Abstract
Background
Trastuzumab-induced cardiotoxicity (TIC) can lead to early discontinuation of adjuvant therapy, however there is limited evidence on long-term survival outcomes in patients with operable human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC) experiencing treatment interruption or discontinuation.
Methods
The primary objective of the study was to evaluate disease-free survival (DFS) in non-metastatic, HER2-positive, female BC patients who experienced treatment interruption or early discontinuation of trastuzumab therapy. Clinical and histopathological data were collected on 400 patients at The Ohio State University, an NCI-designated comprehensive cancer center between January 2005 and December 2015. Treatment interruption was defined as any delay of ≥2 weeks during trastuzumab therapy, including permanent cessation prior to completing planned therapy. TIC was defined as LVEF < 50% or > 15 points decline from baseline as evaluated by 2D echocardiogram after initiation of (neo) adjuvant therapy. DFS was defined as the time from diagnosis to first recurrence (loco-regional or distant recurrence) including second primary BC or death. Overall survival (OS) was defined as the time from diagnosis to death or last known follow up. OS/DFS estimates were generated using Kaplan-Meier methods and compared using Log-rank tests. Cox proportional hazard models were used to calculate adjusted hazard ratios (aHR) for OS/DFS.
Results
A total of 369 patients received trastuzumab therapy; 106 (29%) patients experienced treatment interruption at least once and 42 (11%) permanently discontinued trastuzumab prior to completing planned therapy. TIC was the most common reason for interruption (66 patients, 62%). The median duration of trastuzumab in patients with treatment interruption was 11.3 months (range: 0.5–16.9) with 24 (23%) patients receiving ≤6 months of therapy. This duration includes the time delay related to treatment interruption. Patients with any treatment interruption had worse DFS (aHR: 4.4, p = 0.001) and OS (aHR: 4.8, p < 0.001) after adjusting for age, stage, grade, ER, node status and TIC.
Conclusions
Treatment interruption or early discontinuation of trastuzumab therapy in early HER2-positive BC, most often from TIC, is an independent prognostic marker for worse DFS and OS in operable HER2-positive BC. Future prospective studies should consider targeting at-risk populations and optimizing cardiac function to avoid interruption in trastuzumab therapy.
Keywords: HER2, Breast cancer, Trastuzumab, Cardio-oncology, Chemotherapy
Introduction
Breast cancer (BC) is a heterogeneous disease broadly categorized into three distinct phenotypes based on hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) overexpression [1]. HER2-Neu gene amplification and/or overexpression accounts for 15–20% of all newly diagnosed cases in the United States, which results in an aggressive biology associated with a higher risk of recurrence compared to HR-positive disease [2]. Trastuzumab, a humanized, monoclonal antibody that targets the extracellular domain IV of HER2 receptor, has dramatically changed the prognosis of patients with early invasive HER2-positive disease. Several trials have consistently shown a decrease in risk of BC recurrence and BC-specific mortality with the addition of 1 year of trastuzumab to standard adjuvant chemotherapy [3–6]. Although trastuzumab is well tolerated, it is associated with cardiotoxicity in some patients, which can result in early discontinuation of adjuvant therapy [7]. Trastuzumab-induced cardiotoxicity (TIC) can present clinically with asymptomatic decline in left ventricular ejection fraction (LVEF) (3.2–19%) or symptomatic heart failure (0.5–5.1%) [8–11]. HER2 is expressed in cardiac myocytes and is essential for the preservation of cardiac function and structure [12]. Although this could explain a potential link, the true mechanism of TIC remains unclear [13]. Patients receiving adjuvant trastuzumab undergo periodic monitoring of LVEF every 3 months while on therapy [14]; those experiencing decline in LVEF ≥16% from baseline or LVEF below normal limits and ≥ 10% decline are asked to withhold treatment for at least 4 weeks and continue LVEF monitoring every 4 weeks until resolution. Older age (> 50 years) and pre-existing cardiovascular (CV) diseases, such as uncontrolled hypertension and congestive heart failure, have been implicated in increasing the risk of TIC [10, 15–19]. The incidence of TIC is also higher in patients receiving concomitant or sequential anthracycline (up to 27%) compared to non-anthracycline regimens [4, 20–22]. Several randomized trials have evaluated shorter duration of adjuvant trastuzumab with a goal of minimizing cardiotoxicity while maintaining efficacy of systemic therapy [23–26]. A recent open-label, phase III randomized trial compared 6 months vs. 12 months of adjuvant trastuzumab therapy and showed that shorter duration was non-inferior to standard 1 year of therapy and resulted in less cardiac toxicity. They demonstrated that a four-year disease-free survival (DFS) was 89% (95% CI 88–91) in both arms. The estimated hazard ratio was 1.05 (95% CI 0.88–1.25) indicating non-inferiority (hazard ratio < 1.29) of 6-months of trastuzumab (p = 0.01) [23]. On the other hand, several other studies, including a meta-analysis of four trials evaluating shorter duration of adjuvant trastuzumab, failed to report non-inferiority compared to a standard 1 year regimen, cautioning against the adoption of this approach in routine clinical practice [24–26].
