Abstract
To determine what exacerbate severity of the COVID-19 among patients without comorbidities and advanced age and investigate potential clinical indicators for early surveillance, we adopted a nested case−control study, design in which severe cases (case group, n = 67) and moderate cases (control group, n = 67) of patients diagnosed with COVID-19 without comorbidities, with ages ranging from 18 to 50 years who admitted to Wuhan Tongji Hospital were matched based on age, sex and BMI. Demographic and clinical characteristics, and risk factors associated with severe symptoms were analysed. Percutaneous oxygen saturation (SpO2), lymphocyte counts, C-reactive protein (CRP) and IL-10 were found closely associated with severe COVID-19. The adjusted multivariable logistic regression analyses revealed that the independent risk factors associated with severe COVID-19 were CRP (OR 2.037, 95% CI 1.078–3.847, P = 0.028), SpO2 (OR 1.639, 95% CI 0.943–2.850, P = 0.080) and lymphocyte (OR 1.530, 95% CI 0.850–2.723, P = 0.148), whereas the changes exhibited by indicators influenced incidence of disease severity. Males exhibited higher levels of indicators associated with inflammation, myocardial injury and kidney injury than the females. This study reveals that increased CRP levels and decreased SpO2 and lymphocyte counts could serve as potential indicators of severe COVID-19, independent of comorbidities, advanced age and sex. Males could at higher risk of developing severe symptoms of COVID-19 than females.
Key words: Coronavirus disease 2019, C-reactive protein, nested case−control study, peripheral oxygen saturation, severe acute respiratory syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19) pandemic has spread rapidly, affecting more than 215 countries globally since the outbreak of the disease, with approximately 5% of patients with COVID-19 and 20% of hospitalised patients with COVID-19 experiencing severe symptoms that required intensive care [1, 2]. Hypoxaemic respiratory failure has been identified as the most common cause of admission of patients to the intensive care unit (ICU), and there is a pressing need for mechanical ventilation therapy in most patients presenting severe symptoms of COVID-19 to avoid negative effects caused by hypoxaemia [3, 4]. In addition to respiratory failure, patients with severe COVID-19 often present with lymphopenia, thrombocytopenia and coagulation abnormalities and are at high risk of rapidly progressing to acute cardiac injury, renal injury, hepatic injury, heart failure, neurologic manifestations, rhabdomyolysis, coagulopathy and shock or even death [5–9]. Therefore, a timely assessment of the condition of patients with severe COVID-19 to facilitate administration of appropriate treatments considerably saves a patient's life.
Emerging data have revealed that people with pre-existing conditions such as cardiovascular diseases, chronic pulmonary disease, diabetes, carcinoma and obesity are more likely to develop severe COVID-19 [10, 11], and that males and the elderly are thought to be prone to developing severe symptoms, which could act as independent risk factors for poor prognosis. Fifty models have been used to predict risks of progression to severity or death in patients with COVID-19 patients, and the prominent prognostic factors that are frequently considered include comorbidities, age, sex, lymphocyte counts and C-reactive protein (CRP), among others [12]. However, most current studies on COVID-19 have been conducted in populations that are predominantly composed of males and the elderly, and often coupled with other comorbidities [13–15]. However, the specific clinical indicators that contribute to progression and severe symptoms in patients with COVID-19, which are independent of comorbidities, advanced age and gender, remain indeterminate.
The aim of the present nested case−control study was to investigate risks associated with suspected severe disease, and retrospectively analyse the clinical parameters in young and middle-aged patients diagnosed with moderate and severe COVID-19 on admission, without comorbidities and based on sex, age and body mass index (BMI). A prediction model combined with clinical data can provide insights that could help mitigate the burden of care, thereby enabling efficient triaging of patients, rational allocation of resources and prompt treatments.
Methods
Study participants
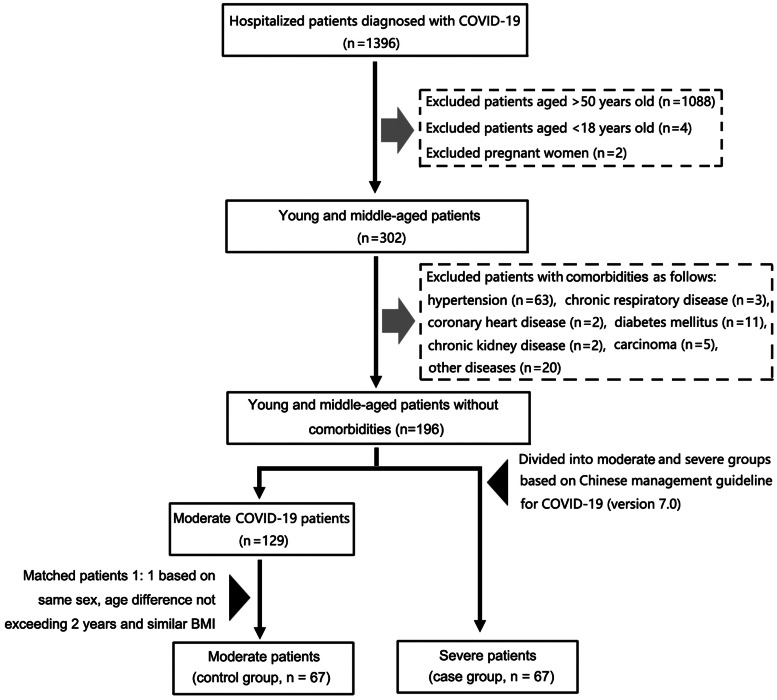
A retrospective nested case−control study of patients with severe COVID-19 was conducted. Clinical data of 1396 patients diagnosed with COVID-19 admitted to the Infection Department of Wuhan Tongji Hospital from 27 January to 12 February 2020 were collected and compiled, and follow-up made until 12 March 2020. All patients were assessed comprehensively based on age, comorbidity and severity of disease at admission. Exclusion criteria included: (1) age >50 years or <18 years old or pregnant women, (2) a history of comorbidities, including hypertension, chronic respiratory disease, coronary heart disease, diabetes, chronic kidney disease, tumour and other diseases (including history of surgery, hepatitis, gout, anaemia, thyroid disease, breast disease, tuberculosis, obesity etc.). Middle-aged patients with no history of comorbidities were divided into patients with moderate COVID-19 (n = 129) and patients with severe COVID-19 (n = 67) with reference to the Chinese management guideline for COVID-19 (version 7.0) [16]. The case group was matched with the control group 1:1, based on same sex, age difference not exceeding 2 years and similar BMI. Finally, 67 patients with severe COVID-19 (case group) and 67 patients with moderate COVID-19 (control group) were enrolled for the study (Fig. 1).
