Abstract
In the present study, SWCNH–COOH and SWCNH–TETA were fabricated using single-walled carbon nanohorns (SWCNHs) via carboxylation and grafting with triethylenetetramine (TETA) for uranium (VI) ion [U(VI)] removal. The morpho-structural characterization of as-prepared adsorbing materials was performed by transmission electron microscopy, X-ray diffractometry, Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS). Several parameters including the pH value of the aqueous solutions, contact time, temperature, and U(VI) concentration were used to evaluate the sorption efficiency of SWCNH–COOH and SWCNH–TETA. The Langmuir isotherm model could well represent the as-obtained adsorption isotherms, and the kinetics was successfully modeled by pseudo-second-order kinetics in the adsorption process. The maximum adsorption capacity of SWCNH–TETA was calculated as 333.13 mg/g considering the Langmuir isotherm model. Thermodynamic studies showed that adsorption proved to be a spontaneous endothermic process. Moreover, SWCNH–TETA exhibited excellent recycling performance and selective adsorption of uranium. Furthermore, the possible mechanism was investigated by XPS and density functional theory calculations, indicating that the excellent adsorption was attributed to the cooperation capability between uranium ions and nitrogen atoms in SWCNH–TETA. This efficient approach can provide a strategy for developing high-performance adsorbents for U(VI) removal from wastewater.
1. Introduction
Contamination due to wastewaters, originating from uranium mining, containing radionuclides and toxic metals is a primary environmental concern.1 Uranium with high toxicity and radioactivity could cause great damage to human health including liver and kidney pathologies and eventually death.2 Therefore, in order to extend the development of the nuclear industry, more and more attention has been paid to the removal and recovery of uranium from surface water and groundwater. The effective recovery of uranium from wastewater has become a critical issue. So far, some approaches, such as solvent extraction,3 chemical precipitation,4 flotation,5 ion-exchange processes,6−8 biological treatment,9,10 and adsorption,11−14 were extensively applied for the uptake of uranium (VI) ions [U(VI)] from aqueous solutions. Among these techniques, adsorption is considered to be one of the most effective technologies because of its excellent efficiency, cost-effectiveness, and simple operation.15,16 Many potential sorbents, such as carbon-based materials, silica, zeolites, polymers, metal–organic frameworks, and chitosan,17−24 were already proposed with promising results on U(VI) removal using adsorption.
Among them, carbon-based nanomaterials, including carbon nanotubes,25,26 graphene,27 carbonaceous nanofibers,28 and carbon spheres,29 are research hotspots in this field because of their highly porous structure, large specific surface area, more surface groups, acid and alkali resistance, radiation resistance, and thermal stability. They have particular advantages in the treatment of radioactive wastewater, but there are some bottlenecks for these technologies. For example, the impurities in the preparation process of carbonaceous nanomaterials are difficult to be separated in practical applications. The metal (salt) catalyst used in the preparation process of carbon nanotubes is not efficiently removable at present. Moreover, the adsorption capacity of the nonfunctionalized carbon-based nanomaterials for uranium is relatively small. The saturated U(VI) adsorption capacities of pure carbon nanotubes (CNTs) and graphene oxide are around 41.48 and 97.5 mg·g–1, respectively.30,31 Therefore, many improved methods were already proposed to optimize the solution to the problems mentioned above. For example, Liu et al.(32) prepared a magnetic phosphoryl functional polyphosphazene base built with a CNT composite (PZS-TPP/CNT/Fe3O4), which had an optimal adsorption of 300 mg·g–1 for U(VI) removal. Zhu et al.(27) grafted fungus hypha onto a layer of two-dimensional graphene oxide sheets and pyrolyzed to form a fungal hyphae/graphene oxide aerogel for efficient removal of U(VI) with an increased adsorption capacity (288.42 mg·g–1). Although the adsorption ability can be increased by various modification methods, this approach is often complicated and costly.
As a new carbon nanomaterial, single-walled carbon nanohorns (SWCNHs) are characterized as being horn-shaped graphitic tubes with a diameter of 3–6 nm and a tube length of 45–55 nm. These tubes, upon aggregation, could form dahlia-like, bud-like, and seed-like structures.33 SWCNHs were prepared by laser ablation of graphite without using a metal catalyst, resulting in SWCNHs with high purity and without metal impurities.34 The preparation process was simple and could be used in the production of larger quantities. The high specific surface area, internal nanometer-scaled space, and abundant pore structure enable SWCNHs to be easily functionalized.35 Therefore, SWCNHs could provide an excellent alternative to other carbon nanomaterials in the field of radioactive element adsorption.
It can also be affirmed that the introduced functional groups on SWCNHs (such as −NH2, −OH, −SH, and −COOH groups) can provide active adsorption sites and thereby efficiently enhance the interaction between the target object and SWCNHs.36 In particular, the amino group was proved to be a superb functional group and widely used in laboratory and industry because of its excellent coordination affinity for U(VI).37 Among various amine-based compounds, triethylenetetramine (TETA) having four active amine groups is a promising candidate to react with SWCNHs.38,39 In addition, COOH was introduced to improve solubility and dispersion in wastewater.
Herein, amino-functionalized SWCNHs (SWCNH–TETA) were obtained via covalent grafting of TETA onto the carboxylated surface of SWCNHs and used for the removal of uranium ions from aqueous solutions. As it was already anticipated, the as-prepared absorbent was shown to have a higher adsorption capacity than only carboxylated SWCNHs (SWCNH–COOH), indicating that SWCNH–TETA is an excellent material to remove uranium ions with increased efficiency. The effects of several parameters such as pH of solutions, adsorption time, U(VI) concentration, and temperature on adsorption efficiency were investigated. Furthermore, the adsorption mechanism, kinetics, isotherm model, and thermodynamics were systematically investigated.
2. Materials and Methods
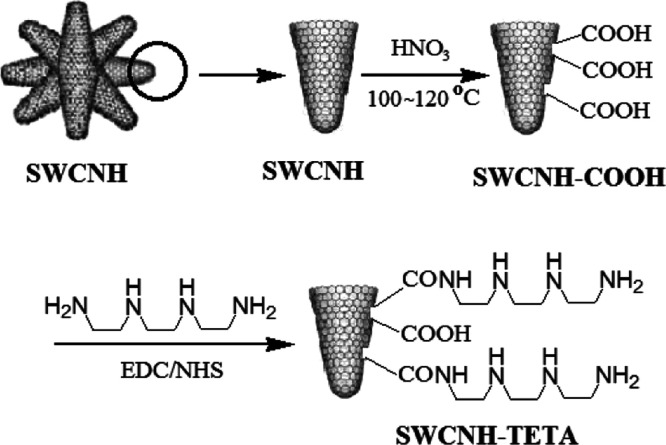
2.1. Synthesis of SWCNH–COOH Composite
150 mg of SWCNHs (S1.1, Supporting Information) was dispersed in 200 mL of nitric acid solution and refluxed at 120 °C for 24 h to obtain carboxylated SWCNH (SWCNH–COOH) dispersion solution. The dispersed solution was centrifuged, washed, and filtered with deionized water until the filtrate was neutral, and then dried by 50 °C for 48 h in vacuum. Finally, SWCNH–COOH was dispersed in deionized water to prepare a 1.0 mg/mL dispersion solution.
