Abstract
Calves with lower concentrations of immunoglobulin G (IgG) in their blood, have a greater risk of developing diseases. There is a lack of knowledge on genetic markers known to be associated with immunological variability or disease resistance. Therefore, the objective of this study was to identify SNP markers associated with passive immunity measures (serum IgG, serum protein, albumin, globulin and total protein concentrations, total solids Brix percentage, zinc sulphate turbidity units) and disease (pneumonia, diarrhoea, crude illness) traits in Irish commercial beef-suckler and dairy calves through genome wide association studies (GWAS). Genotyping was performed on DNA samples from beef-suckler (n = 698) and dairy (n = 1178) calves, using the IDBv3 chip. Heritability of passive immunity associated traits (range 0.02–0.22) and the disease traits (range 0.03–0.20) were low-to-moderate. Twenty-five and fifteen SNPs approached genome wide significance (P < 5 × 10−5) for the passive immunity and the disease traits, respectively. One SNP “ARS-BFGL-BAC-27914” reached Bonferroni genome wide significance (P < 1.15 × 10−6) for an association with serum IgG concentration in beef calves. Further work will evaluate these SNPs in larger cattle populations and assess their contribution to genomic selection breeding strategies, aimed towards producing more disease resistant livestock.
Subject terms: Genetics, Zoology, Biomarkers, Diseases
Introduction
High morbidity and mortality rates in beef and dairy calves result in significant economic losses for farmers and a reduction in animal welfare1. Internationally, calf mortality rates in the first year of life range from 5 to 11% in dairy and beef enterprises2–4. In Ireland, the combined mortality rate of beef and dairy calves from 0 to 12 months of age is 5.8%5. Furthermore, in a large scale, Irish observational study, Todd et al.6 reported that 20% of beef-suckler calves and 30% of dairy calves were treated for at least one disease event, from birth to 6 months of age, and higher treatment rates have been observed internationally7. Internationally, the diseases responsible for the majority of the morbidity and mortality, in beef and dairy calves less than 6 months of age, are diarrhoea and pneumonia4,8–10. Calves with lower plasma or serum immunoglobulin G (IgG) concentrations, or failure of passive transfer are at a greater risk of developing these diseases6,11–13. Since there is no trans-placental transfer of immunoglobulins or leukocytes in cattle, the calf is born without detectable antibodies13–15. Immunoglobulins and other macromolecules (e.g. maternal leukocytes, growth factors, hormones, cytokines) in colostrum ingested by the calf after birth, are absorbed and transported through enterocytes, and subsequently deposited into the circulatory system of the neonatal calf in the first 24 h of life14. There are many different tests that can assess passive transfer, some of which measure IgG directly (IgG ELISA) and others that act as a proxy for IgG concentration, which include serum protein, albumin, globulin and total protein concentrations, total solids Brix percentage, and zinc sulphate turbidity (ZST) units6. Passive immunity test results are generally categorised for failure of passive transfer (FPT) using test-specific cut-off values13.
The efficiency of passive transfer is strongly dependent on genetic, environmental and management factors such as the quantity and the quality of colostrum that the calf receives, and the length of time from birth to colostrum ingestion13,16,17. Differences between breeds in serum immunoglobulin concentration of neonatal calves have been observed18–22. We have previously observed that genes involved in the blood systemic immune response, particularly in the development of immune competence, are differentially expressed between beef and dairy calves during the first week of life22. Additionally, susceptibility to pneumonia has previously been demonstrated to be heritable in pre-weaned Holstein calves in the United States23,24. A further point which illustrates a genetic basis for immunity in cattle is that natural antibody (IgG and IgM) concentration in serum of Canadian Holstein cows has been estimated to be moderately heritable and associated with several DNA marker variants25.
Breeding programs to improve the genetic merit of both production and health traits of livestock have been extremely successful, both internationally and in Ireland, particularly in the dairy sector26–28. An important goal of these programmes is to breed robust animals which display resistance to disease29. In Ireland, health traits (mastitis, somatic cell count and lameness) currently make up 4% of the Economic Breeding Index (EBI), which is the index used for the selection of profitable dairy cattle in Ireland30. The beef Euro-Star Index, a relatively new index incorporating two overall indexes, the Replacement Index and the Terminal Index, is used for the selection of profitable beef animals in Ireland. Currently, cow survival and calf mortality make up 8% and 1%, respectively, of the Replacement Index, and mortality makes up 3% of the Terminal Index31; however, disease resistance or improved immune function traits are not included. This is partly due to limited phenotypic reporting and the current lack of knowledge on genetic markers known to be associated with immunological variability or disease resistance, to incorporate into these genetic breeding programmes (e.g. EBI, beef Euro-Star Index). Consequently, the discovery and addition of SNPs conferring resistance to disease and to general improved immune response capabilities, including calf passive immunity, would be extremely beneficial to these Irish breeding indexes, as it may result in the augmentation of selection of healthier cattle. Therefore, the objectives of the current study were to perform GWAS for passive immunity and disease-related traits in Irish beef-suckler and artificially-reared dairy-bred calves, and to identify SNP markers associated with superior immunity and disease resistance.
Methods
Ethical approval
Project and individual authorisations, in accordance with European Union (Protection of Animals used for Scientific Purposes) Regulations 2012 (S.I. No. 543 of 2012) as amended and Directive 2010/63/EU, were obtained (Health Products Regulatory Authority, Dublin, Ireland (AE19132-P006)). All study procedures were also reviewed and approved by the Teagasc Animal Ethics Committee (TAEC-97).
Animal details
Beef-suckler (n = 698) and dairy (n = 1178) calves used in this study were from commercial suckler beef (n = 29) and dairy farms (n = 32) in Ireland and were part of a larger study, examining passive immunity status in Irish suckler beef and dairy calves6. The suckler-bred calves included a mix of pure-bred and cross-bred Limousin, Simmental, Charolais, Aberdeen Angus, Hereford, Belgium Blue, Parthenaise, Saler, Shorthorn and Blonde d'Aquitaine. The dairy-bred calves included a mix of pure-bred and cross-bred Holstein–Friesian and Jersey, and beef × dairy breeds. All calves in the present study were born and resided at a beef or dairy farm in which at least ten calves per farm were available for genotyping.
Passive immunity traits measurement and profiling
Passive immunity traits were profiled from analyses performed on the calves’ serum samples (total IgG, total protein, albumin, specific gravity, globulin, total solids percentage from a Brix refractometer, ZST). All serum sample analyses performed in this study have been described in detail6; however, for clarity, they are summarised briefly here. Blood samples were collected from heifer and bull calves, aged between 1 and 21 days (as the half-life for IgG in colostrum fed calves is 28.5 days32), by jugular venepuncture, into 8.5 ml vacutainers (BD Vacutainer Serum Separator Tube II Advance 367,958 no anticoagulant, Unitech, Dublin, Ireland) using an 18-gauge needle. Samples were allowed to clot and then stored at 4 °C for 24 h. Serum was harvested following centrifugation (1600 × g for 10 min at 4 oC) and subsequently frozen at− 20 °C.
