Abstract
RNA interference is a powerful tool for dissecting gene function. In Caenorhabditis elegans, ingestion of double stranded RNA causes strong, systemic knockdown of target genes. Further insight into gene function can be revealed by tissue-specific RNAi techniques. Currently available tissue-specific C. elegans strains rely on rescue of RNAi function in a desired tissue or cell in an otherwise RNAi deficient genetic background. We attempted to assess the contribution of specific tissues to polyunsaturated fatty acid (PUFA) synthesis using currently available tissue-specific RNAi strains. We discovered that rde-1 (ne219), a commonly used RNAi-resistant mutant strain, retains considerable RNAi capacity against RNAi directed at PUFA synthesis genes. By measuring changes in the fatty acid products of the desaturase enzymes that synthesize PUFAs, we found that the before mentioned strain, rde-1 (ne219) and the reported germline only RNAi strain, rrf-1 (pk1417) are not appropriate genetic backgrounds for tissue-specific RNAi experiments. However, the knockout mutant rde-1 (ne300) was strongly resistant to dsRNA induced RNAi, and thus is more appropriate for construction of a robust tissue-specific RNAi strains. Using newly constructed strains in the rde-1(null) background, we found considerable desaturase activity in intestinal, epidermal, and germline tissues, but not in muscle. The RNAi-specific strains reported in this study will be useful tools for C. elegans researchers studying a variety of biological processes.
Keywords: fatty acid desaturase, RNA interference
RNA interference (RNAi) is an evolutionarily ancient defense mechanism against viruses and transposable elements . Caenorhabditis elegans has been a powerful system for discovering the molecular mechanisms underlying the RNAi phenomena and its role in gene regulation (Fire et al. 1998; Grishok 2005; Hammond 2005). The discovery that double stranded RNA (dsRNA) expressed by bacteria and ingested by the worm could effectively silence target genes revolutionized the use of RNAi as a tool for high-throughput, large-scale genetic knockdown studies in C. elegans (Timmons and Fire 1998; Fraser et al. 2000; Ashrafi et al. 2003; Yigit et al. 2006).
Genetic screens for mutants that are resistant to RNAi have been essential for elucidating the mechanism of processing exogenously introduced double stranded RNAs and inducing gene silencing (Zhuang and Hunter 2012). The feeding method of RNAi delivery is successful in C. elegans because of two membrane proteins called SID-1 and SID-2 that facilitate the uptake of double stranded RNA into cells (Winston et al. 2002; Winston et al. 2007). Both mutant strains grow and reproduce normally but are resistant to system-wide RNAi delivered by the feeding method. Other screens for viable mutants with RNAi deficiency (RDE mutants) revealed genes coding for many highly-conserved activities required for RNAi, including RDE-4, a dsRNA binding protein which forms a complex with Dicer to bind long dsRNA and cleave it into ∼22 bp interfering RNAs (22G siRNAs) (Tabara et al. 2002; Knight and Bass 2001; Parker et al. 2006; Raman et al. 2017).
In C. elegans, RDE-1 is the primary Argonaute component of the RNA induced silencing complex (RISC), which degrades the passenger strand of the siRNA and uses the remaining strand to target mRNA for use as a template for synthesis of secondary siRNAs (Tabara et al. 1999; Parrish and Fire 2001; Steiner et al. 2009). Production of the siRNAs amplifies the signal and are used as the final targeting signal for degradation of newly formed mRNAs. The C. elegans genome encodes multiple homologs of certain components of the RNAi machinery, including four RNA-dependent RNA polymerases (RdRPs), including RRF-1, and a large family of Argonaute proteins (Smardon et al. 2000; Sijen et al. 2001; Yigit et al. 2006; Xu et al. 2018). The RdRPs amplify silencing by using the primary siRNA/mRNA complex as a template for synthesizing secondary siRNAs. RDE-3, a member of the polymerase beta nucleotidyltransferase superfamily is required for siRNA accumulation (Chen et al. 2005). RDE-10 and RDE-11 form a complex that promotes secondary siRNA amplification (Yang et al. 2012).
Identifying mutants that are resistant to the induction of RNAi by double stranded RNA has typically involved screening with a visible phenotype such as lethality, movement defects, or suppression of a fluorescent transgene (Tabara et al. 1999; Yigit et al. 2006; Winston et al. 2002). Mutant strains in which the treated worms failed to display the knockdown phenotype were considered RNAi resistant. These methods have proven efficient and invaluable for the discovery of RNAi pathway genes. However, the qualitative nature of these visible phenotypes makes them insufficient to determine if a specific mutation in an RNAi pathway gene completely inhibits RNAi capacity, or only enough to prevent the visible phenotype.
Gene knockdown by RNAi is useful for elucidating gene function, but it does not provide information about the function of genes in specific tissues. To assess tissue specific gene function, investigators have employed tissue specific transgenic rescue of key RNAi machinery (Sijen et al. 2001; Qadota et al. 2007). Accurate analysis of experiments performed in tissue specific systems depends on RNAi functioning only in the desired cells and tissues. This is achieved by expressing a transgene under the control of a tissue-specific promoter in the background of an RNAi-deficient mutant. Any residual RNAi activity in off-target tissues can lead to erroneous conclusions, thus it is essential that the mutant strain used is completely RNAi-deficient and it is also essential that the promoter is expressed only in specific cell types.
Our objective was to determine various tissue contributions to the overall degree of fatty acid desaturation in the nematode C. elegans using tissue-specific RNAi strains and feeding RNAi directed toward two fatty acid desaturase genes. We used a biochemical method employing gas chromatography-mass spectrometry (GC-MS) to monitor the flux of fatty acid desaturation reactions from substrates to products as a quantitative approach to measuring the RNAi silencing capacity in previously characterized C. elegans mutant strains. Our quantitative method revealed that some C. elegans strains deemed “RNAi deficient” retained substantial RNAi silencing capacity when exposed to the feeding method of RNAi delivery, and therefore the available strains used for tissue-specific RNAi are not useful for determining tissue-specificity of gene function. We present here a collection of new strains for tissue-specific RNAi and report that intestine and epidermal tissues produce the highest amounts of desaturated fatty acids, while the germline also shows activity of omega-3 and delta-5 fatty acid desaturases.
