Abstract

Microporous silica (MS) materials are a kind of an emerging and promising adsorbent precursor. MS prepared from vermiculite has the advantages of easy preparation, low cost, and low layer charge. In this study, organo-MS (OMS) modified by a typical gemini surfactant 1,2-bis(hexadecyldimethylammonio)ethane dibromide (G16) is first synthesized and proved to have effective retention capacity toward cationic dyes. Fourier transform infrared spectroscopy, TG-DTG, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and Brunauer–Emmett–Teller are used to explore the structural characters of adsorbents. Gradient adsorption of compound MS (MS and OMS) in a binary dye system [methylene blue (MB) and crystal violet (CV)] was investigated. In a single system, the relationship between the adsorption capacity and influencing factors (dye concentration, contact time, temperature, and pH), adsorption kinetics, isotherms, as well as thermodynamics was comprehensively compared to reveal the adsorption mechanism. The adsorption values of MB and CV on MS and OMS are 308 mg g–1 (R = 77.0%, 15 min) and 250 mg g–1 (R = 83.3%), respectively, which may be caused by various intermolecular interactions (electrostatic or hydrophobic interactions) between the dye and adsorbent surface. In a binary system, the improved first spectroscopy method is used to calculate the individual concentration of the dye in the binary system. The total removal efficiency of gradient adsorption reaches as high as 89.5% (MB) and 86.4% (CV). In addition, compound MS can be effectively regenerated by HCl solution for several cycles.
1. Introduction
Dyes have greatly enhanced human life in the fields of fashion, catering, real estate, transportation, and so forth. However, human beings and other organisms are suffering from serious environmental concerns due to the dye industry while enjoying good quality of life.1 According to reports, about 7 × 104 tons of organic dyes are directly released into the environment every year. Even in the presence of trace amounts, most of them can induce serious diseases.2 From the perspective of human health and environmental protection, these effluents must be pretreated before being discharged.3 Among the common effluent dyes, methylene blue (MB) and crystal violet (CV), shown in Figure 1, are two cationic dyes widely used in the industry.4,5 These cationic dyes are more toxic to anionic substances, so the purification methods for MB and CV are more stringent and instant.
Figure 1.
Chemical structures of surfactant (16-2-16), MB, and CV.
Most sewage in the environment is complex containing more than one pollutant, which increases the degree of difficulty for purification approaches. Among the popular treatment methods, such as precipitation, filtration, membrane separation, and adsorption,6−8 adsorption has the advantages of high efficiency, low cost, and convenient operation, which make it the best choice for dye removal. Previous studies have demonstrated the competitive adsorption effects of MB and CV in the binary system, in which the formation of MB-coated adsorbent aggregates inhibited CV removal. Moreover, as basic dyes of triphenylmethane type, CV has the same ionic charge with MB. The triangular structure of CV, however, is significantly different from linear structured MB, which impedes the dual removal of CV and MB in the binary system. Therefore, to avoid the competition effect is an urgent issue for the removal of MB and CV from water.9,10
It is important to find a suitable adsorption process for multicomponent systems.3 Gradient adsorption, as a new method for wastewater purification, has been proven to significantly improve the adsorption efficiency in binary systems. The gradient adsorption process is characterized by two steps, where the multicomponent system is purified by one kind of adsorbent to reach equilibrium and then treated by the other one to achieve the second adsorption equilibrium.11,12 Considering the prospect of practical applications, it is necessary to explore suitable value-added adsorbents.
For the sake of dye adsorbents, commercial activated carbon is popular for its notable porosity and huge surface area. The high price and strict operation conditions of activated carbon, however, limit its application at a large scale. With the evolution of nanotechnology, a variety of nanoadsorbents in different forms and dimensionalities (D) of nanoparticles (0D), nanofibers and nanotubes (1D), nanosheets (2D), and nanoflowers (3D) have been explored.13,14 Among them, ordered microporous silica (i.e. silica nanosheets) presents good physical properties to be used as an adsorbent, such as high specific surface area, controlled pore size, uniform layered structure, tunable morphology, and favorable surface properties for functionalization, and it is also a nontoxic and environmentally friendly material.15 Especially, silica nanosheets (MS) with large surface area, high silica purity, and exchangeable cations are easily functionalized through simple activation reactions, thus serving as an alternative adsorbent for organic pollutants.16
In recent years, popular modification methods of MS materials include grafting of metal oxides,17 amine functionalization,18 biopolymer functionalization19 surfactant modification,20 and so forth. An organic modification process creates a hydrophobic surface and an enlarged interlayer space, enhancing the affinity of the resultant toward hydrophobic organic pollutants.15 In addition, the combination of MS and specific modifiers (such as gemini surfactants with two hydrophobic heads) can further improve the adsorption performance. The modification process confers suitable hydrophobicity and functional groups on the resultant organic microporous silica (OMS), which could modulate the precursor property as well as reach a composite effect of the organic modifier MS precursor, resulting in enhanced adsorbents. Gemini surfactant-modified OMS has been successfully applied to remove a variety of organic pollutants.13 Therefore, MS as an admirable functional material is receiving more and more attention, which urgently needs to tap more potential applications.
The purpose of this work is to explore a new adsorption method that can effectively remove pollutants in the binary system. Gradient adsorption is adopted for researching the removal ability of compound adsorbents for the first time. MB and CV are chosen as target pollutants, and MS and OMS modified by G16 are employed as adsorbents. The obtained adsorbent is characterized by a series of analysis techniques, adsorption isotherms and kinetic models, and thermodynamic experiments. The improved first-order derivative spectra method is introduced to calculate the single dye concentration in a binary dye system. By using composite adsorbents and renewable adsorbents, costs can be greatly reduced in the subsequent adsorption industry. This study is expected to throw light on addressing the challenge about the treatment of multicomponent wastewater.
2. Results and Discussion
2.1. Characterization of the Adsorbents
2.1.1. FT-IR Analysis
Fourier transform infrared (FT-IR) spectra of G16, MS, and OMS are measured to obtain clear information about the basic chemical structure (Figure 2). In the case of G16, two bands that emerged at 2933 and 2873 cm–1 are related to the antisymmetric and symmetric stretching vibrations of C–H bonds.21 The bending vibration of C–H could be observed at 1478 cm–1. Notably, the peaks at 3425 and 1634 cm–1 are due to the water molecules adsorbed in G16. In the case of OMS-1 and OMS-2, the broad bands at 3425 and 1634 cm–1 are due to the stretching and bending vibrations of the hydrogen-bonded water molecules in the interlayer.21,22 The characteristic bands at 1077, 801, and 985 cm–1 correspond to the antisymmetric and symmetric vibration of Si–O and the bending vibration of Si–OH, respectively.23 In the OMS spectrum, three additional bands are observed at 2933, 2873, and 1478 cm–1, corresponding to the antisymmetrical, symmetrical tensile, and flexural vibrations of the C–H bond, respectively.23 All these results indicate that the modifiers have successfully adsorbed on the MS surface or intercalated into the MS interlayer.
