Fifteen 4-(2-methoxyphenyl)piperazin-1-ium salts with organic anions exhibit a range of hydrogen-bonded supramolecular assemblies in the form of finite aggregates, a chain of rings, ribbons, sheets and three-dimensional networks.
Keywords: synthesis, piperazines, crystal structure, molecular conformation, absolute configuration, disorder, hydrogen bonding, supramolecular assembly
Abstract
Fifteen 4-(2-methoxyphenyl)piperazin-1-ium salts containing organic anions have been prepared and structurally characterized. In the isostructural 4-chlorobenzoate and 4-bromobenzoate salts, C11H17N2O+·C7H4ClO2 − (I) and C11H17N2O+·C7H4BrO2 − (II), and the 4-iodobenzoate salt C11H17N2O+·C7H4IO2 − (III), the ions are linked by N—H⋯O hydrogen bonds, forming centrosymmetric R 4 4(12) four-ion aggregates; a similar aggregate is formed in the 2-chlorobenzoate salt (V), isomeric with (I). In the 2-fluorobenzoate salt C11H17N2O+·C7H4FO2 − (IV), and the isomorphous pair of salts, the 2-bromobenzoate (VI), isomeric with (II) and 2-iodobenzoate (VII), isomeric with (III), N—H⋯O and C—H⋯π(arene) interactions link the components into three-dimensional arrays. Four-ion R 4 4(12) aggregates are also found in the 2-methylbenzoate, 4-aminobenzoate and 4-nitrobenzoate salts, C11H17N2O+·C8H7O2 − (VIII), C11H17N2O+·C7H6NO2 − (IX) and C11H17N2O+·C7H4NO4 − (X), but those in (IX) are linked into complex sheets by an additional N—H⋯O hydrogen bond. In the 3,5-dinitrobenzoate salt, C11H17N2O+·C7H3N2O6 −·2H2O (XI), N—H⋯O and O—H⋯O hydrogen bonds link the components into a complex ribbon structure. In the picrate salt, C11H17N2O+·C6H2N3O7 − (XII), the four-ion aggregates are linked into chains of rings by C—H⋯O hydrogen bonds. In the hydrogen maleate salt, C11H17N2O+·C4H3O4 − (XIII), two- and three-centre hydrogen bonds link the ions into a ribbon structure while both anions contain very short but asymmetric O—H⋯O hydrogen bonds, having O⋯O distances of 2.4447 (16) and 2.4707 (17) Å. O—H⋯O Hydrogen bonds link the anions in the hydrogen fumarate salt (XIV), isomeric with (XIII), into chains that are linked into sheets via N—H⋯O hydrogen bonds. In the hydrogen (2R,3R)-tartrate salt, C11H17N2O+·C4H5O6 −·1.698H2O (XV), the anions are linked into sheets by O—H⋯O hydrogen bonds. Comparisons are made with the structures of some related compounds.
Chemical context
We have recently reported the molecular and supramolecular structures of the recreational drug N-(4-methoxyphenyl)piperazine (4-MeOPP) (Kiran Kumar et al., 2020 ▸) and those of a range of salts formed by 4-MeOPP with organic acids (Kiran Kumar, Yathirajan, Foro et al., 2019 ▸; Kiran Kumar et al. 2020 ▸), as well as those of a number of N-aroyl derivatives (Kiran Kumar, Yathirajan, Sagar et al., 2019 ▸). We have also reported the structures of some salts of N-(4-fluorophenyl)piperazine (4-FPP) (Harish Chinthal, Yathirajan, Archana et al., 2020 ▸; Harish Chinthal, Yathirajan, Kavitha et al., 2020 ▸). As a continuation of this study, we have now investigated a number of salts of the isomeric N-(2-methoxyphenyl)piperazine (2-MeOPP), which has been used as a building block in the synthesis of both 5-HT1A receptor ligands (Orjales et al., 1995 ▸) and dopamine D2 and D3 ligands (Hackling et al., 2003 ▸) and also as a building block for the synthesis of derivatives exhibiting antidepressant-like activity (Waszkielewicz et al., 2015 ▸). Here we report the syntheses and structures of the salts (I)–(XI) (Figs. 1 ▸–11 ▸
▸
▸
▸
▸
▸
▸
▸
▸
▸) formed between 2-MeOPP and eleven aromatic carboxylic acids, along with a redetermination of the salt (XII) (Fig. 12 ▸) formed with 2,4,6-trinitrophenol (picric acid) where the reported structure (Verdonk et al., 1997 ▸; CSD refcode NEBGIK) shows signs of unmodelled disorder, and we report here also the structures of three acid salts (XIII)–(XV) (Figs. 13 ▸–15 ▸
▸) formed with some aliphatic dicarboxylic acids. All of the salts (I)–(XV) were straightforwardly prepared by the acid–base reactions and subsequent crystallizations of equimolar mixtures of 2-MeOPP with the appropriate organic acid.
Figure 1.
The independent components of compound (I) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 2.
The independent components of compound (II) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 3.
The independent components of compound (III) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 4.
The independent components of compound (IV) showing the atom-labelling scheme and the disorder in the anion; the major disorder component is drawn using full lines and the minor disorder component is drawn using broken lines. Displacement ellipsoids are drawn at the 30% probability level.
Figure 5.
The independent components of compound (V) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 6.
The independent components of compound (VI) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 7.
The independent components of compound (VII) showing the atom-labelling scheme and the disorder in the carboxylate group; the major disorder component is drawn using full lines and the minor disorder component is drawn using broken lines. Displacement ellipsoids are drawn at the 30% probability level.
Figure 8.
The independent components of compound (VIII) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 9.
The independent components of compound (IX) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 10.
The independent components of compound (X) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 11.
The independent components of compound (XI) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 12.
The independent components of compound (XII) showing the atom-labelling scheme and the disorder in one of the nitro groups, where the dominant disorder component is drawn using full lines, and the two minor disorder components are drawn using broken lines. Displacement ellipsoids are drawn at the 30% probability level.
Figure 13.
The independent components of compound (XIII) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 14.
The independent components of compound (XIV) showing the atom-labelling scheme and the disorder in one of the anions. The major disorder component is drawn using full lines and the minor disorder component is drawn using broken lines. Displacement ellipsoids are drawn at the 30% probability level. The atoms marked ‘a’ or ‘b’ are at the symmetry positions (2 − x, 1 − y, 2 − z) and (−x, −y, 2 − z), respectively. The H atoms bonded to atoms O32, O34 and O42 have occupancies 0.286 (9), 0.214 (9) and 0.5, respectively, as do their inversion-related equivalents.
Figure 15.
The independent components of compound (XV) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Structural commentary
Compounds (I) and (II) (Figs. 1 ▸ and 2 ▸) are isostructural in space group P
 . Although the 4-iodobenzoate analogue (III) (Fig. 3 ▸) also crystallizes in the same space group, it is not isostructural with (I) and (II). Among the 2-halobenzoate salts, in the 2-fluorobenzoate (IV) the anion is disordered over two sets of atomic sites having occupancies 0.907 (8) and 0.093 (8) (Fig. 4 ▸). There is a significant peak, 1.15 e Å−3, in the final difference map for compound (V): it was originally thought that this might represent a partial-occupancy water molecule, although no associated H atoms could be located, but its distance from atom O32 is only 2.35 Å, which would require an unusually short O—H⋯O hydrogen bond for this assignment to be plausible. Consistent with this, examination of the refined, solvent-free structure of (V) using PLATON (Spek, 2020 ▸) showed that the structure contains no solvent-accessible void spaces. Compounds (VI) and (VII) are isomorphous, but whereas the components of (VI) are fully ordered (Fig. 6 ▸), in (VII) the carboxylate group in the anion is disordered over two sets of atomic sites having occupancies 0.54 (9) and 0.46 (9) (Fig. 7 ▸); hence, these isomorphous compounds cannot be regarded as strictly isostructural (cf. Acosta et al., 2009 ▸; Yépes et al., 2012 ▸; Shreekanth et al., 2020 ▸), because of the disorder in (VII). The structures of (VI) and (VII) are mutually inverse for the crystals selected for data collection, but this has no chemical significance. Compounds (VIII)–(X) (Figs. 8 ▸–10 ▸
▸) all crystallize in solvent-free form, but the 3,5-dinitrobenzoate salt (XI) is a dihydrate (Fig. 11 ▸). The structure of the picrate salt (XII) was reported a number of years ago (Verdonk et al., 1997 ▸), but the deposited anisotropic displacement parameters suggest the presence of unmodelled disorder in one of the nitro groups. Accordingly, we have redetermined this structure and found, indeed, that one of the nitro groups is disordered over three sets of atomic sites having occupancies 0.850 (5), 0.080 (4) and 0.069 (4) (Fig. 12 ▸).
. Although the 4-iodobenzoate analogue (III) (Fig. 3 ▸) also crystallizes in the same space group, it is not isostructural with (I) and (II). Among the 2-halobenzoate salts, in the 2-fluorobenzoate (IV) the anion is disordered over two sets of atomic sites having occupancies 0.907 (8) and 0.093 (8) (Fig. 4 ▸). There is a significant peak, 1.15 e Å−3, in the final difference map for compound (V): it was originally thought that this might represent a partial-occupancy water molecule, although no associated H atoms could be located, but its distance from atom O32 is only 2.35 Å, which would require an unusually short O—H⋯O hydrogen bond for this assignment to be plausible. Consistent with this, examination of the refined, solvent-free structure of (V) using PLATON (Spek, 2020 ▸) showed that the structure contains no solvent-accessible void spaces. Compounds (VI) and (VII) are isomorphous, but whereas the components of (VI) are fully ordered (Fig. 6 ▸), in (VII) the carboxylate group in the anion is disordered over two sets of atomic sites having occupancies 0.54 (9) and 0.46 (9) (Fig. 7 ▸); hence, these isomorphous compounds cannot be regarded as strictly isostructural (cf. Acosta et al., 2009 ▸; Yépes et al., 2012 ▸; Shreekanth et al., 2020 ▸), because of the disorder in (VII). The structures of (VI) and (VII) are mutually inverse for the crystals selected for data collection, but this has no chemical significance. Compounds (VIII)–(X) (Figs. 8 ▸–10 ▸
▸) all crystallize in solvent-free form, but the 3,5-dinitrobenzoate salt (XI) is a dihydrate (Fig. 11 ▸). The structure of the picrate salt (XII) was reported a number of years ago (Verdonk et al., 1997 ▸), but the deposited anisotropic displacement parameters suggest the presence of unmodelled disorder in one of the nitro groups. Accordingly, we have redetermined this structure and found, indeed, that one of the nitro groups is disordered over three sets of atomic sites having occupancies 0.850 (5), 0.080 (4) and 0.069 (4) (Fig. 12 ▸).
The solvent-free 1:1 acid salt (XIII) derived from maleic acid crystallizes with Z′ = 2 (Fig. 13 ▸). A search for possible additional crystallographic symmetry revealed none, although the atomic coordinates of the two cations and the two anions are related by the approximate, but non-crystallographic translation (x,  + y, z). In sharp contrast to compound (XIII), the 1:1 salt (XIV) derived from fumaric acid, which is isomeric with maleic acid, crystallizes with two independent hydrogen fumarate anions, each lying across a centre of inversion: one of the anions is fully ordered but the other is disordered over two sets of atomic sites having occupancies 0.572 (9) and 0.428 (9) (Fig. 14 ▸). The 1:1 acid salt (XV) derived from (2R,3R)-tartaric acid crystallizes as a dihydrate (Fig. 15 ▸).
+ y, z). In sharp contrast to compound (XIII), the 1:1 salt (XIV) derived from fumaric acid, which is isomeric with maleic acid, crystallizes with two independent hydrogen fumarate anions, each lying across a centre of inversion: one of the anions is fully ordered but the other is disordered over two sets of atomic sites having occupancies 0.572 (9) and 0.428 (9) (Fig. 14 ▸). The 1:1 acid salt (XV) derived from (2R,3R)-tartaric acid crystallizes as a dihydrate (Fig. 15 ▸).
In none of the salts reported does the cation exhibit any internal symmetry: hence all are conformationally chiral but, with the exception of compounds (VI) and (VII), the space groups indicate that equal numbers of both conformational enantiomers are present. For all compounds except (VII), the reference cation was selected to be one for which the ring-puckering angles θ (Cremer & Pople, 1975 ▸) is close to zero, as calculated for the atom sequence (N1,C2,C3,N4,C5,C6). For the crystal of (VII) chosen for data collection, the value of this angle is 177.2 (5)°, confirming that this salt and (VI) have opposite absolute structures. In all of the cations, the piperazine ring adopts a chair conformation with the N-aryl substituent in an equatorial site. In the 2-methoxyphenyl units, the methoxy C atom is always close to coplanar with the adjacent aryl ring: the displacement of this atom from the plane of the ring ranges from 0.038 (5) Å in compound (I) to 0.288 (5) Å in compound (VII). Associated with this near planarity, the two exocyclic C—C—O angles differ in each compound by ca 10°, as is usually observed in planar or near-planar alkoxyarenes (Seip & Seip, 1973 ▸; Ferguson et al., 1996 ▸).
The two independent ions in compound (XIII) both contain a very short O—H⋯O hydrogen bond (Table 1 ▸): while these are both nearly linear, the two O—H distances in each are significantly different, as established both by refinement of the atomic coordinates for the H atom, and from the final difference maps.
Table 1. Hydrogen bonds and short inter-ion contacts (Å, °).
Cg1, Cg2 and Cg3 represent the centroids of the rings (C31–C36), (C21–C26) and (C41–C46), respectively.
| Compound | D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|---|
| (I) | N1—H11⋯O31 | 1.02 (2) | 1.60 (2) | 2.616 (3) | 176 (2) |
| N1—H12⋯O32i | 0.92 (3) | 1.88 (3) | 2.792 (3) | 173 (2) | |
| C3—H3A⋯Cg1i | 0.97 | 2.96 | 3.881 (3) | 160 | |
| (II) | N1—H11⋯O31 | 0.89 (4) | 1.75 (4) | 2.620 (4) | 168 (3) |
| N1—H12⋯O32i | 0.88 (4) | 1.91 (4) | 2.786 (4) | 175 (4) | |
| (III) | N1—H11⋯O31 | 0.88 (2) | 1.83 (2) | 2.684 (3) | 163 (2) |
| N1—H11⋯O32 | 0.88 (2) | 2.60 (2) | 3.060 (3) | 113.6 (17) | |
| N1—H12⋯O32i | 0.91 (3) | 1.84 (3) | 2.746 (3) | 176 (3) | |
| C33—H33⋯O32ii | 0.93 | 2.57 | 3.327 (3) | 139 | |
| C2—H2B⋯Cg2iii | 0.97 | 2.77 | 3.482 (2) | 131 | |
| (IV) | N1—H11⋯O31 | 0.99 (3) | 1.72 (3) | 2.694 (4) | 167 (3) |
| N1—H11⋯O32 | 0.99 (3) | 2.51 (3) | 3.131 (4) | 120.9 (19) | |
| N1—H12⋯O32iii | 0.88 (3) | 1.83 (3) | 2.679 (4) | 161 (3) | |
| N1—H11⋯O41 | 0.99 (3) | 1.77 (5) | 2.67 (4) | 151 (3) | |
| N1—H11⋯O42 | 0.99 (3) | 2.52 (5) | 3.20 (4) | 126 (2) | |
| N1—H12⋯O42iii | 0.88 (3) | 1.83 (5) | 2.63 (4) | 151 (3) | |
| C34—H34⋯Cg2iv | 0.93 | 2.74 | 3.543 (5) | 145 | |
| C44—H44⋯Cg2iv | 0.93 | 2.99 | 3.73 (4) | 137 | |
| C26—H26⋯Cg3v | 0.93 | 2.96 | 3.754 (17) | 144 | |
| (V) | N1—H11⋯O31 | 0.97 (4) | 1.74 (3) | 2.682 (4) | 162 (3) |
| N1—H12⋯O32i | 0.92 (4) | 1.79 (4) | 2.700 (5) | 170 (4) | |
| C5—H5B⋯Cg1ii | 0.97 | 2.87 | 3.554 (4) | 128 | |
| C34—H34⋯Cg2vi | 0.93 | 2.93 | 3.658 (7) | 136 | |
| (VI) | N1—H11⋯O31 | 0.75 (4) | 1.98 (4) | 2.726 (4) | 170 (4) |
| N1—H12⋯O32vii | 0.88 (3) | 1.86 (3) | 2.712 (4) | 163(3 | |
| C25—H25⋯O32viii | 0.93 | 2.56 | 3.488 (4) | 173 | |
| C26—H26⋯Cg1viii | 0.93 | 2.93 | 3.697 (4) | 141 | |
| (VII) | N1—H11⋯O31 | 0.89 | 1.80 | 2.66 (3) | 162 |
| N1—H11⋯O33 | 0.89 | 1.93 | 2.80 (3) | 165 | |
| N1—H12⋯O31ix | 0.89 | 1.97 | 2.83 (3) | 162 | |
| N1—H12⋯O33ix | 0.89 | 1.74 | 2.60 (3) | 161 | |
| C25—H25⋯O34x | 0.93 | 2.50 | 3.43 (3) | 174 | |
| C26—H26⋯Cg1x | 0.93 | 2.93 | 3.716 (5) | 143 | |
| (VIII) | N1—H11⋯O31 | 1.010 (15) | 1.673 (15) | 2.6696 (19) | 168.6 (13) |
| N1—H12⋯O32i | 0.963 (16) | 1.745 (16) | 2.7077 (17) | 178.2 (10) | |
| (IX) | N1—H11⋯O31 | 1.068 (15) | 1.547 (15) | 2.6048 (15) | 169.7 (14) |
| N1—H12⋯O32i | 0.942 (15) | 1.861 (15) | 2.7797 (15) | 164.4 (14) | |
| N34—H34⋯O32xi | 0.914 (16) | 2.155 (16) | 3.0535 (18) | 167.5 (14) | |
| (X) | N1—H11⋯O31 | 0.974 (16) | 1.677 (16) | 2.6500 (19) | 176.8 (15) |
| N1—H11⋯O32 | 0.974 (16) | 2.581 (17) | 3.2169 (17) | 123.0 (12) | |
| N1—H12⋯O32i | 0.948 (17) | 1.837 (17) | 2.7709 (18) | 168.2 (16) | |
| (XI) | N1—H11⋯O31 | 0.929 (16) | 1.771 (16) | 2.6837 (16) | 166.8 (15) |
| N1—H12⋯O41 | 0.911 (16) | 1.939 (16) | 2.8324 (19) | 165.5 (14) | |
| O41—H41⋯O32xii | 0.84 (2) | 1.99 (2) | 2.8156 (19) | 168 (2) | |
| O41—H42⋯O51 | 0.90 (2) | 1.91 (2) | 2.810 (2) | 172 (2) | |
| O51—H51⋯O31i | 0.90 (2) | 1.91 (2) | 2.810 (2) | 172 (2) | |
| O51—H52⋯O22xii | 0.77 (2) | 2.25 (2) | 2.9544 (19) | 153 (2) | |
| C25—H25⋯O36i | 0.93 | 2.58 | 3.433 (2) | 153 | |
| (XII) | N1—H11⋯O33 | 0.868 (18) | 2.224 (18) | 2.9120 (19) | 136.1 (16) |
| N1—H12⋯O31i | 0.900 (18) | 1.833 (18) | 2.7142 (18) | 165.9 (16) | |
| N1—H12⋯O32i | 0.900 (19) | 2.593 (17) | 3.154 (2) | 121.2 (13) | |
| C6—H6A⋯O34xiii | 0.97 | 2.56 | 3.423 (2) | 148 | |
| (XIII) | O33—H33⋯O32 | 1.07 (2) | 1.37 (2) | 2.4447 (16) | 177.7 (16) |
| O43—H43⋯O42 | 1.00 (2) | 1.48 (2) | 2.4707 (17) | 174.0 (17) | |
| N11—H111⋯O32 | 0.927 (17) | 1.891 (17) | 2.8122 (18) | 172.3 (16) | |
| N11—H112⋯O41xiv | 0.930 (17) | 1.848 (17) | 2.7725 (17) | 172.9 (13) | |
| N21—H211⋯O42 | 0.975 (15) | 1.821 (15) | 2.7926 (16) | 174.5 (14) | |
| N21—H212⋯O31 | 0.895 (15) | 2.283 (15) | 2.9776 (17) | 134.4 (12) | |
| N21—H212⋯O34xv | 0.895 (15) | 2.428 (15) | 3.1170 (18) | 134.1 (12) | |
| C16—H16A⋯O34xv | 0.97 | 2.55 | 3.341 (2) | 138 | |
| C16—H16B⋯O44xv | 0.97 | 2.52 | 3.338 (2) | 141 | |
| C25—H25B⋯Cg4xvi | 0.97 | 2.92 | 3.8440 (16) | 159 | |
| (XIV) | N1—H11⋯O31 | 0.89 | 2.01 | 2.894 (5) | 171 |
| N1—H11⋯O33 | 0.89 | 1.73 | 2.584 (7) | 160 | |
| N1—H12⋯O41 | 0.89 | 1.97 | 2.8251 (15) | 161 | |
| O32—H32⋯O32xvii | 0.82 | 1.54 | 2.355 (7) | 176 | |
| O34—H34⋯O34xvii | 0.82 | 2.03 | 2.820 (9) | 161 | |
| O42—H42⋯O42xviii | 0.82 | 1.62 | 2.4352 (12) | 177 | |
| (XV) | N1—H11⋯O31 | 0.79 (4) | 2.40 (4) | 3.028 (4) | 137 (3) |
| N1—H11⋯O36xii | 0.79 (4) | 2.43 (4) | 2.977 (4) | 128 (3) | |
| N1—H11⋯O35xix | 0.79 (4) | 2.50 (3) | 2.942 (3) | 117 (3) | |
| N1—H12⋯O41 | 0.89 (4) | 1.91 (4) | 2.792 (5) | 168 (3) | |
| O33—H33⋯O34xx | 0.77 (4) | 2.14 (4) | 2.800 (3) | 144 (4) | |
| O34—H34⋯O31xx | 0.82 (4) | 2.11 (4) | 2.836 (3) | 148 (3) | |
| O36—H36⋯O32ii | 0.81 (4) | 1.68 (4) | 2.478 (3) | 167(3 | |
| O41—H41⋯O33xxi | 0.82 (5) | 1.94 (5) | 2.753 (4) | 167 (3) | |
| O41—H42⋯O31xii | 0.87 (5) | 1.90 (5) | 2.766 (4) | 169 (3) | |
| O51—H51⋯O41 | 0.98 (4) | 1.80 (5) | 2.776 (5) | 172 (9) | |
| O51—H52⋯O22xii | 0.97 (7) | 2.22 (7) | 3.054 (7) | 144 (6) | |
| O51—H52⋯N4xii | 0.97 (7) | 2.48 (6) | 3.307 (6) | 143 (5) | |
| C23—H23⋯Cg2xxii | 0.93 | 2.91 | 3.722 (4) | 147 |
Symmetry codes: (i) 1 − x, 1 − y, 1 − z; (ii) 1 + x, y, z; (iii) x, 1 − y, − + z; (iv) −
+ z; (iv) − + x,
+ x,  − y, −
− y, − + z; (v)
+ z; (v)  + x, −
+ x, − + y, z; (vi) −1 + x, y, 1 + z; (vii)
+ y, z; (vi) −1 + x, y, 1 + z; (vii)  + x,
+ x,  − y, 1 − z; (Viii)
− y, 1 − z; (Viii)  − x, 1 − y, −
− x, 1 − y, − + z; (ix) −
+ z; (ix) − + x,
+ x,  − y, 1 − z; (x)
− y, 1 − z; (x)  − x, 1 − y,
− x, 1 − y,  + z; (xi) x,
+ z; (xi) x,  − y, −
− y, − + z; (xii) −1 + x, y, z; (xiii) x, 1 + y, z; (xiv) x, −1 + y, z; (xv) −x, 1 − y, 1 − z; (xvi) 1 − x, −y, −z; (xvii) 1 − x, 1 − y, 2 − z; (xviii) 1 − x, −y, 2 − z; (xix) 2 − x,
+ z; (xii) −1 + x, y, z; (xiii) x, 1 + y, z; (xiv) x, −1 + y, z; (xv) −x, 1 − y, 1 − z; (xvi) 1 − x, −y, −z; (xvii) 1 − x, 1 − y, 2 − z; (xviii) 1 − x, −y, 2 − z; (xix) 2 − x,  + y, 1 − z; (xx) 2 − x, −
+ y, 1 − z; (xx) 2 − x, − + y, 1 − z; (xxi) −1 + x, 1 + y, z; (xxii) 1 − x,
+ y, 1 − z; (xxi) −1 + x, 1 + y, z; (xxii) 1 − x,  + y, −z.
+ y, −z.
Supramolecular features
The supramolecular assembly in the salts (I)–(XV) is based on N—H⋯O and O—H⋯O hydrogen bonds augmented in a number of cases by C—H⋯O and C—H⋯π(arene) hydrogen bonds. In general, we have discounted hydrogen bonds having D—H⋯A angles that are significantly less than 140°, as the interaction energies associated with such contacts are likely to be very low, so that these cannot be regarded as structurally significant (Wood et al., 2009 ▸). We have also discounted short contacts involving the H atoms of the methyl groups, as such groups are likely to be undergoing very rapid rotation about the adjacent C—O bonds (Riddell & Rogerson, 1996 ▸, 1997 ▸). Most of the C—H⋯π(arene) contacts have H⋯Cg distances in excess of 2.85 Å, and we have therefore only considered the effects of such contacts in the assembly of compounds (III) and (IV), where these distances are below 2.80 Å. It should perhaps be conceded here that these are somewhat arbitrary judgments, made with the primary aim of avoiding over-interpretation of the longer contacts and over-complication of the crystal structure descriptions.
In each of the isostructural pair of compounds (I) and (II), two N—H⋯O hydrogen bonds (Table 1 ▸) link the ionic components into a centrosymmetric four-ion aggregate, characterized by an  (12) (Etter, 1990 ▸; Etter et al., 1990 ▸; Bernstein et al., 1995 ▸) motif (Fig. 16 ▸). A similar motif occurs in the structure of compound (III) (Fig. 17 ▸), but the different orientations of the unit-cell outline in Figs. 16 ▸ and 17 ▸, illustrate the different arrangements of the components in compounds (I) and (II) on the one hand and compound (III) on the other. In (III), the four-ion aggregates are linked into chains by a C—H⋯π(arene) interaction, but the C—H⋯O contact in (III) has a very small D—H⋯A angle and is thus not structurally significant (Wood et al., 2009 ▸).
(12) (Etter, 1990 ▸; Etter et al., 1990 ▸; Bernstein et al., 1995 ▸) motif (Fig. 16 ▸). A similar motif occurs in the structure of compound (III) (Fig. 17 ▸), but the different orientations of the unit-cell outline in Figs. 16 ▸ and 17 ▸, illustrate the different arrangements of the components in compounds (I) and (II) on the one hand and compound (III) on the other. In (III), the four-ion aggregates are linked into chains by a C—H⋯π(arene) interaction, but the C—H⋯O contact in (III) has a very small D—H⋯A angle and is thus not structurally significant (Wood et al., 2009 ▸).
Figure 16.
Part of the crystal structure of compound (I) showing the formation of a centrosymmetric four-ion aggregate. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted. The atoms marked with an asterisk (*) are at the symmetry position (1 − x, 1 − y, 1 − z).
Figure 17.
Part of the crystal structure of compound (III) showing the formation of a centrosymmetric four-ion aggregate. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted. The atoms marked with an asterisk (*) are at the symmetry position (1 − x, 1 − y, 1 − z).
The hydrogen bonding involving the two disorder components in compound (IV) are very similar (Table 1 ▸) and thus only the major component needs to be considered here. The combination of two N—H⋯O hydrogen bonds and one C—H⋯π(arene) hydrogen bond, involving atom C34 as the donor, links the ions into a three-dimensional network, whose formation is readily analysed in terms of three one-dimensional sub-structures (Ferguson et al., 1998a
▸,b
▸; Gregson et al., 2000 ▸). In addition to the N—H⋯O hydrogen bond forming the ion pair, which defines the selected asymmetric unit, we consider in turn the linking of these ion pairs by the action of the N—H⋯O hydrogen bond involving atom H12, acting alone; by that of the C—H⋯π(arene) hydrogen bond acting alone; and finally by that of the two hydrogen bonds in combination. The ion pairs are linked by a second N—H⋯O hydrogen bond to form a  (6) chain running parallel to the [001] direction (Fig. 18 ▸), and they are linked by the C—H⋯π(arene) hydrogen bond to form a chain running parallel to [101] (Fig. 19 ▸). The N—H⋯O and C—H⋯π hydrogen bonds, acting alternately, generate a chain running parallel to the [112] direction (Fig. 20 ▸), and the combination of chains running parallel to [001], [101] and [112] suffices to generate a three-dimensional structure. In the 2-chlorobenzoate analogue, compound (V), two independent N-H⋯O hydrogen bonds again link the ions into a centrosymmetric
(6) chain running parallel to the [001] direction (Fig. 18 ▸), and they are linked by the C—H⋯π(arene) hydrogen bond to form a chain running parallel to [101] (Fig. 19 ▸). The N—H⋯O and C—H⋯π hydrogen bonds, acting alternately, generate a chain running parallel to the [112] direction (Fig. 20 ▸), and the combination of chains running parallel to [001], [101] and [112] suffices to generate a three-dimensional structure. In the 2-chlorobenzoate analogue, compound (V), two independent N-H⋯O hydrogen bonds again link the ions into a centrosymmetric  (12) motif, of the type observed in compounds (I)–(III). There are two C—H⋯π(arene) contacts in (V), but these are both long, and probably not structurally significant.
(12) motif, of the type observed in compounds (I)–(III). There are two C—H⋯π(arene) contacts in (V), but these are both long, and probably not structurally significant.
Figure 18.
Part of the crystal structure of compound (IV) showing the linking of the ion pairs by a further N—H⋯O hydrogen bond to form a  (6) chain running parallel to [001]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the minor disorder component and the H atoms bonded to C atoms have been omitted.
(6) chain running parallel to [001]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the minor disorder component and the H atoms bonded to C atoms have been omitted.
Figure 19.

Part of the crystal structure of compound (IV) showing the linking of the ions pairs by a C—H⋯π(arene) hydrogen bond to form a chain parallel to [101]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the minor disorder component and the H atoms not involved in the motif shown have been omitted.
Figure 20.
Part of the crystal structure of compound (IV) showing the alternating action of N—H⋯O and C—H⋯π(arene) hydrogen bonds in linking the ion pairs into a chain parallel to [112]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the minor disorder component and the H atoms not involved in the motif shown have been omitted.
The ion pairs in compounds (VI) and (VII) are again linked into three-dimensional arrays, by a combination of N—H⋯O and C—H⋯O hydrogen bonds, as opposed to the N—H⋯O and C—H⋯π(arene) interactions in the structure of (IV). An N—H⋯O hydrogen bond links ion pairs which are related by the 21 screw axis along (x, 1/4, 1/2) to form a  (4) chain along [100] (Fig. 21 ▸). In addition, the ion pairs which are related by the 21 screw axis along (1/4, 1/2, z) are linked by a C—H⋯O hydrogen bond to form a
(4) chain along [100] (Fig. 21 ▸). In addition, the ion pairs which are related by the 21 screw axis along (1/4, 1/2, z) are linked by a C—H⋯O hydrogen bond to form a  (12) chain along [001] (Fig. 22 ▸), while the alternating action of the N—H⋯O and C—H⋯O hydrogen bonds generates a chain running parallel to the [010] direction (Fig. 23 ▸). The combination of chains along [100], [010] and [001] thus generates a three-dimensional array.
(12) chain along [001] (Fig. 22 ▸), while the alternating action of the N—H⋯O and C—H⋯O hydrogen bonds generates a chain running parallel to the [010] direction (Fig. 23 ▸). The combination of chains along [100], [010] and [001] thus generates a three-dimensional array.
Figure 21.
Part of the crystal structure of compound (VI) showing the formation of a  (4) chain running parallel to [100], in which ion pairs are linked by a further N—H⋯O hydrogen bond. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
(4) chain running parallel to [100], in which ion pairs are linked by a further N—H⋯O hydrogen bond. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
Figure 22.

Part of the crystal structure of compound (VI) showing the formation of a  (12) chain running parallel to [001], in which ion pairs are linked by a C—H⋯O hydrogen bond. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms not involved in the motif shown have been omitted.
(12) chain running parallel to [001], in which ion pairs are linked by a C—H⋯O hydrogen bond. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms not involved in the motif shown have been omitted.
Figure 23.
Part of the crystal structure of compound (VI) showing the formation of a chain running parallel to [010], in which ion pairs are linked by alternating N—H⋯O and C—H⋯O hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms not involved in the motif shown have been omitted.
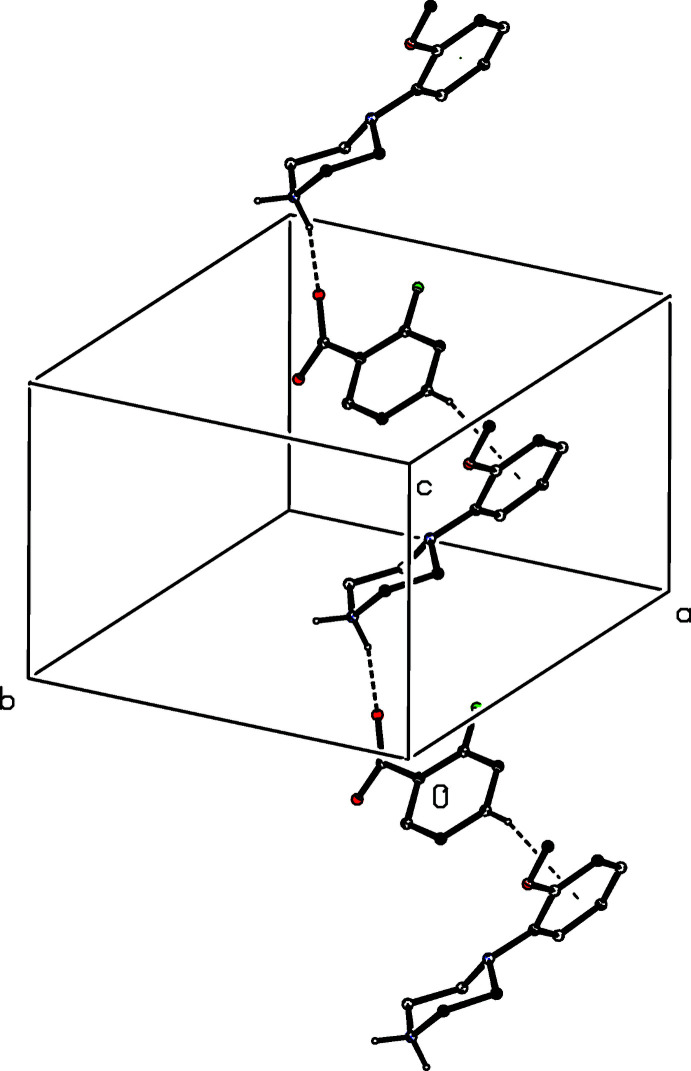
The ions in compound (VIII) are linked by two N—H⋯O hydrogen bonds to form an  (12) four-ion aggregate analogous to those observed in compounds (I)–(III) and (V). Similar four-ion aggregates are also found in compounds (IX) and (X), but in (IX) they are linked by a further N—H⋯O hydrogen bond, involving the amino group, to form a complex sheet lying parallel to (100) (Fig. 24 ▸). In the dihydrate (XI), each water molecule acts as a single acceptor and a double donor of hydrogen bonds (Table 1 ▸), and supramolecular aggregation takes the form of a complex ribbon running parallel to the [100] direction (Fig. 25 ▸). In the picrate salt (XII), a combination of two independent N—H⋯O hydrogen bonds links the components into a centrosymmetric four-ion aggregate of
(12) four-ion aggregate analogous to those observed in compounds (I)–(III) and (V). Similar four-ion aggregates are also found in compounds (IX) and (X), but in (IX) they are linked by a further N—H⋯O hydrogen bond, involving the amino group, to form a complex sheet lying parallel to (100) (Fig. 24 ▸). In the dihydrate (XI), each water molecule acts as a single acceptor and a double donor of hydrogen bonds (Table 1 ▸), and supramolecular aggregation takes the form of a complex ribbon running parallel to the [100] direction (Fig. 25 ▸). In the picrate salt (XII), a combination of two independent N—H⋯O hydrogen bonds links the components into a centrosymmetric four-ion aggregate of  (16) type, where the two acceptor are the phenolic atom O31 and one of the nitro O atoms (Fig. 26 ▸). Aggregates of this type are weakly linked into a chain of rings by a C—H⋯O hydrogen bond.
(16) type, where the two acceptor are the phenolic atom O31 and one of the nitro O atoms (Fig. 26 ▸). Aggregates of this type are weakly linked into a chain of rings by a C—H⋯O hydrogen bond.
Figure 24.

Part of the crystal structure of compound (IX) showing the formation of a hydrogen-bonded sheet lying parallel to (100). Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
Figure 25.
Part of the crystal structure of compound (XI) showing the formation of a hydrogen-bonded ribbon running parallel to [100]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
Figure 26.
Part of the crystal structure of compound (XII) showing the formation of a centrosymmetric four-ion aggregate. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted and only the major disorder component is shown. The atoms marked with an asterisk (*) are at the symmetry position (1 − x, 1 − y, 1 − z).
In compound (XIII), where Z′ = 2, each of the anions contains a very short O—H⋯O hydrogen bond, although in each of these interactions the two O—H distances are significantly different (Table 1 ▸). The supramolecular assembly depends upon three independent two-centre N—H⋯O hydrogen bonds and one three-centre N—H⋯(O)2 hydrogen bond. These link the ions into a ribbon, or molecular ladder, running parallel to the [010] direction and in which  (14) rings centred at (0, n + 1/2, 1/2) alternate with
(14) rings centred at (0, n + 1/2, 1/2) alternate with  (30) rings centred at (0, n, 1/2), where n represents an integer in each case (Fig. 27 ▸). Analysis of the supramolecular assembly in compound (XIV) is complicated by the combination of centrosymmetric anions and the disorder exhibited by one of them. However, since the hydrogen bonds involving the two disorder components are very similar, only the major disorder components need to be considered here. The ordered anions are linked by O—H⋯O hydrogen bonds into a chain along (x, 0, 1) and the disordered anions are similarly linked into a chain along (x, 1/2, 1). The two types of chain, which alternate along the [010] direction, are linked by the cations to form a sheet of
(30) rings centred at (0, n, 1/2), where n represents an integer in each case (Fig. 27 ▸). Analysis of the supramolecular assembly in compound (XIV) is complicated by the combination of centrosymmetric anions and the disorder exhibited by one of them. However, since the hydrogen bonds involving the two disorder components are very similar, only the major disorder components need to be considered here. The ordered anions are linked by O—H⋯O hydrogen bonds into a chain along (x, 0, 1) and the disordered anions are similarly linked into a chain along (x, 1/2, 1). The two types of chain, which alternate along the [010] direction, are linked by the cations to form a sheet of  (26) rings lying parallel to (001) (Fig. 28 ▸). In the structure of compound (XV), the anions are linked by three independent O—H⋯O hydrogen bonds, in which both of the hydroxyl groups as well as the carboxyl group act as donors, to form a sheet lying parallel to (001), in which both
(26) rings lying parallel to (001) (Fig. 28 ▸). In the structure of compound (XV), the anions are linked by three independent O—H⋯O hydrogen bonds, in which both of the hydroxyl groups as well as the carboxyl group act as donors, to form a sheet lying parallel to (001), in which both  (18) and
(18) and  (20) rings can be identified (Fig. 29 ▸). The cations and the water molecules are tethered to this sheet, markedly increasing its complexity but without changing the dimensionality of the overall assembly. The result is a thick tripartite sheet, occupying the whole domain 0 < z < 1.0 and having a hydrogen-bonded layer in the centre with the aryl groups on the outside surfaces: there are no direction-specific interactions between adjacent sheets.
(20) rings can be identified (Fig. 29 ▸). The cations and the water molecules are tethered to this sheet, markedly increasing its complexity but without changing the dimensionality of the overall assembly. The result is a thick tripartite sheet, occupying the whole domain 0 < z < 1.0 and having a hydrogen-bonded layer in the centre with the aryl groups on the outside surfaces: there are no direction-specific interactions between adjacent sheets.
Figure 27.
Part of the crystal structure of compound (XIII) showing the formation of a hydrogen-bonded ribbon of  (14) and
(14) and  (30) rings running parallel to [010]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
(30) rings running parallel to [010]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
Figure 28.
Part of the crystal structure of compound (XIV) showing the formation of a hydrogen-bonded sheet of  (26) rings lying parallel to [001]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
(26) rings lying parallel to [001]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
Figure 29.
Part of the crystal structure of compound (XV) showing the formation of a hydrogen-bonded sheet of anions lying parallel to (001). Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the H atoms bonded to C atoms have been omitted.
In summary, therefore, the hydrogen-bonded assembly is finite, or zero-dimensional in compounds (I)–(III), (V), (VIII) and (X); one-dimensional in (XI), (XII) and (XIII); two-dimensional in (IX), (XIV) and (XV); and three–dimensional in (IV), (VI) and (VII).
Database survey
It is of interest briefly to compare the structures of the compounds reported here with those of some closely related examples, in particular the salts formed by the isomeric N-(4-methoxyphenyl)piperazine (4-MeOPP) and the analogous N-(4-fluorophenyl)piperazine (4-FPP). The salts formed between 4-MeOPP and the benzoic acids 4-XC6H4COOH, where X = H, F, Cl, and Br, all crystallize as stoichiometric monohydrates and they are all isomorphous in space group P
 (Kiran Kumar, Yathirajan, Foro et al., 2019 ▸), a combination of N—H⋯O, O—H⋯O, C—H⋯O and C—H⋯π(arene) hydrogen bonds links the components into complex sheets. By contrast, compounds (I)–(III) reported here all crystallize in solvent-free form and all form finite centrosymmetric four-ion aggregates (Figs. 16 ▸ and 17 ▸). The salt formed between 4-MeOPP and 4-aminobenzoate crystallizes as a monohydrate (Kiran Kumar et al., 2020 ▸), as compared with the solvent free analogues (IX) reported here, and the components are linked by a combination of N—H⋯O, O—H⋯O and C—H⋯π(arene) hydrogen bonds to form a three-dimensional assembly, as compared with the two-dimensional assembly in (IX). The 3,5-dinitrobenzoate salt with 4-MeOPP crystallizes in solvent-free form (Kiran Kumar et al., 2020 ▸), as opposed to the dihydrate (XI) reported here, and the component ions are linked into the simple
(Kiran Kumar, Yathirajan, Foro et al., 2019 ▸), a combination of N—H⋯O, O—H⋯O, C—H⋯O and C—H⋯π(arene) hydrogen bonds links the components into complex sheets. By contrast, compounds (I)–(III) reported here all crystallize in solvent-free form and all form finite centrosymmetric four-ion aggregates (Figs. 16 ▸ and 17 ▸). The salt formed between 4-MeOPP and 4-aminobenzoate crystallizes as a monohydrate (Kiran Kumar et al., 2020 ▸), as compared with the solvent free analogues (IX) reported here, and the components are linked by a combination of N—H⋯O, O—H⋯O and C—H⋯π(arene) hydrogen bonds to form a three-dimensional assembly, as compared with the two-dimensional assembly in (IX). The 3,5-dinitrobenzoate salt with 4-MeOPP crystallizes in solvent-free form (Kiran Kumar et al., 2020 ▸), as opposed to the dihydrate (XI) reported here, and the component ions are linked into the simple  (12) motif found here for compounds (I)–(III), (VIII) and (X). The picrate salt of 4-MeOPP exhibits orientational disorder in one of the nitro groups (Kiran Kumar et al., 2020 ▸), as observed in compound (XII) here, but the supramolecular aggregation is more complex than the simple aggregate found for (XII), in that a combination of N—H⋯O and C—H⋯π(arene) hydrogen bonds generates a sheet structure. The anion in the hydrogen maleate salt of 4-MeOPP, which crystallizes with Z′ = 1 (Kiran Kumar, Yathirajan, Foro et al., 2019 ▸) unlike the Z′ = 2 for compound (XIII), contains a very short, but unsymmetrical O—H⋯O hydrogen bond, and the ions are linked into a chain of rings by a combination of two-centre N—H⋯O and three-centre N—H⋯(O,O) hydrogen bonds. By contrast with compound (XIV) reported here where there are two independent hydrogen fumarate anions each lying across a centre of inversion, in the hydrogen fumarate salt of 4-MeOPP, there is only one type of anion, although this exhibits some orientational disorder and Z′ = 1: a combination of N—H⋯O and O—H⋯O and C—H⋯π(arene) hydrogen bonds links the ions into a three-dimensional structure, as opposed to the two-dimensional structure of (XIV). Finally, we note some salts formed by 4-FPP with organic acids (Harish Chinthal, Yathirajan, Archana et al., 2020 ▸; Harish Chinthal, Yathirajan, Kavitha et al., 2020 ▸). The 2-fluorobenzoate crystallizes as a stoichiometric monohydrate, and the 2-bromobenzoate as a partial hydrate, while the 2-iodobenzoate crystallizes in solvent-free form (Harish Chinthal, Yathirajan, Kavitha et al., 2020 ▸), in contrast to compounds (IV)–(VII), which are all solvent-free, and the 3,5-dinitrobenzoate salt of 4-FPP is also solvent-free, as opposed to the dihydrate (XI). The 1:1 acid salt formed between (2R,3R)-tartaric acid and 4-FPP crystallizes as a monohydrate (Harish Chinthal, Yathirajan, Archana et al., 2020 ▸), whereas the analogous compound (XV) crystallizes as a 1.70 (hydrate).
