Abstract
Nanocomposite materials based on metal nanoparticles and graphene oxide (GO) have gained increasing attention for their wide range of potential applications in various materials science fields. In this study, an efficient photocatalyst based on GO/ZnO nanocomposites with embedded metal nanoparticles was successfully synthesized via a simple one-pot method. The synthesized nanocomposites were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared (FTIR) spectroscopy. The photocatalytic activity of the synthesized nanocomposites was tested in the degradation of methylene blue (MB) dyes, as a model of water pollutants. A catalytic activity of 84% was achieved using a nanocomposite with a percentage of 3.125% GO, after 90 min sunlight irradiation. Furthermore, embedded copper and silver nanoparticles were used as dopants to study their effects on the activity of the photocatalyst. The GO–ZnO–Cu nanocomposite showed that the activity toward MB degradation was decreased by 50%, while a significant increase in the activity of MB degradation was achieved by the GO–ZnO–Ag nanocomposite. The removal efficiency of MB by the GO–ZnO–Ag nanocomposite reached 100% after 40 min of sunlight irradiation. Thus, the GO–ZnO–Ag nanocomposite has the potential to be an efficient adaptable photocatalyst for the photodegradation of organic dyes in industrial wastewater.
1. Introduction
A serious problem of water pollution was raised with the rapid evolution of industries and a large amount of pollutants they produced such as organic solvents, oils, and dyes that were discharged to water resources. The discharge of this type of pollutants to water resources leads to a shortage of fresh and clean water that is indispensable for the sustainability of the global population.1,2 Thus, water pollution remains at the forefront of the central societal apprehensions.3 That is why new technologies have been developed for the photodegradation of organic pollutants.
The photodegradation of dyes using semiconductor materials abetted by sunlight irradiation is the most effective method for wastewater treatment.4,5 This type of photodegradation is combined with advanced oxidation processes (AOPs), which are based on chemical treatment of organic pollutants by oxidation reactions with hydroxyl radicals (•OH). The high oxidation potential (2.8 V) of •OH makes it capable to fully oxidize organic dyes at room temperature.6 Andreozzi et al.7 discussed the most common techniques that belong to AOPs, namely, hydrogen peroxide/UV irradiation (H2O2/UV), ozone/UV irradiation (O3/UV), hydrogen peroxide/ozone (H2O2/O3), and metal oxide-catalyzed/UV oxidation. The degradation reaction occurs by attacking the organic compounds with •OH, which can be converted to small oxidized byproducts, as shown in eq 1, where the in situ formed H2O2 oxidizing agent played a substantial rule in additional hydroxyl radical •OH generation.8,9
| 1 |
TiO2 and ZnO have gained a considerable significance for their exceptional photoactivity and attracted the attention of researchers, mainly due to their lower cost, exceptional photosensitivity as well as chemical, physical, and photocatalytic stability.10,11
ZnO semiconductors retain exemplary photosensitivity due to unique properties such as wide band gap (3.37 eV), high intrinsic electron mobility (300 cm2/V·s), and high exciton binding energy (60 meV);12 therefore, they have been used as an efficient photocatalyst for the degradation of organic pollutants.13 Many studies showed that ZnO is considered as a more effective photocatalyst than TiO2.14 Kumaresan et al.15 developed a hydrothermal route to obtain different one- and two-dimensional (2D) nanostructures of ZnO by changing the pH. Spindle nanorods, hexagonal disks, nanorods, and flower nanorods have been introduced by adjusting the pH values to 7, 9, 11, and 13, respectively. The photoactivity of the prepared sample showed that the optimum result was achieved using the two-dimensional structures such as hexagonal disks for the degradation of about 94% of rhodamine B (RhB) dye after 120 min exposure to light irradiation. Recently, Kiriarachchi et al. developed a fast and simple route for the synthesis of sea urchin-like three-dimensional (3D) ZnO nanostructures. The synthesized ZnO nanostructures show superior photocatalytic efficiency for the degradation of common organic dyes.16 The 3D nanostructure ZnO has gained considerable attention by serving in photocatalysis and sensing applications due to its unique physical properties such as high surface area, low aggregation, and superior carrier mobility.
