Abstract
In this study, a highly efficient two-component [3 + 2] cycloaddition reaction of substituted aryl aldehydes with 4-toluenesulfonylmethyl isocyanide (TosMIC) in the presence of 2 equiv of potassium phosphate as a base to 5-substituted oxazoles were established in a isopropanol medium under microwave irradiation. However, using 1 equiv of K3PO4 as a base resulted in the diastereoselective synthesis of 4,5-disubstituted oxazolines under identical reaction conditions. The foremost benefits of these protocols are the moderate-to-excellent yields with good functional group compatibility, simple experimental procedure, inexpensive readily available starting materials, nonchromatographic purification, and high bond-forming efficiency. The synthetic manipulation reported herein represents a cleaner route to the sustainable preparation of 5-substituted oxazoles and diastereoselective 4,5-disubstituted oxazolines derivatives.
1. Introduction
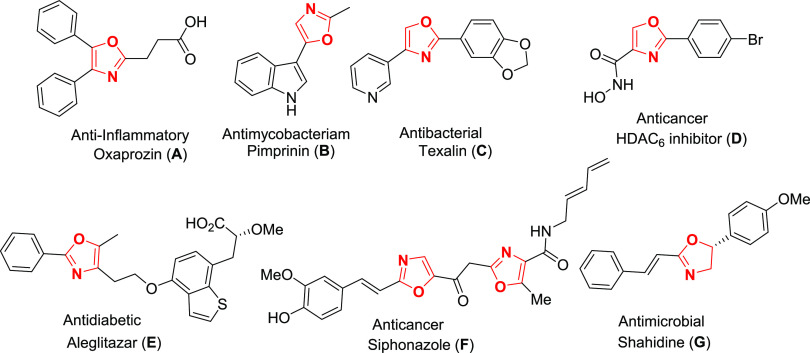
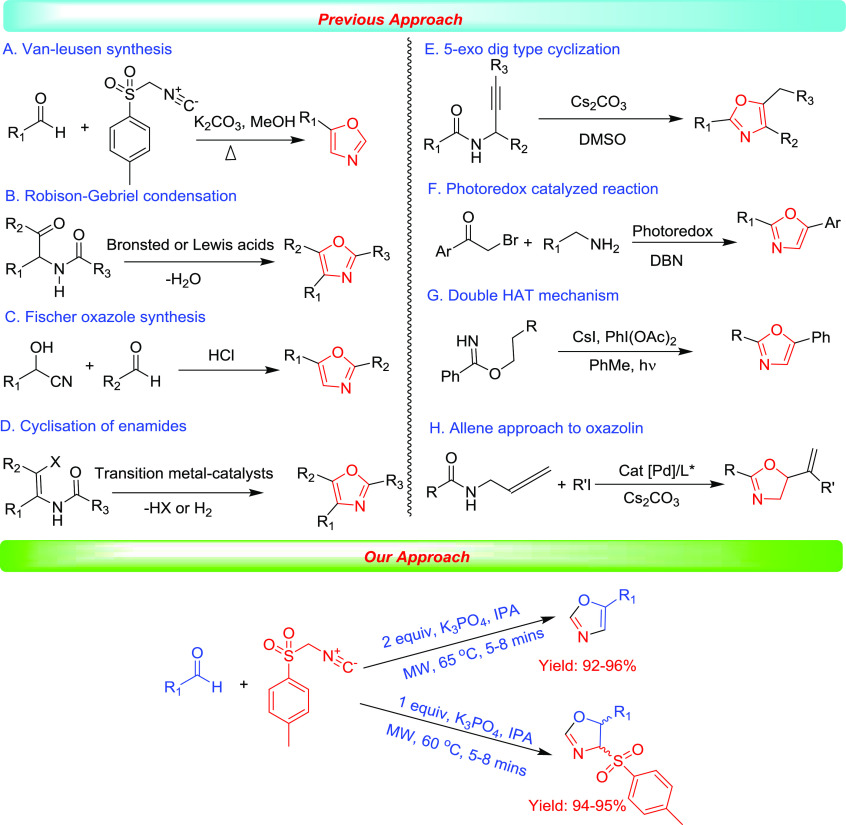
Diverse heterocyclic small molecules are enormously used in biological systems as drug molecules to combat various diseases. Researchers in pharmaceutical industries and academia have a pronounced attention in the synthesis of small heterocyclic molecules, as they show innovative roles in all levels of biology including cell growth, sensing, and proliferation.1 Heterocyclic small molecules are enormously significant for studying cell biology and the treatment of diverse diseases as they control the function of enzymes, receptors, and protein–protein interactions.2,3 In this perspective, oxazoles and oxazolines are five-membered heterocyclic moieties containing nitrogen and oxygen as heteroatoms, which established an important class of drug candidates in organic chemistry. Predominantly, substituted oxazoles and oxazolines containing heterocycles could bind diverse enzymes and receptors via noncovalent interactions and in the biological system to display a wide variety of biological activities.4−8 Numerous oxazole-containing drugs such as oxaprozin (A), a nonsteroidal anti-inflammatory drug (NSAID); pimprinin (B), an antimycobacterium agent; texalin (C), an antibacterial natural product; oxazole hydroxamate (D), a HDAC6 inhibitor; aleglitazar (E), an antidiabetic agent; siphonazole (F), an anticancer agent; and shahidine, an antimicrobial oxazoline drug (G), are extensively used in clinical exercises (Figure 1).9−18 In view of their extensive bioactivities, several synthetic protocols have been developed by organic chemists.19,20 In 1972, for the first time, Van Leusen and his group discovered a two-step [3 + 2] cycloaddition reaction from substituted aldehyde with 4-toluenesulfonylmethyl isocyanide (TosMIC) under a refluxing methanol solvent using K2CO3 as a base to 5-substituted oxazoles (Scheme 1A).21 Subsequently, in 1999, Kulakarni and Ganesan modified the Van Leusen protocol for the oxazole synthesis using ambersep 900 OH– as resin in a dimethoxyethane/MeOH-refluxing solvent (Table S1, entry 1).22 In 2009, Ludivine et al. utilized the 1,8-diazabicyclo[5.4.0]undec-7-ene-polystyrnene as a mild base in an acetonitrile solvent at room temperature for oxazoline synthesis (Table S1, entry 2).23 Very recently, Ramanathan et al. used imidazole as a base in a water medium for the diastereoselective synthesis of oxazolines and 5-substituted oxazoles (Table S1, entry 3).24
Figure 1.
Selected biologically active oxazole and oxazoline derivatives.
Scheme 1. Oxazoles and Oxazolines Syntheses: Previous and Present Works.
