Abstract
Background: The benefit of adjuvant chemotherapy varies widely among patients with stage II/III gastric cancer (GC), and tools predicting outcomes for this patient subset are lacking. We aimed to develop and validate a nomogram to predict recurrence-free survival (RFS) and the benefits of adjuvant chemotherapy after radical resection in patients with stage II/III GC.
Methods: Data on patients with stage II/III GC who underwent R0 resection from January 2010 to August 2014 at Fujian Medical University Union Hospital (FMUUH) (n = 1,240; training cohort) were analyzed by Cox regression to identify independent prognostic factors for RFS. A nomogram including these factors was internally and externally validated in FMUUH (n = 306) and a US cohort (n = 111), respectively.
Results: The multivariable analysis identified age, differentiation, tumor size, number of examined lymph nodes, pT stage, pN stage, and adjuvant chemotherapy as associated with RFS. A nomogram including the above 7 factors was significantly more accurate in predicting RFS compared with the 8th AJCC-TNM staging system for patients in the training cohort. The risk of peritoneal metastasis was higher and survival after recurrence was significantly worse among patients calculated by the nomogram to be at high risk than those at low risk. The nomogram's predictive performance was confirmed in both the internal and external validation cohorts.
Conclusion: A novel nomogram is available as a web-based tool and accurately predicts long-term RFS for GC after radical resection. The tool can also be used to determine the benefit of adjuvant chemotherapy by comparing scores with and without this intervention.
Keywords: gastric cancer, recurrence patterns, adjuvant chemotherapy benefit, nomogram, web-based tool
Introduction
Gastric cancer (GC) is the fifth most common malignancy worldwide and ranks third in cancer-related mortality (1). Radical gastrectomy is still the main treatment. However, even with radical resection, postoperative recurrence is common, affecting ~18 to 45.5% of patients (2–5). Patients with stage I GC have good prognosis and low recurrence rates, while outcomes for those with stage II/III GC patients vary widely and can be challenging to predict (3, 6–8).
Postoperative recurrence is the leading cause of death among patients with stage II/III GC (2, 8). Currently, the most widely used system for estimating survival and risk of recurrence is the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system. However, for patients with non-metastatic GC, the AJCC-TNM system considers only two variables (pT, pN), and its ability to predict recurrence is still limited (2, 9). A predictive model including 6 variables that significantly better predicts overall survival (OS) than the AJCC-TNM staging system was developed by Han et al. but its predictive power for recurrence in patients with stage II/III GC was not evaluated (10). To better plan follow-up and treatment strategies for these patients, an individualized predictive tool to predict recurrence would be of value.
Another challenge in treating patients with stage II/III GC is determining who will benefit from adjuvant (postoperative) chemotherapy. While this treatment strategy has become standard for these patients (11–13) and has improved their long-term prognosis, whether all patients with stage II/III GC need adjuvant chemotherapy has been questioned (14, 15). A model to predict its benefit in patients with stage II/III GC was introduced by Jiang et al. but its predictive performance was not ideal (concordance index was 0.686), and it was not externally validated using Western data (16).
Therefore, in the present study, we used data from a large-volume center in China to construct a nomogram that can effectively predict postoperative recurrence and chemotherapy benefit in patients with stage II/III GC after radical surgery. The model was internally and externally validated using data from our center and a Western cohort, respectively. This is the first predictive model, which is available as a web-based tool, for postoperative recurrence and chemotherapy benefits based on international, multicenter data.
Materials and Methods
Datasets
The database at Fujian Medical University Union Hospital (FMUUH) was reviewed following approval from the Institutional Review Board (IRB). The inclusion criteria were as follows: histologically confirmed primary gastric cancer, no distant metastasis, and R0 gastrectomy performed between January 2010 and August 2014. The exclusion criteria included receiving neoadjuvant chemotherapy, pathologic stage I disease according to the 8th-AJCC-TNM staging system, remnant gastric cancer, and postoperative death within 3 months.
To examine the generalizability of the model, data on patients that satisfied the aforementioned inclusion and exclusion criteria were obtained from FMUUH between September 2014 and August 2015 (internal validation) and from the Mayo Clinic between January 2005 and December 2012 (external validation) following IRB approval.
Tumor stage, including pT, pN, and final stage, was determined according to the 8th AJCC classification system (17).
Follow-Up and Treatment
Follow-up visits for both cohorts generally consist of clinic visits every 3 months for the first 2 years and every 6 months for years 3 to 5. Most routine patient follow-up appointments include a physical examination, laboratory tests, chest radiography, abdominal ultrasonography, or CT, and an annual or biannual endoscopic examination for patients with a remnant stomach (18). Disease recurrence was diagnosed with radiologic findings on cross-sectional imaging or biopsies of suspicious lesions (3).
For those who could tolerate adjuvant chemotherapy, adjuvant chemotherapy was routinely recommended for patients with pathological stage II and III disease (19). The adjuvant chemotherapy consisted of either single-agent 5-fluorouracil (5-FU) or a combination of 5-FU and cisplatin/oxaliplatin or paclitaxel (19). To simplify of the nomogram, patients were classified as having received chemotherapy or not, regardless of the number of cycles (16).
Definition and Categorization of Recurrence
Recurrences were categorized by site involved as previously described: (2, 3, 20) locoregional, peritoneal, distant, or multiple. Multiple recurrences were defined as the presence of recurrent disease in 2 or more sites. Early recurrence was defined as recurrence occurring within 12 months (3). Patients for which the exact site or sites of recurrence were unknown because of diagnosis in other hospitals were excluded from the analysis of recurrence patterns.
