Abstract
The increasing use of eHealth has ushered in a new era of patient-centred cancer care that moves beyond the traditional in-person care model to real-time, dynamic, and technology-assisted assessments and interventions. eHealth has the potential to better the delivery of cancer care through improved patient–provider communication, enhanced symptom and toxicity assessment and management, and optimised patient engagement across the cancer care continuum. In this Review, we provide a brief, narrative appraisal of the peer reviewed literature over the past 10 years related to the uses of patient-centred eHealth to improve cancer care delivery. These uses include the addressal of symptom management, health-related quality of life, and other patient-reported outcomes across cancer care. In addition, we discuss the challenges of, and opportunities for, accessibility, scalability, and implementation of these technologies, important areas for further development, and future research directions.
Introduction
Improvements in early cancer detection and treatment efficacy have led to an unprecedented number of cancer survivors in the USA. The National Cancer Institute defines a cancer survivor as someone affected by cancer from the time of diagnosis until the end of life.1 In January, 2019, there were an estimated 17 million cancer survivors in the USA, accounting for approximately 5% of the population, and the number of cancer survivors continues to grow. By 2029, cancer survivors are expected to exceed 21 million people.2 The benefit of cancer survival is offset by the challenges that patients, survivors, their families, and care partners face across the care continuum, which spans from diagnosis and treatment planning to treatment, symptom monitoring and management, surveillance, and end-of-life care. Many patients with cancer and survivors of cancer have chronic and debilitating treatment-related side-effects that can persist well beyond active treatment.3,4 Side-effects occur concurrently with emotional responses to treatment burden, fear of progression or recurrence, and functional limitations. Not surprisingly, about 30% of patients with cancer report clinically elevated (ie, more severe) symptoms of psychological distress (eg, depression and anxiety) during their care,5 and disease severity, treatment-related symptoms and dysfunction, and premorbid psychological problems are robust predictors of psychological distress. Additionally, more than 60% of cancer survivors are aged 65 years or older.6 Thus, a large proportion have age-related comorbidities, functional decline, and other age-related stressors (eg, social isolation and financial burden) that can exacerbate cancer care challenges.7 These treatment-related, emotional, and age-related stressors can negatively affect health-related quality of life (HRQOL), other patient-reported outcomes (PROs; eg, treatment satisfaction and symptom burden), treatment compliance, and health outcomes.
Over the past few decades, the National Academy of Sciences has published influential reports documenting the need to effectively address the physical, emotional, social, financial, and care coordination needs of patients with cancer and survivors of cancer.8,9 These reports highlight how cancer care can be complex, expensive, and fragmented. Despite having evidence-based resources to address the needs of patients with cancer and survivors, programmes might not always be accessible, and survivors of cancer are often neglected as they progress from primary treatment to adjuvant and follow-up care. Therefore, the development and implementation of pragmatic, patient-centred care delivery approaches across the care continuum are crucial. These approaches will enable better management of the growing population of survivors of cancer and ultimately improve PROs and systems-level outcomes (eg, visits to emergency departments, hospital readmissions, length of hospital stay, and treatment satisfaction). With advances in health information technology, there is a timely opportunity to optimise research on cancer care delivery and address the multiple challenges faced by patients, their families, and care partners.
For this Review, eHealth refers to the broad use of health information and communication technologies and networks to enhance patient-centred care delivery. Other terms commonly used interchangeably with eHealth include telehealth, telemedicine, and teleoncology; in this Review, these terms are encompassed within the definition of eHealth. Within eHealth, mHealth refers to the use of mobile and wireless devices (eg, computers, tablets, and smartphones) with health applications that support patient care, education, and research. We also include health information technologies, which refer to technologies that support the collection, aggregation, and management of health information (eg, electronic health records and online patient portals).10,11
The increasing use of eHealth has ushered in a new era of patient-centred cancer care that transcends the in-person care model to real-time, dynamic, and technology-assisted assessments and interventions. eHealth has the potential to improve patient–provider communication, enhance symptom and toxicity assessment and management, and optimise patient engagement across the care continuum. A crucial component of eHealth programmes is the recording of PROs. Moreover, these technologies might facilitate care access for underserved groups or rural communities that face challenges in accessing routine in-person care. However, use of eHealth in cancer care delivery presents several dissemination and implementation challenges that must be considered. In this Review, we provide a narrative literature review of the use of patient-centred eHealth to improve cancer care delivery, symptom management, HRQOL, and other PROs (table). We review sample studies selected on the basis of the relevance and representativeness of the current science. This Review is neither scoping, nor meta-analytic; rather, it is complementary to existing systematic and meta-analytic reviews of the current topic,40-44 which describe the potential of eHealth interventions for improving cancer outcomes41,43,44 and highlight remaining gaps in the published literature, including external validity,40 managing and integrating electronic information,42 tailoring eHealth resources to be more disease-specific,44 and the need for more high-quality and large-scale trials.41 We also discuss the challenges to and opportunities for patient access, scalability, and implementation of these technologies, and we conclude by underlining important areas for future directions.
Table:
Select studies using eHealth and mHealth interventions in cancer care
| Study design | Study population | Intervention or treatment |
Control condition |
Intervention duration |
Follow-up duration |
Key outcomes | |
|---|---|---|---|---|---|---|---|
| Using eHealth to link PROs with clinical cancer care | |||||||
| Judson et al (2013)12 | Feasibility study | 286 patients with lung, gynaecological, breast, and genitourinary cancers (all stages) receiving chemotherapy | eHealth platform for collecting weekly PRO data (eg, symptoms and the toxic effects of treatment) | NA | 214 weeks | 214 weeks | Monthly compliance with eHealth PRO assessments was high, but weekly compliance was low; compliance was greatest during the initial 12 weeks |
| Girgis et al (2017)13 | Feasibility study | 35 patients with cancer (all stages) either currently receiving or soon to be receiving cancer treatment | eHealth platform for collecting PRO data | NA | 3 months | 3 months | eHealth PRO assessments were acceptable and feasible, with most patients willing to participate |
| Bae et al (2018)14 | Feasibility study | 101 patients with cancer (all stages) undergoing treatment with chemotherapy | mHealth application for collecting daily PRO data during chemotherapy | NA | 4 weeks | 4 weeks | Daily mHealth PRO assessments were feasible and accessible during chemotherapy |
| Hauth et al (2019)15 | Feasibility study | 21 patients with cancer (all stages) undergoing radiotherapy | mHealth application for collecting PRO data during and after radiotherapy | NA | 5 weeks | 5 weeks | mHealth PRO assessments were feasible and acceptable during radiotherapy |
| Klagholz and colleagues16 (2018) | Feasibility study | 129 cancer caregivers | eHealth platform for collecting PRO data | NA | 6 months | 6 months | eHealth PRO assessments were acceptable and feasible for cancer caregivers |