(12) motif found here for compounds (I)–(III), (VIII) and (X). The picrate salt of 4-MeOPP exhibits orientational disorder in one of the nitro groups (Kiran Kumar et al., 2020 ▸), as observed in compound (XII) here, but the supramolecular aggregation is more complex than the simple aggregate found for (XII), in that a combination of N—H⋯O and C—H⋯π(arene) hydrogen bonds generates a sheet structure. The anion in the hydrogen maleate salt of 4-MeOPP, which crystallizes with Z′ = 1 (Kiran Kumar, Yathirajan, Foro et al., 2019 ▸) unlike the Z′ = 2 for compound (XIII), contains a very short, but unsymmetrical O—H⋯O hydrogen bond, and the ions are linked into a chain of rings by a combination of two-centre N—H⋯O and three-centre N—H⋯(O,O) hydrogen bonds. By contrast with compound (XIV) reported here where there are two independent hydrogen fumarate anions each lying across a centre of inversion, in the hydrogen fumarate salt of 4-MeOPP, there is only one type of anion, although this exhibits some orientational disorder and Z′ = 1: a combination of N—H⋯O and O—H⋯O and C—H⋯π(arene) hydrogen bonds links the ions into a three-dimensional structure, as opposed to the two-dimensional structure of (XIV). Finally, we note some salts formed by 4-FPP with organic acids (Harish Chinthal, Yathirajan, Archana et al., 2020 ▸; Harish Chinthal, Yathirajan, Kavitha et al., 2020 ▸). The 2-fluorobenzoate crystallizes as a stoichiometric monohydrate, and the 2-bromobenzoate as a partial hydrate, while the 2-iodobenzoate crystallizes in solvent-free form (Harish Chinthal, Yathirajan, Kavitha et al., 2020 ▸), in contrast to compounds (IV)–(VII), which are all solvent-free, and the 3,5-dinitrobenzoate salt of 4-FPP is also solvent-free, as opposed to the dihydrate (XI). The 1:1 acid salt formed between (2R,3R)-tartaric acid and 4-FPP crystallizes as a monohydrate (Harish Chinthal, Yathirajan, Archana et al., 2020 ▸), whereas the analogous compound (XV) crystallizes as a 1.70 (hydrate).
Synthesis and crystallization
All reagents were obtained commercially, and all were used as received. For the synthesis of compounds (I)–(XV), solutions of N-(2-methoxyphenyl)piperazine (100 mg, 0.52 mmol) in methanol (10 ml) were mixed with an equimolar quantity of the appropriate acid [4-chlorobenzoic acid (82 mg) for (I), 4-bromobenzoic acid (103 mg) for (II), 4-iodobenzoic acid (129 mg) for (III), 2-fluorobenzoic acid (73 mg) for (IV), 2-chlorobenzoic acid (82 mg) for (V), 2-bromobenzoic acid (103 mg) for (VI), 2-iodobenzoic acid (129 mg) for (VII), 2-methylbenzoic acid (71 mg) for (VIII), 4-aminobenzoic acid (72 mg) for (IX), 4-nitrobenzoic acid (97 mg) for (X), 3,5-dinitrobenzoic acid (110 mg) for (XI), picric acid (120 mg) for (XII), maleic acid (61 mg) for (XIII), fumaric acid (61 mg) for (XIV) and (2R,3R)-tartaric acid (78 mg) for (XV)] also dissolved in methanol (10 ml). These mixtures were then heated briefly at 323 K with magnetic stirring and then set aside to crystallize at room temperature. The resulting products were then collected by filtration and dried in air. Crystals suitable for single-crystal X-ray diffraction were grown by slow evaporation, at ambient temperature and in the presence of air, of solutions in acetone/acetonitrile (initial composition 1:1, v/v) for (I), methanol/acetonitrile (1:6, v/v) for (II), methanol/acetonitrile (1:1, v/v) for (III), ethyl acetate/acetone (2:1, v/v) for (IV) and (V), methanol/ethyl acetate (1:7, v/v) for (VI) and (VII), methanol for (VIII), (X), and (XIII)–(XV), methanol/ethyl acetate (3:2, v/v) for (IX), and methanol/ethyl acetate (1:1, v/v) for (XI) and (XII). M.p. (I) 374–378 K, (II) 390–394 K, (III) 422–428 K, (IV) 384–387 K, (V) 396–389 K, (VI) 396–399 K, (VII) 402–408 K, (VIII) 389–393 K, (IX) 441–445 K, (X) 408–412 K, (XI) 437–442 K, (XII) 430–435 K, (XIII) 390–396 K, (XIV) 435–437 K, (XV) 407–411 K.
Refinement
Crystal data, data collection and refinement details are summarized in Table 2 ▸. Two bad outlier reflections [(1,4,0) and (1,2,2)] were removed from the dataset for compound (V), and one bad outlier reflection (0, ,13) was removed from the dataset for compound (XV) before the final refinements. For compound (IV), calculation of the Flack x parameter (Flack, 1983 ▸) using 1089 quotients of the type [(I
+) − (I
−)]/[(I
+) + (I
−)] (Parsons et al., 2013 ▸) gave a value 0.2 (3) in the absence of significant resonant scattering, the correct orientation of the structure of (IV) with respect to the polar axis directions remains uncertain. The correct absolute configurations for compounds (VI) and (VII) were established from the Flack x parameters: for (VI)
x = 0.004 (5) calculated using 919 coefficients, and for (VII)
x = 0.004 (10) calculated using 1045 coefficients. For the minor disorder component in compound (IV), the bonded distances and the 1,3-non-bonded distances were restrained to be the same as the corresponding distances in the major disorder components, subject to s.u. values of 0.01 and 0.02 Å, respectively, and the anisotropic displacement parameters for corresponding pairs of atoms in the two disorder components were constrained to be the same, giving occupancies of 0.907 (8) and 0.093 (8). Similar distance restraints were applied to the disordered carboxylate group in compound (VII), where the displacement parameters for the disordered O atoms were subjected to similarity restraints, giving occupancies of 0.53 (9) and 0.47 (9). The disordered nitro group in compound (XII) was modelled over three sets of atomic sites, with similar restraints to those imposed in (VII) giving occupancies of 0.860 (5), 0.080 (4) and 0.069 (4). All H atoms, apart from those in the minor disorder component of compound (IV) and in the partial-occupancy water molecule in compound (V), were located in difference maps. The H atoms bonded to C atoms, apart from those in the disordered anion of compound (XIV) which were permitted to ride at the locations found in difference maps, were then treated as riding atoms in geometrically idealized positions with C—H distances of 0.93 Å (alkenyl and aromatic), 0.96 Å (CH3), 0.97 Å (CH2) or 0.98 Å (aliphatic C—H), and with U
iso(H) = kU
eq(C), where k = 1.5 for the methyl groups, which were permitted to rotate but not to tilt, and 1.2 for all other H atoms bonded to C atoms: the H atoms in the minor disorder component of compound (IV) were included on exactly the same basis. For the H atoms bonded to N atoms, these were treated as riding atoms in the disordered structures (VII) and (XIV) with N—H distances of 0.89 Å and U
isoH = 1.2U
eq(N), but in all other compounds, the atomic coordinates of the H atoms bonded to N atoms were refined with U
isoH = 1.2U
eq(N), giving the N—H distances shown in Table 1 ▸. For the H atoms bonded to O atoms in compounds (XI), (XIII) and (XV), the atomic coordinates were refined with U
iso(H) = 1.5U
eq(O), giving the O—H distances shown in Table 1 ▸, but the partial occupancy H atoms bonded to O atoms in compound (XIV) were treated as riding atoms with O—H = 0.82 Å and U
iso(H) = 1.5U
eq(O).
,13) was removed from the dataset for compound (XV) before the final refinements. For compound (IV), calculation of the Flack x parameter (Flack, 1983 ▸) using 1089 quotients of the type [(I
+) − (I
−)]/[(I
+) + (I
−)] (Parsons et al., 2013 ▸) gave a value 0.2 (3) in the absence of significant resonant scattering, the correct orientation of the structure of (IV) with respect to the polar axis directions remains uncertain. The correct absolute configurations for compounds (VI) and (VII) were established from the Flack x parameters: for (VI)
x = 0.004 (5) calculated using 919 coefficients, and for (VII)
x = 0.004 (10) calculated using 1045 coefficients. For the minor disorder component in compound (IV), the bonded distances and the 1,3-non-bonded distances were restrained to be the same as the corresponding distances in the major disorder components, subject to s.u. values of 0.01 and 0.02 Å, respectively, and the anisotropic displacement parameters for corresponding pairs of atoms in the two disorder components were constrained to be the same, giving occupancies of 0.907 (8) and 0.093 (8). Similar distance restraints were applied to the disordered carboxylate group in compound (VII), where the displacement parameters for the disordered O atoms were subjected to similarity restraints, giving occupancies of 0.53 (9) and 0.47 (9). The disordered nitro group in compound (XII) was modelled over three sets of atomic sites, with similar restraints to those imposed in (VII) giving occupancies of 0.860 (5), 0.080 (4) and 0.069 (4). All H atoms, apart from those in the minor disorder component of compound (IV) and in the partial-occupancy water molecule in compound (V), were located in difference maps. The H atoms bonded to C atoms, apart from those in the disordered anion of compound (XIV) which were permitted to ride at the locations found in difference maps, were then treated as riding atoms in geometrically idealized positions with C—H distances of 0.93 Å (alkenyl and aromatic), 0.96 Å (CH3), 0.97 Å (CH2) or 0.98 Å (aliphatic C—H), and with U
iso(H) = kU
eq(C), where k = 1.5 for the methyl groups, which were permitted to rotate but not to tilt, and 1.2 for all other H atoms bonded to C atoms: the H atoms in the minor disorder component of compound (IV) were included on exactly the same basis. For the H atoms bonded to N atoms, these were treated as riding atoms in the disordered structures (VII) and (XIV) with N—H distances of 0.89 Å and U
isoH = 1.2U
eq(N), but in all other compounds, the atomic coordinates of the H atoms bonded to N atoms were refined with U
isoH = 1.2U
eq(N), giving the N—H distances shown in Table 1 ▸. For the H atoms bonded to O atoms in compounds (XI), (XIII) and (XV), the atomic coordinates were refined with U
iso(H) = 1.5U
eq(O), giving the O—H distances shown in Table 1 ▸, but the partial occupancy H atoms bonded to O atoms in compound (XIV) were treated as riding atoms with O—H = 0.82 Å and U
iso(H) = 1.5U
eq(O).
Table 2. Experimental details.
| (I) | (II) | (III) | (IV) | (V) | |
|---|---|---|---|---|---|
| Crystal data | |||||
| Chemical formula | C11H17N2O+·C7H4ClO2 − | C11H17N2O+·C7H4BrO2 − | C11H17N2O+·C7H4IO2 − | C11H17N2O+·C7H4FO2 − | C11H17N2O+·C7H4ClO2 − |
| M r | 348.82 | 393.27 | 440.27 | 332.37 | 348.82 |
| Crystal system, space group | Triclinic, P

|
Triclinic, P

|
Triclinic, P

|
Monoclinic, C c | Monoclinic, P21/c |
| Temperature (K) | 296 | 296 | 296 | 296 | 296 |
| a, b, c (Å) | 7.401 (1), 7.888 (1), 15.410 (3) | 7.4313 (5), 7.9163 (5), 15.5212 (9) | 7.1129 (4), 11.2722 (7), 12.5923 (8) | 19.940 (1), 10.2705 (7), 9.0148 (7) | 7.9974 (8), 27.611 (2), 8.5972 (9) |
| α, β, γ (°) | 100.28 (2), 94.40 (1), 94.14 (1) | 101.565 (5), 94.780 (5), 92.691 (5) | 69.852 (5), 74.681 (5), 79.121 (5) | 90, 109.663 (8), 90 | 90, 106.40 (1), 90 |
| V (Å3) | 879.2 (2) | 889.54 (10) | 908.82 (10) | 1738.5 (2) | 1821.2 (3) |
| Z | 2 | 2 | 2 | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 0.24 | 2.33 | 1.78 | 0.09 | 0.23 |
| Crystal size (mm) | 0.44 × 0.28 × 0.16 | 0.42 × 0.42 × 0.12 | 0.48 × 0.24 × 0.14 | 0.48 × 0.36 × 0.22 | 0.48 × 0.20 × 0.12 |
| Data collection | |||||
| Diffractometer | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD |
| Absorption correction | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) |
| T min, T max | 0.884, 0.963 | 0.258, 0.756 | 0.534, 0.779 | 0.884, 0.963 | 0.747, 0.973 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 6241, 3763, 2318 | 5996, 3739, 2989 | 6342, 3897, 3203 | 6204, 3343, 2786 | 13275, 3410, 2060 |
| R int | 0.019 | 0.018 | 0.012 | 0.012 | 0.030 |
| (sin θ/λ)max (Å−1) | 0.650 | 0.652 | 0.660 | 0.658 | 0.607 |
| Refinement | |||||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.053, 0.131, 1.01 | 0.043, 0.115, 1.05 | 0.026, 0.065, 1.02 | 0.036, 0.100, 1.03 | 0.067, 0.216, 1.03 |
| No. of reflections | 3763 | 3739 | 3897 | 3343 | 3410 |
| No. of parameters | 224 | 224 | 224 | 256 | 223 |
| No. of restraints | 0 | 0 | 0 | 25 | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.19, −0.27 | 0.84, −0.55 | 0.52, −0.70 | 0.24, −0.14 | 1.15, −0.30 |
| Absolute structure | – | – | – | Flack x determined using 1089 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) | – |
| Absolute structure parameter | – | – | – | 0.2 (3) | – |
| (VI) | (VII) | (VIII) | (IX) | (X) | |
|---|---|---|---|---|---|
| Crystal data | |||||
| Chemical formula | C11H17N2O+·C7H4BrO2 − | C11H17N2O+·C7H4IO2 − | C11H17N2O+·C8H7O2 − | C11H17N2O+·C7H6NO2 − | C11H17N2O+·C7H4NO4 − |
| M r | 393.28 | 440.27 | 328.40 | 329.39 | 359.38 |
| Crystal system, space group | Orthorhombic, P212121 | Orthorhombic, P212121 | Triclinic, P

|
Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 293 | 293 | 296 | 296 | 296 |
| a, b, c (Å) | 6.9824 (2), 13.2292 (4), 19.4903 (7) | 7.0101 (4), 13.3796 (6), 19.5524 (6) | 7.826 (1), 10.320 (2), 12.055 (3) | 14.922 (1), 7.6951 (5), 15.560 (1) | 7.5174 (5), 7.9761 (5), 29.860 (2) |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 78.37 (2), 78.27 (2), 73.83 (2) | 90, 106.911 (8), 90 | 90, 97.322 (6), 90 |
| V (Å3) | 1800.35 (10) | 1833.87 (14) | 904.6 (3) | 1709.4 (2) | 1775.8 (2) |
| Z | 4 | 4 | 2 | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 2.30 | 1.76 | 0.08 | 0.09 | 0.10 |
| Crystal size (mm) | 0.50 × 0.50 × 0.48 | 0.50 × 0.50 × 0.48 | 0.48 × 0.48 × 0.40 | 0.48 × 0.44 × 0.16 | 0.50 × 0.50 × 0.40 |
| Data collection | |||||
| Diffractometer | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD |
| Absorption correction | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) |
| T min, T max | 0.297, 0.331 | 0.373, 0.431 | 0.883, 0.968 | 0.830, 0.986 | 0.855, 0.961 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 13089, 3895, 2640 | 7500, 3735, 3036 | 6091, 3838, 2600 | 6720, 3668, 2606 | 13660, 3934, 2879 |
| R int | 0.033 | 0.019 | 0.013 | 0.014 | 0.019 |
| (sin θ/λ)max (Å−1) | 0.654 | 0.655 | 0.653 | 0.651 | 0.658 |
| Refinement | |||||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.035, 0.077, 0.94 | 0.032, 0.071, 1.05 | 0.042, 0.119, 1.06 | 0.039, 0.112, 1.10 | 0.040, 0.111, 1.03 |
| No. of reflections | 3895 | 3735 | 3838 | 3668 | 3934 |
| No. of parameters | 224 | 237 | 226 | 231 | 242 |
| No. of restraints | 0 | 17 | 0 | 0 | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H-atom parameters constrained | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.29, −0.53 | 0.46, −0.65 | 0.16, −0.16 | 0.15, −0.25 | 0.17, −0.15 |
| Absolute structure | Flack x determined using 919 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) | Flack x determined using 1045 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) | – | – | – |
| Absolute structure parameter | 0.004 (5) | 0.004 (10) | – | – | – |
| (XI) | (XII) | (XIII) | (XIV) | (XV) | |
|---|---|---|---|---|---|
| Crystal data | |||||
| Chemical formula | C11H17N2O+·C7H3N2O6 −·2H2O | C11H17N2O+·C6H2N3O7 − | C11H17N2O+·C4H3O4 − | C11H17N2O+·C4H3O4 − | C11H17N2O+·C4H5O6 −·1.698H2O |
| M r | 440.41 | 421.33 | 308.33 | 308.33 | 372.97 |
| Crystal system, space group | Triclinic, P

|
Triclinic, P

|
Triclinic, P

|
Triclinic, P

|
Monoclinic, P21 |
| Temperature (K) | 296 | 296 | 296 | 296 | 296 |
| a, b, c (Å) | 7.8448 (6), 11.4635 (9), 12.0747 (9) | 9.4151 (5), 9.8721 (5), 10.9572 (5) | 11.1076 (6), 11.1164 (6), 13.7649 (7) | 7.8546 (4), 8.9626 (6), 11.2056 (8) | 7.479 (1), 7.065 (1), 17.788 (3) |
| α, β, γ (°) | 94.406 (7), 105.075 (8), 93.717 (7) | 77.524 (4), 81.360 (5), 81.002 (5) | 80.353 (5), 78.353 (5), 74.406 (5) | 79.043 (5), 87.715 (5), 85.840 (5) | 90, 101.58 (2), 90 |
| V (Å3) | 1041.33 (14) | 974.97 (9) | 1591.76 (16) | 772.15 (9) | 920.8 (2) |
| Z | 2 | 2 | 4 | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 0.11 | 0.12 | 0.10 | 0.10 | 0.11 |
| Crystal size (mm) | 0.48 × 0.48 × 0.44 | 0.48 × 0.48 × 0.24 | 0.48 × 0.40 × 0.36 | 0.48 × 0.48 × 0.34 | 0.36 × 0.32 × 0.12 |
| Data collection | |||||
| Diffractometer | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD | Oxford Diffraction Xcalibur with Sapphire CCD |
| Absorption correction | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) |
| T min, T max | 0.892, 0.951 | 0.805, 0.973 | 0.863, 0.966 | 0.867, 0.967 | 0.956, 0.987 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 7353, 4419, 3409 | 12926, 4279, 3276 | 11727, 6817, 4221 | 5533, 3307, 2608 | 3655, 2895, 2062 |
| R int | 0.016 | 0.017 | 0.012 | 0.009 | 0.022 |
| (sin θ/λ)max (Å−1) | 0.654 | 0.656 | 0.657 | 0.655 | 0.658 |
| Refinement | |||||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.039, 0.108, 1.06 | 0.040, 0.119, 1.07 | 0.042, 0.121, 1.03 | 0.036, 0.105, 1.06 | 0.039, 0.081, 0.97 |
| No. of reflections | 4419 | 4279 | 6817 | 3307 | 2895 |
| No. of parameters | 300 | 317 | 415 | 240 | 263 |
| No. of restraints | 0 | 85 | 0 | 6 | 4 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H-atom parameters constrained | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.23, −0.17 | 0.24, −0.27 | 0.15, −0.17 | 0.20, −0.15 | 0.14, −0.17 |
Supplementary Material
Crystal structure: contains datablock(s) global, I, II, III, IV, V, VI, VII, VIII, IX, X, XI, XII, XIII, XIV, XV. DOI: 10.1107/S2056989020014097/hb7950sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020014097/hb7950Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989020014097/hb7950IIsup3.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989020014097/hb7950IIIsup4.hkl
Structure factors: contains datablock(s) IV. DOI: 10.1107/S2056989020014097/hb7950IVsup5.hkl
Structure factors: contains datablock(s) V. DOI: 10.1107/S2056989020014097/hb7950Vsup6.hkl
Structure factors: contains datablock(s) VI. DOI: 10.1107/S2056989020014097/hb7950VIsup7.hkl
Structure factors: contains datablock(s) VII. DOI: 10.1107/S2056989020014097/hb7950VIIsup8.hkl
Structure factors: contains datablock(s) VIII. DOI: 10.1107/S2056989020014097/hb7950VIIIsup9.hkl
Structure factors: contains datablock(s) IX. DOI: 10.1107/S2056989020014097/hb7950IXsup10.hkl
Structure factors: contains datablock(s) X. DOI: 10.1107/S2056989020014097/hb7950Xsup11.hkl
Structure factors: contains datablock(s) XI. DOI: 10.1107/S2056989020014097/hb7950XIsup12.hkl
Structure factors: contains datablock(s) XII. DOI: 10.1107/S2056989020014097/hb7950XIIsup13.hkl
Structure factors: contains datablock(s) XIII. DOI: 10.1107/S2056989020014097/hb7950XIIIsup14.hkl
Structure factors: contains datablock(s) XIV. DOI: 10.1107/S2056989020014097/hb7950XIVsup15.hkl
Structure factors: contains datablock(s) XV. DOI: 10.1107/S2056989020014097/hb7950XVsup16.hkl
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950Isup17.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IIsup18.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IIIsup19.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IVsup20.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950Vsup21.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950VIsup22.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950VIIsup23.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950VIIIsup24.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IXsup25.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950Xsup26.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIsup27.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIIsup28.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIIIsup29.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIVsup30.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
CHC thanks the University of Mysore for research facilities.
supplementary crystallographic information
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Crystal data
| C11H17N2O+·C7H4ClO2− | Z = 2 |
| Mr = 348.82 | F(000) = 368 |
| Triclinic, P1 | Dx = 1.318 Mg m−3 |
| a = 7.401 (1) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 7.888 (1) Å | Cell parameters from 3770 reflections |
| c = 15.410 (3) Å | θ = 2.6–27.8° |
| α = 100.28 (2)° | µ = 0.24 mm−1 |
| β = 94.40 (1)° | T = 296 K |
| γ = 94.14 (1)° | Plate, orange |
| V = 879.2 (2) Å3 | 0.44 × 0.28 × 0.16 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3763 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2318 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.019 |
| ω scans | θmax = 27.5°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −9→8 |
| Tmin = 0.884, Tmax = 0.963 | k = −10→6 |
| 6241 measured reflections | l = −19→19 |
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Refinement
| Refinement on F2 | Primary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.053 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.131 | w = 1/[σ2(Fo2) + (0.0498P)2 + 0.3034P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 3763 reflections | Δρmax = 0.19 e Å−3 |
| 224 parameters | Δρmin = −0.27 e Å−3 |
| 0 restraints |
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.3863 (3) | 0.5719 (3) | 0.61980 (13) | 0.0537 (6) | |
| H11 | 0.349 (3) | 0.654 (3) | 0.5787 (16) | 0.064* | |
| H12 | 0.494 (4) | 0.526 (3) | 0.6072 (17) | 0.064* | |
| C2 | 0.2453 (4) | 0.4243 (3) | 0.61032 (16) | 0.0595 (7) | |
| H2A | 0.2389 | 0.3567 | 0.5509 | 0.071* | |
| H2B | 0.1276 | 0.4677 | 0.6194 | 0.071* | |
| C3 | 0.2901 (4) | 0.3104 (3) | 0.67711 (14) | 0.0492 (6) | |
| H3A | 0.1964 | 0.2154 | 0.6709 | 0.059* | |
| H3B | 0.4051 | 0.2623 | 0.6665 | 0.059* | |
| N4 | 0.3016 (3) | 0.4131 (2) | 0.76645 (11) | 0.0417 (5) | |
| C5 | 0.4446 (3) | 0.5568 (3) | 0.77727 (15) | 0.0456 (6) | |
| H5A | 0.5614 | 0.5111 | 0.7685 | 0.055* | |
| H5B | 0.4511 | 0.6234 | 0.8369 | 0.055* | |
| C6 | 0.4046 (4) | 0.6728 (3) | 0.71121 (15) | 0.0489 (6) | |
| H6A | 0.2928 | 0.7261 | 0.7232 | 0.059* | |
| H6B | 0.5023 | 0.7641 | 0.7173 | 0.059* | |
| C21 | 0.3080 (3) | 0.3202 (3) | 0.83759 (14) | 0.0402 (5) | |
| C22 | 0.2709 (3) | 0.4034 (3) | 0.92205 (14) | 0.0401 (5) | |
| C23 | 0.2740 (3) | 0.3164 (3) | 0.99224 (16) | 0.0500 (6) | |
| H23 | 0.2531 | 0.3740 | 1.0482 | 0.060* | |
| C24 | 0.3080 (4) | 0.1439 (3) | 0.97981 (17) | 0.0565 (7) | |
| H24 | 0.3088 | 0.0854 | 1.0272 | 0.068* | |
| C25 | 0.3405 (4) | 0.0595 (3) | 0.89756 (18) | 0.0588 (7) | |
| H25 | 0.3615 | −0.0569 | 0.8889 | 0.071* | |
| C26 | 0.3420 (3) | 0.1471 (3) | 0.82735 (16) | 0.0498 (6) | |
| H26 | 0.3663 | 0.0889 | 0.7721 | 0.060* | |
| O22 | 0.2258 (2) | 0.5712 (2) | 0.92836 (10) | 0.0522 (4) | |
| C27 | 0.2117 (4) | 0.6682 (3) | 1.01422 (15) | 0.0539 (6) | |
| H27A | 0.1780 | 0.7816 | 1.0093 | 0.081* | |
| H27B | 0.3267 | 0.6779 | 1.0488 | 0.081* | |
| H27C | 0.1207 | 0.6107 | 1.0426 | 0.081* | |
| C31 | 0.2111 (3) | 0.8543 (3) | 0.37936 (15) | 0.0450 (6) | |
| C32 | 0.1780 (4) | 0.8004 (3) | 0.28892 (17) | 0.0597 (7) | |
| H32 | 0.1867 | 0.6850 | 0.2643 | 0.072* | |
| C33 | 0.1321 (4) | 0.9151 (4) | 0.23404 (17) | 0.0622 (7) | |
| H33 | 0.1104 | 0.8772 | 0.1732 | 0.075* | |
| C34 | 0.1190 (3) | 1.0848 (3) | 0.27049 (16) | 0.0498 (6) | |
| Cl34 | 0.06196 (11) | 1.23102 (10) | 0.20197 (5) | 0.0724 (3) | |
| C35 | 0.1471 (5) | 1.1410 (3) | 0.35980 (18) | 0.0712 (9) | |
| H35 | 0.1353 | 1.2559 | 0.3843 | 0.085* | |
| C36 | 0.1932 (5) | 1.0247 (3) | 0.41347 (17) | 0.0697 (9) | |
| H36 | 0.2127 | 1.0631 | 0.4744 | 0.084* | |
| C37 | 0.2650 (4) | 0.7306 (3) | 0.43954 (18) | 0.0529 (6) | |
| O31 | 0.2833 (4) | 0.7908 (3) | 0.52081 (13) | 0.0955 (8) | |
| O32 | 0.2879 (3) | 0.5797 (2) | 0.40612 (13) | 0.0704 (6) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0757 (16) | 0.0508 (13) | 0.0397 (12) | 0.0186 (11) | 0.0112 (11) | 0.0142 (10) |
| C2 | 0.079 (2) | 0.0591 (16) | 0.0380 (14) | 0.0075 (14) | −0.0055 (13) | 0.0050 (12) |
| C3 | 0.0626 (16) | 0.0437 (13) | 0.0383 (13) | 0.0038 (12) | −0.0001 (11) | 0.0014 (10) |
| N4 | 0.0525 (12) | 0.0389 (10) | 0.0326 (10) | 0.0038 (9) | 0.0011 (9) | 0.0048 (8) |
| C5 | 0.0538 (15) | 0.0426 (13) | 0.0393 (13) | 0.0030 (11) | 0.0035 (11) | 0.0057 (10) |
| C6 | 0.0641 (17) | 0.0412 (13) | 0.0419 (13) | 0.0079 (12) | 0.0088 (12) | 0.0063 (10) |
| C21 | 0.0399 (13) | 0.0386 (12) | 0.0416 (13) | 0.0046 (10) | 0.0003 (10) | 0.0072 (10) |
| C22 | 0.0420 (13) | 0.0385 (12) | 0.0402 (13) | 0.0065 (10) | 0.0027 (10) | 0.0075 (10) |
| C23 | 0.0566 (16) | 0.0540 (15) | 0.0411 (14) | 0.0043 (12) | 0.0040 (11) | 0.0131 (11) |
| C24 | 0.0690 (18) | 0.0498 (15) | 0.0544 (16) | 0.0031 (13) | −0.0027 (13) | 0.0240 (13) |
| C25 | 0.0725 (19) | 0.0388 (13) | 0.0652 (18) | 0.0073 (12) | −0.0056 (14) | 0.0143 (13) |
| C26 | 0.0602 (16) | 0.0420 (13) | 0.0458 (14) | 0.0077 (11) | 0.0015 (12) | 0.0046 (11) |
| O22 | 0.0747 (12) | 0.0445 (9) | 0.0404 (9) | 0.0210 (8) | 0.0131 (8) | 0.0070 (7) |
| C27 | 0.0599 (17) | 0.0488 (14) | 0.0502 (15) | 0.0053 (12) | 0.0115 (13) | −0.0012 (12) |
| C31 | 0.0465 (14) | 0.0442 (13) | 0.0452 (14) | 0.0083 (11) | 0.0072 (11) | 0.0075 (11) |
| C32 | 0.0740 (19) | 0.0551 (16) | 0.0502 (16) | 0.0264 (14) | 0.0077 (14) | 0.0010 (12) |
| C33 | 0.0737 (19) | 0.0767 (19) | 0.0376 (14) | 0.0250 (15) | 0.0068 (13) | 0.0065 (13) |
| C34 | 0.0507 (15) | 0.0572 (15) | 0.0455 (14) | 0.0055 (12) | 0.0047 (12) | 0.0197 (12) |
| Cl34 | 0.0783 (5) | 0.0800 (5) | 0.0671 (5) | 0.0054 (4) | −0.0011 (4) | 0.0394 (4) |
| C35 | 0.119 (3) | 0.0433 (15) | 0.0511 (17) | 0.0156 (16) | −0.0029 (16) | 0.0085 (12) |
| C36 | 0.120 (3) | 0.0498 (15) | 0.0375 (14) | 0.0171 (16) | −0.0043 (15) | 0.0048 (12) |
| C37 | 0.0586 (16) | 0.0494 (15) | 0.0546 (16) | 0.0122 (12) | 0.0095 (13) | 0.0153 (12) |
| O31 | 0.175 (2) | 0.0646 (13) | 0.0529 (13) | 0.0405 (14) | −0.0007 (14) | 0.0189 (10) |
| O32 | 0.0899 (15) | 0.0484 (11) | 0.0781 (13) | 0.0266 (10) | 0.0171 (11) | 0.0138 (10) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Geometric parameters (Å, º)
| N1—C6 | 1.481 (3) | C24—C25 | 1.370 (4) |
| N1—C2 | 1.485 (3) | C24—H24 | 0.9300 |
| N1—H11 | 1.02 (3) | C25—C26 | 1.385 (3) |
| N1—H12 | 0.92 (3) | C25—H25 | 0.9300 |
| C2—C3 | 1.516 (3) | C26—H26 | 0.9300 |
| C2—H2A | 0.9700 | O22—C27 | 1.421 (3) |
| C2—H2B | 0.9700 | C27—H27A | 0.9600 |
| C3—N4 | 1.461 (3) | C27—H27B | 0.9600 |
| C3—H3A | 0.9700 | C27—H27C | 0.9600 |
| C3—H3B | 0.9700 | C31—C36 | 1.373 (3) |
| N4—C21 | 1.423 (3) | C31—C32 | 1.380 (3) |
| N4—C5 | 1.471 (3) | C31—C37 | 1.515 (3) |
| C5—C6 | 1.512 (3) | C32—C33 | 1.386 (3) |
| C5—H5A | 0.9700 | C32—H32 | 0.9300 |
| C5—H5B | 0.9700 | C33—C34 | 1.369 (4) |
| C6—H6A | 0.9700 | C33—H33 | 0.9300 |
| C6—H6B | 0.9700 | C34—C35 | 1.364 (3) |
| C21—C26 | 1.389 (3) | C34—Cl34 | 1.750 (2) |
| C21—C22 | 1.406 (3) | C35—C36 | 1.384 (3) |
| C22—O22 | 1.377 (2) | C35—H35 | 0.9300 |
| C22—C23 | 1.380 (3) | C36—H36 | 0.9300 |
| C23—C24 | 1.384 (3) | C37—O32 | 1.239 (3) |
| C23—H23 | 0.9300 | C37—O31 | 1.251 (3) |
| C6—N1—C2 | 110.65 (19) | C22—C23—H23 | 119.8 |
| C6—N1—H11 | 107.0 (14) | C24—C23—H23 | 119.8 |
| C2—N1—H11 | 109.8 (15) | C25—C24—C23 | 119.8 (2) |
| C6—N1—H12 | 109.8 (17) | C25—C24—H24 | 120.1 |
| C2—N1—H12 | 106.9 (16) | C23—C24—H24 | 120.1 |
| H11—N1—H12 | 113 (2) | C24—C25—C26 | 120.1 (2) |
| N1—C2—C3 | 110.5 (2) | C24—C25—H25 | 120.0 |
| N1—C2—H2A | 109.6 | C26—C25—H25 | 120.0 |
| C3—C2—H2A | 109.6 | C25—C26—C21 | 121.5 (2) |
| N1—C2—H2B | 109.6 | C25—C26—H26 | 119.3 |
| C3—C2—H2B | 109.6 | C21—C26—H26 | 119.3 |
| H2A—C2—H2B | 108.1 | C22—O22—C27 | 117.86 (17) |
| N4—C3—C2 | 109.35 (19) | O22—C27—H27A | 109.5 |
| N4—C3—H3A | 109.8 | O22—C27—H27B | 109.5 |
| C2—C3—H3A | 109.8 | H27A—C27—H27B | 109.5 |
| N4—C3—H3B | 109.8 | O22—C27—H27C | 109.5 |
| C2—C3—H3B | 109.8 | H27A—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.3 | H27B—C27—H27C | 109.5 |
| C21—N4—C3 | 116.59 (17) | C36—C31—C32 | 117.8 (2) |
| C21—N4—C5 | 113.53 (18) | C36—C31—C37 | 120.7 (2) |
| C3—N4—C5 | 110.53 (18) | C32—C31—C37 | 121.5 (2) |
| N4—C5—C6 | 110.35 (19) | C31—C32—C33 | 121.2 (2) |
| N4—C5—H5A | 109.6 | C31—C32—H32 | 119.4 |
| C6—C5—H5A | 109.6 | C33—C32—H32 | 119.4 |
| N4—C5—H5B | 109.6 | C34—C33—C32 | 119.2 (2) |
| C6—C5—H5B | 109.6 | C34—C33—H33 | 120.4 |
| H5A—C5—H5B | 108.1 | C32—C33—H33 | 120.4 |
| N1—C6—C5 | 110.38 (19) | C35—C34—C33 | 120.9 (2) |
| N1—C6—H6A | 109.6 | C35—C34—Cl34 | 119.4 (2) |
| C5—C6—H6A | 109.6 | C33—C34—Cl34 | 119.7 (2) |
| N1—C6—H6B | 109.6 | C34—C35—C36 | 119.0 (2) |
| C5—C6—H6B | 109.6 | C34—C35—H35 | 120.5 |
| H6A—C6—H6B | 108.1 | C36—C35—H35 | 120.5 |
| C26—C21—C22 | 117.5 (2) | C31—C36—C35 | 121.9 (2) |
| C26—C21—N4 | 123.2 (2) | C31—C36—H36 | 119.1 |
| C22—C21—N4 | 119.20 (19) | C35—C36—H36 | 119.1 |
| O22—C22—C23 | 123.4 (2) | O32—C37—O31 | 124.7 (2) |
| O22—C22—C21 | 115.91 (18) | O32—C37—C31 | 119.0 (2) |
| C23—C22—C21 | 120.7 (2) | O31—C37—C31 | 116.3 (2) |
| C22—C23—C24 | 120.4 (2) | ||
| C6—N1—C2—C3 | −56.8 (3) | C24—C25—C26—C21 | −1.1 (4) |
| N1—C2—C3—N4 | 58.6 (3) | C22—C21—C26—C25 | −0.3 (4) |
| C2—C3—N4—C21 | 168.3 (2) | N4—C21—C26—C25 | −177.6 (2) |
| C2—C3—N4—C5 | −60.1 (3) | C23—C22—O22—C27 | 10.9 (3) |
| C21—N4—C5—C6 | −167.25 (18) | C21—C22—O22—C27 | −171.5 (2) |
| C3—N4—C5—C6 | 59.6 (2) | C36—C31—C32—C33 | 1.3 (4) |
| C2—N1—C6—C5 | 55.6 (3) | C37—C31—C32—C33 | −178.7 (2) |
| N4—C5—C6—N1 | −56.8 (3) | C31—C32—C33—C34 | −0.2 (4) |
| C3—N4—C21—C26 | 13.9 (3) | C32—C33—C34—C35 | −1.3 (4) |
| C5—N4—C21—C26 | −116.3 (2) | C32—C33—C34—Cl34 | 179.8 (2) |
| C3—N4—C21—C22 | −163.3 (2) | C33—C34—C35—C36 | 1.4 (5) |
| C5—N4—C21—C22 | 66.5 (3) | Cl34—C34—C35—C36 | −179.6 (2) |
| C26—C21—C22—O22 | −175.8 (2) | C32—C31—C36—C35 | −1.2 (5) |
| N4—C21—C22—O22 | 1.6 (3) | C37—C31—C36—C35 | 178.9 (3) |
| C26—C21—C22—C23 | 1.9 (3) | C34—C35—C36—C31 | −0.2 (5) |
| N4—C21—C22—C23 | 179.3 (2) | C36—C31—C37—O32 | −176.7 (3) |
| O22—C22—C23—C24 | 175.4 (2) | C32—C31—C37—O32 | 3.3 (4) |
| C21—C22—C23—C24 | −2.1 (4) | C36—C31—C37—O31 | 3.2 (4) |
| C22—C23—C24—C25 | 0.6 (4) | C32—C31—C37—O31 | −176.7 (3) |
| C23—C24—C25—C26 | 1.0 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-chlorobenzoate (I). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 1.02 (2) | 1.60 (2) | 2.616 (3) | 176 (2) |
| N1—H12···O32i | 0.92 (3) | 1.88 (3) | 2.792 (3) | 173 (2) |
| C3—H3A···Cg1i | 0.97 | 2.96 | 3.881 (3) | 160 |
Symmetry code: (i) −x+1, −y+1, −z+1.
