Abstract
Trichloroethylene (TCE) is used as a solvent in various industrial processes. During its use, TCE vaporizes and its vapor pollutes the working atmosphere. Its recovery is very important and activated carbon may be used for this purpose. In the present study, experiments were conducted with activated carbon particles for adsorption and desorption of TCE vapor. The adsorption isotherms were measured over a temperature range of 30–100 °C. Also, the effects of particle sizes (dp; 355, 500, and 710 μm), initial concentration of TCE vapor (100, 150, 200, and 250 ppm), and temperature (30, 50, and 100 °C) on the adsorption isotherms of TCE on activated carbon with air as the carrier stream were investigated, which were not reported earlier. From the experimental results, it was found that as the particle size decreases the adsorption capacity increases because of the increase in surface area with decrease in size of particles. The effect of the initial concentration of TCE vapor showed proportionality with adsorption capacity. The increase in temperature showed increase in the adsorption capacity. The adsorption isotherms obtained from the experimental results were compared with model isotherms viz. Langmuir and Freundlich. The Langmuir and Freundlich isotherm models showed accurate fits with R2 values of 0.99067 and 0.99142, respectively, suggesting a hybrid adsorption mechanism involving monolayer and multilayer adsorption. From the desorption study, it was found that the recovery of TCE–vapor from activated carbon is possible, and hence its reuse. This study confirms the suitability of activated carbon as an adsorbent for the removal of TCE vapors emitted from industrial and domestic sources. The details of the experiments and results are discussed in this article.
1. Introduction
The emission of toxic volatile organic compounds (VOCs) into the atmosphere from different industries has become an alarming concern today as it affects not only the environment but also human health. Some VOCs are toxic and carcinogenic in nature, known to cause ozone depletion and contribute to smog formation when combined with other air pollutants.1 Pollution control authorities worldwide have levied stringent emission guidelines to restrict the VOCs released from various industries. Hence, VOC removal using sustainable and energy-efficient techniques has become vital and challenging.2
VOCs viz. benzene, toluene, and many more pose numerous health and environmental risks.3−5 Trichloroethylene (TCE) is a chlorinated VOC, widely used in automobile and paint industries as a solvent to remove grease from metal parts and also as a raw material to make other chemicals, especially the refrigerant HFC-134a. It also finds wide applications in domestic products viz. adhesives, ink remover, and so forth.6,7 Owing to its high solubility in water (1.1 g L–1 at 25 °C), TCE is commonly found in groundwater and soils. TCE is persistent and nonbiodegradable in nature and is known to cause liver and kidney damage in humans. Additionally, it is potentially carcinogenic. Because of these, TCE is listed as a priority pollutant by the environmental protection agency in the US and the European Union.7−9
Several methods have been studied for the effective removal of VOCs in the last few decades.10 Among them, adsorption has been accepted as the most favored process as it is reasonably easy to handle, nontoxic, and inexpensive facilitating the process. The VOC adsorption on activated carbons is highly efficient at low and moderate concentrations.10−12 Activated carbon is considered as one of the most efficient adsorbents for the removal of organic compounds, owing to its high surface area, hydrophobicity, easy availability, extensive pore size distribution, and pore volume.10−13 The objective of this study is to determine the efficiency of activated carbon as an adsorbent for the adsorption and recovery of TCE vapor from the air.
The granular activated carbon is widely used in the air purification systems for the treatment of VOCs emitted from the exhaust of industries and automobiles, as it consists of a larger internal surface and smaller pores.14 Different morphologies of activated carbon such as activated carbon fiber (ACF),15 activated carbon monolith (ACM),16 activated carbon beds (ACB),17 carbon nanotubes (CNT), etc.,18 have been extensively used to study the effective adsorption of TCE and other VOCs in their vapor form. The nonpolar nature of TCE makes it easy to get adsorbed on the hydrophobic and nonpolar activated carbon.19,20 Zhou et al.21 studied the adsorption of SO2, NO, and CO2 on ACF along with the competitive adsorption of the mixture of gases. They observed that the individual SO2 gas showed better adsorption over the other gases. Also, the competitive adsorption reduced the adsorption capacity by half over the individual compound adsorption. Ryu et al.22 carried out the adsorption of toluene and gasoline vapor on activated carbon at different temperatures and pressures. The adsorption capacity for toluene (3.002 mol/Kg) was higher than that for gasoline (2.079 mol/Kg) over the entire temperature and pressure range as the affinity of toluene toward AC was higher than that of gasoline.
Erto et al.23 carried out the adsorption of a binary mixture of TCE/PCE on activated carbon and used a model to examine the behavior. The PCE and TCE showed competitive behavior for adsorption sites where PCE molecules displacing the TCE molecules showed better adsorption in the presence of TCE. Salih et al.24 studied the influence of iron oxide nanoparticles on activated carbon for the adsorption of TCE. Miyake et al.25 studied the adsorption of TCE vapor stripped from groundwater on the ACF as an adsorbent and examined the consequence of the presence of water vapor on the adsorption of TCE. TCE in the presence of N2 as a carrier stream was passed through activated carbon at concentrations (0.6–6.5 mg TCE/L N2). The presence of water vapor largely affected the characteristic energy of adsorption of TCE on the activated carbon but has little effect on the limiting adsorption volume. Karanfil and Dastgheib26 studied the effect of different surface-modified activated carbons on the adsorption of TCE vapor and TCE in the presence of water vapor. The adsorption capacity of TCE vapor was approximately 100 mg/g on macrocarbon AC. They also determined that the adsorption of TCE depends on the physical properties of the adsorbent such as hydrophobicity, pore volume, and pore size distribution.
None of the earlier investigators studied the adsorption isotherms of TCE vapor on activated carbon particles using air as a carrier gas. In the present study, experiments were conducted to investigate the effect of different operating conditions viz. the particle size of activated carbon, initial concentration of TCE–vapor in the air stream, and temperature on the adsorption isotherms. The adsorption isotherms were compared with classical isotherms viz. Freundlich and Langmuir adsorption isotherms to determine the best fit. Also, desorption of TCE–vapor from the activated carbon particles was studied usin TG-DTA to find the regeneration time, optimum temperature for desorption, and degree of recovery.
1.1. Adsorption Isotherms
For the optimum design of an adsorption system and to determine its characteristics, adsorption isotherm is crucial. It is the equilibrium relationship between the amounts of adsorbate adsorbed by a substance, qe (mg g–1), at a given pressure, P (Pa), of the adsorbate.27
Model adsorption isotherms, viz. the Langmuir isotherm and Freundlich isotherm were used for comparing the experimental results to obtain the accurate adsorption isotherms.
