Abstract
Various Hydra species have been employed as model organisms since the 18th century. Introduction of transgenic and knock-down technologies made them ideal experimental systems for studying cellular and molecular mechanisms involved in regeneration, body-axis formation, senescence, symbiosis, and holobiosis. In order to provide an important reference for genetic studies, the Hydra magnipapillata genome (species name has been changed to H. vulgaris) was sequenced a decade ago (Chapman et al., 2010) and the updated genome assembly, Hydra 2.0, was made available by the National Human Genome Research Institute in 2017. While H. vulgaris belongs to the non-symbiotic brown hydra lineage, the green hydra, Hydra viridissima, harbors algal symbionts and belongs to an early diverging clade that separated from the common ancestor of brown and green hydra lineages at least 100 million years ago (Schwentner and Bosch 2015; Khalturin et al., 2019). While interspecific interactions between H. viridissima and endosymbiotic unicellular green algae of the genus Chlorella have been a subject of interest for decades, genomic information about green hydras was nonexistent. Here we report a draft 280-Mbp genome assembly for Hydra viridissima strain A99, with a scaffold N50 of 1.1 Mbp. The H. viridissima genome contains an estimated 21,476 protein-coding genes. Comparative analysis of Pfam domains and orthologous proteins highlights characteristic features of H. viridissima, such as diversification of innate immunity genes that are important for host-symbiont interactions. Thus, the H. viridissima assembly provides an important hydrozoan genome reference that will facilitate symbiosis research and better comparisons of metazoan genome architectures.
Keywords: green hydra, Hydra viridissima A99, whole genome sequencing, de novo assembly, symbiosis
The Cnidaria is an evolutionarily ancient and well-defined phylum, characterized by the possession of nematocytes (Brusca et al. 2016). Cnidarian species belong to the Medusozoa, which comprises the Hydrozoa, the Scyphozoa, the Cubozoa, and the Anthozoa (Figure 1A). Although cnidarian morphology exhibits astonishingly diverse forms and life styles, those of fresh water hydrozoans of the genus Hydra are relatively simple. Hydra possess only a polyp stage, while other medusozoans exhibit alternation of polyp and medusa stages.
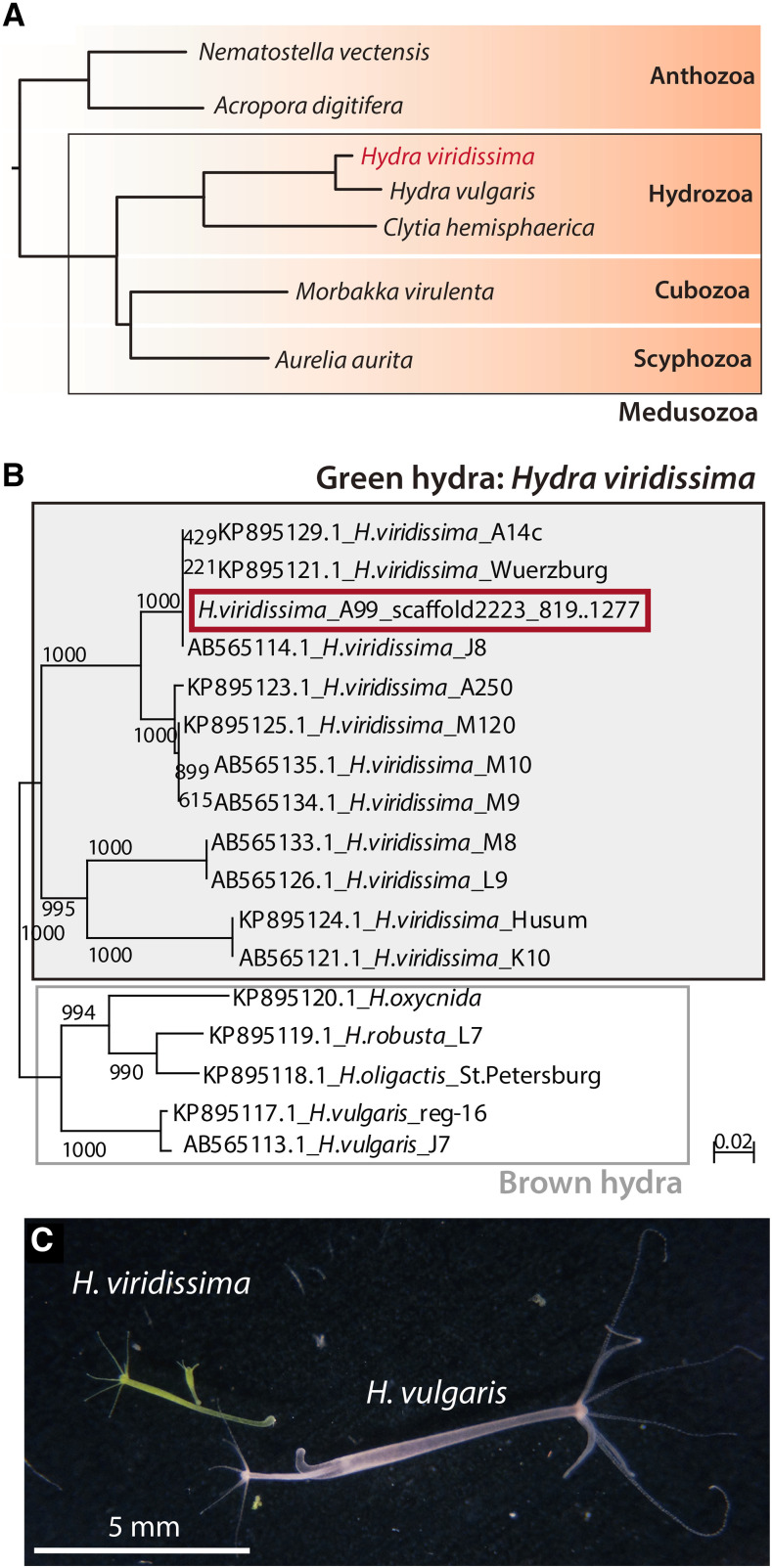
Figure 1.
Phylogeny and morphology of green hydra Hydra viridissima. (A) Phylogenetic position of H. viridissima (red) within the phylum Cnidaria. (B) Relationship of Hydra viridissima strain A99 (red) with other H. viridissima strains and brown hydra species, based on phylogenetic analysis with the NJ method using cytochrome c oxidase subunit I (COI) gene sequences. The genomic region in H. viridissima A99 and Genbank IDs in other strains used in the phylogenetic analysis are indicated. (c) Photographs of H. viridissima (left) and H. vulgaris (right). H. viridissima is smaller than H. vulgaris, and green due to symbiotic Chlorella in its endodermal epithelial cells.
With its simple body structure and easy laboratory cultivation, Hydra has been an experimental model for studying cellular and molecular mechanisms underlying the formation of the body axis (Bode 2011), regeneration (Trembley et al. 1744; Bode 2003; Holstein et al. 2003), and also holobiotic relationships with microbiota (Deines and Bosch 2016). Introduction of transgenic and knock-down technologies further promoted these studies (Wittlieb et al. 2006). In order to provide genetic information for these studies, the Hydra magnipapillata (now classified as H. vulgaris) genome was sequenced in 2010 (Chapman et al. 2010), and an improved version was published in 2017 (Hydra 2.0 Web Portal: https://research.nhgri.nih.gov/hydra/).
While H. vulgaris belongs to the non-symbiotic brown hydra lineage, the green hydra, Hydra viridissima, establishes a mutualistic relationship with microalgae and exchanges metabolites with its symbionts (Figure 1C) (Muscatine 1965; Cernichiari et al. 1969; Mews 1980; McAuley 1991). While symbiosis with dinoflagellates is observed in many marine cnidarians, such as corals, jellyfish, and sea anemones, H. viridissima harbors the green alga, Chlorella (Douglas and Huss 1986; Huss et al. 1993/1994; Davy et al. 2012). According to several phylogenetic reconstructions, Hydra viridissima belongs to the basally branching lineage in the genus Hydra (Martínez et al. 2010; Schwentner and Bosch 2015) and its genome is much smaller than those of brown hydra species (Zacharias et al. 2004). Although all hydra species have a similar body plan, green and brown hydras are evolutionarily distant, and little is known about the genetics that enable green hydras to support this unique symbiosis with Chlorella.
Recent advances in genome sequencing have facilitated comparative analyses of cnidarian genomes. In addition to H. vulgaris (H. magnipapillata), genomes of representative species in each subgroup of cnidarians have been sequenced, including another hydrozoan, Clytia hemisphaerica (Leclère et al. 2019), the scyphozoan jellyfishes, Aurelia aurita (Khalturin et al. 2019; Gold et al. 2019) and Nemopilema nomurai (Kim et al. 2019), the cubozoan box jellyfish, Morbakka virulenta (Khalturin et al. 2019), the anthozoan sea anemones, Nematostella vectensis (Putnam et al. 2007), Aiptasia (Baumgarten et al. 2015), and various coral species, including Acropora digitifera (Shinzato et al. 2011). Here we report a draft assembly of the ∼284-Mbp genome of Hydra viridissima strain A99 as another high-quality Hydra reference genome. We report significant characteristics of the green hydra genome, including transposable elements, innate immunity-related genes, and genes that determine its body plan.
Materials and Methods
Hydra and extraction of DNA
The Australian Hydra viridissima strain A99, which was kindly provided by Dr. Richard Campbell, at the University of California at Irvine, was used in this study. Polyps were maintained at 18° on a 12-hour light/dark cycle and fed with Artemia two or three times a week. DNA for genome sequencing were isolated from about 1000 polyps that were clonally cultured. Before genomic DNA extraction, symbiotic algae in H. viridissima were removed by photobleaching with 5 μM DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea), as described previously (Pardy 1976; Habetha et al. 2003). To remove contamination from other organisms, polyps were starved and treated with antibiotics (50 mg/L ampicillin, rifampicin, neomycin, and streptomycin) for one week.