2.2. Preparation of the SWCNH–TETA Composite
SWCNH–COOH and TETA as raw materials were reacted with the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) catalyst, under protection of a nitrogen atmosphere, at 80 °C for 12 h. Then, the products were cooled to 20 °C, centrifuged, and repeatedly washed with pure water. Finally, they were dried in vacuum at 50 °C. Here, the mass ratio of SWCNH to TETA was 5:1, while the molar proportion of EDC to NHS was 1:1 and the weight proportion of EDC and NHS to TETA was 1:1. The experimental flow is shown in Scheme 1.
Scheme 1. Preparation Process of SWCNH–COOH and SWCNH–TETA.
3. Results and Discussion
3.1. Morpho-Structural Characterization of SWCNH-Based Materials
The morphologies and microstructures of as-obtained carbon-based materials were characterized first using transmission electron microscopy (TEM) (Figure 1). The SWCNH–COOH (Figure 1A) exhibits a transparent and dahlia-like structure, which is evenly dispersed without apparent agglomeration. Moreover, the surface of SWCNH–COOH is clean, with few impurities, indicating that the acidification treatment is efficient. On the other hand, many random needle-like structures are distributed on the surface and roughen the surface of SWCNH–TETA (Figure 1B). The structure of the amino-functionalized SWCNH is looser, which could significantly increase the specific surface area of the carbon-based material, which is advantageous considering the adsorption performances.
Figure 1.
TEM images of SWCNH–COOH (A) and SWCNH–TETA (B).
Typical X-ray diffraction (XRD) patterns of SWCNH–COOH and SWCNH–TETA are exhibited in Figure 2A. An intensive diffraction peak could be observed at a 2θ value of 26.3° and a smaller peak at 43.2°, corresponding to the (002) and (101) reflections of graphitic carbon (JCPDS card no. 41-1487), respectively. The (002) peaks of SWCNH–COOH and SWCNH–TETA are broad, suggesting the presence of some graphitization and the existence of amorphous structures in the carbonaceous material.40
Figure 2.
XRD spectra (A) and Raman spectra (B) of SWCNH–COOH and SWCNH–TETA.
Raman spectroscopy is the most direct and nondestructive technique to characterize the structure and quality of carbonaceous materials, especially to determine the defects and the ordered and/or the disordered structures. Two remarkable peaks are exhibited at about 1346 and 1586 cm–1 (Figure 2B), corresponding to the well-defined D and G bands, respectively. Generally, the G band represents the stretching E2g vibrational modes of the benzenoid framework of SWCNH, and the D band is ascribed to the sp3-hybridized carbon atom on the surface of the carbon-based materials.41 The frequencies of D and G bands in the SWCNH–TETA are similar to those observed in the case of SWCNH–COOH, indicating that both retain an excellent graphitized structure. It is generally supposed that the intensity proportion of these two bands (ID/IG) can be regarded as an indicator of graphitization of carbon materials.42 SWCNH–TETA has an increased ID/IG value in comparison with SWCNH–COOH (0.92 vs 1.07), indicating that TETA was successfully grafted onto the surface of SWCNH and more defect structures were introduced in the process, which can be beneficial for the removal of U(VI).
X-ray photoelectron spectroscopy (XPS) was applied to investigate the chemical composition and states of SWCNH-based materials. Atomic concentrations in SWCNH derivatives obtained from XPS are shown in Table S1. The results indicated that an increase in nitrogen was observed, suggesting that TETA was successfully grafted onto the SWCNH surface via adsorption or chemical bonding. In addition, the elemental content analysis of XPS also indicated that a reduction in oxygen appeared, indicating that the amidation reaction can cause a decrease in the oxygen content.
The wide-range XPS spectra of SWCNH–COOH and SWCNH–TETA are shown in Figure 3A. In the SWCNH–TETA spectrum, a small peak (N 1s) appeared, and the peak of the O element is reduced, compared with the XPS spectrum of SWCNH–COOH, which could be attributed to the introduction of nitrogen element during the amidation reaction.
Figure 3.
(A) XPS spectra of SWCNH–COOH and SWCNH–TETA. High-resolution XPS spectra of SWCNH–TETA: (B) C 1s, (C) N 1s, and (D) O 1s.
The C 1s spectrum of SWCNH–TETA (Figure 3B) mainly shows four peaks, which represent the signals of C–C/C=C (284.5 eV), C–N (285.4 eV), C–O (alkanol/epoxide, 286.3 eV), and C=O (288.2 eV).38
The N 1s XPS spectrum of SWCNH–TETA is shown in Figure 3C, which is split into three peaks at 399.1, 400.3, and 401.9 eV, respectively. The peak at 399.1 eV represents the nitrogen atoms of free NH2 in TETA and the peak at 400.3 eV corresponds to the amide group of CONH, indicating that TETA was covalently grafted onto the surface of SWCNH–COOH. The peak with 401.9 eV is present because of the N atoms in NH3+.43−45
The O 1s spectrum of SWCNH–TETA (Figure 3D) is deconvoluted into four peaks at 531.2, 531.9, 532.5, and 533.5 eV, which are ascribed to C=O, CONH, C–OH, and C–O, respectively.43 It can be concluded from the results from XPS investigations that SWCNH–TETA was successfully obtained.
The Fourier-transform infrared (FT-IR) spectra of SWCNH–COOH and SWCNH–TETA are recorded in Figure S1. The FT-IR spectrum of SWCNH–COOH shows peaks at 1727, 1626, 1167, and 1039 cm–1, which can be attributed to the C=O stretching vibration, C=C stretching vibration, C–O stretching and bending vibrations, respectively. The results indicated that SWCNH–COOH contains a large number of hydroxyl, carboxyl, and epoxy groups with strongly hydrophilic properties. After modification with TETA, two new strong peaks at 2915 and 2845 cm–1 resulted from the symmetric and asymmetric stretching vibrations of CH2 groups of alkyl chains in TETA. The peaks at 1727 and 1626 cm–1 can be attributed to the stretching vibrations of C=O and C=C groups, respectively. The bands corresponding to vibrations of N–H and C–N are observed at 1436 and 1342 cm–1, respectively.46−48 The FT-IR spectra indicate that TETA molecules are successfully grafted onto the SWCNH surface by chemical bonding.
