Abstract
The liver is the commonest site of metastatic disease for patients with colorectal cancer, with at least 25% developing colorectal liver metastases (CRLM) during the course of their illness. The management of CRLM has evolved into a complex field requiring input from experienced members of a multi-disciplinary team involving radiology (cross sectional, nuclear medicine and interventional), Oncology, Liver surgery, Colorectal surgery, and Histopathology. Patient management is based on assessment of sophisticated clinical, radiological and biomarker information. Despite incomplete evidence in this very heterogeneous patient group, maximising resection of CRLM using all available techniques remains a key objective and provides the best chance of long-term survival and cure. To this end, liver resection is maximised by the use of downsizing chemotherapy, optimisation of liver remnant by portal vein embolization, associating liver partition and portal vein ligation for staged hepatectomy, and combining resection with ablation, in the context of improvements in the functional assessment of the future remnant liver. Liver resection may safely be carried out laparoscopically or open, and synchronously with, or before, colorectal surgery in selected patients. For unresectable patients, treatment options including systemic chemotherapy, targeted biological agents, intra-arterial infusion or bead delivered chemotherapy, tumour ablation, stereotactic radiotherapy, and selective internal radiotherapy contribute to improve survival and may convert initially unresectable patients to operability. Currently evolving areas include biomarker characterisation of tumours, the development of novel systemic agents targeting specific oncogenic pathways, and the potential re-emergence of radical surgical options such as liver transplantation.
Keywords: Colorectal, Cancer, Liver, Metastases, Management, Review
Core Tip: The management of colorectal liver metastases is a complex evolving field requiring input from an experienced multi-disciplinary team involving radiology (cross sectional, nuclear medicine and interventional), Oncology, Liver surgery, Colorectal surgery, and Histopathology. Patient management is based on clinical, radiological and biomarker information. Despite incomplete evidence in this very heterogeneous patient group, maximising resection of colorectal liver metastases using all available techniques remains a key objective and provides the best chance of long-term survival. For unresectable patients, optimal systemic and locoregional chemotherapeutic, biological and radiotherapeutic treatments improve survival, and may convert initially unresectable patients to operability.
INTRODUCTION
Colorectal cancer (CRC) represents a major worldwide health care burden, as the second most common cancer diagnosed in women and third most common in men, and accounting for 10% of all annually diagnosed cancers and cancer-related deaths worldwide[1].
As result of improvements in detection through screening[2], better referral pathways[3], centralisation of services[4], effective primary surgery[5], development of systemic chemotherapy[6], biological agents[7], and understanding of tumour biology[8], survival rates following diagnosis have improved[9].
Nevertheless, at least 25%-50% of patients with CRC develop colorectal liver metastases (CRLM) during the course of their illness.
From a historical perspective, the surgical management approach to CRLM has undergone a significant evolution. Starting from an era prior to the 1930s during which liver surgery for malignancy presented insurmountable challenges for technical and oncological reasons, tentative attempts at liver resection for malignancy were made in the subsequent decades resulting in early reports establishing proof of principle that long term survival following resection of CRLM was possible[10,11]. These results were confirmed and emphasised by larger landmark studies firmly establishing liver surgery as a potentially curative treatment for CRLM[12,13].
The era since has been characterised by progress in understanding of tumour biology as well as surgical and oncological developments. These overlapping and interdependent factors have directed the modern management of CRLM to a multidisciplinary approach involving radiology (cross sectional, nuclear medicine and interventional), Oncology, Liver surgery, Colorectal surgery, Histopathology, and Specialist nursing[14]. The paramount importance of the MDT cannot be overemphasised as it represents the forum where key management decisions are made after consideration of information spanning many different disciplines, with demonstrable benefits in terms of significant treatment alterations[15,16], numbers of patient offered resection[17,18], and ultimately translating into improved survival[19,20].
In the following review, we present modern management of CRLM. In order to assist the reader, section contents are provided below: (1) Diagnosis and staging of CRLM post resection of CRC; (2) Tumour characterisation and biomarkers in CRC; (3) Systemic and locoregional chemotherapy and targeted agents in CRLM management; (4) Surgical management of resectable CRLM; and (5) Histopathological assessment of resected CRLM.
SECTION 1: DIAGNOSIS, STAGING, AND SURVEILLANCE OF COLORECTAL LIVER METASTASES POST RESECTION
The detection of CRLM is achieved during staging investigations in the case of synchronous CRLM and by post CRC resection surveillance programmes in the case of metachronous CRLM. The section below discusses the timing and epidemiology of metachronous CRLM, an understanding of which is essential in judging the effectiveness of post CRC resection surveillance practice. The section also describes current optimal staging of CRLM, and finally current practice as it applies to surveillance after resection of CRLM.
CRLM epidemiology
Colorectal cancer is the third most common cancer worldwide and accounts for 10% of all cancers. It is a major cause of morbidity and the second most common cause of cancer related mortality[1].
Although it is regularly reported that approximately 50% of patients with colorectal cancer develop liver metastases, either as synchronous or metachronous disease[21-25], this is likely an exaggeration of true incidence originating from an historic autopsy study of patients who died with colorectal cancer[26]. Large epidemiological studies from multiple European centres demonstrate the incidence of both synchronous and metachronous liver metastases in patients with colorectal cancer to be lower, at approximately 25%[27-31]. The incidence of synchronous liver metastases in epidemiological studies ranges from 13.8%–17.1%[27,29,30] and the rate of metachronous liver metastases in these studies ranges from 7.6%–15.1%[27,29,30,32]. The interval between primary diagnosis and the detection of metastatic disease used in the literature ranges from the time of primary resection[29], to 3 mo[29,33], or 6 mo after diagnosis, and this lack of a consensus regarding the definition of metachronous metastases may partly explain the reported variation[24,32]. Further confounders include evolution in the sensitivity of pre-operative staging, and the reported increase in synchronous disease[28]. CRLM occur more frequently in male patients and in patients with left sided CRC, relating to embryological origin of the primary tumour[27,30].
With regards to metachronous disease, most recurrences occur early in follow up: 76%-85.3% occur within a year and 83%-97.5% within 3 years, with 30%-40% of patients having disease confined to the liver[33,34]. Approximately 2% of patients will develop liver metastases between 5 and 10 years after resection of the primary tumour[27,29,33].
CRC surveillance programmes
Surveillance programmes accompanied the widespread introduction of liver resection for CRLM, to detect recurrent disease early, with a view to improve survival. A meta-analysis of five randomised controlled trials published in 2002 supported this hypothesis by demonstrating a survival benefit associated with more intensive follow up regimes[35]. This encouraged the introduction of more intensive surveillance programmes, although a subsequent large multicentre randomised control trial performed in the United Kingdom by Primrose et al[36] failed to replicate these findings. In this study, intensive surveillance regimes with computed tomography (CT) with or without carcino-embryonic antigen (CEA) resulted in an increased rate of surgical treatment with curative intent, but this failed to translate to improved survival when compared to the minimal surveillance group[36]. Interestingly, the reported incidence of metachronous disease in this study was markedly lower than that reported in the previous meta-analysis (8.4% vs 32%). The stage-specific case mix and risk of recurrence within tumour stage across studies remained similar but one explanation for this reported difference was possibly superior pre-operative staging. This would provide an explanation for the previously reported improved benefit of more intensive follow up programmes with early recurrence in these older studies representing undetected residual disease[36]. A further meta-analysis published in 2016 of 15 randomised controlled trials came to a similar conclusion to that of Primrose et al[36] and demonstrated no overall survival benefit with more intensive follow up regimes[36,37].
In summary, surveillance programmes with either regular CEA or CT increase the likelihood of detecting recurrent disease and result in an increased proportion of patients undergoing surgical treatment with curative intent. This has not, however, been shown to translate into improved patient survival in trials. This counter-intuitive finding may partially be explained by the failure of randomised trials to detect small differences: If 25% of patients develop CRLM post CRC resection, of which 25% are operable, and of which 25% are 10 year survivors, the difference in overall survival in a surveillance group may prove beyond detection. In practice, the real world observation of lives saved following resection of metachronous CRLM has resulted in the continued adoption of surveillance programmes using CT and serum CEA, although the additional value of the latter has been difficult to demonstrate in trials[36].
CRLM characterisation and staging
Imaging has an important role in defining optimal treatment of CRLM. Knowing the size, location and vascular relationships of CLRM is essential prior to treatment planning and assessment of neoadjuvant response. Imaging techniques include ultrasound, CT, magnetic resonance imaging (MRI) and fluoro-18-deoxyglucose (FDG) positron emission tomography (PET-CT).
Ultrasound: Ultrasound has a limited role in pre-operative evaluation as it has a low sensitivity (64%) for CRLM compared with other imaging modalities[38]. In recent years contrast-enhanced ultrasound (CEUS) has become widely used to characterise liver lesions based on dynamic assessment of tumour vascularity. CEUS has a reported sensitivity of 80%–90%, comparable to CT and is significantly more sensitive than grey-scale ultrasound for detecting small CRLM less than 10 mm[39,40]. Nevertheless, CEUS does not offer comprehensive information needed for surgical planning as compared to CT or MRI. Intra-operative ultrasound (IOUS) has an established role in lesion detection and mapping of major hepatic vessels during surgery. IOUS has been shown to identify new lesions in 16% of patients and alter clinical management in 9%[41]. Contrast enhanced IOUS has higher sensitivity and specificity than traditional IOUS particularly for detection of “disappearing” lesions in the setting of neoadjuvant therapy[42,43].
Computed tomography: CT is the modality of choice for detection of liver and extrahepatic metastases. The high spatial resolution of CT combined with isotropic pixel size enables reformatted images in various planes, which enables better delineation of tumour and adjacent vascular structures for accurate segmental localisation[44]. The portal venous phase (approximately 60-70 s after administration of contrast agent) is the most reliable phase for detection of CRLM with a detection rate of 85% and a positive predictive value of 96%[45]. CRLM are typically hypovascular with variable heterogeneity depending on size and previous treatment. Since CRLM are hypovascular, arterial phase imaging does not improve detection but is helpful for pre-surgical or pre-embolisation planning[46]. The performance of CT is somewhat limited in detecting CRLM < 10 mm which are interpreted as too small to characterise[47]. In addition fatty liver is not uncommon post chemotherapy which can further limit detection of liver metastases.
Magnetic resonance imaging: Compared to CT, MRI has superior soft tissue contrast which makes it an invaluable tool for detection and characterisation of CRLM particularly those below < 10 mm[48]. CRLM are typically T1-hypointense, mildly T2-hyperintense with heterogeneous but predominantly rim enhancement in the arterial phase and hypo-enhancement in portal venous and delayed phases. Two advances which have revolutionised the role of MRI in the last decade are diffusion weighted imaging (DWI) and the use of hepatocyte-specific contrast agents. DWI measures the mobility of water molecules in tissues. Apparent diffusion coefficient values are quantitative estimates of diffusion restriction. CRLM show restricted diffusion of water molecules due to their hypercellular nature which manifests as high signal intensity lesions with low apparent diffusion coefficient values. Addition of DWI improves sensitivity and specificity for lesion detection and characterisation[49,50]. Hepatocyte-specific contrast agents are highly sensitive for detection of small lesions, which may be virtually occult on other sequences[51]. This also allows for detection of “disappearing” lesions which can mimic complete response to neoadjuvant therapy[52]. Gadobenate dimeglumine (MultiHance, Bracco) and gadoxetate disodium (Eovist, Bayer) are both hepatocyte-specific contrast agents which are preferentially taken up by hepatocytes and excreted into the biliary tree. In the delayed hepatobiliary phase (10–120 min after administration) normal hepatocytes are hyperintense compared to liver metastases, which do not retain the contrast agent. DWI has similar sensitivity and specificity as MRI with extracellular contrast agent but lower sensitivity than MRI with hepatocyte-specific contrast agent[53].
Positron emission tomography/computed tomography: There is lack of clinical evidence to show that Fluorine18 labelled Positron Emission Tomography/Computed Tomography (18FDG PET-CT) has significant impact on the clinical management of localised non-metastatic colorectal cancer preoperatively[54]. Its role in the initial assessment colorectal cancer, therefore, is not yet established[55]. Most centres do not carry out a routine 18FDG PET-CT at this stage.
18FDG PET-CT is considered to be very accurate and sensitive in the detection of CRLM, especially those greater than 10 mm[56]. However, small liver metastases (< 10 mm) and liver metastases from some mucinous adenocarcinomas can be missed[57-59].
18FDG PET-CT has been found to be accurate in identifying extrahepatic metastasis. Some studies suggest addition of 18FDG PET-CT can lead to change in management in over one-third of patients avoiding unnecessary metastasectomy[60-62], with a significant impact on survival[63]. However, other studies have disputed this and found only a modest 8% change in surgical management with 6% of false positive findings[64,65]. The role of 18FDG PET-CT in addition to standard imaging of CT chest, abdomen and pelvis, and MR liver in presurgical patients remains uncertain. Some authors have proposed it could be used as problem solving modality[66] to identify extrahepatic metastases in high risk patients[48]. Despite its shortcomings, 18FDG PET-CT remains part of our imaging algorithm prior to hepatic metastasectomy.
There is insufficient evidence for the use 18FDG PET-CT on routine surveillance, however, it does have a supplementary role in the context of rising CEA if CT fails to identify the site of disease[67].
Surveillance after resection of CRLM
Given that over half of patients undergoing liver resection for CRLM develop recurrence[68], that approximately half of these are hepatic only[69], and in the light of favourable outcomes after re-hepatectomy (see section 4) for intra hepatic recurrence, there is an intuitive and logical justification for surveillance following resection of CRLM. However, there is considerable heterogeneity in surveillance practice[70], and concerns have been raised regarding the implications of irradiation[71] and health care costs[72].
Defining optimal surveillance requires a knowledge of when recurrence occurs, and how best to detect it. In a retrospective multi-institution cohort study of 2320 patients undergoing initial hepatectomy for CRLMs, Hallet et al[73] reported that 89.1% of recurrences developed within 3 years. Recurrence was intrahepatic in 46.2%, extrahepatic in 31.8% and combined intra/extrahepatic in 22%.
Despite this concentration of recurrence in the early years, and many surveillance protocols suggesting follow up for 5 years[69,74], there is consistent evidence of recurrence occurring beyond 5 years in a significant minority of patients. Pulitanò et al[75] reported that whilst 93% of recurrences occurred within the first 5 years of follow-up, 11% of patients who were disease-free at 5 years developed later recurrence. Similarly, Tomlinson et al[76] found that of patients who were found to be disease free at 5 years, 23% had a documented first recurrence after 5 years, and Viganò et al[77] reported that 15% of the patients disease-free at 5 years developed later recurrence.
Heterogeneity applies not only to length of surveillance but also to surveillance type, reflecting the lack of evidence in this area.
However, in a prospective study of 76 patients, Bhattacharjya et al[78] reported that the use of CT or tumour markers CEA alone failed to demonstrate early recurrence in 12 and 18 patients respectively, and that the combination of tumour markers and CT detected significantly more recurrence than either modality alone, thus supporting the combination of CT and CEA in the follow-up of patients with resected colorectal liver metastases.
In an attempt to rationalise surveillance in long term survivors, Galjart et al[74] produced a stratification risk score based on primary nodal status and disease free interval between primary and CRLM resection to determine surveillance intensity. The authors found that in patients who were disease free after 5 years, recurrence rate beyond 5 years was 3% in the low risk group, but 12% in the high-risk group.
The role of other modalities such as MRI or PET-CT in post-operative surveillance is not defined but is predominantly used to investigate, confirm and characterise recurrence where it is suspected from CT and CEA results.
In conclusion recurrence after resection of CRLM is frequent and occurs mostly in the first 3 years post resection. Nevertheless, up to 23% of patients who are diseased free at 5 years may develop recurrence thereafter, such that protocols ending surveillance at 5 years would miss those patients. Generating good evidence for optimal length, frequency, and type of surveillance is likely to be challenging, and surveillance protocols are likely to be determined by clinician/patient preference as well as health care system resource issues.
SECTION 2: TUMOUR CHARACTERISATION AND BIOMARKERS IN COLORECTAL CANCER
The development of liver resection for CRLM has stimulated attempts to identify prognostic factors to aid in patient selection. Such factors have included primary CRC characteristics (tumour site, TNM stage), CRLM characteristics (size of largest liver metastasis, number of lesions, grade of differentiation, margin status), and other factors such as CEA, presence of additional extra-hepatic disease, and time interval between the emergence of CRC and CRLMs[79-82]. The limitations of individual factors in prognostication prompted their combination to produce risk scores such as the Fong score[83], however even this was found wanting in terms of prognostication[84-86]. It seems likely that the prognostic shortcomings of clinical criteria reflect the fact that they are merely surrogate markers for the underlying molecular biological markers that truly determine tumour biology.
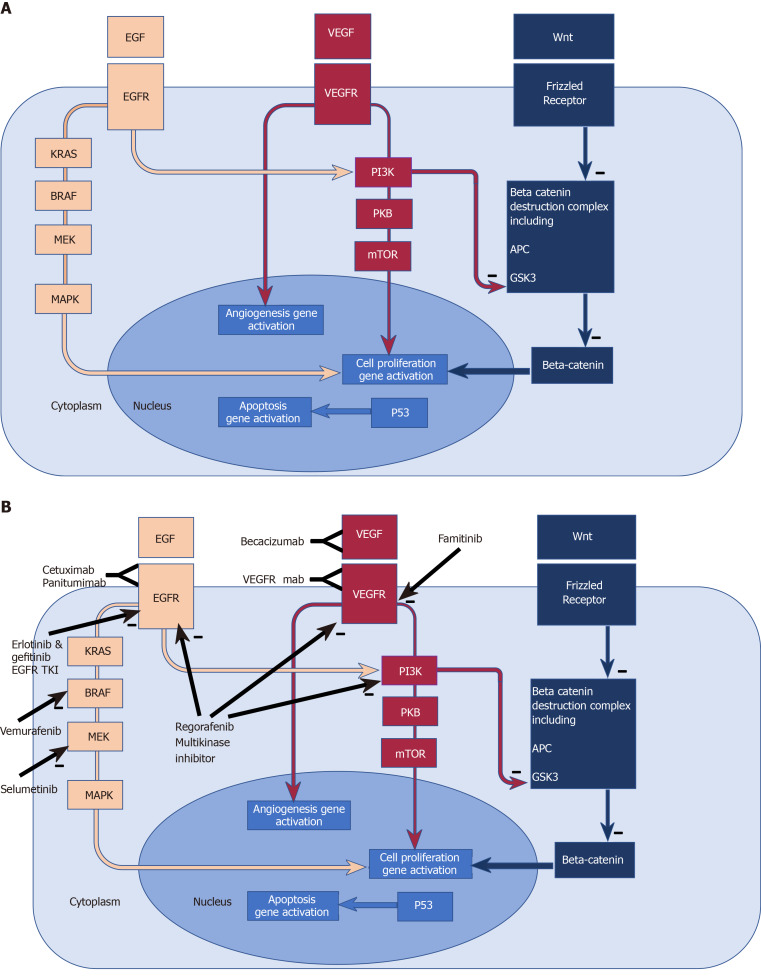
Although a detailed account of current CRC biomarkers is beyond the scope of this review, the following summaries and Figure 1 give an impression of some of the key CRC oncogenic pathways (Figure 1A) and the biomarkers KRAS, NRAS, BRAF, TP53, PIK3CA, APC, and Mismatch Repair Deficiency (MMRD), chosen for their prominence, and also because they inform the rationale for current chemotherapy and biological targeting treatments (Figure 1B).
Figure 1.
Biomarkers, molecular pathways, existing and emerging therapeutic targets in colorectal cancer. A: Biomarkers and molecular pathways in colorectal cancer. Epidermal Growth Factor Receptor (EGFR) pathway: EGFR is a transmembrane receptor tyrosine kinase[340]. EGF binding to the extracellular domain results in activation of down- stream intracellular signalling pathways such as RAS-RAF-MEK-MAPK, and the PI3K PKB mTOR pathway, amongst others, which favour cell proliferation and survival[341-344]; Angiogenesis pathway: Vascular endothelial growth factors influence angiogenesis in health and disease via binding to the vascular endothelial growth factor receptor. Deregulated angiogenesis impacts on progression in solid tumours, thus providing potential anti-angiogenic therapies[345]; Wnt pathway: The Wnt genes are vast family of highly conserved genes with wide ranging roles in development, cell proliferation and migration and tumorigenesis[346]. Beta catenin accumulation in the cytoplasm and nucleus leads to cell proliferation. Excess beta catenin accumulation is prevented by its destruction by the “beta catenin destruction complex” (a multiprotein assembly containing adenomatous polyposis coli and GSK3). Wnt binding to its receptor frizzled leads to impaired function of the Beta catenin destruction complex and hence beta catenin accumulation and cell proliferation[347]. Mutations in adenomatous polyposis coli prevent the formation of the beta catenin complex, and therefore allow beta catenin accumulation and cell proliferation. PI3K inhibits the function of GSK3[112], thereby impairing the beta catenin destruction complex, hence contributing to the tumorigenic accumulation of beta catenin. B: Existing and emerging therapeutic targets in colorectal cancer pathways. B: Cetuximab and Panitumumab are monoclonal antibodies targeting the EGFR, thus blocking activation of downstream signalling pathways. Mutated and constitutively active downstream effectors (such as RAS and RAF) confer resistance to EGFR blockade. Erlotinib and gefitinib are EGFR Tyrosine kinase inhibitors and are associated with improved PFS when combined to Bevacizumab in the DREAM trial[156]. Vemurafenib is a RAF inhibitor which in combination with EGFR blockade[157] has shown marked responses in some case reports[158]. Selumetinib is a MEK kinase inhibitor showing tumour response in some patients with KRAS mutant colorectal cancers progressing on Oxaliplatin[159]. Regorafenib inhibits is a multi-kinase inhibitor[153], with OS benefit in randomised double blind control trials[154,155]. Bevacizumab is a Monoclonal antibody against VEGFA with the most prominently established role in the treatment of metastatic colorectal cancer. Famitinib is a multiple tyrosine kinase inhibitor and targets the vascular endothelial growth factor receptor tyrosine kinase. Monoclonal antibodies targeting the VEGF receptors are also under investigation[160]. EGF: Epidermal Growth Factor; EGFR: Epidermal Growth Factor Receptor; EGFR TKI: Epidermal Growth Factor Receptor tyrosine kinase inhibitor; K-ras: K-Ras protein (product of the proto-oncogene KRAS); BRAF: BRAF protein (product of the proto-oncogene BRAF; MEK: Mitogen activated protein kinase which activates MAPK (mitogen-activated protein kinase); VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; PI3K: phosphoinositide 3-kinase; PKB: Protein kinase B; mTOR: Mammalian target of Rapamycin; Wnt: Wnt protein product of proto-oncogne Wnt; Frizzled Receptor: Receptor for Wnt; APC: Protein product of the tumour suppressor gene APC (Adenomatous polyposis coli); GSK3: Glycogen synthase kinase 3.
KRAS
KRAS is a GTP-binding protein and the first member of the KRAS-BRAF-MEK-MAPK pathway which is activated following binding of ligand to Epidermal Growth Factor Receptor (EGFR).
