Abstract
Mammalian sperm must spend a minimum period of time within a female reproductive tract to achieve the capacity to fertilize oocytes. This phenomenon, termed sperm ‘capacitation’, was discovered nearly seven decades ago and opened a window into the complexities of sperm–female interaction. Capacitation is most commonly used to refer to a specific combination of processes that are believed to be widespread in mammals and includes modifications to the sperm plasma membrane, elevation of intracellular cyclic AMP levels, induction of protein tyrosine phosphorylation, increased intracellular Ca2+ levels, hyperactivation of motility, and, eventually, the acrosome reaction. Capacitation is only one example of post-ejaculatory modifications to sperm (PEMS) that are widespread throughout the animal kingdom. Although PEMS are less well studied in non-mammalian taxa, they likely represent the rule rather than the exception in species with internal fertilization. These PEMS are diverse in form and collectively represent the outcome of selection fashioning complex maturational trajectories of sperm that include multiple, sequential phenotypes that are specialized for stage-specific functionality within the female. In many cases, PEMS are critical for sperm to migrate successfully through the female reproductive tract, survive a protracted period of storage, reach the site of fertilization and/or achieve the capacity to fertilize eggs. We predict that PEMS will exhibit widespread phenotypic plasticity mediated by sperm–female interactions. The successful execution of PEMS thus has important implications for variation in fitness and the operation of post-copulatory sexual selection. Furthermore, it may provide a widespread mechanism of reproductive isolation and the maintenance of species boundaries. Despite their possible ubiquity and importance, the investigation of PEMS has been largely descriptive, lacking any phylogenetic consideration with regard to divergence, and there have been no theoretical or empirical investigations of their evolutionary significance. Here, we (i) clarify PEMS-related nomenclature; (ii) address the evolutionary origin, maintenance and divergence in PEMS in the context of the protracted life history of sperm and the complex, selective environment of the female reproductive tract; (iii) describe taxonomically widespread types of PEMS: sperm activation, chemotaxis and the dissociation of sperm conjugates; (iv) review the occurence of PEMS throughout the animal kingdom; (v) consider alternative hypotheses for the adaptive value of PEMS; (vi) speculate on the evolutionary implications of PEMS for genomic architecture, sexual selection, and reproductive isolation; and (vii) suggest fruitful directions for future functional and evolutionary analyses of PEMS.
Keywords: spermatozoa, morphogenesis, capacitation, hyperactivation, motility, seminal proteins, female reproductive tract, post-copulatory sexual selection, sperm competition, fertility
I. INTRODUCTION
Between the 1870s and the 1950s, numerous research programs employed an in vitro approach to the study of fertilization in echinoderms, amphibians and mammals. These studies were fruitful in elucidating, for example, interactions between sperm and eggs, and between sperm and the cumulus cells surrounding the egg in mammals. However, and despite numerous erroneous claims to the contrary (reviewed by Austin, 1951; but see Moricard, 1950 as discussed in Austin, 1951), the goal of achieving fertilization in vitro in a mammal was stymied. The breakthrough came in a pair of studies published by C. R. ‘Bunny’ Austin and Min Chueh Chang in 1951. Working with rabbits and rats, and seemingly inspired by previous investigations of the timing of fertilization relative to insemination (i.e. delays were not attributable to the time required by sperm to reach the site of fertilization, nor for large numbers of sperm to accumulate there; e.g. Hammond, 1934) both Austin (1951) and Chang (1951) showed that sperm must spend some minimum threshold amount of time within the female reproductive tract (FRT) before fertilization can occur. Specifically, sperm introduced into the periovarian sac of the rat or the Fallopian tubes of the rabbit required several hours to lapse before fertilization was observed (Austin, 1951; Chang, 1951). However, fertilization occurred swiftly and efficiently if sperm had first spent 5 h within the uterus of another rabbit (Chang, 1951). Both authors concluded that sperm must be physiologically modified within the FRT in some manner necessary to acquire the capacity to fertilize oocytes. In the following year, Austin (1952) confirmed the results under the condition of natural insemination in rats, and he coined the term ‘capacitation’. Without the discovery of capacitation, the application of in vitro fertilization to assist reproduction by humans experiencing fertility problems (Pacey, 2009) and by threatened and endangered species (Roldan & Gomendio, 2009) may not have been possible (Visconti, 2009).
Capacitation in mammals has subsequently been investigated intensively [see online Supporting Information, Appendix S1 (Section VII.5); Gervasi & Visconti, 2016]. The term is now used widely to refer to a combination of cellular processes that include specific molecular modifications to the sperm plasma membrane, increased Ca2+ permeability, the elevation of intracellular cyclic AMP levels, hyperactivation of motility, the induction of sperm protein tyrosine phosphorylation and, eventually, the acrosome reaction (Vadnais, Galantino-Homer, & Althouse, 2007; Gadella & Boerke, 2016; Gervasi & Visconti, 2016). These modifications occur naturally within the FRT, but can also be induced in vitro. Although capacitation is frequently suggested to be exclusive to mammals, evidence suggests that this phenomenon is more taxonomically widespread (e.g. Nixon et al., 2016a, 2019b). This point depends in part, of course, on the definition of ‘capacitation’. Numerous reports in the literature describe an array of post-ejaculatory modifications to sperm (PEMS) occurring in the FRT in a diversity of internally fertilizing taxa, of which capacitation as described for mammals represents but one example. Because there is no cohesive field of study encompassing such modifications, the descriptions are mostly anecdotal and lack consistent nomenclature. Nevertheless, there is cause to postulate that critical modifications to sperm within the FRT are the rule rather than the exception for nearly all taxa with internal fertilization. Moreover, recent evidence suggests female-induced modifications to sperm, mediated by contact with ovarian/oviductal fluid released with eggs and forming a boundary layer around them, may be widespread among externally fertilizing species as well (Evans & Sherman, 2013). Alterations to sperm form and function resulting from sperm–female interactions may therefore have derived from deeply ancestral processes.
The depth of knowledge about the biology of PEMS varies greatly among taxa, with the highest resolution studies having been directed at capacitation (including hyperactivation) in mammals, the attachment of proteins manufactured by male accessory reproductive glands to the head and/or flagellum of sperm and their cleavage from sperm within the FRT of Drosophila, the shedding of extracellular envelopes by lepidopteran sperm, and the activation of frog and toad sperm by egg jelly. These systems stand out for having been the subject of molecular genetic analyses and, in rare cases, the subject of experimental approaches (e.g. RNA interference (RNAi) knockdown or CRISPR–Cas9 knockouts; e.g. Fricke, Bretman, & Chapman, 2009; Findlay et al., 2014). By contrast, studies of PEMS in other taxa are predominantly descriptive, including ultrastructural, gross morphological or behavioural data (e.g. flagellar beat frequency and swimming trajectory) acquired using transmission electron, scanning electron or light microscopy to compare sperm that have or have not had the opportunity to interact with the FRT. Importantly, studies to discern whether PEMS occur have not been carried out for the overwhelming majority of taxa.
Given that PEMS are potentially widespread and an important determinant of reproductive outcomes, yet in most cases understudied and poorly understood, we had seven goals in crafting this review. First, we clarify PEMS-related nomenclature. Second, we promote a perspective of sperm having a more protracted life history and maturational trajectory than traditionally considered, while highlighting the FRT as the principle selective environment for sperm over the majority of their life history in species with internal fertilization. Third, we describe two taxonomically widespread types of PEMS: sperm activation and the dissociation of sperm conjugates. Fourth, we review the occurrence and diversity of PEMS throughout the animal kingdom. Fifth, we consider alternative hypotheses for the adaptive value of PEMS. Sixth, we address the evolutionary implications of PEMS for genomic architecture, sexual selection and speciation. Finally, we suggest fruitful directions for future functional and evolutionary analyses of PEMS. Whereas our goal was to be exhaustive in reviewing examples of PEMS throughout the animal kingdom, the expansive biology related to PEMS restricted us to providing citations that could serve as entry points to further exploration for numerous, more general aspects of reproductive biology.
II. DEFINING PEMS AND SUGGESTED NOMENCLATURE
In order to facilitate a more cohesive and taxon-independent field of enquiry, we encourage adoption of ‘PEMS’ when referring to biochemical, physiological and/or structural modifications to sperm occuring after ejaculation but excluding modifications to sperm that are attributable to sperm–egg interactions (Karr, Swanson, & Snook, 2009). These modifications may be male-mediated, for example being triggered by non-sperm seminal fluid constituents, or by being intrinsic to sperm (i.e. ‘programmed’ modifications that do not occur until after ejaculation). Alternatively, PEMS may be female-mediated, resulting, for example, from (i) female-derived proteins, carbohydrates or lipids binding to sperm, or female-derived exosomes fusing with sperm (Aalberts, Stout, & Stoorvogel, 2014; Corrigan et al., 2014); (ii) female-mediated cleavage or dissolution of sperm components; and/or (iii) post-translational modifications to sperm proteins. Sperm also undergo changes within the FRT as a consequence of aging, degradation or destruction that has been shown to occur within the FRT across diverse taxa (e.g. Brinton, Burgdorfer & Oliver, 1974; Picard, 1980; Longo et al., 1993; Viscuso et al., 1996; Burighel & Martinucci, 1994a; Sutovsky, 2003; Pizzari et al., 2008). We do not consider such phenomena to be PEMS. Rather, PEMS include those highly regulated and consistently observed phenomena that are necessary for sperm to progress (e.g. within the FRT), survive and/or eventually fertilize ova.
The majority of PEMS will be associated with alterations to sperm behaviour, such as hyperactivation in eutherian mammals and the widespread phenomena of activation and chemotaxis (see Section IV.1). However, one should not conversely presume that all changes to sperm behaviour involve PEMS, since many determinants of sperm behaviour, including flagellar conformation, beat frequency and velocity, can all be influenced by interactions with the environment (e.g. temperature, viscosity, architecture) and hence may arise without biochemical, physiological or structural modifications to sperm (Werner & Simmons, 2008; Yang & Lu, 2011; Lüpold & Pitnick, 2018).
The abbreviation ‘PEMS’ applies equally well to all animals irrespective of their mode of reproduction (e.g. external, internal, spermcasting, direct or indirect spermatophore transfer) and is not restricted to the single, operational criterion of any specific modification being critical to sperm achieving the capacity for fertilization (i.e. capacitation, sensu stricto). This latter attribute is important, because it is hypothetically possible for sperm to have the capacity to fertilize despite failure to undergo some PEMS. Moreover, most investigations, across diverse animal taxa, do not include explicit demonstrations that the modifications to sperm are required for fertilization competency, despite it being evident that most of the examples would meet this criterion. For example, fertilization would not be possible in any species with conjugated sperm or sperm surrounded by a glycocalyx or outer vestment prior to successful execution of the PEMS. In other cases, sperm would not migrate properly to the site of storage/fertilization or experience prolonged survival within the FRT without activation or other PEMS. Hence, demonstrating capacitation, sensu stricto, is unlikely to be a priority for most investigators [but note that a specific PEMS being a necessary prerequisite for fertilization has been shown for some non-mammalian species: e.g. hydrozoa (O’Rand, 1972, 1974); frogs (Shivers & James, 1970a); toads (Krapf et al., 2007, 2009)].
We suggest the canonical adoption of the term ‘capacitation’ to refer to the specific case of PEMS that involves molecular modifications to the sperm plasma membrane, increased Ca2+ permeability, the elevation of intracellular cyclic AMP levels, hyperactivation of motility, the induction of sperm protein tyrosine phosphorylation and, eventually, the acrosome reaction that is widespread in eutherian mammals (e.g. Gervasi & Visconti, 2016; see Section VII 5c in Appendix S1) and has recently been shown also to occur in a crocodile (Nixon et al., 2016a, 2019b; see Section VII.4 in Appendix S1). This suggestion is consistent with the term’s application by many contemporary reproductive biologists, and underlies the commonly proferred opinion that capacitation is a phenomenon predominantly restricted to mammals (e.g. Nixon et al., 2016a, 2019b). This explicitly restrictive use of ‘capacitation’ will avoid confusion that has arisen by some studies applying the term more generally to PEMS. For example, ‘capacitation’ was used to refer to sperm modification in the FRT in taxa including ticks (Oliver & Brinton, 1971), spiders (Brown, 1985), cockroaches (Hughes & Davey, 1969), grasshoppers (Longo et al., 1993), flies (Makielski, 1966), butterflies (Friedländer, Jeshtadi, & Reynolds, 2001), prosobranch snails (Bojat, Sauder, & Haase, 2001), pulmonate snails (Selmi, Bigliardi, & Giusti, 1989), octopuses (Tosti et al., 2001) and frogs (Shivers & James, 1970a). The inconsistent application of ‘capacitation’ may explain why no general term for the phenomenon of PEMS is applied in most relevant studies on non-mammalian species, whereas other studies use expressions such as “capacitation-like”, “reminiscent of capacitation”, “sperm reaction” and “sperm conditioning”. Finally, some PEMS have been described for mammal species that occur independently of, or in addition to, the traditionally described modifications associated with capacitation (e.g. rotation of the sperm head into the ‘thumbtack’ or ‘T’ orientation in Australian marsupials and the dissociation of sperm pairs in New World marsupials or the conjugatated sperm of some flying squirrels, rodents and primates; Monclus & Fornes, 2016). Additional mammalian PEMS undoubtedly remain to be discovered, and delineating which qualify as capacitation is unlikely to advance a general understanding of the phenomena of sperm modification. One goal of this review is to demonstrate that capacitation in mammals is simply one example of a much larger phenomenon common to most, if not all, animals with internal fertilization (and some with external fertilization) and to encourage a more cohesive field of investigation into the molecular, cellular and evolutionary biology of PEMS.
III. ELEMENTS OF THE LIFE HISTORY OF SPERM
(1). Sperm maturation rarely ends in the testes
Spermatogenesis is the origin and development of spermatozoa from germ cells. The post-meiotic portion of this process, spermiogenesis, is defined as the morphogenesis of haploid, round spermatids into spermatozoa within the testes (Gilbert & Barresi, 2016). It is widely recognized that sperm may become modified or complete maturation after leaving the testes. In mammals, the epididymides are specialized for sperm modification, with proteins, glycoproteins and RNA potentially being added, lost and modified and the lipid component of membranes being altered as sperm pass through epididymides of monotremes (e.g. Djakiew & Jones, 1983; Nixon et al., 2011, 2016b), marsupials (e.g. Temple-Smith & Bedford, 1980) and eutherian mammals (e.g. Bedford, 1979; Baker et al., 2005; Sullivan & Saez, 2013; Aalberts, Stout, & Stoorvogel, 2014; Skerget et al., 2015; Machtinger, Laurent, & Baccarelli, 2016; Sharma et al., 2018; Nixon et al., 2019a). Although less thoroughly studied, similar changes to sperm may occur within the Wolffian duct of reptiles (e.g. Esponda & Bedford, 1987) and birds (e.g. Esponda & Bedford, 1985; Morris et al., 1987; Nixon et al., 2014) and the seminal vesicles of insects (e.g. Riemann & Giebultowicz, 1992; T. L. Karr, personal communication). For insects, sperm may be additionally modified further downstream in the ejaculatory duct as they are combined with secretions from the male accessory reproductive glands and other secretory organs (see Section V.3h in Appendix S1). Finally, as evidenced by descriptions of PEMS in diverse taxa (see Section V), the final steps in sperm maturation for many species take place within the female. The precise nature of sperm maturation processes occurring in males and females is expected to be evolutionarily dynamic as they are largely determined by sperm–female interactions, which are themselves expected to evolve rapidly (Pitnick, Wolfner, & Suarez, 2009b).
Among all cell types present in metazoan taxa, sperm have a truly unique biology. They are the only cells that are cast forth from the soma to spend their lives in a ‘foreign’ environment. In the case of species with internal fertilization, the ‘free-living’ portion of a sperm’s life takes place inside the FRT and can last for hours, days, months or years. A robust understanding of the biology of PEMS thus requires explicit consideration of the protracted life history of sperm from a behavioural ecology perspective. How are sperm designed to maximize their survival as they navigate a spatially and temporally heterogeneous selective environment? Resolving structure–function relationships from an evolutionary perspective requires examination of fitness variation in the context of the underlying mechanisms at play within the selective environment (or an appropriate proxy), and these criteria have rarely been met in studies of spermatozoa (Lüpold & Pitnick, 2018).
Given the protracted life history of sperm, and the fitness consequences of properly executing PEMS and otherwise being properly designed for compatibility with the FRT, there are several reasons why it might be adaptive for sperm to complete their maturation within the FRT. First, the function of some PEMS may be to deliver male-derived materials to the female and, hence, sperm changes associated with delivery cannot be completed until sperm have reached the proper place and time within the FRT (see Section VI.6). Second, sperm must perform numerous functions within the FRT, and there may be no single optimal design serving all functions (see Section VI for a full discussion). Selection may have shaped the maturational trajectory of sperm to match their functional life history, as it has with evolution of the soma. Further, it would be advantageous to coordinate the timing of PEMS with critical events occurring within the FRT, and hence for sperm to use FRT characteristics as proximate triggers for the modifications. Third, because of FRT variation within species (e.g. Lüpold et al., 2013), it may be adaptive for sperm to delay their maturation until they are in the FRT and then exhibit some plasticity to conform to the specific biochemical, physiological and/or morphogical FRT conditions in which they find themselves. Adaptive plasticity in sperm form and function has been demonstrated for diverse taxa, including insects, ascidians, fish and birds (e.g. Pizzari et al., 2003; Rudolfsen et al., 2006; Thomas & Simmons, 2007; Crean & Marshall, 2008; Ota et al., 2010), albeit not explicitly with regard to PEMS.
(2). The female reproductive tract is a complex, interactive and selective environment
There is tremendous variation among species in female reproductive ecology, remating behaviour and FRT morphology, physiology and biochemistry (Eberhard, 1996; Pitnick, Wolfner, & Suarez, 2009b; Orr & Brennan, 2015; McDonough et al., 2016). There is also growing evidence of extensive within-population genetic variation in FRT traits that influence sperm performance and fate (Lüpold et al., 2012, 2016) and that such traits diversify rapidly (e.g. Simmons & Fitzpatrick, 2019). As a consequence, the FRT may generate diversifying selection on sperm, including PEMS. For example, one of the most robust patterns in comparative reproductive biology is the co-diversification of sperm and FRT morphology, as observed in diverse taxa including several families of flies and beetles, moths, snails, frogs, birds and mammals (reviewed in Pitnick, Wolfner, & Suarez, 2009b). In fact, among species of diving beetles (Dytiscidae), the evolutionary remodelling of different components of the FRT explains a significant amount of the variation in sperm length, sperm-head shape, the presence or absence of conjugation, and conjugate size and length (Higginson et al., 2012b).
The selective forces underlying widespread sperm–FRT co-diversification are not well understood. Each sex-specific trait can theoretically generate selection on the other (i.e. coevolution) as a consequence of both natural/ecological selection (Reinhardt, Dobler, & Abbott, 2015b) and post-copulatory sexual selection (Birkhead, Møller, & Sutherland, 1993; Keller & Reeve, 1995; Eberhard, 1996; Yasui, 1997; Snook, 2005; Pitnick, Wolfner, & Suarez, 2009b; Orr & Brennan, 2015; Firman et al., 2017; Lüpold & Pitnick, 2018). There is also some empirical evidence, albeit limited, suggesting that FRT design may evolve first, with sperm form then evolutionarily tracking such changes (i.e. compensatory evolution; Miller & Pitnick, 2002; Higginson et al., 2012b). Regardless, virtually all such selection is expected to be mediated by FRT traits (morphological, cellular, biochemical and immune) that interact directly with the ejaculate to influence the migration of sperm, their maintenance and modification, and their relative competitiveness for fertilization. Because the secretory biology of the FRT has historically been understudied and generally is poorly characterized and understood, the specific mechanisms underlying the majority of these interactions have yet to be determined. Fortunately, interest in the molecular and functional biology of FRT secretions is rapidly growing and, thanks to recent advances in proteomics and transcriptomics, our understanding of sperm–FRT interactions is expanding (McDonough et al., 2016).
Taxonomically diverse examples of sperm–female interactions critical to sperm performance and survival within the FRT are being revealed. Genetic manipulation of the secretory cells of the spermathecae and parovaria in Drosophila melanogaster supports the importance of FRT secretions for fertility, sperm storage and normal ovulation (Anderson, 1945; Allen & Spradling, 2008; Schnakenberg, Matias, & Siegal, 2011). In both the honeybee, Apis mellifera, and in the boll weevil, Anthonomus grandis, secretions of the spermathecal gland have similarly been shown to contribute to sperm activation and their continued motility (Koeniger, 1970; Ruttner & Koeniger, 1971; Villavaso, 1975). The sperm of A. mellifera can survive for decades within the FRT, and recent proteomic analyses have revealed that spermathecal fluid contains a large, integrated network of proteins that includes enzymes of energy metabolism and antioxidant defence (Baer et al., 2009a, 2009b; Poland et al., 2011). Ovarian fluid in a fish with internal fertilization, the guppy Poecilia reticulata, has been shown experimentally to reduce the temporal decline in sperm viability (Gasparini & Evans, 2013). In the Chinese soft-shelled turtle, Pelodiscus sinensis, spermatogenesis is seasonal and, following spermiation, sperm spend many months within the male epididymis and the female oviduct, respectively (Zhang et al., 2008). The epithelial cells of both tissues have distinctive secretory functions that are believed to contribute to the protection and nourishment of sperm (Han et al., 2008; Bian et al., 2013). In mammals, the complex epithelial folds, channels, microgrooves and mucous of the FRT create a highly selective environment through which sperm must navigate, significantly reducing the population of sperm that enter the oviduct from the uterotubal junction (Coy et al., 2012; Holt & Fazeli, 2015, 2016a; Tung et al., 2015).
