Abstract
Thr hypothalamic-pituitary-adrenal (HPA) axis, engages biological pathways throughout the brain and body which promote adaptation and survival to changing environmental demands. Adaptation to environmental challenges is compromised when these pathways are no longer functioning optimally. The physiological and behavioral mechanisms through which HPA axis function influences stress adaptation and resilience are not fully elucidated. Our understanding of stress biology and disease must take into account the complex interactions between the endocrine system, neural circuits, and behavioral coping strategies. In addition, further consideration must be taken concerning influences of other aspects of physiology, including the circadian clock which is critical for regulation of daily changes in HPA activity. While adding a layer of complexity, it also offers targets for intervention. Understanding the role of HPA function in mediating these diverse biological responses will lead to important insights about how to bolster successful stress adaptation and promote stress resilience.
Keywords: Neurobiology, neuroendocrine, brain, allostasis, circadian rhythms
1. Introduction
1.1. Defining Stress
The term “stress” has a complicated meaning. Stress not only refers to challenges imposed on an organism by the external or internal environment but is also used to describe the processes engaged by an organism to cope with such demands. Stress can be defined as any stimulus or experience which threatens homeostasis [1; 2]. Throughout evolutionary history, these threats were largely imposed by challenges originating in the external environment, such as predation, infection, and starvation. However, while human physiology evolved in these conditions, modern advances in technology greatly reduced the likelihood that one would frequently face such direct challenges to survival. In place of these direct threats to well-being, stress in modern society is most commonly experienced in response to internally-generated challenges. Importantly, the same biological systems are engaged whether a person is facing a real threat to survival or instead perceives a situation as threatening. The near constant engagement of these systems, in the absence of actual danger from the environment, causes wear and tear on tissues and organ systems throughout the body, increasing susceptibility to disease and accelerating the detrimental processes of aging [3; 4].
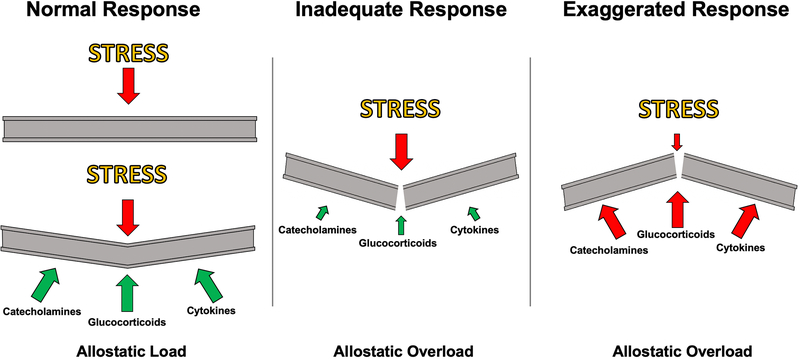
The physiological response to stress prepares the body for action in the face of threatening events through the coordinated regulation of biological systems throughout the organism. The biological systems involved in the stress response are highly conserved between vertebrates, and largely rely on the catecholamine and glucocorticoid secretion into the circulation to appropriately synchronize tissue and organ-specific responses to stress [5]. Acutely, these molecules activate classic “fight or flight” processes, by increasing energy availability by stimulating cytokine production, mobilizing glucose in peripheral tissues, increasing cardiovascular tone, while suppressing digestion and reproductive functions [3; 6]. In the face of a threatening situation, these responses increase chances of survival, while reducing investment in processes not directly related to that immediate goal. However, if stress responses are engaged in an exaggerated fashion (e.g. too readily, too frequently), or inadequately, these same processes can cause a breakdown in normal bodily function and predispose an organism to disease (Figure 1) [3; 7].
Figure 1. Illustration of interactions between stress and physiological responses can affect stress resilience.
Stress can lead to strain on an organism, and the response (e.g. catecholamines, glucocorticoids, cytokines) supports adaptation to this increased “allostatic load” (left). However, if the stress response is inadequate (middle), or exaggerated/protracted (right), the increased load can lead to the physiological systems being placed into a state of “allostatic overload”. In fact, the very stress resposnes that have evolved to enable stress adaptation may lead to allostatic load and overload themselves, even in the face of a small additional stressor (right), which may be a substrate for stress resilience.
1.2. Allostasis and allostatic load
The term “allostasis” has been conceived to describe the active physiological process of adapting to stress [8]. While homeostasis maintains individual physiological parameters (e.g. blood pressure, body temperature, blood pH) reactively within a relatively constant range of values necessary for life, allostatic processes are engaged in both an anticipatory and reactive manner to maintain homeostatic equilibrium when pressure from the internal or external environment requires one or more of these vital physiological parameters shift outside of their normal range [3; 9]. For example, in anticipation of a threatening situation, and subsequent exposure to a threat, an organism may need to maintain elevated blood pressure and circulating glucose levels in order to escape from a predator or other environmental hazard. The systems that mediate allostasis include the hypothalamic-pituitary adrenal (HPA) axis, the autonomic nervous system, metabolic systems, and the immune system [7]. While helpful in isolated situations of survival, the frequent, prolonged, or inadequate engagement of these systems can lead to wear and tear on tissues and organ systems, an effect defined as “allostatic load”. To continue the example above, while acute increases in blood pressure are helpful in mobilizing a response to an acute threat, chronically elevated blood pressure can lead to hypertension and increased risk for heart failure [3; 7]. The relationship between allostatic load and physiological performance follows an inverted “U” shaped dose-response curve, by which physiological responses to a given challenge that are chronically either inadequate or too excessive can lead to a breakdown in bodily function and the development of disease states (Figure 1) [3; 4]. Understanding the links between dysregulation of allostatic systems and the development of stress-related diseases is one of the key aims of current stress research efforts.
2. The hypothalamic-pituitary-adrenal axis
2.1. Overview
The biological stress response is largely dependent on the release of endocrine signals into the circulation which are produced in response to neuronal transmission at key neuroendocrine junctions. In vertebrates, this coordinated cascade is carried out by the HPA axis. In short, after a stressor is perceived via sensory inputs to the central nervous system, corticotrophin-releasing hormone (CRH) is synthesized and secreted from neurons in the paraventricular nucleus of the hypothalamus (PVH) into the median eminence, reaching proopiomelanocortin (POMC)-containing cells in the anterior lobe of the pituitary via the hypophyseal portal system. This stimulates the synthesis and secretion of adrenocorticotrophic hormone (ACTH) into the circulating blood, which, in-turn, stimulates the synthesis and secretion of glucocorticoids from the adrenal zona fasciculata into the circulation [1; 10]. The primary glucocorticoids secreted by the adrenal gland in response to stress are cortisol in humans and other primates, and corticosterone (CORT) in murine animals. While it has many different biological effects, CORT acts primarily to increase metabolic rate in peripheral tissues by stimulating glucose mobilization [6]. The metabolic effects of CORT work synergistically with the sympathetic nervous system to promote survival in the fight-or-flight response. A key characteristic of the neuroendocrine HPA response is that it functions as a negative feedback loop. Specifically, CORT exerts an inhibitory effect at the hypothalamus and pituitary, which decrease secretion of CRH and ACTH, respectively [11; 12]. This inhibition occurs directly via the binding of CORT to glucocorticoid receptors (GR) in these regions, and also indirectly via GR binding in upstream limbic brain regions which have inhibitory projections to the PVH and pituitary [10; 13].
2.2. Corticotropin-releasing hormone
CRH is a critical upstream effector of pituitary and adrenal hormone secretion into the circulating blood. This is accomplished through projections from CRH-producing neurosecretory neurons in the hypothalamus to the median eminence, reaching POMC-containing corticotrophin neurons of the anterior pituitary via the hypophyseal portal system [1]. Acting on type 1 CRH receptors (CRHR1) in the anterior pituitary, CRH stimulates the synthesis and secretion of ACTH into the circulating blood. CRHR1 is a G-protein coupled receptor (GPCR) that acts primarily through Gαs coupling [14], and stimulates ACTH synthesis through cAMP-mediated increases in POMC expression [15]. POMC is then synthesized into ACTH through proteolytic cleavage by prohormone convertase enzymes and secreted upon cellular stimulation [16].
In addition to the PVH, CRH is produced in multiple extrahypothalamic regions of the mammalian brain [17], mediating key autonomic and behavioral responses to stress. In support of this, CRH projections from limbic and brainstem structures appear to play a role in locomotor, metabolic, and emotional function during the stress response [18; 19; 20]. Furthermore, selective inactivation of the CRHR1 gene in the forebrain (while pituitary CRHR1 is maintained) results in reduced anxiety-like behavior in response to stress [21].
2.3. Adrenocorticotropic hormone
ACTH is one of many peptide hormones synthesized and secreted by POMC-containing neurons of the anterior pituitary into the circulating blood. This hormone is a critical mediator of glucocorticoid release, acting at the level of the adrenal zona fasciculata to induce the synthesis and secretion of CORT into the bloodstream [16; 22]. The effects of ACTH on glucocorticoid regulation are mediated by type 2 melanocortin receptors (MCR2) in the adrenal cortex [23]. The MCR2 is a GPCR that, when bound to ACTH stimulates adenylyl cyclase and increases intracellular cAMP to promote the synthesis of CORT from 11-deoxycorticosterone by the enzyme 11β-hydroxylase [24; 25]. Importantly, genetic knockout studies have shown that MCR2-deficient mice have undetectable levels of CORT, while retaining normal levels of circulating ACTH [23]. In addition to its roles in glucocorticoid synthesis, ACTH plays a key role in adrenal growth and health. In support of this, ACTH deficiency decreases, and ACTH treatment increases the volume of the adrenal zona fasciculata through effects on cell differentiation [16]. Furthermore, ACTH levels are highly correlated with the expression of antioxidant enzymes, suggesting a protective role of ACTH on adrenal function [26].
2.4. Glucocorticoids
CORT is synthesized and released into the circulating blood by the adrenal cortex (zona fasciculata). This endocrine signal acts on nearly every tissue in the body and nervous system to generally increase energy mobilization, and importantly, also acts as a negative feedback signal to the hypothalamus and pituitary to inhibit HPA output [6; 27]. CORT acts on both mineralocorticoid receptors (MR) and GR, which can modulate both metabolic and neurobehavioral aspects of the stress response [28]. The MR is thought to play an important role in electrolyte homeostasis in the periphery, where aldosterone is the preferred ligand; however CORT is the preferred ligand in the central nervous system [28].The MR binds to CORT with a ten-fold higher affinity than the GR, and thus, even under basal (non-stressed) conditions, it is thought that 80% of MRs are occupied by CORT [29]. Although research into the function of the MR in the brain has been sparse, there is evidence to suggest that these receptors play a regulatory role in the neuroendocrine stress response and also in glutamatergic synaptic transmission [30; 31].
