Abstract
Maternal and paternal obesity and type 2 diabetes are recognized risk factors for the development of metabolic dysfunction in offspring, even when offspring follow a healthy lifestyle. Multiple studies demonstrate that regular physical activity in mothers and fathers has striking beneficial effects on offspring health, including preventing the development of metabolic disease in rodent offspring as they age. Here we review the benefits of maternal and paternal exercise to combat the development of metabolic dysfunction in adult offspring, focusing on offspring glucose homeostasis and adaptations to metabolic tissues. We discuss recent findings on the roles of the placenta and sperm in mediating the effects of parental exercise on offspring metabolic health and discuss mechanisms hypothesized to underlie these beneficial changes. Given the worldwide epidemics of obesity and type 2 diabetes, translation of these findings to humans would provide hope that regular exercise during the reproductive years may limit the vicious cycles of increased metabolic risk being propagated across generations.
A. Introduction
In 2019 the worldwide prevalence of type 2 diabetes was estimated to be 9.3% or 463 million people, and unfortunately, this number is expected to increase to 693 million by 20451. Individual health consequences aside, the currently staggering prevalence of type 2 diabetes has already placed an enormous strain on health care systems, public health, and the global economy; burdens that will undoubtedly be exacerbated if these increases in type 2 diabetes occur as predicted. Obesity is a major risk factor for type 2 diabetes, and the two diseases share many points of overlap in their causes and outcomes, as well as their treatments. Rates of obesity and type 2 diabetes increase as people age, and although these metabolic diseases are often considered preventable, in reality they are complex and arise from a combination of genetic susceptibility and environmental factors. In recent years it has become well established that risk patterns for both obesity and type 2 diabetes can originate as a consequence of alterations in growth and metabolism during critical windows of prenatal and early postnatal development 2. In addition, there is emerging evidence that the father’s metabolic status can affect the health of his offspring 3. Given that rates of obesity and type 2 diabetes are increasing among women and men of child-bearing age, it is important to understand means to reduce the risk of parental transmission of metabolic disease to their offspring.
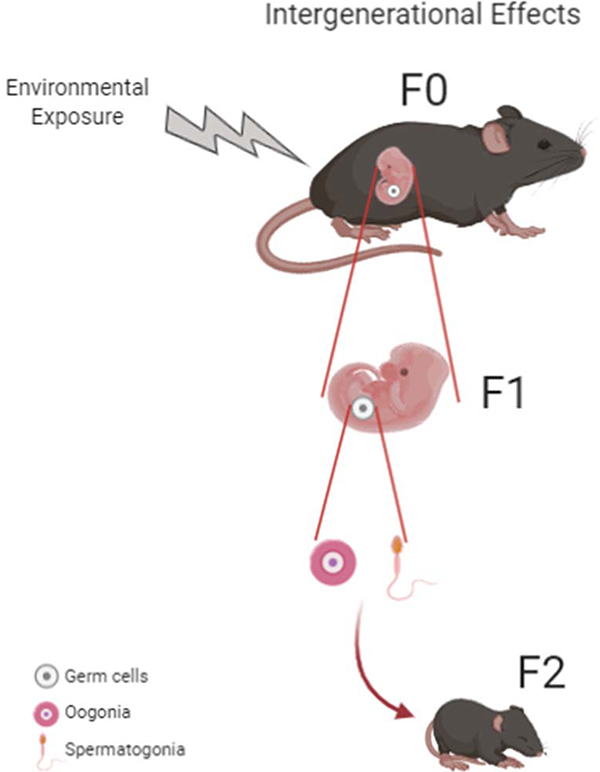
It has long been recognized that exercise has important health benefits for individuals with type 2 diabetes, and regular physical exercise can delay or prevent the onset of this disease 4,5. While there has been extensive investigation into the physiological effects of exercise and the mechanisms of action of exercise on the individual performing the physical activity, there has been less investigation aimed at understanding the effects of parental exercise on offspring health. In this review, we will focus on research investigating the effects of maternal and paternal exercise on offspring metabolic health in adulthood, the time in life when metabolic diseases typically surface. In addition, we will review current literature aimed at understanding the mechanisms mediating offspring phenotype in response to maternal and paternal exercise. While we provide an overview of studies in human subjects, given the generational length of rodents vs human (~2 vs 80 years) 6 and the ability to obtain tissues for molecular studies, most research into the effects of maternal and paternal exercise on the metabolic health of adult offspring have been done using rodent models, and that will be our focus here. The terminology F0 (mother or father), F1 (offspring of F0), and F2 (offspring of F1 and “grand-offspring” of F0) will be used to describe the generations studied. Intergenerational effects are defined as those mediated by direct exposure during pregnancy affecting F1 and F2 offspring (maternal exposure) (Figure 1) or exposure before mating affecting F1 offspring (paternal exposure); while transgenerational effects require persistence of the phenotype, even without direct exposures, affecting F3 offspring (maternal exposure) or F2 offspring (paternal exposure) 2.
Fig 1: Intergenerational Effects of Environmental Exposure.
In females, intergenerational transmission occurs when a dam receives an environmental exposure during pregnancy, affecting not only F0, but also the F1 and F2 generations. Exposures during pregnancy can directly affect the embryo and its progenitor germs cells. During F1 maturation, these germ cells will become the adult gametes that create the F2 generation.
B. Maternal Nutrition and Exercise Effects on Offspring Health
Human Studies of Maternal Nutrition and Offspring Metabolism.
During pregnancy, insults to the intrauterine environment such as maternal under-nutrition, over-nutrition and obesity are significant factors that contribute to the development of obesity and type 2 diabetes in offspring 2,7. Currently, the prevalence of obesity in pregnant women hovers around 30% in western countries, with approximately 40% of women gaining an excessive amount of weight during their pregnancy 8. Conversely, in poorer regions of the world like South-central and Southeast Asia and Sub-Saharan Africa, the rates of low maternal weight and maternal malnutrition range between 15 and 20% 9. Both extremes of the maternal weight spectrum present risks to the mother and are associated with a suite of complications for offspring, with potentially life-long consequences.
Human studies exploring the effects of famine during pregnancy show that individuals exposed to maternal under-nutrition in utero have decreased glucose tolerance in their adult life 10,11 and are more prone to developing obesity and diabetes 12. The detrimental effects of maternal over-nutrition and obesity on the metabolic health of offspring have been similarly well-established 13–17. Maternal obesity during pregnancy in humans is associated with numerous health outcome in offspring including: increased risk of certain structural, congenital abnormalities in infants 16; increased BMI, waist circumference and adiposity in early childhood 17; and increased risk of developing diabetes during adulthood 15. Thus, a poor maternal nutritional environment can impact the intrauterine milieu in a manner that results in numerous effects that are metabolic in nature and are present throughout the lifetime of the offspring.
Human Studies of Maternal Exercise and Offspring Metabolism.
The effects of exercise during pregnancy on maternal and fetal outcomes have been extensively investigated in humans, with a wealth of research showing that exercise during pregnancy is safe and beneficial to both the mother and fetus (e.g. reviews 18,19). As shown in Table 1, which summarizes four different meta-analysis studies, both aerobic and strength training reduce the risk of excessive gestational weight gain, gestational diabetes and hypertension in mothers 18–21. As in all human exercise studies, it should be noted that there are limitations on the accuracy of daily exercise data by self-report questionnaires, although this has been improved in recent years with wearable devices.
Table 1:
Effects of exercise in women during pregnancy in systematic review and meta-analysis studies.
| Study (PMID) | Population | Gestational Age for Enrolment | Training Cessation | Protocol | Mode of Exercise | Major Findings |
|---|---|---|---|---|---|---|
| Davenport et al, 2018 (30337463) | 106 studies met the criteria: N=273,182. 65 RCTs, 9 non-RCTs, 13 cohorts, 11 cross-sectional and 8 case-control studies. Studies dates: up to 6 January 2017. 27 countries and five continents. |
Anytime during pregnancy | To the end of the 3rd trimester | Majority of the studies identified the exercise as moderate intensity. Frequency of exercise ranged from 1–7 days/week, duration ranged from 10–90min/session. | Walking, swimming, cycling, water gymnastics, resistance training, stretching, yoga or pelvic floor muscle training. | Reduced risk of developing GDM, GH and PE. Pregnant women need to accumulate at least 600 MET-min/week of moderate-intensity exercise (for example: 140 min of brisk walking, water aerobics, stationary cycling or resistance training). |
| Ming et al, 2018 (30419848) | 8 RCTs studies met the criteria: N=3256. Studies dates: 2011–2014. European countries. |
6 – 20 weeks | To the end of 3rd trimester | Light-moderate intensity exercise performed 3 times/week (only one study performed exercise once/week), duration of 35–60min each time. | Aerobic and/or resistance training/muscle strength. | Reduced risk for gestational diabetes & decreased gestational weight gain |
| Beetham et al, 2019 (31391016) | 13 studies met the criteria: 5 RCTs studies N=623, 8 cohorts studies N=7225. Studies dates: 1983–2018. RCTs: Canada, Brazil, New Zealand, China. Cohorts: Australia, USA, Denmark, United Kingdom. |
10 – 20 wks (2 studies were retrospectives; 1 not reported) | During the 3rd trimester | Vigorous/high-intensity exercise. RCTs: performed a minimum of 3 times/week duration of 25–60min each time. Cohorts: performed 1–3 times/week (1 study was unable to evaluate), duration of more than 3min to 180min each time. |
Walking, running, swimming, circuit training, interval training, weight lifting, or plyometrics. | Increased gestational age & reduced risk of prematurity |
| Wang et al, 2019 (31277127) | 23 RCTs studies met the criteria: N=4462. Studies dates: 1999–2017. Europe, USA, Asia, South America. |
1st trimester | To the end of the 3rd trimester | Light-moderate intensity ranged from 1–2 to 4–5 times per week. | Aerobic exercises, strength training, walking, cycling, or weight training. | Reduced risk for gestational weight gain, especially with exercise 3 times/week, duration of 30–45min/session |
RCT = Randomized controlled trials. GDM = gestational diabetes mellitus. GH = gestational hypertension. PE = pre-eclampsia. MET=metabolic equivalent task.