After several rounds of washing in sterilized culture medium, polyps were lysed in DNA extraction buffer (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 25 mM EDTA, pH 8.0, 0.5% SDS) and digested with 100 mg/L Proteinase K. Genomic DNA was extracted using the standard phenol-chloroform method with 100 mg/L RNaseA treatment. The quantity of DNA was determined using a NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA), and the quality of high molecular-weight DNA was checked using agarose gel electrophoresis.
Sequencing of genomic DNA
In paired-end library preparations for genome sequencing, genomic DNA was fragmented with a Focused-ultrasonicator M220 (Covaris Inc., Woburn, MA, USA). A paired-end library (average insert size: 540 bp) and mate-pair libraries (average insert sizes: 3.2, 4.6, 7.8 and 15.2 kb) were prepared using Illumina TruSeq DNA LT Sample Prep Kits and Nextera Mate Pair Sample Preparation Kits (Illumina Inc., San Diego, CA, USA), following the manufacturers’ protocols. These libraries were quantified by Real-Time PCR (Applied Biosystems StepOnePlus, Thermo Fisher Scientific) and quality checked using capillary electrophoresis on a Bioanalyzer. Genome sequencing was performed using the Illumina Miseq system with 600-cycle chemistry (2 × 300 bp). Genome sequencing statistics is shown in Table S1A.
RNA extraction and sequencing
Total RNA was extracted from about 1000 polyps in six different conditions (with or without symbiotic algae, in light or dark conditions, and treated with antibiotics or DMCU with symbiotic algae) using Trizol reagent (Thermo Fisher Scientific) and an RNeasy Mini kit (QIAGEN, Hilden, Germany). The quantity of RNA was determined with a NanoDrop (Thermo Fisher Scientific). Quality of total RNA was checked with a BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA). For mRNA-seq, libraries were produced using an Illumina TruSeq Stranded mRNA Sample Prep Kit and were sequenced on HiSeq 2000 instruments using 2 × 150-cycle chemistry. mRNA-sequencing statistics are shown in Table S1B.
Assembly and gene prediction
Sequencing reads of genomic DNA were assembled using the Newbler Assembler, version 2.8 (Roche, Penzberg, Germany), and subsequent scaffolding was performed with SSPACE (Boetzer et al. 2011). Gaps inside scaffolds were closed with paired-end and mate-pair data using GapCloser of the Short Oligonucleotide Analysis Package (Luo et al. 2012). Then one round of Haplomerger2 processing pipeline (Huang et al. 2017) was applied to eliminate redundancy in scaffolds and to merge haplotypes. For gene model prediction, we used a species-specific gene prediction model that was trained based on mapping of the Hydra viridissima transcriptome and raw RNAseq reads against the genome assembly. Mapping and gene structure annotation were performed using the PASA pipeline v2.01 and were used to train AUGUSTUS software (Haas et al. 2003; Stanke et al. 2006). Genome completeness was evaluated using BUSCO (Benchmarking Universal Single-Copy Ortholog) (Seppey et al. 2019). RNA-Seq transcripts were mapped to the genome assembly with BWA.
Genome size estimation
Genome size was estimated from raw paired-end reads by k-mer distribution analysis. Jellyfish v2.0.0 was used to count k-mers and their frequencies (Marçais and Kingsford 2011). The Hydra viridissima genome size was estimated from k-mer distribution frequencies using the GenomeScope web tool (Vurture et al. 2017) (http://qb.cshl.edu/genomescope/).
Analysis of repetitive elements
Repetitive elements in the draft genome assembly of Hydra viridissima were identified de novo with RepeatScout version 1.0.5 (http://www.repeatmasker.org/RepeatModeler) and RepeatMasker version 4.0.6 (http://www.repeatmasker.org). Repetitive elements were filtered by length and occurrence so that only sequences longer than 50 bp and present more than 10 times in the genome were retained. The resulting sets of repetitive elements were annotated using BLASTN and BLASTX searches against RepeatMasker.lib (35,996 nucleotide sequences) and RepeatPeps.lib (10,544 peptides) bundled with RepeatMasker version 4.0.6. The results of both searches were combined, and BLASTX results were given priority in cases where BLASTN and BLASTX searches gave conflicting results.
Analysis of Hydra viridissima genes
For comparative analysis of H. viridissima genes among cnidarians, protein sequences were obtained from Hydra 2.0 web portal (https://research.nhgri.nih.gov/hydra/) and the Compagen server (http://www.compagen.org) for Hydra vulgaris (H. magnipapillata) strain 105, from JGI (https://genome.jgi.doe.gov/Nemve1/Nemve1.home.html) for Nematostella vectensis (Putnam et al. 2007), from MARIMBA (available at http://marimba.obs-vlfr.fr/organism/Clytia/hemisphaerica) for Clytia hemisphaerica (Leclère et al. 2019), from the genome project website of OIST Marine Genomics Unit (https://marinegenomics.oist.jp/gallery/gallery/index) for Acropora digitifera (Shinzato et al. 2011), for Morbakka virulenta and for the Atlantic Ocean strain of Aurelia aurita (Khalturin et al. 2019). We used protein models derived from the Hydra 2.0 assembly of the H. vulgaris genome for all comparative analyses with H. viridissima as this assembly has higher continuity (scaffold N50 ∼1Mbp) and BUSCO values than the originally published assembly (Chapman et al., 2010). For comparative reasons, statistics and results obtained with the Hydra 1.0 assembly (Chapman et al., 2010) and Hydra 2.0 assembly (https://research.nhgri.nih.gov/hydra/) are shown side by side in Table 1 and Tables 4-7.
Table 1. Comparison of the genome assembly statistics of cridarians.
| Hydrozoa | Scyphozoa | Cubozoa | Anthozoa | |||||
|---|---|---|---|---|---|---|---|---|
| Species | Hydra viridissimaa | Hydra vulgaris (Hydra magnipapillata) | Clytia hemisphaericab | Aurelia auritac | Morbakka virulentac | Acropora digitiferad | Nematostella vectensise | |
| Geographical origin | Laboratory strain A99 | Laboratory strain 105 | Villefranche (Atlantic) | Baltic Sea (Atlantic) | Seto Inland Sea (Pacific) | Okinawa (Pacific) | Laboratory strain | |
| ver. 1f | ver. 2g | |||||||
| Genome size (Mbp) | 284 | 852 | 854 | 445 | 377 | 952 | 420 | 457 |
| Number of Scaffolds | 2,677 | 20,914 | 5,525 | 7,644 | 2,710 | 4,538 | 2,420 | 10,804 |
| Longest scaffold (Mbp) | 5.1 | 0.91 | 4.4 | 2.9 | 4.4 | 14.5 | 2.5 | 3.3 |
| Scaffold N50 (Mbp) | 1.1 | 0.1 | 1.0 | 0.4 | 1.0 | 2.2 | 0.5 | 0.5 |
| GC content (%) | 24.7 | 25.4 | 25.4 | 35 | 34.7 | 31.4 | 39 | 39 |
| Repeats (%) | 37.5 | 57 | 60.3 | 41 | 44.7 | 37.4 | 12.9 | 26 |
| Gap rate (%) | 16.8 | 7.82 | 8.0 | 16.6 | 6.63 | 11.9 | 15.2 | 16.6 |
| Number of genes | 21,476 | 31,452 | 33,820 | 26,727 | 28,625 | 24,278 | 23,668 | 27,273 |
| Mean gene length (bp) | 7,637 | 6,873 | 12,378 | 5,848 | 10,215 | 21,444 | 8,727 | 4,500 |
| Mean exon length (bp) | 209 | 247 | N/A | 281 | 368 | 350 | 316 | 208 |
| Mean intron length (bp) | 838 | N/A | N/A | N/A | 1391 | 3572 | 1057 | 800 |
| BUSCO (complete) % | 83.9 | 76.8 | 80.2 | 86.4 | 79.8 | 81.5 | 74.5 | 91.4 |
This study. b Leclère et al., 2019, cKhalturin et al., 2019. dShinzato et al., 2011. ePutnam et al., 2007, fGenome assembly version 1, Chapman et al.,2010, gGenome assembly version 2, Hydra2.0 Web Portal (https://research.nhgri.nih.gov/hydra/).
Table 4. The number of NACHT/NB-ARC domain-containing proteins in cnidarians and the combination of repeat domains.
| Hvir | Hvul v1 | Hvul v2 | Ch | Aa | Mv | Nv | Ad | |
|---|---|---|---|---|---|---|---|---|
| Total | 264 | 89 | 101 | 56 | 58 | 24 | 6 | 489 |
| TPR | 103 | 38 | 29 | 3 | 8 | 4 | 2 | 57 |
| WD40 | 2 | 4 | 2 | 3 | 2 | 3 | 1 | 8 |
| LRR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 125 |
| Ank | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
Hvir: Hydra viridissima A99, Hvul v1: Hydra vulgaris (Chapman et al., 2010),Hvul v2: Hydra vulgaris (Hydra2.0), Ch: Clytia hemisphaerica, Mv: Morbakka virulenta, Aa: Aurelia aurita, Nv, Nematostella vectensis, Ad: Acropora digitifera,
Table 7. Number of homeodomain-containing genes in the Hydra viridissima genome.