KRAS mutation leads to constitutive activation of the pathway and is one mechanism in EGFR blockade resistance. Once acquired, KRAS mutation persists with 96% concordance between primary tumours and metastases[87].
KRAS mutation (predominantly at codon 12[88] and 13[89]) is present in approximately 30% of colorectal cancers, and associated with more aggressive disease and more frequent recurrence after resection of colorectal liver metastases[90], although the poor prognostic effect of mutant KRAS may be limited to left sided primary tumours[81].
In terms of its implications for treatment of colorectal liver metastases, it has been reported that mutant KRAS is associated with a higher incidence of positive margins[91], with some authors reporting better outcomes in mutant KRAS patients whose metastases were resected with wider margins in anatomical (rather than non-anatomical) resections[92]. However, these results have been challenged with the alternative interpretation that the increased recurrence rate in the non-anatomical group may have been related to a higher proportion of radiofrequency ablation (RFA) treated tumours[91]. Thus it may be that the higher recurrence rate seen in mutant KRAS patients after resection of colorectal liver metastases is not directly caused by the higher positive margin rate, but that the two are manifestations of underlying aggressive biology[93].
BRAF
BRAF is part of the mitogen-activated protein kinase cascade (MAPK), downstream from KRAS.
BRAF mutation, most commonly at the V600E codon[94], is found in 5%-15% of colorectal cancer patients[94] and is associated with aggressive disease, resistance to EGFR blockade[95], worse overall survival (OS) in patients with non-metastatic primary colorectal cancer[96], and patients with metastatic colorectal cancer treated with palliative chemotherapy[97].
As a result of aggressive and often multisite disease associated with BRAF mutation, the incidence of BRAF mutation in patients undergoing resection of CRLM is low (2%-4%). Those patients with BRAF mutation who do undergo liver resection have a worse overall survival in comparison to patients with wild type BRAF[98]. The most recent and largest case control study[99] suggests this effect is not due to more frequent recurrence, but to the lethal multisite recurrence pattern in those patients in whom disease recurs.
In spite of these findings, in those patients with BRAF mutation who do undergo liver resection, long term survival (37% 5 years, and median survival 40 mo) is reported, and compares favourably with systemic chemotherapy[98,99], such that liver resection in these highly selected patients is still deemed indicated, though with appropriate counselling regarding outcome.
TP53 and combination mutations
TP53 is a tumour suppressor gene, the product of which (P53) plays crucial roles in the regulation of the cell cycle, induction of apoptosis, and Deoxyribonucleic acid (DNA) repair[100].
The incidence of TP53 mutation in patients with CRLM ranges between 40%-60%[101].
Although many studies have associated altered P53 activity with advanced stage[102] and poor survival in primary CRC[103], reports are conflicting in relation to the prognostic significance of mutant in patients undergoing resection of CRLM with Tanaka et al[104] identifying it as a predictor of poor survival, in contradiction of other studies[105]. Thus, although mutation undoubtedly has a key role in the early stages of CRC oncogenesis, its part in CRLM specifically is less clear.
The discrepancy in reported studies may also be in part explained by interactions between P53 and other mutations, as suggested by the poor prognosis associated with the combination of P53 and KRAS[106] in patients undergoing liver resection for CRLM.
Phospoinositide3-kinase catalytic subunit alpha
Phospoinositide3-kinase catalytic subunit alpha (PIK3CA) encodes the subunit of phosphoinositide-3 kinase, which controls downstream genes involved in cell proliferation and survival[107]. PIK3CA mutations result in loss of apoptosis, increased tumour invasiveness[108], and resistance to EGFR blockade[109].
Mutant PIK3CA is reported in 20% of patients with CRLM and associated with shorter time to relapse following resection[110], and significantly worse OS in patients harbouring the combination of mutation in PIK3CA and the Adenomatous Polyposis Coli gene (APC)[111]. As further discussed in Figure 1 and in the APC section below, mutant phosphoinositide-3 kinase inhibits the function of glycogen synthase kinase 3[112], thereby impairing the beta catenin destruction complex, hence contributing to the tumorigenic accumulation of beta catenin.
APC
APC is one component of a protein complex (the beta catenin destruction complex) which degrades beta catenin. Thus APC mutations allow the accumulation beta catenin in the cytoplasm and nucleus, resulting in activation of genes promoting cell proliferation and tumorigenesis[113].
APC mutation is reported in 50% of patients with CRLM, and, whilst not prognostic on its own, is associated with significantly worse OS in patients harbouring the combination of mutation in PIK3CA and APC[111].
This effect may be mediated by the fact that mutant PIK3CA inhibits the function of Glycogen synthase kinase 3[112], another component of the beta catenin destruction complex, thereby contributing to the tumorigenic accumulation of beta catenin.
MMRD
The mismatch repair system is a group of enzymes which repair errors which accumulate during DNA replication. When the proteins of the mismatch repair system do not function correctly, errors or mutations occur in the DNA. As a result, tumours which are mismatch repair deficient have high levels of mutation or are “hypermutated”. The most common mismatch repair protein which is altered in colorectal cancer is MLH1 which may be mutated in the germline (approximately 15% of cases), or absent due to promoter hypermethylation (sporadic, 85% of cases). Other proteins which are frequently affected include MSH2, MSH6 and PSM2. Mismatch repair deficiency in tumours can be assessed using protein immunohistochemistry or by examining microsatellites on DNA using Polymerase chain reaction (microsatellite instability)–these tests are highly concordant[114].
Sporadic mismatch repair deficient tumours are more common in older patients and in the right colon, and in early stage cancers. Hypermutation leads to production of high levels of immune stimulating neoantigens and increased immune infiltrates, which in early stage cancers confers a good prognosis. However, in later stages the positive prognostic effect of mismatch repair deficiency becomes lost by a process of immune editing. Mismatch repair deficient tumours are considered chemo refractory and sporadic mismatch repair deficient cancers are often associated with BRAF mutations which confer a further negative prognosis. However, the advent of immune checkpoint blockade with anti-PD-1 and anti-CTLA4 inhibitory antibodies has heralded a new era for the small number of patients with advanced MMRD colon cancers[115,116]. Treatment with novel immunotherapy drugs may lead to long term remission for these patients.
Interestingly, MMRD colon cancer may less commonly metastasise to the liver than non MMRD colon cancer. Many MMR tumours downregulated HLA expression as a mechanism of immune evasion, and HLA negative tumours are less common in liver metastases. This is believed to be due to the presence of natural killer cells in the liver which eliminate cells with an absent “self” phenotype[117].
SECTION 3: SYSTEMIC AND LOCOREGIONAL CHEMOTHERAPY AND TARGETED AGENTS IN COLORECTAL LIVER METASTASES MANAGEMENT
Introduction
The role of chemotherapy in the overall management of colorectal liver metastases is evolving and complex, consistent with the multitude of different but sometimes overlapping contexts in which chemotherapy may be considered.
Although evidence exists to guide management in some scenarios, even then decision making remains nuanced in the face of heterogeneity within randomised trial groups, as well as patient specific factors such as individual chemotherapy tolerance, and risks associated with comorbidities.
Seen from the perspective of maximising the chance of liver resection, as the treatment which offers the best chance of long-term survival, these different contexts may be classified into three broad categories, although it is acknowledged that these may overlap: (1) Patients with unequivocally unresectable disease; (2) Those with up-front resectable disease; and (3) Those patients between these 2 ends of the spectrum, whose disease is deemed initially unresectable, but with the potential of conversion to resectability by downsizing chemotherapy.
The section below discusses chemotherapeutic options for the three categories above in the scenario of metachronous colorectal liver metastases, with synchronous metastases discussed in a later separate section (see section 4).
Prior to describing options for these broad patient groups, we discuss chemotherapy related hepato-toxicity, as this has a significant influence on decision making.
Chemotherapy related toxicity
Chemotherapy associated hepato-toxicity presents in three main entities: Steatosis, steato-hepatitis, and sinusoidal obstruction syndrome.
Steatosis: Liver changes associated with fat accumulation in hepatocytes are termed “non-alcoholic fatty liver disease”. Whilst indolent in most patients, a progressive form of “non-alcoholic fatty liver disease” can lead to steato-hepatitis, and thereafter progress to fibrosis and ultimately cirrhosis[118]. 30%-40% of patients treated with 5-Fluorouracil develop reversible steatosis demonstrated radiologically and histologically[119-121]. Steatosis is associated with increased complications post liver resection, though not increased mortality[122].
Steato-hepatitis: Steato-hepatitis is hypothesised to be the end result of the “two hit theory” where the first insult (steatosis) is compounded a second insult in the form of reactive oxygen species. Irinotecan is the drug predominantly associated with steatohepatitis, with high BMI patients particularly at risk, presumably as result of pre-existing steatosis[122]. In terms of its impact on liver surgery, patients with steatohepatitis have been shown to have not only more frequent post-operative complications, but also significantly increased 90d mortality rate (15% vs 2%for patients without steatohepatitis[122].
Chemotherapy-associated hepatic sinusoidal obstruction syndrome: Sinusoidal obstruction syndrome (SOS) was first recognised in the context of bone marrow transplantation and treatments involving combinations of several cytotoxic drugs[123]. In the context of chemotherapy for colorectal liver metastases oxaliplatin is the predominant drug associated with SOS, with 78% of patients receiving oxaliplatin having evidence of sinusoidal injury[124]. SOS is associated with Increased morbidity post liver resection, though not mortality[125].
Chemotherapy duration: As well as the type of agent, there is some evidence that the length of chemotherapy course may impact on perioperative complications. In terms of minimising chemotherapy associated hepato-toxicity, Karoui et al[125] found that patient receiving fewer than 6 chemotherapy cycles experienced significantly fewer post liver resection complications than those who had received more than 6 cycles (19% vs 54% complication rate) although there was no impact on mortality rates.
In the context of other evidence discussed below, hepatoxicity may influence choice of chemotherapeutic agent, for example with a caution in relation to the reported increased mortality associated with irinotecan in patients with pre-existing steatosis who are potential surgical candidates.
Chemotherapy for patients with unequivocally unresectable disease
The subgroup of patients with liver unresectable metastasis represents a very heterogeneous group, and therefore a careful multidisciplinary evaluation of patient and tumour’s characteristics as well as treatment toxicities is crucial in the decision-making process. In this setting, patients may be distinguished into three different subgroups: (1) Patients with good performance status but with tumour burden related symptomatic disease; (2) Patients with good performance status but without symptoms related to tumour burden; and (3) Patients with poor performance status. In the first case, the objective of treatment is the tumour shrinkage with the aim of symptom control, whereas in the second subgroup the objective is disease control with improvement of OS and preservation of quality of life. In the third group, best supportive care represents the most appropriate option because active treatment will not be tolerated.
Although a comprehensive description of systemic treatment options for metastatic disease is beyond the scope of this review, this section provides a summary of the current indications for first-line medical treatment in metastatic CRC.
According to international guidelines[126] chemotherapy plus target agents (anti-EGFR or anti-vascular endothelial growth factor) provide the best first line treatment for patients with appropriate performance status. In particular, doublet therapy based on fluoropyrimidines (5-FU/capecitabine) and oxaliplatin or irinotecan (FOLFOX/XELOX/FOLFIRI) represents the standard of care in order to improve survival[127-129] More recently, triplet chemotherapy with FOLFOXIRI has been associated with a further 25% increase in median OS, although at the expense of greater toxicity[130,131]. As trials show no difference in the outcomes when using oxaliplatin or irinotecan-based doublets, the choice is mainly related to the different safety profile[132]. In addition, biological agents could be added to chemotherapy according to tumour (RAS mutational status, sidedness) and patient characteristics.
EGFR blockade: The key evidence in favour of EGFR blockade in the context of colorectal liver metastases comes from randomised trials demonstrating improved OS and progression free survival (PFS) in patients treated with EGFR blockade added to conventional chemotherapy compared with chemotherapy alone. Summarising this evidence, a meta-analysis of randomised trials showed that combining cetuximab or panitumumab to oxaliplatin or irinotecan regimens increased response rates in patients with initially inoperable CLM[133]. In terms of the relative efficacy of oxaliplatin vs irinotecan based regimens in combination with EGFR blockade, the CELIM study comparing the efficacy of FOLFOX + cetuximab to FOLFIRI + cetuximab, showed no significant difference in efficacy between the 2 regimens[134]. In a trial comparing triplet chemotherapy (FOLFOXIRI) + panitumumab to FOLFOXIRI alone, EGFR blockade was associated with improved response rates though no difference in PFS or OS[135].
In terms of the efficacy of EGFR blockade alone, Cetuximab alone was found to be less effective alone than in combination with Irinotecan in the BOND study[136].
In terms of patient selection for EGFR based therapy, CRC harbouring mutations in KRAS[137] and NRAS[138] genes which result in constitutive activation of the downstream signalling cascade have been demonstrated to be insensitive to treatment with anti-EGFR blockade. Furthermore, some RAS wild type CRC may also prove insensitive to EGFR blockade, possibly due to the presence of other mutations in downstream genes, including that of BRAF, present in 9% of CRC, and associated with poor prognosis[139], or amplification of receptor tyrosine kinase genes[140] or mutations in the EGF receptor itself[141]. In addition, there is growing evidence that primary tumour sidedness may also affect response to EGFR blockade, with right sided tumours failing to benefit, even when RAS wild type, as discussed further below[142].
Anti-angiogenic agents: Bevacizumab is the only anti- vascular endothelial cell growth factor agent approved in first line setting for metastatic CRC. Several trials have demonstrated that bevacizumab improves overall response rate, PFS and OS when added to irinotecan based regimens and PFS when added to oxaliplatin based regimens[143,144] regardless of RAS status. Furthermore, a meta-analysis of 6 randomized clinical trials assessing bevacizumab in patients with metastatic CRC reported improved PFS and OS[145]. In terms of combining bevacizumab with triplet chemotherapy, the phase II OLIVIA trial studied the addition of bevacizumab to FOLFOX or FOLFOXIRI in patients with initially unresectable liver and demonstrated improved PFS, overall response rate and R0 rates in the FOLFOXIRI + bevacizumab group[146], with confirmation of these results in the phase III TRIBE trial[147].
Factors influencing choice of targeted therapy: In considering the choice between EGFR blockade and antiangiogenic agents in combination with chemotherapy in RAS WT patients, evidence is somewhat conflicting.
Whilst the FIRE 3 trial[148] (comparing FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer), and the PEAK trial[149] (comparing FOLFOX plus panitumumab vs FOLFOX plus bevacizumab) both reported improved OS in the EGFR blockade group, the CALGB 80405 trial showed no difference in OS between EGFR blockade and anti-angiogenic agents[150].
Combination of EGFR blockade with anti- angiogenic agents was examined in the PACCE trial which suggested prohibitive increased toxicity[151], and although this was not confirmed in the combination CAIRO 2 study[152], concerns regarding toxicity have led to an avoidance of the combination of EGFR blockade with anti-angiogenics.
The choice of which targeted therapy is best added to conventional chemotherapy may also be influenced by the sidedness of the primary tumour. It is increasingly recognised that right and left sided colon cancers have different biological and clinical behaviours which impact on their response to systemic treatment. In a systematic review of 6 randomised trials examining treatment regimens for RAS wild type colon cancer, Arnold et al[142] found that right sided tumours had worse prognosis, that EGFR blockade benefit was restricted to left sided tumours, that there may be possible adverse effect of EGFR blockade to right sided tumours, and that right sided tumours may benefit more from anti-angiogenic therapies, thus giving rise to the consideration of triplet therapy combined with bevacizumab for right sided tumours.
Novel agents: Novel agents targeting other aspects of known oncogenic pathways (Figure 1B) are also in varying stages of assessment. These include multi-kinase inhibitors, agents targeting other steps in the EGF receptor signalling pathway, antiangiogenic agents, and immune checkpoint inhibitors.
Multi-kinase inhibitors such as regorafenib inhibits a wide range kinases impacting on several oncogenic pathways[153], and has shown OS benefit in randomised double blind control trials[154,155].
EGFR pathway blockade using EGFR tyrosine kinase inhibition by agents such as erlotinib or gefitinib has been associated improved PFS when combined to bevacizumab in the DREAM trial[156].
The BRAF mutation, present in 10% of colorectal cancers, and associated with aggressive disease and poor prognosis has been targeted by the agent vemurafenib in combination with EGFR blockade[157] with marked responses in some case reports[158].
MEK kinase has been targeted by the inhibitor selumetinib with tumour response shown in some patients with KRAS mutant colorectal cancers progressing on oxaliplatin[159].
The potential for exploiting anti-angiogenic pathway is also under investigation with other agents such as famitinib which inhibits multiple receptor tyrosine kinases, and monoclonal antibodies targeting the VEGF receptors[160].
Pembrolizumab is an immune checkpoint inhibitor which impacts on cytotoxic immune responses. In a phase 2 study mismatch-repair status predicted clinical benefit of immune checkpoint blockade with pembrolizumab[115].
Chemotherapy for patients with up-front resectable disease
In patients with up-front resectable colorectal liver metastases, the role of chemotherapy has been investigated in both neoadjuvant and adjuvant roles.
Neoadjuvant chemotherapy: In the context of initially resectable liver metastases, neoadjuvant chemotherapy may have theoretical advantages or objectives such as assessing chemo-responsiveness to inform future treatment strategy, provide tumour shrinkage to increase chance of R0 resection, and to eliminate undetectable micro-metastases. Weighed against these potential advantages are the disadvantages of chemotoxicity, and hepatoxicity in particular. In the midst of these conflicting principles, 2 randomised trials provide evidence.
The first, the EORTC 40983 trial[161], which compared liver resection alone to FOLFOX (6 cycles preop) - liver resection - FOLFOX (6 cycles post op). At 3 years the study showed a significantly better 8% higher PFS in the peri-operative chemo group, but no difference in OS, and significantly more complications in the chemotherapy group (25% vs 16%). Moreover, the long term outcome[162] showed no OS benefit in the chemotherapy group. The absence of OS benefit has been attributed to the fact that with a sample size of 364, the trial was powered to detect a PFS, but insufficiently powered for OS. In comparison, trials such as the MOSAIC trial[163] included a relatively large sample size of 2246, and was able to detect a 4.2% OS benefit at 6 years of follow-up for patients treated with FOLFOX over those treated with Leucovorin 5-FU after resected stage III colon cancer.
Thus, despite improved PFS in the peri-operative chemo group, the absence of OS survival and the increased complication rate has not led to peri-operative chemotherapy being used routinely in patients with initially resectable liver metastases. Moreover, in a meta-analysis of 18 studies, neo-adjuvant chemotherapy in resectable colorectal liver metastases was not associated with a survival benefit[164].
The evidence for targeted therapies in the perioperative context is, if anything, weaker. Primrose et al[165] compared 2 perioperative systemic regimens (FOLFOX – surgery - FOLFOX vs cetuximab + FOLFOX – surgery – cetuximab + FOLFOX) in patients with initially resectable colorectal liver metastases, and found a significantly inferior disease free survival (DFS) in the cetuximab group (20.1 vs 14.5 mo). Although some confounding factors have been suggested (possible different baseline characteristics between groups, 11% missing outcome data, and more ablations and more positive margins in cetuximab group), these findings argue against EGFR blockade in patients with upfront resectable liver metastases.
Peri-operative or neoadjuvant bevacizumab in upfront resectable disease has not been investigated.
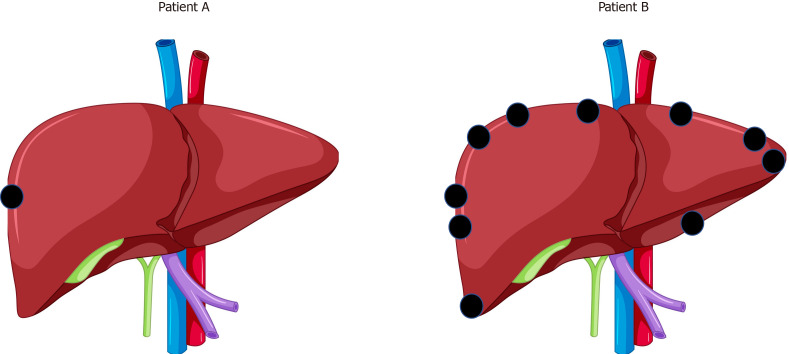
In practice the use of neoadjuvant chemotherapy in the context of upfront resectable CRLM is influenced not only by the evidence above, but also by nuances in individual case presentations which blur the boundaries of what is meant by “upfront resectable”. Adam et al[166] allude to the concept of patients who may be “technically “resectable, but in whom a poor oncological outcome is suspected. A hypothetical example is shown in Figure 2. Both patient A (with a single superficial CRLM) and patient B (with 10 superficial CRLM) are “technically” resectable, but there would likely be consensus amongst MDTs that whilst patient A would best be recommended for upfront liver resection, patient B would best be served by neoadjuvant chemotherapy in the first instance.
Figure 2.
A hypothetical example is shown. A: Patient A (with a single superficial colorectal liver metastases); B: Patient B (with 10 superficial colorectal liver metastases).
In the context of better defining patients who are technically resectable but may have a poor oncological outcome, Fong et al[83] developed a preoperative oncological score including five factors: Node-positive disease, disease-free interval from primary to metastases < 12 mo, > 1 hepatic lesion, > 5 cm in the highest hepatic lesion diameter and carcinoembryonic antigen level > 200 ng/mL. Patients with ≤ 2 criteria showed a better outcome, while chemotherapy might be considered in case of patients with ≥ 3 criteria.
This highlights the heterogeneity of “upfront resectable” patients, and MDTs may take additional factors than those included in the Fong score into account in decision making, resulting in a “case by case” approach.
Adjuvant chemotherapy: There is no level I evidence for the use of adjuvant therapy in patients with resected colorectal liver metastases, However, meta-analysis of available trials suggests that there may be a benefit to this approach[167]. Included in this meta-analysis are the report from Portier et al[168] who compared Surgery alone vs surgery with followed by 6 mo of systemic adjuvant fluorouracil and folinic acid, and demonstrated an improved DFS at 5 years of 33.5% for patients in the chemotherapy group vs 26.7% for patients in the control group, though no OS survival benefit.
Kim et al[169] compared the outcome of 3 different adjuvant chemotherapy regimens (oxaliplatin/ fluoropyrimidine (group I), irinotecan/fluoropyrimidine (group II) and fluoropyrimidine alone (group III). Median DFS was 23.4 mo in group I and significantly better than the combined other groups, 14.1 mo in group II and 16.3 mo in group III (P = 0.03).
The EORTC 40983 trial[161] also provides some evidence of chemotherapy benefit in PFS, although it is difficult to establish whether this was attributable to adjuvant chemotherapy, as the trial group also received neo-adjuvant treatment.
Thus, in the context of adjuvant chemotherapy for resected colorectal liver metastases there is some, though limited, evidence for improved DFS with certain agents. As a theoretical consideration, adjuvant treatment allows chemotherapy delivery and avoids the increased liver surgery complications associated with neo-adjuvant chemotherapy.