The proteomics and transcriptomics of the mammalian oviduct microenvironment has revealed anatomic regions with distinct, hormonally regulated molecular profiles (Buhi, Alvarez, & Kouba, 2000). Recent transcriptome studies of the oviduct in the pig and human have established the sensitivity of oviduct epithelial cells and secretions to respond differentially to the presence of sperm (Alminana et al., 2014; Artemenko et al., 2015). The oviductal epithelium of eutherian mammals also plays an important role in sperm storage and capacitation, including hyperactive motility (Coy et al., 2012). Interestingly, proteins involved in oviduct–sperm binding, carbohydrates in the apical cells of the epithelium and glycosylated proteins in the sperm head, all exhibit pronounced variation among species, suggesting species specificity in the biochemistry of this ejaculate–female interaction (Suarez, 2008; Talevi & Gualtieri, 2010). Finally, across taxa as diverse as polychaete worms, scale insects, mites and ticks, crustacea, clams, snails, ascidians, frogs, snakes, birds and eutherian mammals, the epithelium of the FRT has been observed to interact directly with sperm through sperm binding or embedding (reviewed in Pitnick, Wolfner, & Suarez, 2009b).
Recognizing this protracted life history of sperm with maturation spanning both sexes, we predict molecular and biochemical continuity between the male reproductive tract (MRT) and the FRT, which should be manifest in patterns of sex- and organ-specific gene expression. We further predict that such continuity will be evolutionarily dynamic with variation across taxa correlated with diversification in the extent of PEMS. We develop this concept and its genomic consequences in greater detail below (see Section VII.1).
IV. TAXONOMICALLY WIDESPREAD FORMS OF PEMS
In addition to capacitation by eutherian mammal sperm, other specific classes of PEMS that are generally well known include sperm activation, chemotaxis, and the dissociation of sperm conjugates. Because these phenomena have been widely investigated and the subject of previous reviews, we only briefly describe them below before proceeding to detailed descriptions of the myriad, lesser-known and often taxonomically restricted forms of PEMS (Section V). Note that in the taxon-specific descriptions of PEMS (see Appendix S1), we mention sperm activation and dissociation of conjugates when they co-occur with other forms of PEMS. However, we have excluded taxonomic groups from Section V for which the only known PEMS are those associated with sperm activation, chemotaxis and conjugate dissociation.
(1). Sperm activation and chemotaxis
For many internally and externally fertilizing species, sperm activation (i.e. the acquisition of full motility) is only achieved after spawning/ejaculation, whereas sperm within the male reproductive tract are observed to be immotile or only weakly motile. The cell signalling mechanisms underlying sperm activation have been the subject of intense investigation and numerous reviews (e.g. Ward & Kopf, 1993; Darszon et al., 1999; Morisawa & Yoshida, 2005; Miller et al., 2016; Tosti & Ménézo, 2016) and so will only be briefly addressed here. Motility is triggered by the binding of ligands to sperm receptors and/or the opening or closing of ion channels. Exposure of sperm to a variety of cations or to changes in osmotic pressure following dilution in fresh water, salt water or within the environment of the FRT have been shown to initiate motility across diverse taxa. Sperm chemokinesis (metabolism and motility) and chemotaxis, which describes changes in sperm flagella waveform (and hence swimming path) in order to move up a chemoattractant gradient, may be further stimulated by molecular signals (e.g. small peptides and other molecules) that are released from unfertilized eggs, the FRT epithelium or found in ovarian fluid or jelly surrounding eggs and then bind to receptors on the sperm’s surface (Miller, 1985; Ward & Kopf, 1993; Eisenbach, 1999; Morisawa & Yoshida, 2005; Eisenbach & Giojalas, 2006; Watanabe et al., 2010; Evans & Sherman, 2013).
Perhaps less well known are the myriad examples of sperm motility being initiated by more dramatic structural PEMS following insemination. For example, in spiders, many insects and some crustacea, sperm do not become motile within the FRT until a rigid outer sheath, coat or glycocalyx has been removed (e.g. Alberti, 1990, 2000; Lupetti, Mercati, & Dallai, 2001; Friedländer, Seth, & Reynolds, 2005; Matzke-Karasz, Smith, & Heb, 2017), or until an accessory membrane has been degraded to permit the flagellum to unkink or uncoil (Dallai, 1972; Dallai et al., 2003, 2004). In the fungus gnat, Sciara coprophila, sperm activation is associated with the evacuation of a large portion of the mitochondrial derivative (Makielski, 1966; Phillips, 1966a, 1966b). The sperm of many tick species must undergo a dramatic metamorphosis and elongation within the FRT before motility is possible (Oliver & Brinton, 1971; Brinton, Burgdorfer, & Oliver Jr., 1974). These examples are described in Appendix S1.
(2). Dissociation of sperm conjugates
Sperm conjugation refers to the phenomenon of inseminated sperm being physically bound to one another (reviewed by Immler, 2008; Pitnick, Hosken, & Birkhead, 2009a; Higginson & Pitnick, 2011; Monclus & Fornes, 2016). Conjugation can be primary, with all ‘sibling’ descendants of each spermatogonium remaining attached to one another rather than dissociating at the end of spermatogenesis. Alternatvely, conjugation can be secondary, with sperm individualizing within the testes and later, within the epididymides, seminal vesicles or the FRT, binding together in a species-specific manner (Higginson & Pitnick, 2011). Conjugation has arisen independently numerous times across diverse taxa, including annelid and polychaete worms, gastropod molluscs, myriapods, spiders, insects, and monotreme, marsupial and placental mammals (Immler, 2008; Pitnick, Hosken, & Birkhead, 2009a; Higginson & Pitnick, 2011; Higginson et al., 2012a; Monclus & Fornes, 2016). It is manifested in diverse ways (e.g. Fig. 1), from paired sperm (e.g. nearly all species of New World opossum; Biggers & Creed, 1962; Moore & Taggart, 1995) to loosely formed sperm trains involving up to hundreds of sperm (e.g. some species of muroid rodent; Immler et al., 2007) to conjugates comprising thousands of sperm that possess a nearly crystalline exactness in their structural organization (e.g. the diving beetle Hygrotus sayi; Higginson & Pitnick, 2011). Ultrastructural analyses have further revealed a diversity of cellular, extracellular and mechanical mechanisms by which conjugation is achieved (reviewed by Afzelius & Dallai, 1987; Hayashi, 1997; Higginson & Pitnick, 2011; Monclus & Fornes, 2016).
Fig. 1.

Sperm conjugates of the whirligig beetle, Dineutus sp.: (A) conjugates from the male ejaculatory duct under differential interference contrast microscopy; (B) conjugates stained with 4′,6-diamidino-2-phenylindole (DAPI) to show the organization of sperm heads along the spermatostyle; (C) conjugate from the female spermatheca in the process of sperm dissociation; (D) bundle of spermless spermatostyles from the spermatheca of a wild-caught female. Photomicrographs by S. Pitnick.
Whereas the adaptive value of conjugation is unknown in most cases, it often operationally facilitates social cooperation among sperm for the purpose of movement through the FRT (reviewed by Immler, 2008; Pizzari & Foster, 2008; Higginson & Pitnick, 2011). For all taxa with sperm conjugation, the dissociation of conjugates prior to fertilization clearly represents a dramatic example of structural PEMS. Such dissociation rarely occurs prior to conjugates arriving in the female’s sperm-storage organ(s), and in many cases only after prolonged storage or immediately prior to fertilization (Rodger & Bedford, 1982; Higginson & Pitnick, 2011). The mechanisms by which sperm within conjugates dissociate from one another are generally unknown and postulated to involve an active female secretion (Higginson & Pitnick, 2011). Nevertheless, the only identified candidate (in the moth Bombyx mori) is a product of the male’s ejaculatory duct (Osanai, Kasuga, & Aigaki, 1989a; Aigaki et al., 1994; Osanai & Isono, 1997).
V. A SURVEY OF PEMS THROUGHOUT THE KINGDOM ANIMALIA
The occurrence of PEMS has been convincingly demonstrated for a multitude of diverse taxa (described in detail in Appendix S1), including hydras, bryozoans, clams (see Fig. 2C, D), snails, octopuses (see Fig. 3), polychaete worms (see Fig. 2A, B), ticks (see Fig. 4), spiders (see Fig. 5), crustaceans, insects [e.g. springtails (see Fig. 6), jumping bristletails (see Fig. 7), grasshoppers, cockroaches, beetles (see Fig. 1), honeybees, butterfles and flies (see Figs 8 and 9)], tunicates (see Fig. 10), fish, salamanders, frogs and toads, turtles (see Fig. 11), crocodiles, birds, monotremes, marsupials (see Fig. 12) and placental mammals. It is important to note that, among the taxon-specific PEMS described in Appendix S1, there is tremendous variation in the extent to which systems have been investigated and in the experimental tools employed. Consequently, we have a relatively sophisticated understanding of the cellular and molecular mechanisms underlying PEMS in model systems such as eutherian mammals (i.e. mouse, rat, rabbit and human) and the fruit fly Drosophila melanogaster. By contrast, our understanding of PEMS for the majority of taxa is restricted to what can be inferred from ultrastructural comparisons between sperm obtained from the MRT and FRT. In the descriptions provided (see Appendix S1), we have striven to be explicit about methods and to share authors’ conclusions and interpretations of their findings. We describe more generalized, taxon-specific aspects of the reproductive biology whenever it was deemed necessary to understand the described PEMS.
Fig. 2.
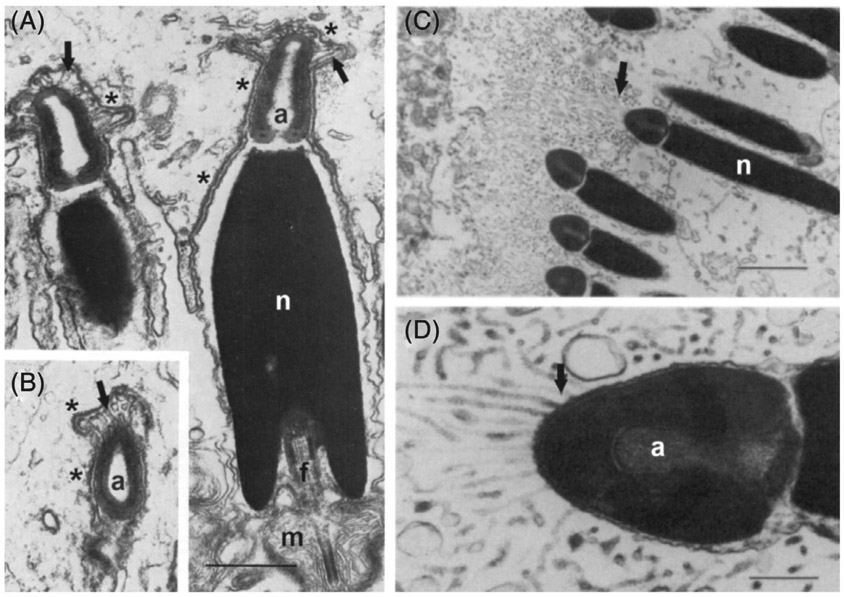
Tranmission electron micrographs showing post-ejaculatory modifications to sperm (PEMS) in the form of digitate processes (examples indicated by arrows) formed from the sperm periacrosomal plasmalemma in order to strengthen contact with (A, B) the female spermathecal cell membrane in the polychaete worm, Spirorbis spirorbis, and (C, D) the female gill filament in the brooding clam, Mysella tumida. a, acrosome; f, flagellum; m, mitochondrion; n, nucleus; *, specialized contacts between sperm and spermathecal cell membranes with scalariform junctions. Adopted with permission from (A, B) Daly & Golding (1977); (C, D) Ó Foighil (1985b). Scale bars: A, 0.5 μm; C, 2 μm; D, 0.4 μm.
Fig. 3.

Scanning electron micrographs of Octopus vulgaris spermatozoon collected from (A) the spermatophore, with an intact outer membrane covering the acrosome, and (B) from the female oviducal gland, following post-ejaculatory modifications to sperm (PEMS) to reveal the corkscrew-shaped acrosome. Inset in A shows magnification of the acrosomal region. Arrows indicate indentations separating the acrosome, nuclear and midpiece regions. Adopted with permission from Tosti et al. (2001). Scale bars: A, 3.0 μm; B, 2.0 μm.
Fig. 4.

Schematic diagram of different morphological stages of post-ejaculatory modifications to sperm (PEMS) of the tick, Amblyomma dissimili. mp, motile processes. Adopted with permission from Reger (1963).
Fig. 5.
Sperm of the spider Caponina alegre (A–D) before and (E) after post-ejaculatory modifications to sperm (PEMS). (A–D) Reconstruction of a synspermium. The image stack used for the three-dimensional reconstruction is stored in MorphDBase (https://www.morphdbase.de?P_Michalik_20120927-M-3.1). (A) Numerous membrane-bound vesicles are attached to the vesicular area, enclosing the spermatozoa. (B) The main cell components of the four fused spermatozoa are coiled within the vesicular area of the syncytium, but not twisted around each other. (C) The prominent extremely elongated nucleus of one spermatozoon is coiled 2.5 times around the centre of the syncytium into which the axoneme finally opens. (D) Cross-section through a synspermium showing the arrangement of the coiled sperm components in the periphery of the syncytium, leaving the centre only filled with the vesicular area. (E) Schematic drawing of the main components of a post-PEMS, mature spermatozoon. AC, acrosomal complex (acrosomal vacuole and acrosomal filament); AF, acrosomal filament; Ax, axoneme; IF, implantation fossa; peN, post-centriolar elongation of nucleus; prcN, precentriolar region of nucleus. Adopted with permission from Lipke & Michalik (2012).
Fig. 6.

(A) Scanning electron micrograph (SEM) of two rolled spermatozoa showing the long extra-acrosomal structure (‘peduncle’) of the collembolan Allacma fusca. Inset, SEM of a single rolled sperm showing the acrosome and peduncle. (B) Schematic reconstruction of a spermatozoon of Orchesella villosa. Sperm components form several spires within the same plasma membrane surrounding material within an ‘extracellular’ cavity. A, acrosome; EAS, extra-acrosomal structure; Ex, extracellular cavity; sp, spermatozoon. Adopted with permission from (A) Fanciulli et al. (2017); (B) Dallai et al. (2004).
Fig. 7.
Schematic drawing of a spermatozoon of the jumping bristletail, Machilis distincta, from the female spermatheca but prior to post-ejaculatory modifications to sperm (PEMS). Adopted with permission from Dallai (1972).
Fig. 8.

(A) Schematic of a ‘mature’ sperm from the testis (top) and the female spermatheca 2 days after insemination (bottom) in the fungus gnat, Sciara coprophila. Discontinuities in the diagrams indicate that the cell is much longer relative to the width than depicted. (B) Changes undergone by the axial filament complex during storage in the female reproductive tract. (C, D) Transmission electron micrograph of transverse section through the subnuclear portion of a ‘mature’ sperm from (C) the male testis and (D) the female spermatheca. A, acrosome; AF, axial filament complex; B, dense body; MC, mitochondrial crystalloid; MH, mitochondrial homogeneous material; N, nucleus. Adopted with permission from (A, B, D) Phillips (1966a); (C) Dallai, Bernini, & Giusti (1973).
Fig. 9.
A model of molecular post-ejaculatory modifications to sperm (PEMS) in Drosophila melanogaster. A network of seminal proteins is required for sex peptide (SP) to bind stably to sperm within the female seminal receptacle. Coloured shapes indicate proteins produced in the male accessory glands. CG1652 and CG1656 require fellow network proteins CG9997 and Antr to be transferred to females. Once deposited in females, Sems and CG17575 are required for SP and CG1656 to localize to the seminal receptacle (SR), the major site of female sperm storage. In the SR, SP and CG1656 bind sperm within 2 h of the start of mating. Also, within the female reproductive tract (FRT), the presence of CG1652 and CG1656 slows the rate at which CG9997 is processed from a 45 kDa form to a 36 kDa form. One additional network protein, Intrepid, is not shown, since its position in the pathway is presently unknown. Loss of any one of these network proteins prevents SP accumulation on sperm in the SR. Following the events shown, the SP C-terminus is cleaved from stored sperm over time. Colours indicate predicted protein functional classes: red/ orange/yellow are serine proteases and protease homologs; pink/purple are cysteine-rich secretory proteins; green are C-type lectins. Adopted with permission from Singh et al. (2018).
Fig. 10.

Schematic drawing illustrating post-ejaculatory modifications to sperm (PEMS) of the tunicate, Diplosoma listerianum. Head of a spermatozoon from the male’s sperm duct (left) and from the female’s ovarian fertilization canal (right). et, endoplasmic tubules; fl, flagellum; m, mitochondrion; dg, dense groove. Adopted with permission from Burighel & Martinucci (1994a).
Fig. 11.

Schematic illustrating post-ejaculatory modifications to the head of the spermatozoon of the Chinese soft-shelled turtle, Pelodiscus sinensis. Adopted with permission from Zhang et al. (2015).
Fig. 12.

Differential interference contrast micrographs of (A, B) many sperm stored within the deep portion of an isthmic crypt, and of (C, D) a single sperm released from storage of a female of the dasyurid marsupial, Sminthopsis crassicaudata, mated about 24–26 h previously. (A, C) The female is preovulatory and all sperm are spear shaped, with the anterior midpiece of the tail lying within lateral folds of the head; (B, D) the female is post-ovulatory and all sperm are T-shaped with the head angulated or perpendicular to the tail (the flagellum in panel D was oscillating and so appears blurred). Adopted with permission from Bedford & Breed (1994).
Several general conclusions can be drawn from this survey (Appendix S1). First, mammals can exhibit multiple types of PEMS, meaning modifications in addition to the well-studied capacitation. Second, modifications to the plasma membrane of sperm and those related to the acrosome reaction are by no means restricted to mammals. Third, some forms of PEMS, such as conjugate dissociation are taxonomically widespread (albeit they may be relatively rare, being found in relatively few species within a taxon; Higginson & Pitnick, 2011). Fourth, insects as a group are especially diverse with regard to the different forms of PEMS exhibited. Fifth, some forms of PEMS are particularly rare, including the attachment of seminal fluid proteins to sperm or the release of sperm-bound material into the FRT. It is important to recognize that such rarity may strictly be a function of limited investigation of these phenomena because they are difficult to observe and to study.
VI. HYPOTHESES FOR THE ADAPTIVE SIGNIFICANCE OF PEMS
Our understanding of variation in PEMS across taxa is too incomplete to draw conclusions about its evolutionary diversification. Despite an extensive literature on capacitation in mammals, our knowledge of its comparative biology is scant (but see Fan, Lefebvre, & Manjunath, 2006; Lefebvre et al., 2007). Because there is variation among mammal species in temporal and mechanistic aspects of the formation of the sperm reservoir, sperm longevity, and in sperm–egg interactions during fertilization (Holt & Fazeli, 2016b), there may be correlated variation among species in the timing and molecular mechanisms of capacitation. In general, theory would predict that PEMS diverge rapidly. Reproductive traits, especially those involved in male–female interactions, tend to evolve quickly (e.g. Swanson & Vacquier, 2002; Haerty et al., 2007), post-copulatory sexual selection is known to be a powerful agent of diversification (Simmons, 2001; Pitnick & Hosken, 2010), and sperm and FRT traits are notorious for evolving rapidly (Pitnick et al., 2009a, 2009b; Simmons & Fitzpatrick, 2019). Congeneric species have been demonstrated to have diverged in sperm–FRT interactions in the case of internal fertilization (Howard et al., 2009; Manier et al., 2013a, 2013b, 2013c), and in sperm–ovarian fluid interactions in the case of external fertilization (Yeates et al., 2013) that critically determine reproductive outcomes. Further, there can be high heritability of both sperm traits and FRT traits that determine sperm handling (Simmons & Moore, 2009; Lüpold et al., 2012, 2013, 2016), and there is even adaptive, within-population variation in sperm–ovarian fluid interactions pertaining to chemoattraction in echinoderms and molluscs (Evans & Sherman, 2013).