The properties of ZnO nanoparticles depend on their size, shape, surface area, crystallinity, and stability, which are controlled by the synthesis methods and conditions. Recently, Guan et al.17 successfully developed a green one-step method using bamboo cellulose nanocrystal (CNC) as templates for the growth of ZnO nanoparticles with modulated morphologies. The synthesized ZnO/CNC hybrids behaved as an exceptional and low-cost adsorbent for efficient adsorption of dyes in wastewater treatment. One of the challenging tasks in the synthesis of nanocellulose–ZnO nanohybrids is to obtain a nanolevel dispersion and high homogeneity. Therefore, more eco-friendly templates are expected to be investigated for improving dispersion and avoiding self-aggregation or microphase separation of these nanohybrids. Graphene oxide (GO) is receiving increasing attention because it possesses similar properties to graphene as well as the special surface structures with the introduced hydroxyl and carboxyl groups for the synthesis of GO-containing nanocomposites. In addition, graphene oxide (GO) provides a large scaffold for anchoring various substances owing to its large specific surface area and a two-dimensional planar conjugated structure. The hybridization of graphene with semiconductor materials has attained significant attention due to its unique electrical and optical properties with superior high surface area, which can promote the photoactivity of semiconductors.18 These types of nanocomposites exhibit high surface area (about 2630 m2/g) and high electrical (106 S/cm) and thermal (5000 W/m·K) conductivities, which can serve well in energy storage, photochemical, and photocatalytic applications. Moreover, a promising attractive character of graphene was achieved by the ease of functionalization.19,20
Electron–hole recombination is one of the major factors limiting the efficiency of ZnO-based photocatalysts. Therefore, several strategies were employed to suppress electron–hole recombination and boost the photocatalytic efficiency of ZnO. One of the successful methods was introducing GO and metal nanoparticles to enhance the photocatalytic activity of ZnO. Darvishi et al.21 prepared GO–CuO nanocomposites by the microwave irradiation technique, where the effect of GO on the photoactivity of the CuO catalyst for methylene blue (MB) degradation after 60 min of UV irradiation has been investigated. The synthesized GO–CuO nanocomposite showed that the efficiency increased by 4.48 times than that achieved by pure CuO. In addition, it has been observed that the degradation rate increases by increasing the ratio of GO. This is mainly due to the synergistic effect between GO and metal oxides that promotes the electron–hole separation22 and enhances the oxidation reaction on the semiconductor surface;23 also, the presence of GO increased the possibility of pollutant adsorption on the surface of the nanocomposite.24
In recent years, noble metal hybridization in semiconductor photocatalysts has attained significant attention because of the ability of noble metals to increase the photoactivity of the semiconductors.25 The hybridization represents an efficient approach to avoid e–/h+ recombination in the semiconductors. Furthermore, it has been concluded that Ag acts as an antenna for visible light, which boosts the efficiency of the catalyst.22 Zhang et al. prepared GO–ZnO and GO–ZnO–Pd by a hydrothermal route, where hybrid photocatalysts with different weight ratios of ZnO and GO were synthesized.26 They demonstrated that the activity of GO composite is higher than ZnO for the photodegradation of MB under UV irradiation. The GO composite that contains Pd nanoparticles gave higher activity than that achieved by ZnO and GO–ZnO. GO was suggested to enhance the photocatalytic activity of ZnO because of its great capability to dye adsorption and charge separation. Furthermore, the junction between Pd and ZnO was believed to effectively separate the photogenerated charges due to the metal–semiconductor diode effect.27,28
The great light absorption capability and high separation efficiency of photogenerated electron–hole pairs among a composite of TiO2, carbon, and Ag nanoparticles enhanced the photocatalytic efficiency of photodegradation of dyes compared with that of the pure TiO2 nanofibers.18
In the literature, the photocatalytic activity of the majority of photocatalysts was tested by different types of organic dyes, which are usually categorized according to their chromophores. Thiazine dyes, mainly methylene blue, are one of the most studied dyes. It is well known that methylene blue has a complex aromatic structure; hydrophilic nature; and high stability against light, temperature, water, chemicals, etc.; therefore, it cannot be degraded through the conventional water treatment process and may cause substantial environment pollution. Photodegradation of dyes can be accomplished by a reductive mechanism followed by an oxidative pathway to form the end products. In certain cases, the photoreduced species might be reoxidized back to colored methylene blue under appropriate conditions (absence of light, high pH, high concentration of dissolved oxygen). The reversibility is highly dependent on the experimental conditions.29
In this work, a photocatalyst (ZnO/GO nanocomposites) is prepared with embedded metal (Ag and Cu) nanoparticles. The embedded copper and silver nanoparticles are used as dopants to study their effects on the activity of the photocatalyst. The photocatalytic activity of the synthesized nanocomposites is evaluated based on the degradation of methylene blue (MB) dye, as a water pollutant model. The results provide evidence that the GO–ZnO–Ag nanocomposite has the potential to be an efficient adaptable photocatalyst for the photodegradation of organic dyes in industrial wastewater. Furthermore, the ZnO–GO–Ag nanocomposites are expected to have a broad range of applications in environmental conservation.
2. Results and Discussion
2.1. Nanocomposite Characterization
Fourier transform infrared (FTIR) spectrum of the prepared GO sample shows many peaks that reveal the presence of oxygen in the sample. Figure 1a shows several peaks at 1069, 1243, 1430, and 1769 cm–1, which correspond to the functional groups of alkoxy C–O, epoxy C–O, tertiary C–OH, and carbonyl C=O, respectively. The peaks in the region between 3300 and 3700 cm–1 can be attributed to the O–H bond stretching vibration of the hydroxyl groups. The peak at 1649 cm–1 may refer to the remaining sp2 bonds in GO.30−33 The FTIR spectra for the samples containing ZnO have peaks in the area between 400 and 460 cm–1 that represent the stretching of oxygen–metal bonds (Figure 1b).34 Also, the intensity of the bands that recognize the bonds containing oxygen was decreased because of the partial reduction of the GO through the annealing of the samples, as shown in Figure 1b. Figure 1b shows that no new bands were observed for doped Ag and Cu nanoparticles because no covalent bonds were formed in the composites. Figure 1b shows noticeable weak bands in the range of 400–600 cm–1, attributed to the E2 mode of ZnO (active Raman) with vibrating masses of ZnO.35 The FTIR spectra confirm the fabrication of the GO–ZnO–metal nanocomposite structure.
Figure 1.

FTIR spectra of (a) GO and (b) GO–ZnO, GO–ZnO–Cu, and GO–ZnO–Ag nanocomposites.
