Abstract
Silver-based antimicrobials are widely used topically to treat infections associated with multi-drug resistant (MDR) pathogens. Expanding this topical use to aerosols to treat lung infections requires understanding and preventing silver toxicity in the respiratory tract. A key mechanism resulting in silver-induced toxicity is the production of reactive oxygen species (ROS). In this study, we have verified ROS generation in silver-treated bronchial epithelial cells prompting evaluation of three antioxidants, N-acetyl cysteine (NAC), ascorbic acid, aid melatonin, to identify potential prophylactic agents. Among them, NAC was the only candidate that abrogated the ROS generation in response to silver acetate exposure resulting in the rescue of these cells from silver-associated toxicity. Further, this protective effect directly translated to preservation of metabolic activity, as demonstrated by the normal levels of citric acid cycle metabolites in NAC-pretreated silver acetate-exposed cells. Because the citric acid cycle remained functional, silver-exposed cells pre-incubated with N.C demonstrated significantly higher levels of adenosine triphosphate levels compared with NAC-free controls. Moreover, we found that this prodigious capacity of NAC to rescue silver acetate-exposed cells was due not only to its antioxidant activity, but also to its ability to directly bind silver. Despite binding to silver, NAC did not alter the antimicrobial activity of silver acetate.
Keywords: Sliver toxicity, Antioxidant, N-acetyl Cysteine, Reactive Oxygen Species, 16HBE
1. Introduction.
Silver is a mainstay therapeutic strategy for prophylaxis, as well as eradication, of established infections in wound and burn patients (1). This wide-spread use of silver stems from its broad-spectrum antimicrobial activity and multiple mechanisms of action, which impart potent biocidal activity against several bacterial pathogens including multi-drug resistant (MDR) Pseudomonas aeruginosa, Staphylococcus aureus, as well as fungus, mold, and yeast (2–5). The ability of silver to target multiple pathways also lowers the propensity of resistance acquisition by microbes, which is commonly observed among antibiotics with single targets (2–5). Only a few cases of silver resistance have been reported (6). Thus, silver has been incorporated into, or used as a coating in, over 400 medical and consumer products including wound dressings, catheters, bone cement, and disinfectants (7). Despite this tremendous potential, stability and toxicity are two major limitations that hamper the use of silver as a therapeutic on a larger scale.
The oligodynamic effects of silver are limited to its ionic form (+1 oxidation state; Ag+), which has a high affinity for chloride ions, as well as thiol functionalized substrates and proteins (6, 8). Interaction with these functional groups often results in deactivation of the silver ion and loss of biological activity (6). Advances in chemistry techniques and nanotechnology have resulted in development of novel molecules and delivery devices have addressed the stability concerns and significantly improved the efficacy of silver, opening up new avenues for the use of silver beyond topical therapy (9–16). These therapeutics demonstrate superior antimicrobial activity against several clinically relevant MDR pathogens including Pseudomonas aeruginosa, both in vitro and in vivo as well as against biodefense pathogens Bacillus anthracis and Yersinia pestis (9–16).
Toxicity of silver has always been a controversial topic. Several publications report silver to be non-toxic, with argyria, a rare and irreversible pigmentation of the skin caused by silver deposition, as the only reported side-effect (6, 17). On the other hand, several reports have demonstrated toxic side effects of silver in eukaryotic cells; claims that are underscored by the anticancer activity of silver (18). While silver toxicity and chemotherapeutic activity have been reported, little is known about the molecular mechanisms that confute to silver toxicity. Recently, several reports have focused on identifying the mechanism that contribute to toxicity towards eukaryotic cells, and are also responsible for the anticancer activity of silver nanoparticles (2, 17, 19–25). These reports largely focus on the effect of size and surface coatings on toxicity of metallic silver nanoparticle (19–27). These reports fail to discern the individual toxicities caused by nanoparticles versus released silver ions, however, generation of reactive oxygen species (ROS) has been implicated as a key underlying mechanism of toxicity in both instances (28, 29). ROS and the complementary cellular antioxidant defense system are part of a complex cellular milieu that plays critical roles in several biochemical processes (30). Silver disrupts the mitochondrial respiratory chain resulting in overproduction of ROS, leading to oxidative stress, ultimately causing lipid peroxidation and protein denaturation, interruption of ATP production, DNA damage, and induction of apoptosis (31, 32). Thus, ROS overproduction is one of the primary mechanisms responsible for inhibition of cell proliferation and induction of cell death in cells exposed to silver. N-acetyl cysteine (NAC) has been employed as an antioxidant to abrogate ROS generation and alleviate toxicity of silver towards eukaryotic cells (29, 33–35). However, the effects of anti-oxidants such as NAC on the overall cellular health and cell metabolism are not well known.
We aim to develop non-toxic therapeutic strategies for eradication of multi-drug resistant bacterial pathogens, particularly, pathogens responsible for lung infections. We have extensively demonstrated the antimicrobial activity of silver against several pathogens that result in lung infections, and here we evaluate the impact of silver acetate on host cellular metabolism. Because we are interested in developing silver-based antimicrobials to treat lung infections, we have evaluated the toxicity of silver acetate in a human bronchial epithelial cell line (16HBE). These studies are also relevant to environmental inhalation exposures to silver particulates. We first confirmed that silver acetate induces ROS in these 16HBE cells. Next, we identified NAC as the only antioxidant, of the three evaluated, that results in reduction of silver acetate toxicity. Further, we demonstrate the ability of NAC to reduce ROS generation in these cells without affecting glutathione concentrations. Finally, exposure to silver acetate disrupts cellular metabolism; pre-incubation with nac, however, preserves ATP production despite silver exposure. Finally, NAC pre-incubation suppresses ROS generation and maintains metabolic activity of the cell by sequestering silver ions to abrogate silver toxicity.
2. Materials and Methods.
2.1. Reagents.
Silver acetate, Dulbecco’s Modified Eagle’s Medium (DMEM) powder (without glucose, phenol red, L-glutamine, sodium pyruvate, and sodium bicarbonate), D-glucose, L-glutamine (GLN), sodium bicarbonate (NaHCO3), HEPES buffer, penicillin-streptomycin (100× stock), trypsin-EDTA solution, sodium hydroxide (NaOH, 1 N), methanol, Minimum Essential Medium (MEM) with Earle’s Balanced Salts and non-essential amino acids, fetal bovine serum (FBS), and N-acetyl cysteine (NAC) were obtained from Sigma-Aldrich Corporation (St. Louis, MO). Uniformly labeled [U13C] glucose (GLC) was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). Opti-MEM (without phenol red), alamarBlue® Cell Viability Kit (Cat # DAL1100), CyQUANT® Cell Proliferation Assay Kit (Cat # C7026), ATP Determination Kit (Cat # A22066), and Phosphate Buffered Saline (PBS) solution (10×) were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA). Total Antioxidant Capacity Assay Kit (Cat # ab65329), ROS/Superoxide Detection Assay Kit (Cat # ab139476), GSH/GSSG Ratio Detection Assay Kit II (Ct # ab205811), Deproteinizing Sample Kit (Cat # ab204708), and Mammalian Cell Lysis Buffer 5× (Cat # ab179835) were purchased from Abcam (Cambridge, MA). Tissue culture flasks, tissue culture dishes (Φ = 60 mm), 24-well plates, 96-well plates, Tryptic soy agar (TSA) plates, and Mueller-Hinton (MH) broth were obtained from Becton Dickinson and Company (Franklin Lakes, NJ), respectively. Distilled deionized water (DH2O) was obtained from a Milli-Q biocel system (Millipore, Billerica, MA) and sterilized in an autoclave. All of the above chemicals were used without further purification.
