The title structure consists of [Ni(CN)4]2– square-planar anions separated by layers of (C10H10N3)+ cations. The crystal packing features N—H⋯N hydrogen bonds, which generate [101] chains.
Keywords: crystal structure, tetracyanonickelate, N-(pyridin-2-yl)pyridinium-2-amine, hydrogen bonding, Hirshfeld surface analysis, crystal structure
Abstract
In the title molecular salt, (C10H10N3)2[Ni(CN)4], the dihedral angle between the pyridine rings in the cation is 1.92 (13)° and the complete anion is generated by a crystallographic centre of symmetry. An intramolecular N—H⋯N hydrogen bond occurs in the cation, which closes an S(6) ring. In the crystal, the components are linked by N—H⋯N and weak C—H⋯N hydrogen bonds, which generate chains propagating in the [101] direction. Weak aromatic π–π stacking interactions are also observed. A Hirshfeld surface analysis and two-dimensional fingerprint plots indicate that the most important contact types in the crystal packing are N⋯H/H⋯N, C⋯H/H⋯C and H⋯H with contributions of 37.2, 28.3 and 21.9%, respectively.
Chemical context
Transition-metal coordination compounds, where CN− ligands play the main structure-forming role, so-called cyanocarbanion or cyanometallate complexes, have been the subject of interest for many years, in particular due to their magnetic properties (Ferlay et al., 1995 ▸; Bretosh et al., 2020 ▸; Benmansour et al., 2012 ▸; Setifi et al., 2009 ▸; Yuste et al., 2009 ▸; Addala et al., 2015 ▸), including spin-crossover behavior (Benmansour et al., 2010 ▸; Yoon et al., 2011 ▸). The square-planar tetracyanonickelate(II) anion [Ni(CN)4]2– has proved to be very versatile and diverse in both coordination chemistry and magnetism.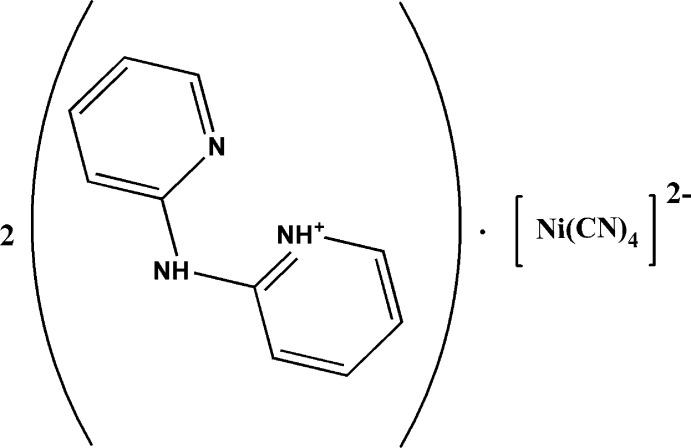
We have been interested in using the tetracyanonickelate(II) anion in combination with other chelating or bridging neutral co-ligands to explore their structural features and properties relevant to the field of molecular materials exhibiting the spin-crossover phenomenon (Setifi et al., 2013 ▸, 2014 ▸; Kucheriv et al., 2016 ▸). During the course of attempts to prepare such complexes with 2,2′-dipyridylamine (dpa), we isolated the title molecular salt, (I), whose molecular and supramolecular structure is described herein.
Structural commentary
The asymmetric unit of (I) contains one (C10H10N3)+ cation and one half of a [Ni(CN)4]2− anion (Fig. 1 ▸). The C—N and C—C bonds lengths in the cation vary from 1.340 (3) to 1.383 (3) Å and from 1.346 (4) to 1.402 (3) Å, respectively. The C—N—C bond angles range from 117.8 (2) to 129.7 (2)° and the N—C—C angles range from 119.0 (2) to 123.4 (2)°. The dihedral angle between the C3–C7/N4 and C8–C12/N5 rings is 1.92 (13)°. These data are comparable to those found for other compounds containing dpa as an organic template (Bowes et al., 2003 ▸; Willett, 1995 ▸). In the cation, the pyridyl nitrogen atoms are arranged on both sides of the central N3 atom and assume a cis conformation (Fig. 1 ▸). The (C10H10N3)+ cation is monoprotonated at the pyridyl-N4 atom, which leads to the the formation of a short and presumably strong intramolecular N4—H4A⋯N5 hydrogen bond (Table 1 ▸), which generates an S(6) ring (Fig. 2 ▸).
