This cohort study uses registry-based data of US organ transplant recipients to assess the incidence of sebaceous carcinoma and patient survival among solid organ transplant recipients from 1987 through 2017.
Key Points
Question
What is the incidence of sebaceous carcinoma (SC) after solid organ transplantation and patient survival after a transplant-associated SC diagnosis?
Findings
This registry-based cohort study of 301 075 US transplant recipients found a 25-fold increased risk for SC overall after transplantation. Lung transplant and posttransplant diagnosis of cutaneous squamous cell carcinoma (caused by UV radiation) were both strong risk factors for SC in the transplant population. Patients with SC and a prior transplant had decreased overall survival compared with other patients with SC in the general population.
Meaning
These findings point to the etiologic importance of immunosuppression and suggest a possible role for UV radiation in SC tumorigenesis.
Abstract
Importance
Risk of sebaceous carcinoma (SC), a rare skin cancer associated with Muir-Torre syndrome, is elevated among solid organ transplant recipients (SOTRs). However, population studies evaluating this association and assessing survival for posttransplant cases are lacking, and further understanding of SC epidemiology in this immunosuppressed population could provide etiologic and clinical insights.
Objective
To assess SC incidence and patient survival after solid organ transplantation.
Design, Setting, and Participants
This cohort study, conducted from January 1, 1987, to December 31, 2017, used data from the Transplant Cancer Match Study, which links transplant and cancer registry data for 17 states and 1 metropolitan area in the United States. Altogether, these registries account for approximately 46% of all US transplants. Data on demographic and transplant characteristics as well as induction and initial maintenance immunosuppressive therapies were obtained from the transplant registry. Standardized incidence ratios (SIRs) comparing SC incidence among SOTRs to the general population were calculated. Incidence rate ratios (IRRs) comparing SC risk between SOTR subgroups were calculated using multivariate Poisson regression. Cox regression was used to compare overall survival between SC cases in SOTRs and other individuals.
Main Outcomes and Measures
Sebaceous carcinoma incidence and overall patient survival after transplantation compared with the general population.
Results
A total of 326 282 transplant procedures were performed for 301 075 patients (No. [%] age at transplant, 126 550 [38.8%] aged 0-44 years; 82 394 [25.3%] aged 45-54 years; 82 082 [25.5%] aged 55-64 years; 35 256 [10.8%] aged ≥65 years; 201 354 male patients [61.7%]; 202 557 White patients [62.1%]). A total of 102 SCs were diagnosed in 301 075 SOTRs, corresponding to a 25-fold increased incidence (SIR, 24.8; 95% CI, 20.2-30.1). Incidence was especially elevated among lung recipients (SIR, 47.7; 95% CI, 20.6-94.0) and after a posttransplant diagnosis of cutaneous squamous cell carcinoma (SIR, 104.0; 95% CI, 62.8-163.0). Among SOTRs, factors independently associated with SC risk included male sex (IRR, 2.46; 95% CI, 1.48-4.07; P < .001), race/ethnicity (non-Hispanic Black vs non-Hispanic White, IRR, 0.28; 95% CI, 0.10-0.77; P = .01), older age (IRR, 7.85; 95% CI, 3.85-16.0; ≥65 vs 0-44 years; P < .001 for trend), use of thymoglobulin induction (IRR, 1.82; 95% CI, 1.16-2.86; P = .009), posttransplant cutaneous squamous cell carcinoma (IRR, 4.60; 95% CI, 2.67-7.94; P < .001), and longer time since transplant (IRR, 8.40; 95% CI, 3.94-17.90; ≥10 vs 0-1.9 years; P < .001 for trend). Muir-Torre syndrome–associated cancers were rare among both SOTRs and others with SC (3.3%-4.1%). Among patients with SC, prior transplantation was associated with increased overall mortality (adjusted hazard ratio, 2.09; 95% CI, 1.45-3.01), although few deaths were attributed to SC (4 of 92 SOTRs [4.3%]; 235 of 3585 non-SOTRs [6.6%]).
Conclusions and Relevance
Among SOTRs, results of this large cohort study suggest that SC was associated with measures of immunosuppression, and overall survival was worse than for other patients with SC. Findings also suggest a possible role for UV radiation in carcinogenesis.
Introduction
Sebaceous carcinoma (SC) incidence in the United States has been increasing since the 1970s.1,2,3 Risk factors for this aggressive cancer3,4 are only partly known and include Muir-Torre syndrome (OMIM 158320) and UV radiation (UVR).1,5 Immunosuppression is also an important risk factor, although the reasons for this association are unclear. Sebaceous carcinoma incidence is increased 8-fold among individuals with AIDS.6 Several single-institution case series have also suggested an increased risk for SC among solid organ transplant recipients (SOTRs).7,8,9 However, population-based analyses assessing SC incidence in the transplant population are lacking.
In the current study, we use population data from the Transplant Cancer Match Study,10,11 which links the US SOTR registry with multiple population-based cancer registries, to assess SC risk and overall patient survival in the transplant population.
