This cohort study assesses whether left ventricular volumes and volume-derived left ventricular ejection fraction are determinants of mortality in aortic regurgitation.
Key Points
Question
Are disk-summation method–derived left ventricular (LV) end-systolic volume index and LV ejection fraction (LVEF) associated with mortality in asymptomatic patients with hemodynamically significant chronic aortic regurgitation?
Findings
In this cohort study of 492 asymptomatic patients with moderately severe to severe aortic regurgitation, besides conventional linear LVEF and LV end-systolic dimension index, LV end-systolic volume index and volume-derived LVEF were robust independent factors associated with mortality. Thresholds of increased mortality risk for linear LVEF and volume-derived LVEF were 60%, and for LV end-systolic dimension index and volume index, 21 to 22 mm/m2 and 40 to 45 mL/m2, respectively; previously reported LV end-systolic volume index threshold of 45 mL/m2 was a robust marker of increased risk of death.
Meaning
In asymptomatic low-risk patients with aortic regurgitation, LV end-systolic volume index and volume-derived LVEF provided similar risk-stratifying power as conventional linear LVEF and LV end-systolic dimension index.
Abstract
Importance
Volumetric measurements by transthoracic echocardiogram may better reflect left ventricular (LV) remodeling than conventional linear LV dimensions. However, the association of LV volumes with mortality in patients with chronic hemodynamically significant aortic regurgitation (AR) is unknown.
Objective
To assess whether LV volumes and volume-derived LV ejection fraction (Vol-LVEF) are determinants of mortality in AR.
Design, Setting, and Participants
This cohort study included consecutive asymptomatic patients with chronic moderately severe to severe AR from a tertiary referral center (January 2004 through April 2019).
Exposures
Clinical and echocardiographic data were analyzed retrospectively. Aortic regurgitation severity was graded by comprehensive integrated approach. De novo disk-summation method was used to derive LV volumes and Vol-LVEF.
Main Outcome and Measures
Associations between all-cause mortality under medical surveillance and the following LV indexes: linear LV end-systolic dimension index (LVESDi), linear LVEF, LV end-systolic volume index (LVESVi), and Vol-LVEF.
Results
Of 492 asymptomatic patients (mean [SD] age, 60 [17] years; 425 men [86%]), ischemic heart disease prevalence was low (41 [9%]), and 453 (92.1%) had preserved linear LVEF (≥50%) with mean (SD) LVESVi of 41 (15) mL/m2. At a median (interquartile range) of 5.4 (2.5-10.1) years, 66 patients (13.4%) died under medical surveillance; overall survival was not different than the age- and sex-matched general population (P = .55). Separate multivariate models, adjusted for age, sex, Charlson Comorbidity Index, and AR severity, demonstrated that in addition to linear LVEF and LVESDi, LVESVi and Vol-LVEF were independently associated with mortality under surveillance (all P < .046) with similar C statistics (range, 0.83-0.84). Spline curves showed that continuous risks of death started to rise for both linear LVEF and Vol-LVEF less than 60%, LVESVi more than 40 to 45 mL/m2, and LVESDi above 21 to 22 mm/m2. As dichotomized variables, patients with LVESVi more than 45 mL/m2 exhibited increased relative death risk (hazard ratio, 1.93; 95% CI, 1.10-3.38; P = .02) while LVESDi more than 20 mm/m2 did not (P = .32). LVESVi more than 45 mL/m2 showed a decreased survival trend compared with expected population survival.
Conclusions and Relevance
In this large asymptomatic cohort of patients with hemodynamically significant AR, LVESVi and Vol-LVEF worked equally as well as LVESDi and linear LVEF in risk discriminating patients with excess mortality. A LVESVi threshold of 45 mL/m2 or greater was significantly associated with an increased mortality risk.
Introduction
Assessment of left ventricular (LV) function is critical for clinical decision-making in patients with valvular heart disease.1 Patients with hemodynamically significant aortic regurgitation (AR) incur excess mortality.2 Transthoracic echocardiography (TTE)–derived prognostic indexes1 in AR are based on LV linear measurements, including ejection fraction (linear LVEF), and LV end-systolic dimension index (LVESDi)1 from studies conducted more than 2 decades ago. Nonetheless, in patients with AR with dilated left ventricles, the extent of true LV remodeling cannot be fully reflected by linear LV dimensions measured in a single view3 and may be better delineated by volumetric measurements using disk-summation methods from 2-dimensional (2-D) TTE.4
The prognostic value of LV volumes in contemporary AR cohorts remains poorly known, likely because measurements are more time consuming, technically demanding (ie, require effort in acquiring nonforeshortened apical views, appropriate endocardial border recognition, and consistency in LV border delineation), and have not been routine practice in many echocardiography laboratories until 2015 when chamber-quantification guidelines strongly recommended measuring LV volumes by the disk-summation method.4 Also, the fact that the disk-summation method underestimates LV volumes against the criterion standard cardiac magnetic resonance (CMR)5 casts doubt as to whether LV volumes may be associated with prognosis in patients with AR. However, CMR has limited availability, is expensive, and is incompatible with most metallic devices; noninvasive 2-D TTE remains the first-line choice to evaluate and follow-up patients with AR in clinical practice.
With advances in technology, 2-D TTE image quality has been improved for visualizing LV endocardial border.6 Therefore, it is of interest to explore whether LV volume is associated with adverse outcomes in contemporary patients with significant AR, particularly asymptomatic patients, in whom decision-making regarding surgical-referral timing is complex, yet critical. Accordingly, in patients with moderately severe to severe chronic AR, we sought to analyze the prognostic value of LV volumes (LV end-systolic volume index [LVESVi] and volume-derived LVEF [Vol-LVEF]) and compare it with currently recommended linear indexes, including LVESDi and linear LVEF.