While shorter duration of trastuzumab therapy is associated with lower incidence of TIC [23], there is limited evidence to describe disease outcomes in patients with interruption and/or early discontinuation of adjuvant trastuzumab secondary to cardiac toxicity. Interruption or early discontinuation may adversely affect disease outcomes in HER2-positive early BC patients independent of planned duration of adjuvant HER2-directed therapy. Herein, we report the results of a single-institution study assessing survival outcomes in patients who experienced treatment interruption or early discontinuation of adjuvant trastuzumab at The Ohio State University Comprehensive Cancer Center (OSUCCC-James).
Materials and methods
Study design
This study was an IRB-approved (OSU 2017C0080) retrospective chart review of clinical and histopathologic data from female patients ≥18 years of age, with HER2-positive, stage I-III BC seen at OSUCCC-James between January 2005 and December 2015. HER2 expression was confirmed per ASCO-CAP guidelines at the time of diagnosis defined by immunohistochemistry of 3+ (uniform, intense membrane staining of > 30% of invasive tumor cells), a fluorescent in situ hybridization (FISH) result of ≥6 HER2 gene copies per nucleus, or a FISH ratio (HER2 gene signals to chromosome 17 centromeric signals) of ≥2.2. Patients with incomplete clinical data, those who had stage IV disease, pathologically in-situ or microinvasive disease at diagnosis (≤1 mm invasive disease in the greatest dimension), and those treated at other institutions were excluded. Treatment interruption was defined as any treatment delay of ≥2 weeks in receiving trastuzumab, including permanent discontinuation prior to completing planned duration of therapy. TIC was defined as LVEF < 50% or > 15 points decline from baseline as evaluated by 2D echocardiogram after initiation of (neo) adjuvant therapy. In concordance with FDA package insert criteria and our institutional guidelines for trastuzumab, patients who experienced a decline in LVEF ≥16% from baseline or LVEF below normal limits and ≥ 10% decline were asked to withhold treatment for at least 4 weeks and referred to a cardiologist to initiate cardioprotective therapy. LVEF monitoring was repeated every 4 weeks until resolution. Trastuzumab re-challenge was only provided to those with a recovery in LVEF to normal.
Data collection
Data was obtained from The Ohio State University Information Warehouse and uploaded into REDCap database [27]. Missing data were populated using manual review of each patient’s electronic medical record. Data were collected on demographic characteristics, biomarker profiles (ER, PR, and HER2) of the tumor, therapy modalities (surgery, chemotherapy type and regimen, and radiotherapy) and duration of trastuzumab therapy, disease recurrence, and survival outcomes.
Statistical methods
Demographic and clinical characteristics as well as treatment modalities were summarized using descriptive statistics. DFS was defined as the time from diagnosis to first recurrence (loco-regional or distant recurrence), including second primary BC or death. OS was defined as the time from diagnosis to death or last known follow up. OS/DFS estimates were generated using Kaplan-Meier methods and compared using Log-rank tests. Cox proportional hazard models were used to calculate adjusted hazard ratios (aHR) for OS/DFS. All data analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) or Stata 14 (StataCorp LLC, College Station, TX).
Results
A total of 400 patients with HER2-positive stage I-III BC were included in this study; 369 (92%) received (neo) adjuvant trastuzumab and were included in survival analyses. Patient demographics, clinical and treatment characteristics of the 400 patients are summarized in Table 1. Patients were predominantly white (83%) and post-menopausal (64%) with a median age of 55 years (range: 26–95) at diagnosis. The majority of patients presented with invasive ductal carcinoma (93%) and grade 3 (61%) disease. Only ten (3%) patients were diagnosed with a lobular histology. One hundred and eighty-six (47%) were diagnosed with node-positive disease and over half received (neo) adjuvant anthracycline (53%). Most common preexisting cardiac risk factors reported in the study were a previous history of smoking (31%), dyslipidemia (33%), or any preexisting cardiac disorders (35%) (Table 1).
Table 1.
