, we sought to implement a novel mouse model of antigen-driven, mixed-granulocytic, severe allergic asthma to determine biomarkers of the disease process and potential therapeutic targets. C57BL/6J wild-type, interleukin-6 knockout (IL-6−/−), and IL-6 receptor knockout (IL-6R−/−), mice were injected with an emulsion of complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) on day 1. Dexamethasone, a lymphocyte-depleting biological, or anti-IL-17A was administered during the intranasal HDM challenge on days 19–22. On day 23, the CFA/HDM model elicited mixed bronchoalveolar lavage (BAL) cellularity (typically 80% neutrophils and 10% eosinophils), airway hyperresponsiveness (AHR) to methacholine, diffusion impairment, lung damage, body weight loss, corticosteroid resistance, and elevated levels of serum amyloid A (SAA), pro-inflammatory cytokines, and T helper type 1/ T helper type 17 (Th1/Th17) cytokines compared with eosinophilic models of HDM-driven allergic airway disease. BAL cells in IL-6- or IL-6R-deficient mice were predominantly eosinophilic and associated with elevated T helper type 2 (Th2) and reduced Th1/Th17 cytokine production, along with an absence of SAA. Nevertheless, AHR remained in IL-6-deficient mice even when dexamethasone was administered. However, combined administration of anti-IL-17A and systemic corticosteroid significantly attenuated both overall and neutrophilic airway inflammation and also reduced AHR and body weight loss. Inhibition of IL-17A combined with systemic corticosteroid treatment during antigen-driven exacerbations may provide a novel therapeutic approach to prevent the pathological pulmonary and constitutional changes that greatly impact patients with the mixed-granulocytic endotype of severe asthma.
Keywords: asthma, corticosteroids, interleukin-17, mice, neutrophils
INTRODUCTION
Asthma is defined as a chronic disorder of the airways that can manifest with hyperresponsiveness to bronchoconstricting agents, typically due to underlying inflammation (41a). Although most asthmatics respond to traditional inhaler treatments consisting of anti-inflammatory corticosteroids and bronchodilators, there is a subset of severe asthmatics who fall under a broader category of “difficult-to-treat” (54). Severe asthmatics are defined as uncontrolled despite treatment with the two aforementioned maintenance inhalers and chronic oral corticosteroid treatment for more than 50% of the previous calendar year (17, 54). Along with respiratory symptoms, these asthmatics also have increased incidences of anxiety and depression and their caregivers endure emotional and financial burdens (61). In addition, these patients disproportionately account for utilization of health-care resources and lost productivity relative to the overall asthmatic population (61). Consequently, there is an unmet need to better define these severe asthmatics through the identification of biomarkers and to evaluate potential therapeutic interventions targeted at novel pathogenic mediators.
Asthma “endotyping” is an approach to patient stratification in which clinical presentation and biomarkers are used to better identify markers, mediators, and targets of disease (51). It has been established that severe asthmatics can be categorized based on the type of inflammation that is suspected to be present, often categorized as “Th2high” and “non-Th2” asthma. Preclinical models (often mice) were of great utility to understand the cells, secreted factors (e.g., cytokines and leukotrienes), and signaling pathways that mediate Th2high allergic asthma. Consequently, small molecule and biological therapeutics targeting eosinophilpoiesis [interleukin-5 (IL-5)], IgE, and Th2 cytokines (IL-4 and IL-13) are now available to effectively treat previously refractory Th2high asthmatics (29). Novel therapies do not exist for non-Th2 asthmatics, as disease mechanisms are likely heterogeneous and remain poorly understood.
To meet the therapeutic needs of treatment-refractory non-Th2 patients, there needs to be a better mechanistic understanding of the pathophysiology in such presentations of severe asthma. It has been the objective of many researchers to address specific non-Th2 endotypes of severe asthma, and preclinical animal models can also be designed to represent these additional and especially severe asthma endotypes (37). Although previous mouse models of asthma have frequently demonstrated an eosinophilic-predominant bronchoalveolar lavage fluid (BALF) cellularity, mimicking a Th2high phenotype, mouse models of non-Th2 asthma are much less common (34), limiting the ability of the scientific community to evaluate biomarkers and potential mediators of disease. To this end, researchers have used combinations of allergens (22), alternative adjuvants polarizing non-Th2 adaptive immune responses (49, 68), adoptive transfer of in vitro-polarized CD4+ T cells (40), superimposed infections (50) or environmental exposures (10), and protracted allergen challenge schemes (41), among others. From these models and from studies of human subjects with severe asthma, several biomarkers and candidate mediators have been proposed, including neutrophils, innate immune responses, Th17 and Th1 cytokines, and certain metabolic processes. Although there are severe asthmatics who do not exhibit antigen sensitivity, many severe asthmatics exhibit antigen-specific reactivity to the most common perennial allergen, house dust mites (Dermatophagoides farina and Dermatophagoides pteronyssinus) (12). Our objective was to develop a novel mouse model of antigen-driven neutrophil-predominant severe allergic asthma to better understand biomarkers and mediators of the disease process, as well as to identify potential targets for novel therapies.
MATERIALS AND METHODS
Mice.
Wild-type C57BL/6J mice (stock number 000664) were purchased from The Jackson Laboratory (Bar Harbor, ME). IL-6−/− mice (46) and IL-6Ra−/− (39) mice on the C57BL/6J background were bred at the University of Vermont. All animals were maintained on a 12-h light/dark cycle and were provided chow (~15% kcal from fat; TestDiet, St. Louis, MO) and water ad libitum in an American Association for the Accreditation of Laboratory Animal Care-accredited facility. Sodium pentobarbital (150 mg/kg by intraperitoneal injection; Wilcox Pharmacy, Rutland, VT) was administered for euthanasia.
Mouse model of mixed-granulocytic severe asthma.
Mice were anesthetized with inhaled isoflurane and injected under the skin of their back with 100 µL of an emulsification containing 50 µL of complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO) supplemented with 4 mg/mL of Mycobacterium tuberculosis extract (BD 231441) and 25 μg (based on protein content) of Dermatophagoides pteronyssinus house dust mite (HDM) extract in saline (Part No. XPB70D3A25, Lot No. 343205; Stallergenes Greer, Lenoir, NC), hereafter referred to as CFA/HDM, on day 1. Antigen challenges were performed by intranasal inhalation of 10 μg HDM in 40 µL of saline on days 19, 20, 21, and 22. Mice were analyzed on day 23.
Adoptive transfer mouse model of airway inflammation.
Pooled mediastinal lymph nodes and spleens from female mice subjected to the model of mixed-granulocytic severe asthma were dissociated through a 70-μm mesh filter (BD Biosciences, San Jose, CA), and lymphocytes were enriched by centrifugation through the lymphocyte separation medium (MP Biomedicals, Irvine, CA). CD4+ T cells were isolated via magnetic negative selection following the manufacturer’s instructions (STEMCELL Technologies, Vancouver, Canada). Washed cells were resuspended in saline, and 2 × 106 cells were adoptively transferred into the retroorbital sinus of isoflurane-anesthetized female C57BL/6J mice. Recipient mice were antigen-challenged immediately following adoptive transfer and on each of the next 4 days via intranasal instillation of 10 μg of HDM extract in 40 µL of saline. Mice were analyzed 1 day following the final HDM exposure.
Mouse models of eosinophilic asthma.
For the intranasal HDM (inHDM) model, isoflurane-anesthetized mice were sensitized by intranasal instillation of 10 μg (by protein) of HDM extract (Stallergens Greer) in 40 μL of saline on days 1 and 8. For the alum and HDM (alum/HDM) model, mice were administered 100 µL of an emulsification containing 50 µL of alum (Imject Alum, Pierce Biotechnology, Rockford, IL) and 25 µg of HDM extract in 100 µL total volume of saline via intraperitoneal injection on days 1 and 8. For both models, antigen challenges were performed by intranasal inhalation of 10 μg of HDM in 40 µL of saline on days 19, 20, 21, and 22. Mice were analyzed on day 23.
Mouse treatments.
Mice were treated with 2.5 mg/kg of dexamethasone (AgriLabs, St. Joseph, MO) or 100–300 μg/mouse of anti-CD90, anti-IL-17A, or manufacturer-recommended isotype control antibodies (Bio X Cell, West Lebanon, NH) by i.p. injection in saline on days 19 and 21, immediately preceding the intranasal HDM challenge.
Assessment of pulmonary responsiveness to methacholine.
Responsiveness to inhaled methacholine was assessed in closed-chested mice. The mice were anesthetized with intraperitoneal sodium pentobarbital (90 mg/kg), the trachea was cannulated with a blunted 18-g needle, and the mice were connected to a flexiVent computer-controlled small-animal ventilator (SCIREQ, Inc., Montreal, Canada). The mice were ventilated at 200 breaths/min with a 0.25 mL tidal volume and a 3 cmH2O positive end-expiratory pressure (PEEP). Next, the mice were paralyzed with an intraperitoneal injection of pancuronium bromide (0.8 µg/kg). The mice were stabilized over about 10 min of regular ventilation at a PEEP of 3 cmH2O. A standard lung volume history was then established by delivering two total lung capacity maneuvers to a pressure limit of 25 cmH2O and holding for 3 s. Next, two baseline measurements of respiratory input impedance (Zrs) were obtained from 2 s multifrequency oscillations at PEEP = 3 cmH2O. This was followed by an inhalation of aerosolized phosphate-buffered saline (PBS) (control) for 10 s, achieved by an in-line piezo electric nebulizer (Aeroneb, Aerogen, Galway, Ireland). Zrs was then measured every 10 s for 3 min (18 measurements of Zrs in total). This complete sequence of maneuvers and measurements was then repeated for aerosol exposures to three ascending doses of aerosolized methacholine (12.5, 25, and 50 mg/mL). Data were fit to the constant phase model of the lung (7) to provide values reflecting airway resistance (RN), tissue damping (G), and tissue elastance (H). Individual data points were excluded when the coefficient of determination to the constant phase model was below 0.85. All data points for a particular dose of methacholine in an individual mouse were excluded when >50% or more of individual data points (>9 of 18) were excluded or when the second data point after a deep breath was excluded. The mean values (± SE) of RN, G, and H in each of the mouse groups, at each incremental methacholine dose, are reported.
Diffusion factor for carbon monoxide.
Mice were anesthetized with intraperitoneal ketamine (90 mg/kg) and xylazine (15 mg/kg) and tracheotomized to allow measurement of lung diffusing capacity (20) by rapid inflation with 0.8 mL of 0.5% neon/0.5% carbon monoxide (CO) balanced with room air. After 9 s, 0.8 mL of air was quickly withdrawn and injected into a gas chromatograph for analysis (MicroGC Fusion, INFICON, East Syracuse, NY). Three measurements were obtained for each mouse and the mean was reported, except in instances where technical limitations allowed only a single measurement.
Serum and bronchoalveolar lavage fluid collection.