Methods
Study Population and Clinical Data
Detailed study flow is shown in eFigure 1 in the Supplement. Between January 2004 and April 2019, all consecutive patients 18 years or older with moderately severe to severe chronic AR by TTE were retrospectively identified from our electronic echo database. All cases were manually reviewed to ascertain eligibility. Exclusion criteria included no research authorization, any typical symptoms (heart failure symptoms, exertional chest pain, exertional dyspnea, exercise intolerance; patients with atypical symptoms [ie, palpitations, fatigue, dizziness] were not excluded), more than mild mitral regurgitation and/or stenosis, more than mild aortic stenosis, prior mitral/aortic surgery, complex cyanotic congenital heart disease, carcinoid heart disease, acute AR (dissection, trauma, active endocarditis), and those whose image quality prohibited volumetric measurements (eFigure 1 in the Supplement). Non-US residents were excluded because of incomplete follow-up. In addition, patients who underwent aortic valve surgery (AVS) within 2 months of baseline TTE or those who underwent AVS exclusively for aortic aneurysms (surgical decisions not directly related to LV size) were excluded. After exclusions, 492 asymptomatic patients with New York Heart Association functional class I constituted the study cohort (325 patients belong to a previous cohort; eFigure 1 in the Supplement).2 All patients had comprehensive cardiology and/or cardiovascular surgery evaluations within 30 days of TTE. Baseline symptoms, independently recorded by treating physicians, were meticulously abstracted from the electronic medical record. Comorbid conditions recorded during AR consultation were electronically extracted using International Classification of Diseases, Ninth and Tenth Revision codes. Charlson Comorbidity Index (CCI) was calculated. This study was approved by the institutional review board at Mayo Clinic, and informed consent waived because of the retrospective nature of this study.
Echocardiography
In patients with multiple TTEs, the first eligible study was used as baseline for analysis. Transthoracic echocardiography was performed by certificated sonographers and reviewed by cardiologists with level III echocardiography training, using commercially available echo systems. Aortic regurgitation quantitative (effective regurgitant orifice area and regurgitant volume) and semiquantitative measurements (vena contracta and time-velocity integral of descending aorta reversed flow) were performed by an integrated/comprehensive approach according to guidelines.7
Maximal linear LV dimensions were obtained in the parasternal long-axis view choosing largest measurements from basal to mid LV. Left ventricular volume was measured de novo for each patient by guideline-recommended4 disk-summation method on 2-D apical 2- and 4-chamber views (without imaging-enhancing agents) by an observer with 7 years of volumetric-measurement experience (L.-T.Y.); the interface between the compacted myocardium and LV cavity was traced at end-diastole and end-systole in both apical 2- and 4-chamber views, unless only single plane was feasible for measuring nonforeshortened LV volumes. Single plane volumes were included in the analysis to reflect clinical practice (eFigure 1 in the Supplement).
Observer Variability
Intraobserver (assessed more than 1 week apart) and interobserver (L.-T.Y. vs E.I.Z. [board-certified sonographer]) variability in measurements of LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were assessed in 30 randomly selected participants using the same baseline TTE clip with both observers blinded to clinical information and assessed by intraclass correlation coefficient. Before tracing, the 2 observers discussed to ascertain consistency in definitions of LV endocardial border based on guideline suggestions. Also, we compared our de novo measurements with that measured prospectively by multiple sonographers at the time of baseline TTE, available in 186 of 492 patients (37.8%).
Outcomes
Our primary end point was all-cause death8 under medical surveillance. Thus, observation time was between baseline TTE and AVS, death, or last follow-up. Mortality status and last follow-up were retrieved using electronic medical records. For participants not known to be deceased, linkage to mortality was done using Accurint (LexisNexis Risk Solutions), a proprietary resource gathering multiple national sources, on November 30, 2019. Participants who were linked to Accurint and not found to be deceased were censored on May 31, 2019. Secondary end points were all-cause death during entire follow-up (observation time between TTE, death, or last follow-up), AVS, and death plus AVS. We sought to test the previously proposed cutoffs: (1) LVESDi cutoff of 20 mm/m2 derived from both asymptomatic/mildly symptomatic cohort9 and combined symptomatic and asymptomatic cohort2 and (2) LVESVi cutoff of 45 mL/m2 derived from an asymptomatic cohort with preserved LVEF.10
Statistical Analysis
Continuous variables, expressed as mean (SD) or median (interquartile range [IQR]) according to data distribution, were compared using the t test or Wilcoxon rank sum test, as appropriate based on distributional assumptions. Categorical data, presented as count and percentages, were compared using χ2 test. Pearson correlation coefficients were estimated to summarize association between LV parameters. Survival was estimated using Kaplan-Meier method and log-rank statistic. Both primary and secondary end points were analyzed using Cox proportional hazard model under medical surveillance; LV parameters of interest (linear LVEF, LVESVi, LVESDi, Vol-LVEF) were tested in different models to avoid collinearity. To compare 4 abovementioned LV parameters, analyses were restricted in patients having all 4 parameters with adjustment for factors affecting LV remodeling (AR severity, sex, age); C statistics were computed. Expected mortality was estimated based on the mortality of participants in the general population of similar age and sex and compared using the 1-sample log-rank test.11 Penalized smoothing splines were used to illustrate the risk of mortality over the range of the LV parameters compared with the cohort as well as comparing with the general population based on age and sex. Statistical analyses were performed using a combination of commercially available software (JMP version 11 and SAS statistical software version 9.4 [SAS Institute]) and the R software package version 3.4.3 (R Foundation). A 2-sided P value less than .05 was considered statistically significant.
Results
Baseline Characteristics
Baseline characteristics for the entire cohort and based on LVESVi are displayed in Table 1. The final study cohort included 492 patients with AR without typical symptoms (mean [SD] age, 60 [17] years; 425 men [86%]; 181 [37%] with bicuspid AV) with biplane volume acquisition in 438 (89%) by the disk-summation method (eFigure 1 in the Supplement) and 54 (11%) with single plane. In this low-risk cohort, prevalence of ischemic heart disease and the CCI were low; 453 patients (92%) had preserved LVEF (≥50%) and 20 (4.1%) and 23 patients (4.7%) presented with LVESD more than 50 mm and indexed LVESD more than 25 mm/m2, respectively. Of 492 cases, 186 (37%) had LV volumes measured prospectively by certified sonographers at the time of baseline TTE. Patients with LVESVi more than 45 mL/m2 were younger, were more often male, and had more bicuspid AV, lower LVEF, larger LV size, and more severe AR (Table 1).