Figure 1.
The molecular structure of (I) with displacement ellipsoids drawn at the 50% probability level. Symmetry code: (i) −x + 1, −y, −z
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N3—H3A⋯N2 | 0.86 | 2.00 | 2.853 (3) | 172 |
| N4—H4A⋯N5 | 0.86 | 1.97 | 2.629 (3) | 132 |
| N4—H4A⋯N1ii | 0.86 | 2.41 | 3.055 (3) | 132 |
| C5—H5⋯N1ii | 0.93 | 2.68 | 3.206 (4) | 117 |
Symmetry code: (ii)  .
.
Figure 2.
Offset and parallel π–π-stacking interactions (broken lines) in the cation–cation chains.
The Ni2+ ion of the anion is located on a crystallographic inversion center and coordinates four terminal (non-bridging) cyanide ligands, exhibiting a square-planar geometry. The bond lengths and angles in the anion are in good agreement with those found in other [Ni(CN)4]2− salts (Paharová et al., 2003 ▸; Karaağaç et al., 2013 ▸).
Supramolecular features
Fig. 3 ▸ shows the packing of (I) in a view along the b-axis direction, in which the organic and inorganic ions form chains propagating in the [101] direction linked by N—H⋯N and C—H⋯N hydrogen bonds. The pyridinium N4 atom in the cation, as well as forming the intramolecular hydrogen bond described above, acts as donor to the cyanate N atom in the anion, in an N4—H4A⋯N1ii [symmetry code: (ii) −x + 1, −y + 1, −z + 1) link (Table 1 ▸). The secondary amino group (N3H) forms a strong N3—H3A⋯N2 hydrogen bond with a cyano group acceptor and the H3A⋯N2 distance is 2.0 Å. Fig. 3 ▸ shows the parallel offset π-stacking contacts between pyridyl groups [centroid–centroid distance of 4.3421 (16) Å] and parallel face-centred π-stacking interactions between the S(6) centroids and pyridyl groups [centroid–centroid distance of 3.487 (2) Å].
Figure 3.
View parallel to the ac plane of the packing in (I) with hydrogen bonds shown as green dashed lines.
Hirshfeld surface analysis
Hirshfeld surface calculations (Spackman & Jayatilaka, 2009 ▸) for (I) were performed in order to further characterize the supramolecular association. The Hirshfeld surfaces and two-dimensional fingerprint plots (McKinnon et al., 2007 ▸) calculated using CrystalExplorer 17.5 (Turner et al., 2017 ▸) are shown in Figs. 4 ▸ and 5 ▸, respectively. The red spots on the Hirshfeld surface represent strong interaction through N—H⋯N and C—H⋯N hydrogen bonding, whereas the blue color represents a lack of interaction. The presence of π–π stacking interactions is indicated by adjacent red and blue triangles on the shape-index surface (Fig. S1a in the supporting information). Areas on the Hirshfeld surface with high curvedness (Fig. S1b) can be related to the planar packing arrangement of the cations. The most abundant intermolecular interactions in the crystal packing (Fig. 5 ▸) are N⋯H/H⋯N, C⋯H/H⋯C and H⋯H with percentage contributions 37.2, 28.3 and 21.9%, respectively. The presence of weak π–π stacking interactions between the cationic rings are reflected in the 4.6 and 3.8% contributions from C⋯C and C⋯N/N⋯C contacts to the Hirshfeld surfaces of the cations. The analysis reveals the lowest contribution of Ni⋯N (1.7%), Ni⋯C (1.3%) and N⋯N (1.2%) contacts.
Figure 4.
Hirshfeld surface of (I) mapped over d norm.
Figure 5.
Two-dimensional fingerprint plots and relative contributions for (I) resolved into all, N⋯H, C⋯H and H⋯H contacts.
Database survey
A search of the Cambridge Structural Database (Version 5.41, last update November, 2019; Groom et al., 2016 ▸), for the tetracyanonickelate moiety revealed 532 hits. Most of them are complexes of [Ni(CN)4]2– anions with different metal–ligand coordination cations. Salts containing tetracyanonickelate anions and organic cations corresponded to 38 hits.
A compound closely related to the title compound is (C10H11N3)·[CuCl4] (Willett, 1995 ▸; CSD refcode ZAMCEV), which crystallizes in the same space group of P
 . In this compound the cation is diprotonated and the pyridyl nitrogen atoms are in a cis conformation and the pyridine rings are significantly twisted away from coplanarity. The tetrachlorocuprate anion takes on a squashed tetrahedral geometry.
