Abstract
In this Method paper, we describe the protocols for selective labeling of GCGR, a member of the class B GPCR family regulating glucose homeostasis, in live cells. A two-step procedure is presented in which a strained alkene chemical reporter is inserted into any desired location within the GPCR in the first step, followed by a robust bioorthogonal ligation reaction with a fluorophore-conjugated tetrazine or tetrazole reagent in the second step. The amber codon suppression strategy was adopted for site-specific incorporation of the strained alkene reporter, either spirohexene or trans-cyclooctene, in HEK293T cells. Subsequently, the inverse electron-demand Diels–Alder reaction with an AF647-conjugated 3,6-di(2-pyridyl)-S-tetrazine (DpTz) was performed with the alkene-encoded GCGR on live-cell surface. Alternatively, a photo-induced cycloaddition with a Cy5-conjugated, sterically shielded tetrazole was carried out, giving rise to faster fluorescent labeling along with excellent selectivity. Owing to their robust reaction kinetics and excellent chemo-selectivity, the bioorthogonal labeling protocols described here could be readily adapted to labeling any accessible protein targets, e.g., membrane proteins, in live cells.
Keywords: Bioorthogonal chemistry, GPCR, chemical reporter, tetrazine ligation, photo-click chemistry, strained alkene, fluorescent probes
1. Introduction to fluorescent labeling of GPCR
The class B G protein-coupled receptors (GPCR) are composed of 15 peptide-binding receptors in humans, including those for secretin, glucagon, glucagon-like peptide 1 (GLP-1), corticotropin-releasing factor (CRF), parathyroid hormone (PTH), and the calcitonin gene-related peptide (CGRP), and are validated drug targets for diabetes, depression, and osteoporosis (Bortolato et al., 2014). A recent surge of structural data of full-length class B GPCRs have shed new light into the structural basis of ligand binding and receptor activation (Jazayeri et al., 2017; Liang et al., 2017; Song et al., 2017; Zhang, H. et al., 2017; Zhang et al., 2018; Zhang, Y. et al., 2017). In contrast, our understanding of the class B GPCR conformational multiplicity and dynamics during receptor activation and signaling to G protein and β-arrestin in native cellular environment remains quite limited (Granier & Kobilka, 2012; Kahsai et al., 2011; Lane et al., 2017; Latorraca et al., 2017). A major barrier for progress in this area has been the lack of a general method to construct functional GPCR biosensors that allow the monitoring of receptor conformation in real-time in live cells (Culhane et al., 2015; Tian et al., 2017). So far, the most successful design strategy involved genetic fusion of two fluorescent proteins (FPs) to generate the FRET-based biosensors for single-cell analysis of GPCR signaling (Clister et al., 2015; Kauk & Hoffmann, 2018; Vilardaga et al., 2003; Violin et al., 2006; Wehbi et al., 2013). While this approach has enabled the collection of temporal and spatial information about GPCR signaling, due to their large size, the FP fusion is restricted to the N- or C-terminus of GPCR and not suitable for intracellular loop regions where the most profound conformational changes occur. In overcoming this limitation, an alternative approach has been developed involving the insertion of a tetra-cysteine peptide tag, which binds to a fluorescein arsenical hairpin binder (FlAsH) to generate an organic fluorophore in situ (Hoffmann et al., 2005). However, there are two drawbacks to this approach: 1) high background signals due to interaction of FlAsH with other thiol-containing proteins, which often requires time-consuming washing, and 2) incorporation of the cysteine-rich peptide sequence may cause incorrect disulfide-bond formation within the receptor, and as a result, alter the receptor function (Lotze et al., 2016).
To construct fluorescence-based GPCR biosensors, we decided to pursue bioorthogonal chemistry strategy because it has been exploited successfully for visualizing lipids (Bumpus & Baskin, 2017; Neef & Schultz, 2009), glycans (Lopez Aguilar et al., 2017; Mahal et al., 1997), proteins (Griffin et al., 1998; Lang & Chin, 2014), and nucleic acids (El-Sagheer & Brown, 2010) in live cells. Indeed, the Sakmar group has reported the successful bioorthogonal labeling of rhodopsin in vitro through genetic encoding of p-acetyl-L-phenylalanine into the extracellular site of rhodopsin followed by selective ligation reaction with a fluorescein-conjugated hydrazide (Ye et al., 2008). The same group also reported fluorescent bioorthogonal labeling of the ghrelin receptor (GhrR) via the reaction of the genetically encoded p-azido-L-phenylalanine and the AF647-conjugated dibenzocyclooctyne probe, and subsequent studies of the conformational dynamics of GhrR in a reconstituted system (Park et al., 2015). Recently, we showed for the first time that the bioorthogonal chemistry tools can be harnessed for construction of functional GPCR biosensors in live mammalian cell (An et al., 2018; Ramil et al., 2017). Specifically, we performed a two-step bioorthogonal labeling procedure: first, a small reactive alkene amino acid is site-selectively incorporated into the extracellular loop 3 (ECL3) of GCGR/GLP-1R in mammalian cells via amber codon suppression; second, the cells overexpressing the alkene-encoded GPCR were treated with a fluorophore-conjugated probe containing either a tetrazine or tetrazole to form the fluorescently labeled GCGR/GLP-1R in live mammalian cells (Figure 1).
Figure 1.