Fig. 1.
A flow diagram representing recruitment of patients with COVID-19 for the study. *Other diseases included surgical histories, hepatitis, gout, anaemia, thyroid diseases, breast diseases, tuberculosis and obesity. COVID-19, Coronavirus disease 2019.
The study was approved by the National Health Commission of China and the Institutional Review Committee of Tongji Hospital (Wuhan) affiliated to Tongji Medical College of Huazhong University of Science and Technology. The ethics committee of the designated hospital waived the requirement for obtaining informed consent.
Definitions
A positive COVID-19 case was defined as a positive high-throughput sequencing of nasopharyngeal swab specimens or positive nucleic acid detection by real-time reverse transcription-polymerase chain reaction (RT-PCR), according to the World Health Organization interim guidelines [17].
Patients with moderate disease were defined as patients diagnosed with COVID-19 presenting with fever and respiratory symptoms, and pneumonia manifestations visible via imaging.
Patients with severe disease were defined as patients diagnosed with COVID-19 and they met any of the following criteria: (1) respiratory distress with RR ⩾30 times/min; (2) peripheral oxygen saturation (SpO2) ≤ 93% at rest and (3) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa).
History of comorbidities was defined as a documented acute or chronic illness, surgical injury, or adverse physical condition prior to infection with COVID-19.
Data collection
We evaluated clinical and laboratory parameters of the two case and control groups of patients during hospitalisation, and all data were extracted from the hospital's electronic medical records. Data were independently collected and reviewed by two or more medical professionals using standardised data sets. Demographic characteristics included sex, age, comorbidities, time from the onset of illness to hospital admission and length of hospital stay; clinical symptoms included fever, diarrhoea, cough, chest distress and fatigue; treatments included antivirals, antibiotics, corticosteroids, high-flow nasal cannula oxygen therapy (HFNC), intravenous immunoglobulin (IVIG), invasive mechanical ventilation (IV), non-invasive mechanical ventilation (NIV), extracorporeal membrane oxygenation (ECMO) and others. Laboratory parameters included physiological parameters (heart rate, blood pressure, respiratory rate and oxygen saturation) and biological parameters (blood cell counts, blood chemistry analysis, assessment of coagulation, liver and kidney function, measurement of electrolytes, markers of inflammation and myocardial injury). All laboratory tests were performed at the laboratory of Wuhan Tongji Hospital.
Statistical analysis
For matched cases, categorical variables were expressed as counts and numerical percentages (%) and analysed using McNemar's test. Continuous variables were expressed as median and inter-quartile range (IQR), paired t-test and Wilcoxon signed-rank test were used as appropriate based on whether the data conformed to a normal distribution. When comparing differences in cases by gender, categorical variables were analysed using chi-squared (χ2) test or Fisher's exact test as needed. Comparisons of continuous variables were made using the Mann−Whitney U test for they did not conform to the normal distribution. Non-parametric Spearman's correlation analysis was used to analyse the correlation between variables and severe COVID-19. Receiver operating characteristic (ROC) curve analysis, single variable and multivariable conditional logistic regression model analyses were used to analyse risk factors associated with severe COVID-19. The variables included in the stepwise multivariable model analyses were risk factors of P value < 0.2 in single variable analysis, while sex and age were included in the adjusted model. Relative risks were estimated by calculating an odds ratio (OR) and an adjusted odds ratio (aOR) at corresponding 95% confidence intervals (CIs). A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistic version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Demographic and clinical characteristics of patients with COVID-19 patients between case and control groups
A total of 134 young and middle-aged patients with COVID-19 were enrolled for the study after exclusion of hospitalised patients with COVID-19 who were over 50 and under 18 years old, as well as pregnant and patients with a history of comorbidities, with a median age of 43 years, median time from onset of illness to hospitalisation of 10 days, and a median time in hospital of 17 days, and 72 (53.7%) were male, 62 (46.3%) were female. The most prevalent symptoms were fever (90.3%), followed by cough (81.3%), chest distress (44.8%), whereas fatigue (29.9%) and diarrhoea (19.4%) were less likely to present in patients with COVID-19. The most common treatment interventions used were antivirals (97%), followed by antibiotics (72.4%).
The length of hospital stay was significantly longer in the severe cases group than in the moderate control group (median time: 20 days vs. 14 days, P = 0.006), which was consistent with the anticipated presumptive results. With reference to treatment modalities, the use of antibiotics (P = 0.015), corticosteroids (P < 0.0001) and NIV was considerably higher in the case group than in the control group. Clinical parameters including heart rate (P = 0.001), respiratory rate (P = 0.001), neutrophils (P = 0.003), lactate dehydrogenase (LDH) (P < 0.0001), CRP (P < 0.0001), procalcitonin (PCT) (P = 0.001), fibrinogen (P = 0.007), IL-8 (P = 0.045), IL-10 (P = 0.019) significantly increased in the case group when compared to the control group. In addition, SpO2 (P < 0.0001), lymphocytes (P < 0.0001) and albumin (P = 0.003) were substantially decreased in the case group when compared to the control group (Table 1).
Table 1.