Figure 2.
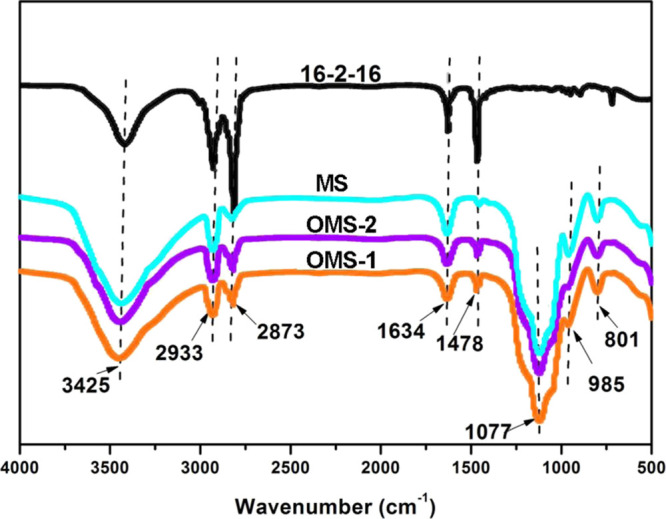
FT-IR spectra of 16-2-16 and adsorbents.
2.1.2. TG-DTG and Elemental Analysis
The thermal stabilities of the prepared MS and OMS are studied by TG-DTG analysis, and the results are shown in Figure 3. Two obvious peaks (57 and 59 °C) at the first stage (T < 200 °C) resulted from desorption of physically adsorbed water.24 Two other mass loss peaks (271 and 391 °C) of OMS are shown in Figure 3b, which are attributed to the elimination of the surface and/or interlayer to physically adsorb G16. In addition, the loading amount of the modifier can be obtained according to the weight losses, and the modifier utilization rate of OMS-1 (89.9%) and OMS-2 (44.9%) can be calculated.
Figure 3.

TG (a) and DTG (b) curves of MS, OMS-1, and OMS-2.
After modification, the carbon content in OMS significantly increased compared to untreated MS (Table S1). The increase in hydrogen and nitrogen contents observed in OMS also indicates that the cationic surfactant molecules have been successfully inserted into MS, which is in contrast to FT-IR results.
2.1.3. SEM and XRD Analysis
The surface morphologies of three adsorbents are investigated using scanning electron microscopy (SEM) images (Figure 4). The morphological structure of MS presents as a typical lamellar structure with small fragments and thick layers stacking tightly (Figure 4a). Compared with the regular, flat layered structure of the original MS, OMS shows a fractured pleated layered structure, which illustrates the successful modification process. Interactions between adjacent G16 on the OMS surface could aggregate into a curled and crumped surface25 Meanwhile, the compact packing of G16 leads to the aggregation of neighboring OMS into a large and irregular shape [as shown in transmission electron microscopy (TEM) images].
Figure 4.
SEM and TEM images of MS (a,d), OMS-1 (b,e), and OMS-2 (c,f).
X-ray diffraction (XRD) patterns can provide information about the internal structure of the adsorbent. MS is similar to amorphous silica, and no obvious diffraction peaks (2θ from 0.5 to 10°) are observed on the low-angle pattern. This may be related to the exchange of cations and hydrated protons between the interlayers.26 Similar to MS, an inconspicuous diffraction peak is observed in that case of OMS (Figure S1).
2.1.4. BET Analysis
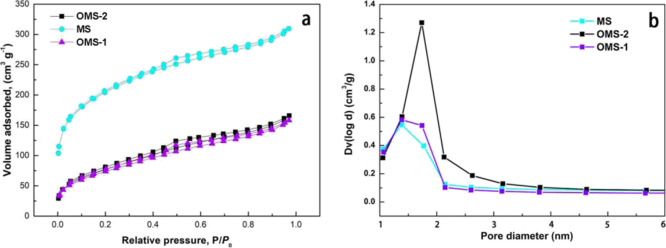
The nitrogen adsorption–desorption isotherms (Figure 5a) are recorded to obtain the specific surface area, pore volume, and pore size of MS and OMS. The results show a typical type IV adsorption–desorption isotherm.27 As can be seen from Table 1, the surface area of MS (640 m2 g–1) is remarkably larger than that of OMS (OMS-1: 230 m2 g–1; OMS-2: 241 m2 g–1); this is due to the introduction of G16 in MS, which occupies the empty space, resulting in the obstruction of N2 molecules into the framework.28 As shown in the pore size distribution curves of MS and OMS (Figure 5b), a narrow pore distribution is finite at around 1.5 nm, locating within the range of microporous size. During the modification process, G16 molecules not only intercalate into the MS interlayer but also adsorb on the exterior surface, leading to the occupation of available pores to some degree. Moreover, the wrinkles and cracks on the surface of OMS could also contribute to its larger pore size compared with MS.28
Figure 5.
Nitrogen sorption isotherms (a) and particle size distribution (b) of MS, OMS-1, and OMS-2.
Table 1. Porous Features of MS, OMS-1, and OMS-2a.
| sample | SBET/m2 g–1 | Vtp/cm3 g–1 | Dp/nm |
|---|---|---|---|
| MS | 640 | 0.324 | 1.1 |
| OMS-1 | 230 | 0.167 | 1.7 |
| OMS-2 | 241 | 0.186 | 1.6 |
SBET/m2 g–1—specific surface area, Vtp/cm3 g–1—total pore volume, and Dp/nm—average pore diameter.
2.2. Single System Adsorption Tests
2.2.1. Effect of Contact Time
Figure 6a,b shows the performances of MS, OMS-1, and OMS-2 for removing MB and CV in the single system as a function of contact time. The adsorption efficiency goes up quickly in the first 5 min, which is attributed to the abundant active sites for combining with dye molecules.29 However, the adsorption has little change when it exceeds 15 min, which owes to the occupation of most of the available adsorption sites by the dye molecules, leaving the adsorbates to exist in solution at a steady state. Notably, from the perspective of cost and reaction equilibrium, a contact time of 30 min is maintained for all the following adsorption experiments.
Figure 6.
Effects on contact time and pH on the adsorption of MB (a,c) and CV (b,d).
2.2.2. Effects of Dye Concentration and Dosage of Surfactants
Batch adsorption of MB and CV with different initial concentrations on MS and OMS is conducted in the single system. For comparison, the removal of dyes by all studied adsorbents is presented in Figure S2. As shown in Figure S2, the adsorption of MB onto MS and OMS increases progressively with the initial concentration increase. High removal efficiency and adsorption capacity are closely associated with plenty of adsorption sites within the adsorbent. The removal efficiency reduces gradually as the initial MB concentration increases, until a saturation of available active sites is occupied by MB molecules.29 Interestingly, the adsorption capacity of pristine MS is higher than OMS-1 and OMS-2, indicating that (i) interactions between MB and the MS surface are stronger than those between G16 and the MS surface and (ii) there exists a competition effect between MB and G16 onto the MS surface; that is, the adsorption of MB molecules is mainly through electrostatic interactions. According to the abovementioned results, 400 mg L–1 of MB is picked as the optimal value in the following experiments.