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Crystal data
| C11H17N2O+·C7H4BrO2− | Z = 2 |
| Mr = 393.27 | F(000) = 404 |
| Triclinic, P1 | Dx = 1.468 Mg m−3 |
| a = 7.4313 (5) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 7.9163 (5) Å | Cell parameters from 3779 reflections |
| c = 15.5212 (9) Å | θ = 2.6–27.6° |
| α = 101.565 (5)° | µ = 2.33 mm−1 |
| β = 94.780 (5)° | T = 296 K |
| γ = 92.691 (5)° | Plate, yellow |
| V = 889.54 (10) Å3 | 0.42 × 0.42 × 0.12 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3739 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2989 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.018 |
| ω scans | θmax = 27.6°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −9→8 |
| Tmin = 0.258, Tmax = 0.756 | k = −10→10 |
| 5996 measured reflections | l = −19→16 |
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Refinement
| Refinement on F2 | Primary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.043 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.115 | w = 1/[σ2(Fo2) + (0.0576P)2 + 0.570P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max < 0.001 |
| 3739 reflections | Δρmax = 0.84 e Å−3 |
| 224 parameters | Δρmin = −0.55 e Å−3 |
| 0 restraints |
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.3878 (4) | 0.5725 (3) | 0.62065 (16) | 0.0514 (6) | |
| H11 | 0.361 (5) | 0.638 (5) | 0.582 (2) | 0.062* | |
| H12 | 0.494 (5) | 0.532 (5) | 0.610 (2) | 0.062* | |
| C2 | 0.2490 (5) | 0.4279 (4) | 0.61017 (19) | 0.0557 (8) | |
| H2A | 0.2421 | 0.3600 | 0.5504 | 0.067* | |
| H2B | 0.1318 | 0.4735 | 0.6198 | 0.067* | |
| C3 | 0.2949 (4) | 0.3139 (4) | 0.67528 (18) | 0.0471 (7) | |
| H3A | 0.2023 | 0.2204 | 0.6683 | 0.057* | |
| H3B | 0.4096 | 0.2641 | 0.6642 | 0.057* | |
| N4 | 0.3062 (3) | 0.4172 (3) | 0.76485 (13) | 0.0385 (5) | |
| C5 | 0.4474 (4) | 0.5584 (3) | 0.77722 (17) | 0.0424 (6) | |
| H5A | 0.5640 | 0.5108 | 0.7685 | 0.051* | |
| H5B | 0.4531 | 0.6256 | 0.8371 | 0.051* | |
| C6 | 0.4075 (4) | 0.6739 (4) | 0.71261 (18) | 0.0470 (7) | |
| H6A | 0.2968 | 0.7303 | 0.7252 | 0.056* | |
| H6B | 0.5051 | 0.7627 | 0.7193 | 0.056* | |
| C21 | 0.3137 (3) | 0.3252 (3) | 0.83448 (17) | 0.0375 (5) | |
| C22 | 0.2745 (3) | 0.4102 (3) | 0.91928 (17) | 0.0387 (6) | |
| C23 | 0.2796 (4) | 0.3234 (4) | 0.98826 (19) | 0.0470 (6) | |
| H23 | 0.2571 | 0.3812 | 1.0443 | 0.056* | |
| C24 | 0.3181 (4) | 0.1505 (4) | 0.9745 (2) | 0.0549 (8) | |
| H24 | 0.3210 | 0.0927 | 1.0212 | 0.066* | |
| C25 | 0.3515 (5) | 0.0658 (4) | 0.8927 (2) | 0.0556 (8) | |
| H25 | 0.3747 | −0.0506 | 0.8832 | 0.067* | |
| C26 | 0.3511 (4) | 0.1526 (4) | 0.8233 (2) | 0.0473 (7) | |
| H26 | 0.3765 | 0.0935 | 0.7680 | 0.057* | |
| O22 | 0.2276 (3) | 0.5771 (2) | 0.92653 (12) | 0.0505 (5) | |
| C27 | 0.2084 (4) | 0.6742 (4) | 1.01265 (19) | 0.0504 (7) | |
| H27A | 0.1755 | 0.7884 | 1.0087 | 0.076* | |
| H27B | 0.3209 | 0.6814 | 1.0488 | 0.076* | |
| H27C | 0.1156 | 0.6182 | 1.0386 | 0.076* | |
| C31 | 0.2104 (4) | 0.8486 (4) | 0.38246 (18) | 0.0423 (6) | |
| C32 | 0.1691 (5) | 0.7880 (4) | 0.2931 (2) | 0.0552 (8) | |
| H32 | 0.1715 | 0.6704 | 0.2697 | 0.066* | |
| C33 | 0.1240 (5) | 0.8992 (4) | 0.2376 (2) | 0.0574 (8) | |
| H33 | 0.0956 | 0.8571 | 0.1774 | 0.069* | |
| C34 | 0.1217 (4) | 1.0726 (4) | 0.27254 (19) | 0.0457 (6) | |
| Br34 | 0.06068 (5) | 1.22764 (5) | 0.19700 (2) | 0.06693 (16) | |
| C35 | 0.1596 (6) | 1.1360 (4) | 0.3608 (2) | 0.0713 (11) | |
| H35 | 0.1558 | 1.2533 | 0.3841 | 0.086* | |
| C36 | 0.2042 (6) | 1.0211 (4) | 0.4153 (2) | 0.0703 (11) | |
| H36 | 0.2305 | 1.0633 | 0.4756 | 0.084* | |
| C37 | 0.2644 (4) | 0.7278 (4) | 0.4432 (2) | 0.0506 (7) | |
| O31 | 0.2859 (5) | 0.7932 (3) | 0.52394 (16) | 0.0910 (10) | |
| O32 | 0.2844 (4) | 0.5748 (3) | 0.41048 (16) | 0.0653 (6) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0781 (19) | 0.0471 (14) | 0.0325 (12) | 0.0163 (13) | 0.0077 (12) | 0.0123 (10) |
| C2 | 0.080 (2) | 0.0522 (17) | 0.0310 (14) | 0.0068 (15) | −0.0080 (14) | 0.0033 (12) |
| C3 | 0.0635 (18) | 0.0421 (15) | 0.0322 (13) | 0.0027 (13) | −0.0021 (12) | 0.0020 (11) |
| N4 | 0.0514 (13) | 0.0347 (11) | 0.0269 (10) | 0.0017 (9) | −0.0025 (9) | 0.0031 (8) |
| C5 | 0.0555 (16) | 0.0378 (14) | 0.0320 (13) | 0.0012 (12) | 0.0000 (11) | 0.0043 (11) |
| C6 | 0.0682 (19) | 0.0364 (14) | 0.0366 (14) | 0.0074 (13) | 0.0075 (13) | 0.0059 (11) |
| C21 | 0.0396 (13) | 0.0370 (13) | 0.0347 (13) | 0.0021 (10) | −0.0032 (10) | 0.0077 (10) |
| C22 | 0.0404 (13) | 0.0393 (14) | 0.0361 (13) | 0.0049 (11) | 0.0001 (11) | 0.0080 (11) |
| C23 | 0.0519 (16) | 0.0534 (16) | 0.0365 (14) | 0.0014 (13) | −0.0001 (12) | 0.0134 (12) |
| C24 | 0.0651 (19) | 0.0513 (17) | 0.0516 (18) | −0.0022 (14) | −0.0066 (14) | 0.0251 (14) |
| C25 | 0.072 (2) | 0.0348 (14) | 0.059 (2) | 0.0056 (13) | −0.0080 (15) | 0.0128 (13) |
| C26 | 0.0577 (17) | 0.0378 (14) | 0.0437 (15) | 0.0041 (12) | −0.0048 (13) | 0.0056 (12) |
| O22 | 0.0738 (14) | 0.0445 (11) | 0.0337 (10) | 0.0191 (10) | 0.0067 (9) | 0.0052 (8) |
| C27 | 0.0581 (18) | 0.0478 (16) | 0.0412 (15) | 0.0055 (13) | 0.0079 (13) | −0.0025 (12) |
| C31 | 0.0473 (15) | 0.0421 (14) | 0.0384 (14) | 0.0069 (11) | 0.0050 (11) | 0.0090 (11) |
| C32 | 0.070 (2) | 0.0458 (16) | 0.0469 (17) | 0.0186 (14) | 0.0023 (15) | 0.0005 (13) |
| C33 | 0.070 (2) | 0.067 (2) | 0.0331 (15) | 0.0162 (16) | 0.0044 (14) | 0.0033 (14) |
| C34 | 0.0459 (15) | 0.0520 (16) | 0.0416 (15) | 0.0018 (12) | −0.0004 (12) | 0.0175 (13) |
| Br34 | 0.0726 (2) | 0.0759 (3) | 0.0590 (2) | −0.00290 (17) | −0.00806 (16) | 0.03751 (18) |
| C35 | 0.125 (3) | 0.0384 (16) | 0.0471 (18) | 0.0068 (18) | −0.0112 (19) | 0.0089 (14) |
| C36 | 0.129 (3) | 0.0435 (17) | 0.0343 (16) | 0.0120 (19) | −0.0120 (18) | 0.0038 (13) |
| C37 | 0.0537 (17) | 0.0488 (17) | 0.0534 (18) | 0.0084 (13) | 0.0048 (14) | 0.0190 (14) |
| O31 | 0.171 (3) | 0.0586 (15) | 0.0454 (14) | 0.0315 (17) | −0.0072 (16) | 0.0173 (11) |
| O32 | 0.0879 (17) | 0.0400 (12) | 0.0704 (15) | 0.0200 (11) | 0.0089 (13) | 0.0127 (10) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Geometric parameters (Å, º)
| N1—C2 | 1.479 (4) | C24—C25 | 1.361 (5) |
| N1—C6 | 1.483 (4) | C24—H24 | 0.9300 |
| N1—H11 | 0.89 (4) | C25—C26 | 1.388 (4) |
| N1—H12 | 0.88 (4) | C25—H25 | 0.9300 |
| C2—C3 | 1.513 (4) | C26—H26 | 0.9300 |
| C2—H2A | 0.9700 | O22—C27 | 1.424 (3) |
| C2—H2B | 0.9700 | C27—H27A | 0.9600 |
| C3—N4 | 1.458 (3) | C27—H27B | 0.9600 |
| C3—H3A | 0.9700 | C27—H27C | 0.9600 |
| C3—H3B | 0.9700 | C31—C36 | 1.363 (4) |
| N4—C21 | 1.418 (3) | C31—C32 | 1.377 (4) |
| N4—C5 | 1.470 (4) | C31—C37 | 1.515 (4) |
| C5—C6 | 1.508 (4) | C32—C33 | 1.384 (4) |
| C5—H5A | 0.9700 | C32—H32 | 0.9300 |
| C5—H5B | 0.9700 | C33—C34 | 1.372 (4) |
| C6—H6A | 0.9700 | C33—H33 | 0.9300 |
| C6—H6B | 0.9700 | C34—C35 | 1.361 (4) |
| C21—C26 | 1.387 (4) | C34—Br34 | 1.905 (3) |
| C21—C22 | 1.413 (4) | C35—C36 | 1.394 (4) |
| C22—O22 | 1.367 (3) | C35—H35 | 0.9300 |
| C22—C23 | 1.382 (4) | C36—H36 | 0.9300 |
| C23—C24 | 1.390 (4) | C37—O32 | 1.237 (4) |
| C23—H23 | 0.9300 | C37—O31 | 1.251 (4) |
| C2—N1—C6 | 110.8 (2) | C22—C23—H23 | 119.8 |
| C2—N1—H11 | 110 (2) | C24—C23—H23 | 119.8 |
| C6—N1—H11 | 112 (2) | C25—C24—C23 | 119.9 (3) |
| C2—N1—H12 | 110 (2) | C25—C24—H24 | 120.1 |
| C6—N1—H12 | 107 (2) | C23—C24—H24 | 120.1 |
| H11—N1—H12 | 106 (3) | C24—C25—C26 | 120.2 (3) |
| N1—C2—C3 | 110.6 (2) | C24—C25—H25 | 119.9 |
| N1—C2—H2A | 109.5 | C26—C25—H25 | 119.9 |
| C3—C2—H2A | 109.5 | C21—C26—C25 | 121.6 (3) |
| N1—C2—H2B | 109.5 | C21—C26—H26 | 119.2 |
| C3—C2—H2B | 109.5 | C25—C26—H26 | 119.2 |
| H2A—C2—H2B | 108.1 | C22—O22—C27 | 117.7 (2) |
| N4—C3—C2 | 109.2 (2) | O22—C27—H27A | 109.5 |
| N4—C3—H3A | 109.8 | O22—C27—H27B | 109.5 |
| C2—C3—H3A | 109.8 | H27A—C27—H27B | 109.5 |
| N4—C3—H3B | 109.8 | O22—C27—H27C | 109.5 |
| C2—C3—H3B | 109.8 | H27A—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.3 | H27B—C27—H27C | 109.5 |
| C21—N4—C3 | 116.6 (2) | C36—C31—C32 | 118.4 (3) |
| C21—N4—C5 | 113.3 (2) | C36—C31—C37 | 120.4 (3) |
| C3—N4—C5 | 110.7 (2) | C32—C31—C37 | 121.2 (3) |
| N4—C5—C6 | 110.4 (2) | C31—C32—C33 | 121.0 (3) |
| N4—C5—H5A | 109.6 | C31—C32—H32 | 119.5 |
| C6—C5—H5A | 109.6 | C33—C32—H32 | 119.5 |
| N4—C5—H5B | 109.6 | C34—C33—C32 | 119.1 (3) |
| C6—C5—H5B | 109.6 | C34—C33—H33 | 120.4 |
| H5A—C5—H5B | 108.1 | C32—C33—H33 | 120.4 |
| N1—C6—C5 | 110.7 (2) | C35—C34—C33 | 121.2 (3) |
| N1—C6—H6A | 109.5 | C35—C34—Br34 | 119.1 (2) |
| C5—C6—H6A | 109.5 | C33—C34—Br34 | 119.7 (2) |
| N1—C6—H6B | 109.5 | C34—C35—C36 | 118.5 (3) |
| C5—C6—H6B | 109.5 | C34—C35—H35 | 120.7 |
| H6A—C6—H6B | 108.1 | C36—C35—H35 | 120.7 |
| C26—C21—C22 | 117.5 (2) | C31—C36—C35 | 121.7 (3) |
| C26—C21—N4 | 123.4 (2) | C31—C36—H36 | 119.1 |
| C22—C21—N4 | 119.0 (2) | C35—C36—H36 | 119.1 |
| O22—C22—C23 | 123.8 (2) | O32—C37—O31 | 124.9 (3) |
| O22—C22—C21 | 115.9 (2) | O32—C37—C31 | 118.9 (3) |
| C23—C22—C21 | 120.3 (2) | O31—C37—C31 | 116.2 (3) |
| C22—C23—C24 | 120.5 (3) | ||
| C6—N1—C2—C3 | −56.5 (3) | C22—C21—C26—C25 | −0.5 (4) |
| N1—C2—C3—N4 | 58.8 (3) | N4—C21—C26—C25 | −177.9 (3) |
| C2—C3—N4—C21 | 168.3 (2) | C24—C25—C26—C21 | −1.2 (5) |
| C2—C3—N4—C5 | −60.2 (3) | C23—C22—O22—C27 | 9.3 (4) |
| C21—N4—C5—C6 | −167.6 (2) | C21—C22—O22—C27 | −172.6 (2) |
| C3—N4—C5—C6 | 59.3 (3) | C36—C31—C32—C33 | 0.6 (5) |
| C2—N1—C6—C5 | 55.0 (3) | C37—C31—C32—C33 | −178.5 (3) |
| N4—C5—C6—N1 | −56.0 (3) | C31—C32—C33—C34 | 0.4 (5) |
| C3—N4—C21—C26 | 14.8 (4) | C32—C33—C34—C35 | −1.3 (5) |
| C5—N4—C21—C26 | −115.4 (3) | C32—C33—C34—Br34 | 179.8 (2) |
| C3—N4—C21—C22 | −162.6 (2) | C33—C34—C35—C36 | 1.1 (6) |
| C5—N4—C21—C22 | 67.1 (3) | Br34—C34—C35—C36 | −179.9 (3) |
| C26—C21—C22—O22 | −176.2 (2) | C32—C31—C36—C35 | −0.7 (6) |
| N4—C21—C22—O22 | 1.4 (4) | C37—C31—C36—C35 | 178.3 (4) |
| C26—C21—C22—C23 | 2.0 (4) | C34—C35—C36—C31 | −0.1 (7) |
| N4—C21—C22—C23 | 179.6 (2) | C36—C31—C37—O32 | −173.7 (3) |
| O22—C22—C23—C24 | 176.1 (3) | C32—C31—C37—O32 | 5.4 (5) |
| C21—C22—C23—C24 | −1.9 (4) | C36—C31—C37—O31 | 6.2 (5) |
| C22—C23—C24—C25 | 0.2 (5) | C32—C31—C37—O31 | −174.8 (3) |
| C23—C24—C25—C26 | 1.4 (5) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-bromobenzoate (II). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.89 (4) | 1.75 (4) | 2.620 (4) | 168 (3) |
| N1—H12···O32i | 0.88 (4) | 1.91 (4) | 2.786 (4) | 175 (4) |
Symmetry code: (i) −x+1, −y+1, −z+1.
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Crystal data
| C11H17N2O+·C7H4IO2− | Z = 2 |
| Mr = 440.27 | F(000) = 440 |
| Triclinic, P1 | Dx = 1.609 Mg m−3 |
| a = 7.1129 (4) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 11.2722 (7) Å | Cell parameters from 3897 reflections |
| c = 12.5923 (8) Å | θ = 3.0–28.0° |
| α = 69.852 (5)° | µ = 1.78 mm−1 |
| β = 74.681 (5)° | T = 296 K |
| γ = 79.121 (5)° | Needle, orange |
| V = 908.82 (10) Å3 | 0.48 × 0.24 × 0.14 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3897 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 3203 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.012 |
| ω scans | θmax = 28.0°, θmin = 3.0° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −6→9 |
| Tmin = 0.534, Tmax = 0.779 | k = −14→14 |
| 6342 measured reflections | l = −16→16 |
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Refinement
| Refinement on F2 | Primary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.026 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.065 | w = 1/[σ2(Fo2) + (0.029P)2 + 0.4404P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max = 0.003 |
| 3897 reflections | Δρmax = 0.52 e Å−3 |
| 224 parameters | Δρmin = −0.70 e Å−3 |
| 0 restraints |
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.6379 (3) | 0.34876 (17) | 0.41481 (15) | 0.0371 (4) | |
| H11 | 0.736 (3) | 0.364 (2) | 0.437 (2) | 0.045* | |
| H12 | 0.545 (4) | 0.417 (2) | 0.405 (2) | 0.045* | |
| C2 | 0.5540 (3) | 0.2342 (2) | 0.50290 (17) | 0.0393 (4) | |
| H2A | 0.4957 | 0.2524 | 0.5752 | 0.047* | |
| H2B | 0.6580 | 0.1651 | 0.5168 | 0.047* | |
| C3 | 0.4004 (3) | 0.1942 (2) | 0.46316 (17) | 0.0405 (4) | |
| H3A | 0.3499 | 0.1181 | 0.5208 | 0.049* | |
| H3B | 0.2923 | 0.2609 | 0.4536 | 0.049* | |
| N4 | 0.4884 (2) | 0.16937 (15) | 0.35326 (13) | 0.0345 (3) | |
| C5 | 0.5578 (3) | 0.2859 (2) | 0.26462 (17) | 0.0414 (5) | |
| H5A | 0.4490 | 0.3523 | 0.2555 | 0.050* | |
| H5B | 0.6108 | 0.2700 | 0.1909 | 0.050* | |
| C6 | 0.7146 (3) | 0.3286 (2) | 0.30021 (18) | 0.0430 (5) | |
| H6A | 0.8271 | 0.2646 | 0.3039 | 0.052* | |
| H6B | 0.7570 | 0.4071 | 0.2428 | 0.052* | |
| C21 | 0.3825 (3) | 0.10145 (18) | 0.31654 (17) | 0.0354 (4) | |
| C22 | 0.4854 (3) | 0.0392 (2) | 0.23523 (18) | 0.0420 (5) | |
| C23 | 0.3903 (4) | −0.0354 (2) | 0.2031 (2) | 0.0544 (6) | |
| H23 | 0.4592 | −0.0769 | 0.1499 | 0.065* | |
| C24 | 0.1939 (4) | −0.0483 (2) | 0.2498 (2) | 0.0605 (7) | |
| H24 | 0.1313 | −0.0995 | 0.2288 | 0.073* | |
| C25 | 0.0906 (4) | 0.0141 (3) | 0.3269 (2) | 0.0594 (7) | |
| H25 | −0.0424 | 0.0064 | 0.3570 | 0.071* | |
| C26 | 0.1844 (3) | 0.0888 (2) | 0.3602 (2) | 0.0465 (5) | |
| H26 | 0.1132 | 0.1309 | 0.4126 | 0.056* | |
| O22 | 0.6793 (2) | 0.05587 (18) | 0.19524 (16) | 0.0594 (5) | |
| C27 | 0.7839 (4) | 0.0197 (3) | 0.0974 (2) | 0.0694 (8) | |
| H27A | 0.9125 | 0.0472 | 0.0736 | 0.104* | |
| H27B | 0.7156 | 0.0583 | 0.0354 | 0.104* | |
| H27C | 0.7949 | −0.0711 | 0.1166 | 0.104* | |
| C31 | 0.9029 (3) | 0.37755 (18) | 0.70670 (17) | 0.0344 (4) | |
| C32 | 1.1047 (3) | 0.3626 (2) | 0.69034 (18) | 0.0395 (4) | |
| H32 | 1.1812 | 0.3648 | 0.6174 | 0.047* | |
| C33 | 1.1957 (3) | 0.3443 (2) | 0.78032 (18) | 0.0411 (5) | |
| H33 | 1.3317 | 0.3347 | 0.7681 | 0.049* | |
| C34 | 1.0812 (3) | 0.34062 (19) | 0.88832 (17) | 0.0405 (5) | |
| I34 | 1.21476 (3) | 0.30958 (2) | 1.02693 (2) | 0.07326 (9) | |
| C35 | 0.8796 (4) | 0.3559 (2) | 0.90646 (19) | 0.0528 (6) | |
| H35 | 0.8034 | 0.3533 | 0.9795 | 0.063* | |
| C36 | 0.7913 (3) | 0.3751 (2) | 0.8158 (2) | 0.0469 (5) | |
| H36 | 0.6552 | 0.3865 | 0.8279 | 0.056* | |
| C37 | 0.8053 (3) | 0.39540 (18) | 0.60869 (18) | 0.0382 (4) | |
| O31 | 0.9072 (2) | 0.36274 (15) | 0.52331 (13) | 0.0466 (3) | |
| O32 | 0.6270 (2) | 0.43759 (15) | 0.62045 (15) | 0.0520 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0383 (9) | 0.0379 (9) | 0.0422 (9) | 0.0006 (7) | −0.0142 (7) | −0.0195 (7) |
| C2 | 0.0428 (11) | 0.0447 (11) | 0.0327 (10) | −0.0010 (9) | −0.0109 (8) | −0.0150 (8) |
| C3 | 0.0397 (11) | 0.0504 (12) | 0.0336 (10) | −0.0068 (9) | −0.0035 (8) | −0.0182 (9) |
| N4 | 0.0372 (8) | 0.0381 (9) | 0.0310 (8) | −0.0066 (7) | −0.0049 (7) | −0.0147 (7) |
| C5 | 0.0568 (13) | 0.0390 (11) | 0.0309 (10) | −0.0104 (9) | −0.0090 (9) | −0.0118 (8) |
| C6 | 0.0477 (12) | 0.0423 (12) | 0.0398 (11) | −0.0116 (9) | −0.0019 (9) | −0.0160 (9) |
| C21 | 0.0393 (10) | 0.0338 (10) | 0.0348 (9) | −0.0033 (8) | −0.0131 (8) | −0.0095 (8) |
| C22 | 0.0502 (12) | 0.0396 (11) | 0.0415 (11) | −0.0021 (9) | −0.0174 (9) | −0.0151 (9) |
| C23 | 0.0783 (17) | 0.0410 (12) | 0.0566 (14) | −0.0021 (11) | −0.0329 (13) | −0.0197 (10) |
| C24 | 0.0796 (19) | 0.0472 (14) | 0.0684 (16) | −0.0216 (13) | −0.0396 (15) | −0.0095 (12) |
| C25 | 0.0530 (14) | 0.0625 (16) | 0.0630 (15) | −0.0234 (12) | −0.0246 (12) | −0.0021 (12) |
| C26 | 0.0418 (12) | 0.0507 (13) | 0.0459 (12) | −0.0068 (10) | −0.0123 (9) | −0.0105 (10) |
| O22 | 0.0477 (9) | 0.0821 (12) | 0.0644 (11) | −0.0033 (8) | −0.0030 (8) | −0.0515 (10) |
| C27 | 0.0792 (19) | 0.0738 (18) | 0.0601 (16) | −0.0034 (15) | 0.0021 (14) | −0.0420 (14) |
| C31 | 0.0364 (10) | 0.0296 (9) | 0.0380 (10) | −0.0024 (8) | −0.0091 (8) | −0.0114 (8) |
| C32 | 0.0393 (11) | 0.0443 (11) | 0.0350 (10) | −0.0024 (9) | −0.0068 (8) | −0.0146 (9) |
| C33 | 0.0371 (11) | 0.0426 (11) | 0.0443 (11) | −0.0024 (9) | −0.0133 (9) | −0.0121 (9) |
| C34 | 0.0551 (13) | 0.0344 (10) | 0.0342 (10) | −0.0054 (9) | −0.0167 (9) | −0.0079 (8) |
| I34 | 0.09118 (16) | 0.08904 (16) | 0.04736 (11) | −0.00653 (11) | −0.03522 (9) | −0.01745 (9) |
| C35 | 0.0542 (14) | 0.0662 (15) | 0.0340 (11) | −0.0080 (12) | 0.0000 (10) | −0.0171 (10) |
| C36 | 0.0369 (11) | 0.0584 (14) | 0.0439 (12) | −0.0037 (10) | −0.0036 (9) | −0.0184 (10) |
| C37 | 0.0392 (11) | 0.0319 (10) | 0.0461 (11) | −0.0028 (8) | −0.0159 (9) | −0.0110 (9) |
| O31 | 0.0426 (8) | 0.0614 (10) | 0.0433 (8) | −0.0034 (7) | −0.0137 (7) | −0.0232 (7) |
| O32 | 0.0399 (8) | 0.0546 (9) | 0.0664 (10) | 0.0106 (7) | −0.0220 (7) | −0.0254 (8) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Geometric parameters (Å, º)
| N1—C6 | 1.482 (3) | C24—C25 | 1.369 (4) |
| N1—C2 | 1.486 (3) | C24—H24 | 0.9300 |
| N1—H11 | 0.88 (2) | C25—C26 | 1.391 (3) |
| N1—H12 | 0.91 (2) | C25—H25 | 0.9300 |
| C2—C3 | 1.510 (3) | C26—H26 | 0.9300 |
| C2—H2A | 0.9700 | O22—C27 | 1.409 (3) |
| C2—H2B | 0.9700 | C27—H27A | 0.9600 |
| C3—N4 | 1.459 (2) | C27—H27B | 0.9600 |
| C3—H3A | 0.9700 | C27—H27C | 0.9600 |
| C3—H3B | 0.9700 | C31—C32 | 1.381 (3) |
| N4—C21 | 1.419 (2) | C31—C36 | 1.387 (3) |
| N4—C5 | 1.469 (3) | C31—C37 | 1.508 (3) |
| C5—C6 | 1.514 (3) | C32—C33 | 1.386 (3) |
| C5—H5A | 0.9700 | C32—H32 | 0.9300 |
| C5—H5B | 0.9700 | C33—C34 | 1.379 (3) |
| C6—H6A | 0.9700 | C33—H33 | 0.9300 |
| C6—H6B | 0.9700 | C34—C35 | 1.378 (3) |
| C21—C26 | 1.385 (3) | C34—I34 | 2.098 (2) |
| C21—C22 | 1.407 (3) | C35—C36 | 1.379 (3) |
| C22—O22 | 1.363 (3) | C35—H35 | 0.9300 |
| C22—C23 | 1.387 (3) | C36—H36 | 0.9300 |
| C23—C24 | 1.379 (4) | C37—O31 | 1.256 (3) |
| C23—H23 | 0.9300 | C37—O32 | 1.256 (2) |
| C6—N1—C2 | 111.24 (15) | C24—C23—H23 | 119.9 |
| C6—N1—H11 | 108.4 (15) | C22—C23—H23 | 119.9 |
| C2—N1—H11 | 108.5 (15) | C25—C24—C23 | 120.2 (2) |
| C6—N1—H12 | 106.8 (15) | C25—C24—H24 | 119.9 |
| C2—N1—H12 | 110.9 (15) | C23—C24—H24 | 119.9 |
| H11—N1—H12 | 111 (2) | C24—C25—C26 | 120.1 (2) |
| N1—C2—C3 | 111.04 (16) | C24—C25—H25 | 120.0 |
| N1—C2—H2A | 109.4 | C26—C25—H25 | 120.0 |
| C3—C2—H2A | 109.4 | C21—C26—C25 | 121.0 (2) |
| N1—C2—H2B | 109.4 | C21—C26—H26 | 119.5 |
| C3—C2—H2B | 109.4 | C25—C26—H26 | 119.5 |
| H2A—C2—H2B | 108.0 | C22—O22—C27 | 119.10 (19) |
| N4—C3—C2 | 109.09 (16) | O22—C27—H27A | 109.5 |
| N4—C3—H3A | 109.9 | O22—C27—H27B | 109.5 |
| C2—C3—H3A | 109.9 | H27A—C27—H27B | 109.5 |
| N4—C3—H3B | 109.9 | O22—C27—H27C | 109.5 |
| C2—C3—H3B | 109.9 | H27A—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.3 | H27B—C27—H27C | 109.5 |
| C21—N4—C3 | 117.03 (15) | C32—C31—C36 | 118.49 (19) |
| C21—N4—C5 | 114.74 (15) | C32—C31—C37 | 120.99 (18) |
| C3—N4—C5 | 109.97 (16) | C36—C31—C37 | 120.52 (18) |
| N4—C5—C6 | 109.74 (17) | C31—C32—C33 | 121.39 (19) |
| N4—C5—H5A | 109.7 | C31—C32—H32 | 119.3 |
| C6—C5—H5A | 109.7 | C33—C32—H32 | 119.3 |
| N4—C5—H5B | 109.7 | C34—C33—C32 | 118.95 (19) |
| C6—C5—H5B | 109.7 | C34—C33—H33 | 120.5 |
| H5A—C5—H5B | 108.2 | C32—C33—H33 | 120.5 |
| N1—C6—C5 | 110.39 (17) | C35—C34—C33 | 120.63 (19) |
| N1—C6—H6A | 109.6 | C35—C34—I34 | 119.56 (15) |
| C5—C6—H6A | 109.6 | C33—C34—I34 | 119.81 (16) |
| N1—C6—H6B | 109.6 | C34—C35—C36 | 119.7 (2) |
| C5—C6—H6B | 109.6 | C34—C35—H35 | 120.1 |
| H6A—C6—H6B | 108.1 | C36—C35—H35 | 120.1 |
| C26—C21—C22 | 118.24 (19) | C35—C36—C31 | 120.8 (2) |
| C26—C21—N4 | 123.33 (18) | C35—C36—H36 | 119.6 |
| C22—C21—N4 | 118.37 (17) | C31—C36—H36 | 119.6 |
| O22—C22—C23 | 124.4 (2) | O31—C37—O32 | 125.09 (19) |
| O22—C22—C21 | 115.38 (17) | O31—C37—C31 | 117.28 (17) |
| C23—C22—C21 | 120.2 (2) | O32—C37—C31 | 117.58 (18) |
| C24—C23—C22 | 120.3 (2) | ||
| C6—N1—C2—C3 | −54.5 (2) | C22—C21—C26—C25 | 1.6 (3) |
| N1—C2—C3—N4 | 58.0 (2) | N4—C21—C26—C25 | −175.6 (2) |
| C2—C3—N4—C21 | 164.91 (17) | C24—C25—C26—C21 | −0.1 (4) |
| C2—C3—N4—C5 | −61.8 (2) | C23—C22—O22—C27 | 14.5 (4) |
| C21—N4—C5—C6 | −163.68 (17) | C21—C22—O22—C27 | −167.1 (2) |
| C3—N4—C5—C6 | 61.9 (2) | C36—C31—C32—C33 | 0.6 (3) |
| C2—N1—C6—C5 | 53.9 (2) | C37—C31—C32—C33 | −179.05 (19) |
| N4—C5—C6—N1 | −57.3 (2) | C31—C32—C33—C34 | 0.2 (3) |
| C3—N4—C21—C26 | 18.2 (3) | C32—C33—C34—C35 | −0.5 (3) |
| C5—N4—C21—C26 | −112.9 (2) | C32—C33—C34—I34 | 178.83 (15) |
| C3—N4—C21—C22 | −159.00 (18) | C33—C34—C35—C36 | 0.0 (4) |
| C5—N4—C21—C22 | 69.9 (2) | I34—C34—C35—C36 | −179.37 (18) |
| C26—C21—C22—O22 | 179.72 (19) | C34—C35—C36—C31 | 0.9 (4) |
| N4—C21—C22—O22 | −2.9 (3) | C32—C31—C36—C35 | −1.1 (3) |
| C26—C21—C22—C23 | −1.8 (3) | C37—C31—C36—C35 | 178.5 (2) |
| N4—C21—C22—C23 | 175.53 (19) | C32—C31—C37—O31 | 18.7 (3) |
| O22—C22—C23—C24 | 178.9 (2) | C36—C31—C37—O31 | −161.0 (2) |
| C21—C22—C23—C24 | 0.6 (3) | C32—C31—C37—O32 | −163.71 (19) |
| C22—C23—C24—C25 | 1.0 (4) | C36—C31—C37—O32 | 16.7 (3) |
| C23—C24—C25—C26 | −1.2 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-iodobenzoate (III). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.88 (2) | 1.83 (2) | 2.684 (3) | 163 (2) |
| N1—H11···O32 | 0.88 (2) | 2.60 (2) | 3.060 (3) | 113.6 (17) |
| N1—H12···O32i | 0.91 (3) | 1.84 (3) | 2.746 (3) | 176 (3) |
| C33—H33···O32ii | 0.93 | 2.57 | 3.327 (3) | 139 |
| C2—H2B···Cg2iii | 0.97 | 2.77 | 3.482 (2) | 131 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x+1, y, z; (iii) −x+1, −y, −z+1.
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Crystal data
| C11H17N2O+·C7H4FO2− | F(000) = 704 |
| Mr = 332.37 | Dx = 1.270 Mg m−3 |
| Monoclinic, Cc | Mo Kα radiation, λ = 0.71073 Å |
| a = 19.940 (1) Å | Cell parameters from 3343 reflections |
| b = 10.2705 (7) Å | θ = 3.0–27.9° |
| c = 9.0148 (7) Å | µ = 0.09 mm−1 |
| β = 109.663 (8)° | T = 296 K |
| V = 1738.5 (2) Å3 | Block, orange |
| Z = 4 | 0.48 × 0.36 × 0.22 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3343 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2786 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.012 |
| ω scans | θmax = 27.9°, θmin = 3.0° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −25→25 |
| Tmin = 0.884, Tmax = 0.963 | k = −13→13 |
| 6204 measured reflections | l = −11→11 |
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Refinement
| Refinement on F2 | H atoms treated by a mixture of independent and constrained refinement |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0593P)2 + 0.1911P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.036 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.100 | Δρmax = 0.24 e Å−3 |
| S = 1.02 | Δρmin = −0.14 e Å−3 |
| 3343 reflections | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 256 parameters | Extinction coefficient: 0.0111 (16) |
| 25 restraints | Absolute structure: Flack x determined using 1089 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| Primary atom site location: difference Fourier map | Absolute structure parameter: 0.2 (3) |
| Hydrogen site location: mixed |
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.40896 (13) | 0.4278 (2) | 0.1329 (3) | 0.0536 (5) | |
| H11 | 0.3741 (17) | 0.499 (3) | 0.119 (3) | 0.064* | |
| H12 | 0.4026 (16) | 0.383 (3) | 0.046 (4) | 0.064* | |
| C2 | 0.39924 (15) | 0.3361 (3) | 0.2509 (3) | 0.0554 (6) | |
| H2A | 0.3543 | 0.2906 | 0.2060 | 0.067* | |
| H2B | 0.3975 | 0.3846 | 0.3419 | 0.067* | |
| C3 | 0.45902 (15) | 0.2383 (3) | 0.3025 (4) | 0.0564 (6) | |
| H3A | 0.4530 | 0.1831 | 0.3844 | 0.068* | |
| H3B | 0.4579 | 0.1834 | 0.2140 | 0.068* | |
| N4 | 0.52785 (12) | 0.30576 (19) | 0.3622 (3) | 0.0482 (5) | |
| C5 | 0.53817 (15) | 0.3831 (3) | 0.2368 (3) | 0.0553 (6) | |
| H5A | 0.5357 | 0.3275 | 0.1481 | 0.066* | |
| H5B | 0.5848 | 0.4237 | 0.2737 | 0.066* | |
| C6 | 0.48133 (16) | 0.4861 (3) | 0.1862 (3) | 0.0569 (7) | |
| H6A | 0.4861 | 0.5445 | 0.2737 | 0.068* | |
| H6B | 0.4877 | 0.5367 | 0.1011 | 0.068* | |
| C21 | 0.58610 (13) | 0.2258 (2) | 0.4497 (3) | 0.0476 (6) | |
| C22 | 0.58740 (14) | 0.1743 (3) | 0.5946 (3) | 0.0511 (6) | |
| C23 | 0.64515 (17) | 0.1007 (3) | 0.6840 (4) | 0.0635 (7) | |
| H23 | 0.6453 | 0.0653 | 0.7791 | 0.076* | |
| C24 | 0.70234 (17) | 0.0792 (3) | 0.6336 (4) | 0.0702 (8) | |
| H24 | 0.7407 | 0.0296 | 0.6946 | 0.084* | |
| C25 | 0.70252 (16) | 0.1304 (3) | 0.4950 (4) | 0.0659 (8) | |
| H25 | 0.7414 | 0.1168 | 0.4618 | 0.079* | |
| C26 | 0.64492 (14) | 0.2029 (3) | 0.4028 (3) | 0.0561 (6) | |
| H26 | 0.6455 | 0.2369 | 0.3077 | 0.067* | |
| O22 | 0.53102 (11) | 0.2053 (2) | 0.6414 (2) | 0.0672 (6) | |
| C27 | 0.5288 (2) | 0.1521 (5) | 0.7843 (5) | 0.1014 (14) | |
| H27A | 0.5278 | 0.0588 | 0.7775 | 0.152* | |
| H27B | 0.4868 | 0.1822 | 0.8031 | 0.152* | |
| H27C | 0.5702 | 0.1791 | 0.8694 | 0.152* | |
| C31 | 0.30015 (19) | 0.8223 (3) | 0.2163 (4) | 0.0505 (8) | 0.907 (8) |
| C32 | 0.3262 (2) | 0.9222 (4) | 0.3203 (5) | 0.0663 (12) | 0.907 (8) |
| F32 | 0.39194 (19) | 0.9110 (3) | 0.4272 (4) | 0.1101 (11) | 0.907 (8) |
| C33 | 0.2910 (3) | 1.0368 (4) | 0.3207 (6) | 0.0868 (15) | 0.907 (8) |
| H33 | 0.3118 | 1.1029 | 0.3919 | 0.104* | 0.907 (8) |
| C34 | 0.2242 (3) | 1.0503 (5) | 0.2129 (6) | 0.0952 (19) | 0.907 (8) |
| H34 | 0.1988 | 1.1265 | 0.2108 | 0.114* | 0.907 (8) |
| C35 | 0.1943 (3) | 0.9527 (7) | 0.1077 (6) | 0.0984 (19) | 0.907 (8) |
| H35 | 0.1487 | 0.9633 | 0.0356 | 0.118* | 0.907 (8) |
| C36 | 0.2312 (2) | 0.8384 (5) | 0.1072 (5) | 0.0735 (12) | 0.907 (8) |
| H36 | 0.2105 | 0.7728 | 0.0352 | 0.088* | 0.907 (8) |
| C37 | 0.34049 (18) | 0.6986 (3) | 0.2127 (4) | 0.0511 (9) | 0.907 (8) |
| O31 | 0.3266 (2) | 0.6424 (3) | 0.0810 (3) | 0.0663 (9) | 0.907 (8) |
| O32 | 0.38350 (17) | 0.6562 (4) | 0.3377 (4) | 0.0779 (12) | 0.907 (8) |
| C41 | 0.2765 (15) | 0.794 (3) | 0.179 (4) | 0.0505 (8) | 0.093 (8) |
| C42 | 0.2927 (19) | 0.900 (3) | 0.276 (4) | 0.0663 (12) | 0.093 (8) |
| F42 | 0.3612 (19) | 0.929 (4) | 0.357 (6) | 0.1101 (11) | 0.093 (8) |
| C43 | 0.248 (2) | 1.005 (4) | 0.262 (6) | 0.0868 (15) | 0.093 (8) |
| H43 | 0.2599 | 1.0754 | 0.3298 | 0.104* | 0.093 (8) |
| C44 | 0.184 (3) | 0.997 (4) | 0.142 (6) | 0.0952 (19) | 0.093 (8) |
| H44 | 0.1518 | 1.0657 | 0.1284 | 0.114* | 0.093 (8) |
| C45 | 0.166 (2) | 0.894 (4) | 0.042 (5) | 0.0984 (19) | 0.093 (8) |
| H45 | 0.1219 | 0.8954 | −0.0390 | 0.118* | 0.093 (8) |
| C46 | 0.2094 (17) | 0.786 (4) | 0.054 (4) | 0.0735 (12) | 0.093 (8) |
| H46 | 0.1963 | 0.7151 | −0.0135 | 0.088* | 0.093 (8) |
| C47 | 0.3262 (19) | 0.679 (3) | 0.198 (4) | 0.0511 (9) | 0.093 (8) |
| O41 | 0.3491 (19) | 0.664 (4) | 0.084 (4) | 0.0663 (9) | 0.093 (8) |
| O42 | 0.359 (2) | 0.640 (5) | 0.334 (4) | 0.0779 (12) | 0.093 (8) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0552 (13) | 0.0560 (13) | 0.0418 (11) | 0.0094 (11) | 0.0060 (9) | −0.0028 (9) |
| C2 | 0.0443 (13) | 0.0595 (15) | 0.0548 (14) | 0.0023 (12) | 0.0065 (11) | 0.0043 (12) |
| C3 | 0.0451 (13) | 0.0491 (14) | 0.0664 (15) | −0.0031 (11) | 0.0074 (11) | 0.0015 (13) |
| N4 | 0.0420 (10) | 0.0461 (11) | 0.0512 (11) | −0.0012 (9) | 0.0086 (8) | 0.0033 (9) |
| C5 | 0.0546 (15) | 0.0621 (15) | 0.0475 (13) | −0.0023 (13) | 0.0151 (11) | 0.0010 (12) |
| C6 | 0.0664 (17) | 0.0532 (15) | 0.0472 (13) | 0.0001 (13) | 0.0139 (12) | 0.0060 (12) |
| C21 | 0.0434 (13) | 0.0395 (12) | 0.0529 (13) | 0.0002 (11) | 0.0070 (10) | −0.0050 (11) |
| C22 | 0.0458 (13) | 0.0413 (13) | 0.0625 (15) | 0.0009 (10) | 0.0134 (11) | 0.0024 (11) |
| C23 | 0.0618 (17) | 0.0523 (15) | 0.0712 (17) | 0.0109 (13) | 0.0154 (13) | 0.0138 (14) |
| C24 | 0.0528 (16) | 0.0609 (17) | 0.083 (2) | 0.0184 (14) | 0.0049 (14) | 0.0046 (16) |
| C25 | 0.0456 (15) | 0.0724 (19) | 0.0751 (18) | 0.0090 (14) | 0.0142 (13) | −0.0172 (16) |
| C26 | 0.0470 (14) | 0.0636 (16) | 0.0541 (14) | 0.0009 (12) | 0.0121 (11) | −0.0126 (13) |
| O22 | 0.0646 (12) | 0.0695 (13) | 0.0737 (13) | 0.0176 (10) | 0.0314 (10) | 0.0251 (10) |
| C27 | 0.102 (3) | 0.109 (3) | 0.115 (3) | 0.029 (2) | 0.064 (2) | 0.052 (3) |
| C31 | 0.0524 (19) | 0.0539 (18) | 0.0516 (18) | 0.0091 (15) | 0.0257 (15) | 0.0149 (15) |
| C32 | 0.061 (3) | 0.075 (2) | 0.069 (3) | 0.014 (2) | 0.030 (3) | 0.0084 (19) |
| F32 | 0.098 (2) | 0.119 (2) | 0.098 (2) | 0.0153 (17) | 0.0134 (17) | −0.0298 (18) |
| C33 | 0.118 (4) | 0.071 (2) | 0.093 (3) | 0.019 (3) | 0.064 (3) | 0.005 (2) |
| C34 | 0.121 (5) | 0.089 (3) | 0.100 (4) | 0.052 (3) | 0.069 (4) | 0.040 (3) |
| C35 | 0.083 (3) | 0.134 (5) | 0.088 (4) | 0.058 (3) | 0.042 (3) | 0.055 (3) |
| C36 | 0.062 (2) | 0.093 (3) | 0.071 (3) | 0.028 (2) | 0.0298 (19) | 0.034 (2) |
| C37 | 0.0469 (19) | 0.0538 (18) | 0.0535 (16) | 0.0080 (15) | 0.0180 (13) | 0.0166 (14) |
| O31 | 0.064 (2) | 0.0682 (17) | 0.0588 (12) | 0.0137 (15) | 0.0102 (14) | 0.0001 (12) |
| O32 | 0.092 (3) | 0.087 (2) | 0.0537 (12) | 0.041 (2) | 0.0226 (16) | 0.0226 (12) |
| C41 | 0.0524 (19) | 0.0539 (18) | 0.0516 (18) | 0.0091 (15) | 0.0257 (15) | 0.0149 (15) |
| C42 | 0.061 (3) | 0.075 (2) | 0.069 (3) | 0.014 (2) | 0.030 (3) | 0.0084 (19) |
| F42 | 0.098 (2) | 0.119 (2) | 0.098 (2) | 0.0153 (17) | 0.0134 (17) | −0.0298 (18) |
| C43 | 0.118 (4) | 0.071 (2) | 0.093 (3) | 0.019 (3) | 0.064 (3) | 0.005 (2) |
| C44 | 0.121 (5) | 0.089 (3) | 0.100 (4) | 0.052 (3) | 0.069 (4) | 0.040 (3) |
| C45 | 0.083 (3) | 0.134 (5) | 0.088 (4) | 0.058 (3) | 0.042 (3) | 0.055 (3) |
| C46 | 0.062 (2) | 0.093 (3) | 0.071 (3) | 0.028 (2) | 0.0298 (19) | 0.034 (2) |
| C47 | 0.0469 (19) | 0.0538 (18) | 0.0535 (16) | 0.0080 (15) | 0.0180 (13) | 0.0166 (14) |
| O41 | 0.064 (2) | 0.0682 (17) | 0.0588 (12) | 0.0137 (15) | 0.0102 (14) | 0.0001 (12) |
| O42 | 0.092 (3) | 0.087 (2) | 0.0537 (12) | 0.041 (2) | 0.0226 (16) | 0.0226 (12) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Geometric parameters (Å, º)
| N1—C2 | 1.482 (4) | C27—H27B | 0.9600 |
| N1—C6 | 1.485 (4) | C27—H27C | 0.9600 |
| N1—H11 | 0.98 (3) | C31—C32 | 1.369 (6) |
| N1—H12 | 0.88 (4) | C31—C36 | 1.405 (5) |
| C2—C3 | 1.508 (4) | C31—C37 | 1.510 (4) |
| C2—H2A | 0.9700 | C32—F32 | 1.345 (5) |
| C2—H2B | 0.9700 | C32—C33 | 1.371 (5) |
| C3—N4 | 1.468 (3) | C33—C34 | 1.366 (7) |
| C3—H3A | 0.9700 | C33—H33 | 0.9300 |
| C3—H3B | 0.9700 | C34—C35 | 1.370 (8) |
| N4—C21 | 1.424 (3) | C34—H34 | 0.9300 |
| N4—C5 | 1.452 (3) | C35—C36 | 1.387 (7) |
| C5—C6 | 1.504 (4) | C35—H35 | 0.9300 |
| C5—H5A | 0.9700 | C36—H36 | 0.9300 |
| C5—H5B | 0.9700 | C37—O32 | 1.243 (3) |
| C6—H6A | 0.9700 | C37—O31 | 1.264 (4) |
| C6—H6B | 0.9700 | C41—C42 | 1.365 (13) |
| C21—C26 | 1.394 (4) | C41—C46 | 1.432 (13) |
| C21—C22 | 1.402 (4) | C41—C47 | 1.518 (12) |
| C22—O22 | 1.365 (3) | C42—F42 | 1.347 (14) |
| C22—C23 | 1.387 (4) | C42—C43 | 1.376 (13) |
| C23—C24 | 1.380 (5) | C43—C44 | 1.364 (15) |
| C23—H23 | 0.9300 | C43—H43 | 0.9300 |
| C24—C25 | 1.357 (5) | C44—C45 | 1.365 (15) |
| C24—H24 | 0.9300 | C44—H44 | 0.9300 |
| C25—C26 | 1.386 (4) | C45—C46 | 1.388 (14) |
| C25—H25 | 0.9300 | C45—H45 | 0.9300 |
| C26—H26 | 0.9300 | C46—H46 | 0.9300 |
| O22—C27 | 1.414 (4) | C47—O42 | 1.245 (13) |
| C27—H27A | 0.9600 | C47—O41 | 1.263 (13) |
| C2—N1—C6 | 111.80 (18) | C21—C26—H26 | 119.3 |
| C2—N1—H11 | 107.6 (17) | C22—O22—C27 | 118.3 (2) |
| C6—N1—H11 | 108.1 (18) | O22—C27—H27A | 109.5 |
| C2—N1—H12 | 107 (2) | O22—C27—H27B | 109.5 |
| C6—N1—H12 | 109 (2) | H27A—C27—H27B | 109.5 |
| H11—N1—H12 | 113 (3) | O22—C27—H27C | 109.5 |
| N1—C2—C3 | 111.3 (2) | H27A—C27—H27C | 109.5 |
| N1—C2—H2A | 109.4 | H27B—C27—H27C | 109.5 |
| C3—C2—H2A | 109.4 | C32—C31—C36 | 116.6 (3) |
| N1—C2—H2B | 109.4 | C32—C31—C37 | 124.3 (3) |
| C3—C2—H2B | 109.4 | C36—C31—C37 | 119.1 (3) |
| H2A—C2—H2B | 108.0 | F32—C32—C31 | 118.7 (3) |
| N4—C3—C2 | 110.1 (2) | F32—C32—C33 | 116.6 (4) |
| N4—C3—H3A | 109.6 | C31—C32—C33 | 124.7 (4) |
| C2—C3—H3A | 109.6 | C34—C33—C32 | 117.5 (5) |
| N4—C3—H3B | 109.6 | C34—C33—H33 | 121.2 |
| C2—C3—H3B | 109.6 | C32—C33—H33 | 121.2 |
| H3A—C3—H3B | 108.2 | C33—C34—C35 | 120.8 (4) |
| C21—N4—C5 | 116.3 (2) | C33—C34—H34 | 119.6 |
| C21—N4—C3 | 114.73 (19) | C35—C34—H34 | 119.6 |
| C5—N4—C3 | 109.4 (2) | C34—C35—C36 | 120.9 (4) |
| N4—C5—C6 | 109.3 (2) | C34—C35—H35 | 119.6 |
| N4—C5—H5A | 109.8 | C36—C35—H35 | 119.6 |
| C6—C5—H5A | 109.8 | C35—C36—C31 | 119.5 (5) |
| N4—C5—H5B | 109.8 | C35—C36—H36 | 120.2 |
| C6—C5—H5B | 109.8 | C31—C36—H36 | 120.2 |
| H5A—C5—H5B | 108.3 | O32—C37—O31 | 123.9 (3) |
| N1—C6—C5 | 111.5 (2) | O32—C37—C31 | 119.0 (3) |
| N1—C6—H6A | 109.3 | O31—C37—C31 | 117.0 (3) |
| C5—C6—H6A | 109.3 | C42—C41—C46 | 120.5 (14) |
| N1—C6—H6B | 109.3 | C42—C41—C47 | 123.0 (16) |
| C5—C6—H6B | 109.3 | C46—C41—C47 | 116.5 (15) |
| H6A—C6—H6B | 108.0 | F42—C42—C41 | 120.1 (19) |
| C26—C21—C22 | 117.7 (2) | F42—C42—C43 | 113 (2) |
| C26—C21—N4 | 122.8 (2) | C41—C42—C43 | 123.7 (16) |
| C22—C21—N4 | 119.3 (2) | C44—C43—C42 | 115.8 (17) |
| O22—C22—C23 | 123.8 (3) | C44—C43—H43 | 122.1 |
| O22—C22—C21 | 116.2 (2) | C42—C43—H43 | 122.1 |
| C23—C22—C21 | 119.9 (2) | C43—C44—C45 | 122.5 (17) |
| C24—C23—C22 | 120.8 (3) | C43—C44—H44 | 118.8 |
| C24—C23—H23 | 119.6 | C45—C44—H44 | 118.8 |
| C22—C23—H23 | 119.6 | C44—C45—C46 | 123.1 (17) |
| C25—C24—C23 | 120.0 (3) | C44—C45—H45 | 118.4 |
| C25—C24—H24 | 120.0 | C46—C45—H45 | 118.4 |
| C23—C24—H24 | 120.0 | C45—C46—C41 | 114.4 (16) |
| C24—C25—C26 | 120.1 (3) | C45—C46—H46 | 122.8 |
| C24—C25—H25 | 119.9 | C41—C46—H46 | 122.8 |
| C26—C25—H25 | 119.9 | O42—C47—O41 | 123 (2) |
| C25—C26—C21 | 121.4 (3) | O42—C47—C41 | 118.2 (19) |
| C25—C26—H26 | 119.3 | O41—C47—C41 | 113.7 (19) |
| C6—N1—C2—C3 | −50.7 (3) | C37—C31—C32—C33 | 177.5 (3) |
| N1—C2—C3—N4 | 55.7 (3) | F32—C32—C33—C34 | 179.8 (4) |
| C2—C3—N4—C21 | 165.0 (2) | C31—C32—C33—C34 | 2.2 (6) |
| C2—C3—N4—C5 | −62.2 (3) | C32—C33—C34—C35 | −0.6 (6) |
| C21—N4—C5—C6 | −165.1 (2) | C33—C34—C35—C36 | −0.5 (7) |
| C3—N4—C5—C6 | 62.9 (3) | C34—C35—C36—C31 | 0.1 (6) |
| C2—N1—C6—C5 | 51.9 (3) | C32—C31—C36—C35 | 1.3 (5) |
| N4—C5—C6—N1 | −57.9 (3) | C37—C31—C36—C35 | −178.8 (4) |
| C5—N4—C21—C26 | −11.4 (3) | C32—C31—C37—O32 | 31.1 (5) |
| C3—N4—C21—C26 | 118.2 (3) | C36—C31—C37—O32 | −148.9 (4) |
| C5—N4—C21—C22 | 164.2 (2) | C32—C31—C37—O31 | −150.8 (4) |
| C3—N4—C21—C22 | −66.3 (3) | C36—C31—C37—O31 | 29.3 (5) |
| C26—C21—C22—O22 | 176.1 (2) | C46—C41—C42—F42 | −159 (5) |
| N4—C21—C22—O22 | 0.3 (3) | C47—C41—C42—F42 | 23 (6) |
| C26—C21—C22—C23 | −1.6 (3) | C46—C41—C42—C43 | 0 (6) |
| N4—C21—C22—C23 | −177.4 (2) | C47—C41—C42—C43 | −179 (4) |
| O22—C22—C23—C24 | −176.3 (3) | F42—C42—C43—C44 | 160 (5) |
| C21—C22—C23—C24 | 1.3 (4) | C41—C42—C43—C44 | 0 (7) |
| C22—C23—C24—C25 | 0.0 (5) | C42—C43—C44—C45 | 0 (8) |
| C23—C24—C25—C26 | −0.9 (4) | C43—C44—C45—C46 | 1 (9) |
| C24—C25—C26—C21 | 0.5 (4) | C44—C45—C46—C41 | −2 (7) |
| C22—C21—C26—C25 | 0.8 (4) | C42—C41—C46—C45 | 1 (6) |
| N4—C21—C26—C25 | 176.4 (2) | C47—C41—C46—C45 | 180 (4) |
| C23—C22—O22—C27 | −4.5 (5) | C42—C41—C47—O42 | 40 (6) |
| C21—C22—O22—C27 | 177.9 (3) | C46—C41—C47—O42 | −138 (5) |
| C36—C31—C32—F32 | 179.9 (4) | C42—C41—C47—O41 | −115 (4) |
| C37—C31—C32—F32 | 0.0 (5) | C46—C41—C47—O41 | 66 (5) |
| C36—C31—C32—C33 | −2.6 (5) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-fluorobenzoate (IV). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.99 (3) | 1.72 (3) | 2.694 (4) | 167 (3) |
| N1—H11···O32 | 0.99 (3) | 2.51 (3) | 3.131 (4) | 120.9 (19) |
| N1—H12···O32i | 0.88 (3) | 1.83 (3) | 2.679 (4) | 161 (3) |
| N1—H11···O41 | 0.99 (3) | 1.77 (5) | 2.67 (4) | 151 (3) |
| N1—H11···O42 | 0.99 (3) | 2.52 (5) | 3.20 (4) | 126 (2) |
| N1—H12···O42i | 0.88 (3) | 1.83 (5) | 2.63 (4) | 151 (3) |
| C34—H34···Cg2ii | 0.93 | 2.74 | 3.543 (5) | 145 |
| C44—H44···Cg2ii | 0.93 | 2.99 | 3.73 (4) | 137 |
| C26—H26···Cg3iii | 0.93 | 2.96 | 3.754 (17) | 144 |
Symmetry codes: (i) x, −y+1, z−1/2; (ii) x−1/2, −y+3/2, z−1/2; (iii) x+1/2, y−1/2, z.