Demographic and clinical characteristics of patients diagnosed with moderate and severe COVID-19 on admission
| Variables | All patients (n = 134) | Cases (n = 67) | Controls (n = 67) | P value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 72 (53.7) | 36 (53.7) | 36 (53.7) | – |
| Female | 62 (46.3) | 31 (46.3) | 31 (46.3) | – |
| Age, year (IQR) | 43 (38, 47) | 43 (37, 47) | 44 (39, 47) | – |
| Time from the onset of illness to hospital admission, days (IQR) | 10 (7, 14) | 10 (7, 13) | 10 (7, 15) | 0.521 |
| Length of hospital stay, days (IQR) | 17 (14, 22) | 20 (15, 25) | 14 (13, 20) | 0.006 |
| Symptom, n (%) | ||||
| Fever (temperature ⩾ 37.3 °C) | 121 (90.3) | 63 (94.0) | 58 (86.6) | 0.267 |
| Diarrhoea | 26 (19.4) | 15 (22.4) | 11 (16.4) | 0.481 |
| Cough | 109 (81.3) | 57 (85.1) | 52 (77.6) | 0.359 |
| Fatigue | 40 (29.9) | 18 (26.9) | 22 (32.8) | 0.541 |
| Chest distress | 60 (44.8) | 32 (47.8) | 28 (41.8) | 0.618 |
| Treatment, n (%) | ||||
| Antibiotics | 97 (72.4) | 55 (82.1) | 42 (62.7) | 0.015 |
| Antiviral treatment | 130 (97.0) | 64 (95.5) | 66 (98.5) | 0.625 |
| Corticosteroids | 46 (34.3) | 33 (49.3) | 13 (19.4) | <0.0001 |
| IVIG | 29 (21.6) | 16 (23.9) | 13 (19.4) | 0.678 |
| HFNC | 2 (1.5) | 2 (3.0) | 0 (0.0) | – |
| NIV | 5 (3.7) | 5 (7.5) | 0 (0.0) | – |
| IV | 2 (1.5) | 1 (1.5) | 1 (1.5) | 1.000 |
| ECMO | 1 (0.7) | 1 (1.5) | 0 (0.0) | – |
| Physiological parameter (IQR) | ||||
| Heart rate, times per min | 90 (80, 101) | 95 (83, 106) | 83 (76, 98) | 0.001 |
| Systolic pressure | 123 (115, 130) | 123 (119, 131) | 121 (112,130) | 0.154 |
| Diastolic pressure | 78 (72, 88) | 81 (71, 89) | 78 (72, 87) | 0.913 |
| RR, breaths per min | 20 (20, 22), n = 133 | 20 (20,23), n = 66 | 20 (19, 20) | 0.001 |
| SpO2, % | 97 (95, 98), n = 126 | 95 (92, 97), n = 60 | 97 (96, 98), n = 66 | <0.0001 |
| Biological parameters (IQR) | ||||
| WBC, × 109/l | 5.3 (4.0, 6.8) | 5.4 (4.0, 7.3) | 5.3 (4.0, 6.5) | 0.078 |
| Neutrophil, × 109/l | 3.7 (2.4, 5.0) | 4.0 (2.8, 5.6) | 3.3 (2.2, 4.2) | 0.003 |
| Lymphocyte, × 109/l | 1.17 (0.83, 1.46) | 0.89 (0.68, 1.33) | 1.3 (1.1, 1.6) | <0.0001 |
| Haemoglobin, g/l | 131 (122, 142) | 131 (118, 142) | 132 (123, 143) | 0.387 |
| Platelet, × 109/l | 234.5 (171.8, 306.0) | 230.0 (165.0, 294.0) | 241.0 (173.0, 324.0) | 0.091 |
| Albumin, g/l | 36.3 (32.9, 39.1) | 34.8 (31.3, 37.6) | 37.9 (33.7, 40.2) | 0.003 |
| LDH, U/l | 272.5 (218.0, 362.3) | 301.0 (242.0, 431.0) | 236.0 (199.0, 302.0) | <0.0001 |
| NaHCO3, mmol/l | 23.9 (22.2, 25.3) | 23.6 (21.8, 25.3) | 24.1 (22.7, 25.4) | 0.806 |
| Urea, mmol/l | 3.6 (2.8, 4.5) | 3.8 (2.7, 4.7) | 3.5 (3.0, 4.2) | 0.510 |
| Creatinine, μmol/l | 65.0 (54.8, 79.0) | 66.0 (52.0, 84.0) | 65.0 (57.0, 78.0) | 0.977 |
| Creatine kinase, U/l | 74.5 (44.0, 163.5), n = 42 | 80.5 (36.5, 316.8), n = 20 | 69.0 (46.8, 116.3), n = 22 | 0.201 |
| CRP, mg/l | 26.2 (3.9, 65.4), n = 132 | 58.9 (13.0, 101.0) | 10.8 (2.1, 31.8), n = 65 | <0.0001 |
| PCT, ng/ml | 0.06 (0.03, 0.98), n = 88 | 0.06 (0.04, 1.53), n = 54 | 0.04 (0.03, 0.07), n = 34 | 0.001 |
| SF, μg/l | 564.8 (212.0, 1069.6), n = 40 | 524.8 (251.7, 1104.2), n = 23 | 604.7 (108.0, 1104.3), n = 17 | 0.941 |
| Fibrinogen, g/l | 5.2 (3.9, 6.0), n = 109 | 5.6 (4.5, 6.5), n = 50 | 4.7 (3.6, 5.7), n = 59 | 0.007 |
| hs-cTnI, pg/ml | 2.7 (1.9, 4.9), n = 94 | 3.1 (1.9, 8.0), n = 48 | 1.9 (1.9, 4.2), n = 46 | 0.093 |
| IL-2R, U/ml | 693.0 (526.0, 886.0), n = 51 | 743.0 (536.0, 1028.0), n = 27 | 654.5 (461.5, 842.5), n = 24 | 0.535 |
| IL-6, pg/ml | 11.3 (3.0, 30.9), n = 51 | 20.3 (5.6, 41.7), n = 27 | 6.5 (1.7, 19.8), n = 24 | 0.088 |
| IL-8, pg/ml | 13.0 (7.8, 22.5), n = 51 | 18.5 (10.3, 22.5), n = 27 | 10.3 (6.9, 24.3), n = 24 | 0.045 |
| IL-10, pg/ml | 5.0 (5.0, 9.0), n = 51 | 7.3 (5.0, 11.4), n = 27 | 5.0 (5.0, 5.0), n = 24 | 0.019 |
| NT-proBNP, pg/ml | 62.0 (19.8, 188.8), n = 86 | 86.5 (22.8, 294.8), n = 44 | 43.0 (29.3, 1.2), n = 42 | 0.117 |
| D-Dimer, μg/ml | 0.57 (0.34, 1.16), n = 127 | 0.68 (0.39, 1.16), n = 63 | 0.44 (0.29, 1.19), n = 64 | 0.294 |
IQR, interquartile range; IVIG, intravenous immunoglobin; HFNC, high-flow nasal cannula oxygen therapy; NIV, non-invasive mechanical ventilation; IV, invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation; RR, respiratory rate; SpO2, peripheral oxygen saturation; WBC, white blood cell count; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCT, procalcitonin; SF, serum ferritin; hs-cTnI, hypersensitive cardiac troponin I; NT-proBNP, N terminal pro B type natriuretic peptide.
