Abstract
Hypertension and kidney involvement are common in patients with autoimmune disease. Sodium intake is linked to hypertension in both human and animal studies. Evidence suggests that dietary salt may be an important environmental factor that promotes autoimmune activity. Therefore, we hypothesized that a long-term high-salt diet would accelerate the progression of autoimmunity, hypertension, and albuminuria during systemic lupus erythematosus (SLE), an autoimmune disease that predominantly affects young women and has a high prevalence of hypertension and renal disease. To test this hypothesis, an established experimental model of SLE (female NZBWF1 mice) that develops hypertension and renal disease was used. SLE mice were fed a high-salt (4% NaCl) or normal (0.4% NaCl) diet for 24 wk beginning at 10 wk of age and ending at 34 wk of age, a time by which female NZBWF1 mice typically have hypertension and exhibit signs of renal disease. Plasma anti-dsDNA autoantibodies were measured as an indicator of active SLE disease, and urinary albumin was monitored longitudinally as a marker of renal disease. Arterial pressure was measured in conscious, freely moving mice at 34 wk of age. Urinary endothelin-1 (ET-1) excretion, renal endothelin A and B receptor protein expression, and renal mRNA expression of NOS1, NOS2, NOX2, MCP-1, TNF-α, serum- and glucocorticoid-regulated kinase 1, and interleukin-2 (IL-2) were assessed to determine the impact on gene products commonly altered by a high-salt diet. SLE mice fed a high-salt diet had increased circulating autoantibodies, but the high-salt diet did not significantly affect albuminuria or arterial pressure. Urinary ET-1 excretion was increased, whereas renal endothelin A receptor and IL-2 expression were decreased in response to a high-salt diet. These data suggest that a chronic high-salt diet may not accelerate cardiovascular and renal consequences commonly associated with SLE.
Keywords: autoimmunity, endothelin, hypertension, interleukin-2, systemic lupus erythematosus
INTRODUCTION
Hypertension is common in patients with autoimmune disease (2, 6, 14, 19, 31, 33, 40, 45) and is a major modifiable risk factor for cardiovascular disease (CVD) prevention. CVD is a leading cause of morbidity and mortality in patients with autoimmune disease (16, 21, 22, 48). Systemic lupus erythematosus (SLE) is characterized by a strong sex bias, female-to-male ratio of 9:1 (37), prevalent hypertension (40, 45), high incidence and prevalence of renal involvement (4, 6, 30, 43), autoantibodies to nuclear antigens (15, 55), and a complex etiology involving genetic, hormonal, and environmental factors (3, 17, 29, 56). Previous studies from our laboratory demonstrated that female NZBWF1 mice, an established murine model of SLE with a complex genetic background, develop many of the same characteristics of disease as humans, including a strong female predilection, anti-dsDNA autoantibodies, glomerulosclerosis, and hypertension (39). This contrasts with other common experimental models of SLE, such as the MRL/lpr model, that arise from a single genetic mutation, impacts both males and females equally, and do not spontaneously develop hypertension. This makes the female NZBWF1 mouse an important model for studying mechanisms of hypertension in the setting of autoimmunity.
Diet, particularly dietary salt intake, is an important environmental factor that could influence autoimmunity and the associated cardiovascular and renal disease risk. Both human and preclinical studies show a positive relationship between salt intake and blood pressure (10, 23), and meta-analyses of clinical studies support the concept of reducing salt (NaCl) intake to decrease blood pressure, particularly in individuals with hypertension (16a). Dietary sodium for adults in the United States averages 3,400 mg plus per day (8). This far exceeds the recommendation of 2,300 mg per day outlined in the 2015–2020 Dietary Guidelines for Americans (8). In addition, long-term high dietary sodium intake is linked to CVD-related mortality (1, 7).
The impact of high dietary salt intake on autoimmunity remains poorly understood. Preclinical studies suggest that high sodium chloride intake interferes with the immunosuppressive effects of regulatory T cells (TREG) (46) on T helper 17 (TH17) cells through the activation of serum- and glucocorticoid-regulated kinase 1 (SGK1) (53). In addition, a high-salt diet has been shown to exacerbate disease in an experimental model of multiple sclerosis (experimental autoimmune encephalitis) (20) and in the MRL/lpr mouse model of SLE (54).
In clinical studies, the impact of a high-salt diet on autoimmunity remains understudied. High salt intake has been reported to increase the ratio of TH17 to TREG cells (44) in patients with rheumatoid arthritis and has been associated with an increase in self-reported disease activity (41). In patients with multiple sclerosis (MS), conflicting data related to high salt consumption have been reported (11, 12). An observational study on patients with MS concluded that patients consuming a medium- or high-salt diet had a greater chance of developing lesions, as assessed by MRI, when compared with those consuming a low-salt diet (11). In contrast, in a randomized clinical trial, higher salt consumption did not have an impact on the activity or course of MS, as assessed by MRI (12). In patients with SLE, a salt-restricted diet (less than 5 g NaCl per day) attenuated the inflammatory response by increasing TREG cells, decreasing T helper 17 (TH17) cells, and decreasing serum interleukin 9 levels (44). Whether long-term high dietary salt intake affects the development of hypertension and/or renal injury during autoimmune diseases like SLE is unclear. Based on the prevalent hypertension among patients with autoimmune disease and preclinical studies supporting a link between sodium and autoimmunity, the current study tested the hypothesis that a long-term high-salt diet will increase blood pressure and albuminuria during SLE in female NZBWF1 mice.