Compared to MR, much more is known about the function of the GR in the brain. The GR is thought to mediate neuroendocrine function under stress-induced levels of CORT, leading to decreases in hypothalamic CRH and pituitary ACTH secretion [32]. This occurs through inhibitory actions of GR in the hypothalamus [33], as well as excitatory effects in key limbic regions of the brain which indirectly inhibit hypothalamic activity, namely the medial prefrontal cortex (mPFC) and hippocampus [34]. The GR exerts its effects through rapid engagement of cellular signaling systems as well as through slower-acting effects on cellular adaptation via control of gene expression [6–8]. The rapid, nongenomic actions of GR in the central nervous system are primarily mediated by membrane-bound receptors [35]. These actions have been well studied in the context of synaptic excitability and glucocorticoid-mediated negative feedback to the HPA axis (discussed below). For example, in synaptosomes isolated from the mPFC, CORT rapidly potentiates evoked glutamate release through GR-mediated increases in SNARE complex proteins [36]. Membrane-bound GR has also been shown to indirectly influence synaptic excitability through rapid induction of endocannabinoid signaling in the hypothalamus [11]. This implies that nongenomic GR signaling plays an important role in the regulation of excitatory synaptic tone. The genomic effects of GR are mediated by receptors located in the cytoplasm [37]. Once bound to glucocorticoids, a conformational change and phosphorylation by MAP kinase induce translocation of the GR to the nucleus, where GR binds to glucocorticoid response elements (GRE) in the promoter region of target genes [38]. The binding of GR to the GRE then induces gene transactivation or transrepression through the recruitment of general transcription factors [39]. A large portion of the human genome (10–20%) is regulated by the genomic effects of the GR [40]. Included in this group are genes related to metabolism, inflammation, synaptic excitability, and neuronal plasticity [41].
2.5. Glucocorticoid Negative Feedback
2.5.1. Direct feedback to the HPA axis
Direct glucocorticoid-mediated negative feedback to the HPA axis at the level of the hypothalamus occurs through both delayed and rapid feedback mechanisms. Studies have shown that glucocorticoids applied to the hypothalamus decrease CRH mRNA levels, which subsequently reduces the downstream secretion of ACTH and CORT [42; 43]. This delayed glucocorticoid mediated negative feedback in the hypothalamus thus occurs as a consequence of genomic actions of the GR. In addition to genomic action, the presence of glucocorticoids in the hypothalamus quickly reduces glutamate-mediated excitatory synaptic currents in CRH neurons via a GR-mediated mechanism [13]. Rapid glucocorticoid-mediated negative feedback is accomplished primarily by stimulation of local endocannabinoid synthesis and release into glutamatergic synapses; causing an overall depression of excitatory neurotransmission [11; 44; 45]. The contribution of endocannabinoid synthesis to glucocorticoid-mediated negative feedback in the hypothalamus is supported by the following: 1) cannabinoid receptor antagonism completely abolishes glucocorticoid-induced suppression of excitatory post-synaptic currents in the PVH [33; 46], and 2) glucocorticoids increase the levels of the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) in hypothalamic tissue preparations in vitro and in vivo [46; 47]. Taken together, these findings implicate that both delayed and rapid glucocorticoid-mediated feedback to the hypothalamus are involved in the regulation of HPA output.
2.5.2. Indirect feedback to the HPA axis
Circulating glucocorticoids also negatively modulate HPA output indirectly, via action in limbic brain regions which send projections to the hypothalamus. This indirect feedback occurs via multi-synaptic connections between the mPFC and hippocampus to the PVH, via a sign-changing relay in the bed nucleus of the stria terminalis (BNST) [48; 49]. The involvement of the mPFC and hippocampus in glucocorticoid-mediated negative feedback was initially discovered in lesion studies, which found that damage to either of these regions leads to potentiation of stress-induced glucocorticoid secretion [50; 51; 52]. Additionally, local glucocorticoid administration in these regions reduces HPA output [53].
Anatomical tracing studies have revealed that both the mPFC and hippocampus exert their inhibitory effect on HPA activity via a relay in the BNST [49]. This region has a dense population of inhibitory GABAergic neurons that project to neurosecretory CRH cells in the hypothalamus, down-regulating their activity [49]. Glucocorticoids increase activity of mPFC and hippocampus pyramidal neurons [34; 54], therefore it is hypothesized that circulating CORT works to decrease HPA output indirectly by stimulating excitatory projection neurons in these regions, increasing the activity of inhibitory neurons of the BNST and thus decreasing the activity of neurosecretory CRH neurons in the PVH. HPA axis output can also be indirectly stimulated by the central nucleus of the amygdala (CeA). The CeA is thought to stimulate neurosecretory neurons of the PVH indirectly via inhibitory GABAergic projections to the BNST. Consistent with this, lesions of the CeA reduce stress-induced glucocorticoid release and stress-induced anxiety-like behavior [55]. Glucocorticoids may also exert negative feedback actions to the PVH through effects on amygdala function. For example, electrophysiological experiments have revealed that application of glucocorticoids suppresses glutamatergic inputs to the basolateral amygdala (BLA) in vitro via effects on non-genomic endocannabinoid signaling [56]. Together, these findings reveal that glucocorticoid-mediated negative feedback to the HPA axis occurs both directly at the level of the PVH and indirectly through the mPFC, hippocampus, and amygdala. Importantly, feedback signals from these brain regions act to modulate HPA output via common connections to the BNST. The multiple mechanisms of glucocorticoid-mediated feedback to the HPA axis highlight the importance of HPA regulation to homeostatic regulation and health. However, these different mechanisms of feedback also highlight the number of possible areas in which dysfunction can emerge to disrupt normal neuroendocrine stress responses and lead to negative health consequences.
3. HPA influences on mood and behavior: Function and dysfunction
3.1. Overview
As discussed above, the effects of stress on physiology and behavior can depend on the frequency and duration of stress exposure. While biological responses to an isolated exposure to stress, referred to as acute stress, generally only have transient effects on metabolic and neural systems, responses to frequent or prolonged exposure to stress, referred to as chronic stress, generally lead to longer lasting changes in the basal functions of these systems. However, we would be remiss if we neglected the fact that a single intense or traumatic stressor can lead to the development of PTSD, which certainly has long-lasting changes in function, and this depends on a variety of risk factors that seem to reduce resilience in humans (detailed meta-analysis; [57]).
The effects of stress on physiology and behavior are highly dependent on the nature of the stressful stimulus experienced. Even though it is likely not possible to completely dichotomize stress into “physical” vs. “psychogenic”, more physical stressors (e.g. blood loss, cold, infection) engage a reflexive systemic response primarily involving the spinal cord and brainstem pathways, while more psychogenic stressors (e.g. restraint, immobilization, novel environment) involve additional processing in forebrain circuits, such as the amygdala, prior to HPA activation [2; 58; 59]. Furthermore, some stressors involve a combination of physical and psychogenic insults (e.g. foot shock, forced swim). The distinction between stressor types has important implications for interpreting the outcomes of stress exposure, as each may signal via different neural circuits. To directly test this hypothesis, a study by Dayas et. al measured the activation of hypothalamic, brainstem, and amygdala sub-regions following exposure to different stressors [60]. Their findings indicated that while all stressors measured similarly increased the activity of hypothalamic CRH neurons, physical stressors (arterial hemorrhage, ether exposure) preferentially engaged neurons in the rostral region of the dorsomedial medulla and the CeA, and psychogenic stressors (noise, restraint) preferentially engaged neurons in the caudal region of the dorsomedial medulla and the medial amygdala (MeA). Furthermore, Pacak et. al showed that different stressor types have different effects on patterns of brain-wide Fos immunoreactivity and neuroendocrine stress responses [2; 61], further illustrating that the nature of the stressful stimulus can have a large impact on ensuing physiological responses.
In addition to stressor type, the impact of stress on physiology and neural circuits is highly dependent on the chronicity and pattern of exposure. Stress habituation is the process by which the physiological or behavioral responses to stress decrease following repeated exposure to the same (homotypic) stressor over time. However, as discussed by Herman, while stress habituation can reduce the overall physiological burden of chronic stress exposure, it does not represent a return to “normal” physiologic status [62]. Rather, these habituated responses are driven by long-term adaptation in central stress circuits, including the hypothalamus [62]. The central processes of stress habituation appear to be regulated in part by glucocorticoids, as MR antagonism on the final day of repeated exposure to restraint stress prevents habituation of stress-induced CORT secretion [63]. Habituation of the HPA response to stress has been observed in response to repeated exposure to both physical and psychogenic stressors, including restraint, forces swim, and cold stress [64]. However, if an animal is exposed to a stressor that is varied (heterotypic) over time, HPA habituation does not occur to the same extent [62; 65]. Additionally, if an animal is exposed to a novel stressor following chronic stress, they display an exaggerated HPA response [62; 66]. This phenomenon is referred to as stress sensitization, and occurs after both homotypic and heterotypic chronic stress exposure [62]. The process of stress sensitization is thought to be driven in part by chronic stress-induced increases in hypothalamic CRH expression and noradrenergic drive to the PVN which occur as a result of chronic stress exposure [65; 67].
3.2. Modeling HPA dysfunction in rodents
In this section, we will describe four (non-exhaustive) approaches to alter HPA function, as well as the wider brain circuits involved in stress responses, in order to understand how they contribute both to normal, and abnormal, neurobehavioral responses to stress.
3.2.1. Adrenalectomy
The necessity and sufficiency of HPA-secreted hormones to alter physiology and behavior can be tested with various models of removal and replacement. The most widely used method of glucocorticoid removal is bilateral adrenalectomy (ADX). This procedure involves surgical removal or destruction of the adrenal glands, and completely abolishes circulating CORT levels following stress [68; 69]. ADX is useful as it allows one to test the necessity of glucocorticoid secretion to physiological or behavioral parameters of interest. Additionally, this method allows one to test the sufficiency of CORT in mediating these measures, as known concentrations of CORT can be reintroduced into the circulation via injection or the subcutaneous implantation of a pellet which continuously releases a tonic amount of CORT. ADX has been well-studied for its effects on peripheral metabolism. In both rats and mice, ADX typically decreases body weight gain and white adipose tissue (WAT) deposition [70; 71; 72]. However, some studies in mice report no change in body weight [73], suggesting that the metabolic effects of glucocorticoid depletion may be species-dependent. Furthermore, replacement of CORT via pellet implantation restores normal body weight and WAT in rats, however CORT-induced WAT increases are preferentially deposited in abdominal fat stores [70; 74]. In the brain, ADX increases basal hypothalamic CRH expression and significantly enhances stress-induced ACTH secretion, effects which are not rescued by CORT replacement [53; 69]. Furthermore, while ADX reduces extracellular glutamate responses to stress in the mPFC and hippocampus (discussed above) [75; 76], patterns of c-fos responses to stress across the brain were not affected by ADX or CORT replacement [77].
While the ADX model has been useful for elucidating some important mechanisms of glucocorticoid necessity and sufficiency, the technique has some important limitations in its use as a model of HPA dysfunction for understanding disease states. First, the surgical procedure used to perform ADX may induce pain or stress itself, which leads to confounds when comparing measures between “baseline” and stressed animals. Additionally, the basal increases in hypothalamic CRH and circulating ACTH are confounding in the interpretation of stress-related measures, as these hormones can have direct effects on the processes in the periphery and brain [16; 78]. Finally, while ADX removes plasma CORT responses to stress, other important functions (e.g. aldosterone, epinephrine) secreted by the adrenal are also lost. For example, while ADX completely abolishes circulating epinephrine levels, extra-adrenal epinephrine secretion from the SNS increases as a compensatory response [79; 80], which can cause significant effects on autonomic functions [81].
3.2.2. Genetic models
In addition to directly removing glucocorticoid secretion, disruption of the HPA axis can be achieved using genetic manipulations which interfere with glucocorticoids signaling. Genetic manipulations are useful tools, as they overcome the need for extensive manipulations that may be stressful or harm the animal. Furthermore, many stress-related health disorders have a heritable component [82; 83], giving genetic methods added translational value in understanding the etiology of these diseases. The GR and FK506 binding protein 51 (FKBP5) are main targets for genetic modulation of HPA function, as polymorphisms in these genes are associated with risk of MDD and PTSD [84; 85; 86]. Genetic GR knockout in mice impairs glucocorticoid negative feedback, resulting in elevated levels of circulating ACTH and CORT [87; 88]. Additionally, forebrain-specific GR knockout mice exhibit increased basal CORT levels and show markers of behavioral despair and anhedonia, similar to characteristics of MDD [89]. In contrast, genetic knockout of FKBP5, a negative modulator of GR signaling, results in decreased circulating CORT levels and enhanced GR-mediated negative feedback to the HPA axis [90]. Beyond GR and the HPA-axis, recent work has shown that GABAergic neurons in the forebrain contribute to stress resilience. Genetic deletion of CRH in these neurons can reduce stress-related changes in neuronal activity, and promotes resilience to stress, suggesting that CRH activity in these neurons may be a critical extra-HPA node the modulates resilience/vulnerability [91].