Materials and Methods
Worm maintenance and RNAi
NGM growth media was supplemented with 100 μg/mL ampicillin, 2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and seeded with the appropriate HT115 RNAi bacteria. RNAi constructs for fat-1, fat-4, and empty vector control were obtained from the Ahringer RNAi library and sequence verified (Fraser et al. 2000). Synchronized L1 larvae were plated onto the RNAi plates and allowed to grow for 2-3 days at 20° or at 25° (for act-5 and dpy-7 experiments) until worms reached young adult stage.
The following strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440): N2, wild type; WM27, rde-1(ne219) V; NL2098, rrf-1(pk1417) I; NL3511, ppw-1(pk1425) I; VS26, rde-10(hj20) I; VS27, rde-11(hj37) IV; WM30, rde-3(ne298) I; WM119, sago-2(tm894) ppw-1(tm914) I; C06A1.4(tm887) F58G1.1(tm1019) II; H10D12.2(tm1144) IV; sago-1(tm1195) V; neIs10 X; WM49, rde-4(ne301) III, HC196, sid-1(qt9) V; WM118, rde-1(ne300) V, neIs9 [myo-3::HA::RDE-1 + rol-6(su1006)]. The WM45, rde-1(ne300) V strain was provided by Craig Mello and the DCL569 strain was provided by Di Chen (Zou et al. 2019).
Construction of new tissue-specific RNAi strains
The transgenes frSi17 and frSi21 are single copy insertions on chromosome II (ttTi5605 location) of pNP160 (mtl-2p::RDE-1_3′rde-1 ttTi5605] and pSO21(col-62p::RDE-1_3′rde-1 ttTi5605) respectively by CRISPR using a self-excising cassette (SEC) (Dickinson et al. 2015). The strain pNP160 was obtained by insertion of the promoter of mtl-2 which is only expressed in the intestine into the pNP154 vector. pSO21 was obtained by insertion of the promoter of col-62 which is only expressed in the adult in the epidermis into the pNP154 vector. pNP154 was made from a vector containing the SEC for single insertion on Chromosome II at the position of ttTi5605 (pAP087, kindly provided by Ari Pani) (Taffoni et al. 2020). pNP160 and pSO21 were injected in rde-1(ne300) at 10 ng/µl together with pDD122 (eft-3p::Cas9) at 40 ng/µl, pCFJ90 (myo-2p::mCherry) at 2.5 ng/µl, pCFJ104 (myo-3p::mCherry) at 5 ng/µl, and #46168 (eef-1A.1p::CAS9-SV40_NLS::3′tbb-2 (Friedland et al. 2013) at 30 ng/µl. Non-fluorescent roller worms were selected then heat shocked to remove the SEC by FloxP as described in (Dickinson et al., 2015). The intestine-specific IG1839 rde-1(ne300) V; frSi17[pNP160(mtl-2p::RDE-1_3′rde-1 ttTi5605] II; frIs7[nlp-29p::GFP, col-12p::DsRed] IV and the adult-skin-specific IG1846 rde-1(ne300) V; frSi21[pSO21(col-62p::RDE-1_3′rde-1 ttTi5605] II; frIs7[nlp-29p::GFP, col-12p::DsRed] IV transgenic strains were subsequently obtained by conventional crosses and all genotypes were confirmed by PCR or sequencing. All constructs were made using Gibson Assembly (NEB Inc., MA) and confirmed by PCR or sequencing.
Fatty acid analysis
To measure fatty acid composition, approximately 1000 young adult stage worms (containing 0-8 embryos) were washed from feeding plates with water on ice and washed once to remove residual bacteria. After settling on ice again, as much water as possible was removed (∼90%). Fatty acids were converted to methyl esters for analysis as previously described (Shi et al. 2013). The worm suspensions were incubated for 1 hr at 70° in 2 ml of 2.5% sulfuric acid in methanol. Following incubation, the reactions were stopped by adding 1ml of water and then mixed thoroughly with 200 μl of hexane to extract the resulting fatty acid methyl esters. We measured relative amount of fatty acid methyl esters by injecting 2 μl of the hexane layer onto an Agilent 7890 GC-5975C MS in scanning ion mode.
Worm size and GFP fluorescence analyses
Size was measured as time of flight = TOF as arbitrary units and expression of the nlp-29p::gfp reporter was quantified with the COPAS Biosort (Union Biometrica). The ratio of GFP/size was then calculated to normalize the fluorescence (Pujol et al. CB 2008). The results shown are representative of at least 3 independent experiments.
Statistics for desaturase index comparisons
At least 3 biological replicates were used for analysis. Within each biological experiment, samples were collected in triplicate. P values shown in Table S1 were determined by comparing the control empty vector to the RNAi knockdown using t-tests, which were corrected for multiple comparisons using the Holm-Sidak method with an alpha value of 0.05.
Determination of desaturation index and percent RNAi deficiency
Desaturation index for each strain was determined by comparing the relative peak areas of individual fatty acid methyl esters using the following formulas.
FAT-1 desaturation index
DI = (20:4n-3 +20:5n-3)/(20:3n-6 + 20:4n-6)
FAT-4 desaturation index
DI = (20:4n-6+20:5n-3)/(20:3n-6 + 20:4n-3)
Data availability
Worm strains, data and reagents are available upon request. Accompanying the manuscript is one supplemental table, Table S1. Supplemental material available at figshare: https://doi.org/10.25387/g3.12968096.
Results
Using polyunsaturated fatty acid desaturation to assess RNAi efficiency
Fatty acids are the building blocks of the lipids that cells use for energy storage, membrane structure, and signaling. C. elegans obtains fatty acids from its bacterial diet, and also has the capacity to generate fatty acids de novo from acetyl-CoA (Watts and Ristow 2017). Saturated fatty acids obtained from the diet or synthesized de novo are further modified by chain elongation and the addition of one or more double bonds, (Watts and Browse 2002) greatly enhancing the structural diversity and utility of fatty acids available to the cell. Based on published RNA sequencing studies (Chikina et al. 2009; Cao et al. 2017) we suspected that fatty acid desaturases are expressed in various tissues in C. elegans, especially in the intestine, the tissue responsible for absorption of dietary fats and modification of synthesized and dietary fats for incorporation into membrane lipids, and as well as for storage lipids contained in lipid droplets and yolk (Watts and Ristow 2017). We reasoned that using tissue specific RNAi strains, and determination of fatty acid composition by GC-MS, we could establish the relative contributions of various tissues to overall fatty acid composition.