Given that exercise is one of the most effective and commonly recommended interventions for the treatment of conditions like obesity and diabetes, it stands to reason that exercise during pregnancy may be similarly efficacious in preventing the intergenerational inheritance of metabolic dysfunction (Figure 2). Most of the human studies investigating offspring health have focused on responses during infancy. Beginning with birth itself, maternal exercise during pregnancy is associated with increased rates of full-term delivery, normalized birth measures, and a reduced risk of macrosomia 22,23. Maternal aerobic exercise during pregnancy can also improve newborn neurobehavioural ability 24 and contribute to improved offspring cardiac autonomic health 25. Maternal aerobic exercise during pregnancy has been shown to improve infant capacity for movement, suggesting that children from exercised mothers may be more physically active; therefore reducing their risk of developing obesity during infancy 26.
Fig 2: Parental Exercise Training Affects Parents and Offspring.
When women exercise before pregnancy, their oocytes may be affected and exercise during pregnancy affects placenta. These alterations result in numerous effects in the F1 newborn, adaptations that may continue into adulthood. When men are exposed to exercise before breeding, their sperm physiology is changed which may lead to changes in F1 as newborns. Whether these changes affect health of the offspring as they age to adulthood is not known. More studies are needed to understand the effects of maternal and paternal exercise on offspring health in adulthood and to determine mechanisms for these effects.
Studies in obese women found that reduced sedentary behaviour during pregnancy decreased infant adiposity as measured by skinfold thickness 27,28. Another report showed that moderate intensity maternal exercise during pregnancy decreased the risk of offspring developing obesity in early childhood as assessed by BMI 29. Similarly, maternal exercise was shown to decrease adiposity in neonates at 6 months postpartum 27. A recent study using randomized controlled trials reported that aerobic physical activity in combination with a healthy diet during pregnancy decreases subcutaneous fat in neonates 48 hours after birth 28. In contrast to these reports, the initiation of an exercise intervention during pregnancy in otherwise sedentary women resulted in offspring with increased total body fat compared to controls 30. The reason for this discrepancy is not clear, although the authors stated that larger studies are needed to investigate the timing of exercise during pregnancy and that there should be follow-up into adolescence, which will better predict the risk of obesity in adulthood 30. This is an important point, as the onset of type 2 diabetes and other metabolic diseases typically occur in adulthood. The effects of maternal exercise on the metabolic health of adult offspring have not been studied in humans; understandable as the long human generation times make it is difficult to carry out randomized controlled trials investigating effects of maternal exercise across the entirety of the F1 lifespan. Therefore, rodent models, which have a much shorter generation timeline, have been used to investigate the effects of maternal environment on offspring metabolic health in adulthood.
Rodent Studies of Maternal Nutrition and Offspring Health.
Similar to human studies 13–15, the detrimental effects of maternal under-nutrition, over-nutrition and obesity on the metabolic health of offspring has been well-established in animal studies 31–36. Under-nutrition in ICR mice during pregnancy has been shown to increase adiposity 35 and cause severe glucose intolerance 36 in F1 offspring. In studies using Sprague-Dawley rats and C57BL6 mice, maternal over-nutrition increased weight gain and adiposity 31,37, induced hyperlipidaemia, and resulted in impaired glucose tolerance in F1 offspring during adolescence 31, with similar metabolic impairments extending through adulthood 33,34,38. The mechanisms responsible for altering offspring phenotypes as a result of these nutritional maternal-fetal exposures is not fully understood, but changes in the intrauterine milieu resulting from altered maternal nutrition can have direct effects on fetal tissues, which can alter the metabolic phenotypes seen in offspring at later stages of life. As examples, studies in C57BL/6 mice, Sprague-Dawley rats, and Wistar rats have shown that maternal high-fat feeding resulted in adult offspring with altered pancreatic function 39, increased adiposity 33,40–42 and decreased glucose tolerance 33,34,41.
Mechanisms altering offspring phenotype may also occur through gene-environment interactions leading to epigenetic changes. Epigenetics is defined as heritable changes in gene activity that do not involve changes in the DNA sequence. DNA methylation, histone modification, and non-coding RNAs are typical examples that can regulate gene expression. Various types of exogenous exposures during the prenatal development can influence epigenetic modifications 7,43,44. Maternal over-nutrition and obesity are well-recognized to influence epigenetic patterns by altering the activity of enzymes or varying epigenetic substrate availability that are involved in the addition or removal of epigenetic marks through the oocyte development or intrauterine environment. Typically, maternal over-nutrition increases the level of DNA methylation at the promoter, resulting in decreased expression of target genes in offspring tissue. For example, maternal high-fat diet during pregnancy increased the level of methylation at the promoter of insulin receptor substrate 2 (Irs2) in C57BL/6 offspring livers, which in turn led to insulin resistance in the adult offspring45. Thus, epigenetic modifications are proposed to be an underlying molecular mechanism for developmental programming of metabolic dysfunction of offspring later in life 7. Therefore, this intergenerational inheritance is likely playing a causal role in the increasing global rates of obesity and diabetes, resulting in a vicious cycle of metabolic disease being propagated across generations.
Maternal Exercise Improves Glucose Homeostasis in Rodent Offspring.
As stated above, the onset of type 2 diabetes and other metabolic diseases typically occur in adulthood, so studies designed to capture the full extent of maternal exercise’s effects on offspring health have largely utilized rodent models. As a result of these rodent studies, over the past decade there has been a significant increase in our understanding of how maternal exercise affects adult offspring metabolic health (Table 2, Figure 3). Treadmill running is one of the two major modes of exercise training that has been used for studies of parental exercise in rodents. Treadmill running has the distinct advantage of controlling the amounts and intensity of exercise but the disadvantage of putting stress on the mothers during the pregnancy. Although it is well-established that treadmill exercise in non-pregnant rodents has many benefits on metabolic health, treadmill exercise can produce high levels of corticosterone and noradrenaline in the kidney and liver, result in modifications of the peripheral clock 46, and cause anxious behaviour 47. Voluntary wheel running (VWR) is the other mode, which has been used by many groups including all of our maternal exercise studies. With VWR exercise, rodents run voluntarily and at higher levels during their dark active phase, presumably causing little or no stress to the animals. Rodents, including pregnant females will typically perform high levels of exercise daily 33,34,48,49, although running distances will significantly decrease at the later stages of pregnancy. While the intensity and time on the wheel cannot always be controlled or recorded with VWR, there are now systems that can precisely measure these parameters. Braking of the wheels can be used to match volume of exercise, allowing for more control of exercise performed.
Table 2:
Characteristics of F1 offspring in response to maternal, paternal and maternal + paternal exercise.
