The structure of the title compound is stabilized by the presence of N—H⋯O and C—H⋯O hydrogen bonds. Other interactions such as C—H⋯π are also important in the analysis of the Hirshfeld surface. Molecular docking studies show this compound to be a potential anticoagulant agent.
Keywords: crystal structure, Hirshfeld surfaces, molecular electrostatic potential, molecular docking
Abstract
The title compound, C21H17N3O5, consists of three rings, A, B and C, linked by amide bonds with the benzene rings A and C being inclined to the mean plane of the central benzene ring B by 2.99 (18) and 4.57 (18)°, respectively. In the crystal, molecules are linked via N—H⋯O and C—H⋯O hydrogen bonds, forming fused R
2
2(18), R
3
4(30), R
4
4(38) rings running along [ 0
0 ] and R
3
3(37) and R
3
3(15) rings along [001]. Hirshfeld analysis was undertaken to study the intermolecular contacts in the crystal, showing that the most significant contacts are H⋯O/O⋯H (30.5%), H⋯C/C⋯H (28.2%) and H⋯H (29.0%). Two zones with positive (50.98 and 42.92 kcal mol−1) potentials and two zones with negative (−42.22 and −34.63 kcal mol−1) potentials promote the N—H⋯O interactions in the crystal. An evaluation of the molecular coupling of the title compound and the protein with enzymatic properties known as human coagulation factor Xa (hfXa) showed the potential for coupling in three arrangements with a similar minimum binding energy, which differs by approximately 3 kcal mol−1 from the value for the molecule Apixaban, which was used as a positive control inhibitor. This suggests the title compound exhibits inhibitory activity.
] and R
3
3(37) and R
3
3(15) rings along [001]. Hirshfeld analysis was undertaken to study the intermolecular contacts in the crystal, showing that the most significant contacts are H⋯O/O⋯H (30.5%), H⋯C/C⋯H (28.2%) and H⋯H (29.0%). Two zones with positive (50.98 and 42.92 kcal mol−1) potentials and two zones with negative (−42.22 and −34.63 kcal mol−1) potentials promote the N—H⋯O interactions in the crystal. An evaluation of the molecular coupling of the title compound and the protein with enzymatic properties known as human coagulation factor Xa (hfXa) showed the potential for coupling in three arrangements with a similar minimum binding energy, which differs by approximately 3 kcal mol−1 from the value for the molecule Apixaban, which was used as a positive control inhibitor. This suggests the title compound exhibits inhibitory activity.
Chemical context
The synthesis of new compounds derived from aminobenzamides has generated a growing interest in the search for molecular systems that can have different physical, chemical or biological properties. Some aminobenzamide derivatives present high efficiencies in their non-linear optical properties (Prasad et al., 2011 ▸). Exceptional insecticidal activity against lepidopterous insects has been shown by some anthranilic diamides (Lahm et al., 2005 ▸) and benzene dicarboxamide derivatives (Tohnishi et al., 2005 ▸; Lahm et al., 2007 ▸). Other substituted benzamides are used as medications for patients with schizophrenia (Racagni et al., 2004 ▸). Some benzamide compounds are used in the area of neurology for their powerful neuroprotective effects (Hirata et al., 2018 ▸). Other diamide compounds analogous to the product obtained in this work have been tested as potential options for antithrombotic treatments by acting as direct inhibitors of coagulation factor Xa (Xing et al., 2018 ▸; Lee et al., 2017 ▸). This study was undertaken with the aim of providing new compounds with possible pharmaceutical applications. The results of the synthesis, crystal-structure determination by single-crystal X-ray diffraction, supramolecular analysis and an evaluation of molecular coupling on human factor Xa of the title compound, (I), a new diamide derived from 3-aminobenzamide, is presented here.
Structural commentary
In the title compound, Fig. 1 ▸, the mean planes of rings A (C1–C6; r.m.s deviation = 0.0127 Å) and C (C15–C20; r.m.s deviation = 0.0086 Å) form dihedral angles of of 2.99 (18) and 4.57 (18)°, respectively, with the mean plane of the central ring B (C8–C13; r.m.s deviation = 0.0072 Å). In turn, the amide groups C8/N2/C7(O3)/C1 (r.m.s deviation = 0.0081 Å) and C15/N3/C14(O4)/C10 (r.m.s deviation = 0.0101 Å), which link the rings, are inclined by 37.72 (15) and 29.35 (16)°, respectively, to ring B. The nitro group forms an angle of 5.3 (2)° with ring A while the methoxy group is approximately coplanar with ring C, forming an angle of 1.1 (5)°. The molecule is formed by three main planes resulting from the planes that form the rings with the B ring resembling a fallen step between A and C. All bond lengths (Allen et al., 1987 ▸) and bond angles are within normal ranges.
Figure 1.
The molecular structure of (I), showing the atom labeling and displacement ellipsoids drawn at the 50% probability level.
Supramolecular features
The crystalline packing in compound (I) is mainly regulated by the presence of N—H⋯O and C—H⋯O hydrogen bonds. The N2—H2N⋯O4i and N3—H3N⋯O3ii interactions [symmetry codes: (i) −x + 1, y +  , −z + 1; (ii) −x, y +
, −z + 1; (ii) −x, y +  , −z + 1] form fused
, −z + 1] form fused  (18),
(18),  (30) and
(30) and  (38) rings (Etter, 1990 ▸) running along [
(38) rings (Etter, 1990 ▸) running along [ 0
0 ] (see Table 1 ▸ and Fig. 2 ▸
a). In turn, playing the role of complementary interactions in crystal growth, other C—H⋯O-type interactions [C19—H19⋯O1iv and C21—H21C⋯O1v; symmetry codes: (iv) x − 1, y, z + 1; (v) −x + 1, y −
] (see Table 1 ▸ and Fig. 2 ▸
a). In turn, playing the role of complementary interactions in crystal growth, other C—H⋯O-type interactions [C19—H19⋯O1iv and C21—H21C⋯O1v; symmetry codes: (iv) x − 1, y, z + 1; (v) −x + 1, y −  , −z + 1] link the molecules at their ends, ensuring their stability in the growth process. Together with the N—H⋯O interactions, they contribute to the formation of additional fused
, −z + 1] link the molecules at their ends, ensuring their stability in the growth process. Together with the N—H⋯O interactions, they contribute to the formation of additional fused  (37) and
(37) and  (15) rings, which run along [001] (see Table 1 ▸, Fig. 2 ▸
b). Weak C21—H21B⋯Cg1 interactions occur, where Cg1 is the centroid of the C1–C6 benzene ring, with a H21B⋯Cg1 distance of 2.83 Å.