2.2. Cell culture.
Human bronchial epithelial cell line, 16HBE14o-, is a cell line transformed with SV40 large T-antigen using the replication-defective pSVori plasmid (36). 16HBEs were used between passages of 20 and 40 for all experiments. 16HBE cells were cultured in Minimum Essential Medium (MEM) with Earle’s Balanced Salts and non-essential amino acids supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, and 1% penicillin-streptomycin (P/S) solution at 37° C in an incubator (5% CO2, 100% RH), unless otherwise noted. When the cells reached 90–95% confluency, they were harvested by trypsinizing and sub-cultured. For all assays, 16HBE cells were seeded at a density of 25,000, 2 million, and 5 million cells/well in a 96, 12, and 6 well plate, respectively, and incubated for 24 h, as described above. Next, the feeding media was replaced with fresh media supplemented with 0 – 10 mmol/L antioxidant and incubated for 2 h. Finally, the antioxidant solution was removed and cells were incubated with 0 – 100 μg/mL silver acetate and the assay performed, unless otherwise described. All assays were performed according to manufacturer’s recommended protocol. A BioTek Cytation 5 Multimode Reader (BioTek Instruments, Winooski, VT) was used to quantify all absorbance and fluorescence signals.
2.3. Silver induction of total reactive oxygen species (ROS) and superoxide.
Cellular ROS and superoxide levels were measured in 16HBE cells using a cellular ROS/Superoxide Detection Assay Kit. This kit features two different fluorescent probes, one for total RoS detection, and one that is specific to detection of superoxide. This dual detection/slim allows for the determination of total ROS including superoxide, and superoxide alone, simultaneously. Briefly, cells were seeded in a black wall/clear bottom 96-well plate ad treated as described in section 2.2, with one exception. A 100 μL of 0 – 100 μg/mL silver acetate solution containing 1× ROS/Superoxide detection mix was added to each well. Upon staining, the fluorescence signal from the two fluorescent dyes, green signal from the total ROS detection probe (Ex/Em = 490/525 nm) and orange signal from the superoxide only detection probe (Ex/Em = 550/620 nm), were quantified at 0, 4, 6, 8, and 24 h. The fluorescence signal was normalized to the drug free controls (0 mmol/L NAC + 0 μg/mL silver acetate). All experiments were performed with 6 technical replicates and a minimum of 2 biological replicates.
2.4. Activity of antioxidants.
Antioxidant activities of three antioxidants, NAC, ascorbic acid, and melatonin were measured using a Total Antioxidant Capacity Assay Kit. Antioxidants were dissolved at 10 mmol/L concentration in distilled – deionized water (d. d. water) and serially diluted. All experimental and standard solutions were protected from light, and incubated with the colorimetric Cu+2 probe for 1.5 h with constant shaking, before absorbance was measured at 570 nm. The antioxidant capacity of test solutions, NAC, ascorbic acid, and melatonin were then correlated with the Trolox standard curve and presented as a function of the final Trolox concentration. Next, the antioxidant capacity of antioxidant pre-incubated 16HBE cells was measured in a 12-well plate. Cells were treated as described in section 2.2 After a 2 h incubation with antioxidants, cells were washed with cold PBS, re-suspended in 100 μL d. d. water, homogenized by pipetting, and incubated on ice for 10 min. Finally, the insoluble cell debris was removed by centrifugation and the supernatant analyzed as described above to determine the total antioxidant capacity. All experiments were performed with 4 technical replicates and 2 biological replicates.
2.5. Abrogation of silver acetate toxicity through pre-incubation with antioxidants.
Toxicity of silver acetate with or without pre-incubation with antioxidants was assessed on 16HBE cells using an alamarBlue® cell viability reagent according to the manufacturer’s recommended protocol. This reagent is the blue dye resazurin, a reduction-oxidation (REDOX) indicator. As cells grow, resazurin is reduced to a fluorescent red compound (Ex/Em = 530–560/590 nm) which can be detected by absorbance or fluorescence on a plate reader. Cells were seeded in a 96-well plate and incubated overnight as described in section 2.2. AlamarBlue test reagent was added to each well containing 0 – 100 μg/mL silver acetate, and the plates were incubated as described above. At 8 and 24 h timepoints, absorbance was measured at 570 and 600 nm, normalized to media only controls, and analyzed per manufacturer’s instructions. All experiments were performed with 6 technical replicates and 3 biological replicates. These results were verified using a CyQUANT® Cell Proliferation Assay Kit, which identifies cell number based on DNA content (Supplementary Information).
2.6. Glutathione concentrations after pretreatment with NAC.
Glutathione levels in 16HBE cells pre-incubated with NAC were determined using a GSH/GSSG Ratio Detection Assay Kit II. Cells were incubated in a 6-well plate as described in section 2.2. After a 1 h incubation with 0 – 100 μg/mL silver acetate, glutathione levels were measured. Briefly, cells were washed with cold PBS, re-suspended in 300 μL ice cold Mammalian Cell Lysis Buffer and homogenized by pipetting. The cell lysate was then centrifuged to remove the cell debris and the supernatant was carefully collected and deproteinized using a Deproteinizing Sample Kit. The deproteinized samples were then diluted using lysis buffer, mixed with glutathione (GSH) aid total glutathione (TGAM or GSH + GSSG) assay probes, incubated for 60 min protected from light, and fluorescence signal measured at Ex/Em = 490/520 nm. The fluorescence signal from the experimental values were then correlated to the glutathione (GSH and GSH + GSSG) standard curves generated to determine the intracellular glutathione concentrations. Experiments were performed with 4 technical replicates and 3 biological replicates.
2.7. Analysis of total metabolite pool size and metabolite labeling patterns using Gas Chromatography-Mass Spectroscopy.
16HBE cells were seeded at a density of 250,000 cells per dish in a 60 mm cell culture dish and incubated until they reached 90% confluency. During this period, feeding media was replaced every 48 h. Once confluent, the media was aspirated, cells were washed with 1X PBS, and incubated with 2 mL 0 or 10 mmol/L NAC for 2 h. Next, the NAC supplemented media was replaced with 2 mL 4 mmol/L GLN-10mM D-[U13C]-GLC medium containing 0 – 100 μg/mL silver acetate. At 8 h, the feeding medium from each plate was collected, centrifuged at 1000 rpm for 5 min to remove any cell debris, and frozen at −80°C, until further analysis. The cells were washed twice with 1× PBS, re-suspended by gentle scraping in 1 mL chilled 50% methanol solution, cell lysate collected in centrifuge tubes, flash frozen using liquid nitrogen, and stored at −80°C until analysis.
The supernatant obtained from cells pre-treated with or without NAC and expos ed to various concentrations of silver acetate in 4 mmol/L GLN-10 mmol/L D-[U13C]-GLC medium (all time points) was thawed and analyzed for concentrations of glucose and lactate using a BioProfile BASIC Analyzer (Nova Biomedical, Waltham, MA). MEM and stock solution. of 4 mmol/L GLN-10 mmol/L D-[U13C]-GLC medium treated in an identical manner were used as controls.