Data are shown as the median (IQR), n (%), n, where n is the actual number with available data. Categorical variables were analysed using McNemar's test and continuous variables were analysed using paired t-test or Wilcoxon signed-rank test as appropriate based on whether the data conformed to a normal distribution.
Demographic and clinical characteristics of patients with COVID-19 based on gender
To investigate the disparity between parameters among patients with COVID-19 based on gender, we further grouped patients by sex. The results of the analyses revealed that apart from haemoglobin, which exhibited distinct gender characteristics, other indicators such as LDH (P = 0.005), creatinine (P < 0.0001), creatine kinase (P = 0.008), CRP (P = 0.001), PCT (P < 0.0001), serum ferritin (SF) (P < 0.0001), fibrinogen (P = 0.01) and hs-cTnI (P = 0.008) levels were significantly higher in males than in females (Table 2). Furthermore, patients of different gender demonstrated significant differences among certain indicators in both the case and control groups (Supplementary Tables S1 and S2). The observation was consistent with the findings of previous studies, which have supported the hypothesis that males are more likely to suffer severe clinical symptoms than females when infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Table 2.
Demographic and clinical characteristics of male and female patients with COVID-19 on admission
| Variables | Male patients (n = 72) | Female patients (n = 62) | P value |
|---|---|---|---|
| Age, year (IQR) | 42 (37, 47) | 45 (39,47) | 0.111 |
| Time from the onset of illness to hospital admission, days (IQR) | 10 (7, 14) | 10 (7, 14) | 0.404 |
| Length of hospital stay, days (IQR) | 18 (14, 24) | 17 (13, 22) | 0.695 |
| Symptom, n (%) | |||
| Fever (temperature ⩾ 37.3 °C) | 65 (90.3) | 56 (90.3) | 0.993 |
| Diarrhoea | 11 (15.3) | 15 (24.2) | 0.193 |
| Cough | 58 (80.6) | 51 (82.3) | 0.801 |
| Fatigue | 19 (26.4) | 21 (33.9) | 0.345 |
| Chest distress | 35 (48.6) | 25 (40.3) | 0.336 |
| Treatment, n (%) | |||
| Antibiotics | 55 (76.4) | 42 (67.7) | 0.264 |
| Antiviral treatment | 69 (95.8) | 61 (98.4) | 0.386 |
| Corticosteroids | 30 (41.7) | 16 (25.8) | 0.054 |
| IVIG | 20 (27.8) | 9 (14.5) | 0.063 |
| HFNC | 2 (2.8) | 0 (0.0) | 0.186 |
| NIV | 1 (1.4) | 4 (6.5) | 0.123 |
| IV | 2 (2.8) | 0 (0.0) | 0.186 |
| ECMO | 1 (1.4) | 0 (0.0) | 0.352 |
| Physiological parameter (IQR) | |||
| Heart rate, per min | 92 (81, 102) | 88 (78, 101) | 0.110 |
| Systolic pressure | 124 (118, 132) | 120 (113, 129) | 0.070 |
| Diastolic pressure | 80 (72, 90) | 76 (70, 86) | 0.070 |
| RR, breaths per min | 20 (20, 21), n = 71 | 20 (20,22) | 0.488 |
| SpO2, % | 96(95, 98), n = 68 | 97 (95, 99), n = 58 | 0.283 |
| Biological parameters (IQR) | |||
| WBC, × 109/l | 5.3 (4.4, 6.7) | 5.4 (3.9, 7.0) | 0.815 |
| Neutrophil, × 109/l | 3.8 (2.5, 5.0) | 3.6 (2.2, 4.7) | 0.693 |
| Lymphocyte, × 109/l | 1.1 (0.78, 1.4) | 1.2 (0.9, 1.5) | 0.269 |
| Haemoglobin, g/l | 138 (129, 146) | 124 (114, 131) | <0.0001 |
| Platelet, × 109/l | 210.5 (162.8, 295.8) | 242.0 (184.0, 312.8) | 0.131 |
| Albumin, g/l | 36.3 (33.1, 38.7) | 36.6 (32.8, 39.8) | 0.560 |
| LDH, U/l | 304.0 (222.0, 424.8) | 247.0 (211.8, 294.3) | 0.005 |
| NaHCO3, mmol/l | 23.6 (21.9, 25.2) | 24.1 (23.0, 25.5) | 0.141 |
| Urea, mmol/l | 3.6 (2.8, 4.5) | 3.6 (2.7, 4.6) | 0.510 |
| Creatinine, μmol/l | 75.0 (65.0, 84.0) | 50.0 (55.5, 65.0) | <0.0001 |
| Creatine kinase, U/l | 139.0 (64.5, 195.5), n = 21 | 52.0 (36.0, 86.0), n = 21 | 0.008 |
| CRP, mg/l | 47.3 (9.6, 92.4), n = 70 | 12.3(2.5, 36.3) | 0.001 |
| PCT, ng/ml | 0.09 (0.04, 0.16), n = 45 | 0.04 (0.02, 0.06), n = 43 | <0.0001 |
| SF, μg/l | 965.6 (677.7, 1536.5), n = 17 | 225.0 (106.1, 604.7), n = 23 | <0.0001 |
| Fibrinogen, g/l | 5.7 (4.5, 6.5), n = 59 | 4.3 (3.7, 5.3), n = 50 | 0.001 |
| hs-cTnI, pg/ml | 3.6 (1.9, 6.5), n = 47 | 1.9 (1.9, 3.5), n = 47 | 0.008 |
| IL-2R, U/ml | 808.5 (530.8, 992.5), n = 24 | 675.0 (474.0, 755.0), n = 27 | 0.160 |
| IL-6, pg/ml | 18.3 (6.0, 42.0), n = 24 | 6.3 (1.9, 23.3), n = 27 | 0.061 |
| IL-8, pg/ml | 15.2 (8.1, 21.0), n = 24 | 11.8 (7.7, 25), n = 27 | 0.651 |
| IL-10, pg/ml | 5.1 (5.0, 10.2), n = 24 | 5.0 (5.0, 7.3), n = 27 | 0.184 |
| NT-proBNP, pg/ml | 62.0 (18.0, 158.8), n = 44 | 62.5 (19.8, 201.0), n = 42 | 0.628 |
| D-Dimer, μg/ml | 0.57 (0.37, 1.17), n = 68 | 0.56 (0.31, 1.14), n = 59 | 0.617 |
IQR, interquartile range; IVIG, intravenous immunoglobin; HFNC, high-flow nasal cannula oxygen therapy; NIV, non-invasive mechanical ventilation; IV, invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation; RR, respiratory rate; SpO2, peripheral oxygen saturation; WBC, white blood cell count; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCT, procalcitonin; SF, serum ferritin; hs-cTnI, hypersensitive cardiac troponin I; NT-proBNP, N terminal pro B type natriuretic peptide.