| Basch et al (2016)17 and Basch et al (2017)18 | Randomised controlled trial | 766 patients with advanced solid tumour malignancies undergoing treatment with chemotherapy | eHealth platform for collecting PRO data between clinical visits, including alerts for physicians and nurses in the case of severe or worsening symptoms | Usual care | 49 months | 49 months | Patients in the intervention group reported improved HRQOL, had less frequent hospital admissions, and remained on chemotherapy longer; these patients also had longer quality-adjusted survival |
| Denis et al (2017)19 | Randomised controlled trial | 133 patients with advanced stage lung cancer | eHealth platform for collecting weekly PRO data, including alerts to physicians in the case of severe or worsening symptoms | Usual care | 12 months | 12 months | Patients in the intervention group had longer overall survival |
| Nixon et al (2018)20 | Markov model to investigate cost-effectiveness | Patients with advanced or metastatic solid tumours receiving systemic therapy | eHealth platform for collecting PRO data | Usual care | N/A | N/A | eHealth PRO assessments were considered good value for improving quality-adjusted life years relative to financial cost |
| Lizee et al (2019)21 | Medico-economic analysis to investigate cost-effectiveness | Patients with advanced stage lung cancer | eHealth platform for collecting weekly PRO data | Usual care | N/A | N/A | eHealth PRO assessments reduced follow-up costs |
| Wagner et al (2015)22 | Implementation study | 636 patients receiving gynaecological oncology outpatient care | HIT-integrated platform for collecting PRO data, including alerts to clinicians for severe symptoms | NA | 27 months | 27 months | Approximately 50% of patients completed the initial assessment; HIT-integrated PRO data collection facilitated referrals to psychosocial and supportive care |
| Garcia et al (2019)23 | Implementation study | 6825 oncology patients | HIT-integrated platform for collecting PRO data, including alerts to clinicians for severe symptoms | NA | 31 months | 31 months | Approximately 50% of patients completed at least two assessments; HIT-integrated PRO data collection facilitated referrals to psychosocial and supportive care |
| eHealth for managing symptom burden in patients with cancer | |||||||
| Somers et al (2015)24 | Feasibility study | 25 patients with breast, lung, prostate, or colorectal cancer who reported persistent pain | mHealth pain coping skills training | NA | 4 weeks | 5 weeks | The mHealth pain intervention was feasible and acceptable to patients with cancer with persistent pain |
| Somers et al (2016)25 | Randomised controlled trial | 30 patients with breast, lung, prostate, or colorectal cancer who reported persistent pain | mHealth pain coping skills training | Traditional in-person pain coping skills training | 4 weeks | 9 weeks | The mHealth pain intervention was feasible and provided benefits similar to an in-person intervention |
| Kelleher et al (2019)26 | Randomised controlled trial | 178 patients with breast, lung, prostate, or colorectal cancer who reported persistent pain | mHealth pain coping skills training | Traditional in-person pain coping skills training | 4 weeks | 3 months | The mHealth pain intervention had better feasibility and was non-inferior compared with an in-person intervention |
| Somers et al (2018)27 | Randomised controlled trial | 68 patients with cancer and pain after haemopoietic stem cell transplant | mHealth pain coping skills training | Usual care | 6 weeks | 10 weeks | The mHealth pain intervention was feasible and acceptable to patients with cancer with pain following haemopoietic stem cell transplant |
| Jim et al (2020)28 | Randomised controlled trial | 44 patients with chronic myeloid leukaemia treated with tyrosine kinase inhibitors with severe fatigue | mHealth cognitive behavioural therapy for fatigue | Wait list* | 18 weeks | 18 weeks | The mHealth fatigue intervention was feasible and acceptable with preliminary efficacy for improving fatigue and HRQOL |
| Wu et al (2018)29 | Randomised controlled trial | 60 patients with advanced prostate cancer treated with androgen deprivation therapy | eHealth cognitive training | Usual care | 8 weeks | 8 weeks | The eHealth intervention was feasible, mostly acceptable, and associated with improved reaction time, but temporarily suppressed memory |
| Yanez et al (2015)30 | Randomised controlled trial | 74 patients with advanced prostate cancer | eHealth cognitive behavioural stress management | eHealth health promotion attention-control group† | 10 weeks | 6 months | The eHealth intervention was feasible, acceptable, and associated with decreased depressive symptoms and improved relaxation self-efficacy |
| Bouchard et al (2018)31 | Randomised controlled trial | 192 patients with advanced prostate cancer | eHealth cognitive behavioural stress management | eHealth health promotion attention-control group† | 10 weeks | 12 months | Both eHealth conditions were feasible to black and non-Hispanic white men, and acceptability was higher for black men; black men in the intervention condition reported the greatest decreases in anxiety related to prostate cancer |
| Greer et al (2017)32 | Randomised controlled trial | 145 patients with advanced cancers and elevated anxiety | mHealth cognitive behavioural therapy for anxiety | mHealth health education application | 12 weeks | 12 weeks | Both mHealth conditions were related to improved anxiety, depression, and HRQOL; patients with high baseline anxiety in the intervention condition reported the greatest decreases in anxiety |
| eHealth in survivors of cancer after treatment | |||||||
| Willems et al (2017)33 | Randomised controlled trial | 462 cancer survivors (all stages) who had completed treatment | eHealth program for managing psychosocial and lifestyle-related issues | Wait list | 6 months | 6 months | The eHealth intervention was associated with reduced depression and fatigue, and improved emotional and social functioning |
| Cockle-Hearne et al (2018)34 | Feasibility study | 135 patients with non-metastatic prostate cancer and mild-to-moderate distress | eHealth cognitive behavioural therapy to decrease anxiety and distress | NA | 4 weeks | 4 weeks | The eHealth intervention was acceptable to patients and associated with decreased levels of distress and improved self-efficacy for coping |
| Kinner et al (2018)35 | Feasibility study | 28 female survivors of ovarian cancer | eHealth psychosocial group intervention to improve mood, HRQOL, and perceived stress | NA | 10 weeks | 10 weeks | The eHealth intervention was highly usable and acceptable, with moderate feasibility, and was preliminarily associated with decreased perceived stress and improved ovarian cancer-specific HRQOL |
| Ritterband et al (2012)36 | Randomised controlled trial | 28 survivors of cancer (all stages) with insomnia | eHealth cognitive behavioural therapy for insomnia | Wait list | 9 weeks | 11 weeks | The eHealth intervention was associated with improved overall insomnia severity, sleep efficiency, sleep onset latency, soundness of sleep, and general fatigue, and feeling restored on awakening |
| Baseman et al (2017)37 | Feasibility Study | 11 survivors of breast cancer (all stages) and their providers | mHealth survivorship care application | NA | One-time | NA | The mHealth intervention was feasible and acceptable for meeting survivorship care objectives and goals |
| Buscemi et al (2018)38 | Feasibility Study | 25 Latina survivors of breast cancer (stage 0-IIIA) | mHealth application to reduce symptom burden and improve HRQOL | NA | 4 weeks | 4 weeks | The mHealth intervention was feasible and acceptable |
| Yanez et al (2019)39 | Randomised controlled trial | 80 Latina survivors of breast cancer (stage 0-IIIA) | mHealth application to reduce symptom burden and improve HRQOL | mHealth application to promote healthy lifestyle | 6 weeks | 8 weeks | Both mHealth applications were feasible and acceptable, and were associated with temporary decreases in symptom burden and improved breast cancer wellbeing |
PROs=patient-reported outcomes. HRQOL=health-related quality of life. HIT=health information technologies.
Patients in this group received treatment as usual until the study duration had ended (18 weeks). These patients were then offered the intervention in the treatment group.
