Abstract
Accumulating evidence suggests that dysregulation of transcriptional enhancers plays a significant role in cancer pathogenesis. Herein, we performed a genome-wide discovery of enhancer elements in colorectal cancer (CRC). We identified PVT1 locus as a previously unrecognized transcriptional regulator in CRC with a significantly high enhancer activity, which ultimately was responsible for regulating the expression of MYC oncogene. High expression of the PVT1 long-non-coding RNA (lncRNA) transcribed from the PVT1 locus was associated with poor survival among patients with stage II and III CRCs (p < 0.05). Aberrant methylation of the PVT1 locus inversely correlated with the reduced expression of the corresponding the PVT1 lncRNA, as well as MYC gene expression. Bioinformatic analyses of CRC-transcriptomes revealed that the PVT1 locus may also broadly impact the expression and function of other key genes within two key CRC-associated signaling pathways – the TGFβ/SMAD and Wnt/β-Catenin pathways. We conclude that the PVT1 is a novel oncogenic enhancer of MYC and its activity is controlled through epigenetic regulation mediated through aberrant methylation in CRC. Our findings also suggest that the PVT1 lncRNA expression is a promising prognostic biomarker and a potential therapeutic target in CRC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-020-01277-4.
Keywords: PVT1, MYC, Enhancer, Epigenetic, Prognostic marker, Colorectal cancer
Main text
Accumulating evidence indicates that the pathogenesis of colorectal cancer (CRC) is influenced by epigenetic modifications. Among these, in the past decade, alterations in enhancer elements have garnered a significant attention [1], and are emerging as important players in cancer pathogenesis and being exploited as potential therapeutic targets. Recently, the FANTOM5 (Functional Annotation of the Mammalian Genome 5) project curated a genome-wide enhancer element atlas from normal tissues and tumor cells, using the cap analysis gene expression (CAGE) and next-generation sequencing (NGS) approaches [2–5]. Herein, we systematically examined the FANTOM5 database and identified the PVT1 locus as an epigenetic enhancer in CRC and provided a novel evidence for its role in specific targeting of the MYC oncogene. Furthermore, we found that PVT1 lncRNA was transcribed by the PVT1 locus via an enhancer-like activity. Multiple in silico datasets were utilized to evaluate the clinical significance of the PVT1 lncRNA. Pooling of seven such datasets improved the overall statistical power of the analysis and minimized potential cohort bias. Our analyses indicated that PVT1 is associated with cancer stemness and modulates key CRC-associated signaling pathways – the TGFβ/SMAD and Wnt/β-Catenin pathways. We additionally noted that high expression of the PVT1 lncRNA associated with poorer survival in CRC patients. Taken together, our data provide first evidence that the PVT1 locus plays a key role in CRC pathogenesis, and that it may serve as a prognostic biomarker and a potential therapeutic target in patients with CRC.