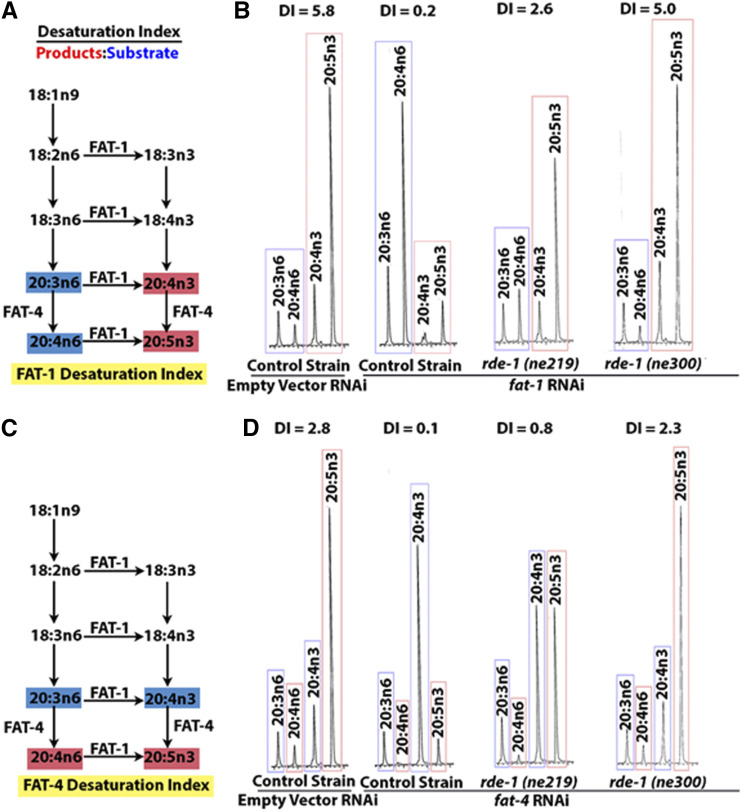
We focused on two of the FA desaturase enzymes, FAT-1, an omega-3 desaturase, and FAT-4, a ∆5 desaturase (Watts and Browse 2002). FAT-1 adds double bonds between the third and fourth carbons of a FA from the methyl end (omega-3), whereas the FAT-4 ∆5 desaturase adds double bonds between the fifth and sixth carbon counting from the carboxyl end of the fatty acid. Figure 1 shows the fatty acid desaturation pathway, highlighting the substrates used by, and products synthesized by FAT-1 (Figure 1A) and FAT-4 desaturases (Figure 1C). C. elegans grown on bacteria expressing double stranded RNA complementary to fat-1 or fat-4 grow normally and reproduce, as do fat-1 and fat-4 null mutants (Watts and Browse 2002).
Figure 1.
Determination of desaturation index. RNAi efficiency was measured by the ability to knock down the fatty acid desaturase genes fat-1 and fat-4 with RNAi by feeding. We calculated the desaturation index for each treatment, defined as the ratio of fatty acid desaturation products (red) to their substrates (blue) (A and C). Representative chromatographs reveal that in RNAi competent worms (control strain), treatment with RNAi against either fat-1 (B) or fat-4 (D) causes accumulation of substrates (blue) and depletion of products (red) leading to a low desaturation index. Example chromatographs of an RNAi “resistant strain” show a similar desaturation indices as the empty vector control treated worms, while chromatographs of the “partially resistant” strain show intermediate activity.
Loss of function mutations and RNAi treatment of fat-1 and fat-4 lead to reduced ratios of specific fatty acid products to substrates, a relationship that can be quantified as the desaturation index. When grown in lab conditions, on plates with E. coli HT115 bacteria, wild type C. elegans typically shows a desaturation index of 4-6 corresponding to FAT-1 activity, meaning the abundance of omega-3 fatty acid products is 4-6 fold more than the omega-6 substrates. For FAT-4, the desaturation index is typically 2.5-3, meaning the ∆5 desaturated products are 2.5-3 fold more abundant than their substrates (see Materials and Methods for desaturase index calculations). In fat-1 or fat-4 null mutants, the corresponding desaturation indices drop to zero because there are no omega-3 or delta-5 unsaturated PUFAs formed in the mutant strains (Watts and Browse 2002). Feeding RNAi is a very efficient means to knock down FAT-1 and FAT-4 activity, and in a wild type background, fat-1 RNAi causes a drop in the desaturation index from 4-6 to 0.2. Similarly, fat-4 RNAi causes a drop in the desaturation index of 2.5-3 down to 0.1 (Figure 1 and Table 1). An RNAi-resistant strain treated with fat-1 or fat-4 RNAi would be expected to show a similar desaturation index as an untreated strain. Figure 1B and 1D show typical chromatographs of the wild type control strain, as well as a strongly resistant mutant strain and a strain that is only partially RNAi resistant when fed fat-1 or fat-4 RNAi.
Table 1. Desaturation indices of strains used in this study.