Total IgG concentration was directly measured in the serum samples using a commercial ELISA (BIO K165 test kit, BioX Diagnostics, Jemelle, Belgium), as described by Dunn, et al.33. A clinical chemistry analyser (Olympus AU400, Tokyo, Japan) and test reagent kits (OSR6132 and OSR6102, Beckman Coulter Ireland Inc., Lismeehan, Co. Clare, Ireland) were used to quantitatively determine serum total protein and albumin concentrations, as described by Early, et al.34. Globulin concentration was calculated for each serum sample as the difference between the total protein and albumin concentration. Serum samples were analysed for ZST units, as described by McEwan et al.35. An optical Brix refractometer with automatic temperature compensation (RSG-100ATC, Grand Index Solution Enterprise Limited, Hong Kong, China) was used to determine total solids percentage by Brix refractometry. A digital hand held refractometer with automatic temperature compensation (DR-303, Index Instruments Ltd, Cambridgeshire, UK) was used to determine total protein concentration which was subsequently referred to as specific gravity.
Health phenotypes for disease traits
Cases of calf pneumonia, diarrhoea, and any other illnesses during the first 6 months of life were observed and recorded by the farmers using standardised recording sheets6. Any calves which were sold before they reached 6 months of age or for which no data were received, were removed from the GWAS analyses. Health traits analysed by GWAS were crude illness, pneumonia and diarrhoea. Crude illness was defined as calves treated for at least one disease event, excluding injury, attributed to any cause (e.g. bovine respiratory disease (BRD), diarrhoea, navel infection, joint infection, lameness). Pneumonia was defined as calves treated for BRD and diarrhoea was defined as calves treated for diarrhoea.
Animal genotyping
DNA was extracted from blood samples collected in 6 ml K3EDTA tubes (Vacuette; Cruinn Diagnostics, Ireland) using the Maxwell 16 Blood DNA kit (Promega, Madison, WI, USA) as per manufacturer’s instructions. Extracted DNA samples were analysed for quality and quantity using a Nanodrop spectrophotometer and normalised to 50 ng/µL for genotyping analysis. Genotyping was performed at Weatherby’s Scientific Ltd. (Johnstown, Naas, Co. Kildare, Ireland) using the IDBv3 chip which contains 50,855 markers36.
Genotype quality control and population substructure correction
All traits were analysed within three separate analysis groups; combined analysis including all beef and dairy calves, dairy calves only analysis group and beef calves only analysis group. Quality control (QC) was carried out on genotypes within the three separate analysis groups using PLINK v1.90b3.44 64-bit37. SNPs were removed from the analyses if they had a genotype call rate of less than 0.95, a minor allele frequency of less than 0.05 or showed a significant (P value < 1 × e−4) deviation from Hardy–Weinberg equilibrium. In the beef and dairy calf combined analysis, no variants were removed due to poor genotype call rates, 2,084 variants were removed as they were out of Hardy–Weinberg equilibrium and 5,825 variants were removed due to minor allele thresholds < 0.05. Following QC in PLINK, 42,946 autosomal variants and 1,876 calves passed all filters and remained for further analysis. In the beef calf analysis, no variants were removed due to missing genotype data, 413 variants were removed as they were out of Hardy–Weinberg equilibrium and 6,854 variants were removed due to minor allele thresholds < 0.05. Following QC in PLINK, 43,588 autosomal variants and 698 calves passed all filters and remained for further analysis. In the dairy calf analysis, no variants were removed due to missing genotype data, 349 variants were removed as they were out of Hardy–Weinberg equilibrium and 6,291 variants were removed due to minor allele thresholds < 0.05. A further two calves were removed from the analysis as they had a recorded breed inconsistent with that of a dairy-bred animal. Following QC in PLINK, 44,215 autosomal variants and 1,176 calves passed all filters and remained for further analysis. The retained genotypes within each of the three analysis groups (combined beef and dairy calves, beef calves only and dairy calves only), were separated into four principal components based on breed population structure using PLINK v1.90b3.44 64-bit37. The principle components were used for population substructure correction of the phenotypic data.
Correcting the phenotype traits for fixed and random effects
The quantitative phenotype distributions were initially visualized using histogram plots generated in Microsoft Excel in order to identify potential outlier records38. The phenotypic records which were more than three standard deviations away from the mean were excluded as outliers (Supplementary Table S1). Calves which were sold before they reached 6 months of age or for which no recording sheets were received were removed from all disease trait analyses (Supplementary Table S1).
Phenotype data were examined for significant fixed effects of population structure principal components, sex, age at blood sample collection, season of birth and task (herd level or calf level study as described by Todd, et al.6) using either a lmer model with the package lme4 version 1.1-18-139 or a glmer model with the package mlmRev version 1.0-640, in R version 3.5.1, for continuous and binary phenotypes, respectively. Non-significant fixed effects were sequentially removed from each phenotype model and the optimal model was selected for each phenotype by examining AIC values and R squared values (package MuMIn version 1.42.1)41 of the models (Supplementary Table S1). Phenotypes were corrected for significant fixed effects and the random effect of farm by obtaining the residuals of the optimal model for each phenotype and carrying these values forward for the GWAS.
Genome-wide association studies and heritability analyses
Heritability estimates and GWAS analyses for each phenotype were performed using GCTA (version 1.91.6 beta1)42. The GWAS were carried out using the mixed linear model association (–mlma) method:
where yij was the adjusted phenotype of the ith individual, bj was the allele substitution effect of the jth SNP marker, SNPij was the genotype of the ith animal for the jth SNP (coded as 0, 1 and 2), gi was the random polygenic effect of the ith individual, and eij was the random residual effect for the ith individual and jth SNP. The polygenic effects (g) followed a normal distribution g ~ N(0, Gσg2), where G was the genomic relationship matrix (calculated as described by 42), and the residuals followed a normal distribution e ~ N(0, Iσe2).
The GWAS resulted in the generation of association statistics for each trait of interest (total IgG, total protein, albumin, specific gravity, globulin, total solids percentage from a Brix refractometer, ZST units, crude illness, pneumonia, diarrhoea) within each analysis group (combined beef and dairy calves, beef calves only and dairy calves only). SNPs were considered significant at the genome wide threshold if they had a Bonferroni P value less than 0.05 (i.e. P value threshold = 0.05/total no. of variants in analysis), whereas SNPs with raw P value s < 5 × 10−5 were considered to be suggestively significant. Manhattan plots were generated in R (version 3.5.1) using the package qqman version 0.1.443. Genes closest to SNPs of interest were obtained using the package Bedtools (version 2.27.1) closest44.