METHODS
Animal model.
All studies were conducted on female NZBWF1 (SLE) mice. Mice were obtained from The Jackson Laboratories (Bar Harbor, ME) between 6 and 8 wk of age and housed at room temperature with access to water ad libitum. At 10 wk of age, mice were either started on a 4% NaCl (high salt) chow or remained on a 0.4% NaCl (control) chow and maintained ad libitum until 34 wk of age. Mice were maintained on a 12-h light/dark cycle. All studies were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center (UMMC) and were in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Sodium intake.
Salt intake was measured by weighing food intake daily and converting grams of NaCl to milliequivalents.
Diet composition.
Mice received Envigo Teklad 8640 (0.4% NaCl) or Envigo Teklad TD.92034 (4% NaCl) chow ad libitum.
Autoantibody production.
Plasma samples were taken at 34 wk of age to assess anti-dsDNA (dsDNA) IgG (total) autoantibodies by using an ELISA test (Alpha Diagnostic International) as per the manufacturer’s instructions and as described previously by our laboratory (39, 51). Results are presented in units per milliliters.
Mean arterial pressure.
Mean arterial pressure (MAP) was assessed in freely moving, conscious mice by indwelling carotid artery catheterization connected to an eight-channel pressure transducer (PowerLab/16SP ADInstruments) for 2 h on two consecutive days beginning ~22 h postsurgery, as previously described by our laboratory (25, 39, 50).
Urinary excretion of albumin and endothelin-1.
Albuminuria, a marker of renal injury, was assessed at 10 wk of age and every 4 wk until 30 wk of age, then weekly until 34 wk of age, using dipstick analysis (Albustix; Siemens). Urinary albumin excretion rate (µg/h) was calculated by measuring albumin with a commercial ELISA test (Alpha Diagnostics International) from 24-h urine samples collected at 34 wk of age, as previously described by our laboratory (25–27). Twenty-four-hour urine samples were collected as previously described (26, 27, 50), and urinary endothelin-1 (ET-1) concentration was determined by ELISA according to the manufacturer’s instructions (no dilutions, Quantiglo; R&D Systems, Minneapolis, MN).
Protein expression.
Endothelin receptor protein expression was evaluated by Western blot analysis as previously described (42). Briefly, samples (200 µg of whole kidney lysates) were loaded onto 7.5% polyacrylamide gels and separated by electrophoresis. Membranes were incubated overnight with specific antibodies: anti-ET-A (1:250, ab85163, Abcam, Inc., Cambridge, MA) and anti-ET-B (1:250, ab117529, Abcam, Inc., Cambridge, MA). The membranes were incubated with secondary antibodies: goat anti-rabbit (1:1000, 170–6515, Bio-Rad, Hercules, CA). Bands of interest were visualized by using an enhanced chemiluminescence reagent and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software; Bio-Rad) as integrated optical density (IOD) after subtraction of background. The IOD was factored for Ponceau red staining (Sigma) to correct for any variations in total protein. The protein abundance was represented as IOD/Ponceau.
RNA expression.
Renal expression of gene products commonly altered by a high-salt diet was determined using quantitative reverse-transcriptase PCR (qRT-PCR). Kidneys were harvested and the poles were used as cortical-enriched samples, or kidneys were longitudinally bisected and the medulla was dissected for medulla-enriched samples. RNA was isolated from renal tissue using the Nucleospin RNA kit (Macherey Nagel, Inc.). The manufacturer’s suggested protocol was followed for RNA extraction, with a few modifications. Briefly, 6 μL of β-mercaptoethanol was added to 600 μL of RA1 with the renal tissue sample. The sample was homogenized with a rotor-stator tissue homogenizer and then applied to the Nucleospin RNA kit homogenizing filter. RNA was eluted in 60 μL of nanopure H2O. RNA preparations were quantified, and the A260/280 ratio was found to be near 2.0 for all samples using a NanoDrop 2000 spectrophotometer. Triplicate samples of 100 ng RNA each were quantified using quantitative reverse-transcriptase real-time PCR (qRT-PCR) using the Bio-Rad CFX Detection System and Brilliant II SYBR green 1-step master mix. Specific DNA oligo primers for GAPDH, TNF-α, NOS1, NOS2, Cybb (NOX2), Ccl2 (MCP-1), interleukin-2 (IL-2), and SGK1 were obtained from Integrated DNA Technologies (PrimeTime Predesigned qPCR Assay primers for intercalating dye protocols). The qRT-PCR thermal profile was as follows: 50°C for 30 min, 95°C for 10 min, 95°C for 30 s, 50°C for 1 min, 72°C for 30 s (40 cycles), and 95°C for 1 min. A melt curve cycle was also used to determine homogeneity of PCR products. Fold change of both 4% and 0.4% NaCl samples was calculated for each specific primer relative to GAPDH.
Statistical analysis.