| Class | Subclass | Familiy | Hvir | Hvul v1 | Hvul v2 | Ch | Aa | Mv | Nv | Ad |
|---|---|---|---|---|---|---|---|---|---|---|
| ANTP | HOXL | Cdx | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 |
| Evx | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Gbx | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Gsx | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Hox1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| Hox2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | ||
| Hox9-13 | 2 | 3 | 3 | 1 | 4 | 5 | 3 | 1 | ||
| Meox | 1 | 1 | 1 | 0 | 1 | 1 | 4 | 1 | ||
| Mnx | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Xlox/Pdx | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| NKL | Barx | 1 | 1 | 1 | 0 | 1 | 1 | 4 | 2 | |
| Dbx | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | ||
| Dlx | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | ||
| Emx | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 1 | ||
| Hhex | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Hlx | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 2 | ||
| Lbx | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Msx | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | ||
| Msxlx | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | ||
| Nedx | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | ||
| Nk1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | ||
| Nk2.1/2.2/4 | 2 | 1 | 1 | 1 | 1 | 3 | 8 | 4 | ||
| Nk3 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ||
| Nk5/Hmx | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | ||
| Nk6 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ||
| Nk7 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ||
| Noto | 1 | 1 | 1 | 2 | 1 | 1 | 6 | 2 | ||
| Ro | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Tlx | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | ||
| PRD | Alx | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | |
| Arx | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | ||
| Dmbx | 1 | 0 | 1 | 1 | 1 | 0 | 7 | 2 | ||
| Gsc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Hbn | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | ||
| Otp | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| Otx | 3 | 3 | 2 | 3 | 4 | 7 | 3 | 4 | ||
| Pax3/7 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | ||
| Pax4/6 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 1 | ||
| Pitx | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Prox | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Rax | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ||
| Repo | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Uncx | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | ||
| Vsx | 2 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | ||
| LIM | Isl | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | |
| Lhx1/5 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Lhx2/9 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | ||
| Lhx6/8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Lmx | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| POU | Hdx | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Pou1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ||
| Pou3 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 2 | ||
| Pou4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Pou6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| SINE | Six1/2 | 0 | 0 | 0 | 1 | 2 | 2 | 2 | 2 | |
| Six3/6 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | ||
| Six4/5 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | ||
| TALE | Irx | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | |
| Meis | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | ||
| Pbx | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| Pknox | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| Tgif | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| CERS | Cers | 2 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | |
| CUT | Onecut | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| HNF | Hnf1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
Hvir: Hydra viridissima A99, Hvul v1: Hydra vulgaris (Chapman et al., 2010), Hvul v2: Hydra vulgaris (Hydra2.0), Ch: Clytia hemisphaerica, Mv: Morbakka virulenta, Aa: Aurelia aurita, Nv, Nematostella vectensis, Ad: Acropora digitifera.
In comparative analyses, domain searches against the Pfam database (Pfam-A.hmm) were performed using HMMER (Finn et al. 2016), and ortholog gene grouping employed OrthoFinder (Emms and Kelly 2015). To classify homeodomain-containing proteins, BLAST searches and phylogenetic analyses were performed. Homeodomain sequences in various animals were obtained from the Homeobox Database (http://homeodb.zoo.ox.ac.uk/families.get?og=All) (Zhong and Holland 2011).
For phylogenetic analysis, multiple alignments were produced with CLUSTALX (2.1) with gap trimming (Larkin et al. 2007). Sequences of poor quality that did not align well were deleted using BioEdit (Hall 1999). Phylogenetic analyses were performed using the Neighbor-Joining method (Saitou and Nei 1987) in CLUSTALX with default parameters (1,000 bootstrap tests and 111 seeds). Representative phylogenetic trees were drawn using FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). Gene/protein IDs used for phylogenetic analysis are shown in the trees (Figs S2 and S3).
Data availability
This whole-genome shotgun sequencing project has been deposited at DDBJ/ENA/GenBank under BioSample ID SAMN09635813 and BioProject ID: PRJNA480404. RNA-seq reads have been deposited at SRA of NCBI (SRX6792700-SRX6792705). Genome sequences, gene models, and a genome browser are also accessible at the website of the OIST Marine Genomics Unit Genome Project (https://marinegenomics.oist.jp/hydraviridissima_A99). A genome browser was established for assembled sequences using the JBrowse 1.12.3 (Skinner et al. 2009). Gene annotations from the protein domain search and BLAST search are likewise shown on the site. Reagents, software and datasets used in this study are listed in the Reagent Table. k-mer frequency distribution plots in the Hydra viridissima A99 genome is found in Figure S1. Phylogenetic analysis of ANTP genes is in Figure S2. Phylogenetic analysis of PRD genes is presented in Figure S3. Sequencing statistics for Hydra viridissima A99 are in Table S1. A summary of repetitive sequences in the Hydra viridissima A99 genome assembly are found in Table S2. Pfam domain-containing genes in the Hydra viridissima A99 genome are available in Table S3. Orthologs enriched in Hydra viridissima A99 (A) and Hydra (B) are in Table S4. Gene IDs of ANTP genes in Hydra viridissima A99 are in Table S5. Supplemental material available at figshare: https://doi.org/10.25387/g3.12911426.
Results and Discussion
Genome architecture of Hydra viridissima
Hydra viridissima appears green because of the symbiotic Chlorella that inhabit endodermal epithelial cells, and it is smaller than the brown hydra, Hydra vulgaris (Figure 1C). We decoded the genome of H. viridissima strain A99, which is closely related to strain A14c, Wuerzburg and J8 (Figure 1B). We previously reported the genome of its specific symbiotic alga, Chlorella sp. A99, and demonstrated that metabolic co-dependency exists between H. viridissima A99 and the symbiont (Hamada et al. 2018).
The genome of H. viridissima A99 was sequenced using the Illumina platform with paired-end and mate pair libraries. Statistics of sequence reads, the assembly, and genome architecture are shown in Table 1. We obtained ∼7,070 Mbp of paired-end sequences, and 4,765, 4,769, 3,669 and 3,551 Mbp for 3.2k, 4.6k, 7.8k, and 15.2k insert-size pate-pair sequences, respectively, comprising a total of ∼23,826 Mbp (Table S1). The size of the H. viridissima genome was estimated at ∼254 Mbp using k-mer analysis (k-mer = 19) based on paired-end sequence data (Fig. S1). This indicates that we achieved more than 90-fold sequence coverage of the genome. On the other hand, the total length of the genome sequence assembly reached 284,265,305 bp. That is, the total assembly closely matched the estimated genome size.
Although genomic DNA was extracted from a clonally propagated culture of hydra polyps maintained in the laboratory, heterozygosity was comparatively high (2.28% of the entire sequence) (Fig. S1). Thus, polyps originally collected from the wild had a high level of heterozygosity. Repetitive sequences constituted 37.5% of the genome and the gap rate was 16.8% of the genome (Table 1; see next section). Scaffolds from the present analysis numbered 2,677 and the scaffold N50 was 1.1 Mbp, with the longest scaffold reaching 5.1 Mbp (Table 1). The GC content of the genome was 24.7% (Table 1), suggesting that H. viridissima has an AT-rich genome similar to that of H. vulgaris (25.4%). Using 67,339,858,036 nucleotides of RNA-sequence data (Table S1), we predicted gene models. The genome was estimated to contain 21,476 protein-coding genes (Table 1). We did not find any gene models with sequence similarities to the symbiotic Chlorella. The mean gene length, exon length, and intron length were 7,637 bp, 209 bp, and 838 bp, respectively (Table 1). Compared to H. vulgaris, the green hydra has a compact genome, with 36.5% fewer genes (Table 1). The BUSCO value for the H. viridissima assembly is 84% for complete gene models and with inclusion of partial sequences, the genome accounts for 91% of the metazoan reference gene set (Table 1). Comparison of H. viridissima genome statistics with those of other cnidarian genomes showed that the H. viridissima genome assembly is comparable or of even better quality in regard to the scaffold N50 and BUSCO completeness (Table 1).
During assembly and gene annotation, we noticed that scaffold2223, composed of 18,375 base pairs (bp), contained almost the entire H. viridissima mitochondrial genome. The mitochondrial genome of H. viridissima strain A99 was linear, as reported by Bridge et al. (1992) and Pan et al. (2014b) for the other green hydras, while in brown hydra, Hydra vulgaris, mitochondrial genome is composed of two linear molecules (Bridge et al. 1992; Pan et al. 2014a; Pan et al. 2014b).
Repetitive sequences in the Hydra viridissima genome
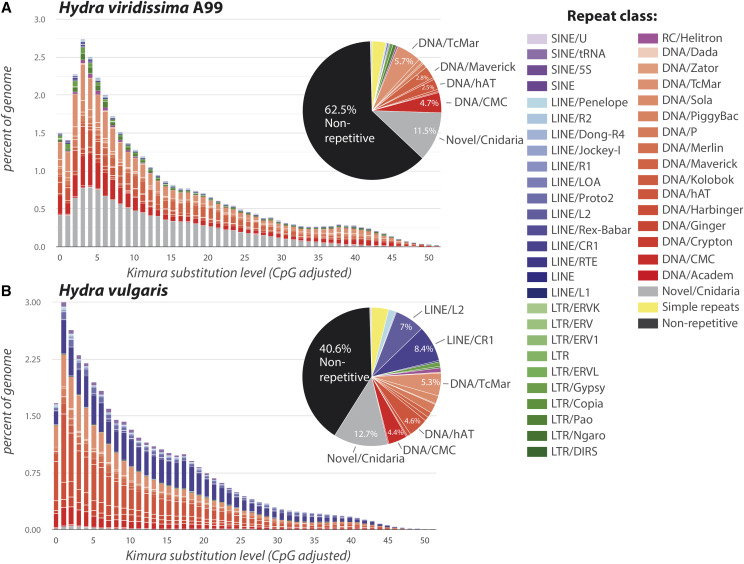
Although the abundance of repetitive sequences in anthozoan genomes is generally low (15∼17%), genomes of medusozoans and hydrozoans have comparatively high levels of repetitive sequences, 60% in H. vulgaris, 41% in Clytia, 45% in Aurelia, and 37% in Morbakka (Table 1). This was also true of H. viridissima (37.5%) (Table 1). DNA transposons were the most abundant type, accounting for approximately 22.41% of the genome (Figure 2A, Table S2). Of these, TcMariner, CMC, Maverick and hAT were the largest components (Figure 2A). On the other hand, percentages of LTR retrotransposons (1.63%) and non-LTR retrotransposons (0.99%) were comparatively low (Figure 2A, Table S2).
Figure 2.
Interspersed Repeat Landscape in Hydra. Components and proportions of repetitive sequences in the genome of (A) Hydra viridissima A99 and (B) H. vulgaris are shown. Classes of repeat are shown in the right column.