Other methods including the Robinson–Gabriel condensation of α-acyl amino ketone in the presence of dehydrating agents such as Bronstead or Lewis acid provided 2,4-disubstitutedoxazoles (Scheme 1B).25 In 2018, Jiang et al. established a convenient copper-catalyzed [2 + 3] cyclization reaction of alpha-hydroxy ketones with arylacetonitriles to furnish 2,4,5-trisubstituted oxazoles (Scheme 1C).26 Several other methods included the transition-metal-catalyzed cyclization of enamide with vinylic functionalization to the corresponding oxazoles (Scheme 1D).27 In 2018, Chang et al. established a Cs2CO3-catalyzed halogen-free 5-exo-dig cyclization reaction for the amalgamation of oxazoles (Scheme 1E).28 In 2019, Li et al. described tandem-oxidative cyclization reactions of α-bromo ketones and amines by a CO2/photoredox co-catalyst for the preparation of substituted oxazoles (Scheme 1F).29 In 2020, Nagib and his group have established a radical cascade strategy for the synthesis of oxazoles via the tandem hydrogen atom transfer (HAT) approach (Scheme 1G).30 In 2018, Ma et al. described a highly effective enantioselective synthesis of asymmetric oxazoline derivatives via palladium-catalyzed reaction of aryl or 1-alkenyl iodides with N-(buta-2,3-dienyl) amides (Scheme 1H).31
However, all these methods suffer a few drawbacks such as the usage of toxic Lewis acid, transition-metal catalysts, longer reaction time, and toxic organic solvents such as CH3OH, CH3CN, and THF.32−39 Henceforth, there is substantial attention in the improvement of alternate methodologies evading the usage of expensive base catalysts, toxic organic solvents, and transition-metal catalysts. Therefore, it is necessary to develop a novel protocol to build these diverse and functionalized oxazoles and oxazolines scaffolds for drug development. In the last decade, the combined application of microwave-assisted irradiation in green solvents has increased considerably owing to the generation of quick products in the nontoxic environment. In this respect, isopropanol is a very attractive nontoxic green polar solvent for several competent organic reactions that could solubilize numerous reactants at room or high temperatures.40−42 Herein, we developed an expedient microwave-assisted simple protocol of 5-substituted oxazoles and diastereoselective 4,5-disubstituted oxazolines derivatives using K3PO4 as a base in an IPA reaction medium.43
2. Result and Discussion
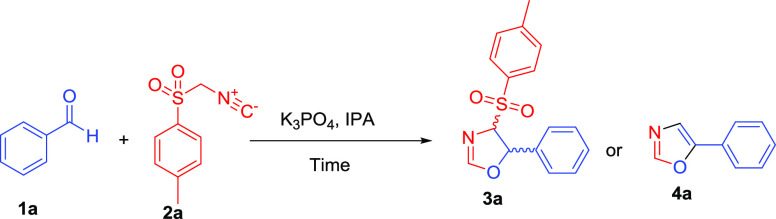
At the beginning of this exploration, we have selected benzaldehyde 1a and 4-toluenesulfonylmethyl isocyanide (TosMIC) 2a as the model substrate. The reaction of benzaldehyde 1a with 4-toluenesulfonylmethyl isocyanide (TosMIC) 2a without a base in a refluxing methanol solvent for 12 h did not yield any 4,5-disubstituted oxazoline 3a and 5-phenyl oxazole 4a products (Table 1, entry 1). Interestingly, employing the 2 equiv of organic bases such as triethylamine, N,N-diisopropylethylamine, imidazole, N-methylmorpholine, and NaHCO3 as inorganic mild bases for the same set of reactions under the refluxing methanol solvent for 6 h yielded 92–95% 4,5-disubstituted oxazolines 3a (Table 1, entries 2–6). Surprisingly, using 2 equiv of K2CO3 or K3PO4 as a base, the same set of reaction yielded 5-phenyl oxazole 4a in 94–95% in a reduced time (Table 1, entries 7 and 8). Because of a higher pKa value of K3PO4 compared to K2CO3 or other organic bases, the [3 + 2] cycloaddition reaction resulted exclusively in 5-phenyl oxazole 4a in a short time.43,44
Table 1. Effect of Base for [3 + 2] Cycloaddition Reaction in CH3OH Solventa.
| entry | base | pKa | equiv | time | oxazoline yield (%)b3a | oxazole yield (%)b4a |
|---|---|---|---|---|---|---|
| 1 | 12 h | 0 | 0 | |||
| 2 | triethylamine | 10.75 | 2 | 6 h | 95 | 0 |
| 3 | N,N-diisopropylethylamine | 10.74 | 2 | 6 h | 95 | 0 |
| 4 | imidazole | 6.9 | 2 | 6 h | 92 | 0 |
| 5 | N-methymorpholine | 7.4 | 2 | 6 h | 93 | 0 |
| 6 | NaHCO3 | 5.95 | 2 | 6 h | 90 | 0 |
| 7 | K2CO3 | 9.1 | 2 | 2 h | 0 | 94 |
| 8 | K3PO4 | 11.74 | 2 | 1.5 h | 0 | 95 |
Reaction was performed using 1a (1.18 mmol), 2a (1.18), and 2.36 mmol base.
Yield of the isolated product.
Next, to improve the efficacy of this synthetic protocol, we have chosen 2 equiv of K3PO4 (pKa = 11.74) as a suitable base for the [3 + 2] cycloaddition of 4-toluenesulfonylmethyl isocyanide (TosMIC) with benzaldehyde for various solvent optimization. Employing 2 equiv of the K3PO4 strong base, we screened several organic and green solvents for the same set of reactions at 60 °C. Using a polar aprotic solvent such as DMF and DMSO did not yield any cyclized product (Table 2, entries 1 and 2). However, using polar aprotic solvents such as THF and CH3CN resulted in the 4,5-disubstituted oxazolines 3a product with 95% yield in 6 h (Table 2, entries 3 and 4). Surprisingly, using 2 equiv of the K3PO4 base for the same set of reactions in a CHCl3 solvent resulted in the formation of 4,5-disubstituted oxazolines 3a and 5-phenyl oxazole 4a in a 1:0.9 ratio (Table 2, entry 5). The use of H2O-IPA as the reaction medium at 60 °C resulted in the formation 5-phenyl oxazole 4a in greater proportion compared to 5-phenyl-4-tosyl-4,5-dihydrooxazole 3a (Table 2, entry 6), whereas at room temperature condition, it obtained the exclusive formation of 5-phenyl-4-tosyl-4,5-dihydrooxazole 3a (Table 2, entry 7). To avoid the toxic and high boiling solvents and shorten the reaction time, we performed the same set reaction in solvents such as EtOH and IPA and resulted in the formation of 5-phenyl oxazole 4a in 92–95% yields (Table 2, entries 8 and 9).