| Strain | Gene (allele) | Protein/Function | Reported RNAi phenotype | Mutation | fat-1 desat. index | fat-4 desat. index |
|---|---|---|---|---|---|---|
| N2 | wild type | susceptible | none | 5.8->0.2 | 2.9->0.1 | |
| NL2098 | rrf-1(pk1417) | RNA dependent polymerase | RNAi deficient in soma (Sijen et al. 2001) | 400 bp deletion | 5.2->0.6 | 2.7->0.3 |
| NL3511 | ppw-1(pk1425) | Argonaute/Piwi | RNAi deficient in germline (Tijsterman et al. 2002) | 1504 bp deletion | 6.0->0.2 | 2.7->0.1 |
| VS26 | rde-10(hj20) | siRNA amplification | RNAi deficient (Yang et al. 2012) | premature stop codon | 5.0->0.8 | 2.3->0.4 |
| VS27 | rde-11(hj37) | siRNA amplification | RNAi deficient (Yang et al. 2012) | premature stop codon | 5.0->0.8 | 2.6->0.3 |
| WM30 | rde-3(ne298) | nucleotidyl transferase | RNAi deficient (Chen et al. 2005) | G366R | 5.2->1.1 | 2.7->0.9 |
| WM119 | MAGO | multiple Argonautes | RNAi deficient (Yigit et al. 2006) | multiple | 4.4->2.0 | 2.9->1.4 |
| WM49 | rde-4(ne301) | dsRNA binding protein | RNAi deficient (Tabara et al. 2002) | unknown | 5.5->4.3 | 2.5->1.5 |
| WM27 | rde-1(ne219) | Argonaute/Piwi | RNAi deficient (Tabara et al. 1999) | E414K | 6.0->2.6 | 2.5->0.8 |
| WM45 | rde-1(ne300) | Argonaute/Piwi | RNAi deficient (Tabara et al. 1999) | premature stop codon | 5.6->6.0 | 2.4->2.3 |
| HC196 | sid-1(qt9) | dsRNA channel | RNAi deficient (Winston et al. 2002) | S536I | 5.2->5.0 | 2.4->2.0 |
The rde-1 (ne219) strain is only partially RNAi resistant
We sought to determine the extent of fatty acid desaturation in various tissues by using the RNAi-deficient strain rde-1(ne219), as well as transgenic strains in which the rde-1 gene was expressed under control of tissue-specific promotors in the rde-1(ne219) mutant background (Qadota et al. 2007). Interpretation of these types of tissue specific RNAi experiments relies on the absence of RNAi activity in the rde-1 mutant strain. We found that the rde-1(ne219) strain, which contains a single glutamate to lysine substitution at a conserved residue, retained considerable RNAi silencing activity. The FAT-1 desaturation index fell from 6.0 to 2.6 during fat-1 RNAi feeding treatment, while the FAT-4 desaturation index fell from 2.5 to 0.8 during fat-4 RNAi treatment (Figure 2A, Table 1). We therefore could not interpret the data from the tissue-specific rescue strains, because of the remaining RNAi capacity of the rde-1(ne219) mutant strain.
Figure 2.
Desaturation indices for FAT -1 and FAT-4 of control (N2) and RNAi-deficient strains FAT-1 and FAT-4 desaturation indices. (A) rde-1 (ne219) and rde-1 (ne300) compared to control (N2) reveal that rde-1 (ne219) is partially resistant to feeding RNAi and rde-1 (ne300) is strongly RNAi resistant. (B) Germline-specific RNAi deficient (ppw-1) and somatic-specific RNAi deficient (rrf-1) strains both show nearly wild-type levels of RNAi efficiency. (C) Screen of RNAi-deficient strains showing RNAi deficiency (sid-1 and rde-4), partial deficiency (MAGO and rde-3) and nearly normal RNAi efficiency (rde-10 and rde-11) when treated with fat-1 and fat-4 RNAi by feeding. Comparisons of RNAi treatment to empty vector treatment showed statistically significant differences (P values reported in Table S1), except for comparisons of the strains depicted on the graphs with NS, not significant. The strains with no significant difference in desaturation index are considered to be completely RNAi resistant.
We obtained a second rde-1 mutation, rde-1(ne300), which is predicted to contain a premature stop codon within the protein’s PIWI domain (Tabara et al. 1999). In contrast to the results with rde-1(ne219), we found the rde-1(ne300) strain to be strongly RNAi deficient. Treatment with fat-1 or fat-4 RNAi did not significantly change desaturation index compared to treatment with empty vector control. Previous reports concluded that rde-1 (ne219) was highly resistant to RNAi against several genes when using lethality and fluorescence as indicators (Tabara et al. 1999; Qadota et al. 2007). Quantification of metabolite products of targeted enzymes, therefore, provides a more sensitive, quantitative method for assessing the RNAi efficiency of reduction-of-function mutations.
Germline and somatic specific RNAi
The apparent tissue-specificity of several different RNA-dependent RNA polymerases and Argonaute proteins enabled the popular method for delineating whether a gene is acting in somatic tissue or in the germ line in C. elegans. Knockdown of a gene of interest in the background of rrf-1, which was reported to be deficient in RNAi in somatic cells (Sijen et al. 2001), has been compared with a knockdown in the ppw-1 background, which is deficient in RNAi in the germ line (Tijsterman et al. 2002). Similar to the tissue-specific RNAi experiments described above, experiments seeking to quantify the relative contributions of somatic or germline activity of FAT-1 and FAT-4 desaturases were uninterpretable, because both strains showed high levels of RNAi efficiency. Remarkably, the strain carrying the rrf-1(pk1417) mutation retained RNAi activity capable of knocking down fat-1 and fat-4 nearly to the same extent as wild type (Figure 2B and Table 1). Several years ago, however, Kumsta and Hansen demonstrated that rrf-1 (pk1417) maintained RNAi capacity in the intestine and in the hypodermis (Kumsta and Hansen 2012). In spite of their careful analysis and clear evidence of RNAi activity in somatic tissues of rrf-1, researchers continue to publish studies using rrf-1 as a mutant lacking RNAi activity in somatic cells (Webster et al. 2017). Our expectation was that most FAT-1 and FAT-4 desaturation activity would be found in the somatic tissues of intestine and epidermis, and we suspect that ample RNAi activity remained in the rrf-1(pk1417) mutants because this strain is not truly deficient in RNAi activity in intestinal and hypodermal tissues, supporting the findings of Kumsta and Hansen (Kumsta and Hansen 2012). Recent studies demonstrated that EGO-1, an RNA-dependent RNA polymerase that acts in the germline, also functions in intestinal tissue, and therefore acts in the RNAi pathway in the absence of RRF-1 (Ravikumar et al. 2019).
Previously-described RNAi-resistant strains show varied susceptibility to RNAi feeding
Our findings that the rde-1(ne219) strain and the rrf-1 strain were not RNAi-deficient as described led us to use our biochemical GC-MS method to quantitatively assess the degree of RNAi deficiency in other strains reported to be RNAi deficient. We performed the RNAi and GC-MS analysis of various RNAi-deficient mutants using empty vector, fat-1, and fat-4 RNAi and calculated the desaturation indices. We found that sid-1 mutants were resistant to RNAi of fat-1 and fat-4 induced by the feeding method, as expected (Figure 2C and Table 1). The RDE-4 strain was partially resistant to RNAi, as was the multiple Argonaute (MAGO) strain, which carries mutations in several Argonaute proteins, including SAGO-1 and SAGO-2 that are known to interact with secondary siRNA (Yigit et al. 2006). The strain carrying a mutation in the gene encoding the nucleotidyltransferase RDE-3 was also partially resistant to fat-1 and fat-4 RNAi. Neither rde-10 (hj20), nor rde-11 (hj37) were significantly RNAi resistant in our study (Figure 2C and Table 1). The range of desaturase activity measured in various strains demonstrated that our method is a precise way to measure the degree of RNAi-deficiency in C. elegans mutant strains.