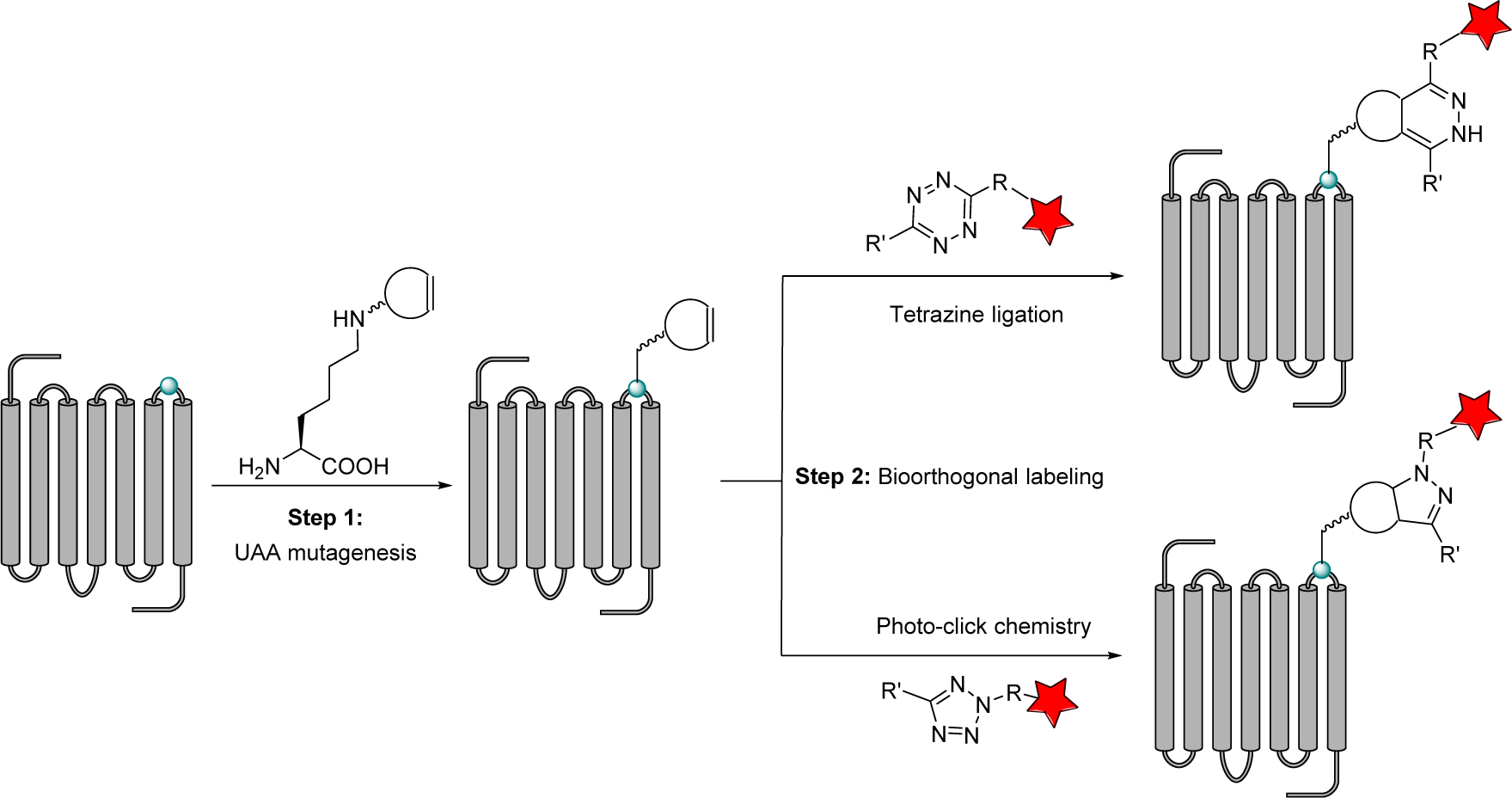
Bioorthogonal labeling of GPCRs using tetrazine ligation or tetrazole-based photo-click chemistry.
2. Site-specific incorporation of alkene reporters into the class B GPCR
Given the vast array of functional groups present in cellular environment, stringent criteria need to be met for chemical reporters and the cognate bioorthogonal probes to be useful for bioorhtogoal labeling (Tian & Lin, 2019). For site-specific incorporation of a chemical reporter into class B GPCRs, we focused on the genetically encodable strained alkenes because of their small size and outstanding reactivity in the strain-promoted cycloaddition reactions such as the tetrazole-based photo-click chemistry (Yu & Lin, 2014; Yu et al., 2012) and the inverse electron demand Diels-Alder reaction (Blackman et al., 2008; Yang et al., 2012). In our initial studies, we found that spiro[2.3]hex-1-ene (Sph) displays high reactivity while maintaining high stability and selectivity (Ramil et al., 2017). In reaction with 3,6-di-(2-pyridyl)-S-tetrazine (DpTz), Sph gave the second-order rate constant of 7900 ± 1000 M−1 s−1, about five times slower than trans-cyclooctene (TCO), a dienophile used widely in tetrazine ligation.
To monitor movement of the extracellular domain (ECD) relative to the seven-transmembrane domain (TMD) during receptor activation, we examined position-55 of the ECD as well as six positions on the extracellular loop 3 (ECL3) of GCGR as potential incorporation sites (Figure 2a) because they are solvent-exposed and not essential for receptor activity. Accordingly, we transfected HEK293T cells with a pair of plasmids: one encoding Methanosarcina mazei pyrrolysyl-tRNA synthetase (MmPylRS) and PylT; and the other encoding GCGR-GFP with the amber codon placed at the desired location. For comparison, we also transfected HEK293T cells with a TCOKRS plasmid that encodes the mutant PylRS recognizing TCO-lysine (TCOK), together with the same set of GCGR-GFP amber constructs. We found that Sph-lysine (SphK) could be efficiently charged into all positions based on the fluorescent cells detected in confocal microscopy, while TCOK appeared to give a lower incorporation efficiency (Figure 2b). The internalization assays with both wild-type and two selected mutants, E55SphK and H372SphK, showed that the SphK-encoded GCGR mutants remain functional and undergo ligand-induced internalization based on confocal microscopy (Ramil et al., 2017).
Figure 2.