(15) rings, which run along [001] (see Table 1 ▸, Fig. 2 ▸
b). Weak C21—H21B⋯Cg1 interactions occur, where Cg1 is the centroid of the C1–C6 benzene ring, with a H21B⋯Cg1 distance of 2.83 Å.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2N⋯O4i | 0.88 | 2.09 | 2.908 (4) | 155 |
| N3—H3N⋯O3ii | 0.88 | 2.21 | 3.056 (4) | 163 |
| C5—H5⋯O2iii | 0.95 | 2.28 | 3.093 (5) | 143 |
| C19—H19⋯O1iv | 0.95 | 2.44 | 3.365 (5) | 164 |
| C21—H21C⋯O1v | 0.98 | 2.64 | 3.490 (5) | 145 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  .
.
Figure 2.
(a) Partial crystal structure of (I), showing the formation of D =  (18), E =
(18), E =  (30) and F =
(30) and F =  (38) fused rings running along [
(38) fused rings running along [ 0
0 ] and (b) G =
] and (b) G =  (37) and H =
(37) and H =  (15) fused rings. These fused rings run along [001]. Symmetry code: (vi) x − 1, y − 1, z + 1.
(15) fused rings. These fused rings run along [001]. Symmetry code: (vi) x − 1, y − 1, z + 1.
Hirshfeld surface analysis
Intermolecular interactions, including hydrogen-bond interactions, are essential in reinforcing the stability of the supramolecular structure. This behavior can be analyzed through the study of Hirshfeld surfaces (HS) using the CrystalExplorer program (Spackman & Jayatilaka, 2009 ▸), which allows the visualization of the interactions within the crystal structure, including N⋯H and C⋯H interactions. To examine in depth the strength and capacity of hydrogen bonds and other intermolecular contacts, one of the Hirshfeld surface analysis tools, the normalized contact distance, d norm, has been used (Turner et al., 2017 ▸). The results show that the most important contributions to the crystal packing are due to the N—H⋯O and C—H⋯O hydrogen bonds. This is evidenced by the more intense red spots on the HS for (I) (see Fig. 3 ▸ a,b). The overall two-dimensional fingerprint plot (Fig. 4 ▸ a), and those delineated into by H⋯O/O⋯H, H⋯H, H⋯C/C⋯H, C⋯C, N⋯C/C⋯N, H⋯N/N⋯H, O⋯O, O⋯C/C⋯O, and N⋯O/O⋯N contacts are illustrated in Fig. 4 ▸ b–j, respectively, together with their relative contributions to the Hirshfeld surface. The most important interaction corresponds to H⋯O/O⋯H contributing 30.5% to the overall crystal packing, as shown in Fig. 4 ▸ b. The pair of spikes in the fingerprint plot have a symmetrical distribution of high-density points with the tip at d e + d i = 1.98 Å. The H⋯H interactions, shown in Fig. 4 ▸ c, contribute 29.0% to the total crystal packing and appear as widely dispersed points of high density due to the large hydrogen-atom content of the molecule with the rounded tip at d e + d i = 1.20 Å. The presence of C—H⋯π interactions is shown as a pair of characteristic wings on the fingerprint plot (Fig. 4 ▸ d), delineated into H⋯C/C⋯H contacts (28.2% contribution to the HS) having the tip at d e + d i = 2.84 Å. These results reveal the importance of H-atom contacts in establishing the packing. The large number of H⋯H, H⋯C/C⋯H and H⋯O/O⋯H interactions suggest that van der Waals interactions and hydrogen bonding play major roles in the crystal packing (Hathwar et al., 2015 ▸).
Figure 3.
(a) A view of the Hirshfeld surface of (I) mapped over d
norm emphasizing the N—H⋯O and C—H⋯O intermolecular interactions and (b) showing the H⋯O/O⋯H and C⋯C (π–π) interactions. Symmetry codes: (i) −x + 1, y +  , −z + 1; (ii) −x, y +
, −z + 1; (ii) −x, y +  , −z + 1; (iii) x − 1, y, z + 1.
, −z + 1; (iii) x − 1, y, z + 1.
Figure 4.
Two-dimensional fingerprints plots for the title compound, showing (a) all interactions, (b) H⋯O/O⋯H, (c) H⋯H, (d) H⋯C/C⋯H, (e) C⋯C, (f) N⋯C/C⋯N, (g) H⋯N/N⋯H, (h) O⋯O, (i) O⋯C/C⋯O, and (j) N⋯O/O⋯N interactions. The d i and d e values are the closest internal and external distances (in Å) of given points on the Hirshfeld surface.
Molecular Electrostatic Potential (MEP)
A study of the molecular electrostatic potential (MEP) of (I) using the Gauss09W (Frisch et al., 2009 ▸) and Gauss View 5.0 programs, at the DFT/B3LYP/6-31G (d, p) level of theory, to obtain a qualitative analysis and the Multiwfn 3.6 program for a quantitative analysis (Lu & Chen, 2012 ▸) of the surface was undertaken. On the potential surface, marked in dark blue, positive regions of low electron density that can suffer nucleophilic attacks in a chemical reaction or can have interactions with nucleophiles in the process of crystalline growth are shown (see Fig. 5 ▸). Two zones show positive potentials of 50.98 and 42.92 kcal mol−1 in regions that follow the directions of the N—H bonds above and below the plane through the rings of the molecule and which promote the formation of the N2—H2N⋯O4i and N3—H3N⋯O3ii hydrogen bonds throughout the crystal. In turn, other areas with high density, being close to the carbonyl oxygen atoms and shown in a reddish color, show values of −42.22 and −34.63 kcal mol−1. Thus, in the first stage of crystal formation, these low and high electronic density regions are intertwined to promote the formation of rings and chains of molecules along the crystal. The other areas with lower values in the regions will be accommodated to enable the formation of other weaker bonds, C5—H5⋯O2iii, C19—H19⋯1iv and C21—H21C⋯O1v, representing the growth characteristics of each crystal.