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Crystal data
| C11H17N2O+·C7H4ClO2− | F(000) = 736 |
| Mr = 348.82 | Dx = 1.272 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.9974 (8) Å | Cell parameters from 4043 reflections |
| b = 27.611 (2) Å | θ = 2.6–28.0° |
| c = 8.5972 (9) Å | µ = 0.23 mm−1 |
| β = 106.40 (1)° | T = 296 K |
| V = 1821.2 (3) Å3 | Needle, orange |
| Z = 4 | 0.48 × 0.20 × 0.12 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3410 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2060 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.030 |
| ω scans | θmax = 25.6°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −9→9 |
| Tmin = 0.747, Tmax = 0.973 | k = −33→33 |
| 13275 measured reflections | l = −10→10 |
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Refinement
| Refinement on F2 | Primary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.067 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.216 | w = 1/[σ2(Fo2) + (0.1138P)2 + 0.7483P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max < 0.001 |
| 3410 reflections | Δρmax = 1.15 e Å−3 |
| 223 parameters | Δρmin = −0.29 e Å−3 |
| 0 restraints |
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.4620 (4) | 0.44249 (11) | 0.3578 (4) | 0.0640 (8) | |
| H11 | 0.368 (5) | 0.4525 (14) | 0.402 (4) | 0.077* | |
| H12 | 0.511 (5) | 0.4709 (16) | 0.336 (4) | 0.077* | |
| C2 | 0.3954 (4) | 0.41253 (14) | 0.2112 (5) | 0.0684 (10) | |
| H2A | 0.3246 | 0.4323 | 0.1241 | 0.082* | |
| H2B | 0.3225 | 0.3869 | 0.2333 | 0.082* | |
| C3 | 0.5449 (4) | 0.39067 (13) | 0.1593 (4) | 0.0614 (9) | |
| H3A | 0.4993 | 0.3699 | 0.0659 | 0.074* | |
| H3B | 0.6122 | 0.4163 | 0.1284 | 0.074* | |
| N4 | 0.6572 (3) | 0.36247 (10) | 0.2916 (3) | 0.0545 (7) | |
| C5 | 0.7325 (5) | 0.39472 (14) | 0.4289 (4) | 0.0666 (10) | |
| H5A | 0.7975 | 0.4204 | 0.3957 | 0.080* | |
| H5B | 0.8118 | 0.3767 | 0.5159 | 0.080* | |
| C6 | 0.5886 (5) | 0.41589 (16) | 0.4871 (5) | 0.0747 (11) | |
| H6A | 0.5290 | 0.3901 | 0.5265 | 0.090* | |
| H6B | 0.6379 | 0.4377 | 0.5769 | 0.090* | |
| C21 | 0.7769 (4) | 0.33160 (12) | 0.2452 (4) | 0.0538 (8) | |
| C22 | 0.7097 (5) | 0.29139 (12) | 0.1470 (4) | 0.0607 (9) | |
| C23 | 0.8208 (7) | 0.26032 (14) | 0.0999 (5) | 0.0798 (12) | |
| H23 | 0.7765 | 0.2342 | 0.0326 | 0.096* | |
| C24 | 1.0003 (7) | 0.26825 (19) | 0.1536 (6) | 0.0962 (16) | |
| H24 | 1.0751 | 0.2470 | 0.1223 | 0.115* | |
| C25 | 1.0676 (6) | 0.30630 (18) | 0.2506 (6) | 0.0869 (13) | |
| H25 | 1.1875 | 0.3109 | 0.2868 | 0.104* | |
| C26 | 0.9558 (5) | 0.33835 (14) | 0.2954 (4) | 0.0672 (10) | |
| H26 | 1.0018 | 0.3648 | 0.3602 | 0.081* | |
| O22 | 0.5338 (3) | 0.28580 (9) | 0.1060 (3) | 0.0770 (8) | |
| C27 | 0.4570 (7) | 0.24698 (17) | 0.0025 (6) | 0.1036 (16) | |
| H27A | 0.3330 | 0.2476 | −0.0157 | 0.155* | |
| H27B | 0.4835 | 0.2502 | −0.0992 | 0.155* | |
| H27C | 0.5027 | 0.2168 | 0.0522 | 0.155* | |
| C31 | 0.1308 (4) | 0.43466 (12) | 0.7353 (4) | 0.0541 (8) | |
| C32 | 0.1418 (4) | 0.38566 (13) | 0.7729 (4) | 0.0606 (9) | |
| Cl32 | 0.29281 (16) | 0.35006 (4) | 0.71550 (13) | 0.0871 (4) | |
| C33 | 0.0387 (6) | 0.36423 (19) | 0.8551 (5) | 0.0886 (13) | |
| H33 | 0.0482 | 0.3312 | 0.8774 | 0.106* | |
| C34 | −0.0776 (7) | 0.3916 (3) | 0.9036 (6) | 0.1056 (17) | |
| H34 | −0.1480 | 0.3772 | 0.9600 | 0.127* | |
| C35 | −0.0935 (6) | 0.4406 (2) | 0.8710 (5) | 0.0955 (15) | |
| H35 | −0.1735 | 0.4591 | 0.9055 | 0.115* | |
| C36 | 0.0107 (5) | 0.46182 (16) | 0.7865 (5) | 0.0760 (11) | |
| H36 | 0.0000 | 0.4948 | 0.7637 | 0.091* | |
| C37 | 0.2417 (5) | 0.45733 (12) | 0.6402 (5) | 0.0643 (9) | |
| O31 | 0.1985 (3) | 0.45110 (11) | 0.4917 (3) | 0.0810 (8) | |
| O32 | 0.3701 (5) | 0.48041 (13) | 0.7200 (4) | 0.1152 (12) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0672 (19) | 0.0561 (18) | 0.075 (2) | −0.0144 (15) | 0.0306 (17) | −0.0193 (16) |
| C2 | 0.058 (2) | 0.067 (2) | 0.075 (2) | −0.0070 (17) | 0.0105 (18) | −0.0203 (19) |
| C3 | 0.063 (2) | 0.065 (2) | 0.051 (2) | 0.0015 (17) | 0.0071 (16) | −0.0152 (16) |
| N4 | 0.0560 (16) | 0.0569 (16) | 0.0480 (15) | −0.0057 (12) | 0.0104 (13) | −0.0120 (12) |
| C5 | 0.066 (2) | 0.073 (2) | 0.055 (2) | −0.0103 (18) | 0.0071 (17) | −0.0133 (17) |
| C6 | 0.084 (3) | 0.083 (3) | 0.058 (2) | −0.012 (2) | 0.022 (2) | −0.024 (2) |
| C21 | 0.060 (2) | 0.0538 (18) | 0.0486 (18) | −0.0018 (15) | 0.0169 (15) | 0.0073 (15) |
| C22 | 0.079 (2) | 0.0516 (19) | 0.057 (2) | 0.0033 (18) | 0.0281 (18) | 0.0030 (16) |
| C23 | 0.113 (4) | 0.056 (2) | 0.082 (3) | 0.013 (2) | 0.045 (3) | 0.0067 (19) |
| C24 | 0.113 (4) | 0.080 (3) | 0.114 (4) | 0.045 (3) | 0.062 (3) | 0.038 (3) |
| C25 | 0.072 (3) | 0.084 (3) | 0.108 (3) | 0.025 (2) | 0.029 (2) | 0.042 (3) |
| C26 | 0.061 (2) | 0.067 (2) | 0.067 (2) | 0.0059 (18) | 0.0090 (18) | 0.0218 (18) |
| O22 | 0.0822 (18) | 0.0675 (16) | 0.0825 (18) | −0.0186 (13) | 0.0251 (14) | −0.0303 (14) |
| C27 | 0.129 (4) | 0.072 (3) | 0.111 (4) | −0.036 (3) | 0.037 (3) | −0.041 (3) |
| C31 | 0.0585 (19) | 0.0577 (19) | 0.0435 (17) | −0.0075 (15) | 0.0100 (15) | 0.0004 (14) |
| C32 | 0.072 (2) | 0.062 (2) | 0.0431 (18) | −0.0134 (17) | 0.0088 (16) | 0.0058 (15) |
| Cl32 | 0.1206 (9) | 0.0538 (6) | 0.0861 (8) | 0.0079 (5) | 0.0279 (6) | 0.0040 (5) |
| C33 | 0.109 (4) | 0.092 (3) | 0.065 (3) | −0.020 (3) | 0.026 (3) | 0.020 (2) |
| C34 | 0.103 (4) | 0.151 (5) | 0.070 (3) | −0.021 (4) | 0.036 (3) | 0.033 (3) |
| C35 | 0.080 (3) | 0.148 (5) | 0.064 (3) | 0.012 (3) | 0.031 (2) | 0.007 (3) |
| C36 | 0.076 (2) | 0.086 (3) | 0.069 (2) | 0.007 (2) | 0.025 (2) | 0.004 (2) |
| C37 | 0.072 (2) | 0.0407 (17) | 0.085 (3) | −0.0034 (17) | 0.030 (2) | 0.0005 (18) |
| O31 | 0.0823 (18) | 0.101 (2) | 0.0683 (18) | 0.0104 (15) | 0.0350 (15) | 0.0168 (15) |
| O32 | 0.130 (3) | 0.094 (2) | 0.132 (3) | −0.062 (2) | 0.053 (2) | −0.024 (2) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Geometric parameters (Å, º)
| N1—C6 | 1.472 (5) | C24—C25 | 1.355 (7) |
| N1—C2 | 1.475 (4) | C24—H24 | 0.9300 |
| N1—H11 | 0.97 (4) | C25—C26 | 1.387 (6) |
| N1—H12 | 0.92 (4) | C25—H25 | 0.9300 |
| C2—C3 | 1.515 (5) | C26—H26 | 0.9300 |
| C2—H2A | 0.9700 | O22—C27 | 1.418 (4) |
| C2—H2B | 0.9700 | C27—H27A | 0.9600 |
| C3—N4 | 1.460 (4) | C27—H27B | 0.9600 |
| C3—H3A | 0.9700 | C27—H27C | 0.9600 |
| C3—H3B | 0.9700 | C31—C36 | 1.385 (5) |
| N4—C21 | 1.421 (4) | C31—C32 | 1.388 (5) |
| N4—C5 | 1.465 (4) | C31—C37 | 1.503 (5) |
| C5—C6 | 1.497 (5) | C32—C33 | 1.363 (5) |
| C5—H5A | 0.9700 | C32—Cl32 | 1.733 (4) |
| C5—H5B | 0.9700 | C33—C34 | 1.354 (7) |
| C6—H6A | 0.9700 | C33—H33 | 0.9300 |
| C6—H6B | 0.9700 | C34—C35 | 1.378 (7) |
| C21—C26 | 1.385 (5) | C34—H34 | 0.9300 |
| C21—C22 | 1.407 (5) | C35—C36 | 1.381 (6) |
| C22—O22 | 1.359 (4) | C35—H35 | 0.9300 |
| C22—C23 | 1.375 (5) | C36—H36 | 0.9300 |
| C23—C24 | 1.395 (6) | C37—O31 | 1.237 (5) |
| C23—H23 | 0.9300 | C37—O32 | 1.238 (5) |
| C6—N1—C2 | 111.8 (3) | C22—C23—H23 | 120.2 |
| C6—N1—H11 | 107 (2) | C24—C23—H23 | 120.2 |
| C2—N1—H11 | 111 (2) | C25—C24—C23 | 121.2 (4) |
| C6—N1—H12 | 110 (2) | C25—C24—H24 | 119.4 |
| C2—N1—H12 | 112 (2) | C23—C24—H24 | 119.4 |
| H11—N1—H12 | 105 (3) | C24—C25—C26 | 119.4 (4) |
| N1—C2—C3 | 110.5 (3) | C24—C25—H25 | 120.3 |
| N1—C2—H2A | 109.5 | C26—C25—H25 | 120.3 |
| C3—C2—H2A | 109.5 | C21—C26—C25 | 121.2 (4) |
| N1—C2—H2B | 109.5 | C21—C26—H26 | 119.4 |
| C3—C2—H2B | 109.5 | C25—C26—H26 | 119.4 |
| H2A—C2—H2B | 108.1 | C22—O22—C27 | 118.8 (3) |
| N4—C3—C2 | 110.3 (3) | O22—C27—H27A | 109.5 |
| N4—C3—H3A | 109.6 | O22—C27—H27B | 109.5 |
| C2—C3—H3A | 109.6 | H27A—C27—H27B | 109.5 |
| N4—C3—H3B | 109.6 | O22—C27—H27C | 109.5 |
| C2—C3—H3B | 109.6 | H27A—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.1 | H27B—C27—H27C | 109.5 |
| C21—N4—C3 | 114.6 (2) | C36—C31—C32 | 117.3 (3) |
| C21—N4—C5 | 115.8 (3) | C36—C31—C37 | 121.1 (3) |
| C3—N4—C5 | 109.0 (3) | C32—C31—C37 | 121.6 (3) |
| N4—C5—C6 | 109.1 (3) | C33—C32—C31 | 122.4 (4) |
| N4—C5—H5A | 109.9 | C33—C32—Cl32 | 118.2 (3) |
| C6—C5—H5A | 109.9 | C31—C32—Cl32 | 119.4 (3) |
| N4—C5—H5B | 109.9 | C34—C33—C32 | 119.1 (5) |
| C6—C5—H5B | 109.9 | C34—C33—H33 | 120.5 |
| H5A—C5—H5B | 108.3 | C32—C33—H33 | 120.5 |
| N1—C6—C5 | 111.8 (3) | C33—C34—C35 | 121.1 (4) |
| N1—C6—H6A | 109.2 | C33—C34—H34 | 119.4 |
| C5—C6—H6A | 109.2 | C35—C34—H34 | 119.4 |
| N1—C6—H6B | 109.2 | C34—C35—C36 | 119.3 (4) |
| C5—C6—H6B | 109.2 | C34—C35—H35 | 120.4 |
| H6A—C6—H6B | 107.9 | C36—C35—H35 | 120.4 |
| C26—C21—C22 | 118.6 (3) | C35—C36—C31 | 120.8 (4) |
| C26—C21—N4 | 123.4 (3) | C35—C36—H36 | 119.6 |
| C22—C21—N4 | 118.0 (3) | C31—C36—H36 | 119.6 |
| O22—C22—C23 | 124.1 (4) | O31—C37—O32 | 126.2 (4) |
| O22—C22—C21 | 115.9 (3) | O31—C37—C31 | 117.9 (3) |
| C23—C22—C21 | 120.0 (4) | O32—C37—C31 | 115.9 (4) |
| C22—C23—C24 | 119.6 (4) | ||
| C6—N1—C2—C3 | −51.8 (4) | C22—C21—C26—C25 | −0.1 (5) |
| N1—C2—C3—N4 | 56.7 (4) | N4—C21—C26—C25 | 178.0 (3) |
| C2—C3—N4—C21 | 166.2 (3) | C24—C25—C26—C21 | 1.3 (6) |
| C2—C3—N4—C5 | −62.2 (3) | C23—C22—O22—C27 | −2.9 (5) |
| C21—N4—C5—C6 | −166.8 (3) | C21—C22—O22—C27 | 177.8 (3) |
| C3—N4—C5—C6 | 62.2 (4) | C36—C31—C32—C33 | 0.7 (5) |
| C2—N1—C6—C5 | 53.2 (4) | C37—C31—C32—C33 | −178.1 (4) |
| N4—C5—C6—N1 | −58.0 (4) | C36—C31—C32—Cl32 | −178.7 (3) |
| C3—N4—C21—C26 | 112.9 (3) | C37—C31—C32—Cl32 | 2.4 (4) |
| C5—N4—C21—C26 | −15.3 (4) | C31—C32—C33—C34 | −0.7 (6) |
| C3—N4—C21—C22 | −68.9 (4) | Cl32—C32—C33—C34 | 178.7 (3) |
| C5—N4—C21—C22 | 162.8 (3) | C32—C33—C34—C35 | 0.2 (7) |
| C26—C21—C22—O22 | 177.9 (3) | C33—C34—C35—C36 | 0.3 (7) |
| N4—C21—C22—O22 | −0.3 (4) | C34—C35—C36—C31 | −0.3 (6) |
| C26—C21—C22—C23 | −1.4 (5) | C32—C31—C36—C35 | −0.2 (5) |
| N4—C21—C22—C23 | −179.6 (3) | C37—C31—C36—C35 | 178.6 (4) |
| O22—C22—C23—C24 | −177.6 (4) | C36—C31—C37—O31 | −101.0 (4) |
| C21—C22—C23—C24 | 1.7 (5) | C32—C31—C37—O31 | 77.8 (4) |
| C22—C23—C24—C25 | −0.5 (6) | C36—C31—C37—O32 | 79.5 (4) |
| C23—C24—C25—C26 | −0.9 (6) | C32—C31—C37—O32 | −101.7 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-chlorobenzoate (V). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.97 (4) | 1.74 (3) | 2.682 (4) | 162 (3) |
| N1—H12···O32i | 0.92 (4) | 1.79 (4) | 2.700 (5) | 170 (4) |
| C5—H5B···Cg1ii | 0.97 | 2.87 | 3.554 (4) | 128 |
| C34—H34···Cg2iii | 0.93 | 2.93 | 3.658 (7) | 136 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x+1, y, z; (iii) x−1, y, z+1.
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Crystal data
| C11H17N2O+·C7H4BrO2− | Dx = 1.451 Mg m−3 |
| Mr = 393.28 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, P212121 | Cell parameters from 3863 reflections |
| a = 6.9824 (2) Å | θ = 5.6–89.3° |
| b = 13.2292 (4) Å | µ = 2.30 mm−1 |
| c = 19.4903 (7) Å | T = 293 K |
| V = 1800.35 (10) Å3 | Block, orange |
| Z = 4 | 0.50 × 0.50 × 0.48 mm |
| F(000) = 808 |
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3895 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2640 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.033 |
| ω scans | θmax = 27.7°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −9→9 |
| Tmin = 0.297, Tmax = 0.331 | k = −16→17 |
| 13089 measured reflections | l = −24→23 |
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.035 | w = 1/[σ2(Fo2) + (0.0418P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.077 | (Δ/σ)max = 0.001 |
| S = 0.94 | Δρmax = 0.28 e Å−3 |
| 3895 reflections | Δρmin = −0.53 e Å−3 |
| 224 parameters | Absolute structure: Flack x determined using 919 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 0 restraints | Absolute structure parameter: 0.004 (5) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.2366 (5) | 0.3584 (2) | 0.50360 (15) | 0.0385 (7) | |
| H11 | 0.156 (5) | 0.338 (3) | 0.5251 (19) | 0.046* | |
| H12 | 0.271 (5) | 0.307 (2) | 0.4774 (17) | 0.046* | |
| C2 | 0.4021 (5) | 0.3893 (3) | 0.54630 (18) | 0.0422 (9) | |
| H2A | 0.4540 | 0.3309 | 0.5699 | 0.051* | |
| H2B | 0.3611 | 0.4380 | 0.5805 | 0.051* | |
| C3 | 0.5535 (5) | 0.4355 (2) | 0.50113 (17) | 0.0351 (8) | |
| H3A | 0.6611 | 0.4572 | 0.5290 | 0.042* | |
| H3B | 0.5993 | 0.3855 | 0.4686 | 0.042* | |
| N4 | 0.4745 (4) | 0.52259 (19) | 0.46388 (14) | 0.0323 (7) | |
| C5 | 0.3155 (5) | 0.4908 (2) | 0.42030 (18) | 0.0356 (9) | |
| H5A | 0.3607 | 0.4421 | 0.3868 | 0.043* | |
| H5B | 0.2651 | 0.5488 | 0.3957 | 0.043* | |
| C6 | 0.1594 (5) | 0.4441 (3) | 0.4630 (2) | 0.0446 (10) | |
| H6A | 0.1064 | 0.4946 | 0.4938 | 0.054* | |
| H6B | 0.0574 | 0.4203 | 0.4334 | 0.054* | |
| C21 | 0.6096 (5) | 0.5876 (2) | 0.43291 (17) | 0.0318 (8) | |
| C22 | 0.7393 (5) | 0.6421 (2) | 0.47504 (16) | 0.0353 (7) | |
| C23 | 0.8583 (5) | 0.7139 (3) | 0.4459 (2) | 0.0493 (10) | |
| H23 | 0.9422 | 0.7502 | 0.4735 | 0.059* | |
| C24 | 0.8539 (6) | 0.7323 (3) | 0.3762 (2) | 0.0574 (11) | |
| H24 | 0.9346 | 0.7808 | 0.3573 | 0.069* | |
| C25 | 0.7323 (5) | 0.6800 (3) | 0.33479 (18) | 0.0482 (9) | |
| H25 | 0.7286 | 0.6931 | 0.2879 | 0.058* | |
| C26 | 0.6140 (5) | 0.6070 (3) | 0.36316 (18) | 0.0392 (9) | |
| H26 | 0.5348 | 0.5698 | 0.3343 | 0.047* | |
| O22 | 0.7279 (4) | 0.62323 (15) | 0.54385 (11) | 0.0412 (6) | |
| C27 | 0.8382 (6) | 0.6854 (3) | 0.58800 (19) | 0.0531 (11) | |
| H27A | 0.9718 | 0.6758 | 0.5784 | 0.080* | |
| H27B | 0.8126 | 0.6675 | 0.6348 | 0.080* | |
| H27C | 0.8048 | 0.7549 | 0.5806 | 0.080* | |
| C31 | 0.0331 (5) | 0.3548 (2) | 0.67134 (16) | 0.0357 (8) | |
| C32 | 0.1964 (5) | 0.3120 (3) | 0.69802 (18) | 0.0444 (9) | |
| Br32 | 0.23614 (7) | 0.16981 (3) | 0.68887 (3) | 0.0746 (2) | |
| C33 | 0.3352 (6) | 0.3685 (4) | 0.7308 (2) | 0.0629 (13) | |
| H33 | 0.4435 | 0.3377 | 0.7491 | 0.076* | |
| C34 | 0.3097 (7) | 0.4713 (4) | 0.7360 (2) | 0.0712 (15) | |
| H34 | 0.4033 | 0.5110 | 0.7566 | 0.085* | |
| C35 | 0.1467 (8) | 0.5153 (4) | 0.7107 (2) | 0.0685 (14) | |
| H35 | 0.1279 | 0.5845 | 0.7158 | 0.082* | |
| C36 | 0.0120 (6) | 0.4583 (3) | 0.6783 (2) | 0.0527 (10) | |
| H36 | −0.0964 | 0.4895 | 0.6605 | 0.063* | |
| C37 | −0.1145 (5) | 0.2952 (3) | 0.6315 (2) | 0.0397 (9) | |
| O31 | −0.0853 (3) | 0.29029 (17) | 0.56785 (12) | 0.0409 (6) | |
| O32 | −0.2506 (4) | 0.2563 (2) | 0.66127 (13) | 0.0792 (9) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0415 (17) | 0.0396 (15) | 0.0344 (17) | −0.0058 (17) | 0.0099 (16) | −0.0041 (12) |
| C2 | 0.048 (2) | 0.044 (2) | 0.035 (2) | 0.0010 (17) | 0.0020 (18) | 0.0005 (17) |
| C3 | 0.0376 (18) | 0.0328 (18) | 0.035 (2) | −0.0018 (16) | −0.0022 (16) | −0.0011 (16) |
| N4 | 0.0320 (15) | 0.0302 (15) | 0.0348 (18) | −0.0001 (13) | −0.0053 (13) | 0.0004 (13) |
| C5 | 0.037 (2) | 0.0373 (19) | 0.033 (2) | −0.0035 (15) | −0.0070 (15) | 0.0017 (15) |
| C6 | 0.0376 (19) | 0.045 (2) | 0.051 (2) | −0.0019 (17) | −0.0041 (18) | 0.0004 (19) |
| C21 | 0.0355 (18) | 0.0291 (16) | 0.031 (2) | 0.0035 (15) | 0.0023 (16) | −0.0034 (15) |
| C22 | 0.0340 (17) | 0.0379 (17) | 0.034 (2) | −0.0008 (18) | −0.0025 (18) | −0.0017 (14) |
| C23 | 0.041 (2) | 0.057 (2) | 0.050 (3) | −0.0128 (19) | −0.0041 (18) | 0.001 (2) |
| C24 | 0.051 (2) | 0.069 (3) | 0.052 (3) | −0.017 (2) | 0.006 (2) | 0.014 (2) |
| C25 | 0.045 (2) | 0.070 (2) | 0.0304 (19) | −0.005 (2) | 0.0032 (18) | 0.0057 (17) |
| C26 | 0.039 (2) | 0.045 (2) | 0.034 (2) | −0.0004 (17) | −0.0016 (17) | −0.0041 (17) |
| O22 | 0.0488 (14) | 0.0450 (12) | 0.0297 (13) | −0.0095 (13) | −0.0065 (13) | −0.0008 (10) |
| C27 | 0.059 (2) | 0.061 (3) | 0.039 (2) | −0.008 (2) | −0.0174 (19) | −0.0053 (19) |
| C31 | 0.0362 (19) | 0.046 (2) | 0.025 (2) | −0.0065 (16) | 0.0051 (15) | −0.0024 (15) |
| C32 | 0.0384 (19) | 0.065 (2) | 0.030 (2) | −0.0035 (17) | 0.0008 (16) | 0.0064 (19) |
| Br32 | 0.0640 (3) | 0.0691 (3) | 0.0907 (4) | 0.0123 (3) | −0.0067 (3) | 0.0203 (3) |
| C33 | 0.045 (2) | 0.107 (4) | 0.037 (2) | −0.014 (3) | −0.0080 (19) | 0.012 (2) |
| C34 | 0.070 (3) | 0.103 (4) | 0.040 (3) | −0.045 (3) | 0.006 (2) | −0.022 (3) |
| C35 | 0.087 (3) | 0.060 (3) | 0.058 (3) | −0.023 (3) | 0.016 (3) | −0.015 (2) |
| C36 | 0.058 (2) | 0.051 (2) | 0.049 (3) | −0.004 (2) | 0.002 (2) | −0.006 (2) |
| C37 | 0.0325 (19) | 0.048 (2) | 0.039 (2) | 0.0034 (17) | −0.0004 (17) | −0.0047 (18) |
| O31 | 0.0475 (14) | 0.0471 (14) | 0.0283 (14) | −0.0077 (12) | 0.0019 (12) | −0.0046 (12) |
| O32 | 0.0561 (18) | 0.131 (3) | 0.0509 (17) | −0.044 (2) | 0.0165 (16) | −0.0198 (16) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Geometric parameters (Å, º)
| N1—C2 | 1.481 (5) | C24—C25 | 1.360 (5) |
| N1—C6 | 1.484 (4) | C24—H24 | 0.9300 |
| N1—H11 | 0.75 (4) | C25—C26 | 1.386 (5) |
| N1—H12 | 0.89 (3) | C25—H25 | 0.9300 |
| C2—C3 | 1.506 (5) | C26—H26 | 0.9300 |
| C2—H2A | 0.9700 | O22—C27 | 1.417 (4) |
| C2—H2B | 0.9700 | C27—H27A | 0.9600 |
| C3—N4 | 1.469 (4) | C27—H27B | 0.9600 |
| C3—H3A | 0.9700 | C27—H27C | 0.9600 |
| C3—H3B | 0.9700 | C31—C32 | 1.375 (5) |
| N4—C21 | 1.412 (4) | C31—C36 | 1.383 (4) |
| N4—C5 | 1.460 (4) | C31—C37 | 1.512 (5) |
| C5—C6 | 1.505 (5) | C32—C33 | 1.381 (5) |
| C5—H5A | 0.9700 | C32—Br32 | 1.910 (4) |
| C5—H5B | 0.9700 | C33—C34 | 1.375 (7) |
| C6—H6A | 0.9700 | C33—H33 | 0.9300 |
| C6—H6B | 0.9700 | C34—C35 | 1.370 (7) |
| C21—C26 | 1.384 (5) | C34—H34 | 0.9300 |
| C21—C22 | 1.419 (4) | C35—C36 | 1.361 (6) |
| C22—O22 | 1.366 (4) | C35—H35 | 0.9300 |
| C22—C23 | 1.385 (5) | C36—H36 | 0.9300 |
| C23—C24 | 1.380 (5) | C37—O32 | 1.226 (4) |
| C23—H23 | 0.9300 | C37—O31 | 1.260 (4) |
| C2—N1—C6 | 111.8 (3) | C24—C23—H23 | 119.6 |
| C2—N1—H11 | 112 (3) | C22—C23—H23 | 119.6 |
| C6—N1—H11 | 107 (3) | C25—C24—C23 | 120.5 (4) |
| C2—N1—H12 | 109 (2) | C25—C24—H24 | 119.7 |
| C6—N1—H12 | 112 (2) | C23—C24—H24 | 119.7 |
| H11—N1—H12 | 104 (3) | C24—C25—C26 | 119.3 (3) |
| N1—C2—C3 | 109.3 (3) | C24—C25—H25 | 120.3 |
| N1—C2—H2A | 109.8 | C26—C25—H25 | 120.3 |
| C3—C2—H2A | 109.8 | C21—C26—C25 | 122.3 (3) |
| N1—C2—H2B | 109.8 | C21—C26—H26 | 118.8 |
| C3—C2—H2B | 109.8 | C25—C26—H26 | 118.8 |
| H2A—C2—H2B | 108.3 | C22—O22—C27 | 117.3 (3) |
| N4—C3—C2 | 110.1 (3) | O22—C27—H27A | 109.5 |
| N4—C3—H3A | 109.6 | O22—C27—H27B | 109.5 |
| C2—C3—H3A | 109.6 | H27A—C27—H27B | 109.5 |
| N4—C3—H3B | 109.6 | O22—C27—H27C | 109.5 |
| C2—C3—H3B | 109.6 | H27A—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.2 | H27B—C27—H27C | 109.5 |
| C21—N4—C5 | 115.8 (3) | C32—C31—C36 | 117.3 (3) |
| C21—N4—C3 | 116.0 (3) | C32—C31—C37 | 123.0 (3) |
| C5—N4—C3 | 110.3 (3) | C36—C31—C37 | 119.6 (3) |
| N4—C5—C6 | 110.3 (3) | C31—C32—C33 | 122.3 (4) |
| N4—C5—H5A | 109.6 | C31—C32—Br32 | 119.4 (3) |
| C6—C5—H5A | 109.6 | C33—C32—Br32 | 118.3 (3) |
| N4—C5—H5B | 109.6 | C34—C33—C32 | 118.6 (4) |
| C6—C5—H5B | 109.6 | C34—C33—H33 | 120.7 |
| H5A—C5—H5B | 108.1 | C32—C33—H33 | 120.7 |
| N1—C6—C5 | 110.2 (3) | C35—C34—C33 | 120.1 (4) |
| N1—C6—H6A | 109.6 | C35—C34—H34 | 119.9 |
| C5—C6—H6A | 109.6 | C33—C34—H34 | 119.9 |
| N1—C6—H6B | 109.6 | C36—C35—C34 | 120.3 (4) |
| C5—C6—H6B | 109.6 | C36—C35—H35 | 119.8 |
| H6A—C6—H6B | 108.1 | C34—C35—H35 | 119.8 |
| C26—C21—N4 | 123.2 (3) | C35—C36—C31 | 121.4 (4) |
| C26—C21—C22 | 117.4 (3) | C35—C36—H36 | 119.3 |
| N4—C21—C22 | 119.2 (3) | C31—C36—H36 | 119.3 |
| O22—C22—C23 | 124.3 (3) | O32—C37—O31 | 124.8 (3) |
| O22—C22—C21 | 116.0 (3) | O32—C37—C31 | 120.3 (3) |
| C23—C22—C21 | 119.6 (3) | O31—C37—C31 | 114.9 (3) |
| C24—C23—C22 | 120.7 (3) | ||
| C6—N1—C2—C3 | −56.0 (4) | N4—C21—C26—C25 | 172.1 (3) |
| N1—C2—C3—N4 | 58.1 (4) | C22—C21—C26—C25 | −3.2 (5) |
| C2—C3—N4—C21 | 165.1 (3) | C24—C25—C26—C21 | 2.4 (5) |
| C2—C3—N4—C5 | −60.7 (3) | C23—C22—O22—C27 | 3.5 (5) |
| C21—N4—C5—C6 | −166.1 (3) | C21—C22—O22—C27 | −172.2 (3) |
| C3—N4—C5—C6 | 59.7 (4) | C36—C31—C32—C33 | 0.2 (5) |
| C2—N1—C6—C5 | 55.3 (4) | C37—C31—C32—C33 | 176.2 (3) |
| N4—C5—C6—N1 | −56.5 (4) | C36—C31—C32—Br32 | −179.1 (3) |
| C5—N4—C21—C26 | −11.1 (4) | C37—C31—C32—Br32 | −3.1 (4) |
| C3—N4—C21—C26 | 120.6 (3) | C31—C32—C33—C34 | −1.0 (6) |
| C5—N4—C21—C22 | 164.2 (3) | Br32—C32—C33—C34 | 178.3 (3) |
| C3—N4—C21—C22 | −64.2 (4) | C32—C33—C34—C35 | 2.0 (6) |
| C26—C21—C22—O22 | 178.3 (3) | C33—C34—C35—C36 | −2.3 (7) |
| N4—C21—C22—O22 | 2.8 (4) | C34—C35—C36—C31 | 1.5 (6) |
| C26—C21—C22—C23 | 2.4 (5) | C32—C31—C36—C35 | −0.5 (6) |
| N4—C21—C22—C23 | −173.2 (3) | C37—C31—C36—C35 | −176.6 (4) |
| O22—C22—C23—C24 | −176.4 (4) | C32—C31—C37—O32 | 91.3 (4) |
| C21—C22—C23—C24 | −0.8 (5) | C36—C31—C37—O32 | −92.8 (5) |
| C22—C23—C24—C25 | 0.0 (6) | C32—C31—C37—O31 | −89.1 (4) |
| C23—C24—C25—C26 | −0.7 (6) | C36—C31—C37—O31 | 86.8 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-bromobenzoate (VI). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.75 (4) | 1.98 (4) | 2.726 (4) | 170 (4) |
| N1—H12···O31i | 0.88 (3) | 1.86 (3) | 2.712 (4) | 163 (3) |
| C25—H25···O32ii | 0.93 | 2.56 | 3.488 (4) | 173 |
| C26—H26···Cg1ii | 0.93 | 2.93 | 3.697 (4) | 141 |
Symmetry codes: (i) x+1/2, −y+1/2, −z+1; (ii) −x+1/2, −y+1, z−1/2.