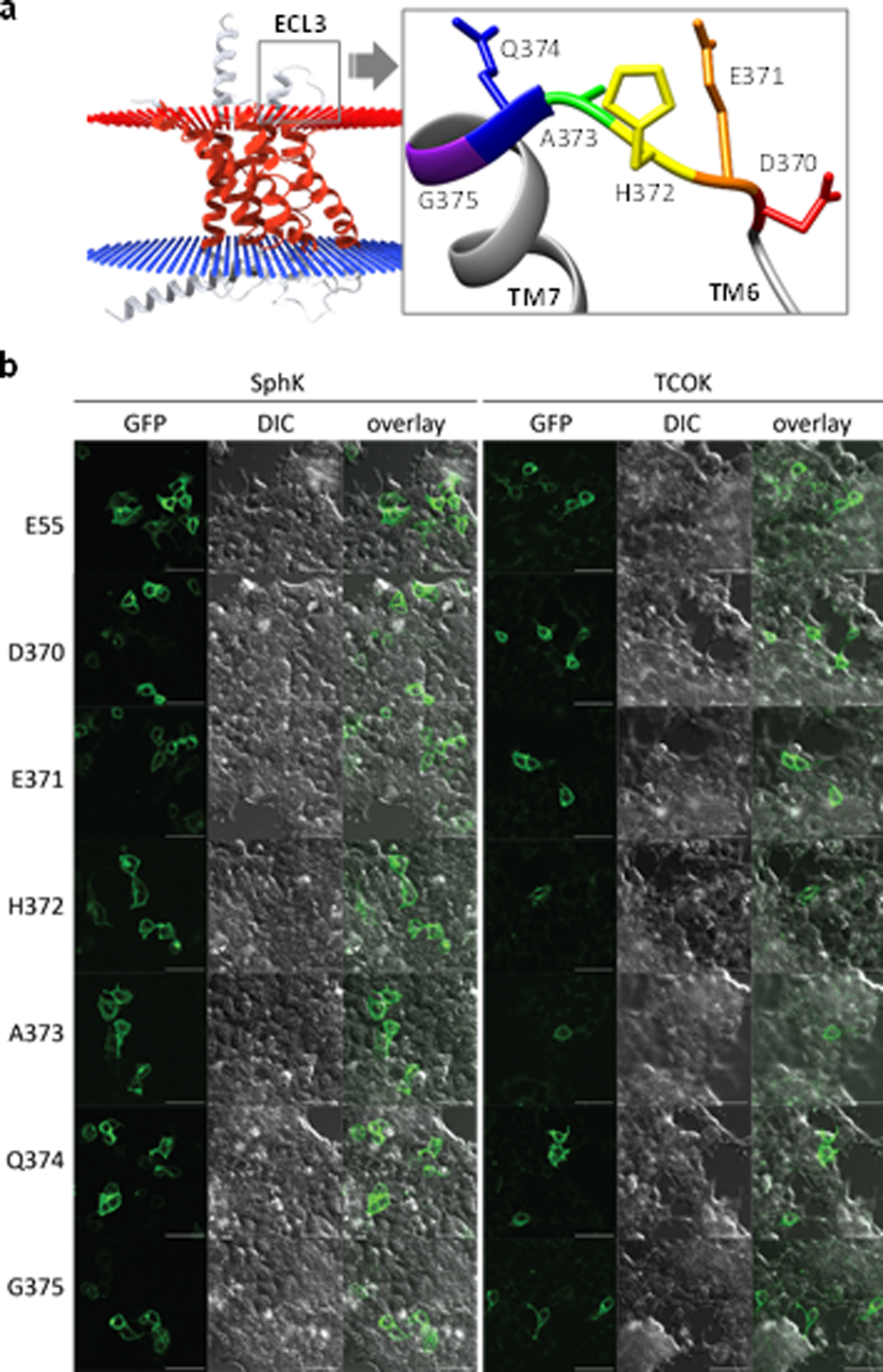
Site-specific incorporation of SphK and TCOK into the various positions of GCGR-GFP in HEK293T cells. (a) Residues on ECL3 of GCGR (PDB: 4L6R) showing their relative position on the exterior surface of the cytoplasmic membrane. (b) Confocal micrographs of HEK293T cells expressing the strained alkene-encoded GCGR-GFP; scale bar = 50 μm.
2.1. Protocol for the incorporation of alkene reporters into GCGR-GFP in HEK293T cells
2.1.1. Materials
HEK293T cells (CRL-11268, ATCC)
Dulbecco’s modified eagle medium (DMEM, 4.5 g/L glucose, 2 mM glutamine; Life Technologies)
Fetal bovine serum (FBS, Life Technologies)
Phosphate buffered saline (PBS, Gibco)
Gentamicin (Life Technologies)
0.05% Trypsin-EDTA (Life Technologies)
35 mm Glass bottom culture dishes, glass diameter: 14 mm, glass thickness: 1.5 (0.16–0.19 mm) (Matsunami Glass; Cat. No. D35-14-1.5-U)
OPTI-MEM reduced serum medium (Gibco)
Polyethylenimine (PEI, Polysciences Inc.)
FluoroBrite DMEM (Life Technologies)
pCMV-MmPylRS-U6-tRNA (Li et al., 2015)
pCMV-TCOKRS-U6-tRNA: TCOKRS carries three mutations: Y306A, L309M and C348A. The first round of mutation, Y306A/L309M, was carried out by PCR using the template pCMV-MmPylRS-U6-PylT and the primer pair:
F: 5’- CCCATGCTTGCTCCAAACCTTGCGAACTACATGCGCAAGCTTGACAGGGCCCTG −3’
R: 5’- CAGGGCCCTGTCAAGCTTGCGCATGTAGTTCGCAAGGTTTGGAGCAAGCATGGG −3’
The second round of PCR was carried out to introduce the C348A mutation using the primer pair:
F: 5’- GAGTTTACCATGCTGAACTTCGCGCAGATGGGATCGGGATGCACA −3’
R: 5’- TGTGCATCCCGATCCCATCTGCGCGAAGTTCAGCATGGTAAACTC −3’
pCMV6-GCGR-GFP (OriGene, CAT#: RG211179); Amber codon was introduced into selected positions in GCGR through site-directed mutagenesis using Platinum Pfx DNA polymerase (Thermo Fisher Scientific) or Phusion high-fidelity DNA polymerase (New England Biolabs) following manufacturers’ instructions
DMSO (molecular biology grade, Fisher Scientific)
Sterile syringe filter w/0.2 μm polyethersulfone membrane (VWR)
Autoclaved water
2.1.2. Reagents
SphK and TCOK: Prepared by following the reported procedures (Yu & Lin, 2014)
2.1.3. Equipment
5% CO2 cell culture incubator (Thermo Electron Corporation)
Benchtop centrifuge (Allegra™ X-22R Centrifuge)
Biosafety cabinet (The Baker Company)
Water bath (VWR)
Inverted microscope (Olympus-CKX31)
Zeiss LSM 710 Confocal Microscope equipped with Plan-Apochromat 20×/0.8 M27 or 40×/1.3 Oil DIC M27 objective
2.1.4. Protocol
Maintain HEK293T cells in DMEM supplemented with 10 % (v/v) FBS at 37 °C in a 5 % CO2 cell culture incubator.