Nomogram Construction, Validation, and Calibration
A Cox proportional hazards regression model was used to identify independent prognostic factors associated with RFS. Variables with p < 0.05 in the univariable analysis were subsequently included in the multivariable analysis, from which a nomogram was formulated in R for predicting the probability of 5-year recurrence-free survival (RFS).
The nomogram was subjected to 1,000 bootstrap resamples for internal and external validation. Its performance in predicting outcomes was evaluated by calculating the concordance index (C-index), area under the curve (AUC), and Akaike information criterion (AIC) (3). The nomogram was calibrated by comparing predicted with observed RFS after bias correction.
Statistical Analysis
Continuous variables are reported as means ± SD or medians (interquartile ranges). Categorical variables were compared using the χ (2) or Fisher's exact test and continuous variables by t-test. RFS was assessed using the Kaplan–Meier method. Post-recurrence survival was defined as the period from the date of recurrence to the date of death or final follow-up. The non-linear relationship between nomogram-derived scores and RFS was modeled using restricted cubic splines (21). The nomogram's clinical usefulness was evaluated by decision curve analysis, which calculates the rate of true and false positives for various risk thresholds (3). The cohort was dichotomized into low-risk and high-risk subgroups by the median nomogram score among patients in the training cohort. Statistical analyses were performed using SPSS v.18.0 for Windows (SPSS Inc., Chicago, IL, USA) and R (https://www.r-project.org/). The R package “DynNom” was used to develop the web-based nomogram. P < 0.05 were considered statistically significant.
Results
Characteristics of the Training, Internal Validation, and External Validation Cohorts
A total of 1,240 patients with stage II/III GC who underwent radical gastrectomy were included in the training cohort. There were 306 and 111 patients were included in the internal and external validation cohort, respectively (Supplementary Figure 1). Table 1 shows the clinical and pathological data of both cohorts. The mean age of the training cohort was significantly younger than the external validation cohort (61.5 ± 11.2 vs. 68.3 ± 15.8 years), and included a higher proportion of male patients (75.5 vs. 56.8%). Adjuvant chemotherapy was administered to more patients in the training cohort (76.8 vs. 36.0%). The baseline characteristics was balanced between the training and internal validation cohorts.
Table 1.
Patient and tumor characteristics in the training and validation cohorts.
| Training cohort, no. (%) | Internal validation cohort, no. (%) | p-value | External validation cohort, no. (%) | p-value* | |
|---|---|---|---|---|---|
| FMUUH, n = 1240 | FMUUH, n = 306 | Mayo clinic, n = 111 | |||
| Age, mean years (SD) | 61.5 (11.2) | 60.4 (11.9) | 0.147 | 68.3 (15.8) | <0.001 |
| Tumor size, mean mm (SD) | 51.1 (24.2) | 52.4 (25.5) | 0.397 | 67.0 (37.3) | <0.001 |
| Examined LNs, mean No (SD) | 36.9 (13.6) | 36.9 (14.6) | 0.999 | 25.2 (15.6) | <0.001 |
| Sex | 0.127 | <0.001 | |||
| Female | 304 (24.5) | 88 (28.8) | 48 (43.2) | ||
| Male | 936 (75.5) | 218 (71.2) | 63 (56.8) | ||
| Tumor location | 0.801 | 0.011 | |||
| Lower | 474 (38.2) | 120 (39.2) | 42 (37.8) | ||
| Middle | 278 (22.4) | 61 (19.9) | 36 (32.4) | ||
| Upper | 316 (25.5) | 79 (25.8) | 28 (25.2) | ||
| Mix | 172 (13.9) | 46 (15.0) | 5 (4.5) | ||
| Differentiation | 0.910 | <0.001 | |||
| Well | 56 (4.5) | 14 (4.6) | 0 (0.0) | ||
| Moderate | 446 (36.0) | 106 (34.6) | 11 (9.9) | ||
| Poor | 738 (59.5) | 186 (60.8) | 100 (90.1) | ||
| Lymphovascular invasion† | 0.493 | NA | |||
| Absent | 804 (64.8) | 192 (62.7) | 0 (0.0) | ||
| Present | 436 (35.2) | 114 (37.3) | 0 (0.0) | ||
| Unknown | NA | NA | 111 (100.0) | ||
| Adjuvant chemotherapy | 0.537 | <0.001 | |||
| Absent | 288 (23.2) | 66 (21.6) | 71 (64.0) | ||
| Present | 952 (76.8) | 240 (78.4) | 40 (36.0) | ||
| pT stage | 0.659 | 0.003 | |||
| T1 | 24 (1.9) | 8 (2.6) | 2 (1.8) | ||
| T2 | 101 (8.1) | 29 (9.5) | 11 (9.9) | ||
| T3 | 572 (46.1) | 144 (47.1) | 64 (57.7) | ||
| T4a | 515 (41.5) | 121 (39.5) | 27 (24.3) | ||
| T4b | 28 (2.3) | 4 (1.3) | 7 (6.3) | ||
| pN stage | 0.113 | 0.028 | |||
| N0 | 195 (15.7) | 43 (14.1) | 30 (27.0) | ||
| N1 | 233 (18.8) | 49 (16.0) | 23 (20.7) | ||
| N2 | 285 (23.0) | 92 (30.1) | 20 (18.0) | ||
| N3a | 322 (26.0) | 79 (25.8) | 24 (21.6) | ||
| N3b | 205 (16.5) | 43 (14.1) | 14 (12.6) | ||
| pTNM stage | 0.493 | 0.031 | |||
| II | 804 (64.8) | 192 (62.7) | 49 (44.1) | ||
| III | 436 (35.2) | 114 (37.3) | 62 (55.9) |
Continuous data represented as mean ± SD and categorical data as n (%).