. In this compound the cation is diprotonated and the pyridyl nitrogen atoms are in a cis conformation and the pyridine rings are significantly twisted away from coplanarity. The tetrachlorocuprate anion takes on a squashed tetrahedral geometry.
Synthesis and crystallization
The title compound was synthesized solvothermally under autogenous pressure using a mixture of iron(II) sulfate heptahydrate (28 mg, 0.10 mmol), 2,2′-dipyridylamine (17 mg, 0.10 mmol) and potassium tetracyanonickelate(II) (24 mg, 0.10 mmol) in mixed solvents of water/ethanol (3:1 v/v, 20 ml). The mixture was sealed in a Teflon-lined autoclave and held at 423 K for 3 d, and then cooled to room temperature at a rate of 10 K per hour (yield 27%). Pale-yellow plates of (I) suitable for single-crystal X-ray diffraction analysis were selected.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All H atoms were positioned geometrically in idealized positions and constrained to ride on their parent atoms, with C—H = 0.93 or N—H = 0.86 Å, and with U iso(H) = 1.2U eq(C,N).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | (C10H10N3)2[Ni(CN)4] |
| M r | 507.21 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 273 |
| a, b, c (Å) | 7.1046 (4), 9.1467 (4), 9.3833 (4) |
| α, β, γ (°) | 100.182 (2), 98.729 (2), 97.444 (2) |
| V (Å3) | 585.49 (5) |
| Z | 1 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.86 |
| Crystal size (mm) | 0.35 × 0.23 × 0.19 |
| Data collection | |
| Diffractometer | Oxford Diffraction Xcalibur with Sapphire CCD detector |
| Absorption correction | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) |
| T min, T max | 0.914, 0.962 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 16272, 3572, 2659 |
| R int | 0.052 |
| (sin θ/λ)max (Å−1) | 0.715 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.048, 0.134, 1.07 |
| No. of reflections | 3572 |
| No. of parameters | 161 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.01, −0.34 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698902001419X/hb7948sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698902001419X/hb7948Isup2.hkl
Figure S1 Hirshfeld surface of (C10H10N3)2[Ni(CN)4] mapped with shape index (a) and curvedness (b). DOI: 10.1107/S205698902001419X/hb7948sup3.tif
CCDC reference: 2040378
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| (C10H10N3)2[Ni(CN)4] | Z = 1 |
| Mr = 507.21 | F(000) = 262 |
| Triclinic, P1 | Dx = 1.439 Mg m−3 |
| a = 7.1046 (4) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 9.1467 (4) Å | Cell parameters from 7173 reflections |
| c = 9.3833 (4) Å | θ = 2.8–27.9° |
| α = 100.182 (2)° | µ = 0.86 mm−1 |
| β = 98.729 (2)° | T = 273 K |
| γ = 97.444 (2)° | Plate, pale yellow |
| V = 585.49 (5) Å3 | 0.35 × 0.23 × 0.19 mm |
Data collection
| Oxford Diffraction Xcalibur Sapphire CCD detector diffractometer | 2659 reflections with I > 2σ(I) |
| Radiation source: Enhance (Mo) X-ray Source | Rint = 0.052 |