Data are shown as the median (IQR), n (%), n, where n is the actual number with available data. Categorical variables were analysed using chi-squared (χ2) test or Fisher's exact test as needed. Comparisons of continuous variables were analysed using the Mann−Whitney U test for they did not conform to the normal distribution.
Independent risk factors associated with severe COVID-19
The correlation between parameters recorded during initial clinical testing on admission and the occurrence of severe COVID-19 symptoms was analysed using Spearman's correlation analysis to further explore risk factors associated with severe COVID-19. The analyses results revealed that there were negative correlations between the levels of SpO2 (r = −0.372, P < 0.0001), lymphocytes (r = −0.356, P < 0.0001) and the occurrence of severe COVID-19; however, the levels of CRP (r = 0.423, P < 0.0001) and IL-10 (r = 0.474, P < 0.0001) were positively correlated with the occurrence of severe COVID-19 (Supplementary Table S3). In addition, ROC curve analyses revealed that SpO2 (AUC = 0.713, 95% CI 0.620–0.807), lymphocytes (AUC = 0.706, 95% CI 0.616–0.795), CRP (AUC = 0.744, 95% CI 0.659–0.829) and IL-10 (AUC = 0.742, 95% CI 0.602–0.881) were potential predictive values for severe COVID-19 (Supplementary Fig. S1, Table S4).
Single variable and multivariable conditional logistic regression model analyses were further performed to elucidate the risk factors for severe COVID-19 after classifying parameters into sub-groups with a cutoff value in the ROC curve analysis. We subsequently identified 13 potential indicators of severe COVID-19 after adjusting for sex and age, with CRP as the most significant indicator (aOR = 2.651, 95% CI 1.599–4.506, P < 0.0001) (Table 3). Furthermore, results of the stepwise multivariable analyses demonstrated that the independent risk factors associated with severe COVID-19 were CRP (OR 1.798, 95% CI 0.975–3.316, P = 0.060), SpO2 (OR 1.630, 95% CI 0.933–2.849, P = 0.086) and lymphocyte (OR 1.521, 95% CI 0.847–2.731, P = 0.160) and after the inclusion of sex and age, CRP (OR 2.037, 95% CI 1.078–3.847, P = 0.028), SpO2 (OR 1.639, 95% CI 0.943–2.850, P = 0.080) and lymphocyte (OR 1.530, 95% CI 0.850–2.723, P = 0.148) substantially influenced progression to severe COVID-19 (Table 4). The results suggest that apart from the effects of comorbidities, sex and age, increased CRP is potentially a key risk factor for the progression of patients from moderate-to-severe COVID-19, and, that decreased SpO2 and lymphocyte counts could be associated with the occurrence of severe symptoms to a lesser extent.
Table 3.
Single variable conditional logistic regression model for risk factors associated with severe COVID-19 on admission
| Variables | Crude odds ratio (95% CI) | P value | Adjusted odds ratioa (95% CI) | P value |
|---|---|---|---|---|
| HR, times per min (>89.5 vs. <89.5) | 1.739 (1.055–2.865) | 0.030 | 1.799 (1.087–2.977) | 0.022 |
| RR, breaths per min (>20.5 vs. <20.5) | 1.722 (1.063–2.791) | 0.027 | 1.744 (1.074–2.830) | 0.024 |
| SpO2, % (<95.5 vs. >95.5) | 2.369 (1.427–3.935) | 0.001 | 2.359 (1.419–3.923) | 0.001 |
| Neutrophil, × 109/l (>3.89 vs. <3.89) | 1.773 (1.088–2.889) | 0.022 | 1.779 (1.091–2.900) | 0.021 |
| Lymphocyte × 109/l (<0.97 vs. >0.97) | 1.434 (1.434–3.806) | 0.001 | 2.319 (1.407–3.823) | 0.001 |
| Albumin, g/l (<36.7 vs. >36.7) | 2.149 (1.273–3.626) | 0.004 | 2.196 (1.299–3.712) | 0.003 |
| LDH, U/l (>290.0 vs. <290.0) | 1.825 (1.126–2.959) | 0.015 | 1.911 (1.155–3.161) | 0.012 |
| CRP, mg/l (>34.8 vs. <34.8) | 2.441 (1.474–4.042) | 0.001 | 2.651 (1.559–4.506) | <0.0001 |
| PCT, ng/ml (>0.11 vs. <0.11) | 1.732 (0.991–3.028) | 0.054 | 2.022 (1.043–3.920) | 0.037 |
| Fibrinogen, g/l (>4.97 vs. <4.97) | 2.503 (1.330–4.708) | 0.004 | 2.668 (1.362–5.226) | 0.004 |
| hs-cTnI, pg/ml (>2.25 vs. <2.25) | 1.962 (1.053–3.655) | 0.034 | 2.185 (1.137–4.199) | 0.019 |
| IL-6, pg/ml (>11.94 vs. <11.94) | 1.912 (0.876–4.177) | 0.104 | 2.032 (0.882–4.683) | 0.096 |
| IL-10, pg/ml (>5.1 vs. <5.1) | 2.635 (1.207–5.754) | 0.015 | 2.724 (1.213–6.117) | 0.015 |
| NT-proBNP, pg/ml (>189.5 vs. <189.5) | 1.949 (1.062–3.575) | 0.031 | 1.939 (1.054–3.566) | 0.033 |
HR, heart rate; RR, respiratory rate; SpO2, peripheral oxygen saturation; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCT, procalcitonin; hs-cTnI, hypersensitive cardiac troponin I; NT-proBNP, N terminal pro B type natriuretic peptide.