Table 1. Baseline Clinical and Echocardiographic Characteristics in All Patients (N = 492).
| Characteristic | Mean (SD) | P value | ||
|---|---|---|---|---|
| Total | LVESVi, mL/m2 | |||
| ≤45 (n = 328) | >45 (n = 164) | |||
| Age, y | 60 (17) | 62 (16) | 55 (18) | <.001 |
| Women | 67 (14) | 55 (17) | 12 (7) | .002 |
| Blood pressure, mm Hg | ||||
| Systolic | 131 (20) | 130 (19) | 133 (22) | .22 |
| Diastolic | 66 (13) | 66 (12) | 65 (14) | .17 |
| Resting heart rate, beats/min | 61 (11) | 61 (11) | 62 (10) | .44 |
| Body surface area, m2 | 2.00 (0.23) | 2.00 (0.23) | 2.02 (0.21) | .33 |
| Medical history, No. (%) (n = 491) | ||||
| Hypertension | 228 (46) | 157 (48) | 71 (43) | .30 |
| Diabetes mellitus | 60 (12) | 39 (12) | 21 (13) | .78 |
| Hyperlipidemia | 202 (41) | 151 (46) | 51 (31) | .001 |
| Prior myocardial infarction | 23 (5) | 12 (4) | 11 (7) | .14 |
| Prior coronary artery bypass grafting | 11 (2) | 7 (2) | 4 (2) | .83 |
| Coronary artery disease | 41 (9) | 24 (7) | 17 (10) | .26 |
| Atrial fibrillation at time of echo | 14 (3) | 11 (3) | 3 (2) | .31 |
| Chronic kidney disease ≥stage 3 | 26 (5) | 12 (4) | 14 (9) | .03 |
| Creatinine, mg/dL | 1.1 (0.6) | 1.09 (0.42) | 1.32 (0.85) | .004 |
| Charlson Comorbidity Index | 0.95 (1.16) | 0.96 (1.13) | 0.93 (1.23) | .83 |
| Echo parameters, No. (%) | ||||
| Bicuspid aortic valve | 181 (37) | 113 (35) | 67 (41) | .17 |
| Linear measurements | ||||
| Linear LVEF, %a | 60 (7) | 62 (5) | 56 (9) | <.001 |
| <50, No. (%) | 39 (8) | 6 (2) | 33 (20) | <.001 |
| LVESD, mm | 39 (6) | 37 (4) | 43 (5) | <.001 |
| >50, No. (%) | 20 (4) | 1 (<1) | 19 (12) | <.001 |
| LVESDi, mm/m2 | 19.8 (3.2) | 18.8 (2.6) | 21.7 (3.5) | <.001 |
| >25, No. (%) | 23 (5) | 4 (1) | 19 (12) | <.001 |
| LVEDD, mm | 60 (6) | 58 (5) | 63 (5) | <.001 |
| >65, No. (%) | 71 (14) | 23 (7) | 48 (29) | <.001 |
| Volumetric measurements, No. (%) | ||||
| LVEDV, mL | 201 (57 | 175 (41 | 253 (48 | <.001 |
| LVEDVi, mL/m2 | 100 (26) | 87 (18) | 125 (21) | <.001 |
| LVESV, mL | 82 (31) | 66 (18) | 115 (24) | NA |
| LVESVi, mL/m2 | 41 (15) | 33 (8) | 57 (12) | NA |
| Volume-derived LVEF, %a | 60 (7) | 62 (6) | 54 (7) | <.001 |
| Left atrial volume index, mL/m2 (n = 471) | 39 (13) | 38 (13) | 41 (14) | .03 |
| LV mass index, mL/m2 (n = 457) | 137 (34) | 128 (29) | 155 (36) | <.001 |
| Tricuspid regurgitation velocity >2.8 m/s, No. (%) | 39 (8) | 24 (7) | 15 (9) | .48 |
| E velocity, m/s (n = 469) | 0.7 (0.2) | 0.7 (0.2) | 0.7 (0.2) | .89 |
| E/e’ (n = 461) | 11 (5) | 11 (4) | 11 (5) | .82 |
| Aortic annulus, mm (n = 491) | 26 (3) | 25 (3) | 27 (3) | <.001 |
| Sinus of Valsalva, mm (n = 472) | 40 (6) | 40 (5) | 41 (6) | .49 |
| Mid-ascending aorta, mm (n = 446) | 40 (6) | 40 (6) | 40 (6) | .83 |
| Aortic regurgitation quantification | ||||
| Regurgitant volume, mL (n = 436) | 68 (23) | 66 (19) | 74 (28) | <.001 |
| EROA, mm2 (n = 408) | 27 (9) | 26 (9) | 30 (10) | <.001 |
| TVI of reversed flow in descending aorta, cm (n = 431) | 14.2 (4.2) | 13.3 (3.7) | 15.9 (4.5) | <.001 |
| Vena contracta, mm (n = 326) | 5.9 (1.5) | 5.8 (1.5) | 6.1 (1.6) | .05 |
| Aortic regurgitation severity (severe) | 245 (50) | 137 (42) | 108 (66) | <.001 |
Abbreviations: E/e’, early mitral diastolic velocity to mitral annulus tissue velocity; EROA, effective regurgitant orifice area; i, index; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; LVESV, left ventricular end-systolic volume; NA, not applicable; TVI, time-velocity integral.
SI conversion factor: To convert creatinine to µmol/L, multiply by 88.4.
Linear LVEF <55% and volume-derived LVEF <55% were in 80 patients (16%) and 109 patients (22%), respectively.
Observer Variability
The intraobserver variability in 30 randomly selected cases was 0.98, 0.95, and 0.73 for LVEDV, LVESV, and Vol-LVEF, respectively. The corresponding interobserver variability was 0.97, 0.97, and 0.71, respectively.
Correlation Between TTE Parameters
There was high negative correlation between linear LVEF and LVESD (r = −0.72) and moderate negative correlation between linear LVEF and LVESDi (r = −0.65). There was high negative correlation between Vol-LVEF and LVESVi (r = −0.71). The correlation between LVESD and LVESV was 0.70 (high positive), but after body surface area normalization (between LVESDi and LVESVi), it was 0.56 (moderate) (eFigure 2 in the Supplement). There was moderate positive correlation between linear LVEF and Vol-LVEF (r = 0.64). As for end-diastolic parameters, the correlation between LV end-diastolic dimension and LVEDV was 0.70 (high positive), but after body surface area normalization (between LV end-diastolic dimension index and LVEDV index [LVEDVi]), it was 0.42 (low positive).
There was also moderate to high positive correlation between LV volumes measured prospectively (by multiple sonographers) and de novo in 186 cases (eTable in the Supplement). Finally, as has been reported,4 there was an inverse association between age and LVESVi (eFigure 3 in the Supplement), while LVESDi was not associated with age. These significant correlations advised that it was reasonable to test linear LVEF, Vol-LVEF, LVESVi, and LVESDi in separate multivariable models.
Surgical Indications and Procedures During Follow-up
At a median (IQR) of 5.4 (2.5-10) years of total follow-up, 121 patients (24.6%) received AVS at a median (IQR) of 13.5 (5.7-40.1) months from baseline TTE. Seven patients with AVS (5.7%) had atypical symptoms at baseline that were interpreted by the physician as class I indications and were analyzed as such. Overall surgical indications included class I in 70 patients (57.9%), class II in 32 patients (26.4%), and surgery based on clinical judgment without guideline-based indications in 19 patients (15.7%). Class I indications included symptoms in 56 (46.3%) and LVEF less than 50% in 14 patients (11.6%). Class II indications were LVESD more than 50 mm or LVESDi more than 25 mm/m2 in 6 patients (5.0%) and LV end-diastolic dimension more than 65 mm in 26 patients (21.5%). The mean (SD) 10-year rate of AVS was 32% (3%) (46% [5%] in those with LVESVi >45 mL/m2 and 25% [3%] in those with LVESVi ≤45 mL/m2; P < .001; eFigure 4 in the Supplement).