Results
Mean, standard deviation, minimum and maximum values for the passive immunity traits in the combined beef-suckler and dairy calf population are shown in Table 1. In the combined analysis of beef-suckler and dairy calves, heritability estimates of the passive immunity associated traits and the disease traits were low-to-moderate (range 0.06–0.19) (Table 2). There were no SNPs which reached Bonferroni genome wide significance. However, there was one SNP in the serum IgG analysis, two SNPs in the albumin analysis, three SNPs in the total protein analysis, three SNPs in the globulin analysis, one SNP in the specific gravity analysis, five SNPs in the total solids percentage from a Brix refractometer analysis, four SNPs in the pneumonia analysis, one SNP in the diarrhoea analysis and two SNPs in the crude illness analysis, which were suggestively significant (P < 5 × 10−5) (Table 3).
Table 1.
Means and standard deviations for the passive immunity traits in the Irish commercial beef-suckler and dairy calves.
| Variable | Mean | S.D | Maximum | Minimum |
|---|---|---|---|---|
| Immunoglobulin G (mg/ml) | 13.35 | 5.17 | 29.72 | 1.50 |
| Albumin | 27.08 | 2.56 | 34.80 | 18.30 |
| Total protein (g/l) | 61.76 | 8.10 | 86.20 | 38.40 |
| Globulin (g/l) | 34.58 | 8.65 | 61.20 | 12.40 |
| Zinc sulphate turbidity (units) | 16.32 | 5.80 | 34.10 | 0.30 |
| Specific gravity (g/dl) | 6.13 | 0.87 | 8.70 | 3.20 |
| Total solids Brix (%) | 8.94 | 0.93 | 11.60 | 6.00 |
S.D standard deviation.
Table 2.
Heritability estimates for passive immunity and disease traits in Irish commercial beef-suckler and dairy calves.
| Variable | Combined beef and dairy heritability | Combined beef and dairy S.E | Beef calves heritability | Beef calves S.E | Dairy calves heritability | Dairy calves S.E |
|---|---|---|---|---|---|---|
| Immunoglobulin G | 0.16 | 0.05 | 0.1 | 0.09 | 0.15 | 0.06 |
| Albumin | 0.19 | 0.05 | 0.00 | 0.06 | 0.22 | 0.06 |
| Total protein | 0.12 | 0.04 | 0.05 | 0.07 | 0.13 | 0.06 |
| Globulin | 0.18 | 0.05 | 0.04 | 0.07 | 0.19 | 0.06 |
| Zinc sulphate turbidity | 0.05 | 0.04 | 0.03 | 0.07 | 0.00 | 0.03 |
| Specific gravity | 0.07 | 0.04 | 0.02 | 0.06 | 0.05 | 0.04 |
| Total solids Brix % | 0.06 | 0.04 | 0.00 | 0.05 | 0.05 | 0.05 |
| Pneumonia | 0.1 | 0.05 | 0.00 | 0.05 | 0.09 | 0.07 |
| Diarrhoea | 0.13 | 0.05 | 0.00 | 0.05 | 0.20 | 0.08 |
| Crude illness | 0.13 | 0.05 | 0.03 | 0.06 | 0.19 | 0.08 |
S.E standard error.
Table 3.
Irish commercial beef-suckler and dairy calves GWAS results for passive immunity and disease traits.
| Population | Variable | Associated SNP | Chromosome | RS number | P value | Closest gene | Distance of SNP to gene |
|---|---|---|---|---|---|---|---|
| Combined dairy and beef | Immunoglobulin G | ARS-BFGL-NGS-114208 | 1 | rs110082431 | 3.76E−05* | ENSBTAG00000045984 | 25,324 |
| Albumin | ARS-BFGL-NGS-11531 | 12 | rs109028090 | 7.87E−06 | DHRS12 | 0 | |
| ARS-BFGL-NGS-100170 | 12 | rs109708871 | 2.68E−05 | SLC10A2 | − 462,315 | ||
| Total protein | BTB-01120104 | 4 | rs42277262 | 1.75E−05 | GNAI1 | −36,497 | |
| UA-IFASA-8558 | 24 | rs41646027 | 3.92E−05 | LPIN2 | 0 | ||
| BTB-00174357 | 4 | rs43383611 | 4.22E−05 | KIAA1324L | 22,817 | ||
| Globulin | UA-IFASA-8558 | 24 | rs41646027 | 3.17E−05 | LPIN2 | 0 | |
| BTB-01120104 | 4 | rs42277262 | 3.34E−05 | GNAI1 | − 36,497 | ||
| BOVINEHD2400010261 | 24 | rs109172808 | 4.44E−05 | LPIN2 | 0 | ||
| Specific gravity | BTB-01120104 | 4 | rs42277262 | 3.03E−06 | GNAI1 | − 36,497 | |
| Total solids Brix % | ARS-BFGL-NGS-69831 | 7 | rs42619441 | 2.71E−05 | ENSBTAG00000038284 | 0 | |
| BTB-01120104 | 4 | rs42277262 | 3.89E−05 | GNAI1 | −36,497 | ||
| ARS-BFGL-NGS-15820 | 11 | rs110788172 | 3.93E−05 | CDKL4 | 0 | ||
| UA-IFASA-8558 | 24 | rs41646027 | 4.05E−05 | LPIN2 | 0 | ||
| BOVINEHD2400010261 | 24 | rs109172808 | 4.80E−05 | LPIN2 | 0 | ||
| Pneumonia | ARS-BFGL-NGS-57317 | 25 | rs110476838 | 8.60E−06 | ENSBTAG00000014417 | 334,478 | |
| ARS-BFGL-NGS-50482 | 2 | rs110785912 | 2.73E−05 | CXCR4 | 447,880 | ||
| BOVINEHD2900007001 | 29 | rs42465360 | 3.60E−05 | SLC6A5 | 338,375 | ||
| HAPMAP52014-BTA-90653 | 5 | rs41593661 | 3.75E−05 | ENSBTAG00000046268 | 30,344 | ||
| Diarrhoea | ARS-BFGL-NGS-114897 | 11 | rs110764285 | 4.40E−05 | NFU1 | 1970 | |
