Abstract
Interleukin-2 (IL-2) controls the homeostasis and function of regulatory T (Treg) cells and defects in the IL-2 pathway contribute to multiple autoimmune diseases. Although recombinant IL-2 therapy has been efficacious in certain inflammatory conditions, the capacity for IL-2 to also activate inflammatory effector responses highlights the need for IL-2-based therapeutics with improved Treg cell-specificity. From a panel of rationally designed murine IL-2 variants, we identified IL-2 muteins with reduced potency and enhanced Treg cell-selectivity due to increased dependence on the IL-2-receptor component CD25. As an Fc-fused homodimer, the optimal Fc.IL-2 mutein induced selective Treg cell enrichment and reduced agonism of effector cells across a wide dose range. Furthermore, despite being a weaker agonist, overall Treg cell growth was greater and more sustained due to reduced receptor-mediated clearance of the Fc.IL-2 mutein compared to Fc-fused wild-type IL-2. Preferential Treg cell enrichment was also observed in the presence of activated pathogenic T cells in the pancreas of non-obese diabetic (NOD) mice, despite a loss of Treg cell-selectivity in an IL-2R-proximal response. These properties facilitated potent and extended resolution of NOD diabetes with infrequent dosing schedules.
One Sentence Summary
A CD25-dependent IL-2 mutein expanded regulatory T cells and controlled spontaneous diabetes in non-obese diabetic (NOD) mice.
INTRODUCTION
Interleukin-2 (IL-2) plays a central role in both the maintenance of normal immune homeostasis as well as the amplification and regulation of antigen-specific immune responses (1, 2). A major factor defining the systemic outcome of IL-2 signaling is whether the dominating IL-2-responsive cell populations are pro-inflammatory lymphocytes or anti-inflammatory regulatory T (Treg) cells expressing the transcription factor forkhead box P3 (Foxp3). These differential cellular responses are dictated by the hierarchy of the IL-2 receptor (IL-2R) expression levels among these lymphocyte subsets. IL-2R is expressed either as an intermediate-affinity dimer, composed of IL-2Rβ (CD122) and the common cytokine receptor gamma chain (CD132), or a high-affinity trimer that includes IL-2Rα (CD25). CD25 has no signaling capacity but functions by binding IL-2 and presenting it to the CD122/CD132 signaling complex (3). Constitutive CD25 expression is largely limited to Foxp3+ Treg cells (4–6), making them highly responsive to limiting amounts of IL-2. In contrast, CD122 and CD132 are found on nearly all T cells and are most highly expressed by memory CD8+ T cells and NK cells (7). Moreover, CD25 is induced on conventional CD4+ and CD8+ T cells after TCR stimulation (8–10), allowing them to compete with Treg cells for IL-2 access. Thus, in addition to its key anti-inflammatory functions through Treg cells, IL-2 can promote inflammation via activation of effector T cells and NK cells. Nevertheless, Treg cells typically express the highest levels of CD25 in inflammatory conditions due to positive feedback driven by Foxp3 enhancement of IL2RA transcription and IL-2R/signal transducer and activator of transcription 5 (STAT5) enhancement of FOXP3 transcription (11–14).
The pro- and anti-inflammatory functions of IL-2 make it an attractive candidate for immunotherapy. In cancer, IL-2 can be used to stimulate CD25−/lo NK cells and effector T cells to boost antitumor immunity. While recombinant IL-2 (aldesleukin) was approved for cancer immunotherapy in 1992, the high doses required for tumor regression result in only limited efficacy and are associated with the drawbacks of significant Treg cell activation and severe side effects such as vascular leak syndrome leading to multi-organ dysfunction (15, 16). Mutated IL-2 fusion proteins, IL-2 mutants, or de novo proteins have been engineered to overcome these limitations by deleting the vasopermeability activity of IL-2 (17–19) or directing IL-2 binding towards the CD122/CD132 heterodimer and away from CD25 (20, 21). Conversely, for autoimmune and inflammatory diseases, the objective has been to leverage IL-2 to enhance Treg cell numbers and function while minimizing activation of pathogenic effector cells. For this, IL-2 is used at the lowest biologically active doses with carefully optimized dosing regimens (22–24). However, balancing the efficacy and the safety of IL-2 therapy in these settings remains a major concern, and development of IL-2-based therapeutics with improved Treg cell-selectivity is needed.
IL-2 mutant proteins (muteins) with decreased CD122 affinity represent one approach for increasing CD25 dependence and enhancing Treg cell-selectivity. Recently, a human IL-2 mutein was developed that selectively activated and expanded functional Treg cells in humanized mice during xenogeneic graft-versus-host disease (GVHD) (25), and a second IL-2 mutein has advanced to clinical trials in GVHD (NCT03422627), systemic lupus erythematosus (SLE) (NCT03451422) and rheumatoid arthritis (RA) (NCT03410056). Despite these advances, detailed mechanistic studies evaluating the therapeutic effect of IL-2 muteins on IL-2 responsive cell populations in the secondary lymphoid organs and inflamed target tissues are lacking. Evaluating IL-2 muteins in mouse disease models is necessary to define their mechanism of action in vivo, and can be used to help develop and guide therapeutically efficacious dosing strategies. To this end, we generated and screened a panel of 28 candidate murine IL-2 muteins, and identified a highly CD25-dependent molecule with potent Treg cell-enhancing properties. We explored the functional mechanisms of enhanced mutein activity, we found that mutein treatment potently arrests autoimmune diabetes in non-obese diabetic (NOD) with greater dosing flexibility compared to WT IL-2.