Methods
SC Incidence
For this cohort study, we used Transplant Cancer Match Study data to perform a retrospective cohort analysis to assess SC incidence among SOTRs in 18 US states and metropolitan regions during January 1, 1987, to December 31, 2017 (see Table 1 note). Altogether, these registries account for approximately 46% of all US transplants. The SOTRs were followed from the date of transplantation until the earliest event: SC diagnosis, death, transplant failure or retransplantation, loss to follow-up, or end of cancer registry coverage. Retransplanted patients were considered unique patients, and follow-up began at the time of retransplant. Sebaceous carcinoma diagnoses were identified using linked cancer registry data based on the International Classification of Diseases for Oncology (version 3) morphology code 8410/3. Six SOTRs with SC diagnosed before transplantation were excluded. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The Transplant Cancer Match Study was approved by the institutional review board at the National Institutes of Health and, as required, by participating cancer registries. The National Cancer Institute institutional review board granted a waiver of consent because the study used preexisting data not involving patient contact and without posing risk to patients.
Table 1. Demographic and Clinical Characteristics of Solid Organ Transplant Recipients in the United States, 1987-2017.
| Characteristic | Transplant procedures, No. (%)a |
|---|---|
| Overall | 326 282 (100) |
| Transplant No. | |
| First | 294 771 (90.3) |
| Second or later | 31 511 (9.7) |
| Sex | |
| Female | 124 928 (38.3) |
| Male | 201 354 (61.7) |
| Race/ethnicity | |
| Non-Hispanic | |
| White | 202 557 (62.1) |
| Black | 58 876 (18.0) |
| Hispanic | 47 369 (14.5) |
| Asian or Pacific Islander | 17 480 (5.4) |
| Age at transplant, y | |
| 0-44 | 126 550 (38.8) |
| 45-54 | 82 394 (25.3) |
| 55-64 | 82 082 (25.2) |
| ≥65 | 35 256 (10.8) |
| Transplanted organ | |
| Kidney | 190 260 (58.3) |
| Liver | 69 306 (21.2) |
| Heart | 30 511 (9.4) |
| Lung | 15 757 (4.8) |
| Other or multiple | 20 448 (6.3) |
| Calendar year of transplant | |
| 1987-1994 | 32 293 (9.9) |
| 1995-2002 | 108 454 (33.2) |
| 2003-2010 | 135 570 (41.5) |
| 2011-2017 | 49 965 (15.3) |
| Posttransplant skin cancer | |
| Squamous cell carcinoma | 7654 (2.3) |
| Basal cell carcinoma | 5223 (1.6) |
There were 326 282 transplants performed for 301 075 patients in 18 state or metropolitan area registries during 1987-2017. Transplant data were available for the following sites (calendar years): California (1988-2012); Colorado (1988-2014); Connecticut (1987-2009); Florida (1987-2009); Georgia (1995-2010); Hawaii (1987-2007); Iowa (1987-2009); Illinois (1987-2013); Kentucky (1995-2011); Michigan (1987-2009); North Carolina (1990-2010); New Jersey (1987-2016); New York (1995-2017); Ohio (1996-2015); Pennsylvania (1987-2013); Seattle, Washington (1987-2014); Texas (1995-2014); and Utah (1987-2008).
From the transplant registry, we obtained data on demographic and transplant characteristics as well as induction and initial maintenance immunosuppressive therapies. We also obtained information reported by transplant centers on the first occurrence of posttransplant diagnoses of cutaneous squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). Ambient UVR data, obtained from the Total Ozone Mapping Spectrometer database managed by the National Aeronautics Space Administration,12 were linked to the transplant registry by residential zip code reported at the time of entry onto the waitlist or transplantation.
We compared SC risk among SOTRs to the general population using the standardized incidence ratio (SIR), defined as the ratio of observed to expected SC cases. For these calculations, we applied cancer incidence rates from the general population (18 cancer registries; see Table 1 footnote) to the transplant population, stratified by sex, 5-year age intervals, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and Asian or Pacific Islander), calendar year, and cancer registry to calculate the expected number of SCs.
We assessed risk factors for SC among SOTRs using Poisson regression. We included variables that were significant in the univariate models (P < .05) as candidates in a multivariable model; 2 variables (posttransplant diagnosis of BCC, ambient UV-B) were no longer significantly associated with SC risk after adjusting for other covariates, and as a result, these 2 variables were removed from the final multivariable model (Table 2).