A novel enhancer, the PVT1 lncRNA, is frequently activated in colorectal cancers, and activates its enhancer potential via oncogenic MYC
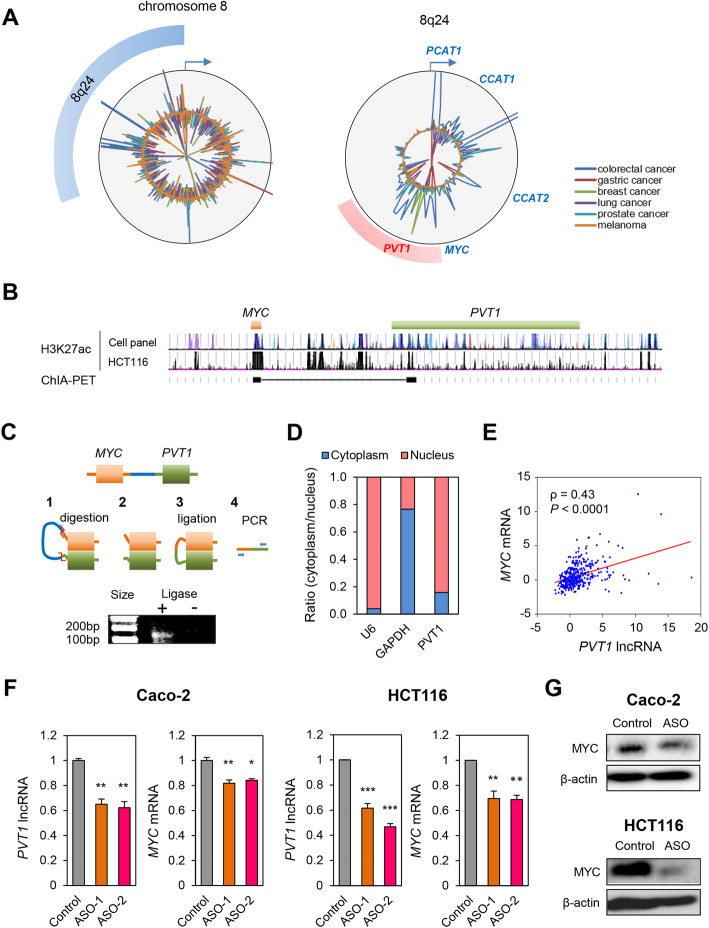
We performed a systematic analysis of 43,011 enhancer elements that were recently reported in the FANTOM5 enhancer database (Supplementary Table 1). We observed that the strongest enhancer activity in CRCs was confined to the classic, CRC-associated chromosome 8q24 region – often referred to as the ‘gene desert’, including the genes such as the PCAT1, CCAT1, CCAT2, PVT1 and MYC (Fig. 1a). In particular, CCAT1-L lncRNA, which is transcribed from the CCAT1 locus, has been shown to stabilize loop structure between CCAT1 and MYC via an “enhancer-like function” [6, 7]. Therefore, we were intrigued by the identification of a novel locus, PVT1, which has previously not been reported as an enhancer region in CRC. In the genome-wide FANTOM5 enhancer database, the PVT1 locus exhibited cancer-specific enhancer activity, especially in CRCs. We analyzed this region carefully using the University of California Santa Cruz (UCSC) genome browser. We discovered that the PVT1 locus indeed exhibited a strong H3K27ac enhancer signal, in a panel of cell lines (GM12878, H1-hESC, HSMM, HUVEC, K562, NHEK and NHLF), as well as in HCT116 CRC cells (Fig. 1b). Next, we analyzed Chromatin Interaction Analysis by Paired-End Tag Sequencing (ChIA-PET) results from the UCSC genome browser and demonstrated that the PVT1 region interacts with the MYC oncogene (Fig. 1b).
Fig. 1.
The PVT1 locus has an enhancer element that targets MYC. a Enhancer activity within chromosome 8, and specifically within the 8q24 region, among six different cancer types (Colorectal cancer: Gastric cancer, Breast cancer, Lung cancer, Prostate cancer, and Melanoma) according to the FANTOM5 database. b Comparison panel of two different analyses; H3K27ac signal in a cell line panel (GM12878, H1-hESC, HSMM, HUVEC, K562, NHEK, NHLF) and in HCT116 (CRC cell line); Chia-PET in K562 leukemia cell line (as revealed by exploring the UCSC genome browser). c 3C assay to detect the PVT1-MYC interaction in HCT116 cells. d RT-qPCR determination of the PVT1 lncRNA, U6 snRNA (nucleus-specific), and GAPDH mRNA (cytoplasm-specific) levels in nuclear and cytosolic extracts from HCT116 cells. e Plot for the PVT1 lncRNA and MYC mRNA expression levels in CRC. f Effect of knockdown of the PVT1 lncRNA on expression levels of the PVT1 lncRNA and MYC mRNA in Caco-2 and HCT116 cells as determined by RT-qPCR. g Western immunoblotting to determine the effect of knockdown of the PVT1 lncRNA on MYC protein levels in Caco-2 and HCT116 cells. *P < 0.05, **P < 0.01, ***P < 0.001
Our results from the FANTOM5 database and the UCSC genome browser analysis lead to the hypothesis that the PVT1 region might have oncogenic enhancer activity that targets the MYC oncogene in CRC cells. Using a chromosome conformation capture (3C) assay in HCT116 cell lysates, we demonstrated that indeed the PVT1 locus formed a loop structure in a cis conformation with MYC (Fig. 1c).