Conversion and down-sizing chemotherapy for patients with initially unresectable disease
The results of downsizing chemotherapy for initially unresectable colorectal liver metastases are well established. In a systematic review including 10 studies of downsizing systemic chemotherapy and rescue liver surgery for initially unresectable CLM, Lam et al[170] reported objective response rate of 64% (range, 43%–79%) of patients after systemic chemo-therapy, with 22.5% of patients converted to a resectable status and macroscopically curative liver resection overall. For those resected patients, median overall survival was 45 (range, 36–60 mo) mo with 19% of patients alive and recurrence-free, thus comparing favourably to chemo alone, and to outcomes for patients undergoing up-front resectable liver metastases.
Downsizing regimens based on oxaliplatin[171] and irinotecan[172] have achieve similar response rates in the range of 50% and rates of liver metastases resection of 33%-40%. Moreover, in a randomised controlled trial comparing FOLFIRI and FOLFOX, the two regimens had identical response rates (55%) and similar levels of clear margin (R0) resections[173]. The triplet combination of folinic acid 5FU, oxaliplatin and irinotecan has also been studied, and in randomised trials comparing FOLFOXIRI to FOLIFIRI[130] and FOLFIRINOX to FOLFOX or FOLFIRI[174], the triplet combination was associated with improved response rates, progression free survival, overall survival, and increased resection rates, but at the expense of greater toxicity.
EGFR blockade in downsizing setting: Given the evidence demonstrating the benefit of adding EGFR blockade to conventional chemotherapy in the setting of unequivocally unresectable colorectal liver metastases[133], the potential for such combination to maximise conversion of initially unresectable liver metastases has also been explored. Thus addition of EGFR blockade to systemic chemotherapy in RAS wild type patients was associated with improved conversion to resectability and R0 rates[175], in comparison to systemic chemotherapy alone. Furthermore, in the CELIM trial comparing cetuximab with either FOLFOX or FOLFIRI, both regimens demonstrated similar high response rates and increased resectability rates[134].
The impact of adding EGFR blockade to triplet chemotherapy has also been studied in The VOLFI trial comparing FOLFOXIRI with panitumumab vs FOLFOXFIRI alone, showing improved response rates and resection rates in the panitumumab group[135].
Antiangiogenic therapy in downsizing: Anti-angiogenic therapies have also been studied in the downsizing context. Wong et al[176] reported a 40% conversion to operability with XELOX and bevacizumab. Similarly, increased resection rates, R0 rates and PFS were associated with addition of Bevacizumab to triplet chemotherapy in the OLIVIA trial[146].
As discussed in the section relating to inoperable colorectal live metastases, the choice of addition of EGFR blockade or antiangiogenic therapy is a complex one, and is influenced not only by RAS status, but also by primary tumour sidedness[142].
In conclusion, an improvement in OS is clearly demonstrated for patients converted to R0 surgery by use of conversion chemotherapy. Radiological response should be evaluated 2-3 moly by RECIST criteria, taking into account the radiological pattern of response to antiangiogenic agents. Timing of surgery is critical in order to avoid overtreatment of lesions which may disappear and to avoid liver toxicity. The benefit derived from adjuvant treatment (chemotherapy alone or in association with target agents) in patients that received complete resection of liver metastasis after conversion therapy is still unclear.
Locoregional intra-arterial therapies
In addition to the systemic agents described above, the option of locoregional chemotherapy, delivered intra-arterially by a variety of means also exists. The following section describes current knowledge of hepatic arterial infusion (HAI) chemotherapy, and trans arterial delivery of irinotecan coated beads (DEBIRI).
Hepatic arterial infusion chemotherapy: The underlying biological rationale for considering hepatic arterial infusion chemotherapy is based on the fact that the blood supply to colorectal liver metastases is predominantly arterial, and that such infusion provides favourable pharmacodynamics allowing high intrahepatic and low systemic concentrations of drugs[177]. The potential role of hepatic arterial chemotherapy, via surgically or percutaneously placed catheters[178], has been studied in varying contexts, including patients with unresectable colorectal liver metastases, but also in downsizing and adjuvant scenarios.
In the unresectable CRLM context, although initial reports from randomised trials of HAI[179] suggested survival benefit , the modest increase in survival was not widely been felt to justify the quality of life cost brought about by the considerable toxicity associated with Floxouridine. However, further studies with newer agents including Oxaliplatin HAI, 5FU/leucovorin intravenously (IV)[180], Oxaliplatin + Irinotecan + 5FU HAI + cetuximab IV[181] in first or second line settings reported median overall survival of 25 to 27 mo, with conversion to operability in 29% and 37% survival at 4 years for those who underwent resection. Thus, although the place of HAI remains uncertain in the first line setting, these results could form the justification for a randomised trial of HAI vs conventional systemic chemotherapy in second line treatment.
In the adjuvant setting one non randomised report[182] studying 2368 consecutive patients after complete resection of CLM suggest a potential significant benefit in OS for patients receiving HAI with significantly improved median OS 67 mo vs 47 mo without HAI (P = 0.001) and 10-year survival (38.0% vs 23.8% without HAI). In terms of randomised data, although one randomized trial demonstrated increased disease-free survival with systemic chemotherapy (5-FU) plus HAI compared to systemic chemotherapy alone (37.4 vs 17.2 mo, P < 0.01)[183] , a meta-analysis did not demonstrate an improved OS[184].
In summary, HAI has progressed a great deal since the early reports associated with prohibitive toxicity, and with improvements in catheter placement options. Non-randomised results suggest a potential benefit, although this needs confirmation in carefully designed trials, some of which are in progress[177,185].
DEBIRI: DEBIRI consists of trans arterial delivery of irinotecan coated beads, theoretically allowing slow drug delivery for prolonged antineoplastic effect.
The mechanism of action of DEBIRI[186] presents a paradox in that intra-arterial delivery implies a regional effect of the drug, although irinotecan is a prodrug that requires activation in healthy liver parenchyma to its active Topo-isomerase 1 inhibiting metabolite. Animal models suggest that although much lower overall doses are given in DEBIRI, drug levels at 24 h are higher in tumour and lower in serum than with either intra-arterial or intravenous administration. Further animal model studies suggest that beads cause ischaemic embolization in the predominantly arterial vascularity of tumours. Although beads alone have little tumour burden reducing effect, there is a dose response to DEBIRI beads suggesting that ischaemia and the drug act in concert. This is perhaps as a result of ischaemia induced acid pH, at which the active form of irinotecan is much more effective, and thus perhaps explaining its sparing of neighbouring liver parenchyma where ischaemia is less marked owing to the predominant portal circulation.
The clinical experience of DEBIRI was reviewed by Akinwande et al[187] in a systematic review including 11 single arm retrospective and prospective phase studies and two prospective randomized control trials involving 850 patients. Overall toxicity rates were reported at 35% with 10% high grade toxicity, and 2 possible treatment related deaths (0.2%). Overall response rate was 56%, in spite of the fact that patients referred for DEBIRI typically had undergone at least 2 prior lines of chemotherapy. Progression free survival and overall survival was 8.1 mo and 16.8 mo, respectively, comparing favourably with comparable cohorts.
Two randomised trials have examined DEBIRI vs conventional chemotherapy. Martin et al[188] compared FOLFOX and bevacizumab to FOLFOX, bevacizumab + DEBIRI (FOLFOX - DEBIRI), and found that the DEBIRI patients had significantly better overall response, and improved median progression-free survival (15.3 mo vs 7.6 mo). Fiorentini et al[189] compared DEBIRI with systemic FOLFIRI, and found that the DEBIRI group had significantly improved OS (OS median 22 and 15 mo respectively, P = 0.031), PFS [median 7 vs 4 mo (P = 0.006)], although the study was criticized for the absence of Cetuximab in the FOLFIRI arm.
In summary, DEBIRI has been shown to be safe in the treatment of colorectal liver metastases and to have promising response rates in the setting of patients who have been exposed to multiple prior lines of chemotherapy, with some early randomised evidence of favourable results in comparison to systemic chemotherapy. Its ideal role, in terms of patient group and optimal context, remains to be determined by future trials.
Radiation based therapies for unresectable CRLM
In addition to chemotherapy in all its forms, unresectable CRLM may be treated by radiation either by selective internal radiotherapy (SIRT) or stereotactic body radiotherapy (SBRT).
Selective internal radiation therapy: The blood supply of metastatic liver tumours is predominantly arterial, in contrast to that of hepatocytes which is mostly portal venous[190,191]. This, together with significant arterial neovascularisation in the tumour bed[192], provides the physiological underpinning of SIRT, which achieves tumour destruction by delivery of radioactive microspheres via its arterial supply. Ytrium-90, which undergoes beta decay, is the most commonly used radionuclide used to label microspheres, on account of favourable penetration characteristics: Mean and maximal penetration are 2.5 and 10 mm respectively, thus delivering maximal irradiation to the tumour whilst sparing surrounding parenchyma[193]. Currently glass and resin-based versions of the sphere are commercially available. A newer sphere which employs Holmium-166 rather than Ytrium-90 is also available and being evaluated[194].
In the context of colorectal liver metastases, interest in SIRT originated from studies done in patients with unresectable liver or liver dominant metastases who had proved refractory to conventional chemotherapy. These studies suggested response to SIRT in the face of prior chemo refractory status[195,196], and in some reports, significantly improved OS in patients who responded to SIRT[197-199].
On the basis of the above and other studies, 3 randomised prospective trials[200-202] were carried out to investigate the potential role of SIRT by comparing FOLFOX + SIRT vs FOLFOX alone as first-line treatment for mCRC with liver-only or liver-predominant metastases. The combined results of the 3 trials were reported by Wasan et al[203]. The overall findings were that there was no OS survival benefit to the addition of SIRT to FOLFOX, but that progression within the liver within the first 12 mo of follow-up was significantly lower in the SIRT group.
It was concluded that given the absence of OS survival, SIRT could not be recommended as first line treatment for mCRC with liver-only or liver-predominant metastases, but that its role in other contexts required investigation. In this perspective Gibbs et al[204] reviewed the outcomes of the FOXFIRE trials with respect to primary tumour sidedness and found that the median OS for patients with right-sided primaries was significantly higher for patients in the SIRT arm compared to the control group, and that left sided primary tumour patients did not benefit from SIRT.
In summary, the current role of SIRT is evolving and will doubtless be further refined as the results of new trials become available. In the United Kingdom, based on a review of current evidence[205], SIRT is commissioned for use in patients with unresectable or ablatable colorectal liver metastases who have progressed or are refractory to both oxaliplatin-based and irinotecan-based chemotherapy, with five or fewer liver tumours, a percentage tumour to liver volume of ≤ 25%, and World Health Organisation (WHO) performance status 0-1[206].
Stereotactic body radiation therapy for colorectal liver metastases: The results of studies suggesting benefit to local ablative therapies such as RFA[207] in the treatment of colorectal liver metastases has prompted investigation of whether similar benefits could be achieved by radiotherapy. Stereotactic body radiation therapy offers an alternative approach to the treatment of liver metastasis by precise targeted delivery of radiation. The potential benefits would be the use of a non-invasive technique, without need for general anaesthetic, and perhaps an opportunity of overcoming the limitations of ablation such as tumour size restriction, and problems such as heat sink effects in tumours situated near vascular structures.
In a systematic review, Petrelli et al[208] analysed the results of a total of 18 studies, encompassing 656 patients, with colorectal liver metastases, numbering 1–2 lesions in most cases, with a size range of 0.7-11.6 cm in size, the majority having received systemic chemotherapy, with a median follow up of two years.
The pooled one and two-year OS were 67.18% and 56.5% respectively, and median PFS and OS were 11.5 and 31.5 mo. The pooled one-year and two-year local control was 67% and 59.3%. In terms of liver related toxicity, pooled grade 1–2 and grade 3–4 liver toxicity[209] were 30.7% and 8.7%, with mild nausea and fatigue reported as other toxicities. There were 4 cases of liver failure (0.6%), and three treatment related deaths (0.004%).
The optimal irradiation dose is likely to be multifactor dependant, but reports suggest improved local control rates after increasing biological equivalent dose, with local control rates of 90% in patients exposed to higher biologically effective dose[210,211], with dose response relation confirmed in a pooled analysis[212]. In terms of lesion size limits, although early reports correlate large tumour size (> 3 cm) with poorer rates of local control[213], more recent studies report local control in tumours 3-6 cm as equivalent to that achieved with tumours less than 3 cm by use of higher irradiation doses[214].
The interpretation of data relating to the effectiveness of SBRT in the treatment of colorectal liver metastases is difficult for a number of reasons: Firstly the studies are subject to case selection bias, and markedly heterogeneous in terms of population and techniques: The study populations vary in age and performance status, number and size of metastases, median follow-up, subsequent chemotherapy delivery, SBRT techniques, and fractionation. Secondly, the absence of randomised trials makes it difficult to assess the hypothesised additional benefit that SBRT may bring to optimal chemotherapy and existing ablation methods.
In this regard, there is a difficult problem with recruitment to such trials, with 2 examples of such studies (the French OLIVER trial (NCT03296839) investigating chemotherapy +/- SBRT[215], and the Dutch RAS01 trial (NCT01233544)[216] comparing systemic chemo + RFA or SBRT) both closed with insufficient recruitment. Undoubtedly part of the problem with recruitment in such areas is the fact that both patients and clinicians may not perceive equipoise. Furthermore, different techniques are often complementary rather than in competition, such that their indication for use may be subtly but importantly different. For example, a tumour adjacent to a large vein may not be appropriate for ablation because of heat sink effect, but potentially a good indication for SBRT.
In summary, the results of SBRT in terms of local control and overall survival are hard to ignore, especially as they are achieved in the context of patients who have exhausted other treatment options. Although formal comparisons with other treatments will be difficult to carry out, ongoing studies to define SBRT technique such as irradiation dose and fractionation will likely deliver ongoing improvements in outcomes and help to define the niche for SBRT in the armamentarium for treatment of colorectal liver metastases.
SECTION 4: SURGICAL MANAGEMENT OF RESECTABLE COLORECTAL LIVER METASTASES
Introduction
The success of liver resection for CRLM in achieving long term survival has driven the investigation of numerous techniques to increase resection rates. In defining ‘resectability’, there is distinction to be made between what is technically feasible, and what is oncologically sensible. In this regard, clinical, biochemical and histopathological factors[79-82] and risk scores such as the Fong score[24] (see section 2) have provided some direction in decision making. From the sole perspective of technicality however, CRLM may be thought of as resectable provided that clear margins are achieved, and that the Future Liver Remnant (FLR) is of sufficient size, with adequate arterial supply, portal venous supply, hepatic venous drainage, and biliary outflow. The techniques used to increase resectability include downsizing chemotherapy (discussed in section 3), portal vein embolization (PVE), Associating Liver Partition and Portal vein Ligation for Staged hepatectomy (ALPPS), and the use of ablation technology. Surgery may be carried out laparoscopically or open, and in selected patients prior to or synchronously with resection of the primary CRC. These considerations are discussed in more detail in this section.
Liver resection for CRLM: General considerations
Biopsy of CRLM: Biopsy of suspected CRLM should be avoided. The problem of needle track seeding with malignant cells following biopsy of malignant liver lesions is well documented in the context of HCC[217]. In terms of this risk in biopsy of CRLM, Rodgers et al[218] reported that out of 43 patients who had undergone CRLM biopsy, 7 (16%) developed needle track seeding[218]. In a similar study Ohlson et al[219] reported a needle track seeding rate in 5 (10%) of 51 biopsied patients. Jones et al[220] reported a 19% rate of needle track seeding and found that following resection of CRLM, biopsied patients had a significantly lower 4 year survival, with biopsy being identified as an independent predictor of poor survival in regression analysis. These findings, taken together with the low percentage (< 2%) of benign lesions resected unnecessarily following incorrect radiological diagnosis of a CRLM argue strongly against pre-operative biopsy of CRLM[221].
Anatomical vs non-anatomical resections: In a systematic review of 2505 patients included in 12 studies, Moris et al[222] found that there was no difference between anatomical and non-anatomical (parenchymal sparing) hepatectomy in terms of peri-operative and long term oncological criteria, thus arguing in favour of a parenchymal sparing approach whenever appropriate.
Resection margins: There is consensus that positive margins after resection of CRLM remains a negative prognostic factor[223] . Although historical practice suggested a liver resection margin of 1cm in resection of CRLM, a Propensity Score Case-Match study from Hamady et al[224] showed that 1 mm cancer-free resection margin achieved 33% 5-year overall disease-free survival, and that additional margin width did not add disease-free survival advantage. Moreover, De Haas et al[225] reported that although patients with involved (R1) margins experienced more recurrences, the contraindication of R1 resection should be revisited in the current era of effective chemotherapy because survival was similar, in their study, to that of R0 resection. Thus, although R0 resection is doubtless preferable, the necessity of R1 resection for lesions near structures that cannot be sacrificed, or for preservation of liver parenchyma, may be accepted in selected patients.
Extra-hepatic disease: Whilst a full review of resection of extra-hepatic disease is beyond the scope of this review, there is consensus in favour of proceeding with liver resection of CRLM in particular scenarios[226,227].
Positive retroperitoneal or coeliac lymphadenopathy is still an absolute contraindication to liver resection, but hepatectomy may be carried out in selected patients with hepato-duodenal ligament lymphadenopathy, albeit with less good 5 year survival than for patients without hilar lymphadenopathy[228].
Although studies relating to resection of pulmonary resection should be interpreted with caution, because of significant patient selection bias, a Liver Met Survey registry study reported that selected patients who had resection of liver and lung metastases had similar overall survival to those who had undergone removal of isolated liver metastases[229].
In terms of peritoneal disease, current studies suggest that in selected patients, cytoreductive surgery in combination with chemotherapy is associated with better survival than with chemotherapy alone, but there is controversy regarding the benefit of hyperthermic intraperitoneal chemotherapy over systemic chemotherapy[230].
Laparoscopic and robotic liver resection: Laparoscopic liver surgery has increased rapidly over the last decade with reports of minor and major liver resections[231,232], ALPPS[233] and both paediatric[234] and adult[235] live donor liver donation.
The international consensus conference on laparoscopic liver resection[236] established a range of recommendations and guidelines with an imperative that the innovators in this field deliver high quality evidence to validate its introduction into standard practice, and randomised clinical trials comparing laparoscopic and open liver resection followed as a result[237,238]. The ORANGE II trial[239] closed prematurely after failing to recruit. The OSLO COMET trial[237] compared laparoscopic with open parenchymal sparing liver resection for minor liver resections in 280 patients. The trial demonstrated a significant reduction in 30 d complications with the laparoscopic approach and a shorter hospital stay of 3 compared to 4 d. There was no difference in resection margin status or overall survival between groups. The significantly increased initial operative costs of the laparoscopic approach were offset by the shorter stays in recovery and hospital stay resulting in no overall difference between the two groups.
Thus the evidence from OSLO COMET trial, case series and cohort studies suggest that laparoscopic liver surgery is not inferior to open liver resection in terms of operative mortality, margin negativity and overall survival for both minor and major resections. Furthermore, there may be benefits in terms of reduced length of stay, reduced post-operative pain, and a reduction in the need for blood transfusion. At this time there remains a significant heterogeneity in adoption of not only laparoscopic but also robotic[239] liver surgery and it is appropriate that these evolving techniques should be performed in high volume centres with expertise in advanced minimally invasive procedures[240].
Liver function and volume assessment
Liver failure after resection has mortality of up to 80%[241], and hence there is much interest in the assessment of liver function, in particular the prediction of function in the future remnant liver (FRL), with a view to maximising safety following liver resection.
Although global clinical liver function assessment systems exist, such as the Childs Pugh score for assessment of liver function in the presence of chronic liver disease, and the MELD score for risk stratification of patients with end-stage liver disease awaiting transplantation, neither the Childs Pugh score[242] or MELD score[243] have proved useful in the context of liver resection in patients without underlying liver disease. Moreover, these scoring systems apply to the whole liver and cannot be used to predict function of a defined part of the liver such as the FRL.
Modern imaging software allows the accurate calculation of volumes of defined parts of the liver, such that the volume of the FRL may be assessed either as an absolute value or as a fraction of the whole liver. Whilst volume alone may be helpful in patients with completely healthy liver, in which case a minimum FRL of 25% has been advocated, in cases where liver parenchyma is suboptimal volume may not correlate with function[244,245], particularly in patients with steatosis, chemotherapy associated liver injury, or after PVE or ALPS. For those on the limit of threshold, decision making is difficult, and thus the shortcoming of purely volumetric assessments has prompted the investigation of dynamic liver function tests, which are discussed below.
Indocyanine Green: Indocyanine Green (ICG) is a tricarbocyanine dye that binds to albumin and is distributed evenly in the blood within minutes of intravenous injection. ICG is taken up by the liver and is excreted in bile without conjugation[246].
Whilst having some value in predicting post op liver failure an death in the context of HCC resection in cirrhotic patients[247], this was not the case in resection of colorectal liver metastases in chemotherapy affected livers[248]. Moreover, ICG clearance provides a global assessment of liver function and does not offer the possibility of assessing parts of the liver, in particular the future remnant liver left in situ after a resection. Although calculating fractional ICG excretion has been reported[249] this assumes homogenous liver function, and in this regard, Hepato-biliary scintigraphy offers potential opportunities.
Hepatobiliary scintigraphy: Hepato-biliary scintigraphy (HBS) uses a gamma camera detection system in combination with cross-sectional imaging to quantitatively assess hepatic processing of a labelled molecule, both globally and/ or regionally in the liver, thus allowing future remnant liver functional assessment. Two main labelled molecules, Galactosyl human Serum Albumin and iminodiacetic acid (IDA) derivatives have been reported on most widely. (1) Technetium-99 m galactosyl human serum albumin scintigraphy: Galactosyl human Serum Albumin is exclusively taken up in the liver by an active transport mechanism on the sinusoidal surface of hepatocytes, and is thereafter degraded in lysosomes without biliary excretion, thus offering the advantage of not being affected by high bilirubin concentrations. Its use has been developed significantly in Japan where it is reported as a useful technique in the prediction of post liver resection liver failure[250], but little used outside Japan owing to availability; (2) Hepatobiliary scintigraphy using IDA derivatives: Technitium labelled IDA derivatives, of which Mebrofenin is the most effective because of its high hepatic specificity and low competitive displacement by bilirubin, are taken up in the liver and then excreted into bile by active transport mechanisms. Protocols reviewed by Rassam et al[251] allow the calculation of hepatic extraction of Tc99 Mebrofenin as a percentage of total dose per minute, adjusted for body surface area. Single-photon emission CT-computed tomography (SPECT-CT) is combined with the extraction data to provide values for total liver or future remnant liver. Early studies determined that pre-operative values calculated for future remnant liver function correlated well with actual future remnant function measured post operative[244], thus suggesting the technique as a valuable pre-operative function assessment of the FLR; (3) Use in predicting post hepatectomy liver failure: Dinant et al[245] studied 46 patients with mixed tumour histology requiring liver resection, with and without underlying liver parenchymal disease. Patients with uptake above 2.5%/min/m2 had a 3% chance of liver failure in comparison to those with uptake below 2.5%/min/m2 who had a 56% chance of liver failure. Moreover, patients with uptake above 2.2%/min/m2 had a 3% chance of mortality whilst those with uptake below 2.2%/min/m2 had a 50% chance of liver failure. The volume of the future remnant was not significantly associated with any of the outcome parameters. Similarly, in a review of 55 high-risk patients undergoing major liver resection, de Graaf et al[252] identified patients who developed postoperative liver failure. Thus, patients with values above and below 2.69%/min/m2 had 2.4% and 57% chance of developing liver failure respectively. Likewise, Chapelle et al[253] studied 88 patients undergoing liver resection and found that post op liver failure was strongly associated with FRL- F but not future remnant volume, and that no liver failure mortality was observed in patients with FRL-F of above 2.3%/min/m2; and (4) Tc 99 Mebrofenin use in post PVE situation and ALPPS: Cieslak et al[254] studied 163 patients undergoing liver resection whose need for PVE was based on FRL-F by Tc99Mebrofenin extraction, with a cut off value of 2.7%/min/m2. The authors noted that 8/29 patients required PVE based on low HBS values in spite of satisfactory volume assessments, thus suggesting that HBS may have prevented post op liver failure in those patients. Similarly, Chapelle et al[255] used a cut off value of 2.3%/min/m2 as cut off for proceeding to PVE and found a lower incidence of post op liver failure than observed in a historical control group. Cieslak et al[256] identified Mebrofenin hepato-biliary scintigraphy parameters which identified non response to PVE at an earlier stage than conventional volume assessment 6 weeks post PVE, thus potentially allowing early selection of patients who may require ALPS. A significant concern with ALPS is the incidence of post op liver failure after the second stage, raising the impression that liver volume does not correlate well with function in the post ALPS setting, perhaps due to functional immaturity of rapidly proliferating hepatocytes. Supporting this notion, in the second stage of ALPS, Olthof et al[257] found that liver volume growth was out of proportion with the increase in function as assessed by HBS, and suggest the use of HBS FLR assessment in this setting rather than just volume. In conclusion, in the context of liver resection for colorectal liver metastases, assessment of the FLR is crucially important to avoid PHLF. Increasing evidence supports the role of hepato-biliary scintigraphy, as a technique which offer a specific functional assessment applicable to defined regions of the liver. Further confirmation and definition of the potential should be forthcoming with the results of a large multicentre prospective trial[258].