Table 2. Multivariate Incidence Rate Ratios (IRRs) for Sebaceous Carcinoma Risk Among US Solid Organ Transplant Recipients.
| Characteristic | Observed cases | Incidence rate per 100 000 person-years | Multivariate IRR (95% CI)a | P value |
|---|---|---|---|---|
| Sex | ||||
| Female | 19 | 2.81 | [Reference] | <.001 |
| Male | 83 | 7.90 | 2.46 (1.48-4.07) | |
| Race/ethnicity | ||||
| Non-Hispanic | .03 | |||
| White | 80 | 7.15 | [Reference] | |
| Black | 4 | 1.46 | 0.28 (0.10-0.77) | |
| Hispanic | 13 | 5.35 | 1.09 (0.60-1.98) | |
| Asian or Pacific Islander | 5 | 5.44 | 1.01 (0.40-2.51) | |
| Age at transplant, y | ||||
| 0-44 | 13 | 1.80 | [Reference] | <.001b |
| 45-54 | 31 | 6.77 | 3.59 (1.87-6.90) | |
| 55-64 | 37 | 9.22 | 4.73 (2.47-9.05) | |
| ≥65 | 21 | 14.5 | 7.85 (3.85-16.0) | |
| Transplanted organ | ||||
| Kidney | 59 | 5.87 | [Reference] | .09 |
| Liver | 13 | 3.43 | 0.52 (0.28-0.98) | |
| Heart | 18 | 9.58 | 0.89 (0.51-1.56) | |
| Lung | 8 | 13.3 | 1.87 (0.87-4.03) | |
| Other or multiple | 4 | 4.21 | 0.92 (0.33-2.57) | |
| Induction therapy with thymoglobulin | ||||
| No | 73 | 5.30 | [Reference] | .009 |
| Yes | 29 | 8.29 | 1.82 (1.16-2.86) | |
| Posttransplant diagnosis of cutaneous squamous cell carcinoma | ||||
| No | 83 | 4.89 | [Reference] | <.001 |
| Yes | 19 | 65.6 | 4.60 (2.67-7.94) | |
| Time since transplant, y | ||||
| 0-1.9 | 10 | 1.88 | [Reference] | <.001b |
| 2-4.9 | 30 | 5.47 | 2.86 (1.39-5.85) | |
| 5-9.9 | 35 | 7.62 | 4.04 (1.98-8.23) | |
| ≥10 | 27 | 14.4 | 8.40 (3.94-17.90) |
Multivariate model adjusted for variables listed in the table.
P value for trend.
Survival Analysis
We compared survival between patients with SC, identified in participating cancer registries, according to whether they had previously received a solid organ transplant (based on linkage to the transplant registry). Fisher exact and χ2 tests were used to assess differences in clinical characteristics between patients with SC both with and without a history of solid organ transplantation, including the frequency of Muir-Torre syndrome–associated cancers (ie, colorectal, bladder, uterine, or ovarian cancers) diagnosed before or after SC diagnosis. The Kaplan-Meier method was used to describe overall survival following SC diagnosis, and Cox regression was used to derive unadjusted and adjusted hazard ratios (HRs) estimating the association of transplant status with overall mortality. To assess cancer-specific survival, we attributed deaths to SC if individuals had a previous diagnosis of SC (including patients with cancer diagnoses in addition to SC) and an International Classification of Diseases, Ninth Revision (ICD-9) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) cause of death indicated as a cancer of “other non-epithelial skin,” “eye and orbit,” or unspecified site.13 Cause of death was also attributed to SC if an individual had an ICD-9 or ICD-10 cancer code for any cancer site if SC was the patient’s only cancer diagnosis.14 This approach for assessing cancer-specific survival was initially developed by Howlader and colleagues to address the issue of misclassification of cancer-specific deaths in Surveillance, Epidemiology, and End Results cancer registries.14
Statistical Analysis
All statistical analyses were performed from January 1, 1987, to December 31, 2017, using Stata software, version 15.0 (StataCorp LLC). All P values were 2 sided, and a significance level was set at .05.
Results
SC Incidence
Overall, we assessed SC incidence following 326 282 transplant procedures performed for 301 075 patients (No. [%] age at transplant, 126 550 [38.8%] aged 0-44 years; 82 394 [25.3%] aged 45-54 years; 82 082 [25.5%] aged 55-64 years; 35 256 [10.8%] aged ≥65 years; 201 354 male patients [61.7%]; 202 557 White patients [62.1%]) in 18 US state and metropolitan registries from January 1, 1987, to December 31, 2017, corresponding to 1 727 927 person-years of follow-up after transplantation (Table 1). A total of 294 771 transplant procedures (90.3%) were a patient’s first transplant. The most commonly transplanted organs were 190 260 kidneys (58.3%) and 69 306 livers (21.2%). During follow-up, 7654 cutaneous SCCs (2.3%) and 5223 BCCs (1.6%) were diagnosed in SOTRs, respectively.