Table 2. Optimizations of Various Solvents for [3 + 2] Cycloaddition Reaction Using K3PO4 Basea.
| entry | solvent | temperature (°C) | time (h) | oxazoline yield (%)c | oxazole yield (%)c |
|---|---|---|---|---|---|
| 1 | DMF | 60 | 6 | 0 | 0 |
| 2 | DMSO | 60 | 6 | 0 | 0 |
| 3 | THF | 60 | 6 | 95 | 0 |
| 4 | CH3CN | 60 | 6 | 95 | 0 |
| 5 | CHCI3 | 60 | 6 | 50 | 40 |
| 6 | IPA-H2O | 60 | 4 | 10 | 80 |
| 7 | IPA | rt | 6 | 94 | 0 |
| 8 | EtOH | 60 | 1.5 | 0 | 92 |
| 9 | IPA | 60 | 1 | 0 | 95 |
| 10 | IPA | MWb, 65 | 0.13 | 0 | 96 |
Reaction was performed using 1a (1.18 mmol), 2a (1.18 mmol), and K3PO4 (2.36 mmol).
Microwave reactions were carried out in a microwave model no. CATA R (Catalyst Systems, Pune) at 65 °C using a power of 350 W.
Yield of the isolated product.
However, the use of microwave irradiation at 65 °C and 350 W for 8 min in an IPA medium resulted in the formation of 5-phenyl oxazole 4a in 96% yield (Table 2, entry 10). Next, we have chosen 2 equiv of the K3PO4 base for the synthesis of the 5-phenyl oxazole 4a derivative with optimized microwave power and time. Additionally, by employing organic bases triethylamine, N,N-diisopropylethylamine, imidazole, and N-methylmorpholine as well as mild base NaHCO3 for the same set of reactions at 60 °C in the IPA solvent for 6 h yielded only 92–95% of 4,5-disubstituted oxazoline 3a (Table S1 and Figure S40). Hence, organic bases such as triethylamine, N,N-diisopropylethylamine, imidazole, and N-methylmorpholine, and mild base NaHCO3 as well as room temperature of the reaction were not efficient to oxidize the 5-phenyl-4-tosyl-4,5-dihydrooxazole 3a to the 5-phenyl oxazoles 4a. Next, we focused our attention to the optimization of the reaction condition using K3PO4 as a base. The results are summarized in Table 3.
Table 3. Reaction Optimization Using K3PO4 Base Equivalentsa.
| entry | base | equiv | temperature (°C) | time (h) | oxazoline yield (%)b3a | oxazole yield (%)b4a |
|---|---|---|---|---|---|---|
| 1 | K3PO4 | 0.3 | 60 | 12 | 70 | 0 |
| 2 | K3PO4 | 0.5 | 60 | 12 | 85 | 0 |
| 3 | K3PO4 | 1.0 | 60 | 12 | 90 | 0 |
| 4 | K3PO4 | 1.5 | 60 | 12 | 65 | 30 |
| 5 | K3PO4 | 1 | MWc, 60 | 0.13 | 94 | 0 |
Reaction was performed using 1a (1.18 mmol) and 2a (1.18 mmol).
Yield of the isolated product.
Microwave reactions were carried out in a microwave model no. CATA R (Catalyst Systems, Pune) using a power of 280 W.
The optimal amounts of tripotassium phosphate bases were also evaluated in the same set of reactions. Using 0.3–1.0 equiv of K3PO4 as a base yielded exclusively the 5-phenyl-4-tosyl-4,5-dihydrooxazole 3a in 70–90% yields (Table 3, entries 1–3). Using 1.5 equiv of K3PO4 for the same set of reaction resulted in the mixture of 5-phenyl-4-tosyl-4,5-dihydrooxazole 3a and 5-phenyl oxazole 4a in a 2:1 ratio (Table 3, entry 4). However, using 1 equiv of the K3PO4 base for the same set of reaction under microwave irradiation at 60 °C for 280 W in 8 min resulted in the formation of 5-phenyl-4-tosyl-4,5-dihydrooxazole 3a in 94% yield (Table 3, entry 5). With the optimal reaction condition in hand, we have studied the substrate scope for the synthesis of substituted oxazoles and oxazolines using various substituted aldehyde (Scheme 2 and Tables 4 and 5). We also studied the kinetics of electron-donating and electron-withdrawing aldehydes for [3 + 2] cycloaddition reactions and are shown in Scheme 2. Diverse para-substituents on the aryl ring of aromatic aldehyde such as −Me, −OMe, −Cl, −CN, −Br, −NO2, and −F are well tolerated for the [3 + 2] cycloaddition reactions, yielding the 5-substituted oxazole products 4 in high yields. Moreover, substitutents present on the o- or m-position of the aromatic ring such as −OH, −NO2, −Cl, and heteroaryl aldehyde also yielded corresponding 5-substituted oxazoles products 4 in good yields (Table 4).
Scheme 2. One-Pot [3 + 2] Cycloaddition Reaction to 5-Substituted Oxazoles 4 and Diastereoselective 4,5-Disubstituted Oxazolines 3.
Table 4. Substrate Scope for 5-Substituted Oxazoles Derivatives 4a.
The reactions were performed with aldehyde (3 mmol), tosmic (3 mmol), and K3PO4 (6 mmol) at 65 °C.
Isolated yield.
Microwave reactions were carried out in a microwave model no. CATA R (Catalyst Systems, Pune) using a power of 350 W.
Table 5. Substrate Scope for the Diastereoselective 4,5-Disubstituted Oxazoline Derivatives 3a.
The reactions were performed with aldehyde (3 mmol), tosmic (3 mmol), and K3PO4 (3 mmol) at 60 °C.
Isolated yield.
Microwave reactions were carried out in a microwave model no. CATA R (Catalyst Systems, Pune) at 60 °C using a power of 280 W.
The formation of oxazoles and oxazolines was examined by the proton NMR spectroscopy. The representative compounds 5-(p-tolyl)-4-tosyl-4,5-dihydrooxazole 3b and 5-(p-tolyl) oxazole 4b product conversion were examined by proton NMR spectroscopy. It has been found that the characteristic vicinal protons Ha and Hb of the oxazoline ring appeared at 6.04 and 5.05 ppm, respectively, in spectra A in Figure 2. Subsequent formation of 5-(p-tolyl)oxazole confirmed by the disappearance of Ha and Hb protons and appearance of two-singlet proton at 8.42 and 7.63 ppm oxazole moieties and with the four aromatic proton of the tolyl moiety in spectra B in Figure 2.
Figure 2.

Representative stepwise monitoring 5-(p-tolyl)-4-tosyl-4,5-dihydrooxazole 3b to 5-(p-tolyl)oxazole 4b by 1H NMR spectroscopy.