Survival
As of November 2019, the follow-up rate was 100%. Median (IQR) total follow-up was 5.4 (2.5-10.1) years and up to 15.6 years (>5 years in 256 [52%]), during which 83 patients (16.9%) died. Of these, 66 died under medical surveillance (our primary end point) and 17 died post-AVS (30-day post-AVS mortality, n = 2). Age-adjusted linear LVEF (hazard ratio [HR] per 10%, 0.61; 95% CI, 0.48-0.80; P < .001), Vol-LVEF (HR per 10%, 0.57; 95% CI, 0.44-0.78; P < .001), and LVESVi (HR per 5 mL/m2, 1.11; 95% CI, 1.03-1.19; P < .001) were univariately associated with death under medical surveillance, while age-adjusted LVESDi (HR per 5 mm/m2, 1.42; 95% CI, 0.98-1.99; P = .06) and LVEDVi (HR per 5 mL/m2, 1.03; 95% CI, 0.99-1.08; P = .09) showed a trend. Multivariate models (Table 2) showed that lower linear LVEF, larger LVESDi, larger LVESVi, and lower Vol-LVEF, as continuous variables, were all separately associated with all-cause death with similar discriminative power (similar C statistics and likelihood ratio χ2 for each model were 0.84 [95% CI, 0.80-0.89], 0.83 [95% CI, 0.78-0.88], 0.84 [95% CI, 0.79-0.89], and 0.84 [95% CI, 0.78-0.89], respectively, and 105, 99, 103, and 105, respectively), while LVEDVi was not. Of note, these C statistics represent only a small change from the C statistic of a baseline model of age, sex, AR severity, and CCI (baseline C statistic, 0.83 [0.78-0.88]), yet these LV parameters were determinants of death independently of demographic factors and AR severity (Table 2). To confirm our results, the end point of death during the entire follow-up was tested, and linear LVEF, LVESVi, and Vol-LVEF were associated with death after adjustment for age, sex, AR severity, CCI, and time-dependent AVS, while LVESDi showed a trend (Table 2).
Table 2. Associations Between Left Ventricular Parameters and Death, AVS, or Combined End Points.
| Determinants | Linear LVEF (per 10%) | LVESDi (per 5 mm/m2) | LVESVi (per 5 mL/m2) | Vol-LVEF (per 10%) | LVEDVi (per 5 mL/m2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| All-cause death under medical surveillance (n = 65)a | 0.65 (0.50-0.83) | <.001 | 1.42 (1.01-1.99) | .046 | 1.12 (1.04-1.20) | .002 | 0.61 (0.47-0.80) | <.001 | 1.05 (1.00-1.10) | .06 |
| All-cause death during total follow-up (n = 82)a | 0.65 (0.52-0.81) | <.001 | 1.38 (1.01-1.89) | .05 | 1.09 (1.02-1.17) | .01 | 0.60 (0.47-0.78) | <.001 | 1.02 (0.98-1.07) | .33 |
| Death and AVS (n = 186)a | 0.72 (0.61-0.84) | <.001 | 1.40 (1.16-1.71) | <.001 | 1.10 (1.05-1.15) | <.001 | 0.67 (0.56-0.81) | <.001 | 1.05 (1.02-1.08) | <.001 |
| AVS (n = 121)a | 0.74 (0.59-0.93) | .009 | 1.49 (1.17-1.89) | .001 | 1.10 (1.05-1.17) | <.001 | 0.70 (0.55-0.90) | .004 | 1.05 (1.02-1.09) | .002 |
| Models using previously reported cutoffs for LVESDi and LVESVi (n = 66 deaths under medical surveillance)b | HR (95% CI) | P value | ||||||||
| Model 1: LVESVi >45 mL/m2 vs ≤45 mL/m2 | 1.93 (1.10-3.38) | .02 | ||||||||
| Model 2: LVESDi >20 mm/m2 vs ≤20 mm/m2 | 1.28 (0.78-2.10) | .32 | ||||||||
| Model 3: LVESVi ≤45 mL/m2 and LVESDi ≤20 mm/m2 | 1 [Reference] | NA | ||||||||
| LVESVi >45 mL/m2 and LVESDi >20 mm/m2 | 2.00 (1.04-3.82) | .04 | ||||||||
Abbreviations: AVS, aortic valve surgery; HR, hazard ratio; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESDi, left ventricular end-systolic dimension index; LVESVi, left ventricular end-systolic volume index; NA, not applicable; Vol-LVEF, volume-derived left ventricular ejection fraction.
All analyses restricted to 490 patients (65 deaths under medical surveillance and 82 deaths during entire follow-up) who had all 5 left ventricular parameters. The adjusted (age, sex, Charlson Comorbidity Index, aortic regurgitation severity) HR, 95% CI, and P value for LVESD per 5-mm increase was 1.24 (1.00-1.54) for all-cause death (P = .05), 1.21 (0.99-1.47) for death during total follow-up (P = .06), 1.33 (1.18-1.52) for death plus AVS (P < .001), and 1.48 (1.26-1.74) for AVS (P < .001), respectively.
All models were adjusted for age, sex, Charlson Comorbidity Index, aortic regurgitation severity; for the end point of death during total follow-up, additional adjustment for time-dependent AVS was done.
Spline curves adjusted for age and sex depict the continuous risk of death by parameter. Within-cohort risk started to increase when LVEF or Vol-LVEF were below 60%, LVESVi above 40 to 45 mL/m2, and LVESDi above 21 to 22 mm/m2 (Figure 1). Compared with the general population (Figure 2), the continuous risk of death increased with decreasing linear LVEF and Vol-LVEF and with increasing LVESVi and LVESDi with similar thresholds as the within-cohort analysis. When previously reported2,10 cutoffs for LVESVi and LVESDi were used to risk-stratify patients, those with LVESVi more than 45 mL/m2 demonstrated a 1.9-fold increased risk of death (95% CI, 1.10-3.38; P = .02) (Table 2 and Figure 3A), while those with LVESDi more than 20 mm/m2 did not (95% CI, 0.78-2.10; P = .32). Those with both LVESVi more than 45 mL/m2 and LVESDi more than 20 mm/m2 demonstrated a 2.0-fold increased risk of death (95% CI, 1.04-3.82; P = .04) (Table 2). Compared with the age- and sex-matched general population, this low-risk asymptomatic total cohort had similar survival expected (95% CI, 0.72-1.18; P = .55) (Figure 3B), yet patients with LVESVi more than 45 mL/m2 exhibited a trend toward decreased survival (95% CI, 0.94-2.31; P = .06) vs LVESVi 45 mL/m2 and less (Figure 3C and D). Regarding secondary end points of AVS or death plus AVS, all 5 LV parameters exhibited independent associations (Table 2).