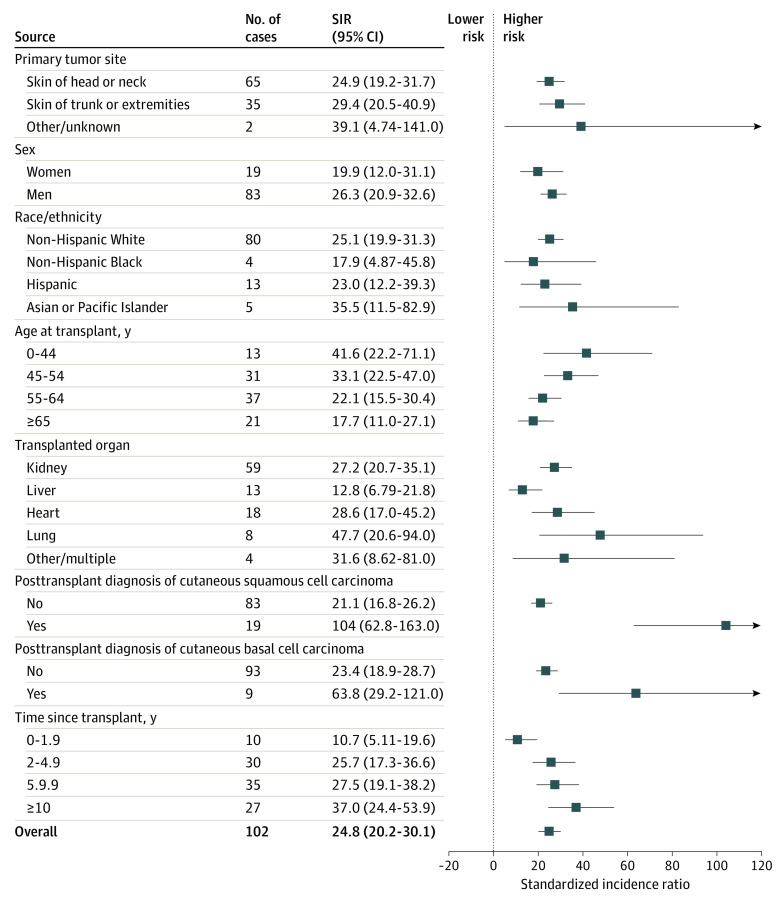
There were 102 SOTRs diagnosed with SC during the study period, corresponding to a 25-fold increased risk compared with the general population (SIR, 24.8; 95% CI, 20.2-30.1) (Figure 1). As shown in Figure 1, SC risk was similarly increased for cancers arising on chronically sun-exposed skin of the head or neck and intermittently sun-exposed skin of the trunk or extremities (SIR 24.9 [95% CI, 19.2-31.7] vs 29.4 [95% CI, 20.5-40.9], respectively). The SC risk was also elevated to a similar degree among male patients (SIR, 26.3; 95% CI, 20.9-32.6) and female patients (SIR, 19.9; 95% CI, 12.0-31.1) and according to race/ethnicity, although wide CIs were observed for these comparisons. Although CIs were similarly wide for transplanted organ type, lung transplant appeared to be associated with the highest elevation in risk (SIR, 47.7; 95% CI, 20.6-94.0), kidney and heart recipients manifested intermediate elevations (SIR, 27.2 [95% CI, 20.7-35.1] and 28.6 [95% CI, 17.0-45.2], respectively), and liver recipients had the lowest elevation in risk (SIR, 12.8; 95% CI, 6.8-21.8). Standardized incidence ratios were also highest among the youngest group (SIR, 41.6; 95% CI, 22.2-71.1; age, 0-44 years at transplant) and decreased with age at transplant. The greatest elevation in SC risk was observed among individuals after a posttransplant diagnosis of cutaneous SCC (SIR, 104.0; 95% CI, 62.8-163.0) or BCC (SIR, 63.8; 95% CI, 29.2-121.0).
Figure 1. Forest Plot of Standardized Incidence Ratios Comparing Sebaceous Carcinoma Risk Among Solid Organ Transplant Recipients to the General Population.
The standardized incidence ratio is the ratio of observed to expected number of cases for each group. The expected number was derived by applying cancer registry rates to the solid organ transplant recipient cohort according to sex, race/ethnicity, attained age, calendar year, and registry area. SIR indicates standardized incidence ratio.
Univariate analyses of SC risk factors are shown in the eTable in the Supplement. In the multivariate analysis, independent SC risk factors included male sex (IRR, 2.46; 95% CI, 1.48-4.07; P < .001), older age at transplant (IRR, 7.85; 95% CI, 3.85-16.0; ≥65 years vs 0-44; P < .001 for trend), thymoglobulin induction (IRR, 1.82; 95% CI, 1.16-2.86; P = .009), posttransplant diagnosis of cutaneous SCC (IRR, 4.60; 95% CI, 2.67-7.94; P < .001), and longer time since transplant (IRR, 8.40; 95% CI, 3.94-17.90; ≥10 years vs 0-1.9 years; P < .001 for trend) (Table 2). Risk of SC was lower for liver recipients (IRR, 0.52; 95% CI, 0.28-0.98 [vs kidney recipients]; P = .04), and for non-Hispanic Black patients (IRR, 0.28; 95% CI, 0.10-0.77 [vs non-Hispanic White patients]; P = .01) (Table 2).