Data are presented as means and standard error. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software). Unpaired, two-tailed Student’s t test was used to determine differences between groups for all normally distributed data. All data were normally distributed, with the exception of urinary albumin excretion. These data were analyzed with a two-tailed Mann–Whitney (nonparametric) test. Statistical significance was defined as P < 0.05.
RESULTS
Sodium intake.
To confirm that the mice were eating the 4% NaCl diet, sodium intake was measured. Sodium intake was significantly higher in female SLE mice fed a 4% NaCl diet compared with female SLE mice fed a 0.4% NaCl diet (5.7 ± 0.6 vs. 0.6 ± 0.1 meq/day, P < 0.01) at all ages (Fig. 1).
Fig. 1.
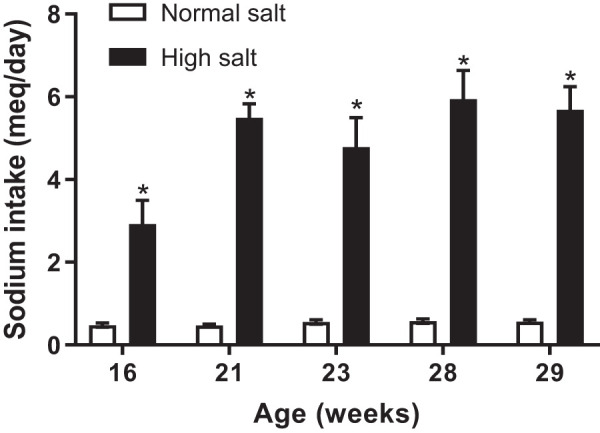
Sodium intake is higher in female systemic lupus erythematosus (SLE) mice fed a high-salt diet. SLE mice fed a 4% NaCl diet consume significantly higher NaCl (*P = 0.003, < 0.0001, 0.0004, < 0.0001, and < 0.0001) compared with SLE mice fed a 0.4% NaCl diet, per 2-tailed, unpaired, t test. n = 13 (4% NaCl) and n = 12 (0.4% NaCl). Error bars represent SE.
Anti-dsDNA IgG autoantibodies.
Anti-dsDNA IgG autoantibodies, a common clinical marker in SLE, are increased in female SLE mice fed a 4% NaCl diet at 34 wk of age (P = 0.0247) compared with those fed a 0.4% NaCl diet (Fig. 2). This suggests that a high-salt diet may promote humoral immunity.
Fig. 2.
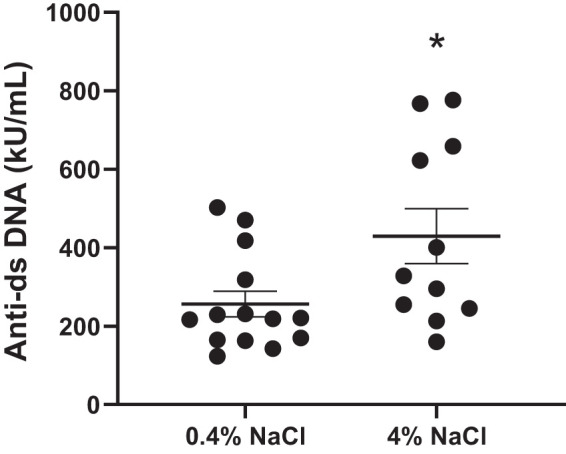
Anti-dsDNA IgG autoantibodies are increased in female systemic lupus erythematosus (SLE) mice fed a high-salt diet at 34 wk of age. Anti-dsDNA IgG autoantibodies are significantly increased in SLE mice fed a 4% NaCl diet by 34 wk of age compared with those fed a 0.4% NaCl diet; *P = 0.0247, 2-tailed, unpaired t test. n = 11 (4% NaCl) and n = 14 (0.4% NaCl). Error bars represent SE.
Mean arterial pressure.
Our laboratory previously reported that female NZBWF1 mice develop hypertension by 34 wk of age. In the current study, MAP was measured in a subgroup of animals at the conclusion of the 24-wk protocol. MAP is not significantly altered in female SLE mice fed a 4% NaCl diet by 34 wk of age compared with those fed a 0.4% NaCl diet (Fig. 3). The level of circulating autoantibodies does not correlate with blood pressure (r = −0.192, P = 0.51).
Fig. 3.
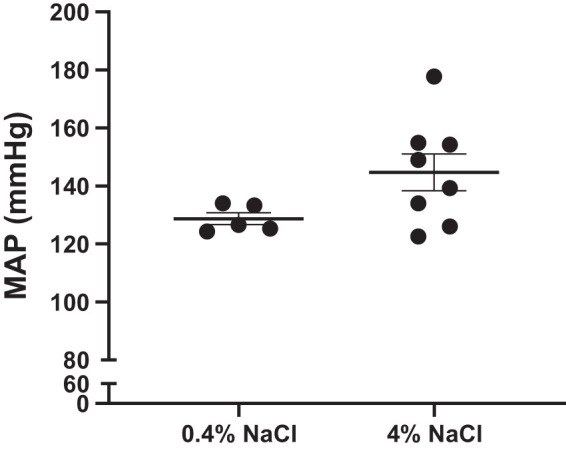
Blood pressure is unchanged in female systemic lupus erythematosus (SLE) mice fed a high-salt diet at 34 wk of age. No significant differences were seen between SLE mice fed a 4% NaCl diet (n = 8) and those fed a 0.4% NaCl diet (n = 5); P = 0.0819, per 2-tailed, unpaired t test. Error bars represent SE. MAP, mean arterial pressure.