1.2. Langmuir Adsorption Isotherm
The Langmuir isotherm assumes the adsorption surface as homogeneous with the monolayer occupying the sites. Also, there is no interaction between the adsorbate molecules. The adsorption occurs until the equilibrium is reached. The nonlinear form of the equation for the Langmuir isotherm is as shown in eq 1.
| 1 |
In eq 1, qe (mg g–1) is the equilibrium adsorption capacity, qm (mg g–1) is the monolayer adsorption capacity of the adsorbent, P (Pa) is pressure, and KL (m3 g–1) is the Langmuir adsorption constant.
Further analysis of the Langmuir adsorption isotherm is done by a dimensionless number, the separation factor, RL. The expression for RL is given as shown in eq 2.
| 2 |
RL is an important factor because it gives the possibility of the occurrence of the reversibility of the adsorption process. C0 is the initial concentration vapor (g/m3). The value of RL = 0 indicates a reversible process and RL = 1 suggests an irreversible process. Thus, the adsorption process is considered as favorable if the value of RL lies between 0 and 1.28
1.3. Freundlich Adsorption Isotherm
The Freundlich isotherm explains the physisorption phenomena assuming multilayer adsorption of molecules on a heterogeneous surface. Also, the model assumes that the energies of the active sites present on the surface are not uniformly distributed. The Freundlich equation is useful at low pressures. The nonlinear equation for Freundlich adsorption isotherm is given using eq 3.
| 3 |
In eq 3, KF is the Freundlich adsorption constant and 1/n gives the measure of adsorption intensity and heterogeneity of the data.29 The high value of KF suggests a larger adsorption capacity of the adsorbent whereas a higher value of n supports in favor of higher adsorption for the respective adsorbate.
2. Materials and Methods
2.1. Materials
Liquid TCE (C2HCl3) was obtained from Merck, with a purity of 99% by mass. The physical properties of TCE are given in Table 1. Commercial grade, granular activated carbon was procured from M/s. SRL Chemicals, Mumbai. According to M/s. SRL Chemicals, Mumbai, the activated carbon was derived from bituminous coal and was steam activated.
Table 1. Physical Properties of TCE9.
| Properties | Value |
|---|---|
| Formula | C2HCl3 |
| Molecular weight (amu) | 131.39 |
| Density (g/cm3) | 1.4642 |
| Solubility in water (mg/L) | 1280 |
| Vapor pressure at 20 °C (mm Hg) | 69 |
| Boiling point (°C) | 87.2 |
2.2. Methods
The following methods were followed in the present experimental study.
2.2.1. Size Reduction and Classification
Size reduction of granular activated carbon was carried out in a ball mill and classifications were carried out using a vibratory sieve shaker with ASTM standard meshes to get three different particle sizes viz., 355, 500, and 710 μm.
2.2.2. Characterization of Activated Carbon Particles
Characterization of all three different particle sizes of activated carbon was carried out using a Brunauer–Emmett–Teller (BET) surface analyzer (make: Thermo-Fisher Scientific, model: Surfer) to obtain the surface area and micropore volume and using a helium pycnometer (make: Thermo-Fisher Scientific, model: Pycnomatic ATC) to obtain the true density of the material. The BET surface area and pore volume of the samples were calculated using N2 at 77 K. The physical properties of the activated carbon particles of activated carbon used in the experimental study are listed in Table 2.
Table 2. Physical Properties of Commercial Activated Carbon Sample Using a N2—BET Surface Area Analyzer.
| Particle size of activated carbon (μm) | BET surface area (m2/g) | Micropore volume (cm3/g) | Real density (g/cm3) |
|---|---|---|---|
| 355 | 1542.778 | 0.315 | 1.8709 |
| 500 | 1233.145 | 0.301 | 1.8709 |
| 710 | 988.354 | 0.286 | 1.8709 |
2.2.3. Adsorption of TCE on Activated Carbon
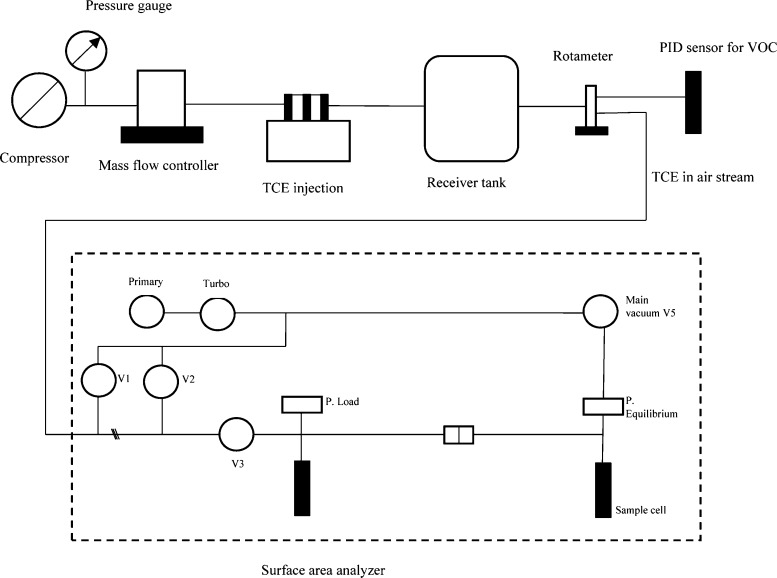
The schematic representation of the experimental setup is shown in Figure 9. The adsorption experiments were carried out using the BET surface area analyzer (make: ThermoFisher, Scientific, model: Surfer) to obtain the adsorption isotherms of the TCE–air mixture on the activated carbon. The adsorption experiments were carried out by the volumetric method in a glass column of 10 mm inner diameter and 160 mm height, containing a fixed amount of activated carbon (50–60 mg). The glass column was connected to the pressure transducers through valves to measure the pressure of the adsorbate gas. TCE mixed with air as a carrier stream was passed through the activated carbon sample in the glass column at increasing pressures of the gas mixture. The internal temperature of the adsorbent on which the adsorption is to be carried out was maintained using a heating mantle. The PT100 sensor recorded the temperature of the system. TCE vapor and air mixture were prepared by mixing dried compressed pure air with a known quantity of TCE. The mixture was allowed to settle (for 1 h) in a receiver storage tank. The concentration of TCE was measured using a calibrated photoionization detector (make: ENDEE, model: multi-channel detector). When a stable reading was observed for a significant amount of time (0.5 h), the TCE was passed into the surface area analyzer setup. The gas was loaded at initial loading pressure and consequently injected into the glass column through the valve. When the required pressure over the sample was reached, the valve was stopped and after a preset time interval, the residual pressure was measured. Similarly, the increasing pressures on the sample were experimentally measured at specified intervals to calculate the amount of TCE adsorbed by the sample until the saturation pressure was reached. The number of moles adsorbed on the sample is computed using the surface area analyzer software from the difference between the initial and final number of moles using the Virial equation for real gases. The adsorption isotherm study was carried on different parameters such as the particle size of activated carbon (710, 500, and 355 μm), initial concentration of TCE in the air (100, 150, 200, and 250 ppm), and temperature of the activated carbon bed (30, 50, and 100 °C) to optimize the adsorption conditions for better efficiency.
