Abstract
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy and is increasing in incidence. Long-term outcomes are optimized when patients undergo margin-negative resection followed by adjuvant chemotherapy. Unfortunately, a significant proportion of patients present with locally advanced, unresectable disease. Furthermore, recurrence rates are high even among patients who undergo surgical resection. The delivery of systemic and/or liver-directed therapies prior to surgery may increase the proportion of patients who are eligible for surgery and reduce recurrence rates by prioritizing early systemic therapy for this aggressive cancer. Nevertheless, the available evidence for neoadjuvant therapy in ICC is currently limited yet recent advances in liver directed therapies, chemotherapy regimens, and targeted therapies have generated increasing interest its role. In this article, we review the rationale for, current evidence for, and ongoing research efforts in the use of neoadjuvant therapy for ICC.
Keywords: Biliary tract cancer, Preoperative therapy, Conversion therapy, Down-staging, Hepatectomy, Liver resection
Core Tip: Liver resection is the primary component of curative-intent treatment for patients with localized intrahepatic cholangiocarcinoma (ICC). However, a majority of patients present with locally advanced disease and even those who undergo resection are at high risk of recurrence. Neoadjuvant therapy may successfully downstage a subset of patients to resectable disease and improve the long-term outcomes of patients treated with multimodality therapy. As such, the benefits of neoadjuvant treatment strategies aimed at down-staging the tumor and increasing resection rates are of great interest. While high-level evidence regarding the efficacy of neoadjuvant therapy in ICC is lacking, emerging evidence from case control series, as well as recent advances in systemic therapies, liver-directed treatments, and targeted therapies based on an improved understanding of cholangiocarcinogenesis have led to increasing interest in its use.
INTRODUCTION
Cholangiocarcinomas (CCA) are a heterogeneous type of biliary tract cancer (BTC) arising from the epithelial cells of the intrahepatic and extrahepatic biliary tracts[1]. CCAs are classified as distal bile duct, perihilar, or intrahepatic based on their anatomic location[2]. Intrahepatic cholangiocarcinoma (ICC) arises distal to the secondary biliary radicals and comprise approximately 20% of all CCAs. ICCs have distinct molecular, anatomic, clinical, and prognostic characteristics compared with other BTCs. Although relatively rare, the incidence of ICC has been increasing over the past decade and ICC is currently the second most common type of primary liver cancer. The optimal management of ICC includes surgical resection; unfortunately, a majority of patients will present with metastatic or locally advanced disease and are therefore not candidates for surgery. Even those patients with localized disease who undergo margin-negative resection are at high risk for recurrence, highlighting the need for effective systemic therapies. While the optimal systemic therapy given following resection (i.e., adjuvant therapy) continues to evolve, there is growing interest in the use of neoadjuvant therapy (NT) for ICC. Such strategies may effectively downstage patients with locally advanced disease in order to achieve surgical resection while prioritizing the early and guaranteed delivery of systemic therapy in order to improve long-term oncologic outcomes for this aggressive cancer. Recent advances in the molecular understanding of ICC, as well as the development of effective systemic and liver-directed therapies, have also increased interest in the use of NT. In this study, we review the rationale for, evidence of, and ongoing research efforts in the use of NT for ICC.
MANAGEMENT OF ICC
Surgical resection
Surgical resection remains the only treatment option with curative intent in the management of ICC[3,4]. Unfortunately, only about 20%-30% of patients present with resectable disease. Given that most patients present with advanced disease, patient selection is critical to ensure that patients will benefit from surgery. All patients require comprehensive evaluation along three domains: Anatomic, biologic, and physical condition. Patients must have appropriate performance status to undergo major liver surgery without prohibitive medical comorbidities. Complete staging with cross-sectional imaging and tumor markers should be performed to ensure the absence of metastatic disease. In general, distant, contralateral hepatic, peritoneal, and lymph node metastases beyond the porta hepatis are contraindications to resection[5]. As such, some guidelines recommend a diagnostic laparoscopy prior to resection[5,6]. Dedicated, liver-protocol, contrast-enhanced imaging is needed to assess resectability. In general, a technically safe hepatic resection requires a future liver remnant (FLR) defined as at least two contiguous liver segments with intact arterial and portal venous inflow, intact hepatic vein outflow and intact biliary drainage. In general, an FLR of at least 20% is necessary for patients with normal liver function. For patients undergoing neoadjuvant chemotherapy or those with significant steatosis, an FLR of at least 30% is required. And for patients with underlying liver cirrhosis, an FLR of at least 40% is required[7]. FLR is traditionally calculated by volumetric analysis using CT, MRI, or Scintigraphy. Several strategies can be employed to improve the FLR, including ligation of the feeding portal vessels or embolization[8,9].
Like most solid organ tumors, obtaining a margin negative (R0) resection is an important oncologic goal and one of the few metrics potentially under control of the surgeon. In a small series of 50 patients with locally advanced tumors by Lang et al[10], the median survival after R0 resection was 46 mo vs 5 mo for R1 resection[10]. In a larger series of 224 patients, Yeh et al[11] reported a median survival of 26.1 mo and 1-year, 3-year, and 5-year OS of 78.5%, 43.3%, and 28.6%, respectively in patients who underwent R0 resection vs a median survival of 11.4, and 1-year, 3-year, and 5-year OS of 47.5%, 6.8%, and 4.5% for patients who had an R1 resection. The results are even worse for R2 resection in which the median survival was 5.8 mo and 1-year, 3-year, and 5-year OS was 24.0 %, 6.0%, and 0%, respectively[11]. These results have been confirmed in larger studies and meta-analyses[12,13]. While an R0 resection margin should clearly be the goal of surgery, the optimal margin width remains controversial with some data suggesting a wider margin (≥ 10 mm) is associated with improved outcomes[14-18]. Portal lymphadenectomy is also recommended as part of the surgical approach to ICC. Lymphadenectomy is essential for accurate staging, determining prognosis, and guiding the use of adjuvant therapies[19,20]. Removal of at least six lymph nodes is recommended by the National Comprehensive Cancer Network[21], although this is commonly not achieved[20]. Limited data support the use of minimally invasive approaches at experienced centers and the use of vascular resection, when indicated, in order to achieve negative margins.
Adjuvant therapy
Even among patients who undergo a complete macro- and microscopic margin negative resection, patients can still experience a high incidence of recurrence. Indeed, ICC is an aggressive malignancy, and overall survival remains poor[22]. For this reason, there has been a longstanding interest in the development of effective adjuvant therapies to reduce cancer recurrence. Fortunately, several recent prospective trials have provided new data on this controversial issue. The French PRODIGE 12–ACCORD 18 Trial was a phase III randomized trial that randomized patients to adjuvant chemotherapy (Gemcitabine/Oxaliplatin, GEMOX) vs observation following R0 or R1 resection of BTCs (43% ICC). The investigators reported no difference in overall survival or relapse-free survival[23]. The BILCAP trial randomized patients with resected ICC to capecitabine or observation following an R0/R1 resection. In this trial, 19% of the patients had ICC, 38% of whom had R1 resection, and 47% had nodal metastases. Patients who received adjuvant capecitabine experienced improved median overall survival (51.1 mo vs 36.4 mo)[24]. The ACTICCA-1 trial[25] and JCOG1202[26] are currently ongoing. Adjuvant radiotherapy and transarterial chemoembolization (TACE) have also been investigated, but the data are still lacking[27-31]. Based on these data, current guidelines recommend the use of adjuvant capecitabine following resection of any BTC, including ICC.