The X-ray diffraction (XRD) pattern for the prepared GO–ZnO composite (Figure 2a) shows peaks for the ZnO nanocrystals at 2θ values of 31.2, 34.3, 36.1, 47.2, 56.5, 62.5, and 68.0°, which correspond to the planes (100), (002), (101), (102), (110), (103), and (112), respectively. The observed high-intensity pixels above 2θ indicate the high purity and wurtzite hexagonal structure of ZnO. Low-intensity peaks are shown in Figure 2a between 2θ = 23 and 27°, indicating a large gap between the graphene layers due to graphite oxidation and formation of functional groups, such as carboxyl, hydroxyl, and epoxy. Moreover, it indicates the presence of partial restoration GO in the form of reduced graphene oxide besides over-riding the presence of GO. The observed strong peaks in Figure 2a are indicative of highly crystallized ZnO. In Figure 2a, peak (002) is not as dominant as other peaks at (101) and (002); this suggests that the ZnO in our synthesized sample is polydirectional.36Figure 2b shows a diffraction peak at 2θ = 12.8° corresponding to the plane (001) of pristine GO; this confirms the presence of GO in the nanocomposite.37 Furthermore, it can be observed that the intensity of the major diffraction peaks of ZnO in the GO–ZnO–Ag and GO–ZnO–Cu nanocomposites (Figure 2b) decreased compared to those observed in GO–ZnO (Figure 1a), which confirm the doping of Ag and Cu in the nanocomposites.38 The XRD pattern of nanosilver composite (Figure 2b) shows two new peaks at 2θ of 37.5 and 42.8° for the planes (111) and (200), respectively, which could be indexed to the face-centered cubic phase metallic silver (JCPDS No. 04-0783). Furthermore, these peaks represent the Ag(0) nanoparticles with weak intensity due to its low concentration in the sample.39 The XRD pattern for nanocopper composite shows two peaks at 2θ of 43.1 and 51.0° (Figure 2b), which indicates the (111) and (200) planes, respectively (JCPDS No. 65-9026), with very weak intensity due to its low concentration in the sample.40 This result confirmed the doping of Ag and Cu onto the structure of GO–ZnO nanocomposite (Figure 2b).
Figure 2.
XRD patterns of (a) GO–ZnO and (b) GO–ZnO–Ag and GO–ZnO–Cu nanocomposites.
Figure 3 displays the scanning electron microscopy (SEM) images of GO–ZnO, GO–ZnO–Ag, and GO–ZnO–Cu nanocomposites. It can be clearly seen from Figure 3a that a massive volume of ZnO nanoparticles is positioned over and between GO sheets without any agglomeration. Moreover, the images (Figure 3a) exhibited a high degree of uniformity of the ZnO nanoparticles. Figure 3b shows the compact GO sheets decorated with numerous Ag and ZnO nanorods in different orientations in addition to a few ZnO nanosheets. The existence of nanorods may enhance the surface area of the synthesized nanocomposite and may result in better adsorption capacity. Figure 3c clearly shows ZnO nanosheets distributed with Cu nanoparticles on the surface and between GO and ZnO sheets.
Figure 3.

SEM images of (a) GO–ZnO, (b) GO–ZnO–Ag, and (c) GO–ZnO–Cu.
Energy dispersive analysis of X-ray (EDAX) spectra for the prepared composites furthermore confirmed the elements that exist in each sample (Figure 4). Figure 4 shows sharp peaks for Zn, which reveal the large quantity of ZnO in each composite. Figure 4a,b shows peaks at 2.98 and 8.10 keV for Ag and Cu, respectively. These two peaks further confirmed the doping of Ag and Cu into the structure of GO–ZnO nanocomposites.
Figure 4.
EDAX spectra of (a) GO–ZnO–Ag and (b) GO–ZnO–Cu.
UV–vis diffuse reflectance spectrum of the GO–ZnO–Ag sample was obtained to determine the effect of Ag and GO on the optical properties of ZnO nanoparticles (Figure 5). It is well known that bare ZnO can absorb only UV light, which is confirmed by a characteristic absorption edge at 420 nm. However, the GO–ZnO–Ag nanocomposites show a broad absorption in the whole region (200–800 nm) in which the strongest absorption was observed to appear in the range of less than 400 nm, while an intense absorption was also found to exist in a visible region at 450 (Figure 5). The enhanced light-harvesting capability of the GO–ZnO–Ag sample is attributed to the synergistic effects of the surface Plasmon absorption of Ag nanoparticles and improved light absorption of GO sheets.
Figure 5.

UV–vis diffuse reflectance spectrum of GO–ZnO–Ag nanocomposites.
2.2. Photocatalytic Activity
The photocatalytic activity of the synthesized GO–ZnO, GO–ZnO–Ag, and GO–ZnO–Cu nanocomposites was evaluated based on the photodegradation efficiency of MB dye under sunlight irradiation. To investigate the efficiency of the prepared nanocomposites for the degradation of MB sample, each nanocomposite was tested under the same conditions as a function of irradiation time. The results are shown in Figure 6, which displays the UV–visible spectra of MB recorded at different time intervals during the photodegradation experiments. The total percentages of photodegradation were evaluated using eq 2.
| 2 |
where C0 represents the initial concentration of MB and C is the concentration of MB after degradation.
Figure 6.
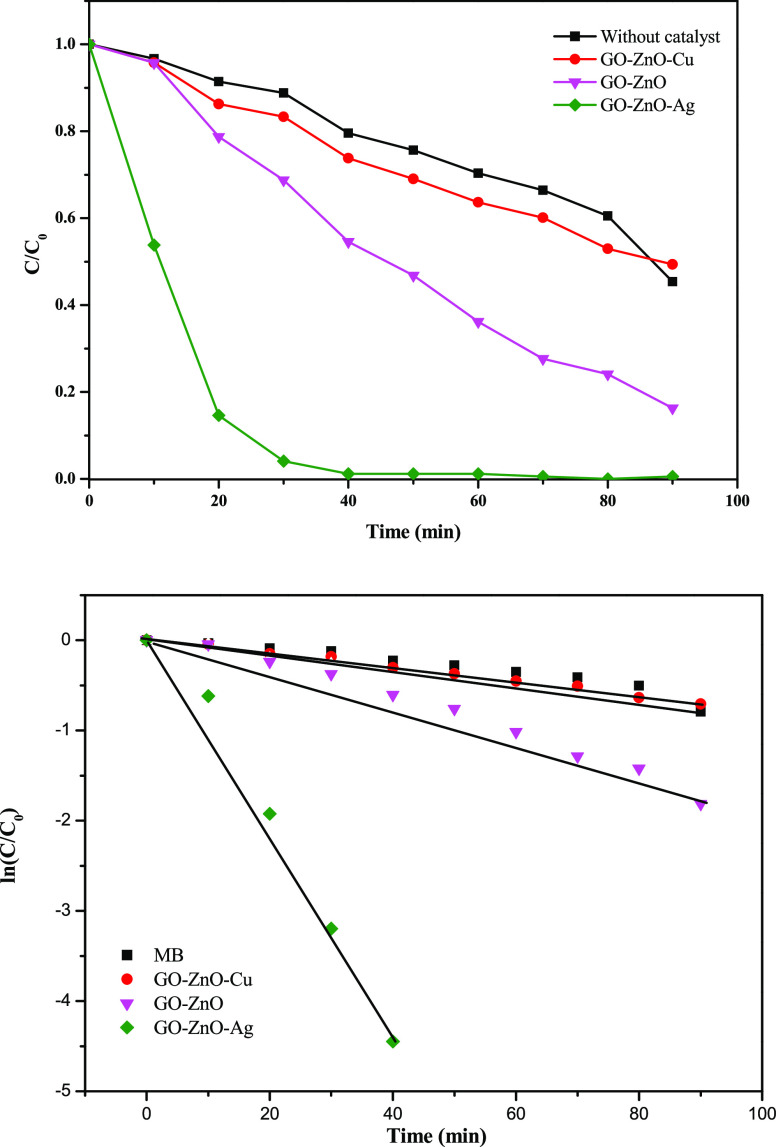
Photocatalytic activity of GO–ZnO, GO–ZnO–Ag, and GO–ZnO–Cu nanocomposites in degrading the MB dye.