Gently remove the growth medium and wash cells with 5 mL of prewarmed PBS.
-
Add 2 mL of 0.05% Trypsin-EDTA (37 °C) to the cells and spread evenly to cover the entire surface. Incubate the plate at 37 °C, 5% CO2 for 5 min to detach cells from the surface.
Note: Make sure that the cells have detached, and clumps have completely dispersed as monitored by a microscope.
Add 8 mL of growth medium and collect all cells into a 15 mL sterile conical tube.
Centrifuge cells at 400 RCF for 5 min at 20 °C to a pellet and then discard the supernatant without disturbing the pellet.
Resuspend the pellet in fresh medium and determine cell number using a hemacytometer.
Seed 6.0 × 105 HEK293T cells into 35 mm glass bottom culture dishes in 2 mL growth medium containing DMEM supplemented with 10% (v/v) FBS and 10 μg/mL gentamicin. Prepare as many cell culture dishes as the number of samples.
Monitor confluency using a microscope and transfect the cells at 70–80% confluency using polyethyleneimine (PEI) reagent.
-
Dilute stock solutions of UAA (SphK/TCOK, 100 mM in DMSO) into 2 mL growth medium to obtain a final concentration of 1 mM UAA, and filter the resulting medium through 0.2 μm polyethersulfone membrane filter.
Note: To dissolve SphK completely, sonicate for about 20 min.
Replace the growth medium in each plate with the 2 mL of UAA containing growth medium.
For SphK incorporation, mix 600 ng of pCMV-GCGR-GFP plasmid containing the amber codon at the desired position and 2400 ng of pCMV-MmPylRS-U6-tRNA in 200 μL OPTI-MEM medium.
For TCOK incorporation, mix 600 ng of pCMV-GCGR-GFP plasmid containing the amber codon at the desired position and 2400 ng of pCMV-TCOKRS-U6-tRNA to 200 μL OPTI-MEM medium.
-
After 5 min, add 9 μg of polyethylenimine to these mixtures and incubate for 10 min at room temperature before adding into the appropriate well.
Note: Warm PEI at 80 °C for 10 sec and vortex the tube for 10 sec to obtain a clear solution.
Incubate cells at 37 °C and 5% CO2 for 24–48 h and perform imaging experiments using a Zeiss LSM 710 equipped with Plan-Apochromat 20×/0.8 M27 or 40×/1.3 Oil DIC M27 objective.
3. Bioorthogonal labeling of GCGR in live cells using tetrazine ligation
The second step in our bioorthogonal labeling strategy involves the introduction of a fluorophore into class B GPCR in live cells using a bioorthogonal reaction. Among various bioorthogonal reactions reported in the literature (Ramil & Lin, 2013), we chose the inverse electron demand Diels–Alder reaction between Sph and DpTz (Figure 3a) because its fast reaction kinetics permits labeling of low-abundance proteins such as class B GPCRs. In the live-cell labeling experiments, we compared the labeling efficiency mediated by SphK and TCOK and found that SphK gave higher labeling yields for all GCGR mutants carrying the genetically encoded alkene reporter. For example, the SphK-encoded cells expressing GCGR-GFP-E55SphK and -D370SphK mutants showed significantly strong and specific labeling with the AF647-conjugated DpTz probe (DpTz-AF647), while the cells expressing the TCOK-encoded GCGR mutants did not (Figure 3b). An explanation is that E55 and D370 are located near the hydrophobic patch that interacts strongly with the more hydrophobic TCO chemical reporter, making it inaccessible for the bioorthogonal labeling reaction (Ramil et al., 2017).
Figure 3.

Bioorthogonal labeling of the strained alkene-encoded GCGR mutants in live HEK293T cells. (a) Scheme for bioorthogonal labeling of a GCGR-GFP mutant encoding SphK at position-370 with DpTz-AF647. The C-terminal GFP is omitted for clarity. (b) Confocal micrographs showing fluorescent labeling of the GCGR-GFP mutants encoding BocK, TCOK, and SphK at position E55 or D370 by DpTz-AF647 in HEK293T cells; Scale bar = 20 μm.
3.1. Protocol for labeling the alkene-encoded GCGR-GFP using tetrazine ligation
Seed 6.0 × 105 HEK293T cells to 35-mm glass-bottom culture dishes in 2 mL DMEM supplemented with 10% (v/v) FBS and 10 μg/mL gentamicin. Prepare six plates for transfection with two plasmids, pCMV-GCGR-E55TAG-GFP and pCMV-GCGR-D370TAG-GFP, and the expression conditions for three different unnatural amino acids: BocK, TCOK, and SphK.
Monitor confluency using an inverted microscope and transfect the cells at 70–80% confluency using polyethyleneimine (PEI) reagent as described previously.
Dilute stock solutions of UAA (SphK/TCOK; 100 mM in DMSO) into 2 mL growth medium to obtain a final concentration of 1 mM, and filter the resulting medium through a 0.2 μm poly(ether sulfone) membrane filter. For BocK, a final concentration of 2 mM in DMEM was used. Add the UAA-containing medium to the appropriate plate.
For BocK and SphK incorporation, mix 600 ng of pCMV-GCGR-GFP plasmid containing amber codon at the desired position and 2400 ng of pCMV-MmPylRS-U6-tRNA in 200 μL OPTI-MEM medium.