Metastatic disease
Unfortunately, most patients with ICC present with metastatic disease as many will not develop symptoms until an advanced stage. As surgical resection is not appropriate in the setting of metastatic disease, treatment goals focus on improving local control, treating symptoms, and extending survival. To that end, various systematic chemotherapy regimens and liver-directed therapies have been tried with varying success[32]. The ABC-02 trial was a prospective randomized trial of 410 patients with locally advanced or metastatic BTCs (intrahepatic or extrahepatic cholangiocarcinoma, gallbladder cancer, or ampullary carcinoma) who were randomized to cisplatin plus gemcitabine (Gem-Cis) or gemcitabine alone. Patients treated with Gem-Cis had significantly better overall survival and progression-free survival, establishing this doublet regimen as the standard chemotherapy for advanced BTC[33]. Recent trials have explored triplet gemcitabine-cisplatin-nab-paclitaxel, although further data are needed[34]. Several mutations in IL-6, ErbB2, K-ras, BRAF, and COX-2, p53, P16, cyclin D1, and DNA repair enzymes have all been linked to ICC and provide a basis for targeted therapies. Most of the morbidity and mortality among patients with metastatic ICC is due to liver disease and liver failure. Therefore, the selective use of liver-directed therapies, even in the presence of metastatic disease, may improve local disease control, health related quality of life, and potentially overall survival[35].
RATIONALE FOR NEOADJUVANT THERAPY
While the use of NT has been increasing in other common cancers, its use in ICC remains relatively rare[36-39]. Thus, a sound rationale for the use of NT in BTCs is necessary as empirical data accrue (Table 1). As previously detailed, only a minority of patients presenting with ICC are eligible for resection; fewer patients successfully undergo an R0 resection. Yet the ability to achieve margin-negative resection is a primary determinant of long term survival outcomes[11,12,40,41]. Therefore, a major impetus for pursuing NT is the potential to downstage locally advanced cancers and convert to resectable disease (Figure 1). Thus, NT given for this purpose is commonly referred to as downstaging or conversion therapy[42].
Table 1.
Rationale for the use of neoadjuvant therapy in intrahepatic cholangiocarcinoma
| Rationale for the use of neoadjuvant therapy in ICC | |
| 1 | Downstaging of locally advanced tumors |
| 2 | Improve margin-negative resection rate |
| 3 | Increase receipt of systemic therapy given challenges in delivering postoperative chemotherapy |
| 4 | Prioritize the early systemic treatment of potential micrometastatic disease |
| 5 | Enhance patient selection for major surgery |
| 6 | Facilitate an in vivo test of chemotherapy’s effectiveness |
ICC: Intrahepatic cholangiocarcinoma.
Figure 1.
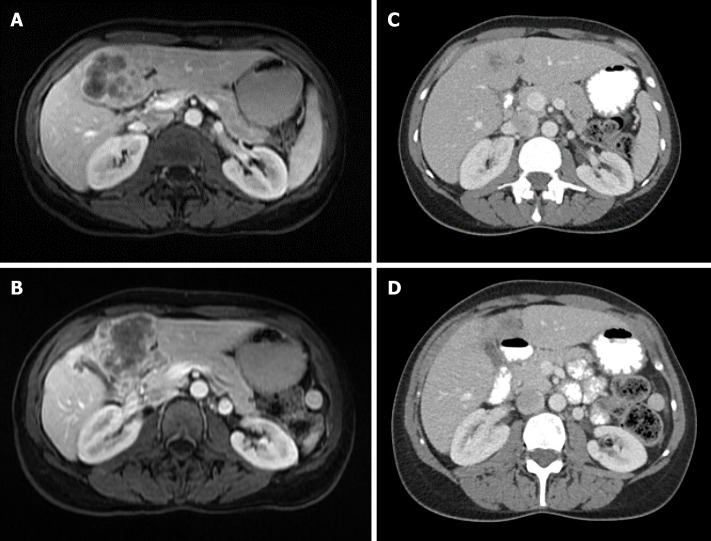
Locally advanced intrahepatic cholangiocarcinoma. A and B: 48F with large, multifocal intrahepatic cholangiocarcinoma who received 5 cycles of neoadjuvant gemcitabine/cisplatin; C and D: She experienced an excellent response and underwent extended left hepatectomy with pathology showing T2N0 moderately differentiated cholangiocarcinoma with negative margins.
A second major motivation for the use of NT is to ensure its early and near-universal use. While current guidelines recommend the use of adjuvant therapy for essentially all patients with resected ICC, a significant proportion of patients are unable to initiate and/or complete adjuvant therapy due to postoperative complications or poor performance status following major liver surgery. For example, in a retrospective series of 72 patients who underwent resection for ICC, only 35% of the patients received chemotherapy[43].
Not only does the delivery of systemic therapies prior to surgery ensure receipt is not prevented by postoperative complications, it also prioritizes the use of systemic therapies for an aggressive cancer with a strong tendency for systemic recurrence. For example, a recent multi-institutional review of patients with resected ICC reported that approximately 22% of patients experienced very early recurrence (defined as within six months of surgery), which ultimately led to poor survival outcomes[43]. It is likely that micrometastatic disease was present in these patients at the time of surgery and early systemic therapy may have been beneficial. Similarly, the use of NT also facilitates the appropriate selection of patients for a major surgery by ensuring that the rapid progression of metastatic disease does not occur while on systemic therapy. Recent prediction models may be useful in determining which patients with ICC may be best suited for NT[44,45]. Finally, given the genomic, phenotypic, and clinical heterogeneity of patients with ICC, monitoring the response to NT radiographically, biochemically, and histopathologically provides important prognostic and therapeutic information. This in vivo test will only become more important with the development of more effective targeted therapies in the era of personalized medicine.
EVIDENCE FOR NEOADJUVANT THERAPY IN ICC
Systemic chemotherapy
There have been no prospective randomized trials evaluating the benefit of neoadjuvant chemotherapy among patients with BTC, including ICC. However, there are some case reports, single-institution studies, and some retrospective data to suggest its benefit (Table 2). Chemotherapy regimens often use a combination of Gem-Cis, based on data extracted from the ABC-02 trial[33]. In some early studies of patients with locally advanced and unresectable tumors receiving Gem-Cis, 36.4% (8/22) in one study[46] and 25.6% (10/39) in another study[47] were able to be appropriately downsized and undergo resection. In a large single-center study of 186 patients with locally advanced ICC, 39/74 (53%) of patients who received NT were able to undergo resection[48]. In a large meta-analysis of 18 studies and 1,880 patients, including eight studies with chemotherapy. Patients who underwent resection following downstaging had significantly longer median survival compared with patients who did not (29 mo vs 12 mo, P < 0.001)[49]. In another systematic review of 132 patients, 27 patients (20.5%) were downstaged to surgical resection candidates[42]. In sum, these data support the use of systemic therapy among patients with locally advanced ICC as an attempt to downstage tumors to become resectable since achieving resection following conversion therapy is associated with improved long-term survival.
Table 2.
Select studies on neoadjuvant systemic chemotherapy for intrahepatic cholangiocarcinoma
| Ref. | Study type | Intervention | Sample size | Conversion to resection | Tumor response |
| Kato et al[46], 2013 | Retrospective | Gemcitabine | 22 | 8 (37%) | 3 PR, 11 SD, 8 PD |
| Kato et al[47], 2015 | Retrospective | Gemcitabine plus cisplatin | 39 | 10 (26%) | 9PR, 21 SD, 9 PD |
| Rayar et al[71], 2017 | Retrospective | Gemcitabine and/or platinums; Y-90 TARE | 45 | 10 (22%) | NR |
| Konstantinidis et al[79], 2017 | Retrospective | Bevacizumab + FUDR HAI | 104 | 8 (8%) | NR |
| Omichi et al[110], 2017 | Retrospective | Gemcitabine based therapy | 43 | 43 (100%) | NR |
| Le Roy et al[48], 2018 | Retrospective | Gemcitabine plus oxaliplatin | 74 | 39 (53%) | 18 PR, 33 SD, 23 PD |
| Sumiyoshi et al[112], 2018 | Retrospective | S-1 + IMRT | 7 | 5 (71%) | 4 PR, 1 SD, 2 PD |
NR: Not reported; PR: Partial response; SD: Stable disease; PD: Disease progression; ICC: Intrahepatic cholangiocarcinoma; TARE: Transarterial radioembolization; IMRT: Intensity-modulated radiation therapy.
