Abstract
Introduction.
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by Severe Acute Respiratory Corona Virus-2 (SARS-CoV-2). The disease was first identified in December 2019 in Wuhan, the capital of China's Hubei province, and has since spread globally, resulting in the ongoing 2019–2020 corona virus pandemic. SARS-CoV-2 is closely related to the original SARS-CoV. It is thought to have a zoonotic origin. The virus is primarily spread between people during close contact, often via small droplets produced by coughing, sneezing or talking. People may also become infected by touching a contaminated surface and then touching their face. COVID-19 patients currently remain the primary source of infection. An epidemiological survey indicated that the general population is susceptible to SARS-CoV-2. The spectrum of this disease ranges from mild to life-threatening. Fever is the most common symptom, although older people and those with comorbidities may experience fever later in the disease. Other common symptoms include cough, loss of appetite, fatigue, shortness of breath, sputum production, and muscle and joint pains. Symptoms such as nausea, vomiting and diarrhea have been observed in varying percentages. Some cases might progress promptly to acute respiratory distress syndrome (ARDS) and/or multiple organ function failure. Asymptomatic carriers and those in the incubation period may also be infectious.
Aim.
To determine the epidemiological and clinical characteristics of patients presenting with COVID-19 at the screening clinic of a tertiary care hospital in Peshawar, Pakistan.
Methodology.
In this descriptive study, we analysed data of patients presenting to a newly established Covid-19 screening clinic in Rehman Medical Institute. Anyone who reported with new onset fever and/or cough was tested for SARS-CoV-2 in the screening clinic. We documented and analysed demographic, epidemiological and clinical characteristics, which included age, sex, travel history, clinical features, comorbidities and laboratory data of patients confirmed by real-time reverse-transcription (RT)-PCR at Rehman Medical Institute, Peshawar, Pakistan from 15 March till 21 April 2020. Paired specimens of throat swabs and nasal swabs were obtained from 845 patients, ribonucleic acid (RNA) was extracted and tested for SARS-CoV-2 by the RT-PCR assay.
Results.
A total of 845 specimens were taken as described above. The positive rate for SARS-CoV-2 was about 14.3%. Male and older population had a significantly higher positive rate. Of the 121 patients infected with SARS-CoV-2, the mean age was 43.19 years (sd, 17.57) and the infections were more frequent among male gender accounting for 85 (70.25 %) patients. Common symptoms included fever (88 patients, 72 %), cough (72 patients, 59.5 %) and shortness of breath (69 patients, 57 %). Twenty-two (18 %) patients had recent travel history outside Pakistan in the previous 14 days, the majority of whom had returned back from Saudi Arabia.
Conclusion.
In this single-centre, prospective, descriptive study, fever, cough and shortness of breath were the most common symptoms. Old age (>50 years), chronic underlying comorbidities and travel history may be risk factors. Therefore, we concluded that viral nucleic acid amplification tests (NAAT) played an important role in identifying SARS-CoV-2 infection in a screening clinic, which helped with isolation and cohorting of these patients.
Keywords: COVID-19, severe acute respiratory syndrome-related coronavirus 2, RT-PCR
Introduction
In December 2019, a novel coronavirus, current reference name severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported from a cluster of patients with pneumonia of unknown cause. This was linked to a local Huanan South China Seafood Market in Wuhan, Hubei Province, China [1]. The virus primarily causes an acute febrile illness, which can proceed to acute respiratory distress syndrome (ARDS). The World Health Organization (WHO) named the disease COVID-19 [2–4]. On 30 January 2020, the WHO declared it a Public Health Emergency of International Concern (PHEI) and on 11 April 2020 it was declared a pandemic [5]. At the time of this writing, the WHO estimates that COVID-19 has already been diagnosed in 3 205 726 people from 210 countries worldwide, claiming nearly 227 290 deaths [6]. In Pakistan, there has been 15 759 confirmed cases and 346 fatalities as of today.
The SARS-CoV-2 belongs to a family of viruses that may cause various symptoms such as pneumonia, fever, difficulty in breathing and pneumonitis [7]. The clinical manifestations include fever, cough, dyspnea, myalgia, fatigue, normal or decreased leukocyte counts and radiographic evidence of pneumonia. Organ dysfunction (such as shock, ARDS, acute cardiac injury and acute kidney injury) and death can occur in severe cases, yet severity seems to be associated with age, biological sex and comorbidities [3].