RESULTS
An optimal Treg cell-selective Fc.IL-2 mutein exhibits reduced IL-2R agonism yet enhanced Treg cell growth stimulation in vivo
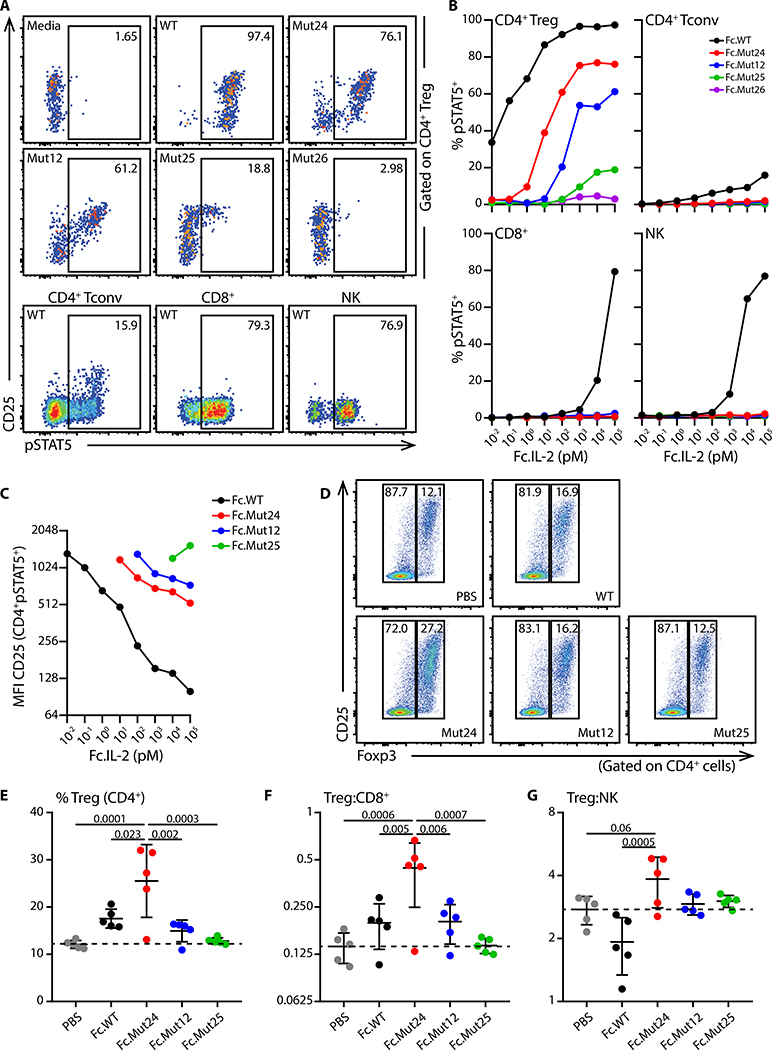
Reducing IL-2 binding to CD122 can increase CD25 dependency (26), and improve Treg cell-selectivity in therapeutic settings. Therefore, we generated constructs for twenty-eight mouse IL-2 variants (IL-2 muteins, table S1), mutating conserved residues with known functional importance at the CD122 contact interface (27–29). To improve pharmacokinetics and tissue distribution, the IL-2 muteins were fused to an IgG2a Fc domain which contained an N297G mutation to abolish effector function (30). We first expressed the Fc.IL-2 fusion constructs in HEK 293T/17 cells and screened the supernatants for activity by measuring phosphorylation of STAT5 in mouse splenic Treg cells (see fig. S1 for representative gating strategies of relevant lymphocyte populations). Fc fused wild-type IL-2 (Fc.WT) showed comparable activity to mouse recombinant IL-2 in stimulating STAT5 phosphorylation in Treg cells, Foxp3−CD4+ T cells, CD8+ T cells, and NK cells, indicating that the IgG2a Fc did not affect its bioactivity or IL-2R-selectivity (fig. S2). The Fc fused muteins (Fc.muteins) ranged from full activity on Treg cells comparable to Fc.WT to complete functional abrogation (fig. S3). Since weaker activity (indicating diminished IL-2/CD122 interaction) should translate into enhanced CD25-dependence and improved Treg cell-selectivity, we selected four mutein candidates exhibiting intermediate (Fc.Mut24 and Fc.Mut12) to weak/negligible (Fc.Mut25 and Fc.Mut26) activity for further evaluation. When titrated on freshly isolated splenocytes, Fc.Mut24 (EC50=11.8pM), Fc.Mut12 (EC50=162.3pM) and Fc.Mut25 (EC50=1046pM) showed reduced signaling activity on Treg cells compared to Fc.WT (EC50=0.058pM), whereas Fc.Mut26 was functionally inactive (Fig. 1, A and B). Unlike Fc.WT, the Fc.muteins did not stimulate Foxp3−CD4+ T cells, CD8+ T cells or NK cells even at high doses (Fig 1, A and B). Although Fc.WT stimulated nearly all Treg cells, response to the Fc.muteins was limited to CD25hi Treg cells; and decreased potency of the Fc.muteins correlated with enhanced CD25 dependence for signaling (Fig 1, A to C). Binding of the Fc.muteins to CD25-expressing HEK cells was similar to Fc.WT (fig. S4), indicating that their reduced signaling potencies were due to impaired CD122 binding. To assess the Treg cell-selectivity of the Fc.muteins under conditions more closely resembling inflamed tissues, we used splenocytes that were pre-activated with anti-CD3 to enhance CD25 expression and IL-2 responsiveness of both Foxp3−CD4+ and CD8+ T cells. Although we observed increased activity on activated effector T cells, the Fc.muteins retained enhanced Treg cell-selectivity and CD25-dependence compared to Fc.WT across a wide dose range (fig. S5).
Fig. 1. Fc.muteins show a weak signaling potency but a high Treg cell-selectivity.
(A to C) B6 splenocytes were stimulated with the indicated purified Fc.IL-2 fusion proteins for 15 min. (A) Representative flow cytometric analyses of Treg cells, Foxp3−CD4+ conventional T cells (Tconv), CD8+ T cells, and NK cells, at 100nM dose of the indicated Fc.IL-2. (B) pSTAT5 dose-response curves for the indicated populations in response to each Fc.mutein. (C) CD25 median fluorescence intensity on CD4+pSTAT5+ cells stimulated with the indicated doses of each Fc.mutein. Data are representative of at least two independent experiments. (D to G) B6 mice were treated with PBS or the indicated Fc.IL-2 (8μg), and inguinal lymph nodes were harvested four days later. (D) Representative flow cytometric analyses of Treg cell frequency. (E) Frequencies of Treg cells and ratios of (F) Treg:CD8+ and (G) Treg:NK are summarized. Data shown as mean ± SD, significance determined by one-way ANOVA followed by Tukey post-test. Representative of at least two independent experiments with n=5 mice/group.
To extend these findings in vivo, we compared responses of IL-2 sensitive lymphocytes in the lymph nodes (LN) of B6 mice after a single treatment with Fc.WT, Fc.Mut12, Fc.Mut24 and Fc.Mut25. Surprisingly, despite its weaker activity in vitro, Fc.Mut24 was more effective than Fc.WT at expanding the Treg cell population relative to Foxp3−CD4+ T cells, CD8+ T cells and NK cells in vivo, whereas Fc.Mut12 showed weaker activity and Fc.Mut25 showed no activity (Fig. 1, D to G). Thus, we selected Fc.Mut24 as the optimal candidate for mechanistic studies of Treg cell enrichment based therapy in autoimmunity.
Fc.Mut24 retains Treg cell-selectivity across a wide dose range
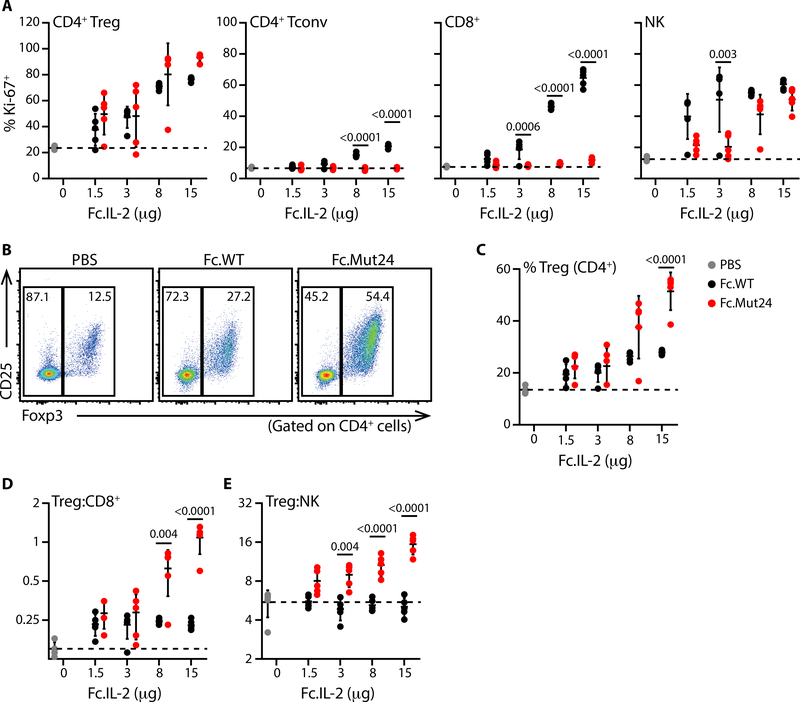
The narrow dose-range necessary to avoid effector cell activation is a barrier to the implementation of IL-2-based therapies in autoimmune diseases. To determine if Fc.Mut24 maintained Treg cell-selectivity over a wider dose-range, we assessed Treg cell and effector lymphocyte responses to increasing doses of Fc.WT or Fc.Mut24 by measuring induction of the proliferation marker Ki-67. Treg cells proliferated in a dose-dependent manner in response to both Fc.WT and Fc.Mut24. However, whereas Fc.WT also activated Foxp3−CD4+ T cells, CD8+ T cells and NK cells, Fc.Mut24 did not activate any effector T cells and only stimulated NK cells at the highest doses tested (Fig. 2A). Consequently, there was a robust dose-dependent increase in the frequency of Treg cells compared to all of the effector populations in Fc.Mut24-treated animals (fig. S6), with Treg cells reaching more than 50% of CD4+ T cells at the highest dose. In contrast, Treg cell frequencies in mice given Fc.WT plateaued at less than 30% (Fig. 2, B to E). Stimulation of NK cells by Fc.Mut24 in vivo is curious given their lack of CD25 expression and the inability of Fc.Mut24 to induce STAT5 phosphorylation in NK cells in vitro. However, NK cells express extremely high levels of CD122, and trans-presentation of IL-2 by CD25-expressing cells to CD122hi cells has been reported (31). Administration of daily low-dose human IL-2, or IL-2/JES6–1A12 immune complexes (IL-2ic) have also been used to enrich Treg cells and treat autoimmunity in mice (32). However, Fc.Mut24 treatment induced significantly greater Treg cell expansion compared to either of these alternative approaches (fig. S7).