From 1H NMR spectra in spectra A in Figure 2, we observed that all the synthesized 4,5-disubstituted oxazoline derivatives 3a–3d are diastereoselective. The 1H NMR spectra of 4,5-disubstituted oxazoline 3b show that the coupling constant value for vicinal JHa,Hb are almost 6 Hz (Supporting Information). The previous literature report and coupling constant for all synthesized 4,5-disubstituted oxazoline derivatives of Ha and Hb confirmed that the synthesized oxazoline derivatives are trans (anti) geometrical formation.45,46 In addition, to examine the limitations and scope of the [3 + 2] cycloaddition reactions, we have reacted aliphatic aldehyde such as butyraldehyde with 4-toluenesulfonylmethyl isocyanide (TosMIC) in the presence of 2 equiv of the potassium phosphate base in the IPA solvent at 60 °C heating for 6 h. However, we failed to obtain the desired oxazole or oxazoline derivatives.
Furthermore, we have established the potential synthetic utility of this synthetic procedure on the gram-scale synthesis using the model reaction. The reaction of 10 mmol 1a with 10 mmol 2a under microwave irradiation at 65 °C and 350 W for 8 min could afford a 1.4 g 5-phenyl oxazoles 4a yield (96%).The present methodology represents the potential applications of the present synthetic protocol for a large-scale synthesis (Scheme 3).
Scheme 3. Gram-Scale Synthesis of 5-Phenyl Oxazoles 4a.
Based on the experimental result of the reaction, a plausible mechanistic pathway for one-pot [3 + 2] cycloaddition reaction is outlined (Scheme 4). To synthesize diverse 5-substituted oxazoles derivatives, a minimum 2 equiv K3PO4 base was required in the IPA solvent. Mechanistically, we believe that the 1 equiv of the strong K3PO4 base abstracts protons from the acidic methylene position of 4-toluenesulfonylmethyl isocyanide (TosMIC) 2 provided the intermediate 2a′, which subsequently reacted with an arylaldehyde 1 to form intermediate b′ and simultaneously undergone one-pot [3 + 2] cycloaddition reaction to form intermediate c′ which under subsequent protonation from 4,5-disubstituted oxazoline derivatives 3d′. In the presence of another 1 equiv of the K3PO4 strong base and heating condition, 4,5-disubstituted oxazoline derivatives 3d′ underwent elimination reaction by the formation of 5-substituted oxazoles 4e′. Here, the tosyl group not only lower the pKa value of TosMIC but also act as a leaving group in 4,5-disubstituted oxazolines that depend upon the solvent, nature of the bases, equivalent of bases, and temperature of the reaction. Hence selectively, we achieved 5-substituted oxazoles using 2 equiv of strong K3PO4 bases in the IPA solvent under microwave irradiation. Using 1 equiv of the strong K3PO4 base, 4,5-disubstituted oxazolines products 3d′ predominates under microwave irradiation for 5–8 mins at 60 °C. We achieved exclusively the synthesis of 4,5-disubstituted oxazolines and 5-substituted oxazoles in a short time using the K3PO4 strong base in the IPA solvent under appropriate microwave irradiation.
Scheme 4. Plausible Reaction Mechanism to 5-Substituted Oxazoles 4.
3. Conclusions
In summary, we have developed a one-pot microwave-assisted [3 + 2] cycloaddition reaction of a substituted arylaldehyde with 4-toluenesulfonylmethyl isocyanide (TosMIC) to diastereoselective 4,5-disubstituted oxazolines and 5-substituted oxazoles by controlling the amount of K3PO4 as the base. Moreover, using organic bases such as triethylamine, N,N-diisopropylethylamine, imidazole, and N-methylmorpholine in heating conditions resulted in 4,5-disubstituted oxazolines. The rapid, simple, one-pot microwave-assisted syntheses of 4,5-disubstituted oxazolines and 5-substituted oxazoles in the IPA solvent make the process environmentally benign and economical. The method is extremely efficient, took a very short time, and simple to perform under mild conditions. We trust that our process accompaniments with associated methodologies by providing an alternative for synthesizing 4,5-disubstituted oxazolines and 5-substituted oxazoles.
4. Experimental Section
4.1. General Methods
All the starting materials, substituted aryl aldehyde, 4-toluenesulfonylmethyl isocyanide (TosMIC), triethylamine, diisopropylethylamine, N-methylmorpholine, imidazole, sodium bicarbonate, potassium carbonate, potassium phosphate tribasic (anhydrous, reagent grade > 98%), and 2-propanol (anhydrous, 99.5%), were purchased from Sigma-Aldrich, and solvents were used from commercial suppliers without further purification. Analyses of 1H NMR and 13C NMR were performed by a Bruker DRX400 spectrometer (400 MHz). Chemical shifts are reported in parts per million (ppm) relative to the internal standard. Coupling constants (J) are given in hertz (Hz). Multiplicities of peaks are given as d (doublet), m (multiplet), s (singlet), and t (triplet). The removal of the solvent was carried out by a rotary evaporator under reduced pressure. IR spectra were recorded on a Bomen DA8 3 FTS spectrometer. GC–MS has been recorded using a Perkin Elmer Clarus 600C spectrometer. Microwave-assisted reactions were carried out in a catalyst scientific microwave oven system (model no: CATA R; Catalyst System, Pune) operating at 2450 MHz equipped with glass vial extension by a condenser used for performing the reaction. The microwave was equipped with a temperature control system (external probe).
4.2. Representative General Procedure for the Synthesis of 5-Phenyl Oxazoles 4a
In a 50 mL round-bottle flask, benzaldehyde 1a (0.125 g, 1.18 mmol, 1.0 equiv), 4-toluenesulfonylmethyl isocyanide (TosMIC) 2 (0.230 g, 1.18 mmol, 1.0 equiv), and 10 mL IPA were added subsequently. Further, in the same round-bottle flask, K3PO4 was charged (0.500 g, 2.36 mmol, 2.0 equiv). The reaction mixture was irradiated in an open vessel fitted with a reflux condenser under 800 rpm stirring at 65 °C and 350 W for 8 min. After completion of the reaction as monitored by TLC, the reaction mixture was cooled to room temperature. The IPA solvent was removed under reduced pressure, and the crude product was diluted with water (15 mL) and extracted with ethyl acetate (15 mL). Further, the organic layer was washed with water (5 mL) and brine (5 mL). The crude product was washed with ice-cooled ether (15 mL) to provide pure products 4a (without column chromatography) in 96% yield (0.16 g).
4.3. Representative General Procedure for the Synthesis of Diastereoselective 5-Phenyl Oxazoline 3a
In a 50 mL round-bottle flask, benzaldehyde 1a (0.125 g, 1.18 mmol, 1.0 equiv), 4-toluenesulfonylmethyl isocyanide (TosMIC), 2 (0.230 g, 1.18 mmol, 1.0 equiv), and 10 mL IPA were added subsequently. In the same round-bottle flask, K3PO4 (0.381 g, 1.18 mmol, 1.0 equiv) was charged. The reaction mixture was irradiated in an open vessel under microwave at 60 °C and 280 W for 8 min. After completion of the reaction as monitored by TLC, the reaction mixture was cooled to room temperature. The IPA solvent was removed using reduced pressure, and the crude product was diluted with water (10 mL) and extracted with ethyl acetate. Further, the organic layer was washed with water (5 mL) and brine (5 mL). The crude product was washed with ice-cooled ether (15 mL) and hexane (10 mL) to provide pure products 3a (without column chromatography) with 94% yield (0.34 g).