We next measured the ability of the PVT1 lncRNA for its ability to drive the expression of MYC in CRC cells. Recent reports suggest that enhancers may produce lncRNAs that can stabilize a cis conformation between enhancers and promoters [7]. This mechanism of enhancer-related lncRNA activity primarily occurs inside the nucleus. We found that majority of the PVT1 lncRNA was indeed present within the nuclear compartment (Fig. 1d). In addition, we were very encouraged to observe that a positive correlation between this noncoding RNA and the MYC gene in the CRC specimens (cohort 1, ρ = 0.43, P < 0.0001, Fig. 1e).
To further investigate the role for the PVT1’s enhancer activity, we next performed knockdown experiments for the PVT1 lncRNA. Although the PVT1 lncRNA knockdown using siRNA has already been previously attempted, such efforts did not result in concomitant suppression of the MYC mRNA [8], because establishing an effective nuclear PVT1 lncRNA knockdown using siRNAs is challenging. To overcome this issue, we established a specific antisense oligonucleotide (ASO) that targets the PVT1 lncRNA and permits its knockdown in the nucleus. Our approach led to successful knockdown of the PVT1 lncRNA in the cancer cell lines (P < 0.01 in Caco-2, P < 0.001 in HCT116), with a simultaneous transcriptional suppression of the MYC mRNA (P < 0.05 in Caco-2, P < 0.01 in HCT116, Fig. 1f) and MYC protein levels (Fig. 1g), suggesting an enhancer-like function.
The PVT1 lncRNA is frequently overexpressed in stage II and III CRCs
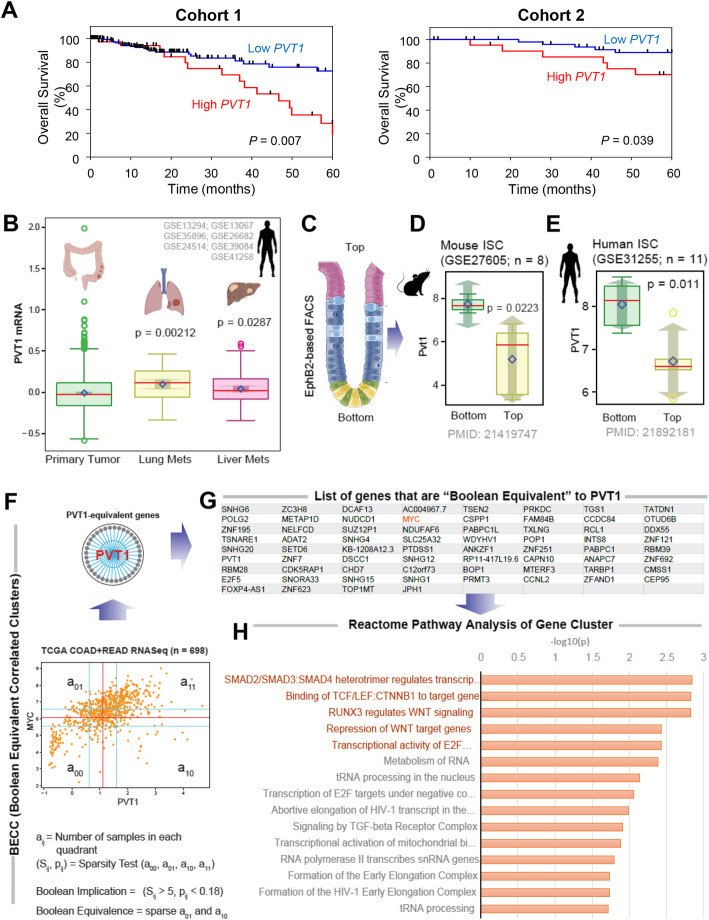
Next, we analyzed the PVT1 lncRNA expression in CRC specimens. We evaluated the expression levels of the PVT1 lncRNA in stage II and III CRCs. In multivariate analysis, high-PVT1 expression emerged as an independent prognostic factor in stage II and III CRC patients (Cohort-1: P = 0.0246; Cohort-2: P = 0.0196, Supplementary Table 2 and 3). Data derived from these clinical cohorts further highlight that the PVT1 lncRNA expression levels may serve as an important prognostic biomarker for stage II and III CRC patients, and can facilitate stratification of appropriate patient subsets that are optimal candidates for benefitting from adjuvant chemotherapy and attenuate recurrence [9].