The adsorption of CV on MS and OMS exhibits similar trends with MB, but the removal of CV by MS is significantly lower than OMS-1 and OMS-2, which may be due to the weak hydrophobic interactions between CV and the hydrophilic MS surface. The adsorption capacity of OMS-1 toward CV is comparable with OMS-2, which manifests that (i) the retention of CV is mainly through the partition process derived from the alkyl chain of G16 instead of electrostatic interactions and (ii) the steric hindrance of CV molecules plays a non-negligible role in the adsorption process. The tighter storage of surfactants within MS can partly impede the attachment of adsorbates, which is in accordance with Brunauer–Emmett–Teller (BET) results. Therefore, 300 mg L–1 of CV is picked as the optimal value in the following experiments.
2.2.3. Effect of Solution pH
Solution pH plays a major role in the adsorption of dye contaminants, which may impact both the aqueous environment and surface binding sites of the adsorbents. As shown in Figure 6c, with the increment of the pH value, the negatively charged surfaces of MS and OMS (indicated by zeta potentials in Table S2) provide ideal affinity toward the cationic MB molecules. Interactions between −N+ of MB and negatively charged adsorbents induce larger absorbing ability. From the results shown in Figure 6c, a pH of 10 is regarded as the optimal value for MB removal.
For CV, the surface zeta potentials of adsorbents (Table S2) are more negative with the pH increase. The optimal adsorption of CV on MS (pH of 4) and OMS (pH of 8) occurred at different conditions. Vibrations of the adsorption capacity resulted from the change of CV species and the number of OH– or H+ in solution.30,31 pH 8 is considered the suitable experimental condition for CV retention.
2.2.4. Adsorption Kinetics
Kinetic models of pseudo-second order and pseudo-first order are applied to estimate the adsorption rate and the essence of interactions between dye molecules and adsorbents (Figure S3).32 The calculated kinetic parameters of MB and CV are presented in Table 2. It is found that R2 of the pseudo-second order model all exceed 0.99, and the calculated values of qe are closer to experimental values than the pseudo-first order model, indicating that the pseudo-second order kinetic model can fit well with the adsorptions of MB and CV, which implies the concentration of the functional site in adsorbents as the decisive factor on the adsorption. Moreover, it also demonstrates that the rate-limiting step may involve in valence forces by exchanging or sharing electrons between dyes and adsorbents.33,34
Table 2. Kinetic Parameters for Adsorption of MB and CV on MS, OMS-1, and OMS-2 (V = 50 mL, Adsorbent Mass = 0.05 g, T = 25 °C).
| pseudo-first-order |
pseudo-second-order |
intra-particle diffusion |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| dyes | adsorbents | qe (mg g–1) | k1 (×10–2) (min–1) | R2 | qe (mg g–1) | k2 (×10–2) (g (mg min)−1) | R2 | kid | C (102) | R2 | SSE |
| MB | MS | 27.03 | 2.94 | 0.7634 | 190.48 | 0.69 | 0.9999 | 5.2 | 1.45 | 0.9799 | 40.36 |
| OMS-1 | 29.17 | 2.82 | 0.7611 | 125.16 | 0.60 | 0.9996 | 25.85 | 0.38 | 0.9423 | 15.71 | |
| OMS-2 | 47.80 | 3.30 | 0.9397 | 141.64 | 0.32 | 0.9997 | 22.55 | 0.45 | 0.9147 | 17.95 | |
| CV | MS | 16.57 | 2.28 | 0.8434 | 99.90 | 1.03 | 0.9997 | 0.49 | 0.92 | 0.9756 | 1.36 |
| OMS-1 | 31.90 | 2.47 | 0.6539 | 247.52 | 0.66 | 0.9999 | 4.78 | 2.19 | 0.9871 | 0.40 | |
| OMS-2 | 35.11 | 2.88 | 0.6458 | 272.48 | 0.59 | 0.9999 | 8.25 | 2.24 | 0.9788 | 1.03 | |
Moreover, obvious division of the intraparticle diffusion model by three linear segments (the diffusion, gradual adsorption, and equilibrium stages, respectively) verifies the participation of the diffusion process (Figure 7). Higher values of kid for MB than CV indicate faster diffusion due to its smaller molecular size. A significant role of intraparticle diffusion is indicated by the high correlation coefficient (R2) and low SSE values. Moreover, the larger the boundary layer (C), the higher the adsorption amounts of dye adsorbed. The thicker C of CV than MB may be caused by its larger molecular size, less steric effect, and stronger aggregations of CV than MB molecules.
Figure 7.
Intraparticle diffusion plots of MB (a) and CV (b) onto MS, OMS-1, and OMS-2.
2.2.5. Adsorption Isotherms
The adsorption isotherms for the adsorption of MB and CV are studied in order to further explore the adsorption behavior of dyes onto adsorbents. Three typical adsorption isotherms Langmuir, Freundlich, and Redlich–Peterson (R–P) models are utilized to fit the experimental data (Supporting Information).35 It is noticed that all R2 of R–P models for MB and CV are higher than 0.99 (Table 3), which are the highest among the three isotherm models, indicating a suitable fit by the model applied. The g values of R–P models are close to unity for CV, suggesting that the removal process is prone to an ideal Langmuir model (monolayer adsorption). In the case of MB, however, higher g values indicate the coexistence of monolayer and multilayer adsorption in the adsorption processes, which may be due to the π–π stacking interaction between linear MB molecules.36,37
Table 3. Adsorption Isotherm Parameters of MB and CV Adsorbed on MS, OMS-1, and OMS-2 (V = 50 mL, Adsorbent Mass = 0.05 g, T = 25 °C).
| Langmuir |
Freundlich |
R–P |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| dyes | adsorbents | qmax (mg g–1) | KL (L mg–1) | R2 | KF (mg g–1) | n | R2 | A (L g–1) | B (×10–4) (L mg–1) | g | R2 |
| MB | MS | 532.51 | 0.016 | 0.9879 | 19.36 | 1.60 | 0.9533 | 6.61 | 2.97 | 1.79 | 0.9978 |
| OMS-1 | 372.30 | 0.010 | 0.9757 | 12.74 | 1.74 | 0.9519 | 3.00 | 5.50 | 1.50 | 0.9924 | |
| OMS-2 | 411.79 | 0.008 | 0.9842 | 10.40 | 1.61 | 0.9557 | 2.68 | 5.40 | 1.90 | 0.9922 | |
| CV | MS | 279.56 | 0.004 | 0.9892 | 3.75 | 1.55 | 0.9737 | 0.99 | 17.2 | 1.12 | 0.9899 |
| OMS-1 | 394.06 | 0.035 | 0.9556 | 45.63 | 2.47 | 0.8975 | 13.11 | 26.6 | 1.04 | 0.9950 | |
| OMS-2 | 393.63 | 0.045 | 0.9651 | 59.48 | 2.79 | 0.9229 | 15.56 | 26.3 | 1.08 | 0.9980 | |
2.2.6. Adsorption Thermodynamics
Adsorption thermodynamics can help understand the influences of temperature and relevant mechanisms in adsorption; batch experiments concerning MB and CV at 298, 308, 318, and 328 K are conducted, and thermodynamics parameters are calculated, including Gibbs free energy (ΔG°), standard entropy (ΔS°), and enthalpy (ΔH°), and all values are presented in Table 4. Negative ΔS° confirms the decreasing randomness of reaction molecules, and negative ΔH° suggests the exothermic nature of the adsorption process for the contaminants.38 Negative ΔG° values demonstrate that the adsorption of CV on OMS is spontaneous, meanwhile the ΔG° values of crude MS in removing MB indicate that the spontaneity is shifty.39 Considering the enthalpy values, physisorption is a key character in all adsorption processes.40
Table 4. Thermodynamic Parameters for the Adsorption of MB and CV on MS, OMS-1, and OMS-2 (V = 50 mL, Adsorbent Mass = 0.05 g).