3.2. pH Influence
The initial pH value plays a very important role in affecting the sorption capacity in the adsorption process. Therefore, the influence of pH value (ranging from 2.5 to 8.0) on the adsorption of uranium ions was investigated (Figure 4A). SWCNH–COOH and SWCNH–TETA had similar tendencies of U(VI) sorption capacities, as the obtained values first increased and then decreased at neutral and/or in a weak basic medium.
Figure 4.
(A) Influence of pH on U(VI) removal for SWCNH–COOH and SWCNH–TETA. (C0 = 60 mg·L–1, V = 50 mL, m = 10 mg, T = 303 K, t = 180 min.) (B) Zeta potential of SWCNH–COOH and SWCNH–TETA.
U(VI) removal is closely related to the distribution of uranium species of the solution, which mostly depended on the pH of the solution. When the pH value was lower than 4.0, U(VI) were mainly present in the form of UO22+ ions, which can cause competition with H3O+. Moreover, the accumulation of H3O+ weakens the electrostatic interaction between uranyl ions and the adsorbent.49 The capacity of U(VI) adsorbed by SWCNH–TETA increases significantly from 116.35 to 205.6 mg/g with the increase of pH value to slightly acidic values (4.0–6.0). When the pH was 6.0, the adsorption capacities of SWCNH–COOH and SWCNH–TETA for uranium reached their maximum, with 65.5 and 205.6 mg/g, respectively. This is because the negative charge on the surface of the adsorbent increases at a relatively higher pH value, giving rise to electrostatic attraction between the cations and the adsorbent. When the pH value was higher than 6, U(VI) easily hydrolyzed and combined with other species to form complexes, such as (UO2)3(OH)7– and UO2(OH)3–, causing electrostatic repulsion.50 Therefore, a decrease of uranyl ion adsorption capacity would occur at a pH higher than 6.0.51 Besides, compared with SWCNH–COOH, SWCNH–TETA exhibited higher sorption capacity within the pH range of 2.5–8.0. This could be attributed to the nitrogen in the amino group, which has stronger electronegativity and coordination ability with U(VI).
To better understand the influence of pH on U(VI) adsorption, the zeta potentials of SWCNH–COOH and SWCNH–TETA as a function of pH values were investigated (Figure 4B). With the increase of pH, the zeta potentials of SWCNH–COOH and SWCNH–TETA gradually reduced, and the corresponding pH values at the point of zero charges (pHPZC) were 4.05 and 3.32, respectively. When the pH was lower than the value of pHPZC, the surface of the material is positively charged, and U(VI) mainly existed in the form of UO22+, causing electrostatic repulsion and weakening of the adsorption of U(VI) at a relatively higher pH value.52
3.3. Adsorption Kinetics
The effects of adsorption time of SWCNH–COOH and SWCNH–TETA on U(VI) removal are compiled in Figure 5. The adsorption rate increased quickly at the early stage because of the availability of a lot of vacant sorption sites. Afterward, the vacant sorption sites were slowly occupied with uranyl ions, which made the rate of sorption gradually stable, and the sorption rate became slower in comparison with the beginning of the process. U(VI) sorption on SWCNH–COOH and SWCNH–TETA reached equilibrium after 60 and 240 min, respectively.
Figure 5.
Influence of time on the adsorption of U(VI) with SWCNH–COOH and SWCNH–TETA. (C0 = 60 mg·L–1, V = 50 mL, m = 10 mg, pH = 6.0, T = 303 K.)
It is generally supposed that adsorption kinetics can be divided into two stages. The initial adsorption stage is rapid and has a significant contribution to equilibrium absorption. Because of the existence of considerable adsorption sites, it is described as a quasi-instantaneous adsorption stage on the external surface. The second stage is regarded to be a gradual sorption stage, in which mainly the intramolecular diffusion controls the absorption rate until the adsorption reaches the equilibrium.
For investigating the dynamics of adsorption, two various kinetic equations, a pseudo-first order and a pseudo-second order, were employed to describe the U(VI) sorption
The pseudo-first-order equation can be seen in eq 1
| 1 |
The pseudo-second-order equation is given in eq 2
| 2 |
where Qe and Qt stand for the capacities of adsorbed U(VI) (mg/g) at equilibrium and at time t, respectively; k1 (min–1) and k2 (g/mg·min) represent the rate constants of kinetic equations; and t is the contact time (min).
The representations of ln(Qe – Qt) versus t and t/Qt versus t are shown in Figure 6A,B. The kinetic parameters (Table 1) are determined for the two models. We can find that the correlation coefficients of pseudo-second-order kinetics R2 (0.9979 and 0.990, respectively) were better than that of pseudo-first-order kinetics. Moreover, the calculative U(VI) adsorption capacities Q2,cal of SWCNH–COOH and SWCNH–TETA (71.43 and 333.33 mg/g, respectively) were close to the experimental values Qe,exp (65.5 and 205.6 mg/g, respectively). Therefore, the pseudo-second-order kinetic model can better describe the adsorption process of U(VI), indicating that the adsorption rate is mainly dominated by chemical reactions.
Figure 6.
(A) Pseudo-first order and (B) pseudo-second order kinetic plots of U(VI) adsorption by SWCNH–COOH and SWCNH–TETA.
Table 1. Kinetic Parameters of U(VI) Adsorbed Using SWCNH–COOH and SWCNH–TETA.
| pseudo-first-order model |
pseudo-second-order model |
||||||
|---|---|---|---|---|---|---|---|
| sample | Qe,exp (mg/g) | Q1,cal (mg/g) | k1 (min–1) | R2 | Q2,cal (mg/g) | k2 (g/mg min) | R2 |
| SWCNH–COOH | 65.5 | 25.30 | 0.034 | 0.9364 | 71.43 | 1.70 × 10–3 | 0.9979 |
| SWCNH–TETA | 205.6 | 396.03 | 0.028 | 0.9685 | 333.33 | 4.41 × 10–5 | 0.990 |
3.4. Sorption Isotherms
The influence of the initial uranyl ion concentration on the adsorption properties of SWCNH–COOH and SWCNH–TETA was investigated at pH 6.0 with the uranyl ion concentrations varying from 20 to 80 mg/L at 303 K.
As shown in Figure 7, with the increase of U(VI) concentration (≤50 mg/L), the adsorption capacity of both SWCNH–COOH and SWCNH–TETA increased. This is because the concentration gradient between the solution and the adsorbent increased with higher concentrations of U(VI). When the U(VI) concentration exceeded 50 mg/L, the value of adsorption capacity remained nearly unchanged (≈205 and ≈63 mg/g for SWCNH–TETA and SWCNH–COOH, respectively). The explanation for this phenomenon is that the binding sites of the as-prepared carbonaceous materials have been occupied successfully, and adsorption reached saturation at these concentrations. Moreover, a higher adsorption capacity was obtained in the case of SWCNH–TETA than that of SWCNH–COOH.