Full-genome sequencing and phylogenic analysis indicated that SARS-Cov-2 was a distinct clade from the beta-coronaviruses associated with humans, in addition to the six known coronaviruses that infect humans: HCoV-229E, HCoV-OC43, SARS-CoV, HCoV-NL63, HCoV-HKU1 and MERS-CoV [2]. It is closely similar to bat coronaviruses, with a homology of 85–96% to a bat SARS-like coronavirus (bat-SL-CoVZC45) at the whole-genome level [8] and it has been postulated that bats may be the primary source, nevertheless no specific animal association has been identified. At this present moment, the origin of SARS-Cov-2 is yet to be completely ascertained. SARS-CoV-2 has a single-stranded positive-sense RNA genome that is 26 to 32 kilobases in length, encoding 27 proteins including an RNA-dependent RNA polymerase (RdRP) and four structural proteins include the spike surface glycoprotein (S), nucleocapsid protein (N), small envelope protein (E) and matrix protein (M) [9, 10]. Conventional modes of transmission of SARS-CoV, MERS-CoV and highly pathogenic influenza consist of respiratory droplets [11], mechanisms that probably occur with SARS-CoV-2 as well. COVID-19 has been found to have higher levels of transmissibility and pandemic risk than the previous SARS-CoV, as the effective reproductive number (R) of COVID-19 (2.9) is estimated to be higher than the reported effective reproduction number (R) of SARS (1.77) at this early stage. Although, the average incubation period of COVID-19 was initially estimated to be 4.8±2.6, ranging from 2 to 11 days [9], currently guidelines from health authorities state an average incubation period of 7 days, ranging from 2 to 14 days [3].
Signs of Covid-19 infection overlap with other viral infections, which makes a clinical diagnosis very tricky. Diagnostic test based on detection of the viral sequence by real-time reverse-transcription (RT)-PCR is the gold standard confirmatory test [12]. People with positive SARS-CoV-2 RNA by respiratory tract specimens are probably an infectious source of COVID-19. The aim of our study was to analyse the epidemiological and clinical characteristics of patients with COVID-19 after diagnosis through detection of viral nucleic acid by RT-PCR.
Methodology
The medical records and data from both outpatients and inpatients, with laboratory-confirmed Covid-19, as reported between 14 March 2020 and 21 April 2020, were collected. Covid-19 symptoms were diagnosed on the basis of the WHO interim guidance [13]. The selection criteria of the population group was based on, any patient presenting with new onset of fever, cough, headaches and body aches, were included in the study. A confirmed case of Covid-19 was defined as a positive result on real-time RT-PCR assay of nasal and oropharyngeal swab specimens [3]. All health-care workers caring for infected patients received comprehensive training and demonstrated competence in implementing infection control practices and procedures. Combined oropharyngeal and nasopharyngeal swab specimens in a single viral-transport medium tube were obtained under transmission based precautions from all patients presenting at the screening clinic. The procedure for collecting throat (oropharyngeal, OP) swabs entails swabbing the posterior pharynx and each tonsil area at least three times separately using a nylon-flocked swab, avoiding the tongue, and immediate placement of the swab into a sterile tube, containing 2–3 ml of viral transport media. All biological samples were sealed and transferred to the laboratory in strict accordance with the standard protocol.
This study was approved by the Ethics Committee of the Rehman Medical Institute, Peshawar and the need for informed consent was waived.
Laboratory confirmation
A real-time RT-PCR, a commercial assay in accordance with the manufacturer's instructions, was used for the quantitative detection of ribonucleic acid from SARS-CoV-2 (Primerdesign COVID-19 genesig Real-Time PCR assay), which was in nasal and oropharyngeal swab specimens from patients suspected of COVID-19. The product contains oligonucleotide primers and dual-labelled hydrolysis probes (TaqMan Technology), as well as control material, along with manual nucleic acid extraction. (MasterPure Complete DNA and RNA Purification Kit, Lucigen). The oligonucleotide primers and probe for the detection of SARS-CoV-2 were selected from the orf1 ab genome region and also targeting RNA-dependent RNA polymerase –RdRp. RNA extracted and purified from nasal and oropharyngeal swabs was reverse transcribed to cDNA and subsequently amplified using real-time PCR instruments: Roche LightCycler 480 II (software version 1.5). Conditions for amplifications were 50 °C for 15 min, 95 °C for 3 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s. The concentration of 0.33 copies/µl of SARS-CoV-2 whole-viral-genome RNA was the limit of detection of this assay. Specimens were processed and results were dispatched within 6–8 h, if delay was expected then specimens were stored at 2–8 °C for up to 72 h after collection. The extracted nucleic acid was stored at −70 °C in a freezer for long-term storage. To ensure the integrity and verification of RT-PCR assay results, an internal control was analysed in parallel for each patient sample, as well as testing one replicate of the positive control and one replicate of the negative control in each batch. A cycle threshold value (Ct-value) less than 37 was defined as a positive test result, and a Ct-value of 40 or more was defined as a negative test result. A medium load, defined as a Ct-value of 37 to less than 40, required confirmation by retesting. Each control was processed as a sample, through nucleic acid isolation and amplification/detection. Controls results (detection cycle or Ct) were generated for the two SARS-CoV-2 targets, and the internal control target. Acceptable control results for the SARSCoV-2 and internal control were required for the run to be acceptable.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges or simple ranges, as appropriate. Categorical variables were summarized as counts and percentages. No imputation was made for missing data. Because the cohort of patients in our study was not derived from random selection, all statistics are deemed to be descriptive only. All analyses were performed using SPSS 22.0.