For TCOK incorporation, mix 600 ng of add 600 ng of pCMV-GCGR-GFP plasmid containing the amber codon at the desired position and 2400 ng of pCMV-TCOKRS-U6-tRNA in 200 μL OPTI-MEM medium.
After 5 min, add 9 μg of polyethylenimine to these mixtures and incubate for 10 min at rt before adding into appropriate cell culture plate.
Incubate cells for an additional 24–48 h and gently wash two times with the growth medium (1 h intervals).
Dilute the stock solution of DpTz-AF647 (1 mM in DMSO) into 1 mL DMEM to obtain a final concentration of 5 μM and filter the resulting solution through a 0.2 μm poly(ethersulfone) membrane.
Replace the medium in the cell culture plate with 1 mL of DpTz-AF647-containing medium and incubate at 37 °C, 5% CO2 for 1 h.
Remove the tetrazine-containing medium and wash the cells two times with growth medium before incubating in 1 mL of growth medium at 37 °C and 5% CO2 for 2 h.
Change the medium to FluoroBrite DMEM before laser scanning confocal microscopy.
Acquire the confocal micrographs using a Zeiss LSM 710 equipped with Plan-Apochromat 20×/0.8 M27 or 40×/1.3 Oil DIC M27 objective with ex. 488/em. 493–598 nm for the GFP channel and ex. 635/em. 640–759 nm for the Alexa Fluor channel.
4. Bioorthogonal labeling of GCGR in live cells using photo-click chemistry
The use of light to generate highly reactive species in situ from stable precursors has attracted substantial interest in the bioorthogonal chemistry field (Herner & Lin, 2016). For example, the photo-induced cycloaddition reaction between the tetrazoles and the electron-deficient or strained alkenes has shown broad utility in labeling proteins in biological systems (Lim & Lin, 2011; Ramil & Lin, 2014; Song et al., 2008; Yu et al., 2010), particularly where the spatiotemporal control is desired (Yu et al., 2011; Yu et al., 2013). However, the in situ generated nitrile imine dipoles also display electrophilic character, which leads to potential side reactions such as hydrolysis or nucleophilic additions with biological thiols (Herner et al., 2016). To overcome this limitation, we designed the sterically shielded tetrazoles in which a pendant group, N-Boc-pyrrole, is added to the ortho positions to prevent the competing nucleophilic additions (An et al., 2018). As a result, the in situ generated, sterically shielded nitrile imine favors the cycloaddition over the nucleophilic additions. Indeed, the half-life of the nitrile imine was determined to be 102 sec in aqueous media, drastically longer than the sterically unshielded nitrile imine. To demonstrate the utility of this photo-click reaction in biological systems, we treated HEK293T cells expressing the Sph-encoded GCGR-GFP mutant with a water-soluble Cy5-conjugated tetrazole followed by a brief photo-irradiation (Figure 4a). The rapid and selective labeling of GCGR in live cells was readily detected by confocal microscopy (Figure 4b), which was further verified by flow cytometry analysis. Compared to tetrazine ligation, photo-click chemistry requires a lower concentration of tetrazole reagent and offers faster (~1 min) labeling of GCGR (An et al., 2018).
Figure 4.
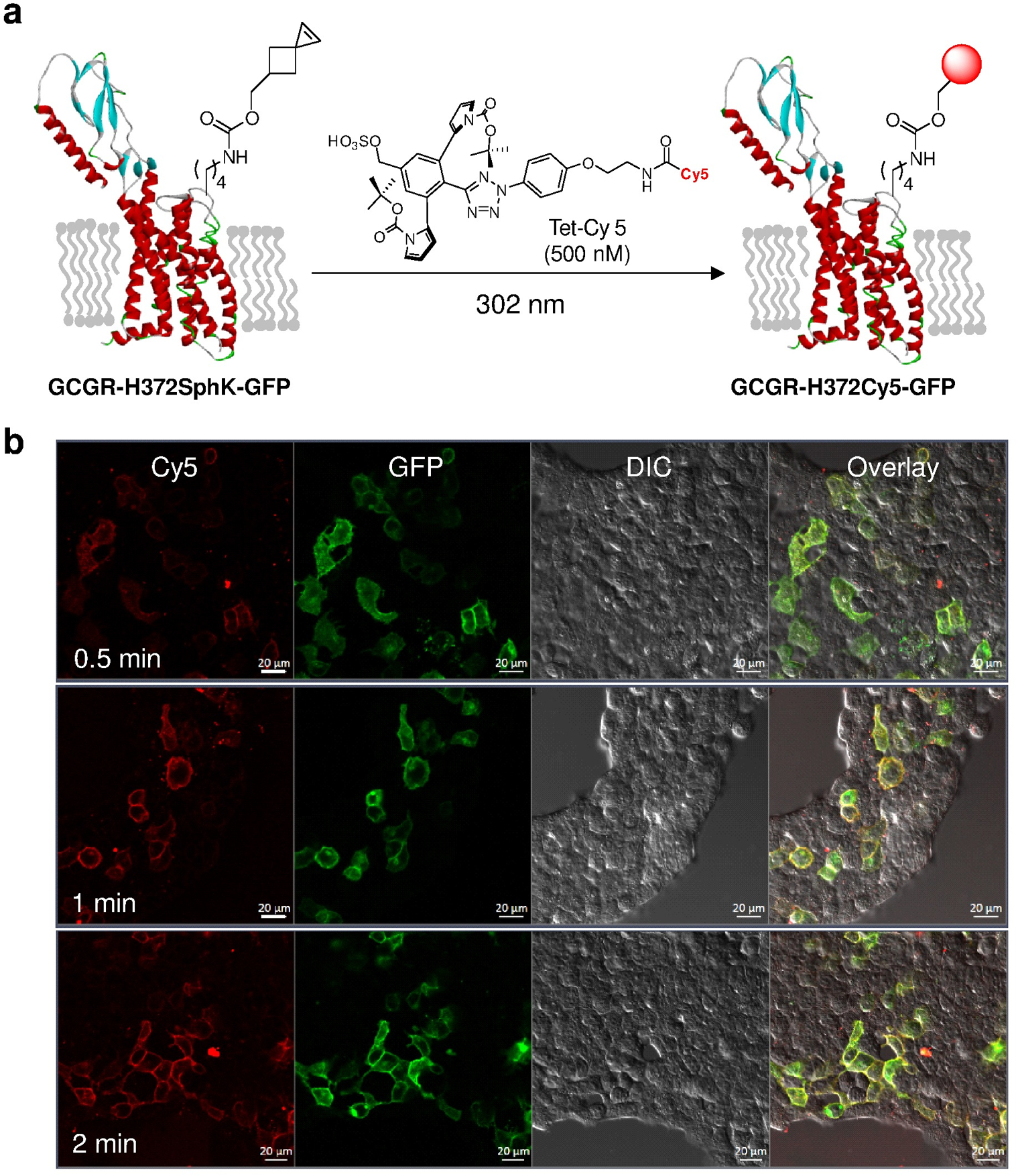
Application of the photo-click chemistry to bioorthogonal labeling of GCGR in live cells. (a) Scheme for bioorthogonal labeling of a GCGR-GFP mutant encoding SphK at position-372 with a water-soluble tetrazole, Tet-Cy5. The C-terminal GFP is omitted for clarity. (b) Confocal micrographs of HEK293T cells expressing GCGR-H372SphK-GFP after photo-irradiation with a handheld 302-nm UV lamp for a period of 0.5, 1, or 2 min in DMEM medium containing 500 nM Tet-Cy5. Scale bar = 20 μm.