Figure 7.
Influence of initial concentrations of U(VI) adsorbed onto SWCNH–COOH and SWCNH–TETA (V = 50 mL, m = 10 mg, pH = 6.0, T = 303 K, t = 180 min).
For investigating the adsorption behavior of the as-prepared adsorbents, the Langmuir (eq 3) and Freundlich (eq 4) equations were used to fit the experimental data and for in-depth interpretation of the adsorption process in solid–liquid systems. The linear forms of these isotherms can be given as eqs 3 and 4.
| 3 |
| 4 |
where Ce stands for the equilibrium concentration (mg/L), Qe represents the equilibrium uptake (mg/g), Qm denotes the maximum amount of metal ion required per unit weight of adsorbent to form a complete monolayer on the surface (mg/g), KL is the Langmuir adsorption equilibrium constant (L/mg), and Kf and n are the Freundlich constant related to the adsorption amount and the adsorption intensity of the sorbent, respectively.
According to the equilibrium data, the linear plots of Ce/Qe versus Ce and ln Qe versus ln Ce are compiled in Figure 8. The related parameters were calculated and are listed in Table 2. The correlation coefficients (R2) with the Langmuir isotherm were 0.973 and 0.986 for SWCNH–COOH and SWCNH–TETA, respectively, which are better than those in the case of Freundlich isotherm. The result shows that the Langmuir isotherm gave better prediction for the equilibrium of U(VI) adsorption.
Figure 8.
(A) Langmuir and (B) Freundlich isotherms for uranyl ions adsorbed onto SWCNH–COOH and SWCNH–TETA.
Table 2. Data of Langmuir and Freundlich Isotherms of Uranyl Ions Adsorbed onto SWCNH–COOH and SWCNH–TETA.
| Langmuir
isotherm |
Freundlich
isotherm |
|||||
|---|---|---|---|---|---|---|
| materials | KL | Qm (mg/g) | R2 | Kf | n | R2 |
| SWCNH–COOH | 0.0502 | 90.9 | 0.973 | 9.964 | 2.058 | 0.9323 |
| SWCNH–TETA | 0.0919 | 333.13 | 0.986 | 73.99 | 3.185 | 0.826 |
Generally, the Langmuir isotherm model assumes the formation of a monolayer onto the surface of the adsorbent, with a finite number of identical sites. From the Langmuir model, the obtained maximum monolayer capacities (Qm) of SWCNH–COOH and SWCNH–TETA were 90.90 and 333.13 mg/g, respectively, which indicated that NH2-functionalized SWCNHs have a better adsorption capacity than carboxyl-modified SWCNHs.
In comparison with the maximum sorption capacities of other nitrogen-containing sorbents from the literature (Supporting Information, Table S2), the Qm value of SWCNH–TETA was found to be higher than that of many other adsorbents except SBA-15-NH2. SWCNH–TETA with such an excellent affinity toward the adsorption of U(VI) shows high applicability in the adsorption and recovery of U(VI).
3.5. Sorption Thermodynamics
The adsorption thermodynamics is fundamental to understand the sorption mechanisms as well as kinetics. Thermodynamic parameters such as ΔH, ΔG, and ΔS can be elucidated using the following equations
| 5 |
| 6 |
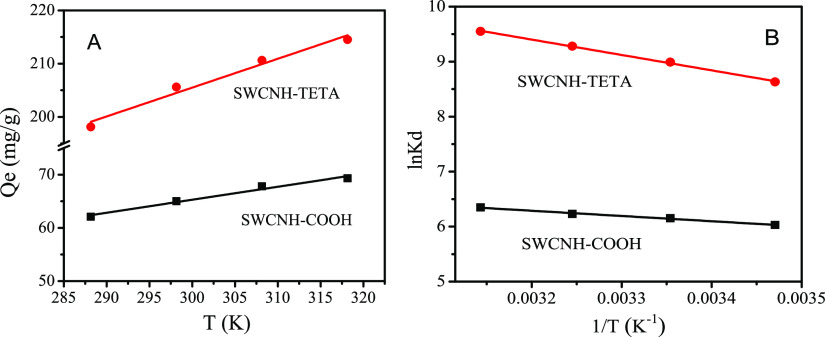
where Kd stands for the distribution coefficient. T is the absolute temperature. ΔH (J/mol), ΔS (J/mol K), and ΔG (J/mol) are the enthalpy change, entropy change, and Gibbs free energy change, respectively. R stands for the universal gas constant (8.314 J/mol K). The effects of temperature on the adsorption of U(VI) can be observed in Figure 9A. It can be noticed that the sorption capacity of U(VI) increases gradually with the increase of temperature, suggesting that the sorption exhibits an endothermic behavior. Therefore, increasing temperature was conducive to adsorption.
Figure 9.
(A) Influence of the temperature on U(VI) removal; (B) thermodynamics of adsorption of U(VI) in the presence of SWCNH–COOH and SWCNH–TETA. (m = 10 mg, C0 = 60 mg/L, V = 50 mL, and pH 6.0).
From the slope and intercept of the straight lines in Figure 9B, the values of ΔH and ΔS were calculated. The as-calculated thermodynamic data are listed in Table 3. The positive values of ΔH indicate that the adsorption processes of U(VI) using SWCNH–COOH and SWCNH–TETA are endothermic, and the positive values of ΔS are indicative of the increased randomness at the adsorbent–adsorbate interface. Moreover, the values of ΔG between 283.15 and 318.15 K were negative, indicating that the adsorption onto the carbonaceous materials was spontaneous and feasible. The values of ΔG decreased with the increase of temperature, which indicated that the adsorption process was more efficient at higher temperatures. Furthermore, the value of ΔG for SWCNH–TETA is lower than that for SWCNH–COOH at the same temperature, demonstrating that the adsorption process of SWCNH–TETA is more favorable than that of its acidic counterpart.