Survival Analysis
We assessed survival among 3677 SC cases according to transplant status (SOTRs, 92 cases; non-SOTRs, 3585 cases). Five states or registries (Florida, Hawaii, Michigan, North Carolina, and Utah) did not provide data on cause of death and were excluded from the analysis. In total, cause of death was available for 92 of the 102 SOTRs with SC. Compared with non-SOTRs with SC, SOTRs with SC were more likely to be male patients (1894 [52.8%] vs 77 [83.7%]; P < .001) and younger at diagnosis (age, ≥65 y, 2435 [67.9%] vs 43 [46.7%]; P < .001), have a primary tumor site on the skin of the trunk or extremities (652 [18.2%] vs 29 [31.5%]; P = .005), and be diagnosed after calendar year 2003 (2140 [59.7%] vs 83 [90.2%]; P < .001) (Table 3). In both groups, the majority of cases were in non-Hispanic White patients (nontransplant vs SOTR, 2702 [75.4%] vs 62 [67.4%]; P = .18) and were diagnosed at the localized stage (2494 [69.6%] vs 70 [76.1%]; P = .39), although these differences were not statistically significant. Treatment (surgery and radiation) and frequency of Muir-Torre syndrome cancers were similar between groups (Table 3).
Table 3. Characteristics of Patients With Sebaceous Carcinoma in the US General Population by Transplant Status.
| Characteristic | No. (%) | P valuea | |
|---|---|---|---|
| Nontransplant patients | Solid organ transplant recipients | ||
| Overall | 3585 (100.0) | 92 (100.0)b | |
| Sex | |||
| Female | 1691 (47.2) | 15 (16.3) | <.001 |
| Male | 1894 (52.8) | 77 (83.7) | |
| Race/ethnicity | |||
| Non-Hispanic | .18 | ||
| White | 2702 (75.4) | 62 (67.4) | |
| Black | 101 (2.8) | 1 (1.1) | |
| Hispanic | 144 (4.0) | 6 (6.5) | |
| Asian or Pacific Islander | 124 (3.5) | 5 (5.4) | |
| Unknown | 514 (14.3) | 18 (19.6) | |
| Age at diagnosis, y | |||
| 0-44 | 157 (4.4) | 4 (4.3) | <.001 |
| 45-54 | 385 (10.7) | 12 (13.0) | |
| 55-64 | 608 (17.0) | 33 (35.9) | |
| ≥65 | 2435 (67.9) | 43 (46.7) | |
| Calendar year of diagnosis | |||
| 1987-1994 | 436 (12.2) | 0 | <.001 |
| 1995-2002 | 1009 (28.1) | 9 (9.8) | |
| 2003-2010 | 1363 (38.0) | 41 (44.6) | |
| 2011-2017 | 777 (21.7) | 42 (45.7) | |
| Primary tumor site | |||
| Skin of head or neck | 2744 (76.5) | 61 (66.3) | .005 |
| Skin of trunk or extremities | 652 (18.2) | 29 (31.5) | |
| Other or unknown | 189 (5.3) | 2 (2.2) | |
| Tumor stage | |||
| Localized | 2494 (69.6) | 70 (76.1) | .39 |
| Regional | 218 (6.1) | 4 (4.3) | |
| Distant | 158 (4.4) | 1 (1.1) | |
| Unknown | 715 (19.9) | 17 (18.5) | |
| Diagnosis of other Muir-Torre syndrome cancersc | |||
| No | 3437 (95.9) | 89 (96.7) | >.99 |
| Yes | 148 (4.1) | 3 (3.3) | |
| Radiation treatmentd | |||
| No | 2795 (93.8) | 82 (93.2) | .83 |
| Yes | 186 (6.2) | 6 (6.8) | |
| Surgical treatmente | |||
| No | 450 (14.1) | 9 (10.1) | .28 |
| Yes | 2733 (85.9) | 80 (89.9) | |
P values were calculated using a Fisher exact test, with the exception of sex, radiation treatment, and surgical treatment, for which P values were calculated using a χ2 test because each group contained ≥5 cases.
Five states/registries (Florida, Hawaii, Michigan, North Carolina, Utah) did not provide data on cause of death and were excluded from the above analysis. In total, cause of death was available for 92 of the 102 transplant recipients (Table 2) with sebaceous carcinoma.
Muir-Torre syndrome cancers include colorectal, urinary bladder, uterine, and ovarian cancers.
Information on radiation treatment was not available for 604 (16.8%) nontransplant patients and 4 (4.3%) solid organ transplant recipients. Cases with missing data were excluded from the χ2 test.
Information on surgical treatment was not available for 402 (11.2%) nontransplant patients and 3 (3.3%) solid organ transplant recipients. Cases with missing data were excluded from the χ2 test.
