Abstract
In this work, a biogenic-mediated approach is successfully used to synthesize a novel heterogeneous Cs2O–MgO/MPC basic nanocomposite. For the first time, the established technicality employs pomegranate seed extract that gives a green capping fuel and reducing mediators during an aqueous solution combustion process of metal ion precursors. The synthesized nanocomposites were identified by X-ray diffraction, Fourier transform infrared, N2 isotherms, field emission scanning electron microscopy, and CO2-TPD analyses. The transesterification process of olive oil was used to evaluate the catalytic performance. The nanocomposite displayed outstanding catalytic efficiency stemming from the boosting of the reactant and product diffusion. The transesterification activity and the optimization design were assessed by applying the response surface methodology. Based on the experimental tests, the finest experimental conditions with a biodiesel yield of 96.1% are 4 h, 4% catalyst amount, an oil/methanol ratio of 1:15, and a temperature of 65 °C. The predicted optimal conditions based on the statistical model are 6 h contact time, 5.2 % catalyst dose, 65 °C reaction temperature, and 1:15 oil/methanol molar ratio, attaining a biodiesel yield of 95.18%. The catalyst reusability has been performed almost continuously up to four cycles, with no loss of the active constituents. The obtained biodiesel demonstrated characteristics close to those of international standards of biodiesel. Besides, the process employed in this study demonstrated significant potential for further development and commercialization and is cheaper than the refined vegetable oil used in traditional approaches of biodiesel manufacturing.
1. Introduction
Economic and environmental accidents and subsequent impacts on human life were substantially affected nowadays.1 The increasing reliance on life on energy is evident, but fossil fuel resources are scarce, and their use contributes to contamination of the environment.2 Several researchers have attempted to explore a sustainable and environmentally friendly fuel comparable in properties to the existing fossil fuels.3−6 The importance of biodiesel production is related to providing energy and preserving a clean and healthy climate. To date, several current research studies have oriented on sustainable and renewable fuels such as biodiesel.7−11 Biodiesel is among the biodegradable fuels that result from an oil or fat and alcohol (methanol or ethanol) transesterification process in the existence of acidic or basic catalysts generating biodiesel and glycerin as a byproduct.12 Biodiesel offers appealing features such as nontoxicity, renewability, environmentally friendliness, more magnificent flash, and cetane number. Biodiesel is associated with reduced carbon monoxide, hydrocarbon, and lower CO2 emissions relative to traditional diesel, thus virtually removing sulfate. Biodiesel and fossil diesel are very identical to one another, so biodiesel in motors needs no significant maintenance costs to change engines with new fuel.13 Although biodiesel would be generated via uncatalytic operations, the use of catalysts reduces costs. The commonly pure acids and bases with great reaction rates are homogeneous catalysts.14 While applying homogeneous catalysts, the steps of acid or base separation and recycling will be applied to the manufacturing process.15 Much research was outlined to find an appropriate heterogeneous catalyst to overcome these problems.16−18 The surface area provided using a support is one of the remarkable variables in tailoring the heterogeneous catalysts.19−21 Dispersing the active constituents on the support produces adequate molecular bonding relevant to the existence and kind of support, active site deposition, and several other working parameters.22 Therefore, various materials have various approaches to fabrication.23,24 Of these, carbon-based materials were used as a support for catalyst manufacture.25
Heterogenic basic catalysts are categorized into metal oxides and their alternatives. Alkali earth metal oxides are commonly used as solid basic catalysts because of their minimal cost and strong basicity.26 The basicity of barium oxide and calcium oxide is typically greater than that of magnesium oxide.27 BaO, however, is poisonous and dissolves facilely in methanol or ethanol and has an undesirable environmental impact. CaO leaching was a significant problem, and Ca2+ leaching resulted in a rapid catalyst deactivation and the development of free fatty acid soap.28 In contrast, MgO has not been readily detached from the reaction process and retains significant performance when reacted with raw materials with a higher content of water.29 However, owing to its low surface area and catalytic performance, there is no economic value for the direct need for pure MgO as a catalyst. Hence, fabrication of MgO with great surface area and catalytic performance is vital.
Among the most potential applications of transesterification is the development of biodiesel from vegetable oils. Still, if vegetable oils of edible quality are considered, this process is not economical because raw materials are key factors contributing to biodiesel cost. Through the process of producing olive oil, however, it often tends to happen that the free fatty acid content of the olive oil meets nonstandard high quantities, which effectively make the oil inedible. The olive oils refining with a high concentration of free fatty acids is vulnerable to refining loss, and their processing is usually not affordable to the industrial food sector. The Al-Jouf region in the Kingdom of Saudi Arabia is famous for olive agriculture and abundance of water. Because of the difference in the quality of both groundwater and the nature of the soil, two types of olive oil with different qualities were produced. One is suitable for human (edible quality) use because of its distinctive taste and high quality and the other type is inedible. Consequently, the use of this inedible olive oil is of commercial benefit to biodiesel development through transesterification. The impact of the competitive nature of the suggested approach on the market because biodiesel production from olive oil is economically feasible without government assistance or tax benefits even under the commercialization trend of significant increases in raw material prices. Besides, if cheaper feed as inedible olive oil could be used in the suggested approach, its productivity is expected to be further enhanced, and industrial availability could be considerably increased.
In this paper, Cs2O–MgO/mesoporous carbon (MPC) as a basic nanocatalyst is practiced for biodiesel development from olive oil via the transesterification reaction. The nanocatalyst was fabricated by a one-pot aqueous solution combustion process in the presence of pomegranate seed extract (PMSE) as the capping reducing/stabilizer agent. Different parameters influencing the biodiesel production efficiency from olive oil, comprising methanol/olive oil molar ratio, contact time, temperature, and catalyst dose, were further examined. The response surface methodology (RSM) was utilized to optimize the biodiesel process and evaluate the influence of the variable connections.
2. Results and Discussion
2.1. Nanocomposite Characterizations
2.1.1. X-ray Diffraction Assessment
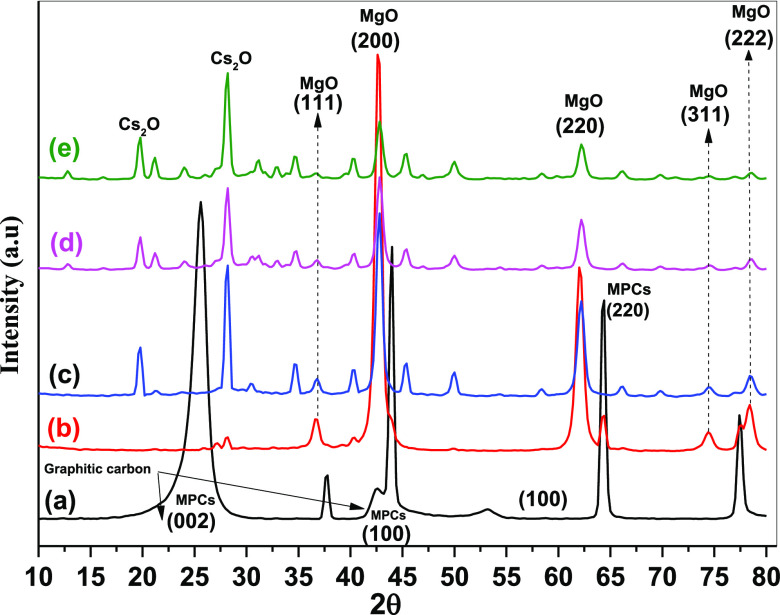
Figure 1 reveals the X-ray diffraction (XRD) patterns of MPCs and Cs2O–MgO/MPC nanocomposite with various Cs2O contents. The pristine MPCs offer two sharp and strong peaks allocated at 25.5 and 44.2 corresponding to the (002) and (101) facets, which can be related to the graphite nature of MPCs.30 The diffraction pattern for MgO/MPC shows characteristics peaks at 36.8, 42.7, 62.3, and 74.6°, assigned to the (111), (200), (220), and (311) facets, respectively, suggesting the development of a cubic MgO.31 It is also worth mentioning that the XRD patterns of Cs2O–MgO/MPCs are closely similar to that of the MgO/MPCs. However, the intensity of the diffraction peak of MgO (PDF#45-0946) became slightly broader and weaker, indicating that the crystal structure of the MgO remained unchanged in the presence of Cs2O. Also, The MPC diffraction peaks were not observed because Cs2O–MgO was incorporated within the MPCs. These findings show that the Cs2O–MgO, as the primary components, would have a strong relationship with the prior synthesizing features. The diffraction peak for Cs2O was observed at 19.7 and 28.1° even in the sample (MgO with 5 mol % Cs). It is worth noting that with increasing Cs2O content, the intensity of the diffraction peaks of MgO markedly decreased. Furthermore, the particle size of the Cs2O–MgO/MPC nanocomposite with different Cs2O contents was calculated using Debye–Scherrer eq 1
| 1 |
where the wavelengths λ and β are related to the line broadening at half the maximum intensity in radian and θ is assigned to the Bragg angle. The average particle size for MgO/MPCs was estimated to be 11.3 nm. The incorporation of Cs2O resulted in an increase in the particle size to 13.1 and 14.6 nm because of the presence of 5 and 20 mol % Cs2O, respectively (Table 1).