| ω scans | θmax = 30.6°, θmin = 2.2° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −10→10 |
| Tmin = 0.914, Tmax = 0.962 | k = −13→13 |
| 16272 measured reflections | l = −13→13 |
| 3572 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.048 | w = 1/[σ2(Fo2) + (0.0546P)2 + 0.3025P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.134 | (Δ/σ)max < 0.001 |
| S = 1.07 | Δρmax = 1.01 e Å−3 |
| 3572 reflections | Δρmin = −0.34 e Å−3 |
| 161 parameters | Extinction correction: SHELXL-2014/7 (Sheldrick 2014, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.091 (17) |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ni1 | 0.5000 | 0.0000 | 0.0000 | 0.04195 (19) | |
| C1 | 0.6743 (4) | 0.0656 (3) | 0.1772 (3) | 0.0508 (6) | |
| N1 | 0.7800 (5) | 0.1080 (3) | 0.2861 (3) | 0.0745 (8) | |
| C2 | 0.3639 (4) | 0.1552 (3) | 0.0568 (2) | 0.0455 (5) | |
| N2 | 0.2831 (4) | 0.2503 (3) | 0.0942 (3) | 0.0624 (6) | |
| N4 | 0.1820 (3) | 0.7199 (2) | 0.3981 (2) | 0.0445 (4) | |
| H4A | 0.1949 | 0.7110 | 0.4886 | 0.053* | |
| N5 | 0.2473 (3) | 0.5418 (2) | 0.5852 (2) | 0.0484 (5) | |
| N3 | 0.2311 (3) | 0.4715 (2) | 0.3342 (2) | 0.0492 (5) | |
| H3A | 0.2354 | 0.3997 | 0.2625 | 0.059* | |
| C3 | 0.1977 (3) | 0.6028 (3) | 0.2949 (3) | 0.0422 (5) | |
| C8 | 0.2592 (3) | 0.4356 (3) | 0.4722 (3) | 0.0443 (5) | |
| C5 | 0.1462 (4) | 0.8522 (3) | 0.3626 (3) | 0.0508 (6) | |
| H5 | 0.1351 | 0.9313 | 0.4365 | 0.061* | |
| C4 | 0.1807 (4) | 0.6192 (3) | 0.1479 (3) | 0.0503 (6) | |
| H4 | 0.1942 | 0.5397 | 0.0753 | 0.060* | |
| C12 | 0.3276 (4) | 0.2629 (3) | 0.6262 (4) | 0.0595 (7) | |
| H12 | 0.3538 | 0.1690 | 0.6407 | 0.071* | |
| C7 | 0.1442 (4) | 0.7522 (3) | 0.1118 (3) | 0.0557 (6) | |
| H7 | 0.1314 | 0.7635 | 0.0144 | 0.067* | |
| C10 | 0.2763 (4) | 0.5097 (3) | 0.7197 (3) | 0.0553 (6) | |
| H10 | 0.2695 | 0.5836 | 0.7998 | 0.066* | |
| C6 | 0.1264 (4) | 0.8713 (3) | 0.2224 (3) | 0.0564 (7) | |
| H6 | 0.1013 | 0.9625 | 0.1994 | 0.068* | |
| C9 | 0.3006 (4) | 0.2930 (3) | 0.4869 (3) | 0.0524 (6) | |
| H9 | 0.3096 | 0.2214 | 0.4055 | 0.063* | |
| C11 | 0.3157 (4) | 0.3727 (4) | 0.7447 (3) | 0.0591 (7) | |
| H11 | 0.3341 | 0.3540 | 0.8397 | 0.071* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.0617 (3) | 0.0339 (2) | 0.0330 (2) | 0.01697 (18) | 0.01155 (18) | 0.00464 (15) |
| C1 | 0.0751 (18) | 0.0380 (12) | 0.0419 (12) | 0.0238 (11) | 0.0105 (12) | 0.0040 (9) |
| N1 | 0.100 (2) | 0.0626 (15) | 0.0527 (14) | 0.0317 (15) | −0.0091 (14) | −0.0053 (12) |
| C2 | 0.0628 (15) | 0.0412 (12) | 0.0328 (10) | 0.0168 (11) | 0.0073 (10) | 0.0028 (9) |
| N2 | 0.0820 (17) | 0.0554 (13) | 0.0494 (12) | 0.0333 (12) | 0.0076 (12) | −0.0032 (10) |
| N4 | 0.0475 (11) | 0.0447 (10) | 0.0424 (10) | 0.0101 (8) | 0.0080 (8) | 0.0096 (8) |
| N5 | 0.0520 (12) | 0.0482 (11) | 0.0468 (11) | 0.0092 (9) | 0.0106 (9) | 0.0115 (9) |
| N3 | 0.0664 (14) | 0.0403 (10) | 0.0421 (10) | 0.0166 (9) | 0.0133 (10) | 0.0022 (8) |