In comparing repetitive elements between H. viridissima and H. vulgaris, it became apparent that DNA transposons (DNA/TcMar, DNA/hAT and DNA/CMC) occupy a similar portion of both Hydra genomes (Figure 2). In addition, novel and potentially cnidarian-specific repetitive elements occupy ∼12% of both genomes. Second, long, interspersed nuclear elements LINE/L2 (∼7%) and LINE/CR1 (8.4%) are large components of the H. vulgaris genome, but they are almost absent in the H. viridissima genome. It was suggested that a burst of retrotransposons occurred in the brown hydra lineage after divergence from the green hydra lineage, and may account for the large genomes of brown hydras (Chapman et al. 2010; Wong et al. 2019). Because H. viridissima occupies a basal position in the Hydra lineage, and the genome of another hydrozoan, Clytia, is smaller (∼450Mbp) and has fewer repetitive elements (41%) than H. vulgaris, the ancestral Hydra genome was likely rather compact, with fewer retrotransposons. Molecular and evolutionary mechanisms involved in the insertion of LINE components in the H. vulgaris genome will be a subject of future studies in relation to diversification and speciation within the Hydra clade.
Innate immunity-related protein genes in the Hydra viridissima genome
Using the Pfam-domain search method, we surveyed genes for protein domains in the H. viridissima genome. We found approximately 4,500 different Pfam domains in this species (Table S3), a number comparable to those of other cnidarians. To identify the domains that are enriched in the H. viridissima genome, we counted the number of genes with each Pfam domain in cnidarian genomes, and selected the domains of which number are ≥2x higher in the green hydra genome than those of non-symbiotic cnidarians and show significant difference based on Chi-Square test (p-value < 0.001) (Table 2A). Then we checked the number of H. viridissima-enriched domains in the genome of the coral, Acropora digitifera, since it is also a symbiotic cnidarian. NACHT and NB-ARC, which have similar structures and functions, were the two most highly enriched domains and TIR occurred in the top 10 (Table 2A). NACHT/NB-ARC and TIR domains are found in the pattern-recognition receptors, Nod-like and Toll-like, which are sensors for pathogen- and damage-associated molecules. It appears that these domains are also enriched in Acropora digitifera, but not in non-symbiotic cnidarians (Table 2A).
Table 2. Number of genes with Pfam domains enriched in the Hydra viridissima genome and comparison of their number in the other cnidarian genomes. A. Domains enriched in Hydra viridissima. B. Domains enriched in Hydra species.
| A. Domain | Hvir | Hvul | Ch | Aa | Mv | Nv | Ad | Chi test* |
|---|---|---|---|---|---|---|---|---|
| NACHT | 161 | 75 | 42 | 23 | 45 | 39 | 458 | 1E-53 |
| NB-ARC | 106 | 28 | 18 | 4 | 20 | 6 | 220 | 5E-53 |
| ATPase_2 | 64 | 19 | 28 | 13 | 14 | 19 | 36 | 7E-35 |
| TIR_2 | 49 | 11 | 19 | 15 | 24 | 17 | 49 | 7E-16 |
| DUF4218 | 47 | 13 | 16 | 0 | 12 | 4 | 5 | 2E-66 |
| Endonuclease_7 | 46 | 9 | 3 | 5 | 1 | 1 | 0 | 1E-189 |
| RAG1 | 42 | 13 | 1 | 0 | 4 | 1 | 0 | 2E-160 |
| TIR | 41 | 7 | 3 | 11 | 15 | 12 | 36 | 1E-22 |
| CbiA | 23 | 7 | 6 | 6 | 7 | 6 | 2 | 8E-19 |
| MarR_2 | 21 | 6 | 4 | 0 | 0 | 4 | 3 | 6E-40 |
| HTH_Tnp_IS630 | 15 | 5 | 1 | 1 | 1 | 1 | 0 | 4E-40 |
| DUF2961 | 14 | 5 | 2 | 3 | 3 | 1 | 3 | 5E-15 |
| TMEM151 | 10 | 4 | 2 | 0 | 3 | 3 | 6 | 4E-06 |
| DUF1294 | 5 | 1 | 1 | 1 | 2 | 0 | 0 | 6E-07 |
| B. Domain | Hvir | Hvul | Ch | Aa | Mv | Nv | Ad | Chi test* |
|---|---|---|---|---|---|---|---|---|
| DDE_3 | 365 | 481 | 16 | 31 | 16 | 6 | 17 | 0E+00 |
| Dimer_Tnp_hAT | 296 | 376 | 11 | 44 | 52 | 21 | 27 | 0E+00 |
| HTH_Tnp_Tc3_2 | 266 | 268 | 15 | 23 | 13 | 0 | 3 | 0E+00 |
| HTH_32 | 185 | 176 | 14 | 25 | 4 | 4 | 9 | 1E-307 |
| ANAPC3 | 182 | 157 | 61 | 37 | 36 | 46 | 44 | 2E-117 |
| HTH_23 | 166 | 200 | 6 | 33 | 15 | 8 | 20 | 1E-225 |
| zf-BED | 116 | 116 | 14 | 8 | 9 | 19 | 5 | 1E-156 |
| HTH_29 | 95 | 121 | 7 | 19 | 1 | 2 | 5 | 8E-148 |
| HTH_psq | 93 | 165 | 3 | 3 | 6 | 1 | 1 | 3E-189 |
| HTH_28 | 85 | 87 | 2 | 6 | 5 | 6 | 11 | 5E-130 |
| SRP_TPR_like | 83 | 87 | 6 | 2 | 1 | 1 | 1 | 2E-163 |
| DUF4806 | 69 | 54 | 6 | 0 | 1 | 0 | 0 | 3E-157 |
| PAX | 61 | 57 | 3 | 23 | 4 | 9 | 8 | 4E-63 |
| BTAD | 32 | 33 | 7 | 1 | 3 | 3 | 3 | 4E-39 |
| Sigma70_r4_2 | 28 | 31 | 2 | 0 | 2 | 1 | 3 | 3E-44 |
| HTH_Tnp_Tc3_1 | 26 | 13 | 1 | 0 | 0 | 0 | 0 | 3E-87 |
| HTH_7 | 21 | 14 | 2 | 0 | 4 | 1 | 1 | 5E-33 |
| CD225 | 21 | 19 | 9 | 2 | 4 | 7 | 7 | 4E-11 |
| DUF2738 | 21 | 12 | 1 | 0 | 0 | 1 | 0 | 3E-56 |
| Sigma70_r4 | 17 | 16 | 1 | 1 | 1 | 0 | 2 | 2E-26 |
| IGFBP | 15 | 21 | 7 | 5 | 4 | 1 | 3 | 1E-09 |
| Sulfate_transp | 15 | 18 | 2 | 4 | 5 | 4 | 5 | 3E-08 |
| DUF1280 | 13 | 31 | 4 | 0 | 4 | 3 | 2 | 3E-17 |
| HTH_3 | 13 | 11 | 1 | 5 | 2 | 2 | 1 | 4E-11 |
| Polysacc_deac_1 | 13 | 14 | 3 | 6 | 1 | 4 | 2 | 5E-08 |
| DUF4817 | 12 | 9 | 2 | 0 | 1 | 0 | 0 | 6E-21 |
| Ca_chan_IQ | 12 | 16 | 5 | 4 | 5 | 2 | 3 | 1E-05 |
| Torsin | 11 | 11 | 2 | 2 | 2 | 5 | 5 | 2E-05 |
| CIDE-N | 10 | 7 | 1 | 2 | 3 | 2 | 1 | 1E-07 |
| GRDP-like | 9 | 7 | 2 | 2 | 1 | 2 | 3 | 2E-05 |
| Transposase_mut | 8 | 10 | 1 | 1 | 3 | 0 | 0 | 2E-08 |
Hvir: Hydra viridissima A99, Hvul: Hydra vulgaris (Hydra2.0), Ch: Clytia hemisphaerica, Mv: Morbakka virulenta, Aa: Aurelia aurita, Nv, Nematostella vectensis, Ad: Acropora digitifera, *Chi-test: evalue of Chi-square test.
Expansion of genes for NACHT-containing proteins and TIR-containing proteins is supported by identification of orthologous protein groups by OrthoFinder. Genes for proteins similar to Nod-like receptor were the most overrepresented group and TIR-only proteins occurred in the top 7 in the H. viridissima genome (Table 3A, Table S4). However, these orthologs were not scored in the Acropora genome, suggesting that these proteins expanded in H. viridissima are different from those expanded in Acropora.