Fig. 2. Fc.Mut24 remains Treg cell-selective across a wide dose range in vivo.
(A) Percentages of Ki-67+ cells in the indicated populations 4d after PBS or Fc.IL-2 treatment are plotted. (B) Representative flow cytometry analyses of Foxp3 and CD25 expression for mice given PBS or Fc.IL-2 (15μg). (C) Graphical summary of Treg cell frequencies and ratios of (D) Treg:CD8+ and (E) Treg:NK in PBS or Fc.IL-2 treated mice. Data shown as mean ± SD, significance determined by two-way ANOVA followed by Tukey post-test. Representative of at least two independent experiments with n=5 mice/group.
Fc.Mut24 induces sustained Treg cell enrichment in vivo
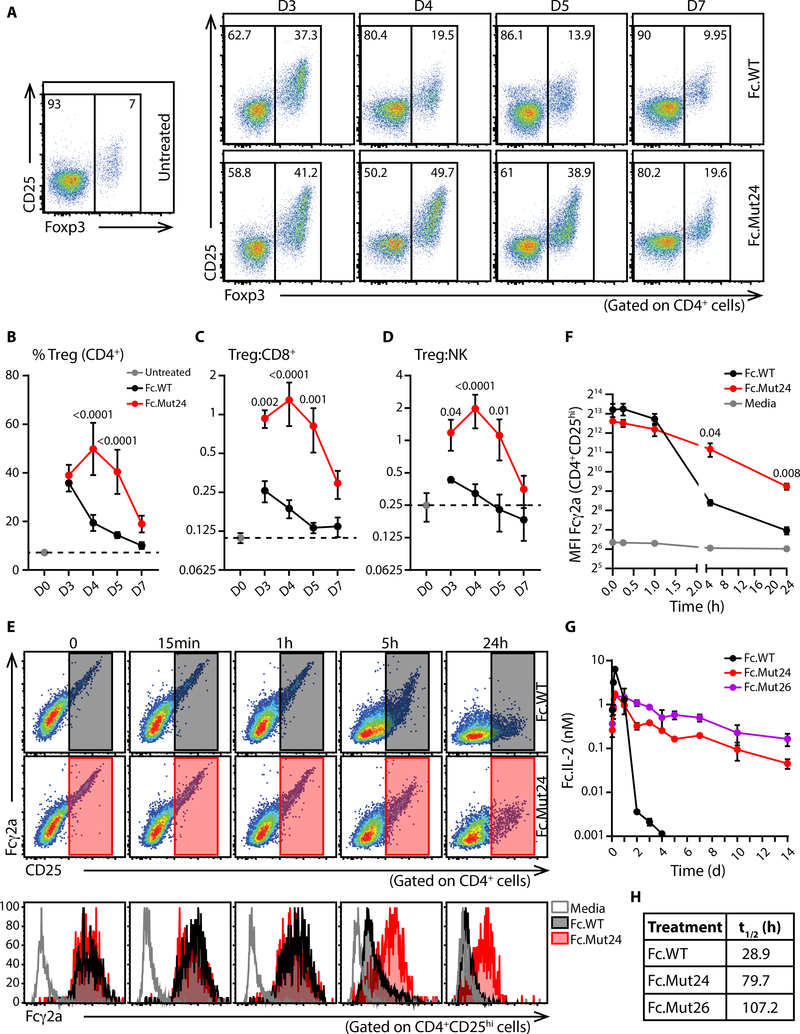
Following a single high-dose (15μg) treatment with Fc.WT, the percentage of Treg cells in the blood peaked on day 3 and declined rapidly to near baseline by day 7. By contrast, the response to treatment with Fc.Mut24 peaked on day 4, then slowly declined but remained elevated compared to baseline for at least a week (Fig. 3, A and B). Furthermore, at all timepoints the ratios of Treg:CD8+ cell and Treg:NK cell were higher in Fc.Mut24-treated mice than in Fc.WT-treated animals, indicating that Treg cell-selectivity is maintained throughout the response (Fig. 3, C and D). At days 5 and 7 post-treatment, Treg cell expansion and selectivity in the LN of Fc.Mut24-treated mice vs Fc.WT-treated mice was similar to that in the blood (fig. S8). Thus, despite being a weaker agonist of IL-2R signaling in vitro, we observed more robust and sustained responses of Treg cells to Fc.Mut24 in vivo.
Fig. 3. Sustained Treg cell enrichment in vivo and lower target mediated clearance of Fc.Mut24.
(A) Representative flow cytometry analyses of Foxp3 and CD25 expression in gated CD4+ T cells at the indicated times after treatment with Fc.IL-2 (15μg). Graphical summaries of (B) Treg cell frequencies, and ratios of (C) Treg:CD8+ and (D) Treg:NK. Data shown as mean ± SD, significance determined by two-way ANOVA followed by Tukey post-test. Representative of two independent experiments with n=4–5 mice/group. (E and F) Anti-CD3/CD28 activated B6 splenocytes were coated with Fc.WT or Fc.Mut24 and cultured for the indicated times. (E) Upper and middle: Representative staining for surface Fc.IL-2 and CD25 on CD4+ cells. Bottom: Analysis of Fcγ2a staining on gated CD4+CD25hi cells. (F) Graphical analysis of median fluorescence intensity of Fcγ2a on gated CD4+CD25hi cells. Data shown as mean ± SD, significance determined by repeated-measures two-Way ANOVA with the Geisser-Greenhouse correction followed by Tukey post-test. Data representative of three biological replicates. (G) Serum levels and (H) calculated in vivo half-life of the indicated Fc.IL-2 molecules in B6 mice after a single 4μg dose. Data shown as mean ± SD, n=2–3 mice/time point.