Compared with the training cohort.
Unavailable for the external validation cohort. FMUUH indicates Fujian Medical University Union Hospital; LN, lymph node; NA, not applicable.
Nomogram Development, Calibration and Internal Validation
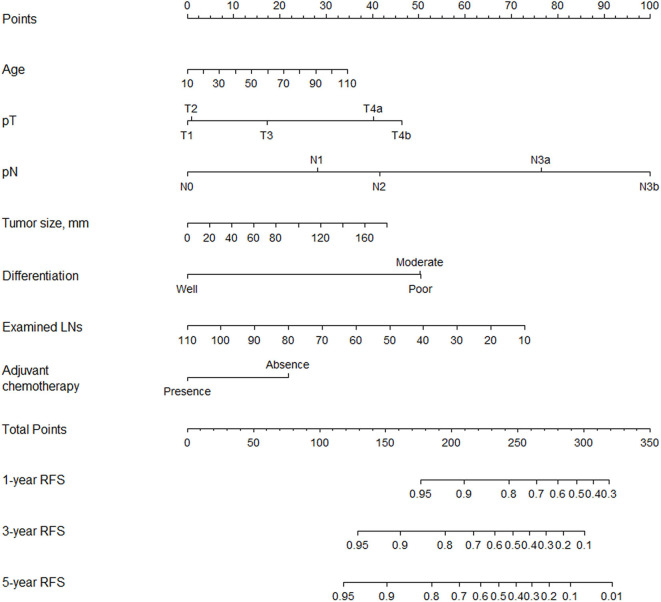
Multivariable analysis identified age, differentiation, tumor size, number of examined lymph nodes, pT stage, pN stage, and adjuvant chemotherapy as associated with RFS (Table 2). We included the above variables in the predictive model to establish a nomogram and made it available online (https://qq406918430.shinyapps.io/DynamicPrediction/) (Figure 1, Supplementary Figure 2). The benefit of chemotherapy can be calculated by using the web-based calculating tool to determine the 5-year RFS probabilities both for the situation in which the patient receives or does not receive it; the difference between the two is the net survival benefit.
Table 2.
Univariable and multivariable analyses of factors associated with recurrence-free survival.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | 1.012 | 1.004–1.020 | 0.005 | 1.009 | 1.001–1.018 | 0.034 |
| Tumor size | 1.014 | 1.011–1.017 | <0.001 | 1.005 | 1.002–1.009 | 0.003 |
| Examined LNs | 0.998 | 0.992–1.005 | 0.646 | 0.984 | 0.977–0.991 | <0.001 |
| Female sex vs. male | 1.059 | 0.863–1.299 | 0.584 | |||
| Tumor location | ||||||
| Lower | 1.000 | – | ||||
| Middle | 1.258 | 0.993–1.592 | 0.057 | – | – | 0.446 |
| Upper | 1.069 | 0.845–1.353 | 0.578 | – | – | 0.892 |
| Mix | 1.660 | 1.280–2.152 | <0.001 | – | – | 0.865 |
| Histologic type | ||||||
| Well | 1.000 | 1.000 | ||||
| Moderate | 3.145 | 1.475–6.707 | 0.003 | 3.037 | 1.419–6.502 | 0.004 |
| Poor | 4.454 | 2.107–9.418 | <0.001 | 3.051 | 1.436–6.484 | 0.004 |
| Lymphovascular invasion* | 1.502 | 1.255–1.798 | <0.001 | – | – | 0.873 |
| Nerve invasion* | 1.319 | 1.085–1.604 | 0.005 | – | – | 0.907 |
| Adjuvant chemotherapy* | 0.745 | 0.610–0.911 | 0.004 | 0.625 | 0.505–0.775 | <0.001 |
| CEA ≥ 5 vs. > 5 ng/mL | 1.287 | 1.060–1.563 | 0.011 | – | – | 0.455 |
| CA19-9 ≥ 37 vs. <37 ng/mL | 1.383 | 1.115–1.717 | 0.003 | – | – | 0.315 |
| pT stage | ||||||
| T1 | 1.000 | 1.000 | ||||
| T2 | 0.891 | 0.333–2.387 | 0.819 | 1.024 | 0.378–2.777 | 0.962 |
| T3 | 1.646 | 0.677–4.005 | 0.272 | 1.465 | 0.596–3.603 | 0.406 |
| T4a | 3.451 | 1.424–8.359 | 0.006 | 2.427 | 0.988–5.958 | 0.053 |
| T4b | 4.655 | 1.705–12.712 | 0.003 | 2.775 | 1.002–7.684 | 0.049 |
| pN stage | ||||||
| N0 | 1.000 | 1.000 | ||||
| N1 | 1.607 | 1.014–2.549 | 0.044 | 1.856 | 1.162–2.963 | 0.010 |
| N2 | 2.411 | 1.576–3.686 | <0.001 | 2.509 | 1.634–3.854 | <0.001 |
| N3a | 5.573 | 3.740–8.305 | <0.001 | 5.373 | 3.582–8.061 | <0.001 |
| N3b | 8.976 | 5.975–13.484 | <0.001 | 9.005 | 5.858–13.843 | <0.001 |
Present vs. absent. CEA, carcinoembryonic antigen; CA, cancer antigen.
Figure 1.
Nomogram for predicting 1-, 3-, and 5-year RFS after radical gastrectomy for stage II/III gastric adenocarcinoma.