4.3.1. 5-Phenyl Oxazole (4a)21−23
Brown liquid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.164 g, 96%; 1H NMR (400 MHz, CDCl3) δ 7.88 (s, 1H), 7.63–7.61 (m, 2H), 7.40–7.37 (m, 2H), 7.31 (d, J = 7.2 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 151.5, 150.5, 128.9, 128.7, 127.67, 124.3, 121.4. MS (m/z, EI+) calcd for C9H7NO (+) 145.05, found 145.13.
4.3.2. 5-(p-Tolyl) Oxazole (4b)22,37
Brown solid; Rf = 0.25 (20% EtOAc/n-hexane); yield: 0.156 g, 94%; mp 59 °C; 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.63 (s, 1H), 7.61 (d, J = 8.4 Hz, 3H), 7.28 (d, J = 8.0 Hz, 2H), 2.33 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 151.9, 150.1, 133.6, 130.1, 125.2, 124.5, 121.7, 21.3. MS (m/z, EI+) calcd for C10H9NO (+) 159.06, found 159.10.
4.3.3. 5-(4-Methoxyphenyl) Oxazole (4c)22,37
Dark brown solid; Rf = 0.25 (20% EtOAc/n-hexane); yield: 0.154 g, 96%; mp 59 °C; 1H NMR (400 MHz, CDCl3) δ 7.87 (s, 1H), 7.58 (d, J = 8.8 Hz, 2H), 7.23 (s, 1H), 6.95 (d, J = 8.4 Hz, 2H), 3.84 (s, 3H). 13C NMR (100 MHZ, CDCl3): δ 159.9, 151.6, 149.9, 125.9, 120.0, 114.4, 55.4. MS (m/z, EI+) calcd for C10H9NO2 (+) 175.06, found 175.13.
4.3.4. 5-(4-Chlorophenyl) Oxazole (4d)22,37
Brown solid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.15 g, 94%; mp 81 °C; 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H), 7.57 (d, J = 8.8 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.34 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 150.6, 150.5, 134.4, 129.2, 126.2, 125.6, 121.8. MS (m/z, EI+) calcd for C9H6ClNO (+) 179.01, found 179.25.
4.3.5. 4-(Oxazol-5-yl) Benzonitrile (4e)32
Brown solid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.151 g, 93%; mp 71 °C; 1H NMR (400 MHz, CDCl3) δ 7.91 (s, 1H), 7.56 (d, J = 8.4 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.34 (s, 1H) 13C NMR (100 MHz, CDCl3) δ 150.6, 150.5, 134.4, 129.2, 126.2, 125.6, 121.8. MS (m/z, EI+) calcd for C10H6N2O (+) 170.04, found 170.06.
4.3.6. 5-(3-Bromophenyl) Oxazole (4f)21,33,37
Yellow powder; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.142 g, 94%; mp 69 °C; 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H), 7.79 (t, J = 1.7 Hz, 1H), 7.58–7.55 (m, 1H), 7.47–7.44 (m, 1H), 7.37 (s, 1H), 7.28 (d, J = 8 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 13C NMR (101 MHz, CDCl3) δ 150.8, 150.1, 131.5, 130.5, 129.6, 127.3, 123.1, 122.9, 122.4. MS (m/z, EI+) calcd for C9H6BrNO (+) 222.96, found 223.07.
4.3.7. 5-(4-Nitrophenyl) Oxazole (4g)22,37
Brown solid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.145 g, 92%; mp 103 °C; 1H NMR (400 MHz, DMSO) δ 8.66 (s, 1H), 8.38 (d, J = 8.8 Hz, 2H), 8.07 (s, 1H), 8.04 (d, J = 8.4 Hz, 2H), 8.04 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 153.9, 149.2, 147.3, 133.7, 126.1, 125.4, 125.0. MS (m/z, EI+) calcd for C9H6N2O3 (+) 190.03, found 190.17.
4.3.8. (E)-5-Styryloxazol (4h)21,35
Brown solid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.154 g, 94%; mp 53 °C; 1H NMR (400 MHz, CDCl3) δ 7.75 (s, 1H), 7.39 (d, J = 8.4 Hz, 2H), 7.28 (t, J = 1.6 Hz, 2H), 7.21 (d, J = 7.2 Hz, 2H), 7.04 (s, 1H), 6.98 (d, J = 5.6 Hz, 2H), 6.82 (d, J = 16.0 Hz, 2H), 13C NMR (100 MHz, CDCl3) δ 150.5, 150.4, 136.2, 130.3, 128.8, 128.4, 126.6, 124.2, 112.9. MS (m/z, EI+) calcd for C11H9NO (+) 171.06, found 171.17.
4.3.9. 3-(Oxazol-5-yl) Phenol (4i)37
Brown solid; Rf = 0.4 (20% EtOAc/n-hexane); yield: 0.153 g, 93%; mp 113 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.21 (brs, 1H), 7.92 (s, 1H), 7.17 (s, 1H), 7.14 (t, J = 8.0 Hz, 1H), 7.04 (d, J = 7.2 Hz, 2H), 6.74 (d, J = 8.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 157.9, 151.5, 150.6, 130.1, 128.7, 121.4, 117.0, 115.4, 111.3. MS (m/z, EI+) calcd for C9H7NO2 (+) 161.04, found 161.15.
4.3.10. 5-(4-Fluorophenyl) Oxazole (4j)22,37
Brown liquid; Rf = 0.4 (20% EtOAc/n-hexane); yield: 0.148 g, 90%; 1H NMR (400 MHz, CDCl3) δ 7.84 (s, 1H), 7.56–7.53 (m, 2H), 7.22 (s, 1H), 7.04 (t, J = 8.4 Hz, 1H), 13C NMR (100 MHz, CDCl3) δ 164.04, 150.47, 126.37, 126.29, 124.06, 124.03, 121.03, 121.01, 116.21, 115. 99. MS (m/z, EI+) calcd for C9H6FNO (+) 163.04, found 163.24.
4.3.11. 5-(4-Bromophenyl) Oxazole (4k)33,37
Brown solid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.14 g, 92%; mp 59 °C; 1H NMR (400 MHz, CDCl3) δ 7.83 (s, 1H), 7.46–7.40 (m, 4H), 7.27 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 150.7, 150.6, 132.2, 126.7, 125.9, 122.6, 122.0. MS (m/z, EI+) calcd for C9H6BrNO (+) 222.96, found 223.07.