Table 3. Thermodynamic Data for U(VI) with SWCNH–COOH and SWCNH–TETA.
| ΔG (KJ/mol) |
||||||
|---|---|---|---|---|---|---|
| sample | ΔH (KJ/mol) | ΔS (J/mol·K) | 288.15 | 298.15 | 308.15 | 318.15 |
| SWCNH–COOH | 7.92 | 77.63 | –14.45 | –15.20 | –15.99 | –16.77 |
| SWCNH–TETA | 23.28 | 152.65 | –20.68 | –22.21 | –23.74 | –25.26 |
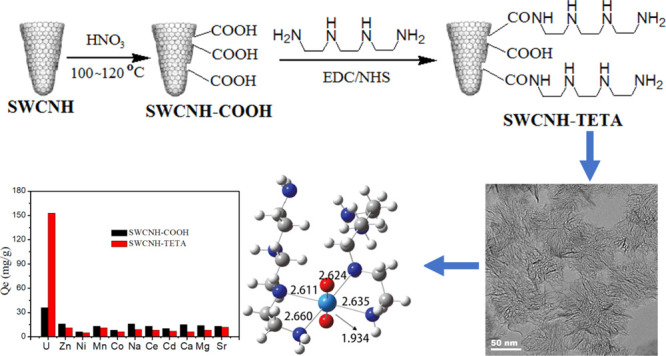
3.6. Selective Adsorption
For investigating the selectivity of the as-prepared carbonaceous materials for the adsorption of uranyl ions, the experiment was conducted under optimal conditions (C0 = 60 mg/L, V = 50 mL, T = 303 K, t = 180 min, and pH = 6.0) with the addition of 10 coexisting metal ions. As shown in Figure 10, the removal capacity of U(VI) of SWCNH–TETA is much higher than those of the coexisting ions. The results have also shown that the coexisting ions had little effect on adsorption, suggesting that SWCNH–TETA exhibited enhanced selectivity for U(VI). However, we found low selective adsorption for metal ions on SWCNH–COOH. The excellent selectivity of SWCNH–TETA could be mainly ascribed to the following two reasons
-
(1)
The essential reason is the strong coordination between nitrogen donor atoms on the adsorbents and uranyl ions. Compared with other metals, uranium has a stronger affinity for complexing with N–H ligands, leading to excellent selectivity of SWCNH–TETA for uranium.53
-
(2)
The average U–N bond distance is markedly smaller than the average distance in the case of other metal–N bonds (i.e. Zn2+, Ni2+, Co2+, Cd2+, Mn2+, and ions), which can lead to a strong affinity of nitrogen-containing functional groups toward U(VI).54 Thus, SWCNH–TETA exhibited promising selectivity for U(VI) from wastewater containing a range of competing metal ions in real wastewater effluents.
Figure 10.
Selective adsorption capacity of coexisting ions on two sorbents (C0 = 60 mg/L, m = 0.01 g, V = 50 mL, T = 303 K, t = 180 min, and pH = 6.0).
3.7. Desorption and Recycle Capability
In order to investigate the reusability of adsorbents in practical applications, 10 mg of adsorbent, SWCNH–TETA, was added to 50 mL of 50 mg/L uranium aqueous solution with pH 6.0 and then shaken at 25 °C for 4 h at a speed of 200 rpm. Finally, the uranium concentrations were measured in the solution before and after adsorption.
The filtered adsorbent was eluted three times with 1 mol/L HCl solution and then rinsed with deionized water several times. Finally, it was dried for the next round of adsorption. As shown in Figure 11, the adsorption capacity of SWCNH–TETA decreased from 205.6 to 179.3 mg/g after repeatedly using five times, and the adsorption capacity of SWCNH–TETA showed a low decrease (maintained above 87.2%), which fully indicates that SWCNH–TETA exhibits excellent reuse performance for U(VI) adsorption.
Figure 11.
Operational stability of SWCNH–TETA.
3.8. Adsorption Mechanism
XPS spectra of both survey and high-resolution scans before and after U(VI) sorption on SWCNH–TETA (Figure 12) were obtained to gain more insights into the interactions during the adsorption mechanisms.
Figure 12.
XPS spectra of SWCNH–TETA before and after adsorption of uranium: (A) survey XPS spectra and (B–D) high-resolution XPS spectra—(B) U 4f, (C) N 1s, and (D) O 1s.
The presence of a new U 4f peak after U(VI) sorption on SWCNH–TETA clearly confirms the successful sorption of U(VI) (Figure 12A). The U 4f spectrum (Figure 12B) shows two characteristic peaks at 382.3 and 393.2 eV, which are assigned to the U 4f peaks reported in the literature and correspond to U 4f7/2 and U 4f5/2, respectively.55 The results showed that the material could adsorb uranium well.
The N 1s and O 1s spectra of SWCNH–TETA before and after U(VI) adsorption are presented in Figure 12C,D. The N 1s peak shifted from 400.2 to 400.7 eV and the O 1s peak moved from 531.9 to 532.5 eV after adsorption of U(VI). This result could be attributed to the reduced electron cloud density of oxygen and nitrogen atoms outside the nucleus when SWCNH–TETA adsorbed U(VI) and indicates the complexation process between U(VI) and the nitrogen- and oxygen-containing functional groups of SWCNH–TETA.56,57
The sorption mechanism of SWCNH–TETA for U(VI) could be attributed to considerable nitrogen-containing and oxygen-containing groups, present on the surface, which is prone to coordinate with UO22+. Moreover, the carboxyl groups on the surface of SWCNH–TETA contributed to the negative charge on the surface.
To understand the mechanism of the sorption behavior deeply, density functional theory (DFT) calculations were used for investigating the coordination of UO22+ with two TETA chains. After all the optimization processes, it was found that there is a tetra- or penta-coordination of UO22+ with the N ligand of TETA (Figure 13). The average U–N bond distance is 2.60 Å in the case of penta-coordination, which is similar to the values of tetra-coordination. Additionally, the bond distances between uranium and the axial oxygen (U–Oax) were 2.017 and 1.934 Å for tetra- and penta-coordination, respectively. The calculated binding energy states between TETA and uranyl ions were 1.37 and 2.14 eV for tetra- and penta-coordination, respectively. The longer U–Oax and the more substantial binding energy indicate that the TETA chains can interact actively with uranyl ions. All theoretical results targeting the applicability showed that the organic TETA chains have high adsorption capacity for uranyl ions.
Figure 13.

Optimized structures of uranyl ion (UO22+) with two TETA chains: (A) penta- and (B) tetra-coordination modes.
4. Conclusions
SWCNH–COOH and SWCNH–TETA were successfully prepared from SWCNHs and TETA by acidification and amination. Their structures and morphologies were characterized in detail by TEM, XRD, XPS, and Raman spectroscopy. The batch static adsorption method was used to deeply research the influence of pH, adsorption time, temperature, and initial concentration on U(VI) adsorption capacity. The results showed that the acidity, the initial uranium concentration, and the temperature of the solution have considerable effects on U(VI) removal.
In comparison with SWCNH–COOH nanosheets, SWCNH–TETA showed a better adsorption capacity for uranium ions. Thermodynamic parameters showed that the adsorption of U(VI) was spontaneous and endothermic. The adsorption isotherms could be well fitted by the Langmuir isotherm equation, and the kinetics was successfully modeled by pseudo-second-order kinetics of the sorption process. Moreover, SWCNH–TETA showed excellent recycling performance and selectivity. Finally, the “after” XPS and DFT calculations showed that the sorption ability was primarily attributed to the interaction between uranium ions and nitrogen atoms in SWCNH–TETA. As expected, the TETA-based composite is a highly efficient uranium ion adsorbent.