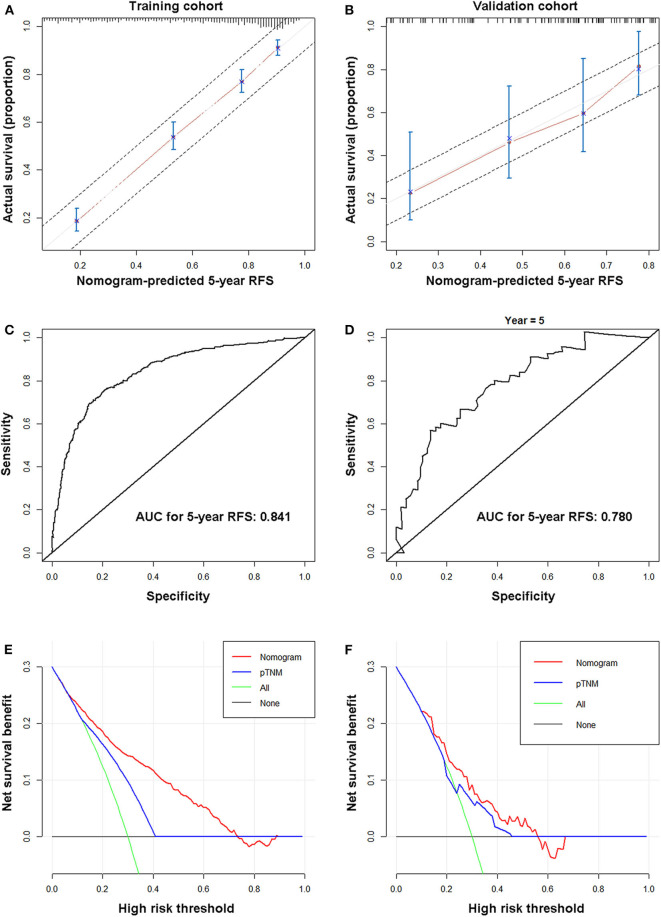
In the training cohort, the calibration curve showed excellent agreement between nomogram-predicted and actual observed 5-year RFS (Figure 2A). Supplementary Figure 3 shows the 5-year RFS rates predicted by the nomogram of each 8th-AJCC-TNM classification. A wide range of predicted survival rates could be determined for each TNM stage, and patients with higher stages had a broader range of predicted survival probabilities. In addition, the model performed better than the 8th AJCC-TNM in predicting 5-year RFS [C-index: 0.774 (95% CI 0.753–0.794) vs. 0.707 (95% CI 0.685–0.729), p < 0.001; AIC: 6,201.097 vs. 6,419.61], with a relatively high bootstrap-corrected C-index (0.774). The analysis of 5-year OS rates yielded similar results (Table 3). The ROC curve of 5-year RFS showed an excellent predictive value (AUC 0.841) (Figure 2B). The restricted cubic splines also confirmed the correlation between nomogram score and risk of recurrence (Supplementary Figure 4).
Figure 2.
Nomogram properties. Calibration (A,C), ROC curves (B,D), and decision curves (E,F) of the nomogram for the training (A,B,E) and validation cohorts (C,D,F).
Table 3.
Comparison of the prognostic accuracies of the nomogram and the 8th AJCC-TNM.
| Nomogram | AJCC 8th TNM | p-value | ||
|---|---|---|---|---|
| Training cohort | ||||
| RFS | C-index (95% CI) | 0.774 (0.753–0.794) | 0.707 (0.685–0.729) | <0.001 |
| AIC | 6,201.097 | 6,419.61 | – | |
| OS | C-index (95% CI) | 0.766 (0.747–0.785) | 0.700 (0.680–0.620) | <0.001 |
| AIC | 7,377.636 | 7,623.727 | – | |
| Internal validation cohort | ||||
| RFS | C-index (95% CI) | 0.770 (0.730–0.810) | 0.732 (0.693–0.770) | 0.013 |
| AIC | 1,249.137 | 1,267.904 | – | |
| OS | C-index (95% CI) | 0.781 (0.742–0.821) | 0.735 (0.696–0.775) | <0.001 |
| AIC | 1,209.733 | 1,236.066 | – | |
| External validation cohort | ||||
| RFS | C-index (95% CI) | 0.686 (0.609–0.763) | 0.596 (0.510–0.684) | <0.001 |
| AIC | 388.265 | 402.46 | – | |
| OS | C-index (95% CI) | 0.692 (0.627–0.757) | 0.593 (0.528–0.658) | <0.001 |
| AIC | 604.176 | 627.198 | – | |
Statistics are for 5-year RFS and OS rates. C-index, Harrell concordance index; AIC, Akaike Information Criterion.
In the internal validation cohort, the same findings were observed in the calibration curve (Supplementary Figure 5A). In addition, the model performed better than the 8th AJCC-TNM in predicting 5-year RFS and 5-year OS (higher C-index and smaller AIC value) (Table 3) with a high AUC for 5-year RFS (0.829) (Supplementary Figure 5B).
Nomogram External Validation and Clinical Applicability
In the external validation cohort, the calibration curve also showed good agreement between the 5-year RFS rates predicted by the nomogram and the actual 5-year RFS rates (Figure 2C). In addition, the model performed better than the 8th AJCC-TNM in predicting 5-year RFS and 5-year OS (higher C-index and smaller AIC value) (Table 3). The ROC curve for 5-year RFS showed an excellent predictive value (AUC: 0.752) (Figure 2D).
Decision curves showed that using the nomograms to predict the 5-year RFS rates provides more benefit than the 8th AJCC-TNM in the three cohorts. (Figures 2E,F, Supplementary Figure 5C).
