Abstract
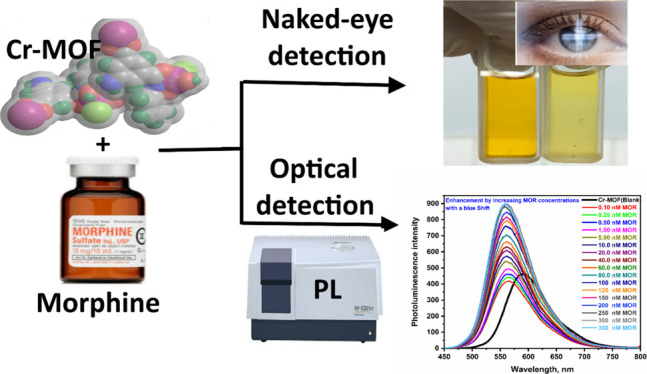
The analytical detection and quantification of abuse drugs such as morphine (MOR) in biological samples are vital missions and remains to attract challenges for forensic toxicology, law enforcement, world antidoping organization, and social health fields. MOR, a benchmark analgesic drug known as “pain killer”, is one of the powerful opioid medications for relieving pain, and overdose of MOR is toxic. In this article, novel promising chromium metal–organic framework nanoparticles [Cr(III)-MOF-NPs] were produced via facile synthesis and characterized using high-resolution transmission electron microscopy, field-emission scanning electron microscopy/energy-dispersive X-ray spectroscopy, mass spectrometry, X-ray photoelectron spectroscopy, elemental analysis, UV–vis, Fourier transform infrared, and thermogravimetry/differential scanning calorimetry, as well as photoluminescence (PL) investigation and magnetic properties. The PL study results revealed that the Cr(III)-MOF-NPs exhibited an emission band at 593 nm. The Cr(III)-MOF-NPs could be used in fast, selective, and sensitive MOR detection and quantification. Under the optimum experimental conditions, with the addition of MOR, a blueshift from 593 to 566 nm occurred with a remarkable PL intensity enhancement, and the color changed from brown to yellow (visually/naked-eye detection). The Cr(III)-MOF-NPs optical chemosensor exhibited a stable response for MOR in a concentration range between 0.1 and 350 nM. The detection and quantification limits were 0.167 and 0.443 nM, respectively, with a correlation coefficient (r2) of 0.96. The developed PL chemosensor showed high selectivity for MOR over other competing interfering matrices. Moreover, the ultrasensitive chemosensor was extensively used for the determination of MOR spiked in different real samples (serum and urine samples) with acceptable recoveries and satisfactory results.
Introduction
Morphine (MOR), one of the most common opioid family members, is derived from the sap of the poppy plant.1 Clinically, MOR is known as the benchmark or gold standard analgesic and one of the best analgesic drugs because of its ability to relieve pain (from moderate to severe pain) in patients of cancer, nonmalignant pain, or undergoing surgical procedures.2−4 Therefore, the World Health Organization (WHO) recommended it as the best opioid drug for pain relief, especially for cancer patients and is commonly used in the USA, UK, some European countries, and worldwide.1 It is also used as the starting material for synthetic opioid drugs as oxymorphine, hydromorphine, and heroin.5 MOR is commonly present in hydrochloride or sulfate forms and is administrated orally, intravenously, intramuscularly, rectally, epidurally, subcutaneously, and intrathecally. The usual dose intravenously/intramuscularly is 0.1–0.2 mg/kg, effective peak is achieved within 0.5–1.0 h, and the action period is up to 4 h. The dose of MOR is controlled according to the age of the patient, not according to their weight, and adjusted corresponding to response.6 About 90% of the MOR dose is excreted within 24 h in urine and contains about 10% in the unmetabolized form.7 The overdose of MOR is toxic and can affect many immune functions and cause a significant disturbance in the central nervous system, blood pressure, and respiratory rate.1,2
In general, MOR is an abused drug without medical recommendation. In sports, it is one of the prohibited doping drugs, and the administration of MOR by athletes is banned from a lot of important organizations such as the International Olympic Committee (IOC) and by the World Anti-Doping Agency (WADA).8−10 The MOR detection in biological samples in the clinical, antidoping, and forensic fields is significant and has been applied by many organizations, as well in many pharmcokinetic studies. The development of fast, simple, selective, sensitive, and convenient approaches and methodologies for the monitoring of MOR in biological fluids is still a challenge.
Many analytical techniques and methodologies were used for MOR detection and quantification such as high-performance liquid chromatography,11 surface plasma resonance,12 immunochromatographic assay,13 fluorescence immunoassays,14 spectrophotometric method,15 chemiluminescence,16 surface-ionization mass spectrometry,17 and electrochemical methods.2−4,18,19 More details about the MOR sensors and detection have been discussed in reference reviews.1,20,21 Although the mentioned methods can offer many advantages, but also have limitations, they frequently require technical expertise, high infrastructure, pretreatment sample steps, are time-consuming, and/or costly. In addition, till date, based on our knowledge, there is no published work for the detection of MOR in terms of used metal–organic framework (MOF) materials.
MOFs are anew families of compounds that have exceptional structures with a large surface area and regular porosities in addition to other extraordinary chemical and physical properties which have made them promising aspirants for environmental, biological, industrial, and societal relevant domain applications such as biosensing, biomedical, separation, catalysis, and so forth.22−32 Recently, a lot of studies have explored the vital potential of the nanometric MOFs (nano-MOFs) in the fields of sensing, biosensing, and biomedical applications. Nano-MOFs, because of their particle size being very small, can improve the interaction with the biological targets and also improve their performances in the release of drugs in the field of drug delivery for many disease treatment protocols.33,34
Chromium MOFs (Cr-MOFs), such as MIL-101(Cr), have been surveyed as one of the extremely imperative prototypical MOFs and used for several fields and applications35 because of their extra high chemical/thermal stability, extraordinary large surface area, pore volume, and various unsaturated Cr-sites. A few number of articles relate to the synthesis and applications of Cr-MOF. For example; Ren et al. reported a Cr-MOF from polyethylene terephthalate bottle wastes via green synthesis and used it for hydrogen storage.35 Segakweng et al. used a carbon Cr-MOF derived for the application of H2 storage and compared it with MOF-5.36 Kayal et al. reported two MIL-101(Cr) derivatives for CH4 and CO2 storage and separation.37,38 Salles et al. reported a molecular study related by the hydrophilic/hydrophobic of MIL-53(Cr)-MOF.39 Guo et al. reported a composite of chromium hydroxide and MIL-101(Cr) MOF for catalyst activity for the isomerization process of fructose from glucose (GLU).40 Hamon et al. reported a modeling and experimental study of mesoporous MIL-100(Cr) MOF for the separation mixtures of CO2 and CH4.41 Rostamnia et al. reported a Cr-MOF based on ethylene diamine for catalyst applications.42 Grall et al. reported a mesoporous Cr-MOF for in vitro biocompatibility nanocarrier applications.28
In this article, to the first date, novel Cr(III)-MOF-NPs were synthesized and fully characterized via numerous microanalytical and spectral tools. The synthesized Cr(III)-MOF-NPs were used as a novel chemosensor for MOR. The sensing mechanism was recognized based on the formation of hydrogen bonding and host–guest interaction between Cr(III)-MOF-NPs and electronegative MOR which enabled the photoinduced transferring of electrons within the Cr(III)-MOF-NP structure causing a photoluminescence (PL) blueshift even in the presence of many competing matrices. The PL emission spectrum of the Cr(III)-MOF-NPs was enhanced professionally as the MOR concentration increased. The outcome results indicated that the Cr(III)-MOF-NPs may be used efficaciously as an auspicious promising chemosensor for the detection of MOR at ultralow levels of concentrations with statistically adequate results. Furthermore, a comparison of the present approach with other published works and the mechanism of interaction was studied. The present optical procedure has all the merits of other presented alternative reports, as it represents a low cost of test, extensive reproducibility, lower detection and quantification limits (LOD/LOQ), significant sensitivity and selectivity, simplicity of the operations/preparation techniques, and portable facilities. Additionally, the applicability of the present approach in different biological samples (urine and serum) was tested.