Construction of new tissue-specific RNAi strains for intestine- and epidermis- specific RNAi
In order to determine the extent of fatty acid desaturation in various tissues, we needed to obtain or construct new strains with the rde-1(null) background. For intestine-specific RNAi, we used the mtl-2 promotor to rescue rde-1 in the rde-1(ne300) strain (Pujol et al. 2008). For epidermis-specific RNAi, we used the col-62 promoter, which is expressed from the L4 stage onward, to rescue rde-1 in the rde-1(ne300) strain (Taffoni et al. 2020). To confirm the intestine-specific RNAi knockdown, we used RNA corresponding to the act-5 gene, which encodes an essential actin known to be expressed in the intestine (MacQueen et al. 2005). When subjected to act-5 RNAi, only the wild-type and the intestine-specific strain arrested their development, while the epidermal-specific strain reached adulthood to the same extent as the rde-1 resistant strain (Figure 3A). To confirm the tissue-specificity of these promoters, we used a transgenic strain containing the nlp-29p::GFP reporter, which is activated in the epidermis upon cuticle disruption (Pujol et al. 2008), including dpy-7 inactivation (Dodd et al. 2018). Only the epidermal-specific and the wild-type strains present a reduced size and an activation of nlp-29p::GFP, while the intestinal-specific strain was similar to the rde-1 resistant strain (Figure 3B). These results confirm the specificity of the intestinal- and epidermal-specific RNAi strains.
Figure 3.
New strains for intestine- and epidermis-specific RNAi knockdown. (A) The intestine-specific RNAi strain shows developmental arrest on act-5(RNAi) like the wild-type strain, while rde-1 null (rde-1(ne300)) and the adult skin-specific RNAi strain reach adult 48 h after transferring synchronized L1 larvae onto RNAi plates at 25°C. (B and C) Similar to the wild-type, the adult skin-specific RNAi strain becomes shorter (B) and presents a high expression of nlp-29 in the epidermis (C) on dpy-7(RNAi), compared to rde-1 null and intestine-specific RNAi strain; the phenotype is observed as in (A) 48 h after transferring synchronized L1 larvae onto RNAi plates at 25°C. Individual data points are shown, with bars depicting the mean and SEM.
Strains for germline- and muscle-specific RNAi
We obtained muscle-specific and germline specific RNAi strains, both of which were made in the rde-1(null) background. The germline-specific strain DCL569 uses a single-copy insertion of a transgene expressing rde-1 under control of the sun-1 promoter (Zou et al. 2019). The muscle-specific strain WM118 expresses rde-1 under control of the myo-3 promoter. We used RNAi constructs corresponding to egg-5, dpy-10, fat-7, and pat-4 to test the phenotypic specificity of the tissue-specific RNAi strains. As expected, we observed sterility after egg-5 knockdown primarily in the wild type and germline-specific RNAi strain. We observed paralyzed worms in wild type and in the muscle-specific RNAi strain after treatment with pat-4 and unc-112 RNAi (Table 2).
Table 2. Efficacy of tissue-specific RNAi strains.
| Wild type | RNAi deficient | Muscle-specific RNAi | Germline-specific RNAi | Intestine-specific RNAi | Skin-specific RNAi | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | Gene | phenotype | # worms | N2 | WM45 rde-1(ne300) | WM118 rde-1(ne300); myo-3p::rde-1(+) | DCL569 rde-1(-); sun-1p::rde-1(+) | IG1839 rde-1(ne300); mtl-2p::rde-1(+) | IG1846 rde-1(ne300); col-62p::rde-1(+) |
| Germline | egg-5 | Sterile, embryo death | 50 per strain | 92 | 0 | 2 | 98 | 0 | 0 |
| Epidermis | dpy-10 | Short and stout | 60-100 per strain | 100 | 0 | 0 | 5 | ND | ND |
| Intestine | fat-7 | Clear intestine | 90 per strain | 100 | 0 | 0 | 4.4 | ND | ND |
| Muscle | unc-112 | paralyzed | 35-90 per strain | 97.4 | 0 | 100 | 4.2 | 0 | 0 |
| Muscle | pat-4 | paralyzed | 100 per strain | 95.2 | 0 | 80 | 6.7 | 0 | 0 |
Desaturase activity occurs in intestine, epidermal, and germline tissues
We performed fat-1 and fat-4 RNAi on wild type, rde-1(ne300), and the tissue-specific RNAi strains and determined the desaturase indices using GC-MS. We found that RNAi activity in intestinal, epidermal and germline tissues partially reduces the desaturation index compared to RNAi performed in rde-1(null) animals, while muscle-specific RNAi showed no detectable change in the desaturation index compared to the rde-1(null) control (Figure 4A-B).
Figure 4.
Desaturation indices for wild type, RNAi-deficient (rde-1(ne300)) and tissue-specific RNAi strains. For both fat-1 RNAi (A) and fat-4 RNAi (B), the greatest reduction of RNAi activity occurred in the intestine-specific RNAi strain, revealing intestine as the major tissue of fatty acid desaturation. Comparisons of RNAi treatment to empty vector treatment showed statistically significant differences (P values reported in Table S1), except for comparisons of the strains depicted on the graphs with NS, not significant.
These experiments reveal the highest desaturase activity in intestinal tissues, with detectable desaturase activity in the germline and in the epidermis, but not in muscle.
Discussion
This study used tissue-specific RNAi paired with a highly quantitative method to determine the tissues in C. elegans that are most active in fatty acid desaturation. Importantly, we demonstrated that several previously isolated RNAi resistant mutants retain significant RNAi capacity against genes involved in fatty acid desaturation. Fatty acid desaturation activity is highly dependent on gene dosage, strains heterozygous for fatty acid desaturase mutations show desaturation indices that are intermediate between wild type and mutant (Watts and Browse 2002). This allows a quantitative analysis of remaining desaturase activity after RNAi induction by feeding of the fat-1 omega-3 desaturase gene or the fat-4 ∆5 desaturase gene. Some mutant strains tested in this study carry point mutations in the gene of interest, and thus the resulting proteins may have some remaining enzymatic function. This demonstrates that our method of assessing RNAi deficiency could be useful for studying the impact of changing specific residues of proteins involved in exogenous RNAi. Of the strains we tested, only rde-1 (ne219), rde-1 (ne300), rde-3 (ne298), rde-4 (ne301), sid-1 (qt9), and the multiple secondary Argonaute mutant strain (MAGO) were resistant to RNAi. And of those, only the null mutant rde-1 (ne300) and sid-1(qt9) were fully resistant to fat-1 and fat-4 RNAi.