Downsizing chemotherapy for conversion of initially inoperable CRLM
The role of downsizing chemotherapy for initially unresectable colorectal liver metastases (discussed in more detail in section 3) is well established, with a systematic review by Lam et al[170] and others[259] reporting a response rate of 64%, with 22.5% of patients converted to curative liver resection overall.
The paradox of this chemotherapeutic success is the phenomenon of the disappearing metastasis, which presents a problem for the surgical team.
Disappearing metastasis: Radiologically disappearing metastasis reported with frequencies ranging from 6%[260], 24%[261], and up to 37%[262], Perhaps reflecting differences in imaging practice between centres and also different chemotherapy regimens. The percentage of patients in whom all CLM disappear radiologically is low (0%-6%).
Metastasis disappearance is usually a radiological phenomenon rather than a biological one: In a systematic review of 11 studies describing disappearing colorectal live metastases, it was found that in 65% of cases of “disappeared metastasis”, a lesion was found at laparotomy. Moreover, of the 35% of lesions not found at laparotomy and therefore not resected, 80% regrew, at site of radiologically disappeared metastasis[263]. Furthermore, there is not a good correlation with complete radiological response and complete pathological response: In Adam et al[264] study, complete pathological response was seen in 4% of patients undergoing liver resection for CLM, but none of these had complete radiological response.
Thus in terms of management of the disappearing liver metastasis the guiding principle is that viable tumour is present at the lesion site in the vast majority of cases, and therefore resecting the target lesion remains the objective. This may be achieved by resecting remnant lesions found at laparotomy visually, by palpation or intra-operative ultrasound. For lesions which are undetectable at laparotomy, a “territorial” resection, encompassing the lesion by reference to fixed landmarks, is sometimes possible. The use of 3D augmented reality imaging software may help in this regard in the future[265].
Some groups have investigated the pre-operative marking of CRLM with Fiducia labels. In a study from Kepenekian et al[266] 76 metastases were marked of which 23 disappeared with preoperative chemotherapy. Four complications were associated with marking: Two intrahepatic haematomas, one fiducial migration and one misplacement. After a median follow-up of 47.7 mo, no needle-track seeding was noted. Four disappearing CRLM were resected, with two local recurrences, and other missing lesions were treated with thermoablation. Thus Fiducia label placement presents an option in the management of disappearing CRLM, although concerns regarding selection of which CRLM to mark, procedural complications, and needle track seeding persist.
In the absence of the above strategies, close surveillance of the target area is the default. In future, such lesions may be targeted by image guided stereo-tactic ablation of the disappeared metastasis site.
Portal vein embolization
Portal vein embolization has been credited to various authors[267-269], but most notably to Makuuchi et al[270] and Kinoshita et al[271] both of whom reported the use of pre-operative PVE to induce hypertrophy prior to liver resection in the 1980s, on the background of prior reports of portal ligation as part of two-stage extended hepatectomies[268]. PVE embolization causes atrophy of the ipsilateral liver segments and a compensatory hypertrophy of the FRL. Assessment of the adequacy of hypertrophy of the FRL remains challenging. Functional liver assessment is well established within Japanese centres[269] but morphological changes in liver volume using CT volumetry as an assessment of hypertrophy remains the mainstay of assessment in many units. When performed, function assessment has traditionally been assessed using indocyanine green clearance however, more recently 99Tc-labelled Mebofenin Hepatobiliary scintigraphy and 99Tc-galactosyl-human serum albumin scintigraphy have been introduced as discussed above.
A systematic review of PVE reported a major complication rate resulting in non-resectability of 0.4% and a mortality rate of 0.1% however, complications in the published literature are likely under reported. Moreover, detailed descriptions of reasons for failure to progress to curative liver resection are frequently lacking in published literature. A systematic review of published cohort series reports an overall failure to proceed to curative liver resection following PVE of 18.7%. The majority of these failures to proceed were due to progression of liver disease (14.2%) and failure to induce sufficient hypertrophy of the FRL (2.8%). The mean time between PVE and liver resection was 36.9 d[268].
The borderline resectability of tumours that necessitate PVE to enable curative resection, combined with concerns regarding the effect of the changes to the liver parenchymal metabolism, gene expression and the microenvironment on tumour growth post-PVE have led some authors to examine the use of chemotherapy during the interval between PVE and resection in an effort to control tumour growth. Cohort series have suggested that continuation of chemotherapy during the interval between PVE and resection does not change hypertrophy of the FLR[272-274]. Some cohort studies have examined the different responses of metastatic disease to neoadjuvant chemotherapy and used this to stratify patients into “slow” and “fast” responders[273,275]. A cohort series has demonstrated subsequent discordant tumour behaviour following PVE between these groups with “slow” responders more likely to demonstrate progression of tumour growth with an accompanying increased risk of failing to progress to curative liver resection[273].
PVE remains an important tool in the armamentarium for management of patients with otherwise unresectable colorectal liver metastases where the FLR would otherwise be insufficient following resection. Despite promising reports from cohort studies, the published literature remains incomplete and frequently lacks detailed descriptions of complications or technical or clinical failure. Moreover, in the most recent systematic review colorectal metastasis comprised 39.6% of the patients and this heterogeneity limits translation of findings to clinical practice[268].
Two-staged hepatectomy
The concept of the “two-staged hepatectomy” was introduced by Adam et al[276], as a technique that could be applied to approximately 4% of patients with conventionally irresectable metastases to make them eligible for liver resection with curative intent. This approach involved a combination of systemic chemotherapy to downstage tumours, with or without PVE, with subsequent planned staged operations that permitted curative resection of large tumour burden that would otherwise have been considered unresectable. The interval between operations enabled hypertrophy of the remnant liver to theoretically reduce the chance of liver insufficiency and patients would receive chemotherapy during the interval between operations in an effort to control tumour growth. The reported results from this small early cohort demonstrated a similar risk of failure to proceed second stage operation when compared to PVE, of 19% (3/16). 54% (7/13) developed recurrent disease after completion of the second stage and the median survival was 31 mo from the second hepatectomy[276]. In current practice, the term, “two-staged hepatectomy” as reported by Adam et al[276] is used less and considered as part of the multimodal approach which has become a mainstay of current practice.
Associating liver partition and portal vein ligation for staged hepatectomy
The development of ALPPS was the result of an unplanned intraoperative decision by Dr Schlitt from Regensburg, Germany. Motivated by a concern for inadequate FLR during a planned extended right hemihepatectomy, a decision was made to perform a hepaticojejunostomy on to the left hepatic duct. In order to do so, liver parenchyma along the falciform ligament was divided, thus devascularising segment IV, and the right portal vein was ligated with the hope of causing hypertrophy of the remnant segments II and III. Post-operatively, rapid hypertrophy was observed within 8 d and resection of the in situ diseased hemiliver was completed[277].
The combination of portal vein ligation, inflammatory injury and the absence of cross portal circulation due to the parenchymal transection has been proposed as the mechanism for the observed more rapid hypertrophy compared to PVE alone.
Despite the enthusiasm for this novel technique, the first case series reported a 12% mortality and significant morbidity[278]. This was in excess of the mortality and morbidity of standard practice with a PVE and two staged approach. The rapid introduction of ALPPS to surgical practice with limited scientific rigor has been heavily criticised[278], leading to attempts to rationalise its use[278,279]. The LIGRO trial was a randomised control trial comparing ALPPS with two staged hepatectomy with PVE[280]. This demonstrated a significant increase in the primary endpoint of resection rates (92% vs 57%) with ALPPS without a significant increase in 30 or 90d mortality between groups. The rate of inadequate hypertrophy in the two stage hepatectomy group was higher in the trial than that reported in cohort series and salvage ALPPS was performed in 24%, however, 90d mortality in both groups remained high (TSH 6.1% vs ALPPS 8.3%)[280].
Advocates for ALPPS suggest that the rate of completion of the second stage is higher with ALPPS compared to the more established two stage approach with PVE. However, this is likely at the expense of a higher peri-operative mortality[281]. More recent case series have suggested that with modifications to the original technique such as; prolonging the interval between operations, performing a more limited or laparoscopic parenchymal transection[282], and the use of the ALPPS risk score[283] the peri-operative mortality can be substantially reduced[284].
Opponents highlight that this remains an experimental, unproven technique that carries a mortality considerably in excess of the 1%-2% mortality observed in high volume units for liver resections following PVE[278] and therefore it should be reserved for highly selected cases such as those considered to be high risk of tumour escape with PVE or as a salvage technique where PVE has failed to produce sufficient FLR hypertrophy. The exact role of ALPPS in the surgical armamentarium remains a matter of debate[284].
Ablation techniques for CRLM
The observation of long term survival after resection of colorectal liver metastases, and evidence suggesting that locoregional resection is oncologically equivalent to major anatomical resection[222] has prompted interest in minimally invasive ablative techniques which might achieve similar results to non-anatomical resection with less morbidity. Radiofrequency ablation and microwave ablation (MWA) have been investigated in the context of a variety of liver tumours including colorectal liver metastases.
Radiofrequency ablation: RFA delivers alternating electrical current to cause ionic agitation, with the resulting heat generation causing denaturation and coagulation of the targeted tissue[285].
The benefit of radiofrequency ablation over systemic chemotherapy alone was suggested by the European Organisation for Research and Treatment of Cancer 40004 CLOCC trial (ClinicalTrials. gov, No. NCT00043004) comparing systemic chemotherapy alone to chemotherapy combined with RFA +/- resection for patients with inoperable CRLM, which showed a significantly improved progression-free survival for patients treated with RFA in the initial analysis[286] and at 9.7 years of median follow up[207].
Whilst this randomised trial provides grounds for a genuine benefit of RFA over chemotherapy alone, comparing the effectiveness of RFA to that of liver resection is difficult, since RFA for CRLM is currently often used in situations where liver resection is not deemed appropriate as a result of unfavourable disease factors or patient comorbidities. In this context, the absence of randomised data makes comparisons of RFA to liver resection subject to a major confounder with an adverse bias against RFA.
Meta-analyses have nevertheless assessed the efficacy of RFA in comparison to liver resection.
In their 2012 meta-analysis, Weng et al[287] acknowledged the confounding factors above, but reported that liver resection was significantly superior to RFA in 3 and 5 year overall and disease free survival. Postoperative morbidity was higher in liver resection, but no significant difference was found in mortality between liver resection and RFA. In a subsequent meta-analysis, Van Amerongen et al[288] reported similar findings. In the most recent systematic review of 18 studies and 2667 patients[289]. Kron et al[289] reported that in 8/18 studies liver resection patients had significantly higher overall survival and disease-free survival, as well as lower local recurrence (LR) rates than RFA treated patients.
Based on HCC results where outcomes for lesions less than 5 cm were oncologically equivalent to resection, and achieved with less morbidity[290], it was hypothesised that RFA may have a particular role in the treatment of small colorectal liver metastases. Berber et al[291] reported that tumor size (> 3 cm), ablation margin, and proximity to hepatic vessels > 4 mm were found to be independent predictors of LR local recurrence after RFA. Hur et al[292] and Ko et al[293] reported similar findings and suggested that for colorectal liver metastases < 3 cm, resection and RFA had similar oncological and local recurrence outcome, but that RFA was less morbid.
However, in contrast to these studies, subgroup analysis from the systemic reviews above does not support idea that CLRM < 3 cm allows results equivalent to resection: Weng et al[287] showed poorer OS for lesions < 3 cm treated with RFA compared to resection. In Van Amerongen’s et al[288] study, subgroup analysis looking exclusively at solitary lesions and lesions of less than 3 cm found that in both cases, there was a significantly higher rate of local recurrence in the RFA group (solitary lesions OR = 7.68, P = 0.001 , and lesions < 3 cm, OR = 8.75, P = 0.001). In Kron’s et al[289] systematic review, 4 studies provided evidence comparing RFA to resection for lesions < 3 cm. Two of the four studies reported worse OS and higher local recurrence for RFA than liver resection[294,295], but the other 2 studies[292,293] found no OS or LR difference between RFA and liver resection.
Thus, the literature provides conflicting results, raising the question of whether other factors within the group of patients with solitary lesions < 3 cm may account for these differing conclusions, including technical and operator factors. In addition, historical case series data may not reflect modifications and technical advancements in tumour ablation such as better lesion targeting due to the advent of navigation systems and appreciation of the importance of ablation zone validation. For example, there is evidence that open RFA has lower recurrence rates than percutaneous RFA[296-299]. In addition, operator learning curve is reported to impact on outcome[298]. Also, operator training may be relevant. In their meta-analysis, Kron et al[289] point out that the clinician carrying out the RFA are surgeons and radiologists in 11% and 33% of studies respectively, with no specified practitioner in 56% of studies, raising another potential confounding factor.
In conclusion, there is randomised evidence showing a benefit for RFA over chemotherapy alone. On the subject of RFA vs liver resection, none of the available evidence is randomised, and significantly confounded by patient selection, with patients undergoing RFA typically having adverse disease characteristics and other additional non cancer related comorbidities. In this light, the finding of worse survival outcomes for RFA patients is not surprising, but difficult to interpret. In terms of local recurrence rates, the role of RFA in colorectal liver metastases < 3 cm remains controversial, with conflicting reports. Whilst such controversy may be settled by randomised trials, in practice this may prove difficult to achieve. The LAVA trial[300] closed due to insufficient recruitment, perhaps due to the perception of non-equipoise on the part of both clinicians and patients. The COLLISION trial[301] and HELARC trial (Trial ID NCT02886104)[302] are currently in progress.
Microwave ablation: MWA produces tissue destruction as a result of heat generated by electromagnetic waves. The theoretical advantages of MWA are faster and greater heat generation than in RFA, penetration through tissues with low conductivity and less heat sink effect[303] in instances of tumours near blood vessels.
In terms of the efficacy of MWA in comparison to chemotherapy alone, no randomised studies have been carried out.
In considering MWA vs liver resection, a small RCT including a total of 30 patients with multiple metastatic colorectal liver metastases, Shibata et al[304] randomised patients to liver resection or MWA, and found equivalent results in OS or DFS at 3 years for both treatment modalities.
In terms of comparing of RFA vs MWA, Although a metanalysis by Huo et al[305] found MW ablation and RF ablation had similar 1 and 5-year overall survival, disease-free survival, local recurrence rate, and adverse events overall for a variety of tumour types including mostly HCC, the studies that related specifically to CLRM suggested a lower local recurrence for MWA. Thus Correa et al[306], in a matched cohort analysis showed patients in the MWA group had lower ablation-site recurrence rates (6% vs 20%; P < 0.01), and similar results were reported by Liu et al[307]. However, the evidence is not unanimous, with some studies finding no difference in local recurrence rates between RFA and MWA[307].
Some studies have examined potential differences in tissue effects between MWA and RFA, and have found less heat sink for MWA[307] in treating lesions near blood vessels, though more complications[308] for peribiliary lesions.
In conclusion, the confounding factors relating to patient selection that make the RFA studies difficult to interpret apply equally to MWA, with the additional fact that there is generally less data for MWA than RFA. No randomised studies comparing MWA to RFA exist, and it may be that the two techniques have complementary rather than competing roles given the suggestion of slightly different tissue consequences in relation to tumours near blood vessels and bile ducts
Management of synchronous CRLM
Introduction: The scenario of synchronous operable CRLM presents another dilemma in opening the options of carrying out liver resection prior to, or synchronously with, the primary CRC. The section below discusses the evidence relating to synchronous and liver first surgery and is followed by a section on the various scenarios where these options may be considered.
Simultaneous liver and colon surgery: The reported experience of a number of studies analysing the outcome of simultaneous resection of a primary colorectal tumour and liver metastases has allowed some guidance of when this approach is appropriate. In a retrospective multicentre study, Reddy et al[309] analysed the outcomes of 610 patients who underwent simultaneous (n = 135) or staged (n = 475) resections. Combined hospital stay was lower after simultaneous resections (median 8.5 vs 14 d, P < 0.0001). Mortality and severe morbidity were similar after simultaneous colorectal resection and minor hepatectomy compared with isolated minor hepatectomy. For major hepatectomy (defined as resection of at least three segments) however, simultaneous colorectal resection increased mortality (8.3% vs 1.4%, P < 0.05) and severe morbidity (36.1% vs 15.1%, P < 0.05). Moreover, combined severe morbidity after staged resections was lower compared to simultaneous resections (36.1% vs 17.6%, P = 0.05). Severe morbidity experienced by patients undergoing combined resection included hepatic failure, intestinal anastomotic leaks, fascial dehiscence, intra-abdominal abscesses, isolated and multiple organ failure, aspiration, and pulmonary embolism. Consistent with these conclusions, Jones et al[310] reported that the rate of major complications was higher in patients undergoing simultaneous resection.
In a single centre retrospective study, De Haas et al[311] reported significantly worse progression free survival in the simultaneous surgery group, but also noted that the staged surgery group had received significantly more chemotherapy, highlighting the difficulty in comparisons in such studies. Similar interpretation questions were raised in a large population study[312] involving 442 and 776 patients undergoing simultaneous and staged resections respectively. The simultaneous resection group had a worse median overall survival, but also had received significantly less chemotherapy.
In a recent meta-analysis involving 32 studies[313], it appeared that the messages from earlier studies relating to avoiding major hepatectomy in the context of simultaneous resections was heeded. In this analysis, the synchronous resection group were found to have a smaller proportion of bilobar disease and underwent less major resections. Consequently, there was no difference between the synchronous and staged resection groups in terms of major morbidity and overall survival.
In conclusion, although interpretation of outcomes is difficult because of patient group heterogeneity, the consistent message from available studies is that simultaneous colon and liver resection can be performed safely in selected patients. Specifically, this excludes liver resections involving 3 or more segments.
Liver-first surgery: The concept of carrying out liver-first surgery in the context of synchronous colorectal liver metastases was first reported by Mentha et al[314]. The scenarios where liver first surgery has been advocated include: (1) Following downsizing of initially unresectable liver metastases with an asymptomatic primary tumour in situ, when it is deemed that there is potentially a limited time window for successful liver resection; (2) In the situation of synchronous operable primary and colorectal liver metastases where the colorectal liver metastases are, by virtue of their size or site, deemed most threatening and may become inoperable during time taken to complete a primary first treatment plan; and (3) In the specific instance of synchronous rectal cancer and liver metastases, where the significant time interval between irradiation of the primary tumour and its resection (3 mo window after long course irradiation) provides an opportunity for metastases to be resected substantially sooner than would be achieved if waiting till after resection of the primary.
Since Mentha’s original report, other studies series have been contributed such as that from Brouquet et al[315] reporting 156 consecutive patients with synchronous resectable CLM of which 72 patients underwent primary-first surgery, 43 combined, and 27 liver-first strategies, with no difference in morbidity, mortality of long term outcome between the groups.
These studies exemplify the problems of interpretation in this area: The numbers of patients reported on by individual centres are inevitably limited, and the patients are inevitably selected for their most appropriate treatment strategy, thus introducing a selection bias in any comparisons between groups.
Although a number of systematic reviews and meta-analyses have since been written, and thus allowed a greater numbers of patient cases to be studied, the issue of selection bias remains.
Thus, in a report from the Livermet survey, Andres et al[316] reported in a total of 787 patients including 58 who underwent liver first surgery. The liver first group included more rectal cancer, neoadjuvant rectal radiotherapy, and underwent more chemotherapy, but overall survival and disease-free survival were similar in both groups.
In their systematic review, Jegatheeswaran et al[317] reported on 4 studies in which patients underwent neoadjuvant chemotherapy first, then liver resection, then resection of the primary tumour. 74% completed the entire treatment protocol. 79% proceeded to liver resection, with disease progression on chemotherapy being the principle reason for not undergoing hepatectomy. In a further systematic review, Lam et al[318] reported very similar findings and conclusions.
In Network meta-analysis reviews, Kelly et al[319] and Gavriilidis et al[313] reviewed 18 studies and 32 studies respectively and found no significant differences in long-term survival and major morbidity were found between the surgical approaches.
In conclusion there are no randomised trials comparing liver-first versus primary-first surgery, and the highly specific nature of individual patient presentation in this context is such that it seems very unlikely that such a trial would be feasible. Nevertheless, in selected patients, the liver first approach results in short- and long-term outcomes that are similar to those achieved when the primary tumour is resected first.
Synchronous liver metastases management scenarios: The definition of synchronous has not been uniform in existing studies, with some authors including only metastases diagnosed at the time of or before diagnosis of the primary tumour, whilst other include metastases diagnosed up to 3 or even 6 mo after diagnosis of the primary. Based on prognostic outcomes, the EGOSLIM consensus group suggested a terminology of synchronous liver metastases (detected at or before diagnosis of the primary tumour), Early metachronous metastases (detected within 12 mo after diagnosis or surgery of the primary) and Late metachronous metastases (detected more than 12 mo after diagnosis or surgery of the primary)[320].
One significant aspect of synchronous presentation of primary tumour and liver metastases is the dilemma of which disease site should be treated first, or whether systemic chemotherapy should be the initial treatment. The conventional order of primary resection followed by liver resection, dictated by chronology in the metachronous situation, may not be the most appropriate in synchronous presentation.