While the use of NT in locally advanced disease is indicated, the routine use of systemic therapy prior to surgery for patients with resectable disease is not well established. Yadav et al[50] evaluated the National Cancer Database and performed a propensity-matched comparison of patients who received NT prior to surgical resection to individuals receiving surgery and adjuvant therapy. Acknowledging the limitations in this type of retrospective review, patients who received NT before surgery experienced improved OS (median OS: 40.3 mo vs 32.8 mo; P = 0.01)[51]. However, in a multi-institutional study of 62 patients who received NT (44% chemotherapy, 29% transarterial therapy) prior to curative-intent resection for ICC, a comparison of propensity-score matched patients who underwent upfront surgery did not find any significant difference in survival[51]. Clearly, more data are needed, preferably through well-designed prospective clinical trials, in order to define the indications for NT among patients with resectable ICC.
TACE
While TACE is routinely used for patients with HCC[52] and neuroendocrine liver metastases[53], its appropriate use in ICC remains undefined. ICC is a hypovascular tumor and thus less responsive to TACE[54,55]. However, since most of the morbidity and mortality associated with ICC results from overwhelming liver disease and liver failure, locoregional therapies such as TACE can be useful in controlling locally advanced disease. As such, in patients with ICC, TACE is traditionally used for those that are not eligible for surgery, as a palliative option, though it has been explored with downstaging intent (Table 3)[56,57]. One of the early successes in the use of TACE as a conversion therapy was reported by Burger et al[58] Seventeen patients with unresectable ICC under conventional TACE using cisplatin, doxorubicin, and mitomycin-C. Six of the patients had previously faced systemic therapy. TACE resulted in 75% tumor necrosis in 8 patients and tumor downstaging in 3 patients. Two of these patients were able to undergo surgical resection[58]. Herber et al[59] investigated the role of TACE using mitomycin C in 15 patients with unresectable disease and noted stable disease in 9 patients, partial response in 1, and tumor progression in 4 patients; no conversions to resection were reported[59]. Gusani et al[60] compared patients who received combination systemic gemcitabine and TACE to gemcitabine alone, and reported significant improvement in survival among patients who received TACE (13.8 mo vs 6.3 mo, respectively; P = 0.0005)[60]. A large retrospective review of 198 patients with advanced ICC undergoing transarterial therapies reported a complete or partial response in 25.5% of patients and stable disease in another 61.5%, suggesting a role for the use of TACE as neoadjuvant treatment (of note, 23.2% of the patients received yttrium-90 radioembolization)[61]. In another large series by Vogl et al[62], there was no difference in survival between different TACE regimens[62].
Table 3.
Select studies on neoadjuvant transarterial chemoembolization for intrahepatic cholangiocarcinoma
| Ref. | Study type | Intervention | Sample size | Conversion to resection | Tumor response |
| Burger et al[58], 2005 | Retrospective | Cisplatin, doxorubicin, and mitomycin-C | 17 | 2 (12%) | NR |
| Herber et al[59],2007 | Retrospective | Mitomycin-C | 15 | BR | 1 PR, 9 SD, 4PD |
| Gusani et al[60], 2008 | Retrospective | Gemcitabine-based | 42 | NR | 20 SD, 15 PD |
| Hyder et al[61], 2013 | Retrospective – multi-institutional | cTACE (64.7%), DEB-TACE (5.6%), bland embolization (6.6%), or Y-90 (23.2%) | 198 | NR | 56 PR, 77 SD, 29 PD |
| Vogl et al[62], 2012 | Retrospective | Mit-C (20.9%), Gem. (7%), Mit-C +Gem (47%), Gem+ Mit-C and Cisplatin (25.1%) | 115 | NR | 10 PR, 66 SD, 39 PD |
| Alibertti et al[64], 2017 | Retrospective | DEB-TACE and PEG-TACE | 127 | 4 (4%) | 19 PR, 101 SD, 7 PD |
| Schiffman et al[65], 2011 | Retrospective | EBIRI or DEB-DOX therapy | 24 | 3 (13%) | 1CR, 1PR, 13 SD, 3 PD |
| Kuhlmann et al[66], 2012 | Prospective | Irinotecan (iDEB-TACE), mitomycin-C (cTACE) | 41 | 1 (4%) | 2 PR, 12 SD, 19 PD |
| Poggi et al[67], 2009 | Retrospective | DEB-TACE | 9 | 3 (33%) | 4 PR, 5 SD |
NR: Not reported; PR: Partial response; SD: Stable disease; PD: Disease progression; ICC: Intrahepatic cholangiocarcinoma; DEB: Drug-eluting bead
Drug-eluting bead chemoembolization (DEB-TACE) allows for the delivery of highly concentrated doses of chemotherapy in addition to conventional chemoembolization. The beads limit the systemic availability and systemic toxicities of chemotherapy. In a retrospective study from Italy, 127 patients with advanced ICC underwent DEB-TACE or polyethylene glycol drug-eluting microspheres (PEG-TACE). Of the 109 patients treated with DEB-TACE, 7% had a partial response, 88% had stable disease, and 5% had progressive disease. Four patients (3.8%) in the DEB-TACE group were downsized and successfully underwent resection[63,64]. Multiple studies have evaluated DEB-TACE for unresectable ICC[65-67]; in general, few conversions to resectability have been reported.
Taken together, these findings suggest a potential role for TACE in the neoadjuvant treatment of patients with locally advanced ICC. However, while radiographic responses are observed, the majority of patients demonstrated stable disease, and conversions to resectable disease are the exception. Future studies may consider combination regimens that aim to enhance the response rate while treating/preventing the risk of systemic disease.
Transarterial radioembolization/selective internal radiation therapy
Transarterial radioembolization with yttrium-90 (Y-90) is an alternative transarterial therapy that is used in the management of locally advanced ICC and may downstage patients to resectability (Table 4). In an early open-label trial of Y-90 for ICC, 24 patients with advanced and unresectable ICC were treated with Y-90. Of the 22 patients with follow-up imaging, 6 patients demonstrated a partial response, 15 had stable disease, and 1 patient had progressive disease; 1 (4%) patient was downstaged and underwent resection[68]. In 2013, Mouli et al[69] reported on a series of 60 patients with ICC treated with Y-90 transarterial radioembolization (TARE). By EASL criteria, 33 patients had partial or complete response disease, and 12 patients showed stable disease. In this cohort, 5 patients successfully underwent an R0 resection[69]. In 2015, Al-Adra et al[70] performed a pooled analysis of several studies reporting on the use of Y-90 for patients with unresectable ICC. In this pooled data of 73 patients, the partial response rate following Y-90 treatment was 28%, and 54% had stable disease at 3 mo; 7 (10%) patients underwent surgical resection post TARE[70].
Table 4.
Select studies on neoadjuvant transarterial radioembolization/selective internal radiation therapy for intrahepatic cholangiocarcinoma
| Ref. | Study type | Intervention | Sample size | Conversion to resection | Tumor response |
| Ibrahim et al[68], 2008 | Prospective | Y-90 | 24 | 1 (4%) | 6PR, 15 SD, 1PD |
| Mouli et al[69], 2013 | Retrospective | Y-90 | 46 | 5 (11%) | 11 PR, 33 SD, 1 PD |
| Rayar et al[71], 2015 | Retrospective | Gemcitabine followed by Y-90 | 10 | 8 (80%) | NR |
| Saxena et al[72], 2010 | Retrospective | Y-90 | 25 | 1 (4%) | 6 PR, 11 SD, 5 PD |
| Rafi et al[73], 2012 | Prospective | Y-90 | 19 | NR | 2 PR, 13 SD, 4 PD |
| Hoffman et al[74], 2012 | Prospective | Y-90 | 33 | NR | 12 PR, 17 SD, 5 PD |
| Riby et al[75], 2020 | Retrospective | Y-90 | 19 | 19 (100%) | NR |
| Edeline et al[76], 2019 | Phase II Trial | GemCis + Y-90 | 26 | 9 (22%) | NR |
NR: Not reported; PR: Partial response; SD: Stable disease; PD: Disease progression; ICC: Intrahepatic cholangiocarcinoma.