Table 3. Top 10 overrepresented orthologs in the Hydra viridissima genome and comparison of their gene number in the other cnidarian genomes. A. Orthologs enriched in the Hydra viridissima genome. B Orthologs enriched in Hydra species.
| A. Ortholog ID | Hvir | Hvul | Ch | Aa | Mv | Nv | Ad | Chi-test* | Annotation |
|---|---|---|---|---|---|---|---|---|---|
| OG0000023 | 152 | 51 | 30 | 19 | 0 | 0 | 0 | 8E-128 | Nod-like receptor like |
| OG0000049 | 98 | 48 | 11 | 4 | 15 | 26 | 1 | 2E-58 | Uncharacterized protein |
| OG0000100 | 73 | 29 | 0 | 0 | 0 | 1 | 0 | 3E-79 | Uncharacterized protein |
| OG0000191 | 29 | 13 | 11 | 0 | 0 | 8 | 0 | 3E-17 | Uncharacterized protein |
| OG0000159 | 24 | 4 | 0 | 8 | 2 | 0 | 0 | 7E-21 | Uncharacterized protein |
| OG0000602 | 23 | 6 | 2 | 0 | 0 | 0 | 0 | 3E-24 | Uncharacterized protein |
| OG0000766 | 23 | 1 | 1 | 0 | 0 | 0 | 1 | 2E-30 | TIR-only protein |
| OG0000525 | 19 | 8 | 1 | 0 | 0 | 0 | 0 | 2E-18 | Uncharacterized protein |
| OG0001051 | 18 | 1 | 0 | 0 | 0 | 0 | 0 | 1E-25 | Uncharacterized protein |
| OG0000975 | 14 | 6 | 2 | 0 | 0 | 0 | 1 | 1E-11 | DDE superfamily endonuclease |
| B. Ortholog ID | Hvir | Hvul | Ch | Aa | Mv | Nv | Ad | Chi-test* | Annotation |
|---|---|---|---|---|---|---|---|---|---|
| OG0000006 | 299 | 339 | 28 | 21 | 29 | 3 | 7 | 2E-262 | HTH domain containing transposase |
| OG0000018 | 204 | 130 | 9 | 14 | 0 | 1 | 0 | 5E-194 | ATP-dependent DNA helicase PIF1-like |
| OG0000015 | 185 | 128 | 46 | 14 | 1 | 0 | 45 | 6E-122 | TPR containing |
| OG0000008 | 184 | 360 | 15 | 9 | 5 | 1 | 3 | 2E-249 | DDE superfamily endonuclease |
| OG0000027 | 166 | 144 | 2 | 4 | 2 | 3 | 2 | 8E-163 | Uncharacterized protein |
| OG0000036 | 95 | 154 | 1 | 1 | 7 | 1 | 1 | 4E-114 | zinc finger domain containing transposase |
| OG0000010 | 82 | 352 | 12 | 38 | 19 | 11 | 2 | 4E-201 | Uncharacterized protein |
| OG0000073 | 77 | 39 | 19 | 1 | 0 | 0 | 0 | 2E-66 | Uncharacterized protein |
| OG0000100 | 73 | 29 | 0 | 0 | 0 | 1 | 0 | 5E-81 | Uncharacterized protein |
| OG0000093 | 61 | 44 | 0 | 1 | 8 | 2 | 0 | 1E-52 | Uncharacterized protein |
Hvir: Hydra viridissima A99, Hvul: Hydra vulgaris (Hydra2.0), Ch: Clytia hemisphaerica, Mv: Morbakka virulenta, Aa: Aurelia aurita, Nv, Nematostella vectensis, Ad: Acropora digitifera, *Chi-test: evalue of Chi-square test.
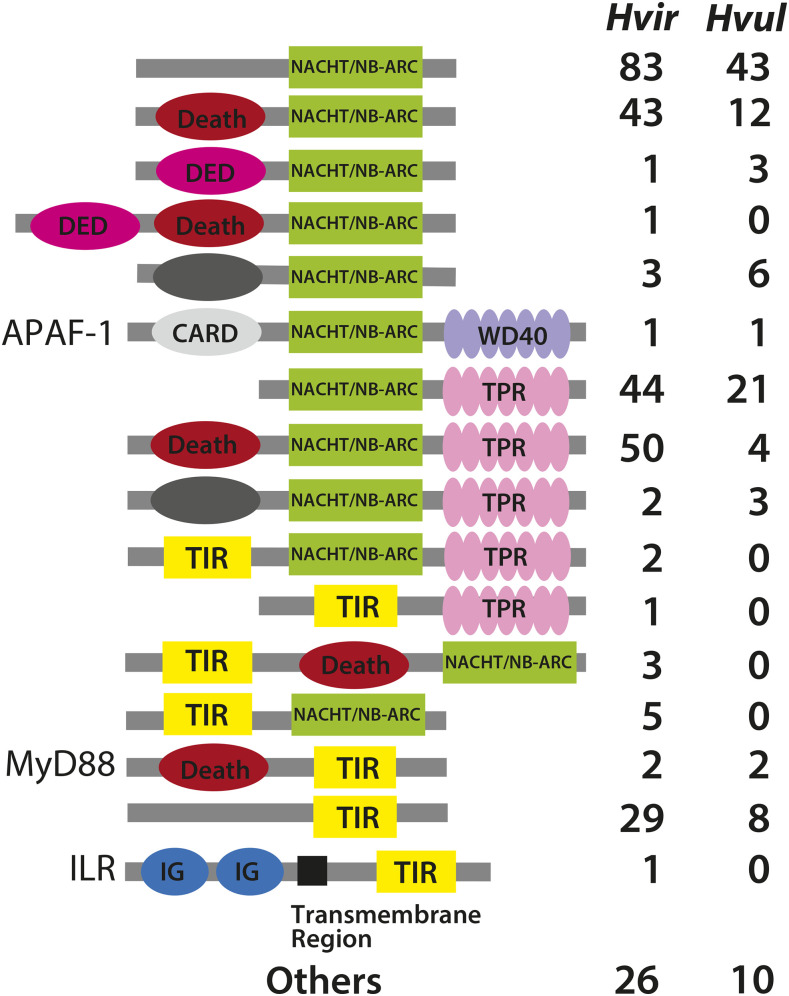
In Acropora, we previously showed unique, complex domain structures of proteins with NACHT/NB-ARC domains (Hamada et al. 2013). Thus, we further examined domain combinations of NACHT/NB-ARC proteins in H. viridissima to determine whether such complex domain structures are also found in this taxon. Basically Nod-like receptors have tripartite domain structures, consisting of effector-binding domains constituted of apoptosis-related domains, such as Death or DED in the N-terminus, NACHT/NB-ARC in the center, and a repeat domain that recognizes pathogen- and damage-associated molecules at the C-terminus. Humans have approximately 20 Nod-like receptor family proteins, and their ligand recognition region is a leucine-rich repeat (LRR). On the other hand, in Nod-like receptors of basal metazoans, not only LRR, but also tetratricopeptide repeats (TPR), WD40 repeats, and ankyrin repeats (Ank) are found as repeat domains. We previously showed that Acropora has all 4 types of Nod-like receptors, and that those with LRR are the most common (Table 4) (Hamada et al. 2013). In other cnidarians examined, only TPR and WD40 are found as repeat domains of Nod-like receptors, suggesting loss of the other types. Especially in H. viridissima, a larger number of genes for Nod-like receptors with TPR were found. In addition, their domain structures in H. viridissima vary widely, compared to those of H. vulgaris (Figure 3). For example, the domain combination of NACHT/NB-ARC with TIR, was found in H. viridissima, but not in H. vulgaris. In addition to NACHT-containing proteins, H. viridissima has more genes for TIR domain-containing proteins such as an interleukin-1 receptor (ILR), which are not found in H. vulgaris.
Figure 3.
Schematic representation of domain structures of NACHT/NB-ARC or TIR-domain-containing proteins identified in Hydra. The domain structures and the number of NACHT/NB-ARC or TIR-domain-containing proteins in Hydra viridissima A99 (Hvir) and H. vulgaris (Hvul) are shown.
As mentioned above, diverse pattern-recognition receptor-related genes are found in both H. viridissima and Acropora digitifera. Their most significant shared attribute is symbiosis, the former with Chlorella and the latter with the dinoflagellate, Symbiodinium. Therefore, it is likely that the evolutionary development of symbiosis by certain cnidarians required expansion and greater sophistication of innate immunity genes. They may participate in recognition and maintenance of symbiotic organisms in cnidarian tissues. On the other hand, the structures (e.g., repeat combination) of the Nod-like receptors most abundant in green hydras and corals are different. This indicates that species-specific adaptations to the environment and particular symbionts occurred independently in these lineages.
Genes enriched in the genus Hydra
We further examined Pfam domains overrepresented specifically in H. viridissima and others present in both H. viridissima and H. vulgaris. This was done using the same criteria as above, that is, that the number of domains is ≥2x higher than those in other cnidarians and that the difference is significant by Chi-Square test (p-value < 0.001). (Table 2B).
Pfam domain searches and ortholog protein grouping demonstrated that H. viridissima and H. vulgaris possess many genes encoding domains that function in DNA binding. For example, genes containing transposase-related domain (DDE_3, Dimer_Tnp_hAT and Transposase_mut) and DNA-binding motif (HTH: helix-turn-helix, zf: zinc finger, Sigma70_r4, CIDE-N, RAG1) were overrepresented in both H. viridissima and H. vulgaris (Table 2B). In addition, ortholog protein grouping suggested that genes for HTH domain-containing transposase, ATP-dependent DNA helicase PIF1-like protein, DDE superfamily endonuclease, and zinc finger domain-containing transposase were overrepresented in both Hydra species (Table 3A). Although the functions of these genes are unknown, they may be involved in genome structure maintenance of Hydra, which contains many transposable elements.
Pfam domain searches also demonstrated that genes for proteins containing Sulfate_transp domain and those containing Polysacc_deac_1 domain are enriched in both H. viridissima and H. vulgaris (Table 2B). Sulfate_transp is found in the sulfate permease family, which is involved in uptake or exchange of inorganic anions, such as sulfate. So far, their functions in Hydra are unknown, but may be related to their limnetic life styles, which require active ion uptake. Polysacc_deac_1 is found in polysaccharide deacetylase, including chitin deacetylase, which is involved in chitin metabolism. It may contribute to construction of the extracellular matrix surrounding the body or structure of nematocytes, or molecular recognition events such as immune responses to pathogens with chitinous cell wall (Balasubramanian et al. 2012; Elieh Ali Komi et al. 2018; Rodrigues et al. 2016).
Gene families for transcription factors and signaling molecules
Using Pfam-supported families, we examined the number of gene families for putative transcription regulator genes and signaling molecules (Table 5), since these genes are essential in development and physiology of metazoans. While major signaling pathways are present in cnidarians, some specialization in Cnidaria is known. For example, Wnt genes, which are important for oral-aboral body axis formation, diversified in the cnidarian lineage (Kusserow et al. 2005; Khalturin et al. 2019). Table 5A shows numbers of putative transcription factor genes in the H. viridissima genome. Zinc finger proteins (C2H2 type) were most abundant, with 105 members, although the abundance of this family has been noted in other cnidarian genomes (Khalturin et al. 2019). There were 33 HLH domain-containing and 50 homeobox domain-containing genes (Homeodomain-containing genes of H. viridissima are discussed in the next section). A similar analysis of putative signaling molecule genes showed that the H. viridissima genome contains 16 fibroblast growth factor (FGF)-like domain genes, 11 transforming growth factor-beta (TGB-β) genes, and 10 Wnt genes (Table 5B). These numbers are comparable to those in H. vulgaris. In general, the number of transcription factor and signaling molecule family members appeared similar among cnidarians, although a few families, such as AT_hook and Hairly-orange of transcription factors (Table 5A) and Interleukin 3 (IL3) families of signaling molecules (Table 5B) were not found in Hydra genomes.
