Abstract
1H-Phenalen-1-one is a very efficient and easy-to-synthesize photosensitizer. Many substitutions have been previously described, but most of them significantly reduce the singlet oxygen quantum yield. The chloromethyl derivative described elsewhere is a good starting point for the synthesis of many useful derivatives because of the methylene bridge that saves its unique photosensitizing properties. Eighteen new phenalenone derivatives have been synthesized, bearing amine, carboxylic acid, alcohol, azide, and other major functional groups in organic chemistry. These reactions were carried out in good-to-excellent yields, and most of these new compounds retained the singlet oxygen quantum yield of the parent molecule. These new derivatives are very promising precursors for a number of applications such as the development of photosensitive antimicrobial agents or materials.
Introduction
1H-Phenalen-1-one, also known as perinaphthenone or simply phenalenone (PN), is an oxygenated polycyclic aromatic hydrocarbon bearing a ketone moiety. This skeleton is found in several plant or fungal compounds, which are thought to play roles in the protection of organisms against external aggressions (e.g., phytoalexins).1 Some of these natural or natural-like phenalenones have demonstrated moderate-to-good activities against fungi,2 as well as against human cancer cells,3 but PN is mostly recognized as a type-II photosensitizer that, upon irradiation with blue light, is able to produce singlet oxygen with a very high quantum yield.4 Several recent reports have described successful photodynamic treatments of fungi,5 bacteria,6 or parasites7 based on the use of phenalenone derivatives. It appears that singlet oxygen production is highly influenced by substitutions on the PN skeleton. Addition of electron-withdrawing moieties5,8 or a methylene bridge9 does not seem to influence the singlet oxygen quantum yield, contrary to electron-donating moieties5,10 or conjugation elongation1 that significantly decreases the photosensitizer capabilities. Based on the previous work of Späth et al., which only described the synthesis of ammonium derivatives,11 we present in this article the functionalization of phenalenone with some of the main organic functions along with an evaluation of the singlet oxygen quantum yields of the synthesized derivatives.
Results and Discussion
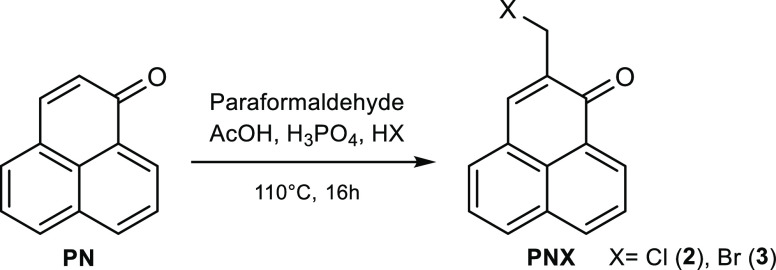
Phenalenone Synthesis and Chloromethylation
PN (1) was synthesized by the method of Song et al.7 and improved with the use of microwave activation (Scheme 1), which allowed a drastic reduction in reaction time from 3 h to 12 min. Multigram-scale reaction leads to a cost-effective production of PN with a very satisfactory purity (further purification is unnecessary).
Scheme 1. Phenalenone Synthesis.
Chloromethylation11 has been optimized by increasing the reaction time from 8 to 16 h (Scheme 2). This modification made it possible to obtain the chloromethyl derivative 2 (PNCl) with a yield of 51%, a noticeable increase compared with the 36% yield as reported in the original protocol. Replacing HCl with HBr allowed us to obtain the bromomethyl derivative 3 (PNBr), although with a lower yield (37%).
Scheme 2. Phenalenone Halomethylation.
Functionalization of the PNXs
PNCl has been previously used in the synthesis of amine and ether derivatives of PN.7,11,12 The halogen moiety is prone to react at room temperature with a variety of anionic compounds (Scheme 3).
Scheme 3. Reactions of PNXs with Various Nucleophiles.
Reaction of PNCl with sodium methoxide in MeOH/CH2Cl2 mixture gave the methyl ether 5 (PNOMe) in 92% yield. Demethylation of PNOMe in the presence of BBr3 resulted in the formation of the corresponding alcohol 6 (PNOH), also in excellent yield (88%) (Scheme 4).
Scheme 4. Alcohol Derivative Synthesis.
The propargylated phenalenone 7 (PNP) was obtained from PNCl by etherification in 86% yield under phase-transfer catalytic conditions using a quaternary ammonium salt as the catalyst and aqueous sodium hydroxide as the base.11
The sulfonate derivative 8 (PNS) was obtained by reaction of Na2SO3 with PNBr in a CHCl3/MeOH/H2O mixture in the presence of a surfactant during 6 h. The synthesis of a few sulfonated phenalenones has been already described, but, because of the experimental conditions (concentrated H2SO4 at 180 °C), regioselectivity seemed to be difficult to control and substitution occurred in position 2 or 5.8,13 Even if, starting from PN, the yield is lower (36%) than those described in the literature (47–55%), the present protocol ensures a rigorous regioselectivity under mild conditions.
The thiocyanate derivative 9 (PNSCN) was obtained by nucleophilic substitution of chlorine with KSCN in 47% yield.
The thioacetate derivative 10 (PNSAc) was synthesized by reaction of PNCl with potassium thioacetate during 4 h in MeOH under an inert atmosphere. After chromatographic separation, PNSAc was isolated in 77% yield. Thioacetate was converted to thiol by treatment of PNSAc with K2CO3 in MeOH, under an inert atmosphere to avoid the formation of a disulfide bond; thiolated phenalenone 11 (PNSH) was obtained in 77% yield (Scheme 5).
Scheme 5. Thiol Derivative Synthesis.
The azide derivative 4 (PNN3) was synthesized by the action of sodium azide in MeOH/H2O with a yield of 93%. This derivative was further modified through Huisgen-1,3-dipolar cycloaddition with propargylated compounds such as methyl propiolate or N-BOC-propargylamine to yield the methylester 12 (PNCOOMe) and carbamate 15 (PNBOC) derivatives, respectively. Conducted at room temperature, these reactions proceeded very quickly and cleanly to afford the corresponding compounds in almost quantitative yields. Acid hydrolysis of PNCOOMe and PNBOC led, respectively, to the carboxylic acid 13 (PNCOOH-A) and the ammonium chloride 16 (PNNH2-A) derivatives without further purification. Another acid derivative (PNCOOH-B, 14) was directly synthesized from PNN3 and 10-undecynoic acid in aqueous media.
The simplest amine derivative 17 (PNNH2-B) was obtained by the classical Staudinger reduction with triphenylphosphine as the reducing agent, a method much more effective than the Gabriel synthesis14 (93% yield instead of 46%) (Scheme 6).
Scheme 6. Synthesis of Amino- and Acid Derivatives.
All these functions can be easily used for the synthesis of new phenalenone derivatives. For example, amino derivatives are easily transformed via the formation of peptide bonds. An adamantane derivative (PNAda, 18) was obtained by reaction of the corresponding acyl chloride with PNNH2-A (16). A maleimide group was added after reaction between NHS-activated 6-maleimidohexanoic acid (MHA) with PNNH2-B (17) to obtain 19 (PNMal). The peptidic couplings were obtained in good yields regardless of the amino-PN used (Scheme 7).
Scheme 7. Examples of Peptidic Coupling.
In addition to adding amino or acid groups, the azide function can be used to attach other functions, for example, sugar moieties. A propargyl-glucose derivative has been attached to PNN3 (4) in good yield, and the resulting product 20 was then deprotected to obtain a new hydrosoluble neutral phenalenone 21 (Scheme 8).
Scheme 8. Attachment of a Glucose Moiety.
The different phenalenone derivatives were characterized by NMR spectroscopy and high-resolution electro-spray mass spectrometry (see the Supporting Information).