Baseline characteristics of patients
| Characteristics | Total (n = 400) |
|---|---|
| n (%) | |
| Age | |
| > 50 ys | 265 (66%) |
| < 50 ys | 135 (34%) |
| BMI | |
| > 30 | 144 (36%) |
| < 30 | 256 (64%) |
| Race | |
| White | 331 (83%) |
| African American | 48 (12%) |
| Other | 21 (5%) |
| Tumor size | |
| < 2 cm | 175 (44%) |
| > 2 cm | 225 (56%) |
| Nodal Status | |
| N0 | 214 (54%) |
| N+ | 186 (47%) |
| ER Positive | |
| Yes | 255 (64%) |
| No | 145 (36%) |
| Grade | |
| I | 15 (4%) |
| II | 137 (34%) |
| III | 245 (61%) |
| Missing | 3 (1%) |
| Pre-existing Cardiovascular risk factors | |
| Smoking | 124 (31%) |
| Hyperlipidemia | 133 (33%) |
| Hypertension | 71 (18%) |
| Diabetes Mellitus | 46 (12%) |
| Heart Failure | 8 (2%) |
| Ischemic Heart Disease | 20 (5%) |
| Arrhythmias | 21 (5%) |
| Valvular diseases | 20 (5%) |
| Adjuvant Systemic Therapy | |
| Trastuzumab | |
| Yes | 369 (92%) |
| No | 31 (8%) |
| Chemotherapy | |
| Anthracyclines | 212 (53%) |
| Non-Anthracyclines | 153 (38%) |
| No chemotherapy | 35 (9%) |
| Hormonal Therapy | |
| Yes | 250 (63%) |
| No | 150 (37%) |
A total of 106 (29%) patients experienced trastuzumab interruption at least once for any cause. The baseline demographic and tumor characteristics of the 369 patients who received (neo) adjuvant trastuzumab, categorized by completion or interruption of therapy, is provided in the supplementary table. Table 2 summarizes treatment interruption and subsequent rechallenges of trastuzumab during 1 year of planned adjuvant therapy. A total of 42 (11%) patients permanently stopped trastuzumab before completing planned therapy. Median duration of therapy in patients with any treatment interruption was 11.3 months, and 24 patients (23%) received less than 6 months of trastuzumab therapy. The most common reason for treatment interruption of trastuzumab was cardiotoxicity (n = 66, 62%). Treatment interruption resulting from neutropenia (n = 16, 15%) during (neo) adjuvant chemotherapy accounts for the majority of non-cardiac events in the study (Table 3). Over half the patients (55%) experiencing any treatment interruption were over 65 years of age. While a higher proportion of patients with treatment interruption reported any anthracycline use, this difference did not reach statistical significance (64% vs. 56%, p = 0.150). Seventy-seven (73%) patients experiencing treatment interruption were referred to a cardiologist with a median time from referral to appointment of nine (range: 0–72) days.
Table 2.
Discontinuation and Re-challenges of Adjuvant Trastuzumab Therapy
| Treatment | Number of Patients (%) |
|---|---|
| Initial Treatment | 369 |
| Completed planned therapy | 263 (71%) |
| First Discontinuation | 106 (29%) |
| Permanently Discontinued | 29 (27%) |
| First Re-challenge | 77 (73%) |
| Completed therapy | 55 (71%) |
| Second Discontinuation | 22 (29%) |
| Permanently Discontinued | 12 (16%) |
| Second Re-challenge | 10 (45.5%) |
| Completed therapy | 9 (90%) |
| Third Discontinuation | 1 (10%) |
| Permanently Discontinued | 1 (10%) |
| Total Permanently Discontinued | 42 (11%) |
Table 3.