Figure 9.
Schematic representation of the experimental setup for the adsorption process (Legends: V1, V2, V3, V4, V5, and V6 are valves, P-load measures the loading pressure of the gas, P-equilibrium measures the equilibrium pressure of the gas in the sample chamber, and Primary and Turbo are the vacuum pumps).
2.2.4. Desorption of TCE from Activated Carbon
Thermogravimetric–differential thermal analysis (TG–DTA) of the exhausted activated carbon sample was carried out using the TG–DTA instrument (Make: Netzsch, Model: STA 449 Jupiter F3) at a 40 mL/min nitrogen flow rate. The simultaneous thermal analyzer NETZSCH STA 449 F3 Jupiter allows the measurement of mass changes and thermal effects between −150 and 2400 °C. The equipment allows high sample loads (up to 35 g) and measurement range (35 g) as well as high resolution (0.1 μg) and low drift. Two easily replaceable identical crucibles were present. The sample was loaded in one of the crucibles and the variation in the mass of the sample along with the difference in temperature between the sample and reference as a function of the time or the temperature was recorded by the associated software when they undergo temperature scanning in a controlled atmosphere. A microbalance is connected with the crucibles. There are two purge ports to provide the desired, controlled atmosphere (inert, oxidizing, reducing, etc.,). The gases that emerge during the analysis were vented out with the continuous flow of this purge gas. The vented out TCE is passed over the PID sensor to measure the intermittent concentration, C. A protective gas stream at higher pressure protects the microbalance from the emerging gases. A thermocouple was used to monitor the temperature accurately. The activated carbon after exhaustion was subjected to TG–DTA analysis.
3. Results and Discussion
The adsorption isotherms were plotted using the surface area analyzer with the amount of TCE adsorbed in mg/g against increasing pressure of the TCE–air mixture. The effects of different process parameters such as the particle size of the adsorbent, initial concentration of TCE in air, and temperature were studied to optimize the adsorption system and find the adsorption capacity of the commercial activated carbon. Additionally, the isotherms were compared with the Freundlich and Langmuir adsorption isotherms to achieve the equilibrium isotherm and find the best fit.
3.1. Effect of Particle Size
To study the effect of particle size of activated carbon on the adsorption capacity, three different particle sizes of activated carbon with average particle diameters 355, 500, and 710 μm were used. Considering the nonpolar nature of TCE, the adsorption of TCE on activated carbon depends on the electrostatic interaction between the TCE molecules and the surface of the activated carbon.30 Thus, the physical properties such as the surface area and micropore volume of the adsorbent will affect the adsorption capacity. Several studies have been carried out on the adsorption of VOCs on activated carbon. The adsorption capacity ranges from a few to several hundred milligrams per gram of activated carbon.20 The experimental conditions were 50 mg mass of the adsorbent, initial concentration of TCE gas of 100 ppm, and a bed temperature of 30 °C to volumetrically evaluate the adsorption capacity. The plot of different adsorption isotherms for the particle size is shown in Figure 1. The relative pressure (P/P0) is represented on the x-axis, which is defined as the ratio of vapor pressure to the saturation pressure of the gas, while the amount of gas adsorbed is represented on the y-axis. P/P0 ranges from 0.05 to 1. For 710 μm, the adsorption capacity achieved was 394.8307 mg/g, whereas for 500 μm it was found to be 413.064 mg/g. However for the finer particle size 355 μm, the adsorption capacity was the highest of 523.252 mg/g. Thus, as the particle size is reduced, an increase in the adsorption capacity was observed. TCE is a highly volatile compound with a small molecular size, hence its adsorption decreases with increasing pore size. This also might be because of the increase in the length of the diffusion path, which results in poor access to the internal micropores of 710 μm particles.31 Furthermore, larger pores might reduce the overlapping adsorption potential that is characteristic of the narrow microporous region, reducing the overall adsorption.32 For the smaller particle size an increased adsorption capacity for TCE was observed because of higher access to micropores. Also, the micropores have narrow pore spaces that allow more TCE molecules to retain in the adsorbent. Larger particles having large pores and thus larger pore spaces will have a lower tendency to retain the TCE molecules.33 The adsorption capacity for the different particle sizes of activated carbon in the decreasing order was 355 μm > 500 μm > 710 μm. Thus, for further experiments, we have used the activated carbon of particle size 355 μm.
Figure 1.
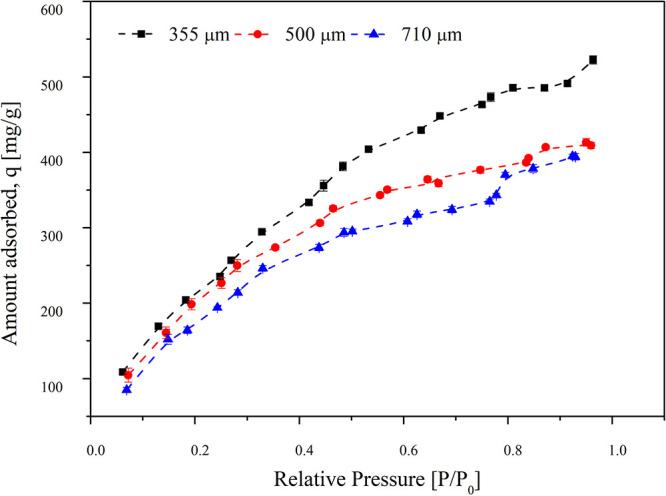
Effect of varying particle sizes of activated carbon on its adsorption capacity for TCE in air. The particle sizes are 355, 500, and 710 μm. The experiments are carried out at 30 °C with 100 ppm initial concentration of TCE in the air (the error bars indicate the standard errors calculated by the pooled standard deviation).