The broad survey of PEMS in diverse taxa presented in Section V does not reveal rates and patterns of diversification. It does, however, suggest that PEMS may have arisen independently numerous times throughout the animal kingdom. Modifications to sperm structure and physiology vary tremendously, as well as in the degree and nature of interaction(s) with the FRT that induce the modifications (albeit female contributions are largely unknown). It is thus likely that PEMS have arisen in response to a diversity of evolutionary selection pressures, and the nature of selection underlying the evolutionary maintenance of PEMS may vary among related species. In considering alternative hypotheses to explain the evolutionary origin and maintenance of species-specific PEMS, it is helpful to recognize that, for internally fertilizing species, sperm perform multiple pre-fertilization actions within the female. Between insemination and fertilization, sperm must successfully migrate and/or be transported to specialized sperm-storage organs [e.g. the spermatheca(e) and/or seminal receptacle] or a site of quasi-specialized, short-term storage (e.g. the sperm reservoir in mammals), successfully compete with competitor sperm for a position within the sperm-storage site and/or to engage in fertilization, survive and remain viable in storage for a period lasting from hours to decades, and exit the storage site and migrate to the site of fertilization at the proper time, all before interacting with an oocyte to form a zygote. Additionally, sperm (or seminal fluid) components may provide material support in the form of nutrients that may increase the number of eggs produced, egg size, or otherwise enhance egg defence or embryonic viability (Simmons & Parker, 1989; Gwynne, 2008). Sperm (and/or seminal fluid) may also provide signals influencing a multitude of female physiological functions that impact, for example, oogenesis, ovulation, immune function, feeding and remating (Ravi Ram et al., 2005; Poiani, 2006; Avila et al., 2011). These functions have been shown (or are expected) to involve some degree of ejaculate-female interaction (Ravi Ram & Wolfner, 2007; Pitnick, Wolfner, & Suarez, 2009b) and may be associated with or reliant upon PEMS.
PEMS should be expected to arise if ‘one size does not fit all’ regarding the optimal design of sperm for the execution of all of the above activities. Selection shapes the development of organisms such that phenotypes change throughout an organism’s life history (e.g. insect holometabolism). When optimal sperm design differs for different functions, selection can respond in three different ways. First, it can favour a single, best compromise phenotype (a ‘jack of all trades and master of none’), which could be achievable in the male reproductive tract. Second, it can favour heteromorphic spermatogenesis within the male, with a division of labour among sperm types, each specialized to perform different functions within the female (Swallow & Wilkinson, 2002; Till-Bottraud et al., 2005). Third, selection can fashion a trajectory that includes multiple, sequential phenotypes that are specialized for stage-specific functionality (i.e. PEMS). To the extent that these are alternative evolutionary outcomes, we predict comparative studies to reveal fewer PEMS in species with sperm heteromorphism. But note that, as demonstrated by some species of Lepidoptera, the second and third strategies can co-occur (see Section V.3g in Appendix S1).
Note that none of the following nine hypotheses are mutually exclusive, and that multiple selection pressures may shape PEMS in any given species. In fact, the known biology of many PEMS is consistent with the predictions of multiple of the hypotheses described below. However, we are unaware of any experimental tests of any of these hypotheses, or of comparative tests of hypotheses that contrast the reproductive biology of related species that differ in the presence or form of PEMS.
(1). H1: economy of sperm transfer
One hypothesis for the origin of PEMS is that males can transfer many more sperm per copulation if a substantive portion of the growth component of sperm morphogenesis occurs post-insemination. This hypothesis, proposed by Brinton, Burgdorfer, & Oliver Jr. (1974), is consistent with the PEMS of some tick species, where sperm increase in size up to tenfold within the FRT (Fig. 4; e.g. Mothes & Seitz, 1981). Enhancing efficiency of sperm transfer has also been postulated as an explanation for sperm conjugation (Dallai & Afzelius, 1985; Afzelius & Dallai, 1987). This hypothesis is unlikely to serve as a general explanation, however, as we are not aware of any other taxa in which PEMS involve increases in sperm size, and it is an unlikely explanation for sperm conjugation (Higginson & Pitnick, 2011). We are also skeptical of this selective explanation for tick PEMS, as it is typically the storage capacity of females that is limiting rather than the number of sperm transferred. Hypothesis 1 predicts that, for taxa with PEMS involving an increase in sperm size or the tight packaging of sperm for transfer, there will be a negative association between the expression of PEMS and the degree of female-biased sexual size dimorphism (as small male size may limit investment per ejaculate).
Another variation of Hypothesis 1, proposed by Matzke-Karasz, Smith, & Heb (2017) as a possible adaptive explanation for the PEMS of ostracod crustacea (i.e. shedding of an outer, fibrous coat) is enhanced organization of transferred and stored sperm in the case of giant sperm and a small FRT.
(2). H2: protecting sperm from stress during transfer and storage
Another hypothesis for PEMS relates to the fact that sperm may be subject to considerable stress, both physical (i.e. shearing forces) during ejaculation and chemical (e.g. reactive oxygen species) during storage in the FRT. Transport through certain regions of the FRT may also be highly selective of sperm due to physical barriers, chemical barriers and leukocytic/phagocytotic responses to copulation (Birkhead, Møller, & Sutherland, 1993; Arnqvist & Rowe, 2005; Suarez, 2006). Due to the often lengthy interval between insemination and fertilization (Birkhead & Møller, 1993; Neubaum & Wolfner, 1999; Orr & Brennan, 2015; Holt & Fazeli, 2016b), sperm may further be subject to oxidative damage in the FRT (Reinhardt et al., 2015a, 2015b). Sperm are largely transcriptionally quiescent and thus unable to deploy a full repertoire of repair mechanisms to respond to these stresses (Dorus & Karr, 2009). If the optimal design of sperm for transfer, transport and/or storage differs from that for fertilization, then selection may have favoured PEMS. Throughout their life history within the FRT, sperm may have to modify attributes that enhance their survival during the early stages in the reproductive process in order to achieve and retain the capacity to fertilize. Hypothesis 2 is generally supported by a diversity of PEMS, including delayed activation of motility, the rigid glycocalyx of many insect sperm, the encapsulation of spider sperm, and thickened periacrosomal membranes that limit premature acrosomal reactions and capacitation in mammals.
(3). H3: aiding sperm reaching a critical location in the FRT
Independent of protection from stressors (H2), PEMS may be hypothesized as adaptations to enhance sperm transport. Two well-studied PEMS support this hypothesis. First, hyperactivation in eutherian mammals has been interpreted as an adaptation to assist sperm in their release from the oviductal sperm reservoir and movement through mucous secretions in reaching the oocyte (Suarez & Pacey, 2006). The second example is sperm conjugation and their dissociation after reaching the site of sperm storage or fertilization (Higginson & Pitnick, 2011). Based on general hydrodynamic and biomechanic principles, conjugation is predicted to enhance sperm motility because it increases force generation with proportionately less drag (e.g. Woolley et al., 2009). It should be noted, however, that only a few studies have quantified the motility of sperm conjugates and these have resulted in inconclusive or mixed results (reviewed by Higginson & Pitnick, 2011).
(4). H4: aiding sperm to remain in a critical location in the FRT
In virtually all species with internal fertilization, sperm must be properly stored within a specialized organ or region of the FRT in order eventually to have an opportunity to encounter an oocyte. Moreover, in many cases sperm competition between males predominantly distills down to competition to occupy limited sperm-storage space within the FRT (e.g. Miller & Pitnick, 2002; Pattarini et al., 2006), and such selection can drive the evolution of extreme sperm traits (Lüpold et al., 2016). That PEMS are important for this is supported by the numerous PEMS that appear to enhance sperm storage, in some cases through fusing with, binding to, or embedding in the epithelial cells of the FRT. Examples include loss of most of the acrosomal membrane from the sperm of the octopus, O. vulgaris, to expose a screw-shaped acrosome, thus permitting sperm to drill into the epithelial cells of the spermatheca (Froesch & Marthy, 1975; Tosti et al., 2001), growth of long slender digitations from the periacrosomal plasma membrane from sperm after embedding in epithelial cells in the gastropod snail, C. montanum (Giusti & Selmi, 1985) and the polychaete worms, S. spirorbis (Daly & Golding, 1977) and P. remota (Alikunhi, 1951; Westheide, 1988), growth of fine, thread-like extensions of the periacrosomal plasmalemma of sperm in the clam, M. tumida, that aid in attachment to the female’s gills (Ó Foighil, 1985b), and adaptations for binding to the oviduct epithelium to form the sperm reservoir in eutherian mammals (Suarez, 2002).
(5). H5: enhancing sperm longevity
As discussed in Section III.2, females of most taxa have specialized organs for sperm storage with associated secretory cells or glands that provide an environment conducive to the long-term viability of sperm. As a consequence, sperm can survive within the FRT from days to decades, depending on the species. The strength of selection for sperm longevity, and hence any associated PEMS that may extend longevity, will depend upon the sperm-storage capacity of females, rate of sperm use (which depends on fecundity and sperm use efficiency), female remating interval, and the mechanisms of sperm competition (see Section VII.2). Putative examples of PEMS that may have been selected to enhance sperm longevity include those associated with sperm remaining in either an inactive or a reduced metabolic state. For example, the sperm of spiders remain coiled and inactive within capsules for prolonged periods within the spermathecae (e.g. Brown, 1985). Note that any species for which PEMS include sperm activating shortly after ejaculation or upon reaching the sperm-storage organs do not support this hypothesis. Any cases of sperm transitioning to an increased flagellar beat frequency may support this hypothesis. If a higher active state is required to fertilize an egg successfully, or to compete for fertilization, then remaining in a state of reduced activity until the right time might be an adaptation to enhance sperm longevity.
(6). H6: delivering male-derived materials to the female in a temporally and/or spatially critical manner
Seminal fluid is biochemically complex and rapidly evolving, as natural and sexual selection can drive ejaculate evolution to serve a multitude of functions. It provides direct material support for sperm and contributes to the FRT environment (and the female as a whole) to facilitate sperm motility and survival. Seminal fluid proteins (SFPs) and other constituents including small molecules, and exosomes or related vesicles, may also provision females with nutrients or hormones used to make eggs or for the female’s own somatic maintenance, and they can modify female gene expression, physiology and (in insects, at least) behaviour in myriad ways that may help or harm the female (Simmons & Parker, 1989; Pitnick, Spicer, & Markow, 1997; Wolfner, 1997; Markow, Coppola, & Watts, 2001; McGraw et al., 2004; Arnqvist & Rowe, 2005; Poiani, 2006; Gwynne, 2008; Avila et al., 2011; Baldini et al., 2013; Aalberts, Stout, & Stoorvogel, 2014; Bromfield et al., 2014; Corrigan et al., 2014; Sirot et al., 2015; Droge-Young et al., 2016).
There may be temporal or functional constraints, however, on the efficacy of molecules within seminal plasma. It seems likely that many may have local effects in the FRT after transfer either because they may not be transported from the site of insemination to the location within the female where they can best function or because they are processed, degraded, or ejected relatively rapidly (notable exceptions include several members of the sex peptide pathway in Drosophila; Peng et al., 2005; Singh et al., 2018). One solution to this problem is to incorporate the molecules into sperm or bind them to the sperm for regulated transport/release/cleavage (i.e. PEMS) within the FRT.
Another potential problem, arising in the case of costly nutritive donations, is that males run the risk of having their mate incorporate the material into eggs that are then fertilized by another male’s sperm (Markow, 1988). One solution to this problem would be to provision sperm with the material for delivery to the oocyte upon fertilization, although such modifications could hamper sperm motility, competitiveness or ability to fertilize. However, we are aware of only a single investigation to test this hypothesis, which did not support it. Karr & Pitnick (1996) examined the amount of spermic material entering the oocyte in 12 species of Drosophila, exhibiting sperm lengths ranging from 36 to 58,290 μm. Whereas the entire sperm enters the oocyte in most of the species, they found that only a small fraction of the sperm enters the oocyte in multiple, independent lineages that have evolved giant sperm. Thus, at least in Drosophila, larger sperm do not appear to have evolved due to selection for post-fertilization provisioning (Karr & Pitnick, 1996). Alternatively, the nutritive material could be incorporated into sperm and then released within the female’s spermatheca(e), which would at least require that a female stores a male’s sperm (i.e. does not eject the sperm from the FRT; e.g. Manier et al., 2010) in order to secure the donation.
Several of the PEMS described in Section V provide general support for Hypothesis 6. First, this explanation was suggested by Dallai et al. (2004) to explain the bizarre arrangement of collembolan sperm, with the flagellum coiled around a central, extracellular cavity containing testicular secretions, and the PEMS occurring inside the spermatheca releasing the secretions (Fig. 6). A second possible example is provided by the PEMS of the fungus gnat, S. coprophila, during which nearly all of the non-paracrystalline component of the mitochondrial derivative (comprising about 50% of the sperm’s volume) is released into the spermatheca (Fig. 8; Makielski, 1966; Phillips, 1966a, 1966b). A third example may be the spermatostyles of some whirligig and carabid beetles. Whereas these substantive rods have only been interpreted as a proximate mechanism facilitating sperm conjugation (e.g. Higginson & Pitnick, 2011), the correct evolutionary interpretation may be that sperm conjugation was favoured so that sperm could cooperate in the delivery of the rods to the female’s spermatheca (Fig. 1). Finally, the best support for the hypothesis that PEMS result from selection on sperm as delivery vehicles comes from investigations of D. melanogaster. For example, of the estimated 200 SFPs that are transferred to females during insemination in D. melanogaster (Findlay et al., 2008; Findlay, MacCoss, & Swanson, 2009; Avila et al., 2011), sex peptide and several other SFPs have been seen to bind to sperm (Fig. 9; Peng et al., 2005; Ravi Ram & Wolfner, 2009; Singh et al., 2018; E. Whittington, A. Singh, S. Pitnick, M. F. Wolfner & S. Dorus, unpublished data) although their retention times on sperm differ.
(7). H7: priming sperm for extragenic contributions to early embryogenesis
Inherent in several of the previous hypotheses is the notion that adaptations specific to sperm transfer, storage or survival may not be conducive to fertilization, leading to the evolution of additional sperm phenotype modifcations prior to encountering an oocyte. A related consideration is that some aspects of the pre-fertilization sperm phenotype may be detrimental to post-fertilization zygote viability. The entire sperm enters the oocyte during fertilization in most animal species (Karr, 1996; Karr & Pitnick, 1996; Krawetz, 2005; Karr, Swanson, & Snook, 2009), with the structure derived from the flagellum being of considerable dimensions and highly persistent throughout early embryogenensis in some taxa (Karr, 1991; Pitnick & Karr, 1998). Some PEMS may have arisen to eliminate sperm proteins or organelles that would be harmful to the zygote or otherwise impede early development (Sutovsky & Song, 2017). Conversely, some PEMS may represent females providing substances to sperm that are beneficial to fertilization or early embryogenesis.
(8). H8: female assessment of sperm quality
Another hypothesis for the existence of PEMS is that they could have arisen through selection on females to enhance zygote viability and fitness through ‘sperm choice’ (Birkhead, 1998). According to this hypothesis, females modify sperm as a mechanism to distinguish high- from low-quality sperm. Those sperm that were able successfully to undergo PEMS, or in the most timely manner, would successfully traverse the FRT and participate in fertilization, whereas unmodified sperm would not (perhaps via a targeted degradation mechanism). According to the ‘good sperm’ model (Yasui, 1997), females accrue indirect genetic benefits through positive covariation between male genetic condition and sperm quality (in this case, their ability to transform properly). Alternatively, the ‘genetic compatibility’ model suggests that females evolve mechanisms to discriminate among sperm based on the compatibility of their haplotypes with the female genome (Jennions, 1997; Neff & Pitcher, 2005).
The best evidence in support of Hypothesis 8 comes from studies of differential chemoattraction of sperm by eggs in the externally fertilizing marine mussel, Mytilus galloprovincialis. Using an elegant method to quantify variation in female-induced acrosome reaction and sperm surface glycan modifications (Kekäläinen et al., 2015), Kekäläinen & Evans (2016) show that the extent to which both processes occur depends upon specific male–female interactions. Further, variation in the response of sperm to chemoattractant cues from different female egg clutches has been shown in M. galloprovincialis to correlate with both fertilization rates (Evans et al., 2012) and offspring fitness (Oliver & Evans, 2014). This example may represent a more widespread phenomenon of sexual selection co-opting self-incompatibility systems to facilitate fertilization by genetically compatible gametes (Kao & McCubbin, 1996; Swanson & Vacquier, 2002; Gillingham et al., 2009; Palumbi, 2009; Evans & Sherman, 2013). With regard to internal fertilization, the FRT is immunologically highly active, particularly after mating and ejaculate transfer (Wira et al., 2005). Moreover, sperm are replete with proteins that function in immunity, and it has been suggested that immunity systems may provide a means for discerning among respective gametes (Dorus, Skerget, & Karr, 2012). It is certainly plausible that sexual selection could co-opt self-incompatibility or immunological systems for use in discriminating among sperm according to other axes of quality (Birkhead, Møller, & Sutherland, 1993; Eberhard, 1996; Holt & Fazeli, 2016a).
In vivo experiments support the ‘good sperm’ over the ‘genetic compatibility’ hypothesis to explain the motility-related PEMS of leaf-cutter ants. It is ancestral to all ant species that queens only mate during the single day of their nuptial flight, after which they have the potential to live for several decades and to produce thousands to millions of offspring (den Boer, Baer, & Dreier, 2009a; den Boer, Boomsma, & Baer, 2009b). Despite the potentially long interval between insemination and fertilization, A. colombica queens have been shown to fertilize close to 100% of their eggs (den Boer, Baer, & Dreier, 2009a). Given this unique biology, and because many more sperm are transferred to females than they are capable of storing in their spermatheca, it was postulated that females have a mechanism of selectively storing only sperm with high viability (Liberti, Baer, & Boomsma, 2016). The greater than 50% enhancement in sperm swimming performance observed in these ants following exposure to FRT extract is a PEMS that was interpreted as a mechanism of sperm choice (Liberti, Baer, & Boomsma, 2016). Notably, this mechanism did not discriminate between sperm of brothers and unrelated males (relative to the queen; Liberti, Baer, & Boomsma, 2016).
VII. EVOLUTIONARY IMPLICATIONS OF PEMS
(1). Genomic consequences of PEMS
The unique biology of sperm, whose prolonged life history includes PEMS in the FRT, requires physiological and biochemical continuity across the sexes. This predicts an evolutionarily dynamic relationship between gene expression within and between the male and female reproductive systems. For example, the extension of mammalian sperm maturation to include the dynamic processes that occur in the epididymis would be expected to require the co-option and deployment of genes ancestrally restricted to the testis (as well as the evolution of entirely new genes). As the duration of sperm maturation evolutionarily expands (to include more extensive PEMS) and contracts (e.g. sperm maturation completed within the male), we predict correlated patterns of sex-biased gene expression evolution amongst spermiogenesis/PEMS-related loci. We note that sex-biased gene expression evolves rapidly, particularly for male-biased genes (Haerty et al., 2007; Zhang et al., 2007; Harrison et al., 2015). An informative example of this involves the creation of a novel SFP (a serine endopeptidase) through the duplication of an existing secreted FRT gene (Sirot et al., 2014). Molecular analyses across seven Drosophila species indicated a switch in sex-biased expression of one of the duplicates from the ancestral pattern of FRT expression to the male accessory gland (Sirot et al., 2014). We further propose that secreted reproductive proteins may be particularly amenable to expressional switches between tissues and/or sexes because they have relatively limited functional constraints within their source tissue and are already evolutionarily optimized to function in the extracellular milieu of the FRT (regardless of whether they are expressed in males or females). Another common feature of the life history of sperm in males and females is the extensive remodelling of the plasma membrane, including changes in protein composition, post-translational protein modification and biochemistry (such as the removal of sterols prior to capacitation). It is easy to envision that the occurence of these modifications (and the underlying mechanisms upon which they rely) could switch, in a bidirectional manner, between the sexes over evolutionary time. Similar scenarios could also pertain to sperm metabolism, including the intrinsic metabolic capacity conferred by males relative to the contribution of female-derived factors to sustain or promote metabolic processes in the FRT.
(2). PEMS and sexual selection
Any traits that impact fertilization success will be subject to strong selection. It is clear from the descriptions above that PEMS are likely to contribute to successful fertility in many species. Because females of most species mate with multiple males within a reproductive cycle (Taylor, Price, & Wedell, 2014), post-copulatory sexual selection is pervasive (Parker, 1970; Simmons, 2001), including sexual selection generated by conflict between the sexes over paternity (Parker, 1979; Arnqvist & Rowe, 2005). Post-copulatory sexual selection is often credited as the principal agent responsible for the rapid evolutionary diversification of seminal fluid and ovarian fluid composition, sperm morphology and FRT design (Lahnsteiner, Weismann, & Patzner, 1995; Snook, 2005; Poiani, 2006; Pitnick et al., 2009a, 2009b). Relatively few studies have quantified genetic variation in ejaculate and FRT traits (Simmons & Moore, 2009), but those that have revealed substantial within-population genetic variation in sex-specific components known to contribute to variation in competitive fertilization success (e.g. Chow, Wolfner, & Clark, 2010; Lüpold et al., 2012, 2013; Zhang, Clark, & Fiumera, 2013).