Photophysical Properties
The photophysical data of all the compounds were obtained in CHCl3 (Table 1). Because of its insolubility in this solvent, PNS (8) was not tested.
Table 1. Photophysical Data of the Different PN.
| compounds | fluorescence lifetime (ns) | Φf | 1O2 lifetime (μs) | ΦΔ |
|---|---|---|---|---|
| PN (1) | 5,7 | <0.01 | 199 | 0,98 |
| PNCl (2) | 1,2 | <0.01 | 201 | 1,04 |
| PNBr (3) | 5,7 | <0.01 | 174 | 0,71 |
| PNN3 (4) | 1,2 | <0.01 | 203 | 0,81 |
| PNOMe (5) | 6,2 | <0.01 | 205 | 0,79 |
| PNOH (6) | 2,6 | <0.01 | 205 | 0,93 |
| PNP (7) | 2,0 | <0.01 | 197 | 1,00 |
| PNS (8) | ND | ND | ND | ND |
| PNSCN (9) | 5,6 | <0.01 | 213 | 0,36 |
| PNSAc (10) | 5,8 | <0.01 | 202 | 0,23 |
| PNSH (11) | 5,6 | <0.01 | 204 | 0,13 |
| PNCOOMe (12) | 5,9 | <0.01 | 189 | 1,14 |
| PNCOOH-A (13) | 3,9 | <0.01 | 201 | 1,30 |
| PNCOOH-B (14) | 4,7 | <0.01 | 201 | 1,11 |
| PNBOC (15) | 2,6 | <0.01 | 203 | 1,07 |
| PNNH2-A (16) | 0,6 | <0.01 | 198 | 1,11 |
| PNNH2-B (17) | 3,7 | <0.01 | 189 | 1,07 |
| PNAda (18) | 3,1 | <0.01 | 193 | 0,89 |
| PNMal (19) | 4,8 | <0.01 | 206 | 1,00 |
| PNGlc-A (20) | 5,6 | <0.01 | 197 | 1,15 |
| PNGlc-B (21) | 3,0 | <0.01 | 178 | 1,15 |
The absorption spectra of the PN derivatives present two main absorption bands: the lower energetic band between 330 and 430 nm corresponding to a n → π* transition and the higher energetic band between 240 and 260 nm corresponding to a π → π* transition. The n → π* transition exhibits a lower extinction coefficient than the π → π* transition. The UV–visible spectrum of PNMal (19) displays an additional band probably because of the presence of the maleimide group (see the Supporting Information).
Singlet oxygen was measured by direct observation of singlet oxygen phosphorescence at 1270 nm. The singlet oxygen quantum yields have been calculated in comparison with the free PN (1) in CHCl3 as reference (QY of 0.98).4 Because of the presence of the methylene bridge,11,12 halogens, ethers, alcohols, amines, acids, and amide moieties do not impact the singlet oxygen quantum yield of the photosensitizers, that is close to unity. On the contrary, sulfur-based derivatives 9, 10, and 11 exhibit significantly lower singlet oxygen quantum yields. To a certain extent, these results are not surprising, as sulfur groups are known to quench singlet oxygen either by physical or chemical process.15 The singlet oxygen lifetime values in CHCl3 are homogeneous and grouped around the average value of 200 μs.
The relative fluorescence quantum yields of all the synthesized derivatives are very weak, less than 1% of that of quinine sulfate. As the first excited state of phenalenone proceeds from a n → π* transition, the fluorescence quantum yield is low because the de-excitation process is for the most part because of an intersystem crossing whose quantum yield is close to unity4 (see the Supporting Information).
In summary, this paper describes the synthesis and the characterization of 21 phenalenone derivatives, of which 18 are original; new reaction conditions or strategies used for some of them led us to improve the yield synthesis. Halogenomethylphenalenones are convenient precursors for a large range of phenalenone derivatives. The methylene bridge allows retaining the singlet oxygen quantum yield of the free PN, but sulfur-based phenalenone derivatives have shown a lower production of singlet oxygen.
Experimental Section
General Information
All reagents and solvents were purchased from Alfa Aesar, TCI, Carlo Erba, Fisher Chemical, VWR, or Sigma Aldrich and were used as received.
Analytical thin-layer chromatography was performed on silica plates (Kieselgel 60 F254, thickness 0.2 mm, Merck) and was revealed by direct observation and under a UV lamp at 345 nm. Purifications by column chromatography were carried out using silica gel 60 (Merck, 0.015–0.040 mm).
NMR analyses were conducted on a Bruker DPX 500 NMR spectrometer, operating at 500 MHz with tetramethylsilane as the reference. s = singlet, d = doublet, dd = double doublet, t = triplet, q = quartet, q5 = quintet.
High-resolution electrospray ionization mass spectra (HR ESI-MS) were performed at the ICOA/CBM (FR2708), Orléans university (France), with a Bruker Q-TOF maXis mass spectrometer coupled to an Ultimate 3000 RSLC chain (Dionex). Melting points were measured on a Kofler bench (Leica VMHB).
FT-IR analyses were realized on a FT-IR/NIR Frontier spectrometer (Perkin Elmer). st. = stretching, b. = bending, opb. = out of plane bending.
Absorption spectra were recorded on a UV-3600 UV–visible double-beam spectrophotometer (Shimadzu, Marne La Vallée, France). Fluorescence spectra were recorded on a Fluorolog FL3-222 spectrofluorometer (HORIBA Jobin Yvon, Longjumeau, France) equipped with a 450 W Xenon lamp, a thermostated cell compartment (25 °C), a UV–visible photomultiplier R928 (Hamamatsu, Japan), and an InGaAs infrared detector (DSS-16A020L Electro-Optical System Inc, Phoenixville, PA, USA). Excitation beam is diffracted by a double-ruled grating SPEX monochromator (1,200 grooves/mm blazed at 330 nm). Emission beam is diffracted by a double-ruled grating SPEX monochromator (1,200 grooves/mm blazed at 500 nm). Singlet oxygen emission was detected through a double-ruled grating SPEX monochromator (600 grooves/mm blazed at 1 μm) and a long-wave pass (780 nm). All spectra were obtained in four sides clear fluorometer quartz cuvettes. All the emission spectra (fluorescence and singlet oxygen luminescence) have been measured from solutions displaying the same absorbance at the excitation wavelength (less than 0.2).
Time-resolved experiments were performed using, for excitation, a pulsed laser diode emitting at 407 nm (LDH-P-C-400M, fwhm <70 ps, 1 MHz) coupled with a PDL 800-D driver (both from PicoQuant GmbH, Berlin, Germany) and, for detection, a SPCM-AQR-15 avalanche photodiode (EG & G, Vaudreuil, Canada) coupled with a 550 nm long-wave pass filter as detection system. Acquisition was performed with a PicoHarp 300 module with a PHR-800 4 channel router (both from PicoQuant GmbH, BERLIN, Germany). Fluorescence decays were recorded using the single photon counting method. Data were collected up to 1,000 counts accumulated in the maximum channel and analyzed using Time Correlated Single Photon Counting (TCSPC) software Fluofit (PicoQuant GmbH, Berlin, Germany) based on iterative reconvolution using a Levensberg-Marquandt algorithm, enabling the obtention of multiexponential profiles (mainly one or two exponentials in our case).