Participants randomly assigned to the control group participated in an eHealth group intervention designed to control for non-specific attention and group effects (ie, contact with a group facilitator and group support). The control group was not designed to improve the primary outcomes (HRQOL and symptom burden), but focused on health promotion topics such as recommendations for physical activity and healthy eating.
The use of eHealth to link PROs with clinical cancer care
During the past decades, there has been growing interest in implementing PROs in cancer care, with the recognition that evaluating and treating a patient’s symptoms, functional status, and overall HRQOL is crucial for providing optimal care. PROs, as defined by the US Food and Drug Administration, are “a report that comes directly from the patient (i.e., study subject) about the status of a patient’s health condition without amendment or interpretation of the patient’s response by a clinician or anyone else”.45 PROs encompass broad constructs and include physical symptoms, emotional functioning, satisfaction with care, adherence to treatment, and HRQOL. Multiple modalities are used to collect PRO data (eg, paper and pencil measures [questionnaires], technology-based assessments, and in-person interviews). Compared with ratings from patients with cancer, clinicians often under-report symptoms and toxic effects,46 and might miss 50% or more of symptoms that patients have.47 The under-reporting of symptoms can lead to poor symptom control, deteriorating physical function, more emergency room admissions and hospital admissions, and great burden on the health system.48 Although the use of PRO data to inform clinical care is not novel, newer eHealth technologies can facilitate data collection, which can then be used more efficiently to improve patient care. For example, systematic PRO assessments based on eHealth, including recording treatment-related side-effects, can attenuate further health deterioration of patients with cancer by facilitating patient monitoring and patient–provider communication.49 To streamline this process even further, health information technologies provide innovative and feasible opportunities for integrating PROs with clinical care to enhance communication with the medical teams, facilitate shared decision making, and assist patients in self-monitoring and symptom management over time.
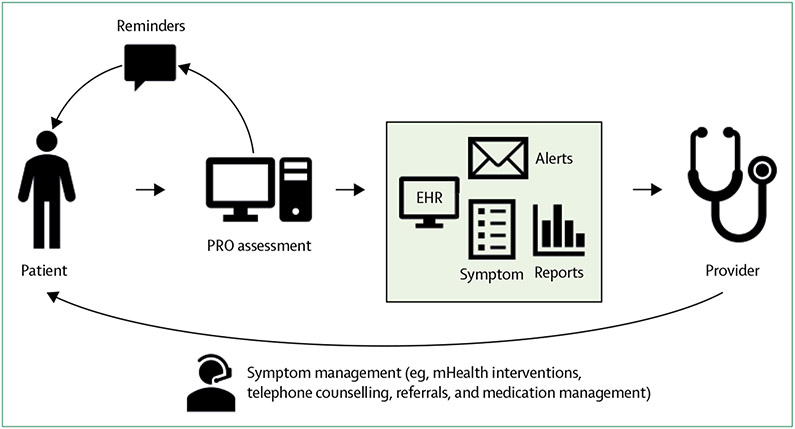
Multiple studies have evaluated the integration of PROs with health information technologies.17,19,25,33 Although procedures varied slightly by study, patients with cancer undergoing routine outpatient treatments were asked to report their side-effects and other PROs using health information technologies, either in the clinic or outside clinic visits. When PROs that can be administered and scored in real-time are integrated with health information technologies, results can be populated immediately into electronic health records and patient portals, and thus provide patients and clinicians with immediate feedback. To avoid burden for both patients and clinicians, PROs incorporated in health information technology platforms must be brief yet valid, with actionable data to guide clinical encounters. PROs such as the Patient Reported Outcomes Measurement Information System (PROMIS) can be electronically administered and scored, and offer assessments that are administered by computer-adaptive testing. Computer-adaptive testing uses item response theory to rapidly assess symptoms with as few items as possible, adding greater measurement precision and reducing patient burden.22 Therefore, integrating PROs, such as PROMIS computer-adaptive testing, into health information technologies can increase the likelihood of patients completing PRO measurements. The resulting clinical data are immediately integrated with electronic health records, and clinicians can be prompted when patients report moderate-to-severe symptoms or worsening toxic effects or concerns, thus creating opportunities for immediate clinical action (figure). Several single-arm studies have established the feasibility and acceptability of these procedures among patients with cancer,12-15 and this work has been extended to the caregivers of patients with cancer.16 In general, when prompted, patients with cancer were willing to use health information technologies to report on their outcomes, and clinicians effectively responded to this PRO information, thus preliminarily supporting the use of these programmes.
Figure: Flow of information during the monitoring of cancer survivors with PROs integrated with health information technologies.
EHR=electronic health record. PRO=patient-reported outcome.
Clinical use of integrating PRO assessments with health information technologies in cancer care
Randomised controlled trials have shown the clinical significance of integrating PROs with health information technologies. Basch and colleagues17 randomly assigned more than 700 patients with advanced cancers undergoing outpatient chemotherapy to weekly PRO monitoring via electronic tablets (experimental condition) or PRO monitoring at the treating physician’s discretion (usual care). In the experimental condition, nurses were notified via email when participants reported severe or worsening symptoms. At 6 months, participants in the experimental group reported significantly less decline in HRQOL, had fewer emergency room admissions and hospitalisations, and continued chemotherapy for longer, compared with usual care.17 At a 7-year follow-up, participants in the experimental group also had significantly longer overall survival.18 The survival benefit associated with weekly PRO monitoring with health information technologies was replicated in a sample of patients with advanced lung cancer.19 These two trials also showed the cost-effectiveness of this monitoring relative to usual care by use of a ratio of institutional costs to the increased number of patients’ quality-adjusted life-years.20,21 Thus, routine PRO monitoring with health information technologies has the potential not only to promote better patient-level outcomes (ie, better HRQOL and longer survival), but also better system-level outcomes (ie, fewer admissions to emergency departments and hospitalisations, and cost-effectiveness). More research is needed to establish the effect of PRO monitoring with health information technologies on important clinical outcomes, such as mortality and the use of health services. Of note, this type of PRO monitoring might be especially crucial for patients with advanced cancers, among whom treatments can be highly toxic and, in some cases, life-threatening (eg, patients participating in early phase clinical trials or initiating immune checkpoint inhibitors). In these situations, clinicians might benefit from these technological tools that enable routine assessment of PROs and are tailored to specific treatment regimens (eg, immunotherapies and kinase inhibitors).50 The use of health information technologies to tailor the delivery of PROs to the patients’ unique clinical characteristics is an area that needs additional investigation.