| Author/year (PMID) | Offspring Gender | Species (strain) | Maternal Exercise | Paternal Exercise | Offspring Age | Offspring BW | Offspring Glucose Metabolism | Tissue (response) |
|---|---|---|---|---|---|---|---|---|
| Carter et al, 2012 (22932781) | Male and Female | Mouse (ICR) | VWR: 7 days before & pregnancy & 14 days during lactation * | - | Adult | No change | Improved | Females: Soleus (↑ glucose uptake) Adipose tissue (↑ glucose uptake) |
| Carter et al, 2013 (23247711) | Female | Rat (Sprague Dawley) | VWR: 7–10 days before & during pregnancy & 12 days during lactation * | - | Adult | Decreased | Improved | Skeletal Muscle (↑ glucose uptake) Heart (↓ glucose uptake) Liver (↓ glucose production) |
| Laker et al, 2014 (24430439) | Female | Mouse (C57BL/6) | VWR: 6 wks before & during pregnancy | - | Adult | No change | Improved | Skeletal Muscle (↓ Pgc1α promoter methylation) |
| Stanford et al, 2015 (25204976) | Male | Mouse (C57BL/6) | VWR: 2 wks before & during pregnancy | - | Adult | Decreased | Improved | Skeletal Muscle (↔ glucose uptake) |
| Quiclet et al, 2016 (27382034) | Male | Rat (Wistar) | Treadmill: 4wks before & first 18 days of pregnancy (submaximal exercise, 5 days/wk) | - | Adult | No change | Worsened | Skeletal Muscle (↓ PKB signaling) Pancreas (↓ secretory capacity) |
| Eclarinal et al, 2016 (27033262) | Male and Female | Mouse (C57BL/6) | VWR: 1 wk before & during pregnancy and 10 days postnatal # | - | Adult | No change | - | ↑ Physical Activity |
| Stanford et al, 2017 (28572303) | Female | Mouse (C57BL/6) | VWR: 2 wks before & during pregnancy | - | Adult | No change | Improved | Liver (↓ glucose production) |
| Quiclet et al, 2017 (28971475) | Male | Rat (Wistar) | Treadmill: 4 wks before & first 18 days of pregnancy (55% VO2 max, 5 days/wk) | - | Young adult | Decreased | Improved | Skeletal Muscle (↓ PKB signaling) |
| Son et al, 2020 (32494609) | Male and Female | Mouse (C57BL/6) | Treadmill: 1 wk before (10min at 10m/min, 3×/wk) & E1.5 to E20.5 of pregnancy (10–14m/min for 40 min daily) | - | Young | Decreased in females. No change in males. |
Improved | BAT (↑ BAT function by ↓ Prdm16 promoter methylation) |
| Falcao-Tebas et al, 2020 (32539156) | Female | Rat (Sprague Dawley) | Treadmill: 4 wks before and until day 19 of pregnancy (60–76% VO2 max before pregnancy and 50–65% VO2 max during pregnancy, frequency not stated) | - | Adult | No change | No change | Skeletal Muscle (↑ insulin-stimulated glucose uptake) Pancreas (↑ beta cell mass and number of islets, and ↑ insulin secretion) |
| Zheng et al, 2020 (32111717) | Male | Mouse (C57BL/6) | VWR: 2 wks before & during pregnancy | - | Adult | No change | Improved | Pancreas (↓ beta cell size) |
| Zheng et al, 2020 (32111717) | Male | Mouse (C57BL/6) | VWR: 2 wks before & during pregnancy | VWR: 3wks before mating | Adult | Decreased | Greater improvement | Pancreas (↓ beta cell size and mass, and islet size) |
| Zheng et al, 2020 (32111717) | Male | Mouse (C57BL/6) | - | VWR: 3wks before mating | Adult | No change | Improved | Pancreas (↓ beta cell size) |
| Stanford et al, 2018 (30344184) | Male and Female | Mouse (C57BL/6) | - | VWR: 3wks before mating | Adult | No change | Improved | Skeletal Muscle (↑ glucose uptake) |
| McPherson et al, 2017 (28208792) | Male | Mouse (C57BL/6NHsd) | - | Swimming: 9 wks before mating (3 × 30 min session/wk) | Adult | No change | Improved | Pancreas (↑ islet cell number and alters pancreatic microRNAs) |
| Murashov et al, 2016 (26506979) | Male and Female | Mouse (C57BL/6) | - | VWR: 12wks before mating | Young adult | Increased | Worsened | Skeletal Muscle (altered metabolic gene expression) |
| McPherson et al, 2015 (25690453) | Female | Mouse (C57BL/6) | - | Swimming: 9 wks before mating (3 × 30 min session/wk) | Adult | No change | Improved | - |
VWR: voluntary wheel running. Pgc1α: Peroxisome proliferator-activated receptor gamma coactivator 1-alpha. PKB: protein kinase B. BAT: Brown Adipose Tissue. Prdm16: PR-domain containing 16.
Males performed exercise during bleeding period (10 days).
Males performed exercise during breeding period (1–4 days).
Pink shading indicates maternal exercise; Blue shading paternal exercise; Purple shading maternal and paternal exercise.
Fig. 3: Targets and Timing of Parental Exercise Differently Affect Offspring Through Inter- or Trans-generational Modes of Inheritance.
A. Inter-generational effects of maternal exercise during pregnancy on F1 and F2 offspring. F0 exercise directly affects the F1 fetus and F2 germ progenitor cells present in the F0 womb. B. Trans-generational effects of maternal exercise during pregnancy on F2 offspring. F0 exercise indirectly affects the development of the F2 fetus through F0-induced changes to F1 metabolic phenotypes, which subsequently influence the F2 generation. C. Inter-generational effects of maternal exercise while non-pregnant on F1 offspring. F0 exercise directly affects maternal oocytes, altering the F1 phenotype. D. Inter-generational effects of paternal exercise on F1 offspring. F0 exercise directly affects the paternal sperm, altering the F1 phenotype. E. Trans-generational effects of paternal exercise on F2 offspring. F0 exercise indirectly affects the development of the F2 fetus through F0-induced metabolic changes to F1 males (F1 phenotype affecting spermatogenesis) or females (F1 phenotype affecting oogenesis or the intrauterine environment).
Our group and several other laboratories have shown that maternal exercise during pregnancy has profound effects on the metabolic health of F1 male and female offspring (Table 2 and Figure 4) 33,34,37,41,50–52. Our study of C57BL6 mice reveals that the maximal benefits of maternal exercise on offspring metabolism occur when dams train both preconception and during gestation 33. Notably, the majority of the effects of maternal exercise on offspring metabolic health are not observed in very young animals, but are present in adult offspring of C57BL6 mice, ICR mice and Sprague-Dawley rats 33,34,50,51. With respect to glucose homeostasis, the beneficial effects of maternal VWR has included improved glucose tolerance in adult male 33,50 and female 34,50,51 offspring, increased insulin sensitivity in adult male 50 and female 50,51 offspring, and decreased insulin concentrations in male 33,37 and female 34 adult offspring, effects that occurred in both C57BL/6 mice and Sprague-Dawley rats. While these studies have consistently reported improved glucose homeostasis with maternal exercise using VWR, one report using Wistar rats came to a different conclusion (Table 2) 53. When mothers performed submaximal treadmill exercise for 4 weeks preconception and during the first 18 days of gestation there was improved glucose tolerance and insulin secretory capacity in the F1 offspring at weaning (3–4 wks of age), whereas the reverse was the case in the adult offspring 53. The reason for the conflicting results is not entirely clear but may be due to species and strain of animals studied, timing of maternal exercise, and/or mode of exercise.
Fig 4: Effects of Maternal and Paternal Exercise Training on F1 Offspring Metabolism.
Maternal exercise improves numerous aspects of offspring metabolism. Whether maternal exercise effects F2 offspring is not known. Most studies show that when sires are exposed to exercise before breeding, F1 offspring have improved metabolism.
Some studies of maternal exercise suggest that male offspring have a greater improvement in glucose tolerance in response to maternal exercise. For example, we found that while male offspring of exercise trained mothers fed both a chow and high-fat diet had improved glucose tolerance, female offspring only had improved glucose tolerance when the trained mothers where fed a high-fat diet 34. We speculate that the lack of an exercise effect in the female offspring from chow-fed mothers was because in contrast to male offspring, there was no deterioration in glucose tolerance in female offspring as they aged. In fact, in offspring from sedentary chow-fed mothers, glucose area under the curve was 40% lower in females compared with male siblings at 52 weeks of age. Thus, females are more glucose tolerant compared with males; therefore, the more minimal effects of maternal exercise on females may be a function of lower glycaemia at baseline.
As discussed above, when sedentary mothers were fed a high-fat diet during pregnancy, both male and female adult offspring had poor metabolic outcomes, most commonly reported as glucose intolerance 32,54–56. When high-fat diet-fed mothers simultaneously performed VWR or treadmill exercise, their offspring had dramatically improved glucose tolerance compared to offspring from sedentary control mothers, despite the otherwise-detrimental effects of a maternal high-fat feeding 33,34,37,38,41,56. In addition to improved glucose tolerance, maternal exercise in chow-fed mothers decreased fasting insulin concentrations in adult offspring, an effect that was even greater in the offspring of mothers who had been fed a high-fat diet 33,34,37,56. In a study that explored the effects of maternal exercise in Sprague-Dawley rats that were mated with high-fat-fed males, maternal treadmill exercise attenuated skeletal muscle insulin resistance and the decrease in pancreatic beta cell mass and insulin secretion observed in the female offspring of obese fathers 57. Taken together, these studies show that maternal exercise has the ability to improve the metabolic health of adult offspring, and importantly, that maternal exercise can prevent the detrimental effects that parental high-fat diets have on offspring health.
Mechanisms of Maternal Exercise Effects on Offspring Metabolism.
The mechanisms underlying for the beneficial effects of maternal exercise on offspring metabolism are only beginning to be elucidated, but we hypothesize that there are many factors involved. It is important to note that the effects of exercise training in pregnant mothers on the mothers themselves has not been extensively investigated, but studying C57/BL6 mice, we have reported normal training adaptations to the mother as shown by increased GLUT4 and hexokinase protein expression in skeletal muscle, traditional markers of training adaptations 33. In contrast, there were no changes in maternal glucose tolerance 33 or maternal body weights 33,37,50,52,53,56 in comparing sedentary and exercise trained pregnant mothers in a number of studies, suggesting the maternal glycaemia and body weight are not a mechanism for changes in offspring phenotype. Litter size, which can affect offspring phenotype, has not been shown to be altered by maternal exercise in most studies 33,41,42,50,52,53,58. In this section, we will discuss other potential mechanisms that may mediate the effects of maternal exercise on offspring metabolism.
Offspring Body Weight and Body Composition.
Body weight is an important factor in regulating glucose tolerance, insulin sensitivity, and overall metabolic health and numerous studies have investigated the effects of maternal exercise on offspring body weight in rodents from the neonatal period to adulthood. In neonates, several studies report that maternal exercise has no effect on litter weight 41,52 or pup body weight 38,50,53; however, two papers report decreased pup body weight 37,42 and one paper reports increased pup body weight 59. Given these conflicting findings, it difficult to determine if neonatal body weight is important contributor to the improved metabolic phenotype of offspring in adulthood.