| Crude illness | ARS-BFGL-NGS-110312 | 12 | rs110793235 | 3.77E−05 | KL | − 74,444 | |
| HAPMAP40647-BTA-110965 | 4 | rs41575187 | 3.91E−05 | DYNC1I1 | 0 | ||
| Beef calves | Immunoglobulin G | ARS-BFGL-BAC-27914 | 20 | rs110897405 | 2.20E−07 | PARP8 | 0 |
| HAPMAP51687-BTA-114691 | 20 | rs41616927 | 1.57E−05 | ISL1 | 85,644 | ||
| ARS-BFGL-NGS-67929 | 2 | rs110780508 | 3.56E−05 | MREG | 0 | ||
| Total protein | ARS-BFGL-BAC-27914 | 20 | rs110897405 | 2.91E−05 | PARP8 | 0 | |
| Globulin | HAPMAP47694-BTA-67030 | 3 | rs43710738 | 1.60E−05 | PTGFRN | 11,015 | |
| ARS-BFGL-BAC-27914 | 20 | rs110897405 | 2.99E−05 | PARP8 | 0 | ||
| BTB-00212876 | 4 | rs43420430 | 4.07E−05 | DPP6 | 0 | ||
| BTA-03263-RS29011028 | 21 | rs29011028 | 4.29E−05 | PPP2R5C | 20,692 | ||
| Zinc sulphate turbidity | BTA-47238-NO-RS | 1 | rs110704582 | 1.00E−05 | PLCH1 | − 144,011 | |
| BTB-00212876 | 4 | rs43420430 | 2.72E−05 | DPP6 | 0 | ||
| HAPMAP47742-BTA-80071 | 7 | rs41656596 | 4.51E−05 | RASA1 | 0 | ||
| ARS-BFGL-NGS-55396 | 10 | rs110351463 | 4.79E−05 | SYNE2 | 0 | ||
| Specific gravity | ARS-BFGL-NGS-4066 | 26 | rs109923400 | 4.53E−05 | ENSBTAG00000003529 | − 5054 | |
| Crude illness | ARS-BFGL-NGS-114450 | 24 | rs109440690 | 1.82E−05 | ATP8B1 | − 131,562 | |
| BOVINEHD0900029149 | 9 | rs109299906 | 2.62E−05 | QKI | − 73,861 | ||
| ARS-BFGL-NGS-43453 | 8 | rs110620477 | 4.58E−05 | SMARCA2 | 0 | ||
| Dairy calves | Immunoglobulin G | HAPMAP54718-RS29022960 | 9 | rs29022960 | 1.56E−05 | ZNF292 | 0 |
| UA-IFASA-8558 | 24 | rs41646027 | 3.78E−05 | LPIN2 | 0 | ||
| BOVINEHD2400010261 | 24 | rs109172808 | 4.15E−05 | LPIN2 | 0 | ||
| Albumin | ARS-BFGL-NGS-11531 | 12 | rs109028090 | 1.48E−05 | DHRS12 | 0 | |
| ARS-BFGL-NGS-6195 | 18 | rs109046420 | 2.27E−05 | BANP | 0 | ||
| HAPMAP39432-BTA-76145 | 6 | rs41596019 | 2.74E−05 | STIM2 | − 184,275 | ||
| Total protein | UA-IFASA-8558 | 24 | rs41646027 | 7.47E−06 | LPIN2 | 0 | |
| ARS-BFGL-NGS-83128 | 11 | rs110743782 | 8.54E−06 | OTOF | 0 | ||
| BOVINEHD2400010261 | 24 | rs109172808 | 9.62E−06 | LPIN2 | 0 | ||
| ARS-BFGL-NGS-11057 | 11 | rs109425927 | 2.17E−05 | OTOF | 0 | ||
| Globulin | UA-IFASA-8558 | 24 | rs41646027 | 5.68E−06 | LPIN2 | 0 | |
| BOVINEHD2400010261 | 24 | rs109172808 | 6.88E−06 | LPIN2 | 0 | ||
| ARS-BFGL-NGS-83128 | 11 | rs110743782 | 4.08E−05 | OTOF | 0 | ||
| Specific gravity | UA-IFASA-8558 | 24 | rs41646027 | 2.62E−05 | LPIN2 | 0 | |
| BOVINEHD2400010261 | 24 | rs109172808 | 2.81E−05 | LPIN2 | 0 | ||
| Total solids Brix % | ARS-BFGL-NGS-83128 | 11 | rs110743782 | 2.91E−06 | OTOF | 0 | |
| ARS-BFGL-NGS-11057 | 11 | rs109425927 | 7.78E−06 | OTOF | 0 | ||
| BOVINEHD2400010261 | 24 | rs109172808 | 1.65E−05 | LPIN2 | 0 | ||
| UA-IFASA-8558 | 24 | rs41646027 | 1.70E−05 | LPIN2 | 0 | ||
| BTB-02047078 | 6 | rs43152213 | 3.30E−05 | ENSBTAG00000040324 | − 406,779 | ||
| Pneumonia | ARS-BFGL-NGS-48754 | 8 | rs108973453 | 7.67E−06 | CAAP1 | 0 | |
| BOVINEHD0600010238 | 6 | rs135767642 | 1.70E−05 | GPRIN3 | 241,903 | ||
| HAPMAP31810-BTA-155140 | 2 | rs42738873 | 4.46E−05 | ARHGAP15 | 0 | ||
| Diarrhoea | BTA-41494-NO-RS | 1 | rs41641198 | 2.27E−05 | TBL1XR1 | 320,947 | |
| BTB-00647119 | 16 | rs41812941 | 2.81E−05 | PRDM2 | 320,495 |
P value = values are significant at the suggestive P value (P < 5 × 10−5), * = additionally significant at the Bonferroni genome wide significance P value threshold (i.e. Bonferroni P value threshold = 0.05/total no. of variants in analysis).
Sample sizes: Combined beef-suckler and dairy calves (Immunoglobulin G n = 1824, Pneumonia n = 1415 (77 case, 1338 control), Diarrhoea n = 1415 (237 case, 1178 control), Crude illness n = 1415 (357 case, 1058 control), Albumin n = 1838, Total protein n = 1838, Globulin n = 1833, Specific gravity n = 1839, Total solids Brix % n = 1836). Beef-suckler calves (Immunoglobulin G n = 679, Crude illness n = 686 (135 case, 551 control), Total protein n = 685, Globulin n = 681, Zinc sulphate turbidity n = 683, Specific gravity n = 686). Dairy calves (Immunoglobulin G n = 1143, Pneumonia n = 727 (33 case, 694 control), Diarrhoea n = 727 (176 case, 551 control), Albumin n = 1153, Total protein n = 1151, Globulin n = 1150, Specific gravity n = 1151, Total solids Brix % n = 1150).