PEMS may similarly prove to be a widespread contributor to post-copulatory sexual selection on males and females. However, to our knowledge there have been no experimental tests of this postulate. There is limited knowledge of the mechanisms by which sperm and constituents of seminal fluid interact with the female and, hence, of the actual targets of post-copulatory sexual selection. Post-mating interactions are notoriously cryptic and likely to be mediated predominantly by molecular interactions between the sexes (Eberhard, 1996; Pitnick, Wolfner, & Suarez, 2009b; McDonough et al., 2016; Firman et al., 2017; Lüpold & Pitnick, 2018). Indeed, there is a growing paradigm in sexual selection theory that emphasizes the contribution of male × female interactions to critical reproductive outcomes (e.g. Clark, Begun, & Prout, 1999; Clark, Dermitzakis, & Civetta, 2000; Tregenza & Wedell, 2000; Nilsson, Fricke, & Arnqvist, 2003; Oh & Badyaev, 2006; Bjork et al., 2007; Ravi Ram & Wolfner, 2007; Chow, Wolfner, & Clark, 2010; Evans & Sherman, 2013; Lüpold et al., 2016; Suarez, 2016).
Failure to modify sperm properly could be a general and widespread mechanism by which females discriminate against the sperm of less-preferred males, as has been shown to be the case with sperm chemoattraction (Kekäläinen & Evans, 2016) and with female sperm ejection and sperm digestion (Manier et al., 2013b; Firman et al., 2017). Similarly, two kinds of studies implicate a role for ovarian fluid in mediating sexual selection within populations. First, ovarian fluid has been shown to affect differentially the behaviour of sperm from males exhibiting alternative reproductive tactics (Alonzo, Stiver, & Marsh-Rollo, 2016; Butts et al., 2017; Lehnert et al., 2017a, 2017b). Second, the chemical composition of ovarian fluid differs among females within populations (Rosengrave et al., 2008; Johnson et al., 2014), with the influence of ovarian fluid on sperm behavior being significantly influenced by female identity, male identity and female × male interaction (Rosengrave et al., 2008). Establishing whether these modifications to sperm following their exposure to ovarian fluid constitute PEMS awaits identification of molecular mechanisms underlying the interactions. However, Gasparini & Pilastro (2011) present experimental data for the guppy, Poecilia reticulata, suggesting that a female preference for genetically unrelated males is mediated by PEMS that influence sperm velocity. Finally, selection on males would favour traits that reduce the proper execution of PEMS by competitor sperm (i.e. resulting from subsequent mating by the female). Such selection has also been postulated to explain mate guarding in the golden-orb-weaving spider, Nephila clavipes (Brown, 1985).
(3). PEMS and reproductive isolation
The rapid diversification of interacting sex-specific traits by sexual selection is believed to be a widespread agent of reproductive isolation, and hence to play an important role in the formation of new species and the maintenance of species boundaries (Coyne & Orr, 2004; Ritchie, 2007; Kraaijeveld, Kraaijeveld-Smit & Mann, 2011). Whereas both empirical and theoretical studies have overwhelmingly addressed either pre-copulatory or post-zygotic isolating mechanisms, there is growing recognition of the potential importance of post-mating/pre-zygotic (PMPZ) reproductive isolation resulting from reproductive incompatibilities or fertilization biases that occur between the start of copulation and successful karyogamy (Coyne & Orr, 2004; Howard et al., 2009; McDonough et al., 2016). In fact, this may be the only kind of isolation restricting gene flow in some species (e.g. Howard et al., 1998; Ahmed-Braimah & McAllister, 2012). Moreover, its occurrence is taxonomically widespread, having been documented in internal fertilizers, broadcast spawners, and plants (as ‘conspecific pollen precedence’).
Identification of the mechanisms that restrict gene flow is a central and long-standing goal of speciation research (Dobzhansky, 1937; Mayr, 1942). Although the causes of PMPZ reproductive isolation are unknown for most systems, recent progress with two model systems, fruit flies and crickets, suggest that underlying mechanisms will tend to be multivariate and multifarious (e.g. Manier et al., 2013a, 2013b, 2013c; Tyler et al., 2013, Avila et al., 2011). To our knowledge, the only study to date that has explicitly investigated a possible role of PEMS in reproductive isolation has been with the congeneric fish, Salmo salar and S. trutta. Ovarian fluid composition is highly divergent in fish, exhibiting substantial differences among species (Lahnsteiner, Weismann, & Patzner, 1995) and even between members of different geographic populations within species (Beirão et al., 2015). Studies comparing ovarian fluid-induced modification to sperm exposed to ovarian fluid derived from the either the same or different populations or congeneric species provide strong support for the conclusion that ovarian fluid composition and interacting sperm traits are evolutionarily co-diversifying (Yeates et al., 2013; Beirão et al., 2015; Devigili et al., 2018). Moreover, Yeates et al. (2013) demonstrated that such co-diversification results in species-specific PEMS (i.e. modifications to sperm flagellar beat and swimming trajectory) that limit the likelihood of hybrid fertilization. We predict that sperm and FRT traits involved in PEMS will tend to coevolve rapidly. If true, then PEMS may represent a powerful and possibly widespread mechanism of PMPZ reproductive isolation. The failure to execute PEMS successfully could restrict the ability of sperm to be stored, to experience sustained longevity and/or to achieve the capacity to fertilize. An illustrative example of this is the rapid diversification and species specificity of the sperm glycosylation system found in amphibian egg jelly (Coppin, Maes, & Strecker, 2002).
VIII. FUTURE DIRECTIONS
Based on our extensive survey of the literature, PEMS appear to be widespread throughout the animal kingdom and there is strong support in well-studied systems that they serve as important determinants of reproductive outcomes. As such, PEMS warrant much wider experimental attention. We propose three investigative goals to advance our understanding of the importance of this general phenomenon. First, comparative analyses of PEMS across strategically selected taxonomic groups and populations are needed to assess the evolution of PEMS. Second, the application of complementary techniques to link the structural and molecular bases of PEMS is required. Last, and most challenging, will be the functional characterization of PEMS, including elucidating male–female interactions that are required for the induction or mediation of PEMS.
There is much to be gained from investigating PEMS in related species to facilitate comparative analyses within a phylogenetic framework (e.g. Adams, 2014; Fuentes-G et al., 2016). With the exception of sperm capacitation studies in mammalian models, few comparative data relating to PEMS are available despite their importance to establishing patterns of PEMS macroevolution. For example, hypotheses about the adaptive diversification in PEMS could be tested by examining the direction and rate variation of phenotypic divergence in PEMS-related characters throughout clades relative to discrete transitions in traits putatively generating selection on PEMS, such as mode of fertilization, features of the mating system and aspects of female reproductive biology. Importantly, if interacting FRT components are identified (see below), then the comparative approach would also facilitate examination of co-diversification of interacting sperm and FRT traits underlying PEMS. Where genomic resources are available for the species examined, important questions related to the molecular evolution of PEMS eventually could be addressed. Do genes underlying PEMS (both male and female expressed) evolve faster or slower than other reproductive genes? Do genes underlying PEMs coevolve and can rate-covariation methods be used to identify PEMS interacting loci (Clark & Aquadro, 2010; Findlay et al., 2014)? Answers to these questions require a far greater understanding of the molecular basis of PEMS.
Intraspecific analyses of PEMS should also be pursued, particularly where this could lend itself to quantitative genetic analyses. Advancing our understanding of the evolutionary diversification of PEMS requires an evaluation of the degree to which PEMS variation is attributable to males, females or male × female interactions and, ideally, how this relates to variation in competitive fertilization success (Birkhead, 1998). Some PEMS are likely to contribute to the variation in reproductive success attributed to differential ‘genetic compatibility’ between males and females (e.g. inbreeding avoidance; Jennions, 1997; Neff & Pitcher, 2005). The failure of PEMS to be executed properly in certain male–female combinations may provide a novel explanation for idiopathic infertility, as first suggested by Chang (1951; see also Matzuk & Lamb, 2008). Along these lines, PEMS-related molecules and mechanisms may prove to be useful candidate targets for the development of long-sought non-hormonal and reversible contraceptives.
The failure of sperm successfully to undergo PEMS within the FRT of hetero-population or heterospecific females (e.g. following a hybrid mating) could be a widespread mechanism contributing to PMPZ reproductive isolation (Howard et al., 2009). We are unaware of any investigations of this possibility. The ideal research program would include a fully factorial cross design using pairs of sibling species that will engage in reciprocal hybrid matings, or else are amenable to artificial insemination, to examine whether PEMS occur in the same way in interspecific as in intraspecific crosses, and to explore whether the failure of PEMS to be executed in hybrid inseminations contributes to gametic isolation (e.g. Howard et al., 2009; Manier et al., 2013b). Combining this approach with molecular approaches (see below) in a hybridizing model system with genomic resources (e.g. sibling Drosophila species), especially when followed up with functional experiments, would be exceptionally powerful.
The vast majority of empirical data supporting PEMS come from microscopy and ultrastructure studies. There is a pressing need to link such observations with underlying molecular changes. Applying a combination of fine-structure and proteomic analyses to compare the sperm from male seminal vesicles and sperm that have experienced prolonged storage in the FRT will prove extremely valuable, especially if applied to taxonomically diverse species. Such studies would further our understanding of the variation in the structural and molecular nature of PEMS. Proteomic studies have played a transformative role in achieving a refined molecular understanding of sperm composition, with sperm having been characterized using proteomics for humans and several model organisms, including the mouse, rat, macaque, fruit fly, mosquito, honeybee and worm, as well as for some non-model species (e.g. Dorus et al., 2006; Baker et al., 2008; Poland et al., 2011; Skerget et al., 2013; Amaral et al., 2014; Ma et al., 2014; Whittington et al., 2015; Degner et al., 2019). As methods for single-cell analysis improve (Budnik et al., 2018), single-cell proteomics could be used to inventory sperm composition across the entire life history of sperm in the FRT. The consequent determination of gains, losses and modifications of sperm (or sperm-associated seminal or female) proteins will provide molecular precision to our understanding of the sequence of changes in sperm as they reside in the FRT. Combining this approach with cryo-electron-tomography (Nicastro, McIntosh, & Baumeister, 2005) to reveal structural changes in the sperm and in the positions or shapes of complexes on or in them, and methods for single-cell measurement of energy consumption (Chen et al., 2015) in sperm will further extend the molecular precision of what is involved in PEMS. This in turn will provide a foundation for systematic efforts to examine the in vivo functional importance of PEMS, in organisms where genetic manipulation is possible, and establish molecular networks governing post-copulatory sperm–female interactions critical to sperm viability and fertility (McDonough et al., 2016).
The ultimate goal of assigning specific functions to PEMS will require molecular advances in our understanding of PEMS in taxa where genetic manipulation (RNAi and CRISPR) and functional assays are tractable (e.g. Bassett & Liu, 2014; Mohr et al., 2014). These methods can be used to remove (or deplete) proteins identified in proteomic studies such as those described above (or other proteins suggested to be involved in PEMS based on other data), from their tissue of synthesis (testis, male reproductive glands, or FRT tissues). They can also be used to prevent production of other potential effectors of PEMS, such as extracellular vesicles that deliver molecules to sperm after the latter have left the testis and/or entered the female (e.g. Aalberts, Stout, & Stoorvogel, 2014; Corrigan et al., 2014; Fereshteh et al., 2019). Effects of removal of the proteins or other effectors can then be examined in terms of the ability of sperm from (or in) knockdown/knockout animals to undergo PEMS, by assaying fertility, sperm fate, and sperm morphology after sperm have left the testis and entered the FRT. Knockdown/out of molecules needed for PEMS would be expected to decrease or abolish fertility (or perhaps to result in abnormalities like polyspermy), to modulate sperm competitive success, and possibly to result in visibly abnormal sperm structures or morphologies, or inappropriate fates or targeting of sperm. For example, such genetic studies have already been useful for examining the function of proteins that bind to sperm and are subsequently cleaved or otherwise released from sperm into the FRT in the well-studied case of Drosophila sex peptide (Peng et al., 2005; Ravi Ram & Wolfner, 2009; Singh et al., 2018). Once genetic studies have pinpointed molecules that are necessary for PEMS, further studies can define those molecules’ active regions, partners, and evolutionary dynamics. Although such studies are likely to be easiest in traditional genetic model organisms, it is expected that analogous studies could be carried out in any organism for which sperm proteomics (or other molecular) analyses can be carried out and in which RNAi and CRISPR techniques are useable, such as insects beyond D. melanogaster. It will likely prove interesting, albeit challenging, to use such approaches to examine the material voided from sperm within the FRT of species such as collembolans (Dallai et al., 2004) and the fungus gnat S. coprophila (Phillips, 1966a), and perhaps on the spermatostyles that accumulate in the female spermatheca of some whirligig and carabid beetles after primary sperm conjugates have disassociated (Breland & Simmons, 1970; Sasakawa, 2007).
Whereas the functional analysis of specific proteins that bind to or are removed from sperm is certainly tractable in many model and non-model organisms, manipulating more complex PEMS, such as major structural transitions or sperm–sperm associations, is likely to prove much more challenging. However, single-cell proteomics may again come to our aid. It will be necessary to isolate and characterize the female-derived molecules responsible for the induction of complex PEMS. Such studies would represent major advances towards achieving an understanding of male–female interactions that mediate reproductive outcomes. This is important from many fundamental perspectives: our understanding of basic reproductive biology and fertility, of the cell biology of gametes and their interaction with the reproductive tract environment and with each other, and of how evolutionary forces shaped these interactions and, in turn, may have been constrained by them. In addition, understanding sperm traits and how they are modified post-ejaculation will likely have direct practical implications. It could provide information that will improve conditions for in vitro sperm maintenance/storage or for assisted reproductive technologies, thus benefitting human reproductive biomedicine or assisting in the recovery or support of threatened species. Alternatively, this infomation could reveal potential new targets for human contraception or for biological control programs against pest or vector insects.
Finally, we hope this review serves to remind readers of the benefits of carrying out descriptive studies of the fascinating reproductive biology of diverse taxa. Such pursuits used to be far more popular than they are now. As one referee pointed out, ‘the rise in molecular and developmental genetics proved so seductive as to suck all the oxygen out of the room’, in the sense of focusing much research emphasis onto a small number of model organisms. Especially in light of the mechanistic insights arising from such research, it is important and instructive to return to examining the varied reproductive strategies used across the animal kingdom. As sagely noted by Grimaldi & Engel (2007, p. 647), “a theory is only as good as what it explains and the evidence (i.e. descriptions) that supports it.”
IX. CONCLUSIONS
(1) Generating mature, functional sperm is an evolutionarily dynamic process that can transcend both male and female reproductive tracts. Understanding the evolution of sperm maturation requires recognizing that sperm have complex and protracted life histories that are shaped by selection generated by the female reproductive tract.
(2) Post-ejaculatory modifications to sperm (PEMS) are a widespread, if not a ubiquitous, component of the life history of sperm in internally fertilizing species. The proper implementation of most PEMS requires interactions between sperm and female reproductive tract molecules and cells.
(3) The widely investigated phenomenon of capacitation in eutherian mammals is only one example of a much more widespread phenomenon. PEMS have likely evolved independently numerous times throughout the kingdom Animalia. A diversity of selection pressures likely underlies the evolutionary origin, maintenance and diversification of PEMS. We advance numerous hypotheses for the adaptive value of PEMS, but note that few data currently exist to test or otherwise discriminate among them.
(4) Few studies have considered PEMS from an evolutionary perspective. Such research initiatives are warranted as variation in PEMS has important implications for our understanding of sex-specific gene expresssion and of post-copulatory sexual selection. Moreover, the rapid evolution of ejaculate–female interactomes underlying PEMS has the potential to generate reproductive isolation between divergent populations or incipient species and hence to be a wideapread ‘engine of speciation’.
Supplementary Material
Appendix S1. A survey of post-ejaculatory modifications to sperm (PEMS) throughout the kingdom Animalia.
X. ACKNOWLEDGMENTS
We would like to thank Sharleen Buel for technical assistance and Susan Suarez, Kirill Borziak, Ethan Degner, Tim Karr, Erin McCullough, Caitlin McDonough, Jane Pascar, Zeeshan Syed and Emma Whittington for fruitful advice, discussion, bringing relevant literature to our attention, and/or helpful comments on earlier drafts. We are also grateful for the detailed and perceptive comments from two anonymous reviewers. We are especially indebted to the myriad biologists whose curiosity and creative exploration of variation in reproductive systems made this synthesis possible and a joy to write. This work was supported by a generous gift by Mike and Jane Weeden to Syracuse University and by grants from the Eunice Shriver National Institute for Child Health and Human Development (R21-HD088910 to S.D., S.P. and M.F.W. and R01-HD038921 to M.F.W.) and the National Science Foundation (DEB-1655840 to S.D., S.P. and M.F.W.),
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
XI. REFERENCES
References used in the online supporting information are identified using asterisks.