Studies investigating mice and rats during weaning, young adulthood or adulthood have shown that maternal exercise either decreases or does not change offspring body weight (Table 2). Our group found that maternal VWR exercise decreased male offspring body weight, but these effects were not observed until the offspring were 52 weeks of age 33. These decreases associated with maternal exercise were observed in offspring from both chow- and high-fat-fed mothers, but were more pronounced in the offspring from mothers that were fed a high-fat diet33. Among female offspring, maternal exercise decreased offspring body weights only beginning at 52 weeks of age, and only under conditions where the mothers were fed a high-fat diet in combination with exercise 34. However, 52 week old offspring from exercise-trained mothers that were fed both chow- or high-fat diet-fed had decreased fat mass as measured by DEXA 34. In adult male offspring, but not in female offspring, maternal VWR exercise in ICR mice increased lean mass and decreased fat mass as measured by NMR 50. Maternal exercise in Sprague-Dawley rats also resulted in lower fat mass in male, but not female adult offspring 52. A study in Long-Evans rats investigating only male offspring showed that maternal VWR exercise during pregnancy decreased offspring body weights starting at weaning and persisting during adult life 60. The effects of maternal exercise on offspring health are also apparent when the offspring themselves are challenged with dietary manipulations, as maternal treadmill running exercise in Wistar rats decreased weight gain and limited fat mass gain in 3-month-old offspring fed a high-fat, high-sucrose diet 42. Taken together, the majority of studies suggest that maternal exercise training decreases offspring body weights, especially as they age, and that these effects may be more pronounced in male offspring. The reason for a sex-specific effect of maternal exercise is not known but may be due to a greater weight gain in males with ageing. While a reduction in body weight and fat mass may be one of the mechanisms through which maternal exercise improves glucose tolerance in offspring, it should be noted that in our work we found that the improved glucose tolerance in the offspring of trained dams preceded lower body weights, suggesting that at least some metabolic changes in offspring occur independent of changes in body weight 33.
Adaptations to Offspring Tissue.
To understand the mechanisms for the profound effects of maternal exercise on offspring glucose tolerance and metabolic health, numerous metabolic tissues have been investigated, primarily using rodent models. Skeletal muscle is critical for glucose homeostasis, since this tissue is responsible for the majority of glucose disposal in response to a glucose load 61. We measured glucose uptake in numerous offspring skeletal muscles (tibialis anterior, gastrocnemius, soleus, extensor digitorum longus), but surprisingly found no effect of maternal VWR exercise on glucose uptake in any of these offspring muscles 33. In contrast, another group investigating Sprague-Dawley rats found that maternal VWR increased glucose uptake in extensor digitorum longus and gastrocnemius muscles in adult offspring 51. The difference in results between these studies could be due to the animal models; our lab used mice while the other study was based on rats. Another important difference is the way each study handled paternal treatment; our lab locked running wheels during breeding whereas in the other study sires had access to running wheel for 10 days during breeding. This paternal exercise may be affecting the offspring metabolic health, as has been previously reported 49. Using ICR mice and in vivo measurements, maternal VWR exercise increased insulin-stimulated glucose uptake in both soleus and adipose tissues in adult female offspring 50. In C57BL/6 mice, maternal VWR exercise prevented maternal high-fat diet-induced hypermethylation of the PGC-1a promoter in offspring skeletal muscle 38. This maternal exercise-induced alteration in methylation status was associated with a decrease in Pgc1a expression and some of its target genes (Glut4, Cox4 and Cyt c), which could contribute to the amelioration of age-associated metabolic dysfunction observed in adult offspring 38. In future studies, it will be important to directly determine how maternal exercise may induce epigenetic alterations in skeletal muscle and how such changes could have downstream implications for offspring physiological functioning.
Because the liver is a major regulator of whole-body glucose homeostasis, we explored the effects of maternal VWR exercise on offspring liver 34. Maternal exercise reversed the detrimental effects of a maternal high-fat diet on liver function, decreasing glucose production in hepatocytes from offspring and increasing expression of genes involved in mitochondrial biogenesis, Krebs cycle, and fatty acid metabolism in the offspring liver 34. In addition, other researchers have shown that maternal VWR exercise during pregnancy protects male adult offspring against hepatic steatosis that otherwise develops when the offspring were fed a high-fat diet in Sprague-Dawley rats 52.
Similar to the liver, maternal high-fat diet during pregnancy has been shown to have detrimental effects on the pancreas of F1 offspring including increased beta cell mass, replication and neogenesis in Swiss mice 62. We found that maternal VWR exercise prevented some of the negative effects that maternal high-fat diet has on the offspring pancreas 49. Another important metabolic tissue is brown adipose tissue (BAT), and maternal treadmill exercise during pregnancy in C57BL/6 mice was shown to improve brown adipose tissue (BAT) and beige adipose function in offspring, decreasing the development of high-fat diet-induced obesity and metabolic dysfunction 58. Given that in both rodents and humans BAT contributes to energy expenditure and may function to counteract weight gain 63, this may prove to be another beneficial effect of maternal exercise on offspring metabolic health.
In addition to tissues directly implicated in glucose metabolism, other tissues have been reported to be regulated by maternal exercise. Maternal exercise by treadmill running in C57BL/6 mice prevented cardiac hypertrophy and dysfunction in offspring from obese mothers64 and maternal VWR exercise resulted in lower incidence of congenital heart defects in the offspring from mothers with pre-gestational diabetes 65. Maternal VWR was also shown to provide protection from neurodegeneration in adult offspring 66.
Taken together, these studies demonstrate that maternal exercise affects multiple tissues in offspring, and it is likely that additional offspring tissues are directly affected by maternal exercise, an important area for future research. Many of the tissues studied thus far (e.g. muscle, liver, pancreas) are highly metabolically active, and while there are few published mechanisms describing the specifics of these effects, alterations to these offspring tissues caused by maternal exercise during pregnancy are likely contributing to the overall improvements in offspring metabolic health.
Placental Responses to Maternal Exercise.
Metabolic phenotypes of offspring are modified by maternal environmental factors including nutritional state, oxygen availability, stress, and endocrinological adaptations 67. The placenta is the central organ connecting the developing fetus to the maternal environment and thus is responsible for conferring the effects of external stimuli and the maternal condition to offspring. The anatomical components of the placenta are the basis for its physiological roles in embryonic offspring maintenance. For instance, its size and vascular nature are necessary for the effective removal of fetal waste and the transmission of hormones, nutrients and oxygen to developing offspring 67. Collection of the post-partum placenta has allowed for the study of placental responses to maternal exercise in human subjects 68. In a study of weight bearing aerobic exercise, mid-trimester placental growth rate was faster and morphometric indexes of placental function were greater in exercise trained pregnant women, indicating that maternal exercise is associated with a balanced increase in fetoplacental growth, decreasing risk of having low-birth-weight outcomes 69. Exercise during pregnancy not only effects placental architecture but also placental gene expression that is essential for adequate nutrient delivery to the fetus and optimal fetoplacental growth. Studies of aerobic exercise during pregnancy 70 and women that were highly active during pregnancy 71,72 revealed that exercise alters the expression of numerous genes including endothelial nitric oxide synthase (eNOS) 70, sodium-coupled neutral amino acid transporter 2 (SNAT2) 71, and fatty acid transport protein 4 (FATP4) 71,72. Although there are experimental limitations, these results suggest that placenta is an organ that can respond to exercise and physical activity during pregnancy, and that exercise interventions can be utilized as means to reduce the risk of developmental disturbances.
Rodent studies have provided important insight into the multiple roles of the placenta with maternal exercise under both normal and high-fat diet conditions. Maternal high-fat diet can disturb the physiological characteristics of the placenta, including placental size, thickness and cell population makeup 73,74 whereas a recent study reported that maternal exercise may provide a moderating environmental factor which protects against the harmful effects of maternal high-fat diet on placental morphology 75. Placental efficiency, as determined by fetal weight divided by the weight of placenta, was decreased by maternal high-fat diet, but this effect was reversed by treadmill exercise training in C57BL/6 mice. Anatomically, maternal high-fat diet decreased labyrinth thickness and increased decidua and junctional zone thickness, effects that were normalized by maternal exercise 75. The expression of placental growth-related genes including insulin growth factor 1 (IGF-1) 76, IGF1 receptor 76, fibroblast growth factor2 (FGF2) 75, FGF2 receptor 75, neurotrophin-4 (NT-4) 77, and placental growth factor (PGF) 78 were increased in the placenta of treadmill exercised trained C57BL/6 mice 75, treadmill trained Wistar rats 76,78 and VWR exercised Wistar rats 77. The expression of placental vascularization-related genes, including Apelin, vascular endothelial factor (VEGF), VEGF receptor 1 and hypoxia inducible factor 1-α (HIF1-α), decreased as a result of maternal high-fat diet, but were recovered by maternal exercise 75,78. Collectively, the data demonstrates that maternal exercise may stabilize morphometrical changes in the placenta that result from a maternal high-fat diet, preventing disturbances to placental function.
Nutritionally regulated genes, including amino acid transporters and genes involved in fatty acid metabolism, are important for developmental processes in offspring 79,80. Maternal high-fat diet decreased the expression of solute carrier family 38 member 1 (Slc38a1), which uptakes glutamine, and Slc38a2, which transports neutral amino acid, effects that were reversed by exercise training 75. Conversely, the expression of lipoprotein lipase (Lpl), Cd36 (fatty acid transporter), glucose transporter 3 (Glut3) and leptin receptor B (Leprb) were increased by maternal high-fat diet, but were normalized by maternal treadmill exercise in C57BL/6 mice, Wistar rats, and Sprague-Dawley rats 75,81,82. In accordance with this expression pattern, maternal treadmill exercise prevented excess placental lipid deposition and hypoxia in C57BL/6 mice 56. Maternal high-fat diet pathogenically increased the placental expression of inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukine-1β (IL-1β), whereas maternal exercise reduced their levels. On the other hand, the level of IL-6 increased in the placenta from trained dams 75, suggesting that maternal exercise not only down-regulates the negative effects of maternal high-fat diet on placental homeostasis, but also up-regulates placental secretion of cytokines, reinforcing its role in the endocrine system.