We analyzed two mutant alleles of the Argonaute encoding gene, rde-1. The rde-1 has been reported to be essential to the success of exogenous RNAi. The primary evidence being that the rde-1 (ne219) allele was completely resistant to pos-1 RNAi, which normally causes lethality (Tabara et al. 1999). However, we provide evidence that the ne219 allele retains significant RNAi processing capacity. The ne219 allele contains a single base pair mutation causing a change from glutamate to lysine in the predicted PAZ RNA binding domain. The ne219 strain must either retain function via residual RDE-1 function, or by bypassing RDE-1 through an unknown mechanism. The deletion allele, rde-1 (ne300) is, in contrast, completely resistant to RNAi against fatty acid desaturase genes, which suggests that RDE-1 function is indispensable. The ne219 allele was used as the RNAi deficient genetic background for tissue specific rescue of RNAi function (Qadota et al. 2007). This technique of tissue-specific RNAi has been cited in over 70 publications, including these recent studies (Shamalnasab et al. 2017; Liu et al. 2017; Jeong et al. 2017; Chun et al. 2017; Han et al. 2017). Our findings indicate that the results of these studies must be carefully interpreted, and future studies of gene knockdowns in specific tissues should not use the rde-1(ne219) background, because this strain is not completely RNAi deficient.
Evidence from RNAi sequencing studies indicate that fat-1 and fat-4 genes are expressed in multiple somatic tissues, including epidermis and intestine (Chikina et al. 2009; Cao et al. 2017). The RNA-dependent RNA polymerase RRF-1 was originally thought to be required for somatic RNAi in C. elegans (Smardon et al. 2000; Sijen et al. 2001). We found that fat-1 and fat-4 were efficiently knocked down in rrf-1 (pk1417), to nearly wild-type levels. The rrf-1 (pk1417) contains a large deletion that eliminates a large conserved region of the protein (Sijen et al. 2001). The mutation is likely a null, which suggests that RRF-1 activity is not required for efficient RNAi in somatic tissues, including the intestine.
There are at least two possible explanations for how silencing in the intestine without RRF-1 is occurring. First, the EGO-1 RdRP has been shown to function in the intestine, substituting for RRF-1 (Ravikumar et al. 2019). A second explanation suggested by Kumsta and Hansen was that in the intestine the concentration of dsRNA, and thus primary siRNA may be high enough that amplification by RdRPs wouldn’t be necessary for silencing. Interestingly, we found that mutants lacking either RDE-10 or RDE-11 were not significantly resistant to fat-1 or fat-4 RNAi. RDE-10 and RDE-11 form a complex that promotes siRNA amplification, which is further evidence that siRNA amplification is not required for efficient silencing of desaturase genes.
We found that the MAGO strain was partially resistant to fat-1 and fat-4 RNAi. The MAGO strain contains mutations in six Argonaute proteins, including SAGO-1 and SAGO-2 that are known to interact with secondary siRNA (Yigit et al. 2006). If RdRP activity is not required for intestinal RNAi, then we would not expect that the SAGOs would be required either. However, it is possible that secondary siRNAs overwhelm the RNAi machinery, preventing silencing by primary siRNAs, but without the SAGOs, the secondary siRNAs produced by RdRP cannot be used for silencing.
Several tissue-specific RNAi strains have been constructed by others that do not rely on the tissue-specific rescue of rde-1 in the rde-1 mutant background. For example, strains have been made in the sid-1(pl3321) mutant background that rescue sid-1 using neuronal, muscle, and intestine-specific promoters (Miles and van Oosten-Hawle 2020; Calixto et al. 2010). We used existing muscle- and germline- specific RNAi strains and constructed new intestine and epidermal-specific strains all made in a rde-1(null) background. With these strains we were able to determine the extent of omega-3 and ∆5 desaturation in various tissues in C. elegans. The lack of RNAi activity in the rde-1(ne300) treated with fat-1RNAi and fat-4RNAi indicates that the rde-1(null) background is appropriate for tissue-specific RNAi experiments.
For both desaturases, we found high desaturase activity in intestinal tissues, as well as significant desaturase activity in the epidermis and the germ line, but not in muscle tissues. Single-cell RNA seq studies reveal fat-1 and fat-4 transcripts in epidermal and germ cells (Chikina et al. 2009; Cao et al. 2017) . In agreement with this, the quantitative tissue-specific method reveals significant expression of desaturase activity in epidermal and germline. Lipid metabolism in the epidermis has recently been recognized as influencing system-wide physiological responses (Kruse et al. 2017). Thus C. elegans fatty acid desaturation efficiency in skin cells could influence barrier formation and cuticle development. Similarly, PUFA synthesis in the germline is important for the proper development of the egg shell permeability barrier (Watts et al. 2018). A limitation of our study was that we did not test neuronal-specific RNAi. Neuronal desaturation could contribute to the overall pool of PUFAs in the worm, since promotor GFP fusion experiments demonstrated expression of fat-1 and fat-4 in several types of neurons (Vásquez et al. 2014)
Our findings emphasize that caution must be used in interpreting previous tissue-specific RNAi studies using the rde-1(ne219) and rrf-1 strains. Similarly, researchers should also be aware that the tissue-specific promotors may also exhibit a low level of expression in other tissues, which may explain the slight leakiness in the DCL569 strain. Expression in unexpected tissues was observed with several neuronal promotors expressed from multicopy arrays (Radetskaya et al. 2019). Transgenic approaches manipulating CRISPR-Cas9 activity in a tissue-specific manner (Shen et al. 2014) or using conditional protein degradation methods may be more precise approaches for tissue-specific knockdown experiments in C. elegans (Nance and Frokjaer-Jensen 2019).