Synthesis of the PN Derivatives
1H-Phenalen-1-one 1 (PN)
PN was synthesized by applying the protocol described elsewhere7 without purification of the black tar to reach a yield of 58%. This protocol has been improved with the use of microwave irradiation. Briefly, 960 mg (7.5 mmol) of naphthalene and 1.25 g of cinnamoyl chloride were dissolved in 7.5 mL of CH2Cl2 and placed in an ice bath for 10 min. AlCl3 (3 g) was slowly added, and the mixture was stirred 10 min at 4 °C. The reaction medium was then irradiated for 12 min at 100 W and then poured into 100 mL of 37% HCl and filtered, and the filtrate was diluted in water before extraction with CH2Cl2 to obtain 780 mg (4.3 mmol, 57%) of a matt yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.64 (dd, J = 1.1, 7.4 Hz, 1H), 8.21 (dd, J = 0.9, 8.0 Hz, 1H), 8.03 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 9.8 Hz, 1H), 7.76 (d, J = 9.6 Hz, 1H), 7.61 (dd, J = 7.1, 8.2 Hz, 1H), 6.74 (d, J = 9.8 Hz, 1H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 185.67, 141.76, 134.89, 132.20, 131.92, 131.34, 130.38, 129.52, 129.28, 127.89, 127.59, 127.14, 126.64. FT-IR: ν (cm–1): 3039 (Aromatic CH st.), 1635, 1622, 1571, 1505 (C=O st., C=C st., CCC b.), 1392, 1356, 1284, 1236, 1183, 1121 (C=C st., CH b., CCC b.), 957, 828, 773, 732, 702, 614 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C13H9O [M + H]+: 181.064791; found, 181.065003. MW: 180.21 g/mol. mp 153 °C.
2-(Chloromethyl)-1H-phenalen-1-one 2 (PNCl)
The procedure described elsewhere11 was improved by increasing the reaction time. Briefly, 9.72 g (54 mmol) of PN, 29.7 g of paraformaldehyde, 216 mL of glacial acetic acid, and 135 mL of 85% phosphoric acid were mixed and warmed at 110 °C until solubilization of the solids. Hydrochloric acid (144 mL, 37%) was added, and the reaction was kept at 110 °C for 16 h. After cooling to room temperature, the solution was poured into 500 mL of ice and water and pH was increased with 5 M NaOH. The supernatant dark solid was filtered and washed with 1 M NaHCO3, and the product was extracted with CH2Cl2 and purified by column chromatography (CH2Cl2/PE 1:1) to yield 6.30 g (27.5 mmol, 51%) of a bright yellow powder (litt. 36%). 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.68 (dd, J = 1.1, 7.4 Hz, 1H), 8.22 (dd, J = 0.9, 8.0 Hz, 1H), 8.05 (d, J = 7.9 Hz, 1H), 7.94 (s, 1H), 7.81 (m, 2H), 7.62 (dd, J = 7.1, 8.2 Hz, 1H), 4.68 (d, J = 0.9 Hz, 2H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 183.49, 140.29, 135.66, 135.17, 132.20, 132.06, 132.00, 130.92, 129.02, 127.31, 127.29, 127.21, 126.81, 41.39. FT-IR: ν (cm–1): 2968, 2924 (Aromatic CH st.), 1637, 1623, 1595, 1578, 1569, 1507 (C=O st., C=C st., CCC b.), 1424, 1403, 1394, 1361, 1270, 1253, 1227, 1191, 1124; 1027 (C=C st., CH b., CCC b.), 949, 937, 847, 790, 769, 670 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C14H10ClO [M + H]+: 229.041469; found, 229.041619. MW: 228.7 g/mol. mp 143 °C.
2-(Bromomethyl)-1H-phenalen-1-one 3 (PNBr)
PN (9.02 g, 50 mmol), 27.5 g of paraformaldehyde, 200 mL of glacial acetic acid, and 125 mL of 85% phosphoric acid were mixed and warmed at 110 °C until solubilization of the solids. Hydrobromic acid (236 mL, 48% ) was added, and the reaction was kept at 110 °C for 16 h. After cooling to room temperature, the solution was poured into 500 mL of ice and water and neutralized with 0.5 M NaOH and then with solid K2CO3. The supernatant dark solid was filtered and washed with 1 M NaHCO3, and the product was extracted with CH2Cl2 and purified by column chromatography (CH2Cl2/PE 1:1) to yield 5.05 g (18.5 mmol, 37%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.69 (dd, J = 0.8, 7.5 Hz, 1H), 8.22 (d, J = 8.1 Hz, 1H), 8.05 (d, J = 8.3 Hz, 1H), 7.92 (s, 1H), 7.81 (m, 2H), 7.62 (dd, J = 7.4, 8.0 Hz, 1H), 4.56 (s, 2H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 183.32, 141.36, 136.36, 135.34, 132.52, 132.27, 132.20, 131.28, 129.25, 127.61, 127.56, 127.45, 127.01, 28.34. FT-IR: ν (cm–1): 3038, 2977 (Aromatic CH st.), 1635, 1621, 1595, 1568, 1507 (C=O st., C=C st., CCC b.), 1417, 1404, 1391, 1358, 1268, 1257, 1227, 1205, 1189, 1111, 1074, 1043, 1026 (C=C st., CH b., CCC b.), 973, 943, 930, 887, 845, 787, 768, 748, 691, 630, 615 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C14H10BrO [M + H]+: 272.990953; found, 272.990960. MW: 273.13 g/mol. mp 174 °C.
2-(Azidomethyl)-1H-phenalen-1-one 4 (PNN3)
PNCl (3.42 g, 15 mmol) and 19.5 g (300 mmol) of NaN3 were dissolved in 1.08 L of MeOH and 120 mL of water. The solution was left under stirring for 24 h at room temperature. MeOH was evaporated, and the aqueous suspension was extracted with CH2Cl2. The organic phase was dried with brine and then over MgSO4 and evaporated. The crude was purified by column chromatography (CH2Cl2/EP 1:1) to yield 3.28 g (14 mmol, 93%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.68 (dd, J = 0.9, 7.4 Hz, 1H), 8.24 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 8.2 Hz, 1H), 7.82 (m, 3H), 7.63 (dd, J = 7.2, 8.1 Hz, 1H), 4.46 (s, 2H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 184.21, 139.21, 135.22, 134.30, 132.09, 132.08, 131.90, 130.82, 129.00, 127.28, 127.26, 127.18, 126.83, 50.05. FT-IR: ν (cm–1): 3049, 2927 (Aromatic CH st.), 2109 (N3 st.), 1628, 1570, 1508 (C=O st., C=C st., CCC b.), 1388, 1247, 1176, 1025 (C=C st., CH b., CCC b.), 908, 838, 769, 700, 661 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C14H10N3O [M + H]+: 236.081838; found, 236.081868. MW: 235.25 g/mol. mp 96 °C.
2-(Methoxymethyl)-1H-phenalen-1-one 5 (PNOMe)
PNCl (1.82 g, 8 mmol) was dissolved in 80 mL of MeOH and 40 mL of CH2Cl2, and 8 mL (20 mmol) of 5 M sodium methoxide in MeOH was slowly added. The solution was stirred for 1 h at room temperature, and the base was neutralized with concentrated HCl, filtered, and evaporated. The crude was purified by column chromatography (eluent: CHCl3/MeOH 99:1) to yield 1.64 g (7.2 mmol, 92%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.64 (dd, J = 0.8, 7.4 Hz, 1H), 8.20 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 8.3 Hz, 1H), 7.82 (s, 1H), 7.77 (t, J = 7.6 Hz, 1H), 7.77 (d, J = 7.0 Hz, 1H), 7.59 (dd, J = 7.3, 8.0 Hz, 1H), 4.55 (d, J = 1.2 Hz, 2H), 3.56 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 184.44, 137.30, 136.72, 134.91, 132.03, 131.39, 131.33 130.39, 129.21, 127.72, 127.07, 127.01, 126.79, 69.37, 59.02. FT-IR: ν (cm–1): 2974, 2935, 2830 (Aromatic CH st.), 1630, 1571, 1507 (C=O st., C=C st., CCC b.), 1447, 1396, 1247, 1191, 1109, 1021 (C=C st., CH b., CCC b.), 918, 839, 772, 672 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C15H13O2 [M + H]+: 225.091006; found, 225.091224. MW: 224.26 g/mol. mp 86 °C.