Several cancer centres have integrated routine PRO monitoring with health information technologies. For example, researchers and clinicians at Northwestern University have implemented assessments of physical symptoms, psychosocial concerns, and informational needs via an online patient portal (MyChart) that is directly linked to electronic health records. Results are immediately populated into electronic health records and messages are generated to notify providers of clinically elevated symptoms (symptoms that are more severe than established severity thresholds). Patients are automatically triaged to appropriate resources on the basis of their individual needs (ie, social work for psychosocial concerns and dietitians for nutrition concerns). These procedures were initiated and refined in the gynaecological oncology outpatient service22 and later rolled-out to all outpatient services in adult oncology.23 Approximately 50% or less of patients with cancer who were invited to complete PRO assessments did so. However, these assessments occurred in real-world settings as part of clinical cancer care rather than in the context of a research study with active recruitment and compensation for participation. These real-world implementations showed the feasibility of applying routine PRO monitoring with health information technologies on a large scale in the context of complex clinic workflows and large volumes of patients. Moreover, these procedures facilitated prompt reporting of actionable PROs and practical needs.22,23
Another real-world example comes from an ongoing cross-institutional effort to harmonise the PRO data collected and integrated into electronic health records across cancer centres at Harvard University, Mayo Clinic, and Northwestern University. These institutions have formed a collaboration (the National Cancer Institute’s IMPACT programme), in which they plan to collect a common set of PRO measures from patients with cancer via patient portals and implement similar strategies for integrating the data into electronic health records (all institutions use Epic Systems to store health records). The goals of this endeavour are to first agree on a common set of PRO measures (eg, PROMIS measures), then develop a common implementation strategy for integrating the PRO data into electronic health records and clinical workflow, and finally rollout the agreed protocol for collecting routine PRO data from patients during cancer care. Ongoing efforts include engagement from key stakeholders, a landscape analysis, and consideration of clinical workflows and available technologies. The use of harmonised measures across cancer centres can facilitate data interpretation, and consideration of complex workflows and technical barriers will guide implementation of these strategies in other settings. Several institutions, including Wake Forest University, Duke University, University of North Carolina at Chapel Hill, University of Miami, and Medical University of South Carolina, are now implementing similar strategies.51
eHealth approaches for managing symptom burden in patients with cancer
Beyond having numerous symptoms and managing the toxic effects of treatment, patients with cancer also express concerns about prognosis, life after treatment, and the effect of their illness on wellbeing and overall HRQOL. Therefore, the time at which a patient is actively receiving treatment is a crucial time for symptom management and psychosocial intervention. The use of eHealth for the delivery of interventions might promote the engagement of patients with cancer in their care and offset clinician burden. With increasing use of mHealth technologies, such as smartphone applications to manage patients’ mild-to-moderate symptoms and concerns, clinicians will have more time to treat patients with severe symptoms. Thus, mHealth has the potential to provide much needed relief to the health-care system, which is crucial because of the growing population of survivors of cancer.2 Given the abundance of smartphones and additional mobile technologies (eg, tablets), there has been a burgeoning number of studies evaluating the feasibility and efficacy of the use of mHealth to deliver evidence-based interventions during active treatment. Although intervention designs vary across studies, a review43 found that most eHealth interventions for managing chemotherapy-related symptoms include education, self-management strategies, tailored information, and communication with clinicians. We reviewed key studies showing that mHealth technologies for symptom management are feasible and efficacious during active cancer treatment.
Pain
One meta-analysis24 concluded that pain is prevalent in more than half of patients during active treatment and in more than 65% of patients with advanced disease.52 Researchers at Duke University have led mHealth programs for the management of pain from cancer, which used a brief mHealth intervention, known as mPCST, that provided training for pain coping skills. Delivered by use of tablet videoconferencing, the intervention was feasible and acceptable to patients with persistent cancer-related and treatment-related pain,24 with benefits similar to a traditional in-person training intervention (eg, decreased pain sensitivity and increased self-efficacy for pain management).25,26 The mPCST intervention was adapted for patients with cancer who had undergone haemopoietic stem cell transplant and reported pain after transplant.27 Following the transplant, the mPCST intervention was feasible and acceptable, and was related to decreased pain, improved self-efficacy, and alleviated pain-related disability as measured by the pain disability index. Notably, the intervention bridged the intensive outpatient and home settings, which allowed for continuity of care and the fostering of strong participant–therapist bonds, and possibly contributed to the high acceptability of the intervention.27 This innovative use of mHealth to bridge inpatient to outpatient settings addressed a major challenge in cancer care and might be extended to multiple settings in future work (eg, after surgery and after inpatient infusions).
Fatigue
Fatigue affects nearly all survivors of cancer,53 and one meta-analysis54 concluded that eHealth interventions can effectively manage fatigue in highly fatigued survivors of cancer. Fatigue is also an important adverse event associated with targeted therapies, including tyrosine-kinase inhibitors,55 that dramatically improve survival rates for patients with cancers such as chronic myeloid leukaemia56 but also can compromise HRQOL. A pilot study documented the feasibility and efficacy of a mobile application intervention to improve fatigue among patients with chronic myeloid leukaemia being treated with targeted therapies compared with a wait list control.28 Patients in the wait list control group received usual care until the study duration ended and were then offered the intervention in the treatment group. Relative to controls, participants randomly assigned to the experimental condition showed greater improvements in fatigue and overall HRQOL.28 Thus, mHealth might be a feasible method to provide symptom management for one of the most common and persistent cancer-related side-effects and might be a key component of symptom management for newer classes of cancer therapies.
Cancer-related cognitive impairment
Cancer-related cognitive impairment affects 17–75% of survivors of cancer57 and is more strongly associated with some cancer treatments than others. For example, among men with advanced prostate cancer, this cognitive impairment is twice as common in men treated with androgen deprivation therapy than those who do not receive this therapy.58 In a pilot study, a cognitive training intervention based on mHealth was compared with usual care in men with advanced prostate cancer on androgen deprivation therapy, with mixed results.29 The intervention was feasible and mostly acceptable, and participants showed improved reaction times but suppressed memory. Thus, the intervention’s efficacy might be limited to specific components of cognitive functioning. Importantly, participants provided feedback on ways to make the mHealth programme more acceptable (eg, by having breaks during training), imparting valuable insight for optimising this and other mHealth interventions.29 Unlike the interventions described for managing pain and fatigue, this mHealth cognitive training intervention did not require facilitation by a clinician and was entirely participant-driven, thus creating opportunities for alleviating clinician burden.
Psychological distress
Preliminary findings from a study of racially diverse men with advanced prostate cancer on androgen deprivation therapy showed the feasibility and preliminary efficacy of a tablet-delivered psychosocial intervention compared with a rigorous health promotion condition focused on attention control and delivered also by tablet.30 Moreover, men who were randomly assigned to the experimental condition reported significantly reduced depressive symptoms and (albeit non-significant) improved distress and functional wellbeing relative to controls.30 Secondary analyses explored racial differences in outcomes and found no differences in feasibility between non-Hispanic black men and non-Hispanic white men.31 However, although both non-Hispanic black men and non-Hispanic white men found the study conditions acceptable, black men rated both conditions more favourably and reported a unique intervention benefit (reduced prostate cancer-specific distress).31 This study showed the feasibility and preliminary efficacy of the use of eHealth to deliver a group-based psychosocial intervention among men with advanced prostate cancer undergoing androgen deprivation therapy and suggests that particular subgroups of patients with cancer (eg, black men) might uniquely benefit. By use of a similar study design and control group, another study evaluated a tablet-delivered psychosocial intervention for reducing anxiety among patients with advanced cancer and at least mild anxiety.32 Participants in both the intervention and active control conditions reported reduced anxiety and depression and improved HRQOL; baseline anxiety modulated the intervention’s effect on anxiety after treatment (ie, participants with more anxiety at baseline who were randomly assigned to the intervention showed the greatest reductions in anxiety).32 This finding highlights the potential benefit of tailoring interventions to individual patient needs.