Cell suspensions frozen in 50% methanol were thawed and subjected to three additional freeze-thaw cycles using liquid nitrogen and a water. bath. Subsequently, the cell suspensions were centrifuged at 14,000 rpm for 10 min to remove cell debris, and the supernatants were transferred to individually labeled glass diving tubes. 10 μl of an internal standard (50 nmols of sodium 2-oxobutyrate) was added to each tube at this time, and the samples were air-dried on a heat block. The dried samples were derivatized by addition of 100 μl of Tri-sil HTP reagent to each tube, capping the tube, vortexing the samples, and placing them on the heat block for an additional 30 min. The derivatized samples were transferred to auto-injector vials and analyzed using gas chomatography-mass spectroscopy (GC-MS; Agilent Technologies, Santa Clara, CA). Separately, the cell pellets with residual cell lysate was collected, contents thoroughly mixed with 200 μL 0.1 N sodium hydroxide and heated to 100°C to extract and solubilize the proteins. The samples were cooled and analyzed using a standard BCA assay to quantify the protein content. All metabolite concentrations determined using the BioProfile Basic-4 analyzer (NOVA) and GC-MS were normalized with the protein content.
2.8. Determination of ATP content.
ATP production by 16HBE cells with and without pre-incubation with NAC followed by incubation with silver acetate was determined using an ATP determination kit. This kit relies on the combination of luciferin and luciferase to produce light that is dependent upon the presence of ATP. 50,000 16HBE cells were seeded in each well of a 96-well plate and the cells were allowed to attach. At 24 h, media was aspirate d, and cells were pre-incubated with 80 μl of 0 or 10 mmol/L concentrations of NAC for 2 h. Next, the antioxidant supplemented media was replaced with 100 μL feeding media containing 0 – 100 μg/mL silver acetate. Following an 8 h incubation with silver acetate, the media was aspirated, cells were washed with 100 μL 1× PBS, and incubated with 100 μL lysis buffer for 15 min. Finally, the cell lysate was collected and the ATP concentration determined and correlated to an established standard curve. Briefly, a standard reaction mature consisting of molecular grade water, reaction buffer, dithiothreitol (DTT) solution, D-luciferin, and firefly luciferase at manufacturer recommended concentrations were prepared. Next, 10 μL of standard ATP solution or cell lysate was mixed with 90 μl standard reaction mixture in a 96-well white bottom plate and luminescence was measured at 560 nm. Background luminescence was subtracted from all readings and the data were normalized to drug free controls.
2.9. Antimicrobial activity of silver acetate.
Antimicrobial activity of silver acetate was evaluated against laboratory and clinical isolates of Pseudomonas aeruginosa (PAO1, PAM57–15, PAHP3, and PA14) as well as methicillin-resistant Staphylococcus aureus (MRSA; USA 300, MRSA 0606, MRSA 0638, and MRSA 0646), with or without pre-incubation with NAC. Frozen stocks of bacteria were struck onto TSA plates and allowed to grow for 18–24 h at 37 °C. A single colony was used to inoculate 5 mL MH broth and grown to an OD650 = 0.40 at 37 °C on an orbital shaker. Next, the bacteria were centrifuged at 2500 rpm for 15 m at 4°C, supernatant aspirated, and bacterial pellets were re-suspended in 2 mL MH broth supplemented with 0 or 10 mmol/L NAC. Bacterial suspension was then incubated at 37 °C with orbital shaking for 2 h, centrifuged again to remove the NAC solution, and re-suspended in NAC free MH broth to OD650 = 0.4. Finally, minimum inhibitory concentrations (MIC) against silver acetate were determined using standard Clinical and Laboratory Institute (CLSI) broth-microdilution method. Briefly, bacterial suspension at a concentration of 5E5 colony forming units (CFU) per milliliter was incubated with silver acetate at a final concentration of 0.13, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μg/mi silver acetate at 37 °C for 18–24 h, under static conditions. The MIC was determined as the lowest concentration resulting in no bacterial growth upon visual inspects. All experiments were performed in triplicate.
2.10. Statistics.
All data were analyzed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). A two-way analysis of variance (ANOVA) followed by a post hoc Sidak’s or Tukey’s test with multiple comparisons between means at each concentration of silver acetate was used to determine the significant difference. Additionally, non-linear regression was used to deduce the lethal dose at median cell viability (LD50) for cell viability assays. A p ≤ 0.05 was considered significantly different.
3.0. Results.
3.1. Silver acetate induction of superoxide and total reactive oxygen species (ROS).
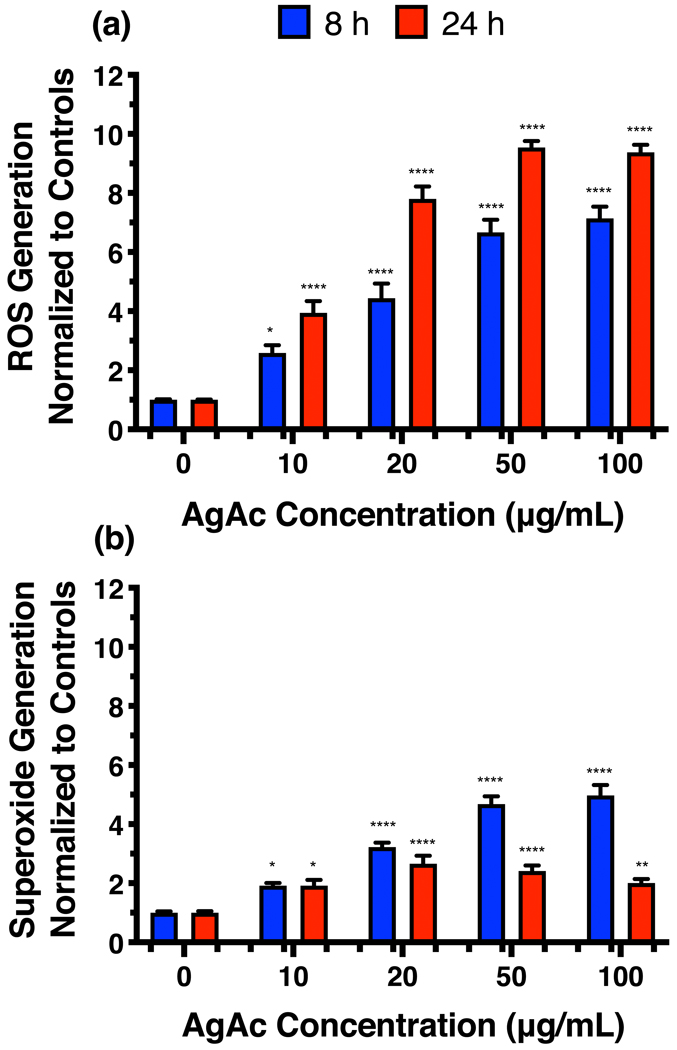
Several publications have demonstrated ROS generation by eukaryotic cells after exposure to silver compounds (18, 28, 29, 31, 32). Silver acetate was chosen for this study due to the biocompatibility of acetate (37). Our results confirm the previously reported findings in the human bronchial cell line, 16HBE; 8 and 24 h exposure to silver acetate results in significantly higher amounts of superoxide ions and total ROS compared with controls (Figure 1).