4.1. Protocol for labeling the SphK-encoded GCGR-GFP using photo-click chemistry
Seed 6.0 × 105 HEK293T cells into 35-mm glass-bottom culture dishes in 2 mL growth medium containing DMEM supplemented with 10% (v/v) FBS and 10 μg/mL gentamicin.
Monitor confluency using an inverted microscope and transfect the cells at 70–80% confluency using a 4:1 ratio of pCMV-MmPylRS-U6-tRNA / pCMV-GCGRH372TAG-GFP plasmids.
Dilute a stock solution of 100 mM SphK in DMSO to obtain a final concentration of 1 mM in 2 mL of the growth medium, then filter the resulting mixture through 0.2 μm polyethersulfone membrane. Add this mixture to the cells followed by the transfection mixture.
In a sterile eppendorf tube, mix 600 ng of pCMV-GCGR-H372TAG-GFP and 2400 ng of pCMV-MmPylRS-U6-tRNA in 200 μL OPTI-MEM serum medium.
-
After 5 min, add 9 μg of polyethylenimine to the mixture and incubate for 10 min at room temperature before adding into the cell culture.
Note: Warm polyethylenimine at 80 °C for 10 sec, vortex for 10 sec to obtain a clear solution.
Incubate cells for an additional 24–48 h and then gently wash the cells with 2 mL of growth medium for two times (1 h each).
Prepare a fresh solution of Tet-Cy5 from 1 mM stock solution in DMSO to obtain a final concentration of 500 nM in 1 mL of growth medium and filter through a 0.2 μm polyethersulfone membrane.
Change the medium to the tetrazole containing medium and expose the cell culture plate to 302 nm UV light for 0.5, 1, and 2 min, respectively.
Remove the tetrazole containing medium and gently wash the cells two times with growth medium before incubating in 1 mL of growth medium at 37 °C and 5% CO2 for 30 min.
Change the medium to FluoroBrite DMEM before laser scanning confocal microscopy.
Acquire confocal images using a Zeiss LSM 710 equipped with Plan-Apochromat 20×/0.8 M27 or 40×/1.3 Oil DIC M27 objective with ex. 488/em. 493–598 nm for the GFP channel and ex. 640/em. 645–759 nm for the Cy5 channel.
5. Precursor techniques
The chemical reporters used in these experiments, SphK and TCOK, were prepared according to the published procedure (Ramil et al., 2017). The corresponding tetrazine reagent, DpTz-AF647, was synthesized by following the literature procedure (Ramil et al., 2017). The water-soluble tetrazole reagent, Tet-Cy5, used in bioorthogonal labeling of GCGR, was prepared according to the published procedure (An et al., 2018).
6. Discussions and Critical Parameters
In constructing the fluorescent-labeled GPCR biosensors, these mutant receptors must retain their functions. To this end, functional assays were carried out during each step of the bioorthogonal labeling. In the first step, the suitable positions within GCGR that can tolerate UAA mutagenesis need to be identified. In our studies, we employed the cAMP response assay to measure the activities of the GCGR mutants encoding BocK at the various loop positions. We found that the ECL3 mutants displayed the varying degrees of activity with the H372BocK mutant showing the highest activity, which is still ten times lower than the wild-type. Furthermore, the mutant GCGR activity can be monitored by the ligand-induced receptor internalization assay. For example, we treated the H372SphK mutant with Cy5-labeled glucagon (GCG-Cy5) and observed the internalization of GCG-Cy5 after its binding to the H372SphK mutant as monitored by confocal microscopy (Ramil et al., 2017). In the second step, after bioorthogonal labeling, the function of the fluorescent-labeled GCGR was also verified by both the cAMP response assay and the receptor internalization assay.
The confluency of the cell culture and the concentrations of the reagents used, including the chemical reporters SphK and TCOK and the cognate tetrazine or tetrazole reagents, are critical for successful bioorthogonal labeling. Over-confluent cell culture may result in detachment of cells from the plate surface. Care needs to be taken while performing the transfection and labeling experiments to avoid disturbance of the adherent cells. The purity of SphK and the related reagents was found to be a crucial factor in the current studies and needs to be evaluated by HPLC or LC-MS before their use. The filtration of the reagents through 0.2 μm polyethersulfone membrane was found to be necessary to avoid the reagent precipitation in the cell culture medium while performing imaging experiments. The optimal concentration for maximum incorporation of SphK was determined to be 1 mM, whereas the optimal concentrations for tetrazine and tetrazole required for GPCR labeling were 5 μM and 500 nM, respectively. For photo-click chemistry mediated labeling, the photo-irradiation time is important and the optimal labeling efficiency was obtained with 1-min photoirradiation.