2-(Hydroxymethyl)-1H-phenalen-1-one 6 (PNOH)
PNOMe (1.64 g, 7.2 mmol) was dissolved in 35 mL of CH2Cl2, and 10 mL (10 mmol) of 1 M BBr3 in CH2Cl2 was slowly added. The orange solution was stirred for 90 min at room temperature. Thirty-five milliliters of 1 M HCl in water was added, and the resulting yellow organic phase was collected, washed with water and brine, dried over MgSO4, and evaporated. The crude was purified by column chromatography (eluent: CHCl3/MeOH 98:2 then 96.5:3.5) to yield 1.33 g (6.33 mmol, 88%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.63 (d, J = 7.3 Hz, 1H), 8.21 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 8.3 Hz, 1H), 7.79 (t, J = 7.7 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.73 (s, 1H), 7.60 (t, J = 7.6 Hz, 1H), 4.72 (s, 2H), 3.03 (s, 1H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 185.88, 138.31, 137.97, 135.26, 132.01, 131.79, 131.77, 130.59, 129.13, 127.44, 127.15, 127.08, 126.83, 62.29. FT-IR: ν (cm–1): 3438 (OH), 2919, 2890, 2851 (Aromatic CH st.), 1635, 1611, 1564, 1509 (C=O st., C=C st., CCC b.), 1450, 1398, 1361, 1263, 1242, 1179, 1125, 1073, 1002, 990 (C=C st., CH b., CCC b.), 892, 844, 778, 764, 724, 691, 666 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C14H11O2 [M + H]+: 211.075356; found, 211.074947. MW: 210.23 g/mol. mp 127 °C.
2-((Prop-2-yn-1-yloxy)methyl)-1H-phenalen-1-one 7 (PNP)
PNCl (5 g, 21.8 mmol), 5 mL (87.2 mmol) of propargyl alcohol, 220 mL of CH2Cl2, 1.1 g (2.97 mmol) of tetrabutylammonium iodide, and 220 mL of 5 M aq. NaOH were vigorously stirred for 24 h at room temperature. After completion of the reaction, 250 mL of CH2Cl2 was added, and the organic phase was washed with water and then with brine, dried over MgSO4, and evaporated. The crude was purified by column chromatography (eluent: CH2Cl2) to yield 4.63 g (18.6 mmol, 86%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.65 (dd, J = 1.0, 7.3 Hz, 1H), 8.21 (dd, J = 0.5, 7.9 Hz, 1H), 8.01 (d, J = 8.2 Hz, 1H), 7.86 (s, 1H), 7.79 (s, 1H), 7.78 (t, J = 7.5 Hz, 1H), 7.60 (dd, J = 7.2, 8.1 Hz, 1H), 4.70 (d, J = 1.3 Hz, 2H), 4.36 (d, J = 2.4 Hz, 2H), 2.50 (t, J = 2.3 Hz, 1H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 184.3, 137.83, 136.14, 134.96, 132.04, 131.53, 131.49, 130.46, 129.18, 127.62, 127.11, 127.07, 126.80, 79.62, 74.82, 66.79, 58.42. FT-IR: ν (cm–1): 3261 (CH acetylenic st.), 2921, 2880, 2858 (Aromatic CH st.), 2111 (CC acetylenic st.), 1638, 1616, 1599, 1580, 1568 (C=O st., C=C st., CCC b.), 1459, 1412, 1395, 1366, 1347, 1263, 1241, 1183, 1158, 1125, 1100, 1020 (C=C st., CH b., CCC b.), 924, 916, 843, 775, 762, 704, 651 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C17H13O2 [M + H]+: 249.091006; found, 249.091015. MW: 248.28 g/mol. mp 130 °C.
Sodium (1-oxo-1H-Phenalen-2-yl)methanesulfonate 8 (PNS)
PNBr (273 mg, 1 mmol), 504 mg (4 mmol) of sodium sulfite, and 369 mg (1 mmol) of tetrabutylammonium iodide were dissolved in a CHCl3/MeOH/H2O (8:1:1) mixture and vigorously stirred at room temperature for 6 h. The solvents were then evaporated, and the crude was adsorbed onto Florisil and purified by column chromatography (eluent: CHCl3/MeOH 7:3 to CHCl3/MeOH/H2O 6:4:1) to yield 295 mg (0.99 mmol, 99%) of a matt yellow powder. 1H-NMR (500 MHz, CD3OD): δ (ppm) 8.64 (dd, J = 0.9, 7.3 Hz, 1H), 8.36 (d, J = 7.6 Hz, 1H), 8.16 (d, J = 8.0 Hz, 1H), 8.15 (s, 1H), 7.94 (d, J = 7.0 Hz, 1H), 7.85 (t, J = 7.7 Hz, 1H), 7.69 (dd, J = 7.2, 8.1 Hz, 1H), 4.22 (s, 2H). 13C-NMR (125 MHz, CD3OD): δ (ppm) 185.92, 144.27, 136.81, 133.71, 133.67, 133.47, 132.89, 132.14, 130.38, 129.00, 128.43, 128.38 (2C), 51.10. FT-IR: ν (cm–1): 3054, 3041 (Aromatic CH st.), 1636, 1618, 1595, 1569, 1508 (C=O st., C=C st., CCC b.), 1407, 1387, 1359, 1281, 1256, 1206, 1171, 1145, 1120, 1058, 1041, 1024 (C=C st., SO st., CH b., CCC b.), 925, 891, 844, 800, 771, 743, 633, 610 (Aromatic −CH opb.). MS: HRMS (ESI–), calcd for C14H9O4S [M]−: 273.022703; found, 273.022380. MW: 296.27 g/mol. mp >260 °C.
2-(Thiocyanatomethyl)-1H-phenalen-1-one 9 (PNSCN)
PNCl (228 mg, 1 mmol) and 485 mg (5 mmol) of potassium thiocyanate were dissolved in 62 mL of MeOH and stirred 48 h at room temperature. The solvent was evaporated, and the crude was purified by column chromatography (eluent: CH2Cl2/petroleum ether 55:45) to yield 117 mg (0.47 mmol, 47%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.57 (d, J = 7.3 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H), 8.30 (d, J = 8.3 Hz, 1H), 8.20 (s, 1H), 8.12 (d, J = 7.0 Hz, 1H), 7.94 (t, J = 7.7 Hz, 1H), 7.78 (dd, J = 7.4, 8 Hz, 1H), 4.29 (s, 2H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 182.24, 141.49, 135.89, 133.29, 133.17, 132.81, 131.72, 130.48, 127.99, 127.56, 127.35, 126.42, 126.19, 113.12, 32.62. FT-IR: ν (cm–1): 3000 (Aromatic CH st.), 2150 (CN st.), 1638, 1622, 1594, 1568, 1507 (C=O st., C=C st., CCC b.), 1404, 1389, 1358, 1268, 1257, 1243, 1227, 1188, 1177, 1121 (C=C st., CH b., CCC b.), 926, 890, 847, 785, 775, 745, 650 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C15H10NOS [M + H]+: 252.047761; found, 252.047774. MW: 251.3 g/mol. mp 135–140 °C.