- Fuentes-G JA, Housworth EA, Weber A & Martins EP (2016). Phylogenetic ANCOVA: estimating changes in evolutionary rates as well as relationships between traits. The American Naturalist 188, 615–627. [DOI] [PubMed] [Google Scholar]
- Aalberts M, Stout TAE & Stoorvogel W, (2014). Prostasomes: extracellular vesicles from the prostate. Reproduction 147, R1–R14. [DOI] [PubMed] [Google Scholar]
- Adams DC (2014). Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Systematic Biology 63, 166–177. [DOI] [PubMed] [Google Scholar]
- Afzelius BA & Dallai R (1987). Conjugated spermatozoa In New Horizons in Sperm Cell Research (ed. Mohri H), pp. 349–355. Japan Science Society, Tokyo. [Google Scholar]
- Ahmed-Braimah YH & McAllister BF (2012). Rapid evolution of assortative fertilization between recently allopatric species of Drosophila. International Journal of Evolutionary Biology 2012, 285468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigaki T, Kasuga H, Nagaoka S & Osanai M (1994). Purification and partial amino acid sequence of initiatorin, a prostatic endopeptidase of the silkworm, Bombyx mori. Insect Biochemistry and Molecular Biology 24, 969–975. [DOI] [PubMed] [Google Scholar]
- *.Aitken RJ & Nixon B (2013). Sperm capacitation: A distant landscape glimpsed but unexplored. Molecular Human Reproduction 19, 785–793. [DOI] [PubMed] [Google Scholar]
- Alberti G (1990). Comparative spermatology of Araneae. Acta Zoologica Fennica 190, 17–34. [Google Scholar]
- Alberti G (2000). Chelicerata In Reproductive Biology of Invertebrates, vol. 9, Part B. Progress in Male Gamete Ultrastructure (ed. Jamieson BGM), pp. 311–388. John Wiley and Sons, Ltd., New York. [Google Scholar]
- Alikunhi KH (1951). On the reproductive organs of Pisione remota (southern), together with a review of the family Pisionidae (Polychaeta). Proceedings of the Indian Academy of Sciences, Section B 33, 14–31. [Google Scholar]
- *.Al-Lawati H, Kamp G & Bienefeld K (2009). Characteristics of the spermathecal contents of old and young honeybee queens. Journal of Insect Physiology 55, 116–121. [DOI] [PubMed] [Google Scholar]
- Allen AK & Spradling AC (2008). The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 135, 311–321. [DOI] [PubMed] [Google Scholar]
- Alminana C, Caballero I, Heath PR, Maleki-Dizaji S, Parrilla I, Cuello C, Gil MA, Vazquez JL, Vazquez JM, Roca J, Marinez EA, Holt WH & Fazeli A (2014). The battle of the sexes starts in the oviduct: modulation of oviductal transcriptome by X- and Y-bearing spermatozoa. BMC Genomics 15, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo SH, Stiver KA & Marsh-Rollo SE (2016). Ovarian fluid allows directional cryptic female choice despite external fertilization. Nature Communications 7, 12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral A, Castillo J Ramalho-Santos J & Oliva R (2014). The combined human sperm proteome: cellular pathways and implications for basic and clinical science. Human Reproduction Update 20, 40–62. [DOI] [PubMed] [Google Scholar]
- Anderson RC (1945). A study of the factors affecting fertility of lozenge females of Drosophila melanogaster. Genetics 30, 280–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Apger-McGlaughon J & Wolfner MF (2013). Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. Journal of Insect Physiology 59, 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist G & Rowe L (2005). Sexual conflict. Princeton University Press, Princeton. [Google Scholar]
- Artemenko K, Horakova J, Steinberger B, Besenfelder U, Brem G, Bergquist J & Mayrhofer C (2015). A proteomic approach to monitor the dynamic response of the female oviductal epithelial cell surface to male gametes. Journal of Proteomics 113, 1–14. [DOI] [PubMed] [Google Scholar]
- *.Au DW-T, Reunov AA & Wu RS-S (1998). Four lines of spermatid development and dimorphic spermatozoa in the sea urchin Anthocidaris crassispina (Echinodermata, Echinoida). Zoomorphology 118, 159–168. [Google Scholar]
- Austin CR (1951). Observations on the penetration of the sperm into the mammalian egg. Australian Journal of Scientific Research. Series B: Biological Sciences 4, 581–596. [DOI] [PubMed] [Google Scholar]
- Austin CR (1952). The ‘capacitation’ of the mammalian sperm. Nature 170, 326. [DOI] [PubMed] [Google Scholar]
- *.Avila FW, Ravi Ram K, Bloch Qazi MC & Wolfner MF (2010). Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186, 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD & Wolfner MF (2011). Insect seminal fluid proteins: identification and function. Annual Review of Entomology 56, 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Aydin H, Sultana A, Li S, Thavalingam A & Lee JE (2016). Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 534, 562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Baccetti B, Rosati F & Bigliardi E (1971). The spermatozoon of Arthropoda. XIII. The cell surface. Journal of Ultrastructure Research 35, 582–605. [DOI] [PubMed] [Google Scholar]
- Baer B Eubel H Taylor NL, O’Toole N & Millar AH (2009a). Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera. Genome Biology 10, R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B, Heaslewood JL, Taylor NL, Eubel H & Millar AH (2009b). The seminal fluid proteome of the honeybee Apis mellifera. Proteomics 9, 2085–2097. [DOI] [PubMed] [Google Scholar]
- Baker MA, Witherdin R, Hetherington L, Cunningham-Smith K & Aitken RJ (2005). Identification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresis. Proteomics 5, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Reeves GM & Aitken RJ (2008). The mouse sperm proteome characterized via IPG strip prefractionation and LC-MS/MS identification. Proteomics 8, 1720–1730. [DOI] [PubMed] [Google Scholar]
- *.Bakst MR & Bauchan G (2015). Apical blebs on sperm storage tubule epithelial cell microvilli: their release and interaction with resident sperm in the Turkey hen oviduct. Theriogenology 83, 1438–1444. [DOI] [PubMed] [Google Scholar]
- Baldini F, Gabrieli P, South A, Valim C, Mancini F & Catteruccia F, (2013). The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biology 11, e1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Báo SN & de Souza W (1993). Ultrastructural and cytochemical studies of the spermatid and spermatozoon of Culex quinquefasciatus (Culicidae). Journal of Submicroscopic Cytology and Pathology 25, 213–222. [PubMed] [Google Scholar]
- Bassett AR & Liu JL (2014). CRISPR/Cas9 and genome editing in Drosophila. Journal of Genetics and Genomics 41, 7–19. [DOI] [PubMed] [Google Scholar]
- *.Bath E, Bowden S, Peters C, Reddy A, Tobias JA, Easton-Calabria E, Seddon N, Goodwin SF & Wigby S (2017). Sperm and sex peptide stimulate aggression in female Drosophila. Nature Ecology and Evolution 1, 0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bedford JM (1970). Sperm capacitation and fertilization in mammals. Biology of Reproduction 2(suppl 1), 128–158. [PubMed] [Google Scholar]
- Bedford JM (1979). Evolution of the sperm maturation and sperm storage functions of the epididymis In The Spermatozoon (eds Fawcett DW and Bedford JM), pp. 7–21. Urban and Schwarzenberg, Baltimore. [Google Scholar]
- *.Bedford JM (1996). What marsupial gametes disclose about gamete function in eutherian mammals. Reproduction Fertility and Development 8, 569–580. [DOI] [PubMed] [Google Scholar]
- Bedford JM & Breed WG (1994). Regulated storage and subsequent transformation of spermatozoa in the fallopian tubes of an Australian marsupial, Sminthopsis crassicaudata. Biology of Reproduction 50, 845–854. [DOI] [PubMed] [Google Scholar]
- *.Beeman RD (1972). Sperm biology in anaspidean mollusks. Echo 5, 19–21. [Google Scholar]
- *.Beeman RD (1977). Gastropoda: Opisthobranchia In Reproduction of Marine Invertebrates, Vol. IV (eds Giese AC and Pearse JS), pp. 115–179. Academic Press, New York. [Google Scholar]
- Beirão J, Purchase CF, Wringe BF & Fleming IA (2015). Inter-population ovarian fluid variation differentially modulates sperm motility in Atlantic cod Gadus morhua. Journal of Fish Biology 87, 54–68. [DOI] [PubMed] [Google Scholar]
- Bian X, Zhang L, Yang L, Yang P, Ullah S, Zhang Q & Chen Q (2013). Ultrastructure of epididymal epithelium and its interaction with the sperm in the soft-shelled turtle Pelodiscus sinensis. Micron 54, 65–74. [DOI] [PubMed] [Google Scholar]
- Biggers JD & Creed RFS (1962). Conjugate spermatozoa of the North American opossum. Nature 196, 1112–1113. [Google Scholar]
- Birkhead TR (1998). Cryptic female choice: criteria for establishing female sperm choice. Evolution 52, 1212–1218. [DOI] [PubMed] [Google Scholar]
- Birkhead TR & Møller AP (1993). Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biological Journal of the Linnean Society 50, 295–311. [Google Scholar]
- Birkhead TR, Møller AP & Sutherland WJ (1993). Why do females make it so difficult for males to fertilize their eggs? Journal of Theoretical Biology 161, 51–60. [Google Scholar]
- *.Bishop JDD (1996). Female control of paternity in the internally fertilizing compound ascidian Diplosoma listerianum. I. Autoradiographic investigation of sperm movements in the female reproductive tract. Proceedings of the Royal Society, Series B 263, 369–376. [Google Scholar]
- *.Bishop JDD & Ryland JS (1991). Storage of exogenous sperm by the compound ascidian Diplosoma listerianum. Marine Biology 108, 111–118. [Google Scholar]
- *.Bishop JDD, Jones CS & Noble LR (1996). Female control of paternity in the internally fertilizing compound ascidian Diplosoma listerianum. II. Investigation of male mating success using RAPD markers. Proceedings of the Royal Society. Series B 263, 401–407. [Google Scholar]
- Bjork A, Starmer WT, Higginson DM, Rhodes CJ & Pitnick S (2007). Complex interactions with females and rival males limit the evolution of sperm offence and defence. Proceedings of the Royal Society, Series B 274, 1779–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bleil JD & Wassarman PM (1983). Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Developmental Biology 95, 317–324. [DOI] [PubMed] [Google Scholar]
- den Boer SPA, Baer B, Dreier S, Aron S, Nash DR & Boomsma JJ (2009a). Prudent sperm use by leaf-cutter ant queens. Proceedings of the Royal Society, Series B B 276, 3945–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer SPA, Boomsma JJ & Baer B (2009b). Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. Journal of Insect Physiology 55, 538–543. [DOI] [PubMed] [Google Scholar]
- *.Boere J, Diaz DE & Holt WV (2011). Sperm motility activation, sperm heterogeneity and sperm-female tract interactions in Bennett’s wallaby (Macfops rufogriseus). Reproduction Fertility and Development 23, 603–617. [DOI] [PubMed] [Google Scholar]
- Bojat NC, Sauder U & Haase M (2001). The spermathecal eptihelium, sperm and their interactions in the hermaphroditic land snail Arianta arbustorum (Pulmonata, Stylommatophora). Zomorphology 120, 149–157. [Google Scholar]
- Breland OP & Simmons E (1970). Preliminary studies of the spermatozoa and the male reproductive system of some whirligig beetles (Coleoptera: Gyrinidae). Entomological News 81, 101–110. [Google Scholar]
- Brinton LP, Burgdorfer W & Oliver JH Jr. (1974). Histology and fine structure of spermatozoa and egg passage in the female tract of Dermacentor andersoni Stiles (Acari: Ixodidae). Tissue & Cell 6, 109–125. [DOI] [PubMed] [Google Scholar]
- Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ & Robertson SA (2014). Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proceedings of the National Academy of Sciences, USA 111, 2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SG (1985). Mating behavior of the golden-orb-weaving spider, Nephila clavipes: II. Sperm capacitation, sperm competition, and fecundity. Journal of Comparative Psychology 99, 167–175. [Google Scholar]
- *.Browne RK, Kaurova SA, Uteshev VK, Shishova NV, McGinnity D, Figiel CR, Mansour N, Agnew D, Wu M, Gakhova EN, Dzyuba, & Cosson J (2015). Sperm motility of externally fertilizing fish and amphibians. Theriogenology 83, 1–13. [DOI] [PubMed] [Google Scholar]
- *.Buckland-Nicks J (1998). Prosobranch parasperm: sterile cells germ cells that promote paternity? Micron 29, 267–280. [Google Scholar]
- Budnik B, Levy E, Harmange G & Slavov N (2018). SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biology 19, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhi WC, Alvarez IM & Kouba AJ (2000). Secreted proteins of the oviduct. Cells, Tissues, Organs 166, 165–179. [DOI] [PubMed] [Google Scholar]
- *.Burger M, Michalik P, Graber W, Jacob A, Nentwig W & Kropf C, (2006). Complex genital system of a Haplogyne spider (Arachnida, Araneae, Tetrablemmidae) indicates internal fertilization and full female control over transfrerred sperm. Journal of Morphology 267, 166–186. [DOI] [PubMed] [Google Scholar]
- Burighel P & Martinucci GB (1994a). Sexual reproduction in the compound ascidian Diplosoma listerianum (Tunicata). I. Metamorphosis, storage and phagocytosis of sperm in female duct. Marine Biology 118, 489–498. [Google Scholar]
- *.Burighel P & Martinucci GB (1994b). Sexual reproduction in the compound ascidian Diplosoma listerianum (Tunicata). II. Sperm penetration through ovary wall and evidence of internal fertilization. Marine Biology 118, 499–510. [Google Scholar]
- *.Burighel P, Martinucci GB & Magri F (1985). Unusual structures in the spermatozoa of the ascidians Lissoclinum perforatum and Diplosoma listerianum (Didemnidae). Cell and Tissue Research 241, 513–521. [Google Scholar]
- Butts IAE, Prokopchuk G, Kaspar V, Cosson J & Pitcher TE (2017). Ovarian fluid impacts flagellar beating and biomechanical metrics of sperm between alternative reproductive tactics. Journal of Experimental Biology 220, 2210–2217. [DOI] [PubMed] [Google Scholar]
- Chang MC (1951). Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697–698. [DOI] [PubMed] [Google Scholar]
- *.Chang MC (1984). The meaning of sperm capacitation. A historical perspective. Journal of Andrology 5, 4550. [DOI] [PubMed] [Google Scholar]
- *.Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK & Partridge L (2003). The sex peptide of Drosophila melanogaster: female post- mating responses analyzed by using RNA interference. Proceedings of the National Academy of Sciences, USA 100, 9923–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DTN, Heymann M, Graden S, Nicastro D & Dogic Z (2015). ATP consumption of eukaryotic flagella measured at a single-cell level. Biophysics Journal 109, 2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Wolfner MF & Clark AG (2010). The genetic basis for male x female interactions underlying variation in reproductive phenotypes of Drosophila. Genetics 186, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NL & Aquadro CF (2010). A novel method to detect proteins evolving at correlated rates: identifying new functional relationships between coevolving proteins. Molecular Biology and Evolution 27, 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Begun DJ & Prout T (1999). Female x male interactions in Drosophila sperm competition. Science 283, 217–220. [DOI] [PubMed] [Google Scholar]
- Clark AG, Dermitzakis ET & Civetta A (2000). Nontransitivity of sperm precedence in Drosophila. Evolution 54, 1030–1035. [DOI] [PubMed] [Google Scholar]
- *.Clements AN & Potter SA (1967). The fine structure of the spermathecae and their ducts in the mosquito Aedes aegypti. Journal of Insect Physiology 13, 1825–1836. [DOI] [PubMed] [Google Scholar]
- *.Cognigni P, Bailey AP & Miguel-Aliaga I (2011). Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metabolism 13, 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Collins AM, Caperna TJ, Williams V, Garett WM & Evans JD (2006). Proteomic analyses of male contributions to honeybee sperm storage and mating. Insect Molecular Biology 15, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cook PA & Wedell N (1999). Non-fertile sperm delay female remating. Nature 397, 486. [Google Scholar]
- Coppin A, Maes E & Strecker G (2002). Species-specificity of amphibia carbohydrate chains: the Bufo viridis case study. Carbohydrate Research 337, 121–132. [DOI] [PubMed] [Google Scholar]
- Corrigan L, Rehai S, Leiblich A, Fan SJ, Perera SM, Patel R, Gandy C, Wainwright SM, Morris JF, Hamdy F, Goberdhan DC & Wilson C (2014). BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. Journal of Cell Biology 206, 671–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy P, García-Vázquez FA, Visconti PE & Avilés M (2012). Roles of the oviduct in mammalian fertilization. Reproduction 144, 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA & Orr HA (2004). Speciation. Sinauer Press, Sunderland. [Google Scholar]
- Crean AJ & Marshall DJ (2008). Gamete plasticity in a broadcast spawning marine invertebrate. Proceedings of the National Academy of Sciences, USA 105, 13508–13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Czarny NA, Mate KE & Rodger JC (2008). Acrosome stability in the spermatozoa of dasyrurid marsupials. Reproduction Fertility and Development 20, 295–302. [DOI] [PubMed] [Google Scholar]
- *.Dallai R (1970). The spermatozoon of Arthropoda XI. Further observations on Collembola In Comparative Spermatology (ed. Baccetti B), pp. 276–279. Academic Press, New York-London. [Google Scholar]
- Dallai R (1972). The arthropod spermatozoon 17. Machilis distincta Janetsch (Insecta, Thysanura). Monitore Zoologico Italiano 6, 37–61. [Google Scholar]
- *.Dallai R (2014). Overview on spermatogenesis and sperm structure of Hexapoda. Arthropod Structure & Development 43, 257–290. [DOI] [PubMed] [Google Scholar]
- Dallai R & Afzelius BA (1985). Membrane specializations in the paired spermatozoa of dytiscid water beetles. Tissue & Cell 17, 561–572. [DOI] [PubMed] [Google Scholar]
- Dallai R, Bernini F & Giusti F (1973). Interdoublet connections in the sperm flagellar complex of Sciara. Journal of Submicroscopic Cytology 5, 137–145. [Google Scholar]
- Dallai R, Fanciulli PP, Frati F, Paccagnini E & Lupetti P (2003). Membrane specializations in the spermatozoa of collembolan insects. Journal of Structural Biology 142, 311–318. [DOI] [PubMed] [Google Scholar]
- Dallai R, Fanciulli PP, Frati F, Paccagnini E & Lupetti P (2004). Sperm winding in Collembola. Pedobiologia 48, 493–501. [Google Scholar]
- *.Dallai R, Zizzari ZV & Fanciulli PP (2008). Fine structure of the spermatheca and of the accessory glands in Orchesella villosa (Collembola, Hexapoda). Journal of Morphology 269, 464–478. [DOI] [PubMed] [Google Scholar]
- Daly JM & Golding DW (1977). A description of the spermatheca of Spirorbis spirorbis (L.) (Polychaeta: Serpulidae) and evidence for a novel mode of sperm transmission. Journal of the Marine Biology Association, UK 57, 219–227. [Google Scholar]
- Darszon A, Labarca P, Nishigaki T & Espinosa F (1999). Ion channels in sperm physiology. Physiological Reviews 79, 481–510. [DOI] [PubMed] [Google Scholar]
- Degner EC & Harrington LC (2016). A mosquito sperm’s journey from male ejaculate to egg: mechanims, molecules, and methods for exploration. Molecular Reproduction and Development 83, 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Degner EC, Ahmed-Braimah YH, Borziak K, Wolfner MF, Harrington LC & Dorus S (2019). Proteins, transcripts, and genetic architecture of seminal fluid and sperm in teh mosquito Aedes aegypti. Molecular and Cellular Proteomics 18(Suppl 1), S6–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devigili A, Fitzpatrick JL, Gasparini C, Ramnarine IW, Pilastro A & Evans JP (2018). Possible glimpses into early speciation: the effect of ovarian fluid on sperm velocity accords with post-copulatory isolation between two guppy populations. Journal of Evolutionary Biology 31, 66–74. [DOI] [PubMed] [Google Scholar]
- Djakiew D & Jones RC (1983). Sperm maturation, fluid transport, and secretion and absorption of protein in the epididymis of the echidna, Tachyglossus aculeatus. Journal of Reproduction and Fertility 68, 445–456. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T (1937). Genetics and the Origin of Species. Columbia University Press, New York. [Google Scholar]
- *.Döring D (1986). On the male reproduction biology of Orchesella cincta (Collembola, Entomobryidae) In Second International Seminar on Apterygota (ed. Dallai R), pp. 171–176. University of Siena, Siena. [Google Scholar]
- Dorus S & Karr TL (2009). Sperm proteomics and genomics In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 435–469. Academic Press, London. [Google Scholar]
- Dorus S, Busby SA, Gerike U, Shabanowitz J, Hunt DF & Karr TL (2006). Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nature Genetics 38, 1440–1445. [DOI] [PubMed] [Google Scholar]
- Dorus S, Skerget S & Karr TL (2012). Proteomic discovery of diverse immunity molecules in mammalian spermatozoa. Systems Biology in Reproductive Medicine 58, 218–228. [DOI] [PubMed] [Google Scholar]
- Droge-Young EM, Belote JM, Eeswara A & Pitnick S (2016). Extreme ecology and an extreme mating system: discrimination among alternative direct benefits models for the evolution of polyandry in the red flour beetle Tribolium castaneum. Behavioral Ecology 27, 575–583. [Google Scholar]
- Eberhard WG (1996). Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press, Princeton. [Google Scholar]
- *.Edwards RG, Bavister BD & Steptoe PC (1969). Early stages of fertilization in vitro of human oocytes matured in vitro. Nature 221, 632–635. [DOI] [PubMed] [Google Scholar]
- *.Edwards RG, Steptoe PC & Purdy JM (1970). Fertilization and cleavage in vitro of preovulator human oocytes. Nature 227, 1307–1309. [DOI] [PubMed] [Google Scholar]
- Eisenbach M (1999). Sperm chemotaxis. Reviews in Reproduction 4, 56–66. [DOI] [PubMed] [Google Scholar]