In practice, the order of treatment is partly dictated by the constraints of the clinical presentation which may be summarised in Figure 3 according to primary symptomatology and liver disease resectability. (1) Asymptomatic primary and unequivocally unresectable liver metastases. In this scenario there is consensus to treat with up front chemotherapy, with a range of systemic chemotherapy agents combined with EGFR blockade and anti-angiogenic agents, guided by principles as described in the section on unresectable metachronous liver metastases, with the intention of achieving maximal response, survival, and perhaps in some cases conversion to a resectable scenario for the liver metastases. For those patients who are converted to operability, liver first surgery may be considered over colon first. In the context of downstaged liver metastases, simultaneous liver and colon resection should be perhaps be avoided on the strength of Liver Met Survey data showing 5-year survival rates were 42% for liver first approach compared with 33% for colon first surgery and 28% for one-stage surgery[320]; (2) Symptomatic primary and unequivocally unresectable liver metastases. In this instance, the objective is to deal with symptoms from the primary, and thereafter offer optimal systemic chemotherapy. However, the differing possible scenarios and lack of evidence leaves much freedom for a pragmatic approach based on diverse clinical circumstances with symptomatology in relation to bleeding, obstruction or perforation. Bleeding may respond to systemic chemotherapy and may be managed by blood transfusion, thus avoiding the need for surgery to the primary tumour. Perforation will usually mandate primary resection unless an entirely palliative approach is appropriate. Obstruction may require resection of the primary tumour, though the options of proximal stoma and stenting may also be appropriate, with relative merits of each outside the scope of this review. Once primary symptoms have been addressed, systemic chemotherapy is indicated with the aim of maximising survival and conversion of liver metastases to an operable scenario, once again guided by principles as described in the section on unresectable metachronous liver metastases; (3) Asymptomatic primary and resectable liver metastases. This scenario is perhaps the one that gives rise to most discussion and controversy. Although the EGOSLIM consensus group recommended systemic chemotherapy first for this scenario, there was not unanimity in this recommendation, and it was pointed out that evidence for such a recommendation was lacking[321]. The only randomised evidence in this area comes from the EORTC 40983 trial[161], comparing surgery alone to FOLFOX–surgery-FOLFOX, which showed an improved DFS in the peri-operative chemotherapy group, though no OS advantage in the early or long term[162]. However, the experimental group received both neoadjuvant and adjuvant chemo, such that it is difficult to state whether any survival advantage was associated with neoadjuvant treatment, adjuvant, or both. Arguing against the value of neoadjuvant treatment, Adam et al[321] showed no benefit associated with neoadjuvant treatment prior to resection of solitary metachronous liver metastases[321]. Although providing some evidence, both Adam’s study and the EORTC trial are nevertheless not directly applicable to the scenario of synchronous liver metastases, as almost 2/3 of the liver metastases in the EORTC trial were metachronous, and Adam’s study relates exclusively to metachronous liver metastases. In a retrospective report Bonney et al[322] studied 1301 patients with synchronous liver metastases, and compared those who received neoadjuvant chemotherapy prior to liver resection to those who underwent liver surgery without neoadjuvant chemotherapy. Neoadjuvant chemotherapy did not affect outcome and was not associated with any survival advantage. Of note, the surgery up front group had a greater number of solitary metastases, and therefore a separate analysis was undertaken to take this into account. The authors found that for patients with solitary CRLM, neither neoadjuvant nor adjuvant chemotherapy was associated with a survival advantage. In contrast, for patients with multiple liver metastases, although neoadjuvant chemotherapy conferred no benefit, adjuvant chemotherapy was found to be associated with a survival advantage. In summary, the evidence base in this scenario is largely lacking, and to some extent conflicting. It would appear that the scenario of “synchronous asymptomatic primary and resectable liver metastases” is not a homogeneous scenario to be treated by a “one size fits all” approach, but a very heterogeneous one, requiring approach flexibility by experienced MDT. Current evidence certainly does not justify neoadjuvant chemotherapy in all cases of synchronous resectable liver metastases; and (4) Symptomatic primary and resectable liver metastases. In this scenario, priority is given to dealing with symptoms from the primary as outlined in the section on symptomatic primary and unequivocally unresectable liver metastases. Thereafter however, once recovered from primary surgery, the next most appropriate treatment depends on the results of restaging. If restaging shows progression with now unresectable liver metastases, clearly chemotherapy is indicated. If restaging suggests disease progression in the liver though still resectable, then a period of systemic chemotherapy may be most appropriate to re-establish disease stability prior to reassessing with a view to liver resection. If restaging suggests stable and resectable metastases, then the scenario becomes similar in principle to the situation of “resectable primary and resectable liver metastases”, where the evidence for neoadjuvant chemotherapy prior to liver resection is not absolute, and there may be circumstances for proceeding to liver resection, with a view to adjuvant chemotherapy after.
Figure 3.
Management options in synchronous colorectal cancer and colorectal liver metastases, according to presentation criteria. CRC: Colorectal cancer; CRLM: Colorectal liver metastases.
Orthotopic liver transplantation for colorectal liver metastases
Some CRLM remain unresectable on account of proximity to vital structures that cannot be sacrificed, or because of insufficient remnant liver volume. However, the favourable results of liver resection in comparison to chemotherapy alone raises the question of whether total hepatectomy, followed by liver transplantation, might have a place in the management of unresectable liver only metastases.
Studies investigating OLT for unresectable CRLM during the 1990s reported poor outcomes in Europe and the United States with 5 year survival of 12%-21%[323,324], and thus much lower than outcome achieved following transplantation for other indications.
In 2006, on a background of improvements in both liver transplantation and CRLM management, and the favourable organ to recipient ratio in Norway, the Oslo University Hospital group initiated a study to reassess the survival of patients with non resectable CRLM after LT (SECA Trial)[325].
21 patients underwent deceased-donor LT, with 1, 3, and 5-year OS of 95%, 68%, and 60% respectively, thus comparing favourably to 19% OS in a comparative retrospective cohort of patients with unresectable CRLM treated with chemotherapy alone[326]. Median time to recurrence was 6 mo and all patients followed for longer than 11 mo experienced recurrence, most frequently in the lungs[327]. Similarly, Toso et al[328] reported the outcomes of 12 patients who underwent OLT for unresectable liver metastases, with a 5 year OS of 50%, and with 4 out of 12 patients showing no sign of recurrence at 48 mo.
The wide inclusion criteria of the SECA 1 study allowed the identification of 4 clinical features associated with a worse survival: Pretransplant tumor diameter > 5.5 cm, a pre-transplant CEA > 80 μg/L, time interval from resection of the primary to transplantation < 2 years, and progression of the metastases under neo-adjuvant chemotherapy. These and other criteria have been used to inform more selective recruitment criteria to the ongoing SECA II trial, with preliminary results showing overall survival at 1, 3, and 5 years of 100%, 83%, and 83%, respectively[329].
Further trial are also in progress, including the TRANSMET (NCT02597348) and SECA III (NCT03494946) trials which compare OLT to optimal systemic chemotherapy in unresectable CRLM.
In conclusion, it appears that in selected patients with unresectable CRLM, OLT is associated with OS Figures which are comparable to those achieved for other OLT indications. The outcome of randomised trials comparing OLT to optimal systemic chemotherapy are eagerly awaited but results favouring OLT would doubtless contribute to the already complex debate regarding organ allocation.
SECTION 5: HISTOPATHOLOGICAL ASSESSMENT OF COLORECTAL LIVER METASTASES
Following resection of CRLM, histopathological assessment is essential, and yields critically important information which directly influences further management. The assessment of margins is an obvious example, affecting decisions regarding re-operation, as well as the timing and intensity of surveillance. Another is the real response to chemotherapy, which may influence oncological management. The following section describes current practice in CRLM histopathological assessment.
Current best practice
The role of histopathology is predominantly one of post-operative assessment of resected liver specimens, with pre-operative biopsy or intra-operative frozen section being required only rarely (the latter usually in the context of lymph nodes suspicious for metastasis, or unexpected subcapsular lesions not identified on preoperative imaging)[330].
Pre-operative assessment: Preoperative percutaneous needle biopsy is avoided where possible due to the risk of tumour seeding along the biopsy needle tract[330,331]. In rare cases were percutaneous liver biopsy is deemed to be necessary, usually in the context of multiple known primary tumours, the test can be modified to mitigate the risk of tumour seeding. Endoscopic ultrasound fine needle aspiration may be technically feasible in some cases, in particular for intra-abdominal lymph node sampling where reasonable yields can usually be obtained to allow for additional immunohisto-chemical assessment, if required.
Routine haematoxylin and eosin (H and E) stained slides are examined in the first instance. A limited panel of immunohistochemical stains such as CK20 and CDX-2 can be used to confirm morphological findings suggestive of colorectal origin[332]. An expanded panel to include CK7 and other specific localising antibodies can be undertaken depending on morphological appearance and clinical history[330,332].
The biopsy report should contain a morphological description of the tumour including the degree of differentiation, details of immunohistochemical analysis, and a conclusion indicating the site, or possible sites, of primary origin. A description of the adjacent/background liver tissue should also be included as the presence of chronic liver disease may influence risk assessment for surgery and chemotherapy[333].
Post-operative assessment: The approach to the resected liver specimen involves both macroscopic and microscopic assessment. (1) Macroscopic assessment: The macroscopic assessment should include the size and weight of the resection specimen, along with a description of the capsule, including areas of disruption or adhesions. Surfaces other than the capsule are painted with ink to allow margin identification. Specimens can be sectioned fresh or fixed, with fixed tissues allowing for more accurate slicing[330]. An example of a fixed specimen is shown in Figure 4. Findings should be carefully correlated with the description in the operation note, especially in the case of complex specimens. Tumour deposit number and size should be assessed. It is also important to correlate with imaging data to ensure that all preoperatively and intraoperatively identified lesions are sampled for microscopic assessment. At least one block should be taken of each metastatic deposit, as well as one block of representative background liver[330]; (2) Microscopic assessment: The microscopic assessment should include a morphological description of any lesions present, including degree of differentiation. An example of the histological appearance of a colorectal metastasis is shown in Figure 5. Immunohistochemistry is not routinely carried out, but should be included if the morphological features are unusual or there is diagnostic uncertainty[330]. Other factors that are assessed include evidence of capsular breach by the tumour, distance to the resection margin (a distance of 1 mm or more being considered “not involved”), the presence or absence of lymphovascular invasion, and the number of involved lymph nodes, if present. The presence or absence of background chronic liver disease should be commented upon[330]. Molecular tests (KRAS, BRAF, NRAS, microsatellite instability) should also be conducted if not previously completed on the primary tumour[333]. The results of these molecular tests can be crucial for the selection of the most appropriate chemotherapy agent, to aid with prognostication, and to establish or exclude a diagnosis of a hereditary tumour syndrome[333]. The effect of preoperative neoadjuvant therapy should be evaluated if applicable. This includes an assessment of tumour response and the presence of chemotherapy-induced injury such as sinusoidal obstruction syndrome (more common with oxaliplatin) or steatohepatitis (more common with irinotecan) in the background liver tissue. The former can be assessed using the chemotherapy-induced sinusoidal injury score[333-347]. In cases where selective internal radiotherapy has been administered, therapeutic microspheres may also be present. If preoperative portal vein embolization has been undertaken then embolic material may be present within portal vein branches, along with variable degrees of parenchymal atrophy due to relative ischaemia, shown in Figure 6. Tumour response to chemotherapy involves assessing the percentage area of viable tumour compared to fibrosis and necrosis. The four tiered system advocated by the American Joint Committee on Cancer based on a modification by Ryan et al[335] is currently recommended by the Royal College of Pathologists for assessment of response in primary colorectal carcinoma (Table 1)[336] . While necrosis, as illustrated in Figure 7, is very common, there is evidence to suggest that the most predictive factor for outcome in the assessment of tumour response to chemotherapy is fibrosis (Figure 8)[333,337].
Figure 4.
Multiple colorectal metastases in a formalin-fixed liver resection specimen showing a pale cut surface with typical lobulated border. Non-capsular surfaces inked for margin identification (scale bar: 5 cm).
Figure 5.
Colorectal metastasis with typical cribriform glandular architecture and central comedonecrosis (Hematoxylin-eosin staining, × 10 magnification).
Figure 6.
Embolic material within a large portal vein branch, with adjacent adenocarcinoma, in a patient who underwent preoperative portal vein embolization (Hematoxylin-eosin staining, × 4 magnification).
Table 1.
Assessment of response to chemotherapy in primary colorectal carcinoma using the Tumour Regression Score (AJCC)
| Evaluation | Tumour regression score |
| No viable cancer cells (complete response) | 0 |
| Single cells or rare small groups of cancer cells (near-complete response) | 1 |
| Residual cancer with evident tumour regression, but more than single cells or rare small groups of cancer cells (partial response) | 2 |
| Extensive residual cancer with no evident tumour regression (poor or no response) | 3 |
Figure 7.
Extensive confluent tumour necrosis with fibrosis in keeping with a partial response to neoadjuvant therapy (Hematoxylin-eosin staining, × 4 magnification).
Figure 8.
Confluent fibrosis and dystrophic calcification without viable residual tumour cells, consistent with pathological complete response to neoadjuvant therapy (Hematoxylin-eosin staining, × 4 magnification).
Future perspectives
Several biomarkers are under development which have both prognostic and predictive potential. One such marker is programmed cell death protein (PD-1), whose dominant ligand is PD-L1, expressed on the surface of activated T cells to regulate proliferation and activation. When carcinoma develops tumour cells may express PD-L1 and thus reduce their immunogenicity. Assessment of PD-L1 expression in tumour cells using immunohistochemistry may therefore provide prognostic information and predict response to treatment with PD-1/PD-L1 inhibitors. This has shown encouraging initial results reported in microsatellite instability-high colorectal carcinoma[337,339].
CONCLUSION
The management of colorectal liver metastases is highly complex owing to multiple treatment modalities. Adding to this complexity is the marked heterogeneity of the patient group, and the nuanced overlap between ‘different’ scenarios. In this context, no single specialty team, let alone individual clinician, is solely equipped to carry out optimal decision making.
Effective management results from careful and informed discussion from an experienced multi-disciplinary team involving radiology (cross sectional, nuclear medicine and interventional), Oncology, Liver surgery, Colorectal surgery, and Histopathology. Furthermore, it is incumbent on such MDTs to remain up to date in what is a fast-evolving field. In the not distant future, geneticists and molecular biologists may be added to the list of specialty representatives required in MDT discussions.
Footnotes
Conflict-of-interest statement: All other authors have nothing to disclose.
Manuscript source: Invited manuscript
Peer-review started: February 27, 2020
First decision: April 7, 2020
Article in press: August 31, 2020
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan H, Mikulic D, Romani A S-Editor: Zhang L L-Editor: A P-Editor: Li JH
Contributor Information
Jack Martin, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Angelica Petrillo, Department of Precision Medicine, Division of Medical Oncology, University of Campania "L. Vanvitelli", Napoli 80131, Italy, & Medical Oncology Unit, Ospedale del Mare, 80147 Napoli Italy.
Elizabeth C Smyth, Department of Oncology, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Nadeem Shaida, Department of Radiology, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB22 0QQ, United Kingdom.
Samir Khwaja, Department of Radiology, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB22 0QQ, United Kingdom.
HK Cheow, Department of Nuclear Medicine, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Adam Duckworth, Department of Pathology, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Paula Heister, Department of Pathology, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Raaj Praseedom, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Asif Jah, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Anita Balakrishnan, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Simon Harper, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Siong Liau, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Vasilis Kosmoliaptsis, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom.
Emmanuel Huguet, Department of Surgery, Addenbrookes Hospital, NIHR Comprehensive Biomedical Research and Academic Health Sciences Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, United Kingdom. emmanuel.huguet@addenbrookes.nhs.uk.
References
- 1.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 3.Neal RD, Din NU, Hamilton W, Ukoumunne OC, Carter B, Stapley S, Rubin G. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2014;110:584–592. doi: 10.1038/bjc.2013.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skipworth RJ, Parks RW, Stephens NA, Graham C, Brewster DH, Garden OJ, Paterson-Brown S. The relationship between hospital volume and post-operative mortality rates for upper gastrointestinal cancer resections: Scotland 1982-2003. Eur J Surg Oncol. 2010;36:141–147. doi: 10.1016/j.ejso.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Cecil TD, Sexton R, Moran BJ, Heald RJ. Total mesorectal excision results in low local recurrence rates in lymph node-positive rectal cancer. Dis Colon Rectum. 2004;47:1145–9; discussion 1149-50. doi: 10.1007/s10350-004-0086-6. [DOI] [PubMed] [Google Scholar]
- 6.Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, Tveit KM, Gibson F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Segelov E, Chan D, Shapiro J, Price TJ, Karapetis CS, Tebbutt NC, Pavlakis N. The role of biological therapy in metastatic colorectal cancer after first-line treatment: a meta-analysis of randomised trials. Br J Cancer. 2014;111:1122–1131. doi: 10.1038/bjc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagland HR, Berg M, Jolma IW, Carlsen A, Søreide K. Molecular pathways and cellular metabolism in colorectal cancer. Dig Surg. 2013;30:12–25. doi: 10.1159/000347166. [DOI] [PubMed] [Google Scholar]
- 9.Snaebjornsson P, Jonasson L, Olafsdottir EJ, van Grieken NCT, Moller PH, Theodors A, Jonsson T, Meijer GA, Jonasson JG. Why is colon cancer survival improving by time? A nationwide survival analysis spanning 35 years. Int J Cancer. 2017;141:531–539. doi: 10.1002/ijc.30766. [DOI] [PubMed] [Google Scholar]
- 10.Woodington GF, Waugh JM. Results of resection of metastatic tumors of the liver. Am J Surg. 1963;105:24–29. doi: 10.1016/0002-9610(63)90263-0. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SM, Adson MA. Surgical treatment of hepatic metastases from colorectal cancers. Arch Surg. 1976;111:330–334. doi: 10.1001/archsurg.1976.01360220026004. [DOI] [PubMed] [Google Scholar]
- 12.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 13.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 14.Weledji EP. Centralization of Liver Cancer Surgery and Impact on Multidisciplinary Teams Working on Stage IV Colorectal Cancer. Oncol Rev. 2017;11:331. doi: 10.4081/oncol.2017.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxenberg J, Papenfuss W, Esemuede I, Attwood K, Simunovic M, Kuvshinoff B, Francescutti V. Multidisciplinary cancer conferences for gastrointestinal malignancies result in measureable treatment changes: a prospective study of 149 consecutive patients. Ann Surg Oncol. 2015;22:1533–1539. doi: 10.1245/s10434-014-4163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Specchia ML, Frisicale EM, Carini E, Di Pilla A, Cappa D, Barbara A, Ricciardi W, Damiani G. The impact of tumor board on cancer care: evidence from an umbrella review. BMC Health Serv Res. 2020;20:73. doi: 10.1186/s12913-020-4930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engstrand J, Kartalis N, Strömberg C, Broberg M, Stillström A, Lekberg T, Jonas E, Freedman J, Nilsson H. The Impact of a Hepatobiliary Multidisciplinary Team Assessment in Patients with Colorectal Cancer Liver Metastases: A Population-Based Study. Oncologist. 2017;22:1067–1074. doi: 10.1634/theoncologist.2017-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thillai K, Repana D, Korantzis I, Kane P, Prachalias A, Ross P. Clinical outcomes for patients with liver-limited metastatic colorectal cancer: Arguing the case for specialist hepatobiliary multidisciplinary assessment. Eur J Surg Oncol. 2016;42:1331–1336. doi: 10.1016/j.ejso.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Lan YT, Jiang JK, Chang SC, Yang SH, Lin CC, Lin HH, Wang HS, Chen WS, Lin TC, Lin JK. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. Int J Colorectal Dis. 2016;31:403–411. doi: 10.1007/s00384-015-2459-4. [DOI] [PubMed] [Google Scholar]