Albuminuria.
The urinary albumin excretion rate, calculated using 24-h urine samples and measured by ELISA at the conclusion of the study, was similar between SLE mice fed a 4% NaCl diet and those fed a 0.4% NaCl diet (Fig. 4).
Fig. 4.
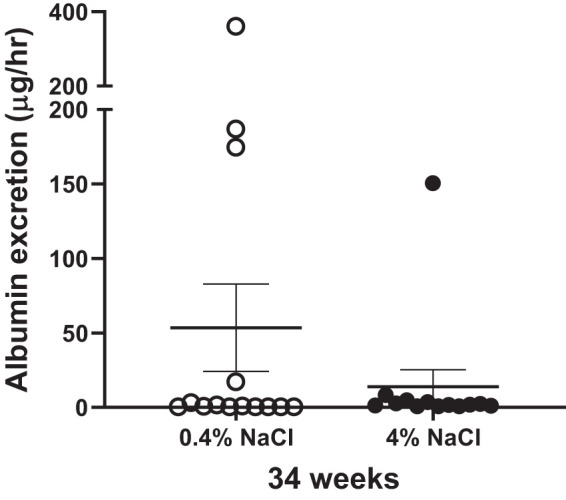
Albumin excretion is unchanged in systemic lupus erythematosus (SLE) mice fed a 4% NaCl diet compared with those fed a 0.4% NaCl diet; P = 0.30, per 2-tailed, Mann–Whitney test. n = 13 (0.4% NaCl) and n = 12 (4% NaCl). Error bars represent SE.
Renal inflammatory markers.
Renal cortical expression of several genes known to be altered during high-salt-diet and/or inflammatory conditions was assessed by qRT-PCR. The data are presented as a fold change relative to the 0.4% NaCl diet-fed mice. mRNA expression of NOS1, NOS2, NOX2, MCP-1, and TNF-α was unchanged in cortex-enriched renal samples from 4% NaCl diet-fed mice relative to those from 0.4% NaCl diet-fed mice. However, renal IL-2, a cytokine associated with T cell differentiation, was significantly lower in 4% NaCl diet-fed SLE mice, (P = 0.03) (Fig. 5). In addition, no significant differences in glomerular scoring were observed between normal-salt (1.61 ± 0.29, n = 13) and high-salt groups (1.18 ± 0.29, n = 10, P = 0.155).
Fig. 5.
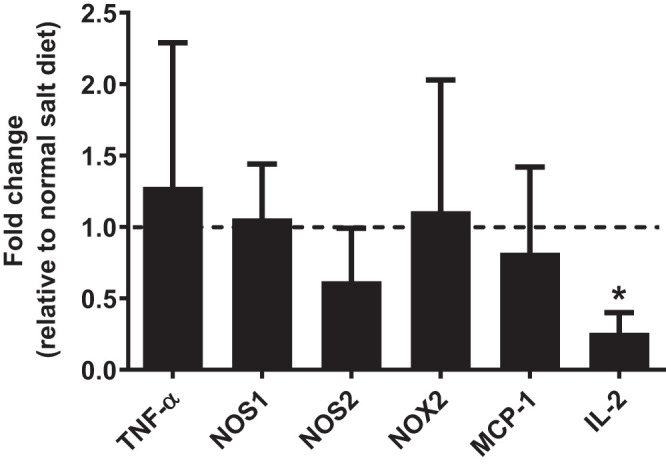
Renal cortex interleukin-2 (IL-2) mRNA levels are decreased in systemic lupus erythematosus (SLE) mice on a chronic high-salt diet. Renal IL-2 mRNA levels significantly decreased in SLE mice fed a 4% NaCl diet compared with those fed a 0.4% NaCl diet; *P = 0.027, per 2-tailed, unpaired t test. Renal mRNA expression of NOS1, NOS2, NOX2, MCP-1, and TNF-α remained unchanged for SLE mice fed a 4% NaCl diet relative to those fed a 0.4% NaCl diet. n = 4 per group. Error bars represent SE.
Endothelin.
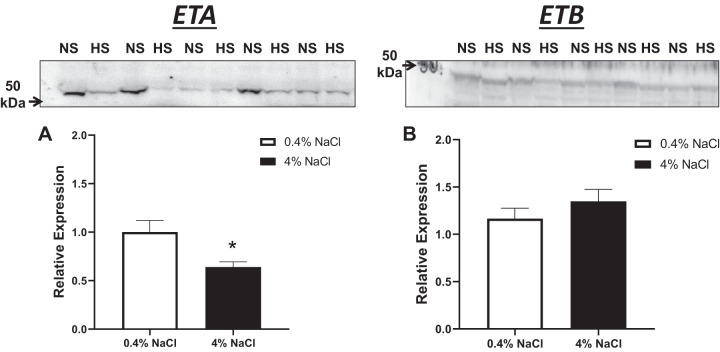
Increases in urinary ET-1 excretion are associated with high-salt diets. Urinary ET-1 excretion is increased at 34 wk of age in SLE mice fed a 4% NaCl diet (P = 0.0002) compared with SLE mice fed a 0.4% NaCl diet (Fig. 6). Renal endothelin A (ETA) receptor protein expression is lower in SLE mice fed a 4% NaCl diet (P = 0.0265) compared with SLE mice fed a 0.4% NaCl diet. No differences were observed in endothelin B (ETB) protein expression (Fig. 7).