3.2. Effect of Concentration of TCE in the Air at the Inlet
To determine the effect of the initial concentration of TCE in the inlet stream, concentrations of 100, 150, 200, and 250 ppm were studied. The other experimental conditions were maintained with the particle size of 355 μm, a bed temperature of 30 °C, and a weight of adsorbent of 50 mg. The results obtained are shown in Figure 2. The adsorption capacities for inlet concentrations of 100, 150, 200, and 250 ppm were 523.252, 575.844, 593.181, and 624.044 mg/g, respectively. With an increase in the concentration of inlet TCE, at increasing pressures, the adsorption capacity of activated carbon shows a steady increase. The increase in adsorption capacity at higher concentrations with increasing pressure might be because of the sticking of the adjacent molecules with the intralayer molecules in the micropores. It results in the formation of stacks of adjacent TCE molecules adhered to the intralayer molecules on the adsorbent surface.34 TCE molecules have a planar shape with a kinetic diameter of 6.6 Å. Hence, it accumulates on the micropores/mesopores (pore diameter <7 Å) of the activated carbon. With an extensive pore volume in the microporous region characteristic of activated carbon, assuming slit-shaped pore geometry, a high affinity for TCE on the activated carbon was expected.24 Erto et al.30 carried out the adsorption of TCE on activated carbon at low concentrations (0–8000 μg/L) and observed an adsorption capacity of 160 mg/g at room temperature.
Figure 2.
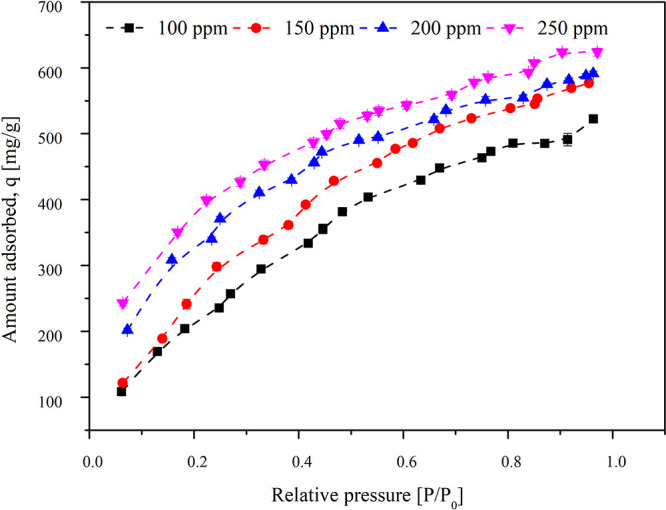
Effect of varying the initial concentration of TCE in the inlet air on the adsorption capacity of activated carbon. The 355 μm particle size of activated carbon was used for the experiment at 30 °C. The concentrations varied are 100, 150, 200, and 250 ppm.
3.3. Effect of Temperature
To study the effect of temperature on adsorption capacity and adsorption efficiency of activated carbon, the experiments were carried out at 30, 50, and 100 °C. The other experimental conditions of a particle size of 355 μm, an initial concentration of TCE in the inlet of 100 ppm, and a weight of the sample of 50 mg were maintained. The results are shown in Figure 3. Increasing the adsorption temperature to 50 °C, an insignificant change was observed at low pressure. The adsorption capacity at equilibrium for an experiment carried out at 50 °C is 489.104 mg/g. Adsorption at 100 °C, however, showed a substantial decrease in adsorption capacity. The maximum adsorption capacity of the activated carbon at 100 °C was 262.245 mg/g. Physical adsorption is an exothermic process; thus, an increase in bed temperature is often accompanied by reduced adsorption capacity. Hence, at any given relative pressure, the adsorption reduces with increasing temperature as heat is evolved during the process and the energy of adsorbate molecules increases. The gas thus migrates back to the gas phase overcoming the Van der Waals’ force. This makes it difficult for the adsorbate to get adsorbed on the surface of the adsorbent as the gases tend to stay in the gaseous state at high pressure.20 Hence, low temperature is favorable for adsorption of TCE.21 The adsorption capacity of activated carbon at 30 °C was observed to be around 523.256 mg/g.
Figure 3.
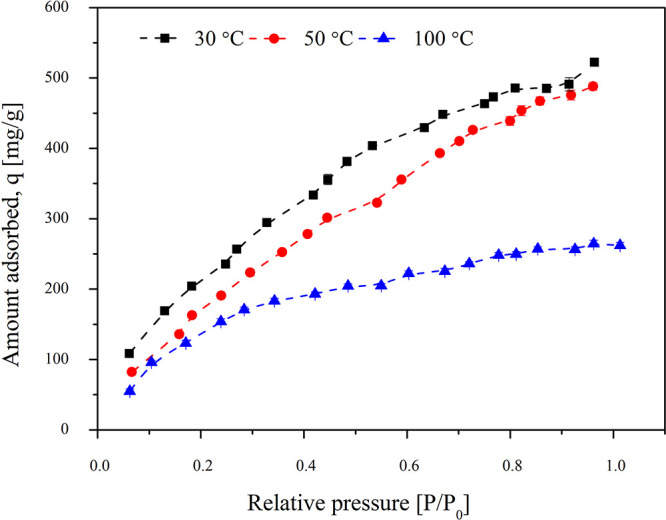
Effect of different adsorption temperatures on the adsorption capacity of the activated carbon. The inlet concentration of TCE in air was 100 ppm and the particle size of activated carbon was 355 μm. The experiments were varied at temperatures 30, 50, and 100 °C.
3.4. Adsorption Isotherms Fitted with Model Isotherms
The adsorption isotherm models were fit into the experimental data to obtain a better understanding of the adsorption of TCE on activated carbon. The nonlinear regression analysis was performed. The adsorption isotherm obtained from the experiments was analyzed using the model isotherm equations of the Langmuir and Freundlich isotherms. The nonlinear plots of Freundlich and Langmuir adsorption isotherms are shown in Figure 4a,b. The values of isotherm parameters obtained from the regression analysis are shown in Table 3. As seen in Figures 4a,b, both the Langmuir isotherm and the Freundlich isotherm were in agreement with the experimental plot, with the R2 values of 0.99142 and 0.99067, respectively. The Langmuir isotherm plot slightly over predicted the adsorption capacity with a standard error of 29.7801%. The Freundlich adsorption isotherm model provided a better value of adsorption capacity of activated carbon. Hence, the occurrence of a hybrid system comprising of monolayer and multilayer adsorption could be possible. A fraction of the active sites available may demonstrate multiple nature of adsorption. Similar results were obtained by Yang et al.35 The KF value of 543.245 mg/g showing strong adsorption capacity and the value of n of 1.789 confirm the physisorption-induced nature of adsorption and high affinity of TCE toward activated carbon. Erdogan and Kopac36 carried out the adsorption of organic vapors on activated carbon prepared using Turkish Kozlu bituminous coal by physical and chemical activation at high temperatures. The highest values of KL = 0.718 (g/g) and KF = 0.766 (g/g) were obtained for adsorption of acetone vapors on the chemically activated carbon using KOH at 800 °C. Chemical activation at higher temperature showed enhanced porosity and adsorption capacity of activated carbon. They carried out similar studies using Zonguldak–Karadon coal chemically activated using KOH, NaOH, ZnCl2, and H3PO4 to determine the effect of the chemical activation on the extent of adsorption. Chemically activated coal samples showed the highest adsorption capacity of 0.63 g/g for acetone,37 0.554 g/g for isopropyl alcohol, and 1.181 g/g for ethyl alcohol.38
Figure 4.