The in vivo half-life of Fc.IL-2 fusion proteins is largely a function of receptor mediated consumption (25, 33). This led us to hypothesize that due to its weaker activity, Fc.Mut24 should exhibit reduced receptor-mediated clearance, resulting in prolonged retention on the surface of CD25+ cells and an extended in vivo half-life. To test this, splenocytes pre-activated with anti-CD3/CD28 were coated with Fc.WT or Fc.Mut24, washed and cultured at 37°C for up to 24h. We then measured the amount of IL-2 fusion protein on the cell surface by staining cells for CD25 and the Fc portion of cell-associated Fc.IL-2. Whereas the amount of Fc.WT on the surface of CD4+CD25hi cells rapidly declined, surface association of Fc.Mut24 was dramatically prolonged, and we detected substantial levels of Fc.Mut24 on the cell surface even after 24h (Fig. 3, E and F). Accordingly, the half-life of Fc.Mut24 in vivo was approximately three times longer than Fc.WT, and was closer to that of the functionally inactive mutein Fc.Mut26 (Fig. 3, G and H). Thus, diminished consumption via IL-2R signaling resulted in extended pharmacokinetics and sustained activity of Fc.Mut24, and this likely explains the potent and prolonged Treg cell enrichment observed after Fc.Mut24 treatment.
Fc.Mut24 treatment blocks T1D development in NOD mice
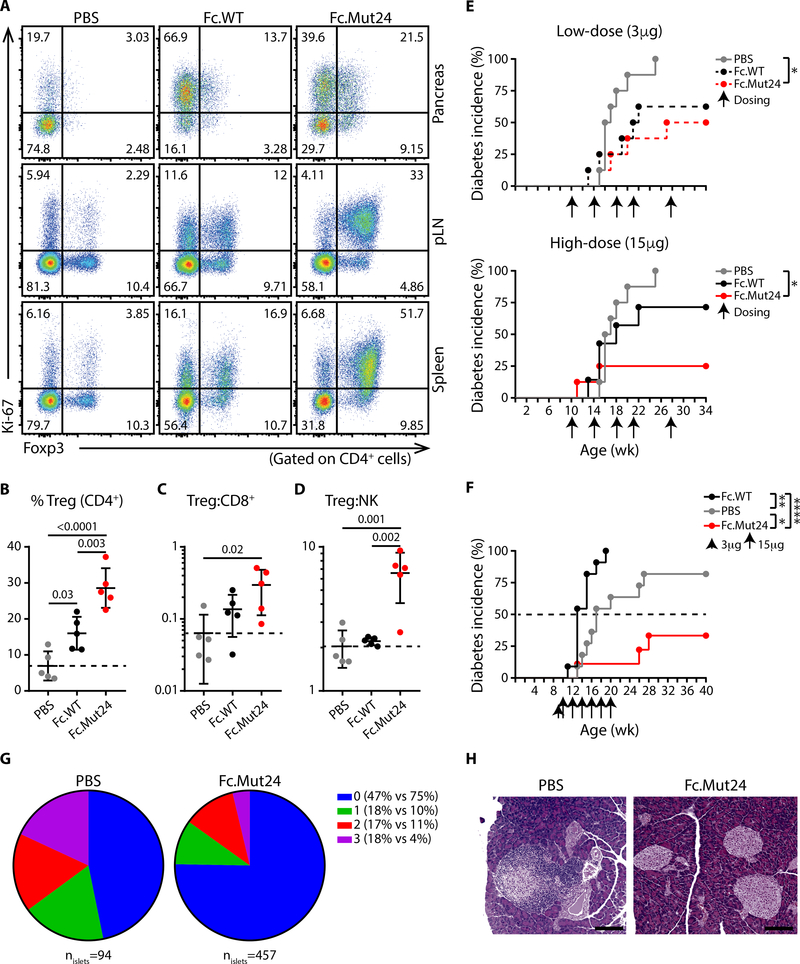
In NOD mice, autoimmunity begins as early as 3 weeks of age (34), and is characterized by infiltration of CD25+ effector T cells and NK cells in the pancreas, which efficiently compete with pancreatic Treg cells for limiting IL-2 to drive pathogenesis (32, 35–37). To determine if the Treg cell-selectivity of Fc.Mut24 is maintained at sites of ongoing IL-2-mediated autoimmunity, we treated 9-week old female NOD mice with a single 15μg dose of Fc.WT, Fc.Mut24, or PBS, and assessed responses of different lymphocyte populations in the pancreas, pancreatic lymph node (pLN), and spleen. Unlike the Treg cell-selective Ki-67 responses to Fc.Mut24 observed in the pLN and spleen, both Fc.Mut24 and Fc.WT induced Ki-67 expression in pancreatic Foxp3−CD4+ and CD8+ T cells to a similar degree (Fig. 4A and fig. S9). However, despite this apparent loss of Treg cell-selectivity in the target organ of the autoimmune response, we still observed dominant expansion of Treg cells in all tissues of Fc.Mut24-treated NOD mice that was superior to that in Fc.WT-treated animals (Fig. 4, A to D).
Fig. 4. Fc.Mut24 induces selective Treg cell enrichment and arrests ongoing autoimmunity in NOD mice.
(A) Representative analyses of Foxp3 and Ki-67 expression in the indicated tissues of female NOD mice 4 days after treatment with PBS, Fc.WT or Fc.Mut24 (15μg). Graphical summaries of (B) Treg cell frequencies and ratios of (C) Treg:CD8+ and (D) Treg:NK in the pancreas after the indicated treatments. Data shown as mean ± SD, significance determined by one-way ANOVA followed by Tukey post-test. Representative of two independent experiments with n=5 mice/group. (E and F) Diabetes incidence in female NOD mice treated with PBS, Fc.WT or Fc.Mut24. Arrows indicate time of dosing. Significance determined by Log-rank (Mantel-Cox) test, *p<0.05, **p<0.01, ****p<0.0001, n=7–8 in (E) and n=9–11 in (F). (G) Pie chart representing the severity of insulitis in disease free mice at the end of the experiment shown in (F) on a severity scale of 0–3. (H) Representative H&E staining of a grade 3 islet from a PBS-treated mouse and three grade 0 islets from an Fc.Mut24-treated mouse (scale bar 100μm).
In NOD mice, daily low-dose IL-2 treatment led to significant protection from type-1 diabetes (T1D) development, whereas frequent high-dose IL-2 treatment actually accelerated disease by potentiating effector T cell and NK cell responses (32). Thus, this is an ideal model to determine if the improved Treg cell-selectivity and pharmacokinetics of Fc.Mut24 translate to better therapeutic efficacy across a wide dose-range and with reduced dosing frequency. Therefore, we treated NOD mice (beginning at 10 weeks of age) with either a low (3μg) or high (15μg) dose of Fc.WT or Fc.Mut24 a total of 5 times over 18 weeks, and monitored blood glucose levels until 34 weeks of age. By 25 weeks, all PBS-treated control mice developed diabetes. In contrast, treatment with 3μg doses of Fc.WT or Fc.Mut24 treatment offered substantial disease protection. Whereas the efficacy of Fc.WT was somewhat reduced at the higher dose, 15μg doses of Fc.Mut24 further enhanced protection against diabetes, with 75% of the mice remaining disease-free throughout the experiment (Fig. 4E). This indicates that clinical efficacy can be achieved by Fc.Mut24 treatment across a wide dose range and with very infrequent dosing relative to prior IL-2 based interventions in this model.