In the analysis of beef-suckler calves, the heritability estimates of the passive immunity associated traits and the disease traits were low (range 0.02–0.10) (Table 2). There was one SNP which reached Bonferroni genome wide significance (ARS-BFGL-BAC-27914) (Table 3, Fig. 1) for an association with serum IgG concentration. This SNP was located within the intron of the PARP8 gene, on chromosome 20 (Table 3, Supplementary Table S2). There were two SNPs in the serum IgG analysis, one SNP in the total protein analysis, four SNPs in the globulin analysis, four SNPs in the ZST analysis, one SNP in the specific gravity analysis and three SNPs in the crude illness analysis, which approached significance (P < 5 × 10−5) (Table 3).
Figure 1.
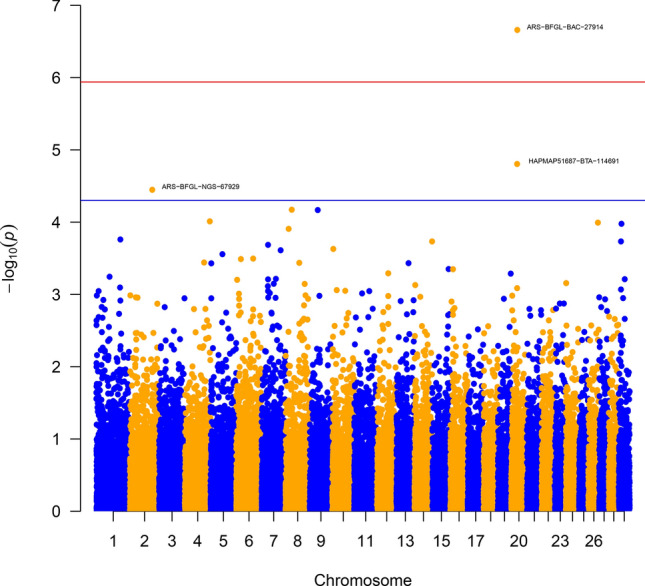
Manhattan plot for immunoglobulin G serum concentration in beef-suckler calves. The blue line indicates the suggestive P value threshold at P < 5 × 10−5. The red line indicates the Bonferroni genome wide significance P value threshold at P < 1.15 × 10−6.
In the analysis of dairy calves, the heritability estimates of the passive immunity associated traits and the disease traits were low to moderate (range 0.05–0.22) (Table 2). The trait with the highest heritability estimate was serum albumin concentration (0.22 ± 0.06) and the trait with the lowest heritability estimate was the serum total solids percentage measured by a Brix refractometer (0.05 ± 0.05) (Table 2). There were no SNPs which reached Bonferroni genome wide significance. However, there were three SNPs associated with serum IgG concentration, three SNPs associated with serum albumin concentration, four SNPs associated with serum total protein content, three SNPs associated with globulin concentration, two SNPs associated with specific gravity, five SNPs associated with total solids percentage from a Brix refractometer, three SNPs associated with incidents of pneumonia and two SNPs associated with diarrhoea occurrence, which were suggestively significant (P < 5 × 10−5) (Table 3).
Discussion
To our knowledge, this is the first study to examine genetic associations with variables measuring the passive immune response and disease traits in beef-suckler and dairy calves in commercial herds. Failure of passive transfer of immunity in neonatal calves leads to greater incidents of disease, longer rearing periods, and increased use of antibiotic and anti-inflammatory treatments45. A meta-analysis and economic study has estimated the total cost of FPT in European beef and dairy production systems to be €80 and €60 per calf, respectively1. Therefore, reducing the prevalence of FPT in calves is warranted to improve animal welfare and augment the economic sustainability of beef and dairy farms. This is the first published study to examine genetic associations with variables measuring the passive immune response and disease traits in beef-suckler and dairy calves in commercial herds.
The GWAS analyses discovered several promising SNPs in all the passive immunity and disease trait analyses, and one SNP which reached genome wide significance in the serum IgG analysis in beef calves. This SNP is located in an intron of the PARP8 gene, on chromosome 20. This gene is responsible for protein–protein interactions, protein-nucleic-acid interactions and the catalysation of the transfer of ADP-ribose from nicotinamide adenine dinucleotide onto target molecules, which consequently modifies the function of the target molecules46,47. It is of particular interest as it is a member of the PARP family and several PARPs are involved in the regulation of the adaptive immune system, inflammation, antiviral processes and activation of immune cells46,47. This gene, PARP8, is implicated in the breed specific development of immune competence in beef calves, as it displayed lower expression in the serum of Charolais-Limousin compared with Limousin-Friesian beef-suckler calves at 48 h post-birth22. Furthermore, increased expression of PARP8 was observed in cultured bovine epithelial and stromal endometrial cells, following exposure to LPS for 6 hours48.
Several SNPs, including ARS-BFGL-BAC-27914, BTB-00212876, BTB-01120104, UA-IFASA-8558, ARS-BFGL-NGS-83128 SNP and ARS-BFGL-NGS-11057, were consistently found as suggestively associated with passive immunity using the several different tests, which increases their reliability as potential predictive markers for passively derived immunity. Interestingly, both the ARS-BFGL-NGS-83128 SNP and the ARS-BFGL-NGS-11057 SNP, which were suggestively associated with numerous indicators of passive immunity in dairy calves (total protein, globulin, total solids percentage from a Brix refractometer and total protein, total solids percentage from a Brix refractometer, respectively), are located within an intron of the OTOF gene, which is linked with a neurosensory non-syndromic recessive hearing loss49.