Rayar et al[71] combined Y-90 with systemic therapy as an option to downstage unresectable ICC. Of the 45 patients treated with the combination regimen, ten were downstaged to potentially resectable, and 8 (17.8%) patients underwent resection[71]. Other studies have reported similarly low conversion rates with neoadjuvant Y-90 treatment[72-74]. In a large retrospective single-institution study of 169 patients, Riby et al[75] compared patients who underwent upfront resection to those who received downstaging chemotherapy with or without selective internal radiation therapy (SIRT) prior to surgical resection. Interestingly, patients with unresectable disease at presentation who became resectable after downstaging had similar median overall survival as patients with resectable disease who underwent upfront surgery (32.3 NT vs 45.9 primary surgery, P = 0.54). In addition, patients who received SIRT as part of their downstaging NT were more likely to undergo an R0 resection[75]. This approach has recently been validated via the MISPHEC trial, which was a prospective, multi-institutional trial of 41 previously untreated patients with locally advanced ICC. The patients received concomitant cisplatin and gemcitabine chemotherapy, followed by TARE with Y-90 microspheres. The response rate was 39% by RECIST criteria and 93% by Choi criteria. 9 patients (22%) were downstaged to resectable candidates, and 8 (20%) of them subsequently underwent an R0 resection. Additionally, subgroup analysis showed that 30% of patients in the trial with disease involving only 1 hemi-liver disease could be downstaged[76].
In summary, these findings suggest that while TARE provides good locoregional control, however the low response rates, when used alone, limits its application as a downstaging treatment for neoadjuvant intent. Recent studies combining TARE with systemic therapy hold promise and should be the subject of future trials.
Hepatic artery infusion
Given the toxicities associated with systemic chemotherapy, hepatic artery infusion (HAI) pumps were initially developed for the management of colorectal liver metastases but, more recently, trialed in patients with ICC (Table 5). One of the earliest experiences with HAI therapy in the management of ICC was performed by the Memorial Sloan Kettering Cancer Center in a phase II trial. Twenty-six patients with unresectable ICC were treated with HAI FUDR therapy. Fourteen patients (53.8%) experienced a partial response, 11 (42.3%) had stable disease, and 1 patient (3.8%) had disease progression[77]. In a follow-up study, the authors added systemic bevacizumab to HAI therapy which resulted in worsened toxicity without improved outcomes[78]. In 2015, Konstantinidis et al[79] reported on large series of 167 patients with advanced/unresectable, 104 of whom had disease confined to the liver, and 63 had regional nodal disease. Patients had either received HAI or HAI plus systemic therapy. Although there was no significant difference in tumor response by RECIST criteria, patients who received HAI plus systemic chemotherapy had better overall survival (30.8 mo) compared with patients who received systemic therapy alone (18.4 mo)[79]. Eight patients from the cohort (4 from each group) underwent resection with curative intent. Despite the low rate of conversion, the results remain promising. In another smaller series reported by Massani et al[80], 11 patients with unresectable disease underwent HAI therapy with fluorouracil and oxaliplatin, 5 patients had a partial response of whom 3 underwent resection. 2 of these patients had more than 70% tumor necrosis on pathology[80].
Table 5.
Select studies on neoadjuvant hepatic artery infusion for intrahepatic cholangiocarcinoma
| Ref. | Study type | Intervention | Sample size | Conversion to resection | Tumor response |
| Jarnagin et al[77], 2009 | Phase II trial | HAI | 26 | 1 (4%) | 14 PR, 11 SD, 1PD |
| Kemeny et al[78], 2011 | Phase II trail | HAI + bevacizumab | 18 | 3 (17%) | 7 PR, 11 SD |
| Konstantinidis et al[79], 2015 | Retrospective | HAI + chemotherapy | 93 | 8 (4%) | NR |
| Massani et al[80], 2015 | Retrospective | HAI | 11 | 3 (27%) | 5 PR, 2 SD, |
| Tanaka et al[113], 2002 | Retrospective | HAI | 11 | 1 (9%) | 7 PR, 2 SD, 2 PD |
| Shitara et al[114], 2008 | Retrospective | HAI | 20 | NR | 1CR, 9PR, 8 SD, 2PD |
| Ghiringhelli et al[115], 2013 | Retrospective | HAI | 12 | 2 (17%) | 8 PR, 3 SD, 1 PD |
NR: Not reported; PR: Partial response; SD: Stable disease; PD: Disease progression; ICC: Intrahepatic cholangiocarcinoma; TACE: Transarterial chemoembolization.
In summary, HAI therapy may induce higher response rates for ICC, especially when combined with systemic chemotherapy. However, conversion rates remain low, and, unlike other transarterial therapies, HAI therapy requires surgical placement of an implanted pump, which carries morbidity and delays the use of systemic therapy. In addition, data supporting its use are largely retrospective and have not been studied among patients undergoing true neoadjuvant intent. Future multi-institutional trials are needed to validate this approach and compare it to other approaches.
FUTURE DIRECTIONS
Targeted therapies
The prognosis of patients with locally advanced, recurrent and/or metastatic ICC remains poor even with contemporary systemic therapy. As such, there is great interest in the development of novel targeted therapies[81]. Next-generation sequencing of patients with advanced and refractory tumors have led to an improved understanding of the genetic changes driving cholangiocarcinogenesis. For example, mutations in KRAS have been identified and are an independent predictor of worse survival after hepatectomy[82]. Mutations in BRAF[83], EGFR[84], PI3K[85], and TP53[86] have also been reported with varying percentages. In addition, there are novel antitumor therapies directed at the fibroblast growth factor receptor 2 fusion protein (FGFR)[87,88], isocitrate dehydrogenase-1 (IDH1), and IDH2[89], BAP1[90], BRAF V600[91], and Her2/neu mutations[82]. Immunotherapies, including anti-PDL1 and anti-CTLA-4 inhibitors have been trialed inBTCs as well including ICC. About 10% of ICC lack mismatch repair mechanisms and as such are good targets in immunotherapy[92,93]. In the Phase II KEYNOTE-158 Study, 41% of patients with cholangiocarcinoma had an objective response[94]. While most of these targeted therapies are currently being investigated in patients with advanced disease and early phase trials, these therapies hold great potential for use in the neoadjuvant and perioperative setting.
Liver transplantation
While liver transplantation (LT) is indicated in the treatment of HCC meeting Milan criteria[95], the role of LT in the management of ICC remains controversial and, in general, remains limited to specialized centers and for patients on clinical trials. A challenge in interpreting the current literature is that most of the early studies combined hilar cholangiocarcinoma and ICC, making the results difficult to interpret. But even in those studies, transplant outcomes for ICC were generally very poor[96,97]. Interestingly, the standardization of transplantation protocols that include strict inclusion criteria and NT regimens has led to increased transplant rates for hilar cholangiocarcinoma[98-100]. Indeed, NT regimens for hilar cholangiocarcinoma are incorporated into the preoperative protocol at most LT centers[101,102]. It is therefore assumed that the development of effective programs for LT for ICC will similarly require effective neoadjuvant therapies.