Results
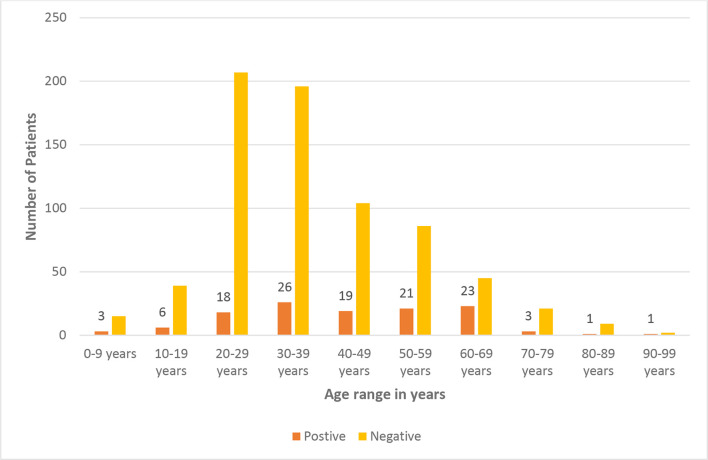
From 15 March to 21 April 2020, 845 cases were tested in the screening clinic for SARS-CoV-2 infection in Rehman Medical Institute, Peshawar, who were suspected or at high risk of infection because of (1) typical respiratory infection symptoms such as new onset fever or cough, or (2) close contact with a SARS-CoV-2 patient. Among those undergoing SARS-COV-2 RT-PCR testing, 601 were male (71.12%), 244 were female (28.88 %). Groups based on age: 0–9 (n=18), 10–19 (n=45), 20–29 (n=225), 30–39 (n=222), 40–49 (n=123), 50–59 (n=107), 60–69 (n=68), 70–79 (n=24), 80–89 (n=10), 90–99 (n=3).
Out of 845 patients, 121 (14.3 %) were SARS-COV-2 RT-PCR positive, 85 (70.25 %) were male while 36 (29.8 %) were female. When we analysed the positive rate between male and female cases from the screening clinic, a significant difference (P<0.01) was observed. The data in age-based groups at the screening clinic, the older (>50 years) patients had a higher positive rate. The median age of the patients was 47 years (interquartile range, 4 to 90); 107 (88.43 %) patients were in the age range of 20 and 69 years (Fig. 1)
Fig. 1.
Age range among patients presenting to the screening clinic of Rehman Medical Institute, Peshawar.
Among the reported symptoms, fever was present in 72.7 % of the patients on arrival at the screening clinic. The second most common symptom was cough (59.5 %) (Table 1). Among the overall positive cases, 39.6% had one of the comorbidities, as shown in Table 2.
Table 1.
Clinical features of SARS-CoV-2 positive cases
|
Signs and symptoms of SARS-COV-2 (n=121) cases n (%) | |
|---|---|
|
Fever |
88 (72.7 %) |
|
Cough |
72 (59.5 %) |
|
Shortness of breath |
69 (57 %) |
|
Muscle ache |
57 (47.1 %) |
|
Sore throat |
44 (36.3 %) |
|
Headache |
38 (31.4 %) |
|
Diarrhea |
21 (17.35 %) |
|
Nausea and vomiting |
15 (12.4 %) |
|
Combined fever, cough and shortness of breath |
12 (9.9 %) |
Table 2.