Several rodent studies reported that maternal exercise affects several signalling pathways in placenta that are known to regulate growth and metabolic processes. Exercise during pregnancy reversed the high-fat diet-induced down-regulation of 5’ adenosine monophosphate-activated protein kinase (AMPK), Akt, extracellular signal-regulated kinase (ERK), and insulin receptor substrate 1 (IRS1) 75, which may be related to the protective effect of maternal exercise on maternal high-fat diet-induced placental dysfunction. Moreover, exercise during pregnancy induced the phosphorylation of numerous proteins involved in transcriptional regulation including 4E-binding protein 1 (4E-BP1), ribosomal protein S6 (rpS6), and mammalian target of rapamycin (mTOR), and the dephosphorylation of signal transducer and activator of transcription 3 (STAT3) 75,81,82. These reports suggest that exercise could play a broad role in placental function through direct regulation of placental signalling mechanisms. Exercise-induced circulating factors, including hormones, cytokines and metabolites, might act as the exercise-mediated molecules involved in placental signal transduction that ultimately contribute to the alterations in placental morphology and function that are observed with exercise.
Collectively, the results of these studies suggest that the placenta is an exercise-sensitive organ that changes its physiological functioning in response to alterations in the maternal environment. These physiological and morphological changes are the basis for the dynamic and communicatory role of the placenta and enable the placenta to transmit maternal information to offspring. These studies also demonstrate that maternal exercise counteracts the harmful effects of maternal high-fat diet on offspring metabolic health by normalizing the alterations to placental morphology, gene expression and signal transduction that are otherwise seen in maternal high-fat diet conditions. Further study is needed to determine the mechanism(s) behind the regulatory role of maternal exercise in placental function and offspring phenotypes.
Other Putative Mediators of Offspring Metabolic Health.
Recently, other factors that may explain the mechanisms of transmission through which maternal exercise affects offspring health have been proposed. Oligosaccharide 3’-sialyllactose (3’-SL) in mothers’ milk has been reported to be an important mediator of health in C57BL/6 mouse offspring and we have investigated the role of 3’-SL in responses to maternal exercise 83. Maternal exercise in global 3’-SL knockout mice (3’-SL−/−) failed to improve glucose metabolic health and cardiac function of offspring, whereas cross-fostering of offspring from trained 3’-SL−/− to trained wild type dams partially restored the benefits of maternal exercise. The supplementation of 3’-SL during the nursing period also improved hepatic and cardiac gene expression in offspring. Although the mechanisms by which maternal exercise changes the composition of milk and how 3’-SL improves offspring metabolism are not known, the effects of maternal exercise on lactation is a potentially consequential topic that should be expanded upon and may ultimately be translated to humans. Another group recently reported that maternal treadmill exercise enhanced brown adipogenesis in C57BL/6 mouse offspring through Apelin, an exercise-induced adipo-myokine 58. Maternal exercise was proposed to increase the level of maternal circulating Apelin, inducing placental hypoxia, which then activated the placental secretion of Apelin. DNA demethylation at the promoter of PR domain containing 16 (Prdm16), a central regulator of browning, was induced by placental Apelin and enhanced brown adipogenesis in offspring. This study of the apelinergic system further highlights the involvement of the placenta as a potential transmitter of the effects of maternal exercise to offspring during prenatal development.
Epigenetic Changes.
Epigenetic alterations such as DNA methylation, histone modifications, and microRNAs regulations during embryological development have been proposed to impact offspring metabolic health 84. Maternal obesity is widely recognized as a detrimental factor that disturbs the normal epigenetic landscape in offspring 7. Since maternal exercise commonly counteracts the unfavourable effects of maternal obesity on offspring health, the benefits of maternal exercise may be attributed to the stabilization of offspring epigenetic status during development. Maternal VWR exercise prevented maternal high-fat diet-induced hypermethylation at the promoter of Pgc-1α in skeletal muscle of C57BL/6 mouse offspring 38. In another study, exercise-induced placental Apelin activated the enzymatic activity of ten-eleven translocation (Tet) to convert 5-methylcytosine to 5-hydroxymethylcytosine of Prdm16 58. Maternal obesity-induced changes in DNA methylation levels are related to histone posttranslational modifications in Wistar rats 85, suggesting that the normalizing effects of maternal exercise on offspring epigenetics are likely to be even broader. Recent advances in the application of omics technology for the systematic characterization of gene expression and histone modifications have great potential for elucidation of a comprehensive mechanism describing the benefits of maternal exercise on offspring metabolism.
C. Paternal Nutrition and Exercise Effects on Offspring Health
While most research has focused on the role of the mother on offspring health, there is now strong evidence that fathers play an important role in the metabolic programming of offspring and that the paternal lineage is responsible for more than just its genetically encoded information 86–90 (Figure 2). In adult men, obesity can impair sperm number and motility 91–93 and decrease live birth rates 91,94. Since offspring development in utero occurs without the physical presence of the father, the effects of paternal diet are likely transmitted to offspring through the sperm 95–97. Thus, paternal lineage can transmit disease risk to the offspring, and strategies to interrupt this intergenerational disease transmission, such as physical activity, my help to stop this harmful cycle 98.
In some rodent models, altered-metabolic phenotypes resulting from paternal diet have been observed in offspring generated via in vitro fertilization, confirming that the relevant dietary information is present in sperm 99–101. Impaired paternal nutrition, either by a high-fat diet or a low protein diet, has similar effects of sperm health parameters 94 and negatively affected embryo metabolism 102,103, fetal growth 102, and the cardiovascular 104 and metabolic health of offspring 87,88. Compared to maternal exercise, there have been relatively few studies investigating the effects of paternal exercise training on offspring metabolic health in rodents (Table 2 and Figure 4). In C57BL/6 mice fed a high-fat diet, 9 weeks of paternal exercise by swim training improved glucose metabolism in 16 week-old male 105 and female 106 offspring. Male offspring were further investigated and showed that paternal exercise in high-fat-fed males resulted in offspring with reduced total adiposity, decreased plasma free fatty acids, increased muscle mass and improved pancreatic function 105. Our group found that high-fat feeding of C57BL/6 for 3 weeks prior to breeding impaired glucose tolerance and increased body fat mass in both male and female adult offspring. We found that paternal VWR exercise attenuated these detrimental effects of paternal high-fat diet on offspring metabolism by improving glucose tolerance, decreasing body fat and increasing glucose uptake in skeletal muscles of adult male and female offspring 49,107. Counter to this, a study focusing on longer duration paternal VWR exercise training had detrimental effects on energy expenditure and whole-body and skeletal muscle glucose metabolism in offspring 108. The reason for the difference between this study and the others 49,105–107 is not known, but could be due to higher volume of paternal exercise performed 108. Nevertheless, though the literature is still sparse and occasionally conflicting, there is a growing body of evidence showing that paternal diet and exercise play a concerted role in defining offspring metabolic phenotypes.
Potential Mechanisms for Paternal Exercise Impacts on Offspring Health.
Both paternal diet and exercise have been identified as factors that affect morphological, physiological, and epigenetic aspects of semen and subsequently offspring metabolic health, but the mechanisms that underlie this intergenerational inheritance are currently ambiguous, as are the specific effects of these factors on offspring outcomes. Published literature points to sperm physiology, epigenetically active molecules contained in sperm, and/or the sperm epigenome as the basis for the mechanism by which the paternal exercise influences offspring health.
Sperm Physiology.
Specific aspects of sperm morphology are necessary for its unique role as a mobile reproductive cell. Human studies have shown that abnormalities in semen morphological parameters, such as sperm concentration, vitality, and motility have been linked to paternal factors such as age, BMI, and diet 93,98,109–111. Some human studies have demonstrated negative correlations between BMI and male reproductive potential 112–114 and paternal obesity has been shown to reduce sperm motility 107 and mitochondrial activity 115, and increase intra-sperm reactive oxygen species and sperm DNA damage 91,116.
Rodent models have been extensively used to investigate mechanisms for modifications of sperm in response to diet-induced obesity and exercise. In mice, paternal high-fat diet decreased sperm functionality during fertilization 91, reduced blastocyst development and implantation 117, and smaller litter sizes 107. While these findings are not shared by all studies investigating paternal high-fat diet 118, paternal diet has consistently been shown to affect sperm physiology, with concomitant alterations to offspring metabolic health 86,100,119 that can be seen in multiple offspring generations 120,121. These alterations to sperm as a result of paternal high-fat diet and their associated consequences on offspring metabolic health are not immutable, as paternal exercise interventions have been shown to reduce or reverse many of the alterations to sperm and offspring that otherwise result from paternal high-fat diet. In sires fed a high-fat diet, 8 weeks of swim training increased sperm motility, improved sperm tail morphology, improved markers of oxidative stress, reduced sperm DNA damage, and increased sperm capacitation 122. In our study where paternal VWR exercise training fully counteracted the deleterious effects of paternal high-fat diet on offspring metabolism 107, paternal VWR exercise did not affect total sperm number or reverse the effects of high-fat diet on litter size. However, similar to the study of paternal swim exercise described above, we found that VWR reversed the high-fat diet-induced reduction of sperm motility 107.