Fig. 6.
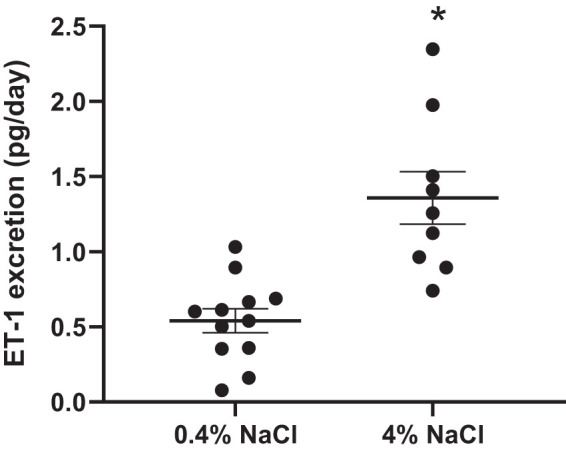
Endothelin-1 (ET-1) excretion is increased in female systemic lupus erythematosus (SLE) mice fed a 4% NaCl diet at 34 wk of age. SLE mice fed a 4% NaCl diet had a significant increase in ET-1 excretion compared with those fed a 0.4% NaCl diet by 34 wk of age; *P = 0.0002, per 2-tailed, unpaired t test. n = 12 (0.4% NaCl) and n = 9 (4% NaCl). Error bars represent SE.
Fig. 7.
Renal endothelin A (ETA) protein expression is reduced in female systemic lupus erythematosus (SLE) mice on a chronic high-salt diet. Relative expression of renal ETA was significantly decreased in SLE mice fed a 4% NaCl diet compared with those fed a 0.4% NaCl diet; *P = 0.0265, per 2-tailed, unpaired t test. n = 11 (0.4% NaCl) and n = 8 (4% NaCl). No differences were seen in endothelin B relative expression. Error bars represent SE. Representative Western blots.
Serum- and glucocorticoid-regulated kinase 1.
SGK1 has been shown to increase the proinflammatory cytokine IL-17 in response to an increase in extracellular NaCl, and the loss of SGK1 attenuates blood pressure during angiotensin II infusion (32). Therefore, renal medullary SGK1 expression was assessed by qRT-PCR. The data show that long-term high salt intake did not impact renal SGK1 expression (Fig. 8).
Fig. 8.
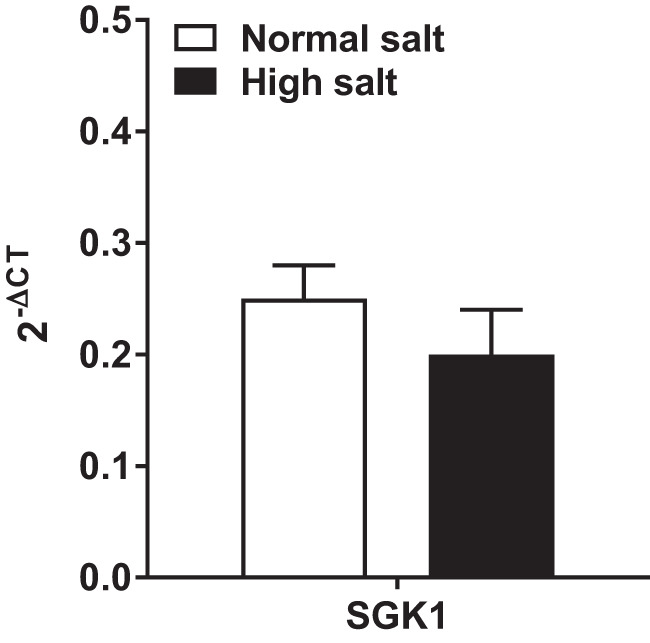
Medullary serum- and glucocorticoid-regulated kinase 1 is not altered in systemic lupus erythematosus (SLE) mice fed a high-salt diet. No significant differences were seen between SLE mice fed a 4% NaCl diet and those fed a 0.4% NaCl diet. n = 11 (0.4% NaCl) and n = 8 (4% NaCl). Error bars represent SE.
DISCUSSION
Patients with SLE develop renal injury and hypertension. The relationship between high dietary sodium, blood pressure, and autoimmunity remains unclear. Understanding the impact of dietary salt intake on the development of hypertension during autoimmunity may help to better inform disease management in this patient population. This study aimed to test whether a long-term high-salt-diet intake exacerbates hypertension and albuminuria in female NZBWF1 mice, an established preclinical model of SLE. This study advances the field by showing that a long-term high-salt diet 1) enhances autoantibody production in female mice with SLE, 2) does not significantly exacerbate autoimmune-associated hypertension or albuminuria, and 3) that renal IL-2 expression and ETA receptor expression are reduced in SLE mice fed a high-salt diet.