Future directions for eHealth programmes in the control of symptoms
There is growing evidence to support the initial efficacy of the use of eHealth for symptom monitoring and management during active cancer treatment; however, there are many opportunities for continued growth and innovation. Although studies show the feasibility and acceptability of mHealth interventions for managing pain, fatigue, and psychosocial distress, there is a scarcity of evidence-based mHealth resources for managing other common symptoms of active treatment (eg, nausea, vomiting, and constipation). Development of such resources, independently or in combination with other mHealth interventions for symptom management, could provide additional support to oncology clinics and particularly to those delivering phase 1 treatments (according to the National Comprehensive Cancer Network guidelines) or treatments with high toxicity. There is a need to move beyond feasibility studies to larger scale efficacy and implementation trials with long-term follow-up and assessment of clinical outcomes (eg, disease progression and survival). To date, very few mHealth interventions for symptom management have been integrated into health information technologies. Furthermore, although there are several mHealth resources publicly available to promote symptom self-management, few focus specifically on cancer disease management59 and most have not been developed on the basis of scientific evidence of their efficacy for improving PROs.60,61
Use of eHealth for increasing access to cancer care and expertise
In addition to facilitating the monitoring and management of patients during active cancer treatment, eHealth platforms provide the crucial benefit of increasing access to cancer care more broadly to hard-to-reach and disenfranchised populations (eg, patients living in rural and low-income areas). When delivering cancer care via eHealth, interactions can either be synchronous (ie, occurring over video technology in real time), asynchronous (ie, not occurring in real time), or a combination of both.62 These approaches allow for flexibility with regard to when services are provided and what information is shared (eg, asynchronous provision of clinical data, subsequent interpretation, followed by synchronous physician–physician or patient–physician consultation). eHealth technologies allow greater access to clinical trials for patients with cancer in rural settings,63-65 which benefits more patients directly and will enhance the generalisability of trial findings to more diverse populations. In addition, the use of mHealth for symptom management might especially benefit patients with cancer with limited access to oncology clinics, supportive care, or behavioural interventions. Thus, making evidence-based mHealth resources publicly available and studying the use of mHealth symptom management in rural settings should be a priority for researchers in this field.
eHealth can also be used to connect local health-care systems with large-scale, academic medical centres and specialists. As a salient example, Project Extension for Community Healthcare Outcomes (ECHO) was initiated at the University of New Mexico as a model of collaborative medical care management and education that connects providers in rural and underserved environments with educational and mentoring resources to improve their ability to manage patients with complex presentations in their communities. Through a collaboration with MD Anderson Cancer Center (Tx, USA), ECHO now seeks to apply the same principles to cancer care.66 Studies show that the low-cost ECHO training model is effective for educating providers in low-resource areas about cancer screening and treatment in the USA and globally.67-69 By empowering and educating providers, it is possible to improve cancer care delivery more broadly and reduce health disparities.
eHealth for survivors of cancer after primary treatment
Many cancer treatment-related symptoms can be chronic, debilitating, and persistent well beyond the period of active treatment (eg, fatigue, pain, and cancer-related cognitive impairment).70 Furthermore, cancer treatments can lead to new debilitating symptoms and the exacerbation of existing conditions. Multiple teams have developed approaches to aid survivors of cancer after treatment and the use of eHealth interventions in this domain is growing.
A common feature of many studies that use eHealth interventions after cancer treatment is a simultaneous focus on multiple domains of HRQOL (eg, fatigue, anxiety, depression, and social support). Largely, these interventions show positive results for improving HRQOL.33-35 Other eHealth interventions have targeted specific symptoms experienced by survivors of cancer. For example, an mHealth intervention that was designed to improve sleep for survivors of cancer with insomnia was associated with decreased insomnia severity, increased sleep efficacy, decreased sleep onset latency, increased soundness of sleep (ie, the quality of sleep was improved), and feeling restored on awakening.36 In another study,37 an mHealth smartphone application (SmartSurvivor) was designed to help survivors of breast cancer develop a survivorship plan. In pilot testing, the application was well received by participants and further work is investigating SmartSurvivor’s efficacy in rural areas.37 eHealth tools to develop and implement care plans for cancer survivorship are an important area for further research, and, in particular, researchers should consider how to integrate these plans with health information technologies to bridge gaps in care as survivors of cancer transition from follow-up with oncology teams to primary care.71
Collectively, these studies show some of the potential benefits of eHealth interventions in the improvement of patient-centred outcomes among survivors of cancer. However, more research that includes larger samples, randomised trial designs, long-term follow-up, and evaluation of clinical outcomes is needed. Furthermore, most work does not have a substantial inclusion of racial and ethnic minorities, rural communities, older patients, and uninsured patients that might have difficulty accessing cancer survivorship care. As an exception, Yanez and colleagues38,39 have investigated the use of an mHealth smartphone application (My Guide) to improve HRQOL in Latina survivors of breast cancer. My Guide was pilot-tested over 4 weeks and showed benefits, including improved breast cancer knowledge and a trend for improved HRQOL.38 In a 6-week randomised controlled trial, My Guide was compared with an attention-control application that promoted healthy lifestyle behaviours. After the intervention, both applications were associated with decreased symptom burden and reduced breast cancer-related concerns.39 Moreover, across both studies, My Guide was feasible and acceptable among Latina breast cancer survivors, thus providing support for the feasibility and acceptability of eHealth programmes in specific populations that might face barriers in accessibility.
Future directions, limitations, and emerging areas for research
eHealth provides a promising opportunity to optimise patient-centred cancer care. Multiple studies have begun to document the acceptability and feasibility of such programmes. However, much of the existing published literature consists of studies with small sample sizes and short follow-up periods that do not report on clinically relevant outcomes (eg, disease activity and disease progression). Furthermore, few studies have considered external validity issues that can produce more generalisable results,40 and very few have integrated eHealth programmes into health information technologies (eg, patient portals and electronic health records) to assist with clinical management. Nonetheless, the fact that these programmes and their administration appear to be feasible and patients consistently report high levels of acceptability suggests that eHealth will have a considerable effect on cancer care as future work in this area is developed and evaluated.