- 20.Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10-year study of outcome following hepatic resection for colorectal liver metastases - The effect of evaluation in a multidisciplinary team setting. Eur J Surg Oncol. 2009;35:302–306. doi: 10.1016/j.ejso.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Donadon M, Ribero D, Morris-Stiff G, Abdalla EK, Vauthey JN. New paradigm in the management of liver-only metastases from colorectal cancer. Gastrointest Cancer Res. 2007;1:20–27. [PMC free article] [PubMed] [Google Scholar]
- 22.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 23.Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg. 1999;86:158–169. doi: 10.1046/j.1365-2168.1999.01013.x. [DOI] [PubMed] [Google Scholar]
- 24.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunne DF, Jones RP, Malik HZ, Fenwick SW, Poston GJ. Surgical management of colorectal liver metastases: a European perspective. Hepat Oncol. 2014;1:121–133. doi: 10.2217/hep.13.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welch JP, Donaldson GA. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg. 1979;189:496–502. doi: 10.1097/00000658-197904000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32:457–465. doi: 10.1007/s10585-015-9719-0. [DOI] [PubMed] [Google Scholar]
- 29.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 32.Landreau P, Drouillard A, Launoy G, Ortega-Deballon P, Jooste V, Lepage C, Faivre J, Facy O, Bouvier AM. Incidence and survival in late liver metastases of colorectal cancer. J Gastroenterol Hepatol. 2015;30:82–85. doi: 10.1111/jgh.12685. [DOI] [PubMed] [Google Scholar]
- 33.Elferink MA, de Jong KP, Klaase JM, Siemerink EJ, de Wilt JH. Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Dis. 2015;30:205–212. doi: 10.1007/s00384-014-2085-6. [DOI] [PubMed] [Google Scholar]
- 34.Chong G, Cunningham D. Improving long-term outcomes for patients with liver metastases from colorectal cancer. J Clin Oncol. 2005;23:9063–9066. doi: 10.1200/JCO.2005.04.4669. [DOI] [PubMed] [Google Scholar]
- 35.Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D FACS Trial Investigators. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–270. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 37.Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200. doi: 10.1002/14651858.CD002200.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–31. doi: 10.1002/jmri.22010. [DOI] [PubMed] [Google Scholar]
- 39.Cantisani V, Ricci P, Erturk M, Pagliara E, Drudi F, Calliada F, Mortele K, D'Ambrosio U, Marigliano C, Catalano C, Marin D, Di Seri M, Longo F, Passariello R. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue® low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med. 2010;31:500–505. doi: 10.1055/s-0028-1109751. [DOI] [PubMed] [Google Scholar]
- 40.Rafaelsen SR, Jakobsen A. Contrast-enhanced ultrasound vs multidetector-computed tomography for detecting liver metastases in colorectal cancer: a prospective, blinded, patient-by-patient analysis. Colorectal Dis. 2011;13:420–425. doi: 10.1111/j.1463-1318.2010.02288.x. [DOI] [PubMed] [Google Scholar]
- 41.van Vledder MG, Pawlik TM, Munireddy S, Hamper U, de Jong MC, Choti MA. Factors determining the sensitivity of intraoperative ultrasonography in detecting colorectal liver metastases in the modern era. Ann Surg Oncol. 2010;17:2756–2763. doi: 10.1245/s10434-010-1108-y. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi M, Hasegawa K, Arita J, Hata S, Aoki T, Sakamoto Y, Sugawara Y, Kokudo N. Contrast-enhanced intraoperative ultrasonography using perfluorobutane microbubbles for the enumeration of colorectal liver metastases. Br J Surg. 2012;99:1271–1277. doi: 10.1002/bjs.8844. [DOI] [PubMed] [Google Scholar]
- 43.Ruzzenente A, Conci S, Iacono C, Valdegamberi A, Campagnaro T, Bertuzzo F, Bagante F, De Angelis M, Guglielmi A. Usefulness of contrast-enhanced intraoperative ultrasonography (CE-IOUS) in patients with colorectal liver metastases after preoperative chemotherapy. J Gastrointest Surg. 2013;17:281–287. doi: 10.1007/s11605-012-2043-y. [DOI] [PubMed] [Google Scholar]
- 44.Tirumani SH, Kim KW, Nishino M, Howard SA, Krajewski KM, Jagannathan JP, Cleary JM, Ramaiya NH, Shinagare AB. Update on the role of imaging in management of metastatic colorectal cancer. Radiographics. 2014;34:1908–1928. doi: 10.1148/rg.347130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soyer P, Poccard M, Boudiaf M, Abitbol M, Hamzi L, Panis Y, Valleur P, Rymer R. Detection of hypovascular hepatic metastases at triple-phase helical CT: sensitivity of phases and comparison with surgical and histopathologic findings. Radiology. 2004;231:413–420. doi: 10.1148/radiol.2312021639. [DOI] [PubMed] [Google Scholar]
- 46.Frankel TL, Gian RK, Jarnagin WR. Preoperative imaging for hepatic resection of colorectal cancer metastasis. J Gastrointest Oncol. 2012;3:11–18. doi: 10.3978/j.issn.2078-6891.2012.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahani DV, Bajwa MA, Andrabi Y, Bajpai S, Cusack JC. Current status of imaging and emerging techniques to evaluate liver metastases from colorectal carcinoma. Ann Surg. 2014;259:861–872. doi: 10.1097/SLA.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 48.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 49.Bruegel M, Holzapfel K, Gaa J, Woertler K, Waldt S, Kiefer B, Stemmer A, Ganter C, Rummeny EJ. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 50.Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 51.Owen JW, Fowler KJ, Doyle MB, Saad NE, Linehan DC, Chapman WC. Colorectal liver metastases: disappearing lesions in the era of Eovist hepatobiliary magnetic resonance imaging. HPB (Oxford) 2016;18:296–303. doi: 10.1016/j.hpb.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 53.Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aubé C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol. 2016;26:4595–4615. doi: 10.1007/s00330-016-4250-5. [DOI] [PubMed] [Google Scholar]
- 54.Cipe G, Ergul N, Hasbahceci M, Firat D, Bozkurt S, Memmi N, Karatepe O, Muslumanoglu M. Routine use of positron-emission tomography/computed tomography for staging of primary colorectal cancer: does it affect clinical management? World J Surg Oncol. 2013;11:49. doi: 10.1186/1477-7819-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, Glanville J, McIntosh H, Renehan A, Weller D, Dunlop M. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1–192, iii-iiv. doi: 10.3310/hta15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deng J, Tang J, Shen N. Meta-analysis of diagnosis of liver metastatic cancers: comparison of (18) FDG PET-CT and gadolinium-enhanced MRI. J Med Imaging Radiat Oncol. 2014;58:532–537. doi: 10.1111/1754-9485.12231. [DOI] [PubMed] [Google Scholar]
- 57.Rohren EM, Paulson EK, Hagge R, Wong TZ, Killius J, Clavien PA, Nelson RC. The role of F-18 FDG positron emission tomography in preoperative assessment of the liver in patients being considered for curative resection of hepatic metastases from colorectal cancer. Clin Nucl Med. 2002;27:550–555. doi: 10.1097/00003072-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Ruers TJ, Langenhoff BS, Neeleman N, Jager GJ, Strijk S, Wobbes T, Corstens FH, Oyen WJ. Value of positron emission tomography with [F-18] fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388–395. doi: 10.1200/JCO.2002.20.2.388. [DOI] [PubMed] [Google Scholar]
- 59.Sahani DV, Kalva SP, Fischman AJ, Kadavigere R, Blake M, Hahn PF, Saini S. Detection of liver metastases from adenocarcinoma of the colon and pancreas: comparison of mangafodipir trisodium-enhanced liver MRI and whole-body FDG PET. AJR Am J Roentgenol. 2005;185:239–246. doi: 10.2214/ajr.185.1.01850239. [DOI] [PubMed] [Google Scholar]
- 60.Engledow AH, Skipworth JR, Pakzad F, Imber C, Ell PJ, Groves AM. The role of 18FDG PET/CT in the management of colorectal liver metastases. HPB (Oxford) 2012;14:20–25. doi: 10.1111/j.1477-2574.2011.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huguet EL, Old S, Praseedom RK, Balan KK, Gibbs P, Jamieson NV. F18-FDG-PET evaluation of patients for resection of colorectal liver metastases. Hepatogastroenterology. 2007;54:1667–1671. [PubMed] [Google Scholar]
- 62.Briggs RH, Chowdhury FU, Lodge JP, Scarsbrook AF. Clinical impact of FDG PET-CT in patients with potentially operable metastatic colorectal cancer. Clin Radiol. 2011;66:1167–1174. doi: 10.1016/j.crad.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 63.Abbadi RA, Sadat U, Jah A, Praseedom RK, Jamieson NV, Cheow HK, Whitley S, Ford HE, Wilson CB, Harper SJ, Huguet EL. Improved long-term survival after resection of colorectal liver metastases following staging with FDG positron emission tomography. J Surg Oncol. 2014;110:313–319. doi: 10.1002/jso.23623. [DOI] [PubMed] [Google Scholar]
- 64.Truant S, Huglo D, Hebbar M, Ernst O, Steinling M, Pruvot FR. Prospective evaluation of the impact of [18F]fluoro-2-deoxy-D-glucose positron emission tomography of resectable colorectal liver metastases. Br J Surg. 2005;92:362–369. doi: 10.1002/bjs.4843. [DOI] [PubMed] [Google Scholar]
- 65.Moulton CA, Gu CS, Law CH, Tandan VR, Hart R, Quan D, Fairfull Smith RJ, Jalink DW, Husien M, Serrano PE, Hendler AL, Haider MA, Ruo L, Gulenchyn KY, Finch T, Julian JA, Levine MN, Gallinger S. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA. 2014;311:1863–1869. doi: 10.1001/jama.2014.3740. [DOI] [PubMed] [Google Scholar]
- 66.Expert Panel on Gastrointestinal Imaging, Kaur H, Hindman NM, Al-Refaie WB, Arif-Tiwari H, Cash BD, Chernyak V, Farrell J, Grajo JR, Horowitz JM, McNamara MM, Noto RB, Qayyum A, Lalani T, Kamel IR. ACR Appropriateness Criteria® Suspected Liver Metastases. J Am Coll Radiol. 2017;14:S314–S325. doi: 10.1016/j.jacr.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 67.Mittal BR, Senthil R, Kashyap R, Bhattacharya A, Singh B, Kapoor R, Gupta R. 18F-FDG PET-CT in evaluation of postoperative colorectal cancer patients with rising CEA level. Nucl Med Commun. 2011;32:789–793. doi: 10.1097/MNM.0b013e3283477dd7. [DOI] [PubMed] [Google Scholar]
- 68.Angelsen JH, Viste A, Løes IM, Eide GE, Hoem D, Sorbye H, Horn A. Predictive factors for time to recurrence, treatment and post-recurrence survival in patients with initially resected colorectal liver metastases. World J Surg Oncol. 2015;13:1–9. doi: 10.1186/s12957-015-0738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metcalfe MS, Mullin EJ, Maddern GJ. Choice of surveillance after hepatectomy for colorectal metastases. Arch Surg. 2004;139:749–754. doi: 10.1001/archsurg.139.7.749. [DOI] [PubMed] [Google Scholar]
- 70.Hyder O, Dodson RM, Mayo SC, Schneider EB, Weiss MJ, Herman JM, Wolfgang CL, Pawlik TM. Post-treatment surveillance of patients with colorectal cancer with surgically treated liver metastases. Surgery. 2013;154:256–265. doi: 10.1016/j.surg.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 72.Dinan MA, Curtis LH, Hammill BG, Patz EF, Jr, Abernethy AP, Shea AM, Schulman KA. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA. 2010;303:1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 73.Hallet J, Sa Cunha A, Adam R, Goéré D, Bachellier P, Azoulay D, Ayav A, Grégoire E, Navarro F, Pessaux P French Colorectal Liver Metastases Working Group, Association Française de Chirurgie (AFC) Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br J Surg. 2016;103:1366–1376. doi: 10.1002/bjs.10191. [DOI] [PubMed] [Google Scholar]
- 74.Galjart B, van der Stok EP, Rothbarth J, Grünhagen DJ, Verhoef C. Posttreatment Surveillance in Patients with Prolonged Disease-Free Survival After Resection of Colorectal Liver Metastasis. Ann Surg Oncol. 2016;23:3999–4007. doi: 10.1245/s10434-016-5388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pulitanò C, Castillo F, Aldrighetti L, Bodingbauer M, Parks RW, Ferla G, Wigmore SJ, Garden OJ. What defines 'cure' after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB (Oxford) 2010;12:244–249. doi: 10.1111/j.1477-2574.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D'Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 77.Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 78.Bhattacharjya S, Aggarwal R, Davidson BR. Intensive follow-up after liver resection for colorectal liver metastases: results of combined serial tumour marker estimations and computed tomography of the chest and abdomen - a prospective study. Br J Cancer. 2006;95:21–26. doi: 10.1038/sj.bjc.6603219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribero D, Viganò L, Amisano M, Capussotti L. Prognostic factors after resection of colorectal liver metastases: from morphology to biology. Future Oncol. 2013;9:45–57. doi: 10.2217/fon.12.159. [DOI] [PubMed] [Google Scholar]
- 80.Jones RP, Brudvik KW, Franklin JM, Poston GJ. Precision surgery for colorectal liver metastases: Opportunities and challenges of omics-based decision making. Eur J Surg Oncol. 2017;43:875–883. doi: 10.1016/j.ejso.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 81.Sasaki K, Margonis GA, Wilson A, Kim Y, Buettner S, Andreatos N, Gani F, Amini N, Spolverato G, Pawlik TM. Prognostic Implication of KRAS Status after Hepatectomy for Colorectal Liver Metastases Varies According to Primary Colorectal Tumor Location. Ann Surg Oncol. 2016;23:3736–3743. doi: 10.1245/s10434-016-5361-6. [DOI] [PubMed] [Google Scholar]
- 82.Bredt LC, Rachid AF. Predictors of recurrence after a first hepatectomy for colorectal cancer liver metastases: a retrospective analysis. World J Surg Oncol. 2014;12:391. doi: 10.1186/1477-7819-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18; discussion 318-21. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, Lodge JP. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. Br J Surg. 2014;101:856–866. doi: 10.1002/bjs.9471. [DOI] [PubMed] [Google Scholar]
- 85.Kumar R, Dennison AR, Robertson V, Jones MJ, Neal CP, Garcea G. Clinical risk scores in the current era of neoadjuvant chemotherapy for colorectal liver metastases. ANZ J Surg. 2018;88:E16–E20. doi: 10.1111/ans.13688. [DOI] [PubMed] [Google Scholar]
- 86.Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809–1824. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 87.Knijn N, Mekenkamp LJ, Klomp M, Vink-Börger ME, Tol J, Teerenstra S, Meijer JW, Tebar M, Riemersma S, van Krieken JH, Punt CJ, Nagtegaal ID. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margonis GA, Kim Y, Spolverato G, Ejaz A, Gupta R, Cosgrove D, Anders R, Karagkounis G, Choti MA, Pawlik TM. Association Between Specific Mutations in KRAS Codon 12 and Colorectal Liver Metastasis. JAMA Surg. 2015;150:722–729. doi: 10.1001/jamasurg.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Margonis GA, Kim Y, Sasaki K, Samaha M, Amini N, Pawlik TM. Codon 13 KRAS mutation predicts patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Cancer. 2016;122:2698–2707. doi: 10.1002/cncr.30085. [DOI] [PubMed] [Google Scholar]
- 90.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102:1175–1183. doi: 10.1002/bjs.9870. [DOI] [PubMed] [Google Scholar]
- 91.Brudvik KW, Vauthey JN. Surgery: KRAS mutations and hepatic recurrence after treatment of colorectal liver metastases. Nat Rev Gastroenterol Hepatol. 2017;14:638–639. doi: 10.1038/nrgastro.2017.129. [DOI] [PubMed] [Google Scholar]
- 92.Margonis GA, Buettner S, Andreatos N, Sasaki K, Ijzermans JNM, van Vugt JLA, Pawlik TM, Choti MA, Cameron JL, He J, Wolfgang CL, Weiss MJ. Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann Surg. 2017;266:641–649. doi: 10.1097/SLA.0000000000002367. [DOI] [PubMed] [Google Scholar]
- 93.Margonis GA, Sasaki K, Kim Y, Samaha M, Buettner S, Amini N, Antoniou E, Pawlik TM. Tumor Biology Rather Than Surgical Technique Dictates Prognosis in Colorectal Cancer Liver Metastases. J Gastrointest Surg. 2016;20:1821–1829. doi: 10.1007/s11605-016-3198-8. [DOI] [PubMed] [Google Scholar]
- 94.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 95.Tie J, Desai J. Targeting BRAF mutant metastatic colorectal cancer: clinical implications and emerging therapeutic strategies. Target Oncol. 2015;10:179–188. doi: 10.1007/s11523-014-0330-0. [DOI] [PubMed] [Google Scholar]
- 96.Taieb J, Le Malicot K, Shi Q, Penault-Llorca F, Bouché O, Tabernero J, Mini E, Goldberg RM, Folprecht G, Luc Van Laethem J, Sargent DJ, Alberts SR, Emile JF, Laurent Puig P, Sinicrope FA. Prognostic Value of BRAF and KRAS Mutations in MSI and MSS Stage III Colon Cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siena S, Rivera F, Taieb J, Peeters M, Prenen H, Koukakis R, Demonty G, Köhne CH. Survival Outcomes in Patients With RAS Wild Type Metastatic Colorectal Cancer Classified According to Köhne Prognostic Category and BRAF Mutation Status. Clin Colorectal Cancer. 2018;17:50–57.e8. doi: 10.1016/j.clcc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Gagnière J, Dupré A, Gholami SS, Pezet D, Boerner T, Gönen M, Kingham TP, Allen PJ, Balachandran VP, De Matteo RP, Drebin JA, Yaeger R, Kemeny NE, Jarnagin WR, D'Angelica MI. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann Surg. 2020;271:147–154. doi: 10.1097/SLA.0000000000002968. [DOI] [PubMed] [Google Scholar]
- 99.Bachet JB, Moreno-Lopez N, Vigano L, Marchese U, Gelli M, Raoux L, Truant S, Laurent C, Herrero A, Le Roy B, Deguelte Lardiere S, Passot G, Hautefeuille V, De La Fouchardiere C, Artru P, Ameto T, Mabrut JY, Schwarz L, Rousseau B, Lepère C, Coriat R, Brouquet A, Sa Cunha A, Benoist S. BRAF mutation is not associated with an increased risk of recurrence in patients undergoing resection of colorectal liver metastases. Br J Surg. 2019;106:1237–1247. doi: 10.1002/bjs.11180. [DOI] [PubMed] [Google Scholar]
- 100.Torén W, Ansari D, Andersson R. Immunohistochemical investigation of prognostic biomarkers in resected colorectal liver metastases: a systematic review and meta-analysis. Cancer Cell Int. 2018;18:217. doi: 10.1186/s12935-018-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakayama M, Oshima M. Mutant p53 in colon cancer. J Mol Cell Biol. 2019;11:267–276. doi: 10.1093/jmcb/mjy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Auvinen A, Isola J, Visakorpi T, Koivula T, Virtanen S, Hakama M. Overexpression of p53 and long-term survival in colon carcinoma. Br J Cancer. 1994;70:293–296. doi: 10.1038/bjc.1994.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Remvikos Y, Tominaga O, Hammel P, Laurent-Puig P, Salmon RJ, Dutrillaux B, Thomas G. Increased p53 protein content of colorectal tumours correlates with poor survival. Br J Cancer. 1992;66:758–764. doi: 10.1038/bjc.1992.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka K, Shimada H, Miura M, Fujii Y, Yamaguchi S, Endo I, Sekido H, Togo S, Ike H. Metastatic tumor doubling time: most important prehepatectomy predictor of survival and nonrecurrence of hepatic colorectal cancer metastasis. World J Surg. 2004;28:263–270. doi: 10.1007/s00268-003-7088-3. [DOI] [PubMed] [Google Scholar]
- 105.Yang Y, Forslund A, Remotti H, Lönnroth C, Andersson M, Brevinge H, Svanberg E, Lindnér P, Hafström L, Naredi P, Lundholm K. P53 mutations in primary tumors and subsequent liver metastases are related to survival in patients with colorectal carcinoma who undergo liver resection. Cancer. 2001;91:727–736. [PubMed] [Google Scholar]
- 106.Chun YS, Passot G, Yamashita S, Nusrat M, Katsonis P, Loree JM, Conrad C, Tzeng CD, Xiao L, Aloia TA, Eng C, Kopetz SE, Lichtarge O, Vauthey JN. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Ann Surg. 2019;269:917–923. doi: 10.1097/SLA.0000000000002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogino S, Lochhead P, Giovannucci E, Meyerhardt JA, Fuchs CS, Chan AT. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology. Oncogene. 2014;33:2949–2955. doi: 10.1038/onc.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 109.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 110.Løes IM, Immervoll H, Sorbye H, Angelsen JH, Horn A, Knappskog S, Lønning PE. Impact of KRAS, BRAF, PIK3CA, TP53 status and intraindividual mutation heterogeneity on outcome after liver resection for colorectal cancer metastases. Int J Cancer. 2016;139:647–656. doi: 10.1002/ijc.30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamashita S, Chun YS, Kopetz SE, Maru D, Conrad C, Aloia TA, Vauthey JN. APC and PIK3CA Mutational Cooperativity Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colorectal Liver Metastases. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002245. [DOI] [PubMed] [Google Scholar]
- 112.Hermida MA, Dinesh Kumar J, Leslie NR. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul. 2017;65:5–15. doi: 10.1016/j.jbior.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 113.Ghosh N, Hossain U, Mandal A, Sil PC. The Wnt signaling pathway: a potential therapeutic target against cancer. Ann N Y Acad Sci. 2019;1443:54–74. doi: 10.1111/nyas.14027. [DOI] [PubMed] [Google Scholar]
- 114.Sinicrope FA. DNA mismatch repair and adjuvant chemotherapy in sporadic colon cancer. Nat Rev Clin Oncol. 2010;7:174–177. doi: 10.1038/nrclinonc.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA., Jr PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 117.Ijsselsteijn ME, Petitprez F, Lacroix L, Ruano D, van der Breggen R, Julie C, Morreau H, Sautès-Fridman C, Fridman WH, de Miranda NFDCC. Revisiting immune escape in colorectal cancer in the era of immunotherapy. Br J Cancer. 2019;120:815–818. doi: 10.1038/s41416-019-0421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533, viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 119.Zeiss J, Merrick HW, Savolaine ER, Woldenberg LS, Kim K, Schlembach PJ. Fatty liver change as a result of hepatic artery infusion chemotherapy. Am J Clin Oncol. 1990;13:156–160. doi: 10.1097/00000421-199004000-00013. [DOI] [PubMed] [Google Scholar]
- 120.Peppercorn PD, Reznek RH, Wilson P, Slevin ML, Gupta RK. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008–2011. doi: 10.1038/bjc.1998.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA. Hepatic toxicity associated with fluorouracil plus levamisole adjuvant therapy. J Clin Oncol. 1993;11:2386–2390. doi: 10.1200/JCO.1993.11.12.2386. [DOI] [PubMed] [Google Scholar]
- 122.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, Risio M, Muratore A, Capussotti L, Curley SA, Abdalla EK. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 123.Reiss U, Cowan M, McMillan A, Horn B. Hepatic venoocclusive disease in blood and bone marrow transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. J Pediatr Hematol Oncol. 2002;24:746–750. doi: 10.1097/00043426-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 124.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, Mentha G, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 125.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 127.Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Lévi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 128.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 129.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 130.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 131.Marques RP, Duarte GS, Sterrantino C, Pais HL, Quintela A, Martins AP, Costa J. Triplet (FOLFOXIRI) versus doublet (FOLFOX or FOLFIRI) backbone chemotherapy as first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;118:54–62. doi: 10.1016/j.critrevonc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 132.Neugut AI, Lin A, Raab GT, Hillyer GC, Keller D, O'Neil DS, Accordino MK, Kiran RP, Wright J, Hershman DL. FOLFOX and FOLFIRI Use in Stage IV Colon Cancer: Analysis of SEER-Medicare Data. Clin Colorectal Cancer. 2019;18:133–140. doi: 10.1016/j.clcc.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petrelli F, Barni S Anti-EGFR agents for liver metastases. Resectability and outcome with anti-EGFR agents in patients with KRAS wild-type colorectal liver-limited metastases: a meta-analysis. Int J Colorectal Dis. 2012;27:997–1004. doi: 10.1007/s00384-012-1438-2. [DOI] [PubMed] [Google Scholar]
- 134.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Köhne CH. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 135.Modest DP, Martens UM, Riera-Knorrenschild J, Greeve J, Florschütz A, Wessendorf S, Ettrich T, Kanzler S, Nörenberg D, Ricke J, Seidensticker M, Held S, Buechner-Steudel P, Atzpodien J, Heinemann V, Seufferlein T, Tannapfel A, Reinacher-Schick AC, Geissler M. FOLFOXIRI Plus Panitumumab As First-Line Treatment of RAS Wild-Type Metastatic Colorectal Cancer: The Randomized, Open-Label, Phase II VOLFI Study (AIO KRK0109) J Clin Oncol. 2019;37:3401–3411. doi: 10.1200/JCO.19.01340. [DOI] [PubMed] [Google Scholar]