Acknowledgments
The authors are grateful for the financial support of the National Natural Science Foundation of China (nos. 41361088, 41867063), Natural Science Foundation of Jiangxi Province (20181BAB203012), Foundation of the Key Laboratory of Radioactive Geology and Exploration Technology Fundamental Science for National Defense of China (2011RGET07, RGET1310), and the Open Project Foundation of Nuclear Engineering Technology Research Center of Ministry of Education (HJSJYB2011-17).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02715.
Experimental section, reagents and materials, batch adsorption experiments, characterization and calculation details, atomic concentrations (from XPS spectra) of different samples, comparison of sorption capacities of N-functionalized sorbents for U(VI), and FTIR spectra of SWCNH–COOH and SWCNH–TETA (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Duan S.; Xu X.; Liu X.; Sun J.; Hayat T.; Alsaedi A.; Li J. Effect of Fe3O4@ PDA morphology on the U (VI) entrapment from aqueous solution. Appl. Surf. Sci. 2018, 448, 297–308. 10.1016/j.apsusc.2018.04.131. [DOI] [Google Scholar]
- Zhang X.; Jiao C.; Wang J.; Liu Q.; Li R.; Yang P.; Zhang M. Removal of uranium (VI) from aqueous solutions by magnetic Schiff base: Kinetic and thermodynamic investigation. Chem. Eng. J. 2012, 198–199, 412–419. 10.1016/j.cej.2012.05.090. [DOI] [Google Scholar]
- Yuan L.; Sun M.; Liao X.; Zhao Y.; Chai Z.; Shi W. Solvent extraction of U(VI) by trioctylphosphine oxide using a room-temperature ionic liquid. Sci. China: Chem. 2014, 57, 1432–1438. 10.1007/s11426-014-5194-8. [DOI] [Google Scholar]
- Mellah A.; Chegrouche S.; Barkat M. The precipitation of ammonium uranyl carbonate (AUC): thermodynamic and kinetic investigations. Hydrometallurgy 2007, 85, 163–171. 10.1016/j.hydromet.2006.08.011. [DOI] [Google Scholar]
- Prasadaao T.; Metilda P.; Gladis J. Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination—an overview. Talanta 2006, 68, 1047–1064. 10.1016/j.talanta.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Banerjee C.; Dudwadkar N.; Tripathi S. C.; Gandhi P. M.; Grover V.; Kaushik C. P.; Tyagi A. K. Nano-cerium vanadate a novel inorganic ion exchanger for removal of americium and uranium from simulated aqueous nuclear waste. J. Hazard. Mater. 2014, 280, 63–70. 10.1016/j.jhazmat.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Song Z.; Huang W.; Zhou Y.; Tian Z.-Q.; Li Z.-M.; Tao D.-J. Thermally regulated molybdate-based ionic liquids toward molecular oxygen activation for one-pot oxidative cascade catalysis. Green Chem. 2020, 22, 103–109. 10.1039/c9gc03646f. [DOI] [Google Scholar]
- Hui W.; Zhou Y.; Dong Y.; Cao Z.-J.; He F.-Q.; Cai M.-Z.; Tao D.-J. Efficient hydrolysis of hemicellulose to furfural by novel superacid SO4H-functionalized ionic liquids. Green Energy Environ. 2019, 4, 49–55. 10.1016/j.gee.2018.06.002. [DOI] [Google Scholar]
- Lovley D. R.; Phillips E. J. P.; Gorby Y. A.; Landa E. R. Microbial reduction of uranium. Nature 1991, 350, 413–416. 10.1038/350413a0. [DOI] [Google Scholar]
- Gupta N. K.; Sengupta A.; Gupta A.; Sonawane J. R.; Sahoo H. Biosorption-an alternative method for nuclear waste management: a critical review. J. Environ. Chem. Eng. 2018, 6, 2159–2175. 10.1016/j.jece.2018.03.021. [DOI] [Google Scholar]
- Liu X.; Cheng C.; Xiao C.; Shao D.; Xu Z.; Wang J.; Hu S.; Li X.; Wang W. Polyaniline (PANI) modified bentonite by plasma technique for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2017, 411, 331–337. 10.1016/j.apsusc.2017.03.095. [DOI] [Google Scholar]
- Chen F.-F.; Huang K.; Fan J.-P.; Tao D.-J. Chemical solvent in chemical solvent: a class of hybrid materials for effective capture of CO2. AIChE J. 2018, 64, 632–639. 10.1002/aic.15952. [DOI] [Google Scholar]
- Zhao H.; Liu X.; Yu M.; Wang Z.; Zhang B.; Ma H.; Wang M.; Li J. A study on the degree of amidoximation of polyacrylonitrile fibers and its effect on their capacity to adsorb uranyl ions. Ind. Eng. Chem. Res. 2015, 54, 3101–3106. 10.1021/ie5045605. [DOI] [Google Scholar]
- An X.-C.; Li Z.-M.; Zhou Y.; Zhu W.; Tao D.-J. Rapid capture and efficient removal of low-concentration SO2 in simulated flue gas by hypercrosslinked hollow nanotube ionic polymers. Chem. Eng. J. 2020, 394, 124859. 10.1016/j.cej.2020.124859. [DOI] [Google Scholar]
- Wang Y.-Q.; Zhang Z.; Liu Y.; Cao X. Adsorption of U(VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem. Eng. J. 2012, 198–199, 246–253. 10.1016/j.cej.2012.05.112. [DOI] [Google Scholar]
- Li W. P.; Han X. Y.; Wang X. Y.; Wang Y. Q.; Wang W. X.; Xu H.; Tan T. S.; Wu W. S.; Zhang H. X. Recovery of uranyl from aqueous solutions using amidoximated polyacrylonitrile/exfoliated Na-montmorillonite composite. Chem. Eng. J. 2015, 279, 735–746. 10.1016/j.cej.2015.05.060. [DOI] [Google Scholar]
- He F.; Qian Y.; Xu J. Performance, mechanism, and kinetics of Fe (III) EDTA reduction by thiourea dioxide[J]. Energy Fuels 2019, 33, 3331–3338. 10.1021/acs.energyfuels.8b03820. [DOI] [Google Scholar]
- Sun Y. B.; Wang Q.; Yang S. T.; Sheng G. D.; Guo Z. Q. Characterization of nano-iron oxyhydroxides and their application in UO22+ removal from aqueous solutions. J. Radioanal. Nucl. Chem. 2011, 290, 643–648. 10.1007/s10967-011-1325-2. [DOI] [Google Scholar]