Following pulmonary function assessment, in some studies, serum was collected from anesthetized mice by cardiac puncture into serum separator tubes (BD Biosciences). Blood was allowed to clot at room temperature for 30 min, tubes were centrifuged, and serum was frozen at −80°C until analysis. Mice were lavaged through an 18-gauge tracheal cannula with 1 mL of room temperature saline. Cells were manually counted immediately in white blood cell stain (0.2 mg/mL crystal violet in 2% acetic acid) using a hemocytometer, the lavage fluid was centrifuged at 200 g for 10 min at room temperature, and cell-free supernatants were snap-frozen for analysis. Cell pellets were resuspended in saline and mounted on slides by cytospin (100,000 cells per slide) for hematoxylin-eosin (H&E) staining. Total protein levels from BAL fluid were measured using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA), and lactate dehydrogenase (LDH) activity was measured using the LDH Detection Assay Kit (Promega, Madison, WI). Protease activity in BAL fluid was measured using a microplate assay in which BAL fluid was incubated in the presence of 10 µg/mL of dye/quencher-ovalbumin (D-12053, Molecular Probes, Eugene, OR) at 37°C for 1 h. Fluorescence intensity (excitation = 485 ± 20 nm and emission = 528 ± 20 nm) induced by the protease-dependent liberation of the quencher (Q) from the BODIPY FL fluorescent dye (D) was read every minute on a BioTek Synergy HTX multi-mode plate reader (Winooski, VT).
In vitro antigen restimulation.
Mediastinal lymph node (MLN) cells were dissociated through a 70-μm mesh filter and processed to single-cell suspensions. Cells were counted with a hemocytometer, and 4 × 106 cells/mL were cultured in RPMI-1640 supplemented with 5% FBS (Cell Generation, Fort Collins, CO), 2,500 μg/mL glucose, 2 mM l-glutamine, 10 μg/mL folic acid, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin and then treated with 15 μg/mL HDM extract (Greer). Supernatants were collected after 96 h of incubation at 37°C in 5% CO2.
Cytokine and SAA protein analysis.
Cytokine or serum amyloid A (SAA) content from BAL fluid, serum, or MLN cell culture supernatants was quantified using ELISA kits for mouse TNFα, IL-1β, and IL-4 (BD Biosciences, San Jose, CA), or IL-5, IL-6, IL-13, IL-17A, IFNγ, and serum amyloid A (SAA)1/2 (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. ELISAs were developed using reagents from R&D Systems and read on a BioTek Synergy HTX multi-mode plate reader.
Quantitative RT-PCR.
Total RNA was extracted from frozen whole lungs or livers using the PrepEase RNA Isolation kit (USB, Cleveland, OH) and reversed transcribed to cDNA using the iScript kit from Bio-Rad. Primers were designed for mouse Saa1, Saa2, and Saa3, and RT-PCR was performed using SYBR Green Supermix (Bio-Rad) and normalized to Gapdh using the ΔΔCT method, as previously described (2).
Lung histology and inflammation scoring.
Lungs were inflated and fixed in 10% neutral buffered formalin at a pressure of 25 cmH2O, and 5-μm sections were cut and mounted on slides before H&E staining. Stained tissue was imaged using an EVOS XL microscope (Life Technologies) at ×20. Representative images are presented. For semiquantitative scoring of lung inflammation, three histological sections per animal, spaced 400 μm apart, were stained with H&E. Systematic uniform random sampling with a grid spacing of 1.5 mm was used to select ×20 imaging locations using the NewCast software package (Visiopharm, Hoersholm, Denmark) coupled to a BX-53 microscope (Olympus USA, Waltham, MA). Photomicrographs were coded and analyzed by independent observers using a 4-point scale in which 0 = healthy, normal parenchymal tissue showing no inflammation, no remodeling; 1 = early signs of inflammation, mostly located around blood vessels and airways and minor increases in alveolar space; 2 = increased inflammation, early signs of remodeling including thickened smooth muscle and increases in alveolar space; and 3 = complete inflammatory cell occlusion and no ventilation possible, substantially increased alveolar space.
Lung CD4+ and CD8+ T cell analysis.
Lungs were collected from naive mice or those subjected to the model of mixed-granulocytic severe asthma and processed to single-cell suspensions using the lung dissociation kit (Miltenyi Biotec, Auburn, CA) and a gentleMACS Dissociator (Miltenyi Biotec), according to the manufacturer’s instructions. One round of program m_lung_01.01 was used before the 37°C incubation and one round of program m_lung_02.01 was used before lysing red blood cells with ammonium-chloride-potassium buffer (8,024 mg/L NH4Cl, 1,001 mg/L KHCO3, 7.722 mg/L EDTA·Na2 2H2O). Cells were washed, counted on a hemocytometer, and resuspended at 1.5 × 106 cells/mL in fluorescence-activated cell sorting (FACS) buffer (2% FBS, 0.1% sodium azide in Dulbecco’s phosphate-buffered saline). One milliliter of cells per tube was pelleted by centrifugation and resuspended in 200 μL of PBS containing 1:1,000 Live/Dead-UV Blue (Invitrogen) and incubated at 4°C for 20 min. In total, 300 μL of FACS Buffer was added per tube, the cells were pelleted by centrifugation, resuspended in 100 μL of FACS buffer containing 1:200 Fc block (antiCD16/CD32; BD PharMingen, San Jose, CA), and incubated at 4°C for 10 min. Then, 800 μL of FACS buffer was added per tube, the cells were pelleted by centrifugation, and resuspended in 100 μL of FACS buffer containing cocktails of the following antibodies: antilymphocyte antigen 6 complex locus G-PacBlue (Invitrogen), antiCD3-FITC (BioLegend, San Diego, CA), antiCD4-APC-Cy7 (BD PharMingen), antiCD8-Alexa647 (BioLegend), and antiCD69-PE-Cy7 (BD PharMingen). Cells were incubated for 30 min at 4°C, washed two times in FACS buffer, resuspended in 300 μL of FACS buffer, read on a Becton Dickenson LSR II flow cytometer, and analyzed using FlowJo Software (Tree Star, Ashland, OR). Cells were first gated for single cells and then for CD3+ cells. The CD69 fluorescence intensity of CD8+ or CD4+ cells was assessed from the CD3+ cell population.
Data acquisition, data availability, and statistical analysis.
All experiments involved multiple mice per group and were replicated. The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Data were analyzed by two-tailed unpaired t test or one-way or two-way ANOVA and Bonferroni or Dunnett’s multiple-comparisons post-hoc test using GraphPad Prism 8.3.1 for Windows (GraphPad Software, Inc., La Jolla, CA). A P value smaller than 0.05 in the t test or multiple-comparisons post-hoc test was considered statistically significant. Significance levels of the indicated comparisons are indicated in the figure legends.
Study approval.
Animal experiments were approved by the University of Vermont’s Institutional Animal Care and Use Committee (Protocol No. 12–018 and No. 18–023), in accordance with the recommendations in the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press (revised 1996). Studies involving potentially hazardous materials were approved by the University of Vermont’s Institutional Biosafety Committee (Protocol No. 09–018).
RESULTS
An antigen-specific adaptive immune response is necessary and sufficient for establishment of the CFA/HDM model of mixed-granulocytic airway inflammation.
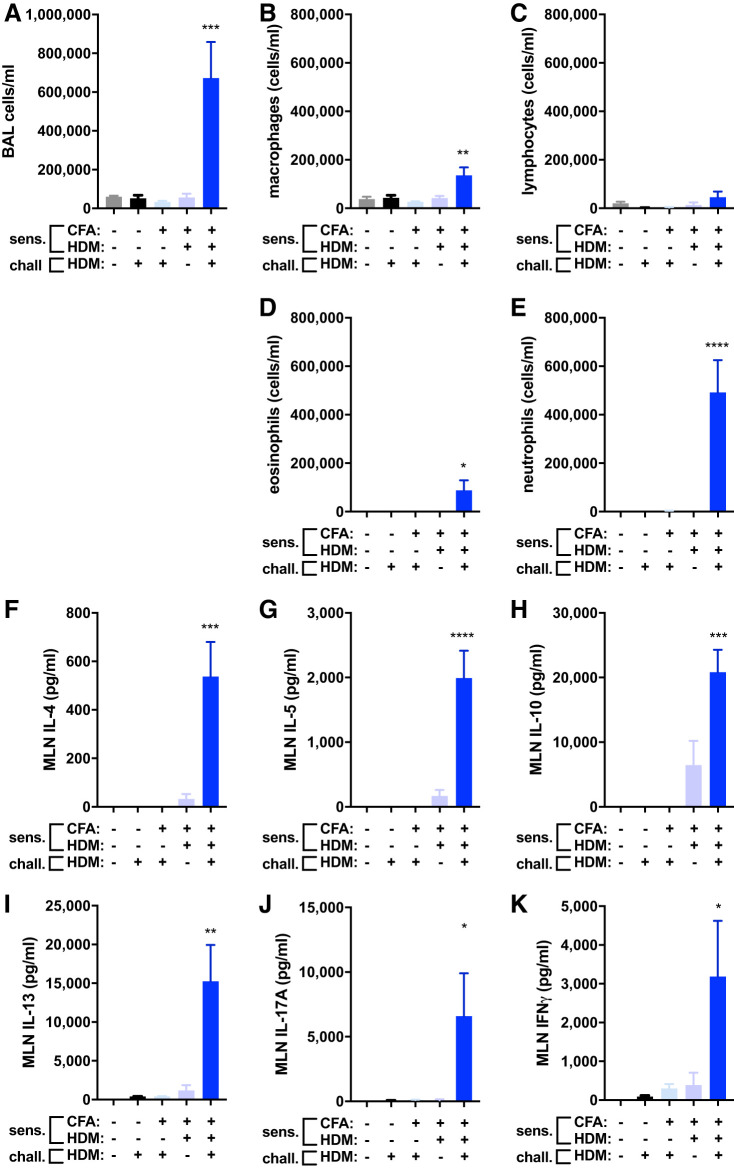
In establishing a mouse model of HDM-provoked severe mixed-granulocytic asthma, C57BL/6J mice were subjected to various perturbations to evaluate the impact of systemic antigen sensitization on day 1, daily inhalational antigen challenge on days 19–22, and subsequent airway inflammation on day 23. Mice were left unexposed (naive), intranasally challenged with HDM, subcutaneously injected with CFA adjuvant alone and intranasally challenged with HDM, subcutaneously injected with a mixture of CFA and HDM antigen extract during sensitization, or subcutaneously injected with a mixture of CFA and HDM antigen extract during sensitization and intranasally challenged with HDM (the CFA/HDM model). Only in mice subjected to sensitization with HDM emulsified in CFA did the intranasal HDM challenge promote airway inflammation (Fig. 1A). Examination of BAL cell types revealed significant elevations in macrophages, eosinophils, and especially neutrophils, with small but insignificant increases in lymphocytes at this time point (Fig. 1, B–E). Interestingly, neutrophils were fivefold more abundant than eosinophils in the lavageable airspaces of the mice subjected to the CFA/HDM model. Mice typically experienced substantial loss of body mass (13.0 ± 2.0%; n = 16) over the course of the intranasal HDM challenges and exhibited lethargy, dehydration, hemorrhagic lung tissue, and erythrocytic BAL fluid. Mediastinal lymph nodes cultured from each group of experimental mice and restimulated in vitro with HDM produced robust quantities of type 2 (Fig. 1, F–I), type 17 (Fig. 1J), and type 1 (Fig. 1K) cytokines. In studies comparing the response of male and female mice to the CFA/HDM model, no sex differences were found. These results demonstrate the requirement for antigen sensitization and challenge to promote mixed-granulocytic airway inflammation dominated by neutrophils and a robust antigen-specific immune response characterized by type 1, type 2, and type 17 cytokines in the CFA/HDM model.