The degradation percentage of the MB dye by GO–ZnO (3.125 wt % GO) after sunlight irradiation for 90 min was 84% (Figure 6a). A superior activity was achieved by the GO–ZnO–Ag nanocomposite with 100% photodegradation of MB after sunlight irradiation for 40 min (Figure 6b). This unique result is attributed to the presence of silver nanoparticles that decreased the recombination of electron–hole pairs and to the effect of one-dimensional (1D) ZnO nanorods that increase the light-harvesting ability, which greatly promoted the photoactivity of the catalyst.41−43 The enhancement of production hydroxyl radicals was influenced by the synergistic effect of the Ag nanoparticles on the activity of the semiconductor (ZnO) by electron and hole trapping.44
An obvious decrease in the photocatalytic activity was demonstrated by the GO–ZnO–Cu nanocomposite, which showed only 50% degradation of MB after sunlight irradiation for 90 min (Figure 6c). This result may be attributed to the electrical conductivity that is higher in silver than in copper.45Figure 7 shows the kinetic plots of the photodegradation reaction of MB in the absence of the catalyst and in the presence of GO–ZnO, GO–ZnO–Ag, and GO–ZnO–Cu nanocomposites.
Figure 7.
Kinetic plots of the photodegradation reaction of MB in the absence of the catalyst and in the presence of GO–ZnO, GO–ZnO–Ag, and GO–ZnO–Cu nanocomposites.
One of the most important parameters that influence the photocatalytic degradation of dye is the pH because it affects the surface charge properties of the photocatalyst and the ionic species in the solution. Soltani et al. reported that at equilibrium in the dark (60 min) when the pH was increased to 6.0, the adsorption was negligible. In a dark place, the catalyst had no adsorption in basic media. However, the removal efficiency of MB was observed in both acidic and basic media under sunlight irradiation.46
The photodegradation reactions for MB follow pseudo-first-order kinetics (Figure 7), and the rate constants (k) of the catalytic reaction are estimated using eq 3, where (t) is the time of sunlight irradiation.47,48
| 3 |
The degradation of MB by the GO–ZnO–Ag photocatalyst shows the fastest kinetics with a rate constant of 0.1112 min–1, which is more than 5 times of the rate constant for the degradation of MB by the GO–ZnO photocatalyst (k = 0.0201 min–1). The rate constants for the degradation of MB by the GO–ZnO–Cu photocatalyst and in the absence of the catalyst were 0.0072 and 0.0041 min–1, respectively.
The total percentage of photodegradation and the photodegradation kinetics show that the photocatalytic activity of GO–ZnO–Ag is significantly higher with fast kinetics than those of GO–ZnO–Cu and GO–ZnO. This result can be attributed to the larger surface area of GO–ZnO–Ag nanocomposite compared to ZnO. The Brunauer–Emmett–Teller (BET) surface area analysis was used to evaluate the surface area of the synthesized nanocomposites. The obtained isotherms of the ZnO, GO–ZnO, and GO–ZnO–Ag samples prepared in ethanol (Figure 8) correspond to a type III isotherm in the Brunauer classification,49 which is characterized by a hysteresis loop, and it does not exhibit any limiting adsorption at high relative pressures. The specific BET surface areas of the ZnO, GO–ZnO, and GO–ZnO–Ag samples were determined to be 13.71, 36.95, and 38.92 m2/g, respectively. Furthermore, the enhanced photocatalytic activity of GO–ZnO–Ag may be attributed to the porous structure, which would enhance the adsorption and trapping of the MB dye within the assembly of the short rods, as confirmed by the SEM images. The GO–ZnO–Cu shows lower photocatalytic activity toward photodegradation of MB; this may be due to the dispersion of Cu nanoparticles on the GO–ZnO nanocomposite surface that had a large distribution and could contain large particles or agglomerates that are not suitable for adsorption of MB on the surface.
Figure 8.

Typical nitrogen adsorption–desorption isotherm plot of synthesized nanocomposites.
The GO–ZnO–Ag nanocomposite was further tested for its photocatalytic recyclability. After four cycles of applications, negligible performance deterioration (less than 3%) was observed for the GO–ZnO–Ag nanocomposite photocatalyst. The stable performance as well as the effective catalytic properties of the GO–ZnO–Ag nanocomposite photocatalyst demonstrated its exceptional catalytic performance.