- Liu Y.; Liu Y.; Cao X.; Hua R.; Wang Y.; Pang C.; Hua M.; Li X. Biosorption studies of uranium (VI) on cross-linked chitosan: isotherm, kinetic and thermodynamic aspects. J. Radioanal. Nucl. Chem. 2011, 290, 231–239. 10.1007/s10967-011-1336-z. [DOI] [Google Scholar]
- Belloni F.; Kütahyali C.; Rondinella V. V.; Carbol P.; Wiss T.; Mangione A. Can carbon nanotubes play a role in the field of nuclear waste management?. Environ. Sci. Technol. 2009, 43, 1250–1255. 10.1021/es802764g. [DOI] [PubMed] [Google Scholar]
- Huynh J.; Palacio R.; Safizadeh F.; Lefèvre G.; Descostes M.; Eloy L.; Guignard N.; Rousseau J.; Royer S.; Tertre E.; Batonneau-Gener I. Adsorption of Uranium over NH2-Functionalized Ordered Silica in Aqueous Solutions. ACS Appl. Mater. Interfaces 2017, 9, 15672–15684. 10.1021/acsami.6b16158. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhao Z.; Yuan D.; Wang Y.; Dai Y.; Zhu Y.; Chew J. W. Introduction of amino groups into polyphosphazene framework supported on CNT and coated Fe3O4 nanoparticles for enhanced selective U(VI) adsorption. Appl. Surf. Sci. 2019, 466, 893–902. 10.1016/j.apsusc.2018.10.097. [DOI] [Google Scholar]
- Abney C. W.; Mayes R. T.; Saito T.; Dai S. Materials for the recovery of uranium from seawater. Chem. Rev. 2017, 117, 13935–14013. 10.1021/acs.chemrev.7b00355. [DOI] [PubMed] [Google Scholar]
- Carboni M.; Abney C. W.; Liu S.; Lin W. Highly porous and stable metal–organic frameworks for uranium extraction. Chem. Sci. 2013, 4, 2396–2402. 10.1039/c3sc50230a. [DOI] [Google Scholar]
- Shao D.; Jiang Z.; Wang X.; Li J.; Meng Y. Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO22+ from aqueous solution. J. Phys. Chem. B 2009, 113, 860–864. 10.1021/jp8091094. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhang Z.; Wang X.; Chen J.; Xu C.; Liu Y.; Yu Z.; Wang X. Fabrication of magnetic Fe/Zn layered double oxide@carbon nanotube composites and their application for U(VI) and 241Am(III) removal. ACS Appl. Nano Mater. 2018, 1, 2386–2396. 10.1021/acsanm.8b00528. [DOI] [Google Scholar]
- Li Y.; Li L.; Chen T.; Duan T.; Yao W.; Zheng K.; Dai L.; Zhu W. Bioassembly of fungal hypha/graphene oxide aerogel as high performance adsorbents for U(VI) removal. Chem. Eng. J. 2018, 347, 407–414. 10.1016/j.cej.2018.04.140. [DOI] [Google Scholar]
- Sun Y.; Wu Z.-Y.; Wang X.; Ding C.; Cheng W.; Yu S.-H.; Wang X. Macroscopic and Microscopic Investigation of U(VI) and Eu(III) Adsorption on Carbonaceous Nanofibers. Environ. Sci. Technol. 2016, 50, 4459–4467. 10.1021/acs.est.6b00058. [DOI] [PubMed] [Google Scholar]
- Yu X.-F.; Liu Y.-H.; Zhou Z.-W.; Xiong G.-X.; Cao X.-H.; Li M.; Zhang Z.-B. Adsorptive removal of U(VI) from aqueous solution by hydrothermal carbon spheres with phosphate group. J. Radioanal. Nucl. Chem. 2014, 300, 1235–1244. 10.1007/s10967-014-3081-6. [DOI] [Google Scholar]
- Liu Y.; Dai Y.; Yuan D.; Wang Y.; Zou L. The preparation of PZS-OH/CNT composite and its adsorption of U(VI) in aqueous solutions. J. Radioanal. Nucl. Chem. 2017, 314, 1747–1757. 10.1007/s10967-017-5578-2. [DOI] [Google Scholar]
- Shao D.; Hou G.; Li J.; Wen T.; Ren X.; Wang X. PANI/GO as a super adsorbent for the selective adsorption of uranium(VI). Chem. Eng. J. 2014, 255, 604–612. 10.1016/j.cej.2014.06.063. [DOI] [Google Scholar]
- Liu Y.; Zhao Z.; Yuan D.; Wang Y.; Dai Y.; Chew J. W. Fast and high amount of U(VI) uptake by functional magnetic carbon nanotubes with phosphate group. Ind. Eng. Chem. Res. 2018, 57, 14551–14560. 10.1021/acs.iecr.8b03864. [DOI] [Google Scholar]
- Gulzar U.; Li T.; Bai X.; Colombo M.; Ansaldo A.; Marras S.; Prato M.; Goriparti S.; Capiglia C.; Proietti Zaccaria R. Nitrogen-doped single-walled carbon nanohorns as a cost-effective carbon host toward high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 5551–5559. 10.1021/acsami.7b17602. [DOI] [PubMed] [Google Scholar]
- Iijima S.; Yudasaka M.; Yamada R.; Bandow S.; Suenaga K.; Kokai F.; Takahashi K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. 10.1016/s0009-2614(99)00642-9. [DOI] [Google Scholar]
- Carli S.; Casarin L.; Syrgiannis Z.; Boaretto R.; Benazzi E.; Caramori S.; Prato M.; Bignozzi C. A. Single walled carbon nanohorns as catalytic counter electrodes for Co(III)/(II) electron mediators in dye sensitized cells. ACS Appl. Mater. Interfaces 2016, 8, 14604–14612. 10.1021/acsami.6b03803. [DOI] [PubMed] [Google Scholar]
- Depan D.; Misra R. D. K. The interplay between nanostructured carbon-grafted chitosan scaffolds and protein adsorption on the cellular response of osteoblasts: Structure–function property relationship. Acta Biomater. 2013, 9, 6084–6094. 10.1016/j.actbio.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Xie L.; Zhong Y.; Xiang R.; Fu G.; Xu Y.; Cheng Y.; Liu Z.; Wen T.; Zhao Y.; Liu X. Sono-assisted preparation of Fe(II)-Al(III) layered double hydroxides and their application for removing uranium (VI). Chem. Eng. J. 2017, 328, 574–584. 10.1016/j.cej.2017.07.051. [DOI] [Google Scholar]
- Vatanpour V.; Haghighat N. Improvement of polyvinyl chloride nanofiltration membranes by incorporation of multiwalled carbon nanotubes modified with triethylenetetramine to use in treatment of dye wastewater. J. Environ. Manage. 2019, 242, 90–97. 10.1016/j.jenvman.2019.04.060. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Fang M.; Pan Y.; Yan S.; Luo Z. Comparison and selection of amine-based absorbents in membrane vacuum regeneration process for CO2 capture with low energy cost. Energy Procedia 2013, 37, 1085–1092. 10.1016/j.egypro.2013.05.205. [DOI] [Google Scholar]