Fig. 1.
Antigen sensitization and challenge are required for establishment of the complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model of mixed-granulocytic airway inflammation. Wild-type mice were naive, intranasally challenged (chall.) with HDM for 4 days, administered CFA and saline during sensitization (sens.) and then challenged with HDM, administered CFA and HDM during sensitization and then challenged with HDM, or administered CFA and HDM during sensitization and then challenged with HDM. Total cells (A), macrophages (B), lymphocytes (C), eosinophils (D), and neutrophils (E) were measured from BAL. IL-4 (F), IL-5 (G), IL-10 (H), IL-13 (I), IL-17A (J), and IFNγ (K) were measured from HDM-restimulated mediastinal lymph node cell cultures. n = 5 mice/group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 compared with naive.
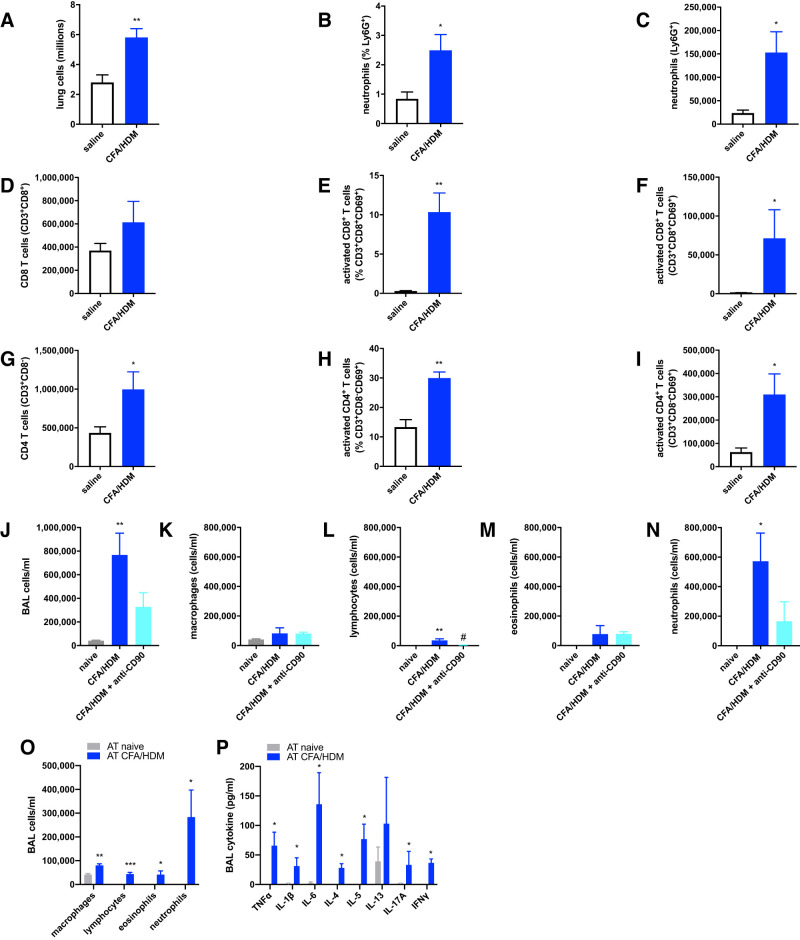
In addition to the airway neutrophils present in BAL fluid, the lung tissues of CFA/HDM mice also contained significantly elevated relative percentages and overall numbers of neutrophils relative to mice that were only administered intranasal saline during the challenge phase (Fig. 2, A–C). Furthermore, the lung tissues of the CFA/HDM mice also contained elevated numbers of activated CD8+ (Fig. 2, D–F) and CD4+ (Fig. 2G–I) T cells, implicating their possible involvement in the phenotype of mice subjected to the CFA/HDM model. Both CD4+ and CD8+ T cells have been reported to exert important functions in HDM-driven models of allergic asthma (48). To evaluate the importance of lymphocytes in the CFA/HDM model, mice were left naive or subjected to the CFA/HDM model without or with administration of a lymphocyte-depleting anti-CD90.2 antibody (30) immediately preceding and during the HDM challenge phase. CFA/HDM mice that were administered the anti-CD90 antibody displayed significantly reduced numbers of total cells, lymphocytes, and neutrophils in the BAL following the HDM challenge relative to the untreated CFA/HDM mice (Fig. 2, J–N). To evaluate the contribution of CD4+ T cells in this model of mixed-granulocytic airway inflammation, CD4+ T cells were enriched from naive mice or those subjected to the CFA/HDM model and adoptively transferred to naive recipients that were then intranasally challenged with HDM. Only in the mice administered CD4+ T cells from CFA/HDM mice did the HDM challenge promote neutrophil-dominated airway inflammation (Fig. 2O) and the release into the lavageable airspaces of proinflammatory, type 2, type 17, and type 1 cytokines (Fig. 2P). These results demonstrate the necessity and sufficiency of CD4+ T cells and the accompanying adaptive immune response to induce the CFA/HDM model of mixed-granulocytic airway inflammation.
Fig. 2.
T-lymphocytes are activated, necessary, and sufficient to confer mixed-granulocytic airway inflammation in the complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model. Wild-type mice were administered saline during the challenge or subjected to the CFA/HDM sensitization and challenge model. Lung total cells (A), neutrophils (B and C), total and activated CD8+ T cells (D–F), and total and activated CD4+ T cells (G–I) were enumerated. n = 4 mice/group. *P ≤ 0.05 and **P ≤ 0.01 compared with saline. Wild-type mice were naive or subjected to the CFA/HDM model without or with administration of a lymphocyte-depleting anti-CD90 antibody during the challenge phase. Total cells (J), macrophages (K), lymphocytes (L), eosinophils (M), and neutrophils (N) were measured from BAL. n = 4 mice/group. *P ≤ 0.05 and **P ≤ 0.01 compared with naive; #P ≤ 0.05 compared with CFA/HDM. CD4+ T cells enriched from naive mice or mice subjected to the CFA/HDM model were administered by adoptive transfer (AT) to naive recipients that were subsequently challenged with intranasal HDM. BAL cells (O) and cytokines (P) were measured after the HDM challenge. n = 4 mice/group. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with AT naive.
The CFA/HDM model of mixed-granulocytic inflammation induces weight loss and an acute-phase response.
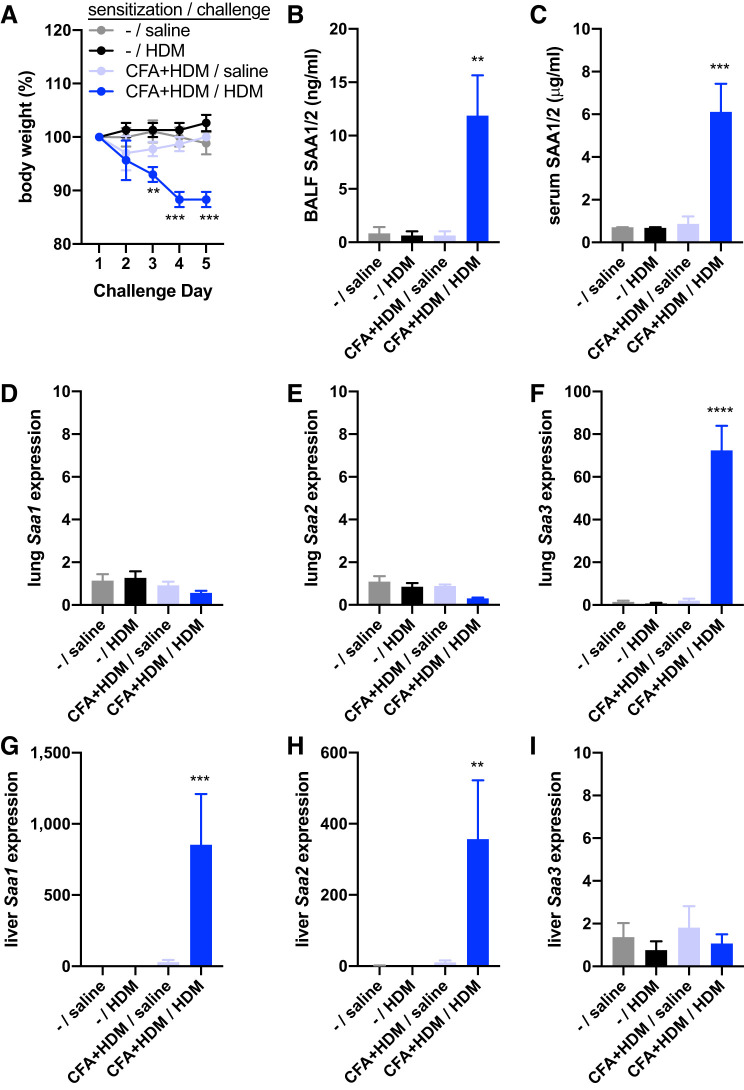
Having noted that mice exposed to the full CFA/HDM model were less active on the day of harvest than other groups of mice, we assessed body weight preceding and over the course of the challenge. Whereas mice that were subjected only to saline instillation during the challenge, the HDM challenge, or the CFA+HDM sensitization and saline challenge maintained body weight over the course of the challenges, mice subjected to the CFA+HDM sensitization and HDM challenge lost significant body weight by the third day of the challenge and continued to exhibit body weight loss throughout the remaining 2 days of the study (Fig. 3A). As acute-phase responses are associated with the overall sickness response and are sufficient to promote constitutional changes leading to weight loss (43), we measured concentrations of the major murine acute-phase protein, serum amyloid A (SAA1/2). Owing to their high degree of sequence similarity, SAA1 and SAA2 are indistinguishable by ELISA (and the ELISA does not cross-react with SAA3). Only in mice subjected to the full CFA/HDM model was SAA1/2 significantly elevated in both the BALF (Fig. 3B) and serum (Fig. 3C). We and others have recognized SAA protein family members as important biomarkers and mediators of pulmonary antigen-specific immune responses and of severe asthma (2–4, 33, 55, 65). Furthermore, SAA1 has recently been identified to interact with HDM allergens to facilitate antigen-specific immune responses (60). Analysis of Saa family member gene expression revealed that only Saa3, the major SAA family member inducibly expressed in peripheral organs of mice, and not Saa1 or Saa2, was significantly elevated in the lungs of mice exposed to the full CFA/HDM model (Fig. 3, D–F). In contrast, Saa1 and Saa2, but not Saa3, were significantly elevated in the liver of mice exposed to the full CFA/HDM model (Fig. 3, G–I). These data support a local inflammatory environment in the lung, as reflected by highly elevated expression of Saa3, and a systemic acute-phase response from the liver, as reflected by highly elevated Saa1 and Saa2 expression in the liver, as well as elevated SAA1/2 serum and BALF concentrations, with the latter likely coming from leakage of plasma from the circulation into the lavageable airspaces, in the CFA/HDM mice. These data support the severity of this model of mixed-granulocytic airway inflammation.