Figure 5.
Three-dimensional representation of the electrostatic potential around a molecule of (I).
Molecular Docking Evaluation
One of the newest synthetic direct-acting compounds to be licensed for the treatment of therapeutic anticoagulation is Apixaban. To evaluate its potential as an anticoagulant agent, a molecular docking study of (I) as a ligand and the human coagulation factor hfXa as the receptor protein was performed. For the molecular coupling calculations, the free software Autodock Vina 4.2.6 (Trott & Olson, 2010 ▸) was used, and as a positive control ligand in the active site, the molecule Apixaban (PDB code 2p16; Pinto et al., 2007 ▸), one of the most important substrates currently accepted by the US Food and Drug Administration (FDA) for antithrombotic treatments (Agnelli et al., 2013 ▸) was used. The Apixaban molecule was also used as a model for the validation and verification of the parameters of the molecular coupling calculations performed. The bonding energy obtained for the new inhibitor was −7.7 and −10.7 kcal mol−1 for the control ligand (Table 2 ▸). Compound (I), as a ligand at the active site of hfXa, presents one hydrogen bond of medium strength between the local N2 atom and the amino acid residue Gly218. However, all hydrophobic residue contacts of the latter in its more stable configuration are also present in the positive control ligand with the exception of Glu217. The results of the molecular coupling calculations show that ligand (I) has two equivalent energy configurations, poses 2 and 3 (see Table 3 ▸), which differ energetically from pose 1 by only 0.1 and 0.3 kcal mol−1, respectively. Thus, pose 3 presents a better 3D conformational arrangement at the active site, this pose being much more similar to that of the control ligand (see Fig. 6 ▸). This behavior implies that two of the three hydrogen bonds presented by the control ligand in the active site Gly216 and Gln192, plus all hydrophobic contacts of ligand (I) in its pose 3, coincide with those presented by the control ligand. For this reason, the difference in binding energy between (I) and the control ligand at the active site is due to the chemical difference between the functional groups present in each structure. The molecular coupling images were generated using the programs PyMOL (Rigsby & Parker, 2016 ▸) and Ligplot+ (Laskowski & Swindells, 2011 ▸).
Table 2. Molecular coupling calculations (kcal mol−1) for the active hfXa site, the title compound, and the Apixaban molecule.
| Compound | Bond energya | No. of hydrogen bondsb | Hydrogen-bonding interaction residuesb | van der Waals interaction residuesb |
|---|---|---|---|---|
| (I) | −7.7 | 1 | Gly218 | Trp215, Cys191, Ser195, Gln192, Cys220, Gly218, Ala190, Gly216, Gly 226, Asp189, Val213, Phe174, Glu217, Glu 146 |
| Apixabanc | −10.7 | 3 | Gly216, Gln192, Glu146 | Cys220, Tyr99, Glu97, Phe174, Thr98, Val213, Trp215, Gly226, Cys191, Asp189, Ser195, Ile227, Ala190, Gly218, Arg143 |
Notes: (a) The binding energy indicates the affinity and binding capacity for the active site of the enzyme protein fXa; (b) The number of hydrogen bonds and all amino acid residues involved in the enzyme-inhibitor complex were determined using AutoDock 4.2.6 (Trott & Olson, 2010 ▸); (c) The Apixaban molecule was used as a positive control ligand for the active site.
Table 3. Equivalent poses of compound (I) in the active hfXa site, as a result of molecular coupling calculations (kcal mol−1).
| Compound | Bond energya | No. of hydrogen bondsb | Hydrogen-bonding interaction residuesb | van der Waals interaction residuesb |
|---|---|---|---|---|
| (I) (pose 1) | −7.7 | 1 | Gly218 | Trp215, Cys191, Ser195, Gln192, Cys220, Gly218, Ala190, Gly216, Gly 226, Asp189, Val213, Phe174, Glu217, Glu 146 |
| (I) (pose 2) | −7.6 | 1 | Gly192 | Phe174, Tyr99, Val213, Gly226, Trp215, Ser195, Asp189, Ala190, Gly218, Gly216, Cys191, Gln192, Ile 227, Lys96 |
| (I) (pose 3) | −7.4 | 2 | Gly 216, Gln192 | Cys220, Glu217, Phe174, Val213, Gly226, Cys191, Ser195, Ala190, Trp215, Gly218, Ile227 |
Notes: (a) The binding energy indicates the affinity and binding capacity for the active site of the enzyme protein fXa; (b) The number of hydrogen bonds and all amino acid residues involved in the enzyme-inhibitor complex were determined using AutoDock 4.2.6 (Trott & Olson, 2010 ▸); (c) The Apixaban molecule was used as a positive control ligand for the active site.
Figure 6.
Models of molecular coupling for inhibition of hfXa by compound (I) in its poses: (a) and (d) pose 1; (b) and (e) pose 2, and (c) and (f) pose 3. Dashed lines indicate hydrogen bonds. Carbon atoms are in black, nitrogen in blue and oxygen in red.