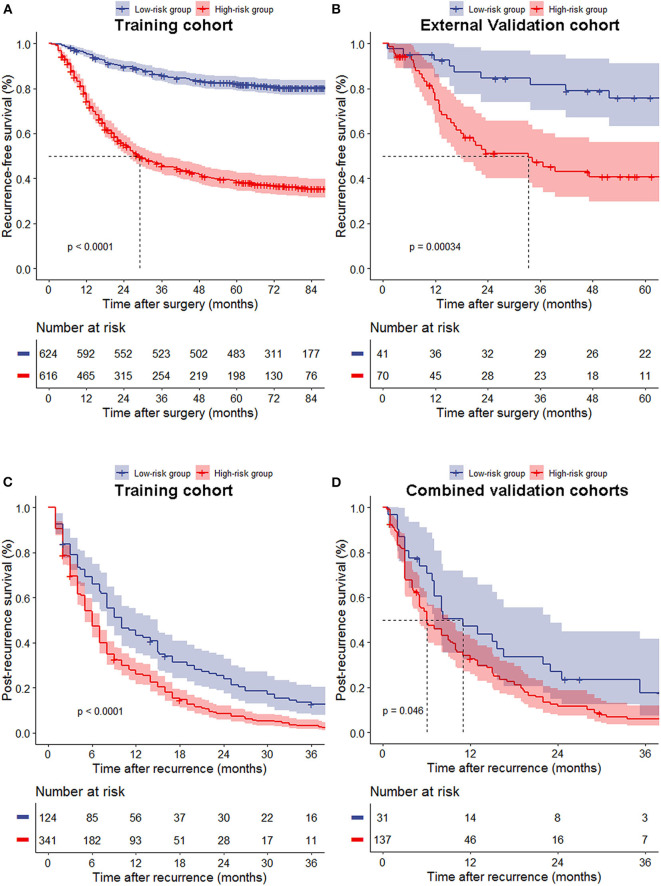
Risk Group Stratification Based on Nomogram Score
The median nomogram score of the training cohort, 212, effectively distinguished populations of different recurrence risk in the training, internal and external validation cohorts (Figures 3A,B, Supplementary Figure 5D). We next analyzed the relationship between risk group and recurrence pattern in patients for whom the exact site(s) of recurrence as known [n = 465 for the training cohort and n = 168 for the combined validation cohorts (due to the small sample size of internal and external cohorts)] and found that the proportion of peritoneal metastasis was significantly higher in the high- (nomogram score >212) vs. the low-risk group (score ≤ 212) in both cohorts [29.3 vs. 20.2% and 43.8 vs. 22.6%, respectively, all p < 0.05], while other recurrence patterns did not significantly differ between the two groups (Figures 4A,B). Within this subset of patients (465 in the training cohort and 168 in the combined validation cohorts), the post-recurrence survival of patients at high risk was inferior to that of low-risk patients in both cohorts (Figures 3C,D).
Figure 3.
Recurrence-free survival (A,B) of all patients and post-recurrence survival (C,D) of patients with recurrence between the low- and high-risk groups in the training (A,C), external validation cohort (B) and combined validation cohorts (D).
Figure 4.
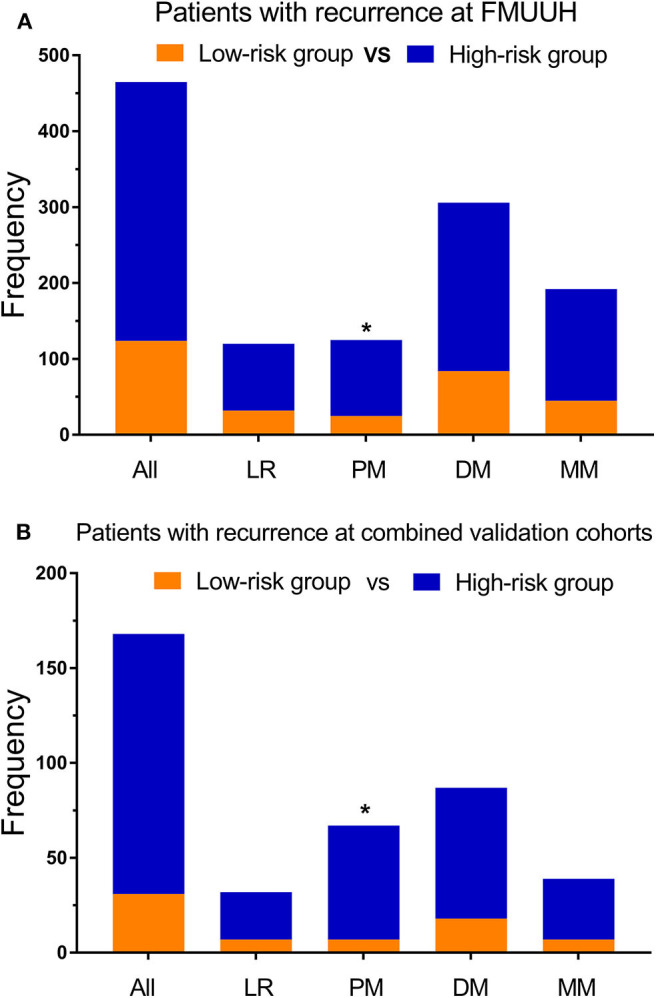
Recurrence patterns among patients determined to be at low vs. high risk using the nomogram. (A) Training cohort and (B) validation cohort. LR, locoregional recurrence; PM, peritoneal metastasis; DM, distant metastasis; MM, multiple metastasis; *p < 0.05.