Adjusted for age and sex. All variables were classified into sub-groups with a cutoff value in the ROC curve analysis.
Table 4.
Multivariable conditional logistic regression model for risk factors associated with severe COVID-19 on admission
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variables | Odds ratio 95% CI |
P value | Odds ratio 95% CI |
P value | Odds ratio 95% CI |
P value |
| CRP, mg/l (>34.8 vs. <34.8) | 1.798 (0.975–3.316) | 0.060 | 2.034 (1.074–3.850) | 0.029 | 2.037 (1.078–3.847) | 0.028 |
| SpO2, % (<95.5 vs. >95.5) | 1.630 (0.933–2.849) | 0.086 | 1.622 (0.933–2.821) | 0.087 | 1.639 (0.943–2.850) | 0.080 |
| Lymphocyte, × 109/l (<0.97 vs. >0.97) | 1.521 (0.847–2.731) | 0.160 | 1.555 (0.876–2.761) | 0.131 | 1.530 (0.850–2.723) | 0.148 |
| Sex (male vs. >female) | – | – | 0.726 (0.417–1.266) | 0.259 | 0.713 (0.406–1.250) | 0.237 |
| Age (year) | – | – | – | – | 0.990 (0.946–1.035) | 0.649 |
Model 1 represents unadjusted CRP, SpO2 and lymphocyte model. Model 2 includes CRP, SpO2 and lymphocyte with an additional covariate of sex. Model 3 includes CRP, SpO2 and lymphocyte with additional covariates of sex and age. CRP, C-reactive protein; SpO2, peripheral oxygen saturation. A total of 92.54% (124/134) cases with available data were included in the analysis.
Interestingly, when further exploring gender-specific independent risk factors associated with severe illness, we found that indicators characterising infection, inflammation and organic damage, including neutrophil, PCT, LDH and IL-10, generally showed greater OR values in male than in female (Supplementary Table S5). And lymphopenia appeared to be a superior risk factor for male patients, whereas increased CRP and decreased SpO2 were the dominant independent risk factors for female patients (Supplementary Table S6).
Correlation between the levels of CRP, SpO2 and lymphocytes on admission and the incidence of patients with severe COVID-19
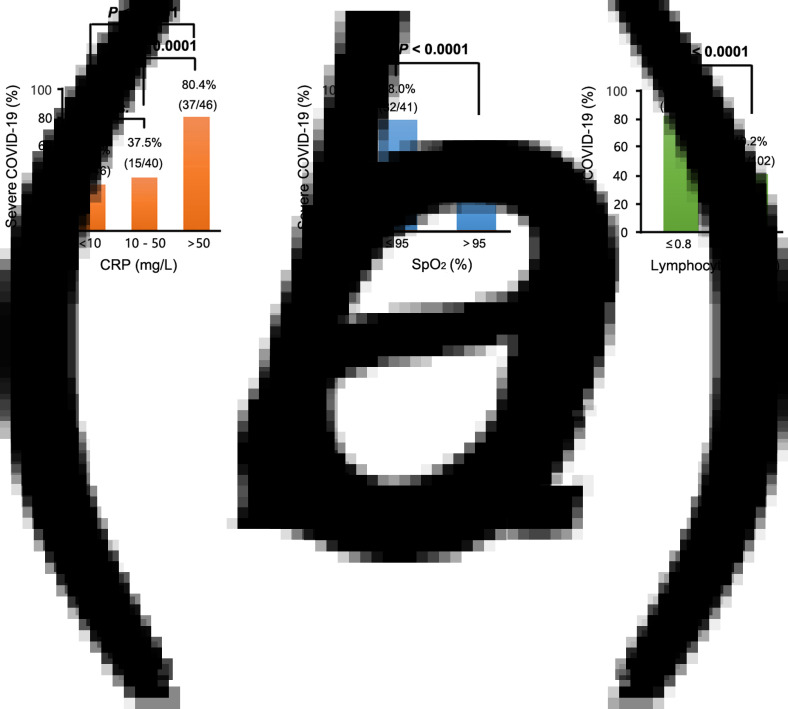
Generally, the clinically normal range for CRP is less than 10 mg/l. CRP was grouped into three levels: <10 mg/ml, 10–50 mg/ml and >50 mg/ml and differences among the three groups evaluated. The results revealed that the incidence of severe COVID-19 was significantly higher in the >50 mg/ml CRP group (37/46, 80.4%) than in <10 mg/ml (12/46, 32.6%) and 10–50 mg/ml (15/40, 37.5%) groups (both P < 0.0001). Similarly, SpO2 was divided into ≤95% and >95% groups based on the clinically normal range. The results of comparisons revealed that incidence of severe COVID-19 was considerably higher in the ≤95% group (32/41, 78.0%) than in the >95% group (28/85, 32.9%) (P < 0.0001). Lymphocyte, which was divided into ≤0.8 and >0.8 groups, also demonstrated higher incidence of severe COVID-19 in the ≤0.8 group (26/32, 81.3%) than in the >0.8 group (41/102, 40.2%) (P < 0.0001) (Fig. 2).
Fig. 2.
Percentages of patients with severe COVID-19 at different concentrations of (a) CRP, (b) SpO2 and (c) lymphocyte. In this study, approximately 98.51% (132/134), 94.03% (126/134) and 100% (134/134) of patients with COVID-19 had examinations for CRP, SpO2 and lymphocyte counts, respectively. A high proportion of patients with severe COVID-19 exhibited high CRP concentrations, low SpO2 concentrations and low lymphocyte counts. With reference to CRP, 10 mg/l is the 99th percentile upper reference limit (99th % URL), 50 mg/l indicates 5 × 99th % URL of CRP, while for SpO2, 95% is the 99th percentile upper reference limit (99th% URL), and for lymphocyte counts, 0.8 × 109/l is the 99th percentile upper reference limit (99th% URL). COVID-19, corona virus disease 2019; CRP, C-reactive protein; SpO2, peripheral oxygen saturation.