Figure 1.
(a) Reactive oxygen species and (b) superoxide levels in human bronchial epithelial (16HBE) cells upon exposure to silver acetate for 8 and 24h. Statistical analysis was completed using 2-way ANOVA with Sidak’s multiple comparisons test. *: p ≤ 0.05, **: p ≤ 0.01, and ****: p ≤ 0.0001.
3.2. Activity of antioxidants.
Results of the Total Antioxidant Capacity assay show that ascorbic acid demonstrated significantly higher antioxidant activity compared with melatonin and NAC (p ≤ 0.0001, Figure 2); NAC demonstrated significantly higher antioxidant activity compared with melatonin (p ≤ 0.001). Cells incubated with ascorbic acid demonstrated significantly higher antioxidant activity over control cells, and NAC incubated cells (p ≤ 0.01), but not melatonin-incubated cells. Surprisingly, NAC incubation did not result in enhanced antioxidant activity, as no significant difference was observed between NAC-incubated cells and melatonin-incubated cells, or control cells.
Figure 2.
Antioxidant activity of (a) N-acetyl cysteine (NAC), ascorbic acid (AA), and melatonin at 10 mmol/L concentration, and (b) cell lysates from cells incubated with no antioxidant, 10 mmol/L N-acetyl cysteine, 10 mmol/L ascorbic acid or 10 mmol/L melatonin for two hours. **: p ≤ 0.01, ***: p ≤ 0.001, and ****: p ≤ 0.0001.
3.3. Abrogation of silver acetate toxicity through pre-incubation with antioxidants.
The effect of antioxidant pre-incubation on silver acetate toxicity towards 16HBE cells using the alamarblue® assay is shown in Figure 3 (and Figure S1 in supplementary information). Cells pre-incubated with 5, 7.5, and 10 mmol/L NAC and up to 50, 75, and 100 μg/mL silver acetate, respectively, demonstrated significantly higher survival over cells that were not pre-incubated with NAC (p ≤ 0.05) at 8 and 24 h. Moreover, a dose response was observed; NAC pre-incubation at concentrations 1.0 mmol/L or higher result in lower silver acetate toxicity when compared with NAC-free controls. The lethal dose at median cell viability (LD50) values for cells exposed to silver acetate with or without NAC pre-incubation are listed in Figure 3. Additionally, the CyQuant cell viability assay demonstrated similar results with 0 mmol/L and 10 mmol/L NAC pre-incubation (Figure S2, supplementary information).
Figure 3.
Viability of human bronchial epithelial (16HBE) cells upon pre-incubation with 0, 2.5, 7.5, and 10 mmol/L concentrations of N-acetyl cysteine (NAC), ascorbic acid, or melatonin for 2 h followed by a 24 h incubation with 0, 10, 20, 30, 40, 50, 75, or 100 μg/mL concentration of silver acetate. Cell viability upon pre-incubation with (a) 2.5 mmol/L antioxidants, (b) 5 mmol/L antioxidants, (c) 7.5 mmol/L antioxidants, (d) 10 mmol/L antioxidants, and (e) lethal dose at median cell viability (LD50) values deduced from cell viability curves upon exposure to silver acetate after pre-incubation with select antioxidants. Statistical analysis was completed using 2-way ANOVA with Tukey’s multiple comparisons test. *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, and ****; p ≤ 0.0001.
Pre-incubation with neither ascorbic acid nor melatonin resulted in increased cellular survival comparable to NAC, as demonstrated by the LD50 values. Thus, of the three anti-oxidants evaluated in this study, only NAC rescued the cells from silver acetate toxicity. Finally, NAC pre-incubation also abrogated the toxicity of silver acetate against human dermal fibroblasts (Figure S3, supplementary information). Thus, NAC was chosen as the molecule of interest for further investigation.
3.4. Silver acetate induction of superoxide and total reactive oxygen species (ROS).
Figure 4 illustrates the suppression of superoxide ions and total ROS in cells preincubated with NAC and exposed to silver acetate for 8 and 24 h. Specifically, cells pre-incubated with 10 mM NAC, upon exposure to 20 and 50 μg/mL silver acetate concentrations, showed significantly lower superoxide and total ROS levels (p ≤ 0.001). Cells incubated with 100 μg/mL silver acetate showed significantly lower superoxide levels at 8 h when pre-incubated with NAC (p ≤ 0.01), but not at 24 h. Surprisingly, NAC pre-incubation initially induced total ROS, which subsided after 8 h, but had no effect on superoxide levels.
Figure 4.
Total reactive oxygen species (a, b) and superoxide (c, d) levels in human bronchial epithelial (16HBE) cells upon pre-incubation with 0 or 10 mmol/L NAC followed by a (a, c) 8 h or (b, d) 24 h exposure to silver acetate. Statistical analysis was completed using 2-way ANOVA with Sidak’s multiple comparisons test. **: p ≤ 0.01 and ****: p ≤ 0.0001.
3.5. Glutathione concentrations after treatment with NAC.
Because NAC is a known precursor of glutathione, the effects of NAC pre-incubation on both oxidized and reduced glutathione concentrations were determined. A dose response and significant reduction in the reduced glutathione concentration was observed with increasing concentrations of silver acetate, with or without NAC pre-treatment (Figure 5). However, this decrease in GSH concentration was not accompanied by an increase in GSSG levels, suggesting the absence of correlation between ROS generation and oxidation of glutathione, after silver acetate incubation. Further, NAC pre-treatment did not result in any changes in total, oxidized, or reduced glutathione concentrations over cells not pre-treated with NAC, with or without silver acetate.
Figure 5.
Levels of reduced (GSH), oxidized (GSSG), and total (GSH+GSSG) glutathione measured in human bronchial epithelial (16HBE) cells upon pre-incubation with 0 or 10 mmol/L NAC followed by exposure to 0, 10, or 100 μg/mL silver acetate (AgAc) for 1 h. Statistical analysis was completed using 2-way ANOVA with Tukey’s multiple comparisons test. ****: p ≤ 0.0001.
3.6. Analysis of total metabolite pool size and metabolite labeling patterns using gas chromatography-mass spectroscopy.
Disruption of the mitochondrial electron transport chain has been linked to the ROS overproduction and cell death upon exposure to silver ions. To further explore the metabolic effects of silver-induced ROS production, we evaluated glucose consumption and its metabolism through the glycolysis pathway. Glucose consumption and lactate production, the end product of glycolysis, were determined using a bioProfile BASIC analyzer (Figure 6). As expected, treatment with increasing concentrations of silver acetate without NAC resulted in increased glucose consumption, resulting in less glucose in the supernatant of the cells, and increased lactate production, resulting in more lactate in the supernatant, until fewer viable cells remained to produce lactate at the higher concentrations of silver acetate. Although incubation with NAC appeared to decrease lactate production, the change was not statistically significant due the high variability of lactate production in the absence of NAC. The addition of NAC did not significantly alter glucose consumption after 8 h until higher concentrations of silver acetate were reached, with the most pronounced decrease seen with the 100 μg/mL treatment. This result suggests that NAC allowed cells to sustain metobolism in the presence of silver acetate at concentrations below 75 μg/mL. Next, we evaluated the effect of NAC incubation on the oxidation of glucose-derived carbon in the TCA cycle. Levels of metabolites involved in the TCA cycle were evaluated using GC-MS and normalized to control cells (exposed to neither silver acetate nor NAC; Figure 7). Cells exposed to silver acetate alone demonstrated little change in lactate levels, but significantly lower levels of TCA cycle intermediates including citrate, fumarate, and malate, as well as glutamate and aspartate, surrogates for α-ketoglutarate and oxaloacetate, respectively, while silver acetate-exposed cells pretreated with NAC showed significantly less attenuation of TCA intermediates (p ≤ 0.05). Finally, lactate levels were significantly higher for cells pre-incubated with NAC and exposed to 50 μg/mL silver acetate only. In addition, pre-incubation with NAC does not appreciably alter the labeling patterns of key metabolites (Figure S4, supplementary information). Thus, these results demonstrated that exposure to silver acetate resulted in mitochondrial stress that can be ameliorated by pre-incubation with NAC.