The photocatalytic mechanism of the ZnO–GO–NP (NP = nanoparticles, Ag and Cu in this study) nanocomposites was investigated in detail by several scientists. The photocatalytic degradation of MB dye by ZnO–GO–NPs under visible light irradiation is explained by three mechanisms proposed. The first one is explained by the addition of GO to the photocatalytic system, which enhances the photocatalytic efficiency of ZnO under visible light irradiation,50,51 where it is believed that the photocatalytic degradation efficiency of MB by ZnO–GO–NPs was improved by combining them with a zero-band gap GO. Furthermore, the large surface area of GO can contribute to the effective adsorption of MB molecules on the photocatalyst surface and increase the formation of π–π* interaction between the MB dye molecules. The second mechanism could be due to the NP doping effect on the ZnO crystal lattice.52 NP doping could minimize the recombination of the excited electrons in the conduction band (CB) with the holes in the valence band (VB), which would increase the production of oxy radicals by reaction with oxygen molecules, leading to the oxidative degradation of MB molecules. The third mechanism is explained by narrowing the band gap of ZnO by doping with NPs.53 The photodegradation of MB by ZnO–GO–NPs is initiated by oxidative and reductive processes, as explained in eqs 4–14. The possible mechanism of the photocatalytic degradation of MB dye is given in Scheme 1.
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
Scheme 1. Mechanism of the Photocatalytic Degradation of MB.

3. Conclusions
In this study, GO–ZnO nanocomposites were prepared by a simple one-pot method. The photoactivity of the prepared nanocomposite was tested for the degradation of MB under sunlight irradiation. A total of 84% of degradation efficiency has been achieved after 90 min under sunlight irradiation. Nanosilver and nanocopper were prepared by keeping the percentage of GO to 3.125% in each sample. GO–ZnO–Ag gave a distinctive result by a full degradation (100%) of MB after only 40 min under sunlight irradiation with a rate constant of 0.1112 min–1. This result was attributed to the morphology of ZnO that appeared in SEM images, which was a mixture of nanoparticles and nanorods that decreased the e––h+ recombination, and to the localized surface plasmon resonance (LSPR) property of the Ag nanoparticles. Ag nanoparticles on the surface of GO–ZnO effectively help visible light harvesting by self-sensitization. The presence of GO, with a superior high surface area, increased the possibility of pollutant adsorption on the surface of the nanocomposite. Thus, the GO–ZnO–Ag nanocomposite has the potential to be an efficient adaptable photocatalyst for the photodegradation of organic dyes in wastewater.
4. Experimental Section
4.1. Materials and Reagents
All materials used in this research were purchased from Sigma-Aldrich and were of analytical grade: graphite (fine powder extra pure), sulfuric acid (H2SO4, 96%), sodium nitrates (NaNO3), potassium permanganate (KMnO4), hydrogen peroxide (H2O2, 30%), hydrochloric acid (HCl), zinc sulfate heptahydrate (ZnSO4·7H2O), ammonium bicarbonate (NH4HCO3), absolute ethanol (C2H5OH), silver nitrate (AgNO3), copper sulfate (CuSO4), hydrazine hydrate (NH2NH2·H2O, 80%), and methylene blue (MB).
4.2. Preparation of Graphene Oxide (GO)
Graphene oxide (GO) was produced using an improved Hummers method from pure graphite powder reported in the literature.19 Graphite powder (4.5 g) was added to 120 mL of sulfuric acid (96%) containing 2.5 g of sodium nitrate (NaNO3). Then, the solution was cooled down to 0 °C with continued cooling for 20 min by an ice bath. Potassium permanganate (KMnO4, 15 g) was added dropwise to the solution with vigorous stirring, maintaining the temperature at 0 °C. Then, the mixture was heated at 40 °C for 2 h with vigorous stirring until the color of the slurry mixture turned to dark green. Deionized water (250 mL) was added dropwise, maintaining the temperature below 50 °C. Then, 20 mL of hydrogen peroxide (H2O2, 30%) was added dropwise to the mixture. The reaction solution started to become brownish yellow, indicating the formation of the GO seeds.15 After 30 min, the mixture was centrifuged at 6000 rpm for 10 min. Finally, the GO was formed, separated from the mixture, and washed with 10% HCl to remove ions, such as SO4–2 and NO3, from the solution, followed by washing with deionized water. The dark brown paste was oven-dried at 50 °C overnight, and then, a black GO solid was formed.
4.3. Preparation of GO–ZnO
The GO/ZnO nanocomposite was produced using a method reported in the literature.15 One hundred microliter of 1.0 M zinc sulfate (ZnSO4) containing GO (250 mg) was added dropwise to 60 mL of 2.0 M ammonium bicarbonate (NH4HCO3). Then, the mixture was placed in a water bath at 60 °C with vigorous stirring; the temperature was kept at 60 °C for 1 h. The obtained mixture was centrifuged and washed with deionized water and absolute ethanol separately to remove the growth solution. The formed solid was then oven-dried at 60 °C overnight.15
4.4. Preparation of a Mixed Shape: GO–ZnO–Ag and GO–ZnO–Cu Nanocomposites
GO–ZnO–Ag and GO–ZnO–Cu nanocomposites were produced using a method reported for GO–ZnO–Pd in the literature.26 The prepared GO–ZnO composite (1.0 g) was added to 100 mL of deionized water and sonicated for 5 min until the composite became suspended. Then, to prepare 5 wt % silver, 10 mL of 0.046 M AgNO3 was added gradually to the mixture with vigorous stirring and then reduced by adding 300 μL of hydrazine hydrate (NH2NH2·H2O, 80%). Then, the solution was irradiated by a conventional microwave for 3.0 min. The prepared mixture was centrifuged and washed with deionized water and absolute ethanol separately to remove the growth solution. The solid was then oven-dried at 60 °C overnight. A similar procedure to the above was used to prepare nanocopper composites, where 10 mL of 0.079 M CuSO4·5H2O was used instead of AgNO3. All prepared samples were calcined using a Thermo Scientific muffle furnace at 450 °C; the temperature was elevated by a rate of (5 °C/min) and kept at 450 °C for 2 h.