Figure 1.
XRD patterns of (a) pure MPC, (b) MgO/MPC, (c) 5Cs2O–MgO/MPC, (d) 10 Cs2O–MgO/MPC, and (e) 20 mol % Cs2O–MgO/MPC nanocomposites.
Table 1. Basicity and Texture Characteristics for the MgO/MPC Nanocomposite Containing Various Contents of Cs2O.
| catalysts | particle size (nm) | basicitya (μmol m–2) | BETb (m2 g–1) | Dp,adsc/nm | Vpd/(cm3g–1)- |
|---|---|---|---|---|---|
| MgO/MPCs | 11.3 | 13 | 475 | 2.8 | 0.64 |
| 5 Cs2O–MgO/MPCs | 13.1 | 421 | 3.6 | 0.57 | |
| 20 Cs2O–MgO/MPCs | 14.6 | 55 | 360 | 2.5 | 0.48 |
Quantity of CO2 desorbed.
Surface area.
Mean pore diameter(BJH).
Total pore volume.
2.1.2. Fourier Transform Infrared Analysis
Fourier transform infrared (FTIR) study was performed to evaluate the functional moieties in MPC, and the connections between them follow the inclusion of MgO and Cs2O in the MPC structure. FTIR spectra of the fabricated nanocomposite are displayed in Figure 2. The results revealed similar bands for all samples. No band designated to NO3– (at 1380 cm–1) was identified in the spectra because the full decomposition of nitrates into the corresponding metal oxides occurs during the solution combustion reaction. The absorption bands centered at 3200 cm–1 correspond to the stretching vibrations of O–H groups because of the physisorption of water molecules.32 The weak band at 2980 cm–1 is related to stretching vibration of C–H aliphatic, and the peaks are allocated at 1680 cm–1 assigned to the C=C group of the unsaturated structure. Besides, the sharp band at 1340 cm–1 can be ascribed to the Mg–O stretching mode. The peaks at 800–1050 cm–1 for the Mg–O bond result in the fruitful fabrication of MgO.33 The intensity of the bands increased, which could be attributed to the interaction between MPCs and Cs2O–MgO nanoparticles. Therefore, the results indicated that the Cs2O–MgO/MPC nanocomposite was successfully synthesized.
Figure 2.
FTIR spectra of the MgO/MPC nanocomposite containing various contents of Cs2O.
2.1.3. Basicity Assessment
The TPD assessment is performed to the fabricated materials owing to assess the basicity strength of the catalysts. Figure 3 describes the corresponding CO2-TPD profiles of the fabricated MgO/MPCs and Cs2O–MgO/MPCs. The quantity of CO2 desorbed is also displayed in Table 1. According to the CO2-TPD profile, three unique CO2-desorption regions emphasized the coexistence of various weak, medium, and reliable basic sites. The presence of a low-temperature region in the range 50–160 °C could be assigned to the adsorption of CO2 onto the surface hydroxyl as a weak basic site exists in the catalyst. The appearance of the desorption peaks in the temperature range 160–350 °C is assigned to the CO2 interaction with moderate basic sites originated from the oxygen in Mg2+ and O2– ion pairs. The intensity of weakly basic sites decreased significantly upon the incorporation of Cs, possibly owing to the promotion of sintering by adding cesium on MgO that eventually declined the hydroxyl surface moieties. Besides, all the fabricated composites display further desorption peak within the temperature range of 350–800 °C, which is associated with the forceful CO2 interaction with influential basic sites.34 Although all the composites demonstrated the existence of super basic sites, their intensity varies with the incorporation of Cs2O. The quantification results of the desorption region indicate that Cs2O–MgO/MPCs exhibit the highest basic strength than the MgO/MPC composite. The overall basicity, along with the dispersion of the basic centers in TPD profiles, increased with the incorporation of Cs2O (Figure 3). The quantity of the overall basicity is evaluated and displayed in Table 1. The robust basicity of Cs2O–MgO/MPCs is attributed mainly to (i) the presence of Cs2O species in close vicinity of MgO, (ii) the boosted surface electron density of actively distributed monovalent Cs by creating a Cs+ O2 ion pair, stemming from the variation in charge of Mg2+ and Cs+ ions, and (iii) the presence of surface defects owing to the incorporation of Cs.35
Figure 3.
CO2-TPD profiles of the MgO/MPC and 20Cs2O–MgO/MPC nanocomposite.
2.1.4. Surface and Textural Assessment
Nitrogen adsorption isotherms and pore size distribution assessments were performed to examine the impact of the incorporation of Cs2O–MgO on the porosity of the MPCs. The BET surface area, pore width, and pore volume of the synthesized materials are displayed in Table 1. Figure 4 displays the N2 adsorption–desorption isotherms of the synthesized composites. It can be shown that all fabricated composites exhibit type IV isotherm, with an H1-type hysteresis loop based on IUPAC classification. The pores formed in the fabricated composites were cylindrical, that is, a reasonable shape for the diffusion of large triglyceride molecules as a fuel source in the biodiesel-manufacturing processes. The influence of Cs2O incorporation on the BET surface area, pore volume, and pore size of all composites is displayed in Table 1. Both 5Cs2O–MgO/MPC and 20Cs2O–MgO/MPC nanocomposites exhibit lower BET surface areas and pore volumes compared with MgO/MPCs; this could be attributed to the accumulation of Cs2O within the MPC and a lack of micropores.36 The pore size distribution profile (inset Figure 4) shows that all composites have a uniform array of pores with a comparatively small distribution of the pore size. Furthermore, some structural changes arising from the incorporation of Cs2O are not noticeable at the expense of uniformity of the pores. Besides, the one-pot aqueous solution combustion process displays great quality and appropriate requirements in terms of the practical surface features for the targeted reaction, contributing to improved performance of this fabricated catalysts over other biodiesel generation catalysts.
Figure 4.
Nitrogen sorption isotherms at 77 K for the MgO/MPC nanocomposite containing various contents of Cs2O (inset represents the pore size distribution).
2.1.5. Morphological Characteristics
The morphological assessment of the 20Cs2O–MgO/MPC nanocomposite is shown utilizing field emission scanning electron microscopy (FESEM). The typical image for the nanocomposite being fabricated is depicted in Figure 5. The nanocomposite conveys mainly spherical-like morphology. Notably, FESEM images of 20Cs2O–MgO/MPCs display an agglomerated characteristic of the microspheres. Figure 5 also displays a porous nature of the synthesized nanocomposites. Such a porous nature of the fabricated materials provides adequate active sites for the catalytic process and enables the diffusion of the reactant molecules in nanocomposite porosity.
Figure 5.

FESEM images of (A,B) 20Cs2O–MgO/MPC nanocomposite prepared by an aqueous solution combustion approach.
2.2. Catalytic Activity Assessment toward Biodiesel Production
The transesterification process is influenced by the alcohol/oil ratio, catalyst dose, contact time, and temperature.