To further evaluate the therapeutic efficacy of Fc.Mut24, we compared Fc.WT and Fc.Mut24 using a dose escalation regimen in which NOD mice were given a single 3μg dose, followed 4 days later by a 12 week course of bi-weekly 15μg treatments. Consistent with previous studies examining frequent high-dose IL-2 administration, in this regimen Fc.WT treatment markedly accelerated disease onset compared to PBS-treated controls. In contrast, durable protection against diabetes was observed in Fc.Mut24-treated mice, that was sustained in the majority of animals for at least 20 weeks following treatment cessation (Fig. 4F). Consistent with arrest of the autoimmune response, histological examination of the mice that were diabetes-free at the end of the study showed a dramatic reduction in the severity of insulitis in the Fc.Mut24-treated mice compared to the surviving PBS-treated controls (Fig. 4, G and H). Thus, Fc.Mut24 treatment results in efficient, targeted, and sustained control of autoimmunity, despite the presence of Fc.mutein-responsive pathogenic T cells at the site of autoimmune inflammation.
DISCUSSION
Defects in Treg cell function and alterations in the IL-2 pathway are associated with autoimmunity (38, 39). These findings have sparked interest in using IL-2 immunotherapy to re-establish tolerance in patients with autoimmune and inflammatory diseases. Administered at low-doses, IL-2 therapy showed promising results of reduced disease activity and improved outcomes in patients with hepatitis C virus-induced vasculitis (40), graft-versus-host disease (41–43), alopecia areata (44), and systemic lupus erythematosus (45–49). Moreover, a recent clinical trial investigating the effects of low-dose IL-2 therapy across 11 autoimmune diseases has shown encouraging signs of efficacy using a dose of 1 MIU/injection in all patients (50). However, although these studies are promising, establishing a “universal” dosage that reliably induces robust Treg cell responses in all patients is likely to be quite challenging, as considerable heterogeneity is observed in responses to low dose IL-2 strategies (24). Differences in the physiopathology of autoimmune diseases may be associated with variable frequencies of CD25-high pathogenic Teff in the target tissue, thereby altering the dose required for optimal Treg selectivity. Furthermore, certain patients may carry intrinsic alterations of the IL-2 pathway that can limit the efficacy of the treatment, as in T1D (51, 52). To avoid potential treatment-induced toxicity and disease exacerbation by activating effector responses, the therapeutic dose-range for IL-2 remains narrow and potentially too low to achieve optimal clinical outcomes. Finally, the short in vivo half-life of IL-2 necessitates very frequent administration. Thus, there is a pressing need for new IL-2-based therapeutics with enhanced Treg-selectivity and improved pharmacokinetics.
We engineered cell-selectivity into mouse IL-2 via mutations at the interface with CD122. Fc.Mut24 contains two amino acid substitutions at conserved CD122 contact residues, replacing neutral-polar residue N103 with a positively charged arginine (R), and hydrophobic V106 with a negatively charged aspartic acid (D). Based on IL-2 and IL-2Rβ mouse:human homologies and structures of the human IL-2/IL-2R quaternary complex, N103 in murine IL-2 (homologous to N88 in human IL-2, N88hIL−2) should form interactions with 4 conserved residues on murine IL-2Rβ (R42, Q71, T74, Y135), and V106 in murine IL-2 (V91hIL−2) should interact with 3 conserved murine IL-2Rβ residues, 2 of which also interact with N88hIL−2 in the human structure (R42, T74, V76) (3, 53). Interestingly, R41 in human IL-2Rβ (R41hIL−2Rb) also contacts V91hIL−2, and is the only residue involved in contacting either N88hIL−2 or V91hIL−2 that is not conserved in murine IL-2Rβ. Given that the analog of the positively charged R41hIL−2Rβ is a hydrophobic L41 in mouse (L41mIL−2Rβ), we speculate that the presence of this leucine may actually increase the strength of interaction with the hydrophobic V106 in murine IL-2, compared to R41hIL−2Rβ-V91hIL−2 interaction in human. This may explain why both the N103R and V106D mutations were required to achieve the substantially reduced signaling potency of Fc.Mut24. Indeed, N88D and N88R mutations dramatically attenuated the function of human IL-2 (25, 26), whereas the homologous N103R mutation in murine IL-2 only modestly affected signaling potency (Fc.Mut11 in fig. S3).
Fc.Mut24 induced robust expansion of Treg cells in multiple locations including the blood, secondary lymphoid tissues, and non-lymphoid autoimmune target tissue of treated mice. Thus, differences in the Treg cell expansion following Fc.WT and Fc.Mut24 treatment are not due to differential effects on Treg cell recruitment to different tissue compartments, but instead reflect the extended response induced by Fc.Mut24. Although Fc.Mut24 did not induce STAT5 phosphorylation by NK cells in vitro, we did observe stimulation of NK cells by high doses of Fc.Mut24 in vivo. This activity may result from trans-presentation of Fc.mut24 to CD25−CD122hi NK cells by CD25-expressing cells, or by soluble or matrix-bound CD25, and this NK response may represent the limit of Treg cell-specificity achieved through selectively targeting IL-2 to CD25+ cells. The in vivo half-life of Fc.IL-2 fusion proteins depends on the rate of IL-2R mediated clearance (25, 33). Consistent with this, we found that the half-life of Fc.WT, Fc.Mut24 and Fc.Mut26 inversely correlated with their signaling potencies, and that the half-life of Fc.Mut24 is almost three times longer than Fc.WT. In vitro, we observed a prolonged association of Fc.Mut24 with surface CD25. The slower clearance of Fc.Mut24 off the surface of CD25-expressing cells would allow for extended bioavailability compared to a rapid consumption of Fc.WT, and the improved pharmacokinetics of Fc.Mut24 likely underpin its extended activity and enhanced Treg cell enrichment compared to Fc.WT.
The increased CD25-dependency and prolonged in vivo half-life of Fc.Mut24 translate into an improved dynamic range of selective Treg cell enrichment and a greater flexibility in therapeutically efficacious dosing strategies. We also found that chronic Treg cell enrichment achieved by frequent dosing was not required to provide lasting protection against autoimmune diabetes, and that disease protection lasted for up to 20 weeks after cessation of treatment. Durable reversal of autoimmunity occurred despite efficient Ki-67 upregulation in pancreatic effector T cells, presumably due to their high CD25 expression. This loss of Treg cell-selectivity in inflamed tissues suggests that systemic Treg cell enrichment may be important for resolving organ-specific autoimmunity.
Human IL-2 muteins with analogous Treg cell-selective properties are being tested for potential use in autoimmune and inflammatory diseases (54, 55). A recent clinical trial treated high-risk first-degree relatives of T1D patients who tested positive for at least two diabetes-related autoantibodies with anti-CD3, and showed significant therapeutic benefit in delaying disease onset (56). Similarly, we started Fc.IL-2 treatment in NOD mice after initiation of autoimmunity but before development of overt hyperglycemia, and our results suggest that IL-2 mutein treatment may be also therapeutically efficacious in at-risk subjects. Thus, Fc.Mut24 is a valuable experimental tool for pre-clinical mechanistic studies, and our findings provide a strong proof-of-concept for IL-2 mutein therapy that help inform future design of clinical trials and dosing strategies. Through optimal and targeted control of the autoimmune response, the IL-2 mutein approach represents a more efficient and flexible alternative than low-dose IL-2 for successful treatment of autoimmunity.
MATERIALS AND METHODS
Study design
The objective of this research was to develop and characterize a Treg-selective IL-2 mutein for pre-clinical mechanistic studies in experimental animals. This was accomplished by generating and screening recombinant Fc.mutein proteins, selecting an optimal candidate, and characterizing its functional activity, pharmacokinetics, and ability to prevent disease in standard murine models. No statistical method was used to predetermine sample sizes, but these were selected based on expected variance and effect size in well-characterized experimental systems, and were large enough to ensure achieve a greater than 80% probability of identifying an effect of >20% in measured variables.