Preclinical studies have shown that excess NaCl increases pathogenic TH17 populations leading to a more severe experimental autoimmune encephalomyelitis, suggesting that excess NaCl may be a driver of autoimmune disease (20). The proposed mechanism by which NaCl increases the TH17 population is through promoting serum- and glucocorticoid-regulated kinase 1 (SGK1)-mediated stabilization of TH17 cells (53). In the current study, no changes were observed in medullary SGK1 expression, suggesting that this pathway may not be a universal mechanism across all autoimmune disorders and models.
Antinuclear autoantibodies, including anti-dsDNA IgG, are present in both human SLE and experimental animal SLE models, where they are pathogenic for the disease. The data in the present study show that a long-term high-salt diet significantly increased circulating anti-dsDNA IgG autoantibody levels in SLE mice. This contrasts with our previously published work on the NZBWF1 murine model showing no effect of a high-salt (8% NaCl) diet for 4 wk on anti-dsDNA IgG autoantibodies (27). Important differences in the experimental design likely account for these disparate findings. For example, our earlier work spanned only 4 wk starting at an age when autoantibodies were already present, whereas the present study was started when the mice were at 10 wk of age before the development of autoantibodies. In addition, the potential impact of utilizing a 4% versus an 8% NaCl diet in the respective studies is not clear. Regardless, the amount of NaCl used in the diets from both studies is very high. The impact of a high-salt diet on autoantibody production in human autoimmune disease is poorly understood.
Clinical data show an increased prevalence of microalbuminuria during high salt intake (18). In our laboratory, ~35%–45% of NZBWF1 mice typically developed albuminuria by 34 wk of age (26, 49, 51). When urinary albumin excretion was calculated taking urine volume into account, there were no significant differences between groups, suggesting that a long-term high-salt diet did not significantly alter the development of albuminuria in SLE mice. We also assessed the impact of chronic high salt intake on the development of glomerulosclerosis in this model, but there were no differences between the groups.
Knowing that autoantibodies drive autoimmune disease, renal injury, and hypertension during SLE, we attempted to gain a better understanding of why the increase in autoantibodies, associated with a long-term high-salt diet, did not exacerbate renal injury and hypertension. To accomplish this, we assessed endothelin system components and the expression of several genes associated with proinflammatory diseases and hypertension. ET-1, acting through two G protein-coupled receptors ETA and ETB, is a powerful regulator of blood pressure and renal excretory function by its vasoactive properties and actions on sodium and water excretion (9, 13, 52). The increased urinary ET-1 excretion in the animals fed a high-salt diet was anticipated, is consistent with what others have reported on animals fed a high-salt diet, and likely reflects the renal production of ET-1 specifically (34–36, 38). Therefore, the data showing that there are changes in the renal endothelin system may be informative about mechanisms.
Previous studies on NZBWF1 mice showed that the expression of ET-1 correlates with renal disease severity and function (38). ETA receptor activation in the renal cortex is typically associated with vasoconstriction that can promote hypertension and renal disease. ETB receptor activation in the renal medulla has powerful natriuretic actions (13, 52). Thus, it is possible that the reduction in renal ETA receptor expression, coupled with higher renal production of ET-1, and unchanged ETB receptor expression, may have blunted the hypertension and renal injury by reducing the effects of ET-1 in the renal cortex, thus shifting toward a more ETB-dominated system that promotes natriuresis. In subsequent studies, it may be more informative to examine the ET receptor expression separately in the cortex and medulla, rather than the whole kidney lysates as used in the present study.
We used real-time PCR to assess the expression of several transcripts that are implicated in chronic inflammatory disorders and that are commonly altered by a high-salt diet. Among the transcripts that were assessed, only IL-2 was changed. IL-2 is a cytokine with complex immunological roles. IL-2 is necessary for T cell differentiation and overall T cell homeostasis where it can promote proinflammatory T cells, and also the development of immunosuppressive TREG cells, depending on the concentration. For example, Taylor et al. (47) showed that low-dose IL-2 treatment in female NZBWF1 mice for 4 wk attenuated MAP and glomerulosclerosis, suggesting a protective effect of low-dose IL-2 administration via TREG expansion. In contrast, infusion of higher levels of IL-2 in mice has been reported to expand cytotoxic T cells, effector T cells, and natural killer cells, contributing to the inflammatory environment (5). Although speculative, the attenuated renal IL-2 expression in the SLE mice fed a 4% NaCl diet may be consistent with a lessening of the inflammatory milieu in the kidneys.
Perspectives and Significance
Taken together, these findings suggest that a long-term high-salt diet does not aggravate hypertension or renal injury in NZBWF1 mice, despite the increase in circulating autoantibodies. The effect of high salt to increase urinary ET-1 excretion and lessen ETA receptor expression may have altered the balance between vasoconstrictive ETA and vasodilatory ETB receptors, thus promoting the natriuretic actions of ET-1 and preserving blood pressure.