2.2.1. Influence of the Methanol/Oil Molar Ratio
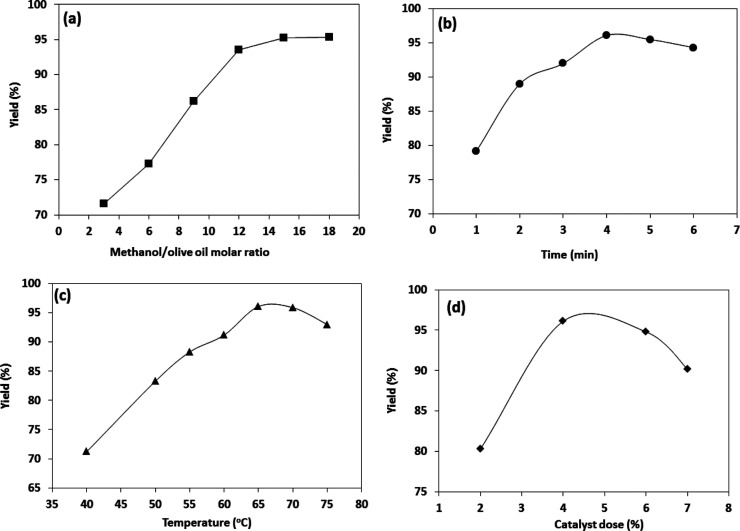
A small volume of methanol could not fulfill the transesterification process. Excessive methanol will increase the expense of the recovery of alcohol. Besides, glycerol becomes harder to isolate from biodiesel when a higher volume of methanol is used.37Figure 6 presents the influence of different parameters on the biodiesel development. Figure 6a examines the impact of varying methanol/olive oil molar ratio (3, 6, 9, 12, 15, and 18) on the biodiesel development applying the prepared 20Cs2O–MgO/MPC nanocomposite. Experimental findings revealed that with increasing methanol/olive oil ratio from 3 to 15, the yield of biodiesel increased from 71.6 to 96.1%, as an increase in the ratio at the beginning of the process could cause the creation of methoxy over the 20Cs2O–MgO/MPC nanocomposite surface, consequently, increasing the biodiesel development performances. However, an additional increase in the methanol/olive oil ratio to 18 resulted in a slight improvement in the biodiesel yield (95.7%). This was due to the difficulty of removing the excess glycerol content in methanol. The excess glycerol prevents the interaction between methanol, 20Cs2O–MgO/MPC nanocomposite, and the reactant, shifting the equilibrium into the backward direction leading to a decrease in the development of biodiesel. Consequently, based on this study, optimum biodiesel yield (96.1%) was gained at the methanol/olive oil molar ratio of 15.
Figure 6.
Impact of (a) methanol/olive oil ratio, (b) time, (c) temperature, and (d) catalyst dose (%) on biodiesel yield (%) from olive oil using the 20Cs2O–MgO/MPC nanocomposite.
2.2.2. Effect of Transesterification Time
Figure 6b contributes to the impact of contact time from 1 to 6 h on biodiesel development at different parameters according to the RSM design. The yield followed a growing trend with time. The performance after 4 h was obtained as 96.1%; after that, the activity slightly declined. A rapid increase in the yield (79.2%) is observed after 1 h. This could be attributed to the existence of a large quantity of ester in the system. Then, after 4 h, the yield increased. Accordingly, an increase in the contact time may assign to ester hydrolysis, thereby increasing the amount of available fatty acids for soap development. Therefore, the contact time of 4 h was stated as the appropriate contact time.
2.2.3. Influence of Temperature
The reaction temperature is a critical factor that drastically alters the transesterification process. The influence of this variable was assessed for the transesterification of olive oil to an ester at 40, 50, 55, 60, 65, 70, and 75 °C at different parameters according to the RSM design. Figure 6c reflects the influence of temperature on the reaction performance. Inspection of Figure 6c disclosed that increase in the transesterification temperature from 40 to 65 °C resulted in a remarkable increase in the yield from 65 to 96.1%. This could be attributed to high temperatures that appeared to enhance the dissolution of glycerin. On the other hand, a further increase in temperature greater than 65 °C decreased the performance because of the evaporation of methanol, thereby decreasing biodiesel generation performance. Therefore, 65 °C is the optimum transesterification temperature. The same trend was observed in the impact of the temperature on the biodiesel production.38,39
2.2.4. Effect of the Catalyst Dose
To emphasize the impact of the catalyst dose on the transesterification yield, a series of experiments with different catalyst dose percents (2, 4, 6, and 7%) were performed at different parameters according to the RSM design. The transesterification yield percent as a function of the amount (%) of catalyst is depicted in Figure 6d. It was found that the yield was increased with an increase in the dose of the catalyst up to 4%. This could be attributed to the increase in the dose that improved the interaction between the nanocomposite and the reactant. Besides, the number of the basic centers steaming from MgO and Cs2O was increased. An additional increase in the catalyst dose up to 7% caused a decrease in transesterification yield to 83%. Therefore, 4% is the optimum dose for transesterification of olive oil.
2.2.5. Effect of the Catalyst Composition
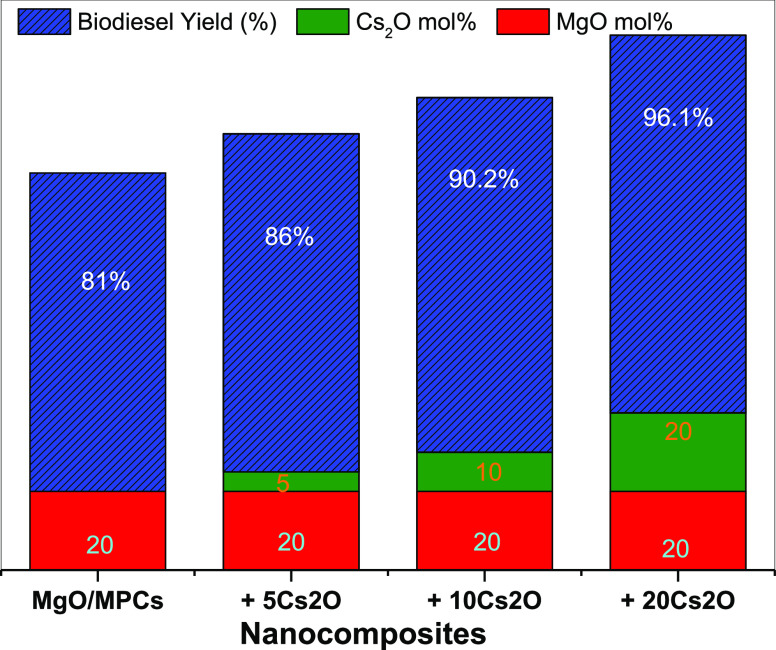
The variation in the biodiesel yield percent as a function of Cs2O content was investigated and is graphically represented in Figure 7. The reaction was performed under the optimum conditions according to different parameters according to the RSM design. As shown in Figure 7, MgO/MPCs containing different mol % of Cs2O showed a significant increase in biodiesel and attained the maximum yield of 96.1% for 20Cs2O–MgO/MPCs. This could be ascribed to the existence of many influential basic sites in 20Cs2O–MgO/MPCs that contribute to the process toward more yield of ester. There is a linear relationship between the biodiesel yields and the basicity of the composites. The CO2-TPD of this composite indicates the existence of extremely basic centers with a considerable quantity of total basicity.
Figure 7.
Influence of the catalyst composition on biodiesel yield (%) from olive oil.
For further understandable comparison, the process parameters and biodiesel yields attained by different MgO containing catalysts for the biodiesel process are presented in Table 2. As displayed in this table, the developed biodiesel in the current investigation has a significant performance compared to other catalysts.40−47
Table 2. Comparison of the Transesterification Performance Process of Different Catalysts Containing MgO.