- 136.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 137.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 138.Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50:307–312. doi: 10.1002/gcc.20854. [DOI] [PubMed] [Google Scholar]
- 139.Clancy C, Burke JP, Kalady MF, Coffey JC. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e711–e718. doi: 10.1111/codi.12427. [DOI] [PubMed] [Google Scholar]
- 140.Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, Sartore-Bianchi A, Scala E, Cassingena A, Zecchin D, Apicella M, Migliardi G, Galimi F, Lauricella C, Zanon C, Perera T, Veronese S, Corti G, Amatu A, Gambacorta M, Diaz LA, Jr, Sausen M, Velculescu VE, Comoglio P, Trusolino L, Di Nicolantonio F, Giordano S, Siena S. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658–673. doi: 10.1158/2159-8290.CD-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP, Minoche A, Seshagiri S, Serrano S, Himmelbauer H, Bellmunt J, Rovira A, Settleman J, Bosch F, Albanell J. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–223. doi: 10.1038/nm.2609. [DOI] [PubMed] [Google Scholar]
- 142.Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–1729. doi: 10.1093/annonc/mdx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 144.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 145.Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89. doi: 10.1186/1471-2407-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702–708. doi: 10.1093/annonc/mdu580. [DOI] [PubMed] [Google Scholar]
- 147.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 148.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 149.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–2247. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 150.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, Berry S, Polite BN, O'Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB, 3rd, Mulkerin DL, Mayer RJ, Blanke C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, Deeter R, Shahin S, Amado RG. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 152.Tol J, Koopman M, Rodenburg CJ, Cats A, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, Mol L, Antonini NF, Punt CJ. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol. 2008;19:734–738. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 153.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 154.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 155.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 156.Tournigand C, Chibaudel B, Samson B, Scheithauer W, Vernerey D, Mésange P, Lledo G, Viret F, Ramée JF, Tubiana-Mathieu N, Dauba J, Dupuis O, Rinaldi Y, Mabro M, Aucoin N, Latreille J, Bonnetain F, Louvet C, Larsen AK, André T, de Gramont A. Bevacizumab with or without erlotinib as maintenance therapy in patients with metastatic colorectal cancer (GERCOR DREAM; OPTIMOX3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2015;16:1493–1505. doi: 10.1016/S1470-2045(15)00216-8. [DOI] [PubMed] [Google Scholar]
- 157.Ducreux M, Chamseddine A, Laurent-Puig P, Smolenschi C, Hollebecque A, Dartigues P, Samallin E, Boige V, Malka D, Gelli M. Molecular targeted therapy of BRAF-mutant colorectal cancer. Ther Adv Med Oncol. 2019;11:1758835919856494. doi: 10.1177/1758835919856494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Z, Dai WP, Zang YS. Complete response with fluorouracil and irinotecan with a BRAFV600E and EGFR inhibitor in BRAF-mutated metastatic colorectal cancer: a case report. Onco Targets Ther. 2019;12:443–447. doi: 10.2147/OTT.S180845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee SY, Oh SC. Advances of Targeted Therapy in Treatment of Unresectable Metastatic Colorectal Cancer. Biomed Res Int. 2016;2016:7590245. doi: 10.1155/2016/7590245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tampellini M, Sonetto C, Scagliotti GV. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs. 2016;25:507–520. doi: 10.1517/13543784.2016.1161754. [DOI] [PubMed] [Google Scholar]
- 161.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 163.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 164.Liu W, Zhou JG, Sun Y, Zhang L, Xing BC. The role of neoadjuvant chemotherapy for resectable colorectal liver metastases: a systematic review and meta-analysis. Oncotarget. 2016;7:37277–37287. doi: 10.18632/oncotarget.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Primrose J, Falk S, Finch-Jones M, Valle J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 166.Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Tabernero J, Teh C, Van Cutsem E Jean-Nicolas Vauthey of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–1239. doi: 10.1634/theoncologist.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brandi G, De Lorenzo S, Nannini M, Curti S, Ottone M, Dall'Olio FG, Barbera MA, Pantaleo MA, Biasco G. Adjuvant chemotherapy for resected colorectal cancer metastases: Literature review and meta-analysis. World J Gastroenterol. 2016;22:519–533. doi: 10.3748/wjg.v22.i2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, Bugat R, Lazorthes F, Bedenne L. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 169.Kim SY, Kim HJ, Hong YS, Jung KH, Park JW, Choi HS, Oh JH, Park SJ, Kim SH, Nam BH, Chang HJ, Kim DY. Resected colorectal liver metastases: does the survival differ according to postoperative chemotherapy regimen? J Surg Oncol. 2009;100:713–718. doi: 10.1002/jso.21403. [DOI] [PubMed] [Google Scholar]
- 170.Lam VW, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19:1292–1301. doi: 10.1245/s10434-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 171.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ, Donohue JH. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 172.Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, Vellone M, Giuliante F, Nuzzo G, Barone C. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 173.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 174.Ychou M, Rivoire M, Thezenas S, Quenet F, Delpero JR, Rebischung C, Letoublon C, Guimbaud R, Francois E, Ducreux M, Desseigne F, Fabre JM, Assenat E. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann Surg Oncol. 2013;20:4289–4297. doi: 10.1245/s10434-013-3217-x. [DOI] [PubMed] [Google Scholar]
- 175.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 176.Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, Mudan S, Brown G, Khan A, Wotherspoon A, Strimpakos AS, Thomas J, Compton S, Chua YJ, Chau I. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–2048. doi: 10.1093/annonc/mdq714. [DOI] [PubMed] [Google Scholar]
- 177.Chapelle N, Matysiak-Budnik T, Douane F, Metairie S, Rougier P, Touchefeu Y. Hepatic arterial infusion in the management of colorectal cancer liver metastasis: Current and future perspectives. Dig Liver Dis. 2018;50:220–225. doi: 10.1016/j.dld.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 178.Deschamps F, Elias D, Goere D, Malka D, Ducreux M, Boige V, Auperin A, de Baere T. Intra-arterial hepatic chemotherapy: a comparison of percutaneous versus surgical implantation of port-catheters. Cardiovasc Intervent Radiol. 2011;34:973–979. doi: 10.1007/s00270-010-9996-6. [DOI] [PubMed] [Google Scholar]
- 179.Rougier P, Laplanche A, Huguier M, Hay JM, Ollivier JM, Escat J, Salmon R, Julien M, Roullet Audy JC, Gallot D. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992;10:1112–1118. doi: 10.1200/JCO.1992.10.7.1112. [DOI] [PubMed] [Google Scholar]
- 180.Ducreux M, Ychou M, Laplanche A, Gamelin E, Lasser P, Husseini F, Quenet F, Viret F, Jacob JH, Boige V, Elias D, Delperro JR, Luboinski M gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol. 2005;23:4881–4887. doi: 10.1200/JCO.2005.05.120. [DOI] [PubMed] [Google Scholar]
- 181.Lévi FA, Boige V, Hebbar M, Smith D, Lepère C, Focan C, Karaboué A, Guimbaud R, Carvalho C, Tumolo S, Innominato P, Ajavon Y, Truant S, Castaing D, De Baere T, Kunstlinger F, Bouchahda M, Afshar M, Rougier P, Adam R, Ducreux M Association Internationale pour Recherche sur Temps Biologique et Chronothérapie (ARTBC International) Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol. 2016;27:267–274. doi: 10.1093/annonc/mdv548. [DOI] [PubMed] [Google Scholar]
- 182.Groot Koerkamp B, Sadot E, Kemeny NE, Gönen M, Leal JN, Allen PJ, Cercek A, DeMatteo RP, Kingham TP, Jarnagin WR, D'Angelica MI. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. J Clin Oncol. 2017;35:1938–1944. doi: 10.1200/JCO.2016.71.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, Bertino JR, Turnbull AD, Sullivan D, Stockman J, Blumgart LH, Fong Y. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 184.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O'Callaghan C, Langer B, Martignoni G, Bouché O, Lazorthes F, Van Cutsem E, Bedenne L, Moore MJ, Rougier P. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 185.Buisman FE, Homs MYV, Grünhagen DJ, Filipe WF, Bennink RJ, Besselink MGH, Borel Rinkes IHM, Bruijnen RCG, Cercek A, D'Angelica MI, van Delden OM, Donswijk ML, van Doorn L, Doornebosch PG, Emmering J, Erdmann JI, IJzerman NS, Grootscholten C, Hagendoorn J, Kemeny NE, Kingham TP, Klompenhouwer EG, Kok NFM, Koolen S, Kuhlmann KFD, Kuiper MC, Lam MGE, Mathijssen RHJ, Moelker A, Oomen-de Hoop E, Punt CJA, Te Riele WW, Roodhart JML, Swijnenburg RJ, Prevoo W, Tanis PJ, Vermaas M, Versleijen MWJ, Veuger FP, Weterman MJ, Verhoef C, Groot Koerkamp B. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases - the multicenter randomized controlled PUMP trial. BMC Cancer. 2019;19:327. doi: 10.1186/s12885-019-5515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lewis AL, Hall B. Toward a better understanding of the mechanism of action for intra-arterial delivery of irinotecan from DC Bead(TM) (DEBIRI) Future Oncol. 2019;15:2053–2068. doi: 10.2217/fon-2019-0071. [DOI] [PubMed] [Google Scholar]
- 187.Akinwande O, Dendy M, Ludwig JM, Kim HS. Hepatic intra-arterial injection of irinotecan drug eluting beads (DEBIRI) for patients with unresectable colorectal liver metastases: A systematic review. Surg Oncol. 2017;26:268–275. doi: 10.1016/j.suronc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 188.Martin RC 2nd, Scoggins CR, Schreeder M, Rilling WS, Laing CJ, Tatum CM, Kelly LR, Garcia-Monaco RD, Sharma VR, Crocenzi TS, Strasberg SM. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121:3649–3658. doi: 10.1002/cncr.29534. [DOI] [PubMed] [Google Scholar]
- 189.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V, Coschiera P. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387–1395. [PubMed] [Google Scholar]
- 190.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 191.Ackerman NB. Experimental studies on the circulation dynamics of intrahepatic tumor blood supply. Cancer. 1972;29:435–439. doi: 10.1002/1097-0142(197202)29:2<435::aid-cncr2820290227>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 192.Bozkurt MF, Salanci BV, Uğur Ö. Intra-Arterial Radionuclide Therapies for Liver Tumors. Semin Nucl Med. 2016;46:324–339. doi: 10.1053/j.semnuclmed.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 193.Campbell AM, Bailey IH, Burton MA. Tumour dosimetry in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol. 2001;46:487–498. doi: 10.1088/0031-9155/46/2/315. [DOI] [PubMed] [Google Scholar]
- 194.Klaassen NJM, Arntz MJ, Gil Arranja A, Roosen J, Nijsen JFW. The various therapeutic applications of the medical isotope holmium-166: a narrative review. EJNMMI Radiopharm Chem. 2019;4:19. doi: 10.1186/s41181-019-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van Hazel GA, Pavlakis N, Goldstein D, Olver IN, Tapner MJ, Price D, Bower GD, Briggs GM, Rossleigh MA, Taylor DJ, George J. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27:4089–4095. doi: 10.1200/JCO.2008.20.8116. [DOI] [PubMed] [Google Scholar]
- 196.Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, De Keukeleire K, Verslype C, Defreyne L, Van Cutsem E, Delatte P, Delaunoit T, Personeni N, Paesmans M, Van Laethem JL, Flamen P. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687–3694. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 197.Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG, Perrone M, Giampalma E, Angelelli B, Fiore F, Lastoria S, Bacchetti S, Gasperini D, Geatti O, Izzo F Italian Society of Locoregional Therapies in Oncology (SITILO) Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324–331. doi: 10.1038/sj.bjc.6605770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seidensticker R, Denecke T, Kraus P, Seidensticker M, Mohnike K, Fahlke J, Kettner E, Hildebrandt B, Dudeck O, Pech M, Amthauer H, Ricke J. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol. 2012;35:1066–1073. doi: 10.1007/s00270-011-0234-7. [DOI] [PubMed] [Google Scholar]
- 199.Kennedy A, Cohn M, Coldwell DM, Drooz A, Ehrenwald E, Kaiser A, Nutting CW, Rose SC, Wang EA, Savin MA. Updated survival outcomes and analysis of long-term survivors from the MORE study on safety and efficacy of radioembolization in patients with unresectable colorectal cancer liver metastases. J Gastrointest Oncol. 2017;8:614–624. doi: 10.21037/jgo.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van Hazel GA, Heinemann V, Sharma NK, Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland AH, Ferguson T, Rodríguez J, Kröning H, Wolf I, Ganju V, Walpole E, Boucher E, Tichler T, Shacham-Shmueli E, Powell A, Eliadis P, Isaacs R, Price D, Moeslein F, Taieb J, Bower G, Gebski V, Van Buskirk M, Cade DN, Thurston K, Gibbs P. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016;34:1723–1731. doi: 10.1200/JCO.2015.66.1181. [DOI] [PubMed] [Google Scholar]
- 201.Dutton SJ, Kenealy N, Love SB, Wasan HS, Sharma RA FOXFIRE Protocol Development Group and the NCRI Colorectal Clinical Study Group. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer. BMC Cancer. 2014;14:497. doi: 10.1186/1471-2407-14-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sharma RA, Wasan HS, Love SB, Dutton S, Stokes JC, Smith JL FOXFIRE Trial Management Group. FOXFIRE: a phase III clinical trial of chemo-radio-embolisation as first-line treatment of liver metastases in patients with colorectal cancer. Clin Oncol (R Coll Radiol) 2008;20:261–263. doi: 10.1016/j.clon.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 203.Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, Peeters M, Findlay M, Weaver A, Mills J, Wilson C, Adams R, Francis A, Moschandreas J, Virdee PS, Dutton P, Love S, Gebski V, Gray A FOXFIRE trial investigators; SIRFLOX trial investigators; FOXFIRE-Global trial investigators, van Hazel G, Sharma RA. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159–1171. doi: 10.1016/S1470-2045(17)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gibbs P, Heinemann V, Sharma NK, Taieb J, Ricke J, Peeters M, Findlay M, Robinson B, Jackson C, Strickland A, Gebski V, Van Buskirk M, Zhao H, van Hazel G SIRFLOX and FOXFIRE Global Trial Investigators. Effect of Primary Tumor Side on Survival Outcomes in Untreated Patients With Metastatic Colorectal Cancer When Selective Internal Radiation Therapy Is Added to Chemotherapy: Combined Analysis of Two Randomized Controlled Studies. Clin Colorectal Cancer. 2018;17:e617–e629. doi: 10.1016/j.clcc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 205.White J, Carolan-Rees G, Dale M, Morgan HE, Patrick HE, See TC, Beeton EL, Swinson DEB, Bell JK, Manas DM, Crellin A, Slevin NJ, Sharma RA. Analysis of a National Programme for Selective Internal Radiation Therapy for Colorectal Cancer Liver Metastases. Clin Oncol (R Coll Radiol) 2019;31:58–66. doi: 10.1016/j.clon.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 206.NHS England Specialised Services Clinical Reference Group for Radiotherapy. Clinical Commissioning Policy: Selective internal radiation therapy (SIRT) for chemotherapy refractory / intolerant metastatic colorectal cancer (adults) [Internet] [cited 2020 Jan 26]; Available from: https://www.england.nhs.uk/wp-content/uploads/2018/12/Selective-internal-radiation-therapy-for-chemotherapy-refractory-intolerant-metastatic-colorectal-cancer.pdf. [Google Scholar]
- 207.Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz MA, Mauer M, Folprecht G, Van Cutsem E, Ducreux M, Nordlinger B; European Organisation for Research and Treatment of Cancer (EORTC); Gastro-Intestinal Tract Cancer Group; Arbeitsgruppe Lebermetastasen und tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG). Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst. 2017;109 [Google Scholar]
- 208.Petrelli F, Comito T, Barni S, Pancera G, Scorsetti M, Ghidini A SBRT for CRC liver metastases. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol. 2018;129:427–434. doi: 10.1016/j.radonc.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 209.Grigorian A, O'Brien CB. Hepatotoxicity Secondary to Chemotherapy. J Clin Transl Hepatol. 2014;2:95–102. doi: 10.14218/JCTH.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Méndez Romero A, Keskin-Cambay F, van Os RM, Nuyttens JJ, Heijmen BJM, IJzermans JNM, Verhoef C. Institutional experience in the treatment of colorectal liver metastases with stereotactic body radiation therapy. Rep Pract Oncol Radiother. 2017;22:126–131. doi: 10.1016/j.rpor.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Joo JH, Park JH, Kim JC, Yu CS, Lim SB, Park IJ, Kim TW, Hong YS, Kim KP, Yoon SM, Park J, Kim JH. Local Control Outcomes Using Stereotactic Body Radiation Therapy for Liver Metastases from Colorectal Cancer. Int J Radiat Oncol Biol Phys. 2017;99:876–883. doi: 10.1016/j.ijrobp.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 212.Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J, Dinniwell R, Brierley J, Kavanagh BD, Dawson LA, Schefter TE. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 213.McPartlin A, Swaminath A, Wang R, Pintilie M, Brierley J, Kim J, Ringash J, Wong R, Dinniwell R, Craig T, Dawson LA. Long-Term Outcomes of Phase 1 and 2 Studies of SBRT for Hepatic Colorectal Metastases. Int J Radiat Oncol Biol Phys. 2017;99:388–395. doi: 10.1016/j.ijrobp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 214.Scorsetti M, Comito T, Clerici E, Franzese C, Tozzi A, Iftode C, Di Brina L, Navarria P, Mancosu P, Reggiori G, Fogliata A, Tomatis S, Torzilli G, Cozzi L. Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol. 2018;13:234. doi: 10.1186/s13014-018-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Servagi S, UNICANCER. Oligometastases of the LIVer Treated With Chemotherapy With ou Without Extracranial Stereotactic Body Radiation Therapy in Patients With Colorectal Cancer - Full Text View - ClinicalTrials.gov [Internet]. [cited 2020 Jan 18] Available from: https://clinicaltrials.gov/ct2/show/NCT03532204. [Google Scholar]
- 216.Hoyer M, Danish Center for Interventional Research in Radiation Oncology. Radiofrequency Ablation Versus Stereotactic Radiotherapy in Colorectal Liver Metastases - Full Text View - ClinicalTrials.gov [Internet]. [cited 2020 Jan 18] Available from: https://clinicaltrials.gov/ct2/show/NCT01233544. [Google Scholar]
- 217.Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol. 2011;66:1007–1014. doi: 10.1016/j.crad.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 218.Rodgers MS, Collinson R, Desai S, Stubbs RS, McCall JL. Risk of dissemination with biopsy of colorectal liver metastases. Dis Colon Rectum. 2003;46:454–8; discussion 458-9. doi: 10.1007/s10350-004-6581-6. [DOI] [PubMed] [Google Scholar]
- 219.Ohlsson B, Nilsson J, Stenram U, Akerman M, Tranberg KG. Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg. 2002;89:757–762. doi: 10.1046/j.1365-2168.2002.02111.x. [DOI] [PubMed] [Google Scholar]
- 220.Jones OM, Rees M, John TG, Bygrave S, Plant G. Biopsy of resectable colorectal liver metastases causes tumour dissemination and adversely affects survival after liver resection. Br J Surg. 2005;92:1165–1168. doi: 10.1002/bjs.4888. [DOI] [PubMed] [Google Scholar]
- 221.Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous--fine needle aspiration of hepatic colorectal metastases. BMJ. 2004;328:507–508. doi: 10.1136/bmj.328.7438.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, Pawlik TM. Parenchymal-Sparing Versus Anatomic Liver Resection for Colorectal Liver Metastases: a Systematic Review. J Gastrointest Surg. 2017;21:1076–1085. doi: 10.1007/s11605-017-3397-y. [DOI] [PubMed] [Google Scholar]
- 223.Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, Kopetz S, Loyer EM, Curley SA, Abdalla EK, Vauthey JN. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hamady ZZ, Lodge JP, Welsh FK, Toogood GJ, White A, John T, Rees M. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg. 2014;259:543–548. doi: 10.1097/SLA.0b013e3182902b6e. [DOI] [PubMed] [Google Scholar]
- 225.de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 226.Carpizo DR, Are C, Jarnagin W, Dematteo R, Fong Y, Gönen M, Blumgart L, D'Angelica M. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16:2138–2146. doi: 10.1245/s10434-009-0521-6. [DOI] [PubMed] [Google Scholar]
- 227.Carpizo DR, D'Angelica M. Liver resection for metastatic colorectal cancer in the presence of extrahepatic disease. Lancet Oncol. 2009;10:801–809. doi: 10.1016/S1470-2045(09)70081-6. [DOI] [PubMed] [Google Scholar]
- 228.Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, Bismuth H, Castaing D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672–3680. doi: 10.1200/JCO.2007.15.7297. [DOI] [PubMed] [Google Scholar]
- 229.Andres A, Mentha G, Adam R, Gerstel E, Skipenko OG, Barroso E, Lopez-Ben S, Hubert C, Majno PE, Toso C. Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br J Surg. 2015;102:691–699. doi: 10.1002/bjs.9783. [DOI] [PubMed] [Google Scholar]
- 230.Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á, Rufián-Peña S, Casado-Adam Á, Cosano-Álvarez A, Briceño-Delgado J. Colorectal peritoneal metastases: Optimal management review. World J Gastroenterol. 2019;25:3484–3502. doi: 10.3748/wjg.v25.i27.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dagher I, O'Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, Lainas P, Laurent A, Nguyen KT, Marvin MR, Thomas M, Ravindra K, Fielding G, Franco D, Buell JF. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250:856–860. doi: 10.1097/SLA.0b013e3181bcaf46. [DOI] [PubMed] [Google Scholar]
- 232.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 233.Melandro F, Giovanardi F, Hassan R, Larghi Laureiro Z, Ferri F, Rossi M, Mennini G, Pawlik TM, Lai Q. Minimally Invasive Approach in the Setting of ALPPS Procedure: a Systematic Review of the Literature. J Gastrointest Surg. 2019;23:1917–1924. doi: 10.1007/s11605-018-04092-x. [DOI] [PubMed] [Google Scholar]
- 234.Samstein B, Cherqui D, Rotellar F, Griesemer A, Halazun KJ, Kato T, Guarrera J, Emond JC. Totally laparoscopic full left hepatectomy for living donor liver transplantation in adolescents and adults. Am J Transplant. 2013;13:2462–2466. doi: 10.1111/ajt.12360. [DOI] [PubMed] [Google Scholar]
- 235.Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant. 2013;13:2467–2471. doi: 10.1111/ajt.12361. [DOI] [PubMed] [Google Scholar]
- 236.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 237.Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018;267:199–207. doi: 10.1097/SLA.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 238.Wong-Lun-Hing EM, van Dam RM, van Breukelen GJ, Tanis PJ, Ratti F, van Hillegersberg R, Slooter GD, de Wilt JH, Liem MS, de Boer MT, Klaase JM, Neumann UP, Aldrighetti LA, Dejong CH ORANGE II Collaborative Group. Randomized clinical trial of open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery after surgery programme (ORANGE II study) Br J Surg. 2017;104:525–535. doi: 10.1002/bjs.10438. [DOI] [PubMed] [Google Scholar]