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Crystal data
| C11H17N2O+·C7H4IO2− | Dx = 1.595 Mg m−3 |
| Mr = 440.27 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, P212121 | Cell parameters from 3735 reflections |
| a = 7.0101 (4) Å | θ = 2.6–27.8° |
| b = 13.3796 (6) Å | µ = 1.76 mm−1 |
| c = 19.5524 (6) Å | T = 293 K |
| V = 1833.87 (14) Å3 | Block, orange |
| Z = 4 | 0.50 × 0.50 × 0.48 mm |
| F(000) = 880 |
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3735 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 3036 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.019 |
| ω scans | θmax = 27.8°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −5→9 |
| Tmin = 0.373, Tmax = 0.431 | k = −17→16 |
| 7500 measured reflections | l = −25→25 |
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.032 | w = 1/[σ2(Fo2) + (0.0306P)2 + 0.7308P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.071 | (Δ/σ)max < 0.001 |
| S = 1.05 | Δρmax = 0.46 e Å−3 |
| 3735 reflections | Δρmin = −0.65 e Å−3 |
| 237 parameters | Absolute structure: Flack x determined using 1045 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 17 restraints | Absolute structure parameter: 0.004 (10) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.7532 (6) | 0.6404 (2) | 0.49690 (15) | 0.0402 (7) | |
| H11 | 0.8436 | 0.6650 | 0.4697 | 0.048* | |
| H12 | 0.7154 | 0.6890 | 0.5249 | 0.048* | |
| C2 | 0.5901 (6) | 0.6069 (4) | 0.4550 (2) | 0.0423 (11) | |
| H2A | 0.6330 | 0.5584 | 0.4215 | 0.051* | |
| H2B | 0.5361 | 0.6634 | 0.4307 | 0.051* | |
| C3 | 0.4399 (6) | 0.5604 (3) | 0.5005 (2) | 0.0387 (10) | |
| H3A | 0.3923 | 0.6100 | 0.5324 | 0.046* | |
| H3B | 0.3338 | 0.5374 | 0.4728 | 0.046* | |
| N4 | 0.5212 (5) | 0.4761 (3) | 0.53831 (17) | 0.0339 (8) | |
| C5 | 0.6810 (6) | 0.5097 (3) | 0.5809 (2) | 0.0396 (10) | |
| H5A | 0.7349 | 0.4530 | 0.6051 | 0.048* | |
| H5B | 0.6356 | 0.5574 | 0.6145 | 0.048* | |
| C6 | 0.8318 (7) | 0.5575 (4) | 0.5374 (3) | 0.0487 (12) | |
| H6A | 0.9335 | 0.5823 | 0.5664 | 0.058* | |
| H6B | 0.8857 | 0.5078 | 0.5068 | 0.058* | |
| C21 | 0.3893 (5) | 0.4115 (3) | 0.5700 (2) | 0.0338 (9) | |
| C22 | 0.2609 (8) | 0.3565 (3) | 0.52887 (19) | 0.0372 (9) | |
| C23 | 0.1420 (7) | 0.2860 (4) | 0.5579 (3) | 0.0517 (12) | |
| H23 | 0.0584 | 0.2496 | 0.5307 | 0.062* | |
| C24 | 0.1478 (8) | 0.2697 (4) | 0.6279 (3) | 0.0583 (14) | |
| H24 | 0.0678 | 0.2219 | 0.6472 | 0.070* | |
| C25 | 0.2675 (8) | 0.3219 (4) | 0.6684 (2) | 0.0527 (12) | |
| H25 | 0.2700 | 0.3102 | 0.7153 | 0.063* | |
| C26 | 0.3866 (6) | 0.3931 (3) | 0.6399 (2) | 0.0418 (11) | |
| H26 | 0.4670 | 0.4296 | 0.6683 | 0.050* | |
| O22 | 0.2722 (6) | 0.3732 (2) | 0.45961 (14) | 0.0432 (7) | |
| C27 | 0.1621 (8) | 0.3095 (4) | 0.4164 (3) | 0.0574 (14) | |
| H27A | 0.0289 | 0.3190 | 0.4257 | 0.086* | |
| H27B | 0.1959 | 0.2411 | 0.4249 | 0.086* | |
| H27C | 0.1874 | 0.3255 | 0.3694 | 0.086* | |
| C31 | 0.9641 (6) | 0.6382 (4) | 0.3324 (2) | 0.0387 (10) | |
| C32 | 0.8007 (6) | 0.6775 (4) | 0.3027 (2) | 0.0421 (11) | |
| I32 | 0.74794 (6) | 0.83201 (2) | 0.30799 (2) | 0.06407 (13) | |
| C33 | 0.6680 (8) | 0.6168 (5) | 0.2703 (3) | 0.0596 (15) | |
| H33 | 0.5591 | 0.6443 | 0.2506 | 0.072* | |
| C34 | 0.7001 (8) | 0.5157 (5) | 0.2677 (3) | 0.0689 (19) | |
| H34 | 0.6110 | 0.4741 | 0.2470 | 0.083* | |
| C35 | 0.8613 (10) | 0.4758 (5) | 0.2953 (3) | 0.0660 (16) | |
| H35 | 0.8834 | 0.4075 | 0.2922 | 0.079* | |
| C36 | 0.9913 (7) | 0.5360 (4) | 0.3277 (3) | 0.0536 (13) | |
| H36 | 1.0999 | 0.5075 | 0.3469 | 0.064* | |
| C37 | 1.1071 (6) | 0.7004 (3) | 0.3715 (2) | 0.0389 (10) | 0.54 (9) |
| O31 | 1.072 (4) | 0.699 (3) | 0.4352 (5) | 0.033 (3) | 0.54 (9) |
| O32 | 1.233 (4) | 0.750 (3) | 0.3440 (12) | 0.065 (5) | 0.54 (9) |
| C38 | 1.1071 (6) | 0.7004 (3) | 0.3715 (2) | 0.0389 (10) | 0.46 (9) |
| O33 | 1.070 (4) | 0.726 (3) | 0.4325 (7) | 0.030 (4) | 0.46 (9) |
| O34 | 1.258 (3) | 0.716 (4) | 0.3417 (13) | 0.065 (6) | 0.46 (9) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0418 (17) | 0.0403 (16) | 0.0384 (16) | −0.005 (3) | 0.013 (2) | −0.0056 (13) |
| C2 | 0.045 (3) | 0.047 (3) | 0.035 (2) | −0.002 (2) | 0.002 (2) | −0.002 (2) |
| C3 | 0.042 (2) | 0.036 (2) | 0.038 (2) | −0.003 (2) | −0.005 (2) | 0.001 (2) |
| N4 | 0.0337 (19) | 0.0347 (19) | 0.0334 (18) | −0.0026 (16) | −0.0045 (15) | −0.0008 (16) |
| C5 | 0.037 (2) | 0.044 (2) | 0.038 (2) | −0.0003 (19) | −0.0061 (19) | −0.004 (2) |
| C6 | 0.040 (2) | 0.053 (3) | 0.054 (3) | −0.001 (2) | −0.005 (2) | −0.006 (3) |
| C21 | 0.031 (2) | 0.034 (2) | 0.036 (2) | 0.0025 (18) | 0.0003 (19) | −0.0023 (19) |
| C22 | 0.037 (2) | 0.038 (2) | 0.0362 (19) | 0.002 (3) | 0.000 (3) | 0.0002 (15) |
| C23 | 0.044 (3) | 0.057 (3) | 0.054 (3) | −0.010 (2) | −0.004 (2) | 0.004 (3) |
| C24 | 0.052 (3) | 0.068 (4) | 0.055 (3) | −0.016 (3) | 0.008 (3) | 0.017 (3) |
| C25 | 0.050 (3) | 0.068 (3) | 0.041 (2) | −0.003 (4) | 0.006 (3) | 0.010 (2) |
| C26 | 0.042 (2) | 0.050 (3) | 0.033 (2) | 0.000 (2) | 0.002 (2) | −0.004 (2) |
| O22 | 0.0477 (19) | 0.0469 (15) | 0.0350 (13) | −0.0054 (18) | −0.0046 (17) | 0.0000 (12) |
| C27 | 0.063 (3) | 0.067 (3) | 0.043 (3) | −0.015 (3) | −0.014 (3) | −0.007 (3) |
| C31 | 0.035 (2) | 0.051 (3) | 0.030 (2) | −0.003 (2) | 0.0036 (18) | −0.004 (2) |
| C32 | 0.041 (3) | 0.055 (3) | 0.030 (2) | −0.0081 (19) | 0.0015 (18) | 0.004 (2) |
| I32 | 0.05832 (19) | 0.0631 (2) | 0.0708 (2) | 0.0097 (3) | −0.0032 (3) | 0.01731 (17) |
| C33 | 0.049 (3) | 0.090 (4) | 0.041 (3) | −0.014 (3) | −0.010 (2) | 0.003 (3) |
| C34 | 0.073 (5) | 0.087 (5) | 0.047 (3) | −0.037 (3) | 0.004 (3) | −0.017 (3) |
| C35 | 0.078 (4) | 0.058 (3) | 0.062 (4) | −0.014 (3) | 0.016 (3) | −0.020 (3) |
| C36 | 0.050 (3) | 0.054 (3) | 0.056 (3) | −0.001 (2) | 0.001 (2) | −0.009 (3) |
| C37 | 0.032 (2) | 0.046 (3) | 0.039 (3) | −0.002 (2) | 0.000 (2) | −0.003 (2) |
| O31 | 0.051 (5) | 0.015 (9) | 0.034 (4) | −0.012 (7) | 0.003 (4) | −0.005 (3) |
| O32 | 0.066 (8) | 0.072 (11) | 0.057 (6) | −0.031 (7) | 0.019 (7) | −0.022 (6) |
| C38 | 0.032 (2) | 0.046 (3) | 0.039 (3) | −0.002 (2) | 0.000 (2) | −0.003 (2) |
| O33 | 0.042 (5) | 0.010 (9) | 0.038 (5) | −0.009 (7) | 0.002 (4) | −0.004 (4) |
| O34 | 0.039 (6) | 0.105 (16) | 0.053 (6) | −0.020 (9) | 0.009 (6) | −0.037 (9) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Geometric parameters (Å, º)
| N1—C6 | 1.470 (6) | C24—C25 | 1.350 (7) |
| N1—C2 | 1.476 (6) | C24—H24 | 0.9300 |
| N1—H11 | 0.8900 | C25—C26 | 1.383 (6) |
| N1—H12 | 0.8900 | C25—H25 | 0.9300 |
| C2—C3 | 1.512 (6) | C26—H26 | 0.9300 |
| C2—H2A | 0.9700 | O22—C27 | 1.428 (6) |
| C2—H2B | 0.9700 | C27—H27A | 0.9600 |
| C3—N4 | 1.464 (5) | C27—H27B | 0.9600 |
| C3—H3A | 0.9700 | C27—H27C | 0.9600 |
| C3—H3B | 0.9700 | C31—C36 | 1.384 (7) |
| N4—C21 | 1.410 (5) | C31—C32 | 1.387 (6) |
| N4—C5 | 1.466 (6) | C31—C37 | 1.511 (6) |
| C5—C6 | 1.500 (7) | C32—C33 | 1.388 (7) |
| C5—H5A | 0.9700 | C32—I32 | 2.103 (5) |
| C5—H5B | 0.9700 | C33—C34 | 1.372 (9) |
| C6—H6A | 0.9700 | C33—H33 | 0.9300 |
| C6—H6B | 0.9700 | C34—C35 | 1.361 (8) |
| C21—C26 | 1.389 (6) | C34—H34 | 0.9300 |
| C21—C22 | 1.413 (6) | C35—C36 | 1.370 (8) |
| C22—O22 | 1.375 (5) | C35—H35 | 0.9300 |
| C22—C23 | 1.382 (6) | C36—H36 | 0.9300 |
| C23—C24 | 1.385 (7) | C37—O32 | 1.226 (9) |
| C23—H23 | 0.9300 | C37—O31 | 1.269 (8) |
| C6—N1—C2 | 111.1 (3) | C22—C23—H23 | 120.1 |
| C6—N1—H11 | 109.4 | C24—C23—H23 | 120.1 |
| C2—N1—H11 | 109.4 | C25—C24—C23 | 121.1 (5) |
| C6—N1—H12 | 109.4 | C25—C24—H24 | 119.5 |
| C2—N1—H12 | 109.4 | C23—C24—H24 | 119.5 |
| H11—N1—H12 | 108.0 | C24—C25—C26 | 119.7 (4) |
| N1—C2—C3 | 109.8 (3) | C24—C25—H25 | 120.2 |
| N1—C2—H2A | 109.7 | C26—C25—H25 | 120.2 |
| C3—C2—H2A | 109.7 | C25—C26—C21 | 121.8 (4) |
| N1—C2—H2B | 109.7 | C25—C26—H26 | 119.1 |
| C3—C2—H2B | 109.7 | C21—C26—H26 | 119.1 |
| H2A—C2—H2B | 108.2 | C22—O22—C27 | 117.1 (4) |
| N4—C3—C2 | 110.1 (4) | O22—C27—H27A | 109.5 |
| N4—C3—H3A | 109.6 | O22—C27—H27B | 109.5 |
| C2—C3—H3A | 109.6 | H27A—C27—H27B | 109.5 |
| N4—C3—H3B | 109.6 | O22—C27—H27C | 109.5 |
| C2—C3—H3B | 109.6 | H27A—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.2 | H27B—C27—H27C | 109.5 |
| C21—N4—C3 | 116.0 (3) | C36—C31—C32 | 117.4 (4) |
| C21—N4—C5 | 116.1 (3) | C36—C31—C37 | 119.1 (4) |
| C3—N4—C5 | 110.4 (3) | C32—C31—C37 | 123.4 (4) |
| N4—C5—C6 | 110.3 (4) | C31—C32—C33 | 121.5 (5) |
| N4—C5—H5A | 109.6 | C31—C32—I32 | 119.8 (3) |
| C6—C5—H5A | 109.6 | C33—C32—I32 | 118.6 (4) |
| N4—C5—H5B | 109.6 | C34—C33—C32 | 118.9 (5) |
| C6—C5—H5B | 109.6 | C34—C33—H33 | 120.6 |
| H5A—C5—H5B | 108.1 | C32—C33—H33 | 120.6 |
| N1—C6—C5 | 111.3 (4) | C35—C34—C33 | 120.6 (5) |
| N1—C6—H6A | 109.4 | C35—C34—H34 | 119.7 |
| C5—C6—H6A | 109.4 | C33—C34—H34 | 119.7 |
| N1—C6—H6B | 109.4 | C34—C35—C36 | 120.3 (6) |
| C5—C6—H6B | 109.4 | C34—C35—H35 | 119.8 |
| H6A—C6—H6B | 108.0 | C36—C35—H35 | 119.8 |
| C26—C21—N4 | 123.4 (4) | C35—C36—C31 | 121.3 (5) |
| C26—C21—C22 | 117.3 (4) | C35—C36—H36 | 119.4 |
| N4—C21—C22 | 119.1 (4) | C31—C36—H36 | 119.4 |
| O22—C22—C23 | 123.4 (4) | O32—C37—O31 | 125.5 (12) |
| O22—C22—C21 | 116.0 (4) | O32—C37—C31 | 123.5 (13) |
| C23—C22—C21 | 120.4 (4) | O31—C37—C31 | 111.0 (13) |
| C22—C23—C24 | 119.7 (5) | ||
| C6—N1—C2—C3 | 56.3 (5) | C24—C25—C26—C21 | −1.1 (8) |
| N1—C2—C3—N4 | −58.4 (5) | N4—C21—C26—C25 | −173.6 (4) |
| C2—C3—N4—C21 | −165.5 (4) | C22—C21—C26—C25 | 1.8 (7) |
| C2—C3—N4—C5 | 59.7 (5) | C23—C22—O22—C27 | −3.7 (7) |
| C21—N4—C5—C6 | 167.0 (4) | C21—C22—O22—C27 | 172.1 (4) |
| C3—N4—C5—C6 | −58.3 (5) | C36—C31—C32—C33 | 0.8 (6) |
| C2—N1—C6—C5 | −55.6 (5) | C37—C31—C32—C33 | −176.6 (4) |
| N4—C5—C6—N1 | 56.2 (5) | C36—C31—C32—I32 | 179.7 (3) |
| C3—N4—C21—C26 | −121.0 (4) | C37—C31—C32—I32 | 2.3 (5) |
| C5—N4—C21—C26 | 11.1 (6) | C31—C32—C33—C34 | 0.0 (7) |
| C3—N4—C21—C22 | 63.7 (5) | I32—C32—C33—C34 | −178.9 (4) |
| C5—N4—C21—C22 | −164.2 (4) | C32—C33—C34—C35 | −1.4 (8) |
| C26—C21—C22—O22 | −177.4 (4) | C33—C34—C35—C36 | 1.8 (8) |
| N4—C21—C22—O22 | −1.8 (6) | C34—C35—C36—C31 | −0.9 (8) |
| C26—C21—C22—C23 | −1.5 (6) | C32—C31—C36—C35 | −0.4 (7) |
| N4—C21—C22—C23 | 174.1 (4) | C37—C31—C36—C35 | 177.1 (5) |
| O22—C22—C23—C24 | 176.1 (5) | C36—C31—C37—O32 | 101 (2) |
| C21—C22—C23—C24 | 0.5 (8) | C32—C31—C37—O32 | −82 (2) |
| C22—C23—C24—C25 | 0.2 (9) | C36—C31—C37—O31 | −82.8 (18) |
| C23—C24—C25—C26 | 0.1 (9) | C32—C31—C37—O31 | 94.6 (18) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-iodobenzoate (VII). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.89 | 1.80 | 2.66 (3) | 162 |
| N1—H11···O33 | 0.89 | 1.93 | 2.80 (3) | 165 |
| N1—H12···O31i | 0.89 | 1.97 | 2.83 (3) | 162 |
| N1—H12···O33i | 0.89 | 1.74 | 2.60 (3) | 161 |
| C25—H25···O34ii | 0.93 | 2.50 | 3.43 (3) | 174 |
| C26—H26···Cg1ii | 0.93 | 2.93 | 3.716 (5) | 143 |
Symmetry codes: (i) x−1/2, −y+3/2, −z+1; (ii) −x+3/2, −y+1, z+1/2.
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Crystal data
| C11H17N2O+·C8H7O2− | Z = 2 |
| Mr = 328.40 | F(000) = 352 |
| Triclinic, P1 | Dx = 1.206 Mg m−3 |
| a = 7.826 (1) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 10.320 (2) Å | Cell parameters from 3838 reflections |
| c = 12.055 (3) Å | θ = 2.7–27.7° |
| α = 78.37 (2)° | µ = 0.08 mm−1 |
| β = 78.27 (2)° | T = 296 K |
| γ = 73.83 (2)° | Block, colourless |
| V = 904.6 (3) Å3 | 0.48 × 0.48 × 0.40 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3838 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2600 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.013 |
| ω scans | θmax = 27.7°, θmin = 2.7° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −5→10 |
| Tmin = 0.883, Tmax = 0.968 | k = −11→13 |
| 6091 measured reflections | l = −15→15 |
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.042 | w = 1/[σ2(Fo2) + (0.0611P)2 + 0.0332P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.119 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.16 e Å−3 |
| 3838 reflections | Δρmin = −0.16 e Å−3 |
| 226 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.057 (5) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.42898 (16) | 0.55387 (12) | 0.64034 (10) | 0.0429 (3) | |
| H11 | 0.3320 (19) | 0.5684 (15) | 0.5920 (12) | 0.051* | |
| H12 | 0.5095 (19) | 0.4646 (16) | 0.6376 (12) | 0.051* | |
| C2 | 0.33370 (18) | 0.55987 (15) | 0.75988 (11) | 0.0449 (3) | |
| H2A | 0.2790 | 0.4832 | 0.7859 | 0.054* | |
| H2B | 0.2384 | 0.6433 | 0.7619 | 0.054* | |
| C3 | 0.46231 (18) | 0.55582 (14) | 0.83911 (11) | 0.0448 (3) | |
| H3A | 0.3967 | 0.5651 | 0.9157 | 0.054* | |
| H3B | 0.5512 | 0.4688 | 0.8430 | 0.054* | |
| N4 | 0.55258 (15) | 0.66676 (12) | 0.79702 (9) | 0.0445 (3) | |
| C5 | 0.6584 (2) | 0.64945 (17) | 0.68405 (12) | 0.0534 (4) | |
| H5A | 0.7448 | 0.5613 | 0.6881 | 0.064* | |
| H5B | 0.7241 | 0.7198 | 0.6581 | 0.064* | |
| C6 | 0.5332 (2) | 0.65903 (17) | 0.60108 (12) | 0.0542 (4) | |
| H6A | 0.4510 | 0.7490 | 0.5945 | 0.065* | |
| H6B | 0.6027 | 0.6466 | 0.5259 | 0.065* | |
| C21 | 0.63967 (18) | 0.69298 (14) | 0.87963 (11) | 0.0434 (3) | |
| C22 | 0.53136 (18) | 0.75748 (14) | 0.97177 (12) | 0.0453 (3) | |
| C23 | 0.6112 (2) | 0.78557 (16) | 1.05340 (13) | 0.0562 (4) | |
| H23 | 0.5396 | 0.8262 | 1.1149 | 0.067* | |
| C24 | 0.7965 (2) | 0.75375 (18) | 1.04446 (14) | 0.0628 (4) | |
| H24 | 0.8488 | 0.7740 | 1.0995 | 0.075* | |
| C25 | 0.9036 (2) | 0.6926 (2) | 0.95519 (15) | 0.0693 (5) | |
| H25 | 1.0283 | 0.6715 | 0.9492 | 0.083* | |
| C26 | 0.8242 (2) | 0.66219 (18) | 0.87324 (14) | 0.0599 (4) | |
| H26 | 0.8973 | 0.6202 | 0.8129 | 0.072* | |
| O22 | 0.34998 (13) | 0.78566 (11) | 0.97381 (9) | 0.0602 (3) | |
| C27 | 0.2347 (2) | 0.85827 (19) | 1.06138 (16) | 0.0734 (5) | |
| H27A | 0.1119 | 0.8784 | 1.0492 | 0.110* | |
| H27B | 0.2676 | 0.9419 | 1.0587 | 0.110* | |
| H27C | 0.2472 | 0.8032 | 1.1350 | 0.110* | |
| C31 | 0.02692 (17) | 0.80006 (14) | 0.37927 (11) | 0.0434 (3) | |
| C32 | 0.0231 (2) | 0.93671 (15) | 0.33658 (14) | 0.0571 (4) | |
| C33 | −0.1295 (3) | 1.01830 (17) | 0.29036 (17) | 0.0752 (5) | |
| H33 | −0.1323 | 1.1089 | 0.2594 | 0.090* | |
| C34 | −0.2735 (2) | 0.9696 (2) | 0.28923 (16) | 0.0774 (6) | |
| H34 | −0.3733 | 1.0266 | 0.2588 | 0.093* | |
| C35 | −0.2708 (2) | 0.8359 (2) | 0.33318 (14) | 0.0660 (5) | |
| H35 | −0.3690 | 0.8019 | 0.3331 | 0.079* | |
| C36 | −0.12087 (18) | 0.75165 (16) | 0.37783 (12) | 0.0523 (4) | |
| H36 | −0.1195 | 0.6609 | 0.4074 | 0.063* | |
| C37 | 0.18760 (19) | 0.70027 (14) | 0.42484 (13) | 0.0482 (4) | |
| O31 | 0.15924 (14) | 0.62406 (13) | 0.51830 (10) | 0.0692 (3) | |
| O32 | 0.34037 (13) | 0.69583 (10) | 0.36497 (10) | 0.0615 (3) | |
| C38 | 0.1751 (3) | 0.99828 (18) | 0.3413 (2) | 0.0863 (6) | |
| H38A | 0.1307 | 1.0954 | 0.3377 | 0.130* | |
| H38B | 0.2688 | 0.9785 | 0.2775 | 0.130* | |
| H38C | 0.2222 | 0.9600 | 0.4116 | 0.130* |
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0422 (6) | 0.0442 (7) | 0.0423 (7) | −0.0085 (5) | −0.0076 (5) | −0.0088 (5) |
| C2 | 0.0437 (7) | 0.0458 (8) | 0.0438 (8) | −0.0139 (6) | −0.0032 (6) | −0.0033 (6) |
| C3 | 0.0488 (8) | 0.0453 (8) | 0.0400 (7) | −0.0170 (6) | −0.0048 (6) | −0.0008 (6) |
| N4 | 0.0510 (7) | 0.0487 (7) | 0.0364 (6) | −0.0207 (5) | −0.0022 (5) | −0.0057 (5) |
| C5 | 0.0586 (9) | 0.0655 (10) | 0.0425 (8) | −0.0319 (8) | 0.0027 (7) | −0.0102 (7) |
| C6 | 0.0678 (10) | 0.0605 (9) | 0.0385 (8) | −0.0287 (8) | −0.0011 (7) | −0.0065 (7) |
| C21 | 0.0503 (8) | 0.0426 (7) | 0.0391 (7) | −0.0187 (6) | −0.0051 (6) | −0.0021 (6) |
| C22 | 0.0480 (8) | 0.0441 (8) | 0.0441 (8) | −0.0156 (6) | −0.0054 (6) | −0.0032 (6) |
| C23 | 0.0658 (10) | 0.0620 (10) | 0.0452 (9) | −0.0237 (8) | −0.0026 (7) | −0.0134 (7) |
| C24 | 0.0649 (10) | 0.0836 (12) | 0.0523 (9) | −0.0336 (9) | −0.0114 (8) | −0.0148 (9) |
| C25 | 0.0497 (9) | 0.0982 (13) | 0.0662 (11) | −0.0248 (9) | −0.0088 (8) | −0.0183 (10) |
| C26 | 0.0517 (9) | 0.0763 (11) | 0.0549 (10) | −0.0196 (8) | −0.0007 (7) | −0.0200 (8) |
| O22 | 0.0496 (6) | 0.0720 (7) | 0.0595 (7) | −0.0088 (5) | −0.0046 (5) | −0.0237 (6) |
| C27 | 0.0582 (10) | 0.0747 (12) | 0.0849 (13) | −0.0085 (9) | 0.0044 (9) | −0.0336 (10) |
| C31 | 0.0417 (7) | 0.0445 (8) | 0.0389 (7) | −0.0049 (6) | −0.0018 (6) | −0.0075 (6) |
| C32 | 0.0583 (9) | 0.0425 (8) | 0.0643 (10) | −0.0064 (7) | 0.0005 (8) | −0.0127 (7) |
| C33 | 0.0775 (12) | 0.0437 (9) | 0.0853 (13) | 0.0025 (8) | −0.0062 (10) | 0.0020 (9) |
| C34 | 0.0550 (10) | 0.0801 (13) | 0.0749 (12) | 0.0088 (9) | −0.0136 (9) | 0.0064 (10) |
| C35 | 0.0473 (9) | 0.0850 (13) | 0.0590 (10) | −0.0117 (8) | −0.0116 (7) | 0.0010 (9) |
| C36 | 0.0460 (8) | 0.0585 (9) | 0.0491 (9) | −0.0137 (7) | −0.0082 (6) | 0.0011 (7) |
| C37 | 0.0462 (8) | 0.0445 (8) | 0.0573 (9) | −0.0121 (6) | −0.0131 (7) | −0.0091 (7) |
| O31 | 0.0594 (7) | 0.0788 (8) | 0.0667 (8) | −0.0227 (6) | −0.0250 (6) | 0.0177 (6) |
| O32 | 0.0422 (6) | 0.0544 (7) | 0.0800 (8) | −0.0033 (5) | −0.0046 (5) | −0.0094 (6) |
| C38 | 0.0885 (13) | 0.0510 (10) | 0.1240 (18) | −0.0242 (10) | −0.0133 (12) | −0.0174 (11) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Geometric parameters (Å, º)
| N1—C6 | 1.4820 (18) | C25—C26 | 1.394 (2) |
| N1—C2 | 1.4866 (18) | C25—H25 | 0.9300 |
| N1—H11 | 1.010 (15) | C26—H26 | 0.9300 |
| N1—H12 | 0.963 (16) | O22—C27 | 1.4300 (19) |
| C2—C3 | 1.5094 (19) | C27—H27A | 0.9600 |
| C2—H2A | 0.9700 | C27—H27B | 0.9600 |
| C2—H2B | 0.9700 | C27—H27C | 0.9600 |
| C3—N4 | 1.4621 (17) | C31—C36 | 1.3861 (19) |
| C3—H3A | 0.9700 | C31—C32 | 1.395 (2) |
| C3—H3B | 0.9700 | C31—C37 | 1.508 (2) |
| N4—C21 | 1.4202 (18) | C32—C33 | 1.402 (2) |
| N4—C5 | 1.4592 (18) | C32—C38 | 1.511 (2) |
| C5—C6 | 1.508 (2) | C33—C34 | 1.359 (3) |
| C5—H5A | 0.9700 | C33—H33 | 0.9300 |
| C5—H5B | 0.9700 | C34—C35 | 1.370 (3) |
| C6—H6A | 0.9700 | C34—H34 | 0.9300 |
| C6—H6B | 0.9700 | C35—C36 | 1.387 (2) |
| C21—C26 | 1.379 (2) | C35—H35 | 0.9300 |
| C21—C22 | 1.413 (2) | C36—H36 | 0.9300 |
| C22—O22 | 1.3634 (16) | C37—O31 | 1.2552 (17) |
| C22—C23 | 1.381 (2) | C37—O32 | 1.2587 (16) |
| C23—C24 | 1.382 (2) | C38—H38A | 0.9600 |
| C23—H23 | 0.9300 | C38—H38B | 0.9600 |
| C24—C25 | 1.368 (2) | C38—H38C | 0.9600 |
| C24—H24 | 0.9300 | ||
| C6—N1—C2 | 111.69 (11) | C25—C24—H24 | 119.8 |
| C6—N1—H11 | 111.2 (8) | C23—C24—H24 | 119.8 |
| C2—N1—H11 | 105.7 (8) | C24—C25—C26 | 119.46 (15) |
| C6—N1—H12 | 109.4 (8) | C24—C25—H25 | 120.3 |
| C2—N1—H12 | 108.3 (9) | C26—C25—H25 | 120.3 |
| H11—N1—H12 | 110.4 (12) | C21—C26—C25 | 121.52 (15) |
| N1—C2—C3 | 110.86 (11) | C21—C26—H26 | 119.2 |
| N1—C2—H2A | 109.5 | C25—C26—H26 | 119.2 |
| C3—C2—H2A | 109.5 | C22—O22—C27 | 117.89 (12) |
| N1—C2—H2B | 109.5 | O22—C27—H27A | 109.5 |
| C3—C2—H2B | 109.5 | O22—C27—H27B | 109.5 |
| H2A—C2—H2B | 108.1 | H27A—C27—H27B | 109.5 |
| N4—C3—C2 | 109.85 (11) | O22—C27—H27C | 109.5 |
| N4—C3—H3A | 109.7 | H27A—C27—H27C | 109.5 |
| C2—C3—H3A | 109.7 | H27B—C27—H27C | 109.5 |
| N4—C3—H3B | 109.7 | C36—C31—C32 | 119.20 (14) |
| C2—C3—H3B | 109.7 | C36—C31—C37 | 117.89 (13) |
| H3A—C3—H3B | 108.2 | C32—C31—C37 | 122.89 (13) |
| C21—N4—C5 | 117.07 (11) | C31—C32—C33 | 117.94 (15) |
| C21—N4—C3 | 113.60 (10) | C31—C32—C38 | 122.05 (15) |
| C5—N4—C3 | 109.82 (11) | C33—C32—C38 | 119.99 (16) |
| N4—C5—C6 | 109.00 (12) | C34—C33—C32 | 122.27 (17) |
| N4—C5—H5A | 109.9 | C34—C33—H33 | 118.9 |
| C6—C5—H5A | 109.9 | C32—C33—H33 | 118.9 |
| N4—C5—H5B | 109.9 | C33—C34—C35 | 119.66 (17) |
| C6—C5—H5B | 109.9 | C33—C34—H34 | 120.2 |
| H5A—C5—H5B | 108.3 | C35—C34—H34 | 120.2 |
| N1—C6—C5 | 110.75 (12) | C34—C35—C36 | 119.66 (17) |
| N1—C6—H6A | 109.5 | C34—C35—H35 | 120.2 |
| C5—C6—H6A | 109.5 | C36—C35—H35 | 120.2 |
| N1—C6—H6B | 109.5 | C31—C36—C35 | 121.23 (15) |
| C5—C6—H6B | 109.5 | C31—C36—H36 | 119.4 |
| H6A—C6—H6B | 108.1 | C35—C36—H36 | 119.4 |
| C26—C21—C22 | 118.20 (13) | O31—C37—O32 | 124.33 (14) |
| C26—C21—N4 | 123.60 (13) | O31—C37—C31 | 117.71 (13) |
| C22—C21—N4 | 118.18 (12) | O32—C37—C31 | 117.92 (13) |
| O22—C22—C23 | 124.39 (13) | C32—C38—H38A | 109.5 |
| O22—C22—C21 | 115.73 (13) | C32—C38—H38B | 109.5 |
| C23—C22—C21 | 119.88 (13) | H38A—C38—H38B | 109.5 |
| C22—C23—C24 | 120.54 (15) | C32—C38—H38C | 109.5 |
| C22—C23—H23 | 119.7 | H38A—C38—H38C | 109.5 |
| C24—C23—H23 | 119.7 | H38B—C38—H38C | 109.5 |
| C25—C24—C23 | 120.39 (15) | ||
| C6—N1—C2—C3 | −52.32 (16) | C22—C21—C26—C25 | 0.3 (2) |
| N1—C2—C3—N4 | 56.11 (15) | N4—C21—C26—C25 | 178.59 (14) |
| C2—C3—N4—C21 | 164.70 (11) | C24—C25—C26—C21 | 0.3 (3) |
| C2—C3—N4—C5 | −62.04 (15) | C23—C22—O22—C27 | 4.9 (2) |
| C21—N4—C5—C6 | −165.56 (12) | C21—C22—O22—C27 | −176.31 (13) |
| C3—N4—C5—C6 | 62.98 (16) | C36—C31—C32—C33 | −2.0 (2) |
| C2—N1—C6—C5 | 53.64 (16) | C37—C31—C32—C33 | 176.40 (15) |
| N4—C5—C6—N1 | −58.51 (17) | C36—C31—C32—C38 | 176.65 (15) |
| C5—N4—C21—C26 | −20.7 (2) | C37—C31—C32—C38 | −4.9 (2) |
| C3—N4—C21—C26 | 109.01 (15) | C31—C32—C33—C34 | 2.0 (3) |
| C5—N4—C21—C22 | 157.56 (13) | C38—C32—C33—C34 | −176.74 (18) |
| C3—N4—C21—C22 | −72.74 (15) | C32—C33—C34—C35 | −0.8 (3) |
| C26—C21—C22—O22 | 179.95 (13) | C33—C34—C35—C36 | −0.3 (3) |
| N4—C21—C22—O22 | 1.60 (18) | C32—C31—C36—C35 | 1.0 (2) |
| C26—C21—C22—C23 | −1.2 (2) | C37—C31—C36—C35 | −177.50 (14) |
| N4—C21—C22—C23 | −179.54 (12) | C34—C35—C36—C31 | 0.2 (2) |
| O22—C22—C23—C24 | −179.86 (14) | C36—C31—C37—O31 | −47.61 (19) |
| C21—C22—C23—C24 | 1.4 (2) | C32—C31—C37—O31 | 133.92 (15) |
| C22—C23—C24—C25 | −0.7 (2) | C36—C31—C37—O32 | 130.15 (14) |
| C23—C24—C25—C26 | −0.2 (3) | C32—C31—C37—O32 | −48.3 (2) |
4-(2-Methoxyphenyl)piperazin-1-ium 2-methylbenzoate (VIII). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 1.010 (15) | 1.673 (15) | 2.6696 (19) | 168.6 (13) |
| N1—H12···O32i | 0.963 (16) | 1.745 (16) | 2.7077 (17) | 178.2 (10) |
Symmetry code: (i) −x+1, −y+1, −z+1.
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Crystal data
| C11H17N2O+·C7H6NO2− | F(000) = 704 |
| Mr = 329.39 | Dx = 1.280 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 14.922 (1) Å | Cell parameters from 3671 reflections |
| b = 7.6951 (5) Å | θ = 2.7–27.8° |
| c = 15.560 (1) Å | µ = 0.09 mm−1 |
| β = 106.911 (8)° | T = 296 K |
| V = 1709.4 (2) Å3 | Plate, orange |
| Z = 4 | 0.48 × 0.44 × 0.16 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3668 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2606 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.014 |
| ω scans | θmax = 27.6°, θmin = 2.7° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −18→15 |
| Tmin = 0.830, Tmax = 0.986 | k = −5→9 |
| 6720 measured reflections | l = −17→20 |
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.039 | w = 1/[σ2(Fo2) + (0.0619P)2 + 0.0395P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.112 | (Δ/σ)max < 0.001 |
| S = 1.10 | Δρmax = 0.15 e Å−3 |
| 3668 reflections | Δρmin = −0.24 e Å−3 |
| 231 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.019 (2) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.60709 (8) | 0.26835 (15) | 0.52995 (8) | 0.0431 (3) | |
| H11 | 0.5586 (10) | 0.2606 (17) | 0.4643 (10) | 0.052* | |
| H12 | 0.5953 (10) | 0.3661 (19) | 0.5615 (10) | 0.052* | |
| C2 | 0.70246 (9) | 0.28650 (18) | 0.51932 (9) | 0.0442 (3) | |
| H2A | 0.7056 | 0.3915 | 0.4858 | 0.053* | |
| H2B | 0.7155 | 0.1883 | 0.4857 | 0.053* | |
| C3 | 0.77450 (9) | 0.29453 (16) | 0.60945 (8) | 0.0385 (3) | |
| H3A | 0.8364 | 0.3050 | 0.6015 | 0.046* | |
| H3B | 0.7635 | 0.3963 | 0.6417 | 0.046* | |
| N4 | 0.77041 (7) | 0.13685 (13) | 0.66227 (7) | 0.0376 (3) | |
| C5 | 0.67672 (9) | 0.11904 (18) | 0.67358 (9) | 0.0433 (3) | |
| H5A | 0.6642 | 0.2174 | 0.7074 | 0.052* | |
| H5B | 0.6741 | 0.0143 | 0.7074 | 0.052* | |
| C6 | 0.60307 (9) | 0.11039 (17) | 0.58382 (9) | 0.0458 (3) | |
| H6A | 0.6131 | 0.0079 | 0.5515 | 0.055* | |
| H6B | 0.5416 | 0.1013 | 0.5928 | 0.055* | |
| C21 | 0.84580 (9) | 0.13068 (16) | 0.74309 (9) | 0.0409 (3) | |
| C22 | 0.93798 (9) | 0.11685 (17) | 0.73737 (10) | 0.0460 (3) | |
| C23 | 1.01328 (10) | 0.1198 (2) | 0.81461 (11) | 0.0600 (4) | |
| H23 | 1.0740 | 0.1142 | 0.8102 | 0.072* | |
| C24 | 0.99867 (13) | 0.1311 (2) | 0.89743 (11) | 0.0714 (5) | |
| H24 | 1.0495 | 0.1345 | 0.9489 | 0.086* | |
| C25 | 0.90983 (13) | 0.1373 (3) | 0.90449 (11) | 0.0732 (5) | |
| H25 | 0.9001 | 0.1412 | 0.9608 | 0.088* | |
| C26 | 0.83373 (11) | 0.1379 (2) | 0.82761 (9) | 0.0559 (4) | |
| H26 | 0.7734 | 0.1432 | 0.8332 | 0.067* | |
| O22 | 0.94654 (7) | 0.10004 (14) | 0.65293 (7) | 0.0603 (3) | |
| C27 | 1.03816 (12) | 0.1063 (3) | 0.64265 (13) | 0.0762 (5) | |
| H27A | 1.0342 | 0.0973 | 0.5801 | 0.114* | |
| H27B | 1.0747 | 0.0114 | 0.6749 | 0.114* | |
| H27C | 1.0675 | 0.2142 | 0.6660 | 0.114* | |
| C31 | 0.38418 (8) | 0.30821 (15) | 0.23323 (8) | 0.0341 (3) | |
| C32 | 0.30092 (9) | 0.39385 (17) | 0.19135 (9) | 0.0410 (3) | |
| H32 | 0.2734 | 0.4636 | 0.2255 | 0.049* | |
| C33 | 0.25815 (9) | 0.37762 (17) | 0.10033 (9) | 0.0429 (3) | |
| H33 | 0.2021 | 0.4355 | 0.0741 | 0.051* | |
| C34 | 0.29810 (9) | 0.27553 (16) | 0.04752 (8) | 0.0373 (3) | |
| C35 | 0.38187 (9) | 0.19061 (17) | 0.08919 (8) | 0.0395 (3) | |
| H35 | 0.4102 | 0.1226 | 0.0551 | 0.047* | |
| C36 | 0.42342 (9) | 0.20608 (16) | 0.18038 (9) | 0.0382 (3) | |
| H36 | 0.4789 | 0.1468 | 0.2069 | 0.046* | |
| C37 | 0.43113 (9) | 0.32513 (16) | 0.33203 (8) | 0.0379 (3) | |
| O31 | 0.50355 (7) | 0.23691 (14) | 0.36411 (6) | 0.0627 (3) | |
| O32 | 0.39544 (7) | 0.42404 (13) | 0.37713 (6) | 0.0526 (3) | |
| N34 | 0.25457 (10) | 0.25415 (18) | −0.04368 (8) | 0.0501 (3) | |
| H341 | 0.2158 (11) | 0.340 (2) | −0.0658 (11) | 0.060* | |
| H342 | 0.2928 (11) | 0.211 (2) | −0.0750 (10) | 0.060* |
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0392 (6) | 0.0463 (7) | 0.0372 (6) | 0.0090 (5) | 0.0006 (5) | −0.0055 (5) |
| C2 | 0.0476 (8) | 0.0477 (8) | 0.0356 (7) | 0.0105 (6) | 0.0093 (6) | 0.0029 (6) |
| C3 | 0.0364 (7) | 0.0412 (7) | 0.0369 (7) | 0.0019 (5) | 0.0090 (5) | 0.0051 (5) |
| N4 | 0.0319 (5) | 0.0418 (6) | 0.0361 (6) | 0.0015 (4) | 0.0054 (4) | 0.0063 (5) |
| C5 | 0.0370 (7) | 0.0447 (7) | 0.0471 (8) | −0.0015 (5) | 0.0106 (6) | 0.0064 (6) |
| C6 | 0.0352 (7) | 0.0449 (8) | 0.0523 (9) | 0.0009 (5) | 0.0048 (6) | −0.0043 (6) |
| C21 | 0.0396 (7) | 0.0402 (7) | 0.0385 (7) | 0.0019 (5) | 0.0045 (6) | 0.0068 (6) |
| C22 | 0.0402 (7) | 0.0477 (8) | 0.0459 (8) | 0.0075 (6) | 0.0061 (6) | 0.0066 (6) |
| C23 | 0.0402 (8) | 0.0653 (10) | 0.0642 (11) | 0.0072 (7) | −0.0012 (7) | 0.0102 (8) |
| C24 | 0.0632 (11) | 0.0846 (13) | 0.0482 (10) | 0.0057 (9) | −0.0124 (8) | 0.0141 (9) |
| C25 | 0.0752 (12) | 0.0985 (14) | 0.0378 (9) | 0.0081 (10) | 0.0037 (8) | 0.0151 (9) |
| C26 | 0.0529 (9) | 0.0727 (10) | 0.0402 (8) | 0.0057 (7) | 0.0105 (7) | 0.0130 (7) |
| O22 | 0.0416 (6) | 0.0838 (8) | 0.0561 (7) | 0.0162 (5) | 0.0150 (5) | 0.0020 (6) |
| C27 | 0.0525 (10) | 0.0953 (14) | 0.0880 (14) | 0.0157 (9) | 0.0320 (9) | 0.0088 (11) |
| C31 | 0.0373 (6) | 0.0343 (6) | 0.0318 (7) | −0.0043 (5) | 0.0119 (5) | −0.0014 (5) |
| C32 | 0.0437 (7) | 0.0454 (7) | 0.0371 (7) | 0.0048 (5) | 0.0165 (6) | −0.0029 (6) |
| C33 | 0.0371 (7) | 0.0497 (8) | 0.0402 (8) | 0.0052 (6) | 0.0086 (6) | 0.0023 (6) |
| C34 | 0.0392 (7) | 0.0411 (7) | 0.0311 (6) | −0.0106 (5) | 0.0093 (5) | −0.0014 (5) |
| C35 | 0.0405 (7) | 0.0435 (7) | 0.0375 (7) | −0.0041 (5) | 0.0162 (6) | −0.0115 (6) |
| C36 | 0.0346 (6) | 0.0407 (7) | 0.0380 (7) | 0.0009 (5) | 0.0086 (5) | −0.0050 (6) |
| C37 | 0.0433 (7) | 0.0375 (6) | 0.0326 (7) | −0.0062 (5) | 0.0108 (6) | −0.0023 (5) |
| O31 | 0.0634 (7) | 0.0731 (7) | 0.0387 (6) | 0.0183 (5) | −0.0053 (5) | −0.0091 (5) |
| O32 | 0.0664 (7) | 0.0579 (6) | 0.0364 (5) | 0.0017 (5) | 0.0197 (5) | −0.0099 (4) |
| N34 | 0.0537 (8) | 0.0590 (8) | 0.0337 (6) | −0.0036 (6) | 0.0067 (5) | −0.0047 (6) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Geometric parameters (Å, º)
| N1—C2 | 1.4864 (18) | C24—H24 | 0.9300 |
| N1—C6 | 1.4875 (18) | C25—C26 | 1.389 (2) |
| N1—H11 | 1.067 (14) | C25—H25 | 0.9300 |
| N1—H12 | 0.942 (15) | C26—H26 | 0.9300 |
| C2—C3 | 1.4997 (16) | O22—C27 | 1.4228 (18) |
| C2—H2A | 0.9700 | C27—H27A | 0.9600 |
| C2—H2B | 0.9700 | C27—H27B | 0.9600 |
| C3—N4 | 1.4768 (15) | C27—H27C | 0.9600 |
| C3—H3A | 0.9700 | C31—C36 | 1.3844 (17) |
| C3—H3B | 0.9700 | C31—C32 | 1.3897 (17) |
| N4—C21 | 1.4235 (16) | C31—C37 | 1.4982 (16) |
| N4—C5 | 1.4654 (16) | C32—C33 | 1.3796 (18) |
| C5—C6 | 1.5067 (18) | C32—H32 | 0.9300 |
| C5—H5A | 0.9700 | C33—C34 | 1.3902 (18) |
| C5—H5B | 0.9700 | C33—H33 | 0.9300 |
| C6—H6A | 0.9700 | C34—N34 | 1.3881 (16) |
| C6—H6B | 0.9700 | C34—C35 | 1.3923 (18) |
| C21—C26 | 1.380 (2) | C35—C36 | 1.3784 (17) |
| C21—C22 | 1.4079 (19) | C35—H35 | 0.9300 |
| C22—O22 | 1.3627 (17) | C36—H36 | 0.9300 |
| C22—C23 | 1.3863 (19) | C37—O31 | 1.2506 (16) |
| C23—C24 | 1.371 (2) | C37—O32 | 1.2542 (15) |
| C23—H23 | 0.9300 | N34—H341 | 0.877 (16) |
| C24—C25 | 1.363 (2) | N34—H342 | 0.913 (16) |
| C2—N1—C6 | 109.66 (10) | C22—C23—H23 | 119.8 |
| C2—N1—H11 | 107.7 (8) | C25—C24—C23 | 120.16 (15) |
| C6—N1—H11 | 111.5 (7) | C25—C24—H24 | 119.9 |
| C2—N1—H12 | 108.2 (9) | C23—C24—H24 | 119.9 |
| C6—N1—H12 | 108.3 (9) | C24—C25—C26 | 120.10 (16) |
| H11—N1—H12 | 111.5 (11) | C24—C25—H25 | 119.9 |
| N1—C2—C3 | 110.42 (11) | C26—C25—H25 | 120.0 |
| N1—C2—H2A | 109.6 | C21—C26—C25 | 121.33 (15) |
| C3—C2—H2A | 109.6 | C21—C26—H26 | 119.3 |
| N1—C2—H2B | 109.6 | C25—C26—H26 | 119.3 |
| C3—C2—H2B | 109.6 | C22—O22—C27 | 117.90 (12) |
| H2A—C2—H2B | 108.1 | O22—C27—H27A | 109.5 |
| N4—C3—C2 | 110.62 (10) | O22—C27—H27B | 109.5 |
| N4—C3—H3A | 109.5 | H27A—C27—H27B | 109.5 |
| C2—C3—H3A | 109.5 | O22—C27—H27C | 109.5 |
| N4—C3—H3B | 109.5 | H27A—C27—H27C | 109.5 |
| C2—C3—H3B | 109.5 | H27B—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.1 | C36—C31—C32 | 117.75 (11) |
| C21—N4—C5 | 115.29 (10) | C36—C31—C37 | 120.43 (11) |
| C21—N4—C3 | 111.66 (9) | C32—C31—C37 | 121.82 (11) |
| C5—N4—C3 | 109.81 (9) | C33—C32—C31 | 121.46 (12) |
| N4—C5—C6 | 110.91 (11) | C33—C32—H32 | 119.3 |
| N4—C5—H5A | 109.5 | C31—C32—H32 | 119.3 |
| C6—C5—H5A | 109.5 | C32—C33—C34 | 120.54 (12) |
| N4—C5—H5B | 109.5 | C32—C33—H33 | 119.7 |
| C6—C5—H5B | 109.5 | C34—C33—H33 | 119.7 |
| H5A—C5—H5B | 108.0 | N34—C34—C33 | 121.17 (12) |
| N1—C6—C5 | 110.38 (10) | N34—C34—C35 | 120.69 (12) |
| N1—C6—H6A | 109.6 | C33—C34—C35 | 118.11 (11) |
| C5—C6—H6A | 109.6 | C36—C35—C34 | 120.86 (11) |
| N1—C6—H6B | 109.6 | C36—C35—H35 | 119.6 |
| C5—C6—H6B | 109.6 | C34—C35—H35 | 119.6 |
| H6A—C6—H6B | 108.1 | C35—C36—C31 | 121.27 (12) |
| C26—C21—C22 | 117.66 (13) | C35—C36—H36 | 119.4 |
| C26—C21—N4 | 123.51 (12) | C31—C36—H36 | 119.4 |
| C22—C21—N4 | 118.83 (12) | O31—C37—O32 | 124.36 (12) |
| O22—C22—C23 | 123.91 (13) | O31—C37—C31 | 117.02 (11) |
| O22—C22—C21 | 115.81 (12) | O32—C37—C31 | 118.62 (11) |
| C23—C22—C21 | 120.28 (14) | C34—N34—H341 | 111.8 (11) |
| C24—C23—C22 | 120.38 (15) | C34—N34—H342 | 114.2 (10) |
| C24—C23—H23 | 119.8 | H341—N34—H342 | 120.4 (15) |
| C6—N1—C2—C3 | −57.43 (14) | C22—C21—C26—C25 | 2.1 (2) |
| N1—C2—C3—N4 | 58.53 (13) | N4—C21—C26—C25 | −177.79 (13) |
| C2—C3—N4—C21 | 172.34 (11) | C24—C25—C26—C21 | 0.6 (3) |
| C2—C3—N4—C5 | −58.48 (13) | C23—C22—O22—C27 | −7.6 (2) |
| C21—N4—C5—C6 | −174.66 (10) | C21—C22—O22—C27 | 172.72 (13) |
| C3—N4—C5—C6 | 58.16 (13) | C36—C31—C32—C33 | 0.31 (19) |
| C2—N1—C6—C5 | 56.92 (14) | C37—C31—C32—C33 | 179.98 (12) |
| N4—C5—C6—N1 | −57.96 (14) | C31—C32—C33—C34 | −0.6 (2) |
| C5—N4—C21—C26 | −10.87 (18) | C32—C33—C34—N34 | 178.21 (12) |
| C3—N4—C21—C26 | 115.36 (14) | C32—C33—C34—C35 | 0.14 (19) |
| C5—N4—C21—C22 | 169.26 (11) | N34—C34—C35—C36 | −177.43 (12) |
| C3—N4—C21—C22 | −64.51 (14) | C33—C34—C35—C36 | 0.65 (19) |
| C26—C21—C22—O22 | 176.36 (13) | C34—C35—C36—C31 | −0.97 (19) |
| N4—C21—C22—O22 | −3.76 (17) | C32—C31—C36—C35 | 0.48 (19) |
| C26—C21—C22—C23 | −3.3 (2) | C37—C31—C36—C35 | −179.20 (11) |
| N4—C21—C22—C23 | 176.56 (11) | C36—C31—C37—O31 | −3.44 (17) |
| O22—C22—C23—C24 | −177.72 (15) | C32—C31—C37—O31 | 176.90 (13) |
| C21—C22—C23—C24 | 1.9 (2) | C36—C31—C37—O32 | 177.29 (12) |
| C22—C23—C24—C25 | 0.8 (3) | C32—C31—C37—O32 | −2.37 (18) |
| C23—C24—C25—C26 | −2.1 (3) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-aminobenzoate (IX). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 1.068 (15) | 1.547 (15) | 2.6048 (15) | 169.7 (14) |
| N1—H12···O32i | 0.942 (15) | 1.861 (15) | 2.7797 (15) | 164.4 (14) |
| N34—H342···O32ii | 0.914 (16) | 2.155 (16) | 3.0535 (18) | 167.5 (14) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x, −y+1/2, z−1/2.