| catalysts | oil | temperature (°C) | time (h) | MeOH/oil (molar ratio) | yield (%) | reference |
|---|---|---|---|---|---|---|
| CaO–MgO | Jatropha | 120 | 3 | 25:1 | 90 | (40) |
| MgAL-LDH | West sunflower | 120 | 4 | 15:1 | 94.6 | (41) |
| MgO/ZSM-5 | spirulina oil | 75 | 1 | 15:1 | 92.1 | (42) |
| MgO | triacetin | 60 | 9 | 6:1 | 18 | (43) |
| MgO/MCM-41 | soybean frying oil | 60 | 24 | 85 | (44) | |
| Sr/MgO | soybean oil | 65 | 0.5 | 12:1 | 93 | (45) |
| Li–MgO | soybean oil | 60 | 2 | 12:1 | 93.9 | (46) |
| MgO/CeM(Si/Ce = 10) | waste cooking oil | 70 | 6 | 9:1 | 94.3 | (47) |
| 20Cs2O– MgO/MPCs | olive oil | 60 | 6 | 15:1 | 96.1 | this work |
2.3. Biodiesel Features
The features of the biodiesel such as flashpoint, kinematic viscosity, density, pour point, and acid value were estimated and matched with the respective United States standard (ASTM D6751) and Europe standard (EN14214) (Table 3).48−50 The obtained characteristics of the produced biodiesel perfectly coincide with universal standard specifications. However, the observed high viscosity could be related to the presence of large amounts of fatty acid-containing lower numbers of the double bond (C18:1). The viscosity of FAMEs increased with decreasing number of double bonds μC18:3 < μC18:2 < μC18:1 < μC18:0.50 Besides, the FTIR spectra of the biodiesel are provided in Figure 8. The FTIR peak at 3300 cm–1 for carboxylic acid (O–H stretching) is very weak, indicating that most free fatty acids have been converted to their corresponding ester. The FTIR bands at 2953 and 2870 cm–1 are ascribed to asymmetric and symmetric starching vibrations of the sp3 C–H bond, respectively. As for the FTIR peak of ester, the obtained intense peak at 1769 cm–1 is consistent with the publication of Rabelo et al.,51 where the carbonyl group of free fatty acid and its corresponding ester was noted in the region 1800–1700 cm–1.52 The significant spectral area enabling to confirm the formation of biodiesel is 900–1500 cm–1 that corresponds to a fingerprint zone. The peak at 1446 cm–1 is assigned to the asymmetric stretch of −CH3 which is only found in biodiesel, while the peak at 1183 cm–1 is ascribed to the stretching of O–CH3 which is typically biodiesel.53 The weak band at 973 cm–1 is assigned to the olefin moieties in the alkyl chains. The peak at 738 cm–1 is ascribed to the rocking C–H bond. This finding is in line with the literature.54−56
Table 3. Biodiesel Features Obtained for Biodiesel Stemmed from Olive Oil.
| property | EN14214 | ASTM D-6751 | biodiesel |
|---|---|---|---|
| flash point (°C) | ≥101 | ≥130 | 139 |
| kinematic viscosity at 40 °C (mm2/s) | 3.5–5.0 | 1.9–6.0 | 5.2 |
| density at 15 °C (kg/m3) | 860–900 | 860–894 | 871 |
| pour point (°C) | –24 | ||
| acid value (mg KOH/g) | max. 0.5 | max. 0.5 | 0.31 |
Figure 8.
FTIR spectrum for biodiesel developed from olive oil using the 20Cs2O–MgO/MPC nanocomposite under optimum conditions.
2.4. Reusability Assessment
The reusability was performed using the 20Cs2O–MgO/MPC nanocomposite for four cycles under optimal conditions according to the RSM design. Recycled run findings indicate that the transesterification performance of the nanocomposite demonstrated stable catalytic performance up to four cycles with a small conversion variance of up to 3% (Figure 9).
Figure 9.
Reusability of the 20Cs2O–MgO/MPC nanocomposite toward biodiesel development from olive oil.
2.5. Optimization of the Transesterification Process by RSM
2.5.1. Fitting Model
The analysis of the variance model (ANOVA), the most reliable approach for assessing the precision of the experiments, has been used. The method also enables the variation of the relationship between the evaluators and the parts to be calculated. RSM explores the relation between the transesterification yield percent and four independent variables of methanol/oil molar ratio (A), contact time (B), temperature (C), and catalyst dose (D). It is worth noting that the model’s predicted values were inconsistent with the actual experimental results for each run, and the quadratic relation is defined in eq 2 with respect to the coded variables.
| 2 |
Linear regression analysis was utilized to evaluate the degree of consistency between the experimental results and the predicted findings stemmed from the statistical model (Figure 10). The acquired regression coefficient (R2 > 0.98) demonstrated robust fit with the theoretical findings and the significance of the model to illustrate the method.
Figure 10.

Relation between the predicted and the actual biodiesel yield (%).
The feasibility of the regression model was analyzed on the basis of the variable stemmed from ANOVA. Table 4 displays the variables of the p value, lack of fit, R2, adjusted R2, predicted R2, and adequate precision to estimate the fitness of the model. The pattern is essential for predicting the yield with the p value less than 0.0001. The model F value is 20.94, which showed an incredibly important model with just about 0.0001% probability to the noise impact of this value. The p values of A, B, C, D, AB, AC, AD, BC, CD, A2, B2, C2, and D2 are less than 0.05, demonstrating the significance of these terms (Table 4). Besides, the estimated value of R2 is 0.8991 that is somewhat in line with the Adj R2 (0.9321), and the amount of Adeq Precision (19.32) implies an appropriate signal and the design could be used to adjust the design space. Thus, the trend in eq 2 is in well-consistent with the experimental results.
Table 4. ANOVA Data for the Response Surface Quadratic Model.
| source | sum of squares | degree of freedom | mean square | F-value | p-value |
|---|---|---|---|---|---|
| model | 2632.7 | 14 | 188.05 | 20.94 | <0.0001 (significant) |
| A—methanol/oil ratio | 44.25 | 1 | 44.25 | 4.93 | 0.0422 |
| B—time | 0.1068 | 1 | 0.1068 | 0.0119 | 0.0146 |
| C—temperature | 2.39 | 1 | 2.39 | 0.2662 | 0.0134 |
| D—catalyst dose | 13.63 | 1 | 13.63 | 1.52 | 0.0369 |
| AB | 7.91 | 1 | 7.91 | 0.8811 | 0.0628 |
| AC | 34.41 | 1 | 34.41 | 3.83 | 0.0691 |
| AD | 8.38 | 1 | 8.38 | 0.9338 | 0.0492 |
| BC | 2.47 | 1 | 2.47 | 0.2749 | 0.0307 |
| BD | 0.0548 | 1 | 0.0548 | 0.0061 | 00387 |
| CD | 0.1286 | 1 | 0.1286 | 0.0143 | 0.0263 |
| A2 | 35.44 | 1 | 35.44 | 3.95 | 0.0355 |
| B2 | 0.0401 | 1 | 0.0401 | 0.0045 | 0.0476 |
| C2 | 53.73 | 1 | 53.73 | 5.98 | 0.0272 |
| D2 | 16.06 | 1 | 16.06 | 1.79 | 0.0200 |
| residual | 134.68 | 15 | 8.98 |
Model: significant. Lack of fit: not significant. R-squared: 0.9811. Adj R-squared: 0.9321. Pred R-squared: .8991. Adeq precision: 19.32.
2.5.2. Interaction Impact between Transesterification Parameters
The 3D response graphs were developed to demonstrate the impacts of the independent variables on the dependent one (Figure 11). The surface enclosed in the smallest ellipse in the contour diagram had indicated the maximum predicted values. Elliptical contours were achieved when the independent parameters were in a complete interaction. A correlation between every two parameters and the highest predicted yield is emphasized by the surface confined in the smallest ellipse in the contour graphs.
Figure 11.
Response 3D surface plots displaying the combination effect of (a) temperature and methanol-to-oil ratio, (b) time and methanol-to-oil ratio, and (c) catalyst dose and temperature.
2.5.2.1. Interaction Impact between Reaction Time and Temperature
The interaction impact showed enhancing biodiesel yield with the incremental increase in reaction time for the selected temperatures (Figure 11a). The yield increased from 50 to around 60.4% for the transesterification temperature of 40 °C, with contact time increasing from 2 to 6 h. The biodiesel yield increased at 60 °C from 81 to around 92.4%, with increasing the time from 2 to 6 h (Figure 11a). The interaction impact of time on the role of temperature also revealed a major effect on the biodiesel yield obtained (Figure 11a). The biodiesel yield enhanced from 50 to 73% at a lower time (2 h), with temperature changes from 40 to 50 °C. Besides, the values increased from 78 to around 95% at the maximum time limit (6 h), with a temperature increase from 40 to 65 °C. The results obtained showed the potential to enhance the influence of time by increasing the temperature and the subsequent evaluation of the impact of temperature by increasing the temperature, which indicated a substantial improvement in the biodiesel yield by increasing the reaction time. The reaction’s temperature has a favorable effect on the yield of 65 °C, after which there is a substantial decrease in yield because of the boiling of methanol.57,58 The process temperature and composite dose effects on yield have interacted. One possible justification is that the reaction’s temperature is the restricted transesterification parameter. It is the little mass transfer resistance with the low catalyst dose, leading to a high yield at 65 °C. However, the resistance to mass transfer is rapidly increased owing to the increased catalyst dose, and the high-temperature methanol evaporation aggravates the tolerance to mass transfer. Time has a proportional impact on biodiesel yield. The yield increases because of the availability of sufficient time to complete the reaction, but afterward, the biodiesel yield began to decline because of the reverse reaction. Such findings also agree with the literature.