Causes of Trastuzumab Discontinuation
| Adverse events | Total (n = 106) |
|---|---|
| n (%) | |
| Cardiomyopahy | 66 (62%) |
| Neutropenia | 16 (15%) |
| Disease Progression | 5 (5%) |
| Patient Preference | 4 (4%) |
| Thrombocytopenia | 3 (3%) |
| Shortness of Breath | 2 (2%) |
| Others | 10 (9%) |
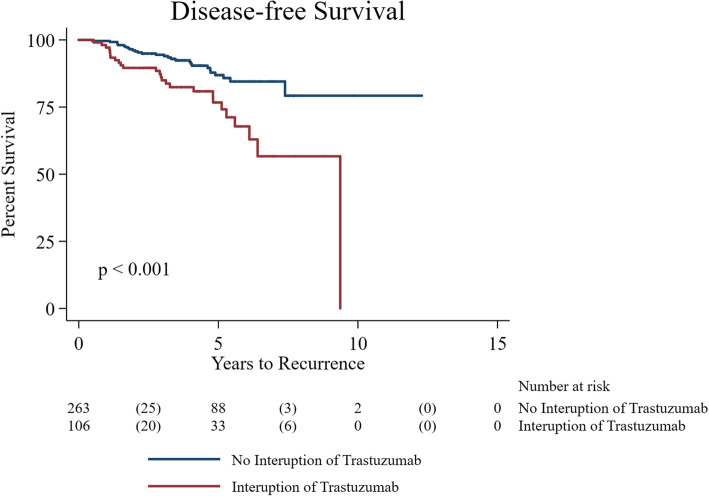
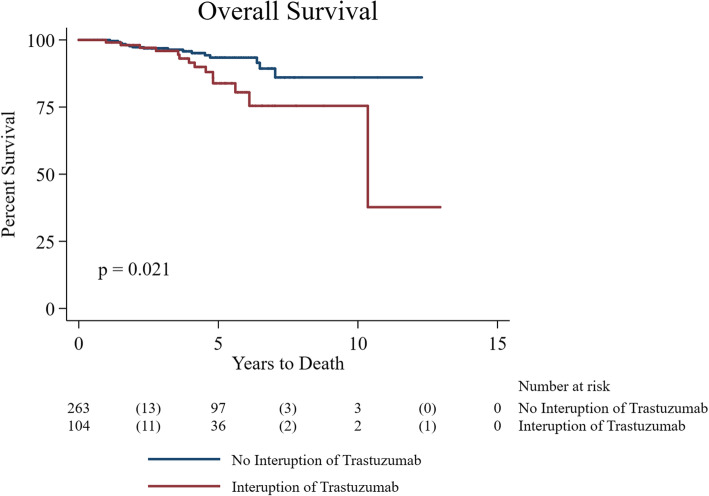
Unadjusted Log-rank tests revealed patients who experienced any interruption in trastuzumab therapy compared to those who had no interruption had worse DFS and OS (p = 0.001 and p = 0.021, respectively) (Figs. 1 and 2). Multivariable analyses confirmed significant worse DFS (aHR: 4.4, 95% CI: 1.8–10.5, p = 0.001) and OS (aHR: 4.8, 95% CI: 2.5–9.2, p < 0.001) after adjusting for age, stage, grade, ER, node status and TIC (Tables 4 and 5). A total of 208 (56%) patients received beta blockers (BB) and/or angiotensin converting enzyme inhibitor (ACEi) therapy prior to or after BC diagnosis. The use of cardioprotective therapy (BB and/or ACEi) was significantly higher in patients with known TIC (89% vs. 11%; p < 0.001). Patients receiving cardioprotective therapy showed worse OS compared to those not on cardioprotective therapy (HR: 2.7, 95% CI: 1.3–5.8, p = 0.010).
Fig. 1.
Kaplan Meier curve showing the disease free survival between patients on trastuzumab and patients who had interruption (unadjusted log-rank results)
Fig. 2.
Kaplan Meier curve showing the overall survival between patients on trastuzumab and patients who had interruption (unadjusted log-rank results)
Table 4.
Multivariate analysis of hazard ratio of DFS with trastuzumab discontinuation
| Variable | Adjusted Hazard Ratio | Std. Err. | 95% CI | P-value |
|---|---|---|---|---|
| Discontinuation Trastuzumab | 4.38 | 1.95 | 1.83–10.46 | 0.001 |
| Age | 0.99 | 0.02 | 0.97–1.03 | 0.750 |
| Stage, 1 or 2 vs Stage 3 | 3.62 | 1.59 | 1.53–8.59 | 0.003 |
| Grade, 1 or 2 vs Grade 3 | 1.39 | 0.56 | 0.63–3.06 | 0.416 |
| ER status, Negative vs Positive | 0.60 | 0.26 | 0.25–1.42 | 0.242 |
| Cardiomyopathy, Yes vs No | 0.25 | 0.14 | 0.08–0.75 | 0.014 |
Table 5.
Multivariate analysis of hazard ratio of OS with trastuzumab discontinuation
| Variable | Adjusted Hazard Ratio | Std. Err. | 95% CI | P-value |
|---|---|---|---|---|
| Discontinuation Trastuzumab | 4.76 | 1.59 | 2.47–9.16 | < 0.001 |
| Age | 0.99 | 0.01 | 0.97–1.01 | 0.302 |
| Stage, 1 or 2 vs Stage 3 | 2.45 | 0.74 | 1.36–4.41 | 0.003 |
| Grade, 1 or 2 vs Grade 3 | 1.69 | 0.53 | 0.91–3.14 | 0.094 |
| ER status, Negative vs Positive | 0.64 | 0.20 | 0.34–1.20 | 0.163 |
| Cardiomyopathy Yes vs No | 0.31 | 0.13 | 0.14–0.70 | 0.004 |
Discussion
This contemporary population-based study in 369 consecutive female patients receiving trastuzumab for early stage HER-2 positive BC between January 2005 and December 2015 showed that 106 (29%) patients experienced treatment interruption of ≥2 weeks, among whom 42 (11%) experienced permanent discontinuation of trastuzumab therapy, largely due to cardiac toxicity.