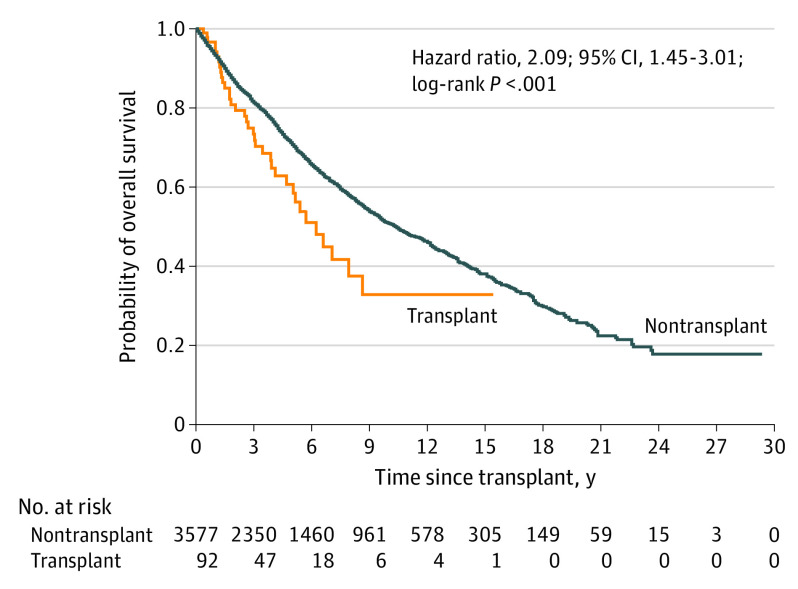
Overall survival was lower for SOTRs compared to non-SOTRs (Figure 2). At 5 and 10 years, overall survival was 61% and 33%, respectively, for SOTRs, compared with 71% and 51% for non-SOTRs. Prior transplant was associated with an elevated overall mortality in an unadjusted Cox model (HR, 1.51; 95% CI, 1.08-2.10; P = .02), and this association persisted and appeared stronger after multivariate adjustment (HR, 2.09; 95% CI, 1.45-3.01; P < .001) (Figure 2). Only a small minority of deaths were specified as due to SC (4 of 92 [4.3%] among SOTRs; 235 of 3585 [6.6%] among non-SOTRs), which precluded an assessment of the association of cancer-specific mortality with transplant status.
Figure 2. Kaplan-Meier Curves and Cox Proportional Hazard Ratios for Overall Survival Following Sebaceous Carcinoma Diagnosis by Transplant Status.
The hazard ratio is adjusted for sex, tumor stage (localized, regional, distant, unknown), and history of radiation treatment (yes or no). The multivariate model is also stratified by other variables that violated the proportionality assumption: race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian or Pacific Islander, unknown), age at diagnosis (0-44, 45-54, 55-64, ≥65 years), primary tumor site (skin of head or neck; skin of trunk or extremities; other/unknown), and history of surgical treatment (yes or no). Cancer data were available for the following sites (calendar years): California (1988-2012); Colorado (1988-2014); Connecticut (1987-2009); Georgia (1995-2010); Illinois (1987-2013); Iowa (1987-2009); Kentucky (1995-2011); New Jersey (1987-2016); New York (1995-2017); Ohio (1996-2015); Pennsylvania (1987-2013); Seattle, Washington (1987-2014); and Texas (1995-2014).
Discussion
This study reports incidence and overall survival for SC over a 30-year period. We observed a 25-fold elevation in risk for SC among patients receiving an organ transplant. This observation appears to confirm the elevation in risk observed in a recent study by D’Arcy and colleagues (SIR, 34.3),11 who also used Transplant Cancer Match Study data to evaluate risk for rare cancer types after transplantation. In contrast to this previous study, our data set included more years of coverage (1987-2017 vs 1987-2014), a greater number of cancer registries (18 vs 17), longer follow-up (1 727 927 vs 1 393 047 person-years), and an increased number of SOTRs (301 075 vs 262 455 patients) and SC cases (102 vs 62 tumors), which resulted in more precise estimates of risk compared with the general population. To our knowledge, this study is also the largest to date to evaluate the association of demographic, transplant, and UVR-related factors with SC risk in the transplant population and the first study to assess the outcome of transplantation on overall survival following an SC diagnosis.
The elevation in SC risk in SOTRs suggests the possibility of a major role for immunosuppression in promoting the development of this cancer. We found that SC risk increased with time since transplant, suggesting the importance of chronic immunosuppression in tumor development. In addition, although CIs were wide and overlapping, the highest and lowest risk estimates for SC were for lung and liver recipients, respectively. Because lung recipients tend to receive the most intensive immunosuppressive therapy and liver recipients the least, these findings suggest that the intensity of immunosuppression is also a modifier of SC risk within the transplant population.15 Finally, SC risk was increased in SOTRs treated with induction therapy using thymoglobulin, a potent T-cell inhibitor that induces strong short-term immunosuppression immediately after transplantation.16 T-cell depletion may be particularly important for SC tumorigenesis as SC risk is also elevated among individuals with AIDS (who have low CD4 T-cell counts).6 Although we did not note associations with other immunosuppressive medications, we assessed maintenance immunosuppressive medications only at baseline and did not assess duration of use or dose.
Of note, both SOTRs and individuals with AIDS have increased risks for other rare cutaneous malignancies that are caused by viruses, including Kaposi sarcoma and Merkel cell carcinoma.6,10,17,18 Thus, our findings suggest that SC might similarly be caused by a virus. In this regard, 2 prior studies each identified high-risk types of human papillomavirus (HPV) in 40% to 60% of eyelid SC tumors.19,20 However, 2 other studies were unable to detect HPV in SC tumors,21,22 whereas a third study found high-risk HPV type 16 in only 1 of 24 eyelid cases.23
We also observed that posttransplant occurrence of cutaneous SCC was associated with a more than 4-fold increased risk for SC relative to other SOTRs, which translated to a 104-fold risk compared with the general population. A posttransplant diagnosis of BCC was similarly associated with an increased risk of SC, although this association was not significant in our multivariate model. Both SCC and BCC are caused by UVR, and the occurrence of these cancers among a subset of SOTRs likely serves as a marker of especially severe chronic solar damage.