Discussion
The present study used data from 2010 to 2014 in a high-volume Eastern cancer center to construct a nomogram, which showed good predictive value for 5-year RFS and 5-year OS among patients with stage II/III GC. Its predictive value was also validated internally and externally using data from the U.S. In addition, the model has good predictive efficacy than the 8th AJCC-TNM. More importantly, because adjuvant chemotherapy was included in the model, it allows simple calculation of the benefit of this treatment for an individual patient. To simplify the nomogram's use, we have made it available as a free web-based calculator.
The nomogram we have developed represents an advance over other recent tools for predicting the outcomes of patients with GC after gastrectomy. Indeed, there have been several nomograms established for GC so far. The model constructed by Han et al. had significantly better predictive value for OS than the AJCC-TNM staging system (10). However, its predictive value for recurrence was not evaluated. The nomogram introduced by Jiang et al. for predicting the disease-free survival (DFS) of patients with stage II/III gastric cancer had limited predictive value, and did not include adjuvant chemotherapy as a variable despite the study's finding that it was an independent prognostic factor for DFS (16). Several scholars recommended that treatment strategy be included in a nomogram (22, 23). These two models also lacked external validation using Western data. Further, a detailed comparison with the other published predictive models (24–28) for RFS is supplemented in Supplementary Table 1. Strengths of the present study include long-term follow-up information, large sample size and patients from Eastern and Western countries.
Although the small sample size, the validation of the nomogram using Western data is a particular advantage because of the clinical and pathological differences in GC between Asia vs. the U.S. and Europe (29). For example, the proportion of diffuse-type GC is higher in Asian patients, while proximal tumors are more frequent in the West. In addition, contributing factors such as environmental exposure and diet differ between geographic regions, as do standards of treatment. Neoadjuvant therapy is preferred in the U.S. and Europe, which is not common in China. In the present study, we focused on the efficacy of postoperative adjuvant chemotherapy and only included patients without neoadjuvant therapy. The postoperative adjuvant chemotherapy consisted of either single-agent 5-fluorouracil (5-FU) or a combination of 5-FU and cisplatin/oxaliplatin or paclitaxel in both China and the U.S. It is interesting that despite the selection bias of including only Western patients who did not receive neoadjuvant therapy, who are presumably the more frail and older patients, the nomogram still demonstrated remarkable predictive value for these patients. In addition, despite the differences in background characteristics, our model still showed strong predictive power in the external validation cohort (AUC 0.780), making it widely applicable.
The ability of the nomogram developed herein to calculate the benefit of adjuvant chemotherapy represents another important step forward. While randomized clinical trials have shown improvements in DFS and OS with adjuvant capecitabine + oxaliplatin (12) and S-1 (13), their benefit for all stage II/III GC patients remains unclear. While Jiang et al. two predictive models allowed calculation of the difference in survival for the same patient if they did or did not receive chemotherapy, their predictive value was not ideal, and required two separate manual calculations (16). In addition, the fact that the model was built on data from two separate groups likely created bias, and it was not validated in a Western population. In contrast, our model was created using data from all stage II/III GC patients together, allows more straightforward calculation of chemotherapy benefit, and showed good predictive performance (C-index: 0.774; AUC: 0.841) that was validated in a Western population. In addition, the model is available as a web-based tool, which makes the calculation more easily (Supplementary Figure 2). We just type in the patient's information on the web page, the 5-year RFS probabilities both for the situation in which the patient receives or does not receive ACT are calculated automatically. The difference between the two is the net survival benefit from the addition of ACT. However, the threshold difference in RFS at which ACT provides a net benefit remains undetermined, calling for further prospective studies to identify a specific cut-off.
The current study also sheds light on postoperative recurrence patterns, which is important for developing appropriate follow-up and treatment strategies. Among the 633 patients with recurrence, those with a nomogram score >212 were more prone to peritoneal metastasis (~30%). Differences in recurrence patterns have been associated with varying pathological stage (8) and Lauren type (2), and increased risk of peritoneal metastasis has previously been linked to gastric signet ring cell histology and neural invasion (9). As the outcomes of patients with peritoneal metastasis are poor, early monitoring is essential, as is reducing its incidence. To that end, early postoperative hyper thermic intraperitoneal chemotherapy (HIPEC) has been shown to reduce peritoneal metastasis (3 vs. 23%, p < 0.05) (30), with similar findings in another randomized controlled trial (RCT) from Russia (31). Therefore, further RCTs determining whether early postoperative HIPEC may be appropriate treatment for patients considered at high risk for peritoneal metastasis, such as those with high nomogram scores, are warranted.
It's known that peri-operative chemotherapy for GC patients is commonly used in the West. While postoperative chemotherapy may seem redundant for patients with neoadjuvant chemotherapy, it may be beneficial, as a study by Schumacher et al. showed that patients with advanced gastric cancer who underwent surgery with neoadjuvant therapy had improved R0 resection rates, but no survival benefit (32). However, the present study and previous studies on the efficacy of adjuvant chemotherapy have excluded patients who received neoadjuvant chemotherapy, so determining its benefit in such patients requires further study.
The present study has several limitations. Many levels of selection bias could result from its retrospective nature and non-random assignment of adjuvant chemotherapy, which was based on clinician experience and patient condition. Second, due to the data limitation and to simplify the model and improve its clinical applicability, we did not explore the effect of the number of chemotherapy cycles on prognosis. Third, due to the data limitation, we only considered the survival benefit of adjuvant chemotherapy and not its side effects, which vary from person to person (33). Previous studies showed that grade 3 or 4 adverse events were reported in 10%~55% patients with different chemotherapy regimens (34–36), which were in the acceptable range. However, PAC should be withheld from patients who were identified in the no-benefit group to avoid unnecessary adverse effects related to PAC. Finally, we did not consider the impact of tumor immune-related indicators and microsatellite instability. Even though an increasing number of studies have shown them to be associated with prognosis lack of benefit of chemotherapy in gastric cancer (14, 15), they are not likely to come into common use recently.