Evidence from randomized trials with adjuvant trastuzumab in combination with an anthracycline –taxane-based regimen have shown a 12–15% asymptomatic decline in LVEF with very few patients reporting permanent discontinuation [10, 11, 28–30]. For instance, secondary analyses of cardiac events in NSABP B31 showed a meager 4% discontinuation of adjuvant trastuzumab [11]. Careful selection of patients with limited to no medical comorbidities in clinical trials is imperative given the concern for long-term toxicity in a curative intent patient population. However, clinicians routinely have to make complex medical decisions weighing the potential benefit of HER2-directed therapy against risks of pre-existing comorbidities in everyday practice. In addition, minority and elderly patents are often underrepresented in therapeutic studies. Our study highlights this ‘real world’ disparity with a significantly higher proportion of patients with treatment interruption or permanent cessation of trastuzumab therapy for early HER2-positive BC, when only half received an anthracycline-based regimen. These results are consistent with other large retrospective series showing higher overall discontinuation of adjuvant trastuzumab in clinical practice, with Wang et al. reporting up to 41% of patients discontinuing planned therapy, most events as a result of TIC [31, 32]. However, a majority of patients reported by Wang et al. were elderly (mean age 71.6 years), emphasizing the need for further investigation of risk factors and risk mitigation strategies in this patient population [33].
Approximately 18% of all patients in the study experienced TIC leading to treatment delays. Consistent with previous studies, TIC was responsible for the majority of treatment interruption (62%) with adjuvant trastuzumab [31, 34, 35]. Shorter (< 6 months) planned duration of adjuvant trastuzumab has been studied in an attempt to reduce risk of CV events while maintaining efficacy of targeted therapy [23, 24, 36]. While 6 months of adjuvant targeted therapy is associated with favorable CV risk, two major phase III randomized adjuvant trials reported conflicting results on non-inferiority in invasive DFS with shorter duration to standard 1 year of therapy [23, 24]. Secondary analyses of US PHARE study revealed that metastases-free survival, a key endpoint, was significantly worse with 6 months vs. 1 year of therapy. However, long term outcomes, especially in patients experiencing treatment interruptions or discontinuation, are lacking from large prospective studies given the small number of events. Our analyses suggest any interruption in trastuzumab treatment as a prognostic factor with up to three times worse DFS and OS compared to patients who finished planned 1 year of therapy or had < 14 days of interruption in treatment. Multivariable analyses confirmed this effect after adjusting for major confounders, including drug-induced cardiotoxicity, indicating that worse outcomes are likely related to cancer recurrence in these patients.
Similar concerns have been reported with early trastuzumab interruption in other studies as well [31–33, 37]. Gong et al. reported higher risk of disease recurrence in patients stopping adjuvant trastuzumab and emphasized that early discontinuation is a stronger prognostic factor for worse survival than cardiotoxicity in this patient population. As in our study, they also reported a high proportion of patients completing therapy with over 85% patients receiving ≥16 doses [32]. Additionally, a retrospective analysis of 5547 patients who received adjuvant trastuzumab demonstrated a significantly higher risk of BC relapse and death in patients who did not complete adjuvant trastuzumab. The 5 year DFS and OS was 94 and 95%, respectively, in those who completed full treatment compared with 80% (HR 3.15; p < 0.001) and 84% (HR 2.12; p = 0.005), respectively, in patients who received treatment interruption in relation to a cardiac event within the treatment period [37, 38]. These results taken together suggest a strong need for early assessment and optimization of CV status of at-risk patients to minimize any interruption in targeted therapy.