PVT1 lncRNA expression increases in CRC metastases, and its expression is widely associated with genes within the TGFβ/SMAD and Wnt/β-catenin pathways
We next evaluated the expression pattern of the PVT1 lncRNA, and the related functional pathways to clarify its clinical significance in data gathered from 7 pooled datasets (see methods). The expression levels of the PVT1 lncRNA were significantly higher in both lung and liver metastases compared with the primary lesions (Fig. 2b). Intriguingly, the PVT1 lncRNA was overexpressed at the bottom of the colorectal crypt compared with the top, both in mice (P = 0.022) and humans (P = 0.011); suggesting that PVT1 lncRNA may either favor or serve as a marker of stemness which exists at the bottom of the crypt (Fig. 2c-e). Together, these findings using unbiased bioinformatic approaches suggest that the PVT1 lncRNA may promote distant metastasis, perhaps via its ability to promote stemness in the colon cells.
Fig. 2.
The PVT1 lncRNA is overexpressed in CRC. PVT1 lncRNA is highly expressed in CRC metastases and within crypt bases; is associated within gene clusters that regulate the TGFβ/SMAD2/3/4 and Wnt/β-Catenin pathways. a Overall Survival plot for patients with high-PVT1 lncRNA expression versus patients with low-PVT1 lncRNA expression in the two cohorts (P < 0.007 in Cohort-1, P < 0.039 in Cohort-2). b Whisker plots showing the levels of expression of PVT1 lncRNA in CRCs (primary and metastases) from 7 pooled datasets. c-e Schematic in C shows the EphB2-based FACS analyses approach used to separate epithelial cells at the bottom of the crypt from those at the top. Mouse (d) and human (e) datasets show whisker plots of the levels of expression of PVT1 lncRNA at the bottom and top of the crypts. f Computational approach to identify clusters of genes that share Boolean Equivalent relationships between each other, in this case identified using the PVT1 lncRNA as ‘seed’ in TCGA COAD dataset (n = 698) (top panel). Number of samples in all four quadrants are used to compute two parameters (S, p). S > 5 and p < 0.05 is used to identify sparse quadrant. Equivalent relationships are discovered when top-left and bottom-right quadrants are sparse (lower panel). g List of genes that are equivalent to the PVT1 lncRNA. MYC is highlighted in red. h Reactome pathway analysis shows pathways that are most prominently enriched (highlighted in red) in the PVT1-equivalent cluster
Finally, we asked what other genes may be impacted by the PVT1 lncRNA, or vice versa. To this end, we analyzed The Cancer Genome Atlas (TCGA) CRC datasets (n = 698; Fig. 2f) using Boolean equivalent correlated clusters (BECC) analysis [10]. We used the PVT1 lncRNA as a seed gene and identified a set of 67 genes (Fig. 2g) that displayed a tight, statistically significant Boolean Equivalent relationship to the PVT1 lncRNA across all 698 CRCs in the dataset, as determined by BooleanNet statistics; and indeed MYC appeared as one of the key genes even in these analysis (Fig. 2f-g). The Reactome pathway analyses revealed that most of the 67 genes served within two major signaling pathways, i.e., TGFβ/SMAD2/3/4 and Wnt/β-Catenin pathways (Fig. 2h). These findings further support our hypothesis that the PVT1 lncRNA widely impacts major signaling pathways in CRC by regulating the expression of key genes, such as the MYC.