Figure 1. Within-Cohort Mortality Risk Under Medical Surveillance.
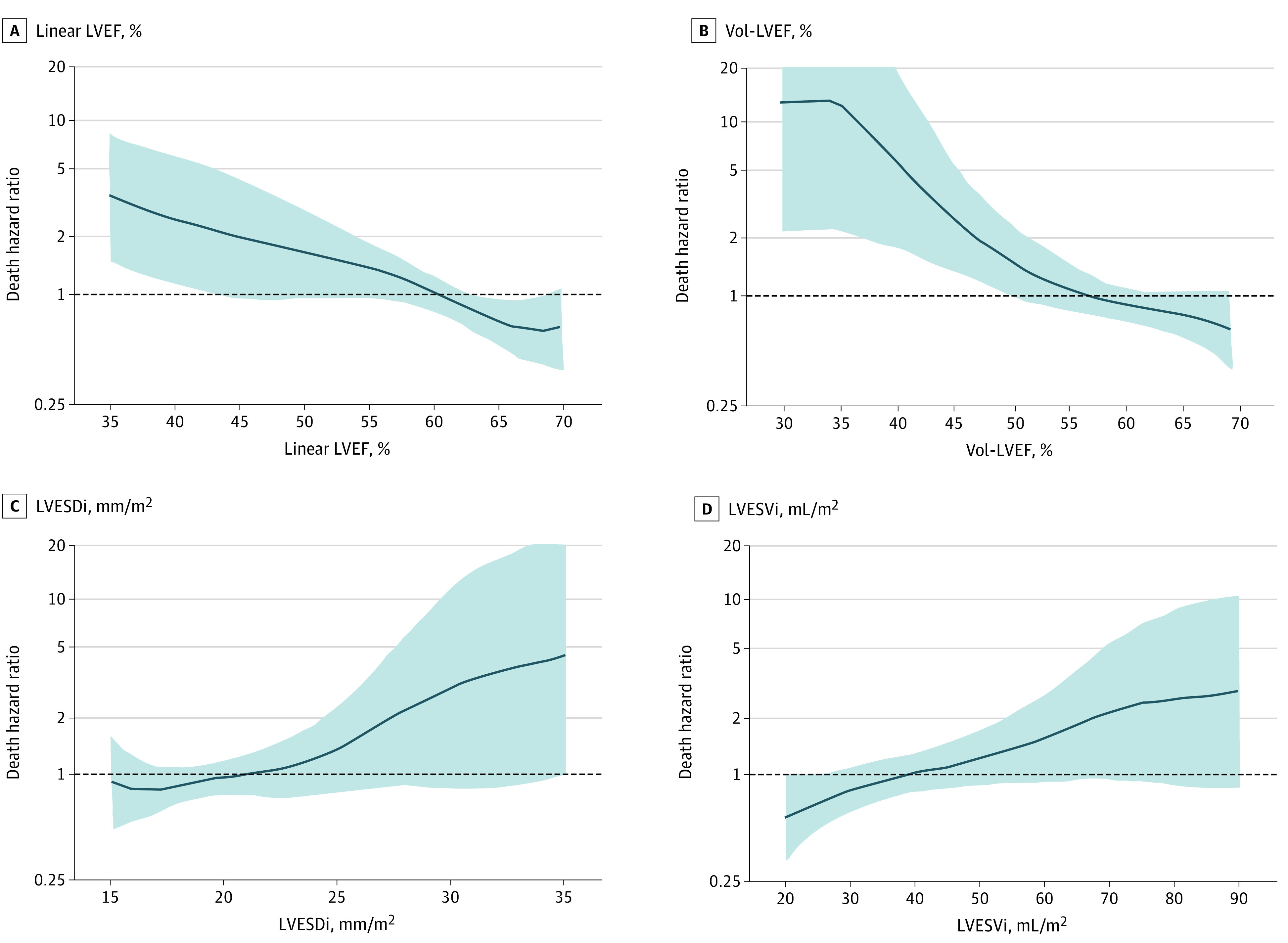
All spline curves were adjusted by age and sex. Risk of death rose with linear left ventricular ejection fraction (LVEF) at <60% (A), volume-derived left ventricular ejection fraction (Vol-LVEF) at <60% (B), left ventricular end-systolic dimension index (LVESDi) between >21 and 22 mm/m2 (C), and left ventricular end-systolic volume index (LVESVi) between >40 and 45 mL/m2 (D).
Figure 2. Mortality Under Medical Surveillance Compared With Population Survival.