7. Summary and Future Directions
As a first step toward the construction of fluorescence-based GPCR biosensors, we present the protocols for successful bioorthogonal labeling of the extracellular loop 3 (ECL3) region of GCGR, a member of the class B GPCR family, in live cells. The site-specific incorporation of spirohexene as a robust chemical reporter and the subsequent fluorescent labeling based on either tetrazine ligation or photo-click chemistry underline the two critical steps involved in the implementation of this bioorthogonal strategy. Given the low copy number of the class B GPCRs expressed on the cell membrane, the successful fluorescent labeling highlights the paramount importance of fast reaction kinetics and high chemoselectivity of bioorthogonal chemical probes among the fitness factors outlined recently (Tian & Lin, 2019). While the protocols described here can be readily adapted to labeling extracellular sites in membrane proteins in live cells, it remains a challenge to use the same set of reagents to label either intracellular protein targets or intracellular sites in membrane proteins because of the challenge in designing cell-permeable reagents that can be washed away from the cytosol after the labeling reaction. Efforts along this line are currently underway with a goal toward constructing GPCR biosensors carrying small organic fluorophores at the intracellular loop region.
Acknowledgments
We gratefully acknowledge the National Institutes of Health (R01GM085092 and R35GM130307) for supporting our work on bioorthogonal chemistry.
References
- An P, Lewandowski TM, Erbay TG, Liu P, & Lin Q (2018). Sterically Shielded, Stabilized Nitrile Imine for Rapid Bioorthogonal Protein Labeling in Live Cells. Journal of the American Chemical Society, 140, 4860–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman ML, Royzen M, & Fox JM (2008). Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. Journal of the American Chemical Society, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, & Marshall FH (2014). Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol, 171, 3132–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus TW, & Baskin JM (2017). Clickable substrate mimics enable imaging of phospholipase D activity. ACS central science, 3, 1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clister T, Mehta S, & Zhang J (2015). Single-cell analysis of G-protein signal transduction. Journal of Biological Chemistry, 290, 6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane KJ, Liu Y, Cai Y, & Yan EC (2015). Transmembrane signal transduction by peptide hormones via family B G protein-coupled receptors. Front Pharmacol, 6, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sagheer AH, & Brown T (2010). Click chemistry with DNA. Chemical Society Reviews, 39, 1388–1405. [DOI] [PubMed] [Google Scholar]
- Granier S, & Kobilka B (2012). A new era of GPCR structural and chemical biology. Nat. Chem. Biol, 8, 670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin BA, Adams SR, & Tsien RY (1998). Specific covalent labeling of recombinant protein molecules inside live cells. Science, 281, 269–272. [DOI] [PubMed] [Google Scholar]
- Herner A, & Lin Q (2016). Photo-Triggered Click Chemistry for Biological Applications. Topics in current chemistry (Cham), 374, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herner A, Marjanovic J, Lewandowski TM, Marin V, Patterson M, Miesbauer L, Ready D, Williams J, Vasudevan A, & Lin Q (2016). 2-Aryl-5-carboxytetrazole as a New Photoaffinity Label for Drug Target Identification. Journal of the American Chemical Society, 138, 14609–14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Gaietta G, Bunemann M, Adams SR, Oberdorff-Maass S, Behr B, Vilardaga JP, Tsien RY, Ellisman MH, & Lohse MJ (2005). A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nature Methods, 2, 171–176. [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Rappas M, Brown AJH, Kean J, Errey JC, Robertson NJ, Fiez-Vandal C, Andrews SP, Congreve M, Bortolato A, Mason JS, Baig AH, Teobald I, Dore AS, Weir M, Cooke RM, & Marshall FH (2017). Crystal structure of the GLP-1 receptor bound to a peptide agonist. Nature, 546, 254–258. [DOI] [PubMed] [Google Scholar]
- Kahsai AW, Xiao K, Rajagopal S, Ahn S, Shukla AK, Sun J, Oas TG, & Lefkowitz RJ (2011). Multiple ligand-specific conformations of the beta2-adrenergic receptor. Nat Chem Biol, 7, 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauk M, & Hoffmann C (2018). Intramolecular and Intermolecular FRET Sensors for GPCRs - Monitoring Conformational Changes and Beyond. Trends Pharmacol. Sci, 39, 123–135. [DOI] [PubMed] [Google Scholar]
- Lane JR, May LT, Parton RG, Sexton PM, & Christopoulos A (2017). A kinetic view of GPCR allostery and biased agonism. Nat Chem Biol, 13, 929–937. [DOI] [PubMed] [Google Scholar]
- Lang K, & Chin JW (2014). Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins. Chemical Reviews, 114, 4764–4806. [DOI] [PubMed] [Google Scholar]
- Latorraca NR, Venkatakrishnan AJ, & Dror RO (2017). GPCR Dynamics: Structures in Motion. Chemical Reviews, 117, 139–155. [DOI] [PubMed] [Google Scholar]
- Li N, Ramil CP, Lim RKV, & Lin Q (2015). A Genetically Encoded Alkyne Directs Palladium-Mediated Protein Labeling on Live Mammalian Cell Surface. ACS Chem. Biol, 10, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SGB, Christopoulos G, Coudrat T, Danev R, Baumeister W, Miller LJ, Christopoulos A, Kobilka BK, Wootten D, Skiniotis G, & Sexton PM (2017). Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature, 546, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RK, & Lin Q (2011). Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells. Acc Chem Res, 44, 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Aguilar A, Briard JG, Yang L, Ovryn B, Macauley MS, & Wu P (2017). Tools for studying glycans: recent advances in chemoenzymatic glycan labeling. ACS chemical biology, 12, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze J, Reinhardt U, Seitz O, & Beck-Sickinger AG (2016). Peptide-tags for site-specific protein labelling in vitro and in vivo. Mol Biosyst, 12, 1731–1745. [DOI] [PubMed] [Google Scholar]