Comorbidities present among SARS-CoV-2 positive cases
|
Comorbidity among SARS-COV-2 (n=121) cases |
n (%) |
|---|---|
|
Hypertension |
15 (12.3 %) |
|
Diabetes mellitus |
13 (10.7 %) |
|
Cardiovascular and cerebrovascular diseases |
9 (7.4 %) |
|
Asthma |
7 (5.7 %) |
|
HBV, HCV or HIV infection |
3 (2.5 %) |
|
Malignant tumours |
1 (0.8 %) |
|
More than one comorbidities |
6 (4.9 %) |
Amongst positive cases, 12 (9.9 %) were healthcare workers (doctors=8; nurses=3; and paramedics=1). Out of 121 positive cases, 35 (28.9 %) had contact with known Covid-19 cases at home or in the community; the rest, 86 (71 %), were not sure about their contact history. Among 121 cases, 25 (20.6 %) travelled intercity and 22 (18 %) had arrived from other countries in the past 14 days. Saudi Arabia (8), Iran (3), Dubai (3), USA (3), UK (3), Germany (1) and Malaysia (1) (Table 3).
Table 3.
Demographic characteristic, Travel history and contact history of SARS-COV-2 (n=121) positive cases
|
Gender |
Age (years) |
Specimen collection date |
Travel history in past 14 days (inside country/intercity) |
Travel history in past 14 days (outside country) |
Contact with Covid-19 positive case in past 14 days |
|---|---|---|---|---|---|
|
Male |
65 |
21/Apr/2020 |
– |
– |
Yes |
|
Male |
26 |
21/Apr/2020 |
– |
– |
Not known |
|
Male |
9 |
21/Apr/2020 |
– |
– |
Yes |
|
Male |
63 |
21/Apr/2020 |
– |
– |
Yes |
|
Male |
67 |
20/Apr/2020 |
– |
– |
Yes |
|
Female |
15 |
20/Apr/2020 |
– |
– |
Yes |
|
Female |
5 |
20/Apr/2020 |
– |
– |
Yes |
|
Male |
50 |
20/Apr/2020 |
– |
– |
Not known |
|
Female |
5 |
20/Apr/2020 |
– |
– |
Yes |
|
Male |
55 |
20/Apr/2020 |
– |
– |
Not known |
|
Male |
50 |
20/Apr/2020 |
– |
– |
Yes |
|
Male |
26 |
20/Apr/2020 |
– |
– |
Not known |
|
Female |
23 |
20/Apr/2020 |
– |
– |
Not known |
|
Male |
55 |
20/Apr/0020 |
– |
– |
Yes |
|
Male |
33 |
19/Apr/2020 |
– |
– |
Not known |
|
Male |
19 |
19/Apr/2020 |
– |
– |
Yes |
|
Female |
22 |
19/Apr/2020 |
– |
– |
Not known |
|
Male |
35 |
19/Apr/2020 |
– |
– |
Not known |
|
Male |
60 |
18/Apr/2020 |
Kohat |
Yes |
|
|
Female |
55 |
18/Apr/2020 |
– |
– |
Not known |
|
Male |
63 |
18/Apr/2020 |
– |
– |
Not known |
|
Male |
36 |
18/Apr/2020 |
– |
– |
Yes |
|
Female |
38 |
18/Apr/2020 |
– |
– |
Yes |
|
Male |
25 |
18/Apr/2020 |
– |
– |
Not known |
|
Male |
37 |
17/Apr/2020 |
Kohat |
Yes |
|
|
Male |
34 |
17/Apr/2020 |
– |
– |
Not known |
|
Male |
44 |
17/Apr/2020 |
– |
– |
Yes |
|
Male |
81 |
17/Apr/2020 |
– |
– |
Not known |
|
Male |
48 |
17/Apr/2020 |
– |
– |
Not known |
|
Male |
25 |
17/Apr/2020 |
– |
– |
Not known |
|
Male |
50 |
17/Apr/2020 |
Kohat |
– |
Not known |
|
Male |
24 |
16/Apr/2020 |
– |
– |
Yes |
|
Male |
70 |
16/Apr/2020 |
– |
– |
Not known |
|
Male |
19 |
16/Apr/2020 |
– |
– |
Not known |
|
Male |
45 |
16/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Female |
57 |
16/Apr/2020 |
– |
– |
Yes |
|
Male |
47 |
16/Apr/2020 |
– |
– |
Yes |
|
Male |
37 |
15/Apr/2020 |
– |
– |
Yes |
|
Male |
9 |
15/Apr/2020 |
– |
– |
Not known |
|
Male |
4 |
15/Apr/2020 |
– |
– |
Yes |
|
Male |
65 |
15/Apr/2020 |
– |
– |
Not known |
|
Male |
28 |
15/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Female |
29 |
15/Apr/2020 |
– |
– |
Yes |
|
Male |
32 |
15/Apr/2020 |
– |
– |
Yes |
|
female |
25 |
15/Apr/2020 |
– |
– |
Yes |
|
Male |
28 |
15/Apr/2020 |
– |
– |
Yes |
|
Male |
65 |
14/Apr/2020 |
– |
– |
Not known |
|
Male |
50 |
14/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Female |
60 |
14/Apr/2020 |
– |
– |
Not known |
|
Male |
58 |
14/Apr/2020 |
– |
– |
Yes |
|
Female |
20 |
14/Apr/2020 |