Evolving technologies
With ongoing technological advances, eHealth programmes should consider moving beyond semidynamic self-tracking and feedback functions to sophisticated user-centred design features. For example, research on gamification (ie, the use of game design elements in non-game contexts) suggests that integration of gamification with eHealth might enhance user engagement and adherence to programmes in oncology.72 More research on the benefits of gamification is needed, including its use for motivating long-term adherence to recommended lifestyle and cancer control activities. There is also growing interest in integrating patient feedback into eHealth platforms to enhance user engagement and ensure that the correct information is reaching the correct patients.73 In early eHealth platforms, all data were made available without customisation, often resulting in cumbersome platforms. Focus has now shifted towards individualised messages and information tailored to a patient’s experience or treatment plan. Patients have also expressed interest in standardising the layout and appearance of applications, so that these platforms can be accessed and understood by all potential users.73 User feedback and formal testing of applications can help developers understand how to design and implement applications with these specifications in mind; these processes will be crucial as more platforms are designed to achieve similar benefits for patients. Artificial intelligence might be another potential tool that researchers can use to act on PRO data and provide intervention. Artificial intelligence is used to synthesise patient data and approximate diagnoses and overall clinical pictures, and has had a role in bioinformatics and wireless and portable devices for the past several decades.74 Researchers should consider leveraging artificial intelligence to further refine existing algorithms developed from PRO data to identify patients in need of intervention. Moreover, researchers should consider the use of artificial intelligence in the provision of high quality, automated symptom management via eHealth. Similarly, ambient intelligence is a system or device that continuously monitors a patient’s health status to promote health maintenance.75 Examples of ambient intelligence include the continuous monitoring of electrocardiograms, electroencephalograms, respiration, and wound healing. As emerging technologies, such as wearable sensor devices that collect real-time data, become more widely used, work should address how eHealth platforms can be linked to wearables and other ambient intelligence, sensor-driven data that are integrated with health information technologies. For example, wearable technologies could be useful for capturing data such as body temperature to monitor neutropenic fever.76
Implementation considerations
Despite the potential benefits of evolving and innovative technologies, one must consider the challenges of implementing the integration of PROs with health information technologies. Successful implementation of this monitoring in clinical cancer care is complex, and reasons for the lack of implementation include considerations of busy clinical workflows (eg, provider burden and IT support) and clinician understanding of how to interpret and respond to PRO data. In addition, triaging patients with identified supportive needs typically occurs within a health system or clinic, with few options for eHealth-delivered or self-management interventions. Thus, access to resources might be problematic. To address these barriers to implementation, researchers must engage key stakeholders (eg, clinicians, administrators, and IT support) to establish steps for developing these PRO monitoring platforms. By engaging providers and other stakeholders early in the implementation process, disruptions to established clinic workflows might be minimised. Reviews of established electronic PRO systems,77,78 guidance for developing one’s own electronic PRO portal,79,80 considerations for selecting PROs and score interpretation, and considerations and decision support for determining appropriate care in response to PRO data80 might all be helpful in this pursuit.
There is a fine balance between fully informing providers with clinically meaningful data versus collecting and reporting vast amounts of information that might overwhelm clinicians. Therefore, integration of patient data into electronic health records requires consideration of how to de-implement existing, time consuming practices that are ineffective or of low-value, and how to simultaneously implement novel, eHealth solutions to optimise clinician and patient needs.81 eHealth can generate massive amounts of data, creating opportunities for analysing big data in oncology. These data can be used to develop algorithms to identify patients at risk of, for example, toxic effects from cancer treatment, poor HRQOL, and non-adherence, and develop evidence-based clinical pathways to optimise care. Big data analyses and interpretation requires expertise that most clinicians and scientists do not have. Therefore, teams and health-care systems must be equipped with the necessary expertise and resources in bioinformatics to manage large data sets. In addition, to promote uptake and stakeholder engagement in the collection of vast amounts of data, clinicians, health system administrators, and others must see the benefit or incremental value in patient-centred care as a result of these technologies. Therefore, documenting the value of decision support tools to help to interpret PROs and the benefits of capturing data outside of in-person clinical encounters is needed, particularly when PROs are used to guide decision making that is consistent with guideline-concordant care.
The need for theoretically guided and reproducible approaches
Another limitation in this work is the scarcity of conceptual models that consider theory-based approaches. For example, the mHealth accountability model postulates that human support can enhance adherence to eHealth interventions by promoting accountability (eg, social presence, goal setting, and monitoring) and legitimacy (eg, expertise, reciprocity, and trustworthiness); the integration of these values into programmes that rely solely on automated functions is a challenge.82 In addition, Ritterband and colleagues83 have developed a theoretical model of behavioural change for web-based interventions. This model suggests that effective eHealth interventions can lead to the initiation and maintenance of desired behavioural changes via non-linear steps (ie, steps toward behavioural change that might differ between people). These behavioural changes involve user characteristics and environmental factors, which in turn affect the use of the technology and the adherence to recommended changes. These steps are also affected by support and website features. Application of these models in eHealth programmes can lead to well designed and reproducible programmes that are scalable and can be widely disseminated if effective.
Diverse and hard-to-reach cancer survivorship populations
A persisting major limitation and challenge, which also presents a crucial opportunity, is that eHealth in the delivery of cancer care needs to give greater attention to diverse patients and consider race, ethnicity, socioeconomic status, and geographical location. Future work should target the development of culturally informed programmes that address the needs of diverse groups, translate existing and future platforms to non-English speakers, and reach patients with cancer and survivors in rural areas or those in disenfranchised communities where access and connectivity remain problematic. As most of the research validating eHealth interventions has focused on non-Hispanic white and English-speaking groups, generalisability and broad implementation are still difficult to achieve. Furthermore, health information technology programmes that have engaged patients with cancer or are integrated into clinical settings via electronic health records have thus far been implemented in large-scale and well resourced medical settings. There must be greater consideration and efforts to implement eHealth interventions in settings such as community oncology clinics, primarily those that treat diverse patients, so that this technology does not contribute to health disparities, but rather helps to foster equity. As the field continues to evolve, disparate communities with poor access to care must be included in studies of health information technology.
The need for dissemination of evidence-based tools
A final challenge to consider is that most commercially available mHealth applications are not based on evidence and their efficacy has not been documented sufficiently. An important future direction is to disseminate evidence-based mHealth applications through commercially available outlets, such as app stores, so that these mHealth interventions have broader reach, especially to patients who are not receiving care in elite, academic medical centres. Nonetheless, we are probably witnessing technological developments that will transform models of cancer care delivery so that the use of technology platforms will become part of routine care. We are at a pivotal point in this transformation at which more research that addresses the existing limitations of eHealth is needed.
Limitations of this Review
Although we did an extensive review of the published literature, we limited our selection of articles to the use of only one search engine (PubMed). We acknowledge that some journals are not indexed on PubMed and we might have missed additional articles. We confined our search to the past 10 years and might have missed some previous studies. Finally, many unfeatured articles are important studies in the field of eHealth and mHealth cancer care. However, because of the scope of this Review, the included studies are deemed both adequate and appropriate.
Supplementary Material
Search strategy and selection criteria.
We identified references through PubMed with the search terms “cancer AND survivorship AND eHealth,” “cancer AND survivorship AND mHealth,” “cancer AND survivorship AND smartphone,” “cancer AND survivorship AND web,” “cancer AND survivorship AND web-based,” and “cancer AND survivorship AND internet” for articles published from Jan 1, 2009 to Sept 30, 2019. We reviewed only papers in English and containing an intervention in cancer survivors (appendix). The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Acknowledgments
This Review was funded, in part, by grants: R18HS026170 (SFG and FJP; Co-PIs); UM1CA233035 (DC; PI); T32CA211034 (Merchant; PI); Sylvester Comprehensive Cancer Center Survivorship Initiative (PG 011640; FJP, PI); and R21CA226671 (BY; PI).
Footnotes
Declaration of interests
BY declares a grant from Bristol-Myers Squibb, which contributed to a funded American Cancer Society grant used to investigate the use of health information technologies for monitoring toxic effects. All other authors declare no competing interests.
Contributor Information
Frank J Penedo, Department of Psychology, Sylvester Comprehensive Cancer Center, University of Miami, Miami, FL, USA.
Laura B Oswald, Health Outcomes and Behavior Program, Moffitt Cancer Center, Tampa, FL, USA.