While these genetic tools are useful in modeling HPA dysregulation, these techniques still possess several drawbacks. First, while polymorphisms in the GR and FKBP5 genes are associated with stress susceptibility, a change in GR or FKBP5 function in humans is different than complete knockout observed in genetic manipulations in mice. For example, global GR knockout is lethal in 90% of mice at the time of birth [88]. Next, in translating the effects of genetic deletion to human health disorders it is difficult to dissociate effects strictly affecting HPA axis function vs those effects caused by interruption of normal developmental processes. For instance, glucocorticoid signaling is involved in processes of neuronal migration and myelination of the developing brain [92; 93]. Furthermore, heritability is estimated to only account for 30–40% of risk for developing either MDD or PTSD [82; 83], indicating that results obtained from genetic knockout studies cannot fully explain mechanisms of stress vulnerability in the human population. While these genetic approaches are important for the physiological dissection of the circuits underlying HPA function and dysfunction, their relevance for many diseases/disorders is likely limited. A more powerful approach may be to more fully characterize the functional changes in other gene mutations outside of classic HPA-axis/stress pathways that are known to modulate resilience and susceptibility to stress-related neuropsychiatric disease. For instance, the val66met polymorphism in brain derived neurotrophic factor (BDNF) is associated with altered stress resilience in humans [94; 95; 96; 97], and work with the as has val66met humanized mouse line aligns well with these findings [98; 99], providing yet another approach to study the effects of extra-HPA factors on stress resilience.
3.2.3. Optogenetic and Chemogenetic models
Contemporary neurobiological approaches that employ genetic tools have allowed for a further dissection of the underlying circuits involved in enhancing resilience or imparting vulnerability. The use of optogenetic and pharmacogenetic approaches has allowed for the temporally restricted manipulation of genetically defined cell-types within different components of the HPA circuitry. Within the HPA, CRH neurons of the PVH contribute to the generation of different stress-induced behaviors, as optogenetic manipulation of these cells can clearly alter their expression and magnitude [100]. Similarly, a local CRH circuit within the PVH has been implicated in both the regulation of the neuroendocrine response to stress, as well as contributing to the activity of PVH targets that are important outputs of this nucleus [101]. Outside of the HPA, similar approaches have provided remarkable new insights into the wider brain networks important in conferring resilience. While the role of the BNST has been well documented in modulation of HPA activity and stress responses via classic neuroanatomical, lesion, and pharmacologic approaches, newer techniques allow for a finer grained dissection of these circuits. Application of optogenetic approaches combined with neuroanatomical tracing has revealed that the anteroventral subdivision of the BNST can inhibit HPA output while also changing passive coping behaviors [102], thus further refining our understanding of the BNST as an important integrative and modulatory node of stress induced changes in physiology and behavior. Use of social defeat models in mice, which causes depressive-like behaviors in a subset of individuals, has uncovered an important role for midbrain dopaminergic (DA) neurons in resilience. Changes in the biophysical characteristics of ventral tegmental area (VTA) DA neurons following social defeat stress reveals that mice resilient to developing depressive-like behaviors have an enhanced hyperpolarization current, and that by optogenetically increasing this current in susceptible mice imparted resilience [103]. In a similar line of work, locus coeruleus (LC ) neurons that project to the VTA have increased firing in resilient mice exposed to social defeat, an effect not observed in susceptible mice. Optogenetic approaches to mimic this increased firing in this circuit can increase resilience in susceptible mice, while pharmacological antagonism of adrenergic signalling in this circuit can block the optogenetic rescue [104]. Related, medium spiny neurons in the nucleus accumbens are implicated in modulating resilience to chronic social defeat, and optogenetic stimulation of these neurons can bidirectionally alter the outcome of this stressor on depressive-like behaviors [105].
3.2.4. Chronic corticosterone via drinking water
Our group and others have shown that chronic treatment with CORT in the drinking water of mice causes changes in HPA activity. For instance, in male mice, the stress-induced rise in CORT is prevented following treatment with oral administration of CORT [106; 107]. Furthermore, in contrast to ADX, which only prevents glucocorticoid secretion, chronic CORT administration also results in reduced hypothalamic CRH mRNA expression and prevents the stress induced rise in ACTH [106]. This reduces confounds of compensatory CRH and ACTH increases observed after ADX. One large advantage of this method is its non-invasive nature as no surgical manipulations or injections are required to achieve downregulation of the HPA stress response, reducing the confounds associated with surgical procedures or injections which may be stressful themselves [108; 109; 110]. An additional benefit is the maintenance of a diurnal CORT rhythm, driven by the diurnal patterns of drinking behavior in mice [106; 111]. The circadian variation in CORT plays a large role in entrainment of central and peripheral clocks, and is known to cause negative effects when absent or flattened [112]. This technique also results in atrophy of the adrenal gland, however reductions are largely localized to the adrenal zona fasciculata (site of glucocorticoid production) while not affecting the adrenal zona glomerulosa (site of aldosterone production) [111; 113]. Thus, the structures supporting other physiological functions of the adrenal gland are maintained while HPA downregulation is achieved, though whether the production or secretion of aldosterone and/or other adrenal factors (e.g. catecholamines) is altered in this model has not been explored. Furthermore, since the adrenal is not removed, this model allows one to investigate recovery, as many of the physiological changes are reversible [113; 114], allowing for the study of long-term consequences of HPA disruption after recovery of normal HPA function. This is a notable advantage in understanding stress-related pathological states, as a history of HPA dysregulation can have long-term effects on health [115; 116]. This method is not without its limitations. For example, this treatment decreases thymus weight, indicating potential impacts on immune function [111; 113]. Also, slight increases in body fat and decreases in lean mass are observed, as well as increases in plasma insulin and leptin, indicating effects on peripheral metabolism [111; 114]. It is also important to note that previous work showed that oral CORT administration at the same dose (25μg/ml) for 20 days resulted in neural structural changes and behavioral despair in mice characteristic of chronic stress exposure [117; 118]. However, these studies used CORT hemisuccinate rather than un-modified pure crystalline CORT, which resulted in plasma CORT levels after drinking well above physiological levels secreted endogenously during stress (>800ng/ml) [117; 118], while the circulating CORT concentrations achieved by drinking using un-modified CORT are well below stress levels (~100ng/ml) [106; 111].
3.3. Impacts of HPA function on Emotional behavior
Given the pervasive impacts of stress on neural function in limbic brain regions such as the mPFC, hippocampus, and amygdala, it is not surprising that stress also exerts a strong influence on emotional behaviors. Importantly, the expression of these behaviors depends on the internal and external context which they are measured. Behavioral responses to acute stress exposure reflect the engagement of processes necessary for survival, generally including increased vigilance and anxiety and decreased exploration and reward-seeking [3]. In rodents, acute stress exposure increases measures of anxiety, including reduced exploration in the open field test and light/dark transition task [119; 120]. Work by Gray et. al revealed that CRH in the amygdala leads to anxiety-like behavior through an endocannabinoid-mediated mechanism [121]. Specifically, following acute stress, CRH activity in the BLA decreases endocannabinoid levels, disinhibiting the amygdala to then initiate anxiety-like behavior [121]. These results are consistent with other studies which show that genetic knockout of the CRH receptor decreases anxiety-like behaviors in rodents [15]. In the short term, anxiogenic effects of stress may be adaptive in that they increase the chance for an animal to escape acute danger [122]. However, chronic engagement of these behavioral responses can lead to the persistence of anxiety even in the absence of acute threat. For example, Nasca et. al showed that, while anxiety-like behaviors measured in a light/dark transition task were increased immediately after exposure to acute restraint stress exposure in mice, chronic restraint stress caused a similar increase in anxiety even in the absence of an acute challenge [119]. While seemingly negative, these prolonged changes in behavior induced by chronic stress exposure can be thought of as adaptive. As discussed by Herman, increases in anxiety and vigilance minimize risk in a hostile environment, and therefore the development of so-called “pathological” behavior in response to chronic stress exposure may be a way for an organism to overcome present danger at the expense of long-term success [62]. In addition to increasing basal measures of anxiety, chronic stress exposure also increases the expression of “depressive-like” behaviors such as increased behavioral despair and anhedonia, a phenomenon that is of high translational relevance to human stress disorders [123; 124]. In both rats and mice, chronic stress exposure increases measures of behavioral despair (e.g. forced swim and tail suspension test immobility) and anhedonia (e.g. sucrose preference and social interaction) [119; 124; 125]. The mechanisms driving these behavioral changes are not well understood, but can be reversed by treatment with antidepressants [124]. Strong links have been made between dysregulation of glutamate signaling in the mPFC and hippocampus and the development of depressive-like behaviors [126; 127]. While chronic restraint stress increases immobility in the forced swim test and decreases social interaction, these effects can be prevented by pharmacological or genetic overexpression of xCT in the ventral hippocampus [125]. This suggests that changes in synaptic excitability in limbic forebrain structures may underlie the expression of pathological behaviors.
While stress can lead to significant changes in emotional behavior which researchers or clinicians might label as “pathological”, not all individuals exposed to stress will develop these negative outcomes. Understanding the factors which determine an individual’s level of stress resilience or vulnerability is an important task in the goal of treating stress-related psychiatric disorders. Stress coping has emerged as a factor strongly linked to stress resilience. As defined by Koolhaas et al. (1999), this concept refers to the behavioral and physiological efforts made to overcome a stressful situation and depends highly on the perceived controllability of the stressor [128]. Stress coping styles are typically categorized as either proactive or reactive/avoidant in nature [128]. Given that the challenges associated with stress can be complex in nature, relying only one coping style may not necessarily be more successful in every situation. HPA activity is also strongly linked to coping style. Höhne et al. showed that in a sample of subjects with remitted MDD, coping strategy predicted ACTH and CORT responses to an acute stress test [129]. In rodents, proactive and reactive coping strategies are associated with low and high HPA axis stress reactivity, respectively [128]. For example, Pérez-Tejada et al. (2013) showed that mice which exhibited more passive coping behaviors in a repeated social defeat paradigm had greater plasma CORT responses than mice which showed more active coping behaviors [130]. Previous work from our group showed that chronic disruption of normal neuroendocrine HPA stress reactivity in mice is associated with altered stress coping responses in a novel open field test [106; 131]. We showed that following chronic oral administration of glucocorticoids, mice were more resistant to the effects of acute swim stress on self-grooming (reactive) behavior and rearing (proactive) behavior compared to vehicle-treated controls [106; 131]. In addition, these coping responses following acute stress exposure were not dependent on acute glucocorticoid secretion, as acute inhibition of CORT synthesis with metyrapone prior to stress had no effect on open field grooming or rearing behaviors, and acute CORT replacement during stress did not rescue normal stress coping after stress in HPA-disrupted mice [131]. These studies indicated that HPA dysregulation may chronically alter the function of neural circuits underlying stress coping behaviors. We specifically tested how HPA disruption affected the activity of the PVH and the paraventricular thalamic nucleus (PVT) in relation to these altered stress coping responses, as pharmacological and pharmacogenetic stimulation of these regions is known to affect these behaviors [100; 132]. Following chronic HPA disruption, acute stress-induced FOS protein expression was blunted in the PVH and PVT, mirroring effects on grooming and rearing behaviors [131]. These findings revealed a role of these brain regions in mediating the interactions between HPA hormones and stress coping, however more detailed interrogation of these mechanisms needs to be performed before causal relationships can be identified. Further exploration of these stress-responsive diencephalic brain circuits may yield important findings about the regulation of stress coping and could be important targets for therapeutics aimed at reducing the negative impacts of stress on health.