Recent studies demonstrate the safety of continuing trastuzumab with mild cardiac dysfunction in the setting of collaborative management with cardiology. In the present study, TIC was defined based on the FDA prescription instructions [14], which recommend to hold therapy for at least 4 weeks in those experiencing a decline in LVEF ≥16% from baseline or if LVEF falls ≥10% below baseline and below the lower limit of normal. Trastuzumab can subsequently be restarted with resolution of cardiac dysfunction. However, since completion of this study, two = single arm prospective trials, SCHOLAR [39] and SAFE-HEaRt [22], have investigated the continuation HER2-targeted therapy while concomitantly starting cardioprotective therapy in patients who develop mildly reduced LVEF. Although larger and randomized trials are needed, both of these studies demonstrate safety data with lack of further deterioration in cardiac function in approximately 90% of cases. In line with this, ESMO has released new guidelines in 2020 [40] which recommend to consider continuation of trastuzumab with initiation of cardioprotective therapy as an option in patients with mild asymptomatic decline in LVEF (level of evidence III, grade of recommendation A). This is defined as an LVEF decrease ≥10% from baseline or a drop in LVEF to ≥40% but < 50%. Given the considerable negative impact of treatment interruption on breast cancer recurrence and survival as demonstrated in our study and others [31–33, 37], we recommend reconsideration of trastuzumab package insert criteria for drug interruption based on mild asymptomatic heart failure. Application of this clinical practice may be offered as an option in patients based on shared decision making between oncologist, cardiologist and patient of risks vs benefits in those who can receive close monitoring.
Cardioprotective therapy, including BB and ACEi, have a well-documented safety profile and are commonly used in practice to improve cardiac outcomes in patients with preexisting heart disease. Current evidence suggests that they may be crucial in preventing treatment interruption of adjuvant trastuzumab in HER2-positive BC patients [41, 42]. In a randomized, double-blinded placebo-controlled trial, Guglin et al. showed that cardiotoxicity-free survival was higher for both lisinopril (HR: 0.53;p = 0.015), and carvedilol (HR: 0.49; p = 0.009) compared to placebo with fewer treatment interruptions in the intervention group [41]. While we report worse OS in patients on cardioprotective therapy, this likely results from a higher proportion of patients receiving BB and/or ACEi therapy for other concurrent illnesses (72%) that may adversely impact survival.
The use of BB and ACEi prior to initiating anti-HER2 therapy needs to be carefully weighed against unintended consequences, such as rising health care costs, over-diagnosis or over-treatment associated with the increased use of cardiac surveillance and prophylactic therapy [43]. Close collaboration between oncologists and cardiologists is needed to guide personalized therapy for vulnerable patients [39, 44–46]. Our institutional guidelines encourage referral to cardio-oncology for at–risk patients prior to initiating trastuzumab therapy and early intervention in patients who experience LVEF decline while on targeted therapy. A majority of patients who experienced treatment interruption (73%) in this study were referred and received timely evaluation by cardio-oncology.
Another strategy to reduce cardiac risk has been to employ non-anthracycline regimens in (neo) adjuvant therapy [47, 48]. Our analyses did not show a significant difference in the use of anthracycline in patients who experienced any treatment interruption compared to controls. This is likely due to careful selection of patients deemed fit to receive adjuvant anthracycline. The advent of adjuvant TDM1 for patients with high risk residual disease and dual anti-HER2 therapies, such as combination with pertuzumab, will further impact the use of adjuvant anthracycline in these patients [49, 50].
Our study is not without limitations. While we have a detailed account of treatment data, reason for trastuzumab interruption/discontinuation, known pre-existing cardiac comorbidities, rechallenge rates and use of cardioprotective therapy, the study is limited by its retrospective nature, including possible selection bias that may impact outcome assessment. Dose titration was at the discretion of the treating clinicians. Additionally, it is a single-institution study with a limited number of events. Given the retrospective nature of chart review, it was challenging to identify time of initiation (before or after onset of TIC) and treatment indication (TIC vs. concurrent cardiac illness) for the use of BB and/or ACEi therapy in patients. This made it difficult to interpret the association of the use of cardioprotective therapy on the incidence of TIC and treatment interruption of trastuzumab. Lastly, our observational study sought to evaluate the association of treatment interruption in adjuvant trastuzumab with long-term cancer outcomes so as to augment patient care and survivorship, but cannot confirm causality.
Conclusion
In summary, treatment interruption or early discontinuation of trastuzumab therapy in early HER2-positive BC is associated with worse DFS and OS irrespective of age, receptor status, stage of disease and treatment-induced cardiotoxicity. The mechanisms and optimal timing of cardioprotective medications as well as best practices in treatment interruption in patients receiving adjuvant trastuzumab therapy requires further investigation in the modern era of cardio-oncology. Growing cooperation between oncologists and cardiologists can foster development of consensus-based guidelines for surveillance, prevention, and care of individuals initiating HER2-directed therapy in BC.
Supplementary Information
Additional file 1: Supplementary Table. Baseline characteristics of patients who received trastuzumab, categorized by completion vs interruption of therapy.
Acknowledgements
- The Ohio State University Center for Clinical and Translational Science grant support (National Center for Advancing Translational Sciences, Grant UL1TR002733).