– |
– |
Not known |
|
Female |
45 |
13/Apr/2020 |
– |
– |
Yes |
|
Male |
30 |
13/Apr/2020 |
Karachi |
– |
Not known |
|
Male |
52 |
13/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Female |
53 |
13/Apr/2020 |
– |
– |
Not known |
|
Female |
17 |
13/Apr/2020 |
– |
– |
Yes |
|
Female |
36 |
12/Apr/2020 |
– |
– |
Yes |
|
Female |
38 |
12/Apr/2020 |
– |
– |
Yes |
|
Female |
67 |
12/Apr/2020 |
– |
– |
Yes |
|
Male |
31 |
11/Apr/2020 |
– |
– |
Yes |
|
Male |
58 |
11/Apr/2020 |
– |
– |
Not known |
|
Male |
28 |
11/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Male |
30 |
10/Apr/2020 |
– |
Not known |
|
|
Female |
30 |
10/Apr/2020 |
– |
Yes |
|
|
Female |
24 |
10/Apr/2020 |
– |
Not known |
|
|
Male |
55 |
10/Apr/2020 |
– |
Not known |
|
|
Female |
65 |
10/Apr/2020 |
– |
Not known |
|
|
Female |
65 |
10/Apr/2020 |
Kohat |
– |
Not known |
|
Male |
42 |
09/Apr/2020 |
– |
– |
Yes |
|
Female |
60 |
09/Apr/2020 |
– |
– |
Not known |
|
Male |
26 |
09/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Male |
90 |
09/Apr/2020 |
– |
– |
Not known |
|
Female |
15 |
08/Apr/2020 |
– |
– |
Yes |
|
Male |
62 |
08/Apr/2020 |
– |
– |
Not known |
|
Male |
54 |
08/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Female |
38 |
08/Apr/2020 |
– |
– |
Not known |
|
Male |
70 |
08/Apr/2020 |
– |
Saudi Arabia |
Not known |
|
Male |
52 |
07/Apr/2020 |
– |
Saudi Arabia |
Not known |
|
Male |
62 |
07/Apr/2020 |
– |
Not known |
|
|
Male |
70 |
06/Apr/2020 |
– |
Not known |
|
|
Male |
32 |
06/Apr/2020 |
Raiwind (Lahore) |
Not known |
|
|
Male |
44 |
06/Apr/2020 |
– |
Not known |
|
|
Male |
46 |
05/Apr/2020 |
– |
Saudi Arabia |
Not known |
|
Male |
63 |
05/Apr/2020 |
– |
– |
Not known |
|
Male |
63 |
05/Apr/2020 |
– |
– |
Not known |
|
Male |
24 |
05/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Male |
60 |
05/Apr/2020 |
– |
Iran |
Not known |
|
Female |
12 |
04/Apr/2020 |
Kohat |
Yes |
|
|
Male |
67 |
04/Apr/2020 |
– |
Not known |
|
|
Female |
61 |
04/Apr/2020 |
– |
Dubai |
Not known |
|
Female |
55 |
04/Apr/2020 |
– |
Saudi Arabia |
Not known |
|
Male |
47 |
03/Apr/2020 |
– |
Dubai |
Not known |
|
Male |
55 |
02/Apr/2020 |
Dera Ismail Khan |
Not known |
|
|
Female |
45 |
02/Apr/2020 |
– |
Not known |
|
|
Male |
30 |
02/Apr/2020 |
– |
United States of America |
Not known |
|
Male |
44 |
02/Apr/2020 |
– |
Iran |
Not known |
|
Male |
44 |
02/Apr/2020 |
– |
United Kingdom |
Not known |
|
Male |
32 |
02/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Male |
47 |
01/Apr/2020 |
Raiwind (Lahore) |
– |
Not known |
|
Male |
65 |
01/Apr/2020 |
– |
Not known |
|
|
Female |
45 |
01/Apr/2020 |
– |
Iran |
Not known |
|
Male |
65 |
01/Apr/2020 |
– |
Saudi Arabia |
Not known |
|
Male |
52 |
01/Apr/2020 |
– |
Not known |
|
|
Male |
60 |
31/Mar/2020 |
Karachi |
Not known |
|
|
Female |
63 |
31/Mar/2020 |
– |
Saudi Arabia |
Not known |
|
Female |
38 |
31/Mar/2020 |
– |
Not known |
|
|
Female |
25 |
30/Mar/2020 |
– |
United Kingdom |
Not known |
|
Male |
30 |
30/Mar/2020 |
– |
Dubai |
Not known |
|
Male |
31 |
30/Mar/2020 |
Raiwind (Lahore) |
Not known |
|
|
Male |
45 |
29/Mar/2020 |
Raiwind (Lahore) |
Not known |
|
|
Male |
52 |
29/Mar/2020 |
– |
United States of America |
Not known |
|
Female |
50 |
29/Mar/2020 |
– |
Saudi Arabia |
Not known |
|
Male |
52 |
29/Mar/2020 |
Karachi |
Not known |
|
|
Male |
45 |
29/Mar/2020 |
Saudi Arabia |
Not known |
|
|
Female |
41 |
30/Mar/2020 |
Germany |
Not known |
|
|
Male |
36 |
30/Mar/2020 |
Kohat |
Not known |
|
|
Male |
39 |
29/Mar/2020 |
Dera Ismail Khan |
Not known |
|
|
Male |
39 |
28/Mar/2020 |
– |