S-((1-oxo-1H-Phenalen-2-yl)methyl) Ethanethioate 10 (PNSAc)
PNCl (1.03 g, 4.5 mmol) and 771 mg (6.75 mmol) of potassium thioacetate were dissolved in 112 mL of MeOH and stirred for 4 h at room temperature under an inert atmosphere. Then, the precipitate was filtered off, and the filtrate was evaporated. The crude was purified by column chromatography (eluent: CH2Cl2) to yield 927 mg (3.46 mmol, 77%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.65 (dd, J = 0.9, 7.3 Hz, 1H), 8.20 (d, J = 7.7 Hz, 1H), 8.00 (d, J = 8.2 Hz, 1H), 7.91 (s, 1H), 7.79 (d, J = 7.5 Hz, 1H), 7.78 (d, 7.3 Hz, 1H), 7.58 (dd, J = 7.4, 8.1 Hz, 1H), 4.10 (s, 2H), 2.33 (s,3H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 195.99, 184.25, 139.99, 136.10, 135.03, 132.02, 131.72, 131.70, 130.83, 129.07, 127.57, 127.20, 127.16, 126.78, 30.46, 28.46. FT-IR: ν (cm–1): 3049, 2981 (Aromatic CH st.), 1675 (CO thioester st.), 1640, 1623, 1597, 1570, 1507 (C=O st., C=C st., CCC b.), 1401, 1388, 1351, 1246, 1253, 1185, 1134, 1102, 1037, 1022, 1003 (C=C st., CH b., CCC b.), 957, 943, 872, 846, 795, 781, 708, 687, 626 (Aromatic −CH opb., CS st.). MS: HRMS (ESI+), calcd for C16H13O2S [M + H]+: 269.063077; found, 269.063190. MW: 268.33 g/mol. mp 105 °C.
2-(Mercaptomethyl)-1H-phenalen-1-one 11 (PNSH)
In 100 mL of argon-purged methanol containing 532 mg (2 mmol) of PNSAc was added 1.1 g (8 mmol) of K2CO3, and the reaction was kept for 3 h under stirring at room temperature. The solution slowly turned orange. After completion of the reaction, a solution of argon-purged HCl/methanol was added until the solution turned back to yellow. The solvent was evaporated, and the crude was purified by column chromatography to yield 347 mg (1.53 mmol, 77%) of a yellow powder. The compound was stored under argon at −22 °C to prevent thiol oxidation. 1H-NMR (500 MHz, CDCl3): 8.67 (d, J = 7.4 Hz, 1H), 8.21 (d, J = 8.1, 1H), 8.01 (d, J = 8.3 Hz, 1H), 7.78 (m, 3H), 7.60 (dd, J = 7.3, 8.0 Hz, 1H), 3.75 (d, J = 8.3 Hz, 2H), 2.12 (t, J = 8.3 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 184.11, 139.37, 138.47, 134.99, 132.03, 131.58, 131.29, 130.80, 129.20, 127.65, 127.20, 127.13, 126.77, 24.00. FT-IR: ν (cm–1): 3047, 2931, 2856 (Aromatic CH st.), 2581 (SH st.), 1634, 1619, 1594, 1579, 1567, 1506 (C=O st., C=C st., CCC b.), 1403, 1391, 1358, 1249, 1191, 1125, 1039, 1026 (C=C st., CH b., CCC b.), 973, 943, 846, 788, 767, 696, 681 (Aromatic −CH opb., CS st.). MS: HRMS (ESI+), calcd for C14H11OS [M + H]+: 227.052512; found, 227.052037. MW: 226.29 g/mol. mp 95 °C.
Methyl 1-((1-oxo-1H-Phenalen-2-yl)methyl)-1H-1,2,3-triazole-4-carboxylate 12 (PNCOOMe)
PNN3 (299 mg, 1.27 mmol), 115 μL (1.28 mmol) of methyl propiolate, 7.6 mg (40 μmol) of copper(I) iodide, 14 μL (80 μmol) of diisopropylethylamine, and 4.6 μL (80 μmol) of glacial acetic acid were dissolved in 4 mL of CH2Cl2 and stirred 90 min at room temperature. The translucent solution turned bright yellow. The formed precipitate was dissolved and purified on a short column to yield 401 mg (1.26 mmol, 99%) of a bright yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.68 (dd, J = 0.9, 7.4 Hz, 1H), 8.41 (s, 1H), 8.25 (d, J = 7.8 Hz, 1H), 8.09 (d, J = 8.3 Hz, 1H), 7.63 (m, 3H), 7.63 (dd, J = 7.3, 8.1 Hz, 1H), 5.62 (s, 2H), 3.93 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 183.87, 161.22, 142.02, 140.10, 135.79, 133.08, 133.03, 132.76, 132.14, 131.29, 128.91, 128.83, 127.52, 127.47, 127.03, 126.69, 52.18, 49.52. FT-IR: ν (cm–1): 3128 (CH3 st.), 3053, 2953 (Aromatic CH st.), 1737 (C=O ester st.), 1640, 1618, 1601, 1568, 1534 (C=O st., C=C st., CCC b.), 1439, 1405, 1395, 1363, 1268, 1248, 1204, 1149, 1126, 1114, 1051, 1016 (C=C st., CO st., CH b., CCC b.), 946, 918, 887, 845, 780, 696, 653 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C18H14N3O3 [M + H]+: 320.102968; found; 320.103102. MW: 319.32 g/mol. mp 207 °C.
4-Carboxy-1-((1-oxo-1H-phenalen-2-yl)methyl)-1H-1,2,3-triazol-1-ium chloride 13 (PNCOOH-A)
PNCOOMe (700 mg, 2.19 mmol) was dissolved in 40 mL of 37% HCl in dioxane (1:1 v/v) and stirred 4 h at 70 °C. Then, the solution was poured into 500 mL of cold water, and the yellow precipitate was filtered. The solid was dissolved in CHCl3/MeOH 90:10 and dried over MgSO4, and the solvent was evaporated to yield 677 mg (1.98 mmol, 90%) of a bright yellow powder. 1H-NMR (500 MHz, DMSO-d6): δ (ppm) 8.75 (s, 1H), 8.51 (d, J = 7.3 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.2 Hz, 1H), 8.07 (d, J = 7.0 Hz, 1H), 7.93 (s, 1H), 7.91 (t, J = 7.7 Hz, 1H), 7.74 (t, J = 7.6 Hz, 1H), 5.61 (s, 2H). 13C-NMR (125 MHz, DMSO-d6): δ (ppm) 182.78, 161.65, 141.11, 139.59, 135.83, 133.18, 132.88, 132.71, 131.67, 130.30, 129.65, 128.11, 127.51, 127.30, 126.39, 126.29, 48.81. FT-IR: ν (cm–1): 3357 (OH st.), 2605, 2522 (Aromatic CH st.), 1701 (C=O acid st.), 1639, 1619, 1597, 1568, 1549, 1510 (C=O st., C=C st., CCC b.), 1426, 1403, 1364, 1338, 1268, 1253, 1225, 1188, 1147, 1125, 1098, 1055, 1016 (C=C st., CO st., CH b., CCC b.), 937, 849, 804, 795, 782, 771, 753, 717, 700 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C17H12N3O3 [M + H]+: 306.087318; found, 306.087284. MW: 341.75 g/mol. mp 140 °C (degradation).