Fig. 3.
The complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model of mixed-granulocytic inflammation induces body weight loss and a systemic acute-phase response. Wild-type mice were unsensitized (–) or sensitized with CFA + HDM. During the challenge phase, mice were intranasally exposed to saline or HDM. Body weight (A) was recorded the day before (day 0), during the challenge (days 1–4), and the day of harvest (day 5). Serum amyloid A 1/2 (SAA1/2) was measured from BALF (B) and serum (C). Expressions of Saa1, Saa2, and Saa3 were measured from lung (D–F) and liver (G–I). n = 4 mice/group. **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 compared with all other groups. Liver Saa1 and Saa2 gene expression values were log10 transformed for statistical analysis.
The CFA/HDM model of mixed-granulocytic inflammation induces methacholine hyperresponsiveness, diffusion impairment, and lung damage.
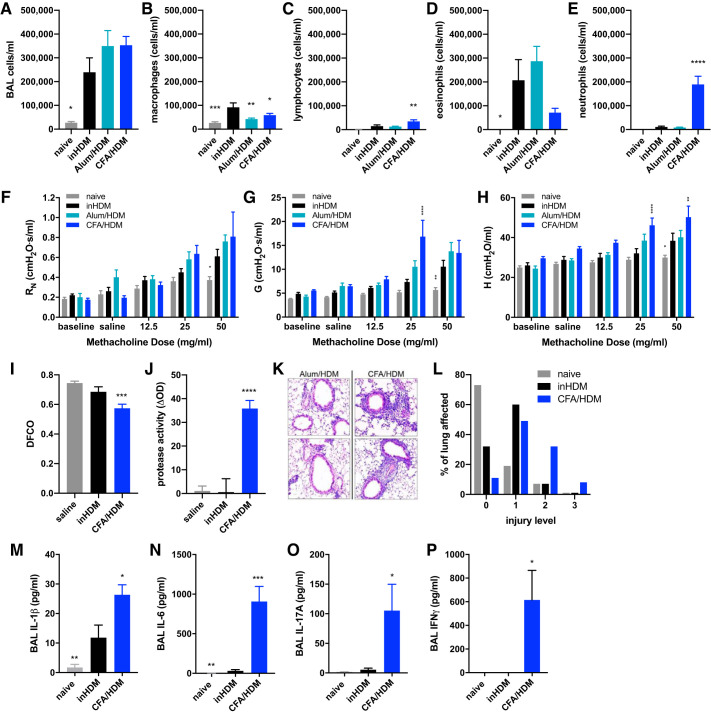
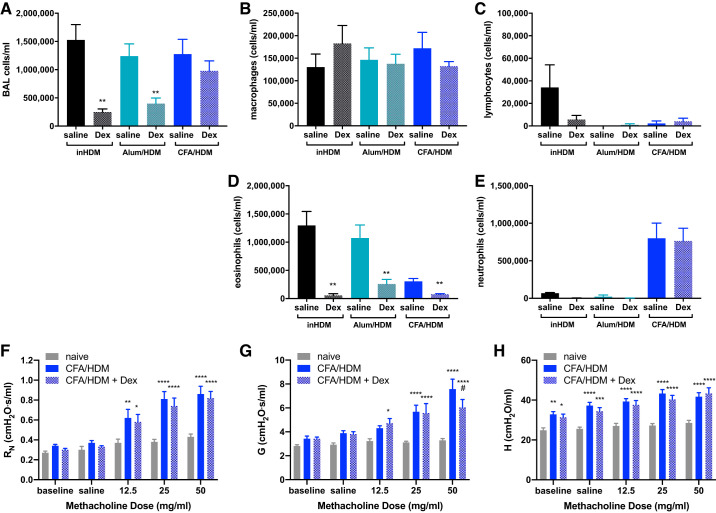
The CFA/HDM model of mixed-granulocytic airway inflammation was next compared with models of eosinophilic airway inflammation induced by HDM sensitization via intranasal exposure (inHDM) or via intraperitoneal injection of HDM in alum adjuvant (alum/HDM). Compared with mice subjected to the inHDM model, the alum/HDM and CFA/HDM models generated substantial airway inflammation that was of similar overall magnitude and differed only from the naive mice (Fig. 4A). Macrophages were lower in the naive, alum/HDM, and CFA/HDM mice compared with the inHDM mice (Fig. 4B), whereas the CFA/HDM model displayed elevated lymphocytes relative to the inHDM model (Fig. 4C). The inHDM and alum/HDM mice displayed eosinophil-predominant airway inflammation (Fig. 4D), whereas the CFA/HDM mice displayed a neutrophil-predominant mixed-granulocytic airway inflammation (Fig. 4E). These same mice were also subjected to assessment of methacholine responsiveness, which contributed to reduced cell recovery in the BAL fluid relative to the data presented in Fig. 1. Comparing with the inHDM model, the alum/HDM model displayed equivalent methacholine hyperresponsiveness, whereas the CFA/HDM model displayed significant increases in airway resistance (Fig. 4F), tissue damping (Fig. 4G), and tissue elastance (Fig. 4H). These results indicate that the CFA/HDM model promotes an asthma-like effect on respiratory physiology that is greater in magnitude than in the eosinophilic HDM models. Furthermore, CFA/HDM mice displayed substantial impairment of diffusion capacity relative to naive or inHDM mice (Fig. 4I), indicative of alveolar damage, which was accompanied by significantly elevated protease activity in the lavageable airspaces (Fig. 4J). The CFA/HDM model induced substantial airspace inflammation and alveolar injury, including enlarged airspaces, relative to the inHDM model of eosinophilic asthma (Fig. 4, K and L). Neutrophilic airway inflammation has been associated with severe asthma (53), protease-mediated alveolar damage in chronic obstructive pulmonary disease (COPD) (44), and asthma-COPD overlap (ACO) (5). Neutrophils can be released from the bone marrow, recruited to a site of tissue injury, and supported in their activities through a number of proinflammatory, type 1, and type 17 cytokines (32). Relative to naive mice or those subjected to the inHDM model of eosinophilic asthma, mice subjected to the CFA/HDM model released elevated levels of IL-1β, IL-6, IL-17A, and IFNγ into the lavageable airspaces (Fig. 4, A–D). These results underscore the severity of the CFA/HDM model of asthma.
Fig. 4.
The complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model of mixed-granulocytic inflammation induces methacholine hyperresponsiveness, diffusion impairment, and lung damage. Wild-type mice were naive, subjected to an intranasal HDM (inHDM) asthma model, a HDM in Alum (Alum/HDM) asthma model, or the CFA/HDM model. Total cells (A), macrophages (B), lymphocytes (C), eosinophils (D), and neutrophils (E) were measured from BAL. Airway resistance (RN; F), tissue damping (G; G), and tissue elastance H; H) were measured. n = 9 or 12 mice/group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 compared with inHDM. Wild-type mice were administered saline during the challenge, subjected to an intranasal HDM (inHDM) asthma model, or subjected to the CFA/HDM model. Diffusion capacity (I), BALF protease activity (J), and lung histology (K and L) were measured. n = 8 or 12 mice/group. ***P ≤ 0.001, and ****P ≤ 0.0001 compared with inHDM. Wild-type mice were naive, subjected to the intranasal HDM (inHDM) model of eosinophilic asthma, or subjected to the CFA/HDM model of mixed-granulocytic asthma. IL-1β (M), IL-6 (N), IL-17A (O), and IFNγ (P) were measured from BAL. n = 4 or 8 mice/group. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with inHDM.
The CFA/HDM model of mixed-granulocytic inflammation induces corticosteroid-resistant airway inflammation and methacholine hyperresponsiveness.
An important indicator of clinical asthma severity is unresponsiveness to corticosteroid treatment. To assess corticosteroid responsiveness of several asthma models, mice were subjected to the eosinophilic inHDM or alum/HDM models or to the mixed-granulocytic CFA/HDM model. Some mice in each of the models were administered 2.5 mg/kg dexamethasone systemically immediately preceding and during the HDM challenge phase (40). The inHDM and alum/HDM groups responded robustly to the dexamethasone, displaying substantially reduced total BAL cell numbers (Fig. 5A), with especially reduced numbers of eosinophils (Fig. 5, B–E). In stark contrast, total BAL cell numbers were unaffected by dexamethasone treatment in the CFA/HDM group (Fig. 5A), and although the small numbers of eosinophils were reduced, the substantial numbers of neutrophils were not reduced by dexamethasone (Fig. 5, B–E). Macrophage and lymphocyte numbers were not significantly affected by dexamethasone in any of the groups (Fig. 5, B–E). A separate cohort of mice was left naive or subjected to the CFA/HDM model without or with dexamethasone treatment. In these mice, the methacholine hyperresponsiveness induced by the CFA/HDM model was largely unaffected by dexamethasone treatment (Fig. 5, F–H), with only tissue damping being modestly decreased at the highest dose of methacholine, but still significantly and substantially elevated relative to naive mice. Together, these results show that the CFA/HDM model is one of severe asthma, with neutrophilic airway inflammation, methacholine hyperresponsiveness, diffusion impairment, lung injury, and corticosteroid unresponsiveness, making the model attractive for assessing targetable mediators of disease.
Fig. 5.
The complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model of mixed-granulocytic inflammation induces corticosteroid-resistant airway inflammation and methacholine hyperresponsiveness. Wild-type mice were subjected to the intranasal HDM (inHDM) or alum and HDM (alum/HDM) models of eosinophilic asthma or to the CFA/HDM model of mixed-granulocytic asthma and were administered saline or 2.5 mg/kg dexamethasone (Dex) during the HDM challenge. Total cells (A), macrophages (B), lymphocytes (C), eosinophils (D), and neutrophils (E) were measured from BAL. n = 4 mice/group. **P ≤ 0.01 compared with saline administration in the same model. Airway resistance (RN; F), tissue damping (G; G), and tissue elastance H; H) were measured. N = 11 or 12 mice/group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 compared with naive; #P ≤ 0.05 compared with CFA/HDM.
IL-6 deficiency promotes eosinophilic airway inflammation without reducing methacholine hyperresponsiveness or conferring corticosteroid sensitivity in the CFA/HDM model of severe asthma.