Acknowledgments
We thank Washington State University students enrolled in MBioS402, Genetics lab, during the years of 2014, 2015, and 2016 for generating preliminary data that inspired this study, Claire Maynard and Pranay Shah for generating strains and Jonathan Ewbank for discussion. We thank Craig Mello for the WM45 strain and Di Chen for the DCL569 strain. Some C. elegans strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). Funding was provided by grants from the National Institutes of Health (R01DK074114 and R01GM133883 to JLW and T32GM083864 to JSW), by the French National Research Agency (ANR-16-CE15-0001-01, ANR-16-CONV-0001 to J. Ewbank and N. P.) and institutional grants from CNRS, AMU and INSERM to the CIML.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25387/g3.12968096.
Communicating editor: J. Ward
Literature Cited
- Ashrafi K., Chang F. Y., Watts J. L., Fraser A. G., Kamath R. S. et al. , 2003. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272. 10.1038/nature01279 [DOI] [PubMed] [Google Scholar]
- Calixto A., Chelur D., Topalidou I., Chen X., and Chalfie M., 2010. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods 7: 554–559. 10.1038/nmeth.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Packer J. S., Ramani V., Cusanovich D. A., Huynh C. et al. , 2017. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357: 661–667. 10.1126/science.aam8940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Simard M. J., Tabara H., Brownell D. R., McCollough J. A. et al. , 2005. A member of the polymerase beta nucleotidyltransferase superfamily is required for RNA interference in C. elegans. Curr. Biol. 15: 378–383. 10.1016/j.cub.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Chikina M. D., Huttenhower C., Murphy C. T., and Troyanskaya O. G., 2009. Global prediction of tissue-specific gene expression and context-dependent gene networks in Caenorhabditis elegans. PLOS Comput. Biol. 5: e1000417 10.1371/journal.pcbi.1000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H., Sharma A. K., Lee J., Chan J., Jia S. et al. , 2017. The Intestinal Copper Exporter CUA-1 Is Required for Systemic Copper Homeostasis in Caenorhabditis elegans. J. Biol. Chem. 292: 1–14. 10.1074/jbc.M116.760876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Pani A. M., Heppert J. K., Higgins C. D., and Goldstein B., 2015. Streamlined Genome Engineering with a Self-Excising Drug Selection Cassette. Genetics 200: 1035–1049. 10.1534/genetics.115.178335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd W., Tang L., Lone J.-C., Wimberly K., Wu C.-W. et al. , 2018. A Damage Sensor Associated with the Cuticle Coordinates Three Core Environmental Stress Responses in <em>Caenorhabditis elegans</em>. Genetics 208: 1467–1482. 10.1534/genetics.118.300827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M. K., Kostas S. A., Driver S. E. et al. , 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811. 10.1038/35888 [DOI] [PubMed] [Google Scholar]
- Fraser A. G., Kamath R. S., Zipperlen P., Martinez-Campos M., Sohrmann M. et al. , 2000. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330. 10.1038/35042517 [DOI] [PubMed] [Google Scholar]
- Friedland A. E., Tzur Y. B., Esvelt K. M., Colaiacovo M. P., Church G. M. et al. , 2013. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 10: 741–743. 10.1038/nmeth.2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishok A., 2005. RNAi mechanisms in Caenorhabditis elegans. FEBS Lett. 579: 5932–5939. 10.1016/j.febslet.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Hammond S. M., 2005. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 579: 5822–5829. 10.1016/j.febslet.2005.08.079 [DOI] [PubMed] [Google Scholar]
- Han S., Schroeder E. A., Silva-Garcia C. G., Hebestreit K., Mair W. B. et al. , 2017. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 544: 185–190. 10.1038/nature21686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong D. E., Lee D., Hwang S. Y., Lee Y., Lee J. E. et al. , 2017. Mitochondrial chaperone HSP-60 regulates anti-bacterial immunity via p38 MAP kinase signaling. EMBO J. 36: 1046–1065. 10.15252/embj.201694781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. W., and Bass B. L., 2001. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293: 2269–2271. 10.1126/science.1062039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse V., Neess D., and Faergeman N. J., 2017. The Significance of Epidermal Lipid Metabolism in Whole-Body Physiology. Trends Endocrinol. Metab. 28: 669–683. 10.1016/j.tem.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Kumsta C., and Hansen M., 2012. C. elegans rrf-1 mutations maintain RNAi efficiency in the soma in addition to the germline. PLoS One 7: e35428 10.1371/journal.pone.0035428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Xiao Y., Ji X. L., Zhang K. Q., and Zou C. G., 2017. The cAMP-PKA pathway-mediated fat mobilization is required for cold tolerance in C. elegans. Sci. Rep. 7: 638 10.1038/s41598-017-00630-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen A. J., Baggett J. J., Perumov N., Bauer R. A., Januszewski T. et al. , 2005. ACT-5 is an essential Caenorhabditis elegans actin required for intestinal microvilli formation. Mol. Biol. Cell 16: 3247–3259. 10.1091/mbc.e04-12-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J., and van Oosten-Hawle P., 2020. Tissue-Specific RNAi Tools to Identify Components for Systemic Stress Signaling. J. Vis. Exp. 159: e61357 10.3791/61357 [DOI] [PubMed] [Google Scholar]
- Nance J., and Frokjaer-Jensen C., 2019. The Caenorhabditis elegans Transgenic Toolbox. Genetics 212: 959–990. 10.1534/genetics.119.301506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. S., Eckert D. M., and Bass B. L., 2006. RDE-4 preferentially binds long dsRNA and its dimerization is necessary for cleavage of dsRNA to siRNA. RNA 12: 807–818. 10.1261/rna.2338706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish S., and Fire A., 2001. Distinct roles for RDE-1 and RDE-4 during RNA interference in Caenorhabditis elegans. RNA 7: 1397–1402. [PMC free article] [PubMed] [Google Scholar]