4.3.12. 5-(Pyridin-2-yl) Oxazole (4l)32
Black liquid; Rf = 0.4 (20% EtOAc/n-hexane); yield: 0.154 g, 90%; 1H NMR (400 MHz, CDCl3) δ 8.55 (d, J = 4.8 Hz, 1H), 7.90 (s, 1H), 7.71–7.66 (m, 1H), 7.63 (s, 1H), 7.58 (d, J = 8 Hz, 1H), 7.17 (dd, J = 4.8, 0.8 Hz 1H), 13C NMR (100 MHz, CDCl3) δ 151.2, 151.0, 149.9, 147.1, 136.9, 124.8, 123.1, 119.4. MS (m/z, EI+) calcd for C8H6N2O (+) 146.05, found 146.18.
4.3.13. 5-(2-Chlorophenyl) Oxazole (4m)22,37
Brown solid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.145 g, 92%; mp 97 °C; 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H), 7.70 (s, 2H), 7.36 (d, J = 8 Hz, 1H), 7.26 (d, J = 7.2 Hz, 1H), 7.20–7.19 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 150.5, 148.2, 130.8, 130.7, 129.3, 127.9, 127.1, 126.5, 126.1. MS (m/z, EI+) calcd for C9H6ClNO (+) 179.01, found 179.11.
4.3.14. 5-(3-Nitrophenyl) Oxazole (4n)
Pale brown solid; Rf = 0.3 (20% EtOAc/n-hexane); yield: 0.142 g, 90%; mp 79 °C; 1H NMR (400 MHz, CDCl3) δ 8.43 (t, J = 1.6 Hz, 1H), 8.14–8.11 (m, 1H), 7.93 (s, 1H), 7.92–7.89 (m, 1H). 7.56 (t, J = 8.4 Hz, 1H), 7.45 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 151.3, 149.4, 148.8, 130.1, 129.8, 129.4, 123.5, 123.1, 119.2. MS (m/z, EI+) calcd for C9H6N2O3 (+) 190.03, found 190.17.
4.3.15. 5-(2,4-Dimethoxyphenyl) Oxazole (4o)32
Yellow solid; Rf = 0.25 (20% EtOAc/n-hexane); yield: 0.146 g, 95%; mp 73 °C; 1H NMR (400 MHz, CDCl3) δ 7.79 (s, 1H), 7.16 (s, 1H), 7.13 (dd, J = 8.4, 2.0 Hz, 1H), 7.05 (d, J = 2.0 Hz, 1H), 6.81 (d, J = 8.4 Hz, 1H), 3.85 (s, 3H), 3.82 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 151.5, 149.9, 149.5, 149.3, 120.7, 120.2, 117.3, 111.4, 107.6, 55.9. MS (m/z, EI+) calcd for C11H11NO3 (+) 205.07, found 205.20.
4.3.16. 5-Phenyl-4-tosyl-4,5-dihydrooxazole (3a)35
White solid; Rf = 0.5 (20% EtOAc/n-hexane); yield: 0.335 g, 94%, mp 171 °C; 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.4 Hz, 2H), 7.40 (t, J = 6.8 Hz, 4H), 7.33 (d, J = 6.8 Hz, 2H), 7.24 (s, 1H), 6.05 (d, J = 6.0 Hz, 1H), 5.05 (d, J = 6.0 Hz, 1H), 2.46 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.5, 145.7, 137.7, 133.1, 129.9, 129.6, 129.1, 92.6, 79.40, 21.8.
4.3.17. 5-(p-Tolyl)-4-tosyl-4,5-dihydrooxazole (3b)23
White solid; Rf = 0.5 (20% EtOAc/n-hexane); yield: 0.313 g, 95%, mp 161 °C; 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.4 Hz, 1H), 7.23 (m, 5H), 6.03 (d, J = 6.0 Hz, 1H), 5.05 (dd, J = 6.0, 1.2 Hz, 1H), 2.47 (s, 3H), 2.38 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.6, 145.7, 139.1, 134.8, 133.2, 129.9, 129.8, 129.6, 125.3, 92.5, 79.5, 21.8, 21.2. MS (m/z, EI+) calcd for C17H17NO3S (+) 315.09, found 315.26.
4.3.18. 5-(4-Methoxyphenyl)-4-tosyl-4,5-dihydrooxazole (3c)36
White solid; Rf = 0.5 (20% EtOAc/n-hexane); Yield = 0.289 g, 95%, mp 155 °C; 1H NMR (400 MHz, DMSO) δ 7.83 (d, J = 8.0 Hz, 1H), 7.67 (s, 1H), 7.48 (d, J = 8 Hz, 1H), 7.21 (d, J = 8 Hz, 1H), 7.21 (d, J = 8 Hz, 1H), 6.96 (d, J = 8 Hz, 1H), 5.86 (d, J = 5.6 Hz, 1H), 5.47 (d, J = 5.6 Hz, 1H), 3.76 (s, 3H), 3.71 (s, 1H), 2.43 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 160.6, 160.3, 145.7, 133.5, 130.2, 130.1, 129.9, 128.2, 114.7, 91.3, 79.3, 55.7, 21.6. MS (m/z, EI+) calcd for C17H17NO4S (+) 331.08, found 331.19.
4.3.19. 2-(4-Tosyl-4,5-dihydrooxazol-5-yl) Phenol (3d)
White solid; Rf = 0.6 (20% EtOAc/n-hexane); yield: 0.306 g, 94%; mp 109 °C; 1H NMR (400 MHz, CDCl3) δ 7.79–7.72 (m, 2H), 7.30 (d, J = 8.8 Hz, 4H), 7.20 (d, J = 6.4 Hz, 2H), 7.14 (s, 1H), 5.95 (d, J = 6.0 Hz, 1H), 4.90 (d, J = 6.0 Hz, 1H), 2.38 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.4, 145.1, 136.2, 135.1, 133.0, 130.0, 129.6, 129.4, 128.8, 127.0, 126.6, 92.5, 78.7, 21.8. MS (m/z, EI+) calcd for C16H15NO4S (+) 317.07, found 317.14.
Acknowledgments
The authors thank DST-Govt of India for funding through DST-SERB-YSS/2015/000450. The authors thank the Chancellor and Vice-Chancellor of VIT for providing an opportunity to carry out this study. K.C. thanks CSIR-Govt of India for funding through grant no. 01(2913)/17/EMR-II. The authors thank VIT for providing “VIT SEED GRANT” for carrying out this research work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04130.
1H NMR, 13C NMR, and GCMS spectra of synthesized compounds (PDF)
The authors declare no competing financial interest.
Dedication
This article is dedicated to Prof. Chung Ming Sun for his enormous contribution in combinatorial chemistry.