Fc.IL-2 muteins
We generated constructs for twenty-eight IL-2 muteins with point mutations introduced at the CD122 interface by site-directed mutagenesis. All muteins also contain P51T and C140A mutations, which improved manufacturability but did not affect IL-2 activity. The P51T mutation was included to reduce cleavage at P51-R52-M53 when IL-2 muteins were expressed in CHO cells; however, we found that expression in HEK 293T/17 does not require the P51T mutation for efficient production. For future work we recommend omitting this mutation. The C140A mutation inhibits disulfide bridging and aggregation during expression. IL-2 muteins were fused via a 4GS linker to the C-terminus of murine IgG2a Fc (N297G, deleted c-terminal K). Fc.IL-2-expressing plasmids (pcDNA3.1) were transiently transfected into HEK 293T/17 adherent cells using PEI 25K (ratio 3:1, PEI to DNA (w/w)). Three days later, supernatants were harvested for in vitro screening. Selected IL-2 muteins were produced and purified by the Biologics Production Facility at Fred Hutchinson Cancer Research Center (Seattle, WA) or by Olympic Protein Technologies (Seattle, WA). Purified IL-2 muteins used in in vivo experiments contained less than 15 EU/mL of endotoxin.
Mice
C57BL/6J (B6) (stock number: 000664) and NOD/ShiLtJ (NOD) (stock number: 001976) mice were purchased from The Jackson Laboratory. All animal experiments were conducted in accordance with policies of the Institutional Animal Care and Use Committee of the Benaroya Research Institute, or the Amgen Institutional Animal Care and Use Committee.
In vitro assays
For pSTAT5 assays, freshly isolated or anti-CD3 (1μg/mL) activated B6 splenocytes (5×105 cells) were stimulated with titrated human recombinant IL-2 (Proleukin), mouse recombinant IL-2, or Fc.IL-2 for 15 min at 37°C in complete RPMI (RPMI supplemented with 10% FBS (heat inactivated), L-glutamine, penicillin-streptomycin, and sodium pyruvate). For Fc.IL-2 association with cell surface CD25, B6 splenocytes were activated with 1μg/mL anti-CD3 and 1μg/mL anti-CD28 for 24h. Cells were washed, rested for 48h, then coated with 1nM Fc.IL-2 for 15 min at RT. Coated splenocytes were thoroughly washed (3 times) to remove excess protein and incubated at 37°C for various time points. All the steps were performed in complete RPMI. For Fc.IL-2 binding to CD25, a plasmid encoding mouse CD25 was transiently transfected into HEK 293T/17 cells using PEI 25K (ratio 3:1, PEI to DNA (w/w)). CD25-expressing HEK 293T/17 cells were coated with titrated Fc.IL-2 for 15 min at RT in DMEM supplemented with sodium pyruvate and 5% FBS. Coated cells were thoroughly washed (4 times) then stained for flow cytometry analysis.
In vivo dosing assays
B6 mice (7–12 weeks old, females or males) and NOD mice (9–10 weeks old, females) were given a single dose of Fc.IL-2, IL-2 immune complex (IL-2ic; mouse recombinant IL-2 + anti-IL-2 (JES6–1A12); molar ratio 2:1, IL-2 to antibody), or PBS by intraperitoneal (IP) injection. Human recombinant IL-2 was administered by daily IP injection for four consecutive days (day 0 through day 3). Analysis was performed four days after initiation of the treatment. For the time course experiment, blood was collected from the saphenous vein of untreated mice (day 0) and on day 3, 4, 5, and 7 after dosing. Inguinal lymph nodes were harvested on day 0, 5, and 7.
PK study
Female C57BL/6 mice (Charles River Laboratories) received a 4μg/mouse subcutaneous dose of Fc.IL-2 in the mid-scapular region. Quantitation of IL-2 in mouse serum was performed using an electrochemiluminescent immunoassay with a biotinylated rat anti-mouse IL-2 as a capture reagent and a ruthenylated goat anti-mouse IgG, Fcγ subclass 2a specific as a detection reagent. Serum concentrations of Fc.WT, Fc.Mut24, and Fc.Mut26 were calculated from individual standard curves for each of the IL-2 variants. Thus, any differences in the ability to detect the IL-2 molecules (which were negligible) are taken into consideration in the analysis. Noncompartmental pharmacokinetic analyses were performed for the mean of three study subjects at each time point using non GLP Watson LIMS (Thermo Fisher Scientific).
NOD mice diabetes study
Female NOD mice were received at 6 weeks of age. Cages were changed once a week for mice younger than 10 weeks old and twice a week after that. Blood glucose levels and body weight were measured weekly. Diabetes was confirmed when blood glucose was above 250mg/dL for two consecutive days. Mice received IP injections of PBS, Fc.WT or Fc.Mut24 at the indicated times. All disease-free mice were euthanized at week 34 (experiment 1) or week 40 (experiment 2).
Flow Cytometry
Single cell suspensions from spleen and lymph nodes were prepared by mechanic disruption and filtered through 70μm strainer. Pancreata were digested enzymatically in plain RPMI supplemented with DNase I (1:200) and liberase TM (1:100) for 30 min at 37°C under agitation. Cell suspensions were filtered through 70μm strainer and immune cells were isolated using Percoll gradient. For blood samples, RBCs were lysed using 1X RBC lysis buffer. Cells were counted using Countess II FL Automated Cell Counter (invitrogen).
Extracellular staining was performed in PBS supplemented with 0.5% BSA at 4°C. For surface mutein staining, samples were incubated first with polyclonal anti-mouse Fcγ2a PE. After 30 min, cells were washed and mouse IgG2a was used for blocking for 15 min. Surface staining antibodies were then added for 30 min.
Intracellular staining was performed using the Foxp3 transcription factor staining buffer set. For pSTAT5 assay, cells were stained using the transcription factor phospho buffer set according to the manufacturer’s instructions. A detailed list of the reagents and antibodies used for cellular staining is provided in Table S2.
Intravascular labeling in NOD mice was done using anti-CD45.1 PE administered by retro-orbital injection shortly before euthanasia (3μg/mouse) for discrimination of vascular and tissue-localized cells in the pancreas.
Flow cytometric data were acquired on BD LSRII flow cytometers using BD FACSDIVA software (version 8.0.1) and analyses were performed with FlowJo software (version 10.5.0).
Histology
Pancreata were fixed in 10% neutral buffered formalin, and embedded in paraffin. 5μm sections (three/mouse, 200μm apart) were stained with hematoxylin and eosin. Islets were scored for the severity of insulitis on a scale of 0–3 (0, no infiltration; 1, peri-islet infiltration; 2, <50% islet infiltration; and 3, >50% infiltration) using Leica DM2500 microscope. Images were taken with SPOT Insight 4.0 Megapixel CCD digital camera using SPOT software (version 5.1).
Statistics
Statistical significance was determined using GraphPad Prism software (version 8.0.0). For titration curves, EC50 was calculated using nonlinear regression assuming a variable slope (four parameters). Statistical differences among groups were calculated as described in the figure legends. Significance is determined by p ≤ 0.5 (*p<0.05, **p<0.01, ****p<0.0001) and all significant comparisons are shown.
Supplementary Material
Table S2. List of key reagents.
Table S3. Raw data file.
Fig. S1. Representative gating strategy of relevant lymphocyte populations.
Fig. S2. Fc.WT shows a comparable activity to mouse recombinant IL-2.
Fig. S3. Fc.mutein activity in Treg cells ranges from full potency to complete functional abrogation.