2.5.2.2. Interaction Impact Between Reaction Time and Catalyst Dose
The produced biodiesel increased from 55.6 to 87.2% at the lower catalyst dose (2%), as the transesterification time increased from 2 to 6 h. Increasing the catalyst dose to 5%, the yield increased from 83.2 to approximately 94.3% as the reaction time changed from 2 to 6 h (Figure 11b). Therefore, the superior influence of time could be achieved using 5% of 20Cs2O–MgO/MPCs as catalyst dose and the more increase in the catalyst amount has a reverse impact.
2.5.2.3. Interaction Impact between Reaction Time and Methanol-to-Oil Ratio
Realistically, transesterification needs triglyceride/methanol (1:3) to produce 3 mol of ester along with 1 mol of glycerol, while the transesterification process is thus reversible, more methanol is commonly required to transfer the equilibrium to the final product. The interaction influence between methanol/oil ratio and transesterification time was investigated at 5% catalyst dose and 65 °C (Figure 11C). The biodiesel yield enhanced at a transesterification time (2 h) from 50 to 70%, with the methanol/oil ratio increasing from 3 to 15. The yield increased from 84.1 to around 93% at the transesterification time (6 h), with the methanol/oil ratio increasing from 3 to 15. Besides, at a constant methanol/oil ratio of 10, the yield increased from 66.5 to around 83% with increasing reaction time from 2 to 6 h. At higher methanol/oil ratio (15:1), the attained yield increased from 70.1 to 93.6%, increasing the transesterification time from 2 to 6 h (Figure 11C). This suggested that at all the transesterification time intervals, the methanol/oil ratio of (15:1) is the exceptional value for the full yield, and increasing the ratio above this ratio is of adverse influence. Summing up, the obtained results do reflect a feasible improvement in the 20Cs2O–MgO/MPC catalyst to attain a yield of 96.1% by tuning the conditions at 6 h, 65 °C, 5.2 wt % as 20Cs2O–MgO/MPC dose, and 1:15 as an oil/methanol ratio.
3. Technoeconomic Assessment
Evaluation of economic viability is an essential predictor for impacting sustainable biodiesel development. Accordingly, by taking into account the Saudi Arabia market price of all components, we evaluate the production cost of 1 ton of biodiesel from inedible olive oil, as shown in Table 5. The cost of raw materials constitutes 60–80% of biodiesel production’s overall cost.59,60 Applying lower-cost raw materials is a significant factor in reducing biodiesel costs. The price of edible and inedible olive oil is enormously different. The cost of biodiesel is predicted to decrease with the success of using waste olive oil. In this work, in the case of using inedible olive oil, the cost of biodiesel production is estimated to be $1.04 kg–1, which is lower than that in the case of edible olive oil ($2.9 kg–1). Future research will concentrate on the possibility of sustainable development and growth in minimizing production costs and, in the meantime, generate more biodiesel, fulfilling the requirements.
Table 5. Manufacturing Cost of Producing 1 ton of Biodiesel Using the 20Cs2O–MgO/MPC Catalyst.
| no. | cost item | components | price ($) | quantity | cost ($) |
|---|---|---|---|---|---|
| 1 | raw material | edible olive oil | 2.5/kg | 1100 | 2750 |
| inedible olive oil | 0.81/kg | 1100 | 891 | ||
| methanol | 0.44/kg | 300 | 132 | ||
| 2 | catalyst | mesopores carbon | 1.5/kg | 6 | |
| MgO–Cs2O | 1.4/kg | 18 | |||
| PSE | 0.1/kg | ||||
| 3 | utility | tap water | 0.33/t | 1′ | 0.33 |
| electricity | 0.12 kw/h | 8 | 0.96 | ||
| manpower | |||||
| total in case edible olive oil | 2901 | ||||
| total in case inedible olive oil | 1042 |
4. Conclusions
Biogenic-mediated synthesis of the Cs2O–MgO/MPC nanocomposite as a powerful catalyst for the generation of biodiesel from olive oil with outstanding durability was successfully fabricated. The transesterification performance and the optimization design were estimated by utilizing the RSM. Based on the experimental tests, the finest experimental circumstances with a biodiesel yield of 96.1% are 4 h, 4% catalyst, an oil/methanol of 1:15, and a temperature of 65 °C. The predicted optimal conditions based on the statistical model are a contact time of 6 h, a catalyst dose of 5.2%, a reaction temperature of 65 °C, and an oil/methanol molar ratio of 1:15 attaining a biodiesel yield of 95.18%. The various physicochemical features emphasized the good quality of the biodiesel achieved according to the international biodiesel standards ASTM D-6571 and EN 14214. Therefore, utilizing 20Cs2O–MgO/MPCs for transesterification of olive oil using methanol as a short-chain alcohol in the absence of any other organic solvent could be desirable in the production of biodiesel with high quality from waste olive oil as a raw material which can be used as an alternative rather than conventional petrodiesel. Besides, the results of technoeconomic assessment display that the inedible olive oil is efficient in the low-cost biodiesel production ($1.04 kg–1) and offers desirable support for a progressively developing industry compared to edible olive oil ($2.9 kg–1). This study may serve the baseline for further development in heterogeneous catalysis for biodiesel production from waste olive oil on a larger scale at the Al-Jouf region in the Kingdom of Saudi Arabia.
5. Materials and Methods
5.1. Materials and Chemicals
Olive oil was derived from olive milling at the city of Sakaka (Jouf, SA). Cesium nitrate (CsNO3), magnesium nitrate (Mg(NO3)2.6H2O), and MPCs were obtained from Sigma-Aldrich. PMSE was obtained from fresh pomegranate fruits. Other solvents are of analytical quality and used with no more treatment.
5.2. Gas Chromatography (GC/MS) of Olive Oil
The gas chromatography (GC) equipment (Shimadzu-QP2020) was utilized to assess the contents of the fatty acid of the applied olive oil. The equipment was fitted with a flame ionization detector (FID) in the presence of helium as the carrier gas. Specifications of the composition of the fatty acid of the olive oil are cited in Table 6.
Table 6. Composition of Fatty Acid Contents of Olive Oil Applied for Developing Biodiesel as Estimated by GC/MS.
| molecular formula | fatty acid | amount (%) |
|---|---|---|
| C16:0 | palmitic | 17.2 |
| C16:1 | palmitoleic acid | 1.3 |
| C18:0 | stearic acid | 4.8 |
| C18:1–trans | oleic acid | 59.1 |
| C18:1–cis | elaidic acid | 2.2 |
| C18:2 | linoleic acid | 13.7 |
| C18:3 | c-linolenic acid | 0.674 |
| C20:0 | arachidic acid | 0.46 |
| C20:1 | paulinic acid | 0.46 |
5.3. Catalyst Fabrication
5.3.1. Preparation of the Cs2O–MgO/MPC Nanocatalyst
Typically, 50 mg of PMSE is placed in a beaker (≈100 mL capacity) and then precisely 40.0 mL of DI water has been added to dissolve the extract. A certain quantity of cesium nitrate (CsNO3), magnesium nitrate (Mg (NO3)2·6H2O), and MPC was then added to the mixture with continuous stirring overnight. A significant darkness of the pink color of PSE solution was found by adding the metal nitrate salts that suggest a characteristic of interaction between PSE and Mg2+ and Cs+ ions. Upon complete dissolution, the mixture was transferred to the crucible. The crucible was then placed in a preheated muffle furnace at 300 °C, starting a 2 h solution combustion reaction. After the combustion process, the C s2O–MgO/MPC nanocatalyst is fabricated.
5.4. Nanocatalyst Characterization
For recording FTIR readings, a Shimadzu IR Tracer-100 FTIR spectrophotometer has been applied. Powder XRD profiles have been recorded using the X-ray diffractometer Maxima—X (D/Max2500VB2+/Pc, Shimadzu Company, Japan) with an X-ray wavelength Cu detector. Raman spectroscopic analysis was conducted. The developed materials’ morphological features were examined applying FESEM (Zeiss FESEM Ultra 60). A NOVA 4200e (Quantachrome Instruments) surface area and pore size analyzer collected the nitrogen adsorption–desorption isotherms at 77 K. FESEM investigation using Zeiss FESEM Ultra 60 was conducted to investigate the morphology of the nanocomposite. CO2 desorption (CO2-TPD) assessment was performed on a Quantachrom Nova Sorbimetric system.