4. HPA dysfunction as a risk factor for disease
4.1. HPA hormones in major depressive disorder and post-traumatic stress disorder
Dysfunction of the HPA axis, particularly in glucocorticoid signaling, is highly associated with stress-related diseases including MDD and PTSD. A large number of depressed patients have increased basal cortisol levels and exhibit pituitary and adrenal enlargement [133; 134]. This elevated HPA output in MDD is thought to be mediated by reduced glucocorticoid-mediated negative feedback, an idea which stems from findings that oral administration of synthetic glucocorticoids fails to reduce cortisol secretion in patients with MDD, whereas the same treatment results in robust suppression of cortisol in healthy individuals [134; 135]. In contrast, a significant portion of individuals with PTSD have lower basal cortisol levels and enhanced glucocorticoid-mediated negative feedback [136; 137]. In addition, lower cortisol levels following awakening have been found in patients with PTSD compared to healthy individuals [138; 139; 140]. The dichotomy of HPA reactivity in MDD and PTSD highlights the inverted “U” shaped relationship between stress mediators and health, however while different patterns of glucocorticoid dysregulation are found between these disorders, it is still unclear how they contribute to negative emotional and cognitive outcomes.
In both MDD and PTSD, changes in structure and function of limbic forebrain regions have been observed. In MDD, decreases in hippocampal volume have been observed in both functional imaging studies [141], and in overall mass from brains collected postmortem from depressed patients [142]. These effects have been attributed to neuronal atrophy and reductions in hippocampal neurogenesis, which also occurs following chronic stress exposure in rodents [143]. Similar decreases in overall size of the mPFC and changes in neuronal morphology have also been observed in MDD [144], indicating similar mechanisms mediating stress-induced changes in each region. One study by Stockmeier and colleagues (2004) showed that while pyramidal cells are reduced in size in the hippocampus in MDD, the density of glial cells is increased, suggesting a role of inflammation in these processes [142]. Importantly, high levels of glucocorticoids mimic the effects of stress on neuronal atrophy and reduced neurogenesis in rodents [145; 146], suggesting a direct link between HPA dysfunction and neural outcomes of MDD in the hippocampus and mPFC. Similar reductions in volumes of the hippocampus and mPFC have been observed in individuals with PTSD compared to healthy individuals [147; 148]. Interestingly, these changes were also observed in comparison to trauma-exposed individuals who did not develop PTSD, suggesting that morphological changes in the hippocampus and mPFC may be a cause, rather than effect of PTSD development [147].
A leading hypothesis for stress-induced neuronal atrophy in the hippocampus and mPFC in human disorders and following stress in rodents relates to changes in synaptic excitability [126; 149; 150]. Abnormal increases in glutamate levels following stress can overstimulate NMDARs, leading to calcium-mediated excitotoxicity and synaptic depression [149; 151]. Furthermore, stress-induced synaptic depression can be mediated through actions of glucocorticoids. In support of this, Yuen and colleagues (2012) showed that prolonged exposure to glucocorticoids facilitated degradation of NMDAR and AMPAR subunits in the mPFC, and GR antagonism blocked repeated stress-induced decreases in excitatory NMDA and AMPA currents [152]. These results suggest that glucocorticoid-mediated synaptic depression may be a compensatory mechanism to reduce excitotoxicity caused by pathological levels of stress and highlight how HPA dysregulation could contribute to increased vulnerability to negative health outcomes related to stress. In addition to glucocorticoids, dysregulation of CRH signaling may also contribute to negative outcomes of stress exposure. In support of this, significantly higher cerebrospinal fluid concentrations of CRH have been observed in individuals with MDD or PTSD, and reduced CRH binding sites have been observed in the frontal cortex of suicide victims [153; 154; 155]. Furthermore, pharmacological or virally-mediated increases in CRH in the brain increase measures of anxiety in rodents, suggesting that CRH signaling is mechanistically linked to control of emotional behavior [156; 157]. However, despite these findings, pharmacological treatments targeting CRHR1 signaling have not been particularly effective in the treatment of MDD, suggesting that the role of dysregulated CRH in human psychiatric disorders may involve other CRH receptor types or interactions with other neurotransmitter systems [158].
4.2. Effects of Disrupted HPA function on Resilience: Timing Matters
Most research on stress and HPA function focuses on the major changes observed in HPA activity following acute and/or chronic stress. However, there is a more subtle aspect of dynamic change in the HPA that does not always receive adequate empirical attention: the influence of time of day on HPA activity, and the importance of this circadian influence on optimal neurobehavioral function and adaptive responses. Circadian rhythms are found in nearly all organisms, from single-celled bacteria to humans. A circadian rhythm is a rhythm that persists in the absence of external cues, and thus is endogenously generated. Current models suggest that circadian rhythms allow organisms to anticipate daily changes in the environment, rather than merely respond to such changes. The circadian system is comprised of both central and peripheral clocks, which work in concert to produce coherent behavioral and physiological rhythms in an organism. In mammals, the master circadian clock is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. This was determined by both lesion studies, which unequivocally demonstrated this necessity of the SCN in generating rhythms [159; 160; 161; 162], and transplants of SCN tissue that restored rhythmicity in hosts whose own rhythms have been eliminated by SCN lesions [163; 164; 165]. Though it is undeniable that the SCN is the master circadian clock, the circadian system can be considered as a hierarchical network of control nodes throughout the brain and body. While the SCN is the master clock it controls a network of oscillators throughout the body, which interact with each other. Given that circadian rhythms have been uncovered in nearly every physiological system, from activity of the HPA axis (details below), to food intake and metabolic processes [166], to immune function [167], and neural plasticity [168], this distributed pseudo-hierarchical model of circadian control is well supported. Taken in this context, it is therefore clear why disruption of the circadian clock has widespread, integrative, and in many cases hormetic effects on the organism, thus affecting the ability of many systems to respond to environmental and/or psychological challenge.
Directly addressing the HPA axis, in all vertebrates that have been examined, including humans, non-human primates, and rodents, circadian fluctuations in glucocorticoid secretion that continue in constant conditions have been observed [161; 169; 170; 171; 172; 173]. In fact, the lack of glucocorticoid rhythms following SCN lesions in rats, were among the first data implicating the SCN as a brain clock [161]. In both nocturnal and diurnal animals, the phase of the rhythm differs, with corticoid secretion beginning to rise before waking, though this may be at the start of the day (in diurnal animals) or start of the night (in nocturnal species) [174; 175; 176]. The SCN is necessary for overt circadian rhythms in CORT. As noted above, lesions of the SCN completely eliminate CORT rhythms [161; 177]. Circadian “forced desynchrony”, which disrupts SCN function can also drive changes in CORT rhythms in rats [178]. Though the adrenals possess the necessary molecular machinery to generate circadian rhythms, adrenal rhythms of clock gene expression are SCN dependent [179]. There are two efferent pathways from the SCN that have been implicated in regulation of the HPA. The first is a monosynaptic projection from the SCN to the CRH neurons in the PVN [180; 181]. Output from the PVN is clearly important in both generating CORT rhythms, as well in response to acute stressors. In addition to this monosynaptic pathway, a multisynaptic SCN-Adrenal cortical pathway has also been described [182]. However, the specific role of this pathway has not yet been fully characterized.
In addition to broad rhythms in CORT and HPA axis activity, it is important to explain the origins of these rhythms in CORT. Heroic work by the Lightman group demonstrate that circulating CORT rhythms are generated by alterations in the profile of pulsatile secretion, occurring in an ultradian fashion, with a period of about 1hr [183; 184; 185]. This ultradian pattern of CORT secretion is driven by feed-forward mechanisms and timed delays in processes at both the pituitary and adrenal levels, and unlike pulsatile rhythms in reproductive hormones, it does not require a pulse generator in the hypothalamus [185]. The significance of these ultradian pulses for overall function is an area of high research activity, with additional findings showing that the adrenal responds optimally to a pulsatile pituitary adrenocorticotropic hormone (ACTH) profile, given that constant ACTH infusion results in significantly reduced CORT levels [186].
Circadian rhythms can be found in many aspects of the HPA axis, and in the brain regions that are important for both cognitive and emotional function. Clock genes, the molecular “gears” of the circadian clock are clearly found at every level of the HPA axis. In the adrenal, clock genes seem to underlie rhythmic responsiveness of the adrenal to pituitary ACTH, and to physiological and physical stressors [179; 187; 188; 189; 190; 191]. A large body of work has illustrated that the core clock protein PER2 is rhythmic in extra-SCN brain areas, including the oval nucleus of the BNST (BNST-OV), and the central (CEA) and basolateral nuclei of the amygdala (BLA) [192; 193]. What makes these rhythms noteworthy is that while BNST-OV and CEA rhythms depend on an intact adrenal, BLA rhythms in PER2 are not affected by adrenalectomy. Indeed, CORT is a key player in this rhythm, as PER2 rhythms in BNST and CEA can be restored with CORT in the drinking water (i.e. a somewhat rhythmic route of administration), but not by subcutaneous pellets (i.e. tonic CORT) [194]. In addition to these subcortical regions, a role for diurnal CORT rhythms has been uncovered in cortical areas, being important for spine turnover in motor cortex and driving performance in motor learning tasks [195]. More recent work has demonstrated that CORT can modulate clock gene expression in the PFC of rats, and these effects can modulate fear learning [196].
4.3. Connecting Circadian Rhythms and HPA Function to Resilience/Vulnerability: A model
We and others have suggested that allostatic load, and overload, contribute to increased vulnerability to stressors, and that buttressed allostatic systems could potentially impart some resilience. But what factors determine how “well” allostatic systems respond to a rapidly changing set of circumstances? Our proposal is that underlying allostasis and the capacity for resilience is an intact and optimally functioning circadian timing system. Our hypothesis is that disruption of the circadian rhythms shifts the organism into a state of higher allostatic load, now being less able to adapt to additional stressors, or changes in stress severity. Evidence exists demonstrating that in rats lacking normal circadian patterns of CORT the HPA axis response is dysregulated, with unreliable/slow termination of the adrenocorticotropic hormone (ACTH) response following the end of a stressor [197]. As noted above, adrenal clock genes also seem to gate normal ACTH sensitivity. Given that CORT is a critical intermediary between the SCN clock and peripheral clocks in many tissues, serving as a humoral synchronizer of these extrahypothalamic clocks further substantiates our proposal that an important relationship exists between disrupted circadian timing and allostatic load. Indeed, a recent review by Rao and Androulakis [198] directly explores the importance of circadian regulation of HPA function in the context of allostatic load.
With respect to the contribution of disrupted clocks to phenotypes that closely mirror instances of high allostatic load, both human and non-human animal studies have been undertaken. Humans who suffer from chronic circadian misalignment show abnormalities in neurobehavioral and physiological function. In a study of flight crews who endure more bouts of jet-lag (short-recovery transmeridian crews) versus crews who fly less transmeridican flights (i.e. more north-south flights; long-recovery crews) show shrunken medial temporal lobes, increased reaction time and poorer performance in visual-spatial cognitive tasks [199]. Physiologically, these populations were also different, in that the short-recovery crews had a significant correlation between salivary cortisol levels and medial temporal cortical volumes, while this correlation was not observed in long-recovery flight crews. The significance of this effect in the temporal lobe with respect to resilience merits some speculation, since in PTSD it has been observed that smaller temporal lobes may impart decreased resilience to negative outcomes of stress [200; 201].