| ΔG° (kJ mol–1) |
|||||||
|---|---|---|---|---|---|---|---|
| dyes | adsorbents | ΔS° (J mol–1 K–1) | ΔH° (kJ mol–1) | T = 298 K | T = 308 K | T = 318 K | T = 328 K |
| MB | MS | –51.11 | –13.18 | 2.05 | 2.56 | 3.07 | 3.58 |
| OMS-1 | –61.90 | –21.58 | –3.13 | –2.51 | –1.89 | –1.27 | |
| OMS-2 | –65.67 | –22.77 | –3.20 | –2.54 | –1.88 | –1.23 | |
| CV | MS | –54.04 | –16.91 | –0.81 | –0.26 | 0.28 | 0.82 |
| OMS-1 | –55.52 | –14.94 | 1.61 | 2.16 | 2.72 | 3.27 | |
| OMS-2 | –49.41 | –13.15 | 1.57 | 2.09 | 2.56 | 3.07 | |
2.3. Gradient Adsorption of the Binary System by Compound MS
MS has the best removal efficiency for MB, whereas two OMS have similar adsorption capacity for CV, so crude MS and OMS-1 are chosen to treat MB and CV solutions from the view of economy and efficiency. In the binary system (MB: 400 mg L–1, CV: 300 mg L–1), pristine MS has a poor removal ability for CV, while OMS shows low adsorption capacity toward MB. Several experiments are carried out using compound adsorbents (the masses of raw MS and OMS-1 are 0.025 g, respectively), and the results display no significant advantage over single system adsorption (the removal efficiency of MB is 80.4%, and the removal efficiency of CV is 53.9%); this is the reason why gradient adsorption is employed for the purification of binary system effluents.
The binary system with a same initial concentration of dye is treated by crude MS primarily, and the results display that the adsorption efficiency of MB is 85.3% and that of CV is 19.0%. After the uptake by OMS-1 (Figure 8), the total removal efficiency of MB reaches up to 89.5 and 86.4% for CV, which are greater than the results of single system treatment. Obviously, the concentration of MB has a great effect on the removal efficiency of CV, declaring that the interactions between MB and adsorbents are stronger than those of CV when limited absorption sites available, which is also partly due to the difference in the dye molecular size. Totally speaking, gradient adsorption is feasible and effective in binary system treatment.
Figure 8.
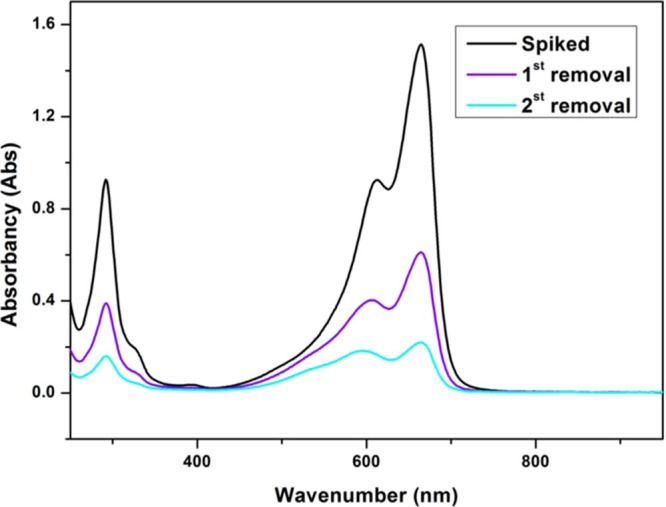
Change of UV–vis spectra of the binary-component system before and after gradient adsorption.
Moreover, in order to better understand the superiority of SiNS-based adsorbents, Table 5 lists the comparison about adsorption performances in the same binary dye system among that and other adsorbents in previous studies. Comprehensively, considering the adsorption capacity and equilibrium time, it can be observed that MS-based adsorbents have comparatively excellent removal ability for dyes versus other adsorbents, consuming less time, and the cost of MS is relatively low.
Table 5. Comparison of Adsorption Performances among MS, OMS, and Other Adsorbents.
| adsorbents | adsorption for MB (mg g–1) | adsorption for CV (mg g–1) | time (min) | references |
|---|---|---|---|---|
| CaO/g-C3N4 based nanocomposite | 612.78 | 458.62 | 240 | (41) |
| H-PAN-ETA membrane | 100 | 20 | (42) | |
| polymer–clay hybrid composite | 155.85 | 137.77 | 250 | (43) |
| Sn (O, S)-NPs-AC | 109.17 | 115.34 | (44) | |
| MS & OMS-1 | 358.0 & 38.1 | 259.2 & 259.2 | 15 & 15 | this study |
2.4. Adsorption Mechanism
As can be seen from Table S2, all zeta potentials of adsorbents reduced as a function of pH. The forms of dyes and adsorbents in lower pH surroundings caused repulsive interaction which plays a dominating role in disturbing their large bonding; therefore, negatively charged surface of adsorbents in basic environments can supply stronger affinity toward cationic dyes due to electrostatic interaction, such as −N+ in MB, and CV will be preferably bonded on negatively charged surfactants in higher pH environment. The change of removal capacity of CV with pH is chiefly due to its zwitterionic character; the negatively charged pristine MS has larger surface area in combination with cationic MB by electrostatic interaction, but it shows low removal capacity toward CV due to the stronger hydrophobicity of CV. The surface area of OMS decreases due to its partly occupied mesopores by 16-2-16, resulting in lower adsorption ability toward MB, but the enhancement of hydrophobicity facilitates higher removal for CV.
FT-IR spectra of the spent adsorbents are depicted in Figure S4. Compared with MS and OMS, additional peaks at 1399, 1343, and 1605 cm–1 (or 1367 and 1594 cm–1) symbolize the stretching vibrations of tertiary amine and aromatic C=C in MB (or aromatic tertiary amine and aromatic C=C in CV).45 A shift of −OH stretching vibration from 3425 to 3432 cm–1 indicates the existence of OH−π interaction.46 CH−π interactions are verified by the shift of −CH2 bending vibrations from 1478 (OMS) to 1492/1484 cm–1 (compound MS/MB or compound MS/CV).