Results and Discussion
Cr(III)-MOF-NP Characterization
According to the reaction scheme (Scheme S1; Supporting Information), the Cr(III)-MOF-NPs were synthesized. A dark brown precipitate (PPT) appeared which was finally filtered, washed well, and dried-out. The structure interpretation and elucidation were performed using many physical and microanalytical instrumentation and investigated in detail.
Field-Emission Scanning Electron Microscopy/Energy-Dispersive X-ray Spectroscopy and High-Resolution Transmission Electron Microscopy Spectroscopy
The field-emission scanning electron microscopy/energy-dispersive X-ray spectroscopy (FE-SEM/EDX) of the Cr(III)-MOF-NPs as well the high-resolution transmission electron microscopy (TEM) images are shown in Figure 1. From the FE-SEM images (Figure 1a–c), the morphology of Cr(III)-MOF-NPs appeared to be nanosheets covered with nanoparticles which ranged from 50 to 100 nm. Moreover, the EDX mapping analysis (Figure 1d and Table S1, Supporting Information) of Cr(III)-MOF-NPs showed the existence of chromium, carbon, chlorine, nitrogen, and oxygen as a constructing block element in every single particle. The EDX analysis revealed that an exceptional element dispersion alongside the cross sections (Figure 1d) also confirmed the Cr(III)-MOF-NP formation. In addition, from (Table S1), the reported EDX results conformed with those elementals calculated theoretically: C, 34.63; N, 7.84; O, 30.75; Cl, 5.68; Cr, 16.66. Found: C, 34.83; N, 7.74; O, 35.77; Cl, 5.27; Cr, 16.39. The TEM images (Figure 1e,f) show the Cr(III)-MOF-NPs with different magnifications. They appear as small nanosheets with the presence of metal nanoparticles in the center of these sheets.
Figure 1.
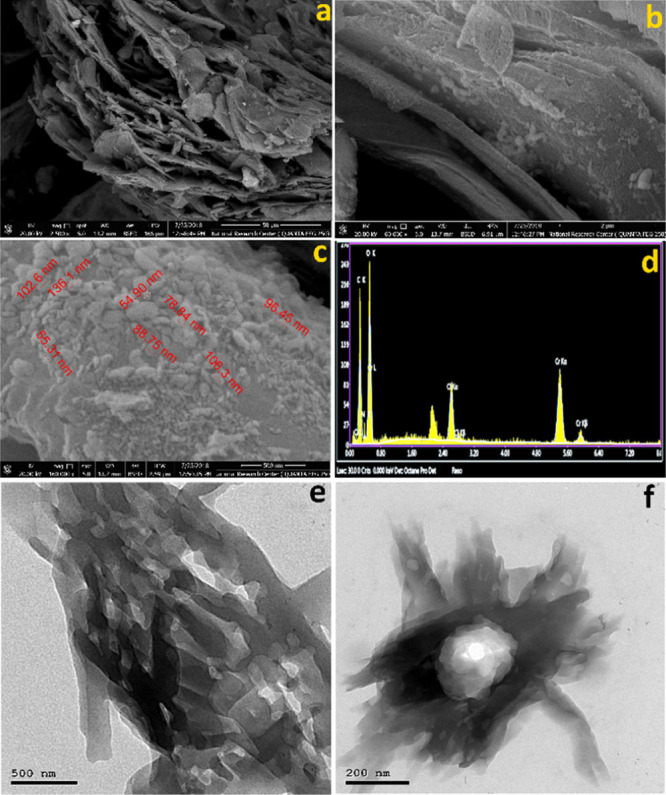
(a–c) FE-SEM images of the chromium MOF [Cr(III)-MOF-NPs] at different magnifications, (d) energy-dispersive X-ray analysis with a single-point EDX mapping analysis of Cr(III)-MOF-NPs, and (e,f) TEM images of the Cr(III)-MOF-NPs at different magnifications.
Elemental Analysis
The elemental C/H/N analysis of the Cr(III)-MOF-NPs (Table S1) is compared with the theoretically calculated analysis. The results were in tremendous consistency with that calculated theoretically for the proposed formula of monomeric Cr(III)-MOF-NP unit; the Anal. Calcd (%): C36H55Cl2Cr4N7O24, (1248.75 g/mol), C, 34.63; H, 4.44; N, 7.84, whereas C/H/N elemental found C, 34.46; H, 4.42; N, 7.96; the melting point was more than 300 °C; and the experimental yield was 55.59%.