GRANTS
This work was supported by American Heart Association Predoctoral Fellowship AHA34380830 to E. L. Dent, Veterans Administration Merit award BX002604 to M. J. Ryan, NIH RO1HL134711 to J. M. Sasser, and P01HL051971, P20GM104357, and U54GM115428 to the Department of Physiology and Biophysics, University of Mississippi Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.J.B. and M.J.R. conceived and designed research; H.J.B., J.M.S., and M.J.R. performed experiments; E.L.D., H.J.B., J.M.S., and M.J.R. analyzed data; E.L.D., H.J.B., J.M.S., and M.J.R. interpreted results of experiments; E.L.D., H.J.B., J.M.S., and M.J.R. prepared figures; E.L.D., H.J.B., and M.J.R. drafted manuscript; E.L.D., H.J.B., J.M.S., and M.J.R. edited and revised manuscript; E.L.D., H.J.B., J.M.S., and M.J.R. approved final version of manuscript.
REFERENCES
- 1.Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc 88: 987–995, 2013. doi: 10.1016/j.mayocp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 30: 493–496, 2003. [PubMed] [Google Scholar]
- 3.Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol 28: 497–505, 2016. doi: 10.1097/BOR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boumpas DT, Austin HA III, Fessler BJ, Balow JE, Klippel JH, Lockshin MD. Systemic lupus erythematosus: emerging concepts. Part 1: Renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med 122: 940–950, 1995. doi: 10.7326/0003-4819-122-12-199506150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311: 1924–1927, 2006. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 6.Budman DR, Steinberg AD. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med 136: 1003–1007, 1976. doi: 10.1001/archinte.1976.03630090033009. [DOI] [PubMed] [Google Scholar]
- 7.Cogswell ME, Frieden TR. Dietary sodium and cardiovascular disease risk. N Engl J Med 375: 2407–2408, 2016. doi: 10.1056/NEJMc1612304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim H-S, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005. doi: 10.1172/JCI23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev 68: 357–418, 2016. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M; Intersalt Cooperative Research Group . Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ 312: 1249–1253, 1996. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 86: 26–31, 2015. doi: 10.1136/jnnp-2014-307928. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald KC, Munger KL, Hartung H-P, Freedman MS, Montalbán X, Edan G, Wicklein E-M, Radue E-W, Kappos L, Pohl C, Ascherio A; BENEFIT Study Group . Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol 82: 20–29, 2017. doi: 10.1002/ana.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurbanov K, Rubinstein I, Hoffman A, Abassi Z, Better OS, Winaver J. Differential regulation of renal regional blood flow by endothelin-1. Am J Physiol Renal Physiol 271: F1166–F1172, 1996. doi: 10.1152/ajprenal.1996.271.6.F1166. [DOI] [PubMed] [Google Scholar]
- 14.Han C, Robinson DW Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 33: 2167–2172, 2006. [PubMed] [Google Scholar]
- 15.Han S, Zhuang H, Shumyak S, Yang L, Reeves WH. Mechanisms of autoantibody production in systemic lupus erythematosus. Front Immunol 6: 228, 2015. doi: 10.3389/fimmu.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M, Shields K, Wasko MC. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 12: 1004–1015, 2013. doi: 10.1016/j.autrev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 16a.Institute of Medicine Sodium and chloride. In: Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: The National Academies Press, 2005, p. 269–357. [Google Scholar]
- 17.Khafagy AM, Stewart KI, Christianson MS, Tao Y, Blanck JF, Shen W. Effect of menopause hormone therapy on disease progression in systemic lupus erythematosus: A systematic review. Maturitas 81: 276–281, 2015. doi: 10.1016/j.maturitas.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Khaledifar A, Gharipour M, Bahonar A, Sarrafzadegan N, Khosravi A. Association between salt intake and albuminuria in normotensive and hypertensive individuals. Int J Hypertens 2013: 523682, 2013. doi: 10.1155/2013/523682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khraishi M, Aslanov R, Rampakakis E, Pollock C, Sampalis JS. Prevalence of cardiovascular risk factors in patients with psoriatic arthritis. Clin Rheumatol 33: 1495–1500, 2014. doi: 10.1007/s10067-014-2743-7. [DOI] [PubMed] [Google Scholar]
- 20.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurmann RD, Mankad R. Atherosclerotic heart disease in women with autoimmune rheumatologic inflammatory conditions. Can J Cardiol 34: 381–389, 2018. doi: 10.1016/j.cjca.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Lai CH, Lai W-W, Chiou M-J, Lin W-C, Yang Y-J, Li C-Y, Tsai L-M. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Ann Rheum Dis 75: 1350–1356, 2016. doi: 10.1136/annrheumdis-2015-207719. [DOI] [PubMed] [Google Scholar]
- 23.Law MR, Frost CD, Wald NJ. Dietary salt and blood pressure. J Hypertens Suppl 9: S37–S41, 1991. [PubMed] [Google Scholar]