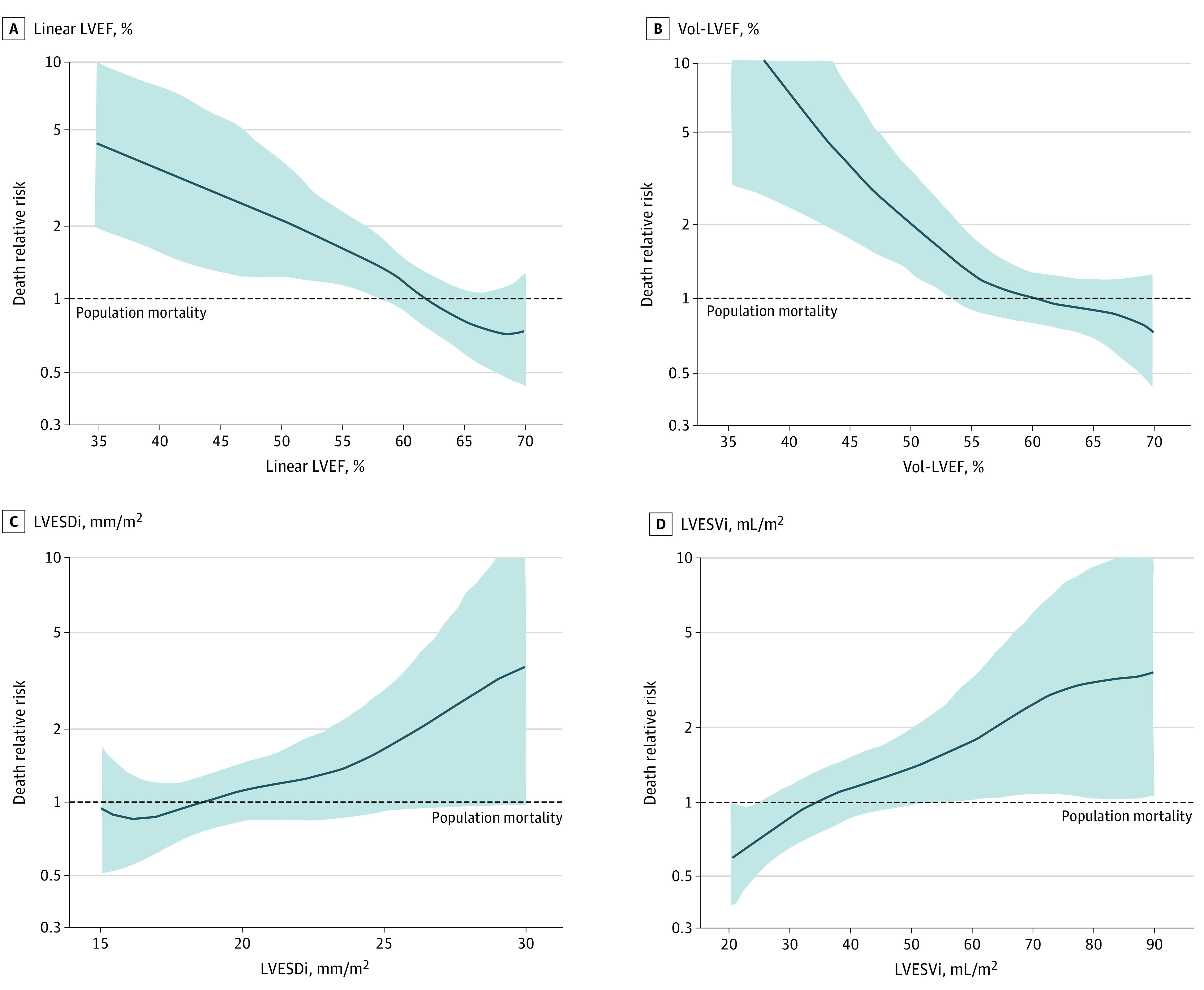
The y-axis of spline curves in each graph represents risks of excess mortality, with risk of 1 line representing age-/sex-matched normal population mortality, and risk >1 indicating excess mortality. Compared with the general population, continuous risk of death increased with decreasing linear left ventricular ejection fraction (LVEF) (A), decreasing volume-derived left ventricular ejection fraction (Vol-LVEF) (B), increasing left ventricular end-systolic dimension index (LVESDi) (C), and increasing left ventricular end-systolic volume index (LVESVi) (D), crossing the risk of 1 line at similar thresholds as within cohort (Figure 1).
Figure 3. Survival Under Medical Surveillance Based on Left Ventricular End-Systolic Volume Index (LVESVi).
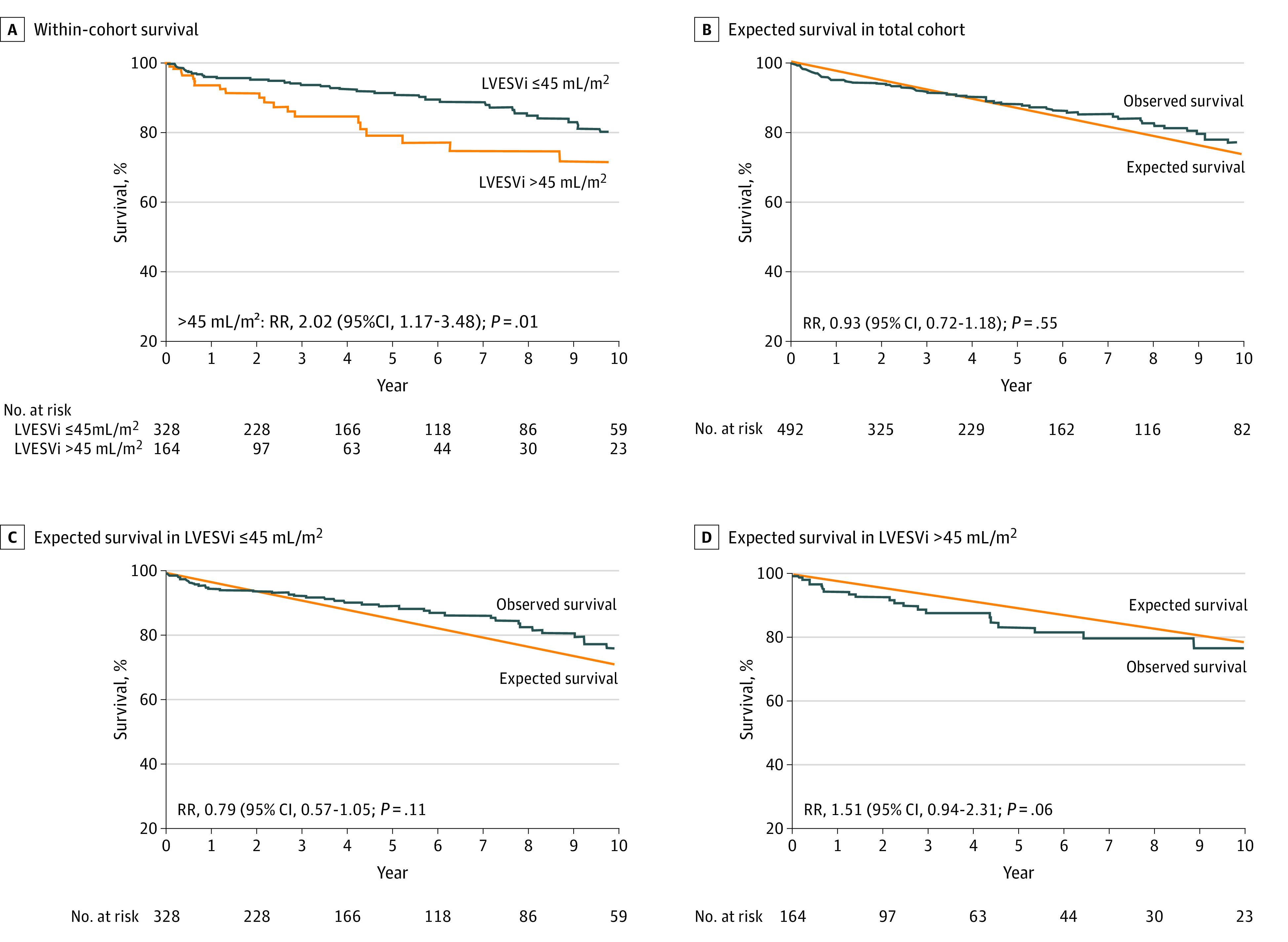
Kaplan-Meier curve adjusted for age and sex showed decreased survival in those with LVESVi >45 mL/m2 (A). The observed survival in the total cohort was similar to population-expected survival (B). Those whose LVESVi was ≤45 mL/m2 had similar observed survival (C), while those with LVESVi >45 mL/m2 showed a trend toward decreased survival compared with the general population (D). HR indicates hazard ratio; RR, relative risk.