Micro RNAs (miRNAs).
Multiple classes of sperm small non-coding RNAs (sncRNAs) can be altered by the paternal environment and subsequently impact offspring metabolic health 123–126. Microinjection studies have confirmed that these sncRNAs present in sperm are indeed capable of transferring aspects of paternally acquired phenotypes to the next generation 94,100,124,125. Micro RNAs (miRNAs) are one class of endogenous sncRNAs that play a role in post-transcriptional gene expression by interacting with specific mRNAs through complementary base pairing 127. miRNAs in sperm function as vectors for the epigenetic programming of offspring by directly modifying sperm before fertilization or by acting as modifiers of the postfertilization processes 128. Studies investigating the combined impact of paternal diet and exercise have shown some level of overlap in the species of miRNA that are altered by both types of paternal exposures, positing them as a potential mechanism through which paternal metabolic phenotypes can be transmitted to offspring. Paternal diet and exercise interventions have been shown to restore some classes of miRNAs altered by paternal high-fat diet, but which specific RNAs were normalized depended on the paternal intervention modality 106,108. Our group has also shown that the paternal environment can alter the RNA payload of sperm, evident in changes to a large number of small RNAs 107. However, between these studies, the effects of paternal diet or exercise on the miRNA profiles in sperm did not completely overlap, potentially as a result of the differences in study protocols. Only changes in miR-465a3p and miR-21 were found to be congruent between studies, suggesting they may be robust regulators that can bridge the interaction of diet and exercise in sperm. Further assessment of the predicted mRNA targets of the sperm miRNAs that are changed by paternal diet and exercise is needed to explain the involvement of paternal miRNAs in offspring developmental programs and metabolic function.
Transfer RNA-Derived Small RNAs (tsRNAs).
tsRNAs are largely made up of two classes of tRNA fragments: tRNA halves (tiRNAs) and tRNA-derived fragments (tRFs). These molecules are derived from mature tRNAs or precursor tRNAs and are regulatory non-coding RNAs involved in gene expression and post-transcriptional regulation129. Paternal dietary manipulations have been shown to cause rapid change in tsRNA populations in human sperm 130, and paternal high-fat diet 86 and paternal exercise 131 have been shown to alter a number of tsRNAs in rodent sperm. We found that paternal high-fat diet increased the abundance of several tsRNAs in sperm, while paternal VWR exercise suppressed the increased tsRNAs that was observed in sedentary high-fat diet-fed sires, leaving the tRF profile similar to that of the sedentary chow-fed fathers 107. Relatedly, we found that exercise training in the chow-fed fathers significantly reduced multiple classes of tsRNAs compared to sedentary controls. The highly abundant fragments tRF-Gly-GCC, tRF-Gly-CCC, and tRF-His-GTG were prominent examples of diet-induced RNAs that were suppressed to baseline levels by exercise training. One interesting tRF is tRF-Gly-GCC, which was upregulated in the sperm of F1 mice generated from Avy/a obese prediabetic sires and was associated with a latent phenotype of metabolic dysfunction in F2 mice 120. However, another group reported that tRF-GCC and tRF-Gly-CCC was significantly increased in sperm by paternal exercise using wheel cage 131. At present, current studies provide a persuasive, though sparse, description of the role that tsRNAs play in paternal-mediated intergenerational inheritance.
DNA Methylation.
Though most genomic DNA is demethylated during gametogenesis and embryo preimplantation, some regions escape this process, suggesting differential methylation resulting from paternal environmental exposures can occur in the regulatory regions of paternally imprinted 132,133 and nonimprinted 134 genes, potentially impacting metabolic phenotypes of offspring. Additionally, miRNAs present in sperm can also target enzymes involved in the DNA methylation/ demethylation cycle, leading to de novo DNA methylation that may alter embryonic development 121,135,136. Although studies are limited, paternal exercise has been shown to alter the global methylome in human 137 and animal sperm 106,108. DNA methylation at the promoter region, which directly represses mRNA expression, was changed in a variety of metabolic genes associated with insulin signalling pathway and the insulin sensitivity of muscle and adipose tissues, as well as in an imprinted gene involved in body weight regulation 108. While these data are far from conclusive, they do support the hypothesis that the paternal environmental exposures, such as physical exercise, can influence the sperm methylome and ultimately influence offspring phenotypes.
Perspective of Paternal Epigenetic Mechanisms.
While the precise mechanism of action remains unclear and the conclusions made by different groups lack consensus, recent studies indicate that miRNAs, tsRNAs and DNA methylation in sperm respond to paternal exercise and have the potential to define the trajectory of offspring metabolic health. Additionally, other epigenetically active aspects of semen, including long non-coding RNAs (lncRNAs), RNA methylation (m6A), and alterations to the protein or exosome profile of seminal plasma have been shown to change as a result of the paternal environment. While there are currently no studies relating these changes in semen to paternal exercise, to varying degrees they have been shown to contribute to offspring programming as a result of other types of paternal exposures 94. Similarly, the positive and/or negative impacts of paternal exercise on offspring metabolic phenotypes remains unclear, as do the effects of paternal exercise on non-metabolic offspring processes. Continued investigations into the role of each mechanism, as well as the broader contribution of paternal exercise to holistic offspring health will be needed before we can define a comprehensive paradigm through which paternal exercise influences future generations.
D. Future Directions and Clinical Translation
Current research, primarily based on studies rodent models, clearly demonstrate that maternal and paternal exercise have important effects on the metabolic health of offspring (Figure 5). There are many critical areas for future investigation using animal-based models. Given emerging data demonstrating that maternal environment effects F2 offspring, it will be of great value to determine the effects of maternal exercise on this generation. Further studies are also needed before we can comprehensively describe the central mechanisms that regulate the beneficial effects of parental exercise on offspring health. For example, it is essential that future research take a holistic approach to analysing epigenetic states like DNA methylation, histone modification, and non-coding RNAs that influence gametes and zygotes from trained parents. The placental-fetal system is another hot spot for offspring developmental programming as it plays an enormous role during the critical windows of prenatal offspring development. Identifying the mediating factors and signalling pathways that connect exercise stimuli to these phenotypic and developmental outcomes is essential for human translation; and given the ever-expanding issues of global obesity, these issues will only become more relevant in the future.
Fig 5: Effects of Exercise Training in Rodents on F0 Gametes and Placenta, and on F1 Newborn and Adults.
When dams are exposed to exercise before and during pregnancy, their oocytes and placenta are affected. While litter size and pup weight are generally not affected by maternal exercise, there are numerous beneficial metabolic changes in offspring, most prevalent in adulthood. Sires that are exposed to exercise have numerous alterations in sperm and these changes likely mediate the beneficial metabolic changes in F1 offspring. There are some sex-specific adaptations in the offspring in response to both maternal and paternal exercise. The beneficial effects of parental exercise offspring may be propagated across subsequent generations, ensuring healthier life cycles for further progeny.
Moving forward, one of the largest hurdles in research on the effects of parental exercise will be the application of knowledge gained from rodent studies to human interventions. As is evident by this review, the role of parental exercise on offspring and grand-offspring health is multi-faceted and complex. However, with the goal of eventually addressing the worldwide obesity and type 2 diabetes epidemics in mind, considerations for the relationship between rodent models and human applications need to be built into the research. The differences intrinsic to rodent and human physiology present another set of obstacles in analogizing human and animal research; however, common serum proteins or metabolites that have been identified in both pregnant rodents and humans could be utilized as predictive factors to evaluate the effects of exercise. The gestational period provides a unique opportunity to promote positive health behaviours that can have both short- and long-term benefits for the mother and child. Based on current research findings and future studies, the employment of parental physical activity may become a primary means for combating the ever-growing issues of obesity and type 2 diabetes that currently threaten the health of our future generations.
Acknowledgements
The authors were supported by NIH grant Awards R01 DK101043 (to L.J.G.), P30 DK036836 (DRC to Joslin Diabetes Center), and by the American Diabetes Association (training grant number 1-17-PMF-009 (to A.B. A-W)). J.K. was supported by individual research fellowships from Sunstar Foundation, JSPS Overseas Research Fellowships, Kanae foundation for the promotion of medical science, and Meiji Yasuda life foundation of health and welfares. We thank Michael F Hirshman for many helpful scientific discussions.
Footnotes
Competing financial interests
The authors have declared that no conflict of interest exists.