9-(1-((1-oxo-1H-Phenalen-2-yl)methyl)-1H-1,2,3-triazol-4-yl)nonanoic acid 14 (PNCOOH-B)
PNN3 (118 mg, 0.5 mmol), 91 mg (0.5 mmol) of 10-undecynoic acid, 19.6 mg of sodium ascorbate, and 2.5 mg of copper(II) sulfate pentahydrate were dissolved in 100 mL of a MeOH/H2O (4:1) mixture and stirred 48 h at room temperature. The solvents were then evaporated, and the crude was purified by column chromatography (eluent: CH2Cl2/EP 1:1) to yield 121 mg (0.29 mmol, 58%) of a poorly soluble yellow powder. 1H-NMR (500 MHz, DMSO-d6): δ (ppm) 8.53 (dd, J = 0.8, 7.5 Hz, 1H), 8.47 (d, J = 7.8 Hz, 1H), 8.26 (d, J = 8.2 Hz, 1H), 8.03 (d, J = 6.9 Hz, 1H), 7.92 (d, J = 8.0 Hz, 1H), 7.90 (d, J = 7.4 Hz, 1H), 7.82 (s, 1H), 7.73 (dd, J = 7.4, 8.0 Hz, 1H), 5.49 (s, 2H), 2.60 (t, J = 7.6 Hz, 2H), 2.09 (m, 2H), 1.56 (q, J = 7.5 Hz, 2H), 1.44 (t, J = 6.5 Hz, 2H), 1.23 (m, 8H). 13C-NMR (125 MHz, DMSO-d6): δ (ppm) 182.72, 175.14, 146.88, 140.50, 135.68, 133.60, 132.85, 132.48, 131.61, 130.19, 128.09, 127.44, 127.22, 126.28, 122.32, 48.02, 34.64, 28.83, 28.67, 28.63, 28.58, 28.48, 24.93, 24.85. FT-IR: ν (cm–1): 2929, 2912, 2849 (Aromatic CH st.), 1695 (C=O acid st.), 1642, 1631, 1602, 1574, 1549 (C=O st., C=C st., CCC b.), 1469, 1431 (1,2,3 triazole ring st.), 1395, 1362, 1327, 1266, 1251, 1209, 1191, 1056, 1014 (C=C st., CH b., CCC b.), 919, 891, 844, 780, 770, 724, 680, 650 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C25H28N3O3 [M + H]+: 418.212518; found, 418.212673. MW: 417.21 g/mol. mp 145 °C.
tert-Butyl ((1-((1-oxo-1H-Phenalen-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)carbamate 15 (PNBOC)
PNN3 (1.45 g, 6.16 mmol), 1.25 g (6.47 mmol) of N-BOC-propargylamine, 38 mg (0.123 mmol) of CuI, 70 μL (0.123 mmol) of DIPEA, and 22.8 μL (0.246 mmol) of acetic acid were placed in an erlenmeyer with the minimum volume of CH2Cl2 (∼20 mL). The reaction was left for 20 min at room temperature under stirring until a deep yellow solid appeared. The crude was evaporated and purified by column chromatography (CHCl3/MeOH 9:1) to yield 2.40 g (6.15 mmol, 99%) of a yellow powder. 1H-NMR (500 MHz, CDCl3): 8.67 (dd, J = 0.8, 7.4 Hz, 1H), 8.24 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 8.3 Hz, 1H), 7.82 (t, J = 7.7 Hz, 1H), 7.77 (m, 2H), 7.70 (s, 1H), 7.62 (dd, J = 7.3, 8.0 Hz, 1H), 5.55 (s, 2H), 5.08 (s, 1H), 4.41 (d, J = 5.4 Hz, 2H), 1.42 (s, 9H). 13C-NMR (125 MHz, CDCl3): 183.83, 155.77, 145.55, 141.10, 135.55, 133.52, 132.68, 132.65, 132.05, 131.08, 128.84, 127.37, 127.34, 126.90, 126.82, 122.96, 79.65, 48.91, 36.29, 28.38 (3C). -FT-IR: ν (cm–1): 3350 (NH st.), 3141, 2972 (Aromatic CH st.), 1687 (CO amide st.), 1641, 1622, 1600, 1570 (C=O st., C=C st., CCC b.), 1529 (CN st., NH b.), 1404 (1,2,3-triazole ring st.), 1395, 1359, 1320, 1265, 1249, 1168, 1127, 1054, 1026, 1013 (C=C st., CH b., CCC b.), 963, 930, 918, 881, 864, 844, 802, 779, 769, 653 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C22H23N4O3 [M + H]+: 391.176467; found, 391.176309. MW: 390.44 g/mol. mp 174 °C.
4-(Ammoniomethyl)-1-((1-oxo-1H-Phenalen-2-yl)methyl)-1H-1,2,3-triazol-1-ium chloride 16 (PNNH2-A)
PNBOC (462 mg, 1.18 mmol) was dissolved in 20 mL of 37% HCl/dioxane (3:1) and left under stirring at room temperature for 2 h. Solvent and acid were then evaporated, and the crude was solubilized in a minimum of methanol. A large volume of diethyl ether was added, causing the formation of a yellow precipitate. The solid was filtered and dried under vacuum to obtain a yellow powder with a quantitative yield (428 mg, 1.18 mmol). 1H-NMR (500 MHz, D2O): 8.26 (s, 1H), 7.75 (dd, J = 1.0, 7.5 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 8.0 Hz, 1H), 7,38 (s, 1H), 7.34 (d, J = 7.4 Hz, 1H), 7.28 (t, J = 7.6 Hz, 1H), 7.25 (dd, J = 7.2, 0.8 Hz, 1H), 5.30 (s, 2H), 4.44 (s, 2H). 13C-NMR (125 MHz, D2O): 184.39, 143.26, 139.65, 136.48, 134.14, 133.62, 130.82, 130.70, 130.67, 126.87, 126.78, 126.60, 126.18, 125.07, 125.00, 49.48, 34.04. FT-IR: ν (cm–1): 3410 (NH st.), 2923 (Aromatic CH St.), 1637, 1618, 1596, 1567, 1508 (C=O st., C=C st., CCC b.), 1404 (1,2,3 triazole ring st.), 1361, 1253, 1226, 1125, 1053 971 (C=C st., CH b., CCC b.), 885, 806, 774 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C17H15N4O [M – H]+: 291.123866; found, 291.124038. MW: 363.24 g/mol. mp 151 °C (degradation).
(1-oxo-1H-Phenalen-2-yl)methanaminium chloride 17 (PNNH2-B)
PNN3 (1.05 g, 4.45 mmol) and 1.34 g (5.12 mmol) of triphenylphosphine were dissolved in 215 mL of THF, and 256 μL of water was added. The solution was stirred for 12 h at 70 °C. The solvent was evaporated, and the crude was purified by column chromatography. The viscous oil obtained was protonated with 3 mL of 37% HCl, evaporated, and crystallized in petroleum ether to yield 1.02 g (4.15 mmol, 93%) of a matt yellow powder (litt.: 46%11). 1H-NMR (500 MHz, CD3OD): δ (ppm) 8.66 (dd, J = 1.1, 7.4 Hz, 1H), 8.41 (dd, J = 0.8, 8.1 Hz, 1H), 8.24 (d, J = 8.3 Hz, 1H), 8.13 (s, 1H), 8.03 (d, J = 6.7 Hz, 1H), 7.90 (t, J = 7.7 Hz, 1H), 7.74 (dd, J = 7.1, 8.2 Hz, 1H), 4.14 (s, 2H). 13C-NMR (125 MHz, CD3OD): δ (ppm) 185.91, 144.40, 137.54, 134.90, 134.71, 133.79, 132.43, 132.19, 129.89, 128.77, 128.60, 128.54, 127.98, 40.77. FT-IR: ν (cm–1): 3374 (NH st.), 2812 (Aromatic CH st.), 1636, 1561, 1508 (C=O st., C=C st., CCC b.), 1472, 1439, 1400, 1376, 1257, 1228, 1121, 1022, 962 (C=C st., CH b., CCC b.), 911, 845, 784, 765, 724, 694 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C14H12NO [M + H]+: 210.091340; found, 210.091153. MW: 245.5 g/mol. mp 210 °C (degradation).