Database survey
A search of the Cambridge Structural Database (CSD, version 5.41, November 2019 update; Groom et al., 2016 ▸) using [3-(benzoyl-λ2-azanyl)phenyl](phenyl-λ2-azanyl)methanone as the main skeleton gave 76 hits. Seven structures containing the [3-(benzoyl-λ2-azanyl)phenyl](phenyl-λ2-azanyl)methanone framework with nitro and methoxy groups as substituents similar to the title compound were found, viz., N-{3-[N′-(2-methoxyphenyl)carbamoyl]-5-methyl-2-methoxyphenyl}-2-methoxy-5-methyl-3-nitrobenzamide (HEXXOY; Yi et al., 2007 ▸), N-{3-[N′-(2-methoxyphenyl)carbamoyl]-5-methyl-2-methoxyphenyl}-2-hydroxy-5-methyl-3-nitrobenzamide (HEXYAL; Yi et al., 2007 ▸), methyl 3-({2-hydroxy-3-[(2-methoxybenzoyl)amino]benzoyl}amino)-2-methoxybenzoate (POQMEP; Liu et al., 2014 ▸), catena-[[μ-methyl 3-({2-oxy-3-[(2-methoxy-3-nitrobenzoyl)amino]benzoyl}amino)-2-methoxybenzoate]aquasodium methanol solvate] (POQMIT; Liu et al., 2014 ▸), methyl 2-methoxy-3-({2-methoxy-3-[(2-methoxy-3-nitrobenzoyl)amino]benzoyl}amino)benzoate (YUXMIO; Yan et al., 2010 ▸), methyl 3-({3-[(2,5-dimethoxy-3-nitrobenzoyl)amino]-2-methoxybenzoyl}amino)-2,5-dimethoxybenzoate (YUXNEL; Yan et al., 2010 ▸), and methyl 3-({3-[(2,5-dimethoxy-3-nitrobenzoyl)amino]-2,5-dimethoxybenzoyl}amino)-2,5-dimethoxybenzoate (YUXNOV; Yan et al., 2010 ▸). The specific characteristics of these diamide systems depend on the presence of the diverse substituents and their position in the rings in such a way that the planarity in the system is maintained between two rings and the third ring is rotated with respect to the first two. In this way, the compounds POQMEP, POQMIT and HEXXOY present a relatively small deviation from planarity, while the systems YUXNOV, YUXNEL, HEXYAL, YUXMIO progressively lose this planarity, ending up with quite large dihedral angles between the planes.
Synthesis and crystallization
3-Amino-N-(4-methoxyphenyl)benzamide (ii) (50.5 mg, 0.129 mmol), previously synthesized, was subjected to an acylation reaction in 3-nitrobenzoyl chloride (a) (50.5 mg, 0.272 mmol) under chloroform reflux conditions to obtain compound (I) [yield 44.7 mg (0.114 mmol), 88.4%; m.p. 502 (1) K].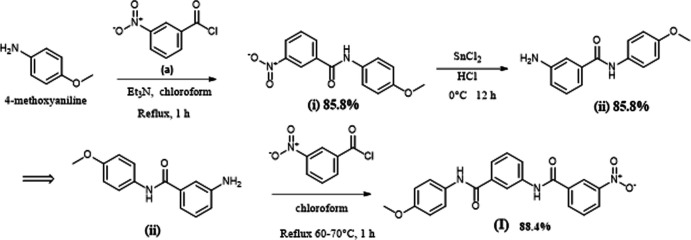
FT–IR (ATR): υ (cm−1) = 3338, 3285 (N—H), 3076, 2969, 1650, 1637 (C=O), 1585 (C=C), 1526, 1510, 1434, 1353 (NO2), 1316, 1301, 1241, 1137, 1026, 898, 829, 819, 721, 683 cm−1. The UV–Vis spectrum for (I) (60 µM) was obtained in DMF with a maximum of 273 nm (0.62 absorption). MS (IE, 40 eV), m/z [M +] calculated for C21H17N3O5 +: 391.12 (100%) and 392.12 (23.1%); found: 391.10 and 392.10 (25.1%).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 4 ▸. All the H atoms were found in a difference-Fourier map and were positioned at geometrically idealized positions, C—H = 0.95 Å (ring), N—H = 0.88 Å and C—H = 0.98 Å (methyl) and were refined using a riding-model approximation, with U iso(H) = 1.2U eq(parent atom) or 1.5U eq(C). After refinement, ROTAX suggested twinning by a 180° about [100]. This law was used to generate an hklf5 format reflection file. Refinement against this file improved R factors and residual Q peaks·BASF refined to 0.090 (4)
Table 4. Experimental details.
| Crystal data | |
| Chemical formula | C21H17N3O5 |
| M r | 391.37 |
| Crystal system, space group | Monoclinic, P21 |
| Temperature (K) | 123 |
| a, b, c (Å) | 7.2948 (3), 7.1242 (3), 16.8297 (7) |
| β (°) | 97.644 (3) |
| V (Å3) | 866.86 (6) |
| Z | 2 |
| Radiation type | Cu Kα |
| μ (mm−1) | 0.91 |
| Crystal size (mm) | 0.50 × 0.40 × 0.18 |
| Data collection | |
| Diffractometer | Oxford Diffraction Gemini S |
| Absorption correction | Multi-scan |
| T min, T max | 0.603, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 3172, 3172, 2982 |
| R int | 0.048 |
| (sin θ/λ)max (Å−1) | 0.620 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.047, 0.126, 1.06 |
| No. of reflections | 3172 |
| No. of parameters | 263 |
| No. of restraints | 3 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.23, −0.30 |