Supplementary Material
References
- Scott D. E.; Bayly A. R.; Abell C.; Skidmore J. Small molecules, big targets: drug discovery faces the protein–protein interaction challenge. Nat. Rev. Drug. Discov. 2016, 15, 533–550. 10.1038/nrd.2016.29. [DOI] [PubMed] [Google Scholar]
- Ruddigkeit L.; van Deursen R.; Blum L. C.; Reymond J. L. Enumeration of 166 Billion Organic Small Molecules in the Chemical Universe Database GDB-17. J. Chem. Inf. Model. 2012, 52, 2864–2875. 10.1021/ci300415d. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Aguilar A.; Bernard D.; Wang S. Small-Molecule Inhibitors of the MDM2–p53 Protein–Protein Interaction (MDM2 Inhibitors) in Clinical Trials for Cancer Treatment. J. Med. Chem. 2015, 58, 1038–1052. 10.1021/jm501092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner K. D.; Hajdin C. E.; Weeks K. M. Principles for targeting RNA with drug-like small molecules. Nat. Rev. Drug. Discov. 2018, 17, 547–558. 10.1038/nrd.2018.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Z.; Zhao Z. L.; Zhou C. H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. 10.1016/j.ejmech.2017.12.044. [DOI] [PubMed] [Google Scholar]
- Ke S.; Zhang Z.; Shi L.; Liu M.; Fang W.; Zhang Y.; Wu Z.; Wan Z.; Long T.; Wang K. An efficient synthesis and bioactivity evaluation of oxazole-containing natural hinduchelins A–D and their derivatives. Org. Biomol. Chem. 2019, 17, 3635–3639. 10.1039/C9OB00352E. [DOI] [PubMed] [Google Scholar]
- Sun L.; Liu Y.; Wang Y.; Li Y.; Liu Z.; Lu T.; Li W. An efficient synthesis of oxazolines via a cascade reaction between azaoxyallyl cations and 1,2-benzisoxazoles. Org. Biomol. Chem. 2019, 17, 7526–7530. 10.1039/C9OB01366K. [DOI] [PubMed] [Google Scholar]
- Delost M. D.; Smith D. T.; Anderson B. J.; Njardarson J. T. From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem. 2018, 61, 10996–11020. 10.1021/acs.jmedchem.8b00876. [DOI] [PubMed] [Google Scholar]
- Aitipamula S.; Wong A. B. H.; Chow P. S.; Tan R. B. H. Novel solid forms of oxaprozin: cocrystals and an extended release drug–drug salt of salbutamol. RSC Adv. 2016, 6, 34110–34119. 10.1039/C6RA01802E. [DOI] [Google Scholar]
- Kakkar S.; Kumar S.; Lim S. M.; Ramasamy K.; Mani V.; Shah S. A. A.; Narasimhan B. Design, synthesis and biological evaluation of 3-(2-aminooxazol-5-yl)-2H-chromen-2-one derivatives. Chem. Cent. J. 2018, 12, 130. 10.1186/s13065-018-0499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R.; Palta K.; Kumar M.; Bhargava M.; Dahiya L. Therapeutic potential of oxazole scaffold: a patent review (2006-2017). Expert Opin. Ther. Pat. 2018, 783–812. 10.1080/13543776.2018.1526280. [DOI] [PubMed] [Google Scholar]
- Zhang M.-Z.; Chen Q.; Mulholland N.; Beattie D.; Irwin D.; Gu Y.-C.; Yang G.-F.; Clough J. Synthesis and fungicidal activity of novel pimprinine analogues. Eur. J. Med. Chem. 2012, 53, 283–291. 10.1016/j.ejmech.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Fang W.; Wan Z.; Wang K.; Yang Q.; Cai X.; Shi L.; Yang Z. Antiviral effects against EV71 of pimprinine and its derivatives isolated from Streptomyces sp. Virol J. 2014, 11, 195. 10.1186/s12985-014-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar S.; Narasimhan B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13, 16. 10.1186/s13065-019-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z. Muscarine, imidaozle, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2013, 30, 869–915. 10.1039/c3np70006b. [DOI] [PubMed] [Google Scholar]
- Robles A. J.; McCowen S.; Cai S.; Glassman M.; Ruiz F. II; Cichewicz R. H.; McHardy S. F.; Mooberry S. L. Structure-Activity Relationships of New Natural Product-Based Diaryloxazoles with Selective Activity against Androgen Receptor-Positive Breast Cancer Cells. J. Med. Chem. 2017, 60, 9275–9289. 10.1021/acs.jmedchem.7b01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger J.; Melesina J.; Marek M.; Romier C.; Oehme I.; Witt O.; Sippl W.; Jung M. Synthesis and Biological Investigation of Oxazole Hydroxamates as Highly Selective Histone Deacetylase 6 (HDAC6) Inhibitors. J. Med. Chem. 2016, 59, 1545–1555. 10.1021/acs.jmedchem.5b01493. [DOI] [PubMed] [Google Scholar]
- Ibrar A.; Khan I.; Abbas N.; Farooq U.; Khan A. Transition-metal-free synthesis of oxazoles: valuable structural fragments in drug discovery. RSC Adv. 2016, 6, 93016–93047. 10.1039/C6RA19324B. [DOI] [Google Scholar]
- Faizi S.; Farooqi F.; Zikr-Ur-Rehman S.; Naz A.; Noor F.; Ansari F.; Ahmad A.; Khan S. A. Shahidine, a novel and highly labile oxazoline from Aegle marmelos: the parent compound of aegeline and related amides. Tetrahedron 2009, 65, 998–1004. 10.1016/j.tet.2008.11.088. [DOI] [Google Scholar]
- Valdomir G.; Fernández M. D. L. A.; Lagunes I.; Padrón J. I.; Martín V. S.; Padrón J. M.; Davyt D. Oxa/thiazole-tetrahydropyran triazole-linked hybrids with selective antiproliferative activity against human tumour cells. New J. Chem. 2018, 42, 13784–13789. 10.1039/C8NJ02388C. [DOI] [Google Scholar]
- Van Leusen A. M.; Hoogenboom B. E.; Siderius H. A novel and efficient synthesis of oxazoles from tosylmethylisocyanide and carbonyl compounds. Tetrahedron Lett. 1972, 13, 2369–2372. 10.1016/S0040-4039(01)85305-3. [DOI] [Google Scholar]
- Kulkarni B. A.; Ganesan A. Solution-phase parallel oxazole synthesis with TosMIC. Tetrahedron Lett. 1999, 40, 5637–5638. 10.1016/S0040-4039(99)01050-3. [DOI] [Google Scholar]