The PVT1 locus is epigenetically regulated and its methylation status inversely correlates with its transcriptional levels in CRC
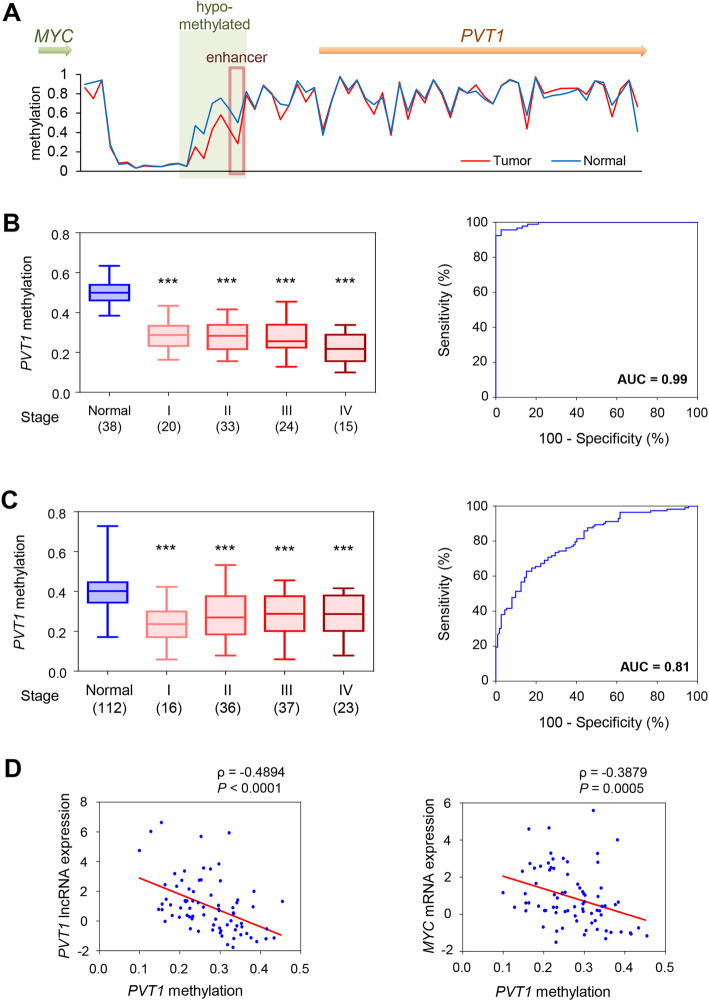
Aberrant methylation is one of the key regulators of enhancer activity in various genes. Interestingly, we observed a significant loss in CpG sequence methylation in the vicinity of the PVT1 locus, including its enhancer cluster (Fig. 3a). Specifically, a CpG site (cg23898497) in the middle of its enhancer region (chr8:128822251–128,823,013) was significantly hypomethylated in CRC vs. normal mucosa, in all disease stages in the cohort-1 (P < 0.001, area under the curve: AUC = 0.99, Fig. 3b) and cohort-3 patients (P < 0.001, AUC = 0.81, Fig. 3c). These results suggest that the PVT1 enhancer activity and the expression of this lncRNA might be controlled through an epigenetic regulation of this region. In support of our other findings, the methylation status of the PVT1 region negatively correlated with its lncRNA expression (ρ = − 0.4894, P < 0.0001), as well as with the MYC gene expression (ρ = − 0.3879, P = 0.0005, Fig. 3d).
Fig. 3.
The PVT1 locus is hypomethylated and methylation state negatively correlated with its lncRNA expression in CRC. a The methylation levels at the PVT1 locus in CRC and normal mucosa determined using a 450 K methylation array derived from TCGA database. b Methylation levels at the CpG site (cg23898497) in CRC compared to normal mucosa in all stages in Cohort-1 (P < 0.001, AUC = 0.99) according to the FANTOM5 database. c Methylation levels at the CpG site (cg23898497) in CRC compared to normal mucosa in all stages in Cohort-3 (P < 0.001, AUC = 0.81) as determined by pyrosequencing. d Plot of the PVT1 methylation versus its lncRNA expression (ρ = − 0.4894, P < 0.0001), and the PVT1 methylation versus MYC expression (ρ = − 0.3879, P = 0.0005) in Cohort-1. ***P < 0.001
Conclusions
Previously, the PVT1 lncRNA was reported as a stabilizer of the MYC protein [8]. In addition, our study indicates that the PVT1 locus may directly controls the MYC mRNA expression as an enhancer. Based on these data, targeting of the PVT1 lncRNA may be a potential therapeutic approach in CRC patients, which could eventually lead to suppression of the oncogenic MYC, at both the RNA and protein levels.