Discussion
In this large contemporary cohort of asymptomatic low-risk patients with hemodynamically significant chronic AR, we report the prognostic value of LVESVi and Vol-LVEF. Our principal findings are (1) asymptomatic patients with AR were low risk, with 10-year survival similar to population expected; (2) LVESVi had an inverse association with age while LVESDi had no association with age; (3) besides conventional linear LVEF and LVESDi, LVESVi and Vol-LVEF were independently associated with death under medical surveillance; (4) after adjustment for age and sex, the within-cohort risk of death increased as both linear LVEF and Vol-LVEF decreased below 60%, and as LVESVi increased above 40 to 45 mL/m2 and LVESDi above 21 to 22 mm/m2; (5) compared with the general population, the risk of death increased continuously as both linear LVEF and Vol-LVEF decreased and both LVESDi and LVESVi increased, with similar risk thresholds; and (6) using proposed cutoffs, patients with LVESVi more than 45 mL/m2 exhibited increased relative death risk (HR, 1.93; 95% CI, 1.10-3.38; P = .02), while LVESDi more than 20 mm/m2 did not (95% CI, 0.78-2.10; P = .32).
Previous Cohorts vs Current Cohort
Patients with AR may remain asymptomatic for years, but when symptoms or reduced LVEF (class I indications) develop, post-AVS outcome is worse than those having surgery for non–class I indications.2 Therefore, it is crucial to investigate determinants of death in asymptomatic patients, and although artificial (because risk is a continuum; Figure 1 and Figure 2), identify thresholds for surgical timing in patients not yet attaining class I surgical indications. Previous studies in asymptomatic AR included younger patients (age 36-46 years) and were conducted more than 2 decades ago where surgical referral parameters were developed with linear TTE measurements.12,13,14 Indeed, volumetric determination of LV function parameters was first recommended in 200515 and reinforced in 20154 by the American Society of Echocardiography. A previous prospective study by Detaint et al10 demonstrated that AR quantification and LVESVi 45 mL/m2 or more were associated with adverse cardiac events in those with asymptomatic AR (enrolled between 1991-2003; only 93 with severe AR). Compared with the study by Detaint et al,10 the present study had similar demographics (ie, sex, age) but lower comorbidity index. Likewise, a lower comorbidity index was observed in the current study vs our previous cohort (mean CCI, 0.91 vs 1.6) where we included symptomatic patients at baseline.16 Therefore, we were able to evaluate volumetric-derived parameters in a low-risk asymptomatic AR population where the 10-year survival was similar to that of the general population.
Prognostic Value of LVESVi and Vol-LVEF
Normal ranges of LV volumes decrease as age increases (ie, inverse association) because of poor adaptation to volume load in older patients (eFigure 3 in the Supplement)17 and age, sex, and body surface area have a strong independent association with LV volumes.4 Yet in our cohort, we demonstrate that after adjustment for age, sex, comorbid conditions, and AR severity, LVESVi and its by-product of Vol-LVEF were independently associated with death under medical surveillance. Indeed, LVEF is an indisputable surrogate for a failing heart that combines elements of systolic function and eccentric hypertrophy,18 whereas LVESVi reflects intrinsic contractile function and thus was more sensitive than LVEDVi in prognostication as we showed herein (Table 2). In this study, risks under medical surveillance (adjusted for age and sex) started to rise approximately at less than 60% for both linear LVEF and Vol-LVEF, more than 40 to 45 mL/m2 for LVESVi, and more than 21 to 22 mm/m2 for LVESDi, which is consistent with recent large studies as pertains to linear LVEF and LVESDi,2,9,19 and represents a novel finding as it relates to LVESVi and Vol-LVEF.
LVESDi or LVESVi in Asymptomatic AR?
We showed that all 4 LV parameters were separately associated with death and secondary end points with similar discrimination power as continuous variables (Table 2). Using previously proposed cutoffs,10 the LVESVi of 45 mL/m2 threshold was strongly linked to increased risk of death (Table 2), while LVESDi of 20 mm/m2 was not; therefore, it is possible that LVESVi is superior to LVESDi as a dichotomous threshold for risk prediction, a notion that needs further corroboration in larger studies, ideally prospectively. Nonetheless, LV volume measurements are influenced by age and highly dependent on image quality. Therefore, using both LVESVi and LVESDi in clinical decision-making in asymptomatic patients with AR seems rational.
Future Directions of Volumetric TTE Parameters
It has been reported that LVEF derived from CMR and TTE volumetric measurement is similar,20 and we showed that Vol-LVEF and linear LVEF were also clinically similar (Table 1), but most importantly, they were equally powerful as determinants of death in asymptomatic AR. It is also known that the disk-summation method, which depend on geometric assumptions, image quality, and proper training, underestimate LV volumes against CMR.5 We assume that this would be the case for our cohort if CMR assessment was available, but our key finding remains: the demonstration that LVESV acquisition is feasible and strongly associated with mortality in asymptomatic AR. Although our observer variability was good, the key for future implementation of volumetric parameters is their scalability to routine clinical practice, ie, the retention of the independent prognostic power of LV volumes when performed during routine clinical practice and by multiple observers.21 Continued technological advances in transducers and fully automated software add promise to improve endocardial border delineation by 2-D TTE.6 Our study, although not a validation study of LV volumes against a criterion standard, provides evidence of the feasibility of measurement of LVESVi and its by-product of Vol-LVEF4,15 in asymptomatic patients with AR and demonstrates a robust association between these parameters and mortality.