(3r,5r,7r)-N-((1-((1-oxo-1H-Phenalen-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)adamantane-1-carboxamide 18 (PNAda)
PNNH2-B (121 mg, 0.33 mmol) was dissolved in a CH2Cl2/DIPEA (2:1) mixture under an inert atmosphere, and 67 mg (0.33 mmol) of 1-adamantanecarbonyl chloride was slowly added. The reaction was left for 5 min at room temperature, and the solvents were distilled under reduced pressure. The crude was purified by column chromatography (CHCl3/MeOH 99:1) to yield 87 mg (0.19 mmol, 58%) of a yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.67 (dd, J = 1.0, 7.4 Hz, 1H), 8.24 (d, J = 8.1 Hz, 1H), 8.07 (d, J = 8.3 Hz, 1H), 7.82 (t, J = 7.7 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.76 (s, 1H), 7.68 (s, 1H), 7.62 (dd, J = 7.2, 8.1 Hz, 1H), 6.28 (t, J = 4.8 Hz, 1H), 5.55 (s, 2H), 4.5 (d, J = 5.4 Hz, 2H), 2.01 (m, 3H), 1.84 (m, 6H), 1.70 (m, 6H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 183.79, 177.98, 144.99, 141.08, 135.55, 133.47, 132.70, 132.63, 132.05, 131.10, 128.83, 127.40, 127.34, 126.89, 126.82, 123.19, 48.96, 40.60, 39.15 (3C), 36.50 (3C), 34.99, 28.10 (3C). FT-IR: ν (cm–1): 2903, 2849 (Aromatic CH st.), 1640, 1598, 1572, 1510 (C=O st., C=C st., CCC b.), 1452, 1405 (1,2,3-triazole ring st.), 1362, 1254, 1183, 1104, 1049 (C=C st., CH b., CCC b.), 812, 778, 662 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C28H29N4O2 [M + H]+: 453.228503; found, 453.228830. MW: 452.56 g/mol. mp 51 °C (degradation).
6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)-N-((1-oxo-1H-phenalen-2-yl)methyl)hexanamide 19 (PNMal)
6-Maleimidohexanoic acid (682 mg, 3.23 mmol), 555 mg (4.84 mmol) of N-hydroxysuccinimide, and 799 mg (3.88 mmol) of dicyclohexylcarbodiimide were dissolved in 20 mL of anhydrous DMF under an inert atmosphere and left to react overnight. The dicyclohexylurea crystals were then discarded, and 660 mg (2.65 mmol) of PNNH2-B and 738 μL (5.3 mmol) of triethylamine were dissolved in 20 mL of anhydrous DMF, and the mixture was added slowly to the reaction. The whole was left for 18 h under an inert atmosphere at room temperature. The solvents were evaporated, the crude was dissolved in CH2Cl2, and the solid was filtered off. The filtrate was evaporated and purified by column chromatography (eluent: CHCl3/MeOH 98:2) to yield 970 mg (2.41 mmol, 91%) of a yellow powder. 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.65 (dd, J = 1.0, 7.4 Hz, 1H), 8.23 (dd, J = 0.8, 8.0 Hz, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.82 (s, 1H), 7.81 (t, J = 7.7 Hz, 1H), 7.80 (d, J = 6.8 Hz, 1H), 7.62 (dd, J = 7.3, 8.2 Hz, 1H), 6.63 (s, 2H), 6.43 (t, J = 5.6 H, 1H), 4.41 (d, J = 6.2 Hz, 2H), 3.47 (t, J = 7.3 Hz, 2H), 2.18 (t, J = 7.6 Hz, 2H), 1.66 (q5, J = 7.6 Hz, 2H), 1.60 (q5, J = 7.5 Hz, 2H), 1.30 (m, 2H). 13C-NMR (125 MHz, CDCl3): δ (ppm) 185.71, 172.67, 170.79 (2C), 140.10, 135.96, 135.27, 134.01 (2C), 132.10, 132.05, 131.87, 130.62, 129.19, 127.48, 127.23, 127.14, 126.90, 49.19, 37.63, 36.52, 28.26, 26.36, 25.04. FT-IR: ν (cm–1): 3276 (NH st.), 2936 (Aromatic CH st.), 1697 (C=O maleimide st.), 1630 1568 (C=O st., C=C st., CCC b.), 1405 (C,C > N symmetric st.), 1362, 1248, 1225, 1131, 1047, (C=C st., CH b., CCC b.), 827, 810, 778 (Aromatic −CH opb.), 695 (ring bending maleimide). MS: HRMS (ESI+), calcd for C24H23N2O4 [M + H]+: 403.165234; found, 403.165045. MW: 402.45 g/mol. mp 148 °C.
(2R,3R,4S,5R,6R)-2-(Acetoxymethyl)-6-((1-((1-oxo-1H-phenalen-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methoxy)tetrahydro-2H-pyran-3,4,5-triyl Triacetate 20 (PNGlc-A)
PNN3 (235 mg, 1 mmol), 380 mg (0.99 mmol) of 2-propynyl-tetra-O-acetyl-β-d-glucopyranoside, 3.8 mg (0.02 mmol) of copper(I) iodide, 7 μL (0.04 mmol) of diisopropylethylamine, and 2.4 μL (0.04 mmol) of glacial acetic acid were dissolved in 10 mL of CH2Cl2 and stirred 72 h at room temperature. The solvent was evaporated, and the crude was purified by column chromatography (eluent: CHCl3/MeOH 99:1) to yield 444 mg (0.71 mmol, 72%) of a bright yellow powder. 1H-NMR (500 MHz, acetone-d6): δ (ppm) 8.60 (dd, J = 1.0, 7.4 Hz, 1H), 8.43 (d, J = 8.1 Hz, 1H), 8.24 (d, J = 8.3 Hz, 1H), 8.09 (s, 1H), 8.01 (d, J = 7.3 Hz, 1H), 7.91 (t, J = 7.6 Hz, 1H), 7.81 (s, 1H), 7.73 (dd, J = 7.3 et 8.2 Hz, 1H), 5.60 (s, 2H), 5.26 (t, J = 9.9 Hz, 1H), 5.05 (dd, J = 9.4, 9.9 Hz, 1H), 4.92 (dd, J = 8.1, 9.4 Hz, 1H), 4.90 (d, J = 12.6 Hz, 1H), 4.86 (d, J = 8.1 Hz, 1H), 4.81 (d, J = 12.6 Hz, 1H), 4.28 (dd, J = 5.0, 12.4 Hz, 1H), 4.15 (dd, J = 2.4, 12.3 Hz, 1H), 3.97 (ddd, J = 2.5, 5.0, 10.0 Hz, 1H), 2.02 (s, 3H), 1.98 (s, 3H), 1.92 (s, 3H), 1.84 (s, 3H). 13C-NMR (125 MHz, acetone-d6): 184.10, 170.81, 170.36, 170.08, 169.72, 144.68, 141.50, 136.50, 135.12, 133.75, 133.45, 133.27, 131.25, 129.80, 128.40, 128.22, 128.06, 127.95, 125.52, 100.14, 73.61, 72.57, 72.18, 69.54, 63.17, 62.83, 49.63, 20.72, 20.68, 20.62, 20.62. FT-IR: ν (cm–1): 2954 (Aromatic CH st.), 1751 (C=O acetyl st.), 1641, 1626, 1599, 1573 (C=O st., C=C st., CCC b.), 1365 (CH acetyl b.), 1218 (CO acetyl st.), 1037 (COC pyranose ring skeletal vibration) 906, 813, 781, 695 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C31H32N3O11 [M + H]+: 622.203135; found, 622.203997. MW: 621.6 g/mol. mp ∼90 °C (degradation).