Joshua P Kronenfeld, Department of Surgery, University of Miami, Miami, FL, USA.
Sofia F Garcia, Department of Medical Social Sciences, Northwestern University, Chicago, IL, USA.
David Cella, Department of Medical Social Sciences, Northwestern University, Chicago, IL, USA.
Betina Yanez, Department of Medical Social Sciences, Northwestern University, Chicago, IL, USA.
References
- 1.The National Cancer Institute. NCI dictionary of cancer terms. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivorship (accessed Oct 15, 2019).
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016; 25: 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008; 112 (suppl 11): 2577–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med 2010; 40: 163–81. [DOI] [PubMed] [Google Scholar]
- 5.Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer 2004; 90: 2297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev 2011; 20: 1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer 2008; 113 (suppl 12): 3519–29. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine and National Research Council. From cancer patient to cancer survivor: lost in transition. 2006. https://www.nap.edu/catalog/11468/from-cancer-patient-to-cancer-survivor-lost-in-transition (accessed March 3, 2020).
- 9.National Academy of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academy of Sciences, 2008. [PubMed] [Google Scholar]
- 10.Miriovsky BJ, Shulman LN, Abernethy AP. Importance of health information technology, electronic health records, and continuously aggregating data to comparative effectiveness research and learning health care. J Clin Oncol 2012; 30: 4243–48. [DOI] [PubMed] [Google Scholar]
- 11.Janssen A, Brunner M, Keep M, et al. Interdisciplinary eHealth practice in cancer care: a review of the literature. Int J Environ Res Public Health 2017; 14: E1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol 2013; 31: 2580–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girgis A, Durcinoska I, Levesque JV, et al. eHealth system for collecting and utilizing patient reported outcome measures for personalized treatment and care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. J Med Internet Res 2017; 19: e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae WK, Kwon J, Lee HW, et al. Feasibility and accessibility of electronic patient-reported outcome measures using a smartphone during routine chemotherapy: a pilot study. Support Care Cancer 2018; 26: 3721–28. [DOI] [PubMed] [Google Scholar]
- 15.Hauth F, Bizu V, App R, et al. Electronic patient-reported outcome measures in radiation oncology: initial experience after workflow implementation. JMIR Mhealth Uhealth 2019; 7: e12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klagholz SD, Ross A, Wehrlen L, Bedoya SZ, Wiener L, Bevans MF. Assessing the feasibility of an electronic patient-reported outcome (ePRO) collection system in caregivers of cancer patients. Psychooncology 2018; 27: 1350–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016; 34: 557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017; 318: 197–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denis F, Lethrosne C, Pourel N, et al. Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. J Natl Cancer Inst 2017; 109: 1. [DOI] [PubMed] [Google Scholar]
- 20.Nixon NA, Spackman E, Clement F, Verma S, Manns B. Cost-effectiveness of symptom monitoring with patient-reported outcomes during routine cancer treatment. J Cancer Policy 2018; 15: 32–36. [Google Scholar]
- 21.Lizée T, Basch E, Trémolières P, et al. Cost-effectiveness of web-based patient-reported outcome surveillance in patients with lung cancer. J Thorac Oncol 2019; 14: 1012–20. [DOI] [PubMed] [Google Scholar]
- 22.Wagner LI, Schink J, Bass M, et al. Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care. Cancer 2015; 121: 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia SF, Wortman K, Cella D, et al. Implementing electronic health record-integrated screening of patient-reported symptoms and supportive care needs in a comprehensive cancer center. Cancer 2019; 125: 4059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somers TJ, Abernethy AP, Edmond SN, et al. A pilot study of a mobile health pain coping skills training protocol for patients with persistent cancer pain. J Pain Symptom Manage 2015; 50: 553–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somers TJ, Kelleher SA, Westbrook KW, et al. A small randomized controlled pilot trial comparing mobile and traditional pain coping skills training protocols for cancer patients with pain. Pain Res Treat 2016; 2016: 2473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelleher SA, Winger JG, Dorfman CS, et al. A behavioral cancer pain intervention: a randomized noninferiority trial comparing in-person with videoconference delivery. Psychooncology 2019; 28: 1671–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somers TJ, Kelleher SA, Dorfman CS, et al. An mHealth pain coping skills training intervention for hematopoietic stem cell transplantation patients: development and pilot randomized controlled trial. JMIR Mhealth Uhealth 2018; 6: e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jim HSL, Hyland KA, Nelson AM, et al. Internet-assisted cognitive behavioral intervention for targeted therapy-related fatigue in chronic myeloid leukemia: results from a pilot randomized trial. Cancer 2020; 126: 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu LM, Amidi A, Tanenbaum ML, et al. Computerized cognitive training in prostate cancer patients on androgen deprivation therapy: a pilot study. Support Care Cancer 2018; 26: 1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanez B, McGinty HL, Mohr DC, et al. Feasibility, acceptability, and preliminary efficacy of a technology-assisted psychosocial intervention for racially diverse men with advanced prostate cancer. Cancer 2015; 121: 4407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouchard LC, Yanez B, Dahn JR, et al. Brief report of a tablet-delivered psychosocial intervention for men with advanced prostate cancer: acceptability and efficacy by race. Transl Behav Med 2018; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greer J, Jacobs JM, Pensak N, et al. Randomized trial of a cognitive-behavioral therapy mobile app for anxiety in patients with incurable cancer. J Clin Oncol 2017; 35 (suppl 31): 10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willems RA, Bolman CA, Mesters I, Kanera IM, Beaulen AA, Lechner L. Short-term effectiveness of a web-based tailored intervention for cancer survivors on quality of life, anxiety, depression, and fatigue: randomized controlled trial. Psychooncology 2017; 26: 222–30. [DOI] [PubMed] [Google Scholar]
- 34.Cockle-Hearne J, Barnett D, Hicks J, Simpson M, White I, Faithfull S. A web-based intervention to reduce distress after prostate cancer treatment: development and feasibility of the getting down to coping program in two different clinical settings. JMIR Cancer 2018; 4: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinner EM, Armer JS, McGregor BA, et al. Internet-based group intervention for ovarian cancer survivors: feasibility and preliminary results. JMIR Cancer 2018; 4: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology 2012; 21: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baseman J, Revere D, Baldwin LM. A mobile breast cancer survivorship care app: pilot study. JMIR Cancer 2017; 3: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buscemi J, Buitrago D, Iacobelli F, et al. Feasibility of a smartphone-based pilot intervention for Hispanic breast cancer survivors: a brief report. Transl Behav Med 2018; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanez B, Oswald LB, Baik SH, et al. Brief culturally informed smartphone interventions decrease breast cancer symptom burden among Latina breast cancer survivors. Psychooncology 2020; 29: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez MA, Rabin BA, Gaglio B, et al. A systematic review of eHealth cancer prevention and control interventions: new technology, same methods and designs? Transl Behav Med 2013; 3: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agboola SO, Ju W, Elfiky A, Kvedar JC, Jethwani K. The effect of technology-based interventions on pain, depression, and quality of life in patients with cancer: a systematic review of randomized controlled trials. J Med Internet Res 2015; 17: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis J, Ray P, Liaw ST. Recent worldwide developments in eHealth and mHealth to more effectively manage cancer and other chronic diseases—a systematic review. Yearb Med Inform 2016; 1: 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moradian S, Voelker N, Brown C, Liu G, Howell D. Effectiveness of Internet-based interventions in managing chemotherapy-related symptoms in patients with cancer: a systematic literature review. Support Care Cancer 2018; 26: 361–74. [DOI] [PubMed] [Google Scholar]