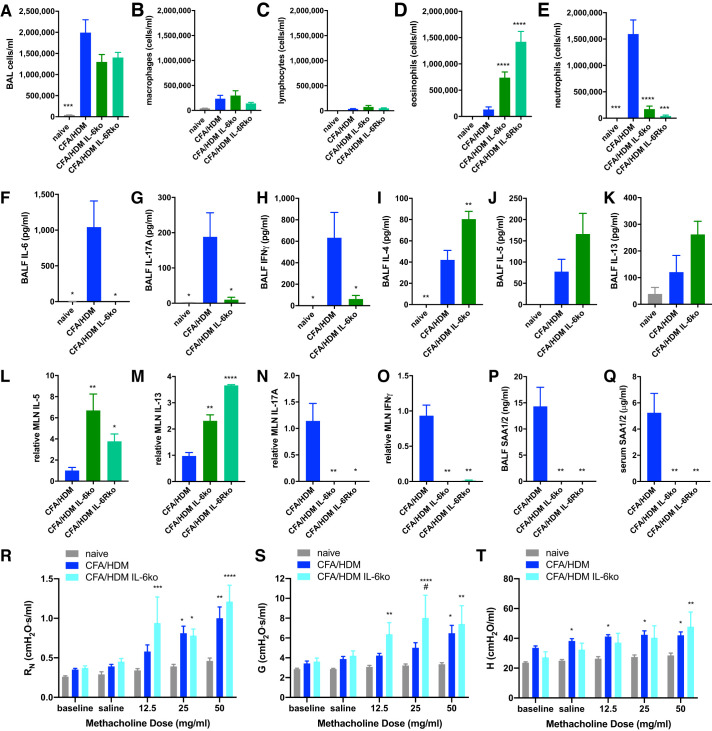
Whereas therapeutic interventions for patients with severe asthma has recently moved toward biologicals, mouse models of severe asthma afford the opportunity to examine the effects of candidate mediators through the use of knockout mice. IL-6 has been implicated as a mediator in a particular endotype of severe asthma (referred to as IL-6high asthma) in which this cytokine is elevated in the circulation (above 3.1 pg/mL) for adults (25, 31, 45, 67) and children (26). Consequently, IL-6- or IL-6R-targeted therapies have been proposed for IL-6high severe asthmatics (45). Whereas IL-6 was not detected in the serum of naive mice, IL-6 levels in the serum of the CFA/HDM mice was 53.23 ± 7.97 pg/mL (n = 4 mice/group). IL-6 is capable of stimulating cells expressing surface IL-6R through the process of “IL-6 classical signaling” (58) and signal to cells that lack IL-6R through IL-6 binding to secreted or shed soluble IL-6R, which then associates with cellular gp130 to induce similar downstream events in the process of “IL-6 trans-signaling” (28). IL-6 trans-signaling has been implicated to cause altered patterns of airway epithelial gene expression compared with the effects of classical signaling and is associated with more severe asthma (27). Although IL-6R is normally present in the serum, its level increased significantly in mice subjected to the CFA/HDM model (11.41 ± 0.44 vs. 17.81 ± 0.97 ng/mL; P ≤ 0.0001; n = 7/group). IL-6R level in BAL fluid from naive mice was 277.07 ± 42.55 pg/mL, whereas the level in BAL fluid from CFA/HDM mice was significantly elevated to 1387.61 ± 262.40 pg/mL (P ≤ 0.001; n = 7/group).
Because of the strong implications for IL-6 as a mediator of a specific endotype of severe asthma and the support for clinical trials to evaluate the efficacy of its neutralization in patients (16, 45), the impact of IL-6- or IL-6R-deficiency in the model was examined. Some wild-type mice were left naive, whereas other wild-type, IL-6 knockout, and IL-6R knockout mice were subjected to the CFA/HDM model. The overall magnitude of the airway inflammatory response evoked by the CFA/HDM model, or the numbers of macrophages or lymphocytes, was unaffected in either strain of knockout mice (Fig. 6, A–C). However, both the IL-6 knockout and IL-6R knockout mice displayed a switch to a predominantly eosinophilic airway inflammation in the CFA/HDM model, in contrast to the neutrophil-predominant response in the wild-type mice (Fig. 6, D and E). An absence of IL-6 was confirmed in the lavageable airspaces of the IL-6 knockout mice, accompanied by decreased IL-17A and IFNγ (Fig. 6, G and H), and an increased type 2 cytokine profile (Fig. 6, I–K). This was surprising considering the well-recognized function of IL-6 in promoting type 2 responses (18). However, studies investigating the contribution of IL-6 to adaptive immune responses have also reported the cytokine to be critical for shaping the type 1 and type 17 responses while inhibiting type 2 responses (24, 38). Consistent with these publications, HDM-restimulated mediastinal lymph node cells (in which antigen-specific CD4+ T cells are abundant) from IL-6 knockout and IL-6R knockout mice produced increased amounts of IL-5 and IL-13, as well as nearly undetectable levels of IL-17A and IFNγ, relative to wild-type mice (Fig. 6, L–O). In support of the critical function of IL-6 in driving acute-phase responses (59), SAA1/2 was undetectable in either the BALF (Fig. 6P) or serum (Fig. 6Q) of IL-6 knockout or IL-6R knockout mice subjected to the CFA/HDM model. Furthermore, the methacholine hyperresponsiveness of the CFA/HDM model was not abrogated in IL-6 knockout mice, and in fact, in some instances, the knockout mice were more responsive than the wild-type mice (Fig. 6, R–T).
Fig. 6.
IL-6 deficiency promotes eosinophilic airway inflammation but does not reduce methacholine hyperresponsiveness in the complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model of severe asthma. Wild-type mice were naive or subjected to the CFA/HDM model of severe asthma. IL-6- or IL-6 receptor (IL-6R)-deficient mice were subjected to the CFA/HDM model of severe asthma. Total cells (A), macrophages (B), lymphocytes (C), eosinophils (D), and neutrophils (E) were measured from bronchoalveolar lavage (BAL). n = 4 or 11 mice/group (A–E). IL-6 (F), IL-17A (G), IFNγ (H), IL-4 (I), IL-5 (J), and IL-13 (K) were measured from BAL. n = 4 mice/group (F–K). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with CFA/HDM (A–K). IL-5 (L), IL-13 (M), IL-17A (N), and IFNγ (O) were measured from HDM-restimulated mediastinal lymph node (MLN) cell culture supernatants. Serum amyloid A 1/2 was measured from BAL (P) and serum (Q). n = 4 mice/group (L–Q). *P ≤ 0.05, **P ≤ 0.01, and ****P ≤ 0.0001 compared with CFA/HDM. Airway resistance (RN; R), tissue damping (G; S), and tissue elastance H; T) were measured. n = 8 or 10 mice/group (R–T). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 compared with naive. #P ≤ 0.05 compared with CFA/HDM.
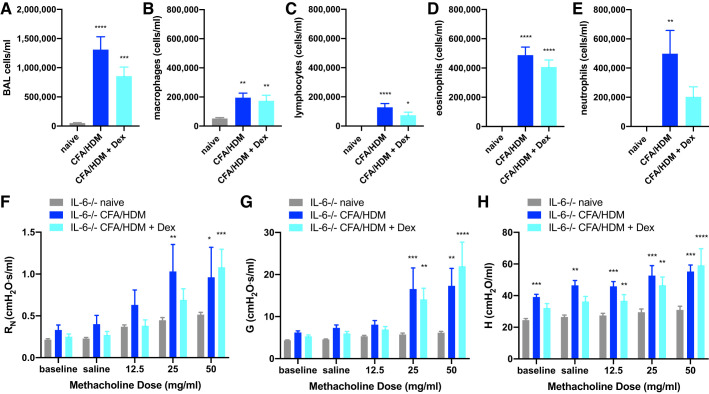
Genetic deficiency in IL-6 signaling conferred through the use of IL-6 knockout or IL-6R knockout mice models the extreme of what neutralization of IL-6 signaling could achieve therapeutically. Furthermore, the switch to eosinophilic airway inflammation and a type 2-predominant response in the IL-6 knockout and IL-6R knockout mice suggest the possibility of corticosteroid sensitivity. Consequently, the impact of superimposing corticosteroid treatment atop IL-6 deficiency in the CFA/HDM model was examined. IL-6 knockout mice were left naive or were systemically administered 2.5 mg/kg dexamethasone immediately preceding and during the HDM challenge phase. Overall cell numbers remained substantially elevated in the IL-6 knockout mice treated with dexamethasone relative to naive mice (Fig. 7A). Although the abundance of macrophages and eosinophils was the same, there were small but insignificant reductions in the number of lymphocytes and neutrophils, the latter being not significantly different from naive mice, in the dexamethasone-treated IL-6 knockout mice (Fig. 7, B–E). These results suggest a potential interaction between IL-6 and corticosteroid sensitivity. When the IL-6 knockout mice were subjected to the methacholine challenge, there was only slight protection against hyperresponsiveness in the IL-6 knockout mice. Specifically, lower doses of methacholine provoked greater airway resistance in the untreated mice than in the dexamethasone-treated mice (Fig. 7F). Whereas there were no differences in tissue damping in the untreated or dexamethasone-treated IL-6 knockout mice (Fig. 7G), there were reductions in tissue elastance at baseline and in response to saline in the dexamethasone-treated mice (Fig. 7H).
Fig. 7.
IL-6 deficiency confers modest corticosteroid sensitivity in the complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model of severe asthma. IL-6-deficient mice were naive or subjected to the CFA/HDM model of severe asthma without or with the administration of 2.5 mg/kg dexamethasone (Dex) during the HDM challenge. Total cells (A), macrophages (B), lymphocytes (C), eosinophils (D), and neutrophils (E) were measured from BAL. Airway resistance (RN; F), tissue damping (G; G), and tissue elastance H; H) were measured. n = 9 or 12 mice/group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 compared with naive. There were no differences between CFA/HDM and CFA/HDM + Dex.
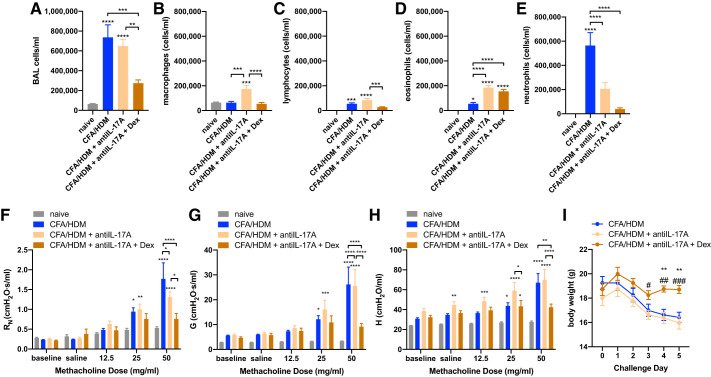
Combined IL-17A neutralization and corticosteroid administration decreases airway inflammation, methacholine hyperresponsiveness, and weight loss in the CFA/HDM model of severe asthma.