United Kingdom |
Not known |
|
Male |
29 |
24/Mar/2020 |
– |
Malaysia |
Not known |
|
Male |
38 |
18/Mar/2020 |
Karachi |
Not known |
|
|
Female |
59 |
15/Mar/2020 |
– |
United States of America |
Not known |
Discussion
The epidemic started in Pakistan with multiple imported cases throughout the country, mostly from Iran, Saudi Arabia and Europe. Pakistan reported its first case on 26 February 2020, a patient who travelled with his family from Iran to Karachi, Pakistan. The province of Khyber-Pakhtunkhwa has reported almost 1984 SARS-CoV-2 positive cases and 104 deaths at this point. Our institute has reported 121 cases out of a total 845 patients tested in the past 1 month, contributing a positivity rate of 14.3% in the tested cohort. In our research, the majority of the positive cases were between 25 to 60 years old, which is similar to previous studies [4]. Some studies have reported a majority age distribution of patients between 25 and 89 years [14] and there have been fewer identified cases among children and infants [15]. Only five positive cases of less than 10 years of age were found in our study (%). The median age of patients in our study was 47 years, which was comparable to the median age of cases in Wuhan reported by Cao [3] and Zhang [16], which were 49.0 and 55.5 years, respectively.
Generally, it was suggested that individuals at risk were mostly immunocompromised, in this case, elderly and those with renal, cardiovascular and hepatic dysfunction [17]. In our study, predominantly male (70.25 %) population were infected, analogous to meta-analysis by Yang et al. [18]. The proportional male to female (M:F) death ratio in confirmed cases is higher in all the countries with available data. Whereas, it is customary to think women are less likely to be infected than men, partly because of their more robust innate and adaptive immune responses [19]. There may be other behavioural and social differences that favour women, with prior studies suggesting women are more likely than men to follow hand-hygiene practices [20] and seek preventive care [21].
Subsequently, 34 (28 %) patients had family members in the same household, also SARS-C0V-2 positive. These findings echo the latest reports, including the outbreak of a family cluster [22], and transmission from an asymptomatic patient [23]. Thus early detection and timely isolation is vital before a single case becomes a cluster. It also raises the concern that the risks and benefits should be considered in home quarantine for confirmed cases, which could result in family case clusters if they transmit the virus to other members of the same household. During the initial phase of the Covid-19 outbreak, the diagnosis of the disease was complicated by the diversity in symptoms, the imaging findings and the severity of disease at the time of presentation. The absence of fever in Covid-19 is more frequent than in SARS-CoV (1 %) and MERS-CoV infection (2 %). Afebrile patients may be missed if the surveillance case definition focuses on fever detection only [24]. The symptoms of COVID-19 are similar to influenza (e.g. fever, cough or fatigue) and outbreaks will usually occur during the time of the year with high incidence of respiratory diseases caused by influenza, respiratory syncytial virus and other respiratory viruses. Vaccines can still be useful in prevention from influenza and will reduce possible confusion with the COVID-19 infection [25]. Contrary to a study in India where almost half (42.9%) of the patients were asymptomatic [26], we reported only 23% such cases.