- Molander G. A.; Febo-Ayala W.; Ludivine J.-G. Condensation Reactions To Form Oxazoline-Substituted Potassium Organotrifluoroborates. Org. Lett. 2009, 11, 3830–3833. 10.1021/ol901395c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham R.; Prakash P.; Mahendran K.; Ramanathan M. A novel and convenient oxidation-controlled procedure for the synthesis of oxazolines from TosMIC and aldehydes in water–Anti biofilm activity. Arab. J. Chem. 2020, 13, 2153–2161. 10.1016/j.arabjc.2018.03.022. [DOI] [Google Scholar]
- Wasserman H. H.; Vinick F. J. Mechanism of the Robinson-Gabriel synthesis of oxazoles. J. Org. Chem. 1973, 38, 2407–2408. 10.1021/jo00953a028. [DOI] [Google Scholar]
- Qi C.; Peng Y.; Wang L.; Ren Y.; Jiang H. Copper-Catalyzed [2 + 3] Cyclization of α-Hydroxyl Ketones and Arylacetonitriles: Access to Multisubstituted Butenolides and Oxazoles. J. Org. Chem. 2018, 83, 11926–11935. 10.1021/acs.joc.8b01822. [DOI] [PubMed] [Google Scholar]
- Panda N.; Mothkuri R. Synthesis of substituted oxazoles from enamides. New J. Chem. 2014, 38, 5727–5735. 10.1039/C4NJ01101E. [DOI] [Google Scholar]
- Zhang L.; Xiao K.; Qiao Y.; Li X.; Song C.; Chang J. Base-Promoted Cycloisomerization for the Synthesis of Oxazoles and Imidazoles. Eur. J. Org. Chem. 2018, 2018, 6913–6918. 10.1002/ejoc.201801351. [DOI] [Google Scholar]
- Zhang X.; He Y.; Li J.; Wang R.; Gu L.; Li G. CO2/Photoredox-Cocatalyzed Tandem Oxidative Cyclization of α-Bromo Ketones and Amines To Construct Substituted Oxazoles. J. Org. Chem. 2019, 84, 8225–8231. 10.1021/acs.joc.9b00283. [DOI] [PubMed] [Google Scholar]
- Chen A. D.; Herbort J. H.; Wappes E. A.; Nakafuku K. M.; Mustafa D. N.; Nagib D. A. Radical cascade synthesis of azoles via tandem hydrogen atom transfer. Chem. Sci. 2020, 11, 2479–2486. 10.1039/C9SC06239D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H.; Yang Z.; Lin W.; Zheng Y.; Ma S. A catalytic highly enantioselective allene approach to oxazolines. Chem. Sci. 2018, 9, 1964–1969. 10.1039/C7SC04079B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimzadeh G.; Kianmehr E.; Mahdavi M. Improvement of the Van Leusen reaction in the presence of β-cyclodextrin: a green and efficient synthesis of oxazoles in water. Zeitschrift für Naturforschung B 2017, 72, 923–926. 10.1515/znb-2017-0005. [DOI] [Google Scholar]
- Savanur H. M.; Kalkhambkar R. G.; Laali K. K. Libraries of C-5 Substituted Imidazoles and Oxazoles by Sequential Van Leusen (VL)–Suzuki, VL–Heck and VL–Sonogashira in Imidazolium-ILs with Piperidine-Appended-IL as Base. Eur. J. Org. Chem. 2018, 2018, 5285–5288. 10.1002/ejoc.201800804. [DOI] [Google Scholar]
- Vinay Kumar K.; Swaroop T.; Rajeev N.; Vinayaka A.; Lingaraju G.; Rangappa K.; Sadashiva M. A One-Pot Tandem Approach for the Synthesis of 5-(Het)aryloxazoles from Substituted (Het)aryl Methyl Alcohols and Benzyl Bromides. Synlett 2016, 27, 1363–1366. 10.1055/s-0035-1561391. [DOI] [Google Scholar]
- Companyo X.; Moyano A.; Rios R. A Mild and Convenient Synthesis of 4-Tosyl-4,5-dihydrooxazoles. Lett. Org. Chem. 2009, 6, 293–296. 10.2174/157017809788489855. [DOI] [Google Scholar]
- Orne D.; Yakushijin K.; Buchi G. A two-step synthesis of imidazoles from aldehydes via 4-tosyloxazolines. Heterocycles 1994, 39, 139–153. [Google Scholar]
- Zhang L.-J.; Xu M.-C.; Liu J.; Zhang X.-M. Tandem cycloaddition–decarboxylation of α-keto acid and isocyanide under oxidant-free conditions towards monosubstituted oxazoles. RSC Adv. 2016, 6, 73450–73453. 10.1039/C6RA16031J. [DOI] [Google Scholar]
- Mathiyazhagan A. D.; Anilkumar G. Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2019, 17, 6735–6747. 10.1039/C9OB00847K. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Liu W.; Zhang D. Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules 2020, 25, 1–18. 10.3390/molecules25071594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseri L.; Razali M.; Pogany P.; Szekely G. Organic Solvents in Sustainable Synthesis and Engineering. Green Chem. 2018, 513–553. 10.1016/B978-0-12-809270-5.00020-0. [DOI] [Google Scholar]
- Saikia A. A.; Rao R. N.; Maiti B.; Balamurali M. M.; Chanda K. Diversity-Oriented Synthesis of Thiazolidine-2-imines via Microwave-Assisted One-Pot, Telescopic Approach and its Interaction with Biomacromolecules. ACS Comb. Sci. 2020, 10.1021/acscombsci.0c00083. [DOI] [PubMed] [Google Scholar]
- Mukku N.; Maiti B. On water catalyst-free synthesis of benzo[d]imidazo[2,1-b] thiazoles and novel N-alkylated 2-aminobenzo[d]oxazoles under microwave irradiation. RSC Adv. 2020, 10, 770–778. 10.1039/C9RA08929B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. K.; Hill A. P.; Redman A. M.; Sneddon H. F. Development of GSK’s acid and base selection guide. Green Chem. 2015, 17, 945–949. 10.1039/C4GC01481B. [DOI] [Google Scholar]
- Qafisheh N.; Mukhopadhyay S.; Joshi A. V.; Sasson Y.; Chuah G.-K.; Jaenicke S. Potassium Phosphate as a High-Performance Solid Base in Phase-Transfer-Catalyzed Alkylation Reactions. Ind. Eng. Chem. Res. 2007, 46, 3016–3023. 10.1021/ie060899e. [DOI] [Google Scholar]
- Suga H.; Ikai K.; Ibata T. Cis and Enantioselective Synthesis of 2-Oxazoline-4-carboxylates through Lewis Acid-Catalyzed Formal [3 + 2] Cycloaddition of 5-Alkoxyoxazoles with Aldehydes. J. Org. Chem. 1999, 64, 7040–7047. 10.1021/jo990525d. [DOI] [Google Scholar]
- Mehedi M. S. A.; Tepe J. J. Diastereoselective One-Pot Synthesis of Oxazolines Using Sulfur Ylides and Acyl Imines. J. Org. Chem. 2019, 84, 7219–7226. 10.1021/acs.joc.9b00883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.