Animal models on circadian desynchronization and stress resilience are still sparse. We have developed a mouse model of chronic circadian desynchronization (CD) in which mice are housed in a 20h light-dark cycle (10hrs light, 10hrs dark; T20) compared to standard 24hr cycles. These mice show metabolic signs of allostatic load, with increased weight, adiposity and leptin levels, as well as an imbalance between insulin and plasma glucose, suggesting a pre-diabetic state. The metabolic changes are accompanied by changes in prefrontal cortex (PFC) cellular morphology, mirroring those observed in chronic stress, with CD animals having shrunken and less complex apical dendritic trees of cells in layer II/III of the medial PFC [202]. The effects are very similar to those observed in 21d of chronic restraint stress in rodents, which results in morphological simplification of prefrontal cortical neurons and impairment in prefrontal mediated behaviors, such as attentional set-shifting or other working memory tasks [203]. Importantly, similar effects are observed in humans [204]. Behaviorally, CD animals show cognitive rigidity in a version of the Morris Water maze, being slower to learn a reversed location of a hidden platform and making more perseverative errors by returning to the original location of the platform [202]. At the same time, CD mice display an “impulsive” like phenotype in the light-dark box, while not showing any outward behavioral anxiety phenotype [202]. Similar work shows that housing mice in a T7 cycle (7hrs light, 7hrs dark) causes particular changes in mood and emotionality, with a direct projection from intrinsically photosensitive retinal ganglion cells to various thalamic regions being implicated [205].
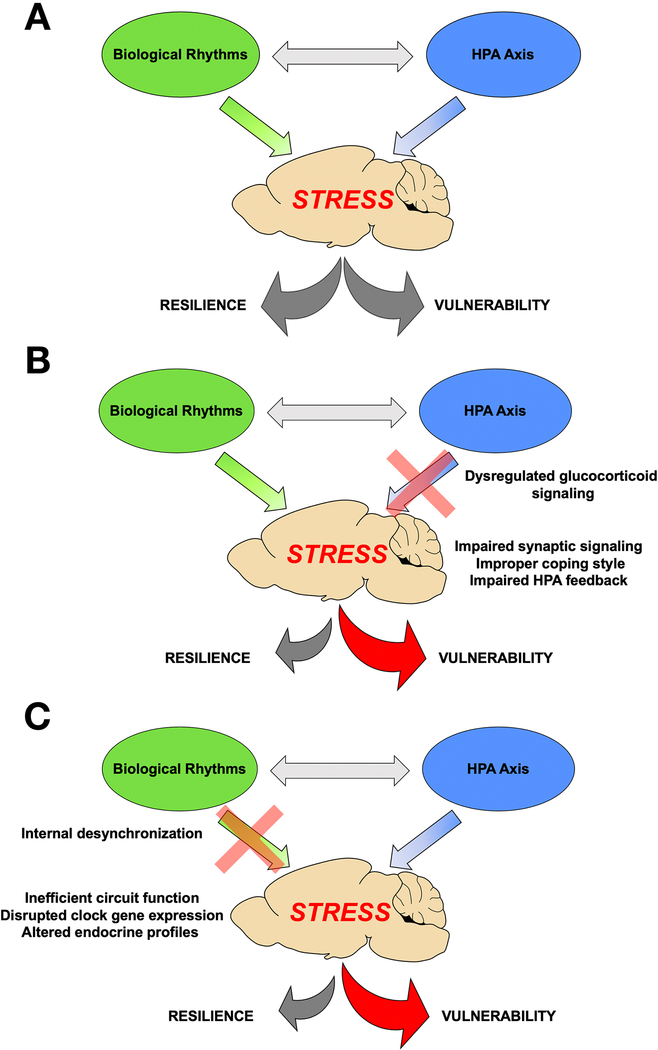
Our model of circadian-allostatic interaction suggests that desynchronization between the master SCN clock and the external environment drives central and peripheral clocks out of phase with each other, leading to the state of internal desynchrony within neural circuits and other critical physiologic systems, such as the HPA axis. This eventually leads to altered neurobehavioral function. A complimentary aspect of this hypothesis is that shorter episodes of circadian disruption could lead to temporary changes in these systems, thereby rendering them more vulnerable to additional perturbation. Thus, both acute and chronic circadian desynchronization results in a whole-body condition that may allow other stressors to overwhelm already compromised networks. Therefore, circadian desynchronization compromises allostatic responses meant to enable organisms adapt to environmental challenge, by disrupting the stress axis, similar to diathesis-stress (e.g. “two hit”) models (Figure 2). Viewing these findings in light of the proposed model could explain many of the epidemiologic findings of increased risk for development of psychiatric, cardiovascular or other physiological syndromes in shift workers or populations undergoing chronic circadian disruption [206; 207; 208; 209].
Figure 2. Hypothalamic Pituitary Adrenal Axis and Biological Rhythms interact to modulate neural, behavioral and physiologicla responses to stress.
Normally functioning HPA responses and biological rhythms promote balance in the neurobehavioral and physiological responses to stress (A). If HPA responses (B) or biological rhythms (C) are disrupted, then the balance can shift form resilience and healthy responses to vulnerability and pathological responses.
Unravelling the mechanisms of this link between circadian rhythms and allostatic load has significant importance for biomedicine, as circadian disruption (e.g. shift work and jet lag) and sleep deprivation are both common in the modern world, and an increasing health concern [210].
5. Summary and Conclusions
The HPA axis is neuroendocrine system which helps organisms adapt to and overcome adverse situations through the secretion of endocrine mediators, including glucocorticoids. This system is subject to extensive physiological regulation and equipped to adjust to differences in stressor type, severity, and chronicity. Dysregulation of HPA axis function is associated with many different metabolic and mental health disorders associated with stress, including MDD and PTSD, highlighting the importance of this system in promoting resilience to adversity. The brain is a major target of stress, and HPA hormones have considerable impacts on neural circuits which regulate cognitive and emotional processing. Furthermore, the ability of HPA hormones to adequately bolster adaptive responses to stress are influenced greatly by circadian rhythms in in all biological processes, which can have profound effects on stress susceptibility.
Our current understanding of the biological factors underlying stress resilience is still relatively nascent, and partially obscured by the vast complexity and individual variability present in physiological, neural, and behavioral pathways. Developing models that consider the interactions between these pathways is necessary in the discovery of novel therapeutic strategies aimed at promoting positive adaptation to stressful life circumstances and decreasing the negative burdens of stress on individuals and on society. Indeed, rodent models, particularly those using chronic psychosocial stress, significantly affect both behavioral and peripheral responses to subsequent physiological challenge [211; 212]. Establishing a unifying theory of stress resilience that incorporates an appreciation of diversity in both biological function and individual experience will be instrumental in improving health outcomes and disease treatment [213].
6. Acknowledgements
This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK119811-01) and a National Science Foundation CAREER Award (1553067) to INK.
7. References
- [1].Smith SM, and Vale WW, The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience 8 (2006) 383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pacak K, and Palkovits M, Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocrine reviews 22 (2001) 502–48. [DOI] [PubMed] [Google Scholar]
- [3].McEwen BS, Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences 840 (1998) 33–44. [DOI] [PubMed] [Google Scholar]
- [4].McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, and Nasca C, Mechanisms of stress in the brain. Nature neuroscience 18 (2015) 1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ulrich-Lai YM, and Herman JP, Neural regulation of endocrine and autonomic stress responses. Nature reviews. Neuroscience 10 (2009) 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Macfarlane DP, Forbes S, and Walker BR, Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. The Journal of endocrinology 197 (2008) 189–204. [DOI] [PubMed] [Google Scholar]
- [7].McEwen BS, Protective and damaging effects of stress mediators. The New England journal of medicine 338 (1998) 171–9. [DOI] [PubMed] [Google Scholar]
- [8].Sterling P, and Eyer J , Allostasis: A New Paradigm to Explain Arousal Pathology in: Fisher S, and Reason J, (Eds.), Handbook of Life Stress, Cognition and Health, John Wiley & Sons, New York, 1988, pp. 629–649. [Google Scholar]
- [9].Ramsay DS, and Woods SC, Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychological review 121 (2014) 225–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ziegler DR, and Herman JP, Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integrative and comparative biology 42 (2002) 541–51. [DOI] [PubMed] [Google Scholar]
- [11].Evanson NK, Tasker JG, Hill MN, Hillard CJ, and Herman JP, Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151 (2010) 4811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Russell GM, Henley DE, Leendertz J, Douthwaite JA, Wood SA, Stevens A, Woltersdorf WW, Peeters BW, Ruigt GS, White A, Veldhuis JD, and Lightman SL, Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. The Journal of neuroscience : the official journal of the Society for Neuroscience 30 (2010) 6106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen YZ, Hua SY, Wang CA, Wu LG, Gu Q, and Xing BR, An electrophysiological study on the membrane receptor-mediated action of glucocorticoids in mammalian neurons. Neuroendocrinology 53 Suppl 1 (1991) 25–30. [DOI] [PubMed] [Google Scholar]
- [14].Markovic D, and Grammatopoulos DK, Focus on the splicing of secretin GPCRs transmembrane-domain 7. Trends in biochemical sciences 34 (2009) 443–52. [DOI] [PubMed] [Google Scholar]
- [15].Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, and Wurst W, Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature genetics 19 (1998) 162–6. [DOI] [PubMed] [Google Scholar]
- [16].Gallo-Payet N, 60 YEARS OF POMC: Adrenal and extra-adrenal functions of ACTH. Journal of molecular endocrinology 56 (2016) T135–56. [DOI] [PubMed] [Google Scholar]
- [17].Swanson LW, Sawchenko PE, Rivier J, and Vale WW, Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36 (1983) 165–86. [DOI] [PubMed] [Google Scholar]
- [18].Bale TL, and Vale WW, CRF and CRF receptors: role in stress responsivity and other behaviors. Annual review of pharmacology and toxicology 44 (2004) 525–57. [DOI] [PubMed] [Google Scholar]
- [19].Korosi A, and Baram TZ, The central corticotropin releasing factor system during development and adulthood. European journal of pharmacology 583 (2008) 204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Steckler T, and Holsboer F, Corticotropin-releasing hormone receptor subtypes and emotion. Biological psychiatry 46 (1999) 1480–508. [DOI] [PubMed] [Google Scholar]
- [21].Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, and Wurst W, Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nature neuroscience 6 (2003) 1100–7. [DOI] [PubMed] [Google Scholar]
- [22].Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, and Myers B, Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Comprehensive Physiology 6 (2016) 603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, Suzuki H, Mitani F, Ogishima T, Shimizu C, Kotaki H, Kakuta S, Sudo K, Koike T, Kubo M, and Iwakura Y, Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proceedings of the National Academy of Sciences of the United States of America 104 (2007) 18205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Penhoat A, Jaillard C, and Saez JM, Corticotropin positively regulates its own receptors and cAMP response in cultured bovine adrenal cells. Proceedings of the National Academy of Sciences of the United States of America 86 (1989) 4978–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gallo-Payet N, and Payet MD, Mechanism of action of ACTH: beyond cAMP. Microscopy research and technique 61 (2003) 275–87. [DOI] [PubMed] [Google Scholar]
- [26].Schimmer BP, Cordova M, Cheng H, Tsao A, and Morris Q, A genome-wide assessment of adrenocorticotropin action in the Y1 mouse adrenal tumor cell line. Molecular and cellular endocrinology 265–266 (2007) 102–7. [DOI] [PubMed] [Google Scholar]
- [27].Kino T, Glucocorticoid Receptor. in: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, and Weickert MO, (Eds.), Endotext, South Dartmouth (MA), 2000. [Google Scholar]
- [28].Joels M, Karst H, DeRijk R, and de Kloet ER, The coming out of the brain mineralocorticoid receptor. Trends in neurosciences 31 (2008) 1–7. [DOI] [PubMed] [Google Scholar]
- [29].Funder JW, Mineralocorticoid receptors: distribution and activation. Heart failure reviews 10 (2005) 15–22. [DOI] [PubMed] [Google Scholar]
- [30].DeRijk RH, Wust S, Meijer OC, Zennaro MC, Federenko IS, Hellhammer DH, Giacchetti G, Vreugdenhil E, Zitman FG, and de Kloet ER, A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. The Journal of clinical endocrinology and metabolism 91 (2006) 5083–9. [DOI] [PubMed] [Google Scholar]