In line with recent studies [16, 17], we found fever (72 %) and cough (59.5 %) were the dominant symptoms; gastrointestinal symptoms (17.35 %) were uncommon, which suggests a difference in viral tropism as compared with SARS-CoV, MERS-CoV and seasonal influenza [27]. These findings have important implications for patient triage and hospital risk zoning. Moreover, fever was more prevalent in patients in the 20–49 age group while cough was more common in patients aged 40 to 79 years. Children aged 1 to 19 usually presented with diarrhea. In one hospital in mainland China, 15 medical workers were reported with COVID-19, 14 of whom were assumed to have been infected by the same patient [28], comparable to our findings of 12 healthcare workers infected during the study time period, but we could not ascertain the source of their infection accurately. Outpatient medical staff could be at higher risk when encountering suspected COVID-19 patients, thus more aggressive PPE protection and proactive patient screening is necessary.
Wang et al. [4] reported findings from 138 cases of COVID-19 among which 64 (46.4 %) had comorbidities. Similarly the prevalence of comorbidities was 38 % in our study, including hypertension (12.3 %), diabetes (10.7 %), cardiovascular and cerebrovascular diseases (9 %) and asthma (7 %) that was consistent with the prevalence of hypertension and diabetes in Chinese COVID-19 studies, which stood at 23.2 % [29] and 10.9 % respectively [30] in adults.
Our findings also suggested that the modes of transmission have changed considerably with the spread of the disease. A large proportion of early reported cases were travellers from both abroad (18.1 %) and inside the country (20.66 %). Among imported cases, they were mostly from countries with high community transmissions. None of the cases in Peshawar had a history of travel from mainland China.
Since RT-PCR is one of the most quickly established laboratory diagnostic method in the COVID-19 pandemic, it served efficiently as a modality to provide us with a result within 2–4 h. However, some investigators and clinicians argued that computed tomography (CT) imaging can be a better modality in identifying the SARS-CoV-2 infection instead, since quite a few severe cases showed progressive multiple peripheral ground-glass opacities in both lungs even when the RT-PCR results were negative [31]. Based on the reasons mentioned above, we suggest that viral NAAT is reliable and widely used laboratory diagnosis for SARS-CoV-2 infection, especially for screening clinics. Clinical characteristics, chest imaging and etiology testing based on viral genes RT-PCR should be used in making a diagnosis [16]. The rising number of cases and mortality risk estimates are demonstrating the dire need for enhanced public health mediations, good hygienic conditions, social distancing and movement limitations to control the COVID-19 pandemic.
Conclusion
The COVID-19 pandemic is a global health emergency that has changed the world in an unprecedented way. It is spreading rapidly throughout the world, including Pakistan, causing varying degrees of illness. Patients mostly presented with fever, cough and shortness of breath and a good proportion of positive cases (23 %) were asymptomatic. The disease has shown a wide spectrum of severity in various studies published so far. Close monitoring and large-scale quarantine and cohorting strategies will be needed to prevent widespread transmission within the community.
There has been a rapid surge in research in response to the outbreak of COVID-19. During this early period, published research has primarily explored the epidemiology, causes, clinical manifestation and laboratory diagnosis. We suggest that both nasopharyngeal and oropharyngeal swabs test for SARS-CoV-2 RNA should be performed to reduce the false-negative rate. More tests, more specimens, and more methods could be considered. Treatment and management options are still in the very early phases of clinical trials.
Limitations
Our study has some notable limitations. First, some cases had incomplete documentation of the exposure history and the variation in the structure of electronic databases among different participating sites and the urgent timeline for data extraction. Since our study was limited by a lack of critically ill patients, more detailed information, such as the final prognosis of patients, could not be obtained.
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
We thank all the patients who consented to this study, and the frontline healthcare professionals who are involved in patient care during this pandemic.