Other interesting SNPs include the ARS-BFGL-NGS-43453 SNP which reached a suggestive association with crude illness in the beef-suckler calves and is located in an intron of the SMARCA2 gene on chromosome 8. This is noteworthy because SMARCA2 is essential for the transcription of interferon-stimulated genes, which are important in the host response to viruses and intercellular pathogens50. Additionally, the BOVINEHD2400010261 SNP which tended to be associated with several passive immunity traits in both the combined beef and dairy calf population and the dairy calf population, is responsible for a missense mutation in the LPIN2 gene, which is associated with a human autosomal recessive, auto-inflammatory disorder called Majeed syndrome51. Furthermore, the UA-IFASA-8558 SNP which was also suggestively associated with several passive immune traits in both the combined beef and dairy calf populations and the dairy calf population is likewise located in the LPIN2 gene, but within an intron. The ARS-BFGL-NGS-50482 SNP which tended to be associated with pneumonia incidence in the combined beef-suckler and dairy calf population was closest to a gene, CXCR4, which was observed to have lower gene expression at 48 h post-birth in the serum of dairy calves (tube fed 5% of their body weight in colostrum, within one hour of birth) and Limousin-Friesian beef-suckler calves (that suckled their dams naturally, within one hour of birth) compared with 0 h (at birth)22. The BOVINEHD0600010238 SNP which was suggestively associated with pneumonia in the dairy calf population was closest to a gene, GPRIN3, which showed lower gene expression in the serum of dairy calves, Charolais-Limousin and Limousin-Friesian beef-suckler calves, at 48 h post-birth compared with at birth, and which showed higher expression in the dairy and the Limousin-Friesian beef-suckler calves serum at 168 h post-birth compared with 72 h post-birth22. The ARS-BFGL-NGS-11531 SNP which tended to be associated with albumin in both the combined beef and dairy calf and the dairy calf population, was located in an intron of the DHRS12 gene. Interestingly, this gene may be involved in the development of the neonatal calves’ immune system as it has shown reduced expression in the serum of Charolais-Limousin beef-suckler calves at 48 h post-birth compared with at birth and it has breed specific expression levels as it was transcriptionally decreased in the serum of Charolais-Limousin compared with Limousin-Friesian beef-suckler calves at 48 h post-birth22 and displayed decreased expression in Jersey relative to Holstein–Friesian calves eight days following gradual weaning52. The SNP marker ARS-BFGL-NGS-67929, which was associated with IgG concentration in the serum of beef calves at a suggestive P value, was located within the MREG gene on chromosome 2. This gene, MREG, has been demonstrated to have higher expression in the serum of Charolais-Limousin and Limousin-Friesian beef-suckler calves at 48 h post-birth compared with at birth, and to have lower expression in the serum of Limousin-Friesian beef-suckler calves at 168 h post-birth compared with 72 h post birth22. Therefore, it appears to play a role in the acquisition of passively derived immunity in beef-suckler calves, and consequently, ARS-BFGL-NGS-67929 is a promising marker SNP for passive immune status. The HAPMAP54718-RS29022960 SNP which was approaching a significant association with serum IgG concentration in dairy calves, was located within an intron of the gene ZNF292 which was observed to be transcriptionally decreased in the serum of Charolais-Limousin beef-suckler calves at 48 h post-birth compared with at birth22. Additionally, the BTB-00174357 SNP which tended to be associated with total serum protein in the combined beef and dairy population was closest to a gene KIAA1324L which showed reduced expression in the serum of Limousin-Friesian beef-suckler calves at 48 h post-birth compared with at birth22. Therefore, as the ARS-BFGL-BAC-27914, ARS-BFGL-NGS-50482, BOVINEHD0600010238, ARS-BFGL-NGS-11531, ARS-BFGL-NGS-67929, HAPMAP54718-RS29022960 and BTB-00174357 SNPs are either closest to, or within, a gene which has been observed to play a role in the development of immune competence in neonatal calves, these SNPs are promising candidates to confer superior immunity to calves.
A study on Canadian-Holstein cows has discovered 23 SNPs to be associated with serum IgG concentration25. One of these SNPs, BTA-03263-RS29011028, was found in the present study to be associated with serum globulin concentration of beef calves, at a suggestive P value (P < 5 × 10−5). Globulin concentration is as a proxy measure for IgG and can reflect the success of passive transfer in calves. This suggests a shared genetic background of immune-related traits across diverse cattle populations, and makes this variant, following validation, a promising candidate for inclusion as a genetic marker for IgG concentration in cattle. This SNP lies in an intergenic region on chromosome 21, with the closest gene being PPP2R5C, which is 20,692 nucleotides downstream of this variant. Apart from BTA-03263-RS29011028, there were no other SNPs significantly associated with IgG concentration in the Canadian study, which were also identified as associated with passive transfer or disease traits in the present study. The primary reason for the inconsistenices in the results between the two studies is likely due to the Canadian study examining the IgG natural antibodies in cows, whereas the present study focussed on passively derived IgG in calves. Other explanations include the effects of the different breeds used (commercial crossbred Irish beef and dairy breeds versus Canadian Holstein), the diverse locations and environmental conditions experienced by the animals, and the different SNP platforms used (IDBv3 SNP chip versus Illumina Bovine SNP50 BeadChip). Additionally, the different tests employed to determine IgG concentrations or passive immunity in serum can vary substantially in their accuracy, sensitivity and/or specificity (precision)6,13. Alternatively, it is plausible that some of SNPs in either the present study or the study on Canadian-Holstein cows25, are not truly related to passive immunity measurements and are simply correlated by chance.
A limitation to discovering reliable markers of disease resistance is the availability of accurate phenotype data53. Most health-related GWAS studies, including the disease traits in the present study, are heavily reliant on accurate disease reporting by producers; however, producers can often misdiagnose or fail to observe a disease case which leads to inaccurate phenotypic data. Schneider et al.54 reported that 60.6% of slaughtered feedlot cattle which never received treatment for BRD had lung lesions present and a study by Wittum et al.55 observed that 68% of slaughtered feedlot steers with no recorded history of BRD presented with lung lesions. Furthermore, health-related phenotypes are generally profoundly influenced by environmental and farm management factors53. In an attempt to control for these environmental and husbandry factors, only farms that had a minimum of 10 calves genotyped were utilised in this study and farm was included as a random factor in the phenotype models.
As immune responses and disease susceptibilities are complex traits which are lowly heritable, and possibly breed and pathogen specific56, they are likely governed by multiple genes. This means that large sample populations may be needed to discover reliable genetic markers, which if selected for, could possibly improve immunity and disease resistance. The relatively small sample size in this study is the probable reason for the lack of identification of a large quantity of SNPs which were significantly associated with passive immune status and disease traits. Additionally, the multitude of different breeds included in this study, particularly within the beef calf population, may have limited the ability to detect genetic associations with passively derived immunity and disease traits, despite the adjustment for breed structure which was performed in the phenotype models. This may account for the heritability estimates for the passive immune traits being substantially lower (range: 0.02–0.22) in this study relative to the heritability estimates for natural antibodies in the Holstein cows in the Canadian study (range: 0.27–0.31)25. This may also explain why many of the SNPs that were suggestively associated with either passive immunity or disease traits were not consistently found within the three populations; beef calves, dairy calves and the combined beef and dairy calf population. Furthermore, it is important to note that SNP-phenotype correlations do not guarantee causality, and consequently it is possible that some of the SNPs which are suggestively significant may be so by chance and may not be truly associated with the passive immunity or disease traits. Equally, SNPs which did not reach the suggestive P value association with the passive immune of disease traits may in fact be associated with those traits, if tested in a larger population.
In conclusion, several suggestive and significant SNP markers associated with passive immunity and disease resistance in Irish commercial beef-suckler and dairy calves, were discovered in this study. These SNPs could be tested in larger alternative beef and dairy populations and following validation, may contribute to Ireland’s national genomic selection breeding programme to select cattle with a greater resistance to disease.