- 239.Troisi RI, Pegoraro F, Giglio MC, Rompianesi G, Berardi G, Tomassini F, De Simone G, Aprea G, Montalti R, De Palma GD. Robotic approach to the liver: Open surgery in a closed abdomen or laparoscopic surgery with technical constraints? Surg Oncol. 2020;33:239–248. doi: 10.1016/j.suronc.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 240.Sheen AJ, Jamdar S, Siriwardena AK. Laparoscopic Hepatectomy for Colorectal Liver Metastases: The Current State of the Art. Front Oncol. 2019;9:442. doi: 10.3389/fonc.2019.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 242.Nagashima I, Takada T, Okinaga K, Nagawa H. A scoring system for the assessment of the risk of mortality after partial hepatectomy in patients with chronic liver dysfunction. J Hepatobiliary Pancreat Surg. 2005;12:44–48. doi: 10.1007/s00534-004-0953-0. [DOI] [PubMed] [Google Scholar]
- 243.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–379. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bennink RJ, Dinant S, Erdogan D, Heijnen BH, Straatsburg IH, van Vliet AK, van Gulik TM. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nucl Med. 2004;45:965–971. [PubMed] [Google Scholar]
- 245.Dinant S, de Graaf W, Verwer BJ, Bennink RJ, van Lienden KP, Gouma DJ, van Vliet AK, van Gulik TM. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med. 2007;48:685–692. doi: 10.2967/jnumed.106.038430. [DOI] [PubMed] [Google Scholar]
- 246.Paumgartner G. The handling of indocyanine green by the liver. Schweiz Med Wochenschr. 1975;105:1–30. [PubMed] [Google Scholar]
- 247.Levesque E, Martin E, Dudau D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016;35:49–57. doi: 10.1016/j.accpm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 248.Wakiya T, Kudo D, Toyoki Y, Ishido K, Kimura N, Narumi S, Kijima H, Hakamada K. Evaluation of the usefulness of the indocyanine green clearance test for chemotherapy-associated liver injury in patients with colorectal cancer liver metastasis. Ann Surg Oncol. 2014;21:167–172. doi: 10.1245/s10434-013-3203-3. [DOI] [PubMed] [Google Scholar]
- 249.Yokoyama Y, Nishio H, Ebata T, Igami T, Sugawara G, Nagino M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg. 2010;97:1260–1268. doi: 10.1002/bjs.7084. [DOI] [PubMed] [Google Scholar]
- 250.Mizutani Y, Hirai T, Nagamachi S, Nanashima A, Yano K, Kondo K, Hiyoshi M, Imamura N, Terada T. Prediction of Posthepatectomy Liver Failure Proposed by the International Study Group of Liver Surgery: Residual Liver Function Estimation With 99mTc-Galactosyl Human Serum Albumin Scintigraphy. Clin Nucl Med. 2018;43:77–81. doi: 10.1097/RLU.0000000000001913. [DOI] [PubMed] [Google Scholar]
- 251.Rassam F, Olthof PB, Bennink RJ, van Gulik TM. Current Modalities for the Assessment of Future Remnant Liver Function. Visc Med. 2017;33:442–448. doi: 10.1159/000480385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.de Graaf W, van Lienden KP, Dinant S, Roelofs JJ, Busch OR, Gouma DJ, Bennink RJ, van Gulik TM. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14:369–378. doi: 10.1007/s11605-009-1085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chapelle T, Op De Beeck B, Huyghe I, Francque S, Driessen A, Roeyen G, Ysebaert D, De Greef K. Future remnant liver function estimated by combining liver volumetry on magnetic resonance imaging with total liver function on (99m)Tc-mebrofenin hepatobiliary scintigraphy: can this tool predict post-hepatectomy liver failure? HPB (Oxford) 2016;18:494–503. doi: 10.1016/j.hpb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cieslak KP, Bennink RJ, de Graaf W, van Lienden KP, Besselink MG, Busch OR, Gouma DJ, van Gulik TM. Measurement of liver function using hepatobiliary scintigraphy improves risk assessment in patients undergoing major liver resection. HPB (Oxford) 2016;18:773–780. doi: 10.1016/j.hpb.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chapelle T, Op de Beeck B, Roeyen G, Bracke B, Hartman V, De Greef K, Huyghe I, Van der Zijden T, Morrison S, Francque S, Ysebaert D. Measuring future liver remnant function prior to hepatectomy may guide the indication for portal vein occlusion and avoid posthepatectomy liver failure: a prospective interventional study. HPB (Oxford) 2017;19:108–117. doi: 10.1016/j.hpb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 256.Cieslak KP, Huisman F, Bais T, Bennink RJ, van Lienden KP, Verheij J, Besselink MG, Busch ORC, van Gulik TM. Future remnant liver function as predictive factor for the hypertrophy response after portal vein embolization. Surgery. 2017;162:37–47. doi: 10.1016/j.surg.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 257.Olthof PB, Tomassini F, Huespe PE, Truant S, Pruvot FR, Troisi RI, Castro C, Schadde E, Axelsson R, Sparrelid E, Bennink RJ, Adam R, van Gulik TM, de Santibanes E. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: Liver volume overestimates liver function. Surgery. 2017;162:775–783. doi: 10.1016/j.surg.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 258.Truant S. Hepato Biliary Scintigraphy to Assess the Risk of Postoperative Liver Failure Hepatectomies - Full Text View - ClinicalTrials.gov [Internet]. [cited 2020 Jan 16] Available from: https://clinicaltrials.gov/ct2/show/NCT02753517. [Google Scholar]
- 259.Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol. 2010;17:492–501. doi: 10.1245/s10434-009-0781-1. [DOI] [PubMed] [Google Scholar]
- 260.Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, Mitry E, Rougier P, Nordlinger B. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 261.van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? J Gastrointest Surg. 2010;14:1691–1700. doi: 10.1007/s11605-010-1348-y. [DOI] [PubMed] [Google Scholar]
- 262.Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg. 2009;250:935–942. doi: 10.1097/sla.0b013e3181b0c6e4. [DOI] [PubMed] [Google Scholar]
- 263.Araujo RLC, Milani JM, Armentano DP, Moreira RB, Pinto GSF, de Castro LA, Lucchesi FR. Disappearing colorectal liver metastases: Strategies for the management of patients achieving a radiographic complete response after systemic chemotherapy. J Surg Oncol. 2020;121:848–856. doi: 10.1002/jso.25784. [DOI] [PubMed] [Google Scholar]
- 264.Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D, Castaing D. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 265.Tsilimigras DI, Ntanasis-Stathopoulos I, Paredes AZ, Moris D, Gavriatopoulou M, Cloyd JM, Pawlik TM. Disappearing liver metastases: A systematic review of the current evidence. Surg Oncol. 2019;29:7–13. doi: 10.1016/j.suronc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 266.Kepenekian V, Muller A, Valette PJ, Rousset P, Chauvenet M, Phelip G, Walter T, Adham M, Glehen O, Passot G. Evaluation of a strategy using pretherapeutic fiducial marker placement to avoid missing liver metastases. BJS Open. 2019;3:344–353. doi: 10.1002/bjs5.50140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg. 2012;255:415–417. doi: 10.1097/SLA.0b013e318248577d. [DOI] [PubMed] [Google Scholar]
- 268.van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25–34. doi: 10.1007/s00270-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nagino M. Fifty-year history of biliary surgery. Ann Gastroenterol Surg. 2019;3:598–605. doi: 10.1002/ags3.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 271.Kinoshita H, Sakai K, Iwasa R, Hirohashi K, Kubo S, Fujio N, Lee KC. Results of preoperative portal vein embolization for hepatocellular carcinoma. Osaka City Med J. 1988;34:115–122. [PubMed] [Google Scholar]
- 272.Goéré D, Farges O, Leporrier J, Sauvanet A, Vilgrain V, Belghiti J. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg. 2006;10:365–370. doi: 10.1016/j.gassur.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 273.Pommier R, Ronot M, Cauchy F, Gaujoux S, Fuks D, Faivre S, Belghiti J, Vilgrain V. Colorectal liver metastases growth in the embolized and non-embolized liver after portal vein embolization: influence of initial response to induction chemotherapy. Ann Surg Oncol. 2014;21:3077–3083. doi: 10.1245/s10434-014-3700-z. [DOI] [PubMed] [Google Scholar]
- 274.van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, van Delden OM, Laméris JS, Gouma DJ. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436–444. doi: 10.1159/000184735. [DOI] [PubMed] [Google Scholar]
- 275.Cauchy F, Aussilhou B, Dokmak S, Fuks D, Gaujoux S, Farges O, Faivre S, Lepillé D, Belghiti J. Reappraisal of the risks and benefits of major liver resection in patients with initially unresectable colorectal liver metastases. Ann Surg. 2012;256:746–52; discussion 752-4. doi: 10.1097/SLA.0b013e3182738204. [DOI] [PubMed] [Google Scholar]
- 276.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 278.Wigmore SJ. ALPPS: The argument against. Eur J Surg Oncol. 2017;43:249–251. doi: 10.1016/j.ejso.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 279.Buac S, Schadde E, Schnitzbauer AA, Vogt K, Hernandez-Alejandro R. The many faces of ALPPS: surgical indications and techniques among surgeons collaborating in the international registry. HPB (Oxford) 2016;18:442–448. doi: 10.1016/j.hpb.2016.01.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Rizell M, Björnsson B. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial) Ann Surg. 2018;267:833–840. doi: 10.1097/SLA.0000000000002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Eshmuminov D, Raptis DA, Linecker M, Wirsching A, Lesurtel M, Clavien PA. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg. 2016;103:1768–1782. doi: 10.1002/bjs.10290. [DOI] [PubMed] [Google Scholar]
- 282.Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 2015;261:e90–e92. doi: 10.1097/SLA.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 283.Linecker M, Stavrou GA, Oldhafer KJ, Jenner RM, Seifert B, Lurje G, Bednarsch J, Neumann U, Capobianco I, Nadalin S, Robles-Campos R, de Santibañes E, Malagó M, Lesurtel M, Clavien PA, Petrowsky H. The ALPPS Risk Score: Avoiding Futile Use of ALPPS. Ann Surg. 2016;264:763–771. doi: 10.1097/SLA.0000000000001914. [DOI] [PubMed] [Google Scholar]
- 284.Chow FC, Chok KS. Colorectal liver metastases: An update on multidisciplinary approach. World J Hepatol. 2019;11:150–172. doi: 10.4254/wjh.v11.i2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Guenette JP, Dupuy DE. Radiofrequency ablation of colorectal hepatic metastases. J Surg Oncol. 2010;102:978–987. doi: 10.1002/jso.21658. [DOI] [PubMed] [Google Scholar]
- 286.Ruers T, Punt C, Van Coevorden F, Pierie JP, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz MA, Mauer M, Van Cutsem E, Lutz MP, Nordlinger B EORTC Gastro-Intestinal Tract Cancer Group, Arbeitsgruppe Lebermetastasen und—tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO) and the National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG) Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23:2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Weng M, Zhang Y, Zhou D, Yang Y, Tang Z, Zhao M, Quan Z, Gong W. Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. PLoS One. 2012;7:e45493. doi: 10.1371/journal.pone.0045493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Fütterer JJ, de Wilt JHW. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis. HPB (Oxford) 2017;19:749–756. doi: 10.1016/j.hpb.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 289.Kron P, Linecker M, Jones RP, Toogood GJ, Clavien PA, Lodge JPA. Ablation or Resection for Colorectal Liver Metastases? A Systematic Review of the Literature. Front Oncol. 2019;9:1052. doi: 10.3389/fonc.2019.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol. 2008;15:2757–2764. doi: 10.1245/s10434-008-0043-7. [DOI] [PubMed] [Google Scholar]
- 292.Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, Cho CH, Ko HK, Lee JT, Kim NK. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197:728–736. doi: 10.1016/j.amjsurg.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 293.Ko S, Jo H, Yun S, Park E, Kim S, Seo HI. Comparative analysis of radiofrequency ablation and resection for resectable colorectal liver metastases. World J Gastroenterol. 2014;20:525–531. doi: 10.3748/wjg.v20.i2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, Curley SA, Zorzi D, Abdalla EK. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–6; discussion 466-7. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 295.Aliyev S, Agcaoglu O, Aksoy E, Taskin HE, Vogt D, Fung J, Siperstein A, Berber E. Efficacy of laparoscopic radiofrequency ablation for the treatment of patients with small solitary colorectal liver metastasis. Surgery. 2013;154:556–562. doi: 10.1016/j.surg.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 296.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Hubert C, Gras J, Goffette P, Grajeda JM, Van Beers BE, Laurence A, Horsmans Y, Sempoux C, Rahier J, Zech F, Gigot JF. Percutaneous and surgical radiofrequency ablation of liver malignancies: a single institutional experience. Acta Gastroenterol Belg. 2007;70:188–194. [PubMed] [Google Scholar]
- 298.Hildebrand P, Leibecke T, Kleemann M, Mirow L, Birth M, Bruch HP, Bürk C. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol. 2006;32:430–434. doi: 10.1016/j.ejso.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 299.Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008;25:445–460. doi: 10.1159/000184736. [DOI] [PubMed] [Google Scholar]
- 300.Gurusamy K, Corrigan N, Croft J, Twiddy M, Morris S, Woodward N, Bandula S, Hochhauser D, Napp V, Pullan A, Jakowiw N, Prasad R, Damink SO, van Laarhoven CJHM, de Wilt JHW, Brown J, Davidson BR. Liver resection surgery versus thermal ablation for colorectal LiVer MetAstases (LAVA): study protocol for a randomised controlled trial. Trials. 2018;19:105. doi: 10.1186/s13063-018-2499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Puijk RS, Ruarus AH, Vroomen LGPH, van Tilborg AAJM, Scheffer HJ, Nielsen K, de Jong MC, de Vries JJJ, Zonderhuis BM, Eker HH, Kazemier G, Verheul H, van der Meijs BB, van Dam L, Sorgedrager N, Coupé VMH, van den Tol PMP, Meijerink MR COLLISION Trial Group. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18:821. doi: 10.1186/s12885-018-4716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Huang M, Sixth Affiliated Hospital SYU. Comparison of Hepatectomy and Local Ablation for Resectable Synchronous and Metachronous Colorectal Liver Metastasis. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) [cited 2020 Jan 22] Available from: https://clinicaltrials.gov/ct2/show/NCT02886104 NLM Identifier: NCT02886104. [Google Scholar]
- 303.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276–284. [PubMed] [Google Scholar]
- 305.Huo YR, Eslick GD. Microwave Ablation Compared to Radiofrequency Ablation for Hepatic Lesions: A Meta-Analysis. J Vasc Interv Radiol. 2015;26:1139–1146.e2. doi: 10.1016/j.jvir.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 306.Correa-Gallego C, Fong Y, Gonen M, D'Angelica MI, Allen PJ, DeMatteo RP, Jarnagin WR, Kingham TP. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21:4278–4283. doi: 10.1245/s10434-014-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Liu Y, Li S, Wan X, Li Y, Li B, Zhang Y, Yuan Y, Zheng Y. Efficacy and safety of thermal ablation in patients with liver metastases. Eur J Gastroenterol Hepatol. 2013;25:442–446. doi: 10.1097/MEG.0b013e32835cb566. [DOI] [PubMed] [Google Scholar]
- 308.van Tilborg AA, Scheffer HJ, de Jong MC, Vroomen LG, Nielsen K, van Kuijk C, van den Tol PM, Meijerink MR. MWA Versus RFA for Perivascular and Peribiliary CRLM: A Retrospective Patient- and Lesion-Based Analysis of Two Historical Cohorts. Cardiovasc Intervent Radiol. 2016;39:1438–1446. doi: 10.1007/s00270-016-1413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, Barbas AS, Abdalla EK, Choti MA, Vauthey JN, Ludwig KA, Mantyh CR, Morse MA, Clary BM. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 310.Jones TJ, Murphy AE, Tameron A, Hussain LR, Grannan K, Guend H, Dunki-Jacobs EM, Lee DY. Trends and Outcomes of Synchronous Resection of Colorectal Metastasis in the Modern Era-Analysis of Targeted Hepatic NSQIP Database. J Surg Res. 2019;238:35–40. doi: 10.1016/j.jss.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 311.de Haas RJ, Adam R, Wicherts DA, Azoulay D, Bismuth H, Vibert E, Salloum C, Perdigao F, Benkabbou A, Castaing D. Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg. 2010;97:1279–1289. doi: 10.1002/bjs.7106. [DOI] [PubMed] [Google Scholar]
- 312.Bogach J, Wang J, Griffiths C, Parpia S, Saskin R, Hallet J, Ruo L, Simunovic M, Serrano PE. Simultaneous versus staged resection for synchronous colorectal liver metastases: A population-based cohort study. Int J Surg. 2020;74:68–75. doi: 10.1016/j.ijsu.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 313.Gavriilidis P, Katsanos K, Sutcliffe RP, Simopoulos C, Azoulay D, Roberts KJ. Simultaneous, Delayed and Liver-First Hepatic Resections for Synchronous Colorectal Liver Metastases: A Systematic Review and Network Meta-Analysis. J Clin Med Res. 2019;11:572–582. doi: 10.14740/jocmr3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872–878. doi: 10.1002/bjs.5346. [DOI] [PubMed] [Google Scholar]
- 315.Brouquet A, Mortenson MM, Vauthey JN, Rodriguez-Bigas MA, Overman MJ, Chang GJ, Kopetz S, Garrett C, Curley SA, Abdalla EK. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 316.Andres A, Toso C, Adam R, Barroso E, Hubert C, Capussotti L, Gerstel E, Roth A, Majno PE, Mentha G. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases: a LiverMetSurvey-based study. Ann Surg. 2012;256:772–8; discussion 778-9. doi: 10.1097/SLA.0b013e3182734423. [DOI] [PubMed] [Google Scholar]
- 317.Jegatheeswaran S, Mason JM, Hancock HC, Siriwardena AK. The liver-first approach to the management of colorectal cancer with synchronous hepatic metastases: a systematic review. JAMA Surg. 2013;148:385–391. doi: 10.1001/jamasurg.2013.1216. [DOI] [PubMed] [Google Scholar]
- 318.Lam VW, Laurence JM, Pang T, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of a liver-first approach in patients with colorectal cancer and synchronous colorectal liver metastases. HPB (Oxford) 2014;16:101–108. doi: 10.1111/hpb.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kelly ME, Spolverato G, Lê GN, Mavros MN, Doyle F, Pawlik TM, Winter DC. Synchronous colorectal liver metastasis: a network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J Surg Oncol. 2015;111:341–351. doi: 10.1002/jso.23819. [DOI] [PubMed] [Google Scholar]
- 320.Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey JN, Påhlman L of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729–741. doi: 10.1016/j.ctrv.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 321.Adam R, Bhangui P, Poston G, Mirza D, Nuzzo G, Barroso E, Ijzermans J, Hubert C, Ruers T, Capussotti L, Ouellet JF, Laurent C, Cugat E, Colombo PE, Milicevic M. Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg. 2010;252:774–787. doi: 10.1097/SLA.0b013e3181fcf3e3. [DOI] [PubMed] [Google Scholar]
- 322.Bonney GK, Coldham C, Adam R, Kaiser G, Barroso E, Capussotti L, Laurent C, Verhoef C, Nuzzo G, Elias D, Lapointe R, Hubert C, Lopez-Ben S, Krawczyk M, Mirza DF LiverMetSurvey International Registry Working Group. Role of neoadjuvant chemotherapy in resectable synchronous colorectal liver metastasis; An international multi-center data analysis using LiverMetSurvey. J Surg Oncol. 2015;111:716–724. doi: 10.1002/jso.23899. [DOI] [PubMed] [Google Scholar]
- 323.Mühlbacher F, Huk I, Steininger R, Gnant M, Götzinger P, Wamser P, Banhegyi C, Piza F. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc. 1991;23:1567–1568. [PubMed] [Google Scholar]
- 324.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726–34; discussion 734-5. [PubMed] [Google Scholar]
- 325.Hagness M, Foss A, Line PD, Scholz T, Jørgensen PF, Fosby B, Boberg KM, Mathisen O, Gladhaug IP, Egge TS, Solberg S, Hausken J, Dueland S. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257:800–806. doi: 10.1097/SLA.0b013e3182823957. [DOI] [PubMed] [Google Scholar]
- 326.Dueland S, Guren TK, Hagness M, Glimelius B, Line PD, Pfeiffer P, Foss A, Tveit KM. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg. 2015;261:956–960. doi: 10.1097/SLA.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 327.Hagness M, Foss A, Egge TS, Dueland S. Patterns of recurrence after liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2014;21:1323–1329. doi: 10.1245/s10434-013-3449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Toso C, Pinto Marques H, Andres A, Castro Sousa F, Adam R, Kalil A, Clavien PA, Furtado E, Barroso E, Bismuth H Compagnons Hépato-Biliaires Group. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver Transpl. 2017;23:1073–1076. doi: 10.1002/lt.24791. [DOI] [PubMed] [Google Scholar]
- 329.Dueland S, Syversveen T, Solheim JM, Solberg S, Grut H, Bjørnbeth BA, Hagness M, Line PD. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann Surg. 2020;271:212–218. doi: 10.1097/SLA.0000000000003404. [DOI] [PubMed] [Google Scholar]
- 330.Wyatt J, Hubscher S, Goldin R. Standards and datasets for reporting cancers Dataset for histopathology reporting of liver resection specimens ( including gall bladder ) and liver biopsies for primary and metastatic carcinoma (2nd ed) 2012. Available from: https://www.rcpath.org/uploads/assets/555302b1-8b11-4d8a-a24431d0693d3287/G167-Dataset-for-histopathological-reporting-of-cancer-of-unknown-primary-CUP-and-malignancy-of-unknown-primary-origin-MUO.pdf. [Google Scholar]
- 331.Jones OM, Rees M, John TG, Bygrave S, Plant G. Biopsy of potentially operable hepatic colorectal metastases is not useless but dangerous. BMJ. 2004;329:1045–1046. doi: 10.1136/bmj.329.7473.1045-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.National Institute for Helath and Care Excellence. Metastatic Malignant Disease of Unknown Primary Origin in Adults: Diagnosis and Management. 2010. Available from: https://www.nice.org.uk/Guidance/CG104. [PubMed] [Google Scholar]
- 333.Moreno Prats M, Sasatomi E, Stevenson HL. Colorectal Liver Metastases: A Pathologist's Guide to Creating an Informative Report and Improving Patient Care. Arch Pathol Lab Med. 2019;143:251–257. doi: 10.5858/arpa.2017-0505-RA. [DOI] [PubMed] [Google Scholar]
- 334.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, Chaussade S, Mentha G, Terris B. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 335.Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, O'Donoghue DP, Moriarty M, Fennelly D, Sheahan K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–146. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 336.Li-Chang HH, Driman DK. Comment on: Pathologic Response to Preoperative Chemotherapy in Colorectal Liver Metastases: Fibrosis, Not Necrosis, Predicts Outcome. Ann Surg Oncol. 2017;24:607. doi: 10.1245/s10434-017-6198-3. [DOI] [PubMed] [Google Scholar]
- 337.Shen Z, Gu L, Mao D, Chen M, Jin R. Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:4. doi: 10.1186/s12957-018-1544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Boland PM, Ma WW. Immunotherapy for Colorectal Cancer. Cancers (Basel) 2017;9 doi: 10.3390/cancers9050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Stevenson HL, Prats MM, Sasatomi E. Chemotherapy-induced Sinusoidal Injury (CSI) score: a novel histologic assessment of chemotherapy-related hepatic sinusoidal injury in patients with colorectal liver metastasis. BMC Cancer. 2017;17:35. doi: 10.1186/s12885-016-2998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 341.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 342.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 343.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): a novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 345.Jászai J, Schmidt MHH. Trends and Challenges in Tumor Anti-Angiogenic Therapies. Cells. 2019;8 doi: 10.3390/cells8091102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Mohammed MK, Shao C, Wang J, Wei Q, Wang X, Collier Z, Tang S, Liu H, Zhang F, Huang J, Guo D, Lu M, Liu F, Liu J, Ma C, Shi LL, Athiviraham A, He TC, Lee MJ. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016;3:11–40. doi: 10.1016/j.gendis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Grainger S, Willert K. Mechanisms of Wnt signaling and control. Wiley Interdiscip Rev Syst Biol Med. 2018:e1422. doi: 10.1002/wsbm.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]