- Eisenbach M & Giojalas LC (2006). Sperm guidance in mammals - an unpaved road to the egg. Nature Reviews in Molecular and Cell Biology 7, 276–285. [DOI] [PubMed] [Google Scholar]
- Esponda P & Bedford JM (1985). Surface of the rooster spermatozoon changes in passing through the Wolffian duct. Journal of Experimental Zoology 234, 441–449. [DOI] [PubMed] [Google Scholar]
- Esponda P & Bedford JM (1987). Post-testicular change in the reptile sperm surface with particular reference to the snake. Natrix fasciata. Journal of Experimental Zoology 241, 123–132. [DOI] [PubMed] [Google Scholar]
- Evans JP & Sherman CDH (2013). Sexual selection and the evolution of egg-sperm interactions in broadcast-spawning invertebrates. Biological Bulletin 224, 166–183. [DOI] [PubMed] [Google Scholar]
- Evans JP, Garcia-Gonzalez F, Almbro M, Robinson O & Fitzpatrick JL (2012). Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proceedings of the Royal Society, Series B 279, 2855–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Lefebvre J & Manjunath P (2006). Bovine seminal plasma proteins and thier relatives: a new expanding superfamily in mammals. Gene 375, 63–74. [DOI] [PubMed] [Google Scholar]
- Fanciulli PP, Mencarelli C, Mercati D, Dallai R & Lupetti P (2017). The peculiar extra-acrosomal structure of the Collembola (Hexapoda) spermatozoa. Micron 101, 114–122. [DOI] [PubMed] [Google Scholar]
- Fereshteh Z, Bathala P, Galileo DS & Marin-DeLeon PA (2019). Detection of extracellular vesicles in the mouse vaginal fluid: their delivery of sperm proteins that stimulate capacitation and modulate fertility. Journal of Cell Physiology 234, 12745–12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Yi XH, MacCoss MJ & Swanson WJ (2008). Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biology 6, 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, MacCoss M & Swanson WJ (2009). Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Research 19, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Sitnik JL, Wang W, Aquadro CA, Clark NL & Wolfner MF (2014). Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PLoS Genetics 10, e1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firman RC, Gasparini C, Manier MK & Pizzari T (2017). Postmating female control: 20 years of cryptic female choice. Trends in Ecology and Evolution 32, 368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Florman HM & Babcock DF (1991). Progress toward understanding the molecular basis of capacitation In Elements of Mammalian Fertilization: Basic Concepts (ed. Wassarman PM), pp. 105–132. CRC Press, Boca Ratan, FL. [Google Scholar]
- *.Florman HM & Fissore RA (2015). Fertilization in mammals In Knobil and Neill’s Physiology of Reproduction, 4th Edition (eds Plant TM and Zeleznik AJ), pp. 149–196. Elsevier, Amsterdam. [Google Scholar]
- *.Florman HM & Storey BT (1982). Mouse gamete interactions: the zona pellucida is the site of the acrosome reaction leading to fertilization in vitro. Developmental Biology 91, 121–130. [DOI] [PubMed] [Google Scholar]
- *.Foelix RF (2011). Biology of Spiders. Oxford University Press, Oxford. [Google Scholar]
- *.Fretter V (1953). The transference of sperm from male to female prosobranch, with reference, also, to pyramidellids. Proceedings of the Linnean Society of London 164, 217–224. [Google Scholar]
- Fricke C, Bretman A & Chapman T (2009). Female nutritional status determines the magnitude and sign of responses to a male ejaculate signal in Drosophila melanogaster. Journal of Evolutionary Biology 23, 157–165. [DOI] [PubMed] [Google Scholar]
- Friedländer M, Jeshtadi A &, Reynolds SE (2001). The structural mechanism of trypsin-induced intrinsic motility in Manduca sexta spermatozoa in vitro. Journal of Insect Physiology 47, 245–255. [DOI] [PubMed] [Google Scholar]
- Friedländer M, Seth RK &, Reynolds SE (2005). Eupyrene and apyrene sperm: dichotomous spermatogensis in Lepidoptera. Advances in Insect Physiology 32, 206–308. [Google Scholar]
- Froesch D & Marthy H-J (1975). The structure and function of the oviducal gland in octopods (Cephalopoda). Proceedings of the Royal Society, Series B 188, 95–101. [DOI] [PubMed] [Google Scholar]
- Gadella BM & Boerke A (2016). An update on post-ejaculatory remodeling of the sperm surface before mammalian fertilization. Theriogenology 85, 113–124. [DOI] [PubMed] [Google Scholar]
- *.Gadella BM & Visconti PE (2006). Regulation of capacitation In The Sperm Cell: Production, Maturation, Fertilization, Regeneration (eds De Jonge C and Barrett C), pp. 134–169. Cambridge, Cambridge UniversityPress. [Google Scholar]
- *.Gao Z, Ruden DM & Lu X (2003). PKD2 cation channel is required for directional sperm movement and male fertility. Current Biology 13, 2175–2178. [DOI] [PubMed] [Google Scholar]
- Gasparini C & Evans JP (2013). Ovarian fluid mediates the temporal decline in sperm viability in a fish with sperm storage. PLoS One 8(5), e64431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini C & Pilastro A (2011). Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proceedings of the Royal Society, Series B 278, 2495–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi MG & Visconti PE (2016). Chang’s meaning of capacitation: a molecular perspective. Molecular Reproduction and Development 83, 860–874. [DOI] [PubMed] [Google Scholar]
- Gilbert SF & Barresi MJ (2016). Developmental Biology, 11th Edition. Sinauer Associates. [Google Scholar]
- Gillingham MA, Richardson DS, Lovlie H, Moynihan A, Worley K & Pizzari T (2009). Cryptic preference for MHCdissimilar females in male red junglefowl, Gallus gallus. Proceedings of the Royal Society, Series B 276, 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gist DH, Dawes SM, Turner TW, Sheldon S & Congdon J (2002). Sperm storage in turtles: a male perspective. Journal of Experimental Zoology 292, 180–186. [DOI] [PubMed] [Google Scholar]
- *.Giuffrida A & Rosati F (1993). Changes in sperm tail of Eyprepacnemis plorans (Insecta, Orthoptera) as a result of in vitro incubation in spermathecal extract. Invertebrate Reproduction and Development 24, 47–52. [Google Scholar]
- Giusti F & Selmi MG (1985). The seminal receptacle and sperm storage in Cochlostoma montanum (Issel) (Gastropoda: Prosobranchia). Journal of Morphology 184, 121–133. [DOI] [PubMed] [Google Scholar]
- Grimaldi DA & Engel MS (2007). Why descriptive science still matters. Bioscience 57, 646–647. [Google Scholar]
- *.Gupta BL (1968). Aspects of the motility in the non-flagellate spermatozoa of freshwater Ostracods In: Aspects of Cell Motility, XXII Symposium of the Society for Experimental Biology (Ed. by Miller PL), pp. 117–129. Oxford: Cambridge University Press. [PubMed] [Google Scholar]
- Gwynne DT (2008). Sexual conflict over nuptial gifts in insects. Annual Review of Entomology 53, 83–101. [DOI] [PubMed] [Google Scholar]
- Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Ravi Ram K, Sirot LK, Levesque L, Artieri CG, Wolfner MF, Civetta A & Singh RS (2007). Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics 177, 1321–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J (1934). The fertilisation of rabbit ova in relation to time. Journal of Experimental Biology 11, 140–161. [Google Scholar]
- Han X, Zhangli L, Li M, Bao H, Hei N & Chen Q (2008). Ultrastructure of anterior uterus of the oviduct and the stored sperm in female soft-shelled turtle, Trionyx sinensis. Anatomical Record 291, 335–351. [DOI] [PubMed] [Google Scholar]
- *.Harrison RA & Gadella BM (2005). Bicarbonate-induced membrane processing in sperm capacitation. Theriogenology 63, 342–351. [DOI] [PubMed] [Google Scholar]
- Harrison PW, Wright AE, Zimmer F, Dean R, Montgomery SH, Pointer MA & Mank JE (2015). Sexual selection drives evolution and rapid turnover of male gene expression. Proceedings of the National Academy of Sciences, USA 112, 4393–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hayakawa Y (2007). Parasperm: morphological and functional studies on nonfertile sperm. Icthyological Research 54, 111–130. [Google Scholar]
- Hayashi F (1997). A trypsin-degradable protein agglutinates fishfly sperm-bundles (Megaloptera: Corydalidae). International Journal of Insect Morphology and Embryology 26, 63–66. [Google Scholar]
- *.Herberstein ME, Schneider JM & Michalik P (2011). Sperm dynamics in spiders. Behavioral Ecology 22, 692–695. [Google Scholar]
- Higginson DM & Pitnick S (2011). Intra-ejaculate sperm interactions: do sperm cooperate? Biological Reviews 87, 249–270. [DOI] [PubMed] [Google Scholar]
- Higginson DM, Miller KB, Segraves KA & Pitnick S (2012a). Convergence, recurrence and diversification of complex sperm traits. Evolution 66–5, 1650–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson DM, Miller KB, Segraves KA & Pitnick S (2012b). Female reproductive tract form drives the evolution of complex sperm morphology. Proceedings of the National Academy of Sciences, USA 109, 4538–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Higginson DM Badyaev AV Segraves KA & Pitnick S (2015). Causes of discordance between allometries at and above species level: an example with aquatic beetles. The American Naturalist 186, 176–186. [DOI] [PubMed] [Google Scholar]
- *.Ho HC & Suarez SS (2003). Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biology of Reproduction 68, 1590–1596. [DOI] [PubMed] [Google Scholar]
- *.Holman L & Snook RR (2006). Spermicide, cryptic female choice and the evolution of sperm form and function. Journal of Evolutionary Biology 19, 1660–1670. [DOI] [PubMed] [Google Scholar]
- *.Holman L & Snook RR (2008). A sterile sperm caste protects brother fertile sperm from female-mediated death in Drosophila pseudoobscura. Current Biology 18, 292–296. [DOI] [PubMed] [Google Scholar]
- Holt WV & Fazeli A, (2015). Do sperm possess a molecular passport? Mechanistic insights into sperm selection in the female reproductie tract. Molecular Human Reproduction 21, 491–501. [DOI] [PubMed] [Google Scholar]
- Holt WV & Fazeli A (2016a). Sperm selection in the female mammalian reproductive tract. Focus on the oviduct: hypotheses, mechanisms, and new opportunities. Theriogenology 85, 105–112. [DOI] [PubMed] [Google Scholar]
- Holt WV & Fazeli A (2016b). Sperm storage in the female reproductive tract. Annual Review of Animal Bioscience 4, 291–310. [DOI] [PubMed] [Google Scholar]
- *.Horrocks AJ, Stewart S, Jackson L & Wishart GJ (2000). Induction of acrosomal exocytosis in chicken spermatozoa by inner perivitelline-derived N-linked glycans. Biochemical and Biophysical Research Communications 278, 84–89. [DOI] [PubMed] [Google Scholar]
- Howard DJ, Gregory PG, Chu J & Cain ML (1998). Conspecific sperm precedence is an effective barrier to hybridization between closely related species. Evolution 52, 511–516. [DOI] [PubMed] [Google Scholar]
- Howard DJ, Palumbi SR, Birge LM & Manier MK (2009). Sperm and speciation In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 367–403. Academic Press, London. [Google Scholar]
- *.Howarth B Jr. (1971). An examination for sperm capacitation in the fowl. Biology of Reproduction 3, 338–341. [DOI] [PubMed] [Google Scholar]
- *.Howarth B Jr. & Palmer MB (1972). An examination of the need for sperm capacitation in the Turkey, Meleagris gallopavo. Journal of Reproduction and Fertility 28, 443–445. [DOI] [PubMed] [Google Scholar]
- Hughes M & Davey KG (1969). The activity of spermatozoa of Periplaneta. Journal of Insect Physiology 15, 1607–1616. [Google Scholar]
- *.Hung P & Suarez SS (2012). Alterations to the bull sperm surface proteins that bind sperm to oviductal epithelium. Biology of Reproduction 87, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immler S (2008). Sperm competition and sperm cooperation: the potential role of diploid and haploid expression. Reproduction 135, 275–283. [DOI] [PubMed] [Google Scholar]
- Immler S, Moore HDM, Breed WG & Birkhead TR (2007). By hook or by crook? Morphometry, competition and cooperation in rodent sperm. PLoS One 2, e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Inoue N, Satouh Y, Ikawa M, Okabe M & Yanagimachi R (2011). Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proceedings of the National Academy of Sciences, USA 108, 20008–20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Jamieson BGM (1987). The Ultrastructure and Phylogeny of Insect Spermatozoa. Cambridge University Press, Cambridge. [Google Scholar]
- *.Jégo P, Joly J & Boisseau C (1980). Les gangues ovulaires des Amphibiens (protéines sécrétées par l’oviducte) et leurs roles dans la fécondation. Reproduction Nutrition Development 20, 57–567. [PubMed] [Google Scholar]
- Jennions MD (1997). Female promiscuity and genetic incompatibility. Trends in Ecology and Evolution 12, 251–253. [DOI] [PubMed] [Google Scholar]
- *.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K & Hirohashi N (2011). Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proceedings of the National Academy of Sciences, USA 108, 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johnson SL & Yund PO (2004). Remarkable longevity of dilute sperm in a free-spawning colonial ascidian. The Biological Bulletin 206, 144–151. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Villarroel M, Rosengrave P, Carne A, Kleffmann T, Lokman PM & Gemmell NJ (2014). Proteomic analysis of Chinook salmon (Onchorhynchus tshaytscha) ovarian fluid. PLoS One 9(8), e104155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johnston SD, Smith B, Pyne M, Stenzel D & Holt WV (2007). One-sided ejaculation of echidna sperm bundles. The American Naturalist 170, E162–E164. [DOI] [PubMed] [Google Scholar]
- *.Joly D, Luck N & Dejonghe B (2008). Adaptation to long sperm in Drosophila: correlated development of the sperm roller and sperm packaging. Journal of Experimental Zoology: Series B 310, 167–178. [DOI] [PubMed] [Google Scholar]
- *.Jones JC & Wheeler RE (1965a). Studies on spermathecal filling in Aedes aegypti (Linnaeus) I. description. Biological Bulletin 134(150), 129. [DOI] [PubMed] [Google Scholar]
- *.Jones JC & Wheeler RE (1965b). Studies on spermathecal filling in Aedes aegypti (Linnaeus). 2. Experimental. Biological Bulletin 129, 532–545. [DOI] [PubMed] [Google Scholar]
- *.de Jonge C (2017). Biological basis for human capacitation - revised. Human Reproduction Update 23, 289–299. [DOI] [PubMed] [Google Scholar]
- *.Kalb JM, DiBenedetto AJ & Wolfner MF (1993). Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proceedings of the National Academy of Sciences, USA 90, 8093–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TH & McCubbin AG (1996). How flowering plants descriminate bewteen self and non-self pollen to prevent inbreeding. Proceedings of the National Academy of Sciences, USA 93, 12059–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr TL (1991). Intracellular sperm/egg interactions in Drosophila: a three-dimensional structural analysis of a paternal product in the developing egg. Mechanisms of Development 34, 101–111. [DOI] [PubMed] [Google Scholar]
- Karr TL (1996). Paternal investment and intracellular sperm-egg interactions during and following fertilization in Drosophila. Current Topics in Developmental Biology 34, 89–115. [DOI] [PubMed] [Google Scholar]
- Karr TL & Pitnick S (1996). The ins and outs of fertilization. Nature 379, 405–406. [DOI] [PubMed] [Google Scholar]
- Karr TL, Swanson WJ & Snook RR (2009). The evolutionary significance of variation in sperm-egg interactions In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 305–365. Academic Press, London. [Google Scholar]
- *.Kaupp UB & Strunker T (2016). Signaling in sperm: more different than similar. Trends in Cell Biology 27, 101–109. [DOI] [PubMed] [Google Scholar]
- Kekäläinen J & Evans JP (2016). Female-induced remote regulation of sperm physiology may provide opportunites for gamete-level mate choice. Evolution 71–2, 238–248. [DOI] [PubMed] [Google Scholar]
- Kekäläinen J, Larma I, Linden M & Evans JP (2015). Lectin staining and flow cytometry reveals female-induced sperm acrosome reaction and surface carbohydrate reorganization. Scientific Reports 5, 15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L & Reeve H (1995). Why do females mate with multiple males? The sexually selected sperm hypothesis. Advances in the Study of Behavior 24, 291–315. [Google Scholar]
- Koeniger G (1970). Die bedeutung der tracheenhülle und der anhangsdrüse der spermatheka für die befruchtungsfähigkeit der spermatozoen in der bienenkönigin. Apidologie 1, 55–71. [Google Scholar]
- *.Köttgen M, Hofherr A, Li W, Chu K,Cook S, Montell C & Watnick T (2011). Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS One 6, e20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kraaijeveld K, Kraaijeveld-Smit FJL & Maan ME (2011). Sexual selection and speciation: the comparative evidence revisited. Biological Reviews 86, 367–377. [DOI] [PubMed] [Google Scholar]
- *.Krapf D, Visconti PE, Arranz SE & Cabada MO (2007). Egg water from the amphibian Bufo arenarum induces capacitation-like changes in homologous spermatozoa. Developmental Biology 306, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf D, O’Brien ED, Cabada MO, Visconti PE & Arranz SE (2009). Egg water from the amphibian Bufo arenarum modulates the ability of homologous sperm to undergo the acrosome reaction in the presence of the vitelline envelope. Biology of Reproduction 80, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf D, O’Brien E, Maidagán PM, Morales ES, Visconti PE & Arranz SE (2014). Calcineurin regulates progressive motility activation of Rhinella (Bufo) arenarum sperm through dephosphorylation of PKC substrates. Journal of Cellular Physiology 229, 1378–1386. [DOI] [PubMed] [Google Scholar]
- Krawetz SA (2005). Paternal contribution: new insights and future challenges. Nature Reviews Genetics 6, 633–642. [DOI] [PubMed] [Google Scholar]
- *.Kubo-Irie M, Irie M, Nakazawa T & Mohri H (2003). Ultrastructure and function of long and short sperm in Cicadidae (Hemiptera). Journal of Insect Physiology 49, 983–991. [DOI] [PubMed] [Google Scholar]
- *.Kuzan FB, Fleming AD & Seidel GE Jr. (1984). Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome reacted) spermatozoa of the rabbit. Fertility and Sterility 41, 766–770. [DOI] [PubMed] [Google Scholar]
- *.La Spina F, Puga Molina LC, Romarowski A, Vitale AM, Falzone TL, Krapf D, Hirohashi N & Buffone MG (2016). Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Developmental Biology 411, 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.LaFlamme BA, Ravi Ram K & Wolfner MF (2012). The Drosophila melanogaster seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genetics 8, e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnsteiner F, Weismann T & Patzner RA (1995). Composition of the ovarian fluid in 4 salmonid species - Oncorhynchus mykiss, Salmo trutta lacusris, Salvelinus alpines and Hucho hucho. Reproduction Nutrition Development 35, 465–474. [DOI] [PubMed] [Google Scholar]
- *.Lambert CC (1982). The ascidian sperm reaction. American Zoologist 22, 841–849. [Google Scholar]
- Lefebvre R, Fan J, Chevalier S, Sullivan R, Carmona E & Manjunath P (2007). Genomic structure and tissue-specific expression of human and mouse genes encoding homologues of the major bovine seminal plasma proteins. Molecular Human Reproduction 13, 45–53. [DOI] [PubMed] [Google Scholar]
- Lehnert SJ, Butts IAE, Flannery EW, Peters KM, Heath DD & Pitcher TE (2017a). Effects of ovarian fluid and genetic differences on sperm performance and fertilization success of alternative reproductive tactics in Chinook salmon. Journal of Evolutionary Biology 30, 1236–1245. [DOI] [PubMed] [Google Scholar]
- Lehnert SJ, Heath DD, Devlin RH & Pitcher TE (2017b). Post-spawning sexual selection in red and white Chinook salmon (Oncorhynbchus tshawytscha). Behavioral Ecology 28, 1–10. [Google Scholar]
- Liberti J, Baer B & Boomsma JJ (2016). Queen reproductive tract secretions enhance sperm motility in ants. Biology Letters 12, 20160722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Liberti J, Baer B & Boomsma JJ (2018). Rival seminal fluid induces enhanced sperm motility in a polyandrous ant. BMC Evolutionary Biology 18, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lin M & Rodger JC (1999). Acrosome formation during sperm transit through the epididymis in two marsupials, the tammar wallaby (Macropus eugenii) and the brushtail possum (Trichosurus vulpecula). Journal of Anatomy 194, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke E & Michalik P (2012). Formation of primary sperm conjugates in a haplogyne spider (Caponiidae, Araneae) with remarks on the evolution of sperm conjugation in spiders. Arthropod Structure and Development 41, 561–573. [DOI] [PubMed] [Google Scholar]
- *.Liu H & Kubli E (2003). Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proceedings of the National Academy of Sciences, USA 100, 9929–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G, Sottile L, Viscuso R, Giuffrida A & Privitera R (1993). Ultrastructural changes in sperm of Eyprepocnemis plorans (Charpentier) (Orthoptera: Acrididae) during storage of gametes in female genital tract. Invertebrate Reproduction and Development 24, 1–6. [Google Scholar]
- Lupetti P, Mercati D & Dallai R (2001). The sperm glycocalyx of Pezotettix giornai (Rossi) (Insecta: Orthoptera) after quick-freeze, deep-etching. Italian Journal of Anatomy and Embryology 106(Suppl 2), 181–188. [PubMed] [Google Scholar]
- Lüpold S & Pitnick S (2018). Sperm form and function: what do we know about the role of sexual selection? Reproduction 155, 229–243. [DOI] [PubMed] [Google Scholar]