Mass Spectrometry
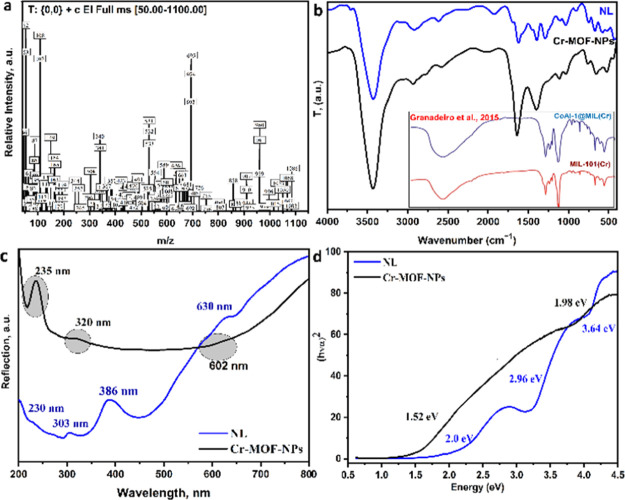
The synthesized Cr(III)-MOF-NPs mass spectrum adopted by the proposed mass fragmentation scheme is shown (Figure 2a and Scheme S2; Supporting Information), respectively. From Figure 2a, it was observed that the m/z peaks were completely matched with the proposed empirical formula. The theoretically molecular ion peak was estimated at 1284.78 m/z, attributable to the mass shortcoming to m/z at 1100. Subsequently, the successive mass fragmentations, as represented in Scheme S2, were well agreeable with the molecular weight (MW) of the suggested structure C36H59Cl2Cr4N7O26. The ion of m/z = 1284.78 under successive mass fragmentation shows a stable peak at m/z = 960.51 by missing 16 water molecules. Afterward, the compound obeyed to additional decomposing, leading to the mass fragmentations with m/z = 695, 532, 193, 165, 149, 108, 107, 93, 66, and 64. Generally, the succeeding mass fragmentations of the Cr(III)-MOF-NPs were completely matching with the values estimated theoretically and with the suggested MW structure.
Figure 2.
(a) Mass spectrum of the Cr(III)-MOF-NPs, (b) Fourier transform infrared spectra of nanolinker (NL) and Cr(III)-MOF-NPs with some reported Cr-MOF, (c) electronic reflection spectra of NL and Cr(III)-MOF-NPs, and (d) band gap energy of NL and Cr(III)-MOF-NPs.
Fourier Transform Infrared
The comparison of NL with Cr(III)-MOF-NPs and MIL-101-Cr-MOF/CoAl-1@MIL(Cr) “reported by Granadeiro et al.,43” spectra is shown in Figure 2b. For Cr(III)-MOF-NPs, the peaks among 3715 and 3100 cm–1 are assigned to NH2 and H2O molecules, respectively. The broad band with high intensity centered at 3426 cm–1 related to the OH vibrations is shown in the presence of a large amount of water within Cr-MOF.44 The band centered around 2550 cm–l is assigned to carbonyl bonds (C–C=O).45 The bands between 1769 and 1530 cm–1 and positioned at 1640 cm–1 are assigned to stretching ν(C=O) and bending ν(NH). The sharp peaks between 1497 and 1305 cm–1 positioned at 1400 cm–1 are assigned to C=C and the symmetric/asymmetric stretching of O–C–O groups, respectively.44,46,47 The narrow and weak peaks between 1170 and 830 cm–1 are related to vibrations of d(C–H) and g(C–H) of the benzene rings ν(CH), respectively.44 The band appeared at 583 cm–1 is assigned to coordinate with oxygen (Cr<−O), which appears in MIL-101(Cr) at 585 cm–1;48 however, the band at 443 cm–1 is assigned to covalent chromium ion–nitrogen bonding ν(Cr–N).49 The presence of the two new bands validated the chelation/complexation of the chromium ion with NL across the N and O atoms.
UV–Vis and Band gap
The comparison of electronic UV–vis as well the band gap energy spectrum of the NL and Cr(III)-MOF-NPs are shown in Figure 2c,d, respectively. From Figure 2c, it is noticed that the Cr(III)-MOF-NPs show three reflection bands: a sharp band at 235 nm, and two broad bands at 320 and 602 nm, appointed to the transition of ligand/metal-charge transferring (LMCT) and because of the charge-transferring intraligand (n−π*/π–π*). However, the NL shows four reflection bands at 230, 303, 386, and 630 nm.
Furthermore, (Figure 2d) shows the energy band gap of the linker and Cr(III)-MOF-NPs and it is noticed that the band gap energy of the linker was reduced in the case of Cr(III)-MOF-NPs (from 2.0, 2.96, and 3.64 to 1.52 and 1.98 eV) because of the high conjugation of the linker which causes the increasing of the highest occupied molecular orbital valance band energy, and therefore, the Cr(III)-MOF-NPs band gap is decreased.
XPS Spectrometry
The X-ray photoelectron spectroscopy (XPS) data analysis revealed the elemental compositions of the synthesized Cr(III)-MOF-NP sample, as shown in Figure 3a,e. The Cr(III)-MOF-NPs sample survey scans (Figure 3a) showed the existence of C, O, N, and Cr as the main building block elements without impurities. The XPS spectrum of Cr 2p (Figure 3b) revealed signals at 585.1 and 575.8 eV ascribed to Cr(III) 2p1/2 and 2p3/2, respectively, which confirmed the presence of Cr(III) in MOF composition. The O 1s XPS peaks, three peaks at 530.20, 531.46, and 532.95 eV (Figure 3c) were displayed for O–Cr–O, C–O, and C=O, respectively. The N 1s XPS spectrum at 398.0 eV (Figure 3d) shows a single peak for the quaternary N. The C 1s XPS spectrum, at 282.69, 284.54, and 285.83 eV (Figure 3e), shows the existence of three signals which are assigned to C–C, C–N, and C=O, correspondingly.47,50
Figure 3.
(a–e) XPS analysis of the chromium MOF [Cr(III)-MOF-NPs]: [(a) survey, (b) Cr 2p, (c) O 1s, (d) N 1s, and (e) C 1s]; (f) TGA/DSC of the [Cr(III)-MOF-NPs].
Thermal Analysis
The thermal behavior of Cr(III)-MOF-NPs, [thermogravimetric analysis/differential scanning calorimetry (TGA/DSC)] plots (Figure 3f) proposed that the Cr(III)-MOF-NPs felt through three-step of collapses, going through a weight-loss of 25.39% a resulting of loss inter-/intra-structure (16) molecules of water in temperature up to 203 °C (theoretically calculated weight loss: 25.24%). The Cr(III)-MOF-NPs collapsed two steps further, resulting in organic phenyl-ring exclusion in optimum temperature at 465.7 °C with a weight loss of 58.64%; whereas the remaining residue (15.97%) is Cr. The above data conformed to that calculated theoretically and those obtained from the EXD data.
Magnetic Properties of Cr(III)-MOF-NPs
The Cr(III)-MOF-NPs magnetization graph (Figure S1; Supporting Information) showed that the saturated magnetization value was 0.10574 emu/g, and the coercivity was 59.425 G, whereas the remanence was 0.0015187 emu/g. From the magnetization graph data and values, it can be noticed that a superparamagnetic behavior of the Cr(III)-MOF-NPs results from the smallest size of the crystallite particles compared with the massive noninteracting magnetic moments. Because of this reason, the Cr(III)-MOF-NPs can be stimulated thermally simply and consequently can be overcome on the magnetic anisotropy.
Finally, from the discussed physical and microanalytical spectral data given above, we can expect the three-dimensional (3D) structure of the Cr(III)-MOF-NP monomeric unit and the 3D-advanced molecular skeleton surface, as shown in (Figure 4a,b), respectively.