- [31].Karst H, Berger S, Turiault M, Tronche F, Schutz G, and Joels M, Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America 102 (2005) 19204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, and Myers B, Neural regulation of the stress response: glucocorticoid feedback mechanisms. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 45 (2012) 292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Di S, Malcher-Lopes R, Halmos KC, and Tasker JG, Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience 23 (2003) 4850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu QS, Gorzalka BB, and Hillard CJ, Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. The Journal of neuroscience : the official journal of the Society for Neuroscience 31 (2011) 10506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Evanson NK, Herman JP, Sakai RR, and Krause EG, Nongenomic actions of adrenal steroids in the central nervous system. Journal of neuroendocrinology 22 (2010) 846–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, and Popoli M, Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PloS one 5 (2010) e8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gray JD, Kogan JF, Marrocco J, and McEwen BS, Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nature reviews. Endocrinology 13 (2017) 661–673. [DOI] [PubMed] [Google Scholar]
- [38].Godowski PJ, Rusconi S, Miesfeld R, and Yamamoto KR, Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature 325 (1987) 365–8. [DOI] [PubMed] [Google Scholar]
- [39].Mitre-Aguilar IB, Cabrera-Quintero AJ, and Zentella-Dehesa A, Genomic and non-genomic effects of glucocorticoids: implications for breast cancer. International journal of clinical and experimental pathology 8 (2015) 1–10. [PMC free article] [PubMed] [Google Scholar]
- [40].Oakley RH, and Cidlowski JA, Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. The Journal of biological chemistry 286 (2011) 3177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Myers B, McKlveen JM, and Herman JP, Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Frontiers in neuroendocrinology 35 (2014) 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sawchenko PE, Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain research 403 (1987) 213–23. [DOI] [PubMed] [Google Scholar]
- [43].Watts AG, Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Frontiers in neuroendocrinology 26 (2005) 109–30. [DOI] [PubMed] [Google Scholar]
- [44].Hill MN, and Tasker JG, Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience 204 (2012) 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tasker JG, and Herman JP, Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 14 (2011) 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, and Tasker JG, Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. The Journal of neuroscience : the official journal of the Society for Neuroscience 26 (2006) 6643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hill MN, Karatsoreos IN, Hillard CJ, and McEwen BS, Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 35 (2010) 1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Herman JP, and Cullinan WE, Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in neurosciences 20 (1997) 78–84. [DOI] [PubMed] [Google Scholar]
- [49].Radley JJ, and Sawchenko PE, A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. The Journal of neuroscience : the official journal of the Society for Neuroscience 31 (2011) 9683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Herman JP, Dolgas CM, and Carlson SL, Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 86 (1998) 449–59. [DOI] [PubMed] [Google Scholar]
- [51].Jacobson L, and Sapolsky R, The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine reviews 12 (1991) 118–34. [DOI] [PubMed] [Google Scholar]
- [52].Sapolsky RM, Krey LC, and McEwen BS, Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proceedings of the National Academy of Sciences of the United States of America 81 (1984) 6174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Diorio D, Viau V, and Meaney MJ, The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 13 (1993) 3839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, and Yan Z, Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America 106 (2009) 14075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, and Pego JM, Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Frontiers in behavioral neuroscience 7 (2013) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Karst H, Berger S, Erdmann G, Schutz G, and Joels M, Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences of the United States of America 107 (2010) 14449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tortella-Feliu M, Fullana MA, Perez-Vigil A, Torres X, Chamorro J, Littarelli SA, Solanes A, Ramella-Cravaro V, Vilar A, Gonzalez-Parra JA, Andero R, Reichenberg PA, Mataix-Cols PD, Vieta E, Fusar-Poli P, Ioannidis P, Stein PMB, Radua J, and Fernandez de la Cruz L, Risk factors for posttraumatic stress disorder: An umbrella review of systematic reviews and meta-analyses. Neuroscience and biobehavioral reviews 107 (2019) 154–165. [DOI] [PubMed] [Google Scholar]
- [58].Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, and Cullinan WE, Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in neuroendocrinology 24 (2003) 151–80. [DOI] [PubMed] [Google Scholar]
- [59].Sawchenko PE, Li HY, and Ericsson A, Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Progress in brain research 122 (2000) 61–78. [DOI] [PubMed] [Google Scholar]
- [60].Dayas CV, Buller KM, Crane JW, Xu Y, and Day TA, Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. The European journal of neuroscience 14 (2001) 1143–52. [DOI] [PubMed] [Google Scholar]
- [61].Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, and Goldstein DS, Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. The American journal of physiology 275 (1998) R1247–55. [DOI] [PubMed] [Google Scholar]
- [62].Herman JP, Neural control of chronic stress adaptation. Frontiers in behavioral neuroscience 7 (2013) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, and Spencer RL, Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. Journal of neuroendocrinology 12 (2000) 1034–42. [DOI] [PubMed] [Google Scholar]
- [64].Grissom N, and Bhatnagar S, Habituation to repeated stress: get used to it. Neurobiology of learning and memory 92 (2009) 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Herman JP, Adams D, and Prewitt C, Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61 (1995) 180–90. [DOI] [PubMed] [Google Scholar]
- [66].Bhatnagar S, and Dallman M, Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84 (1998) 1025–39. [DOI] [PubMed] [Google Scholar]
- [67].Makino S, Smith MA, and Gold PW, Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology 136 (1995) 3299–309. [DOI] [PubMed] [Google Scholar]
- [68].Gotlieb N, Albaz E, Shaashua L, Sorski L, Matzner P, Rosenne E, Amram B, Benbenishty A, Golomb E, and Ben-Eliyahu S, Regeneration of Functional Adrenal Tissue Following Bilateral Adrenalectomy. Endocrinology 159 (2018) 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Scherer IJ, Holmes PV, and Harris RB, The importance of corticosterone in mediating restraint-induced weight loss in rats. Physiology & behavior 102 (2011) 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Castonguay TW, Dallman MF, and Stern JS, Some metabolic and behavioral effects of adrenalectomy on obese Zucker rats. The American journal of physiology 251 (1986) R923–33. [DOI] [PubMed] [Google Scholar]
- [71].Jackson E, Stolz D, and Martin R, Effect of adrenalectomy on weight gain and body composition of yellow obese mice (Ay/a). Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 8 (1976) 452–5. [DOI] [PubMed] [Google Scholar]
- [72].Dubuc PU, and Wilden NJ, Adrenalectomy reduces but does not reverse obesity in ob/ob mice. International journal of obesity 10 (1986) 91–8. [PubMed] [Google Scholar]
- [73].van den Buuse M, Morris M, Chavez C, Martin S, and Wang J, Effect of adrenalectomy and corticosterone replacement on prepulse inhibition and locomotor activity in mice. British journal of pharmacology 142 (2004) 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, and Akana SF, Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology 145 (2004) 2633–8. [DOI] [PubMed] [Google Scholar]
- [75].Moghaddam B, Bolinao ML, Stein-Behrens B, and Sapolsky R, Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain research 655 (1994) 251–4. [DOI] [PubMed] [Google Scholar]
- [76].Lowy MT, Gault L, and Yamamoto BK, Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. Journal of neurochemistry 61 (1993) 1957–60. [DOI] [PubMed] [Google Scholar]
- [77].Helmreich DL, Cullinan WE, and Watson SJ, The effect of adrenalectomy on stress-induced c-fos mRNA expression in the rat brain. Brain research 706 (1996) 137–44. [DOI] [PubMed] [Google Scholar]
- [78].Heinrichs SC, and Koob GF, Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. The Journal of pharmacology and experimental therapeutics 311 (2004) 427–40. [DOI] [PubMed] [Google Scholar]
- [79].Ricordi C, Shah SD, Lacy PE, Clutter WE, and Cryer PE, Delayed extra-adrenal epinephrine secretion after bilateral adrenalectomy in rats. The American journal of physiology 254 (1988) E52–3. [DOI] [PubMed] [Google Scholar]
- [80].Sudo A, Effects of adrenalectomy and chronic guanethidine treatment on tissue adrenaline concentrations in swimming-exposed rats. Japanese journal of pharmacology 45 (1987) 197–201. [DOI] [PubMed] [Google Scholar]
- [81].Remme WJ, The sympathetic nervous system and ischaemic heart disease. European heart journal 19 Suppl F (1998) F62–71. [PubMed] [Google Scholar]
- [82].Heim C, and Binder EB, Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental neurology 233 (2012) 102–11. [DOI] [PubMed] [Google Scholar]
- [83].Stein MB, Jang KL, Taylor S, Vernon PA, and Livesley WJ, Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. The American journal of psychiatry 159 (2002) 1675–81. [DOI] [PubMed] [Google Scholar]
- [84].Spijker AT, and van Rossum EF, Glucocorticoid receptor polymorphisms in major depression. Focus on glucocorticoid sensitivity and neurocognitive functioning. Annals of the New York Academy of Sciences 1179 (2009) 199–215. [DOI] [PubMed] [Google Scholar]
- [85].Lian Y, Xiao J, Wang Q, Ning L, Guan S, Ge H, Li F, and Liu J, The relationship between glucocorticoid receptor polymorphisms, stressful life events, social support, and post-traumatic stress disorder. BMC psychiatry 14 (2014) 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, and Ressler KJ, Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299 (2008) 1291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cole TJ, Harris HJ, Hoong I, Solomon N, Smith R, Krozowski Z, and Fullerton MJ, The glucocorticoid receptor is essential for maintaining basal and dexamethasone-induced repression of the murine corticosteroid-binding globulin gene. Molecular and cellular endocrinology 154 (1999) 29–36. [DOI] [PubMed] [Google Scholar]
- [88].Cole TJ, Myles K, Purton JF, Brereton PS, Solomon NM, Godfrey DI, and Funder JW, GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Molecular and cellular endocrinology 173 (2001) 193–202. [DOI] [PubMed] [Google Scholar]
- [89].Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, and Muglia LJ, Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proceedings of the National Academy of Sciences of the United States of America 102 (2005) 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Bull DR, Ionescu IA, Heinzmann JM, Knapman A, Siebertz A, Depping AM, Hartmann J, Hausch F, Schmidt MV, Holsboer F, Ising M, Cox MB, Schmidt U, and Rein T, FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biological psychiatry 70 (2011) 928–36. [DOI] [PubMed] [Google Scholar]
- [91].Dedic N, Kuhne C, Gomes KS, Hartmann J, Ressler KJ, Schmidt MV, and Deussing JM , Deletion of CRH From GABAergic Forebrain Neurons Promotes Stress Resilience and Dampens Stress-Induced Changes in Neuronal Activity. Front Neurosci 13 (2019) 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Maggi R, Dondi D, Piccolella M, Casulari LA, and Martini L, New insight on the molecular aspects of glucocorticoid effects in nervous system development. Journal of endocrinological investigation 36 (2013) 775–80. [DOI] [PubMed] [Google Scholar]
- [93].Meyer JS, Early adrenalectomy stimulates subsequent growth and development of the rat brain. Experimental neurology 82 (1983) 432–46. [DOI] [PubMed] [Google Scholar]
- [94].Pitts BL, Whealin JM, Harpaz-Rotem I, Duman RS, Krystal JH, Southwick SM, and Pietrzak RH, BDNF Val66Met polymorphism and posttraumatic stress symptoms in U.S. military veterans: Protective effect of physical exercise. Psychoneuroendocrinology 100 (2019) 198–202. [DOI] [PubMed] [Google Scholar]