Th17 responses, and IL-17A itself, have been implicated as important contributors to severe, neutrophilic asthma (11, 33) and are regulated by IL-6 (38). Although IL-17A is not detectable in serum of naive mice, CFA/HDM mice had low but significantly elevated serum IL-17A levels (7.70 ± 2.64 pg/mL; n = 4 or 5/group). As the CFA/HDM model promotes a type-17 adaptive immune response that is substantially inhibited by the constitutive absence of IL-6 or IL-6R during both allergen sensitization and challenge, we investigated the impact of IL-17A neutralization during the HDM challenge in the CFA/HDM model. Wild-type mice were left naive or subjected to the CFA/HDM model. CFA/HDM mice were left untreated or administered IL-17A-neutralizing antibody without or with 2.5 mg/kg dexamethasone immediately preceding and during the HDM challenge phase. The overall magnitude of the airway inflammatory response evoked by the CFA/HDM model was significantly decreased only in the mice that were administered combined anti-IL-17A and dexamethasone (Fig. 8A). Anti-IL-17A alone increased the number of macrophages. Both anti-IL-17A and IL-17A plus dexamethasone increased the number of eosinophils. As anticipated, neutrophil numbers in the BAL fluid were substantially elevated in the CFA/HDM model and were significantly reduced in both the anti-IL-17A and the anti-IL-17A plus dexamethasone groups, to the greatest degree in the latter (Fig. 8, B–E). When anti-IL-17A plus dexamethasone mice were subjected to the methacholine challenge, there was significant protection against hyperresponsiveness observed in central airway resistance (Fig. 8F), tissue damping (Fig. 8G), and tissue elastance (Fig. 8H), effects that were not observed in mice administered anti-IL-17A alone. Finally, there was substantial protection against the weight loss caused by the CFA/HDM model only when mice were administered a combination of antiIL-17A and dexamethasone. (Fig. 8I). These results suggest that aggressive targeting of IL-17A in combination with corticosteroid treatment could provide pulmonary and systemic therapeutic benefit in the setting of severe mixed-granulocytic asthma.
Fig. 8.
Combined IL-17A neutralization and corticosteroid administration decreases inflammation and methacholine hyperresponsiveness in the complete Freund’s adjuvant and house dust mite antigen (CFA/HDM) model of severe asthma. Wild-type mice were naive or subjected to the CFA/HDM model without or with the administration of IL-17A-neutralizing antibody without or with 2.5 mg/kg dexamethasone (Dex) during the HDM challenge. Total cells (A), macrophages (B), lymphocytes (C), eosinophils (D), and neutrophils (E) were measured from BAL. Airway resistance (RN; F), tissue damping (G; G), and tissue elastance H; H) were measured. Body weight was measured on the day preceding the HDM challenge (day 0), throughout the HDM challenge period (days 1–4), and immediately preceding assessment of lung function and BAL on day 5 (I). n= 8 mice/group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 compared with naive or to group indicated by brackets (A–H). **P ≤ 0.01 compared with CFA/HDM; #P ≤ 0.05, ##P ≤ 0.01, and ###P ≤ 0.001 compared with CFA/HDM + antiIL-17A (I).
DISCUSSION
Mouse models of asthma are designed to embody relevant processes affecting asthma patients. This can be accomplished through the use of mouse models that reflect the natural history of asthma development, which are focused on recapitulating the route, dose, and agent of sensitization. Mouse models may also be used to examine the therapeutic potential of particular interventions to determine which are useful and which are not. At the intersection of these two extremes is the use of mouse asthma models to better understand biomarkers, mediators, and potential endotypes of disease (37). The CFA/HDM model of severe mixed-granulocytic asthma developed and explored herein does not attempt to offer a model for understanding the natural history of severe asthma. Certainly, the relevance of subcutaneous injections of house dust mite antigens with CFA containing M. tuberculosis lysate to the natural history of asthma is slight. Nevertheless, the model may afford insight into biomarkers, mediators, and targets for therapeutic intervention in individuals with an asthma endotype reminiscent of that present in this model. Specifically, elevations in innate immune cytokines such as IL-1β and IL-6, mixed-granulocytic neutrophil-predominant airway inflammation, and Th1/Th17 responses are present in the CFA/HDM model. This is in contrast to Th2high presentations of asthma in which Th2 cytokines, IgE, and eosinophils are important and targetable disease mediators.
We are not the first to have incorporated CFA-promoted antigen sensitization into mouse asthma models. However, others who have done so reported a lower magnitude of neutrophil infiltration into lavageable airspaces following an antigen challenge than we found (13, 14). Our CFA formulation used an additional amount of M. tuberculosis lysate and was thoroughly sonicated to produce a thick emulsion, as is frequently performed to model autoimmunity, such as in experimental allergic/autoimmune encephalomyelitis, a mouse model of multiple sclerosis in which Th17 responses and IL-17A are also important. Our preliminary studies revealed the importance of these two elements, as insufficient sonication or omission of the additional M. tuberculosis lysate produced eosinophil-predominant BAL cellularity upon the HDM challenge, in contrast to the neutrophil-predominant mixed-granulocytic response upon execution of the full CFA/HDM model.
The CFA/HDM model of antigen-driven severe mixed-granulocytic asthma addresses an unmet need for novel models and therapeutics to address this specific asthma endotype (61). Whereas a neutrophilic severe asthma endotype seems to be responsive to antibiotics, implicating a bacterial cause of disease, the mixed-granulocytic asthma endotype appears to involve distinct mediators. The CFA/HDM model exhibits a requirement for HDM allergen during both the sensitization and challenge phases. As the HDM extract is produced from whole house dust mites, the contribution of antigens derived from their bacterial microbiome and their potential cross-reactivity to those in the bacterial lysates contained in CFA were considered. However, administration of CFA containing no HDM during the sensitization phase, followed by HDM exposure during the challenge phase, did not promote airway inflammation or antigen-driven cytokine production from mediastinal lymph node cells, in marked contrast to mice administered HDM in CFA during sensitization and subsequently challenged with HDM. Furthermore, the requirement of CD90+ lymphocytes for substantial neutrophilic airway inflammation, as well as the sufficiency of CD4+ T cells to confer neutrophilic airway inflammation, proinflammatory cytokine production, and a mixed type-1/2/17 response after HDM challenge, indicates the necessity and sufficiency of the T cell response in the CFA/HDM model to confer pathological features of disease.
Evidence that the CFA/HDM model is one of severe asthma comes from the overall magnitude of the methacholine hyperresponsiveness elicited, the mixed-granulocytic airway inflammation, acute-phase response, weight loss, the model’s insensitivity to systemic corticosteroids, and the lung damage, enlarged airspaces, and protease activity present contributing to impaired diffusion capacity. As recently reported (63), structural changes in alveoli, such as alveolar size increases as are present in the CFA/HDM model, are likely to be the basis for the increase in compliance and loss of elastic recoil seen in some asthmatics. The aforementioned characteristics of the CFA/HDM model are reminiscent of findings in asthma-COPD overlap (ACO), a contemporary name for the concept that a subset of patients share several aspects of the natural history, pathophysiological presentation, and responsiveness to therapeutics, but are not clearly defined by either disease alone (69). Lacking a formal definition, ACO is best identified in patients with an asthma or atopic history, with smoking exposure, but incompletely reversible airflow limitation and reduced diffusing capacity. ACO is oftentimes exacerbated by exposure to specific antigens and displays mixed inflammatory infiltrates in the lung (neutrophils and eosinophils), unresponsiveness to corticosteroids, and Th1/Th17-type cytokines (5). The mediators dictating disease presentation in ACO remain poorly understood, although elevated serum levels of IL-6 have been described (62). Despite the absence of cigarette smoke exposure, the CFA/HDM model could be relevant to understanding the pathogenesis of ACO. Although there are no formal guidelines regarding treatment of ACO, current practice includes inhaled corticosteroids as primary treatment in this patient population (9). However, our model would suggest that this may be an ineffective tactic based on the pathophysiology described.
Especially elevated in the CFA/HDM model relative to the eosinophilic intranasal HDM model of allergic asthma were IL-1β, IL-6, IL-17A, and IFNγ. IL-1β contributes to augmented glycolysis in the setting of allergic asthma (47) and to Th17 responses (35, 36). IL-6 has been implicated in severe allergic asthma in humans (16, 45), serving as an inflammatory cytokine; promoter of Th1 (24), Th2 (18), and Th17 (8) responses; and inducer of neutrophil growth, recruitment, and survival (70). Neutrophils and the cytoplasts of dead neutrophils have been reported to promote Th17 differentiation and neutrophilic inflammation in severe asthma (28). Both the classic and trans-signaling pathways have emerged as rational targets for novel therapies in specific presentations of asthma with elevated IL-6 (21, 27, 42, 56, 57, 64). However, IL-6 overexpression in the lung causes decreased asthma-like pathologies in antigen-sensitized and antigen-challenged mice, whereas IL-6 deficiency promotes augmented eosinophilic inflammation, Th2 responses, and AHR (66), implicating a more important contribution to non-Th2high asthma endotypes. IL-17A affects lung epithelial cells to induce granulocyte colony-stimulating factor, which promotes neutrophilpoiesis, and CXC chemokines that induce neutrophil recruitment to the lung and airspaces (1). IFNγ has been generally associated with Th1 responses that can be induced by immunomodulatory agents to suppress pathogenic Th2 responses (52). However, both Th1 responses (19) and IFNγ itself have been implicated in mouse models and human studies to cause severe asthma-like pathological changes independent of a Th2 response (49).
Using IL-6 or IL-6R knockout mice, which lack expression of these proteins during both the sensitization and challenge phases, a robust eosinophilic airway inflammation was manifested in the CFA/HDM model. This was associated with decreased IL-17A and IFNγ production and increased IL-5 and IL-13 production from HDM-restimulated mediastinal lymph node cells. These results suggest that in the absence of IL-6, the sensitization with CFA and HDM induces a strong Th2 response and a suppressed Th1/17 response, similar to what has been previously reported in mice immunized with antigen in incomplete Freund’s adjuvant, which does not induce IL-6 production (23). Perhaps not surprisingly, as eosinophilic airway inflammation is itself closely associated with allergic asthma, the switch to eosinophil-predominant airway inflammation in IL-6 knockout mice subjected to the CFA/HDM model did not decrease methacholine hyperresponsiveness. However, it is surprising that even in the presence of systemic corticosteroid treatment, IL-6 deficiency did not substantially decrease airway hyperresponsiveness. Perhaps IL-6- or IL-6R-deficient mice subjected to the CFA/HDM model could represent a preclinical model of Th2high severe asthma that could be of future utility. It seems likely that the important functions of IL-6 in the CFA/HDM model take place during antigen-specific T cell polarization upon initial sensitization, and although IL-6 is elevated during the challenge phase, its contribution to disease at that time may be limited. This calls to question the impact of IL-6 targeting in severe asthma, as the timing may be too late to affect the pathogenic Th17 response.