Table 5. Number of putative transcription factor genes (A) and signaling molecule genes (B) in the Hydra viridissima genome.
| A. Domain | Hvir | Hvul v1 | Hvul v2 | Ch | Aa | Mv | Nv | Ad |
|---|---|---|---|---|---|---|---|---|
| ARID | 8 | 7 | 9 | 10 | 10 | 8 | 5 | 8 |
| AT_hook | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 |
| bZIP_1 | 26 | 26 | 30 | 26 | 22 | 25 | 36 | 29 |
| bZIP_2 | 25 | 22 | 23 | 31 | 24 | 27 | 32 | 17 |
| CUT | 1 | 0 | 1 | 3 | 1 | 1 | 2 | 1 |
| DM | 6 | 6 | 5 | 7 | 9 | 11 | 12 | 7 |
| Ets | 9 | 11 | 11 | 13 | 21 | 14 | 16 | 12 |
| Forkhead | 17 | 17 | 16 | 19 | 15 | 26 | 34 | 22 |
| GATA | 4 | 4 | 5 | 3 | 7 | 7 | 4 | 5 |
| Hairy_orange | 0 | 0 | 0 | 1 | 4 | 2 | 6 | 7 |
| HLH | 33 | 36 | 34 | 44 | 52 | 50 | 72 | 53 |
| HMG_box | 30 | 33 | 33 | 31 | 30 | 29 | 33 | 27 |
| Homeobox | 50 | 44 | 49 | 70 | 88 | 82 | 153 | 96 |
| Hormone_recep | 9 | 9 | 9 | 12 | 12 | 9 | 20 | 9 |
| P53 | 2 | 1 | 3 | 2 | 1 | 2 | 3 | 3 |
| PAX | 61 | 23 | 57 | 3 | 4 | 23 | 9 | 8 |
| Pou | 2 | 3 | 2 | 3 | 4 | 4 | 6 | 4 |
| RHD_DNA_bind | 2 | 1 | 3 | 5 | 2 | 2 | 3 | 2 |
| SRF-TF | 2 | 2 | 2 | 4 | 3 | 2 | 3 | 1 |
| T-box | 6 | 7 | 7 | 11 | 10 | 9 | 14 | 10 |
| TF_AP-2 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | 2 |
| zf-C2H2 | 105 | 123 | 121 | 244 | 233 | 118 | 169 | 90 |
| zf-C2HC | 1 | 2 | 2 | 3 | 2 | 3 | 3 | 4 |
| zf-C4 | 9 | 8 | 8 | 11 | 8 | 9 | 19 | 12 |
| B. Domain | Hvir | Hvul v1 | Hvul v2 | Ch | Aa | Mv | Nv | Ad |
|---|---|---|---|---|---|---|---|---|
| Cbl_N | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 1 |
| Cbl_N2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| Cbl_N3 | 1 | 1 | 1 | 2 | 2 | 1 | 0 | 1 |
| DIX | 1 | 1 | 3 | 3 | 3 | 3 | 4 | 2 |
| FGF | 16 | 12 | 16 | 10 | 18 | 13 | 13 | 13 |
| Focal_AT | 1 | 2 | 3 | 1 | 1 | 1 | 0 | 0 |
| G-alpha | 29 | 28 | 27 | 29 | 29 | 32 | 37 | 22 |
| G-gamma | 2 | 2 | 3 | 1 | 7 | 5 | 4 | 3 |
| IL3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| PDGF | 1 | 2 | 0 | 5 | 2 | 3 | 1 | 6 |
| Phe_ZIP | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| Rabaptin | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| RGS | 13 | 13 | 12 | 14 | 16 | 16 | 13 | 11 |
| RGS-like | 2 | 3 | 2 | 1 | 2 | 1 | 0 | 1 |
| STAT_alpha | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 1 |
| STAT_bind | 2 | 1 | 3 | 1 | 1 | 1 | 1 | 1 |
| STAT_int | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| TGF_beta | 11 | 11 | 11 | 7 | 9 | 9 | 7 | 10 |
| TGFb_propeptide | 8 | 10 | 9 | 4 | 6 | 8 | 6 | 9 |
| wnt | 10 | 13 | 11 | 12 | 15 | 17 | 26 | 15 |
Hvir: Hydra viridissima A99, Hvul v1: Hydra vulgaris (Chapman et al., 2010),Hvul v2: Hydra vulgaris (Hydra2.0), Ch: Clytia hemisphaerica, Mv: Morbakka virulenta, Aa: Aurelia aurita, Nv, Nematostella vectensis, Ad: Acropora digitifera.
Hox and Para-Hox genes in Hydra viridissima
Among transcription factors, homeodomain-containing proteins have been intensively investigated as key molecules in the developmental toolkit. They are highly diversified and participate in a wide variety of developmental processes in metazoans. In particular, those in Cnidarians that are shared by the common ancestors of deuterostomes and protostomes are important to understand body plan evolution of bilaterians (Ferrier and Holland 2001; Chourrout et al. 2006; Ferrier 2016; DuBuc et al. 2018). While many orthologous genes of known homeodomain-containing proteins, including Hox and ParaHox genes, have been identified in cnidarians, cnidarian-specific specializations, such as loss of some homeodomain protein genes and fragmentation of the Hox cluster have been reported (Kamm et al. 2006; Steele et al. 2011; Chapman et al. 2010; Leclère et al. 2019). To understand the evolutionary trajectory of homeobox protein genes in the Hydra lineage, we classified them into ANTP (HOXL and NKL), PRD, LIM, POU, PROS, SINE, TALE, CERS, or ZF using bi-directional BLAST searches against sequences of homeodomains in other animals, using HomeoDB (Zhong and Holland 2011) (Table 6, Table S5) and phylogenetic analysis for ANPT- and PRD-class genes (Figs. S2 and S3), referring to the Hox genes previously identified in other cnidarians (Schummer et al. 1992; Chourrout et al. 2006; Leclère et al. 2019; Khalturin et al. 2019).
Table 6. Number of genes for the subclass of homeodomain-containing proteins in cnidarians.
| Class | Medusozoa | Anthozoad | ||||||
|---|---|---|---|---|---|---|---|---|
| Hydrozoa | ||||||||
| Hvir | Hvul v1 | Hvul v2 | Ch | Mv | Aa | Nv | Ad | |
| ANTP-HOXL | 5 | 7 | 7 | 6 | 13 | 13 | 17 | 9 |
| ANTP-NKL | 9 | 8 | 11 | 20 | 21 | 20 | 65 | 33 |
| PRD | 21 | 16 | 16 | 18 | 25 | 28 | 43 | 31 |
| LIM | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 4 |
| TALE | 4 | 3 | 4 | 5 | 4 | 10 | 6 | 5 |
| SINE | 2 | 2 | 2 | 4 | 5 | 4 | 6 | 4 |
| POU | 2 | 2 | 2 | 3 | 4 | 4 | 6 | 4 |
| CERS | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| CUT | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| HNF | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| PROS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 48 | 44 | 48 | 62 | 79 | 85 | 151 | 94 |
Hvir: Hydra viridissima A99, Hvul v1: Hydra vulgaris (Chapman et al., 2010), Hvul v2: Hydra vulgaris (Hydra2.0), Ch: Clytia hemisphaerica, Mv: Morbakka virulenta, Aa: Aurelia aurita, Nv, Nematostella vectensis, Ad: Acropora digitifera.
In the H. viridissima genome, we identified 48 homeodomain-containing genes in the genome, 5 ANTP-HOXL, 9 ANTP-NKL, 21 PRD, 4 LIM, 4 TALE, 2 SINE, 2 POU, and 1 CERS; however, we failed to find CUT, HNF, PROS and ZF classes. This tendency toward gene loss is shared by the two other hydrozoans, H. vulgaris and Clytia hemishpaerica (Table 6). Among cnidarians, anthozoan genomes (Nematostella and Acropora) apparently contain the most homeodomain-containing genes, while scyphozoans (Aurelia) and cubozoans (Morbakka) have intermediate numbers, and hydrozoan genomes contain the fewest. CUT class genes are not found in medusozoan genomes and all cnidarian genomes lack PROS and ZF class genes altogether. In addition, NKL genes are less abundant in Hydra and HOXL genes are less abundant in hydrozoans generally, than in other cnidarians.