References
- 1.Cho NH, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138, 271–281 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Sales VM, Ferguson-Smith AC & Patti ME Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab 25, 559–571 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharp GC & Lawlor DA Paternal impact on the life course development of obesity and type 2 diabetes in the offspring. Diabetologia 62, 1802–1810 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, et al. Effect of lifestyle intervention in patients with type 2 diabetes: a meta-analysis. Metabolism 64, 338–347 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Schellenberg ES, Dryden DM, Vandermeer B, Ha C & Korownyk C Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Annals of internal medicine 159, 543–551 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Dutta S & Sengupta P Men and mice: Relating their ages. Life Sci 152, 244–248 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE & Saffery R Intrauterine programming of obesity and type 2 diabetes. Diabetologia 62, 1789–1801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaillard R Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol 30, 1141–1152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desyibelew HD & Dadi AF Burden and determinants of malnutrition among pregnant women in Africa: A systematic review and meta-analysis. PloS one 14, e0221712 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumey LH, Khalangot MD & Vaiserman AM Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: a retrospective cohort study. Lancet Diabetes Endocrinol 3, 787–794 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Ravelli AC, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Roseboom T, de Rooij S & Painter R The Dutch famine and its long-term consequences for adult health. Early human development 82, 485–491 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Boney CM, Verma A, Tucker R & Vohr BR Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115, e290–296 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Reynolds RM, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. Bmj 347, f4539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahti-Pulkkinen M, et al. Consequences of being overweight or obese during pregnancy on diabetes in the offspring: a record linkage study in Aberdeen, Scotland. Diabetologia (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stothard KJ, Tennant PW, Bell R & Rankin J Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. Jama 301, 636–650 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Kaar JL, et al. Maternal obesity, gestational weight gain, and offspring adiposity: the exploring perinatal outcomes among children study. J Pediatr 165, 509–515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport MH, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med 52, 1367–1375 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wen D, Liu X & Liu Y Impact of exercise on maternal gestational weight gain: An updated meta-analysis of randomized controlled trials. Medicine (Baltimore) 98, e16199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beetham KS, et al. The effects of vigorous intensity exercise in the third trimester of pregnancy: a systematic review and meta-analysis. BMC pregnancy and childbirth 19, 281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ming WK, et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC pregnancy and childbirth 18, 440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyer C, Reoyo OR & May L The Influence of Prenatal Exercise on Offspring Health: A Review. Clin Med Insights Womens Health 9, 37–42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiebe HW, Boule NG, Chari R & Davenport MH The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol 125, 1185–1194 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Clapp JF 3rd, Lopez B & Harcar-Sevcik R Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. Am J Obstet Gynecol 180, 91–94 (1999). [DOI] [PubMed] [Google Scholar]
- 25.May LE, Scholtz SA, Suminski R & Gustafson KM Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early human development 90, 33–38 (2014). [DOI] [PubMed] [Google Scholar]
- 26.McMillan AG, May LE, Gaines GG, Isler C & Kuehn D Effects of Aerobic Exercise during Pregnancy on 1-Month Infant Neuromotor Skills. Med Sci Sports Exerc 51, 1671–1676 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Patel N, et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int J Obes (Lond) 41, 1018–1026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Poppel MNM, et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: the DALI randomised controlled trial. Diabetologia 62, 915–925 (2019).- This study demonstrated that aerobic physical activity in concurrence with a healthy diet during pregnancy decreases fat in human neonates.
- 29.Mourtakos SP, et al. Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children. BMC pregnancy and childbirth 15, 66 (2015).- By analyzing 5,125 children, this study showed that moderate exercise during pregnancy decreases the risk of offspring to be overweight during childhood.
- 30.Chiavaroli V, et al. Exercise in pregnancy: 1-year and 7-year follow-ups of mothers and offspring after a randomized controlled trial. Scientific reports 8, 12915 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Simar D, Lambert K, Mercier J & Morris MJ Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology 149, 5348–5356 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Masuyama H & Hiramatsu Y Effects of a High-Fat Diet Exposure in Utero on the Metabolic Syndrome-Like Phenomenon in Mouse Offspring through Epigenetic Changes in Adipocytokine Gene Expression. Endocrinology 153, 2823–2830 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Stanford KI, et al. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 64, 427–433 (2015).- This study investigated the best time for exercise training during pregnancy, and determined that maternal exercise in mice before and during pregnancy improves male offspring metabolic health.
- 34.Stanford KI, et al. Maternal Exercise Improves Glucose Tolerance in Female Offspring. Diabetes 66, 2124–2136 (2017).- This study revealed that maternal exercise in mice improves systemic metabolism and liver function in female offspring.
- 35.Isganaitis E, et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes 58, 1192–1200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez-Chillaron JC, et al. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes 54, 702–711 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Raipuria M, Bahari H & Morris MJ Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PloS one 10, e0120980 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laker RC, et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring. Diabetes 63, 1605–1611 (2014).- These authors determined that maternal exercise in mice prevents epigenetic alterations caused by maternal over-nutrition in muscle of female offspring.
- 39.Graus-Nunes F, et al. Pregestational maternal obesity impairs endocrine pancreas in male F1 and F2 progeny. Nutrition 31, 380–387 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Hartil K, et al. Maternal substrate utilization programs the development of the metabolic syndrome in male mice exposed to high fat in utero. Pediatr Res 66, 368–373 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vega CC, et al. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 39, 712–719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quiclet C, et al. Maternal exercise modifies body composition and energy substrates handling in male offspring fed a high-fat/high-sucrose diet. J Physiol 595, 7049–7062 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perng W, Oken E & Dabelea D Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 62, 1779–1788 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Ou XH, Zhu CC & Sun SC Effects of obesity and diabetes on the epigenetic modification of mammalian gametes. J Cell Physiol 234, 7847–7855 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, et al. A Maternal High-Fat Diet Induces DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Frontiers in endocrinology 10, 871 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki H, et al. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Scientific reports 6, 27607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svensson M, et al. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol Stress 5, 8–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YJ, Kim HJ, Lee WJ & Seong JK A comparison of the metabolic effects of treadmill and wheel running exercise in mouse model. Lab Anim Res 36, 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng J, et al. Maternal and paternal exercise regulate offspring metabolic health and beta cell phenotype. BMJ open diabetes research & care 8(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carter LG, et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab 303, E1061–E1068 (2012).- This paper demonstrated that maternal exercise increases insulin-stimulated glucose uptake in soleus and adipose tissues in female offspring mice, improving their metabolic health.
- 51.Carter LG, Qi NR, de Cabo R & Pearson KJ Maternal exercise improves insulin sensitivity in mature rat offspring. Med. Sci. Sports Exerc 45, 832–840 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheldon RD, et al. Gestational exercise protects adult male offspring from high-fat diet-induced hepatic steatosis. J Hepatol 64, 171–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quiclet C, et al. Short-term and long-term effects of submaximal maternal exercise on offspring glucose homeostasis and pancreatic function. Am J Physiol Endocrinol Metab 311, E508–518 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Bayol SA, Simbi BH & Stickland NC A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567, 951–961 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isganaitis E, et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 63, 688–700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez-Twinn DS, et al. Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Scientific reports 7, 44650 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Falcao-Tebas F, Marin EC, Kuang J, Bishop DJ & McConell GK Maternal exercise attenuates the lower skeletal muscle glucose uptake and insulin secretion caused by paternal obesity in female adult rat offspring. J Physiol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Son JS, et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Science Advances 6, eaaz0359 (2020).- By analyzing methylation changes in the Prdm16 promoter of adipose tissue, these authors discovered that maternal exercise in mice improves brown/beige adipose tissue function, protecting offspring from obesity.
- 59.Eclarinal JD, et al. Maternal exercise during pregnancy promotes physical activity in adult offspring. Faseb j (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moser VC, et al. Impacts of maternal diet and exercise on offspring behavior and body weights. Neurotoxicology and teratology 63, 46–50 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Baron AD, Brechtel G, Wallace P & Edelman SV Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol 255, E769–E774 (1988). [DOI] [PubMed] [Google Scholar]
- 62.Gniuli D, et al. Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny. J Lipid Res 49, 1936–1945 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Seale P & Lazar MA Brown fat in humans: turning up the heat on obesity. Diabetes 58, 1482–1484 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beeson JH, et al. Maternal exercise intervention in obese pregnancy improves the cardiovascular health of the adult male offspring. Molecular metabolism 16, 35–44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saiyin T, et al. Maternal voluntary exercise mitigates oxidative stress and incidence of congenital heart defects in pre-gestational diabetes. J Cell Mol Med 23, 5553–5565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herring A, et al. Exercise during pregnancy mitigates Alzheimer-like pathology in mouse offspring. FASEB J 26, 117–128 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Sferruzzi-Perri AN & Camm EJ The Programming Power of the Placenta. Front Physiol 7, 33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petroff MG, Phillips TA, Ka H, Pace JL & Hunt JS Isolation and culture of term human trophoblast cells. Methods Mol Med 121, 203–217 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Clapp JF 3rd, Kim H, Burciu B & Lopez B Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol 183, 1484–1488 (2000). - By analyzing pregnant women who performed weight bearing aerobic exercise, this study showed that maternal exercise is associated with normal fetoplacental growth, decreasing the risk of having low-birth-weight outcomes.
- 70.Ramirez-Velez R, Bustamante J, Czerniczyniec A, Aguilar de Plata AC & Lores-Arnaiz S Effect of exercise training on eNOS expression, NO production and oxygen metabolism in human placenta. PloS one 8, e80225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brett KE, Ferraro ZM, Holcik M & Adamo KB Prenatal physical activity and diet composition affect the expression of nutrient transporters and mTOR signaling molecules in the human placenta. Placenta 36, 204–212 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Hutchinson KA, et al. Physical Activity During Pregnancy Is Associated with Increased Placental FATP4 Protein Expression. Reprod Sci (2020). [DOI] [PubMed] [Google Scholar]
- 73.Howell KR & Powell TL Effects of maternal obesity on placental function and fetal development. Reproduction 153, R97–R108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Myatt L & Maloyan A Obesity and Placental Function. Semin Reprod Med 34, 42–49 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Son JS, et al. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J Physiol 597, 3333–3347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mangwiro YTM, et al. Maternal exercise in rats upregulates the placental insulin-like growth factor system with diet- and sex-specific responses: minimal effects in mothers born growth restricted. J Physiol 596, 5947–5964 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fragoso J, et al. Maternal physical activity-induced adaptive transcriptional response in brain and placenta of mothers and rat offspring. J Dev Orig Health Dis 11, 108–117 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Mangwiro YT, et al. Maternal exercise and growth restriction in rats alters placental angiogenic factors and blood space area in a sex-specific manner. Placenta 74, 47–54 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Berti C, et al. Pregnancy and Infants’ Outcome: Nutritional and Metabolic Implications. Crit Rev Food Sci Nutr 56, 82–91 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Lin G, et al. Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino Acids 46, 1605–1623 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Mangwiro YTM, et al. Exercise initiated during pregnancy in rats born growth restricted alters placental mTOR and nutrient transporter expression. J Physiol 597, 1905–1918 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song L, et al. Prenatal exercise reverses high-fat-diet-induced placental alterations and alters male fetal hypothalamus during late gestation in ratsdagger. Biol Reprod 102, 705–716 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Harris JE, et al. Exercise-induced 3’-sialyllactose in breast milk is a critical mediator to improve metabolic health and cardiac function in mouse offspring. Nat Metab, doi: 10.1038/s42255-42020-40223-42258 Online ahead of print (2020).- These authors proposed that oligosaccharide 3’-sialyllactose in exercise trained mothers milk is an important mediator to improve glucose metabolic health and cardiac function of offspring.