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Crystal data
| C11H17N2O+·C7H4NO4− | F(000) = 760 |
| Mr = 359.38 | Dx = 1.344 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.5174 (5) Å | Cell parameters from 3934 reflections |
| b = 7.9761 (5) Å | θ = 2.7–27.9° |
| c = 29.860 (2) Å | µ = 0.10 mm−1 |
| β = 97.322 (6)° | T = 296 K |
| V = 1775.8 (2) Å3 | Block, yellow |
| Z = 4 | 0.50 × 0.50 × 0.40 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3934 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2879 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.019 |
| ω scans | θmax = 27.9°, θmin = 2.7° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −9→8 |
| Tmin = 0.855, Tmax = 0.961 | k = −10→10 |
| 13660 measured reflections | l = −38→38 |
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.040 | w = 1/[σ2(Fo2) + (0.0457P)2 + 0.4848P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.111 | (Δ/σ)max = 0.001 |
| S = 1.03 | Δρmax = 0.17 e Å−3 |
| 3934 reflections | Δρmin = −0.15 e Å−3 |
| 242 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0175 (15) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.44452 (18) | 0.51582 (18) | 0.42036 (4) | 0.0456 (3) | |
| H11 | 0.359 (2) | 0.579 (2) | 0.4356 (6) | 0.055* | |
| H12 | 0.486 (2) | 0.426 (2) | 0.4397 (6) | 0.055* | |
| C2 | 0.5986 (2) | 0.6202 (2) | 0.41168 (5) | 0.0509 (4) | |
| H2A | 0.6633 | 0.6571 | 0.4402 | 0.061* | |
| H2B | 0.5558 | 0.7188 | 0.3946 | 0.061* | |
| C3 | 0.72308 (19) | 0.5222 (2) | 0.38567 (5) | 0.0445 (4) | |
| H3A | 0.8229 | 0.5926 | 0.3799 | 0.053* | |
| H3B | 0.7707 | 0.4266 | 0.4034 | 0.053* | |
| N4 | 0.62657 (15) | 0.46482 (15) | 0.34309 (4) | 0.0388 (3) | |
| C5 | 0.47731 (19) | 0.3566 (2) | 0.35153 (5) | 0.0431 (3) | |
| H5A | 0.5234 | 0.2588 | 0.3685 | 0.052* | |
| H5B | 0.4140 | 0.3187 | 0.3230 | 0.052* | |
| C6 | 0.3495 (2) | 0.4492 (2) | 0.37760 (5) | 0.0468 (4) | |
| H6A | 0.2950 | 0.5410 | 0.3595 | 0.056* | |
| H6B | 0.2548 | 0.3740 | 0.3841 | 0.056* | |
| C21 | 0.73290 (19) | 0.39011 (18) | 0.31211 (5) | 0.0405 (3) | |
| C22 | 0.6595 (2) | 0.3763 (2) | 0.26645 (5) | 0.0474 (4) | |
| C23 | 0.7580 (3) | 0.3012 (2) | 0.23580 (6) | 0.0602 (5) | |
| H23 | 0.7093 | 0.2922 | 0.2057 | 0.072* | |
| C24 | 0.9269 (3) | 0.2400 (3) | 0.24948 (7) | 0.0697 (5) | |
| H24 | 0.9919 | 0.1898 | 0.2286 | 0.084* | |
| C25 | 1.0000 (2) | 0.2525 (3) | 0.29365 (7) | 0.0695 (5) | |
| H25 | 1.1143 | 0.2108 | 0.3029 | 0.083* | |
| C26 | 0.9025 (2) | 0.3280 (2) | 0.32471 (6) | 0.0546 (4) | |
| H26 | 0.9533 | 0.3368 | 0.3547 | 0.066* | |
| O22 | 0.49191 (17) | 0.43933 (18) | 0.25585 (4) | 0.0657 (4) | |
| C27 | 0.4027 (3) | 0.4122 (3) | 0.21158 (6) | 0.0739 (6) | |
| H27A | 0.2862 | 0.4633 | 0.2088 | 0.111* | |
| H27B | 0.4715 | 0.4612 | 0.1900 | 0.111* | |
| H27C | 0.3902 | 0.2940 | 0.2061 | 0.111* | |
| C31 | 0.16123 (19) | 0.82005 (18) | 0.52883 (4) | 0.0392 (3) | |
| C32 | −0.0213 (2) | 0.79178 (19) | 0.52353 (5) | 0.0450 (4) | |
| H32 | −0.0716 | 0.7179 | 0.5014 | 0.054* | |
| C33 | −0.1305 (2) | 0.8720 (2) | 0.55077 (5) | 0.0458 (4) | |
| H33 | −0.2535 | 0.8531 | 0.5473 | 0.055* | |
| C34 | −0.05131 (19) | 0.98058 (18) | 0.58316 (5) | 0.0414 (3) | |
| C35 | 0.1295 (2) | 1.01111 (19) | 0.58934 (5) | 0.0441 (4) | |
| H35 | 0.1794 | 1.0853 | 0.6115 | 0.053* | |
| C36 | 0.2358 (2) | 0.92921 (19) | 0.56193 (5) | 0.0435 (3) | |
| H36 | 0.3590 | 0.9477 | 0.5658 | 0.052* | |
| C37 | 0.2801 (2) | 0.73155 (19) | 0.49920 (5) | 0.0458 (4) | |
| O31 | 0.20537 (17) | 0.67769 (17) | 0.46209 (4) | 0.0656 (4) | |
| O32 | 0.44235 (15) | 0.71810 (15) | 0.51349 (4) | 0.0570 (3) | |
| N34 | −0.1638 (2) | 1.06966 (19) | 0.61219 (5) | 0.0541 (4) | |
| O41 | −0.32042 (19) | 1.0270 (2) | 0.61086 (5) | 0.0826 (4) | |
| O42 | −0.09646 (19) | 1.18199 (18) | 0.63615 (4) | 0.0731 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0481 (7) | 0.0554 (8) | 0.0341 (6) | 0.0041 (6) | 0.0078 (5) | −0.0034 (6) |
| C2 | 0.0574 (9) | 0.0535 (9) | 0.0410 (8) | −0.0073 (8) | 0.0034 (7) | −0.0110 (7) |
| C3 | 0.0444 (8) | 0.0490 (9) | 0.0395 (7) | −0.0089 (7) | 0.0027 (6) | −0.0033 (7) |
| N4 | 0.0394 (6) | 0.0450 (7) | 0.0321 (6) | −0.0041 (5) | 0.0041 (5) | −0.0029 (5) |
| C5 | 0.0409 (8) | 0.0510 (9) | 0.0372 (7) | −0.0061 (7) | 0.0039 (6) | −0.0064 (6) |
| C6 | 0.0412 (8) | 0.0611 (10) | 0.0377 (7) | −0.0022 (7) | 0.0038 (6) | −0.0026 (7) |
| C21 | 0.0424 (8) | 0.0419 (8) | 0.0383 (7) | −0.0049 (6) | 0.0089 (6) | −0.0004 (6) |
| C22 | 0.0532 (9) | 0.0523 (9) | 0.0375 (7) | 0.0025 (7) | 0.0093 (6) | 0.0007 (7) |
| C23 | 0.0696 (11) | 0.0717 (12) | 0.0422 (9) | 0.0004 (9) | 0.0182 (8) | −0.0069 (8) |
| C24 | 0.0610 (11) | 0.0838 (14) | 0.0703 (12) | −0.0019 (10) | 0.0314 (10) | −0.0169 (11) |
| C25 | 0.0408 (9) | 0.0878 (14) | 0.0816 (13) | 0.0024 (9) | 0.0144 (9) | −0.0132 (11) |
| C26 | 0.0400 (8) | 0.0695 (11) | 0.0543 (9) | −0.0039 (8) | 0.0058 (7) | −0.0040 (8) |
| O22 | 0.0696 (7) | 0.0899 (10) | 0.0349 (6) | 0.0288 (7) | −0.0039 (5) | −0.0076 (6) |
| C27 | 0.0843 (13) | 0.0919 (15) | 0.0406 (9) | 0.0168 (12) | −0.0106 (9) | −0.0046 (9) |
| C31 | 0.0456 (8) | 0.0401 (8) | 0.0318 (7) | 0.0060 (6) | 0.0049 (6) | 0.0060 (6) |
| C32 | 0.0504 (9) | 0.0470 (8) | 0.0359 (7) | −0.0014 (7) | −0.0010 (6) | 0.0003 (6) |
| C33 | 0.0392 (8) | 0.0526 (9) | 0.0454 (8) | −0.0017 (7) | 0.0052 (6) | 0.0076 (7) |
| C34 | 0.0467 (8) | 0.0430 (8) | 0.0360 (7) | 0.0057 (7) | 0.0121 (6) | 0.0071 (6) |
| C35 | 0.0498 (9) | 0.0438 (8) | 0.0385 (7) | −0.0012 (7) | 0.0044 (6) | −0.0019 (6) |
| C36 | 0.0402 (8) | 0.0468 (8) | 0.0432 (8) | 0.0014 (7) | 0.0046 (6) | 0.0019 (7) |
| C37 | 0.0556 (9) | 0.0455 (8) | 0.0372 (7) | 0.0100 (7) | 0.0089 (7) | 0.0053 (7) |
| O31 | 0.0698 (8) | 0.0835 (9) | 0.0428 (6) | 0.0201 (7) | 0.0044 (6) | −0.0148 (6) |
| O32 | 0.0520 (7) | 0.0674 (8) | 0.0526 (7) | 0.0152 (6) | 0.0111 (5) | 0.0023 (6) |
| N34 | 0.0606 (9) | 0.0598 (9) | 0.0456 (7) | 0.0109 (7) | 0.0207 (6) | 0.0082 (7) |
| O41 | 0.0611 (8) | 0.1020 (11) | 0.0923 (10) | 0.0029 (8) | 0.0395 (7) | −0.0035 (9) |
| O42 | 0.0905 (10) | 0.0772 (9) | 0.0558 (7) | 0.0075 (8) | 0.0258 (7) | −0.0159 (7) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Geometric parameters (Å, º)
| N1—C2 | 1.476 (2) | C24—H24 | 0.9300 |
| N1—C6 | 1.4798 (18) | C25—C26 | 1.390 (2) |
| N1—H11 | 0.974 (18) | C25—H25 | 0.9300 |
| N1—H12 | 0.947 (18) | C26—H26 | 0.9300 |
| C2—C3 | 1.508 (2) | O22—C27 | 1.4204 (19) |
| C2—H2A | 0.9700 | C27—H27A | 0.9600 |
| C2—H2B | 0.9700 | C27—H27B | 0.9600 |
| C3—N4 | 1.4551 (17) | C27—H27C | 0.9600 |
| C3—H3A | 0.9700 | C31—C32 | 1.380 (2) |
| C3—H3B | 0.9700 | C31—C36 | 1.381 (2) |
| N4—C21 | 1.4275 (18) | C31—C37 | 1.510 (2) |
| N4—C5 | 1.4626 (18) | C32—C33 | 1.384 (2) |
| C5—C6 | 1.505 (2) | C32—H32 | 0.9300 |
| C5—H5A | 0.9700 | C33—C34 | 1.376 (2) |
| C5—H5B | 0.9700 | C33—H33 | 0.9300 |
| C6—H6A | 0.9700 | C34—C35 | 1.370 (2) |
| C6—H6B | 0.9700 | C34—N34 | 1.4690 (19) |
| C21—C26 | 1.375 (2) | C35—C36 | 1.379 (2) |
| C21—C22 | 1.409 (2) | C35—H35 | 0.9300 |
| C22—O22 | 1.3562 (19) | C36—H36 | 0.9300 |
| C22—C23 | 1.384 (2) | C37—O32 | 1.2443 (18) |
| C23—C24 | 1.373 (3) | C37—O31 | 1.2528 (19) |
| C23—H23 | 0.9300 | N34—O42 | 1.2157 (19) |
| C24—C25 | 1.366 (3) | N34—O41 | 1.2217 (19) |
| C2—N1—C6 | 110.74 (11) | C22—C23—H23 | 119.7 |
| C2—N1—H11 | 111.7 (10) | C25—C24—C23 | 120.23 (17) |
| C6—N1—H11 | 108.4 (10) | C25—C24—H24 | 119.9 |
| C2—N1—H12 | 109.0 (10) | C23—C24—H24 | 119.9 |
| C6—N1—H12 | 109.8 (10) | C24—C25—C26 | 119.65 (17) |
| H11—N1—H12 | 107.1 (14) | C24—C25—H25 | 120.2 |
| N1—C2—C3 | 110.53 (13) | C26—C25—H25 | 120.2 |
| N1—C2—H2A | 109.5 | C21—C26—C25 | 121.52 (16) |
| C3—C2—H2A | 109.5 | C21—C26—H26 | 119.2 |
| N1—C2—H2B | 109.5 | C25—C26—H26 | 119.2 |
| C3—C2—H2B | 109.5 | C22—O22—C27 | 118.37 (13) |
| H2A—C2—H2B | 108.1 | O22—C27—H27A | 109.5 |
| N4—C3—C2 | 109.89 (12) | O22—C27—H27B | 109.5 |
| N4—C3—H3A | 109.7 | H27A—C27—H27B | 109.5 |
| C2—C3—H3A | 109.7 | O22—C27—H27C | 109.5 |
| N4—C3—H3B | 109.7 | H27A—C27—H27C | 109.5 |
| C2—C3—H3B | 109.7 | H27B—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.2 | C32—C31—C36 | 119.48 (13) |
| C21—N4—C3 | 116.07 (11) | C32—C31—C37 | 120.70 (13) |
| C21—N4—C5 | 111.75 (11) | C36—C31—C37 | 119.81 (13) |
| C3—N4—C5 | 110.07 (11) | C31—C32—C33 | 120.84 (14) |
| N4—C5—C6 | 110.72 (12) | C31—C32—H32 | 119.6 |
| N4—C5—H5A | 109.5 | C33—C32—H32 | 119.6 |
| C6—C5—H5A | 109.5 | C34—C33—C32 | 117.93 (14) |
| N4—C5—H5B | 109.5 | C34—C33—H33 | 121.0 |
| C6—C5—H5B | 109.5 | C32—C33—H33 | 121.0 |
| H5A—C5—H5B | 108.1 | C35—C34—C33 | 122.65 (14) |
| N1—C6—C5 | 110.67 (12) | C35—C34—N34 | 118.04 (14) |
| N1—C6—H6A | 109.5 | C33—C34—N34 | 119.30 (14) |
| C5—C6—H6A | 109.5 | C34—C35—C36 | 118.40 (14) |
| N1—C6—H6B | 109.5 | C34—C35—H35 | 120.8 |
| C5—C6—H6B | 109.5 | C36—C35—H35 | 120.8 |
| H6A—C6—H6B | 108.1 | C35—C36—C31 | 120.68 (14) |
| C26—C21—C22 | 118.14 (14) | C35—C36—H36 | 119.7 |
| C26—C21—N4 | 123.33 (13) | C31—C36—H36 | 119.7 |
| C22—C21—N4 | 118.52 (13) | O32—C37—O31 | 125.68 (14) |
| O22—C22—C23 | 124.42 (14) | O32—C37—C31 | 117.82 (13) |
| O22—C22—C21 | 115.75 (13) | O31—C37—C31 | 116.50 (14) |
| C23—C22—C21 | 119.83 (15) | O42—N34—O41 | 123.48 (15) |
| C24—C23—C22 | 120.63 (16) | O42—N34—C34 | 118.43 (14) |
| C24—C23—H23 | 119.7 | O41—N34—C34 | 118.09 (15) |
| C6—N1—C2—C3 | −55.83 (17) | C24—C25—C26—C21 | 0.4 (3) |
| N1—C2—C3—N4 | 58.62 (16) | C23—C22—O22—C27 | 6.8 (3) |
| C2—C3—N4—C21 | 171.70 (12) | C21—C22—O22—C27 | −172.93 (16) |
| C2—C3—N4—C5 | −60.13 (16) | C36—C31—C32—C33 | 0.3 (2) |
| C21—N4—C5—C6 | −170.14 (12) | C37—C31—C32—C33 | 179.66 (13) |
| C3—N4—C5—C6 | 59.35 (15) | C31—C32—C33—C34 | 0.1 (2) |
| C2—N1—C6—C5 | 54.67 (17) | C32—C33—C34—C35 | −0.2 (2) |
| N4—C5—C6—N1 | −56.40 (16) | C32—C33—C34—N34 | 179.10 (13) |
| C3—N4—C21—C26 | 19.2 (2) | C33—C34—C35—C36 | −0.1 (2) |
| C5—N4—C21—C26 | −108.17 (16) | N34—C34—C35—C36 | −179.38 (13) |
| C3—N4—C21—C22 | −162.02 (14) | C34—C35—C36—C31 | 0.5 (2) |
| C5—N4—C21—C22 | 70.65 (17) | C32—C31—C36—C35 | −0.6 (2) |
| C26—C21—C22—O22 | 179.94 (15) | C37—C31—C36—C35 | −179.95 (13) |
| N4—C21—C22—O22 | 1.1 (2) | C32—C31—C37—O32 | −157.78 (15) |
| C26—C21—C22—C23 | 0.2 (2) | C36—C31—C37—O32 | 21.5 (2) |
| N4—C21—C22—C23 | −178.64 (15) | C32—C31—C37—O31 | 22.1 (2) |
| O22—C22—C23—C24 | −179.71 (18) | C36—C31—C37—O31 | −158.53 (15) |
| C21—C22—C23—C24 | 0.0 (3) | C35—C34—N34—O42 | 9.5 (2) |
| C22—C23—C24—C25 | 0.0 (3) | C33—C34—N34—O42 | −169.80 (14) |
| C23—C24—C25—C26 | −0.1 (3) | C35—C34—N34—O41 | −170.64 (15) |
| C22—C21—C26—C25 | −0.4 (3) | C33—C34—N34—O41 | 10.0 (2) |
| N4—C21—C26—C25 | 178.42 (16) |
4-(2-Methoxyphenyl)piperazin-1-ium 4-nitrobenzoate (X). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.974 (16) | 1.677 (16) | 2.6500 (19) | 176.8 (15) |
| N1—H11···O32 | 0.974 (16) | 2.581 (17) | 3.2169 (17) | 123.0 (12) |
| N1—H12···O32i | 0.948 (17) | 1.837 (17) | 2.7709 (18) | 168.2 (16) |
Symmetry code: (i) −x+1, −y+1, −z+1.
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Crystal data
| C11H17N2O+·C7H3N2O6−·2H2O | Z = 2 |
| Mr = 440.41 | F(000) = 464 |
| Triclinic, P1 | Dx = 1.405 Mg m−3 |
| a = 7.8448 (6) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 11.4635 (9) Å | Cell parameters from 4419 reflections |
| c = 12.0747 (9) Å | θ = 2.6–27.7° |
| α = 94.406 (7)° | µ = 0.11 mm−1 |
| β = 105.075 (8)° | T = 296 K |
| γ = 93.717 (7)° | Block, yellow |
| V = 1041.33 (14) Å3 | 0.48 × 0.48 × 0.44 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 4419 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 3409 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.016 |
| ω scans | θmax = 27.7°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −9→10 |
| Tmin = 0.892, Tmax = 0.951 | k = −9→14 |
| 7353 measured reflections | l = −15→14 |
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.039 | w = 1/[σ2(Fo2) + (0.0554P)2 + 0.1343P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.108 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.23 e Å−3 |
| 4419 reflections | Δρmin = −0.17 e Å−3 |
| 300 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.052 (4) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.65966 (17) | 0.38158 (10) | 0.34243 (10) | 0.0387 (3) | |
| H11 | 0.718 (2) | 0.3310 (14) | 0.3931 (14) | 0.046* | |
| H12 | 0.541 (2) | 0.3638 (13) | 0.3304 (13) | 0.046* | |
| C2 | 0.7170 (2) | 0.37246 (12) | 0.23459 (12) | 0.0437 (3) | |
| H2A | 0.6800 | 0.2946 | 0.1947 | 0.052* | |
| H2B | 0.8452 | 0.3838 | 0.2531 | 0.052* | |
| C3 | 0.63768 (19) | 0.46357 (11) | 0.15669 (11) | 0.0383 (3) | |
| H3A | 0.6811 | 0.4588 | 0.0884 | 0.046* | |
| H3B | 0.5097 | 0.4481 | 0.1325 | 0.046* | |
| N4 | 0.68560 (14) | 0.58186 (8) | 0.21770 (8) | 0.0317 (2) | |
| C5 | 0.61652 (19) | 0.58963 (11) | 0.31932 (11) | 0.0374 (3) | |
| H5A | 0.4888 | 0.5723 | 0.2961 | 0.045* | |
| H5B | 0.6431 | 0.6686 | 0.3579 | 0.045* | |
| C6 | 0.7004 (2) | 0.50306 (12) | 0.40043 (11) | 0.0422 (3) | |
| H6A | 0.8278 | 0.5219 | 0.4251 | 0.051* | |
| H6B | 0.6557 | 0.5085 | 0.4682 | 0.051* | |
| C21 | 0.64076 (16) | 0.67242 (10) | 0.14284 (10) | 0.0311 (3) | |
| C22 | 0.74860 (16) | 0.69609 (11) | 0.06906 (11) | 0.0342 (3) | |
| C23 | 0.71254 (19) | 0.78402 (12) | −0.00441 (12) | 0.0421 (3) | |
| H23 | 0.7837 | 0.7991 | −0.0533 | 0.051* | |
| C24 | 0.5696 (2) | 0.84922 (12) | −0.00446 (13) | 0.0466 (4) | |
| H24 | 0.5458 | 0.9083 | −0.0535 | 0.056* | |
| C25 | 0.4631 (2) | 0.82783 (12) | 0.06667 (13) | 0.0476 (4) | |
| H25 | 0.3677 | 0.8721 | 0.0660 | 0.057* | |
| C26 | 0.49877 (18) | 0.73919 (12) | 0.14005 (12) | 0.0399 (3) | |
| H26 | 0.4260 | 0.7245 | 0.1881 | 0.048* | |
| O22 | 0.88764 (13) | 0.62807 (9) | 0.07539 (9) | 0.0471 (3) | |
| C27 | 1.0094 (2) | 0.65658 (16) | 0.01004 (16) | 0.0601 (4) | |
| H27A | 1.1050 | 0.6069 | 0.0266 | 0.090* | |
| H27B | 1.0556 | 0.7372 | 0.0301 | 0.090* | |
| H27C | 0.9495 | 0.6448 | −0.0706 | 0.090* | |
| C31 | 1.07262 (16) | 0.14162 (10) | 0.58115 (10) | 0.0328 (3) | |
| C32 | 1.23171 (17) | 0.09894 (11) | 0.57551 (11) | 0.0361 (3) | |
| H32 | 1.2888 | 0.1233 | 0.5216 | 0.043* | |
| C33 | 1.30429 (17) | 0.01961 (11) | 0.65116 (12) | 0.0384 (3) | |
| C34 | 1.22367 (18) | −0.02069 (11) | 0.73110 (12) | 0.0406 (3) | |
| H34 | 1.2719 | −0.0765 | 0.7792 | 0.049* | |
| C35 | 1.06862 (18) | 0.02555 (11) | 0.73629 (11) | 0.0376 (3) | |
| C36 | 0.99163 (17) | 0.10646 (11) | 0.66424 (11) | 0.0356 (3) | |
| H36 | 0.8874 | 0.1369 | 0.6712 | 0.043* | |
| C37 | 0.98662 (19) | 0.22652 (11) | 0.49671 (11) | 0.0402 (3) | |
| O31 | 0.84578 (15) | 0.26458 (10) | 0.51102 (9) | 0.0585 (3) | |
| O32 | 1.05696 (16) | 0.24991 (11) | 0.42026 (10) | 0.0655 (3) | |
| N33 | 1.47682 (18) | −0.02225 (14) | 0.64945 (12) | 0.0578 (4) | |
| O33 | 1.57628 (16) | 0.04182 (15) | 0.61300 (12) | 0.0825 (4) | |
| O34 | 1.51295 (19) | −0.11651 (13) | 0.68590 (15) | 0.0890 (5) | |
| N35 | 0.98229 (19) | −0.01443 (13) | 0.82304 (11) | 0.0558 (4) | |
| O35 | 1.0381 (2) | −0.09880 (14) | 0.87252 (13) | 0.0929 (5) | |
| O36 | 0.86187 (19) | 0.03867 (14) | 0.84155 (11) | 0.0794 (4) | |
| O41 | 0.28832 (16) | 0.36547 (12) | 0.31241 (12) | 0.0639 (3) | |
| H41 | 0.225 (3) | 0.322 (2) | 0.341 (2) | 0.096* | |
| H42 | 0.230 (3) | 0.431 (2) | 0.306 (2) | 0.096* | |
| O51 | 0.13836 (19) | 0.58148 (13) | 0.29313 (13) | 0.0722 (4) | |
| H51 | 0.152 (3) | 0.630 (2) | 0.356 (2) | 0.087* | |
| H52 | 0.051 (3) | 0.593 (2) | 0.250 (2) | 0.087* |
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0413 (6) | 0.0353 (6) | 0.0404 (6) | 0.0049 (5) | 0.0087 (5) | 0.0154 (5) |
| C2 | 0.0584 (9) | 0.0332 (7) | 0.0429 (7) | 0.0107 (6) | 0.0162 (6) | 0.0094 (6) |
| C3 | 0.0516 (8) | 0.0308 (6) | 0.0324 (6) | 0.0051 (5) | 0.0098 (6) | 0.0057 (5) |
| N4 | 0.0371 (6) | 0.0279 (5) | 0.0311 (5) | 0.0029 (4) | 0.0096 (4) | 0.0077 (4) |
| C5 | 0.0470 (8) | 0.0315 (6) | 0.0363 (7) | 0.0019 (5) | 0.0155 (6) | 0.0049 (5) |
| C6 | 0.0536 (8) | 0.0414 (7) | 0.0320 (6) | 0.0004 (6) | 0.0118 (6) | 0.0079 (5) |
| C21 | 0.0327 (6) | 0.0270 (6) | 0.0318 (6) | 0.0005 (5) | 0.0052 (5) | 0.0060 (5) |
| C22 | 0.0338 (6) | 0.0321 (6) | 0.0365 (7) | 0.0022 (5) | 0.0077 (5) | 0.0080 (5) |
| C23 | 0.0484 (8) | 0.0401 (7) | 0.0390 (7) | 0.0001 (6) | 0.0115 (6) | 0.0141 (6) |
| C24 | 0.0540 (9) | 0.0357 (7) | 0.0460 (8) | 0.0060 (6) | 0.0022 (7) | 0.0161 (6) |
| C25 | 0.0445 (8) | 0.0382 (7) | 0.0580 (9) | 0.0130 (6) | 0.0057 (7) | 0.0111 (6) |
| C26 | 0.0375 (7) | 0.0381 (7) | 0.0459 (7) | 0.0054 (5) | 0.0125 (6) | 0.0087 (6) |
| O22 | 0.0455 (6) | 0.0506 (6) | 0.0572 (6) | 0.0148 (4) | 0.0270 (5) | 0.0240 (5) |
| C27 | 0.0554 (10) | 0.0656 (10) | 0.0750 (11) | 0.0128 (8) | 0.0392 (9) | 0.0226 (9) |
| C31 | 0.0363 (7) | 0.0269 (6) | 0.0337 (6) | −0.0015 (5) | 0.0072 (5) | 0.0046 (5) |
| C32 | 0.0371 (7) | 0.0359 (6) | 0.0353 (6) | −0.0037 (5) | 0.0113 (5) | 0.0042 (5) |
| C33 | 0.0327 (7) | 0.0377 (7) | 0.0425 (7) | 0.0049 (5) | 0.0064 (5) | 0.0007 (6) |
| C34 | 0.0440 (8) | 0.0335 (7) | 0.0413 (7) | 0.0048 (5) | 0.0038 (6) | 0.0107 (6) |
| C35 | 0.0418 (7) | 0.0367 (7) | 0.0347 (7) | −0.0030 (5) | 0.0107 (5) | 0.0100 (5) |
| C36 | 0.0340 (7) | 0.0360 (6) | 0.0375 (7) | 0.0032 (5) | 0.0099 (5) | 0.0070 (5) |
| C37 | 0.0465 (8) | 0.0338 (7) | 0.0375 (7) | −0.0006 (6) | 0.0055 (6) | 0.0098 (5) |
| O31 | 0.0658 (7) | 0.0649 (7) | 0.0502 (6) | 0.0306 (6) | 0.0137 (5) | 0.0242 (5) |
| O32 | 0.0711 (8) | 0.0732 (8) | 0.0619 (7) | 0.0061 (6) | 0.0257 (6) | 0.0399 (6) |
| N33 | 0.0409 (7) | 0.0721 (10) | 0.0583 (8) | 0.0138 (7) | 0.0086 (6) | 0.0005 (7) |
| O33 | 0.0407 (7) | 0.1309 (13) | 0.0809 (9) | 0.0122 (7) | 0.0210 (6) | 0.0204 (9) |
| O34 | 0.0697 (9) | 0.0731 (9) | 0.1238 (13) | 0.0407 (7) | 0.0150 (8) | 0.0152 (8) |
| N35 | 0.0564 (8) | 0.0689 (9) | 0.0457 (7) | −0.0032 (7) | 0.0170 (6) | 0.0226 (6) |
| O35 | 0.1110 (12) | 0.0950 (11) | 0.0922 (10) | 0.0168 (9) | 0.0434 (9) | 0.0664 (9) |
| O36 | 0.0691 (8) | 0.1213 (12) | 0.0645 (8) | 0.0177 (8) | 0.0387 (7) | 0.0319 (8) |
| O41 | 0.0520 (7) | 0.0584 (7) | 0.0919 (9) | 0.0049 (5) | 0.0336 (6) | 0.0231 (7) |
| O51 | 0.0665 (8) | 0.0786 (9) | 0.0674 (8) | 0.0321 (7) | 0.0051 (7) | 0.0044 (7) |
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Geometric parameters (Å, º)
| N1—C2 | 1.4826 (18) | C26—H26 | 0.9300 |
| N1—C6 | 1.4860 (18) | O22—C27 | 1.4257 (17) |
| N1—H11 | 0.929 (16) | C27—H27A | 0.9600 |
| N1—H12 | 0.908 (16) | C27—H27B | 0.9600 |
| C2—C3 | 1.5143 (17) | C27—H27C | 0.9600 |
| C2—H2A | 0.9700 | C31—C32 | 1.3856 (19) |
| C2—H2B | 0.9700 | C31—C36 | 1.3903 (17) |
| C3—N4 | 1.4694 (16) | C31—C37 | 1.5237 (17) |
| C3—H3A | 0.9700 | C32—C33 | 1.3820 (18) |
| C3—H3B | 0.9700 | C32—H32 | 0.9300 |
| N4—C21 | 1.4300 (15) | C33—C34 | 1.3759 (19) |
| N4—C5 | 1.4638 (16) | C33—N33 | 1.4696 (19) |
| C5—C6 | 1.5086 (18) | C34—C35 | 1.372 (2) |
| C5—H5A | 0.9700 | C34—H34 | 0.9300 |
| C5—H5B | 0.9700 | C35—C36 | 1.3803 (17) |
| C6—H6A | 0.9700 | C35—N35 | 1.4727 (17) |
| C6—H6B | 0.9700 | C36—H36 | 0.9300 |
| C21—C26 | 1.3866 (18) | C37—O32 | 1.2297 (17) |
| C21—C22 | 1.4080 (17) | C37—O31 | 1.2615 (18) |
| C22—O22 | 1.3709 (16) | N33—O34 | 1.2204 (19) |
| C22—C23 | 1.3881 (17) | N33—O33 | 1.2231 (19) |
| C23—C24 | 1.387 (2) | N35—O36 | 1.2150 (19) |
| C23—H23 | 0.9300 | N35—O35 | 1.2214 (18) |
| C24—C25 | 1.369 (2) | O41—H41 | 0.84 (2) |
| C24—H24 | 0.9300 | O41—H42 | 0.91 (3) |
| C25—C26 | 1.3936 (19) | O51—H51 | 0.88 (2) |
| C25—H25 | 0.9300 | O51—H52 | 0.77 (2) |
| C2—N1—C6 | 110.76 (10) | C23—C24—H24 | 119.5 |
| C2—N1—H11 | 110.5 (10) | C24—C25—C26 | 119.49 (13) |
| C6—N1—H11 | 108.2 (9) | C24—C25—H25 | 120.3 |
| C2—N1—H12 | 113.0 (10) | C26—C25—H25 | 120.3 |
| C6—N1—H12 | 106.6 (10) | C21—C26—C25 | 121.23 (13) |
| H11—N1—H12 | 107.6 (13) | C21—C26—H26 | 119.4 |
| N1—C2—C3 | 110.82 (11) | C25—C26—H26 | 119.4 |
| N1—C2—H2A | 109.5 | C22—O22—C27 | 117.66 (11) |
| C3—C2—H2A | 109.5 | O22—C27—H27A | 109.5 |
| N1—C2—H2B | 109.5 | O22—C27—H27B | 109.5 |
| C3—C2—H2B | 109.5 | H27A—C27—H27B | 109.5 |
| H2A—C2—H2B | 108.1 | O22—C27—H27C | 109.5 |
| N4—C3—C2 | 110.21 (10) | H27A—C27—H27C | 109.5 |
| N4—C3—H3A | 109.6 | H27B—C27—H27C | 109.5 |
| C2—C3—H3A | 109.6 | C32—C31—C36 | 119.65 (11) |
| N4—C3—H3B | 109.6 | C32—C31—C37 | 120.23 (11) |
| C2—C3—H3B | 109.6 | C36—C31—C37 | 120.12 (12) |
| H3A—C3—H3B | 108.1 | C33—C32—C31 | 118.99 (12) |
| C21—N4—C5 | 115.30 (10) | C33—C32—H32 | 120.5 |
| C21—N4—C3 | 112.57 (9) | C31—C32—H32 | 120.5 |
| C5—N4—C3 | 109.48 (10) | C34—C33—C32 | 122.75 (12) |
| N4—C5—C6 | 109.39 (11) | C34—C33—N33 | 117.76 (12) |
| N4—C5—H5A | 109.8 | C32—C33—N33 | 119.48 (13) |
| C6—C5—H5A | 109.8 | C35—C34—C33 | 116.72 (12) |
| N4—C5—H5B | 109.8 | C35—C34—H34 | 121.6 |
| C6—C5—H5B | 109.8 | C33—C34—H34 | 121.6 |
| H5A—C5—H5B | 108.2 | C34—C35—C36 | 122.99 (12) |
| N1—C6—C5 | 110.28 (11) | C34—C35—N35 | 117.58 (12) |
| N1—C6—H6A | 109.6 | C36—C35—N35 | 119.42 (12) |
| C5—C6—H6A | 109.6 | C35—C36—C31 | 118.82 (12) |
| N1—C6—H6B | 109.6 | C35—C36—H36 | 120.6 |
| C5—C6—H6B | 109.6 | C31—C36—H36 | 120.6 |
| H6A—C6—H6B | 108.1 | O32—C37—O31 | 125.98 (13) |
| C26—C21—C22 | 118.27 (11) | O32—C37—C31 | 118.05 (13) |
| C26—C21—N4 | 123.70 (11) | O31—C37—C31 | 115.95 (12) |
| C22—C21—N4 | 118.02 (11) | O34—N33—O33 | 124.40 (15) |
| O22—C22—C23 | 123.48 (12) | O34—N33—C33 | 118.57 (15) |
| O22—C22—C21 | 116.04 (10) | O33—N33—C33 | 117.02 (14) |
| C23—C22—C21 | 120.48 (12) | O36—N35—O35 | 124.28 (14) |
| C24—C23—C22 | 119.57 (13) | O36—N35—C35 | 118.33 (13) |
| C24—C23—H23 | 120.2 | O35—N35—C35 | 117.39 (15) |
| C22—C23—H23 | 120.2 | H41—O41—H42 | 102 (2) |
| C25—C24—C23 | 120.95 (12) | H51—O51—H52 | 108 (2) |
| C25—C24—H24 | 119.5 | ||
| C6—N1—C2—C3 | −54.19 (15) | C36—C31—C32—C33 | 1.65 (18) |
| N1—C2—C3—N4 | 56.54 (15) | C37—C31—C32—C33 | −178.27 (11) |
| C2—C3—N4—C21 | 169.85 (11) | C31—C32—C33—C34 | 1.13 (19) |
| C2—C3—N4—C5 | −60.51 (14) | C31—C32—C33—N33 | −177.30 (12) |
| C21—N4—C5—C6 | −169.80 (10) | C32—C33—C34—C35 | −2.7 (2) |
| C3—N4—C5—C6 | 62.06 (13) | N33—C33—C34—C35 | 175.77 (12) |
| C2—N1—C6—C5 | 55.87 (15) | C33—C34—C35—C36 | 1.5 (2) |
| N4—C5—C6—N1 | −59.79 (14) | C33—C34—C35—N35 | −178.66 (12) |
| C5—N4—C21—C26 | −21.15 (17) | C34—C35—C36—C31 | 1.1 (2) |
| C3—N4—C21—C26 | 105.44 (14) | N35—C35—C36—C31 | −178.69 (12) |
| C5—N4—C21—C22 | 157.91 (11) | C32—C31—C36—C35 | −2.72 (18) |
| C3—N4—C21—C22 | −75.51 (14) | C37—C31—C36—C35 | 177.21 (11) |
| C26—C21—C22—O22 | 179.73 (11) | C32—C31—C37—O32 | 4.20 (18) |
| N4—C21—C22—O22 | 0.62 (16) | C36—C31—C37—O32 | −175.73 (13) |
| C26—C21—C22—C23 | −0.10 (18) | C32—C31—C37—O31 | −177.24 (13) |
| N4—C21—C22—C23 | −179.21 (11) | C36—C31—C37—O31 | 2.83 (18) |
| O22—C22—C23—C24 | −179.50 (13) | C34—C33—N33—O34 | 25.3 (2) |
| C21—C22—C23—C24 | 0.3 (2) | C32—C33—N33—O34 | −156.20 (15) |
| C22—C23—C24—C25 | −0.2 (2) | C34—C33—N33—O33 | −153.41 (14) |
| C23—C24—C25—C26 | −0.1 (2) | C32—C33—N33—O33 | 25.1 (2) |
| C22—C21—C26—C25 | −0.20 (19) | C34—C35—N35—O36 | 168.36 (14) |
| N4—C21—C26—C25 | 178.85 (12) | C36—C35—N35—O36 | −11.8 (2) |
| C24—C25—C26—C21 | 0.3 (2) | C34—C35—N35—O35 | −11.0 (2) |
| C23—C22—O22—C27 | 5.6 (2) | C36—C35—N35—O35 | 168.77 (14) |
| C21—C22—O22—C27 | −174.26 (12) |
4-(2-Methoxyphenyl)piperazin-1-ium 3,5-dinitrobenzoate dihydrate (XI). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.929 (16) | 1.771 (16) | 2.6837 (16) | 166.8 (15) |
| N1—H12···O41 | 0.911 (16) | 1.939 (16) | 2.8324 (19) | 165.5 (14) |
| O41—H41···O32i | 0.84 (2) | 1.99 (2) | 2.8156 (19) | 168 (2) |
| O41—H42···O51 | 0.90 (2) | 1.91 (2) | 2.810 (2) | 172 (2) |
| O51—H51···O31ii | 0.90 (2) | 1.91 (2) | 2.810 (2) | 172 (2) |
| O51—H52···O22i | 0.77 (2) | 2.25 (2) | 2.9544 (19) | 153 (2) |
| C25—H25···O36ii | 0.93 | 2.58 | 3.433 (2) | 153 |
Symmetry codes: (i) x−1, y, z; (ii) −x+1, −y+1, −z+1.