| Absolute structure | Flack x determined using 524 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | 0.2 (3) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020013730/dj2013sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020013730/dj2013Isup2.hkl
Synthesis and characterization of (I). DOI: 10.1107/S2056989020013730/dj2013sup3.pdf
Supporting information file. DOI: 10.1107/S2056989020013730/dj2013Isup4.cml
CCDC reference: 2016019
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| C21H17N3O5 | Dx = 1.499 Mg m−3 |
| Mr = 391.37 | Melting point: 502(1) K |
| Monoclinic, P21 | Cu Kα radiation, λ = 1.54184 Å |
| a = 7.2948 (3) Å | Cell parameters from 3172 reflections |
| b = 7.1242 (3) Å | θ = 5.3–73.1° |
| c = 16.8297 (7) Å | µ = 0.91 mm−1 |
| β = 97.644 (3)° | T = 123 K |
| V = 866.86 (6) Å3 | Fragment cut from large slab, pale yellow |
| Z = 2 | 0.50 × 0.40 × 0.18 mm |
| F(000) = 408 |
Data collection
| Oxford Diffraction Gemini S diffractometer | 2982 reflections with I > 2σ(I) |
| Radiation source: sealed tube | Rint = 0.048 |
| ω scans | θmax = 73.1°, θmin = 5.3° |
| Absorption correction: multi-scan | h = −8→6 |
| Tmin = 0.603, Tmax = 1.000 | k = −8→8 |
| 3172 measured reflections | l = −20→20 |
| 3172 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.047 | w = 1/[σ2(Fo2) + (0.066P)2 + 0.3205P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.126 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.23 e Å−3 |
| 3172 reflections | Δρmin = −0.30 e Å−3 |
| 263 parameters | Absolute structure: Flack x determined using 524 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 3 restraints | Absolute structure parameter: 0.2 (3) |
Special details
| Experimental. CrysAlisPro, Agilent Technologies, Version 1.171.37.35 (release 13-08-2014 CrysAlis171 .NET) (compiled Aug 13 2014,18:06:01) Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a two-component twinAfter refinement ROTAX suggested twinning by a 180 rotation about direct 1 0 0.This law was used to generate a hklf5 formated reflection file.Refinement against this file improved R factors and residual Q peaks.BASF refined to 0.090 (4) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.6697 (5) | 0.7564 (5) | 0.02367 (18) | 0.0376 (8) | |
| O2 | 0.5804 (5) | 0.9765 (5) | 0.09701 (19) | 0.0350 (7) | |
| O3 | 0.2455 (4) | 0.5231 (5) | 0.35551 (16) | 0.0282 (6) | |
| O4 | 0.2775 (4) | 0.5306 (4) | 0.64175 (16) | 0.0263 (6) | |
| O5 | −0.1675 (4) | 0.3265 (4) | 0.93736 (17) | 0.0277 (7) | |
| N1 | 0.6116 (4) | 0.8119 (5) | 0.0852 (2) | 0.0246 (7) | |
| N2 | 0.3987 (4) | 0.8014 (5) | 0.37262 (18) | 0.0197 (7) | |
| H2N | 0.4792 | 0.8744 | 0.3531 | 0.024* | |
| N3 | 0.0451 (4) | 0.7137 (5) | 0.67641 (19) | 0.0198 (7) | |
| H3N | −0.0181 | 0.8174 | 0.6646 | 0.024* | |
| C1 | 0.4599 (5) | 0.5945 (6) | 0.2656 (2) | 0.0200 (8) | |
| C2 | 0.5006 (5) | 0.7309 (6) | 0.2117 (2) | 0.0201 (7) | |
| H2 | 0.4757 | 0.8598 | 0.2199 | 0.024* | |
| C3 | 0.5793 (5) | 0.6713 (6) | 0.1453 (2) | 0.0214 (8) | |
| C4 | 0.6237 (5) | 0.4862 (7) | 0.1322 (2) | 0.0249 (8) | |
| H4 | 0.6775 | 0.4510 | 0.0860 | 0.030* | |
| C5 | 0.5880 (5) | 0.3544 (6) | 0.1879 (2) | 0.0263 (9) | |
| H5 | 0.6220 | 0.2271 | 0.1817 | 0.032* | |
| C6 | 0.5021 (5) | 0.4077 (6) | 0.2532 (2) | 0.0223 (8) | |
| H6 | 0.4718 | 0.3150 | 0.2899 | 0.027* | |
| C7 | 0.3569 (5) | 0.6363 (6) | 0.3358 (2) | 0.0212 (8) | |
| C8 | 0.3212 (5) | 0.8658 (6) | 0.4411 (2) | 0.0187 (8) | |
| C9 | 0.2817 (5) | 0.7434 (6) | 0.5012 (2) | 0.0186 (7) | |
| H9 | 0.3051 | 0.6129 | 0.4968 | 0.022* | |
| C10 | 0.2080 (5) | 0.8120 (6) | 0.5675 (2) | 0.0190 (8) | |
| C11 | 0.1753 (5) | 1.0041 (6) | 0.5751 (2) | 0.0207 (8) | |
| H11 | 0.1239 | 1.0508 | 0.6202 | 0.025* | |
| C12 | 0.2191 (5) | 1.1265 (6) | 0.5156 (2) | 0.0229 (8) | |
| H12 | 0.1986 | 1.2574 | 0.5207 | 0.028* | |
| C13 | 0.2922 (5) | 1.0588 (6) | 0.4492 (2) | 0.0213 (8) | |
| H13 | 0.3225 | 1.1433 | 0.4093 | 0.026* | |
| C14 | 0.1800 (5) | 0.6713 (6) | 0.6309 (2) | 0.0199 (8) | |
| C15 | −0.0029 (5) | 0.6049 (6) | 0.7415 (2) | 0.0185 (8) | |