- Pujol N., Cypowyj S., Ziegler K., Millet A., Astrain A. et al. , 2008. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr. Biol. 18: 481–489. 10.1016/j.cub.2008.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H., Inoue M., Hikita T., Koppen M., Hardin J. D. et al. , 2007. Establishment of a tissue-specific RNAi system in C. elegans. Gene 400: 166–173. 10.1016/j.gene.2007.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radetskaya O., Lane R. K., Friedman T., Garrett A., Nguyen M. et al. , 2019. The PMK-3 (p38) Mitochondrial Retrograde Response Functions in Intestinal Cells to Extend Life via the ESCRT Machinery. bioRxiv (Preprint posted October 22, 2019) 10.1101/797308 [DOI] [Google Scholar]
- Raman P., Zaghab S. M., Traver E. C., and Jose A. M., 2017. The double-stranded RNA binding protein RDE-4 can act cell autonomously during feeding RNAi in C. elegans. Nucleic Acids Res. 45: 8463–8473. 10.1093/nar/gkx484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar S., Devanapally S., and Jose A. M., 2019. Gene silencing by double-stranded RNA from C. elegans neurons reveals functional mosaicism of RNA interference. Nucleic Acids Res. 47: 10059–10071. 10.1093/nar/gkz748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamalnasab M., Dhaoui M., Thondamal M., Harvald E. B., Faergeman N. J. et al. , 2017. HIF-1-dependent regulation of lifespan in Caenorhabditis elegans by the acyl-CoA-binding protein MAA-1. Aging (Albany NY) 9: 1745–1769. 10.18632/aging.101267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z., Zhang X., Chai Y., Zhu Z., Yi P. et al. , 2014. Conditional knockouts generated by engineered CRISPR-Cas9 endonuclease reveal the roles of coronin in C. elegans neural development. Dev. Cell 30: 625–636. 10.1016/j.devcel.2014.07.017 [DOI] [PubMed] [Google Scholar]
- Shi X., Li J., Zou X., Greggain J., Rodkaer S. V. et al. , 2013. Regulation of lipid droplet size and phospholipid composition by stearoyl-CoA desaturase. J. Lipid Res. 54: 2504–2514. 10.1194/jlr.M039669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen T., Fleenor J., Simmer F., Thijssen K. L., Parrish S. et al. , 2001. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107: 465–476. 10.1016/S0092-8674(01)00576-1 [DOI] [PubMed] [Google Scholar]
- Smardon A., Spoerke J. M., Stacey S. C., Klein M. E., Mackin N. et al. , 2000. EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr. Biol. 10: 169–178. 10.1016/S0960-9822(00)00323-7 [DOI] [PubMed] [Google Scholar]
- Steiner F. A., Okihara K. L., Hoogstrate S. W., Sijen T., and Ketting R. F., 2009. RDE-1 slicer activity is required only for passenger-strand cleavage during RNAi in Caenorhabditis elegans. Nat. Struct. Mol. Biol. 16: 207–211. 10.1038/nsmb.1541 [DOI] [PubMed] [Google Scholar]
- Tabara H., Sarkissian M., Kelly W. G., Fleenor J., Grishok A. et al. , 1999. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99: 123–132. 10.1016/S0092-8674(00)81644-X [DOI] [PubMed] [Google Scholar]
- Tabara H., Yigit E., Siomi H., and Mello C. C., 2002. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell 109: 861–871. 10.1016/S0092-8674(02)00793-6 [DOI] [PubMed] [Google Scholar]
- Taffoni C., Omi S., Huber C., Mailfert S., Fallet M. et al. , 2020. Microtubule plus-end dynamics link wound repair to the innate immune response. eLife 9: e45047 10.7554/eLife.45047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsterman M., Okihara K. L., Thijssen K., and Plasterk R. H., 2002. PPW-1, a PAZ/PIWI protein required for efficient germline RNAi, is defective in a natural isolate of C. elegans. Curr. Biol. 12: 1535–1540. 10.1016/S0960-9822(02)01110-7 [DOI] [PubMed] [Google Scholar]
- Timmons L., and Fire A., 1998. Specific Interference by ingested dsRNA. Nature 395: 854 10.1038/27579 [DOI] [PubMed] [Google Scholar]
- Vásquez V., Krieg M., Lockhead D., and Goodman M. B., 2014. Phospholipids that Contain Polyunsaturated Fatty Acids Enhance Neuronal Cell Mechanics and Touch Sensation. Cell Rep. 6: 70–80. 10.1016/j.celrep.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L., and Browse J., 2002. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99: 5854–5859. 10.1073/pnas.092064799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L., and Ristow M., 2017. Lipid and Carbohydrate Metabolism in Caenorhabditis elegans. Genetics 207: 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. S., Morton D. G., Kemphues K. J., and Watts J. L., 2018. The biotin-ligating protein BPL-1 is critical for lipid biosynthesis and polarization of the Caenorhabditis elegans embryo. J. Biol. Chem. 293: 610–622. 10.1074/jbc.M117.798553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C. M., Pino E. C., Carr C. E., Wu L., Zhou B. et al. , 2017. Genome-wide RNAi Screen for Fat Regulatory Genes in C. elegans Identifies a Proteostasis-AMPK Axis Critical for Starvation Survival. Cell Rep. 20: 627–640. 10.1016/j.celrep.2017.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston W. M., Molodowitch C., and Hunter C. P., 2002. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295: 2456–2459. 10.1126/science.1068836 [DOI] [PubMed] [Google Scholar]
- Winston W. M., Sutherlin M., Wright A. J., Feinberg E. H., and Hunter C. P., 2007. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc. Natl. Acad. Sci. USA 104: 10565–10570. 10.1073/pnas.0611282104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Feng X., Chen X., Weng C., Yan Q. et al. , 2018. A Cytoplasmic Argonaute Protein Promotes the Inheritance of RNAi. Cell Rep. 23: 2482–2494. 10.1016/j.celrep.2018.04.072 [DOI] [PubMed] [Google Scholar]
- Yang H., Zhang Y., Vallandingham J., Li H., Florens L. et al. , 2012. The RDE-10/RDE-11 complex triggers RNAi-induced mRNA degradation by association with target mRNA in C. elegans. Genes Dev. 26: 846–856. 10.1101/gad.180679.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigit E., Batista P. J., Bei Y., Pang K. M., Chen C. C. et al. , 2006. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 127: 747–757. 10.1016/j.cell.2006.09.033 [DOI] [PubMed] [Google Scholar]
- Zhuang J. J., and Hunter C. P., 2012. RNA interference in Caenorhabditis elegans: uptake, mechanism, and regulation. Parasitology 139: 560–573. 10.1017/S0031182011001788 [DOI] [PubMed] [Google Scholar]
- Zou L., Wu D., Zang X., Wang Z., Wu Z. et al. , 2019. Construction of a germline-specific RNAi tool in C. elegans. Sci. Rep. 9: 2354 10.1038/s41598-019-38950-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Worm strains, data and reagents are available upon request. Accompanying the manuscript is one supplemental table, Table S1. Supplemental material available at figshare: https://doi.org/10.25387/g3.12968096.