Figure 4.

(a) 3D structural representation of the Cr(III)-MOF-NPs monomeric unit and (b) advanced molecular surface representation of the Cr(III)-MOF-NP monomeric unit.
PL Study and Application
PL spectra for the Cr(III)-MOF-NPs were investigated. The emission/excitation spectra were performed via auto survey mode in excitation wavelengths between 220 and 550 with a scan interval of 20 nm and emission wavelengths between 240 and 900 nm with an emission/excitation slide width of 5 nm at room temperature (RT). From the excitation/emission spectra (Figure 5a), under optimal conditions, after excitation at 416 nm, the Cr(III)-MOF-NPs displayed an emission band at 593 nm. Also, the PL spectra of the Cr(III)-MOF-NPs were investigated manually at various excitation wavelengths and recorded, as shown in Figure S2, Supporting Information. The fluorescent behavior of the Cr(III)-MOF-NP scan was attributed to the molecular orbital transitions (π–π* and π–n) and due to ligand/LMCT.
Figure 5.
(a) Excitation (red line) and emission (black line) spectra of Cr(III)-MOF-NPs, (b) PL spectra response for the behavior of Cr(III)-MOF-NPs toward 1.0 nM of MOR, [inset (b)] a photography image for providing the sensing activity of the Cr(III)-MOF-NPs against 1.0 nM of MOR, (c) PL spectra response for the behavior of Cr(III)-MOF-NPs toward different concentrations of MOR, (d) histogram of the PL enhancement efficiency against different concentrations of MOR, (e) linear relationship (calibration graph) between the PL intensity of Cr(III)-MOF-NPs and the logarithm MOR concentrations (log[MOR]), (f) PL intensity of the Cr(III)-MOF-NPs toward the MOR against different types of interfering analyte histogram, and [inset (f)] PL intensity spectrum of the Cr(III)-MOF-NPs toward the MOR against different types of interfering analytes.
Moreover, the Cr(III)-MOF-NPs as a spectrofluorimetric chemosensor for MOR were studied. The PL spectrum (at λex = 416 nm) of the Cr(III)-MOF-NPs (0.1 μM) was investigated against (1.0 nM) of MOR (Figure 5b). As shown in Figure 5b, the Cr(III)-MOF-NPs PL intensity was enhanced followed by a remarkable blueshift from 593 to 566 nm. Additionally, as shown from the photographic photo (inset in Figure 5b), the color of diluted Cr(III)-MOF-NP solution changed from brown to yellow color which makes the Cr(III)-MOF-NPs to be used as an MOR-indicator which can be seen by naked eye (visual detection). Also, the PL of the Cr(III)-MOF-NP spectrum and PL enhancement efficiency histogram against different MOR concentrations were studied, and the data are shown in Figure 5c,d, correspondingly. From these figures, the Cr(III)-MOF-NPs PL spectrum shifted to blue from 593 to 566 nm significantly, and the intensity enhanced remarkably as the MOR concentrations increased from 0.1 to 350 nM, and these obtained results prove that the Cr(III)-MOF could be worked as a spectrofluorimetric chemosensor for MOR fast detection and quantification. Different pH conditions for the determination of MOR using Cr(III)-MOF-NPs in acidic, neutral, and basic media were tested. The results revealed that the PL intensities of the Cr(III)-MOF-NPs chemosensor is better in the neutral medium than the acidic and basic medium. Therefore, the optimum pH condition for this study is the neutral medium.
Analytical Merits and Method Validation
Calibration Curve
Under the optimum conditions, the calibration graph of the Cr(III)-MOF-NPs PL intensities at λex = 416 nm, against the MOR concentrations in the range between 0.1 and 350.0 nM, are shown in Figure 5e. From Figure 5e, the PL spectra were significantly dependent on the increase of the MOR concentrations that the PL λ566 increase and then attain the saturation at MOR concentration 350.0 nM. The calibration plot showed a well linear relationship over a range between 0.1 and 300.0 nM, and the fitting equation can be expressed as
The LOD for the chemosensor was found to be 0.167 nM, whereas the LOQ was 0.443 nM. The condensed of the regression of data analysis of the PL intensity is shown (Table S2; Supporting Information). The LOD/LOQ lower values and wide concentration linear range are a confirmation for the ultrahigh sensitivity of the proposed chemosensor. Furthermore, the LOD/LOQ values were improved than the other published articles previously,2−4,14,15,18,19 and the comparison is summarized in Table S3.
Selectivity
The Cr(III)-MOF-NPs PL behavior toward MOR and some interfering analytes based on the suggested photoluminescent approach was investigated to prove the specific selectivity and the recognition ability for MOR. The PL spectrum of Cr(III)-MOF-NPs (0.1 μM) was studied against some cationic and ionic species (Na+, Ca2+, and Cl–) and some organic materials (oxalic acid, ascorbic acid, uric acid, GLU, caffeine, and methamphetamine) at a concentration of 100 nM. The results of the enhancement efficiency histogram are shown in Figure 5f; additionally, the PL spectrum of Cr(III)-MOF-NPs against the above analytes is shown in Figure 5g and inset in Figure 5f. From Figure 5f,g, the PL intensities were scientifically enhanced with a remarkable blueshift in the case of MOR and do not give any noticeable responses for anyone of the other interfering species. Therefore, it can be deduced that the Cr(III)-MOF-NPs is exceedingly selective for MOR throughout the enhancement effect with the remarkable blueshift.
Accuracy, Precision, Reducibility, and Repeatability
The present study is a general statistical evaluation for establishing the applicability and effectiveness of the suggested spectrofluorimetric methodology based on Cr(III)-MOF-NPs to quantify the MOR. This study was carried out at four concentration levels 0.5, 5, 50, and 100 nM, and each experiment was repeated three times in the same/different days (repeatability/reproducibility study). The PL intensity histogram of Cr(III)-MOF-NPs against different concentrations of MOR and summarized results of the “relative-error percent (RE %), mean (X), standard deviation (SD), and coefficient of variation (CV)” is shown in Figures S3 and S4, and Table S4, Supporting Information, respectively.
From the obtained table data and histogram, the computed values of the RE % were between 0.965 and 1.029%; interday and 0.954 and 1.002%; intraday, which indicates the exciting accuracy, repeatability, and reducibility of the suggested methodology. The calculated X values were very close from the target; the SD values were between 0.034 and 0.992%; interday and 0.01 and 1.50%; intraday, whereas the CV values were between 0.001 and 0.98%; interday and 0.006 and 2.25%; intraday. The RE %, CV, and SD lower values revealed the tremendous precision, repeatability, and reducibility of the proposed approach.