Supplementary information
Acknowledgements
This research was supported under the Department of Agriculture, Food and the Marine Research Stimulus Fund (11/S/131) with Dr. B. Earley as the Principal Investigator. Research partners included University College Dublin, Irish Cattle Breeding Federation, and Animal Health Ireland (Technical Working Group on Calf Health, CalfCare). The authors acknowledge: the participating farmers and their Teagasc KT advisors for their contribution to the research; the technical (Butler, O.), administrative staff (Doggert, D., Gilsenan, A., Mulligan, J., Murphy, M., Weldon, M.) and under-graduate students (Aili, M., Browne, E., Calvin, E., Ceriani, F., Conway, A., Doyle, P., Drought, Y., Hoch, A., Kane, S., Kervick, M., Lefevre, H., LeRet, S., Loayan, Mas, V., Mulligan, J., Palmer, R., Scacchi, A., van Giersbergen, M.) with data collection at Teagasc, Grange.
Author contributions
B.E. conceived the study. B.E., S.W. and M.M.G. designed the study. C.G.T. collected the blood samples, phenotype data, and performed the passive immunity tests. C.S. isolated and prepared the DNA for genotyping. J.C.M.C., M.C.M.C., C.G.T. and C.S. collated the genotype and phenotype data. D.J. and R.M. performed the genotype quality control and population substructure correction, corrected the phenotype traits for fixed and random effects and performed the GWAS and heritability analyses. D.J. wrote the paper. All authors critically reviewed the intellectual content of the manuscript, contributed to the main article text, and gave their approval for the final version to be published.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75870-4.
References
- 1.Raboisson D, Trillat P, Cahuzac C. Failure of passive immune transfer in calves: a meta-analysis on the consequences and assessment of the economic impact. PLoS ONE. 2016;11:e0150452. doi: 10.1371/journal.pone.0150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Compton CWR, et al. Invited review: a systematic literature review and meta-analysis of mortality and culling in dairy cattle. J. Dairy Sci. 2017;100:1–16. doi: 10.3168/jds.2016-11302. [DOI] [PubMed] [Google Scholar]
- 3.Ring SC, McCarthy J, Kelleher MM, Doherty ML, Berry DP. Risk factors associated with animal mortality in pasture-based, seasonal-calving dairy and beef herds. J. Anim. Sci. 2018;96:35–55. doi: 10.1093/jas/skx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos, R. et al. Mortality and morbidity of beef calves in free-range farms in Alentejo, Portugal—a preliminary study. Veterinary Medicine International, 3616284 (2019). [DOI] [PMC free article] [PubMed]
- 5.DAFM. AIM Bovine Statistics Report, 2018, https://www.agriculture.gov.ie/media/migration/animalhealthwelfare/animalidentificationandmovement/AIMBovineStatisticsReport2018100519.pdf. Accessed March 16th, 2020.
- 6.Todd CG, et al. An observational study on passive immunity in Irish suckler beef and dairy calves: tests for failure of passive transfer of immunity and associations with health and performance. Prev. Vet. Med. 2018;159:182–195. doi: 10.1016/j.prevetmed.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen MW, et al. Mortality and health treatment rates of dairy calves in automated milk feeding systems in the Upper Midwest of the United States. J. Dairy Sci. 2017;100:9186–9193. doi: 10.3168/jds.2017-13198. [DOI] [PubMed] [Google Scholar]
- 8.Conneely M, et al. Effects of milk feeding volume and frequency on body weight and health of dairy heifer calves. Livest. Sci. 2014;161:90–94. doi: 10.1016/j.livsci.2013.12.022. [DOI] [Google Scholar]
- 9.Johnston D, et al. Electronic feeding behavioural data as indicators of health status in dairy calves. Irish J. Agric. Food Res. 2016;55:159–168. doi: 10.1515/ijafr-2016-0016. [DOI] [Google Scholar]
- 10.Dubrovsky SA, et al. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019;102:7306–7319. doi: 10.3168/jds.2018-14774. [DOI] [PubMed] [Google Scholar]
- 11.Godden S. Colostrum management for dairy calves. Vet. Clin. N. Am. Food Anim. Pract. 2008;24:19–39. doi: 10.1016/j.cvfa.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furman-Fratczak K, Rzasa A, Stefaniak T. The influence of colostral immunoglobulin concentration in heifer calves’ serum on their health and growth. J. Dairy Sci. 2011;94:5536–5543. doi: 10.3168/jds.2010-3253. [DOI] [PubMed] [Google Scholar]
- 13.McGee M, Earley B. Review: passive immunity in beef-suckler calves. Animal. 2019;13:810–825. doi: 10.1017/S1751731118003026. [DOI] [PubMed] [Google Scholar]
- 14.Cervenak J, Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet. Immunol. Immunopathol. 2009;128:171–177. doi: 10.1016/j.vetimm.2008.10.300. [DOI] [PubMed] [Google Scholar]
- 15.Cortese V. Neonatal immunology. Vet. Clin. N. Am. Food Anim. Pract. 2009;25:221–227. doi: 10.1016/j.cvfa.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon R, Harte F. A survey of factors affecting calf blood serum immunoglobulin level. Irish J. Agric. Res. 1987;26:1–7. [Google Scholar]
- 17.Lorenz I, Mee J, Earley B, More S. Calf health from birth to weaning. I. General aspects of disease prevention. Irish Vet. J. 2011;64:10. doi: 10.1186/2046-0481-64-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earley B, et al. Serum immunoglobulin concentrations in suckled calves and dairy-herd calves. Irish J. Agric. Food Res. 2000;39:401–407. [Google Scholar]
- 19.Ballou MA. Immune responses of Holstein and Jersey calves during the preweaning and immediate postweaned periods when fed varying planes of milk replacer. J. Dairy Sci. 2012;95:7319–7330. doi: 10.3168/jds.2012-5970. [DOI] [PubMed] [Google Scholar]
- 20.Villarroel A, Miller TB, Johnson ED, Noyes KR, Ward JK. Factors affecting serum total protein and immunoglobulin G concentration in replacement dairy calves. Adv. Dairy Res. 2013;1:106. doi: 10.4172/2329-888x.1000106. [DOI] [Google Scholar]
- 21.Johnston D, et al. Characterisation of haematological profiles and whole blood relative gene expression levels in Holstein-Friesian and Jersey bull calves undergoing gradual weaning. Animal. 2016;10:1547–1556. doi: 10.1017/S1751731115002438. [DOI] [PubMed] [Google Scholar]
- 22.Surlis C, et al. Blood immune transcriptome analysis of artificially fed dairy calves and naturally suckled beef calves from birth to 7 days of age. Sci. Rep. 2018;8:15461. doi: 10.1038/s41598-018-33627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neibergs HL, et al. Susceptibility loci revealed for bovine respiratory disease complex in pre-weaned holstein calves. BMC Genom. 2014 doi: 10.1186/1471-2164-15-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quick AE, Ollivett TL, Kirkpatrick BW, Weigel KA. Genomic analysis of bovine respiratory disease and lung consolidation in preweaned Holstein calves using clinical scoring and lung ultrasound. J. Dairy Sci. 2020;103:1632–1641. doi: 10.3168/jds.2019-16531. [DOI] [PubMed] [Google Scholar]
- 25.de Klerk B, et al. A genome-wide association study for natural antibodies measured in blood of Canadian Holstein cows. BMC Genom. 2018;19:694–694. doi: 10.1186/s12864-018-5062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Ruiz A, et al. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl. Acad. Sci. USA. 2016;113:E3995–E4004. doi: 10.1073/pnas.1519061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouquet A, Juga J. Integrating genomic selection into dairy cattle breeding programmes: a review. Animal. 2013;7:705–713. doi: 10.1017/s1751731112002248. [DOI] [PubMed] [Google Scholar]
- 28.Berry DP. Genetics of bovine respiratory disease in cattle: can breeding programs reduce the problem? Anim. Health Res. Rev. 2014;15:151–156. doi: 10.1017/s1466252314000292. [DOI] [PubMed] [Google Scholar]
- 29.Raszek MM, Guan LL, Plastow GS. Use of genomic tools to improve cattle health in the context of infectious diseases. Front. Genet. 2016;7:30–30. doi: 10.3389/fgene.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry, D. P. et al. The economic breeding index: A generation on. Technical report to the Irish Cattle Breeding Federation (2007). https://www.icbf.com/wp/wp-content/uploads/2013/06/The_Economic_breeding_a_generation_on_Dec_2007.pdf. Accessed April 04th, 2020.