Author contributions
M.K. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. H.K., S.K. and M.N. contributed equally as co-authors. M.K.: conceptualized and designed the study, methodology. collected data, and revised the manuscript. H.K.: acquisition, statistical analysis, reviewed and revised the manuscript. S.K.: conceptualization, writing – original draft, interpretation of data, critically reviewed the manuscript for important intellectual content. M.N.: administrative, technical, or material support, reviewed and revised the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
Ethical approval was obtained from institutional ethical approval review board before start of the study. Written informed as well as oral consent was taken from the subjects. Consent for publication was taken from institution as well as all authors of the manuscript. Informed consent was obtained from all individuals included in this study.
Footnotes
Abbreviations: ARDS, acute respiratory distress syndrome; Bat-SL-CoVZC45, Bat SARS-like coronavirus; COVID-19, Coronavirus disease 2019; Ct-value, cycle threshold value; HCoV-229E, human coronavirus 229E; HCoV-HKU1, human coronavirus HKU1; MERS-CoV, Middle East respiratory syndrome-related coronavirus; NAAT, nucleic acid amplification tests; PHEI, Public Health Emergency of International Concern; RdRP, RNA-dependent RNA polymerase; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, Severe Acute Respiratory Corona Virus-2; WHO, World Health Organization.
References
- 1.China Wuhan Municipal Health Commission Wuhan Municipal Health Commission briefing on the pneumonia epidemic situation 31 Dec 2019 [in Chinese]. 2020 [cited 2020 Feb 20]. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Coronavirus disease 2019 (COVID-19) Situation Report – 98. 2020 [cited 2020 Apr 27]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 7.WMHC Wuhan Municipal Health and Health Commission’s Briefing on the Current Pneumonia Epidemic Situation in Our City. 2020. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989. Accessed 1 Feb 2020
- 8.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu A, Peng Y, Huang B, Ding X, Wang X, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sexton NR, Smith EC, Blanc H, Vignuzzi M, Peersen OB, et al. Homology-based identification of a mutation in the coronavirus RNA-dependent RNA polymerase that confers resistance to multiple mutagens. J Virol. 2016;90:7415–7428. doi: 10.1128/JVI.00080-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong JH, Kim KH, Jeong SH, Park JW, Lee SM, et al. Comparison of sputum and nasopharyngeal swabs for detection of respiratory viruses. J Med Virol. 2014;86:2122–2127. doi: 10.1002/jmv.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. January 28, 2020
- 14.Da R, Showkat A, Sa R, Ma M. The Covid-19 pandemic: a study of the current evidence in India. Purakala with Issn 0971-2143. Is An Ugc Care Journal. 2020 Apr 14;31:1–6. [Google Scholar]
- 15.Wang C, Prevalence WX. Nosocomial infection and psychological prevention of novel coronavirus infection. Chin General Pract Nurs. 2020;18:2–3. [Google Scholar]
- 16.Chen N, Zhou M, Dong X, Qu J, Gong F, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Guan X, Wu P, Wang X, Zhou L, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Zheng Y, Gou X, Pu K, Chen Z, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Inf Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson HD, Sholcosky D, Gabello K, Ragni R, Ogonosky N. Sex differences in public restroom handwashing behavior associated with visual behavior prompts. Percept Mot Skills. 2003;97:805–810. doi: 10.2466/pms.2003.97.3.805. [DOI] [PubMed] [Google Scholar]
- 21.Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–152. [PubMed] [Google Scholar]
- 22.Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. The Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel A, Jernigan DB, 2019-nCoV CDC Response Team Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak - United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta N, Agrawal S, Ish P, Mishra S, Gaind R, et al. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020 Apr 10;90 doi: 10.4081/monaldi.2020.1294. [DOI] [PubMed] [Google Scholar]
- 27.Leung WK, To K-F, Chan PKS, Chan HLY, Wu AKL, et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/s0016-5085(03)01215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinese Academy of Sciences Wuhan coronavirus has strong ability to infect humans. Press release. Jan 21. 2020.
- 29.Hu S, Gao R, Liu L, ZHU M, WANG W, et al. Summary of the 2018 report on cardiovascular diseases in China. Chin Circulation J. 2019;34:209. [Google Scholar]
- 30.Liu M, Liu S-W, Wang L-J, Bai Y-M, Zeng X-Y, et al. Burden of diabetes, hyperglycaemia in China from to 2016: findings from the 1990 to 2016, global burden of disease study. Diabetes Metab. 2019;45:286–293. doi: 10.1016/j.diabet.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Fang Y, Zhang H, Xie J, Lin M, Ying L, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]