Our study findings suggest that non-Hispanic Black patients had a lower risk for SC than non-Hispanic White patients among the SOTR population. However, SC risk for other transplant groups with increased melanin pigment (eg, Hispanic and Asian or Pacific Islander) was similar to non-Hispanic White patients, suggesting that the amount of melanin pigment may not have a substantial association with overall risk for this cancer type. Given the relatively small number of cases in each racial/ethnic group, future studies will be necessary to evaluate the effect of race/ethnicity and skin pigmentation on SC risk.
Sebaceous carcinoma risk in the general population has been associated with measures of ambient UVR exposure based on geographic location of residence.1 We used a similar approach and found, somewhat paradoxically, that ambient UV-B (but not UV-A) was associated with decreased SC risk among patients with organ transplant in our univariate analysis. However, this association was of borderline significance, and it was attenuated and no longer significant after multivariate adjustment. We note that ambient UVR measures may not accurately capture an individual’s actual UVR exposure, which is influenced by occupation, sun-seeking behaviors such as leisure time activities, and use of sun-protective measures.24,25,26 Misclassification of exposure based on a single residence may also occur if individuals frequently migrate between areas with disparate ambient UVR. Definitive assessment of the role of UVR in tumor development will require additional epidemiologic studies assessing UVR exposure in SOTRs with SC along with tumor-based studies from this immunosuppressed population assessing for UVR signatures in cancer driver genes.
Germline testing data for Muir-Torre syndrome was not available for our study population. Patients with SC and Muir-Torre syndrome are at risk for gastrointestinal and genitourinary malignancies. In our study, the number of Muir-Torre syndrome–associated cancers among patients with SC was very low and did not differ by transplant status (<5% among cases with and without a prior transplant), suggesting that most cases of SC in SOTRs are not due to an underlying germline variant affecting DNA mismatch repair.
With respect to outcome after a diagnosis of SC, results suggest that overall mortality was 2-fold higher among SOTRs compared with nontransplant patients in our adjusted analysis. Although immunosuppression is associated with reduced survival from other cancers,27,28 which is consistent with a role of immunity in helping to control cancer after diagnosis, our findings suggest that SC-specific mortality was very uncommon in our population and similar in SC cases vs those without a prior transplant (4.3% vs 6.6% of all deaths, respectively). These results suggest that much of the mortality in patients with SC arises from comorbid conditions other than the cancer, eg, related to age or (among SOTRs) complications of transplantation including graft failure, infection, and cardiovascular disease.
Limitations
A limitation of the current study was that the number of deaths attributed to SC was too few to perform an analysis of cancer-specific survival. Another limitation of our study was the modest number of SC diagnoses, which may have prevented us from detecting some associations between patient characteristics and SC risk. Despite this limitation, our study found a highly elevated SC risk overall and for multiple subgroups, and we were able to identify several relevant risk factors, including demographic and transplant-related characteristics and a posttransplant diagnosis of cutaneous SCC. There is also the potential for misclassification of tumors in cancer registries as SC, but this is probably very infrequent, because cancer registries typically have access to pathology reports, and there are unique morphologic (sebocyte differentiation) and immunohistochemical features (adipophilin positivity29) that allow pathologists to diagnose this cancer.
Conclusions
In conclusion, the elevated incidence of SC that we observed among SOTRs highlights the importance of immunosuppression in tumor development. Additional studies are needed to better understand the immunologic deficits that predispose to SC and investigate the possible etiologic role of HPV or other viruses in tumor development.