SEM–EDS images of compound MS/MB, compound MS/CV, and compound MS/MB/CV are visualized in Figure S5. The mappings of the N element are denser in compound MS/CV, in agreement with the higher adsorption amounts of CV than MB. Similar S distribution (derived from MB molecules) is observed at the surfaces, verifying the coverage of MB on the adsorbent surfaces due to π–π stacking interaction provided by adsorbed MB or CV molecules.
As a consequence, the electrostatic interaction takes a position on MB removal; hydrophobicity interaction has a great impact on CV adsorption, and H-bond together with van der Waals forces shows a notable effect in the adsorption.
2.5. Desorption Tests
For a large-scale industrial application, stability and reusability are significant characters for ideal adsorbents; MS and OMS-1 are used to test the ability of consecutive adsorption–desorption for 3 cycles. The total MB adsorption decreased from 358.0 to 340.4, 332.1, and 330.8 mg g–1 according to cycle 0, 1, 2, and 3. In case of CV, the adsorption capacity varied from 259.2 to 240.5, 229.1, and 223.2 mg g–1, respectively (Figure S6). Although the adsorption efficiency dropped as regeneration cycles increase, there still exists high removal efficiency of compound MS for MB (R = 82.7%) and CV (R = 74.4%) after the third regeneration by 0.1 mol L–1 HCl solution. The results reveal that the compound adsorbents were reversible by simultaneously removing mixed dyes from water with high efficiency; the decreased efficiency is because of the destruction of the structure of the adsorbents and the residue of dyes adsorbed. Based on the above results, compound adsorbents are proved to be renewable for multicomponent dye removal.
3. Discussion
To correlate the structure of MS-based adsorbents with specific targeted contaminants, to verify the applicability of the improved first-order derivative spectra method in the multicomponent system, and to provide guidance for gradient adsorption, experiments are specifically designed from the aspects of (i) modifier and precursor selection, (ii) adsorbate system and detection, (iii) adsorption and regeneration processes, and (iv) adsorption mechanisms.
Modification of MS with G16 endows a more curled and aggregated surface of the resultant, contributing to decreased surface areas and increased pore sizes. Combining the modifier with MS is mainly through two ways: the one is physical adsorption on the MS surface and the other is intercalation into the MS interlayer spaces through ion-exchange reactions.
In the single adsorption system, MS and OMS exhibit rapid adsorption characters in equilibrium within 30 min. Interestingly, MS and OMS present different affinity toward MB and CV. The functionalization of G16 impedes MB adsorption through a competition effect between the modifier and MB. MB retention is mainly affected by the solution concentration and pH values, with the process obeying pseudo-second order, R–P isotherm, and exothermic nature. Moreover, the adsorption of MB is described well by the intraparticle diffusion model, where the diffusion process plays an important role in MB retention. Results indicate that MB adsorption is mainly through electrostatic interactions with the MS surface. In the case of CV, the existence of G16 enhances the partition process, which is a benefit for the adsorption of CV. The steric hindrance of CV, however, enlarges the boundary layer and becomes a non-negligible factor for adsorption. Pseudo-second order and R–P models are applied to fit CV adsorption.
Due to different affinity of MS and OMS toward MB and CV, gradient adsorption is specifically designed for binary dye systems. The improved first derivative spectra method is applicable to determine the single concentration in the multicomponent system. The total removal efficiency of MB reaches up to 89.5 and 86.4% for CV, which are higher than that in the single system (the removal efficiency of MB is 80.4%, and the removal efficiency of CV is 53.9%). Moreover, the MS-based adsorbents could be regeneration for at least three cycles. Totally speaking, gradient adsorption is feasible and effective for binary system treatment.
4. Conclusions
MS together with OMS modified by G16 was used to get rid of MB and CV in single and binary dye systems, separately. The results show that in this process, single-layer and multilayer adsorptions coexist, and electrostatic and hydrophobic interactions play a leading role in sewage clarity. All the experimental data about dyes in this study are adsorbed according to the pseudo-secondary model, which fits well with the R–P model and shows a natural exotherm. Gradient adsorption and the improved first order derivative spectra method for concentration calculation in the binary dye system were employed successfully. Raw MS and OMS-1 showed removal capacities of 308 mg g–1 (77.0%) and 250 mg g–1 (83.3%) for MB and CV. When it comes to the binary system, the total removal efficiency of compound MS increased up to 89.5% (MB) and 86.4% (CV), and MB had stronger affinity toward studied adsorbents. Comprehensively considering the adsorption capacity and equilibrium time, MS-based adsorbents have comparatively excellent removal ability for dyes versus other adsorbents, consuming less time, and the cost of MS is relatively low. The excellent adsorption capacity (3 cycles) of the regenerated adsorbent further proves the low cost and high efficiency of MS. More importantly, this study proved that gradient adsorption can be successful and effectively purify the sewage of the binary pollutant system.
5. Experimental Section
5.1. Materials
Vermiculite (Vt) supplied from Sigma-Aldrich and hydrochloric acid (HCl, 36–38%) from Beijing Chemical Works were used to prepare MS. 1,2-Bis(hexadecyldimethylammonio)ethane dibromide (G16) was prepared according to the previous literature.47 MB and CV were purchased from Beijing Chemical Works. Deionized water (18 MΩ·cm) was used throughout the experiments.
5.2. Preparation of MS and Organo-MS
MS is prepared through acid leaching of Vt. 5 g of Vt is dispersed into 200 mL of HCl solution (3 mol L–1) and reacted for 6 h under the condition of 60 °C and 180 rpm. The final MS could be obtained by centrifuging the supernatant, washing with deionized water, and drying overnight.
Detailed procedures of modifying MS are presented as follows: 1 g of MS is combined with a certain dosage of modifier (0.5 and 1 g of G16, respectively) after separately dissolving in water and shaking (200 rpm) for 1 h. After mixing MS and G16 solution, reacting for 3 h under the same condition, the products can be obtained by centrifuging and removing the supernatant, washing with deionized water, and drying to a constant weight. The resultant OMS is labeled as OMS-1 (0.5 g of G16) and OMS-2 (1 g of G16).
5.3. Characterization of Adsorbents
FT-IR spectroscopy was performed on a Nicolet Magna 560 E. S. P. spectrometer at a resolution of 4 cm–1 (4000–400 cm–1). XRD patterns (Shimadzu XRD-600) were collected under Cu Kα radiation at 40 kV and 40 mA, scanning from 0.5 to 10° (2θ). Scanning electron microscopy (SEM, SU8010, Japan) and elemental analysis (Vario MACRO cube) were conducted; thermogravimetric analysis (TG, METLER TOLEDO) was performed in a temperature over 25–800 °C with a heating rate of 10 °C min–1 under a nitrogen atmosphere. The specific surface areas of samples were acquired by a Quadrasorb-IQ-2-XR surface area analyzer by the BET method under a relative pressure (P/P0) ranging from 0.05 to 0.15. Zetasizer Nano ZS was used to measure zeta potential values of the resultant absorbents. All the samples tested were dried at 60 °C to constant weight.