Supplementary Information
Additional file 1: Supplementary Table 1
Additional file 2: Supplementary Table 2–7
Acknowledgements
We thank Dr. Carson Harrod and, Dr. Margaret Hinshelwood for carefully proofreading and editing the manuscript.
Abbreviations
- CRC
Colorectal cancer
- FANTOM5
Functional Annotation of the Mammalian Genome 5
- CAGE
Cap analysis gene expression
- NGS
Next-generation sequencing
- UCSC
University of California, Santa Cruz
- ChIA-PET
Chromatin Interaction Analysis by Paired-End Tag Sequencing
- 3C
Chromosome conformation capture
- RISC
RNA-induced silencing complex
- ASO
Antisense oligonucleotide
- TCGA
The Cancer Genome Atlas
- BECC
Boolean equivalent correlated clusters
Authors’ contributions
Conceived and designed experiments: KS, TO, TM; Performed experiments: KS, TO, TM; Analyzed data: KS, TO, ST, DS, PG, AG; Contributed reagents, materials and other analytical tools: TN, TI, HU, AY, TF; Wrote the manuscript: KS, PG, AG. The author(s) read and approved the final manuscript.
Funding
The present work was supported by the grants CA72851, CA181572, CA184792, CA187956, CA202797, CA238042, UG3TR002968 and GM138385 from the National Institute of Health. This work was also supported by a grant from Uehara Memorial Foundation and 20 K17653 from JSPS.
Availability of data and materials
All data derived from public database are available from these sites.
FANTOM5_Human_Enhancer_Tracks: http://slidebase.binf.ku.dk/human_enhancers/presets
TCGA_Research_Network: http://cancergenome.nih.gov/
cBioPortal: http://www.cbioportal.org/index.do
UCSC_Genome_Browser: http://genome.ucsc.edu/
All other data are contained within this article.
Ethics approval and consent to participate
All study-related procedures were performed as per the Declarations of Helsinki, wherein a written informed consent was obtained from each patient, and the institutional review boards of all participating institutions involved approved the study.
Consent for publication
Not applicable. The manuscript does not contain any individual personal data.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shin HY. Targeting super-enhancers for disease treatment and diagnosis. Mol Cell. 2018;41:506–514. doi: 10.14348/molcells.2018.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, Chen Y, Zhao X, Schmidl C, Suzuki T, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–461. doi: 10.1038/nature12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FANTOM5: http://fantomgscrikenjp/ Accessed in July 1, 2015.
- 4.Lizio M, Harshbarger J, Shimoji H, Severin J, Kasukawa T, Sahin S, Abugessaisa I, Fukuda S, Hori F, Ishikawa-Kato S, et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16:22. doi: 10.1186/s13059-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FANTOM5_Human_Enhancer_Tracks: http://slidebasebinfkudk/human_enhancers/presets Accessed in December 2, 2019.
- 6.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, Zhang S, Wang HB, Ge J, Lu X, et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014;24:513–531. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell TC, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, et al. Duration of adjuvant chemotherapy for stage III Colon Cancer. N Engl J Med. 2018;378:1177–1188. doi: 10.1056/NEJMoa1713709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabydeen SA, Desai A, Sahoo D. Unbiased Boolean analysis of public gene expression data for cell cycle gene identification. Mol Biol Cell. 2019;30:1770–1779. doi: 10.1091/mbc.E19-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1
Additional file 2: Supplementary Table 2–7
Data Availability Statement
All data derived from public database are available from these sites.
FANTOM5_Human_Enhancer_Tracks: http://slidebase.binf.ku.dk/human_enhancers/presets
TCGA_Research_Network: http://cancergenome.nih.gov/
cBioPortal: http://www.cbioportal.org/index.do
UCSC_Genome_Browser: http://genome.ucsc.edu/
All other data are contained within this article.