As IL-17A production from Th17 cells is downstream of IL-6 signaling, we went on to examine the impact of IL-17A neutralization in the CFA/HDM model. Anti-IL-17A administered during the allergen challenge significantly decreased neutrophil numbers in the lavageable airspaces, implicating an important function for IL-17A in the modulation of neutrophils in the CFA/HDM model. However, overall inflammatory cell numbers remained as high as in untreated mice subjected to the CFA/HDM model, with macrophages and eosinophils constituting the substantially elevated cell populations. CFA/HDM mice administered that were anti-IL-17A displayed no significant decreases in methacholine hyperresponsiveness in the parameters of tissue damping or tissue elastance and only a small but significant attenuation of airway resistance at the highest dose of methacholine, relative to untreated CFA/HDM mice. However, combined treatment with anti-IL-17A and systemic corticosteroid significantly decreased overall BAL cellularity and especially neutrophils in the CFA/HDM model, as well as methacholine hyperresponsiveness, particularly airway resistance, and also tissue damping and tissue elastance at the highest doses of methacholine. In addition to the beneficial effects in the lungs, combined treatment with anti-IL-17A and corticosteroid promoted systemic constitutional benefit to the mice subjected to the CFA/HDM regimen, as evidenced by maintenance of body weight throughout the protocol. Subjective assessment of mouse fur appearance and recoil, as well as blood viscosity, suggested that the mice were better hydrated and perhaps nourished when they were administered the combined therapy. The combination of an add-on biological therapy and corticosteroid administration is commonplace in the treatment of Th2high asthma (71) and would likely be used in novel non-Th2 severe asthma therapies as well.
Although possibly only one of the several approaches that may be useful, perhaps the combined anti-IL-17A plus corticosteroid treatment represents an optimal therapeutic strategy. IL-17A production from antigen-specific Th17 cells stimulates airway epithelial cells to induce neutrophil growth, recruitment, and survival cytokines that can be attenuated through antibody-mediated IL-17A antagonism (72). Although this is effective in reducing the abundance of inflammatory neutrophils in the airspaces, only when combined with corticosteroid treatment is lung function improved. This combined approach functions at a point in severe asthma pathogenesis wherein it can be effective, at least in this acute preclinical model. IL-17A also has direct effects on airway smooth muscle, wherein it promotes contraction (15). Furthermore, combined IL-17A targeting and corticosteroid treatment has been reported to confer protection in a diesel exhaust particle-induced model of severe asthma (10). It remains unclear how mice receiving anti-IL-17A treatment “reestablish” corticosteroid responsiveness. Whether a similar combined therapeutic strategy can be applied to a protracted model of HDM challenge in mice subjected to the CFA/HDM protocol or to the condition of the chronically remodeled lungs of severe asthmatics remains to be determined. Nevertheless, the CFA/HDM protocol offers a novel model of the pathophysiological changes present in specific endotypes of severe asthma, which promises to be useful for future evaluation of disease biomarkers and mediators.
GRANTS
This work was supported by NIH R56AI116255 (M. Rincon), R01HL10729 (M. E. Poynter), R01HL133920 (M. E. Poynter), and R01HL142081 (M. E. Poynter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.P. conceived and designed research; K.E.M., M.M.M., L.F.R., C.J.W., K.E.V.D.V., J.L.A., D.G.C., B.J.S., and M.E.P. performed experiments; K.E.M., M.M.M., L.F.R., K.E.V.D.V., J.L.A., D.G.C., B.J.S., and M.E.P. analyzed data; K.E.M., D.G.C., B.J.S., M.R., and M.E.P. interpreted results of experiments; K.E.M. and M.E.P. drafted manuscript; K.E.M., D.G.C., B.J.S., M.R., and M.E.P. edited and revised manuscript; K.E.M., M.M.M., L.F.R., C.J.W., J.L.A., D.G.C., B.J.S., M.R., and M.E.P. approved final version of manuscript; M.E.P. prepared figures.
ACKNOWLEDGMENTS
We thank Nirav Daphtary, Minara Aliyeva, and Dr. Jason H.T. Bates for technical and computational support in the generation and interpretation of flexiVent data. We thank Drs. Anne Dixon, Charles Irvin, and David Kaminsky for helpful discussions. Flow cytometry studies were conducted at the Harry Hood Bassett Flow Cytometry and Cell Sorting Facility of the Larner College of Medicine at the University of Vermont, which was supported by NIH S10OD018175.
REFERENCES
- 1.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol 72: 495–516, 2010. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 2.Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC, Poynter ME. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol 187: 64–73, 2011. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ather JL, Dienz O, Boyson JE, Anathy V, Amiel E, Poynter ME. Serum amyloid A3 is required for normal lung development and survival following influenza infection. Sci Rep 8: 16571, 2018. doi: 10.1038/s41598-018-34901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ather JL, Fortner KA, Budd RC, Anathy V, Poynter ME. Serum amyloid A inhibits dendritic cell apoptosis to induce glucocorticoid resistance in CD4(+) T cells. Cell Death Dis 4: e786, 2013. doi: 10.1038/cddis.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacharier LB, Mori A, Kita H. Advances in asthma, asthma-COPD overlap, and related biologics in 2018. J Allergy Clin Immunol 144: 906–919, 2019. doi: 10.1016/j.jaci.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Bates JHT, Irvin CG, Farré R, Hantos Z. Oscillation mechanics of the respiratory system. Compr Physiol 1: 1233–1272, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 9.Bonten TN, Kasteleyn MJ, de Mutsert R, Hiemstra PS, Rosendaal FR, Chavannes NH, Slats AM, Taube C. Defining asthma-COPD overlap syndrome: a population-based study. Eur Respir J 49: 1602008, 2017. doi: 10.1183/13993003.02008-2016. [DOI] [PubMed] [Google Scholar]
- 10.Brandt EB, Khurana Hershey GK. A combination of dexamethasone and anti-IL-17A treatment can alleviate diesel exhaust particle-induced steroid insensitive asthma. J Allergy Clin Immunol 138: 924–928.e2, 2016. doi: 10.1016/j.jaci.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 188: 1294–1302, 2013. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 12.Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, Demoly P. Respiratory allergy caused by house dust mites: What do we really know? J Allergy Clin Immunol 136: 38–48, 2015. doi: 10.1016/j.jaci.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Kim YK, Jeon SG, Kim SH, Kim SS, Park HW, Min KU, Kim YY, Cho SH. Influence of the adjuvants and genetic background on the asthma model using recombinant Der f 2 in mice. Immune Netw 13: 295–300, 2013. doi: 10.4110/in.2013.13.6.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng KC, Lee KM, Krug MS, Watanabe T, Suzuki M, Choe IS, Yoo TJ. House dust mite-induced sensitivity in mice. J Allergy Clin Immunol 101: 51–59, 1998. doi: 10.1016/S0091-6749(98)70193-9. [DOI] [PubMed] [Google Scholar]
- 15.Chesne J, Braza F, Chadeuf G, Mahay G, Cheminant MA, Loy J, Brouard S, Sauzeau V, Loirand G, Magnan A. Prime role of IL-17A in neutrophilia and airway smooth muscle contraction in a house dust mite-induced allergic asthma model. J Allergy Clin Immunol 135: 1643–1645.e3, 2015. doi: 10.1016/j.jaci.2014.12.1872. [DOI] [PubMed] [Google Scholar]
- 16.Chu DK, Al-Garawi A, Llop-Guevara A, Pillai RA, Radford K, Shen P, Walker TD, Goncharova S, Calhoun WJ, Nair P, Jordana M. Therapeutic potential of anti-IL-6 therapies for granulocytic airway inflammation in asthma. Allergy Asthma Clin Immunol 11: 14, 2015. doi: 10.1186/s13223-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet L-P, Brightling C, Chanez P, Dahlen S-E, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 43: 343–373, 2014. [Erratum Eur Respir J 43:1216, 2014]. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 18.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 39: 531–536, 2002. doi: 10.1016/S0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 19.Erjefält JS. Unravelling the complexity of tissue inflammation in uncontrolled and severe asthma. Curr Opin Pulm Med 25: 79–86, 2019. doi: 10.1097/MCP.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 20.Fallica J, Das S, Horton M, Mitzner W. Application of carbon monoxide diffusing capacity in the mouse lung. J Appl Physiol (1985) 110: 1455–1459, 2011. doi: 10.1152/japplphysiol.01347.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farahi N, Paige E, Balla J, Prudence E, Ferreira RC, Southwood M, Appleby SL, Bakke P, Gulsvik A, Litonjua AA, Sparrow D, Silverman EK, Cho MH, Danesh J, Paul DS, Freitag DF, Chilvers ER. Neutrophil-mediated IL-6 receptor trans-signaling and the risk of chronic obstructive pulmonary disease and asthma. Hum Mol Genet 26: 1584–1596, 2017. doi: 10.1093/hmg/ddx053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goplen N, Karim MZ, Liang Q, Gorska MM, Rozario S, Guo L, Alam R. Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma. J Allergy Clin Immunol 123: 925–932.e11, 2009. doi: 10.1016/j.jaci.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heeger PS, Forsthuber T, Shive C, Biekert E, Genain C, Hofstetter HH, Karulin A, Lehmann PV. Revisiting tolerance induced by autoantigen in incomplete Freund’s adjuvant. J Immunol 164: 5771–5781, 2000. doi: 10.4049/jimmunol.164.11.5771. [DOI] [PubMed] [Google Scholar]
- 24.Hercor M, Anciaux M, Denanglaire S, Debuisson D, Leo O, Andris F. Antigen-presenting cell-derived IL-6 restricts the expression of GATA3 and IL-4 by follicular helper T cells. J Leukoc Biol 101: 5–14, 2017. doi: 10.1189/jlb.1HI1115-511R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilmarinen P, Tuomisto LE, Niemelä O, Danielsson J, Haanpää J, Kankaanranta T, Kankaanranta H. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J 48: 1052–1062, 2016. doi: 10.1183/13993003.02198-2015. [DOI] [PubMed] [Google Scholar]