| C16 | 0.0253 (5) | 0.4121 (6) | 0.7483 (2) | 0.0217 (8) | |
| H16 | 0.0805 | 0.3463 | 0.7085 | 0.026* | |
| C17 | −0.0274 (5) | 0.3165 (6) | 0.8134 (2) | 0.0217 (8) | |
| H17 | −0.0067 | 0.1850 | 0.8181 | 0.026* | |
| C18 | −0.1096 (5) | 0.4096 (6) | 0.8715 (2) | 0.0218 (8) | |
| C19 | −0.1431 (6) | 0.6016 (6) | 0.8636 (2) | 0.0249 (9) | |
| H19 | −0.2038 | 0.6659 | 0.9021 | 0.030* | |
| C20 | −0.0880 (5) | 0.6982 (6) | 0.7997 (2) | 0.0236 (9) | |
| H20 | −0.1082 | 0.8297 | 0.7954 | 0.028* | |
| C21 | −0.1360 (6) | 0.1300 (7) | 0.9478 (3) | 0.0312 (10) | |
| H21A | −0.1834 | 0.0882 | 0.9967 | 0.047* | |
| H21B | −0.2001 | 0.0623 | 0.9015 | 0.047* | |
| H21C | −0.0031 | 0.1045 | 0.9524 | 0.047* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0561 (19) | 0.0329 (18) | 0.0277 (15) | 0.0003 (16) | 0.0204 (14) | −0.0033 (15) |
| O2 | 0.0502 (18) | 0.0226 (18) | 0.0357 (17) | −0.0006 (14) | 0.0189 (14) | 0.0045 (14) |
| O3 | 0.0308 (14) | 0.0276 (16) | 0.0280 (14) | −0.0096 (14) | 0.0108 (11) | −0.0054 (13) |
| O4 | 0.0270 (13) | 0.0223 (15) | 0.0321 (14) | 0.0074 (12) | 0.0132 (11) | 0.0065 (13) |
| O5 | 0.0359 (15) | 0.0235 (16) | 0.0254 (14) | −0.0018 (13) | 0.0099 (11) | 0.0038 (13) |
| N1 | 0.0271 (16) | 0.0230 (19) | 0.0244 (17) | −0.0037 (14) | 0.0065 (13) | −0.0036 (15) |
| N2 | 0.0203 (14) | 0.0194 (17) | 0.0210 (14) | −0.0040 (12) | 0.0087 (11) | 0.0000 (13) |
| N3 | 0.0208 (14) | 0.0169 (16) | 0.0221 (15) | 0.0033 (13) | 0.0043 (11) | 0.0014 (13) |
| C1 | 0.0181 (16) | 0.023 (2) | 0.0190 (17) | −0.0018 (15) | 0.0037 (13) | −0.0007 (16) |
| C2 | 0.0188 (16) | 0.0200 (19) | 0.0216 (17) | −0.0012 (15) | 0.0027 (13) | −0.0014 (16) |
| C3 | 0.0216 (17) | 0.021 (2) | 0.0215 (18) | −0.0018 (15) | 0.0030 (14) | −0.0031 (16) |
| C4 | 0.0231 (17) | 0.027 (2) | 0.0251 (18) | −0.0018 (16) | 0.0046 (14) | −0.0064 (18) |
| C5 | 0.026 (2) | 0.019 (2) | 0.034 (2) | 0.0037 (16) | 0.0035 (16) | −0.0049 (17) |
| C6 | 0.0236 (18) | 0.017 (2) | 0.0263 (19) | −0.0004 (15) | 0.0021 (14) | 0.0023 (17) |
| C7 | 0.0186 (16) | 0.023 (2) | 0.0218 (17) | 0.0017 (16) | 0.0039 (13) | 0.0038 (16) |
| C8 | 0.0151 (16) | 0.021 (2) | 0.0202 (17) | −0.0026 (14) | 0.0022 (13) | −0.0001 (16) |
| C9 | 0.0188 (15) | 0.0165 (19) | 0.0213 (18) | −0.0012 (14) | 0.0053 (13) | −0.0019 (16) |
| C10 | 0.0156 (15) | 0.0189 (19) | 0.0227 (18) | −0.0002 (15) | 0.0030 (13) | −0.0011 (17) |
| C11 | 0.0197 (16) | 0.021 (2) | 0.0227 (17) | 0.0001 (16) | 0.0061 (13) | −0.0013 (17) |
| C12 | 0.0227 (17) | 0.0156 (19) | 0.030 (2) | 0.0000 (15) | 0.0027 (15) | −0.0003 (17) |
| C13 | 0.0207 (16) | 0.019 (2) | 0.0239 (18) | −0.0027 (15) | 0.0038 (14) | 0.0058 (16) |
| C14 | 0.0181 (16) | 0.0194 (19) | 0.0219 (18) | −0.0013 (15) | 0.0018 (13) | −0.0022 (16) |
| C15 | 0.0164 (15) | 0.020 (2) | 0.0189 (17) | −0.0005 (14) | 0.0023 (13) | −0.0014 (16) |
| C16 | 0.0226 (18) | 0.019 (2) | 0.0244 (19) | 0.0015 (16) | 0.0065 (15) | −0.0033 (17) |
| C17 | 0.0225 (17) | 0.0146 (19) | 0.0282 (19) | −0.0001 (15) | 0.0045 (14) | 0.0008 (17) |
| C18 | 0.0202 (17) | 0.024 (2) | 0.0211 (17) | −0.0045 (15) | 0.0015 (14) | 0.0017 (17) |
| C19 | 0.0290 (19) | 0.024 (2) | 0.0235 (18) | 0.0011 (16) | 0.0090 (15) | −0.0035 (17) |
| C20 | 0.0258 (18) | 0.021 (2) | 0.0245 (19) | 0.0027 (16) | 0.0061 (15) | 0.0013 (17) |
| C21 | 0.031 (2) | 0.030 (2) | 0.033 (2) | −0.0023 (19) | 0.0060 (17) | 0.012 (2) |
Geometric parameters (Å, º)
| O1—N1 | 1.235 (5) | C8—C9 | 1.394 (5) |
| O2—N1 | 1.216 (5) | C8—C13 | 1.401 (6) |
| O3—C7 | 1.221 (5) | C9—C10 | 1.390 (5) |
| O4—C14 | 1.229 (5) | C9—H9 | 0.9500 |
| O5—C18 | 1.372 (5) | C10—C11 | 1.398 (6) |
| O5—C21 | 1.425 (5) | C10—C14 | 1.498 (5) |
| N1—C3 | 1.465 (5) | C11—C12 | 1.396 (6) |
| N2—C7 | 1.346 (5) | C11—H11 | 0.9500 |
| N2—C8 | 1.425 (5) | C12—C13 | 1.388 (5) |
| N2—H2N | 0.8800 | C12—H12 | 0.9500 |
| N3—C14 | 1.359 (5) | C13—H13 | 0.9500 |
| N3—C15 | 1.424 (5) | C15—C16 | 1.391 (5) |
| N3—H3N | 0.8800 | C15—C20 | 1.396 (5) |
| C1—C2 | 1.388 (6) | C16—C17 | 1.388 (6) |
| C1—C6 | 1.389 (6) | C16—H16 | 0.9500 |