- 84.Neri C & Edlow AG Effects of Maternal Obesity on Fetal Programming: Molecular Approaches. Cold Spring Harbor perspectives in medicine 6, a026591 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marco A, Kisliouk T, Tabachnik T, Weller A & Meiri N DNA CpG Methylation (5-Methylcytosine) and its derivative (5-Hydroxymethylcytosine) Alter Histone Posttranslational Modifications at the Pomc Promoter, Affecting the Impact of Perinatal Diet on Leanness and Obesity of the Offspring. Diabetes 65, 2258–2267 (2016). [DOI] [PubMed] [Google Scholar]
- 86.de Castro Barbosa T, et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Molecular metabolism 5, 184–197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ng SF, et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467, 963–966 (2010). [DOI] [PubMed] [Google Scholar]
- 88.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucas ES & Watkins AJ The Long-Term Effects of the Periconceptional Period on Embryo Epigenetic Profile and Phenotype; The Paternal Role and His Contribution, and How Males Can Affect Offspring’s Phenotype/Epigenetic Profile. Advances in experimental medicine and biology 1014, 137–154 (2017). [DOI] [PubMed] [Google Scholar]
- 90.Li L, Law C, Lo Conte R & Power C Intergenerational influences on childhood body mass index: the effect of parental body mass index trajectories. The American journal of clinical nutrition 89, 551–557 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Bakos HW, Henshaw RC, Mitchell M & Lane M Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertility and sterility 95, 1700–1704 (2011). [DOI] [PubMed] [Google Scholar]
- 92.Chavarro JE, et al. Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertility and sterility 95, 1794–1797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kort HI, et al. Impact of body mass index values on sperm quantity and quality. J Androl 27, 450–452 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Bodden C, Hannan AJ & Reichelt AC Diet-Induced Modification of the Sperm Epigenome Programs Metabolism and Behavior. Trends Endocrinol Metab 31, 131–149 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Bakos HW, Thompson JG, Feil D & Lane M Sperm DNA damage is associated with assisted reproductive technology pregnancy. International journal of andrology 31, 518–526 (2008). [DOI] [PubMed] [Google Scholar]
- 96.Bertolini M, et al. Morphology and morphometry of in vivo- and in vitro-produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 58, 973–994 (2002). [DOI] [PubMed] [Google Scholar]
- 97.Seli E, Gardner DK, Schoolcraft WB, Moffatt O & Sakkas D Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertility and sterility 82, 378–383 (2004). [DOI] [PubMed] [Google Scholar]
- 98.Su L & Patti ME Paternal Nongenetic Intergenerational Transmission of Metabolic Disease Risk. Current diabetes reports 19, 38 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Huypens P, et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 48, 497–499 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Chen Q, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).- This paper demonstrated that tsRNAs present in paternal sperm are capable of directing the metabolic programming of F1 offspring, resulting from the dietary status of the F0 fathers.
- 101.Sharma U & Rando OJ Metabolic Inputs into the Epigenome. Cell Metab 25, 544–558 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Binder NK, Hannan NJ & Gardner DK Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PloS one 7, e52304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lambrot R, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nature communications 4, 2889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watkins AJ & Sinclair KD Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. Am J Physiol Heart Circ Physiol 306, H1444–1452 (2014). [DOI] [PubMed] [Google Scholar]
- 105.McPherson NO, Lane M, Sandeman L, Owens JA & Fullston T An Exercise-Only Intervention in Obese Fathers Restores Glucose and Insulin Regulation in Conjunction with the Rescue of Pancreatic Islet Cell Morphology and MicroRNA Expression in Male Offspring. Nutrients 9(2017). [Google Scholar]
- 106.McPherson NO, Owens JA, Fullston T & Lane M Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am J Physiol Endocrinol Metab 308, E805–821 (2015).- These authors demonstrated that exercise training in obese male mice normalizes the X-linked microRNA profile in sperm, and increases insulin sensitivity in female offspring.
- 107.Stanford KI, et al. Paternal Exercise Improves Glucose Metabolism in Adult Offspring. Diabetes 67, 2530–2540 (2018).- This paper discovered that paternal exercise training normalizes the detrimental effects of paternal high fat diet on sperm motility, sperm microRNA profile, and glucose tolerance in offspring.
- 108.Murashov AK, et al. Paternal long-term exercise programs offspring for low energy expenditure and increased risk for obesity in mice. Faseb j 30, 775–784 (2016).This paper demonstrates that high-volume, long-term paternal exercise results in offspring that are more susceptible to the negative effects of high fat diet on metabolism.
- 109.Gaskins AJ, Colaci DS, Mendiola J, Swan SH & Chavarro JE Dietary patterns and semen quality in young men. Hum Reprod 27, 2899–2907 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hammoud AO, et al. Male obesity and alteration in sperm parameters. Fertil Steril 90, 2222–2225 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Veron GL, et al. Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil Steril 110, 68–75 e64 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Campbell JM, Lane M, Owens JA & Bakos HW Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod Biomed Online 31, 593–604 (2015). [DOI] [PubMed] [Google Scholar]
- 113.Hammoud AO, Carrell DT, Gibson M, Peterson CM & Meikle AW Updates on the relation of weight excess and reproductive function in men: sleep apnea as a new area of interest. Asian J Androl 14, 77–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sallmen M, Sandler DP, Hoppin JA, Blair A & Baird DD Reduced fertility among overweight and obese men. Epidemiology 17, 520–523 (2006). [DOI] [PubMed] [Google Scholar]
- 115.Fariello RM, et al. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int 110, 863–867 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Fullston T, et al. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod 27, 1391–1400 (2012). [DOI] [PubMed] [Google Scholar]
- 117.Mitchell M, Bakos HW & Lane M Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril 95, 1349–1353 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Gomez-Elias MD, et al. Association between high-fat diet feeding and male fertility in high reproductive performance mice. Scientific reports 9, 18546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watkins AJ, et al. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc Natl Acad Sci U S A 115, 10064–10069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cropley JE, et al. Male-lineage transmission of an acquired metabolic phenotype induced by grand-paternal obesity. Molecular metabolism 5, 699–708 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fullston T, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J 27, 4226–4243 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Palmer NO, Bakos HW, Owens JA, Setchell BP & Lane M Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol Endocrinol Metab 302, E768–780 (2012). [DOI] [PubMed] [Google Scholar]
- 123.Klastrup LK, Bak ST & Nielsen AL The influence of paternal diet on sncRNA-mediated epigenetic inheritance. Mol Genet Genomics 294, 1–11 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Shi J, Rassoulzadegan M, Tuorto F & Chen Q Sperm RNA code programmes the metabolic health of offspring. Nat Rev Endocrinol 15, 489–498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gapp K, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 17, 667–669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodgers AB, Morgan CP, Leu NA & Bale TL Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A 112, 13699–13704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pogue AI, Clement C, Hill JM & Lukiw WJ Evolution of microRNA (miRNA) Structure and Function in Plants and Animals: Relevance to Aging and Disease. J Aging Sci 2(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grandjean V & Rassoulzadegan M [Epigenetic inheritance of the sperm: an unexpected role of RNA]. Gynecol Obstet Fertil 37, 558–561 (2009). [DOI] [PubMed] [Google Scholar]
- 129.Li S, Xu Z & Sheng J tRNA-Derived Small RNA: A Novel Regulatory Small Non-Coding RNA. Genes (Basel) 9(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Natt D, et al. Human sperm displays rapid responses to diet. PLoS Biol 17, e3000559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Short AK, et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl Psychiatry 7, e1114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soubry A, et al. Obesity-related DNA methylation at imprinted genes in human sperm: Results from the TIEGER study. Clin Epigenetics 8, 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Potabattula R, et al. Male obesity effects on sperm and next-generation cord blood DNA methylation. PloS one 14, e0218615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wei Y, et al. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc Natl Acad Sci U S A 111, 1873–1878 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rodgers AB, Morgan CP, Bronson SL, Revello S & Bale TL Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33, 9003–9012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sato F, Tsuchiya S, Meltzer SJ & Shimizu K MicroRNAs and epigenetics. FEBS J 278, 1598–1609 (2011). [DOI] [PubMed] [Google Scholar]
- 137.Denham J, O’Brien BJ, Harvey JT & Charchar FJ Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics 7, 717–731 (2015). [DOI] [PubMed] [Google Scholar]