Fig. S4. Fc.muteins retain their ability to bind CD25.
Fig. S5. Fc.muteins retain Treg cell-selectivity across a wide dose range on activated T cells.
Fig. S6. Fc.Mut24 remains Treg cell-selective across a wide dose range in vivo.
Fig. S7. Fc.Mut24 expands Treg cells more specifically and effectively than daily low-dose IL-2 and IL-2ic in vivo.
Fig. S8. Sustained Treg cell enrichment following Fc.Mut24 treatment.
Fig. S9. Reduced Treg cell-selectivity of Fc.Mut24 in pancreatic cells.
Table S1. Panel of Fc.IL-2 muteins.
Acknowledgments:
We thank the Campbell, Long, Buckner and Cerosaletti lab members and C. Pfleger for helpful discussions. We thank C. Stefani and A. Lacy-Hulbert for providing HEK cells and transfection reagents. We thank Benaroya Research Institute core laboratories for technical assistance: P. Johnson and R. Vernon (Histology/Imaging core), K. Arumuganathan, A. Wojno, T. Nguyen (Flow Cytometry core), K. Schwedhelm, J. Thorpe, M. Maerz, and A. Kus (Human Immunophenotyping core), A. Burich, C. Toledano and the vivarium personnel (Animal Resources).
Funding: This work was funded in part by JDRF 2-SRA-2016-305-S-B and Amgen, Inc. to M.A.G. and NIH grant R01AI136475 to D.J.C.
Footnotes
Competing interests: M.A.G., K.C. and J.P. own stock in Amgen, Inc. M.A.G has served as a consultant to Amgen, Inc. All other authors declare that they have no competing interests.
Data and materials availability: All data necessary to understand and evaluate the conclusions of the paper are provided in the manuscript and supplementary materials. Fc.IL2 encoding plasmids are available from D.J.C. under a materials transfer agreement with the Benaroya Research Institute.
REFERENCES AND NOTES
- 1.Malek TR, The biology of interleukin-2. Annu. Rev. Immunol 26, 453–479 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Trotta E, Simeonov DR, Marson A, Bluestone JA, Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol 3, eaat1482 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Rickert M, Garcia KC, Structure of the quaternary complex of interleukin-2 with its α, β and γc receptors. Science. 310, 1159–1163 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M, Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol 155, 1151–1164 (1995). [PubMed] [Google Scholar]
- 5.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY, A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6, 1142–1151 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA, CD4 + CD25 high Regulatory Cells in Human Peripheral Blood. J. Immunol 167, 1245–1253 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Boyman O, Sprent J, The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol 12, 180–190 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Cantrell DA, Smith KA, Transient expression of interleukin 2 receptors: Consequences for T cell growth. J. Exp. Med 158, 1895–1911 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HP, Imbert J, Leonard WJ, Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev 17, 349–366 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Shatrova AN, Mityushova EV, Vassilieva IO, Aksenov ND, Zenin VV, Nikolsky NN, Marakhova II, Time-dependent regulation of IL-2R α-chain (CD25) expression by TCR signal strength and IL-2-induced STAT5 signaling in activated human blood T lymphocytes. PLoS One. 11, e0167215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY, Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 445, 771–775 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ, Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 109, 4368–4375 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudensky AY, Regulatory T cells and Foxp3. Immunol. Rev 241, 260–268 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Arvey A, Chinen T, Van Der Veeken J, Gasteiger G, Rudensky AY, Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 158, 749–763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim GC, Radvanyi L, The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev 25, 377–390 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Wrangle JM, Patterson A, Johnson CB, Neitzke DJ, Mehrotra S, Denlinger CE, Paulos CM, Li Z, Cole DJ, Rubinstein MP, IL-2 and beyond in cancer immunotherapy. J. Interf. Cytokine Res 38, 45–68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu P, Mizokami M, Ruoff G, Khawli LA, Epstein AL, Generation of low-toxicity interleukin-2 fusion proteins devoid of vasopermeability activity. Blood. 101, 4853–4861 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Gillies SD, Lan Y, Hettmann T, Brunkhorst B, Sun Y, Mueller SO, Lo KM, A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin. Cancer Res 17, 3673–3685 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Laurent J, Touvrey C, Gillessen S, Joffraud M, Vicari M, Bertrand C, Ongarello S, Liedert B, Gallerani E, Beck J, Omlin A, Sessa C, Quaratino S, Stupp R, Gnad-Vogt US, Speiser DE, T-cell activation by treatment of cancer patients with EMD 521873 (Selectikine), an IL-2/anti-DNA fusion protein. J. Transl. Med 11, 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P, Pande VS, Fathman CG, Boyman O, Garcia KC, Exploiting a natural conformational switch to engineer an interleukin-2 “superkine.” Nature. 484, 529–533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva DA, Yu S, Ulge UY, Spangler JB, Jude KM, Labão-Almeida C, Ali LR, Quijano-Rubio A, Ruterbusch M, Leung I, Biary T, Crowley SJ, Marcos E, Walkey CD, Weitzner BD, Pardo-Avila F, Castellanos J, Carter L, Stewart L, Riddell SR, Pepper M, Bernardes GJL, Dougan M, Garcia KC, Baker D, De novo design of potent and selective mimics of IL-2 and IL-15. Nature. 565, 186–191 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klatzmann D, Abbas AK, The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol 15, 283–294 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Ye C, Brand D, Zheng SG, Targeting IL-2: an unexpected effect in treating immunological diseases. Signal Transduct. Target. Ther 3, 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seelig E, Howlett J, Porter L, Truman L, Heywood J, Kennet J, Arbon EL, Anselmiova K, Walker NM, Atkar R, Pekalski ML, Rytina E, Evans M, Wicker LS, Todd JA, Mander AP, Bond S, Waldron-Lynch F, The DILfrequency study is an adaptive trial to identify optimal IL-2 dosing in patients with type 1 diabetes. JCI Insight 3, e99306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson LB, Bell CJM, Howlett SK, Pekalski ML, Brady K, Hinton H, Sauter D, Todd JA, Umana P, Ast O, Waldhauer I, Freimoser-Grundschober A, Moessner E, Klein C, Hosse RJ, Wicker LS, A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J. Autoimmun 95, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanafelt AB, Lin Y, Shanafelt MC, Forte CP, Dubois-Stringfellow N, Carter C, Gibbons JA, Cheng SL, Delaria KA, Fleischer R, Greve JM, Gundel R, Harris K, Kelly R, Koh B, Li Y, Lantz L, Mak P, Neyer L, Plym MJ, Roczniak S, Serban D, Thrift J, Tsuchiyama L, Wetzel M, Wong M, Zolotorev A, A T-cell-selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nat. Biotechnol 18, 1197–1202 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Zurawski SM, Zurawski G, Mouse interleukin-2 structure-function studies: substitutions in the first alpha-helix can specifically inactivate p70 receptor binding and mutations in the fifth alpha-helix can specifically inactivate p55 receptor binding. EMBO J 8, 2583–2590 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zurawski SM, Imler JL, Zurawski G, Partial agonist/antagonist mouse interleukin-2 proteins indicate that a third component of the receptor complex functions in signal transduction. EMBO J 9, 3899–3905 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zurawski SM, Vega F Jr., Doyle EL, Huyghe B, Flaherty K, McKay DB, Zurawski G, Definition and spatial location of mouse interleukin-2 residues that interact with its heterotrimeric receptor. EMBO J 12, 5113–5119 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen FW, Stevenson R, Li C, Salimi-Moosavi H, Liu L, Wen J, Luo Q, Daris K, Buck L, Miller S, Ho SY, Wang W, Chen Q, Walker K, Wypych J, Narhi L, Gunasekaran K, Engineering an IgG scaffold lacking effector function with optimized developability. J. Biol. Chem 292, 1865–1875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wuest SC, Edwan JH, Martin JF, Han S, Perry JSA, Cartagena CM, Matsuura E, Maric D, Waldmann TA, Bielekova B, A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med 17, 604–609 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA, Central Role of Defective Interleukin-2 Production in the Triggering of Islet Autoimmune Destruction. Immunity. 