Recovery Study and Real Sample Applications
The currently proposed methodology was examined to quantizing the MOR concentration content in different biological urine and serum samples. The MOR spiked in the serum/urine samples at four concentrations levels 0.5, 5, 50, and 100 nM, and the reading was repeated three times; the summary of results is reported in Table 1. The statistical evaluations of the obtained data via calculation of the percent of recovery (% RC) and relative SD (% RSD) reveal that the average percent of RC ± RSD was 97.98 ± 1.65% for serum samples and 97.23 ± 1.14% for urine samples. The present data prove that the proposed approach is effective and applicable, for quantizing MOR in different biological samples and will be promising future analytical tools related to MOR quantification.
Table 1. Determination of MOR in Different Real Samples Using the Cr(III)-MOF-NPs Chemosensor.
| sample | spiked MOR (nM) | found (nM) | X | RSD | RC % | ||
|---|---|---|---|---|---|---|---|
| serum samples | 0.5 | 0.498 | 0.474 | 0.484 | 0.485 | 0.012 | 97.04 |
| 5 | 4.743 | 4.791 | 5.09 | 4.875 | 0.188 | 97.49 | |
| 50 | 48.6 | 48.4 | 51.72 | 49.57 | 1.862 | 99.15 | |
| 100 | 103.5 | 95.62 | 95.67 | 98.26 | 4.524 | 98.26 | |
| urine samples | 0.5 | 0.468 | 0.484 | 0.529 | 0.494 | 0.032 | 98.74 |
| 5 | 4.851 | 4.827 | 4.812 | 4.83 | 0.02 | 96.59 | |
| 50 | 48.15 | 47.58 | 48.43 | 48.05 | 0.434 | 96.1 | |
| 100 | 94.82 | 95.45 | 102.2 | 97.48 | 4.08 | 97.48 | |
Mechanism of Sensing
Hence, the Cr(III)-MOF-NPs exhibited a PL emission band at 593 nm at excitation λex = 416 nm (Figure 5a). With the construction of the Cr(III)-MOF-NPs/MOR sensing platform, it can be observed that a characteristic rapid host–guest interaction occurred between MOR and the Cr(III)-MOF-NPs, causing a blueshift from 593 to 566 nm with a remarkable PL enhancement (Figure 5b–d). Moreover, visually (naked-eye detection), the color clearly changed from brown to yellow with the addition of MOR (inset in Figure 5b). The above sensing behavior of Cr(III)-MOF-NPs toward MOR provided an extraordinary PL response and can be interpreted because of the hydrogen bonding51 that is responsible about the change in the optical behavior or may be because of the affinity as well the reactivity of MOR toward Lewis acids in Cr(III)-MOF-NPs.54−56 Consequently, MOR is an electroactive molecule, has nitrogen and oxygen atoms with high negative energy, and these atoms are electron-donating sites. These strong electronegativity criteria enabled the photoinduced transferring of electrons within the Cr(III)-MOF-NP structure causing a PL blueshift.24,52−54 Finally, a fashionable host–guest interaction (Lewis acid/base interactions) between the host and guest sites [Cr(III)-MOF-NPs and MOR, respectively] lead to the development and stimulation of an appropriate fast, selective, and sensitive MOR chemosensor for detection and quantification.
Conclusions
In this article, novel and promising chromium(III) MOF nanoparticles [Cr(III)-MOF-NPs] were synthesized and characterized. The characterization revealed that the morphology of Cr(III)-MOF-NPs appeared to be nanosheets covered with nanoparticles ranging from 50 to 100 nm. The XPS and element mapping by spatially-resolved EDX showed the presence of chromium, carbon, chlorine, nitrogen, and oxygen as building block elements in every single particle. The mass spectrum supported with mass fragmentation revealed that the m/z peaks estimated at 1284.78 completely matched with the proposed empirical formula. Other obtained microanalytical results prove the structure and morphology of the prepared MOF. Moreover, a spectrofluorimetric investigation was carried out for Cr(III)-MOF-NPs. The results revealed that the Cr(III)-MOF-NPs could be used as a promising chemosensor for MOR detection. The proposed approach “Cr(III)-MOF-NP-chemosensor” was statistically evaluated and was fit for purpose without interference with the other interfering matrix and lower in LOD and LOQ other published reports. Additionally, the Cr(III)-MOF-NP-chemosensor was applicable for biological real samples for MOR detection and quantification.
Experimental Section
Materials
See details in the Supporting Information.
Instruments
See details in the Supporting Information.
Procedures
Synthesis of the Cr(III)-MOF-NPs
In 15 mL of distilled H2O, dissolved CrCl3·6H2O (0.533 g, 2.0 mmol) was slowly added to the NL, which was synthesized according to ref (26). At 80 °C, the system was stirred and refluxed for two days (Scheme S1). The solution color transformed from reddish-brown suspension to a dark brown PPT. The dark brown PPT was filtered, washed well, and dried.
PL Measurement General Procedure
At RT, (1 μM) stock solution of Cr(III)-MOF-NPs was prepared in dimethyl sulfoxide. Subsequently, the (0.1 μM) working solutions were diluted with distd H2O. The Cr(III)-MOF-NPs solution (0.1 μM) was subjected for consequential for the PL measurement. The PL spectrum of Cr(III)-MOF-NPs (0.1 μM) against various fresh solutions of MOR and other analytes was executed in a separate trail formerly in a mixture together.
MOR Determination Using Cr(III)-MOF-NPs
The PL spectrum of Cr(III)-MOF-NPs (0.1 μM) versus recently prepared different MOR concentrations was recorded. Upon the optimization of PL measurement conditions, a linear relationship was established between the PL intensities of Cr(III)-MOF-NPs at λem = 593 nm and MOR in a range of concentrations between 100 μM and 350.0 nM. Also, according to the deterioration equation: “Y = a + bX, where Y is the Cr(III)-MOF-NP PL intensity at λem; a is the intercept; b is the curve slope, whereas X is the MOR concentrations in nM”. The equation parameters as well the correlation coefficient (r2) data analysis fitted according to the least-squares method. Furthermore, the LOD and LOQ were calculated from the formula: “LOQ = 10 × (S/b) & LOD = 3.3 × (S/b),55,56 where S is the value of the standard error of Cr(III)-MOF-NPs PL intensities, and b is the curve slope of the calibration graph”.
MOR Detection in Real Samples
The urine and serum real samples were obtained from a medical laboratory. The samples were treated and handled according to standard ethics, precautions, recommendations, and guidelines.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. DF-424-247-1441. The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04249.