One of the earliest successful experiences was reported by Goss et al[103] In their review of 127 patients who underwent LT for primary sclerosing cholangitis (PSC), ten patients (8%) had incidental CCA on explant pathology. More importantly, they had equivalent survival to those without ICC (100%, 83%, and 83% at 1, 2, and 5 years, respectively)[103] A recent large multicenter trial by Sapisochin et al[104] found that patients with tumors less than 2cm did not have any added risk of recurrence. 13 patients (8 TACE, 3 RFA and 2 PEI) received some neoadjuvant therapy. Preoperative treatment had no association with outcomes[104]. A more recent larger experience with pre-transplant regimens for ICC was reported by Lunsford et al[105] Patients with non-metastatic locally advanced ICC were treated with a gemcitabine-based chemotherapy regimen. After six months of radiographic response or stability, patients were listed for transplantation. The median duration to transplantation was 26 mo. Overall survival was 100% (95%CI: 100-100) at 1 year, 83.3% (27.3-97.5) at 3 years, and 83.3% (27.3-97.5) at 5 years. Three patients developed recurrent disease at a median of 7.6 mo (IQR 5.8-8.6) after transplantation, with 50% (95%CI: 11.1-80.4) recurrence-free survival at 1, 3, and 5 years[105]. Rayar et al[71] reported on another case of an unresectable lesion that was downstaged with multimodal therapy, including Y-90 TARE, systemic chemotherapy, and external beam radiation. The patient was transplanted after several months of disease stability. Although several barriers exist, these recent studies highlight the potential for improved outcomes for patients with advanced ICC using LT. Future research will require the design of effective multidisciplinary programs for patients that incorporate NT protocols, not only to bridge patients while on the waitlist, but also to ensure appropriate oncologic selection for transplantation.
Biomarkers
The design and validation of effective NT protocols also require the identification of appropriate biomarkers to guide its use and gauge response to therapy. The discovery of somatic mutations in ICC has led to renewed interest in the use of these as potential prognostic and predictive biomarkers[106,107]. For example, KRAS mutations, one of the most frequently seen mutations in ICC, is also associated with worse survival after resection in some studies[83,108]. MUC-44 has been linked to poor outcomes in mass forming ICC subtype[109]. The neutrophil-to-lymphocyte (N:L) ratio has equally been associated with worse survival in ICC[110]. CEA and CA-19 have very wide-ranging sensitivities and specificities in ICC[111]. Recent studies have highlighted the ability of machine learning to identify which patients with ICC may be best suited for NT[44,45]. Despite these recent advances, research on biomarkers for ICC is still lacking, and future trials are needed.
Ongoing trials
While interest in neoadjuvant approaches in other gastrointestinal cancers continues to drive new clinical trials, prospective trials of NT for ICC remain limited. An ongoing trial (NCT03579771) evaluating the benefit of neoadjuvant gemcitabine, cisplatin, and nab-paclitaxel is currently accruing. Another trial (NCT03867370) aims to investigate the benefit of neoadjuvant Toripalimab (a PD-1/PD-L1 immune checkpoint inhibitor) in patients with resectable HCC and ICC, although this study has not started accrual yet.
CONCLUSION
In conclusion, a sound rationale for the use of NT exists in ICC, particularly among patients with locally advanced disease. Given the importance of a margin-negative resection on overall prognosis, NT with a downstaging intent should be given in these patients. While numerous systemic and transarterial therapies have been reported in limited series, strong evidence for the superiority of one approach over another is lacking. Recent evidence has offered support for the use of combination strategies (e.g., systemic therapy with TARE) in order to augment the response and increase the proportion of patients downstaged to resectability. Future comparative effectiveness studies are needed to evaluate the optimal neoadjuvant approach, and ongoing research into targeted therapies for ICC may offer new opportunities for personalized neoadjuvant treatment. Finally, while margin-positive resection rates and disease recurrence is common even among patients with resectable disease, the evidence of NT in this patient population is extremely limited. Thus, the role of routine NT in patients with resectable ICC should be limited to patients with high-risk disease and preferably as part of a clinical trial. In the meantime, oncologic surgery that includes a margin-negative resection with formal lymphadenectomy followed by adjuvant chemotherapy remains the recommended approach.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Manuscript source: Invited manuscript
Peer-review started: August 3, 2020
First decision: August 9, 2020
Article in press: September 8, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mocchegiani F S-Editor: Yan JP L-Editor: A P-Editor: Wang LL
Contributor Information
Clifford Akateh, Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Aslam M Ejaz, Department of Surgery, The Ohio State University, Columbus, OH 43210, United States.
Timothy Michael Pawlik, Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Jordan M Cloyd, Department of Surgery, The Ohio State University, Columbus, OH 43210, United States. jordan.cloyd@osumc.edu.
References
- 1.Pawlik TMC, Jordan M. Dillhoff, Mary. Intrahepatic cholangiocarcinoma: diagnosis and management. Cham: Springer International Publishing; 2019. [Google Scholar]
- 2.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 3.Ejaz A, Cloyd JM, Pawlik TM. Advances in the Diagnosis and Treatment of Patients with Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2020;27:552–560. doi: 10.1245/s10434-019-07873-z. [DOI] [PubMed] [Google Scholar]
- 4.Cloyd JM, Ejaz A, Pawlik TM. The Landmark Series: Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2020;27:2859–2865. doi: 10.1245/s10434-020-08621-4. [DOI] [PubMed] [Google Scholar]
- 5.Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson AB, 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Schmidt C, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Zhu AX, Hoffmann KG, Darlow S. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15:563–573. doi: 10.6004/jnccn.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A, Gupta S, Wallace MJ, Aloia TA. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217:126–33; discussion 133-4. doi: 10.1016/j.jamcollsurg.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung U, Simpson AL, Araujo RL, Gönen M, McAuliffe C, Miga MI, Parada EP, Allen PJ, D'Angelica MI, Kingham TP, DeMatteo RP, Fong Y, Jarnagin WR. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014;219:620–630. doi: 10.1016/j.jamcollsurg.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang H, Sotiropoulos GC, Frühauf NR, Dömland M, Paul A, Kind EM, Malagó M, Broelsch CE. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005;241:134–143. doi: 10.1097/01.sla.0000149426.08580.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh CN, Hsieh FJ, Chiang KC, Chen JS, Yeh TS, Jan YY, Chen MF. Clinical effect of a positive surgical margin after hepatectomy on survival of patients with intrahepatic cholangiocarcinoma. Drug Des Devel Ther. 2015;9:163–174. doi: 10.2147/DDDT.S74940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MX, Bi XY, Li ZY, Huang Z, Han Y, Zhao JJ, Zhao H, Cai JQ. Impaction of surgical margin status on the survival outcome after surgical resection of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Surg Res. 2016;203:163–173. doi: 10.1016/j.jss.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg. 2014;18:562–572. doi: 10.1007/s11605-013-2447-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XF, Bagante F, Chakedis J, Moris D, Beal EW, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Groot Koerkamp B, Guglielmi A, Itaru E, Pawlik TM. Perioperative and Long-Term Outcome for Intrahepatic Cholangiocarcinoma: Impact of Major Versus Minor Hepatectomy. J Gastrointest Surg. 2017;21:1841–1850. doi: 10.1007/s11605-017-3499-6. [DOI] [PubMed] [Google Scholar]