| C1—C7 | 1.511 (5) | C17—C18 | 1.383 (6) |
| C2—C3 | 1.389 (5) | C17—H17 | 0.9500 |
| C2—H2 | 0.9500 | C18—C19 | 1.392 (6) |
| C3—C4 | 1.383 (6) | C19—C20 | 1.380 (6) |
| C4—C5 | 1.376 (6) | C19—H19 | 0.9500 |
| C4—H4 | 0.9500 | C20—H20 | 0.9500 |
| C5—C6 | 1.389 (6) | C21—H21A | 0.9800 |
| C5—H5 | 0.9500 | C21—H21B | 0.9800 |
| C6—H6 | 0.9500 | C21—H21C | 0.9800 |
| C18—O5—C21 | 117.5 (3) | C9—C10—C14 | 116.1 (4) |
| O2—N1—O1 | 122.6 (4) | C11—C10—C14 | 123.2 (4) |
| O2—N1—C3 | 119.7 (3) | C12—C11—C10 | 119.2 (4) |
| O1—N1—C3 | 117.7 (4) | C12—C11—H11 | 120.4 |
| C7—N2—C8 | 124.3 (3) | C10—C11—H11 | 120.4 |
| C7—N2—H2N | 117.8 | C13—C12—C11 | 120.6 (4) |
| C8—N2—H2N | 117.8 | C13—C12—H12 | 119.7 |
| C14—N3—C15 | 125.8 (3) | C11—C12—H12 | 119.7 |
| C14—N3—H3N | 117.1 | C12—C13—C8 | 119.9 (4) |
| C15—N3—H3N | 117.1 | C12—C13—H13 | 120.0 |
| C2—C1—C6 | 120.1 (4) | C8—C13—H13 | 120.0 |
| C2—C1—C7 | 123.0 (4) | O4—C14—N3 | 123.0 (4) |
| C6—C1—C7 | 116.7 (4) | O4—C14—C10 | 121.4 (3) |
| C1—C2—C3 | 117.3 (4) | N3—C14—C10 | 115.5 (4) |
| C1—C2—H2 | 121.3 | C16—C15—C20 | 119.2 (4) |
| C3—C2—H2 | 121.3 | C16—C15—N3 | 123.4 (4) |
| C4—C3—C2 | 123.4 (4) | C20—C15—N3 | 117.4 (3) |
| C4—C3—N1 | 118.6 (4) | C17—C16—C15 | 119.7 (4) |
| C2—C3—N1 | 117.9 (4) | C17—C16—H16 | 120.2 |
| C5—C4—C3 | 118.2 (4) | C15—C16—H16 | 120.2 |
| C5—C4—H4 | 120.9 | C18—C17—C16 | 121.0 (4) |
| C3—C4—H4 | 120.9 | C18—C17—H17 | 119.5 |
| C4—C5—C6 | 120.0 (4) | C16—C17—H17 | 119.5 |
| C4—C5—H5 | 120.0 | O5—C18—C17 | 124.9 (4) |
| C6—C5—H5 | 120.0 | O5—C18—C19 | 115.6 (4) |
| C5—C6—C1 | 120.9 (4) | C17—C18—C19 | 119.4 (4) |
| C5—C6—H6 | 119.5 | C20—C19—C18 | 119.9 (4) |
| C1—C6—H6 | 119.5 | C20—C19—H19 | 120.0 |
| O3—C7—N2 | 124.7 (4) | C18—C19—H19 | 120.0 |
| O3—C7—C1 | 120.0 (4) | C19—C20—C15 | 120.8 (4) |
| N2—C7—C1 | 115.2 (3) | C19—C20—H20 | 119.6 |
| C9—C8—C13 | 119.7 (3) | C15—C20—H20 | 119.6 |
| C9—C8—N2 | 121.8 (4) | O5—C21—H21A | 109.5 |
| C13—C8—N2 | 118.4 (4) | O5—C21—H21B | 109.5 |
| C10—C9—C8 | 120.1 (4) | H21A—C21—H21B | 109.5 |
| C10—C9—H9 | 119.9 | O5—C21—H21C | 109.5 |
| C8—C9—H9 | 119.9 | H21A—C21—H21C | 109.5 |
| C9—C10—C11 | 120.5 (4) | H21B—C21—H21C | 109.5 |
| C6—C1—C2—C3 | 1.6 (5) | C9—C10—C11—C12 | −0.6 (5) |
| C7—C1—C2—C3 | −173.7 (3) | C14—C10—C11—C12 | 175.0 (3) |
| C1—C2—C3—C4 | −2.2 (5) | C10—C11—C12—C13 | 0.7 (6) |
| C1—C2—C3—N1 | 175.9 (3) | C11—C12—C13—C8 | 0.5 (6) |
| O2—N1—C3—C4 | −177.1 (4) | C9—C8—C13—C12 | −2.0 (6) |
| O1—N1—C3—C4 | 3.1 (5) | N2—C8—C13—C12 | −179.2 (3) |
| O2—N1—C3—C2 | 4.7 (5) | C15—N3—C14—O4 | −0.2 (6) |
| O1—N1—C3—C2 | −175.2 (3) | C15—N3—C14—C10 | −178.0 (3) |
| C2—C3—C4—C5 | 0.0 (6) | C9—C10—C14—O4 | 28.3 (5) |
| N1—C3—C4—C5 | −178.1 (3) | C11—C10—C14—O4 | −147.5 (4) |
| C3—C4—C5—C6 | 2.8 (6) | C9—C10—C14—N3 | −153.8 (3) |
| C4—C5—C6—C1 | −3.3 (6) | C11—C10—C14—N3 | 30.4 (5) |
| C2—C1—C6—C5 | 1.1 (6) | C14—N3—C15—C16 | −26.6 (6) |
| C7—C1—C6—C5 | 176.7 (3) | C14—N3—C15—C20 | 155.5 (4) |
| C8—N2—C7—O3 | 1.3 (6) | C20—C15—C16—C17 | −1.4 (6) |
| C8—N2—C7—C1 | −178.4 (3) | N3—C15—C16—C17 | −179.3 (3) |
| C2—C1—C7—O3 | 142.4 (4) | C15—C16—C17—C18 | 0.7 (6) |
| C6—C1—C7—O3 | −33.1 (5) | C21—O5—C18—C17 | 1.8 (6) |
| C2—C1—C7—N2 | −37.9 (5) | C21—O5—C18—C19 | 180.0 (4) |
| C6—C1—C7—N2 | 146.6 (3) | C16—C17—C18—O5 | 179.4 (3) |
| C7—N2—C8—C9 | 36.2 (5) | C16—C17—C18—C19 | 1.3 (6) |
| C7—N2—C8—C13 | −146.6 (4) | O5—C18—C19—C20 | 179.3 (3) |
| C13—C8—C9—C10 | 2.1 (5) | C17—C18—C19—C20 | −2.4 (6) |
| N2—C8—C9—C10 | 179.3 (3) | C18—C19—C20—C15 | 1.7 (6) |
| C8—C9—C10—C11 | −0.9 (5) | C16—C15—C20—C19 | 0.3 (6) |
| C8—C9—C10—C14 | −176.8 (3) | N3—C15—C20—C19 | 178.3 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2N···O4i | 0.88 | 2.09 | 2.908 (4) | 155 |
| N3—H3N···O3ii | 0.88 | 2.21 | 3.056 (4) | 163 |
| C5—H5···O2iii | 0.95 | 2.28 | 3.093 (5) | 143 |
| C19—H19···O1iv | 0.95 | 2.44 | 3.365 (5) | 164 |
| C21—H21C···O1v | 0.98 | 2.64 | 3.490 (5) | 145 |
Symmetry codes: (i) −x+1, y+1/2, −z+1; (ii) −x, y+1/2, −z+1; (iii) x, y−1, z; (iv) x−1, y, z+1; (v) −x+1, y−1/2, −z+1.