- Lüpold S, Manier MK, Berben KS, Smith KJ, Daley BD, Buckley SH, Belote JM & Pitnick S (2012). How multivariate ejaculate traits determine competitive fertilization success in Drosophila melanogaster. Current Biology 22, 1667–1672. [DOI] [PubMed] [Google Scholar]
- Lüpold S, Pitnick S, Berben KS, Blengini C, Belote JM & Manier MK (2013). Female mediation of competitive fertilization success in Drosophila melanogaster. Proceedings of the National Academy of Sciences USA 110, 10693–10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüpold S, Manier MK, Puniamoorthy N, Schoff C, Starmer WT, Luepold SH, Belote JM & Pitnick S (2016). How sexual selection can drive the evolution of costly sperm ornamentation. Nature 533, 535–538. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhu Y, Li C, Xue P, Zhao Y, Chen S, Yang F & Miao L (2014). Characterisation of Caenorhabditis elegans sperm transcriptome and proteome. BMC Genomics 15, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R, Laurent LC & Baccarelli AA (2016). Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Human Reproduction Update 22, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Magarey GM & Mate KE (2003). Fertilization follwing intracytoplasmic sperm injection of in vivo and in vitro matured oocytes from an Australian marsupial, the tammar wallaby (Macropus eugenii). Zygote 11, 339–346. [DOI] [PubMed] [Google Scholar]
- Makielski SK (1966). The structure and maturation of the spermatozoa of Sciara coprophila. Journal of Morphology 118, 11–42. [DOI] [PubMed] [Google Scholar]
- Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT & Pitnick S (2010). Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 328, 354–357. [DOI] [PubMed] [Google Scholar]
- Manier MK, Belote JM, Berben KS, Lüpold S, Ala-Honkola O, Collins WF & Pitnick S (2013a). Rapid diversification of sperm precedence traits and processes among three sibling Drosophila species. Evolution 67–8, 2348–2362. [DOI] [PubMed] [Google Scholar]
- Manier MK, Lüpold S, Belote JM, Starmer WT, Berben KS, Ala-Honkola O, Collins WF & Pitnick S (2013b). Postcopulatory sexual selection generates speciation phenotypes in Drosophila. Current Biology 23, 1853–1862. [DOI] [PubMed] [Google Scholar]
- Manier MK, Lüpold S, Pitnick S & Starmer WT (2013c). An analytical framework for determining the fertilization set and investigating fertilization bias from multiple sperm-storage organs. The American Naturalist 182, 552–561. [DOI] [PubMed] [Google Scholar]
- *.Mann T (1984). Spermatophores. Springer Verlag, New York. [Google Scholar]
- *.Mann T, Martin AW Jr. & Thiersch JB (1970). Male reproductive tract, spermatophores and spermatophoric reaction in the giant octopus of the North Pacific, Octopus dofleini martini. Proceedings of the Royal Society, Series B 175, 31–61. [DOI] [PubMed] [Google Scholar]
- *.Manning A (1967). The control of sexual receptivity in female Drosophila. Animal Behaviour 15, 239–250. [DOI] [PubMed] [Google Scholar]
- Markow TA (1988). Drosophila males provide a material contribution to offspring sired by other males. Functional Ecology 2, 77–79. [Google Scholar]
- Markow TA, Coppola A & Watts TD (2001). How Drosophila make eggs: it is elemental. Proceedings of the Royal Society, Series B 268, 1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mate KE & Rodger JC (1996). Capacitation and the acrosome reaction in marsupial spermatozoa. Reproduction Fertility and Development 8, 595–603. [DOI] [PubMed] [Google Scholar]
- *.Mate KE, Sidhu KS, Molinia FC, Glazier AM & Rodger JC (2000). Sperm binding and penetration of the zona pellucida in vitro but not sperm-egg fusion in an Australian marsupial, the brushtail possum (Trichosurus vulpecula). Zygote 8, 189–196. [DOI] [PubMed] [Google Scholar]
- Matzke-Karasz R, Smith RJ & Heb M (2017). Removal of extracellular coat from giant sperm in female receptacle induces motility in Mytilocyris mytiloides (Cyprididae, Ostracoda, Crustacea). Cell and Tissue Research 368, 171–186. [DOI] [PubMed] [Google Scholar]
- Matzuk MM & Lamb DJ (2008). The biology of infertility: research advances and clinical challenges. Nature Medicine 14, 1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E (1942). Systematics and the Origin of Species. Columbia University Press, New York. [Google Scholar]
- McDonough C, Whittington E, Pitnick S & Dorus S (2016). Male and female reproductive system proteomics: elucidating the molecular basis of postmating/ prezygotic reproductive barriers. Journal of Proteomics 135, 26–37. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG & Wolfner MF (2004). Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Current Biology 14, 1509–1514. [DOI] [PubMed] [Google Scholar]
- *.Michalik P (2007). Spermatozoa and spermiogenesis of Liphistius cf. phuketensis (Mesothelae, Araneae, Arachnida) with notes on phylogenetic implications. Arthropod Structure and Development 36, 327–335. [DOI] [PubMed] [Google Scholar]
- *.Michalik P, Haupt J & Alberti G (2004). On the occurrence of coenospermia in mesothelid spiders (Araneae: Heptathelidae). Arthropod Structure and Development 33, 173–181. [DOI] [PubMed] [Google Scholar]
- *.Michalik P, Reiher W, Tintelnot-Suhm M, Coyle FA & Alberti G, (2005). Female genital system of the folding-trapdoor spider Antrodiaetus unicolor (Hentz, 1842) (Antrodiaetidae, Araneae): ultrastructural study of form and function with notes on reproductive biology of spiders. Journal of Morphology 263, 284–309. [DOI] [PubMed] [Google Scholar]
- Miller RL (1985). Sperm chemo-orientation in the Metazoa In Biology of Fertilization. Vol. 2, Biology of the Sperm (eds Metz CB and Monroy A), pp. 275–337. Academic Press, New York. [Google Scholar]
- Miller GT & Pitnick S (2002). Sperm-female coevolution in Drosophila. Science 298, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF, Hill RZ, Bautista DM, Kirichok Y & Lishko PV (2016). Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Miranda PV, Allaire A, Sosnik J & Visconti PE (2009). Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biology of Reproduction 80, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr SE, Smith JA, Shamu CE, Neumüller RA & Perrimon N (2014). RNAi screening comes of age: improved techniques and complementary approaches. Nature Reviews Molecular and Cell Biology 15, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monclus MA & Fornes MW (2016). Sperm conjugation in mammal reproductive function: different names for the same phenomenon? Molecular Reproduction and Development 83, 884–896. [DOI] [PubMed] [Google Scholar]
- *.Moore HD & Taggart DA (1993). In vitro fertilization and embryo culture in the grey short-tailed opossum, Monodelphis domestica. Journal of Reproduction and Fertility 98, 267–274. [DOI] [PubMed] [Google Scholar]
- Moore HD & Taggart DA (1995). Sperm pairing in the opossum increases the efficiency of sperm movement in a viscous environment. Biology of Reproduction 52, 947–953. [DOI] [PubMed] [Google Scholar]
- Moricard R (1950). Penetration of the seprmatozoon in vitro into the mammalian ovum oxydo potential level. Nature 165, 763. [DOI] [PubMed] [Google Scholar]
- Morisawa M & Yoshida M (2005). Activation of motility and chemotaxis in the spermatozoa: from invertebrates to humans. Reproductive Medicine and Biology 4, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Howarth B Jr., Crim JW, Rodriguez de Cordoba S, Esponda P & Bedford JM (1987). Specificity of sperm-binding Wolffian duct proteins in the rooster and their persistence on spermatozoa in the female host glands. Journal of Experimental Zoology 242, 189–198. [DOI] [PubMed] [Google Scholar]
- *.Morrow EH (2004). How the sperm lost its tail: the evolution of aflagellate sperm. Biological Reviews 79, 795–814. [DOI] [PubMed] [Google Scholar]
- Mothes U & Seitz K-A (1981). The transformation of male sex cells of Tetranychus urticae K (Acari, Tetranychidae) during passage from the testis to the oocytes: an electron microscopic study. International Journal of Invertebrate Reproduction 4, 81–94. [Google Scholar]
- *.Muro Y, Hasuwa H, Isotani A, Miyata H, Yamagata K, Ikawa M, Yanagimachi R & Okabe M (2016). Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biology of Reproduction 94, 80. [DOI] [PubMed] [Google Scholar]
- *.Nakanishi T, Ikawa M, Yamada S, Parvinen M, Baba T, Nishimune Y & Okabe M (1999). Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Letters 449, 277–283. [DOI] [PubMed] [Google Scholar]
- *.Ndiaye M, Mattei X & Thiaw OT (1997). Maturation of mosquito spermatozoa during their transit throughout the male and female reproductive systems. Tissue & Cell 29(6), 675–678. [DOI] [PubMed] [Google Scholar]
- Neff BD & Pitcher TE (2005). Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Molecular Ecology 14, 19–38. [DOI] [PubMed] [Google Scholar]
- Neubaum DM & Wolfner MF (1999). Wise, winsome, or weird? Mechanisms of sperm storage in female animals. Current Topics in Developmental Biology 41, 67–97. [DOI] [PubMed] [Google Scholar]
- *.Newport G (1851). On the impregnation of the ovum in the amphibian. Philosophical Transactions of the Royal Society of London (First Series Part 1) 141, 169–242. [Google Scholar]
- Nicastro D, McIntosh JR & Baumeister W (2005). 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. Proceedings of the National Academy of Sciences, USA 102, 15889–15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Fricke C & Arnqvist G (2003). The effects of male and female genotype on variance in male fertilization success in the red flour beetle (Tribolium castaneum). Behavioral Ecology and Sociobiology 53, 227–233. [Google Scholar]
- Nixon B, Ecroyd HW, Dacheux J-L & Jones RC (2011). Monotremes provide a key to understanding the evolutionary significance of epididymal sperm maturation. Journal of Andrology 32, 665–671. [DOI] [PubMed] [Google Scholar]
- Nixon B, Ewen KA, Krivanek KM, Clulow J, Kidd G, Ecroyd HW & Jones RC (2014). Post-testicular sperm maturation and identification of an epididymal protein in the Japanese quail (Coturnix coturnix japonica). Reproduction 147, 265–277. [DOI] [PubMed] [Google Scholar]
- Nixon B, Anderson AL, Smith ND, McLeod R & Johnston SD (2016a). The Australian saltwater crocodile (Crocodylus porosus) provides evidence that the capacitation of spermatozoa may extend beyond the mammalian lineage. Proceedings of the Royal Society, Series B 283, 20160495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B, Ecroyd HW, Dacheux J-L, Dacheux F, Labas V,Johnston SD & Jones RC (2016b). Formation and dissociation of sperm bundles in monotremes. Biology of Reproduction 95(91), 1–11. [DOI] [PubMed] [Google Scholar]
- Nixon B, De luliis GN, Hart HM, Zhou W, Mathe A, Bernstein IR, Anderson AL, Stanger SJ, Skerrett-Byrne DA, Jamaluddin MFB, Almazi JG, Bromfield EG, Larsen MR & Dun MD (2019a). The proteomic profiling of mouse epididymosomes reveals their contributions to post-testicular sperm maturation. Molecular and Cellular Proteomics 18, S91–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B, Johnston SD, Skerrett-Byrne DA Anderson AL, Stanger SJ, Bromfield EG, Martin JH, Hansbro PM & Dun MD (2019b). Modification of crocodile seprmatozoa refutes the tenet that post-testicular sperm maturation is restricted to mammals. Molecular and Cellular Proteomics 18, S59–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ó Foighil D (1985a). Fine structure of Lasaea subviridis and Mysella tumida sperm (Bivalvia: Galeommatacea). Zoomorphology 105, 125–132. [Google Scholar]
- Ó Foighil D (1985b). Sperm transfer and storage in the brooding bivalve Mysella tumida. Biological Bulletin 169, 602–614. [Google Scholar]
- O’Rand MG (1972). In vitro fertilization and capacitation-like interaction in the hydroid Campanularia flexuosa. Journal of Experimental Zoology 182, 299–305. [DOI] [PubMed] [Google Scholar]
- O’Rand MG (1974). Gamete interaction during fertilization in Campanularia - the female epithelial cell surface. American Zoologist 14, 487–493. [Google Scholar]
- *.O’Rand MG & Miller RL (1974). Spermatozoan vesicle loss during penetration of the female gonangium in the hydroid Campanularia flexuosa. Journal of Experimental Zoology 188, 179–193. [DOI] [PubMed] [Google Scholar]
- Oh KP & Badyaev AV (2006). Adaptive genetic complementarity in mate choice coexists with selection for elaborate sexual traits. Proceedings of the Royal Society, Series B 273, 1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ohto U, Ishida H, Krayukhina E, Uchiyama S, Inoue N & Shimizu T(2016). Structure of IZUMO1-JUNO reveals sperm-oocyte recognition during mammalian fertilization. Nature 534, 566–569. [DOI] [PubMed] [Google Scholar]
- *.Okabe M (2014). Mechanism of fertilization: a modern view. Experimental Animals 63, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Okabe M, Adachi T, Takada K, Oda H, Yagasaki M, Kohama Y & Mimura T (1987). Capacitation-related changes in antigen distribution on mouse sperm heads and its relation to fertilization rate in vitro. Journal of Reproductive Immunology 11, 91–100. [DOI] [PubMed] [Google Scholar]
- Oliver M & Evans JP (2014). Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate. Proceedings of the Royal Society, Series B 281, 20140148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Oliver JH Jr. & Brinton LP (1971). Sperm maturation in ticks: an example of capacitation in invertebrates? Proceedings of the Third International Congress of Acarology, Junk W, The Hague, pp. 733–737. [Google Scholar]
- Orr TJ & Brennan PLR (2015). Sperm storage: distinguishing selective processes and evaluating criteria. Trends in Ecology and Evolution 30, 261–272. [DOI] [PubMed] [Google Scholar]
- Osanai M & Isono M (1997). Dissociation of eusperm bundles by acids, especially by succinate accumulated in the spermatophore of the silkmoth, Bombyx mori. Invertebrate Reproduction and Development 31, 99–108. [Google Scholar]
- Osanai M, Kasuga H & Aigaki T (1989a). Induction of motility of apyrene sperm bundles of the silkmoth, Bombyx mori, by initiatorin and trypsin. Invertebrate Reproduction and Development 15, 97–103. [Google Scholar]
- *.Osanai M Kasuga H & Aigaki T (1989b). Isolations ofeupyrene sperm bundles and apyrene spermatozoa from seminal fluid of the silkmoth, Bombyx mori. Journal of Insect Physiology 35, 401–408. [Google Scholar]
- Ota K, Heg D, Hori M & Kohda M (2010). Sperm phenotypic plasticity in a cichlid: a territorial male’s counterstrategy to spawning takeover. Behavioral Ecology 21, 1293–1300. [Google Scholar]
- Pacey AA (2009). Sperm, human fertility and society In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 565–597. Academic Press, London. [Google Scholar]
- Palumbi SR (2009). Speciation and the evolution of gamete recognition genes: pattern and process. Heredity 102, 66–76. [DOI] [PubMed] [Google Scholar]
- Parker GA (1970). Sperm competition and its evolutionary consequences in the insects. Biological Reviews 45, 526–567. [Google Scholar]
- Parker GA (1979). Sexual selection and sexual conflict In Sexual Selection and Reproductive Competition in Insects (eds Blum MS and Blum NA), pp. 123–166. Academic Press, New York. [Google Scholar]
- Pattarini JM, Starmer WT, Bjork A & Pitnick S (2006). Mechanisms underlying the sperm quality advantage in Drosophila melanogaster. Evolution 60, 2064–2080. [PubMed] [Google Scholar]
- *.Pemberton AJ, Noble LR & Bishop JDD (2003). Frequency dependence in matings with water-borne sperm. Journal of Evolutionary Biology 16, 289–301. [DOI] [PubMed] [Google Scholar]
- Peng J, Chen S, Büsser S, Liu H, Honegger T & Kubli E (2005). Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Current Biology 15, 207–213. [DOI] [PubMed] [Google Scholar]
- Phillips DM (1966a). Fine structure of Sciara coprophila sperm. Journal of Cell Biology 30, 499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DM (1966b). Observations on spermiogenesis in the fungus gnat Sciara coprophila sperm. Journal of Cell Biology 30, 477–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard A (1980). Spermatogenesis and sperm-spermatheca relations in Spirorbis spirorbis (L.). International Journal of Invertebrate Reproduction 2, 73–83. [Google Scholar]
- *.Pincus G & Enzmann EV (1934). Can mammalian eggs undergo normal development in vitro? Proceedings of the National Academy of Sciences, USA 20, 121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S & Hosken DJ (2010). Postcopulatory sexual selection In Evolutionary Behavioral Ecology (eds Westneat DF and Fox CW), pp. 379–399. Oxford University Press, New York. [Google Scholar]
- Pitnick S & Karr TL (1998). Paternal products and by-products in Drosophila development. Proceedings of the Royal Society, Series B 265, 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pitnick S, Markow TA & Spicer GS (1995a). Delayed male maturity is a cost of producing large sperm in Drosophila. Proceedings of the National Academy of Sciences, USA 92, 10614–10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pitnick S, Spicer GS & Markow TA (1995b). How long is a giant sperm? Nature 375, 109. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Spicer GS & Markow TA (1997). Phylogenetic examination of male ejaculatory donations in Drosophila. Evolution 51, 833–845. [DOI] [PubMed] [Google Scholar]
- *.Pitnick S, Markow TA & Spicer GS (1999). Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution 53, 1804–1822. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Hosken DJ & Birkhead TR (2009a). Sperm morphological diversity In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 69–149. Academic Press, London. [Google Scholar]
- Pitnick S, Wolfner MF & Suarez SS (2009b). Ejaculate-female and sperm-female interactions In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 247–304. Academic Press, London. [Google Scholar]
- Pizzari T & Foster KR (2008). Sperm sociality: cooperation, altruism, and spite. PLoS Biology 6, e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Cornwallis CK, Løvlie H & Birkhead TR (2003). Sophisticated sperm allocation in male fowl. Nature 426, 70–74. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Dean R, Pacey A & Moore H (2008). Bonsall. &, M. B, The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends in Ecology and Evolution 23, 131–140. [DOI] [PubMed] [Google Scholar]
- Poiani A (2006). Complexity of seminal fluid: a review. Behavioral Ecology and Sociobiology 60, 289–310. [Google Scholar]
- Poland V, Eubel H, King M, Solheim C, Millar AH & Baer B (2011). Stored sperm differs from ejaculated sperm by proteome alterations associated with energy metabolism in the honeybee Apis mellfera. Molecular Ecology 20, 2643–2654. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K & Wolfner MF (2007). Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integrative and Comparative Biology 47, 427–445. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K & Wolfner MF (2009). A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proceedings of the National Academy of Sciences, USA 106, 15384–15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ravi Ram K, Ji S & Wolfner MF (2005). Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochemistry and Molecular Biology 35, 1059–1071. [DOI] [PubMed] [Google Scholar]
- *.Reger JF (1961). The fine-structure of spermatids from the tick, Amblyomma dissimili. Journal of Ultrastructure Research 5, 584–599. [DOI] [PubMed] [Google Scholar]
- *.Reger JF (1962). A fine-structure study on spermiogenesis in the tick, Amblyomma dissimiie with special reference to the development of motile processes. Journal of Ultrastructure Research 7, 550–565. [DOI] [PubMed] [Google Scholar]
- Reger JF (1963). Spermiogenesis in the tick, Amblyomma dissimili, as revealed by electron microscopy. Journal of Ultrastructure Research 8, 607–621. [DOI] [PubMed] [Google Scholar]
- *.Reger JF (1970). Some aspects of the fine structure of filiform spermatozoa (ostracod, Cypridopsis sp.) lacking tubule sub-structure In: Comparative Spermatology (Ed. by Baccetti B), pp. 237–245. New York: Academic Press. [Google Scholar]
- *.Reger JF (1974). The origin and fine structure of cellular processes in spermatozoa of the tick, Dermacentor andersoni. Journal of Ultrastructure Research 48, 420–434. [Google Scholar]
- Reinhardt K, Breunig HG, Uchugonova A & König K (2015a). Sperm metabolism is altered during storage by female insects: evidence from two-photon autofluorescence lifetime measurements in bedbugs. Proceedings of the Royal Society, Series B 12, 20150609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K, Dobler R & Abbott J (2015b). An ecology of sperm: sperm diversification by natural selection. Annual Review of Ecology and Systematics 46, 435–459. [Google Scholar]
- *.Renieri T & Talluri MV (1974). Sperm modification in the female ducts of a grasshopper. Monitore Zoologico Italiano 8, 1–9. [Google Scholar]
- *.Richings NM, Shaw G, Temple-Smith PD & Renfree MB (2004). Intra-cytoplasmic sperm injection in a marsupial. Reproduction 128, 595–605. [DOI] [PubMed] [Google Scholar]
- *.Riemann JG (1970). Metamorphosis of sperm of the cabbage looper, Trichoplusia ni, during passage from the testes to the female spermathecae In Comparative Spermatology (ed. Baccetti B), pp. 321, 137–331. Accademia Nazionale Dei Lincei, Quaderono N. [Google Scholar]
- *.Riemann JG & Giebultowicz JM (1991). Secretion in the upper vas deferens of the gypsy moth correlated with the circadian rhythm of sperm release from the testes. Journal of Insect Physiology 37, 53–62. [Google Scholar]
- Riemann JG & Giebultowicz JM (1992). Sperm maturation in the vasa deferentia of the gypsy-moth, Lymantria dispar (Lepidoptera, Lymantriidae). International Journal of Insect Morphology andf Embryology 21, 271–284. [Google Scholar]