eTable. Univariate Incidence Rate Ratios for Sebaceous Carcinoma Risk Among Solid Organ Transplant Recipients
References
- 1.Sargen MR, Mai ZM, Engels EA, et al. Ambient ultraviolet radiation and sebaceous carcinoma incidence in the United States, 2000–2016. J Natl Cancer Inst Cancer Spectrum. 2020;4(2). doi: 10.1093/jncics/pkaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathi R, Chen Z, Li L, Bordeaux JS. Incidence and survival of sebaceous carcinoma in the United States. J Am Acad Dermatol. 2016;75(6):1210-1215. doi: 10.1016/j.jaad.2016.07.046 [DOI] [PubMed] [Google Scholar]
- 3.Dasgupta T, Wilson LD, Yu JB. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer. 2009;115(1):158-165. doi: 10.1002/cncr.23952 [DOI] [PubMed] [Google Scholar]
- 4.Muqit MM, Roberts F, Lee WR, Kemp E. Improved survival rates in sebaceous carcinoma of the eyelid. Eye (Lond). 2004;18(1):49-53. doi: 10.1038/sj.eye.6700523 [DOI] [PubMed] [Google Scholar]
- 5.North JP, Golovato J, Vaske CJ, et al. Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat Commun. 2018;9(1):1894. doi: 10.1038/s41467-018-04008-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009;23(3):385-393. doi: 10.1097/QAD.0b013e3283213046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harwood CA, Swale VJ, Bataille VA, et al. An association between sebaceous carcinoma and microsatellite instability in immunosuppressed organ transplant recipients. J Invest Dermatol. 2001;116(2):246-253. doi: 10.1046/j.1523-1747.2001.01233.x [DOI] [PubMed] [Google Scholar]
- 8.Harwood CA, McGregor JM, Swale VJ, et al. High frequency and diversity of cutaneous appendageal tumors in organ transplant recipients. J Am Acad Dermatol. 2003;48(3):401-408. doi: 10.1067/mjd.2003.97 [DOI] [PubMed] [Google Scholar]
- 9.Hoss E, Nelson SA, Sharma A. Sebaceous carcinoma in solid organ transplant recipients. Int J Dermatol. 2017;56(7):746-749. doi: 10.1111/ijd.13490 [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891-1901. doi: 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Arcy ME, Castenson D, Lynch CF, et al. Risk of rare cancers among solid organ transplant recipients. J Natl Cancer Inst. 2020;djaa078. Published online May 27, 2020. doi: 10.1093/jnci/djaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Aeronautics Space Administration Total Ozone Mapping Spectrometer Data Product: Erythemal UV Exposure. Goddard Space Flight Center; 2004. [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) SEER cause-specific death classification. Accessed January 20, 2020. https://seer.cancer.gov/causespecific/
- 14.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584-1598. doi: 10.1093/jnci/djq366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614. doi: 10.1056/NEJMra064928 [DOI] [PubMed] [Google Scholar]
- 16.Kho MM, Bouvy AP, Cadogan M, Kraaijeveld R, Baan CC, Weimar W. The effect of low and ultra-low dosages thymoglobulin on peripheral T, B and NK cells in kidney transplant recipients. Transpl Immunol. 2012;26(4):186-190.doi: 10.1016/j.trim.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 17.Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107(2):dju382. doi: 10.1093/jnci/dju382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194. doi: 10.1002/ijc.23487 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi N, Furihata M, Ohtsuki Y, Ueno H. Search for accumulation of p53 protein and detection of human papillomavirus genomes in sebaceous gland carcinoma of the eyelid. Virchows Arch. 1994;424(5):503-509. doi: 10.1007/BF00191436 [DOI] [PubMed] [Google Scholar]
- 20.Tetzlaff MT, Curry JL, Ning J, et al. Distinct biological types of ocular adnexal sebaceous carcinoma: HPV-driven and virus-negative tumors arise through nonoverlapping molecular-genetic alterations. Clin Cancer Res. 2019;25(4):1280-1290. doi: 10.1158/1078-0432.CCR-18-1688 [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Fernandez F, Kaltreider SA, Patnaik BD, et al. Sebaceous carcinoma. tumor progression through mutational inactivation of p53. Ophthalmology. 1998;105(3):497-506. doi: 10.1016/S0161-6420(98)93034-2 [DOI] [PubMed] [Google Scholar]
- 22.Kwon MJ, Shin HS, Nam ES, et al. Comparison of HER2 gene amplification and KRAS alteration in eyelid sebaceous carcinomas with that in other eyelid tumors. Pathol Res Pract. 2015;211(5):349-355. doi: 10.1016/j.prp.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 23.Liau JY, Liao SL, Hsiao CH, Lin MC, Chang HC, Kuo KT. Hypermethylation of the CDKN2A gene promoter is a frequent epigenetic change in periocular sebaceous carcinoma and is associated with younger patient age. Hum Pathol. 2014;45(3):533-539. doi: 10.1016/j.humpath.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 24.Glanz K, Buller DB, Saraiya M. Reducing ultraviolet radiation exposure among outdoor workers: state of the evidence and recommendations. Environ Health. 2007;6:22. doi: 10.1186/1476-069X-6-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabriel P, Schmitz S Gender differences in occupational distributions among workers. Accessed January 20, 2020. https://www.bls.gov/opub/mlr/2007/06/art2full.pdf
- 26.Bränström R, Kasparian NA, Chang YM, et al. Predictors of sun protection behaviors and severe sunburn in an international online study. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2199-2210. doi: 10.1158/1055-9965.EPI-10-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Arcy ME, Coghill AE, Lynch CF, et al. Survival after a cancer diagnosis among solid organ transplant recipients in the United States. Cancer. 2019;125(6):933-942. doi: 10.1002/cncr.31782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33(21):2376-2383. doi: 10.1200/JCO.2014.59.5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostler DA, Prieto VG, Reed JA, Deavers MT, Lazar AJ, Ivan D. Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: an immunohistochemical study of 117 cases. Mod Pathol. 2010;23(4):567-573. doi: 10.1038/modpathol.2010.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Univariate Incidence Rate Ratios for Sebaceous Carcinoma Risk Among Solid Organ Transplant Recipients