Notably, CRP was significantly higher in males than in females (P = 0.001) in relation to the comparisons of key clinical parameters in patients with COVID-19 based on gender (Table 2). Therefore, further analyses of the proportion of males and females at each level of CRP were conducted. The results revealed that no matter in moderate or severe group, the higher the CRP level on admission, the greater the proportion of male, which was more significant in severe cases (P = 0.008) (Supplementary Fig. S1). The observation suggested that CRP, as a risk factor for the occurrence of severe COVID-19, could have a more pronounced elevation in male patients than in female patients.
Discussion
Elderly patients with coexisting conditions have undergone immense suffering due to their weak immunity as a result of infection with COVID-19, and previous studies have described advanced age and pre-existing conditions as independent risk factors of COVID-19 [18–21]. Unlike previous studies, this study focused on young and middle-aged patients with no influence of previous comorbidities, and we established that increased CRP and decreased SpO2 and lymphocyte counts were significantly associated with adverse progression in patients with COVID-19, thereby exacerbating the risk of developing severe COVID-19. Furthermore, in comparison to females, males exhibited remarkably higher levels of multiple indicators associated with inflammation, myocardial injury and kidney injury, including LDH, creatinine, creatine kinase, fibrinogen, CRP, PCT, SF and hs-cTnI than the females.
Patients with severe COVID-19 were matched to moderate controls based on age, sex and BMI, to minimise the potential influence of common relevant demagogic confounders on the results. The case group had significantly longer hospital stays and a considerable need for assisted ventilation, as well as tendency to require more aggressive therapeutic interventions such as antibiotics and corticosteroids when compared to the control group, which was consistent with previous findings [22, 23]. COVID-19 is characterised by disruption of the endothelial barrier and decreased or impaired function of the alveolar-capillary oxygen transport and diffusion [12]. However, patients with severe COVID-19 often present with acute respiratory distress syndrome (ARDS) described by bilateral pulmonary infiltrates, profound hypoxemia and pulmonary oedema [24], which could explain the high demand for ventilation in severe cases. Recent studies on COVID-19 interventions and standard treatments have revealed that glucocorticoids could be remarkably effective in reducing mortality rates and the need for mechanical ventilation in patients with severe disease [25]. Consequently, proactive treatment regimens are expected to influence prognosis of patients with severe COVID-19.
Conditional logistic regression analyses were performed based on Spearman's correlation and ROC curve analyses to identify the risk factors associated with severe COVID-19. The 13 risk factors identified in the single variable analyses could be associated with severity of COVID-19. Notably, increased CRP and IL-10, and decreased SpO2 and lymphocyte counts demonstrated a high correlation with severe cases, thereby serving as potential predictive values, and the observation was consistent with the findings of previous studies [26, 27]. However, only CRP, SpO2 and lymphocyte counts were identified as independent risk factors in the multivariable analyses, with lymphocyte counts as specific risk factors for male and CRP and SpO2 for female. And the incidence of severe COVID-19 was considerably influenced by levels of the above indicators. CRP is a key and most sensitive acute-phase reaction protein, which usually increases substantially during the early stages of an infection or injury of organs, and the magnitude of increase is positively correlated with the degree of infection. The dramatic increase in CRP levels can serve as a potential biomarker to guide prognosis and treatment of inflammation. Increased CRP levels predict severity in patients with COVID-19, signifies an increased risk of death, which often features in predictive models for patient prognosis [28, 29]. SpO2 is an indicator of respiratory functions and demonstrates variations in arterial oxygenation to indicate hypoxemia in patients to a certain extent. Patients with severe COVID-19 are regularly affected by hypoxemia despite being under assisted ventilation due to respiratory dysfunction and impaired ventilation, resulting in difficulties in the maintenance of SpO2 at normal levels. Previous studies have revealed that SpO2 coupled with other indicators, facilitate assessment of risks associated with poor progression in patients to mitigate substantial impairments induced by refractory hypoxemia and respiratory failure during early stage [30].
Lymphopenia has been extensively reported in patients with severe COVID-19 [31]. SARS-CoV-2 directly attacks lymphocytes, while humoral and cellular immunity causes viral inflammation, which prevents lymphangiogenesis and accelerates lymphocyte apoptosis [32], leading to a progressive decrease in lymphocyte counts in patients. As high as 83% of hospitalised patients were described to have lymphopenia [33], and being the most common haematologic abnormality observed in patients, the parameter could lack specificity in predicting progression of patients' condition from moderate-to-severe COVID-19. Cytokine storms are underlying mechanisms in the pathogenesis of severe COVID-19, and are regularly mediated by multiple complex inflammatory cytokines, which could be associated with inhibition of antiviral adaptive response indicated by the dramatic increases in cytokines including IL-10, and coupled with lymphopenia [34]. The bilateral immunomodulatory properties of IL-10 can regulate pro-inflammatory signals and exert immunosuppressive effects through antigen-presenting cells (APC) cells, as well as stimulate T and B lymphocytes [35]. IL-10 is highly expressed in influenza and is more prominent in adaptive immune response [36], thus reflecting the level of immune response and cytokine storm trends in patients with severe disease to a certain extent. Overall, previous studies form a theoretical basis for the findings of this study, suggesting that CRP and SpO2 are prognostic factors for severe COVID-19, and lymphocyte counts and IL-10 provide useful information.
Moreover, the findings indicated that males exhibited considerably higher levels of multiple inflammatory factors and markers of cardiac and renal injury than the females regardless of comorbidities and advanced age, which could imply that the male population is more vulnerable to infection with SARS-CoV-2. Previous studies have demonstrated that angiotensin-converting enzyme 2 (ACE2), which is a receptor for SARS-CoV-2, is widely distributed in various organs, in which the virus attacks bronchial epithelium and lung cells [37]. Upregulation of ACE2 receptors can increase susceptibility to SARS-CoV-2 and exacerbates COVID-19 progression. In general, males express higher levels of ACE2 than females, especially in the lungs, which could be associated with hormonal differences [38]. In addition, chronic smoking, which is prevalent among the male population, has an effect on ACE2 expression [39]. In this study, this finding could explain the variations in susceptibility to COVID-19 among patients of different gender, suggesting that treatments and interventions should be adopted based on gender.