- The authors would like to thank Alyson McGoldrick (Division of Medical Oncology, The Ohio State University) for her assistance in the preparation and editing of this manuscript
- The Stefanie Spielman Fund for Breast Cancer Research.
Abbreviations
- ACEi
Angiotensin converting enzyme inhibitor
- aHR
Adjusted Hazards Ratio
- BB
Beta blocker
- BC
Breast cancer
- DFS
Disease-free survival
- FISH
Fluorescence in situ hybridization
- HER2
Human epidermal growth factor receptor 2
- HR
Hormone receptor
- LVEF
Left ventricular ejection fraction
- OS
Overall survival
- TIC
Trastuzumab-induced cardiomyopathy
Authors’ contributions
Concept and design: Sagar Sardesai, Bhuvaneswari Ramaswamy. Data analysis and interpretation: Mahmoud Kassem, Evan Morgan, Marilly Palettas, Julie Stephens. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of work: All authors.
Funding
No funding.
Availability of data and materials
The data that support the findings of this study are available from The Ohio State University Information Warehouse but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of The Ohio State University IRB.
Ethics approval and consent to participate
This study was an IRB-approved (OSU 2017C0080) retrospective chart review and received a full waiver for patient’s consent.
Consent for publication
It is a retrospective chart review and we received a full waiver for patient’s consent.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information accompanies this paper at 10.1186/s40959-020-00081-9.
References
- 1.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 2.Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, et al. Pathologic findings from the National Surgical Adjuvant Breast and bowel project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8(1):103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 3.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Romond EH, Suman VJ, Jeong J-H, Sledge G, Geyer CE, Jr, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong J, Yan AT, Cramarossa G, Chan KK. Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Can J Cardiol. 2014;30(8):869–878. doi: 10.1016/j.cjca.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the north central Cancer treatment group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26(8):1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan-Chiu E, Yothers G, Romond E, Geyer CE, Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23(31):7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 12.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17(5):627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- 13.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drig Administration. Herceptin (trastuzumab) Prescribing Information (www.fda.gov) 2010 [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf.
- 15.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the Trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 16.Guarneri V, Lenihan DJ, Valero V, Durand J-B, Broglio K, Hess KR, et al. Long-term cardiac tolerability of Trastuzumab in metastatic breast Cancer: the M.D. Anderson Cancer center experience. J Clin Oncol. 2006;24(25):4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 17.Nowsheen S, Aziz K, Park JY, Lerman A, Villarraga HR, Ruddy KJ, et al. Trastuzumab in female breast Cancer patients with reduced left ventricular ejection fraction. J Am Heart Assoc. 2018;7(15):e008637. doi: 10.1161/JAHA.118.008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60(24):2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 19.Jawa Z, Perez RM, Garlie L, Singh M, Qamar R, Khandheria BK, et al. Risk factors of trastuzumab-induced cardiotoxicity in breast cancer: a meta-analysis. Medicine (Baltimore) 2016;95(44):e5195. doi: 10.1097/MD.0000000000005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewer MS, O'Shaughnessy JA. Cardiac toxicity of Trastuzumab-related regimens in HER2-overexpressing breast Cancer. Clinical Breast Cancer. 2007;7(8):22–29. [PubMed] [Google Scholar]
- 21.Tripathy D, Slamon DJ, Cobleigh M, Arnold A, Saleh M, Mortimer JE, et al. Safety of treatment of metastatic breast Cancer with Trastuzumab beyond disease progression. J Clin Oncol. 2004;22(6):1063–1070. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- 22.Lynce F, Barac A, Geng X, Dang C, Yu AF, Smith KL, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175(3):595–603. doi: 10.1007/s10549-019-05191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earl HM, Hiller L, Vallier A-L, Loi S, Howe D, Higgins HB, et al. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results. 2018. [Google Scholar]
- 24.Pivot X, Romieu G, Debled M, Pierga J-Y, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 25.Joensuu H, Fraser J, Wildiers H, Huovinen R, Auvinen P, Utriainen M, et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2–positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 2018;4(9):1199–1206. doi: 10.1001/jamaoncol.2018.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyawali B, Niraula S. Duration of adjuvant trastuzumab in HER2 positive breast cancer: overall and disease free survival results from meta-analyses of randomized controlled trials. Cancer Treat Rev. 2017;60:18–23. doi: 10.1016/j.ctrv.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell SD, Blackwell KL, Lawrence J, Pippen JE, Jr, Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and bowel project B-31 and the north central Cancer treatment group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–21. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 29.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, De Azambuja E, Procter M, Suter TM, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 30.Perez E, Suman V, Davidson N, Kaufman P, Martino S, Dakhil S, et al. Exploratory analysis from NCCTG N9831: do clinical and laboratory characteristics predict cardiac toxicity of trastuzumab when administered as a component of adjuvant therapy? Breast Cancer Res Treat. 2005;94(Suppl 1):S2038.