Figure 6.
Levels of (a) glucose consumption and (b) lactate production in human bronchial epithelial cells (16HBE) upon pre-incubation with 0 or 10 mmol/L N-acetyl cysteine (NAC) followed by an 8 h exposure to silver acetate, determined using a BioProfile BASIC analyzer. Statistical analysis was completed using 2-way ANOVA with Sidak’s multiple comparisons test. *: p ≤ 0.05.
Figure 7.

Effect of N-acetyl cysteine (NAC) pre-incubation (2h) on the TCA cycle metabolite pool after exposure to silver (8 h) in human bronchial epithelial (16HBE) cells; Levels of (a) lactate, (b) citrate, (c) glutamate, (d) aspartate, (e) fumarate, and (f) malate in the cell lysate determined using tandem gas chromatography - mass chromatography. *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 001, and ****: p ≤ 0.0001.
3.7. Determination of ATP content.
The downstream effect of TCA cycle salvage by NAC was measured in terms of ATP production (Figure 8). Cells pre-incubated with 10 mmol/L NAC demonstrated significantly higher ATP production upon exposure to silver acetate compared with cells exposed to silver acetate alone (p ≤ 0.0001). Thus, NAC pre-incubation rescued cells from disruption of the TCA cycle and electron transport chain to maintain ATP production at a comparable rate to the control group.
Figure 8.
ATP production in human bronchial epithelial (16HBE) cells pre-incubated with 0 or 10 mmol/L N-acetyl cysteine (NAC) followed by exposure to silver acetate for 8 h. ****: p ≤ 0.0001.
3.8. Antimicrobial activity of silver acetate with or without NAC pretreatment.
Antimicrobial activity of silver acetate with or without pre-incubation with NAC was measured using a standard CLSI broth-microdilution method (Table 1). The minimum inhibitory concentration (MIC) of silver acetate did not change when the bacteria were pre-incubated with 0 or 10 mmol/L NAC, demonstrating the selectivity of NAC to rescue eukaryotic cells without altering its antimicrobial activity.
Table 1.
Minimum inhibitory concentration (MIC) of silver acetate (AgAc) against laboratory and clinical isolates of P. aeruginosa and MRSA upon 2 h pre-incubation with 0 or 10 mmol/L NAC.
| Bacteria | MIC of AgAc with 0 mmol/L NAC pre-incubation (μg/mL) | MIc of AgAc with 10 mmol/L NAC pre-incubation (μg/mL) |
|---|---|---|
| PA O1 | 1 | 1 |
| PA M57–15 | 0.25 | 0.25 |
| PA HP3 | 1 | 1 |
| PA 14 | 1 | 1 |
| USA 300 | 2 | 2 |
| MRsa 0606 | 2 | 2 |
| MRSA 0638 | 2 | 2 |
| MRSA 0646 | 2 | 2 |
4.0. Discussion.
The multiple mechanisms of action that make silver an attractive broad-spectrum antimicrobial may also contribute to its toxicity towards eukaryotic cells. Several reports have demonstrated that exposure to silver at low concentrations results in apoptosis, and exposure at high concentrations results in necrosis (32, 35). Cell death, via apoptosis or necrosis, after silver exposure is often a result of ROS generation (32).
Mitochondria are considered the primary sources of ROS (30, 38). Generation of ROS in the mitochondria is a tightly regulated process that relies on the electron transport chain (ETC) for ROS formation and cellular ROS scavengers such as enzymes, superoxide dismutase (SOD), as well as antioxidants such as, glutathione, for countering the generated ROS (30). Under physiological conditions, glucose is converted to pyruvate during glycolysis, yielding two molecules of ATP, which is then followed by oxidative phosphorylation to yield 36 molecules of ATP and ROS (39). Until recently, ROS was generally regarded as a byproduct of this process. However, in the last two decades, normal ROS levels have been implicated as second messengers in signal transduction pathways (40, 41). Upon exposure to chemicals including Ag+ ions, an imbalance of ROS generation results in oxidative stress causing damage to three classes of biologic macromolecules - lipids, proteins, and nucleic acids (38, 40). High levels of oxidative stress induce cell death via one of two pathways: apoptosis or programmed necrosis (42).
Our results demonstrate a significant increase in the ROS and superoxide levels in human cells after exposure to silver acetate (Figure 4). The downstream effects of ROS are complex, including activation of several pathways that ultimately lead to cell death (30, 38, 42). Mitochondrial DNA is highly susceptible to oxidative damage by mtROS due to its close proximity and a lack of protection by histones (38). To that end, the level of oxidatively modified bases in mtDNA has been found to be 10–20 fold higher than in nuclear DNA (38). This mtROS also results in lipid peroxidation to the mitochondrial membrane (38). The downstream effect of these processes results in pyroptosis, a mode of induced cell death (41), necrosis (38, 43), and caspase mediated apoptosis (38). These observations are consistent with our results; exposure to silver acetate results in ROS-induced oxidative stress leading to depletion of ATP (Figure 8) and cell death.
The surplus of superoxide and ROS interacts with critical proteins also resulting in activation of pathways that lead to cell death. Superoxide damages and deactivates iron-sulfur proteins such as aconitases, ultimately blocking the conversion of citrate to isocitrate by aconitases in the TCA cycle (30, 38). These effects are further exacerbated by the production of Fe2+ and hydrogen peroxide upon interaction between superoxide and thiol containing proteins. Released hydrogen peroxide can cause further oxidative damage to DNA, lipids, and proteins (38, 40). This inhibition of aconitases after exposure to ROS is typically associated with accumulation of citrate (30); however, our results show a reduction in citrate levels. This depletion of citrate can be attributed to the inactivation of pyruvate dehydrogenase kinase after exposure to mtROS (30). Pyruvate dehydrogenase kinase 2 (PDHK2), a major player of the pyruvate dehydrogenase complex (PDC) is responsible for catalytic conversion of pyruvate into acetyl-coA, a precursor of citrate in the TCA cycle. The mechanism of deactivation of these enzymes is not limited to the mitochondrial ROS; these proteins, including pyruvate dehydrogenase (PDH), can also be inhibited upon interaction with metal ions (44). Thus, the combined deactivation of these two enzymes results in depletion of acetyl-coA, citrate, and isocitrate, key metabolites for the TCA cycle, ultimately resulting in disruption of the TCA cycle. The downstream effects of the loss of citrate directly impacts the TCA cycle, as demonstrated by reduced levels of downstream metabolites, glutamate, aspartate, fumarate, and malate (Figure 7). In response to this TCA cycle breakdown, there is a significant reduction in the ATP produced after exposure to silver acetate (Figure 8).