4.5. Photocatalytic Experiment
The photocatalytic activity of the prepared nanocomposites was evaluated by utilizing MB dye as a pollutant model. The photocatalyst (50 mg) was dispersed in 100 mL of methylene blue (3.13 × 10–5 M, and the initial pH value of the solution was nearly 6.5). Then, the mixture of the catalyst/MB was sonicated for 2 min using an OMNI SONIC RUPTOR 400 ultrasonicator, followed by stirring for 1 h in the dark to reach adsorption–desorption equilibrium. Then, sunlight using a PECCEL PEC-l01 portable solar simulator illuminated the solution. The solar simulator is equipment irradiating light very similar to sunlight. The wavelength interval was 300–1400 nm. Samples were taken at different time intervals to analyze the concentration variation of the MB dye. The filtered sample was analyzed by monitoring the maximum absorption peak at 664 nm (λmax for MB) using a UV-2500PC Shimadzu UV–vis spectrophotometer. The photodegradation efficiencies were calculated based on the UV–vis absorption of the MB dye at different irradiation times.
4.6. Characterization
The prepared composites have been characterized by an FTIR spectrometer (Bruker ALPHA) and a UV–visible spectrophotometer (UV-2500PC Shimadzu). The XRD pattern was obtained using an XRD-6000 Shimadzu X-ray diffractometer Cu Kα radiation (wavelength λ = 0.154 nm). An FEI Quanta 450 scanning electron microscope (SEM) was used to investigate the morphology of the synthesized samples. The synthesized nanocomposites were activated at 100 °C overnight, and then, the BET surface area was obtained at 77 K by nitrogen adsorption–desorption isotherms.
Acknowledgments
The financial support from the Deanship of Research/Jordan University of Science and Technology is appreciatively acknowledged.
The authors declare no competing financial interest.
References
- Shen Y.; Li L.; Xiao K.; Xi J. Constructing three-dimensional hierarchical architectures by integrating carbon nanofibers into graphite felts for water purification. ACS Sustainable Chem. Eng. 2016, 4, 2351–2358. 10.1021/acssuschemeng.6b00030. [DOI] [Google Scholar]
- Zhang P.; Li J.; Lv L.; Zhao Y.; Qu L. Vertically aligned graphene sheets membrane for highly efficient solar thermal generation of clean water. ACS Nano 2017, 11, 5087–5093. 10.1021/acsnano.7b01965. [DOI] [PubMed] [Google Scholar]
- Romão J.; Barata D.; Habibovic P.; Mul G.; Baltrusaitis J. High throughput analysis of photocatalytic water purification. Anal. Chem. 2014, 86, 7612–7617. 10.1021/ac501426f. [DOI] [PubMed] [Google Scholar]
- Fang X.; Bando Y.; Gautam U. K.; Ye C.; Golberg D. Inorganic semiconductor nanostructures and their field-emission applications. J. Mater. Chem. 2008, 18, 509–522. 10.1039/B712874F. [DOI] [Google Scholar]
- Xu L.; Chu W.; Gan L. Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer. Chem. Eng. J. 2015, 263, 435–443. 10.1016/j.cej.2014.11.065. [DOI] [Google Scholar]
- Glaze W. H.; Kang J. W. Advanced oxidation processes. Description of a kinetic model for the oxidation of hazardous materials in aqueous media with ozone and hydrogen peroxide in a semibatch reactor. Ind. Eng. Chem. Res. 1989, 28, 1573–1580. 10.1021/ie00095a001. [DOI] [Google Scholar]
- Andreozzi R.; Caprio V.; Insola A.; Marotta R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. 10.1016/S0920-5861(99)00102-9. [DOI] [Google Scholar]
- Thiruvenkatachari R.; Vigneswaran S.; Moon I. S. A review on UV/TiO2 photocatalytic oxidation process (Journal Review). Korean J. Chem. Eng. 2008, 25, 64–72. 10.1007/s11814-008-0011-8. [DOI] [Google Scholar]
- Pera-Titus M.; García-Molina V.; Baños M. A.; Giménez J.; Esplugas S. Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl. Catal., B 2004, 47, 219–256. 10.1016/j.apcatb.2003.09.010. [DOI] [Google Scholar]
- Siriwong C.; Wetchakun N.; Inceesungvorn B.; Channei D.; Samerjai T.; Phanichphant S. Doped-metal oxide nanoparticles for use as photocatalysts. Prog. Cryst. Growth Charact. Mater. 2012, 58, 145–163. 10.1016/j.pcrysgrow.2012.02.004. [DOI] [Google Scholar]
- Kumar S. G.; Rao K. S. R. K. Polymorphic phase transition among the titania crystal structures using a solution-based approach: from precursor chemistry to nucleation process. Nanoscale 2014, 6, 11574–11632. 10.1039/C4NR01657B. [DOI] [PubMed] [Google Scholar]
- Wang Z. L. Zinc Oxide Nanostructures: Growth, properties and Applications. J. Phys.: Condens. Matter 2004, 16, R829–R858. 10.1088/0953-8984/16/25/R01. [DOI] [Google Scholar]