5.4. Single System Adsorption Tests
Adsorption experiments in the single system were carried out as following steps: 0.05 g of adsorbent was mixed with 50 mL of dye solution and shaken at 200 rpm for 2 h (under different temperatures). The absorbance of dye solution was measured by a UV–vis spectrophotometer after centrifugation, and the adsorption capacity of the adsorbent was calculated through the absorbance.48
The adsorption kinetics, isotherms, and thermodynamics were conducted and calculated by changing the initial reaction time, concentration, and temperature, respectively.49−53
5.5. Gradient Adsorption for the Binary System
In order to acquire the single adsorbate concentration in the binary system, the improved first-order derivative spectra method was applied in this research.54 It can be seen from Figure 9 that the adsorption spectra of MB and CV solution are partially overlapped, which is inconvenient to determine the solute concentration separately. In their first-order derivative spectra, the absorbance of MB can be determined at 677 nm in the presence of CV, where CV exhibits approximately zero absorbance at this region. At 509 nm, CV accurately with minimum interference corresponding to MB can be quantified. As the first-order derivative spectra of CV is zero at 677 nm, its absorbance could be obtained via calculating the difference of absorbance between MB and the mixture at 677 nm.28 Therefore, the absorbance of MB (CV) at 677 nm (509 nm) was selected to determine the concentration of single dye in the binary system. The absorbance of mixed dyes was acquired by a UV–vis spectrophotometer, and the absorbance of solution was computed by the equations presented below
| 1 |
| 2 |
where A(509nm), AMB(509nm), and ACV(509nm) stand for the total absorbance of the binary system and MB and CV solution at 509 nm, respectively; A(677nm) and AMB(677nm) correspond to the total absorbance of the binary system and MB solution at 677 nm, respectively.
Figure 9.
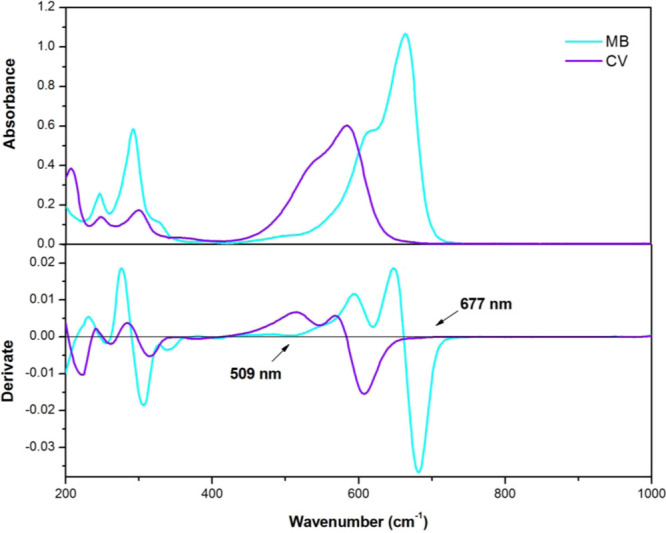
UV–vis spectra and the first derivate spectra of MB and CV.
Procedures of gradient adsorption
0.1 g of MS is added into 100 mL of dye mixture; the condition (T = 25 °C, pH = 10) of solution is adjusted to the maximum adsorption value for MB. The absorbances of the supernatant are recorded at 677 and 509 nm. 50 mL of supernatant is taken out and adjusted to the optimized value for the removal of CV, into which 0.05 g of OMS is added for the second adsorption stage. The supernatant absorbance is measured at 677 and 509 nm.
5.6. Regeneration Tests
The spent adsorbents are mixed with 0.1 mol L–1 HCl solution and then agitated (180 rpm) under room temperature for 0.5 h, with a mass ratio of solid to liquid of 1:100.
Acknowledgments
This research was funded by the Wuhan University of Technology–Tibet University Joint Innovation Fund, grant number LZJ2020003, the Reform and Development Funds for Local Region Universities from China Government in 2020, grant number ZCKJ 2020-11, and the National Natural Science Foundation of China, grant number 11765019.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04437.
SEM images of MS, OMS-2, and OMS-1; XRD patterns of MS, OMS-1, and OMS-2; effect of initial concentration on the removal of MB and CV onto three adsorbents; pseudo-second-order plots of MB and CV onto MS, OMS-1, and OMS-2; elemental analysis data of MS, OMS-1, and OMS-2; and zeta potentials (mV) of MS, OMS-1, and OMS-2 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Thue P. S.; Sophia A. C.; Lima E. C.; Wamba A. G. N.; de Alencar W. S.; Dos Reis G. S.; Rodembusch F. S.; Dias S. L. P. Synthesis and characterization of a novel organic-inorganic hybrid clay adsorbent for the removal of acid red 1 and acid green 25 from aqueous solutions. J. Cleaner Prod. 2018, 171, 30–44. 10.1016/j.jclepro.2017.09.278. [DOI] [Google Scholar]
- Zheng N.-C.; Wang Z.; Long J.-Y.; Kong L.-J.; Chen D.-Y.; Liu Z.-Q. Shape-dependent adsorption of CeO2 nanostructures for superior organic dye removal. J. Colloid Interface Sci. 2018, 525, 225–233. 10.1016/j.jcis.2018.03.087. [DOI] [PubMed] [Google Scholar]
- Chen B.; Zhao H.; Chen S.; Long F.; Huang B.; Yang B.; Pan X. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem. Eng. J. 2019, 356, 69–80. 10.1016/j.cej.2018.08.222. [DOI] [Google Scholar]
- Mouni L.; Belkhiri L.; Bollinger J.-C.; Bouzaza A.; Assadi A.; Tirri A.; Dahmoune F.; Madani K.; Remini H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. 10.1016/j.clay.2017.11.034. [DOI] [Google Scholar]
- Du C.; Song Y.; Shi S.; Jiang B.; Yang J.; Xiao S. Preparation and characterization of a novel Fe3O4-graphene-biochar composite for crystal violet adsorption. Sci. Total Environ. 2020, 711, 134662. 10.1016/j.scitotenv.2019.134662. [DOI] [PubMed] [Google Scholar]
- Trubetskaya A.; Kling J.; Ershag O.; Attard T. M.; Schröder E. Removal of phenol and chlorine from wastewater using steam activated biomass soot and tire carbon black. J. Hazard. Mater. 2019, 365, 846–856. 10.1016/j.jhazmat.2018.09.061. [DOI] [PubMed] [Google Scholar]
- Yang H.; Bai L.; Wei D.; Yang L.; Wang W.; Chen H.; Niu Y.; Xue Z. Ionic self-assembly of poly(ionic liquid)-polyoxometalate hybrids for selective adsorption of anionic dyes. Chem. Eng. J. 2019, 358, 850–859. 10.1016/j.cej.2018.10.100. [DOI] [Google Scholar]