- [95].Felmingham KL, Zuj DV, Hsu KCM, Nicholson E, Palmer MA, Stuart K, Vickers JC, Malhi GS, and Bryant RA, The BDNF Val66Met polymorphism moderates the relationship between Posttraumatic Stress Disorder and fear extinction learning. Psychoneuroendocrinology 91 (2018) 142–148. [DOI] [PubMed] [Google Scholar]
- [96].Fiocco AJ, D’Amico D, de Beaumont L, Poirier J, and Lupien S, Association between BDNF Polymorphism and Hypothalamic-Pituitary-Adrenal Activity in Later Adulthood. Gerontology (2019) 1–7. [DOI] [PubMed] [Google Scholar]
- [97].Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, and Kaitz M, BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology 34 (2009) 382–8. [DOI] [PubMed] [Google Scholar]
- [98].Bath KG, and Lee FS, Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci 6 (2006) 79–85. [DOI] [PubMed] [Google Scholar]
- [99].Yu H, Wang DD, Wang Y, Liu T, Lee FS, and Chen ZY, Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. The Journal of neuroscience : the official journal of the Society for Neuroscience 32 (2012) 4092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, and Bains JS, Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nature communications 7 (2016) 11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jiang Z, Rajamanickam S, and Justice NJ, Local Corticotropin-Releasing Factor Signaling in the Hypothalamic Paraventricular Nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience 38 (2018) 1874–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Johnson SB, Emmons EB, Anderson RM, Glanz RM, Romig-Martin SA, Narayanan NS, LaLumiere RT, and Radley JJ, A Basal Forebrain Site Coordinates the Modulation of Endocrine and Behavioral Stress Responses via Divergent Neural Pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience 36 (2016) 8687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, and Han MH, Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344 (2014) 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhang H, Chaudhury D, Nectow AR, Friedman AK, Zhang S, Juarez B, Liu H, Pfau ML, Aleyasin H, Jiang C, Crumiller M, Calipari ES, Ku SM, Morel C, Tzavaras N, Montgomery SE, He M, Salton SR, Russo SJ, Nestler EJ, Friedman JM, Cao JL, and Han MH, alpha1- and beta3-Adrenergic Receptor-Mediated Mesolimbic Homeostatic Plasticity Confers Resilience to Social Stress in Susceptible Mice. Biological psychiatry 85 (2019) 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iniguez SD, O’Donnell P, Kravitz A, and Lobo MK, Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biological psychiatry 77 (2015) 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kinlein SA, Wilson CD, and Karatsoreos IN, Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Frontiers in psychiatry 6 (2015) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shahanoor Z, Sultana R, Baker MR, and Romeo RD, Neuroendocrine stress reactivity of male C57BL/6N mice following chronic oral corticosterone exposure during adulthood or adolescence. Psychoneuroendocrinology 86 (2017) 218–224. [DOI] [PubMed] [Google Scholar]
- [108].Finnerty CC, Mabvuure NT, Ali A, Kozar RA, and Herndon DN, The surgically induced stress response. JPEN. Journal of parenteral and enteral nutrition 37 (2013) 21S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Meijer MK, Spruijt BM, van Zutphen LF, and Baumans V, Effect of restraint and injection methods on heart rate and body temperature in mice. Laboratory animals 40 (2006) 382–91. [DOI] [PubMed] [Google Scholar]
- [110].Stuart SA, and Robinson ES, Reducing the stress of drug administration: implications for the 3Rs. Scientific reports 5 (2015) 14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, and McEwen BS, Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151 (2010) 2117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dedovic K, and Ngiam J, The cortisol awakening response and major depression: examining the evidence. Neuropsychiatric disease and treatment 11 (2015) 1181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Cassano AE, White JR, Penraat KA, Wilson CD, Rasmussen S, and Karatsoreos IN, Anatomic, hematologic, and biochemical features of C57BL/6NCrl mice maintained on chronic oral corticosterone. Comparative medicine 62 (2012) 348–60. [PMC free article] [PubMed] [Google Scholar]
- [114].Kinlein SA, Shahanoor Z, Romeo RD, and Karatsoreos IN, Chronic Corticosterone Treatment During Adolescence Has Significant Effects on Metabolism and Skeletal Development in Male C57BL6/N Mice. Endocrinology 158 (2017) 2239–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Barahona MJ, Sucunza N, Resmini E, Fernandez-Real JM, Ricart W, Moreno-Navarrete JM, Puig T, Farrerons J, and Webb SM, Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing’s syndrome. The Journal of clinical endocrinology and metabolism 94 (2009) 3365–71. [DOI] [PubMed] [Google Scholar]
- [116].Resmini E, Persistent comorbitities in Cushing’s syndrome after endocrine cure. Advances in Endocrinology 2014 (2014). [Google Scholar]
- [117].Gourley SL, Swanson AM, and Koleske AJ, Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. The Journal of neuroscience : the official journal of the Society for Neuroscience 33 (2013) 3107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gourley SL, Kiraly DD, Howell JL, Olausson P, and Taylor JR, Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biological psychiatry 64 (2008) 884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nistico R, and McEwen BS, Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proceedings of the National Academy of Sciences of the United States of America 112 (2015) 14960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lezak KR, Missig G, and Carlezon WA Jr., Behavioral methods to study anxiety in rodents. Dialogues in clinical neuroscience 19 (2017) 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, and Hill MN, Corticotropin-Releasing Hormone Drives Anandamide Hydrolysis in the Amygdala to Promote Anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience 35 (2015) 3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Rodgers RJ, Cao BJ, Dalvi A, and Holmes A, Animal models of anxiety: an ethological perspective. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 30 (1997) 289–304. [DOI] [PubMed] [Google Scholar]
- [123].Slattery DA, and Cryan JF, The ups and downs of modelling mood disorders in rodents. ILAR journal 55 (2014) 297–309. [DOI] [PubMed] [Google Scholar]
- [124].Krishnan V, and Nestler EJ, Animal models of depression: molecular perspectives. Current topics in behavioral neurosciences 7 (2011) 121–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Nasca C, Bigio B, Zelli D, de Angelis P, Lau T, Okamoto M, Soya H, Ni J, Brichta L, Greengard P, Neve RL, Lee FS, and McEwen BS, Role of the Astroglial Glutamate Exchanger xCT in Ventral Hippocampus in Resilience to Stress. Neuron 96 (2017) 402–413 e5. [DOI] [PubMed] [Google Scholar]
- [126].Sanacora G, Treccani G, and Popoli M, Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62 (2012) 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Musazzi L, Treccani G, and Popoli M, Glutamate hypothesis of depression and its consequences for antidepressant treatments. Expert review of neurotherapeutics 12 (2012) 1169–72. [DOI] [PubMed] [Google Scholar]
- [128].Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, and Blokhuis HJ, Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and biobehavioral reviews 23 (1999) 925–35. [DOI] [PubMed] [Google Scholar]
- [129].Hohne N, Poidinger M, Merz F, Pfister H, Bruckl T, Zimmermann P, Uhr M, Holsboer F, and Ising M, Increased HPA axis response to psychosocial stress in remitted depression: the influence of coping style. Biological psychology 103 (2014) 267–75. [DOI] [PubMed] [Google Scholar]
- [130].Perez-Tejada J, Arregi A, Gomez-Lazaro E, Vegas O, Azpiroz A, and Garmendia L, Coping with chronic social stress in mice: hypothalamic-pituitary-adrenal/ sympathetic-adrenal-medullary axis activity, behavioral changes and effects of antalarmin treatment: implications for the study of stress-related psychopathologies. Neuroendocrinology 98 (2013) 73–88. [DOI] [PubMed] [Google Scholar]
- [131].Kinlein SA, Phillips DJ, Keller CR, and Karatsoreos IN, Role of corticosterone in altered neurobehavioral responses to acute stress in a model of compromised hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology 102 (2019) 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Li Y, Li S, Wei C, Wang H, Sui N, and Kirouac GJ, Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacology, biochemistry, and behavior 95 (2010) 121–8. [DOI] [PubMed] [Google Scholar]
- [133].Gillespie CF, and Nemeroff CB, Hypercortisolemia and depression. Psychosomatic medicine 67 Suppl 1 (2005) S26–8. [DOI] [PubMed] [Google Scholar]
- [134].Pariante CM, and Lightman SL, The HPA axis in major depression: classical theories and new developments. Trends in neurosciences 31 (2008) 464–8. [DOI] [PubMed] [Google Scholar]
- [135].Pariante CM, The glucocorticoid receptor: part of the solution or part of the problem? Journal of psychopharmacology 20 (2006) 79–84. [DOI] [PubMed] [Google Scholar]
- [136].Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL Jr., and Mason JW, Low urinary cortisol excretion in patients with posttraumatic stress disorder. The Journal of nervous and mental disease 178 (1990) 366–9. [DOI] [PubMed] [Google Scholar]
- [137].Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, and Mason JW, Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. The American journal of psychiatry 150 (1993) 83–6. [DOI] [PubMed] [Google Scholar]
- [138].Wessa M, Rohleder N, Kirschbaum C, and Flor H, Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology 31 (2006) 209–15. [DOI] [PubMed] [Google Scholar]
- [139].Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, and Marmar CR, PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology 30 (2005) 373–81. [DOI] [PubMed] [Google Scholar]
- [140].Mason JW, Giller EL, Kosten TR, Ostroff RB, and Podd L, Urinary free-cortisol levels in posttraumatic stress disorder patients. The Journal of nervous and mental disease 174 (1986) 145–9. [DOI] [PubMed] [Google Scholar]
- [141].Videbech P, and Ravnkilde B, Hippocampal volume and depression: a meta-analysis of MRI studies. The American journal of psychiatry 161 (2004) 1957–66. [DOI] [PubMed] [Google Scholar]
- [142].Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, and Rajkowska G, Cellular changes in the postmortem hippocampus in major depression. Biological psychiatry 56 (2004) 640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].McEwen BS, Nasca C, and Gray JD, Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41 (2016) 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, and Stockmeier CA, Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological psychiatry 45 (1999) 1085–98. [DOI] [PubMed] [Google Scholar]
- [145].Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, and Sousa N, Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience 25 (2005) 7792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wellman CL, Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of neurobiology 49 (2001) 245–53. [DOI] [PubMed] [Google Scholar]
- [147].Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, and Werner A, A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and biobehavioral reviews 30 (2006) 1004–31. [DOI] [PubMed] [Google Scholar]
- [148].Sherin JE, and Nemeroff CB, Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues in clinical neuroscience 13 (2011) 263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Popoli M, Yan Z, McEwen BS, and Sanacora G, The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience 13 (2011) 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, and Abdallah CG, Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neuroscience letters 649 (2017) 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, and Lombroso PJ, Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. The Journal of neuroscience : the official journal of the Society for Neuroscience 29 (2009) 9330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Yuen EY, Wei J, Liu W, Zhong P, Li X, and Yan Z, Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73 (2012) 962–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, and Charney DS, Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. The American journal of psychiatry 154 (1997) 624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Nemeroff CB, Owens MJ, Bissette G, Andorn AC, and Stanley M, Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 45 (1988) 577–9. [DOI] [PubMed] [Google Scholar]