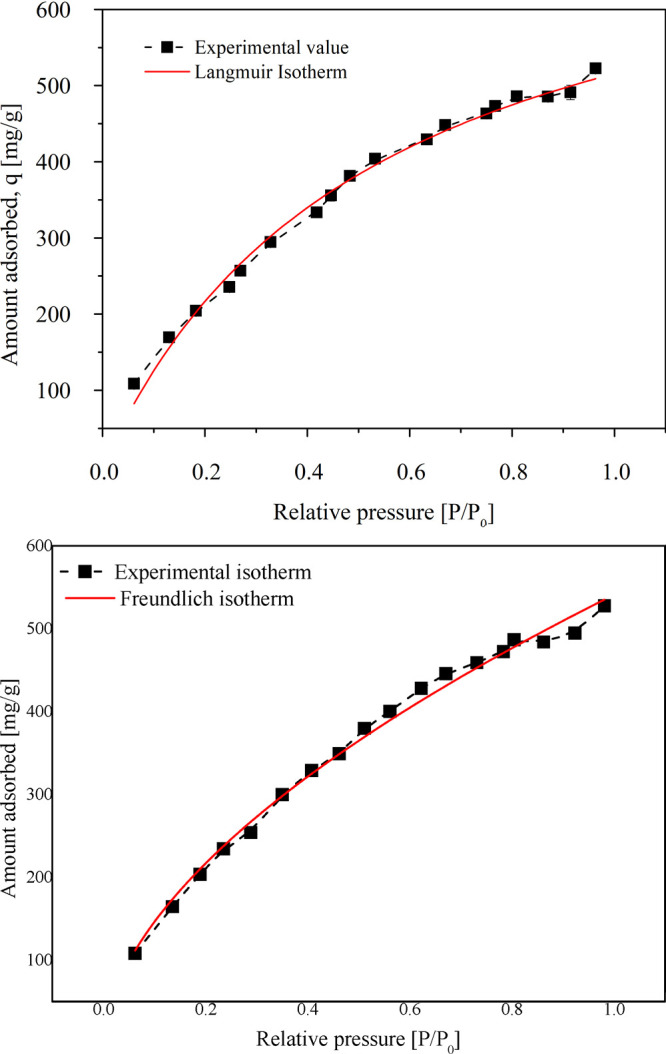
(a) Adsorption isotherm of 100 ppm TCE in the air on 355 μm particle size activated carbon at 30 °C fitted using the Langmuir adsorption isotherm model. (b) Adsorption isotherm of 100 ppm TCE in the air on 355 μm particle size activated carbon at 30 °C fitted using the Freundlich adsorption isotherm model.
Table 3. Values for Isotherm Model Data Fit for the Experimental Data.
| Isotheres | Parameters | Value | Standard Error |
|---|---|---|---|
| Langmuir isotherm | R2 | 0.99067 | |
| qm (mg/g) | 787.711 | 29.78234 | |
| KL (m3/mg) | 1.89552 | 0.14988 | |
| Freundlich isotherm | R2 | 0.99142 | |
| KF [(mg/g) (m3/mg)1/n] | 543.24561 | 6.3314 | |
| n | 1.78932 | 0.05245 |
3.5. Desorption of Adsorbed TCE Vapor
It was observed that all the samples were completely desorbed within 30–35 min. The complete desorption was achieved at a temperature of approximately 350 °C.
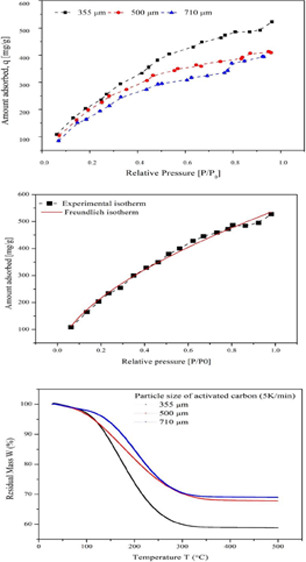
Figure 5 shows the desorption of different concentrations of TCE–vapor-loaded activated carbon samples. The samples with a higher concentration of adsorbed TCE (250 ppm) showed faster desorption. However, the desorption curve became less steep as the concentration of TCE adsorbed decreased. Also, the time required for complete desorption was comparatively higher for the lower concentration adsorbed sample. The weak forces between the TCE molecules and the carbon surface resulted in the fast and easy desorption, which suggests the physical nature of adsorption.38 The desorption of TCE at higher temperatures may cause some oxidation of TCE, which might contaminate the TCE affecting its reusability.39 The TPD curves are shown in Figure 6 depicting the effect of particle size of the adsorbent on the residual mass of TCE. The particle size 355 μm showed higher mass loss (49.093%) than the other two particle sizes (32.295 and 31.103%, respectively) because of the increase in the micropore volume. As the adsorption capacity was higher for 355 μm AC, the desorption was expected to be higher. The complete weight loss for the three samples was obtained below 350 °C. The desorption temperature mainly depends on the boiling point of the VOCs and their volatility. Also, for a higher concentration of TCE in the sample, the temperature and time required for complete desorption will be high.40,41
Figure 5.
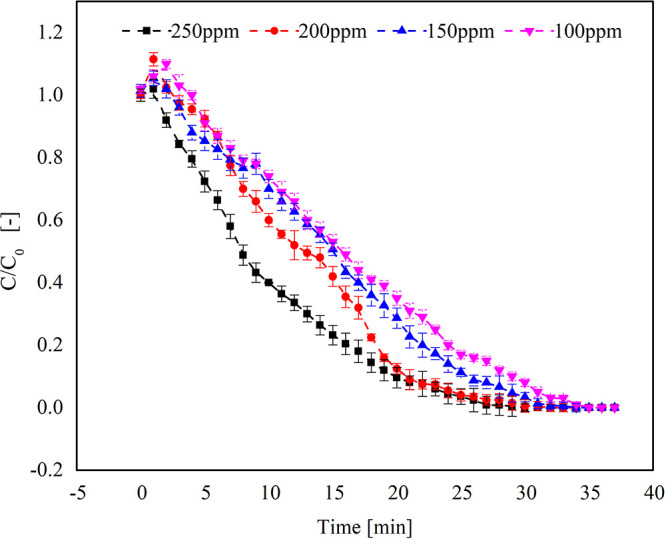
Desorption of the activated carbon sample loaded with different concentrations of TCE.