Limitations
This is a single tertiary referral center retrospective study. The ideal confirmation of asymptomatic status would likely require exercise testing,22 which was not performed in most patients; thus, the asymptomatic status in this study is mostly as reported by patients, yet it represents the scenario we encounter in daily practice. Also, we did not exclude patients with atypical symptoms (7% of our cohort), yet these atypical symptoms could have prompted surgery at the physicians’ discretion. Although we excluded those whose LV volume was unmeasurable, there were only 34 such patients (eFigure 1 in the Supplement). Although most patients referred to our institution will have surgery here and we performed comprehensive detection of surgeries from our surgical database, we cannot completely exclude the possibility that a medical-surveillance patient actually had AVS elsewhere before death. While we excluded patients who underwent AVS within 2 months of echocardiography, 19 patients underwent surgery based on clinical judgment shortly after 2 months (eFigure 4 in the Supplement), which could have influenced our mortality results. Given our low-risk cohort, there were a relatively small number of deaths, which limited the precision of point estimates and made analysis of post-AVS survival impossible. Our results should not be interpreted as suggesting that conventional LV indexes (linear LVEF, LVESDi) are inferior to volume-derived parameters, but rather, that all 4 parameters offer opportunities to identify patients at risk of excess death in a low-risk asymptomatic AR population. Also, we did not use heart failure hospitalization or cardiac death as end points; retrospective analysis of cause-of-death data derived from death certificates is subject to inconsistencies and biases,8 yet we used the most robust patient outcome, all-cause mortality.
Conclusions
In asymptomatic patients with hemodynamically significant chronic AR, LVESVi and Vol-LVEF, as well as conventional TTE linear indexes, demonstrated independent robust associations with all-cause mortality, with risks that began to rise when linear LVEF and Vol-LVEF were lower than 60%, LVESDi higher than 21 to 22 mm/m2, and LVESVi higher than 40 to 45 mL/m2. A LVESVi threshold of 45 mL/m2 was a strong marker of death in this cohort. Our findings indicate that LVESVi and Vol-LVEF could be considered in clinical decision-making algorithms for these patients.
eFigure 1. Study flow
eFigure 2. Correlations between LVESVi and LVESDi
eFigure 3. Correlations between LVESVi, LVESDi and age
eFigure 4. Incidence of surgery
eTable. Comparison between LV volumes measured prospectively and retrospectively
References
- 1.Nishimura RA, Otto CM, Bonow RO, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57-e185. doi: 10.1016/j.jacc.2014.02.536 [DOI] [PubMed] [Google Scholar]
- 2.Yang LT, Michelena HI, Scott CG, et al. Outcomes in chronic hemodynamically significant aortic regurgitation and limitations of current guidelines. J Am Coll Cardiol. 2019;73(14):1741-1752. doi: 10.1016/j.jacc.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 3.Dujardin KS, Enriquez-Sarano M, Rossi A, Bailey KR, Seward JB. Echocardiographic assessment of left ventricular remodeling: are left ventricular diameters suitable tools? J Am Coll Cardiol. 1997;30(6):1534-1541. doi: 10.1016/S0735-1097(97)00329-X [DOI] [PubMed] [Google Scholar]
- 4.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol. 2012;59(20):1799-1808. doi: 10.1016/j.jacc.2012.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata Y, Kado Y, Onoue T, et al. Impact of image quality on reliability of the measurements of left ventricular systolic function and global longitudinal strain in 2D echocardiography. Echo Res Pract. 2018;5(1):27-39. doi: 10.1530/ERP-17-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303-371. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34(3):618-620. doi: 10.1016/S0735-1097(99)00250-8 [DOI] [PubMed] [Google Scholar]
- 9.Mentias A, Feng K, Alashi A, et al. Long-term outcomes in patients with aortic regurgitation and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2016;68(20):2144-2153. doi: 10.1016/j.jacc.2016.08.045 [DOI] [PubMed] [Google Scholar]
- 10.Detaint D, Messika-Zeitoun D, Maalouf J, et al. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. JACC Cardiovasc Imaging. 2008;1(1):1-11. doi: 10.1016/j.jcmg.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 11.Therneau TM, Grambsch PM, Pankratz VS. Technical Report Series No. 66: Penalized Survival Models and Frailty. Department of Health Sciences Research, Mayo Clinic; 2000. [Google Scholar]
- 12.Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84(4):1625-1635. doi: 10.1161/01.CIR.84.4.1625 [DOI] [PubMed] [Google Scholar]
- 13.Borer JS, Hochreiter C, Herrold EM, et al. Prediction of indications for valve replacement among asymptomatic or minimally symptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation. 1998;97(6):525-534. doi: 10.1161/01.CIR.97.6.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tornos MP, Olona M, Permanyer-Miralda G, et al. Clinical outcome of severe asymptomatic chronic aortic regurgitation: a long-term prospective follow-up study. Am Heart J. 1995;130(2):333-339. doi: 10.1016/0002-8703(95)90450-6 [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 16.Yang LT, Pellikka PA, Enriquez-Sarano M, et al. Diastolic blood pressure and heart rate are independently associated with mortality in chronic aortic regurgitation. J Am Coll Cardiol. 2020;75(1):29-39. doi: 10.1016/j.jacc.2019.10.047 [DOI] [PubMed] [Google Scholar]
- 17.Popović ZB, Desai MY, Griffin BP. Decision making with imaging in asymptomatic aortic regurgitation. JACC Cardiovasc Imaging. 2018;11(10):1499-1513. doi: 10.1016/j.jcmg.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 18.Bristow MR, Kao DP, Breathett KK, et al. Structural and functional phenotyping of the failing heart: is the left ventricular ejection fraction obsolete? JACC Heart Fail. 2017;5(11):772-781. doi: 10.1016/j.jchf.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Meester C, Gerber BL, Vancraeynest D, et al. Do guideline-based indications result in an outcome penalty for patients with severe aortic regurgitation? JACC Cardiovasc Imaging. 2019;12(11 pt 1):2126-2138. doi: 10.1016/j.jcmg.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann R, von Bardeleben S, Kasprzak JD, et al. Analysis of regional left ventricular function by cineventriculography, cardiac magnetic resonance imaging, and unenhanced and contrast-enhanced echocardiography: a multicenter comparison of methods. J Am Coll Cardiol. 2006;47(1):121-128. doi: 10.1016/j.jacc.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 21.Antoine C, Benfari G, Michelena HI, et al. Clinical outcome of degenerative mitral regurgitation: critical importance of echocardiographic quantitative assessment in routine practice. Circulation. 2018;138(13):1317-1326. doi: 10.1161/CIRCULATIONAHA.117.033173 [DOI] [PubMed] [Google Scholar]
- 22.Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popović ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines: incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging. 2014;7(2):352-362. doi: 10.1161/CIRCIMAGING.113.001177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study flow
eFigure 2. Correlations between LVESVi and LVESDi
eFigure 3. Correlations between LVESVi, LVESDi and age
eFigure 4. Incidence of surgery
eTable. Comparison between LV volumes measured prospectively and retrospectively