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Crystal data
| C11H17N2O+·C6H2N3O7− | Z = 2 |
| Mr = 421.33 | F(000) = 440 |
| Triclinic, P1 | Dx = 1.435 Mg m−3 |
| a = 9.4151 (5) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 9.8721 (5) Å | Cell parameters from 4279 reflections |
| c = 10.9572 (5) Å | θ = 2.9–27.8° |
| α = 77.524 (4)° | µ = 0.12 mm−1 |
| β = 81.360 (5)° | T = 296 K |
| γ = 81.002 (5)° | Plate, orange |
| V = 974.97 (9) Å3 | 0.48 × 0.48 × 0.24 mm |
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 4279 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 3276 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.017 |
| ω scans | θmax = 27.8°, θmin = 2.9° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −12→12 |
| Tmin = 0.805, Tmax = 0.973 | k = −12→12 |
| 12926 measured reflections | l = −13→13 |
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.040 | w = 1/[σ2(Fo2) + (0.0587P)2 + 0.1566P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.119 | (Δ/σ)max < 0.001 |
| S = 1.07 | Δρmax = 0.24 e Å−3 |
| 4279 reflections | Δρmin = −0.27 e Å−3 |
| 317 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 85 restraints | Extinction coefficient: 0.021 (3) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.55402 (16) | 0.61143 (13) | 0.19946 (11) | 0.0462 (3) | |
| H11 | 0.480 (2) | 0.5879 (18) | 0.2530 (16) | 0.055* | |
| H12 | 0.6047 (19) | 0.6597 (18) | 0.2347 (16) | 0.055* | |
| C2 | 0.64890 (19) | 0.48431 (16) | 0.17048 (14) | 0.0534 (4) | |
| H2A | 0.5924 | 0.4256 | 0.1420 | 0.064* | |
| H2B | 0.6880 | 0.4312 | 0.2461 | 0.064* | |
| C3 | 0.77123 (17) | 0.52432 (16) | 0.07001 (13) | 0.0497 (4) | |
| H3A | 0.8326 | 0.5761 | 0.1013 | 0.060* | |
| H3B | 0.8296 | 0.4405 | 0.0492 | 0.060* | |
| N4 | 0.71457 (12) | 0.61034 (12) | −0.04332 (10) | 0.0418 (3) | |
| C5 | 0.62911 (16) | 0.73798 (15) | −0.01273 (13) | 0.0457 (3) | |
| H5A | 0.5947 | 0.7967 | −0.0883 | 0.055* | |
| H5B | 0.6891 | 0.7898 | 0.0201 | 0.055* | |
| C6 | 0.50188 (16) | 0.70175 (17) | 0.08392 (14) | 0.0490 (4) | |
| H6A | 0.4457 | 0.7868 | 0.1043 | 0.059* | |
| H6B | 0.4398 | 0.6532 | 0.0499 | 0.059* | |
| C21 | 0.82215 (14) | 0.63009 (17) | −0.14998 (13) | 0.0446 (3) | |
| C22 | 0.87678 (16) | 0.51580 (19) | −0.20905 (14) | 0.0525 (4) | |
| C23 | 0.97820 (18) | 0.5326 (2) | −0.31534 (16) | 0.0665 (5) | |
| H23 | 1.0151 | 0.4568 | −0.3534 | 0.080* | |
| C24 | 1.02430 (19) | 0.6610 (3) | −0.36469 (17) | 0.0741 (6) | |
| H24 | 1.0912 | 0.6719 | −0.4366 | 0.089* | |
| C25 | 0.97236 (19) | 0.7728 (2) | −0.30852 (19) | 0.0706 (5) | |
| H25 | 1.0036 | 0.8594 | −0.3426 | 0.085* | |
| C26 | 0.87250 (17) | 0.75717 (19) | −0.19997 (15) | 0.0556 (4) | |
| H26 | 0.8396 | 0.8330 | −0.1610 | 0.067* | |
| O22 | 0.82269 (13) | 0.39384 (13) | −0.15675 (12) | 0.0685 (4) | |
| C27 | 0.8927 (2) | 0.2687 (2) | −0.1938 (2) | 0.0859 (7) | |
| H27A | 0.8424 | 0.1918 | −0.1492 | 0.129* | |
| H27B | 0.8921 | 0.2773 | −0.2827 | 0.129* | |
| H27C | 0.9909 | 0.2521 | −0.1747 | 0.129* | |
| C31 | 0.29075 (13) | 0.15664 (13) | 0.64803 (12) | 0.0362 (3) | |
| O31 | 0.27727 (12) | 0.22396 (11) | 0.73386 (9) | 0.0512 (3) | |
| C32 | 0.32774 (14) | 0.20872 (13) | 0.51527 (12) | 0.0361 (3) | |
| N32 | 0.35214 (13) | 0.35445 (12) | 0.47084 (11) | 0.0445 (3) | |
| O32 | 0.3202 (2) | 0.43611 (12) | 0.54139 (13) | 0.0911 (5) | |
| O33 | 0.40574 (16) | 0.38892 (13) | 0.36249 (11) | 0.0705 (4) | |
| C33 | 0.34703 (14) | 0.12760 (14) | 0.42520 (12) | 0.0381 (3) | |
| H33 | 0.3709 | 0.1669 | 0.3409 | 0.046* | |
| C34 | 0.33077 (14) | −0.01230 (14) | 0.46070 (13) | 0.0393 (3) | |
| N34 | 0.35475 (14) | −0.09763 (14) | 0.36584 (13) | 0.0501 (3) | |
| O34 | 0.38287 (16) | −0.04191 (13) | 0.25521 (11) | 0.0731 (4) | |
| O35 | 0.34933 (15) | −0.22433 (12) | 0.40011 (12) | 0.0699 (4) | |
| C35 | 0.29789 (14) | −0.07370 (14) | 0.58638 (13) | 0.0413 (3) | |
| H35 | 0.2907 | −0.1689 | 0.6101 | 0.050* | |
| C36 | 0.27649 (15) | 0.00877 (14) | 0.67410 (13) | 0.0404 (3) | |
| N36 | 0.24422 (18) | −0.05867 (14) | 0.80615 (13) | 0.0590 (4) | 0.850 (5) |
| O36 | 0.3154 (3) | −0.1672 (2) | 0.84426 (18) | 0.0830 (8) | 0.850 (5) |
| O37 | 0.1395 (3) | −0.0058 (3) | 0.8665 (2) | 0.1155 (12) | 0.850 (5) |
| N37 | 0.24422 (18) | −0.05867 (14) | 0.80615 (13) | 0.0590 (4) | 0.069 (4) |
| O38 | 0.1277 (16) | −0.090 (3) | 0.853 (2) | 0.094 (5) | 0.069 (4) |
| O39 | 0.3392 (18) | −0.077 (3) | 0.8764 (17) | 0.081 (5) | 0.069 (4) |
| N38 | 0.24422 (18) | −0.05867 (14) | 0.80615 (13) | 0.0590 (4) | 0.080 (4) |
| O310 | 0.219 (3) | −0.1764 (12) | 0.8390 (16) | 0.074 (4) | 0.080 (4) |
| O311 | 0.275 (3) | −0.0034 (19) | 0.8882 (12) | 0.084 (5) | 0.080 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0592 (8) | 0.0466 (7) | 0.0358 (6) | −0.0182 (6) | 0.0091 (5) | −0.0164 (5) |
| C2 | 0.0759 (11) | 0.0414 (8) | 0.0405 (8) | −0.0091 (7) | 0.0051 (7) | −0.0101 (6) |
| C3 | 0.0557 (9) | 0.0483 (8) | 0.0401 (8) | 0.0020 (7) | −0.0023 (6) | −0.0066 (6) |
| N4 | 0.0405 (6) | 0.0504 (7) | 0.0326 (6) | −0.0034 (5) | 0.0021 (5) | −0.0102 (5) |
| C5 | 0.0490 (8) | 0.0473 (8) | 0.0384 (7) | −0.0022 (6) | −0.0017 (6) | −0.0083 (6) |
| C6 | 0.0478 (8) | 0.0562 (9) | 0.0443 (8) | −0.0028 (7) | 0.0012 (6) | −0.0200 (7) |
| C21 | 0.0335 (7) | 0.0627 (9) | 0.0359 (7) | −0.0058 (6) | −0.0026 (5) | −0.0075 (6) |
| C22 | 0.0393 (7) | 0.0748 (11) | 0.0445 (8) | −0.0083 (7) | 0.0029 (6) | −0.0190 (7) |
| C23 | 0.0459 (9) | 0.1026 (15) | 0.0524 (9) | −0.0098 (9) | 0.0095 (7) | −0.0289 (10) |
| C24 | 0.0457 (9) | 0.1197 (18) | 0.0492 (10) | −0.0162 (10) | 0.0118 (7) | −0.0081 (11) |
| C25 | 0.0446 (9) | 0.0875 (14) | 0.0697 (11) | −0.0201 (9) | 0.0023 (8) | 0.0086 (10) |
| C26 | 0.0427 (8) | 0.0673 (10) | 0.0532 (9) | −0.0103 (7) | −0.0022 (7) | −0.0040 (8) |
| O22 | 0.0631 (7) | 0.0668 (8) | 0.0754 (8) | −0.0114 (6) | 0.0235 (6) | −0.0334 (6) |
| C27 | 0.0654 (12) | 0.0845 (14) | 0.1171 (18) | −0.0103 (10) | 0.0152 (12) | −0.0576 (13) |
| C31 | 0.0339 (6) | 0.0377 (7) | 0.0377 (7) | −0.0051 (5) | −0.0007 (5) | −0.0108 (5) |
| O31 | 0.0628 (7) | 0.0526 (6) | 0.0424 (5) | −0.0143 (5) | 0.0048 (5) | −0.0213 (5) |
| C32 | 0.0350 (6) | 0.0334 (6) | 0.0394 (7) | −0.0052 (5) | −0.0022 (5) | −0.0074 (5) |
| N32 | 0.0503 (7) | 0.0376 (6) | 0.0439 (7) | −0.0078 (5) | −0.0004 (5) | −0.0063 (5) |
| O32 | 0.1554 (15) | 0.0395 (6) | 0.0721 (9) | −0.0210 (8) | 0.0280 (9) | −0.0198 (6) |
| O33 | 0.1042 (10) | 0.0583 (7) | 0.0468 (6) | −0.0345 (7) | 0.0120 (6) | −0.0028 (5) |
| C33 | 0.0364 (6) | 0.0435 (7) | 0.0342 (6) | −0.0030 (5) | −0.0035 (5) | −0.0092 (5) |
| C34 | 0.0364 (7) | 0.0417 (7) | 0.0434 (7) | −0.0035 (5) | −0.0048 (5) | −0.0177 (6) |
| N34 | 0.0508 (7) | 0.0505 (7) | 0.0550 (8) | −0.0020 (6) | −0.0089 (6) | −0.0249 (6) |
| O34 | 0.1083 (11) | 0.0670 (8) | 0.0462 (7) | −0.0009 (7) | −0.0053 (6) | −0.0262 (6) |
| O35 | 0.0909 (9) | 0.0500 (7) | 0.0782 (8) | −0.0137 (6) | −0.0071 (7) | −0.0319 (6) |
| C35 | 0.0401 (7) | 0.0345 (7) | 0.0500 (8) | −0.0062 (5) | −0.0032 (6) | −0.0102 (6) |
| C36 | 0.0411 (7) | 0.0395 (7) | 0.0382 (7) | −0.0061 (6) | 0.0004 (5) | −0.0050 (5) |
| N36 | 0.0847 (11) | 0.0435 (8) | 0.0447 (8) | −0.0127 (7) | 0.0056 (7) | −0.0060 (6) |
| O36 | 0.1144 (18) | 0.0577 (12) | 0.0612 (10) | 0.0080 (12) | −0.0148 (11) | 0.0118 (8) |
| O37 | 0.153 (2) | 0.0750 (15) | 0.0736 (12) | 0.0185 (14) | 0.0626 (14) | 0.0073 (10) |
| N37 | 0.0847 (11) | 0.0435 (8) | 0.0447 (8) | −0.0127 (7) | 0.0056 (7) | −0.0060 (6) |
| O38 | 0.116 (10) | 0.076 (9) | 0.079 (9) | −0.015 (9) | 0.010 (9) | −0.003 (9) |
| O39 | 0.095 (9) | 0.072 (9) | 0.051 (8) | 0.024 (8) | −0.021 (7) | 0.025 (8) |
| N38 | 0.0847 (11) | 0.0435 (8) | 0.0447 (8) | −0.0127 (7) | 0.0056 (7) | −0.0060 (6) |
| O310 | 0.119 (10) | 0.042 (7) | 0.056 (7) | −0.011 (8) | −0.004 (8) | −0.001 (6) |
| O311 | 0.148 (10) | 0.055 (8) | 0.045 (7) | −0.019 (8) | 0.014 (8) | −0.015 (6) |
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Geometric parameters (Å, º)
| N1—C6 | 1.484 (2) | C25—C26 | 1.397 (2) |
| N1—C2 | 1.4866 (19) | C25—H25 | 0.9300 |
| N1—H11 | 0.869 (18) | C26—H26 | 0.9300 |
| N1—H12 | 0.900 (19) | O22—C27 | 1.417 (2) |
| C2—C3 | 1.506 (2) | C27—H27A | 0.9600 |
| C2—H2A | 0.9700 | C27—H27B | 0.9600 |
| C2—H2B | 0.9700 | C27—H27C | 0.9600 |
| C3—N4 | 1.4661 (18) | C31—O31 | 1.2450 (15) |
| C3—H3A | 0.9700 | C31—C32 | 1.4430 (18) |
| C3—H3B | 0.9700 | C31—C36 | 1.4492 (19) |
| N4—C21 | 1.4274 (17) | C32—C33 | 1.3749 (18) |
| N4—C5 | 1.4601 (18) | C32—N32 | 1.4583 (17) |
| C5—C6 | 1.508 (2) | N32—O32 | 1.2089 (16) |
| C5—H5A | 0.9700 | N32—O33 | 1.2161 (16) |
| C5—H5B | 0.9700 | C33—C34 | 1.3777 (19) |
| C6—H6A | 0.9700 | C33—H33 | 0.9300 |
| C6—H6B | 0.9700 | C34—C35 | 1.3892 (19) |
| C21—C26 | 1.382 (2) | C34—N34 | 1.4454 (17) |
| C21—C22 | 1.411 (2) | N34—O34 | 1.2260 (17) |
| C22—O22 | 1.358 (2) | N34—O35 | 1.2326 (17) |
| C22—C23 | 1.388 (2) | C35—C36 | 1.3623 (19) |
| C23—C24 | 1.376 (3) | C35—H35 | 0.9300 |
| C23—H23 | 0.9300 | C36—N36 | 1.4643 (19) |
| C24—C25 | 1.367 (3) | N36—O36 | 1.199 (2) |
| C24—H24 | 0.9300 | N36—O37 | 1.214 (2) |
| C6—N1—C2 | 111.24 (11) | C25—C24—H24 | 119.8 |
| C6—N1—H11 | 109.0 (11) | C23—C24—H24 | 119.8 |
| C2—N1—H11 | 110.2 (11) | C24—C25—C26 | 120.14 (18) |
| C6—N1—H12 | 109.4 (11) | C24—C25—H25 | 119.9 |
| C2—N1—H12 | 108.7 (11) | C26—C25—H25 | 119.9 |
| H11—N1—H12 | 108.3 (15) | C21—C26—C25 | 120.64 (18) |
| N1—C2—C3 | 110.41 (12) | C21—C26—H26 | 119.7 |
| N1—C2—H2A | 109.6 | C25—C26—H26 | 119.7 |
| C3—C2—H2A | 109.6 | C22—O22—C27 | 119.05 (13) |
| N1—C2—H2B | 109.6 | O22—C27—H27A | 109.5 |
| C3—C2—H2B | 109.6 | O22—C27—H27B | 109.5 |
| H2A—C2—H2B | 108.1 | H27A—C27—H27B | 109.5 |
| N4—C3—C2 | 110.51 (13) | O22—C27—H27C | 109.5 |
| N4—C3—H3A | 109.5 | H27A—C27—H27C | 109.5 |
| C2—C3—H3A | 109.5 | H27B—C27—H27C | 109.5 |
| N4—C3—H3B | 109.5 | O31—C31—C32 | 126.64 (12) |
| C2—C3—H3B | 109.5 | O31—C31—C36 | 121.75 (12) |
| H3A—C3—H3B | 108.1 | C32—C31—C36 | 111.56 (11) |
| C21—N4—C5 | 115.52 (11) | C33—C32—C31 | 123.98 (12) |
| C21—N4—C3 | 113.44 (11) | C33—C32—N32 | 116.28 (11) |
| C5—N4—C3 | 109.59 (11) | C31—C32—N32 | 119.70 (11) |
| N4—C5—C6 | 109.95 (12) | O32—N32—O33 | 122.28 (13) |
| N4—C5—H5A | 109.7 | O32—N32—C32 | 119.94 (12) |
| C6—C5—H5A | 109.7 | O33—N32—C32 | 117.78 (12) |
| N4—C5—H5B | 109.7 | C32—C33—C34 | 119.57 (12) |
| C6—C5—H5B | 109.7 | C32—C33—H33 | 120.2 |
| H5A—C5—H5B | 108.2 | C34—C33—H33 | 120.2 |
| N1—C6—C5 | 109.85 (12) | C33—C34—C35 | 121.15 (12) |
| N1—C6—H6A | 109.7 | C33—C34—N34 | 119.23 (12) |
| C5—C6—H6A | 109.7 | C35—C34—N34 | 119.56 (12) |
| N1—C6—H6B | 109.7 | O34—N34—O35 | 122.70 (13) |
| C5—C6—H6B | 109.7 | O34—N34—C34 | 118.86 (13) |
| H6A—C6—H6B | 108.2 | O35—N34—C34 | 118.41 (13) |
| C26—C21—C22 | 118.49 (13) | C36—C35—C34 | 118.47 (12) |
| C26—C21—N4 | 122.91 (14) | C36—C35—H35 | 120.8 |
| C22—C21—N4 | 118.58 (13) | C34—C35—H35 | 120.8 |
| O22—C22—C23 | 123.92 (16) | C35—C36—C31 | 125.23 (12) |
| O22—C22—C21 | 116.04 (12) | C35—C36—N36 | 117.48 (12) |
| C23—C22—C21 | 120.03 (16) | C31—C36—N36 | 117.20 (12) |
| C24—C23—C22 | 120.29 (18) | O36—N36—O37 | 124.53 (18) |
| C24—C23—H23 | 119.9 | O36—N36—C36 | 118.10 (16) |
| C22—C23—H23 | 119.9 | O37—N36—C36 | 117.11 (16) |
| C25—C24—C23 | 120.37 (16) | ||
| C6—N1—C2—C3 | 54.43 (18) | O31—C31—C32—N32 | −0.5 (2) |
| N1—C2—C3—N4 | −56.58 (17) | C36—C31—C32—N32 | −177.72 (11) |
| C2—C3—N4—C21 | −168.88 (12) | C33—C32—N32—O32 | 170.90 (15) |
| C2—C3—N4—C5 | 60.37 (16) | C31—C32—N32—O32 | −11.3 (2) |
| C21—N4—C5—C6 | 168.83 (12) | C33—C32—N32—O33 | −9.62 (19) |
| C3—N4—C5—C6 | −61.53 (15) | C31—C32—N32—O33 | 168.23 (13) |
| C2—N1—C6—C5 | −55.55 (16) | C31—C32—C33—C34 | 0.0 (2) |
| N4—C5—C6—N1 | 59.07 (15) | N32—C32—C33—C34 | 177.78 (11) |
| C5—N4—C21—C26 | 17.82 (19) | C32—C33—C34—C35 | −1.2 (2) |
| C3—N4—C21—C26 | −109.92 (16) | C32—C33—C34—N34 | −178.48 (12) |
| C5—N4—C21—C22 | −160.45 (13) | C33—C34—N34—O34 | −3.1 (2) |
| C3—N4—C21—C22 | 71.82 (17) | C35—C34—N34—O34 | 179.59 (14) |
| C26—C21—C22—O22 | −179.42 (14) | C33—C34—N34—O35 | 175.20 (13) |
| N4—C21—C22—O22 | −1.1 (2) | C35—C34—N34—O35 | −2.1 (2) |
| C26—C21—C22—C23 | −0.3 (2) | C33—C34—C35—C36 | 2.4 (2) |
| N4—C21—C22—C23 | 178.00 (14) | N34—C34—C35—C36 | 179.67 (12) |
| O22—C22—C23—C24 | 178.03 (17) | C34—C35—C36—C31 | −2.6 (2) |
| C21—C22—C23—C24 | −1.0 (3) | C34—C35—C36—N36 | −179.06 (13) |
| C22—C23—C24—C25 | 1.0 (3) | O31—C31—C36—C35 | −176.00 (13) |
| C23—C24—C25—C26 | 0.4 (3) | C32—C31—C36—C35 | 1.36 (19) |
| C22—C21—C26—C25 | 1.7 (2) | O31—C31—C36—N36 | 0.5 (2) |
| N4—C21—C26—C25 | −176.60 (14) | C32—C31—C36—N36 | 177.86 (12) |
| C24—C25—C26—C21 | −1.7 (3) | C35—C36—N36—O36 | 44.8 (3) |
| C23—C22—O22—C27 | 13.9 (3) | C31—C36—N36—O36 | −132.0 (2) |
| C21—C22—O22—C27 | −167.10 (17) | C35—C36—N36—O37 | −129.6 (3) |
| O31—C31—C32—C33 | 177.16 (13) | C31—C36—N36—O37 | 53.7 (3) |
| C36—C31—C32—C33 | −0.05 (18) |
4-(2-Methoxyphenyl)piperazin-1-ium 2,4,6-trinitrophenolate (XII). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O33 | 0.868 (18) | 2.224 (18) | 2.9120 (19) | 136.1 (16) |
| N1—H12···O31i | 0.900 (18) | 1.833 (18) | 2.7142 (18) | 165.9 (16) |
| N1—H12···O32i | 0.900 (19) | 2.593 (17) | 3.154 (2) | 121.2 (13) |
| C6—H6A···O34ii | 0.97 | 2.56 | 3.423 (2) | 148 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x, y+1, z.
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Crystal data
| C11H17N2O+·C4H3O4− | Z = 4 |
| Mr = 308.33 | F(000) = 656 |
| Triclinic, P1 | Dx = 1.287 Mg m−3 |
| a = 11.1076 (6) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 11.1164 (6) Å | Cell parameters from 6817 reflections |
| c = 13.7649 (7) Å | θ = 2.6–27.9° |
| α = 80.353 (5)° | µ = 0.10 mm−1 |
| β = 78.353 (5)° | T = 296 K |
| γ = 74.406 (5)° | Block, orange |
| V = 1591.76 (16) Å3 | 0.48 × 0.40 × 0.36 mm |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 6817 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 4221 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.012 |
| ω scans | θmax = 27.9°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −14→11 |
| Tmin = 0.863, Tmax = 0.966 | k = −14→10 |
| 11727 measured reflections | l = −17→16 |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Refinement
| Refinement on F2 | Primary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.042 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.121 | w = 1/[σ2(Fo2) + (0.0662P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 6817 reflections | Δρmax = 0.15 e Å−3 |
| 415 parameters | Δρmin = −0.16 e Å−3 |
| 0 restraints |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N11 | 0.34622 (12) | 0.18907 (13) | 0.37745 (11) | 0.0623 (4) | |
| H111 | 0.2917 (16) | 0.2421 (16) | 0.4214 (12) | 0.075* | |
| H112 | 0.3338 (15) | 0.1085 (16) | 0.3969 (11) | 0.075* | |
| C12 | 0.47834 (15) | 0.19077 (16) | 0.38284 (12) | 0.0642 (4) | |
| H12A | 0.4969 | 0.1564 | 0.4493 | 0.077* | |
| H12B | 0.4870 | 0.2768 | 0.3702 | 0.077* | |
| C13 | 0.57059 (14) | 0.11410 (15) | 0.30673 (11) | 0.0570 (4) | |
| H13A | 0.6560 | 0.1195 | 0.3080 | 0.068* | |
| H13B | 0.5675 | 0.0265 | 0.3231 | 0.068* | |
| N14 | 0.53919 (10) | 0.16040 (11) | 0.20732 (8) | 0.0467 (3) | |
| C15 | 0.41371 (12) | 0.14755 (14) | 0.20256 (12) | 0.0562 (4) | |
| H15A | 0.4102 | 0.0602 | 0.2201 | 0.067* | |
| H15B | 0.3954 | 0.1744 | 0.1352 | 0.067* | |
| C16 | 0.31747 (14) | 0.22734 (15) | 0.27418 (13) | 0.0616 (4) | |
| H16A | 0.3182 | 0.3151 | 0.2542 | 0.074* | |
| H16B | 0.2335 | 0.2182 | 0.2724 | 0.074* | |
| C121 | 0.63719 (12) | 0.12322 (13) | 0.12699 (10) | 0.0455 (3) | |
| C122 | 0.74375 (13) | 0.17360 (14) | 0.10986 (11) | 0.0516 (4) | |
| C123 | 0.83917 (14) | 0.14329 (16) | 0.02954 (12) | 0.0636 (4) | |
| H123 | 0.9099 | 0.1764 | 0.0188 | 0.076* | |
| C124 | 0.83016 (15) | 0.06460 (16) | −0.03458 (12) | 0.0669 (5) | |
| H124 | 0.8946 | 0.0451 | −0.0884 | 0.080* | |
| C125 | 0.72766 (15) | 0.01536 (16) | −0.01957 (12) | 0.0654 (4) | |
| H125 | 0.7218 | −0.0375 | −0.0632 | 0.078* | |
| C126 | 0.63143 (14) | 0.04385 (14) | 0.06097 (11) | 0.0576 (4) | |
| H126 | 0.5618 | 0.0092 | 0.0709 | 0.069* | |
| O122 | 0.74475 (10) | 0.25199 (11) | 0.17621 (8) | 0.0716 (3) | |
| C127 | 0.84336 (17) | 0.31480 (17) | 0.15906 (15) | 0.0774 (5) | |
| H17A | 0.8314 | 0.3660 | 0.2115 | 0.116* | |
| H17B | 0.9234 | 0.2540 | 0.1580 | 0.116* | |
| H17C | 0.8424 | 0.3671 | 0.0960 | 0.116* | |
| N21 | 0.32610 (11) | 0.68791 (12) | 0.35465 (9) | 0.0506 (3) | |
| H211 | 0.2716 (14) | 0.7345 (14) | 0.4078 (11) | 0.061* | |
| H212 | 0.3068 (13) | 0.6135 (14) | 0.3614 (11) | 0.061* | |
| C22 | 0.45843 (14) | 0.66671 (15) | 0.37162 (11) | 0.0559 (4) | |
| H22A | 0.4692 | 0.6135 | 0.4343 | 0.067* | |
| H22B | 0.4753 | 0.7466 | 0.3763 | 0.067* | |
| C23 | 0.55121 (13) | 0.60521 (13) | 0.28769 (10) | 0.0510 (4) | |
| H23A | 0.6371 | 0.5971 | 0.2979 | 0.061* | |
| H23B | 0.5404 | 0.5216 | 0.2872 | 0.061* | |
| N24 | 0.53089 (10) | 0.68033 (10) | 0.19212 (8) | 0.0443 (3) | |
| C25 | 0.40353 (12) | 0.68965 (15) | 0.17454 (11) | 0.0548 (4) | |
| H25A | 0.3914 | 0.6062 | 0.1757 | 0.066* | |
| H25B | 0.3920 | 0.7357 | 0.1094 | 0.066* | |
| C26 | 0.30813 (13) | 0.75658 (14) | 0.25435 (11) | 0.0549 (4) | |
| H26A | 0.3179 | 0.8414 | 0.2509 | 0.066* | |
| H26B | 0.2231 | 0.7622 | 0.2434 | 0.066* | |
| C221 | 0.63168 (12) | 0.64911 (13) | 0.11180 (10) | 0.0454 (3) | |
| C222 | 0.74589 (13) | 0.68107 (14) | 0.11058 (11) | 0.0505 (4) | |
| C223 | 0.84489 (15) | 0.65473 (16) | 0.03183 (13) | 0.0660 (5) | |
| H223 | 0.9205 | 0.6760 | 0.0311 | 0.079* | |
| C224 | 0.83202 (17) | 0.59725 (17) | −0.04533 (13) | 0.0722 (5) | |
| H224 | 0.8991 | 0.5797 | −0.0977 | 0.087* | |
| C225 | 0.72259 (17) | 0.56616 (17) | −0.04537 (12) | 0.0735 (5) | |
| H225 | 0.7143 | 0.5275 | −0.0977 | 0.088* | |
| C226 | 0.62268 (16) | 0.59194 (16) | 0.03260 (11) | 0.0641 (4) | |
| H226 | 0.5477 | 0.5703 | 0.0317 | 0.077* | |
| O222 | 0.75012 (10) | 0.73834 (11) | 0.18954 (8) | 0.0683 (3) | |
| C227 | 0.85515 (17) | 0.78893 (18) | 0.18703 (14) | 0.0806 (6) | |
| H27A | 0.8449 | 0.8259 | 0.2473 | 0.121* | |
| H27B | 0.9316 | 0.7230 | 0.1816 | 0.121* | |
| H27C | 0.8601 | 0.8521 | 0.1305 | 0.121* | |
| C31 | 0.21393 (13) | 0.42548 (13) | 0.52990 (11) | 0.0491 (3) | |
| O31 | 0.31437 (10) | 0.44663 (10) | 0.48375 (9) | 0.0721 (3) | |
| O32 | 0.17455 (10) | 0.33051 (9) | 0.52076 (8) | 0.0630 (3) | |
| C32 | 0.13422 (14) | 0.51635 (13) | 0.59942 (11) | 0.0527 (4) | |
| H32 | 0.1710 | 0.5799 | 0.6060 | 0.063* | |
| C33 | 0.01974 (14) | 0.52237 (13) | 0.65358 (11) | 0.0515 (4) | |
| H331 | −0.0094 | 0.5890 | 0.6919 | 0.062* | |
| C34 | −0.06961 (14) | 0.44152 (13) | 0.66358 (11) | 0.0493 (4) | |
| O33 | −0.03904 (10) | 0.34388 (9) | 0.61545 (8) | 0.0587 (3) | |
| H33 | 0.0541 (18) | 0.3376 (15) | 0.5724 (13) | 0.088* | |
| O34 | −0.17286 (11) | 0.46868 (11) | 0.71631 (9) | 0.0830 (4) | |
| C41 | 0.21753 (13) | 0.92641 (13) | 0.50681 (10) | 0.0472 (3) | |
| O41 | 0.31284 (10) | 0.95022 (9) | 0.45083 (8) | 0.0676 (3) | |
| O42 | 0.18296 (10) | 0.82652 (9) | 0.50841 (8) | 0.0661 (3) | |
| C42 | 0.14234 (14) | 1.02060 (13) | 0.57458 (11) | 0.0554 (4) | |
| H42 | 0.1724 | 1.0926 | 0.5676 | 0.067* | |
| C43 | 0.03981 (13) | 1.01999 (13) | 0.64339 (11) | 0.0536 (4) | |
| H431 | 0.0104 | 1.0921 | 0.6756 | 0.064* | |
| C44 | −0.03624 (14) | 0.92597 (13) | 0.67828 (11) | 0.0525 (4) | |
| O43 | −0.01234 (11) | 0.82687 (10) | 0.63191 (9) | 0.0756 (4) | |
| H43 | 0.066 (2) | 0.8219 (18) | 0.5806 (15) | 0.113* | |
| O44 | −0.12061 (12) | 0.94251 (11) | 0.74866 (9) | 0.0869 (4) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N11 | 0.0566 (8) | 0.0488 (8) | 0.0713 (10) | −0.0164 (6) | 0.0283 (7) | −0.0197 (7) |
| C12 | 0.0610 (10) | 0.0759 (11) | 0.0497 (9) | −0.0144 (8) | 0.0086 (8) | −0.0156 (8) |
| C13 | 0.0480 (9) | 0.0685 (10) | 0.0474 (9) | −0.0093 (7) | 0.0035 (7) | −0.0095 (7) |
| N14 | 0.0351 (6) | 0.0597 (7) | 0.0451 (7) | −0.0136 (5) | 0.0027 (5) | −0.0138 (6) |
| C15 | 0.0391 (8) | 0.0619 (9) | 0.0705 (10) | −0.0165 (7) | 0.0024 (7) | −0.0226 (8) |
| C16 | 0.0389 (8) | 0.0622 (10) | 0.0817 (12) | −0.0128 (7) | 0.0077 (8) | −0.0233 (9) |
| C121 | 0.0390 (7) | 0.0525 (8) | 0.0417 (8) | −0.0080 (6) | −0.0006 (6) | −0.0092 (6) |
| C122 | 0.0445 (8) | 0.0603 (9) | 0.0483 (8) | −0.0159 (7) | 0.0038 (7) | −0.0111 (7) |
| C123 | 0.0460 (9) | 0.0804 (11) | 0.0586 (10) | −0.0186 (8) | 0.0103 (7) | −0.0097 (9) |
| C124 | 0.0546 (10) | 0.0855 (12) | 0.0474 (9) | −0.0003 (9) | 0.0066 (7) | −0.0172 (9) |
| C125 | 0.0637 (11) | 0.0742 (11) | 0.0550 (10) | 0.0003 (8) | −0.0073 (8) | −0.0296 (8) |
| C126 | 0.0500 (9) | 0.0654 (10) | 0.0600 (10) | −0.0121 (7) | −0.0065 (7) | −0.0211 (8) |
| O122 | 0.0621 (7) | 0.0907 (8) | 0.0735 (8) | −0.0425 (6) | 0.0152 (6) | −0.0346 (7) |
| C127 | 0.0680 (11) | 0.0796 (12) | 0.0960 (14) | −0.0367 (10) | −0.0123 (10) | −0.0126 (10) |
| N21 | 0.0459 (7) | 0.0451 (7) | 0.0560 (8) | −0.0138 (6) | 0.0135 (6) | −0.0152 (6) |
| C22 | 0.0519 (9) | 0.0666 (10) | 0.0432 (8) | −0.0120 (7) | 0.0029 (7) | −0.0064 (7) |
| C23 | 0.0446 (8) | 0.0558 (9) | 0.0463 (8) | −0.0096 (7) | 0.0019 (6) | −0.0049 (7) |
| N24 | 0.0362 (6) | 0.0554 (7) | 0.0402 (6) | −0.0131 (5) | 0.0009 (5) | −0.0085 (5) |
| C25 | 0.0415 (8) | 0.0714 (10) | 0.0518 (9) | −0.0156 (7) | −0.0011 (7) | −0.0133 (7) |
| C26 | 0.0394 (8) | 0.0578 (9) | 0.0632 (10) | −0.0095 (7) | 0.0004 (7) | −0.0103 (8) |
| C221 | 0.0416 (8) | 0.0494 (8) | 0.0431 (8) | −0.0128 (6) | 0.0017 (6) | −0.0076 (6) |
| C222 | 0.0456 (8) | 0.0556 (9) | 0.0497 (9) | −0.0182 (7) | 0.0020 (7) | −0.0063 (7) |
| C223 | 0.0464 (9) | 0.0781 (11) | 0.0677 (11) | −0.0225 (8) | 0.0116 (8) | −0.0063 (9) |
| C224 | 0.0671 (11) | 0.0813 (12) | 0.0551 (10) | −0.0155 (9) | 0.0207 (9) | −0.0132 (9) |
| C225 | 0.0785 (12) | 0.0898 (13) | 0.0520 (10) | −0.0218 (10) | 0.0078 (9) | −0.0288 (9) |
| C226 | 0.0621 (10) | 0.0839 (12) | 0.0530 (10) | −0.0279 (9) | 0.0021 (8) | −0.0242 (9) |
| O222 | 0.0581 (7) | 0.0936 (8) | 0.0664 (7) | −0.0409 (6) | 0.0024 (5) | −0.0248 (6) |
| C227 | 0.0703 (12) | 0.0969 (14) | 0.0911 (14) | −0.0433 (10) | −0.0245 (10) | −0.0056 (11) |
| C31 | 0.0442 (8) | 0.0477 (8) | 0.0536 (9) | −0.0101 (6) | −0.0053 (7) | −0.0065 (7) |
| O31 | 0.0473 (6) | 0.0693 (7) | 0.0955 (9) | −0.0180 (5) | 0.0097 (6) | −0.0181 (6) |
| O32 | 0.0595 (7) | 0.0564 (6) | 0.0738 (7) | −0.0217 (5) | 0.0168 (5) | −0.0306 (5) |
| C32 | 0.0555 (9) | 0.0422 (8) | 0.0653 (10) | −0.0176 (7) | −0.0070 (8) | −0.0143 (7) |
| C33 | 0.0582 (9) | 0.0408 (8) | 0.0558 (9) | −0.0123 (7) | 0.0004 (7) | −0.0180 (7) |
| C34 | 0.0545 (9) | 0.0416 (8) | 0.0479 (8) | −0.0125 (6) | 0.0058 (7) | −0.0107 (6) |
| O33 | 0.0557 (6) | 0.0550 (6) | 0.0687 (7) | −0.0246 (5) | 0.0121 (5) | −0.0255 (5) |
| O34 | 0.0722 (8) | 0.0720 (8) | 0.0981 (9) | −0.0285 (6) | 0.0364 (7) | −0.0352 (7) |
| C41 | 0.0459 (8) | 0.0441 (8) | 0.0486 (8) | −0.0095 (6) | −0.0021 (7) | −0.0065 (6) |
| O41 | 0.0577 (7) | 0.0555 (6) | 0.0801 (8) | −0.0158 (5) | 0.0177 (6) | −0.0139 (6) |
| O42 | 0.0704 (7) | 0.0537 (6) | 0.0733 (7) | −0.0257 (5) | 0.0230 (6) | −0.0303 (5) |
| C42 | 0.0574 (9) | 0.0424 (8) | 0.0688 (10) | −0.0190 (7) | 0.0029 (8) | −0.0180 (7) |
| C43 | 0.0555 (9) | 0.0433 (8) | 0.0620 (9) | −0.0114 (7) | 0.0019 (7) | −0.0214 (7) |
| C44 | 0.0541 (9) | 0.0486 (8) | 0.0521 (9) | −0.0119 (7) | 0.0051 (7) | −0.0161 (7) |
| O43 | 0.0816 (8) | 0.0597 (7) | 0.0857 (9) | −0.0368 (6) | 0.0332 (7) | −0.0343 (6) |
| O44 | 0.0886 (9) | 0.0869 (9) | 0.0816 (9) | −0.0369 (7) | 0.0373 (7) | −0.0372 (7) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Geometric parameters (Å, º)
| N11—C16 | 1.487 (2) | N24—C221 | 1.4199 (15) |
| N11—C12 | 1.490 (2) | N24—C25 | 1.4566 (17) |
| N11—H111 | 0.927 (17) | C25—C26 | 1.5045 (18) |
| N11—H112 | 0.930 (16) | C25—H25A | 0.9700 |
| C12—C13 | 1.5068 (19) | C25—H25B | 0.9700 |
| C12—H12A | 0.9700 | C26—H26A | 0.9700 |
| C12—H12B | 0.9700 | C26—H26B | 0.9700 |
| C13—N14 | 1.4556 (18) | C221—C226 | 1.383 (2) |
| C13—H13A | 0.9700 | C221—C222 | 1.4027 (18) |
| C13—H13B | 0.9700 | C222—O222 | 1.3629 (17) |
| N14—C121 | 1.4155 (16) | C222—C223 | 1.3863 (19) |
| N14—C15 | 1.4541 (16) | C223—C224 | 1.376 (2) |
| C15—C16 | 1.5031 (19) | C223—H223 | 0.9300 |
| C15—H15A | 0.9700 | C224—C225 | 1.350 (2) |
| C15—H15B | 0.9700 | C224—H224 | 0.9300 |
| C16—H16A | 0.9700 | C225—C226 | 1.384 (2) |
| C16—H16B | 0.9700 | C225—H225 | 0.9300 |
| C121—C126 | 1.3896 (19) | C226—H226 | 0.9300 |
| C121—C122 | 1.4034 (18) | O222—C227 | 1.4172 (17) |
| C122—O122 | 1.3673 (17) | C227—H27A | 0.9600 |
| C122—C123 | 1.3853 (19) | C227—H27B | 0.9600 |
| C123—C124 | 1.377 (2) | C227—H27C | 0.9600 |
| C123—H123 | 0.9300 | C31—O31 | 1.2281 (15) |
| C124—C125 | 1.357 (2) | C31—O32 | 1.2793 (16) |
| C124—H124 | 0.9300 | C31—C32 | 1.4904 (19) |
| C125—C126 | 1.3889 (19) | C32—C33 | 1.3287 (18) |
| C125—H125 | 0.9300 | C32—H32 | 0.9300 |
| C126—H126 | 0.9300 | C33—C34 | 1.4814 (19) |
| O122—C127 | 1.4123 (16) | C33—H331 | 0.9300 |
| C127—H17A | 0.9600 | C34—O34 | 1.2185 (16) |
| C127—H17B | 0.9600 | C34—O33 | 1.2977 (16) |
| C127—H17C | 0.9600 | O33—H33 | 1.074 (19) |
| N21—C22 | 1.4848 (19) | C41—O41 | 1.2400 (15) |
| N21—C26 | 1.4849 (19) | C41—O42 | 1.2643 (15) |
| N21—H211 | 0.974 (16) | C41—C42 | 1.4855 (19) |
| N21—H212 | 0.894 (14) | C42—C43 | 1.3269 (18) |
| C22—C23 | 1.5064 (18) | C42—H42 | 0.9300 |
| C22—H22A | 0.9700 | C43—C44 | 1.4762 (19) |
| C22—H22B | 0.9700 | C43—H431 | 0.9300 |
| C23—N24 | 1.4592 (17) | C44—O44 | 1.2046 (16) |
| C23—H23A | 0.9700 | C44—O43 | 1.3044 (16) |
| C23—H23B | 0.9700 | O43—H43 | 1.00 (2) |
| C16—N11—C12 | 111.87 (11) | C22—C23—H23A | 109.6 |
| C16—N11—H111 | 111.2 (11) | N24—C23—H23B | 109.6 |
| C12—N11—H111 | 107.9 (10) | C22—C23—H23B | 109.6 |
| C16—N11—H112 | 106.9 (10) | H23A—C23—H23B | 108.1 |
| C12—N11—H112 | 110.6 (10) | C221—N24—C25 | 116.82 (11) |
| H111—N11—H112 | 108.4 (13) | C221—N24—C23 | 114.50 (11) |
| N11—C12—C13 | 110.06 (13) | C25—N24—C23 | 110.09 (10) |
| N11—C12—H12A | 109.6 | N24—C25—C26 | 109.32 (12) |
| C13—C12—H12A | 109.6 | N24—C25—H25A | 109.8 |
| N11—C12—H12B | 109.6 | C26—C25—H25A | 109.8 |
| C13—C12—H12B | 109.6 | N24—C25—H25B | 109.8 |
| H12A—C12—H12B | 108.2 | C26—C25—H25B | 109.8 |
| N14—C13—C12 | 110.14 (12) | H25A—C25—H25B | 108.3 |
| N14—C13—H13A | 109.6 | N21—C26—C25 | 110.30 (12) |
| C12—C13—H13A | 109.6 | N21—C26—H26A | 109.6 |
| N14—C13—H13B | 109.6 | C25—C26—H26A | 109.6 |
| C12—C13—H13B | 109.6 | N21—C26—H26B | 109.6 |
| H13A—C13—H13B | 108.1 | C25—C26—H26B | 109.6 |
| C121—N14—C15 | 117.32 (11) | H26A—C26—H26B | 108.1 |
| C121—N14—C13 | 115.49 (11) | C226—C221—C222 | 117.72 (12) |
| C15—N14—C13 | 110.45 (11) | C226—C221—N24 | 123.68 (12) |
| N14—C15—C16 | 108.98 (12) | C222—C221—N24 | 118.57 (12) |
| N14—C15—H15A | 109.9 | O222—C222—C223 | 124.36 (13) |
| C16—C15—H15A | 109.9 | O222—C222—C221 | 115.74 (12) |
| N14—C15—H15B | 109.9 | C223—C222—C221 | 119.90 (14) |
| C16—C15—H15B | 109.9 | C224—C223—C222 | 120.46 (14) |
| H15A—C15—H15B | 108.3 | C224—C223—H223 | 119.8 |
| N11—C16—C15 | 110.40 (13) | C222—C223—H223 | 119.8 |
| N11—C16—H16A | 109.6 | C225—C224—C223 | 120.36 (14) |
| C15—C16—H16A | 109.6 | C225—C224—H224 | 119.8 |
| N11—C16—H16B | 109.6 | C223—C224—H224 | 119.8 |
| C15—C16—H16B | 109.6 | C224—C225—C226 | 119.96 (15) |
| H16A—C16—H16B | 108.1 | C224—C225—H225 | 120.0 |
| C126—C121—C122 | 117.83 (12) | C226—C225—H225 | 120.0 |
| C126—C121—N14 | 123.65 (12) | C221—C226—C225 | 121.60 (14) |
| C122—C121—N14 | 118.45 (12) | C221—C226—H226 | 119.2 |
| O122—C122—C123 | 124.19 (13) | C225—C226—H226 | 119.2 |
| O122—C122—C121 | 115.76 (11) | C222—O222—C227 | 119.00 (12) |
| C123—C122—C121 | 120.05 (14) | O222—C227—H27A | 109.5 |
| C124—C123—C122 | 120.56 (14) | O222—C227—H27B | 109.5 |
| C124—C123—H123 | 119.7 | H27A—C227—H27B | 109.5 |
| C122—C123—H123 | 119.7 | O222—C227—H27C | 109.5 |
| C125—C124—C123 | 120.29 (14) | H27A—C227—H27C | 109.5 |
| C125—C124—H124 | 119.9 | H27B—C227—H27C | 109.5 |
| C123—C124—H124 | 119.9 | O31—C31—O32 | 123.42 (13) |
| C124—C125—C126 | 120.03 (14) | O31—C31—C32 | 117.74 (13) |
| C124—C125—H125 | 120.0 | O32—C31—C32 | 118.84 (12) |
| C126—C125—H125 | 120.0 | C31—O32—H33 | 111.0 (7) |
| C125—C126—C121 | 121.23 (14) | C33—C32—C31 | 131.17 (13) |
| C125—C126—H126 | 119.4 | C33—C32—H32 | 114.4 |
| C121—C126—H126 | 119.4 | C31—C32—H32 | 114.4 |
| C122—O122—C127 | 119.05 (12) | C32—C33—C34 | 131.27 (13) |
| O122—C127—H17A | 109.5 | C32—C33—H331 | 114.4 |
| O122—C127—H17B | 109.5 | C34—C33—H331 | 114.4 |
| H17A—C127—H17B | 109.5 | O34—C34—O33 | 120.32 (13) |
| O122—C127—H17C | 109.5 | O34—C34—C33 | 119.31 (13) |
| H17A—C127—H17C | 109.5 | O33—C34—C33 | 120.37 (12) |
| H17B—C127—H17C | 109.5 | C34—O33—H33 | 108.8 (9) |
| C22—N21—C26 | 111.73 (11) | O41—C41—O42 | 122.92 (13) |
| C22—N21—H211 | 106.8 (8) | O41—C41—C42 | 117.54 (12) |
| C26—N21—H211 | 111.6 (9) | O42—C41—C42 | 119.54 (12) |
| C22—N21—H212 | 108.9 (10) | C41—O42—H43 | 112.1 (8) |
| C26—N21—H212 | 110.2 (10) | C43—C42—C41 | 131.25 (13) |
| H211—N21—H212 | 107.5 (12) | C43—C42—H42 | 114.4 |
| N21—C22—C23 | 110.82 (12) | C41—C42—H42 | 114.4 |
| N21—C22—H22A | 109.5 | C42—C43—C44 | 131.86 (13) |
| C23—C22—H22A | 109.5 | C42—C43—H431 | 114.1 |
| N21—C22—H22B | 109.5 | C44—C43—H431 | 114.1 |
| C23—C22—H22B | 109.5 | O44—C44—O43 | 120.81 (13) |
| H22A—C22—H22B | 108.1 | O44—C44—C43 | 119.49 (13) |
| N24—C23—C22 | 110.15 (12) | O43—C44—C43 | 119.69 (12) |
| N24—C23—H23A | 109.6 | C44—O43—H43 | 111.0 (11) |
| C16—N11—C12—C13 | −52.90 (17) | C23—N24—C25—C26 | 62.57 (15) |
| N11—C12—C13—N14 | 56.09 (17) | C22—N21—C26—C25 | 54.13 (15) |
| C12—C13—N14—C121 | 161.93 (12) | N24—C25—C26—N21 | −58.61 (15) |
| C12—C13—N14—C15 | −61.98 (15) | C25—N24—C221—C226 | −20.0 (2) |
| C121—N14—C15—C16 | −162.19 (12) | C23—N24—C221—C226 | 110.76 (16) |
| C13—N14—C15—C16 | 62.61 (16) | C25—N24—C221—C222 | 157.98 (13) |
| C12—N11—C16—C15 | 54.36 (16) | C23—N24—C221—C222 | −71.22 (16) |
| N14—C15—C16—N11 | −58.23 (16) | C226—C221—C222—O222 | 179.36 (13) |
| C15—N14—C121—C126 | −19.4 (2) | N24—C221—C222—O222 | 1.2 (2) |
| C13—N14—C121—C126 | 113.61 (16) | C226—C221—C222—C223 | −0.2 (2) |
| C15—N14—C121—C122 | 157.54 (13) | N24—C221—C222—C223 | −178.34 (13) |
| C13—N14—C121—C122 | −69.47 (16) | O222—C222—C223—C224 | −179.56 (15) |
| C126—C121—C122—O122 | 179.46 (13) | C221—C222—C223—C224 | 0.0 (2) |
| N14—C121—C122—O122 | 2.4 (2) | C222—C223—C224—C225 | 0.2 (3) |
| C126—C121—C122—C123 | −0.3 (2) | C223—C224—C225—C226 | −0.1 (3) |
| N14—C121—C122—C123 | −177.42 (14) | C222—C221—C226—C225 | 0.3 (2) |
| O122—C122—C123—C124 | −179.23 (15) | N24—C221—C226—C225 | 178.33 (15) |
| C121—C122—C123—C124 | 0.5 (2) | C224—C225—C226—C221 | −0.2 (3) |
| C122—C123—C124—C125 | −0.2 (3) | C223—C222—O222—C227 | 8.1 (2) |
| C123—C124—C125—C126 | −0.2 (3) | C221—C222—O222—C227 | −171.41 (14) |
| C124—C125—C126—C121 | 0.5 (2) | O31—C31—C32—C33 | 175.34 (16) |
| C122—C121—C126—C125 | −0.2 (2) | O32—C31—C32—C33 | −4.3 (3) |
| N14—C121—C126—C125 | 176.77 (14) | C31—C32—C33—C34 | −0.5 (3) |
| C123—C122—O122—C127 | 5.1 (2) | C32—C33—C34—O34 | −177.55 (16) |
| C121—C122—O122—C127 | −174.63 (14) | C32—C33—C34—O33 | 1.4 (3) |
| C26—N21—C22—C23 | −52.60 (16) | O41—C41—C42—C43 | −177.97 (16) |
| N21—C22—C23—N24 | 55.64 (15) | O42—C41—C42—C43 | 1.8 (3) |
| C22—C23—N24—C221 | 164.83 (11) | C41—C42—C43—C44 | 0.5 (3) |
| C22—C23—N24—C25 | −61.17 (14) | C42—C43—C44—O44 | 173.80 (18) |
| C221—N24—C25—C26 | −164.61 (11) | C42—C43—C44—O43 | −7.0 (3) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen maleate (XIII). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O33—H33···O32 | 1.07 (2) | 1.37 (2) | 2.4447 (16) | 177.7 (16) |
| O43—H43···O42 | 1.00 (2) | 1.48 (2) | 2.4707 (17) | 174.0 (17) |
| N11—H111···O32 | 0.927 (17) | 1.891 (17) | 2.8122 (18) | 172.3 (16) |
| N11—H112···O41i | 0.930 (17) | 1.848 (17) | 2.7725 (17) | 172.9 (13) |
| N21—H211···O42 | 0.975 (15) | 1.821 (15) | 2.7926 (16) | 174.5 (14) |
| N21—H212···O31 | 0.895 (15) | 2.283 (15) | 2.9776 (17) | 134.4 (12) |
| N21—H212···O34ii | 0.895 (15) | 2.428 (15) | 3.1170 (18) | 134.1 (12) |
| C16—H16A···O34ii | 0.97 | 2.55 | 3.341 (2) | 138 |
| C16—H16B···O44ii | 0.97 | 2.52 | 3.338 (2) | 141 |
| C25—H25B···Cg4iii | 0.97 | 2.92 | 3.8440 (16) | 159 |
Symmetry codes: (i) x, y−1, z; (ii) −x, −y+1, −z+1; (iii) −x+1, −y, −z.