Figure 6.
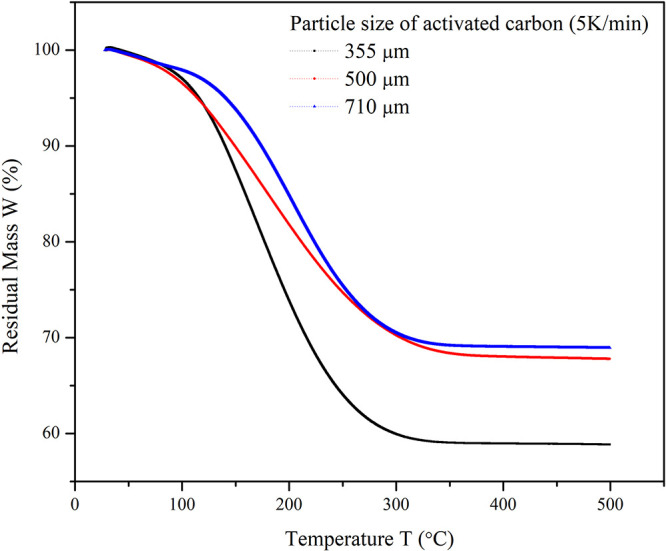
TPD curve of the residual mass of TCE on activated carbon of particle sizes 355, 500, and 710 μm.
Figure 7 shows that no significant effect was observed with the change in the purging gas. Nitrogen, argon, and carbon dioxide gases were used to study the effect on the residual mass. The effect of the heating rate of desorption is shown in Figure 8. It was observed that the heating rate has less impact on the desorption rate. From the analysis of the initial and residual mass of activated carbon particles, it was found that complete recovery of TCE vapor is possible by using activated carbon particles.
Figure 7.
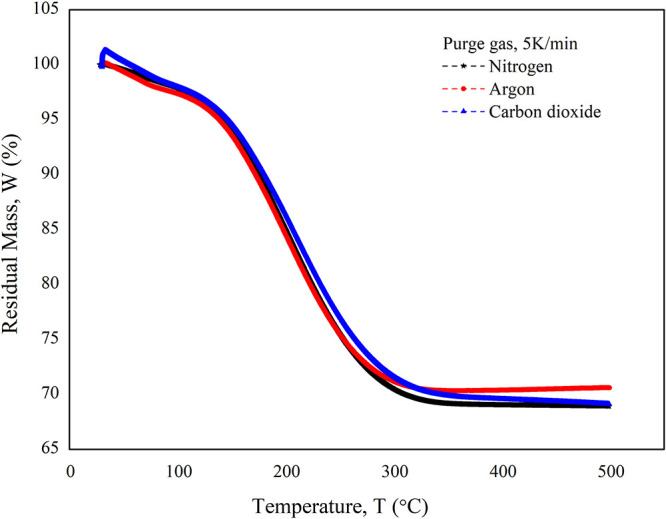
Effect of different purging gases on the TPD curve.
Figure 8.
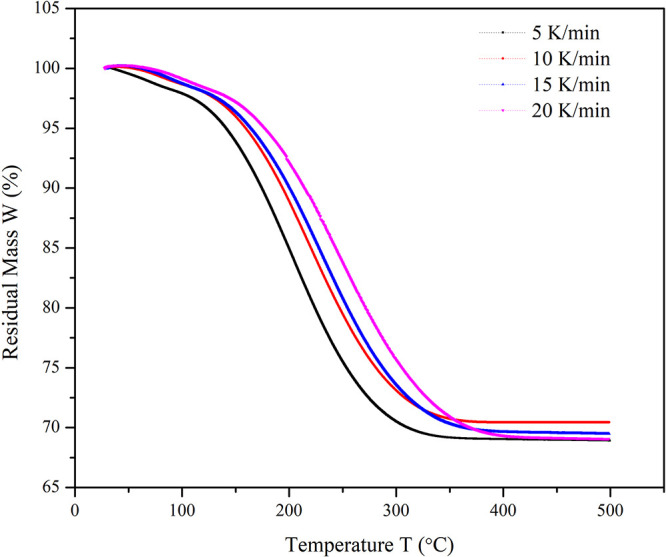
TPD curve of the residual mass of TCE on activated carbon at an increasing heating rate (5–20 K/min).
4. Conclusions
The adsorption and desorption characteristics of TCE vapor on and from activated carbon were studied. It was observed that the physicochemical as well as the operating conditions affect the adsorption capacity of activated carbon. The effect of particle size (and also the surface area) on the adsorption capacity of activated carbon was also studied. It was observed that the reduced particle size showed better adsorption owing to enhanced access to micropore volume. An increase in the initial concentration of TCE in the air at the inlet increased the adsorption capacity showing that the available pore volume of the commercial activated carbon is satisfactory for TCE removal at low initial concentrations. The increase in the temperature showed a decrease in the adsorption capacity of activated carbon, confirming the exothermic nature of adsorption. The adsorption capacity for activated carbon at 100 °C was observed to be the minimum. Finally, the results were analyzed using different model adsorption isotherms, such as Langmuir and Freundlich isotherms, where the Freundlich isotherm showed the best regression fit. The RL value calculated using eq 2 was of the order of 5 × 10–3, thus indicating the adsorption process is reversible. Also, the time (35 min) and temperature (350 °C) necessary for the complete desorption and regeneration of the activated carbon was established.
Moreover, from the desorption of adsorbed TCE vapor from activated carbon particles based on the TG–DTA analysis, it was found that complete recovery of TCE vapor is possible by using activated carbon particles. Thus, the bed of activated carbon particles is very much effective for the recovery of TCE vapor from its working atmosphere to maintain the vapor concentration within the permissible limit and maintain a clean environment as well as to prevent loss of the solvent by completely desorbing the adsorbed TCE vapor.
In the future, we will carry out the adsorption of TCE on a fixed and fluidized bed of activated carbon particles. Recently, hydrodynamics of activated carbon particles of different sizes and at different temperatures in gas–solid-fluidized bed were studied.12 The adsorption and desorption of TCE on activated carbon in cycles will be studied to evaluate its effect on the activation of the carbon sample. Also, the analysis of desorbed TCE will be carried out to assess the reusability of TCE obtained.