- Zhao M.; Tang Z.; Liu P. Removal of methylene blue from aqueous solution with silica nano-sheets derived from vermiculite. J. Hazard. Mater. 2008, 158, 43–51. 10.1016/j.jhazmat.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Rytwo G.; Hitzky E. R. Enthalpies of adsorption of methylene blue and crystal violet to montmorillonite. J. Therm. Anal. 2003, 71, 751–759. 10.1023/a:1023309806214. [DOI] [Google Scholar]
- Shaban M.; Abukhadra M. R.; Khan A. A. P.; Jibali B. M. Removal of Congo red, methylene blue and Cr(VI) ions from water using natural serpentine. J. Taiwan Inst. Chem. Eng. 2018, 82, 102–116. 10.1016/j.jtice.2017.10.023. [DOI] [Google Scholar]
- Sano Y.; Sugahara K.; Choi K.; Korai Y.; Mochida I. Two-step adsorption process for deep desulfurization of diesel oil. Fuel 2005, 84, 903–910. 10.1016/j.fuel.2004.11.019. [DOI] [Google Scholar]
- Pulido-Novicio L.; Hata T.; Kurimoto Y.; Doi S.; Ishihara S.; Imamura Y. Adsorption capacities and related characteristics of wood charcoals carbonized using a one-step or two-step process. J. Wood Sci. 2001, 47, 48–57. 10.1007/bf00776645. [DOI] [Google Scholar]
- Godwin P. M.; Pan Y. F.; Xiao H.; Afzal M. T. Progress in the preparation and application of modified biochar for improving heavy metal ion removal from water. J. Bioresour. Bioprod. 2019, 4, 31–42. 10.21967/jbb.v4i1.180. [DOI] [Google Scholar]
- Homaeigohar S. The nanosized dye adsorbents for water treatment. Nanometerials 2020, 10, 295. 10.3390/nano10020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.; Tang Z.; Liu P. Removal of methylene blue from aqueous solution with silica nano-sheets derived from vermiculite. J. Hazard. Mater. 2008, 158, 43–51. 10.1016/j.jhazmat.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Huang W.; Zhang Y.; Li D. Adsorptive removal of phosphate from water using mesoporous materials: a review. J. Environ. Manage. 2017, 193, 470–482. 10.1016/j.jenvman.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Bacelo H.; Pintor A. M. A.; Santos S. C. R.; Boaventura R. A. R.; Botelho C. M. S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. 10.1016/j.cej.2019.122566. [DOI] [Google Scholar]
- Otalvaro J. O.; Avena M.; Brigante M. Adsorption of organic pollutants by amine functionalized mesoporous silica in aqueous solution. Effects of pH, ionic strength and some consequences of APTES stability. J. Environ. Chem. Eng. 2019, 7, 103325. 10.1016/j.jece.2019.103325. [DOI] [Google Scholar]
- Mu R.-J.; Wang L.; Du Y.; Yuan Y.; Ni Y.; Wu C.; Pang J. Synthesis of konjac glucomannan-silica hybrid materials with honeycomb structure and its application as activated carbon support for Cu(II) adsorption. Mater. Lett. 2018, 226, 75–78. 10.1016/j.matlet.2018.04.133. [DOI] [Google Scholar]
- Zhao Y. X.; Ding M. Y.; Chen D. P. Adsorption properties of mesoporous silicas for organic pollutants in water. Anal. Chim. Acta 2005, 542, 193–198. 10.1016/j.aca.2005.04.005. [DOI] [Google Scholar]
- Pouya E. S.; Abolghasemi H.; Esmaieli M.; Fatoorehchi H.; Hashemi S. J.; Salehpour A. Batch adsorptive removal of benzoic acid from aqueous solution onto modified natural vermiculite: Kinetic, isotherm and thermodynamic studies. J. Ind. Eng. Chem. 2015, 31, 199–215. 10.1016/j.jiec.2015.06.024. [DOI] [Google Scholar]
- Guan W.-m.; Li J.-h.; Qian T.-t.; Wang X.; Deng Y. Preparation of paraffin/expanded vermiculite with enhanced thermal conductivity by implanting network carbon in vermiculite layers. Chem. Eng. J. 2015, 277, 56–63. 10.1016/j.cej.2015.04.077. [DOI] [Google Scholar]
- Wang L.; Wang X.; Yin J.; Wang C. Insights into the physicochemical characteristics from vermiculite to silica nanosheets. Appl. Clay Sci. 2016, 132–133, 17–23. 10.1016/j.clay.2016.05.006. [DOI] [Google Scholar]
- Su X.; Ma L.; Wei J.; Zhu R. Structure and thermal stability of organo-vermiculite. Appl. Clay Sci. 2016, 132–133, 261–266. 10.1016/j.clay.2016.06.011. [DOI] [Google Scholar]
- Aziz A.; Ouali M. S.; Elandaloussi E. H.; De Menorval L. C.; Lindheimer M. Chemically modified olive stone: A low-cost sorbent for heavy metals and basic dyes removal from aqueous solutions. J. Hazard. Mater. 2009, 163, 441–447. 10.1016/j.jhazmat.2008.06.117. [DOI] [PubMed] [Google Scholar]
- Zatta L.; Ramos L. P.; Wypych F. Acid-activated montmorillonites as heterogeneous catalysts for the esterification of lauric acid acid with methanol. Appl. Clay Sci. 2013, 80–81, 236–244. 10.1016/j.clay.2013.04.009. [DOI] [Google Scholar]
- Huang Z.; Liu J.; Xiao Z.; Fu H.; Fan W.; Xu B.; Dong B.; Liu D.; Dai F.; Sun D. A MOF-derived coral-like NiSe@NC nanohybrid: an efficient electrocatalyst for the hydrogen evolution reaction at all pH values. Nanoscale 2018, 10, 22758–22765. 10.1039/c8nr06877a. [DOI] [PubMed] [Google Scholar]
- Younker J. M.; Walsh M. E. Impact of salinity and dispersed oil on adsorption of dissolved aromatic hydrocarbons by activated carbon and organoclay. J. Hazard. Mater. 2015, 299, 562–569. 10.1016/j.jhazmat.2015.07.063. [DOI] [PubMed] [Google Scholar]
- Smith J. A.; Jaffe P. R.; Chiou C. T. Effect of ten quaternary ammonium cations on tetrachloromethane sorption to clay from water. Environ. Sci. Technol. 1990, 24, 1167–1172. 10.1021/es00078a003. [DOI] [Google Scholar]
- Zhang L.; Gao H.; Liao Y. Preparation and application of Poly(AMPS-co-DVB) to remove Rhodamine B from aqueous solutions. React. Funct. Polym. 2016, 104, 53–61. 10.1016/j.reactfunctpolym.2016.05.001. [DOI] [Google Scholar]
- Guo Y.; Zhao J.; Zhang H.; Yang S.; Qi J.; Wang Z.; Xu H. Use of rice husk-based porous carbon for adsorption of Rhodamine B from aqueous solutions. Dyes Pigm. 2005, 66, 123–128. 10.1016/j.dyepig.2004.09.014. [DOI] [Google Scholar]