- 15.Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P, Chiche L, Nuzzo G, Regimbeau JM. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824–29; discussion 830. doi: 10.1097/SLA.0b013e318236c21d. [DOI] [PubMed] [Google Scholar]
- 16.Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Marsh JW, Pawlik TM. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2015;22:4020–4028. doi: 10.1245/s10434-015-4472-9. [DOI] [PubMed] [Google Scholar]
- 17.Ma KW, Cheung TT, She WH, Chok KS, Chan AC, Ng IO, Chan SC, Lo CM. The effect of wide resection margin in patients with intrahepatic cholangiocarcinoma: A single-center experience. Medicine (Baltimore) 2016;95:e4133. doi: 10.1097/MD.0000000000004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang H, Lu W, Li B, Meng X, Dong J. Influence of surgical margins on overall survival after resection of intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore) 2016;95:e4621. doi: 10.1097/MD.0000000000004621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XF, Xue F, Dong DH, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Lv Y, Pawlik TM. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann Surg. 2020 doi: 10.1097/SLA.0000000000003788. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XF, Chen Q, Kimbrough CW, Beal EW, Lv Y, Chakedis J, Dillhoff M, Schmidt C, Cloyd J, Pawlik TM. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time?A National Database Analysis. J Gastrointest Surg. 2018;22:668–675. doi: 10.1007/s11605-017-3652-2. [DOI] [PubMed] [Google Scholar]
- 21.Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, Anders R, Are C, Brown D, Chang DT, Cloyd J, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Hammond LJ, Darlow SD. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw. 2019;17:302–310. doi: 10.6004/jnccn.2019.0019. [DOI] [PubMed] [Google Scholar]
- 22.Spolverato G, Vitale A, Cucchetti A, Popescu I, Marques HP, Aldrighetti L, Gamblin TC, Maithel SK, Sandroussi C, Bauer TW, Shen F, Poultsides GA, Marsh JW, Pawlik TM. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121:3998–4006. doi: 10.1002/cncr.29619. [DOI] [PubMed] [Google Scholar]
- 23.Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, Boudjema K, Fartoux L, Bouhier-Leporrier K, Jouve JL, Faroux R, Guerin-Meyer V, Kurtz JE, Assénat E, Seitz JF, Baumgaertner I, Tougeron D, de la Fouchardière C, Lombard-Bohas C, Boucher E, Stanbury T, Louvet C, Malka D, Phelip JM. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37:658–667. doi: 10.1200/JCO.18.00050. [DOI] [PubMed] [Google Scholar]
- 24.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 25.Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J, Schrum S, Shannon J, Vettorazzi E, Wege H. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015:15(1). doi: 10.1186/s12885-015-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakachi K, Konishi M, Ikeda M, Mizusawa J, Eba J, Okusaka T, Ishii H, Fukuda H, Furuse J Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group. A randomized Phase III trial of adjuvant S-1 therapy vs. observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT) Jpn J Clin Oncol. 2018;48:392–395. doi: 10.1093/jjco/hyy004. [DOI] [PubMed] [Google Scholar]
- 27.Song S, Kim K, Chie EK, Kim S, Park HJ, Yi NJ, Suh KS, Ha SW. Locoregional recurrence after curative intent resection for intrahepatic cholangiocarcinoma: implications for adjuvant radiotherapy. Clin Transl Oncol. 2015;17:825–829. doi: 10.1007/s12094-015-1312-0. [DOI] [PubMed] [Google Scholar]
- 28.Gil E, Joh JW, Park HC, Yu JI, Jung SH, Kim JM. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: a retrospective study. World J Surg Oncol. 2015;13:227. doi: 10.1186/s12957-015-0637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia AY, Wu JX, Zhao YT, Li YX, Wang Z, Rong WQ, Wang LM, Jin J, Wang SL, Song YW, Liu YP, Ren H, Fang H, Wang WQ, Liu XF, Yu ZH, Wang WH. Intensity-modulated radiotherapy following null-margin resection is associated with improved survival in the treatment of intrahepatic cholangiocarcinoma. J Gastrointest Oncol. 2015;6:126–133. doi: 10.3978/j.issn.2078-6891.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen ZT, Zhou H, Li AM, Li B, Shen JS, Zhu XX. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:93541–93550. doi: 10.18632/oncotarget.19972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen WF, Zhong W, Liu Q, Sui CJ, Huang YQ, Yang JM. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg. 2011;35:2083–2091. doi: 10.1007/s00268-011-1171-y. [DOI] [PubMed] [Google Scholar]
- 32.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 34.Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824–830. doi: 10.1001/jamaoncol.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sebastian NT, Tan Y, Miller ED, Williams TM, Noonan AM, Hays JL, Abdel-Misih S, Diaz DA. Association of Liver-Directed Local Therapy With Overall Survival in Adults With Metastatic Intrahepatic Cholangiocarcinoma. JAMA Netw Open. 2019;2:e1911154. doi: 10.1001/jamanetworkopen.2019.11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cloyd JM, Prakash L, Vauthey JN, Aloia TA, Chun YS, Tzeng CW, Kim MP, Lee JE, Katz MHG. The role of preoperative therapy prior to pancreatoduodenectomy for distal cholangiocarcinoma. Am J Surg. 2019;218:145–150. doi: 10.1016/j.amjsurg.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Cloyd JM, Wang H, Overman M, Zhao J, Denbo J, Prakash L, Kim MP, Shroff R, Javle M, Varadhachary GR, Fogelman D, Wolff RA, Koay EJ, Das P, Maitra A, Aloia TA, Vauthey JN, Fleming JB, Lee JE, Katz MHG. Influence of Preoperative Therapy on Short- and Long-Term Outcomes of Patients with Adenocarcinoma of the Ampulla of Vater. Ann Surg Oncol. 2017;24:2031–2039. doi: 10.1245/s10434-017-5777-7. [DOI] [PubMed] [Google Scholar]
- 38.Cloyd JM, Shen C, Santry H, Bridges J, Dillhoff M, Ejaz A, Pawlik TM, Tsung A. Disparities in the Use of Neoadjuvant Therapy for Resectable Pancreatic Ductal Adenocarcinoma. J Natl Compr Canc Netw. 2020;18:556–563. doi: 10.6004/jnccn.2019.7380. [DOI] [PubMed] [Google Scholar]
- 39.Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704–3721. doi: 10.3748/wjg.v25.i28.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellis MC, Cassera MA, Vetto JT, Orloff SL, Hansen PD, Billingsley KG. Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. HPB (Oxford) 2011;13:59–63. doi: 10.1111/j.1477-2574.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, Cappellani A, Malfermoni G, Iacono C. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 42.Fruscione M, Pickens RC, Baker EH, Martinie JB, Iannitti DA, Hwang JJ, Vrochides D. Conversion therapy for intrahepatic cholangiocarcinoma and tumor downsizing to increase resection rates: A systematic review. Curr Probl Cancer. 2020:100614. doi: 10.1016/j.currproblcancer.2020.100614. [DOI] [PubMed] [Google Scholar]
- 43.Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, Serra C, Brandi G, Pinna AD. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252:107–114. doi: 10.1097/SLA.0b013e3181e462e6. [DOI] [PubMed] [Google Scholar]
- 44.Tsilimigras DI, Hyer JM, Paredes AZ, Diaz A, Moris D, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Soubrane O, Koerkamp BG, Endo I, Pawlik TM. A Novel Classification of Intrahepatic Cholangiocarcinoma Phenotypes Using Machine Learning Techniques: An International Multi-Institutional Analysis. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-08696-z. [DOI] [PubMed] [Google Scholar]
- 45.Tsilimigras DI, Mehta R, Moris D, Sahara K, Bagante F, Paredes AZ, Moro A, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Soubrane O, Koerkamp BG, Endo I, Pawlik TM. A Machine-Based Approach to Preoperatively Identify Patients with the Most and Least Benefit Associated with Resection for Intrahepatic Cholangiocarcinoma: An International Multi-institutional Analysis of 1146 Patients. Ann Surg Oncol. 2020;27:1110–1119. doi: 10.1245/s10434-019-08067-3. [DOI] [PubMed] [Google Scholar]
- 46.Kato A, Shimizu H, Ohtsuka M, Yoshidome H, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Kimura F, Miyazaki M. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20:318–324. doi: 10.1245/s10434-012-2312-8. [DOI] [PubMed] [Google Scholar]