Acknowledgments
The authors sincerely thank the staff members of the Materials Development Section of the Alkali Metals and Material Division (AMMD), Bhabha Atomic Research Center (BARC), Mumbai for their support during the experimental works.
The authors declare no competing financial interest.
References
- Yang C.; Miao G.; Pi Y.; Xia Q.; Wu J.; Li Z.; Xiao J. Abatement of Various Types of VOCs by Adsorption/Catalytic Oxidation: A Review. Chem. Eng. J. 2019, 370, 1128–1153. 10.1016/j.cej.2019.03.232. [DOI] [Google Scholar]
- Petrusovà Z.; Machanovà K.; Karolina S. Separation of Organic Compounds from Gaseous Mixtures by Vapor Permeation. Sep. Purif. Technol. 2019, 217, 95–107. 10.1016/j.seppur.2019.02.028. [DOI] [Google Scholar]
- Sui H.; Liu H.; An P.; He L.; Li X.; Cong S. Application of Silica Gel in Removing High Concentrations Toluene Vapor by Adsorption and Desorption Process. J. Taiwan Inst. Chem. Eng. 2017, 74, 218–224. 10.1016/j.jtice.2017.02.019. [DOI] [Google Scholar]
- Gironi F.; Piemonte V. VOCs Removal from Dilute Vapour Streams by Adsorption onto Activated Carbon. Chem. Eng. J. 2011, 172, 671–677. 10.1016/j.cej.2011.06.034. [DOI] [Google Scholar]
- Zhang G.; Liu Y.; Zheng S.; Hashisho Z. Adsorption of Volatile Organic Compounds onto Natural Porous Minerals. J. Hazard. Mater. 2019, 364, 317–324. 10.1016/j.jhazmat.2018.10.031. [DOI] [PubMed] [Google Scholar]
- Nakano Y.; Hua L. Q.; Nishijima W.; Shoto E.; Okada M. Biodegradation of Trichloroethylene (TCE) Adsorbed on Granular Activated Carbon (GAC). Water Res. 2000, 34, 4139–4142. 10.1016/S0043-1354(00)00199-8. [DOI] [Google Scholar]
- Wei Z.; Seo Y. Trichloroethylene (TCE) Adsorption Using Sustainable Organic Mulch. J. Hazard. Mater. 2010, 181, 147–153. 10.1016/j.jhazmat.2010.04.109. [DOI] [PubMed] [Google Scholar]
- Zou W.; Gao B.; Ok Y. S.; Dong L. Integrated Adsorption and Photocatalytic Degradation of Volatile Organic Compounds (VOCs) Using Carbon-Based Nanocomposites: A Critical Review. Chemosphere 2019, 218, 845–859. 10.1016/j.chemosphere.2018.11.175. [DOI] [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile: Trichloroethylene (TCE). https://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=173&tid=30 (accessed Aug 31, 2020).
- Lillo-Ródenas M. A.; Cazorla-Amorós D.; Linares-Solano A. Behaviour of Activated Carbons with Different Pore Size Distributions and Surface Oxygen Groups for Benzene and Toluene Adsorption at Low Concentrations. Carbon 2005, 43, 1758–1767. 10.1016/j.carbon.2005.02.023. [DOI] [Google Scholar]
- Ghoshal A. K.; Manjare S. D. Selection of Appropriate Adsorption Technique for Recovery of VOCs: An Analysis. J. Loss Prev. Process. Ind. 2002, 15, 413–421. 10.1016/S0950-4230(02)00042-6. [DOI] [Google Scholar]
- Nikam S.; Mandal D. A Study on Fluidization of Activated Carbon Particles in Gas-Solid Fluidized Bed. Ind. Eng. Chem. 2020, 10.1080/00194506.2020.1803149. [DOI] [Google Scholar]
- Lillo-Ródenas M. A.; Fletcher A. J.; Thomas K. M.; Cazorla-Amorós D.; Linares-Solano A. Competitive Adsorption of a Benzene–Toluene Mixture on Activated Carbons at Low Concentration. Carbon 2006, 44, 1455–1463. 10.1016/j.carbon.2005.12.001. [DOI] [Google Scholar]
- Bansal R. C.; Goyal M.. Activated Carbon Adsorption; CRC Press, 2005. [Google Scholar]
- Kumita M.; Yamawaki N.; Shinohara K.; Higashi H.; Kodama A.; Kobayashi N.; Seto T.; Otani Y. Methanol Adsorption Behaviors of Compression-Molded Activated Carbon Fiber with PTFE. Int. J. Refrig. 2018, 94, 127–135. 10.1016/j.ijrefrig.2018.07.036. [DOI] [Google Scholar]
- Silas K.; Ghani W. A. W. A. K.; Choong T. S. Y.; Rashid U. Activated Carbon Monolith Co3O4 Based Catalyst: Synthesis, Characterization and Adsorption Studies. Environ. Technol. Innov. 2018, 12, 273–285. 10.1016/j.eti.2018.10.008. [DOI] [Google Scholar]
- Jahandar Lashaki M.; Atkinson J. D.; Hashisho Z.; Phillips J. H.; Anderson J. E.; Nichols M. The Role of Beaded Activated Carbon’s Pore Size Distribution on Heel Formation during Cyclic Adsorption/Desorption of Organic Vapors. J. Hazard. Mater. 2016, 315, 42–51. 10.1016/j.jhazmat.2016.04.071. [DOI] [PubMed] [Google Scholar]
- Bosnick K.; Ban S.; Hiebert W.; Shi Z.; Huang C.; Lister R.; Mleczko M. Organic Vapor Adsorption on in Situ Grown Carbon Nanotube Films. Carbon 2011, 49, 3639–3644. 10.1016/j.carbon.2011.04.067. [DOI] [Google Scholar]
- Luo L.; Ramirez D.; Rood M. J.; Grevillot G.; Hay K. J.; Thurston D. L. Adsorption and Electrothermal Desorption of Organic Vapors Using Activated Carbon Adsorbents with Novel Morphologies. Carbon 2006, 44, 2715–2723. 10.1016/j.carbon.2006.04.007. [DOI] [Google Scholar]
- Zhang X.; Gao B.; Creamer A. E.; Cao C.; Li Y. Adsorption of VOCs onto Engineered Carbon Materials: A Review. J. Hazard. Mater. 2017, 338, 102–123. 10.1016/j.jhazmat.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Yi H.; Tang X.; Deng H.; Liu H. Thermodynamics for the Adsorption of SO2, NO and CO2 from Flue Gas on Activated Carbon Fiber. Chem. Eng. J. 2012, 200–202, 399–404. 10.1016/j.cej.2012.06.013. [DOI] [Google Scholar]