Conclusion
Using data from Asia and the U.S., we established and validated a predictive model that can effectively predict 5-year RFS and the benefit of adjuvant chemotherapy after radical resection of stage II/III GC. We showed that the model has significantly better predictive power and clinical applicability than the AJCC-TNM staging system. The tool is available as a simple calculator via the web, making it easier for clinicians to apply. Further large-scale, prospective, external validation is warranted.
Data Availability Statement
The dataset analyzed for this study is available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Fujian Medical University Union Hospital Ethics Committee and Mayo Clinic Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JL, B-bX, MT, and C-mH conceived the study, analyzed the data, and drafted the manuscript. C-hZ, PL, J-wX, and J-bW helped critically revise the manuscript for important intellectual content. PL, J-wX, J-bW, J-xL, and Q-yC helped collect data and design the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all the medical staff who contributed to the maintenance of the medical record database.
Footnotes
Funding. This study was funded by the Scientific and technological innovation joint capital projects of Fujian Province (2017Y9004); the Scientific and technological innovation joint capital projects of Fujian Province (2017Y9011); the second batch of special support funds for Fujian Province innovation and entrepreneurship talents (2016B013); Construction Project of Fujian Province Minimally Invasive Medical Center [Grant No. (2017)171]; the Scientific and technological innovation joint capital projects of Fujian Province (2018Y9041); Natural Science Foundation of Fujian Province (2019J01155); Fujian Provincial Science and Technology innovation joint fund project plan (2018Y9005); and Fujian Provincial Health Technology project (2019-ZQN-37).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.574611/full#supplementary-material
Data collection flow chart.
Web-based calculating tool that can be used to calculate an individualized estimate of 5-year RFS probabilities both with and without adjuvant chemotherapy (ACT) and then estimate the net survival benefit from the addition of ACT for patient with stage II/III gastric cancer.
The box plot represents the distribution of nomogram-predicted 5-year RFS according to 8th-AJCC-TNM classification.
Restricted cubic spline plots of recurrence hazard ratio vs. nomogram score.
Calibration (A), ROC curve (B), decision curves (C) of the nomogram in the internal validation cohort and recurrence-free survival (D) for the low- and high-risk groups in the internal validation cohort.
Comparison of prognostic models for recurrence-free survival.
References
- 1.Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. (2014) 40:250–60. 10.1111/apt.12814 [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren histologic type is the most important factor associated with pattern of recurrence following resection of gastric adenocarcinoma. Ann Surg. (2018) 267:105–13. 10.1097/SLA.0000000000002040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu BB, Lu J, Zheng ZF, Xie JW, Wang JB, Lin JX, et al. The predictive value of the preoperative C-reactive protein-albumin ratio for early recurrence and chemotherapy benefit in patients with gastric cancer after radical gastrectomy: using randomized phase III trial data. Gastric Cancer. (2019) 22:1016–28. 10.1007/s10120-019-00936-w [DOI] [PubMed] [Google Scholar]
- 4.Chang JS, Kim KH, Yoon HI, Hyung WJ, Rha SY, Kim HS, et al. Locoregional relapse after gastrectomy with D2 lymphadenectomy for gastric cancer. Br J Surg. (2017) 104:877–84. 10.1002/bjs.10502 [DOI] [PubMed] [Google Scholar]
- 5.Ito S, Ohashi Y, Sasako M. Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial. BMC Cancer. (2018) 18:449. 10.1186/s12885-018-4341-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo N, Han K, Hyung WJ, Chung YE, Park CH, Kim J-H, et al. Stratification of postsurgical computed tomography surveillance based on the extragastric recurrence of early gastric cancer. Ann Surg. (2019) 272:319–25. 10.1097/SLA.0000000000003238 [DOI] [PubMed] [Google Scholar]
- 7.Yamada S, Hatta W, Shimosegawa T, Takizawa K, Oyama T, Kawata N, et al. Different risk factors between early and late cancer recurrences in patients without additional surgery after noncurative endoscopic submucosal dissection for early gastric cancer. Gastrointest Endosc. (2019) 89:950–60. 10.1016/j.gie.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Lee HH, Seo HS, Jung YJ, Park CH. Stage-specific difference in timing and pattern of initial recurrence after curative surgery for gastric cancer. Surg Oncol. (2019) 30:81–6. 10.1016/j.suronc.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 9.Lee D, Son SY, Kim YB, Han SU, Hur H. Neural invasion is a significant contributor to peritoneal recurrence in signet ring cell gastric carcinoma. Ann Surg Oncol. (2018) 25:1167–75. 10.1245/s10434-018-6371-3 [DOI] [PubMed] [Google Scholar]
- 10.Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. (2012) 30:3834–40. 10.1200/JCO.2012.41.8343 [DOI] [PubMed] [Google Scholar]
- 11.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. (2010) 303:1729–37. 10.1001/jama.2010.534 [DOI] [PubMed] [Google Scholar]
- 12.Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC):5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. (2014) 15:1389–96. 10.1016/S1470-2045(14)70473-5 [DOI] [PubMed] [Google Scholar]
- 13.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. (2007) 357:1810–20. 10.1056/NEJMoa072252 [DOI] [PubMed] [Google Scholar]