Funding Statement
This work was funded by Banco de la República, Colombia grant CI 1169. Universidad del Valle grant .
References
- Agilent (2014). CrysAlis PRO. Agilent Technologies Ltd, Yarnton, England.
- Agnelli, G., Buller, H. R., Cohen, A., Curto, M., Gallus, A. S., Johnson, M., Porcari, A., Raskob, G. E. & Weitz, J. I. (2013). N. Engl. J. Med. 368, 699–708.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- Etter, M. C. (1990). Acc. Chem. Res. 23, 120–126.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Frisch, M. J., et al. (2009). GAUSSIAN09. Gaussian Inc., Wallingford, CT, USA.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hathwar, V. R., Sist, M., Jørgensen, M. R. V., Mamakhel, A. H., Wang, X., Hoffmann, C. M., Sugimoto, K., Overgaard, J. & Iversen, B. B. (2015). IUCrJ, 2, 563–574. [DOI] [PMC free article] [PubMed]
- Hirata, Y., Sasaki, T., Kanki, H., Choong, C.-J., Nishiyama, K., Kubo, G., Hotei, A., Taniguchi, M. & Mochizuki, H. (2018). Sci. Rep. 8, 1400, 1–9. [DOI] [PMC free article] [PubMed]
- Lahm, G. P., Selby, T. P., Freudenberger, J. H., Stevenson, T. M., Myers, B. J., Seburyamo, G., Smith, B. K., Flexner, L., Clark, C. E. & Cordova, C. (2005). Bioorg. Med. Chem. Lett. 15, 4898–4906. [DOI] [PubMed]
- Lahm, G. P., Stevenson, T. M., Selby, T. P., Freudenberger, J. H., Cordova, D., Flexner, L., Bellin, C. A., Dubas, C. M., Smith, B. K., Hughes, K. A., Hollingshaus, J. G., Clark, C. E. & Benner, E. A. (2007). Bioorg. Med. Chem. Lett. 17, 6274–6279. [DOI] [PubMed]
- Laskowski, R. & Swindells, M. (2011). J. Chem. Inf. Model. 51, 2778–2786. [DOI] [PubMed]
- Lee, S., Lee, W., Nguyen, T., Um, I., Bae, J. & Ma, E. (2017). Int. J. Mol. Sci. 18, 1144–1170. [DOI] [PMC free article] [PubMed]
- Liu, J., Sun, C., Ma, W., Lu, Y.-J., Yu, L., Zhang, K. & Zeng, H. (2014). RSC Adv. 4, 54469–54473.
- Lu, T. & Chen, F. (2012). J. Mol. Graphics Modell. 38, 314–323. [DOI] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Pinto, D., Orwat, M., Koch, S., Rossi, K., Alexander, R., Smallwood, A., Wong, P., Rendina, A., Luettgen, J., Knabb, R., He, K., Xin, B., Wexler, R. R. & Lam, P. Y. S. (2007). J. Med. Chem. 50, 5339–5356. [DOI] [PubMed]
- Prasad, L. G., Krishnakumar, V., Nagalakshmi, R. & Manohar, S. (2011). Mater. Chem. Phys. 128, 90–95.
- Racagni, G., Canonico, P. L., Ravizza, L., Pani, L. & AMoRe, M. (2004). Neuropsychobiology, 50, 134–143. [DOI] [PubMed]
- Rigsby, R. & Parker, A. (2016). Biochem. Mol. Biol. Educ. 44, 433–437. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spackman, M. A. & Jayatilaka, D. (2009). CrystEngComm, 11, 19–32.
- Tohnishi, M., Nakao, H., Furuya, T., Seo, A., Kodama, H., Tsubata, K., Fujioka, S., Kodama, H., Hirooka, T. & Nishimatsu, T. (2005). J. Pestic. Sci. 30, 354–360.
- Trott, O. & Olson, A. (2010). J. Comput. Chem. 31, 455–461. [DOI] [PMC free article] [PubMed]
- Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). CrystalExplorer17. The University of Western Australia. http://https://crystalexplorer. scb. uwa. edu. au/.
- Xing, J., Yang, L., Zhou, J. & Zhang, H. (2018). Bioorg. Med. Chem. 26, 5987–5999. [DOI] [PubMed]
- Yan, Y., Qin, B., Ren, C., Chen, X., Yip, Y. K., Ye, R., Zhang, D., Su, H. & Zeng, H. (2010). J. Am. Chem. Soc. 132, 5869–5879. [DOI] [PubMed]
- Yi, H.-P., Wu, J., Ding, K.-L., Jiang, X.-K. & Li, Z.-T. (2007). J. Org. Chem. 72, 870–877. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020013730/dj2013sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020013730/dj2013Isup2.hkl
Synthesis and characterization of (I). DOI: 10.1107/S2056989020013730/dj2013sup3.pdf
Supporting information file. DOI: 10.1107/S2056989020013730/dj2013Isup4.cml
CCDC reference: 2016019
Additional supporting information: crystallographic information; 3D view; checkCIF report