Proposed mechanism of Cr(III)-MOF-NP synthesis; proposed fragmentation scheme of Cr(III)-MOF-NPs; magnetization curve; PL emission spectra at different excitation wavelengths; histogram of evaluation of interday accuracy, intraday accuracy, and precision; theoretically elemental calculated, elemental analysis, and EDX analysis; theoretically elemental calculated, elemental analysis, and EDX analysis; and comparison between the Cr(III)-MOF-NP chemosensor and some published methods for the determination of MOR (PDF)
Author Contributions
The research was conceived by S.M.S. S.M.S performed the experiments. M.A. and S.M.S. contributed to data analyses and writing the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Abraham P.; Renjini S.; Vijayan P.; Nisha V.; Sreevalsan K.; Anithakumary V. Review—Review on the Progress in Electrochemical Detection of Morphine Based on Different Modified Electrodes. J. Electrochem. Soc. 2020, 167, 037559. 10.1149/1945-7111/ab6cf6. [DOI] [Google Scholar]
- Abraham P.; Renjini S.; Nancy T. E. M.; Kumary V. A. Electrochemical Synthesis of Thin-Layered Graphene Oxide-Poly(CTAB) Composite for Detection of Morphine. J. Appl. Electrochem. 2020, 50, 41–50. 10.1007/s10800-019-01367-2. [DOI] [Google Scholar]
- Bahrami G.; Ehzari H.; Mirzabeigy S.; Mohammadi B.; Arkan E. Fabrication of a Sensitive Electrochemical Sensor Based on Electrospun Magnetic Nanofibers for Morphine Analysis in Biological Samples. Mater. Sci. Eng., C 2020, 106, 110183. 10.1016/j.msec.2019.110183. [DOI] [PubMed] [Google Scholar]
- Yousefi N.; Irandoust M.; Haghighi M. New and Sensitive Magnetic Carbon Paste Electrode for Voltammetry Determination of Morphine and Methadone. J. Iran. Chem. Soc. 2020, 17, 2909. 10.1007/s13738-020-01962-7. [DOI] [Google Scholar]
- Dehdashtian S.; Gholivand M. B.; Shamsipur M.; Kariminia S. Construction of a Sensitive and Selective Sensor for Morphine Using Chitosan Coated Fe3O4 Magnetic Nanoparticle as a Modifier. Mater. Sci. Eng., C 2016, 58, 53–59. 10.1016/j.msec.2015.07.049. [DOI] [PubMed] [Google Scholar]
- Earles M. P. Pharmacy in History by G. E. Trease, London, Bailliere, Tindall & Cox, 1964, pp. vii, 265, illus., 50s. Med. Hist. 1966, 10, 93–94. 10.1017/s0025727300010735. [DOI] [Google Scholar]
- Milne R. W.; Nation R. L.; Somogyi A. A. The Disposition of Morphine and Its 3- and 6-Glucuronide Metabolites in Humans and Animals, and the Importance of the Metabolites to the Pharmacological Effects of Morphine. Drug Metab. Rev. 1996, 28, 345–472. 10.3109/03602539608994011. [DOI] [PubMed] [Google Scholar]
- Thevis M.; Geyer H.; Tretzel L.; Schänzer W. Sports Drug Testing Using Complementary Matrices: Advantages and Limitations. J. Pharm. Biomed. Anal. 2016, 130, 220–230. 10.1016/j.jpba.2016.03.055. [DOI] [PubMed] [Google Scholar]
- https://www.wada-ama.org/en/resources/the-code/world-anti-doping-code? (last accessed July 5, 2020).
- https://www.wada-ama.org/en/content/what-is-prohibited (last accessed July 5, 2020).
- Tagliaro F.; Franchi D.; Dorizzi R.; Marigo M. High- Performance Liquid Chromatographic Determination of Morphine in Biological Samples, an Overview of Separation Methods and Detection Techniques. J. Chromatogr. 1989, 488, 215–228. 10.1016/s0378-4347(00)82947-3. [DOI] [PubMed] [Google Scholar]
- Ke H.; Du X.; Wang L.; Wang X.; Zhu J.; Gao Y.; Peng B.; Hao H.; Cai N. Detection of Morphine in Urine Based on a Surface Plasmon Resonance Imaging Immunoassay. Anal. Methods 2020, 12, 3038–3044. 10.1039/d0ay00648c. [DOI] [PubMed] [Google Scholar]
- Li W.; Li X.; Yang T.; Guo X.; Song Y. Detection of Saliva Morphine Using Surface-Enhanced Raman Spectroscopy Combined with Immunochromatographic Assay. J. Raman Spectrosc. 2020, 51, 642–648. 10.1002/jrs.5822. [DOI] [Google Scholar]
- Zhang C.; Yu X.; Shi X.; Han Y.; Guo Z.; Liu Y. Development of Carbon Quantum Dot–Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine in Hot Pot Soup Base. Food Anal. Methods 2020, 13, 1042–1049. 10.1007/s12161-020-01700-y. [DOI] [Google Scholar]
- Farahani A.; Sereshti H. An Integrated Microfluidic Device for Solid-Phase Extraction and Spectrophotometric Detection of Opium Alkaloids in Urine Samples. Anal. Bioanal. Chem. 2020, 412, 129–138. 10.1007/s00216-019-02214-1. [DOI] [PubMed] [Google Scholar]
- Montgomery M. T.; Conlan X. A.; Theakstone A. G.; Purcell S. D.; Barnett N. W.; Smith Z. M. Extraction and Determination of Morphine Present on the Surface of Australian Food Grade Poppy Seeds Using Acidic Potassium Permanganate Chemiluminescence Detection. Food Anal. Methods 2020, 13, 1159–1165. 10.1007/s12161-020-01729-z. [DOI] [Google Scholar]
- Usmanov D.; Akhunov S.; Khasanov U.; Rotshteyn V.; Kasimov B. Direct Detection of Morphine in Human Urine by Surface-Ionization Mass Spectrometry. Eur. J. Mass Spectrom. 2020, 26, 153–157. 10.1177/1469066719875655. [DOI] [PubMed] [Google Scholar]
- Beitollahi H.; Nejad F. G. Magnetic Core–Shell Graphene Oxide/Fe3O4@SiO2 Nanocomposite for Sensitive and Selective Electrochemical Detection of Morphine Using Modified Graphite Screen Printed Electrode. J. Anal. Chem. 2020, 75, 127–134. 10.1134/S1061934820010049. [DOI] [Google Scholar]