The effect of silver on mitochondria is one of the primary mechanisms of silver toxicity. This initial insult to the mitochondria initiates several downstream signaling pathways that contribute to silver toxicity. For instance, DNA damage caused by ROS insult induces p53 stabilization, ultimately resulting in activation of caspase cascade (38, 45). Similarly, thiol residues on several cysteine containing proteins act as a switch, which upon interaction with ROS can result in activation or repression of multiple transcription factors (30). Further, TP53-induced glycolysis and apoptosis regulator (TIGAR), upregulated by p53, inhibits glycolysis, and shifts the glucose flux into the pentose phosphate pathway (PPP) (38). Similarly, inhibition of pyruvate kinase isoform (PMK2) forces activation of the PPP (30). This shift to the PPP, which is responsible for generating majority of the NADPH, is key defense mechanism activated by the cell in response to the oxidative damage. The products of the PPP are subsequently shuttled back into the glycolytic pathway. In addition to activation of the PPP, PMK2 is also thought to play a critical role in the antioxidant defense system by diverting 3-phosphoglycerate to glutathione synthesis, via the phosphoserine synthesis, pathway (30, 46). Activation of this pathway should result in an increase in the reduced glutathione (GSH) levels, which further deactivates ROS to form the oxidized glutathione GSSG) (40). Our results indicate a reduction in the reduced form of glutathione after exposure of human cells to silver acetate (Figure 5) and are consistent with the results obtained by Arora et al. after exposing cells to silver nanoparticles (32). However, the expected increase in the GSSG levels is not observed. This unexpected behavior is likely caused by the high affinity of silver ions towards the thiol groups present on glutathione. The silver glutathione complex blocks the ability of GSH to neutralize ROS. Thus, silver associated toxicity is a direct consequence of a combination of silver interaction with cellular components and ROS generation. These two processes are closely related, often complementary, and their individual effects cannot be separated. The downstream response of all these mechanisms results in a Warburg-like effect, where the glucose consumed by the cells is not completely oxidized and is secreted as lactate (Figure 6). For use of silver as an effective antimicrobial, we must address all of these toxicity mechanisms without altering its antimicrobial activity.
The multiple cell-death mechanisms activated by ROS generated by exposure to silver have garnered attention of several researchers (28, 29, 32, 34). Antioxidants have been explored to check the ROS generated after exposure to metals (31). N-acetyl cysteine (NAC) is one of the most common antioxidants investigated to abrogate the toxicity of silver, as well as other metal ions (29, 33–35, 47). Several groups have demonstrated successful rescue of mammalian cells upon pre-exposure to NAC followed by exposure to metal ions (33, 35, 46, 47). NAC pre-incubation results in significant roS suppression upon exposure to silver acetate (Figure 4). However, it is unclear if the reduction in the ROS levels is a direct consequence of the ROS scavenging activity of NaCor a downstream effect of silver-NAC interactions. In order to elucidate the mechanism of rescue and develop a future potential therapeutic that can ameliorate toxicity of silver-based therapeutics, such as silver antimicrobial bandages, we investigated several antioxidants and their ability to rescue mammalian cells from silver toxicity. Of all the evaluated antioxidants, NAC is the only antioxidant that demonstrates significant rescue (Figure 3, Supplementary Fig. S1, S2, and S3). Melatonin, which demonstrates only slightly lower antioxidant capacity compared with NAC, does not result in rescue of cells from silver-associated toxicity. Although ascorbic acid has a higher antioxidant capacity than NAC, ascorbic acid failed to rescue the cells from silver toxicity. Indeed, among the antioxidants tested, NAC exclusively rescues cells from silver associated toxicity; the ROS levels in cells pre-incubated with NAC followed by up to 50 μg/mL silver are similar to the negative controls, cells that received neither NAC nor silver. The failure of ascorbic acid to rescue silver-exposed cells suggests that antioxidant activity is not the only mechanism through which NAC pre-treatment resulted in lower ROS levels.
Unique structural properties position NAC to abrogate the multiple toxicity mechanisms induced by silver cations. NAC is a precursor for glutathione, however, the glutathione levels of cells incubated with NAC are similar to those of control cells. Thus, the reduction in ROS is not directly influenced by glutathione. We hypothesized that the thiol groups present on NAC bind with silver, which would prevent it from binding wit, DNA, and generating ROS. We confirmed the ability of NAC to bind with silver using 1H nuclear magnetic resonance (NMR) spectroscopy (Figure S5). The distinct shifts between the two spectra indicate that silver has a significant interaction with NAC. Further, NAC and silver self-assemble into a hydrogel, confirming the interaction between NAC and silver. Similar NAC-based hydrogels after interaction with silver, gold, and copper have been reported by Casuso et al (48). In addition, NAC is known as an antiapoptotic agent, and inhibits several apoptotic pathways including, NF-κB, MEK/ERK, and the JNK pathway, promoting cell survival (49, 50). The downstream effect of NAC treatment is underscored by the significant improvement in the metabolic state of the cell. Cells treated with NAC demonstrate normal levels of TCA cycle metabolites after silver exposure. These cells also demonstrate normal lactate production and ATP levels. The ability of NAC to rescue eukaryotic cells is not limited to bronchial epithelial cells; human dermal fibroblasts also demonstrate similar cell viability patterns. Finally, the ability of NAC to rescue eukaryotic cells from silver associated cell death does not affect the antimicrobial activity of silver; when P. aeruginosa and MRSA isolates were pre-exposed to 10 mmol/L NAC, the MIC of AgAc was identical to that of NAC-free AgAc (Table 1). Moreover, NAC is frequently used as a mucolytic in patients suffering from cystic fibrosis (CF) (51), chronic obstructive pulmonary disorder (COPD) (52), and chronic b’ orchitis (53, 54). The benefits of NAC have led to its inclusion in The World Health Organization’ (WHO) list of essential medicines (55). Thus, a silver/NAC combination presents a unique therapeutic strategy that can effectively eradicate bacterial infections without causing toxicity to eukaryotic cells.
The affinity of silver to sulfur has long been known, and follows the trends outlined by the hard soft acid base (HSAB) theory, with silver being a soft acid and sulfur being a soft base. It is therefore interesting to question whether other thiol containing compounds could also act in the same ways as NAC to protect eukaryotic cells from silver toxicity, while maintaining the antibacterial activity of silver. In a study by Liau et al., researchers assessed the antimicrobial activity of silver nitrate in conjunction with compounds containing thiol groups. The authors found that thiol containing compounds L-cysteine and sodium thioglycollate neutralized the antibacterial activity of silver nitrate against PAO1. Reduced glutathione was also able to neutralize antibacterial activity, but at a higher concentration (0.5% w/v) than seen for both L-cysteine and sodium thioglycollate (0.1% w/v). Interestingly, NAC was shown to neutralize the antimicrobial activity of silver nitrate as well (56). However, the results with silver acetate presented here show that silver acetate remains effective as an antimicrobial even in he presence of NAC. Taken together, these observations indicate that although both silver nitrate and silver acetate exhibit antibacterial activity, the remaining structure of the compound, in this case, nitrate versus acetate, is extremely important when determining if thiol containing compounds can alleviate toxicity, while maintaining antimicrobial activity.