28, 687–697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell CJM, Sun Y, Nowak UM, Clark J, Howlett S, Pekalski ML, Yang X, Ast O, Waldhauer I, Freimoser-Grundschober A, Moessner E, Umana P, Klein C, Hosse RJ, Wicker LS, Peterson LB, Sustained in vivo signaling by long-lived IL-2 induces prolonged increases of regulatory T cells. J. Autoimmun 56, 66–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson JA, Wong FS, Wen L, The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J. Autoimmun 66, 76–88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D, Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J. Exp. Med 210, 1153–1165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James CR, Buckle I, Muscate F, Otsuka M, Nakao M, Oon JS, Steptoe RJ, Thomas R, Hamilton-Williams EE, Reduced interleukin-2 responsiveness impairs the ability of Treg cells to compete for IL-2 in nonobese diabetic mice. Immunol. Cell Biol 94, 509–519 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Godoy GJ, Olivera C, Paira DA, Salazar FC, Ana Y, Stempin CC, Motrich RD, Rivero VE, T regulatory cells from non-obese diabetic mice show low responsiveness to IL-2 stimulation and exhibit differential expression of anergy-related and ubiquitination factors. Front. Immunol 10, 2665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baecher-Allan C, Hafler DA, Human regulatory T cells and their role in autoimmune disease. Immunol.Rev 212, 203–216 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Malek TR, Yu A, Zhu L, Matsutani T, Adeegbe D, Bayer AL, IL-2 family of cytokines in T regulatory cell development and homeostasis. J. Clin. Immunol 28, 635–639 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D, Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med 365, 2067–2077 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Koreth J, Matsuoka KI, Kim HT, McDonough SM, Bindra B, Alyea EP III, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ, Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med 365, 2055–2066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu M-F, Liu H, Melenhorst J, Barrett AJ, Ito S, Foster A, Savoldo B, Yvon E, Carrum G, Ramos CA, Krance RA, Leung K, Heslop HE, Brenner MK, Bollard CM, Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin. Cancer Res 20, 2215–2225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whangbo JS, Kim HT, Mirkovic N, Leonard L, Poryanda S, Silverstein S, Kim S, Reynolds CG, Rai SC, Verrill K, Lee MA, Margossian S, Duncan C, Lehmann L, Huang J, Nikiforow S, Alyea EP, Armand P, Cutler CS, Ho VT, Blazar BR, Antin JH, Soiffer RJ, Ritz J, Koreth J, Dose-escalated interleukin-2 therapy for refractory chronic graft-versus-host disease in adults and children. Blood Adv 3, 2550–2561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, Lacour J-P, Passeron T, Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA dermatology. 150, 751 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Humrich JY, von Spee-Mayer C, Siegert E, Alexander T, Hiepe F, Radbruch A, Burmester G-R, Riemekasten G, Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann. Rheum. Dis 74, 792 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Von Spee-Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, Enghard P, Sawitzki B, Hiepe F, Radbruch A, Burmester GR, Riemekasten G, Humrich JY, Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis 75, 1407–1415 (2016). [DOI] [PubMed] [Google Scholar]
- 47.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, Li Z, Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med 22, 991–993 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Humrich JY, von Spee-Mayer C, Siegert E, Bertolo M, Rose A, Abdirama D, Enghard P, Stuhlmüller B, Sawitzki B, Huscher D, Hiepe F, Alexander T, Feist E, Radbruch A, Burmester GR, Riemekasten G, Low-dose interleukin-2 therapy in refractory systemic lupus erythematosus: an investigator-initiated, single-centre phase 1 and 2a clinical trial. Lancet Rheumatol 1, e44–e54 (2019). [DOI] [PubMed] [Google Scholar]
- 49.He J, Zhang R, Shao M, Zhao X, Miao M, Chen J, Liu J, Zhang X, Zhang X, Jin Y, Wang Y, Zhang S, Zhu L, Jacob A, Jia R, You X, Li X, Li C, Zhou Y, Yang Y, Ye H, Liu Y, Su Y, Shen N, Alexander J, Guo J, Ambrus J, Lin X, Yu D, Sun X, Li Z, Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis 79, 141–149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenzwajg M, Lorenzon R, Cacoub P, Pham HP, Pitoiset F, El Soufi K, Rlbet C, Bernard C, Aractingi S, Banneville B, Beaugerie L, Berenbaum F, Champey J, Chazouilleres O, Corpechot C, Fautrel B, Mekinian A, Regnier E, Saadoun D, Salem JE, Sellam J, Seksik P, Daguenel-Nguyen A, Doppler V, Mariau J, Vicaut E, Klatzmann D, Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis 78, 209–217 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Koreth J, Ritz J, Tsokos GC, Pugliese A, Malek TR, Rosenzwajg M, Klatzmann D, Low-dose Interleukin-2 in the Treatment of Autoimmune Disease. Oncol. Hematol. Rev 10, 157–163 (2014). [Google Scholar]
- 52.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang Z, Pihoker C, Sanda S, Greenbaum C, Buckner JH, Defects in IL-2R Signaling Contribute to Diminished Regulatory T-Cells of Type 1 Diabetic Subjects. Diabetes 59, 407–415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA, Crystal structure of the IL-2 signaling complex: Paradigm for a heterotrimeric cytokine receptor. Proc. Natl. Acad. Sci. U. S. A 103, 2788–2793 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tchao N, Gorski KS, Yuraszeck T, Sohn SJ, Ishida K, Wong H, Park K, Amg 592 is an investigational IL-2 mutein that induces highly selective expansion of regulatory T cells. Blood. 130, 696 (2017).28798058 [Google Scholar]
- 55.Gorski KS, Stern J, Hsu Y-H, Anderson A, Boedigheimer M, Tchao N, Phenotype of foxp3+ regulatory t-cells expanded by the il-2 mutein, amg 592 in healthy subjects in phase 1, first-in-human study. Ann. Rheum. Dis 77, 243 (2018). [Google Scholar]
- 56.Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler A-G, Greenbaum CJ, An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med 381, 603–613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. List of key reagents.
Table S3. Raw data file.
Fig. S1. Representative gating strategy of relevant lymphocyte populations.
Fig. S2. Fc.WT shows a comparable activity to mouse recombinant IL-2.
Fig. S3. Fc.mutein activity in Treg cells ranges from full potency to complete functional abrogation.
Fig. S4. Fc.muteins retain their ability to bind CD25.
Fig. S5. Fc.muteins retain Treg cell-selectivity across a wide dose range on activated T cells.
Fig. S6. Fc.Mut24 remains Treg cell-selective across a wide dose range in vivo.
Fig. S7. Fc.Mut24 expands Treg cells more specifically and effectively than daily low-dose IL-2 and IL-2ic in vivo.
Fig. S8. Sustained Treg cell enrichment following Fc.Mut24 treatment.
Fig. S9. Reduced Treg cell-selectivity of Fc.Mut24 in pancreatic cells.
Table S1. Panel of Fc.IL-2 muteins.