- Ho Y. S.; Mckay G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. 10.1016/s0032-9592(98)00112-5. [DOI] [Google Scholar]
- Yang Q.; Wang Y.; Wang J.; Liu F.; Hu N.; Pei H.; Yang W.; Li Z.; Suo Y.; Wang J. High effective adsorption/removal of illegal food dyes from contaminated aqueous solution by Zr-MOFs (UiO-67). Food Chem. 2018, 254, 241–248. 10.1016/j.foodchem.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Silva T. L.; Cazetta A. L.; Souza P. S. C.; Zhang T.; Asefa T.; Almeida V. C. Mesoporous activated carbon fibers synthesized from denim fabric waste: Efficient adsorbents for removal of textile dye from aqueous solutions. J. Cleaner Prod. 2018, 171, 482–490. 10.1016/j.jclepro.2017.10.034. [DOI] [Google Scholar]
- Huang C.-H.; Chang K.-P.; Ou H.-D.; Chiang Y.-C.; Chang E.-E.; Wang C.-F. Characterization and application of Ti-containing mesoporous silica for dye removal with synergistic effect of coupled adsorption and photocatalytic oxidation. J. Hazard. Mater. 2011, 186, 1174–1182. 10.1016/j.jhazmat.2010.11.125. [DOI] [PubMed] [Google Scholar]
- Foo K. Y.; Hameed B. H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. 10.1016/j.cej.2009.09.013. [DOI] [Google Scholar]
- Wu Y.; Zhang L.; Gao C.; Ma J.; Ma X.; Han R. Adsorption of copper ions and methylene blue in a single and binary system on wheat straw. J. Chem. Eng. Data 2009, 54, 3229–3234. 10.1021/je900220q. [DOI] [Google Scholar]
- Monte Blanco S. P. D.; Scheufele F. B.; Módenes A. N.; Espinoza-Quiñones F. R.; Marin P.; Kroumov A. D.; Borba C. E. Kinetic, equilibrium and thermodynamic phenomenological modeling of reactive dye adsorption onto polymeric adsorbent. Chem. Eng. J. 2017, 307, 466–475. 10.1016/j.cej.2016.08.104. [DOI] [Google Scholar]
- Bhattacharya A. K.; Naiya T. K.; Mandal S. N.; Das S. K. Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem. Eng. J. 2008, 137, 529–541. 10.1016/j.cej.2007.05.021. [DOI] [Google Scholar]
- Chen L.; Zuo L.; Jiang Z.; Jiang S.; Liu K.; Tan J.; Zhang L. Mechanisms of shale gas adsorption: Evidence from thermodynamics and kinetics study of methane adsorption on shale. Chem. Eng. J. 2019, 361, 559–570. 10.1016/j.cej.2018.11.185. [DOI] [Google Scholar]
- Younis S. A.; Abd-Elaziz A.; Hashem A. I. Utilization of a pyrrole derivative based antimicrobial functionality impregnated onto CaO/g-C3N4 for dyes adsorption. RSC Adv. 2016, 6, 89367. 10.1039/c6ra10143g. [DOI] [Google Scholar]
- Yun J.; Wang Y.; Liu Z.; Li Y.; Yang H.; Xu Z.-l. High efficient dye removal with hydrolyzed ethanolamine-Polyacrylonitrile UF membrane: Rejection of anionic dye and selective adsorption of cationic dye. Chemosphere 2020, 259, 127390. 10.1016/j.chemosphere.2020.127390. [DOI] [PubMed] [Google Scholar]
- Preetha B. K.; Vishalakshi B. Microwave assisted synthesis of karaya gum based montmorillonite nanocomposite: Characterization, swelling and dye adsorption studies. Int. J. Biol. Macromol. 2020, 154, 739–750. 10.1016/j.ijbiomac.2020.03.107. [DOI] [PubMed] [Google Scholar]
- Sharifpour E.; Haddadi H.; Ghaedi M. Optimization of simultaneous ultrasound assisted toxic dyes adsorption conditions from single and multi-components using central composite design: Application of derivative spectrophotometry and evaluation of the kinetics and isotherms. Ultrason. Sonochem. 2017, 36, 236–245. 10.1016/j.ultsonch.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Rytwo G.; Nir S.; Margulies L. Interactions of monovalent organic cations with montmorillonite: Adsorption studies and model calculations. Soil Sci. Soc. Am. J. 1995, 59, 554–564. 10.2136/sssaj1995.03615995005900020041x. [DOI] [Google Scholar]
- Oki M.; Iwamure H. Steric effects on the O-H···π interaction in 2-hydroxybiphenyl. J. Am. Chem. Soc. 1967, 89, 576–579. 10.1021/ja00979a019. [DOI] [Google Scholar]
- Awad A. M.; Shaikh S. M. R.; Jalab R.; Gulied M. H.; Nasser M. S.; Benamor A.; Adham S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. 10.1016/j.seppur.2019.115719. [DOI] [Google Scholar]
- Xu Y.; Khan M. A.; Wang F.; Xia M.; Lei W. Novel multi amine-containing Gemini surfactant modified montmorillonite as adsorbents for removal of phenols. Appl. Clay Sci. 2018, 162, 204–213. 10.1016/j.clay.2018.06.023. [DOI] [Google Scholar]
- Wu F.-C.; Tseng R.-L.; Huang S.-C.; Juang R.-S. Characteristics of pseudo-second-order kinetic model for liquid-phase adsorption: a mini-review. Chem. Eng. J. 2009, 151, 1–9. 10.1016/j.cej.2009.02.024. [DOI] [Google Scholar]
- Foo K. Y.; Hameed B. H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. 10.1016/j.cej.2009.09.013. [DOI] [Google Scholar]
- Belbel A.; Kharroubi M.; Janot J.-M.; Abdessamad M.; Haouzi A.; Lefkaier I. K.; Balme S. Preparation and characterization of homoionic montmorillonite modified with ionic liquid: Application in dye adsorption. Colloids Surf., A 2018, 558, 219–227. 10.1016/j.colsurfa.2018.08.080. [DOI] [Google Scholar]
- Li Y.-H.; Di Z.; Ding J.; Wu D.; Luan Z.; Zhu Y. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res. 2005, 39, 605–609. 10.1016/j.watres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Hong S., Wen C., He J., Gan F., Ho Y.-S.. Adsorption thermodynamics of Methylene Blue onto bentonite, J. Hazard. Mater. [DOI] [PubMed] [Google Scholar]
- Andronic L.; Duta A. Photodegradation processes in two-dyes systems-Simultaneous analysis by first-order spectra derivative method. Chem. Eng. J. 2012, 198–199, 468–475. 10.1016/j.cej.2012.06.019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.