- 31.ICBF. https://www.icbf.com/wp/?p=9778. Accessed April 27th, 2020.
- 32.Murphy JM, Hagey JV, Chigerwe M. Comparison of serum immunoglobulin G half-life in dairy calves fed colostrum, colostrum replacer or administered with intravenous bovine plasma. Vet. Immunol. Immunopathol. 2014;158:233–237. doi: 10.1016/j.vetimm.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn A, et al. Comparison of single radial immunodiffusion and ELISA for the quantification of immunoglobulin G in bovine colostrum, milk and calf sera. J. Appl. Anim. Res. 2018;46:758–765. doi: 10.1080/09712119.2017.1394860. [DOI] [Google Scholar]
- 34.Earley B, McDonnell B, O'Riordan EG. Effect of floor type on the performance, physiological and behavioural responses of finishing beef steers. Acta Vet. Scand. 2015;57:73–73. doi: 10.1186/s13028-015-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwan AD, Fisher EW, Selman IE, Penhale WJ. A turbidity test for the estimation of immune globulin levels in neonatal calf serum. Clin. Chim. Acta. 1970;27:155–163. doi: 10.1016/0009-8981(70)90390-6. [DOI] [PubMed] [Google Scholar]
- 36.Mullen, M. P. et al. Development of a custom SNP chip for dairy and beef cattle breeding, parentage and research. Interbull Bulletin (2013).
- 37.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remenyi D, Dan Remenyi GOJE, Onofrei G, English J. An Introduction to Statistics Using Microsoft Excel. London: Academic Publishing Limited; 2009. [Google Scholar]
- 39.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 40.Bates, D., Maechler, M. & Bolker, B. mlmRev: Examples from Multilevel Modelling Software Review. https://CRAN.R-project.org/package=mlmRev (2014).
- 41.Bartoń, K. MuMIn: Multi-ModelInference. https://CRAN.R-project.org/package=MuMIn (2018).
- 42.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner SD. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. BioRxiv. 2014 doi: 10.1101/005165. [DOI] [Google Scholar]
- 44.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stilwell G, Carvalho RC. Clinical outcome of calves with failure of passive transfer as diagnosed by a commercially available IgG quick test kit. Can. Vet. J. 2011;52:524–526. [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen MS, Chang P. Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nat. Chem. Biol. 2018;14:236–243. doi: 10.1038/nchembio.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai P. Biology of poly(ADP-Ribose) polymerases: the factotums of cell maintenance. Mol. Cell. 2015;58:947–958. doi: 10.1016/j.molcel.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 48.Oguejiofor CF, Cheng Z, Abudureyimu A, Fouladi-Nashta AA, Wathes DC. Global transcriptomic profiling of bovine endometrial immune response in vitro. I. Effect of lipopolysaccharide on innate immunity. Biol. Reprod. 2015;93:100. doi: 10.1095/biolreprod.115.128868. [DOI] [PubMed] [Google Scholar]
- 49.Iwasa Y-I, et al. OTOF mutation analysis with massively parallel DNA sequencing in 2,265 Japanese sensorineural hearing loss patients. PLoS ONE. 2019;14:e0215932. doi: 10.1371/journal.pone.0215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dudek AH, Pfaff F, Bolte H, Waguia Kontchou C, Schwemmle M. Partial inactivation of the chromatin remodelers smarca2 and smarca4 in virus-infected cells by caspase-mediated cleavage. J. Virol. 2018;92:e00343–00318. doi: 10.1128/jvi.00343-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferguson PJ, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J. Med. Genet. 2005;42:551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston D, et al. Characterisation of the whole blood mRNA transcriptome in Holstein-Friesian and Jersey Calves in response to gradual weaning. PLoS ONE. 2016;11:e0159707. doi: 10.1371/journal.pone.0159707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berry DP, Bermingham ML, Good M, More SJ. Genetics of animal health and disease in cattle. Irish Vet. J. 2011;64:5–5. doi: 10.1186/2046-0481-64-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider M, Tait R, Busby W, Reecy J. An evaluation of bovine respiratory disease complex in feedlot cattle: Impact on performance and carcass traits using treatment records and lung lesion scores. J. Anim. Sci. 2009;87:1821–1827. doi: 10.2527/jas.2008-1283. [DOI] [PubMed] [Google Scholar]
- 55.Wittum TE, Woollen NE, Perino LJ, Littledike ET. Relationships among treatment for respiratory tract disease, pulmonary lesions evident at slaughter, and rate of weight gain in feedlot cattle. J. Am. Vet. Med. Assoc. 1996;209:814–818. [PubMed] [Google Scholar]
- 56.Tizioto PC, et al. Immunological response to single pathogen challenge with agents of the bovine respiratory disease complex: an RNA-sequence analysis of the bronchial lymph node transcriptome. PLoS ONE. 2015;10:e0131459. doi: 10.1371/journal.pone.0131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