- Sierra-Fernandez A.; De la Rosa-García S.; Gomez-Villalba L. S.; Gómez-Cornelio S.; Rabanal M. E.; Fort R.; Quintana P. Synthesis, photocatalytic, and antifungal properties of MgO, ZnO and Zn/Mg oxide nanoparticles for the protection of calcareous stone heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. 10.1021/acsami.7b06130. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Anandan S.; Hembram K.; Narasinga Rao T. Efficient ZnO-based visible-light-driven photocatalyst for antibacterial applications. ACS Appl. Mater. Interfaces 2014, 6, 13138–13148. 10.1021/am502915v. [DOI] [PubMed] [Google Scholar]
- Kumaresan N.; Ramamurthi K.; Babu R. R.; Sethuraman K.; Babu S. M. Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl. Surf. Sci. 2017, 418, 138–146. 10.1016/j.apsusc.2016.12.231. [DOI] [Google Scholar]
- Kiriarachchi H. D.; Abouzeid K. M.; Bo L.; El-Shall M. S. Growth mechanism of Sea Urchin ZnO Nanostructure in Aqueous Solutions and Their Photocatalytic Activity for the Degradation of Organic Dyes. ACS Omega 2019, 4, 14013–14020. 10.1021/acsomega.9b01772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y.; Yu H.-Y.; Abdalkarim S. Y. H.; Wang C.; Tang F.; Marek J.; Che W.-L.; Militky J.; Yao J.-M. Green one-step synthesis of ZnO/cellulose nanocrystal hybrids with modulated morphologies and superfast absorption of cationic dyes. Int. J. Biol. Macromol. 2019, 132, 51–61. 10.1016/j.ijbiomac.2019.03.104. [DOI] [PubMed] [Google Scholar]
- Wang D.; Choi D.; Li J.; Yang Z.; Nie Z.; Kou R.; Hu D.; Wang C.; Saraf L. V.; Zhang J.; Aksay I. A.; Liu J. Self-assembled TiO2–graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 2009, 3, 907–914. 10.1021/nn900150y. [DOI] [PubMed] [Google Scholar]
- Chen D.; Feng H.; Li J. Graphene oxide: preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. 10.1021/cr300115g. [DOI] [PubMed] [Google Scholar]
- Dai L.; Xue Y.; Qu L.; Choi H.-J.; Baek J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. 10.1021/cr5003563. [DOI] [PubMed] [Google Scholar]
- Darvishi M.; Mohseni-Asgerani G.; Seyed-Yazdi J. Simple microwave irradiation procedure for the synthesis of CuO/Graphene hybrid composite with significant photocatalytic enhancement. Surf. Interfaces 2017, 7, 69–73. 10.1016/j.surfin.2017.02.007. [DOI] [Google Scholar]
- Bai X.; Wang L.; Zong R.; Lv Y.; Sun Y.; Zhu Y. Performance enhancement of ZnO photocatalyst via synergic effect of surface oxygen defect and graphene hybridization. Langmuir 2013, 29, 3097–3105. 10.1021/la4001768. [DOI] [PubMed] [Google Scholar]
- Subramanian V.; Wolf E. E.; Kamat P. V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. 10.1021/ja0315199. [DOI] [PubMed] [Google Scholar]
- Bhunia S. K.; Jana N. R. Reduced graphene oxide-silver nanoparticle composite as visible light photocatalyst for degradation of colorless endocrine disruptors. ACS Appl. Mater. Interfaces 2014, 6, 20085–20092. 10.1021/am505677x. [DOI] [PubMed] [Google Scholar]
- Rosu M.-C.; Coros M.; Pogacean F.; Magerusan L.; Socaci C.; Turza A.; Pruneanu S. Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 2017, 70, 13–20. 10.1016/j.solidstatesciences.2017.05.013. [DOI] [Google Scholar]
- Zhang L.; Du L.; Yu X.; Tan S.; Cai X.; Yang P.; Gu Y.; Mai W. Significantly enhanced photocatalytic activities and charge separation mechanism of Pd-decorated ZnO–graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 3623–3629. 10.1021/am405872r. [DOI] [PubMed] [Google Scholar]
- Sun P.; Liu R.; Ma R.; Xie Z.; Su F.; Gong Y.; Mu Z.; Li L.; Wei Y.; Wan Q. Branched CdO/ZnO Core/Shell Heterogeneous Structure and Its Enhanced Photoelectrocatalytic Performance. ACS Omega 2018, 3, 11517–11525. 10.1021/acsomega.8b00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydeen S. S.; Kumar R. R.; Sambathkumar S.; Kottaisamy M.; Vasantha V. S. Facile Synthesis of ZnO/AC Nanocomposites using Prosopis Juliflora for Enhanced Photocatalytic Degradation of Methylene Blue and Antibacterial Activity. Optik 2020, 224, 165426 10.1016/j.ijleo.2020.165426. [DOI] [Google Scholar]
- Yan X.; Ohno T.; Nishijima K.; Abe R.; Ohtani B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. 10.1016/j.cplett.2006.08.081. [DOI] [Google Scholar]
- Basahel S. N.; Mokhtar M.; Alsharaeh E. H.; Ali T. T.; Mahmoud H. A.; Narasimharao K. Photocatalytic Degradation of p-Nitrophenol in Aqueous Suspension by Using Graphene/ZrO2 Catalysts. Nanosci. Nanotechnol. Lett. 2016, 8, 448–457. 10.1166/nnl.2016.2172. [DOI] [Google Scholar]
- Govindhan P.; Pragathiswaran C.; Chinnadurai M. Facile synthesis of GO/ZnO–Ag nanocomposite and evaluation of rhodamine B dye under sun light. J. Mater. Sci.: Mater. Electron. 2017, 28, 354–362. 10.1007/s10854-016-5530-1. [DOI] [Google Scholar]
- Shen J.; Yan B.; Shi M.; Ma H.; Li N.; Ye M. One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 2011, 21, 3415–3421. 10.1039/c0jm03542d. [DOI] [Google Scholar]