As mentioned previously, studies on the hydrogel forming capabilities of thiol containing compounds with silver nitrate have been performed and can give important insight into the extent of thiol group and silver compound interaction. Research shows that hydrogels can be formed between silver nitrate and either L-cysteine or NAC; however, initiator salts must be added in order to form a gel with L-cysteine, indicating a significant difference in how the silver interacts with each thiol containing ligand (57).
These previously published studies in addition to the work presented here outline an important point; the choice of silver compound and thiol containing ligand appears to play a role in altering the interaction between the silver cation and sulfur atoms. These choices may alter the level of toxicity that silver exhibits, presumably to both prokaryotic and eukaryotic cell types. Further studies are currently underway to investigate additional silver compounds and other thiol containing ligands to determine if the combinations similarly abrogate silver toxicity, yet maintain antibacterial activty, as seen here with silver acetate and NAC.
5.0. Conclusion.
We report here a novel mechanism that results in rescue of mammalian cells from silver-associated toxicity without altering its antimicrobial activity. Cells incubated with silver acetate demonstrate high levels of ROS, which causes disruption of the TCA cycle and reduction in ATP production, ultimately leading to cell death via apoptosis or necrosis. On the other hand, cells pre-incubated with NAC followed by silver acetate do not demonstrate signs of oxidative stress, show a normal metabolic state, as well as ATP production, which translates to lower silver toxicity. Thus, the silver/NAC combination has tremendous potential as a future therapeutic with potent antimicrobial activity with a large therapeutic window.
Supplementary Material
Highlights.
N-acetyl cysteine (NAC) pre-incubation of cells abrogates silver acetate toxicity.
NAC, but not ascorbic acid or melatonin, abrogates silver acetate toxicity.
NAC pre-incubation preserves metabolism in silver-exposed cells.
NAC abrogates toxicity by reducing reactive oxygen species and binding silver.
NAC pre-incubation does not affect the antimicrobial activity of silver acetate.
Acknowledgements.
The authors thank Dr. Dieter Gruenert (University of California, San Francisco, CA) for providing the 16HBE cell line, Dr. Carol Tamminga (University of Texas Southwestern Medical Center, Dallas, TX) for proving human dermal fibroblasts, and Dr. Thomas Ferkol (Washington University, St. Louis, MO) for providing PAM57–15 isolate of P. aeruginosa.
Funding. This work was supported by the National Institutes of Health [HHSN268201000046C] and by departmental support funds at the University of Texas Southwestern Medical Center and Texas A&M University Health Science Center.
Footnotes
Declaration of interests
Drs. Kush Shah, Qingquan Chen and Carolyn Cannon submitted a provisional patent on April 4, 2019 titled “Delivery Devices for Localized Delivery of Antimicrobial, Antiinflammatory, and Antioxidant Agents.” This provisional patent includes some of the data presented in this manuscript. Drs. Parth Shah, Andrew Mullen, and Ralph DeBerardinis have no financial interests or personal relationships which may be considered as potential competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58(2):185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action o. the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74(7):2171–8. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirnay JP, De Vos D, Cochez C, Bilocq F, Pirson J, Struelens M, Duinslaeger L, Cornelis P, Zizi M, Vanderkelen A. Molecular epidemiology of Pseudomonas aeruginosa colonization in a burn unit: persistence of a multidrug-resistant clone and a silver sulfadiazine-resistant clone. J Clin Microbiol. 2003;41(3):1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27(2–3):341–53. [DOI] [PubMed] [Google Scholar]
- 5.Wright JB, Lam K, Hansen D, Burrell RE. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control. 1999;27(4):344–50. [DOI] [PubMed] [Google Scholar]
- 6.Shah PN, Lin LY, Smolen JA, Tagaev JA, Gunsten SP, Han DS, Heo GS, Li Y, Zhang F, Zhang S, Wright BD, Panzner MJ, Youngs WJ, Brody SL, Wooley KL, Cannon CL. Synthesis, Characterization, and In Vivo Efficacy of Shell Cross-Linked Nanoparticle Formulations Carrying Silver Antimicrobials as Aerosolized Therapeutics. ACS Nano. 2013;7(6):4977–87. doi: 10.1021/nn400322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consumer Products Inventory: The Project on Emerging Nanotechnologies [Internet]. Woodrow Wilson Center and Virginia Tech University; 2018. [cited 08/05/2018]. [Google Scholar]
- 8.Zhang F, Smolen JA, Zhang S, Li R, Shah PN, Cho S, Wang H, Raymond JE, Cannon CL, Wooley KL. Degradable polyphosphoester-based silver-loaded nanoparticles as therapeutics for bacterial lung infections. Nanoscale. 2015;7(6):2265–70. doi: 10.1039/c4nr07103d. [DOI] [PubMed] [Google Scholar]
- 9.Cannon CL, Hogue LA, Vajravelu RK, Capps GH, kncevic A, Hindi KM, Kascatan-Nebioglu A, Walter MJ, Brody SL, Youngs WJ. In vitro and murine efficacy and toxicity studies of nebulized SCC1, a methylated caffeine-silver(I) complex, for treatment of pulmonary infections. Antimicrob Agents Chemother. 2009;53(8):3285–93. doi: 10.1128/AAC.00314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindi KM, Siciliano TJ, Durmus S, Panzner MJ, Medvetz DA, Reddy DV, Hogue LA, Hovis CE, Hilliard JK, Mallet RJ, Tessier CA, Cannon CL, Youngs WJ. Synthesis, stability, and antimicrobial studies of electronically tuned silver acetate N-heterocyclic carbenes. J Med Chem. 2008;51(6):1577–83. doi: 10.1021/jm0708679. [DOI] [PubMed] [Google Scholar]
- 11.Kascatan Nebioglu A, Melaiye A, Hindi K, Durmus S, Panzner M, Hogue L, Mallett R, Hovis C, Coughenour M, Crosby S, Milsted A, Ely D, Tessier C, Cannon C, Youngs W. Synthesis from Caffeine of a MixedN-Heterocyclic Carbene-Silver Acetate Complex Active against Resistant Respiratry Pathogens. Journal of medicinal chemistry. 2006;49(23):6811–8. [DOI] [PubMed] [Google Scholar]
- 12.Kascatan-Nebioglu A, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ. N-Heterocyclic carbene–silver complexes: A new class of antibiotics. Coordination Chemistry Reviews. 2007;251(5):884–95. doi: 10.1016/j-.ccr.2006.08.019. [DOI] [Google Scholar]