5.5. Biodiesel Production Procedure
The transesterification approach was chosen to produce biodiesel from the olive oil. The oil was commercially obtainable and was purchased from local distributors (Jouf, Saudi Arabia). A reflux system equipped with a closed upper part condenser was used to prevent methanol evaporation while better attempting to control the temperature of the process. Typically, a certain amount of the catalyst has been introduced to the oil and methanol mixture. At the reflux temperature, the blend was agitated. Biodiesel and glycerol were obtained after the stipulated contact time, and the mix was allowed to isolate overnight. The catalyst was separated from the mix by filtration. The final product after isolating the catalysts was rinsed with water to remove glycerol. Through filtration, the catalyst was isolated from the mixture. The product had been rinsed with water after removing the catalysts to isolate glycerol. The biodiesel collected was retained for the physicochemical investigations. The yield of biodiesel was evaluated from eq 3(11,61,62)
| 3 |
5.6. Optimum Conditions for Biodiesel Production
The impacts of variable settings on biodiesel development, such as reaction time (1, 2, 3, 4, 5, and 6 h), temperature (40, 50, 55, 60, 65, 70, and 75 °C), catalyst dose (2, 4, 6, and 7%), and methanol/olive oil molar ratio (3, 6, 9, 12, 15, and 18), were addressed.
5.7. Biodiesel Characteristics
After the biodiesel was prepared, biodiesel features such as kinematic viscosity, density, flash point, cloud point, pour point, acid number, and oxidation stability were assessed based on global standards.63,64
5.8. Reusability Experiment
The 20Cs2O–MgO/MPC nanocomposite was separated from the mixture by filtration, rinsed several times with methanol to eliminate any organic traces on the catalyst surface, and dried in an oven for 4 h at 120 °C. This was recycled for subsequent transesterification reactions. A similar approach is adopted for all recycled experiments.
5.9. Optimization Assessment
Optimization of the transesterification process was performed through a three-factor experiment to investigate the impacts of the independent variables including methanol/oil molar ratio (A), contact time (B), temperature (C), and catalyst dose (D) on the ester yield applying the RSM of the experiments. The RSM is designed and performed by the regression and graphical analysis of the data pertaining Design-Expert software (state Ease Inc., Minneapolis, MN, USA). The coded and uncoded levels of the independent variables used for the transesterification of olive oil are given in Table 7.65,66 Consequently, the entire sets of 30 experiments were conducted separately for getting the experimental response of yield. The data were analyzed by adopting the second-order polynomial in eq 4..
| 4 |
where Y is the predicted response of the yield, Xi and Xj are the independent parameters, ao, ai, aii, and aij are the constant, linear, quadratic, and interactive coefficients, respectively.
Table 7. RSM-CCD for Transesterification.
| coded |
||||||
|---|---|---|---|---|---|---|
| factor | name | units | minimum | maximum | low | high |
| A | CH3OH:Oil | 3.00 | 18.00 | –1 ↔ 3.00 | +1 ↔ 18.00 | |
| B | time | h | 1.0000 | 6 | –1 ↔ 1.00 | +1 ↔ 6.00 |
| C | temperature | °C | 40.00 | 65.00 | –1 ↔ 40.00 | +1 ↔ 65.00 |
| D | catalyst dose | % | 2.0000 | 7.00 | –1 ↔ 1.00 | +1 ↔ 7.00 |
Acknowledgments
The authors are grateful to Jouf University, the Dean of Scientific Research, for supporting this project fund under grant number (40/311).
The authors declare no competing financial interest.
References
- Sierra-Cantor J. F.; Guerrero-Fajardo C. A. Methods for improving the cold flow properties of biodiesel with high saturated fatty acids content: A review. Renewable Sustainable Energy Rev. 2017, 72, 774–790. 10.1016/j.rser.2017.01.077. [DOI] [Google Scholar]
- Altaie M. A. H.; Janius R. B.; Rashid U.; Taufiq-Yap Y. H.; Yunus R.; Zakaria R.; et al. Performance and exhaust emission characteristics of direct-injection diesel engine fueled with enriched biodiesel. Energy Convers. Manage. 2015, 106, 365–372. 10.1016/j.enconman.2015.09.050. [DOI] [Google Scholar]
- Moradi G. R.; Dehghani S.; Khosravian F.; Arjmandzadeh A. The optimized operational conditions for biodiesel production from soybean oil and application of artificial neural networks for estimation of the biodiesel yield. Renewable Energy 2013, 50, 915–920. 10.1016/j.renene.2012.08.070. [DOI] [Google Scholar]
- Sundus F.; Fazal M. A.; Masjuki H. H. Tribology with biodiesel: A study on enhancing biodiesel stability and its fuel properties. Renewable Sustainable Energy Rev. 2017, 70, 399–412. 10.1016/j.rser.2016.11.217. [DOI] [Google Scholar]
- Rahmani Vahid B.; Haghighi M.; Alaei S.; Toghiani J. Reusability enhancement of combustion synthesized MgO/MgAl2O4 nanocatalyst in biodiesel production by glow discharge plasma treatment. Energy Convers. Manage. 2017, 143, 23–32. 10.1016/j.enconman.2017.03.075. [DOI] [Google Scholar]
- Shaban M.; Hosny R.; Rabie A. M.; Shim J.-J.; Ahmed S. A.; Betiha M. A.; et al. Zinc aluminate nanoparticles: Preparation, characterization and application as efficient and economic catalyst in transformation of waste cooking oil into biodiesel. J. Mol. Liq. 2020, 302, 112377. 10.1016/j.molliq.2019.112377. [DOI] [Google Scholar]
- Chuah L. F.; Klemeš J. J.; Yusup S.; Bokhari A.; Akbar M. M.; Chong Z. K. Kinetic studies on waste cooking oil into biodiesel via hydrodynamic cavitation. J. Cleaner Prod. 2017, 146, 47–56. 10.1016/j.jclepro.2016.06.187. [DOI] [Google Scholar]
- Hajjari M.; Tabatabaei M.; Aghbashlo M.; Ghanavati H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renewable Sustainable Energy Rev. 2017, 72, 445–464. 10.1016/j.rser.2017.01.034. [DOI] [Google Scholar]
- Leng L.; Han P.; Yuan X.; Li J.; Zhou W. Biodiesel microemulsion upgrading and thermogravimetric study of bio-oil produced by liquefaction of different sludges. Energy 2018, 153, 1061–1072. 10.1016/j.energy.2018.04.087. [DOI] [Google Scholar]
- Betiha M. A.; Negm N. A.; El-Sayed E. M.; Mostafa M. S.; Menoufy M. F. Capability of synthesized sulfonated aromatic cross-linked polymer covalently bonded montmorillonite framework in productivity process of biodiesel. J. Cleaner Prod. 2020, 261, 120995. 10.1016/j.jclepro.2020.120995. [DOI] [Google Scholar]
- Negm N. A.; Betiha M. A.; Alhumaimess M. S.; Hassan H. M. A.; Rabie A. M. Clean transesterification process for biodiesel production using heterogeneous polymer-heteropoly acid nanocatalyst. J. Cleaner Prod. 2019, 238, 117854. 10.1016/j.jclepro.2019.117854. [DOI] [Google Scholar]