- *.Riemann JG & Thorson BJ (1971). Sperm maturation in the male and female genital tracts of Anagasta kühniella (Lepidoptera: Pyralididae). International Journal of Insect Morphology andf Embryology 1, 11–19. [Google Scholar]
- Ritchie MG (2007). Sexual selection and speciation. Annual Review of Ecology, Evolution and Systematics 38, 79–102. [Google Scholar]
- *.Robison WG (1970). Unusual arrangement of microtubules in relation to mechanisms of sperm movement In Comparative Spermatology (ed. Baccetti B), pp. 311–320. Academic Press, New York. [Google Scholar]
- *.Rodger JC (1994). Prefertilization gamete maturation events in marsupials. Reproduction Fertility and Development 6, 473–483. [DOI] [PubMed] [Google Scholar]
- Rodger JC & Bedford JM (1982). Separation of sperm pairs and sperm-egg interaction in the opossum, Virginiana didephis. Journal of Reproduction and Fertility 64, 171–179. [DOI] [PubMed] [Google Scholar]
- Roldan ERS & Gomendio M (2009). Sperm and conservation In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 539–564. Academic Press, London. [Google Scholar]
- Rosengrave P, Gemmell NJ, Metcalf V, McBride K & Montgomerie R (2008). A mechanism for cryptic female choice in Chinook salmon. Behavioral Ecology 19, 1179–1185. [Google Scholar]
- Rosengrave P, Taylor H, Montgomerie R, Metcalf V, McBride K & Gemmell NJ (2009). Chemical composition of seminal and ovarian fluids of Chinook salmon (Oncorhynchus tshawytscha) and their effects on sperm motility traits. Comparative Biochemistry and Physiology, Part A 152, 123–129. [DOI] [PubMed] [Google Scholar]
- *.Rothschild L (1961). Structure and movements of tick spermatozoa (Arachnida: Acari). Quarterly Journal of Microscopic Science 102, 239–247. [Google Scholar]
- Rudolfsen G, Figenschou L, Folstad I, Tveiten H & Figenschou M (2006). Rapid adjustments of sperm characteristics in relation to social status. Proceedings of the Royal Society, Series B 273, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttner F & Koeniger G (1971). Die füllung der spermatheka der bienenköniginaktive wanderung oder passiver transport der spermatozoen? Zeitschrift für Vergleichende Physiologie 72, 411–422. [Google Scholar]
- *.Sahara K & Takemura Y (2003). Application of artificial insemination technique to eupyrene and/or apyrene sperm in Bombyx mori. Journal of Expermental Zoology 297A, 196–200. [DOI] [PubMed] [Google Scholar]
- *.Saling PM & Storey BT (1979). Mouse gamete interaction during fertilization in vitro: chlortetracycline as a fluorescent probe for the mouse acrosome reaction. Journal of Cell Biology 83, 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa K (2007). Sperm bundle and reproductive organs of carabid beetles tribe Pterostichini (Coleoptera: Carabidae). Naturwissenschaften 94, 384–391. [DOI] [PubMed] [Google Scholar]
- *.Sastry AN (1979). Pelecypoda (excluding Ostreidae) Pp. 113–292 in Reproduction of Marine Invertebrates, Vol. V. Giese AC and Pearse JS, eds. New York: Academic Press. [Google Scholar]
- Schnakenberg SL, Matias WR & Siegal ML (2011). Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biology 9, e1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schubert LF, Krüger S, Moritz GB & Schubert V (2017). Male reproductive system and spermatogenesis of Limodromus assimilis (Paykull 1790). PLoS One 12, e0180492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi MG, Bigliardi E & Giusti F (1989). Morphological modifications in stored heterospermatozoa of Oxyloma elegans (Pulmonata: Stylommatophora). Journal of Ultrastructure and Molecular Structure Research 102, 82–86. [Google Scholar]
- *.Setiadi D, Lin M &, Rodger JC (1997). Posttesticular development of spermatozoa of the tammar wallaby (Macropus eugenii). Journal of Anatomy 190, 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sever DM (2002). Female sperm storage in amphibians. Journal of Experimental Zoology 292, 165–179. [DOI] [PubMed] [Google Scholar]
- Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL & Rando OJ (2018). Small RNAs are trafficked from the epididymis to developing mammalian sperm. Developmental Cell 46, 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Shepherd J, Levine S & Hall JD (1982a). Maturation of tick spermatozoa in vitro. International Journal of Invertebrate Reproduction 4, 311–321. [Google Scholar]
- *.Shepherd J, Oliver JH Jr. &, Hall JD (1982b). A polypeptide from male accessory glands which triggers maturation of tick spermatozoa. International Journal of Invertebrate Reproduction 5, 129–137. [Google Scholar]
- Shivers CA & James JM (1970a). Capacitation of frog sperm. Nature 227, 183–184. [DOI] [PubMed] [Google Scholar]
- *.Shivers CA & James JM (1970b). Morphology and histochemistry of the oviduct and egg-jelly layers in the frog, Rana pipiens. Anatomical Record 166, 541–556. [DOI] [PubMed] [Google Scholar]
- *.Shivers CA & James JM (1971). Fertilization of antiserum-inhibited frog eggs with “capacitated” sperm. Biology of Reproduction 5, 229–235. [DOI] [PubMed] [Google Scholar]
- *.Sidhu KS, Mate KE, Molinia FC, Glazier AM & Rodger JC (1999a). Secretory proteins from the female reproductive tract of the brushtail possum (Trichosurus vulpecula): binding to sperm and effects on sperm survival in vitro. Reproduction Fertility and Development 11, 329–336. [DOI] [PubMed] [Google Scholar]
- *.Sidhu KS, Mate KE, Molinia FC & Rodger JC (1999b). Induction of thumbtack sperm during coculture with oviduct epithelial cell monolayers in a marsupial, the brushtail possum (Trichosurus vulpecula). Biology of Reproduction 61, 1356–1361. [DOI] [PubMed] [Google Scholar]
- *.Silberglied DR, Shepherd JG & Dickinson JL (1984). Eunuchs: the role of apyrene sperm in Lepidoptera? The American Naturalist 12, 255–265. [Google Scholar]
- Simmons LW (2001). Sperm Competition and its Evolutionary Consequences in the Insects. Princeton University Press, Princeton. [Google Scholar]
- Simmons LW & Fitzpatrick JL (2019). Female genitalia evolve more rapidly and divergently than male genitalia. Nature Communications 10, 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Simmons LW & Moore AJ (2009). Evolutionary quantitative genetics of sperm In Sperm Biology: An Evolutionary Perspective (eds Birkhead TR, Hosken DJ and Pitnick S), pp. 405–434. Academic Press, London. [Google Scholar]
- Simmons LW & Parker GA (1989). Nuptial feeding in insects: mating effort versus paternal investment. Ethology 81, 332–343. [Google Scholar]
- Simmons LW, Roberts JD & Dziminski MA (2009). Egg jelly influences sperm motility in the externally fertilizing frog, Crinia georgiana. Journal of Evolutionary Biology 22, 225–229. [DOI] [PubMed] [Google Scholar]
- Singh A, Buehner NA, Lin H, Baranowski KJ, Findlay GD & Wolfner MF (2018). Long-term interaction between Drosophila sperm and sex peptide is mediated by other seminal proteins that bind only transiently to sperm. Insect Biochemistry and Molecular Biology 102, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Findlay GD, Sitnik JL, Frasheri D, Avila FW & Wolfner MF (2014). Molecular characterization and evolution of a gene family encoding both female- and male-specific reproductive porteins in Drosophila. Molecular Biology and Evolution 31, 1554–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Wong A, Chapman A & Wolfner MF (2015). Sexual conflict and seminal fluid proteins: A dynamic landscape of sexual interactions. Cold Spring Harbor Perspectives in Biology 7, a017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sistina Y, Lin M, Mate KE, Robinson ES & Rodger JC (1993a). The unique stability of the marsupial sperm acrosomal membranes examined by unprotected freeze-thawing and treatment with the detergent triton X-100. Reproduction Fertility and Development 5(1), 14. [DOI] [PubMed] [Google Scholar]
- *.Sistina Y, Lin M, Mate KE & Rodger JC (1993b). Induction of the marsupial acrosome reaction in vitro by treatment with diacylglycerols. Journal of Reproduction and Fertility 99, 335–341. [DOI] [PubMed] [Google Scholar]
- Skerget S, Rosenow M, Polpitiya A, Petritis K, Dorus S & Karr TL (2013). The Rhesus macaque (Macaca mulatta) sperm proteome. Molecular & Cellular Proteomics 12, 3052–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerget S, Rosenow M, Polpitiya A, Petritis K & Karr TL (2015). Sperm proteome maturation in the mouse epididymis. PLoS One 10, e0140650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Smith RJ, Matzke-Karasz R, Kamiya T & De Deckker P (2016). Sperm lengths of non-marine cypridoidean ostracods (Crustacea). Acta Zoologica 97, 1–17. [DOI] [PubMed] [Google Scholar]
- Snook RR (2005). Sperm in competition: not playing by the numbers. Trends in Ecology and Evolution 20, 46–53. [DOI] [PubMed] [Google Scholar]
- Snook RR & Karr TL (1998). Only long sperm are fertilization-competent in six sperm-heteromorphic Drosophila species. Current Biology 8, 291–294. [DOI] [PubMed] [Google Scholar]
- *.Stockley P, Gage MJG, Parker GA & Møller AP (1997). Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. The American Naturalist 149, 933–954. [DOI] [PubMed] [Google Scholar]
- *.Suarez SS (2002). Formation of a reservoir of sperm in the oviduct. Reproduction in Domestic Animals 37, 140–143. [DOI] [PubMed] [Google Scholar]
- Suarez SS (2006). Gamete and zygote transport In Knobil and Neill’s Physiology of Reproduction (ed. Neill JD), pp. 113–145. Elsevier, New York. [Google Scholar]
- Suarez SS (2008). Control of hyperactivation in sperm. Human Reproduction Update 14, 647–657. [DOI] [PubMed] [Google Scholar]
- Suarez SS (2016). Mammalian sperm interactions with the female reproductive tract. Cell and Tissue Research 363, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS & Pacey AA (2006). Sperm transport in the female reproductive tract. Human Reproduction Update 12, 23–37. [DOI] [PubMed] [Google Scholar]
- Sullivan R & Saez F (2013). Epididymosomes, prstasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 146, R21–R35. [DOI] [PubMed] [Google Scholar]
- Sutovsky P (2003). Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microscopy Research and Technique 61, 88–102. [DOI] [PubMed] [Google Scholar]
- Sutovsky P & Song WH (2017). Post-fertilisation sperm mitophagy: the tale of Mitochondrial Eve and Steve. Reproduction Fertilisation and Development 30, 56–63. [DOI] [PubMed] [Google Scholar]
- Swallow JG & Wilkinson GS (2002). The long and short of sperm polymorphisms in insects. Biological Reviews 77, 153–182. [DOI] [PubMed] [Google Scholar]
- Swanson WJ & Vacquier VD (2002). The rapid evolution of reproductive proteins. Nature Reviews Genetics 3, 137–144. [DOI] [PubMed] [Google Scholar]
- *.Takami Y & Sota T (2007). Sperm competition promotes diversity of sperm bundles in Ohomopterus ground beetles. Naturwissenschaften 94, 543–550. [DOI] [PubMed] [Google Scholar]
- *.Takemura Y, Sahara K, Mochida Y & Ohnuma A (2006). Apyrene sperm from the triploid donors restore fecundity of cryopreserved semen in Bombyx mori. Journal of Insect Physiology 52, 1021–1026. [DOI] [PubMed] [Google Scholar]
- Talevi R & Gualtieri R (2010). Molecules involved in sperm-oviduct adhesion and release. Theriogenology 73, 796–801. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Price TAR & Wedell N (2014). Polyandry in nature: a global analysis. Trends in Ecology and Evolution 29, 376–383. [DOI] [PubMed] [Google Scholar]
- *.Temkin MH (1994). Gamete spawning and fertilization in the gymnolaemate bryozoan Membranipora membranacea. Biological Bulletin 187, 143–155. [DOI] [PubMed] [Google Scholar]
- *.Temkin MH & Bortolami SB (2004). Waveform dynamics of spermatozeugmata during the transfer from paternal to maternal individuals of Membranipora membranacea. Biological Bulletin 206, 35–45. [DOI] [PubMed] [Google Scholar]
- Temple-Smith P,. D. & Bedford JM (1980). Sperm maturation and the formation of sperm pairs in the epididymis of the opossum, Didelphis virginiana. Journal of Experimental Zoology 214, 161–171. [DOI] [PubMed] [Google Scholar]
- *.Thaler CD, Miyata H, Haimo LT & Cardullo RA (2013). Waveform generation is controlled by phosphorylation and swimming direction is controlled by Ca2þ in sperm from the mosquito Culex quinquefasciatus. Biology of Reproduction 89, 135. [DOI] [PubMed] [Google Scholar]
- Thomas ML & Simmons LW (2007). Male crickets adjust the viability of their sperm in response to female mating status. The American Naturalist 170, 190–195. [DOI] [PubMed] [Google Scholar]
- Till-Bottraud I, Joly D, LaChaise D & Snook RR (2005). Pollen and sperm heteromorphism: convergence across kingdoms? Journal of Evolutionary Biology 18, 1–18. [DOI] [PubMed] [Google Scholar]
- Tosti E & Ménézo Y (2016). Gamete activation: basic knowledge and clinical applications. Human Reproduction Update 22, 420–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti E, Di Cosmo A, Cuomo A, Di Cristo C & Gragnaniello G (2001). Progesterone induces activation in Octopus vulgaris spermatozoa. Molecular Reproduction and Development 59, 97–105. [DOI] [PubMed] [Google Scholar]
- Tregenza T & Wedell N (2000). Genetic compatibility, mate choice and patterns of parentage. Molecular Ecology 2000, 1013–1027. [DOI] [PubMed] [Google Scholar]
- Tung CK, Hu L, Fiore AG, Ardon F, Hickman DG, Gilbert RO, Suarez SS & Wu M (2015). Microgrooves and fluid flows provide preferential passageways for sperm over pathogen Tritrichomonas foetus. Proceedings of the National Academy of Sciences, USA 112, 5431–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Turner E & Montgomerie R (2002). Ovarian fluid enhances sperm movement in Arctic charr. Journal of Fish Biology 60, 1570–1579. [Google Scholar]
- Tyler F, Harrison XA, Bretman A, Veen T, Rodríguez-Munõz R & Tregenza T (2013). Multiple post-mating barriers to hybridization in field crickets. Molecular Ecology 22, 1640–1649. [DOI] [PubMed] [Google Scholar]
- *.Uhl G (1994). Ultrastructure of the accessory glands in female genitalia of Pholcus phalangioides (Fuesslin, 1771) (Pholcidae; Araneae). Acta Zoologica Stockholm 75, 13–25. [Google Scholar]
- *.Uhl G (1996). Sperm storage secretion of female cellar spiders (Pholcus phalangioides; Araneae): a gel-electrophoretic analysis. Journal of Zoology, London 240, 153–161. [Google Scholar]
- *.Uhl G (2000). Two distinctly different sperm storage organs in female Dysdera erythrina (Araneae: Dysderidae). Arthropod Structure and Development 29, 163–169. [DOI] [PubMed] [Google Scholar]
- *.Ursprung H & Schabtach E (1965). Fertilization in tunicates: loss of the paternal mitochondrion prior to sperm entry. Journal of Experimental Zoology 159, 379–384. [DOI] [PubMed] [Google Scholar]
- Vadnais ML, Galantino-Homer HL & Althouse GC (2007). Current concepts of molecular events during bovine and porcine spermatozoa capacitation. Archives of Andrology 53, 109–123. [DOI] [PubMed] [Google Scholar]
- Villavaso EJ (1975). The role of the spermathecal gland of the boll weevil, Anthonomus grandis. Journal of Insect Physiology 21, 1457–1462. [Google Scholar]
- Visconti PE (2009). Understanding the molecular basis of sperm capacitation through kinase design. Proceedings of the National Academy of Sciences, USA 106, 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscuso R, Barone N, Sottile L & Narcisi L (1996). Spermiolytic activity of the epithlelium of the spermathecal duct of Rhacocleis annulata Fieber (Orthoptera: Tettigoniidae). Internaltional Journal of Insect Morphology andf Embryology 25, 135–144. [Google Scholar]
- *.Viscuso R, Narcisi L, Sottile L & Barone N (1998). Structure of spermatodesms of Orthoptera Tettinonioidae. Tissue & Cell 30, 453–463. [DOI] [PubMed] [Google Scholar]
- *.Viscuso R, Narcisi L, Sottile L & Violetta Brundo M (2001). Role of male accessory glands in spermatodesm reorganization in Orthoptera Tettigonioidae. Tissue & Cell 33, 33–39. [DOI] [PubMed] [Google Scholar]
- *.Viscuso R, Violetta Brundo M & Sottile L (2002). Mode of transfer of spermatozoa in Orthoptera Tettigonioidae. Tissue & Cell 34, 337–348. [DOI] [PubMed] [Google Scholar]
- *.Vöcking O, Uhl G & Michalik P (2013). Sperm dynamics in spiders (Araneae): ultrastructural analysis of the sperm activation process in the garden spider Argiope bruennichi (Scopoli, 1772). PLoS One 8(9), e72660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wake MH & Dickie R (1998). Oviductal structure and function and reproductive modes in amphibians. Journal of Experimental Zoology 282, 477–506. [PubMed] [Google Scholar]
- Ward CR & Kopf GS (1993). Molecular events mediating sperm activation. Developmental Biology 158, 9–34. [DOI] [PubMed] [Google Scholar]
- *.Watanabe A & Onitake K (2002). The urodele egg-coat as the apparatus adapted for the internal fertilization. Zoological Science 19, 1341–1347. [DOI] [PubMed] [Google Scholar]
- *.Watanabe T, Itoh T, Watanabe A & Onitake K (2003). Characteristics of sperm motility induced on the egg-jelly in the internal fertilization of the newt, Cynops pyrrhogaster. Zoological Science 20, 345–342. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kubo H, Takeshima S, Nakagawa M, Ohta M, Kamimura S, Takayama-Watanabe E, Watanabe A & Onitake K (2010). Identification of the sperm motility-initiating substance in the newt, Cynops pyrrhogaster, and its possible relationship with the acrosome reaction during internal fertilization. International Journal of Developmental Biology 54, 591–597. [DOI] [PubMed] [Google Scholar]
- *.Watnick TJ, Jin Y, Matunis E, Kernan MJ & Montell C (2003). A flagellar polycystin-2 homolog required for male fertility in Drosophila. Current Biology 13, 2179–2184. [DOI] [PubMed] [Google Scholar]
- *.Webber HH (1977). Gastropoda: Prosobranchia In Reproduction of Marine Invertebrates, Vol. IV (eds Giese AC and Pearse JS), pp. 1–97. Academic Press, New York. [Google Scholar]
- Werner M & Simmons LW (2008). Insect sperm motility. Biological Reviews 83, 191–208. [DOI] [PubMed] [Google Scholar]
- Westheide W (1988). The ultrastructure of the spermatozoon in Pisioine remota (Annelida: Polychaeta) and its transformation in the receptaculum seminis. Journal of Submicroscopic Cytology and Pathology 20, 169–178. [PubMed] [Google Scholar]
- Whittington E, Zhao Q, Borziak K, Walters JR & Dorus S (2015). Characterisation of the Manduca sexta sperm proteome: genetic novelty underlying sperm composition in Lepidoptera. Insect Biochemistry and Molecular Biology 62, 183–193. [DOI] [PubMed] [Google Scholar]
- *.Williams M, Barratt CL Hill CJ, Warren MA,Dunphy B & Cooke ID (1992). Recovery of artificially inseminated spermatozoa from the fallopian tubes of a woman undergoing total abdominal hysterectomy. Human Reproduction 7, 506–509. [DOI] [PubMed] [Google Scholar]
- *.Wingstrand KG (1988). Comparative spermatology of the Crustacea Entomostraca. 2. Subclass Ostracoda. Biologiske Skrifter Kongelige Danske Videnskabernes Selskab 32, 1–149. [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA & Shen L (2005). Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunology Reviews 206, 306–335. [DOI] [PubMed] [Google Scholar]
- Wolfner MF (1997). Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochemistry and Molecular Biology 27, 179–192. [DOI] [PubMed] [Google Scholar]
- Woolley DM, Crockett RF, Groom WDI & Revell SG (2009). A study of synchronisation between the flagella of bull spermatozoa, with related observations. The Journal of Experimental Biology 212, 2215–2223. [DOI] [PubMed] [Google Scholar]
- *.Yanagimachi R (1982). Requirement of extracellular calcium ions for various stages of fertilization and fertilization-related phenomena in the hamster. Gamete Research 5, 323–344. [Google Scholar]
- Yang Y & Lu X (2011). Drosophila sperm motility in the reproductive tract. Biology of Reproduction 84, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Yapici N, Kim YJ, Ribeiro C & Dickson BJ (2008). A receptor that mediates the post- mating switch in Drosophila reproductive behaviour. Nature 451, 33–37. [DOI] [PubMed] [Google Scholar]
- Yasui Y (1997). A ‘good sperm’ model can explain the evolution of costly multiple mating by females. The American Naturalist 149, 573–584. [Google Scholar]
- *.Yasuzumi G (1979). Spermatogenesis in animals as revealed by electron microscopy. Some modifications of cell surface in developing spermatids of the grasshopper. Monitore Zoologico Italian 13, 265–277. [Google Scholar]
- Yeates SE, Diamond SE, Einum S, Emerson BC, Holt WV & Gage MJG (2013). Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming behavior. Evolution 67(12), 3523–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sturgill D, Parisi M, Kumar S & Oliver B (2007). Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450, 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Han XK, Qi YY, Liu Y & Chen QS (2008). Seasonal effects on apoptosis and proliferation of germ cells in the testes of the Chinese soft-shelled turtle, Pelodiscus sinensis. Theriogenology 69, 1148–1158. [DOI] [PubMed] [Google Scholar]
- Zhang R, Clark AG & Fiumera AC (2013). Natural genetic variation in male reproductive genes contributes to nontransitivity of sperm competitive ability in Drosophila melanogaster. Molecular Ecology 22, 1400–1415. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang P, Bian X, Zhang Q, Ullah S, Waqas Y, Chen X, Liu Y, Chen W, Le Y, Chen B, Wang S & Chen Q (2015). Modification of sperm morphology during long-term sperm storage in the reproductive tract of the Chinese soft-shelled turtle, Pelodiscus sinensis. Scientific Reports 5, 16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. A survey of post-ejaculatory modifications to sperm (PEMS) throughout the kingdom Animalia.