- 14.Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the classic randomized controlled study. Ann Surg. (2019) 270:309–16. 10.1097/SLA.0000000000002803 [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al. Immunoscore signature: a prognostic and predictive tool in gastric cancer. Ann Surg. (2018) 267:504–13. 10.1097/SLA.0000000000002116 [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Li T, Liang X, Hu Y, Huang L, Liao Z, et al. Association of adjuvant chemotherapy with survival in patients with stage II or III gastric cancer. JAMA Surg. (2017) 152:e171087 10.1001/jamasurg.2017.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin MB, Edge S, Greene F. AJCC Cancer Staging Manual. Chicago, IL: American College of Surgeons via Springer Nature; (2017) p. 203–20. [Google Scholar]
- 18.Xu BB, Lu J, Zheng ZF, Huang CM, Zheng CH, Xie JW, et al. Comparison of short-term and long-term efficacy of laparoscopic and open gastrectomy in high-risk patients with gastric cancer: a propensity score-matching analysis. Surg Endosc. (2019) 33:58–70. 10.1007/s00464-018-6268-z [DOI] [PubMed] [Google Scholar]
- 19.Japanese Gastric Cancer A Japanese gastric cancer treatment guidelines 2014. Gastric Cancer. (2017) 20:1–19. 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Xu BB, Zheng ZF, Xie JW, Wang JB, Lin JX, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer. (2019) 22:536–45. 10.1007/s10120-018-0892-0 [DOI] [PubMed] [Google Scholar]
- 21.Trepanier M, Erkan A, Kouyoumdjian A, Nassif G, Albert M, Monson J, et al. Examining the relationship between lymph node harvest and survival in patients undergoing colectomy for colon adenocarcinoma. Surgery. (2019) 166:639–47. 10.1016/j.surg.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 22.Dikken JL, Coit DG, Baser RE, Gönen M, Goodman KA, Brennan MF, et al. Performance of a nomogram predicting disease-specific survival after an R0 resection for gastric cancer in patients receiving postoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. (2014) 88:624–9. 10.1016/j.ijrobp.2013.11.213 [DOI] [PubMed] [Google Scholar]
- 23.Hirabayashi S, Kosugi S, Isobe Y, Nashimoto A, Oda I, Hayashi K, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol. (2014) 25:1179–184. 10.1093/annonc/mdu125 [DOI] [PubMed] [Google Scholar]
- 24.Jeong SH, Kim RB, Park SY, Jung EJ, Ju YT, Jeong CY, et al. Nomogram for predicting gastric cancer recurrence using biomarker gene expression. Eur J Surg Oncol. (2019) 46:195–201. 10.1016/j.ejso.2019.09.143 [DOI] [PubMed] [Google Scholar]
- 25.Wang SB, Qi WX, Chen JY, Xu C, Kirova YM, Cao WG, et al. Competing risk nomogram predicting initial loco-regional recurrence in gastric cancer patients after D2 gastrectomy. Radiat Oncol. (2019) 14:128. 10.1186/s13014-019-1332-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muneoka Y, Akazawa K, Ishikawa T, Ichikawa H, Nashimoto A, Yabusaki H, et al. Nomogram for 5-year relapse-free survival of a patient with advanced gastric cancer after surgery. Int J Surg. (2016) 35:153–9. 10.1016/j.ijsu.2016.09.080 [DOI] [PubMed] [Google Scholar]
- 27.Lai JF, Kim S, Kim K, Li C, Oh SJ, Hung WJ, et al. Prediction of recurrence of early gastric cancer after curative resection. Ann Surg Oncol. (2009) 16:1896–902. 10.1245/s10434-009-0473-x [DOI] [PubMed] [Google Scholar]
- 28.Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg. (2005) 241:247–55. 10.1097/01.sla.0000152019.14741.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. (2010) 251:640–6. 10.1097/SLA.0b013e3181d3d29b [DOI] [PubMed] [Google Scholar]
- 30.Beeharry MK, Zhu ZL, Liu WT, Yao XX, Yan M, Zhu ZG. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study. BMC Cancer. (2019) 19:932 10.1186/s12885-019-6125-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reutovich MY, Krasko OV, Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol. (2019) 45:2405–11. 10.1016/j.ejso.2019.07.030 [DOI] [PubMed] [Google Scholar]
- 32.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for research and treatment of cancer randomized trial 40954. J Clin Oncol. (2010) 28:5210–8. 10.1200/JCO.2009.26.6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyman DM, Eaton AA, Gounder MM, Smith GL, Pamer EG, Hensley ML, et al. Nomogram to predict cycle-one serious drug-related toxicity in phase I oncology trials. J Clin Oncol. (2014) 32:519–26. 10.1200/JCO.2013.49.8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang YK, Chang HM, Yook JH, Ryu MH, Park I, Min YJ, et al. Adjuvant chemotherapy for gastric cancer: a randomised phase 3 trial of mitomycin-C plus either short-term doxifluridine or long-term doxifluridine plus cisplatin after curative D2 gastrectomy (AMC0201). Br J Cancer. (2013) 108:1245–51. 10.1038/bjc.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. (2019) 393:1948–57. 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 36.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. (2012) 379:315–21. 10.1016/S0140-6736(11)61873-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data collection flow chart.
Web-based calculating tool that can be used to calculate an individualized estimate of 5-year RFS probabilities both with and without adjuvant chemotherapy (ACT) and then estimate the net survival benefit from the addition of ACT for patient with stage II/III gastric cancer.
The box plot represents the distribution of nomogram-predicted 5-year RFS according to 8th-AJCC-TNM classification.
Restricted cubic spline plots of recurrence hazard ratio vs. nomogram score.
Calibration (A), ROC curve (B), decision curves (C) of the nomogram in the internal validation cohort and recurrence-free survival (D) for the low- and high-risk groups in the internal validation cohort.
Comparison of prognostic models for recurrence-free survival.
Data Availability Statement
The dataset analyzed for this study is available from the corresponding author on reasonable request.