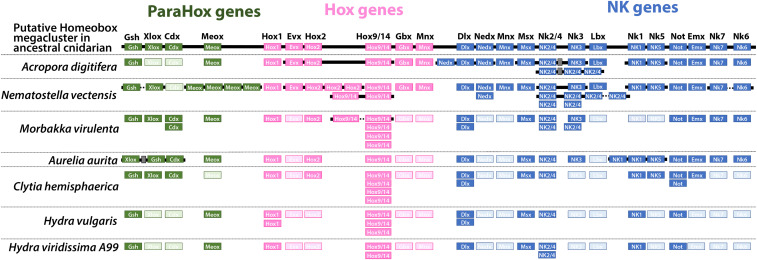
H.viridissima and H. vulgaris possess the same ANTP genes (Figure 4, Table 7, Table S5), suggesting a reason for the same body plan in these Hydra species, although the body size of H. viridissima is smaller. As previously reported (Leclère et al. 2019; Khalturin et al. 2019; Gauchat et al. 2000; Quiquand et al. 2009), ParaHox genes Gsh and Meox are present in Hydra, whereas Xlox and Cdx are missing, unlike other medusozoans (Table 7, Figure 4). On the other hand, Hox gene composition is quite similar among medusozoans. They have Hox1 and Hox9-14, but lack Hox2, Evx, Gbx, Mnx, unlike anthozoans. Medusozoans have lost many NKL genes, Nedx, Hlx, Mnx, Msx, and Lbx compared to anthozoans. In addition, Dbx, Hlx, Nk3, Nk6 and Nk7 are not found in hydrozoans, nor are Nk5, Exm or Msxlx in Hydra (Table 7, Figure 4). In addition, some degree of synteny conservation of HOXL genes and NKL genes is found in Anthozoa, but not in Medusozoa (Figure 4), suggesting complete fragmentation of the homeobox gene cluster in the common ancestor of medusozoans. Nematostella expresses Gbx, Hlx, Nk3 and Nk6 in the pharyngeal or mesenteric region (Gbx in pharyngeal endoderm (Matus et al. 2006), Hlx and Nk6 in pharyngeal ectoderm, and Nk3 in nutrient-storing somatic gonads in mesentery (Steinmetz et al. 2017)). Anthozoans have a pharynx and a mesentery that structurally supports the pharynx and serves as the site of gamete production in the gastrovascular cavity, while these tissues are not found in Hydra. Loss of these genes reflects the simplification of body structure in the Hydra lineage.
Figure 4.
ParaHox, Hox and NK genes in cnidarians. The putative Homeobox megacluster in the last common ancestor of cnidarians (top) and homeobox genes and their cluster structures in extant cnidarians are represented. ParaHox genes (green boxes); Hox genes (pink boxes); NK genes (blue boxes). Empty boxes indicate lost genes. Horizontal lines (black) indicate chromosome fragments.
Conclusions
In this study, we report the first genome assembly of H. viridissima, which is one the most basal species in the genus Hydra and the only species with symbiotic algae. Compared to H. vulgaris, H. viridissima has a compact genome one-third the size and with 36.5% fewer genes (Table 1). In addition, the H. viridissima genome has fewer repetitive sequences. RNA transposons, in particular, are almost absent (Figure 2). On the other hand, the repertoire of transcription factor genes, including homeodomain-containing genes in H. viridissima is quite similar to that in H. vulgaris (Table 5), reflecting the common body plan in these species. Comparative analysis of homeodomain genes among cnidarians indicates gradual simplification of the ANTP gene repertoire in the Hydra lineage (Tables 6 and 7, Figure 4), which is likely to reflect the simple body structure of Hydra and the absence of jellyfish and planula stages. In addition, we found diverse innate immunity genes in the H. viridissima genome that are also observed in corals (Table 4, Figure 3), indicating a common feature involved in algal symbiosis. The H. viridissima genome presented here provides a Hydra genome comparable in quality to those of other cnidarians, including medusozoans and anthozoans, which will hopefully facilitate further studies of cnidarian genes, genomes, and genetics to understand basal metazoan evolution and strategies to support algal symbiosis in cnidarians.
Acknowledgments
This research was supported by funding from the Okinawa Institute of Science and Technology (OIST) to the Marine Genomics Unit (NS) and by the Okayama University Dispatch Project for Female Faculty members (MH). MH was supported by the Japanese Society for Promotion of Science Funding (15K07173, 18K06364). We thank Dr. Steven D. Aird for editing the manuscript, Ms. Kanako Hisata (OIST) for creating the genome browser, Dr. Chuya Shinzato (OIST, Tokyo University) for providing valuable advice and help with genome sequencing and assembly, and Prof. Thomas C. G. Bosch (Kiel University) for valuable discussion. We also thank the DNA Sequencing Section and the IT Section of OIST for excellent technical support. Computation for this work was partially performed on the NIG supercomputer at the ROIS National Institute of Genetics.
Footnotes
Supplemental material available at figshare: 10.1534/g3.120.401411.
Communicating editor: M.-A. Félix
Literature Cited
- Balasubramanian P. G., Beckmann A., Warnken U., Schnölzer M., Schüler A. et al. , 2012. Proteome of Hydra nematocyst. J. Biol. Chem. 287: 9672–9681. 10.1074/jbc.M111.328203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten S., Simakov O., Esherick L. Y., Liew Y. J., Lehnert E. M. et al. , 2015. The genome of Aiptasia a sea anemone model for coral symbiosis. Proc. Natl. Acad. Sci. USA 112: 11893–11898. 10.1073/pnas.1513318112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode H., 2011. Axis Formation in Hydra. Annu. Rev. Genet. 45: 105–117. 10.1146/annurev-genet-102209-163540 [DOI] [PubMed] [Google Scholar]
- Bode H. R., 2003. Head regeneration in Hydra. Dev. Dyn. 226: 225–236. 10.1002/dvdy.10225 [DOI] [PubMed] [Google Scholar]
- Boetzer M., Henkel C. V., Jansen H. J., Butler D., and Pirovano W., 2011. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27: 578–579. 10.1093/bioinformatics/btq683 [DOI] [PubMed] [Google Scholar]
- Bridge D., Cunningham C. W., Schierwater B., DeSalle R., and Buss L. W., 1992. Class-level relationships in the phylum Cnidaria: evidence from mitochondrial genome structure. Proc. Natl. Acad. Sci. USA 89: 8750–8753. 10.1073/pnas.89.18.8750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca R. C., Moore W., and Shuster S. M., 2016. Invertebrates, Sinauer Associates, Inc., Publishers, Sunderland, Massachusetts. [Google Scholar]
- Cernichiari E., Muscatine L., and Smith D. C., 1969. Maltose Excretion by the Symbiotic Algae of Hydra viridis. Proc. R. Soc. Lond. B Biol. Sci. 173: 557–576. 10.1098/rspb.1969.0077 [DOI] [Google Scholar]
- Chapman J. A., Kirkness E. F., Simakov O., Hampson S. E., Mitros T. et al. , 2010. The dynamic genome of Hydra. Nature 464: 592–596. 10.1038/nature08830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourrout D., Delsuc F., Chourrout P., Edvardsen R. B., Rentzsch F. et al. , 2006. Minimal ProtoHox cluster inferred from bilaterian and cnidarian Hox complements. Nature 442: 684–687. 10.1038/nature04863 [DOI] [PubMed] [Google Scholar]
- Davy S. K., Allemand D., and Weis V. M., 2012. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol. Rev. 76: 229–261. 10.1128/MMBR.05014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deines P., and Bosch T. C. G., 2016. Transitioning from Microbiome Composition to Microbial Community Interactions: The Potential of the Metaorganism Hydra as an Experimental Model. Front. Microbiol. 7: 1610 10.3389/fmicb.2016.01610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. E., and Huss V. A. R., 1986. On the characteristics and taxonomic position of symbiotic Chlorella. Arch. Microbiol. 145: 80–84. 10.1007/BF00413031 [DOI] [Google Scholar]
- DuBuc T. Q., Stephenson T. B., Rock A. Q., and Martindale M. Q., 2018. Hox and Wnt pattern the primary body axis of an anthozoan cnidarian before gastrulation. Nat. Commun. 9: 2007 10.1038/s41467-018-04184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elieh Ali Komi D., Sharma L., and Dela Cruz C. S., 2018. Chitin and Its Effects on Inflammatory and Immune Responses. Clin. Rev. Allergy Immunol. 54: 213–223. 10.1007/s12016-017-8600-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms D. M., and Kelly S., 2015. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16: 157 10.1186/s13059-015-0721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier D. E. K., 2016. Evolution of Homeobox Gene Clusters in Animals: The Giga-Cluster and Primary vs. Secondary Clustering. Front. Ecol. Evol. 4: 36 10.3389/fevo.2016.00036 [DOI] [Google Scholar]
- Ferrier D. E. K., and Holland P. W. H., 2001. Ancient origin of the Hox gene cluster. Nat. Rev. Genet. 2: 33–38. 10.1038/35047605 [DOI] [PubMed] [Google Scholar]
- Finn R. D., Coggill P., Eberhardt R. Y., Eddy S. R., Mistry J. et al. , 2016. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44: D279–D285. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchat D., Mazet F., Berney C., Schummer M., Kreger S. et al. , 2000. Evolution of Antp-class genes and differential expression of <em>Hydra Hox/paraHox</em> genes in anterior patterning. Proc. Natl. Acad. Sci. USA 97: 4493–4498. 10.1073/pnas.97.9.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. A., Katsuki T., Li Y., Yan X., Regulski M. et al. , 2019. The genome of the jellyfish Aurelia and the evolution of animal complexity. Nat. Ecol. Evol. 3: 96–104. 10.1038/s41559-018-0719-8 [DOI] [PubMed] [Google Scholar]
- Haas B. J., Delcher A. L., Mount S. M., Wortman J. R., Smith R. K. Jr. et al. , 2003. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 31: 5654–5666. 10.1093/nar/gkg770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habetha M., Anton-Erxleben F., Neumann K., and Bosch T. C., 2003. The Hydra viridis/Chlorella symbiosis. Growth and sexual differentiation in polyps without symbionts. Zoology 106: 101–108. 10.1078/0944-2006-00104 [DOI] [PubMed] [Google Scholar]
- Hall T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symposium Series 41: 95–98. [Google Scholar]
- Hamada M., Schröder K., Bathia J., Kürn U., Fraune S. et al. , 2018. Metabolic co-dependence drives the evolutionarily ancient Hydra-Chlorella symbiosis. eLife 7: e35122 10.7554/eLife.35122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M., Shoguchi E., Shinzato C., Kawashima T., Miller D. J. et al. , 2013. The complex NOD-like receptor repertoire of the coral Acropora digitifera includes novel domain combinations. Mol. Biol. Evol. 30: 167–176. 10.1093/molbev/mss213 [DOI] [PubMed] [Google Scholar]