| C3 | 0.0382 (12) | 0.0411 (11) | 0.0485 (12) | 0.0077 (9) | 0.0070 (9) | 0.0123 (9) |
| C8 | 0.0402 (12) | 0.0451 (12) | 0.0495 (13) | 0.0045 (9) | 0.0081 (10) | 0.0160 (10) |
| C5 | 0.0544 (15) | 0.0406 (12) | 0.0582 (15) | 0.0106 (10) | 0.0111 (12) | 0.0090 (11) |
| C4 | 0.0538 (15) | 0.0525 (14) | 0.0450 (13) | 0.0137 (11) | 0.0095 (11) | 0.0069 (10) |
| C12 | 0.0574 (16) | 0.0509 (15) | 0.0743 (19) | 0.0069 (12) | 0.0059 (14) | 0.0294 (14) |
| C7 | 0.0589 (16) | 0.0636 (16) | 0.0505 (14) | 0.0141 (13) | 0.0117 (12) | 0.0228 (12) |
| C10 | 0.0572 (16) | 0.0640 (16) | 0.0456 (13) | 0.0076 (13) | 0.0120 (12) | 0.0126 (12) |
| C6 | 0.0593 (16) | 0.0494 (14) | 0.0670 (17) | 0.0147 (12) | 0.0115 (13) | 0.0242 (13) |
| C9 | 0.0585 (16) | 0.0417 (12) | 0.0576 (15) | 0.0091 (11) | 0.0097 (12) | 0.0109 (11) |
| C11 | 0.0514 (15) | 0.0747 (19) | 0.0545 (15) | 0.0034 (13) | 0.0068 (12) | 0.0291 (14) |
Geometric parameters (Å, º)
| Ni1—C2 | 1.865 (2) | C8—C9 | 1.399 (3) |
| Ni1—C2i | 1.865 (2) | C5—C6 | 1.346 (4) |
| Ni1—C1 | 1.867 (3) | C5—H5 | 0.9300 |
| Ni1—C1i | 1.867 (3) | C4—C7 | 1.365 (4) |
| C1—N1 | 1.145 (4) | C4—H4 | 0.9300 |
| C2—N2 | 1.136 (3) | C12—C9 | 1.373 (4) |
| N4—C3 | 1.340 (3) | C12—C11 | 1.381 (4) |
| N4—C5 | 1.355 (3) | C12—H12 | 0.9300 |
| N4—H4A | 0.8600 | C7—C6 | 1.399 (4) |
| N5—C8 | 1.326 (3) | C7—H7 | 0.9300 |
| N5—C10 | 1.337 (3) | C10—C11 | 1.371 (4) |
| N3—C3 | 1.355 (3) | C10—H10 | 0.9300 |
| N3—C8 | 1.383 (3) | C6—H6 | 0.9300 |
| N3—H3A | 0.8600 | C9—H9 | 0.9300 |
| C3—C4 | 1.402 (3) | C11—H11 | 0.9300 |
| C2—Ni1—C2i | 180.0 | N4—C5—H5 | 119.4 |
| C2—Ni1—C1 | 89.06 (10) | C7—C4—C3 | 119.8 (2) |
| C2i—Ni1—C1 | 90.94 (10) | C7—C4—H4 | 120.1 |
| C2—Ni1—C1i | 90.94 (10) | C3—C4—H4 | 120.1 |
| C2i—Ni1—C1i | 89.06 (10) | C9—C12—C11 | 119.7 (3) |
| C1—Ni1—C1i | 180.0 | C9—C12—H12 | 120.2 |
| N1—C1—Ni1 | 178.8 (2) | C11—C12—H12 | 120.2 |
| N2—C2—Ni1 | 178.6 (2) | C4—C7—C6 | 119.5 (2) |
| C3—N4—C5 | 121.3 (2) | C4—C7—H7 | 120.3 |
| C3—N4—H4A | 119.4 | C6—C7—H7 | 120.3 |
| C5—N4—H4A | 119.4 | N5—C10—C11 | 122.9 (3) |
| C8—N5—C10 | 117.8 (2) | N5—C10—H10 | 118.5 |
| C3—N3—C8 | 129.7 (2) | C11—C10—H10 | 118.5 |
| C3—N3—H3A | 115.1 | C5—C6—C7 | 119.1 (2) |
| C8—N3—H3A | 115.1 | C5—C6—H6 | 120.4 |
| N4—C3—N3 | 119.7 (2) | C7—C6—H6 | 120.4 |
| N4—C3—C4 | 119.0 (2) | C12—C9—C8 | 117.4 (3) |
| N3—C3—C4 | 121.3 (2) | C12—C9—H9 | 121.3 |
| N5—C8—N3 | 117.0 (2) | C8—C9—H9 | 121.3 |
| N5—C8—C9 | 123.4 (2) | C10—C11—C12 | 118.8 (3) |
| N3—C8—C9 | 119.6 (2) | C10—C11—H11 | 120.6 |
| C6—C5—N4 | 121.3 (2) | C12—C11—H11 | 120.6 |
| C6—C5—H5 | 119.4 |
Symmetry code: (i) −x+1, −y, −z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N3—H3A···N2 | 0.86 | 2.00 | 2.853 (3) | 172 |
| N4—H4A···N5 | 0.86 | 1.97 | 2.629 (3) | 132 |
| N4—H4A···N1ii | 0.86 | 2.41 | 3.055 (3) | 132 |
| C5—H5···N1ii | 0.93 | 2.68 | 3.206 (4) | 117 |
Symmetry code: (ii) −x+1, −y+1, −z+1.