- 25.Mathis KW, Venegas-Pont M, Flynn ER, Williams JM, Maric-Bilkan C, Dwyer TM, Ryan MJ. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol 305: R711–R719, 2013. doi: 10.1152/ajpregu.00602.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathis KW, Venegas-Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59: 673–679, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathis KW, Venegas-Pont M, Masterson CW, Wasson KL, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt-sensitive. Am J Physiol Regul Integr Comp Physiol 301: R1281–R1285, 2011. doi: 10.1152/ajpregu.00386.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity 1: 219–229, 1994. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 30.Nakano M, Ueno M, Hasegawa H, Watanabe T, Kuroda T, Ito S, Arakawa M. Renal haemodynamic characteristics in patients with lupus nephritis. Ann Rheum Dis 57: 226–230, 1998. doi: 10.1136/ard.57.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55: 829–835, 2006. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Norlander AE, Saleh MA, Pandey AK, Itani HA, Wu J, Xiao L, Kang J, Dale BL, Goleva SB, Laroumanie F, Du L, Harrison DG, Madhur MS. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2: e92801, 2017. doi: 10.1172/jci.insight.92801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panoulas VF, Douglas KMJ, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 46: 1477–1482, 2007. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 34.Pollock DM. Renal endothelin in hypertension. Curr Opin Nephrol Hypertens 9: 157–164, 2000. doi: 10.1097/00041552-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Pollock DM, Allcock GH, Krishnan A, Dayton BD, Pollock JS. Upregulation of endothelin B receptors in kidneys of DOCA-salt hypertensive rats. Am J Physiol Renal Physiol 278: F279–F286, 2000. doi: 10.1152/ajprenal.2000.278.2.F279. [DOI] [PubMed] [Google Scholar]
- 36.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 37.Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 39: 257–268, 2010. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter CM. Role of endothelin in chronic renal failure–developments in renal involvement. Rheumatology (Oxford) 45, Suppl 3: iii36–iii38, 2006. doi: 10.1093/rheumatology/kel278. [DOI] [PubMed] [Google Scholar]
- 39.Ryan MJ, McLemore GR Jr. Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 292: R736–R742, 2007. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 40.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jiménez-Jáimez J, Díaz-Chamorro A, Jiménez-Alonso J; Grupo Lupus Virgen de las Nieves . Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 38: 1026–1032, 2011. doi: 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 41.Salgado E, Bes-Rastrollo M, de Irala J, Carmona L, Gómez-Reino JJ. High sodium intake is associated with self-reported rheumatoid arthritis: a cross sectional and case control analysis within the SUN cohort. Medicine (Baltimore) 94: e0924, 2015. doi: 10.1097/MD.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasser JM, Moningka NC, Cunningham MW Jr, Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Regul Integr Comp Physiol 298: R740–R746, 2010. doi: 10.1152/ajpregu.90875.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxena R, Mahajan T, Mohan C. Lupus nephritis: current update. Arthritis Res Ther 13: 240, 2011. doi: 10.1186/ar3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scrivo R, Massaro L, Barbati C, Vomero M, Ceccarelli F, Spinelli FR, Riccieri V, Spagnoli A, Alessandri C, Desideri G, Conti F, Valesini G. The role of dietary sodium intake on the modulation of T helper 17 cells and regulatory T cells in patients with rheumatoid arthritis and systemic lupus erythematosus. PLoS One 12: e0184449, 2017. doi: 10.1371/journal.pone.0184449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaharir SS, Mustafar R, Mohd R, Mohd Said MS, Gafor HA. Persistent hypertension in lupus nephritis and the associated risk factors. Clin Rheumatol 34: 93–97, 2015. doi: 10.1007/s10067-014-2802-0. [DOI] [PubMed] [Google Scholar]
- 46.Sharif K, Amital H, Shoenfeld Y. The role of dietary sodium in autoimmune diseases: The salty truth. Autoimmun Rev 17: 1069–1073, 2018. [Erratum in Autoimmun Rev 18: 214, 2019.] doi: 10.1016/j.autrev.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Taylor EB, Sasser JM, Maeda KJ, Ryan MJ. Expansion of regulatory T cells using low-dose interleukin-2 attenuates hypertension in an experimental model of systemic lupus erythematosus. Am J Physiol Renal Physiol 317: F1274–F1284, 2019. doi: 10.1152/ajprenal.00616.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Doornum S, Brand C, King B, Sundararajan V. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum 54: 2061–2068, 2006. doi: 10.1002/art.21932. [DOI] [PubMed] [Google Scholar]
- 49.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56: 643–649, 2010. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venegas-Pont M, Mathis KW, Iliescu R, Ray WH, Glover PH, Ryan MJ. Blood pressure and renal hemodynamic responses to acute angiotensin II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 301: R1286–R1292, 2011. doi: 10.1152/ajpregu.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1282–R1289, 2009. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vignon-Zellweger N, Heiden S, Miyauchi T, Emoto N. Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci 91: 490–500, 2012. doi: 10.1016/j.lfs.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 53.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517, 2013. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H, Huang X, Qiu H, Zhao M, Liao W, Yuan S, Xie Y, Dai Y, Chang C, Yoshimura A, Lu Q. High salt promotes autoimmunity by TET2-induced DNA demethylation and driving the differentiation of Tfh cells. Sci Rep 6: 28065, 2016. doi: 10.1038/srep28065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 48-49: 10–13, 2014. doi: 10.1016/j.jaut.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P, Lu Q. Genetic and epigenetic influences on the loss of tolerance in autoimmunity. Cell Mol Immunol 15: 575–585, 2018. doi: 10.1038/cmi.2017.137. [DOI] [PMC free article] [PubMed] [Google Scholar]