- Ryu Y.-K.; Lee H.-J.; Yoo H.-K.; Lee C.-H. Adsorption Equilibria of Toluene and Gasoline Vapors on Activated Carbon. J. Chem. Eng. Data 2002, 47, 1222–1225. 10.1021/je020044i. [DOI] [Google Scholar]
- Erto A.; Lancia A.; Musmarra D. A Modelling Analysis of PCE/TCE Mixture Adsorption Based on Ideal Adsorbed Solution Theory. Sep. Purif. Technol. 2011, 80, 140–147. 10.1016/j.seppur.2011.04.021. [DOI] [Google Scholar]
- Salih H. H.; Patterson C. L.; Sorial G. A.; Sinha R.; Krishnan R. The Implication of Iron Oxide Nanoparticles on the Removal of Trichloroethylene by Adsorption. Chem. Eng. J. 2012, 193–194, 422–428. 10.1016/j.cej.2012.03.040. [DOI] [Google Scholar]
- Miyake Y.; Sakoda A.; Yamanashi H.; Kaneda H.; Suzuki M. Activated Carbon Adsorption of Trichloroethylene (TCE) Vapor Stripped from TCE-Contaminated Water. Water Res. 2003, 37, 1852–1858. 10.1016/S0043-1354(02)00564-X. [DOI] [PubMed] [Google Scholar]
- Karanfil T.; Dastgheib S. A. Trichloroethylene Adsorption by Fibrous and Granular Activated Carbons: Aqueous Phase, Gas Phase, and Water Vapor Adsorption Studies. Environ. Sci. Technol. 2004, 38, 5834–5841. 10.1021/es0497936. [DOI] [PubMed] [Google Scholar]
- Yan F.; Chu Y.; Zhang K.; Zhang F.; Bhandari N.; Ruan G.; Dai Z.; Liu Y.; Zhang Z.; Kan A. T.; Tomson M. B. Determination of Adsorption Isotherm Parameters with Correlated Errors by Measurement Error Models. Chem. Eng. J. 2015, 281, 921–930. 10.1016/j.cej.2015.07.021. [DOI] [Google Scholar]
- Langmuir I. The Adsorption Of Gases On Plane Surfaces Of Glass, Mica And Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. 10.1021/ja02242a004. [DOI] [Google Scholar]
- Karanfil T.; Kilduff J. E. Role of Granular Activated Carbon Surface Chemistry on the Adsorption of Organic Compounds. 1. Priority Pollutants. Environ. Sci. Technol. 1999, 33, 3217–3224. 10.1021/es981016g. [DOI] [Google Scholar]
- Erto A.; Andreozzi R.; Di Natale F.; Lancia A.; Musmarra D. Experimental and Statistical Analysis of Trichloroethylene Adsorption onto Activated Carbon. Chem. Eng. J. 2010, 156, 353–359. 10.1016/j.cej.2009.10.034. [DOI] [Google Scholar]
- Silvestre-Albero A.; Silvestre-Albero J.; Sepúlveda-Escribano A.; Rodríguez-Reinoso F. Ethanol Removal Using Activated Carbon: Effect of Porous Structure and Surface Chemistry. Microporous Mesoporous Mater. 2009, 120, 62–68. 10.1016/j.micromeso.2008.10.012. [DOI] [Google Scholar]
- Su Y.-f.; Cheng Y.-l.; Shih Y.-h. Removal of Trichloroethylene by Zerovalent Iron/Activated Carbon Derived from Agricultural Wastes. J. Environ. Manage. 2013, 129, 361–366. 10.1016/j.jenvman.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Kutluay S.; Baytar O.; Şhin Ö. Equilibrium, Kinetic and Thermodynamic Studies for Dynamic Adsorption of Benzene in Gas Phase onto Activated Carbon Produced from Elaeagnus Angustifolia Seeds. Int. J. Chem. Environ. Eng. 2019, 7, 102947. 10.1016/j.jece.2019.102947. [DOI] [Google Scholar]
- Mohan N.; Kannan G. K.; Upendra S.; Subha R.; Kumar N. S. Breakthrough of Toluene Vapours in Granular Activated Carbon Filled Packed Bed Reactor. J. Hazard. Mater. 2009, 168, 777–781. 10.1016/j.jhazmat.2009.02.079. [DOI] [PubMed] [Google Scholar]
- Yang X.; Yi H.; Tang X.; Zhao S.; Yang Z.; Ma Y.; Feng T.; Cui X. Behaviors and Kinetics of Toluene Adsorption-desorption on Activated Carbons with Varying Pore Structure. J. Environ. Sci. 2018, 67, 104–114. 10.1016/j.jes.2017.06.032. [DOI] [PubMed] [Google Scholar]
- Erdogan F. O.; Kopac T.. Highly Effective Activated Carbons from Turkish–Kozlu Bituminous Coal by Physical and KOH Activation and Sorption Studies with Organic Vapors. Int. J. Chem. React. Eng. 2019, 17, ( (5), ). 10.1515/ijcre-2018-0071. [DOI] [Google Scholar]
- Erdogan F.; Kopac T.. Investigation of acetone adsorption characteristics of activated carbons obtained from Zonguldak-Karadon coal at room temperature. 2020. 352211. 10.17341/gazimmfd.686415. [DOI] [Google Scholar]
- Erdoğan F. O.; Kopac T.. Adsorption Behavior of Alcohol Vapors on Zonguldak-Karadon Coal Derived Porous Carbons. 2019. 10.1080/15567036.2019.1666191. [DOI] [Google Scholar]
- Popescu M.; Joly J. P.; Carré J.; Danatoiu C. Dynamical Adsorption and Temperature-Programmed Desorption of VOCs (Toluene, Butyl Acetate and Butanol) on Activated Carbons. Carbon 2003, 41, 739–748. 10.1016/S0008-6223(02)00391-3. [DOI] [Google Scholar]
- Son H. K.; Sivakumar S.; Rood M. J.; Kim B. J. Electrothermal Adsorption and Desorption of Volatile Organic Compounds on Activated Carbon Fiber Cloth. J. Hazard. Mater. 2016, 301, 27–34. 10.1016/j.jhazmat.2015.08.040. [DOI] [PubMed] [Google Scholar]
- Ushiki I.; Kikuchi K.; Takahashi N.; Sato Y.; Ito Y.; Inomata H. Desorption Behavior of Various Volatile Organic Compounds from Activated Carbon in Supercritical Carbon Dioxide: Measurement and Kinetic Modeling. J. Supercrit. Fluids 2017, 121, 41–51. 10.1016/j.supflu.2016.11.007. [DOI] [Google Scholar]