- 31.Neugut AI, Hillyer GC, Kushi LH, Lamerato L, Leoce N, Ambrosone CB, et al. Non-initiation and early discontinuation of adjuvant trastuzumab in women with localized HER2-positive breast cancer. Breast Cancer. 2014;21(6):780–785. doi: 10.1007/s12282-014-0543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong IY, Verma S, Yan AT, Ko DT, Earle CC, Tomlinson GA, et al. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population-based study. Breast Cancer Res Treat. 2016;157(3):535–544. doi: 10.1007/s10549-016-3823-y. [DOI] [PubMed] [Google Scholar]
- 33.Wang SY, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP, et al. Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival. Breast Cancer Res Treat. 2014;146(2):411–419. doi: 10.1007/s10549-014-3029-0. [DOI] [PubMed] [Google Scholar]
- 34.Anouti K, Rustam L, Anouti L, Gnall E, Burke J, Rodriguez R, et al. Left atrial intramural hematoma after percutaneous coronary intervention. J Am Coll Cardiol. 2020;75(11 Supplement 1):3294. doi: 10.1016/S0735-1097(20)33921-8. [DOI] [Google Scholar]
- 35.Yu AF, Yadav NU, Lung BY, Eaton AA, Thaler HT, Hudis CA, et al. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat. 2015;149(2):489–495. doi: 10.1007/s10549-014-3253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purmonen TT, Pänkäläinen E, Turunen JH, Asseburg C, Martikainen JA. Short-course adjuvant trastuzumab therapy in early stage breast cancer in Finland: cost-effectiveness and value of information analysis based on the 5-year follow-up results of the FinHer trial. Acta Oncol. 2011;50(3):344–352. doi: 10.3109/0284186X.2011.553841. [DOI] [PubMed] [Google Scholar]
- 37.Rushton M, Lima I, Tuna M, Johnson C, Ivars J, Pritchard K, Hawken S, Dent S. Impact of stopping trastuzumab in early breast cancer: a population-basedstudy in Ontario, Canada. J Natl Cancer Inst. 2020;28:djaa054. 10.1093/jnci/djaa054. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 38.Blaes AH, Dang C. Trastuzumab: weighing the benefits and the risks? J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leong D, Cosman T, Alhussein M, Tyagi N. Safety of Continuing Trastuzumab Despite Mild Cardiotoxicity: A Phase I Trial JACC. Cardio Oncol. 2019;1(1):1–10. doi: 10.1016/j.jaccao.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W, et al. Randomized trial of Lisinopril versus Carvedilol to prevent Trastuzumab Cardiotoxicity in patients with breast Cancer. J Am Coll Cardiol. 2019;73(22):2859–2868. doi: 10.1016/j.jacc.2019.03.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gujral DM, Lloyd G, Bhattacharyya S. Effect of prophylactic betablocker or ACE inhibitor on cardiac dysfunction & heart failure during anthracycline chemotherapy ± trastuzumab. Breast. 2018;37:64–71. doi: 10.1016/j.breast.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Levis BE, Binkley PF, Shapiro CL. Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol. 2017;18(8):e445–ee56. doi: 10.1016/S1470-2045(17)30535-1. [DOI] [PubMed] [Google Scholar]
- 44.Parent S, Pituskin E, Paterson DI. The cardio-oncology program: a multidisciplinary approach to the Care of Cancer Patients with Cardiovascular Disease. Can J Cardiol. 2016;32(7):847–851. doi: 10.1016/j.cjca.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Steingart RM, Yu AF, Dang CT. Trastuzumab cures Cancer and disrupts the practice of cardiology. JACC: CardioOncology. 2019;1(1):11–13. doi: 10.1016/j.jaccao.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Robert N, Eiermann W, Pienkowski T, Crown J, Martin M, Pawlicki M, et al. BCIRG 006: docetaxel and trastuzumab-based regimens improve DFS and OS over AC-T in node positive and high risk node negative HER2 positive early breast cancer patients: quality of life (QOL) at 36 months follow-up. J Clin Oncol. 2007;25(18_suppl):19647. doi: 10.1634/theoncologist.2013-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Waldron-Lynch M, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27–35. doi: 10.1016/j.ejca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Von Minckwitz G, Procter M, De Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table. Baseline characteristics of patients who received trastuzumab, categorized by completion vs interruption of therapy.
Data Availability Statement
The data that support the findings of this study are available from The Ohio State University Information Warehouse but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of The Ohio State University IRB.