- 13.Panzner MJ, Hindi KM, Wright BD, Taylor JB, Han DS, Youngs WJ, Cannon CL. A theobromine derived silver N-heterocyclic carbene: synthesis, characterization, and antimicrobial efficacy studies on cystic fibrosis relevant pathogens. Dalton Trans. 2009(35):7308–13. doi: 10.1039/b907726j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leid JG, Ditto AJ, Knapp A, Shah PN, Wright BD, Blust R, Christensen L, Clemons CB, Wilber JP, Young GW, Kang AG, Panzner MJ, Cannon CL, Yun YH, Youngs WJ, Seckinger NM, Cope EK. In vitro antimicrobial studies of silver carbene complexes: activity of free and nanoparticle carbene formulations against clinical isolates of pathogenic bacteria. J Antimicrob Chemother. 2012;67(1):138–48. doi: 10.1093/jac/dkr408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright BD, Shah PN, McDonald LJ, Shaeffer ML, Wagers PO, Panzner MJ, Smolen J, Tagaev J, Tessier CA, Cannon CL, Youngs WJ. Synthesis, characterization, and antimicrobial activity of silver carbene complexes derived from 4,5,6,7-tetrachlorobenzimidazole against antibiotic resistant bacteria. Dalton Trans. 2012;41(21):6500–6. doi: 10.1039/c2dt00055e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hindi KM, Ditto AJ, Panzner MJ, Medvetz DA, Han DS, Hovis CE, Hilliad JK, Taylor JB, Yun YH, Cannon CL, Youngs WJ. The antimicrobial efficacy of s stained release silver-carbene complex-loaded L-tyrosine polyphosphate nanoparticles: characterization, in vitro and in vivo studies. Biomaterials. 2009;30(22):3771–9. doi: 10.1016/j.biomaterials.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadrup N, Lam HR. Oral toxicity of silver ions, silver nanoparticles and colloidal silver--a review. Regul Toxicol Pharmacol. 2014;68(1):1–7. doi: 10.1016/j.yrtph.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar MK, Vadivel V, Charan Raja MR, Mahapatra SK. Potential anti-proliferative activity of AgNPs synthesized using M. longifolia in 4T1 cell line through ROS generation and cell membrane damage. J Photochem Photobiol B. 2018;186:160–8. doi: 10.1016/j.jphotobiol.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Piao MJ, Kim KC, Choi JY, Choi J, Hyun JW. Silver nanoparticles down-regulate Nrf2-mediated 8-oxoguanine DNA glycosylase 1 through inactivation of extracellular regulated kinase and protein kinase B in human Chang liver cells. Toxicol Lett. 2011;207(2):143–8. doi: 10.1016/j.toxlet.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011;201(1):92–100. doi: 10.1016/j.toxlet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Eom HJ, Choi J. p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ Sci Technol. 2010;44(21):8337–42. doi: 10.1021/es1020668. [DOI] [PubMed] [Google Scholar]
- 22.Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100(4):1033–43. doi: 10.1002/jbm.a.34053. [DOI] [PubMed] [Google Scholar]
- 23.AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3(2):279–90. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 24.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112(43):13608–19. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 25.McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22(1):116–27. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kittler S, Greulich C, Diendorf J, Koller M, Epple M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chemistry of Materials. 2010;22(16):4548–54. doi: 10.1021/cm100023p. [DOI] [Google Scholar]
- 27.Lu W, Senapati D, Wang S, Tovmachenko O, Singh AK, Yu H, Ray PC. Effect of Surface Coating on the Toxicity of Silver Nanomaterials on Human Skin Keratinocytes. Chem Phys Lett. 2010;487(1–3). doi: 10.1016/j.cplett.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onodera A, Nishiumi F, Kakiguchi K, Tanaka A, Tanabe N, Honma A, Yayama K, Yoshioka Y, Nakahira K, Yonemura S, Yanagihara I, Tsutsumi Y, Kawai Y. Short-term changes in intracellular ROS localisation after the silver nanoparticles exposure depending on particle size. Toxicol Rep. 2015;2:574–9. doi: 10.1016/j.toxrep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179(3):130–9. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Mullarky E, Cantley LC. Diverting Glycolysis to Combat Oxidative Stress In: Nakao K, Minato N, Uemoto S, editors. Innovative Medicine: Basic Research and Development: Springer; 2015. p. 3–24. [PubMed] [Google Scholar]
- 31.Pulido MD, Parrish AR. Metal-induced apoptosis: mechanisms. Mutat Res. 2003;533(1–2):227–41. [DOI] [PubMed] [Google Scholar]
- 32.Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol Lett. 2008;179(2):93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40(4):328–46. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, Ryu DY. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 2009;23(6):1076–84. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85(7):743–50. doi: 10.1007/s00204010-0545-5. [DOI] [PubMed] [Google Scholar]
- 36.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10(1):38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 37.Pfortmueller CA, Fleischmann E. Acetate-buffered crystalloid fluids: current knowledge, a systematic review. Journal of critical care. 2016;35:96–104. [DOI] [PubMed] [Google Scholar]
- 38.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913–22. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 39.Lodish HF. Molecular cell biology. 5th ed New York: W.H. Freeman and Company; 2003. xxxiii, 973, 79 p. p. [Google Scholar]
- 40.Kondoh H, Lleonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22(1):85–90. doi: 10.14670/HH-22.85. [DOI] [PubMed] [Google Scholar]
- 41.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: A nexus of cellar homeostasis. Redox Biol. 2015;6:472–85. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117(6):773–86. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Samikkannu T, Chen CH, Yih LH, Wang AS, Lin SY, Chen TC, Jan KY. Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chem Res Toxicol. 2003;16(3):409–14. doi: 10.1021/tx025615j. [DOI] [PubMed] [Google Scholar]
- 45.Kondoh H. Cellular life span and the Warburg effect. Exp Cell Res. 2008;314(9):1923–8. doi: 10.1016/j.yexcr.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347–54. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EH, Jang H, Roh JL. A Novel Polyphenol Conjugate Sensitizes Cisplatin-Resistant Head and Neck Cancer Cells to Cisplatin via Nrf2 Inhibition. Mol Cancer Ther. 2016;15(11):2620–9. doi: 10.1158/1535-7163.MCT-16-0332. [DOI] [PubMed] [Google Scholar]
- 48.Casuso P, Carrasco P, Loinaz I, Grande HJ, Odriozola I. Converting drugs into gelators: supramolecular hydrogels from N-acetyl-L-cysteine and coinage-metal salts. Org Biomol Chem. 2010;8(23):5455–8. doi: 10.1039/c0ob00311e. [DOI] [PubMed] [Google Scholar]
- 49.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157(4):1630–7. PubMd PMID: 8759749. [PubMed] [Google Scholar]
- 50.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suk JS, Boylan NJ, Trehan K, Tang BC, Schneider CS, Lin JM, Boyle MP, Zeitlin PL, Lai SK, Cooper MJ, Hanes J. N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Mol Ther. 2011;19(11):1981–9. doi: 10.1038/mt.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadowska AM, Verbraecken J, Darquennes K, De Backer WA. Role of N-acetylcysteine in the manage ment of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(4):425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mokhtari V, Afsharian P, Shahhoseini M, Kalantar SM, Moini A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017;19(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stey C, Steurer J, Bachmann S, Medici TC, Tramer MR. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur Respir J. 2000;16(2):253–62. [DOI] [PubMed] [Google Scholar]
- 55.WHO Model Lists of Essential Medicines [Internet]. World Health Organization; 2017. [cited August 2018]. [Google Scholar]
- 56.Liau S, Read D, Pugh W, Furr J, Russell A. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterialaction of silver ions. Letters in applied microbiology. 1997;25(4):279–83. [DOI] [PubMed] [Google Scholar]
- 57.Khizhnyak SD, Komarov PV, Ovchinnikov MM, Zherenkova LV, Pakhomov PM. Mechanism of gelation in low-concentration aqueous solutions of silver nitrate with l-cysteine and its derivatives. Soft matter. 2017;13(30):5168–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.