Funding Statement
This work was funded by Ministère de l’Enseignement Supérieur et de la Recherche Scientifique Algeria grant . Direction Générale de la Recherche Scientifique et du Développement Technologique Algeria grant . Université Ferhat Abbas Sétif 1 grant .
References
- Addala, A., Setifi, F., Kottrup, K. G., Glidewell, C., Setifi, Z., Smith, G. & Reedijk, J. (2015). Polyhedron, 87, 307–310.
- Benmansour, S., Atmani, C., Setifi, F., Triki, S., Marchivie, M. & Gómez-García, C. J. (2010). Polyhedron, 254, 1468–1478.
- Benmansour, S., Setifi, F., Triki, S. & Gómez-García, C. J. (2012). Inorg. Chem. 51, 2359–2365. [DOI] [PubMed]
- Bowes, K. F., Ferguson, G., Lough, A. J. & Glidewell, C. (2003). Acta Cryst. B59, 100–117. [DOI] [PubMed]
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bretosh, K., Béreau, V., Duhayon, C., Pichon, C. & Sutter, J.-P. (2020). Inorg. Chem. Front. 7, 1503–1511.
- Ferlay, S., Mallah, T., Ouahès, R., Veillet, P. & Verdaguer, M. A. (1995). Nature, 378, 701–703.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Karaağaç, D., Kürkçüoğlu, G. S., Yeşilel, O. Z., Hökelek, T. & Süzen, Y. (2013). Inorg. Chim. Acta, 406, 73–80.
- Kucheriv, O. I., Shylin, S. I., Ksenofontov, V., Dechert, S., Haukka, M., Fritsky, I. O. & Gural’skiy, I. A. (2016). Inorg. Chem. 55, 4906–4914. [DOI] [PubMed]
- McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. (2007). Chem. Commun. pp. 3814–3816. [DOI] [PubMed]
- Oxford Diffraction (2009). CrysAlis CCD and CrysAlis RED. Oxford Diffraction Ltd, Abingdon, England.
- Paharová, J., Černák, J., Boča, R. & Žák, Z. (2003). Inorg. Chim. Acta, 346, 25–31.
- Setifi, F., Benmansour, S., Marchivie, M., Dupouy, G., Triki, S., Sala-Pala, J., Salaün, J.-Y., Gómez-García, C. J., Pillet, S., Lecomte, C. & Ruiz, E. (2009). Inorg. Chem. 48, 1269–1271. [DOI] [PubMed]
- Setifi, F., Charles, C., Houille, S., Thétiot, F., Triki, S., Gómez-García, C. J. & Pillet, S. (2013). Polyhedron, 61, 242–247.
- Setifi, F., Milin, E., Charles, C., Thétiot, F., Triki, S. & Gómez-García, C. J. (2014). Inorg. Chem. 53, 97–104. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, M. A. & Jayatilaka, D. (2009). CrystEngComm, 11, 19–32.
- Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). CrystalExplorer17. University of Western Australia.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Willett, R. D. (1995). Acta Cryst. C51, 1517–1519.
- Yoon, J. H., Ryu, D. W., Choi, S. Y., Kim, H. C., Koh, E. K., Tao, J. & Hong, C. S. (2011). Chem. Commun. 47, 10416–10418. [DOI] [PubMed]
- Yuste, C., Bentama, A., Marino, N., Armentano, D., Setifi, F., Triki, S., Lloret, F. & Julve, M. (2009). Polyhedron, 28, 1287–1294.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698902001419X/hb7948sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698902001419X/hb7948Isup2.hkl
Figure S1 Hirshfeld surface of (C10H10N3)2[Ni(CN)4] mapped with shape index (a) and curvedness (b). DOI: 10.1107/S205698902001419X/hb7948sup3.tif
CCDC reference: 2040378
Additional supporting information: crystallographic information; 3D view; checkCIF report