- Salimi A.; Hallaj R.; Khayatian G.-R. Amperometric Detection of Morphine at Preheated Glassy Carbon Electrode Modified with Multiwall Carbon Nanotubes. Electroanalysis 2005, 17, 873–879. 10.1002/elan.200403166. [DOI] [PubMed] [Google Scholar]
- Gandhi S.; Suman P.; Kumar A.; Sharma P.; Capalash N.; Suri C. Recent Advances in Immunosensor for Narcotic Drug Detection. BioImpacts 2015, 5, 207–213. 10.15171/bi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghavi B. J.; Wolfbeis O. S.; Hirsch T.; Swami N. S. Nanomaterial-Based Electrochemical Sensing of Neurological Drugs and Neurotransmitters. Microchim. Acta 2015, 182, 1–41. 10.1007/s00604-014-1308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheta S. M.; El-sheikh S. M.; Osman D. I.; Salem A. M.; Ali O. I.; Harraz F. A.; Shousha W. G.; Shoeib M. A.; Shawky S. M.; Dionysiou D. D. A Novel HCV Electrochemical Biosensor Based on a Polyaniline@Ni-MOF Nanocomposite. Dalton Trans. 2020, 49, 8918–8926. 10.1039/d0dt01408g. [DOI] [PubMed] [Google Scholar]
- Basaleh A. S.; Sheta S. M. Novel Advanced Nanomaterial Based on Ferrous Metal–Organic Framework and Its Application as Chemosensors for Mercury in Environmental and Biological Samples. Anal. Bioanal. Chem. 2020, 412, 3153–3165. 10.1007/s00216-020-02566-z. [DOI] [PubMed] [Google Scholar]
- Sheta S. M.; El-Sheikh S. M.; Abd-Elzaher M. M. A Novel Optical Approach for Determination of Prolactin Based on Pr-MOF Nanofibers. Anal. Bioanal. Chem. 2019, 411, 1339–1349. 10.1007/s00216-018-01564-6. [DOI] [PubMed] [Google Scholar]
- Osman D. I.; El-sheikh S. M.; Sheta S. M.; Ali O. I.; Salem A. M.; Shousha W. G.; El-khamisy S. F.; Shawky S. M. Nucleic Acids Biosensors Based on Metal-Organic Framework (MOF): Paving the Way to Clinical Laboratory Diagnosis. Biosens. Bioelectron. 2019, 141, 111451. 10.1016/j.bios.2019.111451. [DOI] [PubMed] [Google Scholar]
- Sheta S. M.; El-sheikh S. M.; Abd-elzaher M. M.; Ghanem M. L.; Salem S. R. A Novel , Fast , High Sensitivity Biosensor for Supporting Therapeutic Decisions and Onset Actions for Chest Pain Cases. RSC Adv. 2019, 9, 20463–20471. 10.1039/c9ra03030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremias F.; Lozan V.; Henninger S. K.; Janiak C. Programming MOFs for Water Sorption: Amino-Functionalized MIL-125 and UiO-66 for Heat Transformation and Heat Storage Applications. Dalton Trans. 2013, 42, 15967. 10.1039/c3dt51471d. [DOI] [PubMed] [Google Scholar]
- Grall R.; Hidalgo T.; Delic J.; Garcia-Marquez A.; Chevillard S.; Horcajada P. In Vitro Biocompatibility of Mesoporous Metal (III; Fe, Al, Cr) Trimesate MOF Nanocarriers. J. Mater. Chem. B 2015, 3, 8279–8292. 10.1039/c5tb01223f. [DOI] [PubMed] [Google Scholar]
- Sheta S. M.; El-Sheikh S. M.; Abd-Elzaher M. M.; Salem S. R.; Moussa H. A.; Mohamed R. M.; Mkhalid I. A. A Novel Biosensor for Early Diagnosis of Liver Cancer Cases Using Smart Nano-Magnetic Metal–Organic Framework. Appl. Organomet. Chem. 2019, 33, e5249 10.1002/aoc.5249. [DOI] [Google Scholar]
- Jeazet H. B. T.; Staudt C.; Janiak C. Metal–Organic Frameworks in Mixed-Matrix Membranes for Gas Separation. Dalton Trans. 2012, 41, 14003. 10.1039/c2dt31550e. [DOI] [PubMed] [Google Scholar]
- Sheta S. M.; El-Sheikh S. M.; Abd-Elzaher M. M.; Wassel A. R. A Novel Nano-Size Lanthanum Metal – Organic Framework Based on 5-Amino-Isophthalic Acid and Phenylenediamine: Photoluminescence Study and Sensing Applications. Appl. Organomet. Chem. 2019, 33, e4777 10.1002/aoc.4777. [DOI] [Google Scholar]
- Sheta S. M.; El-Sheikh S. M.; Abd-Elzaher M. M. Promising Photoluminescence Optical Approach for Triiodothyronine Hormone Determination Based on Smart Copper Metal – Organic Framework Nanoparticles. Appl. Organomet. Chem. 2019, 33, e5069 10.1002/aoc.5069. [DOI] [Google Scholar]
- Simon-Yarza T.; Mielcarek A.; Couvreur P.; Serre C. Nanoparticles of Metal-Organic Frameworks: On the Road to In Vivo Efficacy in Biomedicine. Adv. Mater. 2018, 30, e1707365 10.1002/adma.201707365. [DOI] [PubMed] [Google Scholar]
- Sheta S. M.; El-Sheikh S. M.; Abd-Elzaher M. M. Simple Synthesis of Novel Copper Metal–Organic Framework Nanoparticles: Biosensing and Biological Applications. Dalton Trans. 2018, 47, 4847–4855. 10.1039/c8dt00371h. [DOI] [PubMed] [Google Scholar]
- Ren J.; Dyosiba X.; Musyoka N. M.; Langmi H. W.; North B. C.; Mathe M.; Onyango M. S. Green Synthesis of Chromium-Based Metal-Organic Framework (Cr-MOF) from Waste Polyethylene Terephthalate (PET) Bottles for Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 18141–18146. 10.1016/j.ijhydene.2016.08.040. [DOI] [Google Scholar]
- Segakweng T.; Musyoka N. M.; Ren J.; Crouse P.; Langmi H. W. Comparison of MOF-5- and Cr-MOF-Derived Carbons for Hydrogen Storage Application. Res. Chem. Intermed. 2016, 42, 4951–4961. 10.1007/s11164-015-2338-1. [DOI] [Google Scholar]
- Kayal S.; Sun B.; Chakraborty A. Study of Metal-Organic Framework MIL-101(Cr) for Natural Gas (Methane) Storage and Compare with Other MOFs (Metal-Organic Frameworks). Energy 2015, 91, 772–781. 10.1016/j.energy.2015.08.096. [DOI] [Google Scholar]
- Kayal S.; Chakraborty A. Activated Carbon (Type Maxsorb-III) and MIL-101(Cr) Metal Organic Framework Based Composite Adsorbent for Higher CH4 Storage and CO2 Capture. Chem. Eng. J. 2018, 334, 780–788. 10.1016/j.cej.2017.10.080. [DOI] [Google Scholar]