- Oppong S. O.; Anku W. W.; Shukla S. K.; Govender P. P. Synthesis and characterisation of neodymium doped-zinc oxide–graphene oxide nanocomposite as a highly efficient photocatalyst for enhanced degradation of indigo carmine in water under simulated solar light. Res. Chem. Intermed. 2017, 43, 481–501. 10.1007/s11164-016-2636-2. [DOI] [Google Scholar]
- Chen G.; Sun H.; Hou S. Electrochemistry and electrocatalysis of myoglobin immobilized in sulfonated graphene oxide and Nafion films. Anal. Biochem. 2016, 502, 43–49. 10.1016/j.ab.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Kumar N.; Srivastava A. K.; Patel H. S.; Gupta B. K.; Das V. G. Facile synthesis of ZnO-reduced graphene oxide nanocomposites for NO2 Gas sensing applications. Eur. J. Inorg. Chem. 2015, 2015, 1912–1923. 10.1002/ejic.201403172. [DOI] [Google Scholar]
- Dhingra V.; Kumar S.; Kumar R.; Garg A.; Chowdhuri A. Room temperature SO2 and H2 gas sensing using hydrothermally grown GO–ZnO nanorod composite films. Mater. Res. Express 2020, 7, 065012 10.1088/2053-1591/ab9ae7. [DOI] [Google Scholar]
- Zhang L.; Du G.; Zhou B.; Wang L. Green synthesis of flower-like ZnO decorated reduced graphene oxide composites. Ceram. Int. 2014, 40, 1241–1244. 10.1016/j.ceramint.2013.06.023. [DOI] [Google Scholar]
- Patil R. S.; Kokate M. R.; Shinde D. V.; Kolekar S. S.; Han S. H. Synthesis and enhancement of photocatalytic activities of ZnO by silver nanoparticles. Spectrochim. Acta, Part A 2014, 122, 113–117. 10.1016/j.saa.2013.09.116. [DOI] [PubMed] [Google Scholar]
- Gao P.; Ng K.; Sun D. D. Sulfonated graphene oxide-ZnO-Ag photocatalyst for fast photodegradation and disinfection under visible light. J. Hazard. Mater. 2013, 262, 826–835. 10.1016/j.jhazmat.2013.09.055. [DOI] [PubMed] [Google Scholar]
- Atchudan R.; Edison T. N. J. I.; Perumal S.; Karthikeyan D.; Lee Y. R. Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J. Photochem. Photobiol., B 2016, 162, 500–510. 10.1016/j.jphotobiol.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Wan J.; Jiang X.; Li H.; Chen K. Facile synthesis of zinc ferrite nanoparticles as non-lanthanide T1 MRI contrast agents. J. Mater. Chem. 2012, 22, 13500–13505. 10.1039/c2jm30684k. [DOI] [Google Scholar]
- Yin Z.; Zhou W.; Gao Y.; Ma D.; Kiely C. J.; Bao X. Supported Pd–Cu bimetallic nanoparticles that have high activity for the electrochemical oxidation of methanol. Chem. – Eur. J. 2012, 18, 4887–4893. 10.1002/chem.201103674. [DOI] [PubMed] [Google Scholar]
- Moussa H.; Girot E.; Mozet K.; Alem H.; Medjahdi G.; Schneider R. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal., B 2016, 185, 11–21. 10.1016/j.apcatb.2015.12.007. [DOI] [Google Scholar]
- Kakhki R. M.; Tayebee R.; Ahsani F. New and highly efficient Ag doped ZnO visible nano photocatalyst for removing of methylene blue. J. Mater. Sci.: Mater. Electron. 2017, 28, 5941–5952. 10.1007/s10854-016-6268-5. [DOI] [Google Scholar]
- Thomann I.; Pinaud B. A.; Chen Z.; Clemens B. M.; Jaramillo T. F.; Brongersma M. L. Plasmon enhanced solar-to-fuel energy conversion. Nano Lett. 2011, 11, 3440–3446. 10.1021/nl201908s. [DOI] [PubMed] [Google Scholar]
- Soltani T.; Entezari M. H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal., A 2013, 377, 197–203. 10.1016/j.molcata.2013.05.004. [DOI] [Google Scholar]
- Türkyılmaz Ş. Ş.; Güy N.; Özacar M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol., A 2017, 341, 39–50. 10.1016/j.jphotochem.2017.03.027. [DOI] [Google Scholar]
- Shanmugam M.; Alsalme A.; Alghamdi A.; Jayavel R. Enhanced photocatalytic performance of the graphene-V2O5 nanocomposite in the degradation of methylene blue dye under direct sunlight. ACS Appl. Mater. Interfaces 2015, 7, 14905–14911. 10.1021/acsami.5b02715. [DOI] [PubMed] [Google Scholar]
- Yu J. C.; Xu A.; Zhang L.; Song R.; Wu L. Synthesis and Characterization of Porous Magnesium Hydroxide and Oxide Nanoplates. J. Phys. Chem. B 2004, 108, 64–70. 10.1021/jp035340w. [DOI] [Google Scholar]
- Thi V. H. T.; Cao T. H.; Pham T. N.; Pham T. T.; Le M. C. Synergistic Adsorption and Photocatalytic Activity under Visible irradiation Using Ag-ZnO/GO nanoparticles Derived at Low Temperature. J. Chem. 2019, 2019, 2979517 10.1155/2019/2979517. [DOI] [Google Scholar]
- Cao S.; Yeung K. L.; Kwan J. K. C.; To P. M. T.; Yu S. C. T. An investigation of the performance of catalytic aerogel filters. Appl. Catal., B 2009, 86, 127–136. 10.1016/j.apcatb.2008.08.019. [DOI] [Google Scholar]
- Adán-Más A.; Wei D. Photoelectrochemical properties of graphene and its derivatives. Nanomaterials 2013, 3, 325–356. 10.3390/nano3030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien H. N.; Luan V. H.; Hoa L. T.; et al. One-pot synthesis of a reduced graphene oxide-zinc sphere composite and its use as a visible light photocatalyst. Chem. Eng. J. 2013, 229, 126–133. 10.1016/j.cej.2013.05.110. [DOI] [Google Scholar]