This study had a few shortcomings that need to be addressed in future studies. Firstly, being a retrospective study, data on patient comorbidities and clinical manifestations were based on dictated information, which could be biased. Secondly, data on certain clinical parameters were incomplete and dynamic laboratory test results were unavailable because of the sudden outbreak of COVID-19 pandemic. Thirdly, the number of young and middle-aged patients with COVID-19 patients with no comorbidities in this study was relatively limited, especially because disease severity was more homogeneous. Therefore, the results obtained in this study should be validated using a larger population. Finally, most of the patients with severe disease recruited in this study survived via treatments; however, further studies are required to obtain additional information on risk factors associated with mortality cases without comorbidities and advanced age.
Nevertheless, this study has the following strengths. To the best of our knowledge, this is the first study that adopted nested case−control study to evaluate patients with COVID-19 independent of disease history, and the effects of advanced age, gender mismatch and BMI differences were excluded where necessary to explore potential clinical risk indicators. A comparison of the clinical examination results of patients with COVID-19 based on genders, but with similarities in age and other demographic information, facilitates designing of individualised treatment regimens and rapid triaging of patients. Additionally, the information could be helpful for early surveillance of the disease to assess risks of laboratory outcomes associated with disease progression among severe cases, to promote effective planning and enhance quality of care.
Conclusions
Overall, this study revealed that increased CRP levels, decreased SpO2 and lymphocyte counts could serve as potential predictors of severe COVID-19, independent of comorbidities, advanced age and sex, and that the greater the change in indicators, the higher the incidence of disease severity. Furthermore, males exhibited substantial increases in indicators of severe COVID-19 and inflammatory markers associated with organ impairment including CRP when compared to the females, suggesting that males are more susceptible to developing severe symptoms of COVID-19 than females.
Acknowledgements
We would like to appreciate all participants recruited in this study and all the fellow clinicians who were on the front lines.
Conflict of interest
The authors have declared that no competing interest exists.
Author contribution
HC, TS and DX designed the whole study. XL contributed to data collection and supervised the laboratory testing results and undertook the data management and analysis. XL and TM took the lead in drafting and interpreting the manuscript. TM, QX, JT, YY and QT participated in the development and revision of statistical methods. All authors reviewed and approved the manuscript for publication.
Financial support
The study was supported by the National Science and Technology Major Project of China (2017ZX10202101-003, 2018ZX10731101-001-005 and 2018ZX09739002-003). The research was designed, conducted, analysed and interpreted by the authors entirely independently of the funding sources.
Ethical Standards
The study was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-C20200101). Owing to the rapid emergence of this infectious disease, written informed consent was waived.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268820002502.
click here to view supplementary material
Data availability statement
The data used to support the findings of this study are available from the authors upon request.
References
- 1.Wu Z and McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Docherty AB et al. (2020) Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369, m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Medicine 8, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Boom W et al. (2020) The search for optimal oxygen saturation targets in critically ill patients: observational data from large ICU databases. Chest 157, 566–573. [DOI] [PubMed] [Google Scholar]
- 5.Levi M et al. (2020) Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematology 7, e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L et al. (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology 77, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T et al. (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiology 5, 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatraju PK et al. (2020) Covid-19 in critically ill patients in the Seattle region – case series. New England Journal of Medicine 382, 2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal P et al. (2020) Clinical characteristics of Covid-19 in New York city. New England Journal of Medicine 382, 2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian J et al. (2020) Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncology 21, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga WJ et al. (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 324, 782–793. [DOI] [PubMed] [Google Scholar]
- 13.Grasselli G et al. (2020) Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 323, 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan WJ et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NHC. National Health Commission of the People's Republic of China. Chinese Management Guideline for COVID-19 (version 7.0). Available at http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (Accessed 25 March 2020).
- 17.WHO. World Health Organization (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. Available at https://apps.who.int/iris/handle/10665/331446 (Accessed 25 March 2020).
- 18.Zhou F et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C et al. (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine 180, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L et al. (2020) Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. Journal of Infection 80, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan WJ et al. (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. European Respiratory Journal 55, 2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arabi YM et al. (2018) Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. American Journal of Respiratory and Critical Care Medicine 197, 757–767. [DOI] [PubMed] [Google Scholar]
- 23.Alhazzani W et al. (2020) Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Critical Care Medicine 48, e440–e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Force ADT et al. (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307, 2526–2533. [DOI] [PubMed] [Google Scholar]
- 25.Siemieniuk RA et al. (2020) Drug treatments for Covid-19: living systematic review and network meta-analysis. BMJ 370, m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie J et al. (2020) Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. medRxiv. 2020.2003.2028.20045997. doi: 10.1101/2020.03.28.20045997. [DOI] [Google Scholar]
- 27.Yan L et al. (2020) A machine learning-based model for survival prediction in patients with severe COVID-19 infection. medRxiv. 2020.2002.2027.20028027. doi: 10.1101/2020.02.27.20028027. [DOI] [Google Scholar]
- 28.Colombi D et al. (2020) Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology 296, E86–E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y et al. (2020) Development and validation of an early warning score (EWAS) for predicting clinical deterioration in patients with coronavirus disease 2019. medRxiv. 2020.2004.2017.20064691. doi: 10.1101/2020.04.17.20064691. [DOI] [Google Scholar]
- 30.Guillamet CV et al. (2020) Toward a Covid-19 score-risk assessments and registry. medRxiv. 2020.2004.2015.20066860. doi: 10.1101/2020.04.15.20066860. [DOI] [Google Scholar]
- 31.Richardson S et al. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 323, 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harapan H et al. (2020) Coronavirus disease 2019 (COVID-19): a literature review. Journal of Infection and Public Health 13, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Morales AJ et al. (2020) Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Medicine and Infectious Disease 34, 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuppalli K and Rasmussen AL (2020) A glimpse into the eye of the COVID-19 cytokine storm. EBioMedicine 55, 102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang W and O'Garra A (2019) IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 50, 871–891. [DOI] [PubMed] [Google Scholar]
- 36.McKinstry KK et al. (2009) IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. Journal of Immunology 182, 7353–7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann M et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y et al. (2020) Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacological Research 157, 104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai G (2020) Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. medRxiv. 2020.2002.2005.20020107. doi: 10.1101/2020.02.05.20020107. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268820002502.
click here to view supplementary material
Data Availability Statement
The data used to support the findings of this study are available from the authors upon request.