- Nayebzadeh H.; Saghatoleslami N.; Haghighi M.; Tabasizadeh M. Influence of fuel type on microwave-enhanced fabrication of KOH/Ca12Al14O33 nanocatalyst for biodiesel production via microwave heating. J. Taiwan Inst. Chem. Eng. 2017, 75, 148–155. 10.1016/j.jtice.2017.03.018. [DOI] [Google Scholar]
- Rahmani Vahid B.; Haghighi M. Urea-nitrate combustion synthesis of MgO/MgAl2O4 nanocatalyst used in biodiesel production from sunflower oil: Influence of fuel ratio on catalytic properties and performance. Energy Convers. Manage. 2016, 126, 362–372. 10.1016/j.enconman.2016.07.050. [DOI] [Google Scholar]
- Islam A.; Taufiq-Yap Y. H.; Chu C.-M.; Chan E.-S.; Ravindra P. Studies on design of heterogeneous catalysts for biodiesel production. Process Saf. Environ. Prot. 2013, 91, 131–144. 10.1016/j.psep.2012.01.002. [DOI] [Google Scholar]
- Liu C.; Lv P.; Yuan Z.; Yan F.; Luo W. The nanometer magnetic solid base catalyst for production of biodiesel. Renewable Energy 2010, 35, 1531–1536. 10.1016/j.renene.2009.10.009. [DOI] [Google Scholar]
- Fazal M. A.; Suhaila N. R.; Haseeb A. S. M. A.; Rubaiee S.; Al-Zahrani A. Influence of copper on the instability and corrosiveness of palm biodiesel and its blends: An assessment on biodiesel sustainability. J. Cleaner Prod. 2018, 171, 1407–1414. 10.1016/j.jclepro.2017.10.144. [DOI] [Google Scholar]
- Korkut I.; Bayramoglu M. Selection of catalyst and reaction conditions for ultrasound assisted biodiesel production from canola oil. Renewable Energy 2018, 116, 543–551. 10.1016/j.renene.2017.10.010. [DOI] [Google Scholar]
- Kaur M.; Malhotra R.; Ali A. Tungsten supported Ti/SiO2 nanoflowers as reusable heterogeneous catalyst for biodiesel production. Renewable Energy 2018, 116, 109–119. 10.1016/j.renene.2017.09.065. [DOI] [Google Scholar]
- Semwal S.; Arora A. K.; Badoni R. P.; Tuli D. K. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 2011, 102, 2151–2161. 10.1016/j.biortech.2010.10.080. [DOI] [PubMed] [Google Scholar]
- Navajas A.; Campo I.; Moral A.; Echave J.; Sanz O.; Montes M.; et al. Outstanding performance of rehydrated Mg-Al hydrotalcites as heterogeneous methanolysis catalysts for the synthesis of biodiesel. Fuel 2018, 211, 173–181. 10.1016/j.fuel.2017.09.061. [DOI] [Google Scholar]
- Alhumaimess M.; Aldosari O.; Alshammari H.; Kamel M. M.; Betiha M. A.; Hassan H. M. A. Ionic liquid green synthesis of CeO2 nanorods and nano-cubes: Investigation of the shape dependent on catalytic performance. J. Mol. Liq. 2019, 279, 649–656. 10.1016/j.molliq.2019.02.014. [DOI] [Google Scholar]
- Grzybowska-Świerkosz B. Effect of Additives on the Physicochemical and Catalytic Properties of Oxide Catalysts in Selective Oxidation Reactions. Top. Catal. 2002, 21, 35–46. 10.1023/A:1020547830167. [DOI] [Google Scholar]
- Mansir N.; Teo S. H.; Rashid U.; Taufiq-Yap Y. H. Efficient waste Gallus domesticus shell derived calcium-based catalyst for biodiesel production. Fuel 2018, 211, 67–75. 10.1016/j.fuel.2017.09.014. [DOI] [Google Scholar]
- Jiménez-López A.; Rodríguez-Castellón E.; Maireles-Torres P.; Díaz L.; Mérida-Robles J. Chromium oxide supported on zirconium- and lanthanum-doped mesoporous silica for oxidative dehydrogenation of propane. Appl. Catal., A 2001, 218, 295–306. 10.1016/s0926-860x(01)00656-1. [DOI] [Google Scholar]
- Seffati K.; Esmaeili H.; Honarvar B.; Esfandiari N. AC/CuFe2O4@CaO as a novel nanocatalyst to produce biodiesel from chicken fat. Renewable Energy 2020, 147, 25–34. 10.1016/j.renene.2019.08.105. [DOI] [Google Scholar]
- Teixeira A. P. C.; Santos E. M.; Vieira A. F. P.; Lago R. M. Use of chrysotile to produce highly dispersed K-doped MgO catalyst for biodiesel synthesis. Chem. Eng. J. 2013, 232, 104–110. 10.1016/j.cej.2013.07.065. [DOI] [Google Scholar]
- Patil P. D.; Deng S. Transesterification of Camelina Sativa Oil Using Heterogeneous Metal Oxide Catalysts. Energy Fuels 2009, 23, 4619–4624. 10.1021/ef900362y. [DOI] [Google Scholar]
- Hung C.-H.; Chen C.-S.; Sheu H.-S.; Chang J.-R. Deactivation and Rejuvenation of Pellet MgO/SiO2 Catalysts for Transesterification of Soybean Oil with Methanol to Biodiesel: Roles of MgO Morphology Change in Catalysis. Ind. Eng. Chem. Res. 2018, 57, 456–469. 10.1021/acs.iecr.7b02859. [DOI] [Google Scholar]
- Banković-Ilić I. B.; Miladinović M. R.; Stamenković O. S.; Veljković V. B. Application of nano CaO–based catalysts in biodiesel synthesis. Renewable Sustainable Energy Rev. 2017, 72, 746–760. 10.1016/j.rser.2017.01.076. [DOI] [Google Scholar]
- Ramadan M.; Hassan H. M. A.; Shahat A.; Elshaarawy R. F. M.; Allam N. K. Ultrahigh performance of novel energy-efficient capacitive deionization electrodes based on 3D nanotubular composites. New J. Chem. 2018, 42, 3560–3567. 10.1039/c7nj03838k. [DOI] [Google Scholar]
- Glaspell G.; Hassan; Elzatahry A.; Fuoco L.; Radwan N. R. E.; El-Shall M. S. Nanocatalysis on Tailored Shape Supports: Au and Pd Nanoparticles Supported on MgO Nanocubes and ZnO Nanobelts. J. Phys. Chem. B 2006, 110, 21387–21393. 10.1021/jp0651034. [DOI] [PubMed] [Google Scholar]
- Rabie A. M.; Betiha M. A.; Park S.-E. Direct synthesis of acetic acid by simultaneous co-activation of methane and CO2 over Cu-exchanged ZSM-5 catalysts. Appl. Catal., B 2017, 215, 50–59. 10.1016/j.apcatb.2017.05.053. [DOI] [Google Scholar]
- Mashayekh-Salehi A.; Moussavi G.; Yaghmaeian K. Preparation, characterization and catalytic activity of a novel mesoporous nanocrystalline MgO nanoparticle for ozonation of acetaminophen as an emerging water contaminant. Chem. Eng. J. 2017, 310, 157–169. 10.1016/j.cej.2016.10.096. [DOI] [Google Scholar]
- Zhang G.; Hattori H.; Tanabe K. Aldol Addition of Acetone, Catalyzed by Solid Base Catalysts: Magnesium Oxide, Calcium Oxide, Strontium Oxide, Barium Oxide, Lanthanum (III) Oxide and Zirconium Oxide. Appl. Catal. 1988, 36, 189–197. 10.1016/s0166-9834(00)80114-1. [DOI] [Google Scholar]
- Cortes-Concepcion J. A.; Patcas F.; Amiridis M. D. Effect of Li on the catalytic activity of MgO for the synthesis of flavanone. Appl. Catal., A 2010, 386, 1–8. 10.1016/j.apcata.2010.07.013. [DOI] [Google Scholar]
- Abdelrahman A. A.; Betiha M. A.; Rabie A. M.; Ahmed H. S.; Elshahat M. F. Removal of refractory Organo-sulfur compounds using an efficient and recyclable {Mo132} nanoball supported graphene oxide. J. Mol. Liq. 2018, 252, 121–132. 10.1016/j.molliq.2017.12.124. [DOI] [Google Scholar]
- Yesilyurt M. K.; Arslan M.; Eryilmaz T. Application of response surface methodology for the optimization of biodiesel production from yellow mustard (Sinapis alba L.) seed oil. Int. J. Green Energy 2019, 16, 60–71. 10.1080/15435075.2018.1532431. [DOI] [Google Scholar]
- Du L.; Ding S.; Li Z.; Lv E.; Lu J.; Ding J. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manage. 2018, 173, 728–734. 10.1016/j.enconman.2018.07.053. [DOI] [Google Scholar]