- [155].Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, and Vale W, Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226 (1984) 1342–4. [DOI] [PubMed] [Google Scholar]
- [156].Dirks A, Groenink L, Bouwknecht JA, Hijzen TH, Van Der Gugten J, Ronken E, Verbeek JS, Veening JG, Dederen PJ, Korosi A, Schoolderman LF, Roubos EW, and Olivier B, Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. The European journal of neuroscience 16 (2002) 1751–60. [DOI] [PubMed] [Google Scholar]
- [157].Flandreau EI, Ressler KJ, Owens MJ, and Nemeroff CB, Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 37 (2012) 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Block SG, and Nemeroff CB, Emerging antidepressants to treat major depressive disorder. Asian journal of psychiatry 12 (2014) 7–16. [DOI] [PubMed] [Google Scholar]
- [159].Moore RY, and Lenn NJ, A retinohypothalamic projection in the rat. J Comp Neurol 146 (1972) 1–14. [DOI] [PubMed] [Google Scholar]
- [160].Stephan FK, and Zucker I, Rat drinking rhythms: central visual pathways and endocrine factors mediating responsiveness to environmental illumination. Physiol Behav 8 (1972) 315–26. [DOI] [PubMed] [Google Scholar]
- [161].Moore RY, and Eichler VB, Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain research 42 (1972) 201–6. [DOI] [PubMed] [Google Scholar]
- [162].Stephan FK, and Zucker I, Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the United States of America 69 (1972) 1583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, and Bittman EL, Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 7 (1987) 1626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ralph MR, Foster RG, Davis FC, and Menaker M, Transplanted suprachiasmatic nucleus determines circadian period. Science 247 (1990) 975–8. [DOI] [PubMed] [Google Scholar]
- [165].Silver R, LeSauter J, Tresco PA, and Lehman MN, A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382 (1996) 810–3. [DOI] [PubMed] [Google Scholar]
- [166].Challet E, The circadian regulation of food intake. Nature reviews. Endocrinology 15 (2019) 393–405. [DOI] [PubMed] [Google Scholar]
- [167].Carroll RG, Timmons GA, Cervantes-Silva MP, Kennedy OD, and Curtis AM, Immunometabolism around the Clock. Trends Mol Med 25 (2019) 612–625. [DOI] [PubMed] [Google Scholar]
- [168].Snider KH, Sullivan KA, and Obrietan K, Circadian Regulation of Hippocampal-Dependent Memory: Circuits, Synapses, and Molecular Mechanisms. Neural Plast 2018 (2018) 7292540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Czeisler CA, and Klerman EB, Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res 54 (1999) 97–130; discussion 130–2. [PubMed] [Google Scholar]
- [170].Dubey AK, Puri CP, Puri V, and Anand Kumar TC, Day and night levels of hormones in male rhesus monkeys kept under controlled or constant environmental light. Experientia 39 (1983) 207–9. [DOI] [PubMed] [Google Scholar]
- [171].Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, and Gallagher TF, Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry 28 (1973) 19–24. [DOI] [PubMed] [Google Scholar]
- [172].Van Cauter E, and Refetoff S, Multifactorial control of the 24-hour secretory profiles of pituitary hormones. Journal of endocrinological investigation 8 (1985) 381–91. [DOI] [PubMed] [Google Scholar]
- [173].Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, and Hellman L, Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. The Journal of clinical endocrinology and metabolism 33 (1971) 14–22. [DOI] [PubMed] [Google Scholar]
- [174].Albers HE, Yogev L, Todd RB, and Goldman BD, Adrenal corticoids in hamsters: role in circadian timing. The American journal of physiology 248 (1985) R434–8. [DOI] [PubMed] [Google Scholar]
- [175].Ottenweller JE, Tapp WN, Pitman DL, and Natelson BH, Adrenal, thyroid, and testicular hormone rhythms in male golden hamsters on long and short days. The American journal of physiology 253 (1987) R321–8. [DOI] [PubMed] [Google Scholar]
- [176].Wong CC, Dohler KD, Geerlings H, and von zur Muhlen A, Influence of age, strain and season on circadian periodicity of pituitary, gonadal and adrenal hormones in the serum of male laboratory rats. Horm Res 17 (1983) 202–15. [DOI] [PubMed] [Google Scholar]
- [177].Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, and Bittman EL, Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140 (1999) 207–18. [DOI] [PubMed] [Google Scholar]
- [178].Wotus C, Lilley TR, Neal AS, Suleiman NL, Schmuck SC, Smarr BL, Fischer BJ, and de la Iglesia HO, Forced desynchrony reveals independent contributions of suprachiasmatic oscillators to the daily plasma corticosterone rhythm in male rats. PloS one 8 (2013) e68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Guo H, Brewer JM, Lehman MN, and Bittman EL, Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. The Journal of neuroscience : the official journal of the Society for Neuroscience 26 (2006) 6406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Buijs RM, Hermes MH, and Kalsbeek A, The suprachiasmatic nucleus-paraventricular nucleus interactions: a bridge to the neuroendocrine and autonomic nervous system. Progress in brain research 119 (1998) 365–82. [DOI] [PubMed] [Google Scholar]
- [181].Vrang N, Larsen PJ, and Mikkelsen JD, Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain research 684 (1995) 61–9. [DOI] [PubMed] [Google Scholar]
- [182].Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, and Kalsbeek A, Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. The European journal of neuroscience 11 (1999) 1535–44. [DOI] [PubMed] [Google Scholar]
- [183].Russell GM, Kalafatakis K, and Lightman SL, The importance of biological oscillators for hypothalamic-pituitary-adrenal activity and tissue glucocorticoid response: coordinating stress and neurobehavioural adaptation. Journal of neuroendocrinology 27 (2015) 378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Spiga F, Walker JJ, Terry JR, and Lightman SL, HPA Axis-Rhythms. Comprehensive Physiology 4 (2014) 1273–98. [DOI] [PubMed] [Google Scholar]
- [185].Walker JJ, Spiga F, Waite E, Zhao Z, Kershaw Y, Terry JR, and Lightman SL, The origin of glucocorticoid hormone oscillations. PLoS biology 10 (2012) e1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Spiga F, Waite EJ, Liu Y, Kershaw YM, Aguilera G, and Lightman SL, ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology 152 (2011) 1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Bittman EL, Doherty L, Huang L, and Paroskie A, Period gene expression in mouse endocrine tissues. Am J Physiol Regul Integr Comp Physiol 285 (2003) R561–9. [DOI] [PubMed] [Google Scholar]
- [188].Kalsbeek A, Ruiter M, La Fleur SE, Van Heijningen C, and Buijs RM, The diurnal modulation of hormonal responses in the rat varies with different stimuli. Journal of neuroendocrinology 15 (2003) 1144–55. [DOI] [PubMed] [Google Scholar]
- [189].Oishi K, Sakamoto K, Okada T, Nagase T, and Ishida N, Humoral signals mediate the circadian expression of rat period homologue (rPer2) mRNA in peripheral tissues. Neuroscience letters 256 (1998) 117–9. [DOI] [PubMed] [Google Scholar]
- [190].Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, and Eichele G, The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4 (2006) 163–73. [DOI] [PubMed] [Google Scholar]
- [191].Ungar F, and Halberg F, Circadian rhythm in the in vitro response of mouse adrenal to adrenocorticotropic hormone. Science 137 (1962) 1058–60. [DOI] [PubMed] [Google Scholar]
- [192].Amir S, Lamont EW, Robinson B, and Stewart J, A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. The Journal of neuroscience : the official journal of the Society for Neuroscience 24 (2004) 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Lamont EW, Robinson B, Stewart J, and Amir S, The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proceedings of the National Academy of Sciences of the United States of America 102 (2005) 4180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Segall LA, Perrin JS, Walker CD, Stewart J, and Amir S, Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience 140 (2006) 753–7. [DOI] [PubMed] [Google Scholar]
- [195].Liston C, and Gan WB, Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proceedings of the National Academy of Sciences of the United States of America 108 (2011) 16074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Woodruff ER, Chun LE, Hinds LR, Varra NM, Tirado D, Morton SJ, McClung CA, and Spencer RL, Coordination between Prefrontal Cortex Clock Gene Expression and Corticosterone Contributes to Enhanced Conditioned Fear Extinction Recall. eNeuro 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Jacobson L, Akana SF, Cascio CS, Shinsako J, and Dallman MF, Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology 122 (1988) 1343–8. [DOI] [PubMed] [Google Scholar]
- [198].Rao R, and Androulakis IP, The physiological significance of the circadian dynamics of the HPA axis: Interplay between circadian rhythms, allostasis and stress resilience. Horm Behav 110 (2019) 77–89. [DOI] [PubMed] [Google Scholar]
- [199].Cho K, Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nature neuroscience 4 (2001) 567–8. [DOI] [PubMed] [Google Scholar]
- [200].Gilbertson MW, Paulus LA, Williston SK, Gurvits TV, Lasko NB, Pitman RK, and Orr SP, Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol 115 (2006) 484–95. [DOI] [PubMed] [Google Scholar]
- [201].Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, and Pitman RK, Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience 5 (2002) 1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, and McEwen BS, Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proceedings of the National Academy of Sciences of the United States of America 108 (2011) 1657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].McEwen BS, Coirini H, Westlind-Danielsson A, Frankfurt M, Gould E, Schumacher M, and Woolley C, Steroid hormones as mediators of neural plasticity. J Steroid Biochem Mol Biol 39 (1991) 223–32. [DOI] [PubMed] [Google Scholar]
- [204].Liston C, McEwen BS, and Casey BJ, Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America 106 (2009) 912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, and Hattar S, Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491 (2012) 594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Davis S, Mirick DK, and Stevens RG, Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 93 (2001) 1557–62. [DOI] [PubMed] [Google Scholar]
- [207].Knutsson A, Health disorders of shift workers. Occup Med (Lond) 53 (2003) 103–8. [DOI] [PubMed] [Google Scholar]
- [208].Lowden A, Moreno C, Holmback U, Lennernas M, and Tucker P, Eating and shift work - effects on habits, metabolism and performance. Scand J Work Environ Health (2010). [DOI] [PubMed] [Google Scholar]
- [209].Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, and Nogawa K, A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring) 16 (2008) 1887–93. [DOI] [PubMed] [Google Scholar]
- [210].Boivin DB, Tremblay GM, and James FO, Working on atypical schedules. Sleep Med 8 (2007) 578–89. [DOI] [PubMed] [Google Scholar]
- [211].Haffner-Luntzer M, Foertsch S, Fischer V, Prystaz K, Tschaffon M, Modinger Y, Bahney CS, Marcucio RS, Miclau T, Ignatius A, and Reber SO, Chronic psychosocial stress compromises the immune response and endochondral ossification during bone fracture healing via beta-AR signaling. Proceedings of the National Academy of Sciences of the United States of America (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Langgartner D, Wachter U, Hartmann C, Groger M, Vogt J, Merz T, McCook O, Fink M, Kress S, Georgieff M, Kunze JF, Radermacher PL, Reber SO, and Wepler M, Effects of Psychosocial Stress on Subsequent Hemorrhagic Shock and Resuscitation in Male Mice. Shock (2018). [DOI] [PubMed] [Google Scholar]
- [213].Karatsoreos IN, Stress: Common themes toward the next frontier. Frontiers in neuroendocrinology 49 (2018) 3–7. [DOI] [PubMed] [Google Scholar]