- Nanaji K.; Upadhyayula V.; Rao T. N.; Anandan S. Environmentally benign synthesis of nanoporous graphene sheets from biowaste for ultrafast supercapacitor application. ACS Sustainable Chem. Eng. 2019, 7, 2516–2529. 10.1021/acssuschemeng.8b05419. [DOI] [Google Scholar]
- Chen J.; Lin C.; Zhang M.; Jin T.; Qian Y. Constructing nitrogen, selenium co-doped graphene aerogel electrode materials for synergistically enhanced capacitive performance. ChemElectroChem 2020, 7, 3311–3318. 10.1002/celc.202000635. [DOI] [Google Scholar]
- Fujimori T.; Urita K.; Aoki Y.; Kanoh H.; Ohba T.; Yudasaka M.; Iijima S.; Kaneko K. Fine nanostructure analysis of single-wall carbon nanohorns by surface-enhanced raman scattering. J. Phys. Chem. C 2008, 112, 7552–7556. 10.1021/jp801416b. [DOI] [Google Scholar]
- Saxena A. P.; Deepa M.; Joshi A. G.; Bhandari S.; Srivastava A. K. Poly(3,4-ethylenedioxythiophene)-ionic liquid functionalized graphene/reduced graphene oxide nanostructures: improved conduction and electrochromism. ACS Appl. Mater. Interfaces 2011, 3, 1115–1126. 10.1021/am101255a. [DOI] [PubMed] [Google Scholar]
- Li F.; Yang Z.; Weng H.; Chen G.; Lin M.; Zhao C. High efficient separation of U (VI) and Th (IV) from rare earth elements in strong acidic solution by selective sorption on phenanthroline diamide functionalized graphene oxide. Chem. Eng. J. 2018, 332, 340–350. 10.1016/j.cej.2017.09.038. [DOI] [Google Scholar]
- Hui W.; He X.-M.; Xu X.-Y.; Chen Y.-M.; Zhou Y.; Li Z.-M.; Zhang L.; Tao D.-J. Highly efficient cycloaddition of diluted and waste CO2 into cyclic carbonates catalyzed by porous ionic copolymers. J. CO2 Util. 2020, 36, 169–176. 10.1016/j.jcou.2019.11.003. [DOI] [Google Scholar]
- Yang Y.; Zhang C.; Huang D.; Zeng G.; Huang J.; Lai C.; Zhou C.; Wang W.; Guo H.; Xue W.; Deng R.; Cheng M.; Xiong W. Boron nitride quantum dots decorated ultrathin porous g-C3N4: intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal., B 2019, 245, 87–99. 10.1016/j.apcatb.2018.12.049. [DOI] [Google Scholar]
- Yang Y.; Zeng G.; Huang D.; Zhang C.; He D.; Zhou C.; Wang W.; Xiong W.; Li X.; Li B.; Dong W.; Zhou Y. Molecular engineering of polymeric carbon nitride for highly efficient photocatalytic oxytetracycline degradation and H2O2 production. Appl. Catal., B 2020, 272, 118970. 10.1016/j.apcatb.2020.118970. [DOI] [Google Scholar]
- Wang W.; Niu Q.; Zeng G.; Zhang C.; Huang D.; Shao B.; Zhou C.; Yang Y.; Liu Y.; Guo H.; Xiong W.; Lei L.; Liu S.; Yi H.; Chen S.; Tang X. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal., B 2020, 273, 119051. 10.1016/j.apcatb.2020.119051. [DOI] [Google Scholar]
- Guo X.; Feng Y.; Li M.; Yu J.; Zhang Y. Uranyl ion adsorption studies on synthesized phosphoryl functionalised MWCNTs: a mechanistic approach. J. Radioanal. Nucl. Chem. 2018, 316, 397–409. 10.1007/s10967-018-5761-0. [DOI] [Google Scholar]
- Liu Y.; Zhao Z.; Yuan D.; Wang Y.; Dai Y.; Zhu Y.; Chew J. W. Introduction of amino groups into polyphosphazene framework supported on CNT and coated Fe3O4 nanoparticles for enhanced selective U(VI) adsorption. Appl. Surf. Sci. 2019, 466, 893–902. 10.1016/j.apsusc.2018.10.097. [DOI] [Google Scholar]
- Dai Z.; Zhang H.; Sui Y.; Ding D.; Hu N.; Li L.; Wang Y. Synthesis and characterization of a novel core–shell magnetic nanocomposite via surface-initiated RAFT polymerization for highly efficient and selective adsorption of uranium(VI). J. Radioanal. Nucl. Chem. 2018, 316, 369–382. 10.1007/s10967-018-5720-9. [DOI] [Google Scholar]
- Dong Z.-m.; Qiu Y.-f.; Dai Y.; Cao X.-h.; Wang L.; Wang P.-f.; Lai Z.-j.; Zhang W.-l.; Zhang Z.-b.; Liu Y.-h.; Le Z.-g. Removal of U(VI) from aqueous media by hydrothermal cross-linking chitosan with phosphate group. J. Radioanal. Nucl. Chem. 2016, 309, 1217–1226. 10.1007/s10967-016-4722-8. [DOI] [Google Scholar]
- Hu R.; Shao D.; Wang X. Graphene oxide/polypyrrole composites for highly selective enrichment of U (VI) from aqueous solutions. Polym. Chem. 2014, 5, 6207–6215. 10.1039/c4py00743c. [DOI] [Google Scholar]
- Yuan D.; Chen L.; Xiong X.; Yuan L.; Liao S.; Wang Y. Removal of uranium (VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheres prepared by a controlled radical polymerization in the presence of DPE. Chem. Eng. J. 2016, 285, 358–367. 10.1016/j.cej.2015.10.014. [DOI] [Google Scholar]
- Feng M.-L.; Sarma D.; Qi X.-H.; Du K.-Z.; Huang X.-Y.; Kanatzidis M. G. Efficient removal and recovery of uranium by a layered organic–inorganic hybrid thiostannate. J. Am. Chem. Soc. 2016, 138, 12578–12585. 10.1021/jacs.6b07351. [DOI] [PubMed] [Google Scholar]
- Yang D.; Song S.; Zou Y.; Wang X.; Yu S.; Wen T.; Wang H.; Hayat T.; Alsaedi A.; Wang X. Rational design and synthesis of monodispersed hierarchical SiO2@layered double hydroxide nanocomposites for efficient removal of pollutants from aqueous solution. Chem. Eng. J. 2017, 323, 143–152. 10.1016/j.cej.2017.03.158. [DOI] [Google Scholar]
- Chen L.; Zhao D.; Chen S.; Wang X.; Chen C. One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium (VI) removal. J. Colloid Interface Sci. 2016, 472, 99–107. 10.1016/j.jcis.2016.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.