- 47.Kato A, Shimizu H, Ohtsuka M, Yoshitomi H, Furukawa K, Takayashiki T, Nakadai E, Kishimoto T, Nakatani Y, Yoshidome H, Miyazaki M. Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer Patients Treated with Gemcitabine Plus Cisplatin Combination Therapy Followed by Radical Surgery. Ann Surg Oncol. 2015;22 Suppl 3:S1093–S1099. doi: 10.1245/s10434-015-4768-9. [DOI] [PubMed] [Google Scholar]
- 48.Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, Vibert E, Castaing D, Adam R, Cherqui D, Sa Cunha A. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 49.Kamarajah S, Giovinazzo F, Roberts KJ, Punia P, Sutcliffe RP, Marudanayagam R, Chatzizacharias N, Isaac J, Mirza DF, Muiesan P, Dasari BV. The role of down staging treatment in the management of locally advanced intrahepatic cholangiocarcinoma: Review of literature and pooled analysis. Ann Hepatobiliary Pancreat Surg. 2020;24:6–16. doi: 10.14701/ahbps.2020.24.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav S, Xie H, Bin-Riaz I, Sharma P, Durani U, Goyal G, Borah B, Borad MJ, Smoot RL, Roberts LR, Go RS, McWilliams RR, Mahipal A. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur J Surg Oncol. 2019;45:1432–1438. doi: 10.1016/j.ejso.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Buettner S, Koerkamp BG, Ejaz A, Buisman FE, Kim Y, Margonis GA, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Marsh JW, IJzermans JN, Pawlik TM. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients-A multi-institutional analysis. J Surg Oncol. 2017;115:312–318. doi: 10.1002/jso.24524. [DOI] [PubMed] [Google Scholar]
- 52.Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers (Basel) 2020;12:1914. doi: 10.3390/cancers12071914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egger ME, Armstrong E, Martin RC 2nd, Scoggins CR, Philips P, Shah M, Konda B, Dillhoff M, Pawlik TM, Cloyd JM. Transarterial Chemoembolization vs Radioembolization for Neuroendocrine Liver Metastases: A Multi-Institutional Analysis. J Am Coll Surg. 2020;230:363–370. doi: 10.1016/j.jamcollsurg.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, Monden M, Kudo M. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676–691. doi: 10.1111/j.1872-034X.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Yoon HK, Sung KB, Ko GY, Gwon DI, Shin JH, Song HY. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113:1614–1622. doi: 10.1002/cncr.23787. [DOI] [PubMed] [Google Scholar]
- 56.Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322–328. doi: 10.1016/j.crad.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, Olthoff K, Christians K, Pappas S, Rilling W, Soulen MC. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117:1498–1505. doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- 58.Burger I, Hong K, Schulick R, Georgiades C, Thuluvath P, Choti M, Kamel I, Geschwind JF. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16:353–361. doi: 10.1097/01.RVI.0000143768.60751.78. [DOI] [PubMed] [Google Scholar]
- 59.Herber S, Otto G, Schneider J, Manzl N, Kummer I, Kanzler S, Schuchmann A, Thies J, Düber C, Pitton M. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2007;30:1156–1165. doi: 10.1007/s00270-007-9032-7. [DOI] [PubMed] [Google Scholar]
- 60.Gusani NJ, Balaa FK, Steel JL, Geller DA, Marsh JW, Zajko AB, Carr BI, Gamblin TC. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): A single-institution experience. Journal of Gastrointestinal Surgery. 2008;12(1):129–137. doi: 10.1007/s11605-007-0312-y. [DOI] [PubMed] [Google Scholar]
- 61.Hyder O, Marsh JW, Salem R, Petre EN, Kalva S, Liapi E, Cosgrove D, Neal D, Kamel I, Zhu AX, Sofocleous CT, Geschwind JF, Pawlik TM. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20:3779–3786. doi: 10.1245/s10434-013-3127-y. [DOI] [PubMed] [Google Scholar]
- 62.Vogl TJ, Naguib NN, Nour-Eldin NE, Bechstein WO, Zeuzem S, Trojan J, Gruber-Rouh T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int J Cancer. 2012;131:733–740. doi: 10.1002/ijc.26407. [DOI] [PubMed] [Google Scholar]
- 63.Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol. 2008;31:883–888. doi: 10.1007/s00270-008-9336-2. [DOI] [PubMed] [Google Scholar]
- 64.Aliberti C, Carandina R, Sarti D, Pizzirani E, Ramondo G, Mulazzani L, Mattioli GM, Fiorentini G. Chemoembolization with Drug-eluting Microspheres Loaded with Doxorubicin for the Treatment of Cholangiocarcinoma. Anticancer Res. 2017;37:1859–1863. doi: 10.21873/anticanres.11522. [DOI] [PubMed] [Google Scholar]
- 65.Schiffman SC, Metzger T, Dubel G, Andrasina T, Kralj I, Tatum C, McMasters KM, Scoggins CR, Martin RC. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol. 2011;18:431–438. doi: 10.1245/s10434-010-1333-4. [DOI] [PubMed] [Google Scholar]
- 66.Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, Fischer R. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437–443. doi: 10.1097/MEG.0b013e3283502241. [DOI] [PubMed] [Google Scholar]
- 67.Poggi G, Amatu A, Montagna B, Quaretti P, Minoia C, Sottani C, Villani L, Tagliaferri B, Sottotetti F, Rossi O, Pozzi E, Zappoli F, Riccardi A, Bernardo G. OEM-TACE: a new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2009;32:1187–1192. doi: 10.1007/s00270-009-9694-4. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, Newman SB, Benson A, 3rd, Omary RA, Salem R. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113:2119–2128. doi: 10.1002/cncr.23818. [DOI] [PubMed] [Google Scholar]
- 69.Mouli S, Memon K, Baker T, Benson AB, 3rd, Mulcahy MF, Gupta R, Ryu RK, Salem R, Lewandowski RJ. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24:1227–1234. doi: 10.1016/j.jvir.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120–127. doi: 10.1016/j.ejso.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, Boucher E, Boudjema K. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102–3108. doi: 10.1245/s10434-014-4365-3. [DOI] [PubMed] [Google Scholar]
- 72.Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–491. doi: 10.1245/s10434-009-0777-x. [DOI] [PubMed] [Google Scholar]
- 73.Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, Kim HS. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36:440–448. doi: 10.1007/s00270-012-0463-4. [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann RT, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr EM, Rauch B, Trumm CT, Jakobs TF, Helmberger TK, Reiser MF, Kolligs FT. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–116. doi: 10.1007/s00270-011-0142-x. [DOI] [PubMed] [Google Scholar]
- 75.Riby D, Mazzotta AD, Bergeat D, Verdure L, Sulpice L, Bourien H, Lièvre A, Rolland Y, Garin E, Boudjema K, Edeline J. Downstaging with Radioembolization or Chemotherapy for Initially Unresectable Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-08486-7. [DOI] [PubMed] [Google Scholar]
- 76.Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, Ayav A, Campillo-Gimenez B, Beuzit L, Pracht M, Lièvre A, Le Sourd S, Boudjema K, Rolland Y, Boucher E, Garin E. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2019;6:51–59. doi: 10.1001/jamaoncol.2019.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jarnagin WR, Schwartz LH, Gultekin DH, Gönen M, Haviland D, Shia J, D'Angelica M, Fong Y, DeMatteo R, Tse A, Blumgart LH, Kemeny N. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589–1595. doi: 10.1093/annonc/mdp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kemeny NE, Schwartz L, Gönen M, Yopp A, Gultekin D, D'Angelica MI, Fong Y, Haviland D, Gewirtz AN, Allen P, Jarnagin WR. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology. 2011;80:153–159. doi: 10.1159/000324704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konstantinidis IT, Groot Koerkamp B, Do RK, Gönen M, Fong Y, Allen PJ, D'Angelica MI, Kingham TP, DeMatteo RP, Klimstra DS, Kemeny NE, Jarnagin WR. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122:758–765. doi: 10.1002/cncr.29824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Massani M, Nistri C, Ruffolo C, Bonariol R, Pauletti B, Bonariol L, Caratozzolo E, Morana G, Bassi N. Intrahepatic chemotherapy for unresectable cholangiocarcinoma: review of literature and personal experience. Updates Surg. 2015;67:389–400. doi: 10.1007/s13304-015-0330-3. [DOI] [PubMed] [Google Scholar]