- Mahal LK, Yarema KJ, & Bertozzi CR (1997). Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science, 276, 1125–1128. [DOI] [PubMed] [Google Scholar]
- Neef AB, & Schultz C (2009). Selective fluorescence labeling of lipids in living cells. Angewandte Chemie International Edition, 48, 1498–1500. [DOI] [PubMed] [Google Scholar]
- Park M, Sivertsen Bjørn B., Els-Heindl S, Huber T, Holst B, Beck-Sickinger Annette G., Schwartz Thue W., & Sakmar Thomas P. (2015). Bioorthogonal Labeling of Ghrelin Receptor to Facilitate Studies of Ligand-Dependent Conformational Dynamics. Chemistry & Biology, 22, 1431–1436. [DOI] [PubMed] [Google Scholar]
- Ramil CP, Dong M, An P, Lewandowski TM, Yu Z, Miller LJ, & Lin Q (2017). Spirohexene-Tetrazine Ligation Enables Bioorthogonal Labeling of Class B G Protein-Coupled Receptors in Live Cells. Journal of the American Chemical Society, 139, 13376–13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramil CP, & Lin Q (2013). Bioorthogonal chemistry: strategies and recent developments. Chemical Communications, 49, 11007–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramil CP, & Lin Q (2014). Photoclick chemistry: a fluorogenic light-triggered in vivo ligation reaction. Current Opinion in Chemical Biology, 21, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Yang D, Wang Y, de Graaf C, Zhou Q, Jiang S, Liu K, Cai X, Dai A, Lin G, Liu D, Wu F, Wu Y, Zhao S, Ye L, Han GW, Lau J, Wu B, Hanson MA, Liu ZJ, Wang MW, & Stevens RC (2017). Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature, 546, 312–315. [DOI] [PubMed] [Google Scholar]
- Song W, Wang Y, Qu J, Madden MM, & Lin Q (2008). A photoinducible 1,3-dipolar cycloaddition reaction for rapid, selective modification of tetrazole-containing proteins. Angew Chem Int Ed Engl, 47, 2832–2835. [DOI] [PubMed] [Google Scholar]
- Tian H, Fürstenberg A, & Huber T (2017). Labeling and Single-Molecule Methods To Monitor G Protein-Coupled Receptor Dynamics. Chemical Reviews, 117, 186–245. [DOI] [PubMed] [Google Scholar]
- Tian Y, & Lin Q (2019). Fitness Factors for Bioorthogonal Chemical Probes. ACS Chem. Biol, 14, 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga JP, Bunemann M, Krasel C, Castro M, & Lohse MJ (2003). Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol, 21, 807–812. [DOI] [PubMed] [Google Scholar]
- Violin JD, Ren XR, & Lefkowitz RJ (2006). G-protein-coupled receptor kinase specificity for beta-arrestin recruitment to the beta2-adrenergic receptor revealed by fluorescence resonance energy transfer. Journal of Biological Chemistry, 281, 20577–20588. [DOI] [PubMed] [Google Scholar]
- Wehbi VL, Stevenson HP, Feinstein TN, Calero G, Romero G, & Vilardaga JP (2013). Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gbetagamma complex. Proc. Natl. Acad. Sci. USA, 110, 1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Seckute J, Cole CM, & Devaraj NK (2012). Live-Cell Imaging of Cyclopropene Tags with Fluorogenic Tetrazine Cycloadditions. Angew. Chem. Intl. Ed, 51, 7476–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Köhrer C, Huber T, Kazmi M, Sachdev P, Yan ECY, Bhagat A, RajBhandary UL, & Sakmar TP (2008). Site-specific Incorporation of Keto Amino Acids into Functional G Protein-coupled Receptors Using Unnatural Amino Acid Mutagenesis. J. Biol. Chem, 283, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Yu Z, Ho LY, & Lin Q (2011). Rapid, photoactivatable turn-on fluorescent probes based on an intramolecular photoclick reaction. Journal of the American Chemical Society, 133, 11912–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Lim RKV, & Lin Q (2010). Synthesis of Macrocyclic Tetrazoles for Rapid Photoinduced Bioorthogonal 1,3-Dipolar Cycloaddition Reactions. Chemistry – A European Journal, 16, 13325–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, & Lin Q (2014). Design of Spiro[2.3]hex-1-ene, a Genetically Encodable Double-Strained Alkene for Superfast Photoclick Chemistry. J. Am. Chem. Soc, 136, 4153–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Ohulchanskyy TY, An P, Prasad PN, & Lin Q (2013). Fluorogenic, two-photon-triggered photoclick chemistry in live mammalian cells. Journal of the American Chemical Society, 135, 16766–16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Pan Y, Wang Z, Wang J, & Lin Q (2012). Genetically Encoded Cyclopropene Directs Rapid, Photoclick-Chemistry-Mediated Protein Labeling in Mammalian Cells. Angew. Chem. Intl. Ed, 51, 10600–10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Qiao A, Yang D, Yang L, Dai A, de Graaf C, Reedtz-Runge S, Dharmarajan V, Zhang H, Han GW, Grant TD, Sierra RG, Weierstall U, Nelson G, Liu W, Wu Y, Ma L, Cai X, Lin G, Wu X, Geng Z, Dong Y, Song G, Griffin PR, Lau J, Cherezov V, Yang H, Hanson MA, Stevens RC, Zhao Q, Jiang H, Wang MW, & Wu B (2017). Structure of the full-length glucagon class B G-protein-coupled receptor. Nature, 546, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Qiao A, Yang L, Van Eps N, Frederiksen KS, Yang D, Dai A, Cai X, Zhang H, Yi C, Cao C, He L, Yang H, Lau J, Ernst OP, Hanson MA, Stevens RC, Wang MW, Reedtz-Runge S, Jiang H, Zhao Q, & Wu B (2018). Structure of the glucagon receptor in complex with a glucagon analogue. Nature, 553, 106–110. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, Tarrasch JT, Li S, Sun Kobilka T, Kobilka BK, & Skiniotis G (2017). Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature, 546, 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]