- 26.Jackson DJ, Bacharier LB, Calatroni A, Gill MA, Hu J, Liu AH, Wheatley LM, Gern JE, Gruchalla RS, Khurana Hershey GK, Kattan M, Kercsmar CM, Kim H, O’Connor GT, Patel S, Pongracic JA, Wood RA, Busse WW. Serum IL-6: A biomarker in childhood asthma? J Allergy Clin Immunol 145: 1701–1704.e3, 2020. doi: 10.1016/j.jaci.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jevnikar Z, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol 143: 577–590, 2019. doi: 10.1016/j.jaci.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamoorthy N, Douda DN, Bruggemann TR, Ricklefs I, Duvall MG, Abdulnour RE, Martinod K, Tavares L, Wang X, Cernadas M, Israel E, Mauger DT, Bleecker ER, Castro M, Erzurum SC, Gaston BM, Jarjour NN, Wenzel S, Dunican E, Fahy JV, Irimia D, Wagner DD, Levy BD; National Heart, Lung, and Blood Institute Severe Asthma Research Program-3 Investigators . Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol 3: eaao4747, 2018. doi: 10.1126/sciimmunol.aao4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 50: 975–991, 2019. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Le Gros GS, Prestidge RL, Watson JD. In-vivo modulation of thymus-derived lymphocytes with monoclonal antibodies in mice. I. Effect of anti-Thy-1 antibody on the tissue distribution of lymphocytes. Immunology 50: 537–546, 1983. [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Hastie AT, Peters MC, Hawkins GA, Phipatanakul W, Li H, Moore WC, Busse WW, Castro M, Erzurum SC, Gaston B, Israel E, Jarjour NN, Levy BD, Wenzel SE, Meyers DA, Fahy JV, Bleecker ER; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program (SARP) Networks . Investigation of the relationship between IL-6 and type 2 biomarkers in patients with severe asthma. J Allergy Clin Immunol 145: 430–433, 2020. doi: 10.1016/j.jaci.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev 99: 1223–1248, 2019. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, Groshong S, Gorska MM, Martin RJ, Alam R. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol 139: 1548–1558 e1544, 2017. doi: 10.1016/j.jaci.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maltby S, Tay HL, Yang M, Foster PS. Mouse models of severe asthma: Understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation. Respirology 22: 874–885, 2017. doi: 10.1111/resp.13052. [DOI] [PubMed] [Google Scholar]
- 35.Martin RA, Ather JL, Daggett R, Hoyt L, Alcorn JF, Suratt BT, Weiss DJ, Lundblad LKA, Poynter ME. The endogenous Th17 response in NO2-promoted allergic airway disease is dispensable for airway hyperresponsiveness and distinct from Th17 adoptive transfer. PLoS One 8: e74730, 2013. doi: 10.1371/journal.pone.0074730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin RA, Ather JL, Lundblad LKA, Suratt BT, Boyson JE, Budd RC, Alcorn JF, Flavell RA, Eisenbarth SC, Poynter ME. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease. Am J Respir Cell Mol Biol 48: 655–664, 2013. doi: 10.1165/rcmb.2012-0423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin RA, Hodgkins SR, Dixon AE, Poynter ME. Aligning mouse models of asthma to human endotypes of disease. Respirology 19: 823–833, 2014. doi: 10.1111/resp.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer A, Debuisson D, Denanglaire S, Eddahri F, Fievez L, Hercor M, Triffaux E, Moser M, Bureau F, Leo O, Andris F. Antigen presenting cell-derived IL-6 restricts Th2-cell differentiation. Eur J Immunol 44: 3252–3262, 2014. doi: 10.1002/eji.201444646. [DOI] [PubMed] [Google Scholar]
- 39.McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, Kozma SC, Drew AF. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol 184: 7219–7228, 2010. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- 40.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 181: 4089–4097, 2008. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy 34: 497–507, 2004. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a.National Heart, Lung, and Blood Institute Guidelines for the Diagnosis and Management of Asthma (EPR-3). Section 2, Definition, Pathophysiology and Pathogenesis of Asthma, and Natural History of Asthma (Online). Bethesda, MD: National Institutes of Health; https://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. [2018 Jan 24]. [Google Scholar]
- 42.Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, Rincon M. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol 183: 1732–1738, 2009. doi: 10.4049/jimmunol.0802923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldenburg HS, Rogy MA, Lazarus DD, Van Zee KJ, Keeler BP, Chizzonite RA, Lowry SF, Moldawer LL. Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. Eur J Immunol 23: 1889–1894, 1993. doi: 10.1002/eji.1830230824. [DOI] [PubMed] [Google Scholar]
- 44.Pandey KC, De S, Mishra PK. Role of proteases in chronic obstructive pulmonary disease. Front Pharmacol 8: 512, 2017. doi: 10.3389/fphar.2017.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, Jarjour NN, Coverstone AM, Castro M, Holguin F, Wenzel SE, Woodruff PG, Bleecker ER, Fahy JV; National Heart, Lung, and Blood Institute Severe Asthma Research Program . Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 4: 574–584, 2016. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 13: 1189–1196, 1994. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian X, Aboushousha R, van de Wetering C, Chia SB, Amiel E, Schneider RW, van der Velden JLJ, Lahue KG, Hoagland DA, Casey DT, Daphtary N, Ather JL, Randall MJ, Aliyeva M, Black KE, Chapman DG, Lundblad LKA, McMillan DH, Dixon AE, Anathy V, Irvin CG, Poynter ME, Wouters EFM, Vacek PM, Henket M, Schleich F, Louis R, van der Vliet A, Janssen-Heininger YMW. IL-1/inhibitory κB kinase ε-induced glycolysis augment epithelial effector function and promote allergic airways disease. J Allergy Clin Immunol 142: 435–450.e10, 2018. doi: 10.1016/j.jaci.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raemdonck K, Baker K, Dale N, Dubuis E, Shala F, Belvisi MG, Birrell MA. CD4+ and CD8+ T cells play a central role in a HDM driven model of allergic asthma. Respir Res 17: 45, 2016. doi: 10.1186/s12931-016-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, Ray P, Ray A. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest 125: 3037–3050, 2015. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravanetti L, Dijkhuis A, Sabogal Pineros YS, Bal SM, Dierdorp BS, Dekker T, Logiantara A, Adcock IM, Rao NL, Boon L, Villetti G, Sterk PJ, Facchinetti F, Lutter R, Group UBS; U-BIOPRED Study Group . An early innate response underlies severe influenza-induced exacerbations of asthma in a novel steroid-insensitive and anti-IL-5-responsive mouse model. Allergy 72: 737–753, 2017. doi: 10.1111/all.13057. [DOI] [PubMed] [Google Scholar]
- 51.Ray A, Camiolo M, Fitzpatrick A, Gauthier M, Wenzel SE. Are we meeting the promise of endotypes and precision medicine in asthma? Physiol Rev 100: 983–1017, 2020. doi: 10.1152/physrev.00023.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Invest 104: 985–993, 1999. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol 38: 942–954, 2017. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest 126: 2394–2403, 2016. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, Israel E, Bleecker ER, Castro M, Erzurum SC, Fahy JV, Gaston BM, Denlinger LC, Mauger DT, Wenzel SE, Comhair SA, Coverstone AM, Fajt ML, Hastie AT, Johansson MW, Peters MC, Phillips BR, Levy BD; National Heart Lung and Blood Institute’s Severe Asthma Research Program-3 Investigators . ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight 2: e93534, 2017. doi: 10.1172/jci.insight.93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson MB, Deshpande DA, Chou J, Cui W, Smith S, Langefeld C, Hastie AT, Bleecker ER, Hawkins GA. IL-6 trans-signaling increases expression of airways disease genes in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 309: L129–L138, 2015. doi: 10.1152/ajplung.00288.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 8: 1237–1247, 2012. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 26: 475–487, 2015. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol 64: 1403–1415, 2016. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Smole U, Gour N, Phelan J, Hofer G, Köhler C, Kratzer B, Tauber PA, Xiao X, Yao N, Dvorak J, Caraballo L, Puerta L, Rosskopf S, Chakir J, Malle E, Lane AP, Pickl WF, Lajoie S, Wills-Karp M. Serum amyloid A is a soluble pattern recognition receptor that drives type 2 immunity. Nat Immunol 21: 756–765, 2020. doi: 10.1038/s41590-020-0698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Svenningsen S, Nair P. Asthma endotypes and an overview of targeted therapy for asthma. Front Med (Lausanne) 4: 158, 2017. doi: 10.3389/fmed.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tommola M, Ilmarinen P, Tuomisto LE, Lehtimäki L, Haanpää J, Niemelä O, Kankaanranta H. Differences between asthma-COPD overlap syndrome and adult-onset asthma. Eur Respir J 49: 1602383, 2017. doi: 10.1183/13993003.02383-2016. [DOI] [PubMed] [Google Scholar]
- 63.Tonga KO, Chapman DG, Farah CS, Oliver BG, Zimmermann SC, Milne S, Sanai F, Jetmalani K, Berend N, Thamrin C, King GG. Reduced lung elastic recoil and fixed airflow obstruction in asthma. Respirology 25: 613–619, 2020. doi: 10.1111/resp.13688. [DOI] [PubMed] [Google Scholar]
- 64.Ullah MA, Revez JA, Loh Z, Simpson J, Zhang V, Bain L, Varelias A, Rose-John S, Blumenthal A, Smyth MJ, Hill GR, Sukkar MB, Ferreira MAR, Phipps S. Allergen-induced IL-6 trans-signaling activates γδ T cells to promote type 2 and type 17 airway inflammation. J Allergy Clin Immunol 136: 1065–1073, 2015. doi: 10.1016/j.jaci.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 65.Vietri L, Fui A, Bergantini L, d’Alessandro M, Cameli P, Sestini P, Rottoli P, Bargagli E. Serum amyloid A: A potential biomarker of lung disorders. Respir Investig 58: 21–27, 2020. doi: 10.1016/j.resinv.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Homer RJ, Chen Q, Elias JA. Endogenous and exogenous IL-6 inhibit aeroallergen-induced Th2 inflammation. J Immunol 165: 4051–4061, 2000. doi: 10.4049/jimmunol.165.7.4051. [DOI] [PubMed] [Google Scholar]
- 67.White SR, Laxman B, Naureckas ET, Hogarth DK, Solway J, Sperling AI, Ober C. Evidence for an IL-6-high asthma phenotype in asthmatic patients of African ancestry. J Allergy Clin Immunol 144: 304–306.e4, 2019. doi: 10.1016/j.jaci.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 180: 720–730, 2009. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodruff PG, van den Berge M, Boucher RC, Brightling C, Burchard EG, Christenson SA, Han MK, Holtzman MJ, Kraft M, Lynch DA, Martinez FD, Reddel HK, Sin DD, Washko GR, Wenzel SE, Punturieri A, Freemer MM, Wise RA. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma-Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am J Respir Crit Care Med 196: 375–381, 2017. doi: 10.1164/rccm.201705-0973WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wright HL, Cross AL, Edwards SW, Moots RJ. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology (Oxford) 53: 1321–1331, 2014. doi: 10.1093/rheumatology/keu035. [DOI] [PubMed] [Google Scholar]
- 71.Zervas E, Samitas K, Papaioannou AI, Bakakos P, Loukides S, Gaga M. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res 4: 00125-2017, 2018. doi: 10.1183/23120541.00125-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zwicky P, Unger S, Becher B. Targeting interleukin-17 in chronic inflammatory disease: a clinical perspective. J Exp Med 217: e20191123, 2020. doi: 10.1084/jem.20191123. [DOI] [PMC free article] [PubMed] [Google Scholar]