- 81.Oliveira DV, Zhang S, Chen X, Calvisi DF, Andersen JB. Molecular profiling of intrahepatic cholangiocarcinoma: the search for new therapeutic targets. Expert Rev Gastroenterol Hepatol. 2017;11:349–356. doi: 10.1080/17474124.2017.1292127. [DOI] [PubMed] [Google Scholar]
- 82.Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861–4870. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robertson S, Hyder O, Dodson R, Nayar SK, Poling J, Beierl K, Eshleman JR, Lin MT, Pawlik TM, Anders RA. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol. 2013;44:2768–2773. doi: 10.1016/j.humpath.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borad MJ, Champion MD, Egan JB, Liang WS, Fonseca R, Bryce AH, McCullough AE, Barrett MT, Hunt K, Patel MD, Young SW, Collins JM, Silva AC, Condjella RM, Block M, McWilliams RR, Lazaridis KN, Klee EW, Bible KC, Harris P, Oliver GR, Bhavsar JD, Nair AA, Middha S, Asmann Y, Kocher JP, Schahl K, Kipp BR, Barr Fritcher EG, Baker A, Aldrich J, Kurdoglu A, Izatt T, Christoforides A, Cherni I, Nasser S, Reiman R, Phillips L, McDonald J, Adkins J, Mastrian SD, Placek P, Watanabe AT, Lobello J, Han H, Von Hoff D, Craig DW, Stewart AK, Carpten JD. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135. doi: 10.1371/journal.pgen.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herberger B, Puhalla H, Lehnert M, Wrba F, Novak S, Brandstetter A, Gruenberger B, Gruenberger T, Pirker R, Filipits M. Activated mammalian target of rapamycin is an adverse prognostic factor in patients with biliary tract adenocarcinoma. Clin Cancer Res. 2007;13:4795–4799. doi: 10.1158/1078-0432.CCR-07-0738. [DOI] [PubMed] [Google Scholar]
- 86.Simbolo M, Vicentini C, Ruzzenente A, Brunelli M, Conci S, Fassan M, Mafficini A, Rusev B, Corbo V, Capelli P, Bria E, Pedron S, Turri G, Lawlor RT, Tortora G, Bassi C, Guglielmi A, Scarpa A. Genetic alterations analysis in prognostic stratified groups identified TP53 and ARID1A as poor clinical performance markers in intrahepatic cholangiocarcinoma. Sci Rep. 2018;8:7119. doi: 10.1038/s41598-018-25669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, Van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36:276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mazzaferro V, El-Rayes BF, Droz Dit Busset M, Cotsoglou C, Harris WP, Damjanov N, Masi G, Rimassa L, Personeni N, Braiteh F, Zagonel V, Papadopoulos KP, Hall T, Wang Y, Schwartz B, Kazakin J, Bhoori S, de Braud F, Shaib WL. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 90.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJA, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goeppert B, Frauenschuh L, Renner M, Roessler S, Stenzinger A, Klauschen F, Warth A, Vogel MN, Mehrabi A, Hafezi M, Boehmer K, von Deimling A, Schirmacher P, Weichert W, Capper D. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod Pathol. 2014;27:1028–1034. doi: 10.1038/modpathol.2013.206. [DOI] [PubMed] [Google Scholar]
- 92.Silva VW, Askan G, Daniel TD, Lowery M, Klimstra DS, Abou-Alfa GK, Shia J. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016;5:62. doi: 10.21037/cco.2016.10.04. [DOI] [PubMed] [Google Scholar]
- 93.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA., Jr Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA., Jr Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. New England Journal of Medicine. 1996;334(11):693–700. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 96.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 97.Goldstein RM, Stone M, Tillery GW, Senzer N, Levy M, Husberg BS, Gonwa T, Klintmalm G. Is liver transplantation indicated for cholangiocarcinoma? Am J Surg. 1993;166:768–71; discussion 771-2. doi: 10.1016/s0002-9610(05)80696-8. [DOI] [PubMed] [Google Scholar]
- 98.Moris D, Kostakis ID, Machairas N, Prodromidou A, Tsilimigras DI, Ravindra KV, Sudan DL, Knechtle SJ, Barbas AS. Comparison between liver transplantation and resection for hilar cholangiocarcinoma: A systematic review and meta-analysis. PLoS One. 2019;14:e0220527. doi: 10.1371/journal.pone.0220527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B, Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 100.Gores GJ, Darwish Murad S, Heimbach JK, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma. Dig Dis. 2013;31:126–129. doi: 10.1159/000347207. [DOI] [PubMed] [Google Scholar]
- 101.Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: the role of liver transplantation. HPB (Oxford) 2008;10:186–189. doi: 10.1080/13651820801992542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong JC, Jones CM, Duffy JP, Petrowsky H, Farmer DG, French S, Finn R, Durazo FA, Saab S, Tong MJ, Hiatt JR, Busuttil RW. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg. 2011;146:683–689. doi: 10.1001/archsurg.2011.116. [DOI] [PubMed] [Google Scholar]
- 103.Goss JA, Shackleton CR, Farmer DG, Arnaout WS, Seu P, Markowitz JS, Martin P, Stribling RJ, Goldstein LI, Busuttil RW. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg. 1997;225:472–81; discussion 481-3. doi: 10.1097/00000658-199705000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sapisochin G, Rodríguez de Lope C, Gastaca M, Ortiz de Urbina J, Suarez MA, Santoyo J, Castroagudín JF, Varo E, López-Andujar R, Palacios F, Sanchez Antolín G, Perez B, Guiberteau A, Blanco G, González-Diéguez ML, Rodriguez M, Varona MA, Barrera MA, Fundora Y, Ferron JA, Ramos E, Fabregat J, Ciria R, Rufian S, Otero A, Vazquez MA, Pons JA, Parrilla P, Zozaya G, Herrero JI, Charco R, Bruix J. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14:660–667. doi: 10.1111/ajt.12591. [DOI] [PubMed] [Google Scholar]
- 105.Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC) Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337–348. doi: 10.1016/S2468-1253(18)30045-1. [DOI] [PubMed] [Google Scholar]
- 106.Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: Molecular markers for diagnosis and prognosis. Surg Oncol. 2017;26:125–137. doi: 10.1016/j.suronc.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 107.Leelawat K, Sakchinabut S, Narong S, Wannaprasert J. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009;9:30. doi: 10.1186/1471-230X-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ikeno Y, Seo S, Iwaisako K, Yoh T, Nakamoto Y, Fuji H, Taura K, Okajima H, Kaido T, Sakaguchi S, Uemoto S. Preoperative metabolic tumor volume of intrahepatic cholangiocarcinoma measured by 18F-FDG-PET is associated with the KRAS mutation status and prognosis. J Transl Med. 2018;16:95. doi: 10.1186/s12967-018-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, Hollingsworth MA, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–229. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 110.Omichi K, Cloyd JM, Yamashita S, Tzeng CD, Conrad C, Chun YS, Aloia TA, Vauthey JN. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162:752–765. doi: 10.1016/j.surg.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 111.Tshering G, Dorji PW, Chaijaroenkul W, Na-Bangchang K. Biomarkers for the Diagnosis of Cholangiocarcinoma: A Systematic Review. Am J Trop Med Hyg. 2018;98:1788–1797. doi: 10.4269/ajtmh.17-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, Matsumoto M, Hata Y, Noda Y, Sui K, Sueda T. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J Surg. 2018;42:2910–2918. doi: 10.1007/s00268-018-4558-1. [DOI] [PubMed] [Google Scholar]
- 113.Tanaka N, Yamakado K, Nakatsuka A, Fujii A, Matsumura K, Takeda K. Arterial chemoinfusion therapy through an implanted port system for patients with unresectable intrahepatic cholangiocarcinoma--initial experience. Eur J Radiol. 2002;41:42–48. doi: 10.1016/s0720-048x(01)00414-4. [DOI] [PubMed] [Google Scholar]
- 114.Shitara K, Ikami I, Munakata M, Muto O, Sakata Y. Hepatic arterial infusion of mitomycin C with degradable starch microspheres for unresectable intrahepatic cholangiocarcinoma. Clin Oncol (R Coll Radiol) 2008;20:241–246. doi: 10.1016/j.clon.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 115.Ghiringhelli F, Lorgis V, Vincent J, Ladoire S, Guiu B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. 2013;59:354–360. doi: 10.1159/000362223. [DOI] [PubMed] [Google Scholar]