- Salles F.; Bourrelly S.; Jobic H.; Devic T.; Guillerm V.; Llewellyn P.; Serre C.; Ferey G.; Maurin G. Molecular Insight into the Adsorption and Diffusion of Water in the Versatile Hydrophilic/Hydrophobic Flexible MIL-53(Cr) MOF. J. Phys. Chem. C 2011, 115, 10764–10776. 10.1021/jp202147m. [DOI] [Google Scholar]
- Guo Q.; Ren L.; Kumar P.; Cybulskis V. J.; Mkhoyan K. A.; Davis M. E.; Tsapatsis M. A Chromium Hydroxide/MIL-101(Cr) MOF Composite Catalyst and Its Use for the Selective Isomerization of Glucose to Fructose. Angew. Chem., Int. Ed. 2018, 130, 5020–5024. 10.1002/ange.201712818. [DOI] [PubMed] [Google Scholar]
- Hamon L.; Heymans N.; Llewellyn P. L.; Guillerm V.; Ghoufi A.; Vaesen S.; Maurin G.; Serre C.; De Weireld G.; Pirngruber G. D. Separation of CO 2-CH 4 Mixtures in the Mesoporous MIL-100(Cr) MOF: Experimental and Modelling Approaches. Dalton Trans. 2012, 41, 4052–4059. 10.1039/c2dt12102f. [DOI] [PubMed] [Google Scholar]
- Rostamnia S.; Alamgholiloo H.; Jafari M. Ethylene Diamine Post-Synthesis Modification on Open Metal Site Cr-MOF to Access Efficient Bifunctional Catalyst for the Hantzsch Condensation Reaction. Appl. Organomet. Chem. 2018, 32, e4370 10.1002/aoc.4370. [DOI] [Google Scholar]
- Granadeiro C. M.; Karmaoui M.; Correia E.; Julião D.; Amaral V. S.; Silva N. J. O.; Cunha-Silva L.; Balula S. S. Cobalt Aluminate Nanoparticles Supported on MIL-101 Structure: Catalytic Performance Investigation. RSC Adv. 2015, 5, 4175–4183. 10.1039/c4ra10498f. [DOI] [Google Scholar]
- Yin D.; Li C.; Ren H.; Shekhah O.; Liu J.; Liang C. Efficient Pd@MIL-101(Cr) Hetero-Catalysts for 2-Butyne-1,4-Diol Hydrogenation Exhibiting High Selectivity. RSC Adv. 2017, 7, 1626–1633. 10.1039/c6ra25722d. [DOI] [Google Scholar]
- Broadhurst C. L.; Schmidt W. F.; Reeves J. B.; Polansky M. M.; Gautschi K.; Anderson R. A. Characterization and Structure by NMR and FTIR Spectroscopy, and Molecular Modeling of Chromium(III) Picolinate and Nicotinate Complexes Utilized for Nutritional Supplementation. J. Inorg. Biochem. 1997, 66, 119–130. 10.1016/S0162-0134(96)00192-4. [DOI] [Google Scholar]
- Jarrah A.; Farhadi S. K6P2W18O62 Encapsulated into Magnetic Fe3O4/ MIL-101 (Cr) Metal–Organic Framework: A Novel Magnetically Recoverable Nanoporous Adsorbent for Ultrafast Treatment of Aqueous Organic Pollutants Solutions. RSC Adv. 2018, 8, 37976–37992. 10.1039/c8ra06287k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhil Vilian A. T.; Dinesh B.; Muruganantham R.; Choe S. R.; Kang S.-M.; Huh Y. S.; Han Y.-K. A Screen Printed Carbon Electrode Modified with an Amino-Functionalized Metal Organic Framework of Type MIL-101(Cr) and with Palladium Nanoparticles for Voltammetric Sensing of Nitrite. Microchim. Acta 2017, 184, 4793–4801. 10.1007/s00604-017-2513-8. [DOI] [Google Scholar]
- Liu Z.; Zhao B.; Zhu L.; Lou F.; Yan J. Performance of MIL-101(Cr)/Water Working Pair Adsorption Refrigeration System Based on a New Type of Adsorbent Filling Method. Materials 2020, 13, 195. 10.3390/ma13010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon D.; Choi J.-H. Crystal structure oftrans-bis(ethane-1,2-diamine-κ2N,N′)bis(thiocyanato-κN)chromium(III) perchlorate from synchrotron data. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2015, 71, 650–653. 10.1107/s2056989015009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Jin Y.; Fu Y.; Zhong Y.; Zhu W.; Ibrahim A. A.; El-Shall M. S. Palladium Nanoparticles Incorporated within Sulfonic Acid-Functionalized MIL-101(Cr) for Efficient Catalytic Conversion of Vanillin. J. Mater. Chem. A 2015, 3, 17008–17015. 10.1039/c5ta03524d. [DOI] [Google Scholar]
- Uttam B.; Kandi R.; Hussain M. A.; Rao C. P. Fluorescent Lower Rim 1,3-Dibenzooxadiazole Conjugate of Calix[4]Arene in Selective Sensing of Fluoride in Solution and in Biological Cells Using Confocal Microscopy. J. Org. Chem. 2018, 83, 11850–11859. 10.1021/acs.joc.8b01761. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Wang J.; Du T.; Zhang W.; Zhu W.; Yang C.; Yue T.; Sun J.; Li T.; Wang J. NH2-MIL-53 (Al) Metal–Organic Framework as the Smart Platform for Simultaneous High-Performance Detection and Removal of Hg 2+. Inorg. Chem. 2019, 58, 12573–12581. 10.1021/acs.inorgchem.9b01242. [DOI] [PubMed] [Google Scholar]
- Pal S.; Bhunia A.; Jana P. P.; Dey S.; Möllmer J.; Janiak C.; Nayek H. P. Microporous La-Metal-Organic Framework (MOF) with Large Surface Area. Chem.—Eur. J. 2015, 21, 2789–2792. 10.1002/chem.201405168. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Wu W.; Zou L.; Feng J.; Gu C.; Li B.; Batten S. R.; Yadav R.; Kumar A. Luminescent Sensing of a New 8-Connected Topological Metal-Organic Framework. Inorg. Chem. Commun. 2016, 70, 160–163. 10.1016/j.inoche.2016.06.007. [DOI] [Google Scholar]
- Abd-Elzaher M. M.; Ahmed M. A.; Farag A. B.; Attia M. S.; Youssef A. O.; Sheta S. M. A Fast and Simple Method for Determination of Testosterone Hormone in Biological Fluids Based on a New Eu(III) Complex Optical Sensor. Sens. Lett. 2017, 15, 977–981. 10.1166/sl.2017.3904. [DOI] [Google Scholar]
- Abd-Elzaher M. M.; Ahmed M. A.; Farag A. B.; Attia M. S.; Youssef A. O.; Sheta S. M. New Optical Sensor for Determination of Hydrochlorothiazide in Pharmaceutical Preparation and Biological Fluids. Sens. Lett. 2017, 15, 525–530. 10.1166/sl.2017.3840. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.