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Crystal data
| C11H17N2O+·C4H3O4− | Z = 2 |
| Mr = 308.33 | F(000) = 328 |
| Triclinic, P1 | Dx = 1.326 Mg m−3 |
| a = 7.8546 (4) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 8.9626 (6) Å | Cell parameters from 3307 reflections |
| c = 11.2056 (8) Å | θ = 2.6–27.8° |
| α = 79.043 (5)° | µ = 0.10 mm−1 |
| β = 87.715 (5)° | T = 296 K |
| γ = 85.840 (5)° | Block, colourless |
| V = 772.15 (9) Å3 | 0.48 × 0.48 × 0.34 mm |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 3307 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2608 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.009 |
| ω scans | θmax = 27.8°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −10→5 |
| Tmin = 0.867, Tmax = 0.967 | k = −11→11 |
| 5533 measured reflections | l = −13→14 |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.036 | w = 1/[σ2(Fo2) + (0.0552P)2 + 0.0881P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.105 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.20 e Å−3 |
| 3307 reflections | Δρmin = −0.15 e Å−3 |
| 240 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 6 restraints | Extinction coefficient: 0.022 (4) |
| Primary atom site location: difference Fourier map |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.47840 (14) | 0.33032 (13) | 0.75754 (9) | 0.0496 (3) | |
| H11 | 0.5555 | 0.3715 | 0.7951 | 0.059* | |
| H12 | 0.4236 | 0.2650 | 0.8131 | 0.059* | |
| C2 | 0.56542 (16) | 0.24842 (16) | 0.66646 (12) | 0.0521 (3) | |
| H2A | 0.6417 | 0.1661 | 0.7071 | 0.063* | |
| H2B | 0.6330 | 0.3178 | 0.6099 | 0.063* | |
| C3 | 0.43454 (16) | 0.18491 (14) | 0.59804 (12) | 0.0478 (3) | |
| H3A | 0.4922 | 0.1350 | 0.5367 | 0.057* | |
| H3B | 0.3740 | 0.1092 | 0.6540 | 0.057* | |
| N4 | 0.31104 (13) | 0.30552 (11) | 0.53920 (9) | 0.0435 (2) | |
| C5 | 0.22526 (17) | 0.38282 (15) | 0.63102 (12) | 0.0499 (3) | |
| H5A | 0.1632 | 0.3105 | 0.6890 | 0.060* | |
| H5B | 0.1438 | 0.4621 | 0.5923 | 0.060* | |
| C6 | 0.35447 (19) | 0.45212 (16) | 0.69650 (13) | 0.0561 (3) | |
| H6A | 0.4148 | 0.5262 | 0.6389 | 0.067* | |
| H6B | 0.2966 | 0.5042 | 0.7565 | 0.067* | |
| C21 | 0.19938 (15) | 0.25100 (13) | 0.46154 (11) | 0.0427 (3) | |
| C22 | 0.26637 (16) | 0.21262 (14) | 0.35255 (11) | 0.0463 (3) | |
| C23 | 0.16107 (18) | 0.15988 (17) | 0.27531 (13) | 0.0576 (3) | |
| H23 | 0.2063 | 0.1337 | 0.2035 | 0.069* | |
| C24 | −0.01099 (19) | 0.14591 (19) | 0.30429 (15) | 0.0649 (4) | |
| H24 | −0.0806 | 0.1102 | 0.2521 | 0.078* | |
| C25 | −0.07834 (18) | 0.18457 (18) | 0.40935 (15) | 0.0644 (4) | |
| H25 | −0.1940 | 0.1763 | 0.4283 | 0.077* | |
| C26 | 0.02619 (17) | 0.23620 (15) | 0.48775 (13) | 0.0532 (3) | |
| H26 | −0.0207 | 0.2615 | 0.5594 | 0.064* | |
| O22 | 0.43698 (12) | 0.23087 (12) | 0.32891 (8) | 0.0587 (3) | |
| C27 | 0.50598 (18) | 0.20342 (16) | 0.21566 (12) | 0.0541 (3) | |
| H27A | 0.6256 | 0.2200 | 0.2105 | 0.081* | |
| H27B | 0.4904 | 0.1001 | 0.2090 | 0.081* | |
| H27C | 0.4486 | 0.2715 | 0.1508 | 0.081* | |
| C31 | 0.7592 (6) | 0.4912 (5) | 0.9702 (5) | 0.0396 (11) | 0.572 (9) |
| O31 | 0.7521 (6) | 0.4322 (5) | 0.8811 (5) | 0.0587 (10) | 0.572 (9) |
| O32 | 0.6295 (6) | 0.5334 (6) | 1.0339 (5) | 0.0540 (9) | 0.572 (9) |
| H32 | 0.5412 | 0.5112 | 1.0068 | 0.081* | 0.286 |
| C32 | 0.9263 (5) | 0.5248 (3) | 1.0179 (3) | 0.0476 (11) | 0.572 (9) |
| H32A | 0.9320 | 0.6190 | 1.0756 | 0.057* | 0.572 (9) |
| C34 | 0.7860 (7) | 0.4556 (8) | 0.9314 (7) | 0.0410 (14) | 0.428 (9) |
| O33 | 0.7520 (8) | 0.4140 (9) | 0.8394 (6) | 0.0583 (12) | 0.428 (9) |
| O34 | 0.6784 (8) | 0.4985 (9) | 1.0107 (5) | 0.0557 (14) | 0.428 (9) |
| H34 | 0.5809 | 0.4886 | 0.9913 | 0.084* | 0.214 |
| C33 | 0.9707 (5) | 0.4644 (5) | 0.9604 (4) | 0.0472 (15) | 0.428 (9) |
| H33A | 1.0572 | 0.4552 | 0.8986 | 0.057* | 0.428 (9) |
| C41 | 0.24159 (13) | 0.01915 (13) | 0.97848 (10) | 0.0383 (3) | |
| O41 | 0.25523 (10) | 0.12259 (12) | 0.89098 (9) | 0.0615 (3) | |
| O42 | 0.36567 (10) | −0.05149 (12) | 1.04016 (8) | 0.0574 (3) | |
| H42 | 0.4557 | −0.0178 | 1.0112 | 0.086* | 0.5 |
| C42 | 0.07112 (13) | −0.03520 (14) | 1.02179 (10) | 0.0393 (3) | |
| H42A | 0.0657 | −0.1221 | 1.0818 | 0.047* |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0464 (6) | 0.0605 (7) | 0.0424 (5) | −0.0165 (5) | −0.0024 (4) | −0.0058 (5) |
| C2 | 0.0406 (7) | 0.0675 (8) | 0.0470 (7) | −0.0047 (6) | −0.0008 (5) | −0.0071 (6) |
| C3 | 0.0443 (7) | 0.0494 (7) | 0.0489 (7) | 0.0028 (5) | −0.0057 (5) | −0.0081 (5) |
| N4 | 0.0452 (6) | 0.0427 (5) | 0.0427 (5) | −0.0007 (4) | −0.0064 (4) | −0.0076 (4) |
| C5 | 0.0524 (7) | 0.0463 (7) | 0.0513 (7) | 0.0047 (5) | −0.0088 (6) | −0.0114 (5) |
| C6 | 0.0666 (9) | 0.0500 (7) | 0.0535 (8) | −0.0074 (6) | −0.0068 (6) | −0.0121 (6) |
| C21 | 0.0432 (6) | 0.0374 (6) | 0.0463 (6) | −0.0015 (5) | −0.0077 (5) | −0.0043 (5) |
| C22 | 0.0416 (6) | 0.0481 (7) | 0.0496 (7) | −0.0042 (5) | −0.0059 (5) | −0.0088 (5) |
| C23 | 0.0527 (8) | 0.0692 (9) | 0.0557 (8) | −0.0046 (6) | −0.0071 (6) | −0.0227 (7) |
| C24 | 0.0501 (8) | 0.0785 (10) | 0.0735 (10) | −0.0069 (7) | −0.0142 (7) | −0.0292 (8) |
| C25 | 0.0400 (7) | 0.0780 (10) | 0.0783 (10) | −0.0063 (7) | −0.0053 (7) | −0.0211 (8) |
| C26 | 0.0451 (7) | 0.0573 (8) | 0.0582 (8) | −0.0007 (6) | −0.0026 (6) | −0.0142 (6) |
| O22 | 0.0458 (5) | 0.0834 (7) | 0.0526 (5) | −0.0150 (5) | 0.0024 (4) | −0.0235 (5) |
| C27 | 0.0530 (8) | 0.0604 (8) | 0.0467 (7) | −0.0043 (6) | 0.0009 (6) | −0.0050 (6) |
| C31 | 0.035 (2) | 0.0441 (19) | 0.040 (3) | −0.0097 (16) | −0.0107 (19) | −0.0044 (15) |
| O31 | 0.0420 (13) | 0.0858 (19) | 0.060 (3) | −0.0113 (11) | −0.0172 (19) | −0.038 (2) |
| O32 | 0.0362 (19) | 0.081 (2) | 0.0480 (18) | −0.0149 (14) | −0.0069 (14) | −0.0143 (13) |
| C32 | 0.037 (2) | 0.0607 (15) | 0.0489 (17) | −0.0106 (11) | −0.0115 (12) | −0.0157 (12) |
| C34 | 0.033 (2) | 0.053 (3) | 0.039 (4) | −0.0108 (19) | −0.008 (2) | −0.011 (2) |
| O33 | 0.0441 (15) | 0.086 (3) | 0.054 (3) | −0.0188 (15) | −0.015 (2) | −0.028 (2) |
| O34 | 0.039 (4) | 0.089 (4) | 0.044 (3) | −0.017 (3) | −0.010 (2) | −0.0188 (19) |
| C33 | 0.033 (2) | 0.068 (2) | 0.044 (2) | −0.0125 (15) | −0.0099 (15) | −0.0141 (18) |
| C41 | 0.0237 (5) | 0.0535 (7) | 0.0386 (6) | −0.0081 (4) | 0.0017 (4) | −0.0091 (5) |
| O41 | 0.0273 (4) | 0.0793 (7) | 0.0671 (6) | −0.0118 (4) | −0.0007 (4) | 0.0162 (5) |
| O42 | 0.0222 (4) | 0.0826 (7) | 0.0590 (6) | −0.0101 (4) | −0.0031 (4) | 0.0110 (5) |
| C42 | 0.0254 (5) | 0.0516 (6) | 0.0407 (6) | −0.0093 (4) | 0.0010 (4) | −0.0058 (5) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Geometric parameters (Å, º)
| N1—C2 | 1.4845 (17) | C24—H24 | 0.9300 |
| N1—C6 | 1.4907 (17) | C25—C26 | 1.3883 (19) |
| N1—H11 | 0.8900 | C25—H25 | 0.9300 |
| N1—H12 | 0.8900 | C26—H26 | 0.9300 |
| C2—C3 | 1.5108 (18) | O22—C27 | 1.4175 (16) |
| C2—H2A | 0.9700 | C27—H27A | 0.9600 |
| C2—H2B | 0.9700 | C27—H27B | 0.9600 |
| C3—N4 | 1.4738 (15) | C27—H27C | 0.9600 |
| C3—H3A | 0.9700 | C31—O31 | 1.221 (4) |
| C3—H3B | 0.9700 | C31—O32 | 1.296 (5) |
| N4—C21 | 1.4318 (15) | C31—C32 | 1.508 (4) |
| N4—C5 | 1.4630 (16) | O32—H32 | 0.8200 |
| C5—C6 | 1.5093 (18) | C32—H32A | 1.1605 |
| C5—H5A | 0.9700 | C34—O33 | 1.207 (6) |
| C5—H5B | 0.9700 | C34—O34 | 1.294 (6) |
| C6—H6A | 0.9700 | C34—C33 | 1.510 (5) |
| C6—H6B | 0.9700 | O34—H34 | 0.8200 |
| C21—C26 | 1.3906 (18) | C33—H33A | 0.9611 |
| C21—C22 | 1.4033 (18) | C41—O41 | 1.2219 (14) |
| C22—O22 | 1.3710 (16) | C41—O42 | 1.2752 (14) |
| C22—C23 | 1.3877 (18) | C41—C42 | 1.4895 (14) |
| C23—C24 | 1.386 (2) | O42—H42 | 0.8200 |
| C23—H23 | 0.9300 | C42—C42i | 1.307 (2) |
| C24—C25 | 1.365 (2) | C42—H42A | 0.9300 |
| C2—N1—C6 | 110.03 (10) | C23—C22—C21 | 120.06 (12) |
| C2—N1—H11 | 109.7 | C24—C23—C22 | 120.53 (13) |
| C6—N1—H11 | 109.7 | C24—C23—H23 | 119.7 |
| C2—N1—H12 | 109.7 | C22—C23—H23 | 119.7 |
| C6—N1—H12 | 109.7 | C25—C24—C23 | 120.04 (13) |
| H11—N1—H12 | 108.2 | C25—C24—H24 | 120.0 |
| N1—C2—C3 | 109.88 (10) | C23—C24—H24 | 120.0 |
| N1—C2—H2A | 109.7 | C24—C25—C26 | 119.89 (13) |
| C3—C2—H2A | 109.7 | C24—C25—H25 | 120.1 |
| N1—C2—H2B | 109.7 | C26—C25—H25 | 120.1 |
| C3—C2—H2B | 109.7 | C25—C26—C21 | 121.48 (13) |
| H2A—C2—H2B | 108.2 | C25—C26—H26 | 119.3 |
| N4—C3—C2 | 111.53 (10) | C21—C26—H26 | 119.3 |
| N4—C3—H3A | 109.3 | C22—O22—C27 | 117.72 (10) |
| C2—C3—H3A | 109.3 | O22—C27—H27A | 109.5 |
| N4—C3—H3B | 109.3 | O22—C27—H27B | 109.5 |
| C2—C3—H3B | 109.3 | H27A—C27—H27B | 109.5 |
| H3A—C3—H3B | 108.0 | O22—C27—H27C | 109.5 |
| C21—N4—C5 | 115.02 (10) | H27A—C27—H27C | 109.5 |
| C21—N4—C3 | 112.31 (9) | H27B—C27—H27C | 109.5 |
| C5—N4—C3 | 109.65 (9) | O31—C31—O32 | 125.7 (4) |
| N4—C5—C6 | 110.19 (11) | O31—C31—C32 | 122.3 (5) |
| N4—C5—H5A | 109.6 | O32—C31—C32 | 112.0 (5) |
| C6—C5—H5A | 109.6 | C31—O32—H32 | 109.5 |
| N4—C5—H5B | 109.6 | C31—C32—H32A | 120.8 |
| C6—C5—H5B | 109.6 | O33—C34—O34 | 126.6 (6) |
| H5A—C5—H5B | 108.1 | O33—C34—C33 | 119.4 (6) |
| N1—C6—C5 | 109.74 (11) | O34—C34—C33 | 114.0 (5) |
| N1—C6—H6A | 109.7 | C34—O34—H34 | 109.5 |
| C5—C6—H6A | 109.7 | C34—C33—H33A | 119.0 |
| N1—C6—H6B | 109.7 | O41—C41—O42 | 125.06 (10) |
| C5—C6—H6B | 109.7 | O41—C41—C42 | 120.88 (10) |
| H6A—C6—H6B | 108.2 | O42—C41—C42 | 114.07 (10) |
| C26—C21—C22 | 118.00 (11) | C41—O42—H42 | 109.5 |
| C26—C21—N4 | 123.21 (11) | C42i—C42—C41 | 122.20 (14) |
| C22—C21—N4 | 118.79 (11) | C42i—C42—H42A | 118.9 |
| O22—C22—C23 | 123.60 (12) | C41—C42—H42A | 118.9 |
| O22—C22—C21 | 116.34 (10) | ||
| C6—N1—C2—C3 | −56.53 (14) | C26—C21—C22—C23 | −0.80 (19) |
| N1—C2—C3—N4 | 56.82 (14) | N4—C21—C22—C23 | −179.82 (11) |
| C2—C3—N4—C21 | 172.63 (10) | O22—C22—C23—C24 | −179.23 (14) |
| C2—C3—N4—C5 | −58.17 (13) | C21—C22—C23—C24 | 0.6 (2) |
| C21—N4—C5—C6 | −172.92 (10) | C22—C23—C24—C25 | 0.2 (2) |
| C3—N4—C5—C6 | 59.39 (13) | C23—C24—C25—C26 | −0.7 (2) |
| C2—N1—C6—C5 | 58.42 (14) | C24—C25—C26—C21 | 0.5 (2) |
| N4—C5—C6—N1 | −60.04 (14) | C22—C21—C26—C25 | 0.3 (2) |
| C5—N4—C21—C26 | −15.65 (17) | N4—C21—C26—C25 | 179.22 (12) |
| C3—N4—C21—C26 | 110.69 (13) | C23—C22—O22—C27 | 4.19 (19) |
| C5—N4—C21—C22 | 163.31 (11) | C21—C22—O22—C27 | −175.63 (11) |
| C3—N4—C21—C22 | −70.35 (14) | O41—C41—C42—C42i | −8.0 (2) |
| C26—C21—C22—O22 | 179.03 (11) | O42—C41—C42—C42i | 172.10 (15) |
| N4—C21—C22—O22 | 0.02 (17) |
Symmetry code: (i) −x, −y, −z+2.
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen fumarate (XIV). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.89 | 2.01 | 2.894 (5) | 171 |
| N1—H11···O33 | 0.89 | 1.73 | 2.584 (7) | 160 |
| N1—H12···O41 | 0.89 | 1.97 | 2.8251 (15) | 161 |
| O32—H32···O32ii | 0.82 | 1.54 | 2.355 (7) | 176 |
| O34—H34···O34ii | 0.82 | 2.03 | 2.820 (9) | 161 |
| O42—H42···O42iii | 0.82 | 1.62 | 2.4352 (12) | 177 |
Symmetry codes: (ii) −x+1, −y+1, −z+2; (iii) −x+1, −y, −z+2.
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Crystal data
| C11H17N2O+·C4H5O6−·1.698H2O | F(000) = 398 |
| Mr = 372.97 | Dx = 1.345 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.479 (1) Å | Cell parameters from 2896 reflections |
| b = 7.065 (1) Å | θ = 3.1–27.9° |
| c = 17.788 (3) Å | µ = 0.11 mm−1 |
| β = 101.58 (2)° | T = 296 K |
| V = 920.8 (2) Å3 | Plate, colourless |
| Z = 2 | 0.36 × 0.32 × 0.12 mm |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Data collection
| Oxford Diffraction Xcalibur with Sapphire CCD diffractometer | 2895 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2062 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.022 |
| ω scans | θmax = 27.9°, θmin = 3.1° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −9→9 |
| Tmin = 0.956, Tmax = 0.987 | k = −9→6 |
| 3655 measured reflections | l = −23→22 |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Refinement
| Refinement on F2 | Primary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.039 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.081 | w = 1/[σ2(Fo2) + (0.0402P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.97 | (Δ/σ)max < 0.001 |
| 2895 reflections | Δρmax = 0.14 e Å−3 |
| 263 parameters | Δρmin = −0.17 e Å−3 |
| 4 restraints | Absolute structure: Flack x determined using 493 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Special details
| Experimental. Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.4545 (5) | 0.3626 (4) | 0.31605 (16) | 0.0472 (7) | |
| H11 | 0.517 (5) | 0.295 (5) | 0.3458 (19) | 0.057* | |
| H12 | 0.341 (5) | 0.371 (6) | 0.3245 (18) | 0.057* | |
| C2 | 0.4519 (5) | 0.2806 (5) | 0.23968 (19) | 0.0514 (9) | |
| H2A | 0.3910 | 0.1588 | 0.2356 | 0.062* | |
| H2B | 0.5760 | 0.2611 | 0.2328 | 0.062* | |
| C3 | 0.3538 (5) | 0.4111 (4) | 0.17851 (18) | 0.0454 (8) | |
| H3A | 0.3539 | 0.3575 | 0.1283 | 0.055* | |
| H3B | 0.2280 | 0.4258 | 0.1839 | 0.055* | |
| N4 | 0.4437 (3) | 0.5952 (4) | 0.18516 (13) | 0.0393 (6) | |
| C5 | 0.4369 (5) | 0.6791 (5) | 0.25945 (16) | 0.0441 (8) | |
| H5A | 0.3106 | 0.6953 | 0.2639 | 0.053* | |
| H5B | 0.4941 | 0.8029 | 0.2633 | 0.053* | |
| C6 | 0.5333 (5) | 0.5553 (4) | 0.32330 (19) | 0.0521 (10) | |
| H6A | 0.6621 | 0.5488 | 0.3217 | 0.063* | |
| H6B | 0.5219 | 0.6093 | 0.3723 | 0.063* | |
| C21 | 0.3879 (5) | 0.7242 (5) | 0.12346 (16) | 0.0416 (8) | |
| C22 | 0.5099 (5) | 0.8649 (5) | 0.11148 (18) | 0.0515 (9) | |
| C23 | 0.4638 (6) | 0.9900 (6) | 0.0511 (2) | 0.0665 (11) | |
| H23 | 0.5453 | 1.0842 | 0.0435 | 0.080* | |
| C24 | 0.2984 (7) | 0.9750 (7) | 0.0025 (2) | 0.0749 (13) | |
| H24 | 0.2678 | 1.0590 | −0.0383 | 0.090* | |
| C25 | 0.1785 (6) | 0.8393 (7) | 0.0131 (2) | 0.0695 (12) | |
| H25 | 0.0662 | 0.8300 | −0.0205 | 0.083* | |
| C26 | 0.2227 (5) | 0.7140 (6) | 0.07395 (18) | 0.0546 (9) | |
| H26 | 0.1390 | 0.6219 | 0.0812 | 0.066* | |
| O22 | 0.6727 (4) | 0.8666 (4) | 0.16178 (15) | 0.0723 (8) | |
| C27 | 0.8158 (6) | 0.9858 (8) | 0.1471 (3) | 0.0936 (15) | |
| H27A | 0.9212 | 0.9712 | 0.1875 | 0.140* | |
| H27B | 0.8463 | 0.9511 | 0.0990 | 0.140* | |
| H27C | 0.7762 | 1.1153 | 0.1449 | 0.140* | |
| C31 | 0.8601 (4) | 0.0130 (4) | 0.38558 (16) | 0.0321 (7) | |
| O31 | 0.8264 (3) | 0.1842 (3) | 0.36938 (12) | 0.0422 (5) | |
| O32 | 0.7484 (3) | −0.1056 (3) | 0.39964 (13) | 0.0484 (6) | |
| C32 | 1.0532 (4) | −0.0580 (4) | 0.38922 (16) | 0.0300 (7) | |
| H32A | 1.0927 | −0.0187 | 0.3423 | 0.036* | |
| O33 | 1.0645 (3) | −0.2564 (3) | 0.39473 (13) | 0.0431 (6) | |
| H33 | 0.985 (5) | −0.285 (6) | 0.414 (2) | 0.065* | |
| C33 | 1.1809 (3) | 0.0309 (4) | 0.45784 (14) | 0.0292 (7) | |
| H33A | 1.1800 | 0.1684 | 0.4506 | 0.035* | |
| O34 | 1.1191 (3) | −0.0087 (3) | 0.52580 (11) | 0.0405 (5) | |
| H34 | 1.162 (5) | −0.109 (6) | 0.544 (2) | 0.061* | |
| C34 | 1.3725 (4) | −0.0400 (4) | 0.46138 (16) | 0.0307 (7) | |
| O35 | 1.4553 (3) | −0.1271 (3) | 0.51524 (11) | 0.0479 (6) | |
| O36 | 1.4319 (3) | −0.0004 (3) | 0.39858 (11) | 0.0351 (5) | |
| H36 | 1.537 (5) | −0.036 (6) | 0.4062 (17) | 0.053* | |
| O41 | 0.0854 (4) | 0.4055 (4) | 0.31999 (16) | 0.0612 (8) | |
| H41 | 0.068 (6) | 0.512 (7) | 0.336 (2) | 0.092* | |
| H42 | −0.004 (6) | 0.335 (7) | 0.329 (2) | 0.092* | |
| O51 | −0.1304 (7) | 0.4856 (9) | 0.1775 (2) | 0.104 (2) | 0.698 (9) |
| H51 | −0.051 (9) | 0.470 (13) | 0.228 (2) | 0.157* | 0.698 (9) |
| H52 | −0.225 (8) | 0.573 (10) | 0.185 (4) | 0.157* | 0.698 (9) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0576 (19) | 0.0355 (16) | 0.0460 (17) | −0.0002 (16) | 0.0043 (15) | 0.0093 (14) |
| C2 | 0.063 (2) | 0.0322 (18) | 0.061 (2) | 0.0033 (17) | 0.0179 (18) | 0.0013 (16) |
| C3 | 0.057 (2) | 0.0362 (19) | 0.0443 (18) | −0.0014 (17) | 0.0143 (15) | −0.0036 (14) |
| N4 | 0.0462 (16) | 0.0352 (14) | 0.0382 (14) | 0.0025 (13) | 0.0127 (12) | 0.0025 (12) |
| C5 | 0.060 (2) | 0.0325 (17) | 0.0389 (17) | −0.0028 (17) | 0.0080 (16) | 0.0031 (15) |
| C6 | 0.065 (2) | 0.040 (2) | 0.0468 (18) | −0.0102 (18) | −0.0007 (17) | 0.0083 (15) |
| C21 | 0.053 (2) | 0.0404 (19) | 0.0355 (16) | 0.0091 (16) | 0.0186 (15) | 0.0017 (15) |
| C22 | 0.064 (2) | 0.047 (2) | 0.0475 (19) | 0.002 (2) | 0.0208 (17) | 0.0074 (18) |
| C23 | 0.093 (3) | 0.054 (2) | 0.062 (2) | 0.005 (2) | 0.037 (2) | 0.015 (2) |
| C24 | 0.104 (4) | 0.067 (3) | 0.058 (2) | 0.024 (3) | 0.026 (2) | 0.026 (2) |
| C25 | 0.074 (3) | 0.077 (3) | 0.053 (2) | 0.018 (3) | 0.003 (2) | 0.009 (2) |
| C26 | 0.056 (2) | 0.057 (2) | 0.0502 (19) | 0.009 (2) | 0.0081 (17) | 0.0031 (19) |
| O22 | 0.0621 (16) | 0.0759 (19) | 0.0786 (17) | −0.0177 (16) | 0.0137 (14) | 0.0213 (16) |
| C27 | 0.078 (3) | 0.091 (3) | 0.118 (4) | −0.030 (3) | 0.034 (3) | 0.010 (3) |
| C31 | 0.0235 (14) | 0.0325 (18) | 0.0411 (15) | 0.0001 (14) | 0.0084 (12) | −0.0038 (14) |
| O31 | 0.0316 (11) | 0.0350 (13) | 0.0616 (13) | 0.0062 (10) | 0.0129 (10) | 0.0057 (11) |
| O32 | 0.0248 (10) | 0.0384 (13) | 0.0869 (16) | −0.0026 (10) | 0.0224 (10) | 0.0027 (12) |
| C32 | 0.0268 (15) | 0.0240 (15) | 0.0419 (15) | 0.0009 (12) | 0.0130 (12) | −0.0027 (13) |
| O33 | 0.0312 (12) | 0.0296 (12) | 0.0749 (16) | −0.0031 (10) | 0.0256 (11) | −0.0055 (11) |
| C33 | 0.0243 (14) | 0.0284 (16) | 0.0364 (15) | 0.0027 (12) | 0.0098 (12) | 0.0044 (13) |
| O34 | 0.0407 (12) | 0.0439 (14) | 0.0424 (11) | 0.0089 (11) | 0.0212 (10) | 0.0037 (11) |
| C34 | 0.0244 (14) | 0.0311 (15) | 0.0368 (15) | −0.0018 (13) | 0.0063 (13) | −0.0025 (14) |
| O35 | 0.0388 (12) | 0.0597 (15) | 0.0442 (12) | 0.0126 (12) | 0.0058 (10) | 0.0128 (12) |
| O36 | 0.0200 (9) | 0.0436 (13) | 0.0435 (11) | 0.0026 (10) | 0.0104 (9) | 0.0042 (10) |
| O41 | 0.0671 (17) | 0.0429 (16) | 0.0847 (18) | −0.0022 (14) | 0.0415 (14) | −0.0044 (13) |
| O51 | 0.104 (4) | 0.137 (5) | 0.072 (3) | 0.042 (4) | 0.020 (2) | 0.004 (3) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Geometric parameters (Å, º)
| N1—C2 | 1.473 (4) | C25—C26 | 1.386 (5) |
| N1—C6 | 1.479 (4) | C25—H25 | 0.9300 |
| N1—H11 | 0.79 (4) | C26—H26 | 0.9300 |
| N1—H12 | 0.90 (4) | O22—C27 | 1.427 (5) |
| C2—C3 | 1.500 (4) | C27—H27A | 0.9600 |
| C2—H2A | 0.9700 | C27—H27B | 0.9600 |
| C2—H2B | 0.9700 | C27—H27C | 0.9600 |
| C3—N4 | 1.458 (4) | C31—O32 | 1.244 (4) |
| C3—H3A | 0.9700 | C31—O31 | 1.257 (4) |
| C3—H3B | 0.9700 | C31—C32 | 1.518 (4) |
| N4—C21 | 1.423 (4) | C32—O33 | 1.406 (3) |
| N4—C5 | 1.458 (4) | C32—C33 | 1.526 (4) |
| C5—C6 | 1.499 (4) | C32—H32A | 0.9800 |
| C5—H5A | 0.9700 | O33—H33 | 0.78 (4) |
| C5—H5B | 0.9700 | C33—O34 | 1.406 (3) |
| C6—H6A | 0.9700 | C33—C34 | 1.508 (4) |
| C6—H6B | 0.9700 | C33—H33A | 0.9800 |
| C21—C26 | 1.368 (4) | O34—H34 | 0.82 (4) |
| C21—C22 | 1.394 (5) | C34—O35 | 1.201 (3) |
| C22—O22 | 1.359 (4) | C34—O36 | 1.312 (3) |
| C22—C23 | 1.381 (5) | O36—H36 | 0.81 (3) |
| C23—C24 | 1.363 (6) | O41—H41 | 0.83 (5) |
| C23—H23 | 0.9300 | O41—H42 | 0.88 (5) |
| C24—C25 | 1.352 (6) | O51—H51 | 0.98 (2) |
| C24—H24 | 0.9300 | O51—H52 | 0.97 (2) |
| C2—N1—C6 | 111.9 (3) | C22—C23—H23 | 120.1 |
| C2—N1—H11 | 106 (2) | C25—C24—C23 | 120.7 (4) |
| C6—N1—H11 | 109 (3) | C25—C24—H24 | 119.7 |
| C2—N1—H12 | 110 (2) | C23—C24—H24 | 119.7 |
| C6—N1—H12 | 107 (3) | C24—C25—C26 | 120.0 (4) |
| H11—N1—H12 | 112 (4) | C24—C25—H25 | 120.0 |
| N1—C2—C3 | 109.9 (3) | C26—C25—H25 | 120.0 |
| N1—C2—H2A | 109.7 | C21—C26—C25 | 120.9 (4) |
| C3—C2—H2A | 109.7 | C21—C26—H26 | 119.6 |
| N1—C2—H2B | 109.7 | C25—C26—H26 | 119.6 |
| C3—C2—H2B | 109.7 | C22—O22—C27 | 119.4 (3) |
| H2A—C2—H2B | 108.2 | O22—C27—H27A | 109.5 |
| N4—C3—C2 | 109.8 (3) | O22—C27—H27B | 109.5 |
| N4—C3—H3A | 109.7 | H27A—C27—H27B | 109.5 |
| C2—C3—H3A | 109.7 | O22—C27—H27C | 109.5 |
| N4—C3—H3B | 109.7 | H27A—C27—H27C | 109.5 |
| C2—C3—H3B | 109.7 | H27B—C27—H27C | 109.5 |
| H3A—C3—H3B | 108.2 | O32—C31—O31 | 125.6 (3) |
| C21—N4—C3 | 116.7 (2) | O32—C31—C32 | 116.1 (2) |
| C21—N4—C5 | 112.4 (2) | O31—C31—C32 | 118.2 (3) |
| C3—N4—C5 | 109.7 (2) | O33—C32—C31 | 112.1 (2) |
| N4—C5—C6 | 110.5 (3) | O33—C32—C33 | 109.6 (2) |
| N4—C5—H5A | 109.5 | C31—C32—C33 | 109.6 (2) |
| C6—C5—H5A | 109.5 | O33—C32—H32A | 108.5 |
| N4—C5—H5B | 109.5 | C31—C32—H32A | 108.5 |
| C6—C5—H5B | 109.5 | C33—C32—H32A | 108.5 |
| H5A—C5—H5B | 108.1 | C32—O33—H33 | 105 (3) |
| N1—C6—C5 | 110.4 (3) | O34—C33—C34 | 111.8 (2) |
| N1—C6—H6A | 109.6 | O34—C33—C32 | 110.2 (2) |
| C5—C6—H6A | 109.6 | C34—C33—C32 | 109.5 (2) |
| N1—C6—H6B | 109.6 | O34—C33—H33A | 108.4 |
| C5—C6—H6B | 109.6 | C34—C33—H33A | 108.4 |
| H6A—C6—H6B | 108.1 | C32—C33—H33A | 108.4 |
| C26—C21—C22 | 118.2 (3) | C33—O34—H34 | 110 (3) |
| C26—C21—N4 | 123.4 (3) | O35—C34—O36 | 125.5 (3) |
| C22—C21—N4 | 118.3 (3) | O35—C34—C33 | 122.5 (3) |
| O22—C22—C23 | 123.9 (4) | O36—C34—C33 | 112.0 (2) |
| O22—C22—C21 | 115.7 (3) | C34—O36—H36 | 106 (2) |
| C23—C22—C21 | 120.5 (3) | H41—O41—H42 | 106 (4) |
| C24—C23—C22 | 119.7 (4) | H51—O51—H52 | 106 (3) |
| C24—C23—H23 | 120.1 | ||
| C6—N1—C2—C3 | −54.9 (4) | C23—C24—C25—C26 | 0.3 (6) |
| N1—C2—C3—N4 | 58.6 (4) | C22—C21—C26—C25 | 0.6 (5) |
| C2—C3—N4—C21 | 169.1 (3) | N4—C21—C26—C25 | −177.5 (3) |
| C2—C3—N4—C5 | −61.7 (3) | C24—C25—C26—C21 | −0.7 (6) |
| C21—N4—C5—C6 | −167.9 (3) | C23—C22—O22—C27 | −8.7 (6) |
| C3—N4—C5—C6 | 60.6 (3) | C21—C22—O22—C27 | 170.4 (4) |
| C2—N1—C6—C5 | 53.7 (4) | O32—C31—C32—O33 | 10.6 (4) |
| N4—C5—C6—N1 | −56.1 (4) | O31—C31—C32—O33 | −169.8 (2) |
| C3—N4—C21—C26 | 22.6 (4) | O32—C31—C32—C33 | −111.3 (3) |
| C5—N4—C21—C26 | −105.3 (3) | O31—C31—C32—C33 | 68.3 (3) |
| C3—N4—C21—C22 | −155.5 (3) | O33—C32—C33—O34 | −66.8 (3) |
| C5—N4—C21—C22 | 76.6 (3) | C31—C32—C33—O34 | 56.6 (3) |
| C26—C21—C22—O22 | −179.1 (3) | O33—C32—C33—C34 | 56.6 (3) |
| N4—C21—C22—O22 | −0.9 (4) | C31—C32—C33—C34 | 179.9 (2) |
| C26—C21—C22—C23 | 0.0 (5) | O34—C33—C34—O35 | 3.2 (4) |
| N4—C21—C22—C23 | 178.2 (3) | C32—C33—C34—O35 | −119.2 (3) |
| O22—C22—C23—C24 | 178.6 (4) | O34—C33—C34—O36 | −178.9 (3) |
| C21—C22—C23—C24 | −0.4 (5) | C32—C33—C34—O36 | 58.7 (3) |
| C22—C23—C24—C25 | 0.3 (6) |
4-(2-Methoxyphenyl)piperazin-1-ium hydrogen (2R,3R)-tartrate 1.698-hydrate (XV) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H11···O31 | 0.79 (4) | 2.40 (4) | 3.028 (4) | 137 (3) |
| N1—H11···O36i | 0.79 (4) | 2.43 (4) | 2.977 (4) | 128 (3) |
| N1—H11···O35ii | 0.79 (4) | 2.50 (3) | 2.942 (3) | 117 (3) |
| N1—H12···O41 | 0.89 (4) | 1.91 (4) | 2.792 (5) | 168 (3) |
| O33—H33···O34iii | 0.77 (4) | 2.14 (4) | 2.800 (3) | 144 (4) |
| O34—H34···O31iii | 0.82 (4) | 2.11 (4) | 2.836 (3) | 148 (3) |
| O36—H36···O32iv | 0.81 (4) | 1.68 (4) | 2.478 (3) | 167 (3) |
| O41—H41···O33v | 0.82 (5) | 1.94 (5) | 2.753 (4) | 167 (3) |
| O41—H42···O31i | 0.87 (5) | 1.90 (5) | 2.766 (4) | 169 (3) |
| O51—H51···O41 | 0.98 (4) | 1.80 (5) | 2.776 (5) | 172 (9) |
| O51—H52···O22i | 0.97 (7) | 2.22 (7) | 3.054 (7) | 144 (6) |
| O51—H52···N4i | 0.97 (7) | 2.48 (6) | 3.307 (6) | 143 (5) |
| C23—H23···Cg2vi | 0.93 | 2.91 | 3.722 (4) | 147 |
Symmetry codes: (i) x−1, y, z; (ii) −x+2, y+1/2, −z+1; (iii) −x+2, y−1/2, −z+1; (iv) x+1, y, z; (v) x−1, y+1, z; (vi) −x+1, y+1/2, −z.
Funding Statement
This work was funded by University Grants Commission, New Delhi grant .
References
- Acosta, L. M., Bahsas, A., Palma, A., Cobo, J., Hursthouse, M. B. & Glidewell, C. (2009). Acta Cryst. C65, o92–o96. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Etter, M. C. (1990). Acc. Chem. Res. 23, 120–126.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Ferguson, G., Glidewell, C., Gregson, R. M. & Meehan, P. R. (1998a). Acta Cryst. B54, 129–138.
- Ferguson, G., Glidewell, C., Gregson, R. M. & Meehan, P. R. (1998b). Acta Cryst. B54, 139–150.
- Ferguson, G., Glidewell, C. & Patterson, I. L. J. (1996). Acta Cryst. C52, 420–423.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Gregson, R. M., Glidewell, C., Ferguson, G. & Lough, A. J. (2000). Acta Cryst. B56, 39–57. [DOI] [PubMed]
- Hackling, A., Ghosh, R., Perachon, S., Mann, A., Höltje, H. D., Wermuth, C. G., Schwartz, J. C., Sippl, W., Sokoloff, P. & Stark, H. (2003). J. Med. Chem. 46, 3883–3899. [DOI] [PubMed]
- Harish Chinthal, C., Yathirajan, H. S., Archana, S. D., Foro, S. & Glidewell, C. (2020). Acta Cryst. E76, 841–847. [DOI] [PMC free article] [PubMed]
- Harish Chinthal, C., Yathirajan, H. S., Kavitha, C. N., Foro, S. & Glidewell, C. (2020). Acta Cryst. E76, 1179–1186. [DOI] [PMC free article] [PubMed]
- Kiran Kumar, H., Yathirajan, H. S., Foro, S. & Glidewell, C. (2019). Acta Cryst. E75, 1494–1506. [DOI] [PMC free article] [PubMed]
- Kiran Kumar, H., Yathirajan, H. S., Harish Chinthal, C., Foro, S. & Glidewell, C. (2020). Acta Cryst. E76, 488–495. [DOI] [PMC free article] [PubMed]
- Kiran Kumar, H., Yathirajan, H. S., Sagar, B. K., Foro, S. & Glidewell, C. (2019). Acta Cryst. E75, 1253–1260. [DOI] [PMC free article] [PubMed]
- Orjales, A., Alonso-Cires, L., Labeaga, L. & Corcóstegui, R. (1995). J. Med. Chem. 38, 1273–1277. [DOI] [PubMed]
- Oxford Diffraction (2009). CrysAlis CCD and CrysAlis RED. Oxford Diffraction Ltd, Abingdon, England.
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Riddell, F. G. & Rogerson, M. (1996). J. Chem. Soc. Perkin Trans. 2, pp. 493–504.
- Riddell, F. G. & Rogerson, M. (1997). J. Chem. Soc. Perkin Trans. 2, pp. 249–256.
- Seip, H. M. & Seip, R. (1973). Acta Chem. Scand. 27, 4024–4027.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Shreekanth, T. K., Yathirajan, H. S., Kalluraya, B., Foro, S. & Glidewell, C. (2020). Acta Cryst. E76, 1605–1610. [DOI] [PMC free article] [PubMed]
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Verdonk, M. L., Voogd, J. W., Kanters, J. A., Kroon, J., den Besten, R., Brandsma, L., Leysen, D. & Kelder, J. (1997). Acta Cryst. B53, 976–983. [DOI] [PubMed]
- Waszkielewicz, A. M., Pytka, K., Rapacz, A., Wełna, E., Jarzyna, M., Satała, G., Bojarski, A. J., Sapa, J., Żmudzki, P., Filipek, B. & Marona, H. (2015). Chem. Biol. Drug Des. 85, 326–335. [DOI] [PubMed]
- Wood, P. A., Allen, F. H. & Pidcock, E. (2009). CrystEngComm, 11, 1563–1571.
- Yépes, A. F., Palma, A., Marchal, A., Cobo, J. & Glidewell, C. (2012). Acta Cryst. C68, o199–o203. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I, II, III, IV, V, VI, VII, VIII, IX, X, XI, XII, XIII, XIV, XV. DOI: 10.1107/S2056989020014097/hb7950sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020014097/hb7950Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989020014097/hb7950IIsup3.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989020014097/hb7950IIIsup4.hkl
Structure factors: contains datablock(s) IV. DOI: 10.1107/S2056989020014097/hb7950IVsup5.hkl
Structure factors: contains datablock(s) V. DOI: 10.1107/S2056989020014097/hb7950Vsup6.hkl
Structure factors: contains datablock(s) VI. DOI: 10.1107/S2056989020014097/hb7950VIsup7.hkl
Structure factors: contains datablock(s) VII. DOI: 10.1107/S2056989020014097/hb7950VIIsup8.hkl
Structure factors: contains datablock(s) VIII. DOI: 10.1107/S2056989020014097/hb7950VIIIsup9.hkl
Structure factors: contains datablock(s) IX. DOI: 10.1107/S2056989020014097/hb7950IXsup10.hkl
Structure factors: contains datablock(s) X. DOI: 10.1107/S2056989020014097/hb7950Xsup11.hkl
Structure factors: contains datablock(s) XI. DOI: 10.1107/S2056989020014097/hb7950XIsup12.hkl
Structure factors: contains datablock(s) XII. DOI: 10.1107/S2056989020014097/hb7950XIIsup13.hkl
Structure factors: contains datablock(s) XIII. DOI: 10.1107/S2056989020014097/hb7950XIIIsup14.hkl
Structure factors: contains datablock(s) XIV. DOI: 10.1107/S2056989020014097/hb7950XIVsup15.hkl
Structure factors: contains datablock(s) XV. DOI: 10.1107/S2056989020014097/hb7950XVsup16.hkl
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950Isup17.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IIsup18.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IIIsup19.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IVsup20.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950Vsup21.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950VIsup22.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950VIIsup23.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950VIIIsup24.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950IXsup25.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950Xsup26.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIsup27.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIIsup28.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIIIsup29.cml
Supporting information file. DOI: 10.1107/S2056989020014097/hb7950XIVsup30.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report