2-((4-((((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-phenalen-1-one 21 (PNGlc-B)
PNGlc-A (300 mg, 0.48 mmol) was dissolved in a mixture of THF/MeOH (1:1) with 1 mL of triethylamine and a drop of water, and the reaction was left for 12 h under stirring at room temperature. The solvents were evaporated, and the crude was purified by column chromatography (CHCl3/MeOH 8:2) and then lyophilized to yield 201 mg (0.44 mmol, 92%) of a bright yellow powder. 1H-NMR (500 MHz, CD3OD): δ (ppm) 8.57 (dd, J = 1.1, 7.4 Hz, 1H), 8.33 (dd, J = 0.7, 8.1 Hz, 1H), 8.16 (s, 1H), 8.14 (d, J = 8.2 Hz, 1H), 7.91 (d, J = 6.8 Hz, 1H), 7.83 (s, 1H), 7.81 (t, J = 7.7 Hz, 1H), 7.66 (dd, J = 7.2, 8.2 Hz, 1H), 5.58 (s, 2H), 4.99 (d, J = 12.5 Hz, 1H), 4.81 (d, J = 12.5 Hz, 1H), 4.39 (d, J = 7.8 Hz, 1H), 3.88 (m, 1H), 3.67 (dd, J = 5.4, 11.9 Hz, 1H), 3.36 (m, 1H), 3.29 (m, 2H), 3.21 (dd, J = 7.8, 9 Hz, 1H). 13C-NMR (125 MHz, CD3OD): δ (ppm) 185.33, 145.94, 143.06, 137.24, 134.75, 134.53, 134.25, 133.69, 131.98, 130.01, 128.63, 128.43 (2C), 128.18, 126.48, 103.79, 78.19, 78.13, 75.17, 71.77, 63.19, 62.94, 50.42. FT-IR: ν (cm–1): 3359 (OH st.), 2875 (Aromatic CH st.), 1637, 1620, 1596, 1567 (C=O st., C=C st., CCC b.), 1404 (1,2,3-triazole ring st.), 1361, 1255 (C=C st., CH b., CCC b.), 1035 (COC pyranose ring skeletal vibration), 812, 779 (Aromatic −CH opb.). MS: HRMS (ESI+), calcd for C23H24N3O7 [M + H]+: 454.160877; found, 454. 160507. MW: 453.45 g/mol. mp 132 °C.
Acknowledgments
The authors acknowledge “Région Nouvelle-Aquitaine” for financial support, Dr. Cyril Colas (ICOA/CBM, Orléans University) for HRMS analysis, and Dr. Michel Guilloton for manuscript editing.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04172.
1H and 13C NMR characterization data; HRMS results; and UV absorbance, fluorescence, and singlet oxygen data of all the compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Flors C.; Nonell S. Light and Singlet Oxygen in Plant Defense Against Pathogens: Phototoxic Phenalenone Phytoalexins †. Acc. Chem. Res. 2006, 39, 293–300. 10.1021/ar0402863. [DOI] [PubMed] [Google Scholar]
- Echeverri F.; Torres F.; Quiñones W.; Escobar G.; Archbold R. Phenylphenalenone Phytoalexins, Will They Be a New Type of Fungicide?. Phytochem. Rev. 2012, 11, 1–12. 10.1007/s11101-010-9205-x. [DOI] [Google Scholar]
- Elsebai M.; Ghabbour H.; Mehiri M. Unusual Nitrogenous Phenalenone Derivatives from the Marine-Derived Fungus Coniothyrium Cereale. Molecules 2016, 21, 178. 10.3390/molecules21020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.; Tanielian C.; Dunsbach R.; Wolff C. Phenalenone, a Universal Reference Compound for the Determination of Quantum Yields of Singlet Oxygen O2(1Δg) Sensitization. J. Photochem. Photobiol. Chem. 1994, 79, 11–17. 10.1016/1010-6030(93)03746-4. [DOI] [Google Scholar]
- Hidalgo W.; Duque L.; Saez J.; Arango R.; Gil J.; Rojano B.; Schneider B.; Otálvaro F. Structure–Activity Relationship in the Interaction of Substituted Perinaphthenones with Mycosphaerella Fijiensis. J. Agric. Food Chem. 2009, 57, 7417–7421. 10.1021/jf901052e. [DOI] [PubMed] [Google Scholar]
- Cieplik F.; Späth A.; Regensburger J.; Gollmer A.; Tabenski L.; Hiller K.-A.; Bäumler W.; Maisch T.; Schmalz G. Photodynamic Biofilm Inactivation by SAPYR—An Exclusive Singlet Oxygen Photosensitizer. Free Radic. Biol. Med. 2013, 65, 477–487. 10.1016/j.freeradbiomed.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Song R.; Feng Y.; Wang D.; Xu Z.; Li Z.; Shao X. Phytoalexin Phenalenone Derivatives Inactivate Mosquito Larvae and Root-Knot Nematode as Type-II Photosensitizer. Sci. Rep. 2017, 7, 42058. 10.1038/srep42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonell S.; González M.; Trull F. R. 1H-Phenalen-1-One-2-Sulfonic Acid: An Extremely Efficient Singlet Molecular Oxygen Sensitizer for Aqueous Media. Afinidad 1993, 448, 445–450. [Google Scholar]
- Bäumler W.; Felgenträger A.; Lehner K.; Maisch T.; Regensburger J.; Santarelli F.. Phenalene-1-One Derivatives, Method for Producing Same and Use Thereof. U.S. Patent 0,039,184 A1, February 6, 2014.
- Sandoval-Altamirano C.; De la Fuente J. R.; Berrios E.; Sanchez S. A.; Pizarro N.; Morales J.; Gunther G. Photophysical Characterization of Hydroxy and Ethoxy Phenalenone Derivatives. J. Photochem. Photobiol. Chem. 2018, 353, 349–357. 10.1016/j.jphotochem.2017.11.049. [DOI] [Google Scholar]
- Späth A.; Leibl C.; Cieplik F.; Lehner K.; Regensburger J.; Hiller K.-A.; Bäumler W.; Schmalz G.; Maisch T. Improving Photodynamic Inactivation of Bacteria in Dentistry: Highly Effective and Fast Killing of Oral Key Pathogens with Novel Tooth-Colored Type-II Photosensitizers. J. Med. Chem. 2014, 57, 5157–5168. 10.1021/jm4019492. [DOI] [PubMed] [Google Scholar]
- Bresolí-Obach R.; Gispert I.; Peña D. G.; Boga S.; Gulias Ó.; Agut M.; Vázquez M. E.; Nonell S. Triphenylphosphonium Cation: A Valuable Functional Group for Antimicrobial Photodynamic Therapy. J. Biophot. 2018, 11, e201800054 10.1002/jbio.201800054. [DOI] [PubMed] [Google Scholar]
- Benniston A. C.; Bunn A. Sulfonation of Phenalenone Revisited: Preparation and Characterisation of Sodium 1H-Phenalene-1-One-5-Sulfonate. J. Chem. Res. 2010, 34, 603–605. 10.3184/030823410X12857507693150. [DOI] [Google Scholar]
- Tabenski I.; Cieplik F.; Tabenski L.; Regensburger J.; Hiller K.-A.; Buchalla W.; Maisch T.; Späth A. The Impact of Cationic Substituents in Phenalen-1-One Photosensitizers on Antimicrobial Photodynamic Efficacy. Photochem. Photobiol. Sci. 2016, 15, 57–68. 10.1039/C5PP00262A. [DOI] [PubMed] [Google Scholar]
- Jensen F.; Greer A.; Clennan E. L. Reaction of Organic Sulfides with Singlet Oxygen. A Revised Mechanism. J. Am. Chem. Soc. 1998, 120, 4439–4449. 10.1021/ja973782d. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.