- 44.Triberti S, Savioni L, Sebri V, Pravettoni G. eHealth for improving quality of life in breast cancer patients: a systematic review. Cancer Treat Rev 2019; 74: 1–14. [DOI] [PubMed] [Google Scholar]
- 45.FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) resource. Silver Spring, MD: US: Food and Drug Administration, 2016. [PubMed] [Google Scholar]
- 46.Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 2016; 24: 3669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 2015; 33: 910–15. [DOI] [PubMed] [Google Scholar]
- 48.Panattoni L, Fedorenko C, Greenwood-Hickman MA, et al. Characterizing potentially preventable cancer- and chronic disease-related emergency department use in the year after treatment initiation: a regional study. J Oncol Pract 2018; 14: e176–85. [DOI] [PubMed] [Google Scholar]
- 49.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014; 32: 1480–501. [DOI] [PubMed] [Google Scholar]
- 50.Yanez B, Bouchard LC, Cella D, et al. Patient-centered engagement and symptom/toxicity monitoring in the new era of tumor next-generation sequencing and immunotherapy: the OncoTool and OncoPRO platforms. Cancer 2019; 125: 2338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung A, Stover AM, Wagner LI, et al. Harmonization of patient-reported outcomes into EHRs at four cancer hospital outpatient clinics for patient care and quality assessment. J Clin Oncol 2017; 35 (suppl 8): 129. [Google Scholar]
- 52.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016; 51: 1070–90. [DOI] [PubMed] [Google Scholar]
- 53.Mock V, Atkinson A, Barsevick A, et al. NCCN Practice guidelines for cancer-related fatigue. Oncology (Williston Park) 2000; 14: 151–61. [PubMed] [Google Scholar]
- 54.Seiler A, Klaas V, Tröster G, Fagundes CP. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: a systematic review and meta-analysis. Psychooncology 2017; 26: 1239–53. [DOI] [PubMed] [Google Scholar]
- 55.Efficace F, Baccarani M, Breccia M, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 2013; 27: 1511–19. [DOI] [PubMed] [Google Scholar]
- 56.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood 2012; 119:1981–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jean-Pierre P Management of cancer-related cognitive dysfunction—conceptualization challenges and implications for clinical research and practice. US Oncol 2010; 6: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez BD, Jim HS, Booth-Jones M, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol 2015; 33: 2021–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bender JL, Yue RY, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res 2013; 15: e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vollmer Dahlke D, Fair K, Hong YA, Beaudoin CE, Pulczinski J, Ory MG. Apps seeking theories: results of a study on the use of health behavior change theories in cancer survivorship mobile apps. JMIR Mhealth Uhealth 2015; 3: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamel LM, Thompson HS, Albrecht TL, Harper FW. Designing and testing apps to support patients with cancer: looking to behavioral science to lead the way. JMIR Cancer 2019; 5: e12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sirintrapun SJ, Lopez AM. Telemedicine in cancer care, volume 38 New York, NY: American Society of Clinical Oncology, 2018: 540–45. [DOI] [PubMed] [Google Scholar]
- 63.Bobb MR, Van Heukelom PG, Faine BA, et al. Telemedicine provides noninferior research informed consent for remote study enrollment: a randomized controlled trial. Acad Emerg Med 2016; 23: 759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark JM, Heifetz LJ, Palmer D, Brown LM, Cooke DT, David EA. Telehealth allows for clinical trial participation and multimodality therapy in a rural patient with stage 4 non-small cell lung cancer. Cancer Treat Res Commun 2016; 9: 139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galsky MD, Shahin M, Jia R, et al. Telemedicine-enabled clinical trial of metformin in patients with prostate cancer. JCO Clin Cancer Inform 2017; 1: 1–10. [DOI] [PubMed] [Google Scholar]
- 66.Arora SH, Hawk E. Ensuring equitable cancer care for all patients. May 10, 2017. https://www.ascopost.com/issues/may-10-2017/ensuring-equitable-cancer-care-for-all-patients/ (accessed March 3, 2020). [Google Scholar]
- 67.Lopez MS, Baker ES, Milbourne AM, et al. Project ECHO: a telementoring program for cervical cancer prevention and treatment in low-resource settings. J Glob Oncol 2016; 3: 658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hariprasad R, Arora S, Babu R, et al. Retention of knowledge levels of health care providers in cancer screening through telementoring. J Glob Oncol 2018; 4: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nethan ST, Hariprasad R, Babu R, Kumar V, Sharma S, Mehrotra R. Project ECHO: a potential best-practice tool for training healthcare providers in oral cancer screening and tobacco cessation. J Cancer Educ 2019; published online May 23 DOI: 10.1007/s13187-019-01549-8. [DOI] [PubMed] [Google Scholar]
- 70.Mayr M, Schmid RM. Pancreatic cancer and depression: myth and truth. BMC Cancer 2010; 10: 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geng Y, Myneni S. Patient engagement in cancer survivorship care through mHealth: a consumer-centered review of existing mobile applications. AMIA Annu Symp Proc 2015; 2015: 580–88. [PMC free article] [PubMed] [Google Scholar]
- 72.Floryan MR, Ritterband LM, Chow PI. Principles of gamification for Internet interventions. Transl Behav Med 2019; 9: 1131–38. [DOI] [PubMed] [Google Scholar]
- 73.Hesse BW, Shneiderman B. eHealth research from the user’s perspective. Am J Prev Med 2007; 32 (suppl 5): S97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel VL, Shortliffe EH, Stefanelli M, et al. The coming of age of artificial intelligence in medicine. Artif Intell Med 2009; 46: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Acampora G, Cook DJ, Rashidi P, Vasilakos AV. A survey on ambient intelligence in health care. Proc IEEE Inst Electr Electron Eng 2013; 101: 2470–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbasi J Wearable digital thermometer improves fever detection. JAMA 2017; 318: 510. [DOI] [PubMed] [Google Scholar]
- 77.Jensen RE, Snyder CF, Abernethy AP, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract 2014; 10: e215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin 2012; 62: 337–47. [DOI] [PubMed] [Google Scholar]
- 79.Holch P, Warrington L, Bamforth LCA, et al. Development of an integrated electronic platform for patient self-report and management of adverse events during cancer treatment. Ann Oncol 2017; 28: 2305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Girgis A, Durcinoska I, Arnold A, Delaney GP. Interpreting and acting on the PRO scores from the Patient-reported Outcomes for Personalized Treatment and Care (PROMPT-Care) eHealth system. Med Care 2019; 57 (suppl 1, 5): S85–91. [DOI] [PubMed] [Google Scholar]
- 81.Norton WE, Kennedy AE, Chambers DA. Studying de-implementation in health: an analysis of funded research grants. Implement Sci 2017; 12: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res 2011; 13: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ritterband LM, Thorndike FP, Cox DJ, Kovatchev BP, Gonder-Frederick LA. A behavior change model for internet interventions. Ann Behav Med 2009; 38: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.