- Holstein T. W., Hobmayer E., and Technau U., 2003. Cnidarians: An evolutionarily conserved model system for regeneration? Dev. Dyn. 226: 257–267. 10.1002/dvdy.10227 [DOI] [PubMed] [Google Scholar]
- Huang S., Kang M., and Xu A., 2017. HaploMerger2: rebuilding both haploid sub-assemblies from high-heterozygosity diploid genome assembly. Bioinformatics 33: 2577–2579. 10.1093/bioinformatics/btx220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss V. A. R., Holweg C., Seidel B., Reich V., Rahat M. et al. , 1993/1994. There is an ecological basis for host/symbiont specificity in Chlorella/Hydra symbioses. Endocytobiosis Cell Res. 10: 35–46. [Google Scholar]
- Kamm K., Schierwater B., Jakob W., Dellaporta S. L., and Miller D. J., 2006. Axial Patterning and Diversification in the Cnidaria Predate the Hox System. Curr. Biol. 16: 920–926. 10.1016/j.cub.2006.03.036 [DOI] [PubMed] [Google Scholar]
- Khalturin K., Shinzato C., Khalturina M., Hamada M., Fujie M. et al. , 2019. Medusozoan genomes inform the evolution of the jellyfish body plan. Nat. Ecol. Evol. 3: 811–822. 10.1038/s41559-019-0853-y [DOI] [PubMed] [Google Scholar]
- Kim H.-M., Weber J. A., Lee N., Park S. G., Cho Y. S. et al. , 2019. The genome of the giant Nomura’s jellyfish sheds light on the early evolution of active predation. BMC Biol. 17: 28 10.1186/s12915-019-0643-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusserow A., Pang K., Sturm C., Hrouda M., Lentfer J. et al. , 2005. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433: 156–160. 10.1038/nature03158 [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A. et al. , 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Leclère L., Horin C., Chevalier S., Lapébie P., Dru P. et al. , 2019. The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat. Ecol. Evol. 3: 801–810. 10.1038/s41559-019-0833-2 [DOI] [PubMed] [Google Scholar]
- Luo R., Liu B., Xie Y., Li Z., Huang W. et al. , 2012. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1: 18 10.1186/2047-217X-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais G., and Kingsford C., 2011. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27: 764–770. 10.1093/bioinformatics/btr011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez D. E., Iñiguez A. R., Percell K. M., Willner J. B., Signorovitch J. et al. , 2010. Phylogeny and biogeography of Hydra (Cnidaria: Hydridae) using mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 57: 403–410. 10.1016/j.ympev.2010.06.016 [DOI] [PubMed] [Google Scholar]
- Matus D. Q., Pang K., Marlow H., Dunn C. W., Thomsen G. H. et al. , 2006. Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc. Natl. Acad. Sci. USA 103: 11195–11200. 10.1073/pnas.0601257103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley, P. J., 1991 Amino acids as a nitrogen source for Chlorella symbiotic with green hydra, pp. 369–376 in Coelenterate Biology: Recent Research on Cnidaria and Ctenophora: Proceedings of the Fifth International Conference on Coelenterate Biology, 1989, edited by R.B. Williams, P.F.S. Cornelius, R.G. Hughes and E.A. Robson. Springer Netherlands, Dordrecht. 10.1007/978-94-011-3240-4_53 10.1007/978-94-011-3240-4_53 [DOI] [Google Scholar]
- Mews L. K., 1980. The Green Hydra Symbiosis. III. The Biotrophic transport of Carbohydrate from Alga to Animal. Proc. R. Soc. Lond. B Biol. Sci. 209: 377–401. 10.1098/rspb.1980.0101 [DOI] [Google Scholar]
- Muscatine L., 1965. Symbiosis of hydra and algae. 3. Extracellular products of the algae. Comp. Biochem. Physiol. 16: 77–92. 10.1016/0010-406X(65)90165-9 [DOI] [PubMed] [Google Scholar]
- Pan H.-C., Fang H.-Y., Li S.-W., Liu J.-H., Wang Y. et al. , 2014a The complete mitochondrial genome of Hydra vulgaris (Hydroida: Hydridae). Mitochondrial DNA 25: 418–419. 10.3109/19401736.2013.809437 [DOI] [PubMed] [Google Scholar]
- Pan H.-C., Qian X.-C., Li P., Li X.-F., and Wang A.-T., 2014b The complete mitochondrial genome of Chinese green hydra, Hydra sinensis (Hydroida: Hydridae). Mitochondrial DNA 25: 44–45. 10.3109/19401736.2013.782017 [DOI] [PubMed] [Google Scholar]
- Pardy R. L., 1976. The morphology of green hydra endosymbionts as influenced by host strain and host environment. J. Cell Sci. 20: 655–669. [DOI] [PubMed] [Google Scholar]
- Putnam N. H., Srivastava M., Hellsten U., Dirks B., Chapman J. et al. , 2007. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317: 86–94. 10.1126/science.1139158 [DOI] [PubMed] [Google Scholar]
- Quiquand M., Yanze N., Schmich J., Schmid V., Galliot B. et al. , 2009. More constraint on ParaHox than Hox gene families in early metazoan evolution. Dev. Biol. 328: 173–187. 10.1016/j.ydbio.2009.01.022 [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Ostermann T., Kremeser L., Lindner H., Beisel C. et al. , 2016. Profiling of adhesive-related genes in the freshwater cnidarian Hydra magnipapillata by transcriptomics and proteomics. Biofouling 32: 1115–1129. 10.1080/08927014.2016.1233325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., and Nei M., 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- Schummer M., Scheurlen I., Schaller C., and Galliot B., 1992. HOM/HOX homeobox genes are present in hydra (Chlorohydra viridissima) and are differentially expressed during regeneration. EMBO J. 11: 1815–1823. 10.1002/j.1460-2075.1992.tb05233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwentner M., and Bosch T. C., 2015. Revisiting the age, evolutionary history and species level diversity of the genus Hydra (Cnidaria: Hydrozoa). Mol. Phylogenet. Evol. 91: 41–55. 10.1016/j.ympev.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Seppey M., Manni M., and Zdobnov E. M., 2019. BUSCO: Assessing Genome Assembly and Annotation Completeness, pp. 227–245 in Gene Prediction: Methods and Protocols, edited by Kollmar M. Springer, New York. [DOI] [PubMed] [Google Scholar]
- Shinzato C., Shoguchi E., Kawashima T., Hamada M., Hisata K. et al. , 2011. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476: 320–323. 10.1038/nature10249 [DOI] [PubMed] [Google Scholar]
- Skinner M. E., Uzilov A. V., Stein L. D., Mungall C. J., and Holmes I. H., 2009. JBrowse: a next-generation genome browser. Genome Res. 19: 1630–1638. 10.1101/gr.094607.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M., Keller O., Gunduz I., Hayes A., Waack S. et al. , 2006. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 34: W435–W439. 10.1093/nar/gkl200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. E., David C. N., and Technau U., 2011. A genomic view of 500 million years of cnidarian evolution. Trends Genet. 27: 7–13. 10.1016/j.tig.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R. H., Aman A., Kraus J. E. M., and Technau U., 2017. Gut-like ectodermal tissue in a sea anemone challenges germ layer homology. Nat. Ecol. Evol. 1: 1535–1542. 10.1038/s41559-017-0285-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembley, A., C. Pronk, J.v.d. Schley, and P. Lyonet, 1744 Mémoires pour servir à l’histoire d’un genre de polypes d’eau douce, à bras en forme de cornes A Leide:: Chez Jean & Herman Verbeek. 10.5962/bhl.title.64073 10.5962/bhl.title.64073 [DOI]
- Vurture G. W., Sedlazeck F. J., Nattestad M., Underwood C. J., Fang H. et al. , 2017. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics 33: 2202–2204. 10.1093/bioinformatics/btx153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittlieb J., Khalturin K., Lohmann J. U., Anton-Erxleben F., and Bosch T. C. G., 2006. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc. Natl. Acad. Sci. USA 103: 6208 10.1073/pnas.0510163103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. Y., Simakov O., Bridge D. M., Cartwright P., Bellantuono A. J. et al. , 2019. Expansion of a single transposable element family is associated with genome-size increase and radiation in the genus Hydra. Proc. Natl. Acad. Sci. USA 116: 22915–22917. 10.1073/pnas.1910106116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias H., Anokhin B., Khalturin K., and Bosch T. C., 2004. Genome sizes and chromosomes in the basal metazoan Hydra. Zoology (Jena) 107: 219–227. 10.1016/j.zool.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Zhong Y., and Holland P. W. H., 2011. HomeoDB2: functional expansion of a comparative homeobox gene database for evolutionary developmental biology. Evol. Dev. 13: 567–568. 10.1111/j.1525-142X.2011.00513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This whole-genome shotgun sequencing project has been deposited at DDBJ/ENA/GenBank under BioSample ID SAMN09635813 and BioProject ID: PRJNA480404. RNA-seq reads have been deposited at SRA of NCBI (SRX6792700-SRX6792705). Genome sequences, gene models, and a genome browser are also accessible at the website of the OIST Marine Genomics Unit Genome Project (https://marinegenomics.oist.jp/hydraviridissima_A99). A genome browser was established for assembled sequences using the JBrowse 1.12.3 (Skinner et al. 2009). Gene annotations from the protein domain search and BLAST search are likewise shown on the site. Reagents, software and datasets used in this study are listed in the Reagent Table. k-mer frequency distribution plots in the Hydra viridissima A99 genome is found in Figure S1. Phylogenetic analysis of ANTP genes is in Figure S2. Phylogenetic analysis of PRD genes is presented in Figure S3. Sequencing statistics for Hydra viridissima A99 are in Table S1. A summary of repetitive sequences in the Hydra viridissima A99 genome assembly are found in Table S2. Pfam domain-containing genes in the Hydra viridissima A99 genome are available in Table S3. Orthologs enriched in Hydra viridissima A99 (A) and Hydra (B) are in Table S4. Gene IDs of ANTP genes in Hydra viridissima A99 are in Table S5. Supplemental material available at figshare: https://doi.org/10.25387/g3.12911426.