- Yahya N. Y.; Ngadi N.; Jusoh M.; Halim N. A. A. Characterization and parametric study of mesoporous calcium titanate catalyst for transesterification of waste cooking oil into biodiesel. Energy Convers. Manage. 2016, 129, 275–283. 10.1016/j.enconman.2016.10.037. [DOI] [Google Scholar]
- Taufiq-Yap Y. H.; Lee H. V.; Yunus R.; Juan J. C. Transesterification of non-edible Jatropha curcas oil to biodiesel using binary Ca–Mg mixed oxide catalyst: effect of stoichiometric composition. Chem. Eng. J. 2011, 178, 342–347. 10.1016/j.cej.2011.10.019. [DOI] [Google Scholar]
- Sayed M. R.; Abukhadra M. R.; Ahmed S. A.; Shaban M.; Javed U.; Betiha M. A.; Shim J.-J.; Rabie A. M. Synthesis of advanced MgAl-LDH based geopolymer as a potential catalyst in the conversion of waste sunflower oil into biodiesel: Response surface studies. Fuel 2020, 282, 118865. 10.1016/j.fuel.2020.118865. [DOI] [Google Scholar]
- Qu S.; Chen C.; Guo M.; Lu J.; Yi W.; Ding J.; Miao Z. Synthesis of MgO/ZSM-5 catalyst and optimization of process parameters for clean production of biodiesel from Spirulina platensis. J. Cleaner Prod. 2020, 276, 123382. 10.1016/j.jclepro.2020.123382. [DOI] [Google Scholar]
- López D. E.; Goodwin J. G.; Bruce D. A.; Lotero E. Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal., A 2005, 295, 97–105. 10.1016/j.apcata.2005.07.055. [DOI] [Google Scholar]
- Georgogianni K. G.; Katsoulidis A. P.; Pomonis P. J.; Kontominas M. G. Transesterification of soybean frying oil to biodiesel using heterogeneous catalysts. Fuel Process. Technol. 2009, 90, 671–676. 10.1016/j.fuproc.2008.12.004. [DOI] [Google Scholar]
- Tantirungrotechai J.; Thepwatee S.; Yoosuk B. Biodiesel synthesis over Sr/MgO solid base catalyst. Fuel 2013, 106, 279–284. 10.1016/j.fuel.2013.01.028. [DOI] [Google Scholar]
- Wen Z.; Yu X.; Tu S.-T.; Yan J.; Dahlquist E. Synthesis of biodiesel from vegetable oil with methanol catalyzed by Li-doped magnesium oxide catalysts. Appl. Energy 2010, 87, 743–748. 10.1016/j.apenergy.2009.09.013. [DOI] [Google Scholar]
- Dehghani S.; Haghighi M. Sono-dispersed MgO over cerium-doped MCM-41 nanocatalyst for biodiesel production from acidic sunflower oil: Surface evolution by altering Si/Ce molar ratios. Waste Manage. 2019, 95, 584–592. 10.1016/j.wasman.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Eryilmaz T.; Yesilyurt M. K. Influence of blending ratio on the physicochemical properties of safflower oil methyl ester-safflower oil, safflower oil methyl ester-diesel and safflower oil-diesel. Renewable Energy 2016, 95, 233–247. 10.1016/j.renene.2016.04.009. [DOI] [Google Scholar]
- Teo S. H.; Goto M.; Taufiq-Yap Y. H. Biodiesel production from Jatropha curcas L. oil with Ca and La mixed oxide catalyst in near supercritical methanol conditions. J. Supercrit. Fluids 2015, 104, 243–250. 10.1016/j.supflu.2015.06.023. [DOI] [Google Scholar]
- Ramírez Verduzco L. F. Density and viscosity of biodiesel as a function of temperature: Empirical models. Renewable Sustainable Energy Rev. 2013, 19, 652–665. 10.1016/j.rser.2012.11.022. [DOI] [Google Scholar]
- Rabelo S. N.; Ferraz V. P.; Ferraz V. P.; Oliveira L. S.; Franca A. S. FTIR analysis for quantification of fatty acid methyl esters in biodiesel produced by microwave-assisted transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964. 10.7763/ijesd.2015.v6.730. [DOI] [Google Scholar]
- Muppaneni T.; Reddy H. K.; Ponnusamy S.; Patil P. D.; Sun Y.; Dailey P.; Deng S. optimization of biodiesel production from palm oil under supercritical ethanol conditions using hexane as co-solvent: A response surface methodology. Fuel 2013, 107, 633–640. 10.1016/j.fuel.2012.11.046. [DOI] [Google Scholar]
- Amini Z.; Ong H. C.; Harrison M. D.; Kusumo F.; Mazaheri H.; Ilham Z. biodiesel production by lipase-catalyzed transesterification of Ocium basilicum L. (sweet basil) seed oil. Energy Convers. Manage. 2017, 132, 82–90. 10.1016/j.enconman.2016.11.017. [DOI] [Google Scholar]
- Knothe G.; Steidley K. R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 184, 1059–1065. 10.1016/j.fuel.2005.01.016. [DOI] [Google Scholar]
- Nisar J.; Razaq R.; Farooq M.; Iqbal M.; Khan R. A.; Sayed M. Enhanced biodiesel production from Jatropha oil using calcined waste animal bones as catalyst. Renewable Energy 2017, 101, 111–119. 10.1016/j.renene.2016.08.048. [DOI] [Google Scholar]
- Hassan H. M. A.; Betiha M. A.; El-Sharkawy E. A.; Elshaarawy R. F. M.; El-Assy N. B.; Essawy A. A.; et al. Highly selective epoxidation of olefins using vanadium (IV) schiff base- amine-tagged graphene oxide composite. Colloids Surf., A 2020, 591, 124520. 10.1016/j.colsurfa.2020.124520. [DOI] [Google Scholar]
- Kim H.-J.; Kang B.-S.; Kim M.-J.; Park Y. M.; Kim D.-K.; Lee J.-S.; et al. transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal. Today 2004, 93–95, 315–320. 10.1016/j.cattod.2004.06.007. [DOI] [Google Scholar]
- Sahani S.; Roy T.; Chandra Sharma Y. Clean and efficient production of biodiesel using barium cerate as a heterogeneous catalyst for the biodiesel production; kinetics and thermodynamic study. J. Cleaner Prod. 2019, 237, 117699. 10.1016/j.jclepro.2019.117699. [DOI] [Google Scholar]
- Kumar D.; Singh B.; Banerjee A.; Chatterjee S. Cement wastes as transesterification catalysts 860 for the production of biodiesel from Karanja oil. J. Cleaner Prod. 2018, 183, 26–34. 10.1016/j.jclepro.2018.02.122. [DOI] [Google Scholar]
- Qu S.; Chen C.; Guo M.; Lu J.; Yi W.; Ding J.; Miao Z. Synthesis of MgO/ZSM-5 catalyst and optimization of process parameters for clean production of biodiesel from Spirulina platensis. J. Cleaner Prod. 2020, 276, 123382. 10.1016/j.jclepro.2020.123382. [DOI] [Google Scholar]
- Seffati K.; Esmaeili H.; Honarvar B.; Esfandiari N. AC/CuFe2O4@CaO as a novel nanocatalyst to produce biodiesel from chicken fat. Renewable Energy 2020, 147, 25–34. 10.1016/j.renene.2019.08.105. [DOI] [Google Scholar]
- Rad A. S.; Nia M. H.; Ardestani F.; Nayebzadeh H. Esterification of waste chicken fat: sulfonated MWCNT toward biodiesel production. Waste Biomass Valorization 2018, 9, 591–599. 10.1007/s12649-016-9732-9. [DOI] [Google Scholar]
- Pandit P. R.; Fulekar M. H. Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renewable Energy 2019, 136, 837–845. 10.1016/j.renene.2019.01.047. [DOI] [Google Scholar]
- Xie W.; Han Y.; Wang H. Magnetic Fe3O4/MCM-41 composite-supported sodium silicate as heterogeneous catalysts for biodiesel production. Renewable Energy 2018, 125, 675–681. 10.1016/j.renene.2018.03.010. [DOI] [Google Scholar]
- Nath B.; Das B.; Kalita P.; Basumatary S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Cleaner Prod. 2019, 239, 118112. 10.1016/j.jclepro.2019.118112. [DOI] [Google Scholar]
- Harsha Hebbar H. R.; Math M. C.; Yatish K. V. Optimization and kinetic study of CaO nanoparticles catalyzed biodiesel production from Bombax ceiba oil. Energy 2018, 143, 25–34. 10.1016/j.energy.2017.10.118. [DOI] [Google Scholar]