Abstract
Quantifying the contribution of individual exposure pathways to a child’s total ingestion of fecal matter could help prioritize interventions to reduce environmental enteropathy and diarrhea. This study used data on fecal contamination of drinking water, food, soil, hands, and objects and second-by-second data on children’s contacts with these environmental reservoirs in rural Bangladesh to assess the relative contribution of different pathways to children’s ingestion of fecal indicator bacteria and if ingestion decreased with the water, sanitation, and hygiene interventions implemented in the WASH Benefits Trial. Our model estimated that rural Bangladeshi children <36 months old consume 3.6–4.9 log10 most probable number E. coli/day. Among children <6 months, placing objects in the mouth accounted for 60% of E. coli ingested. For children 6–35 months old, mouthing their own hands, direct soil ingestion, and ingestion of contaminated food were the primary pathways of E. coli ingestion. The amount of E. coli ingested by children and the predominant pathways of E. coli ingestion were unchanged by the water, sanitation, and hygiene interventions. These results highlight contaminated soil, children’s hands, food, and objects as primary pathways of E. coli ingestion and emphasize the value of intervening along these pathways.
Keywords: diarrhea, environmental enteropathy, fecal contamination, E. coli, multiple pathways, child health, exposure, Bangladesh
Introduction
Exposure to human and animal fecal contamination causes enteric infections and fecal-oral diseases including diarrhea. Repeated exposure to high levels of fecal contamination may also contribute to environmental enteropathy, a subclinical condition of the small intestine associated with blunted intestinal villi and crypt hyperplasia.1 Environmental enteropathy and enteric infections contribute to malnutrition, which caused 1.4 million deaths in 2015.2 Malnutrition is also associated with delayed and reduced schooling, and long-term cognitive impairment.3,4
Interventions to interrupt exposure to fecal contamination typically focus on water disinfection, provision household sanitation, and handwashing.5 In settings heavily contaminated with feces, interventions focused on improving water, sanitation, and hygiene conditions may have had limited effects on reducing diarrhea or improving growth because they did not sufficiently reduce exposure to feces.6,7 These interventions may not have caused a sufficient reduction in fecal contamination along the targeted pathways to observe a reduction in diarrhea;8−10 or transmission occurred along pathways that are typically not addressed by water, sanitation, and hygiene programs. For example, there is evidence that inadequate child feces management impairs child growth;11 exposure to animal feces and soil is associated with increased risk of diarrhea, markers of environmental enteropathy, and growth faltering;12−15 and objects carry fecal contamination that may be ingested by children when objects are mouthed.16,17
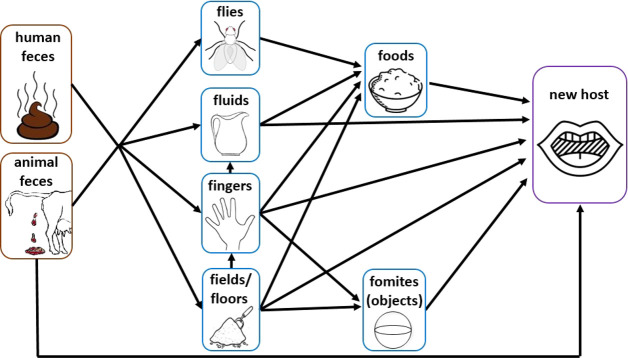
An understanding of the degree to which different fecal exposure pathways (Figure 1) contribute to children’s ingestion of fecal matter can help prioritize pathways for intervention. Additionally, modeling children’s ingestion of E. coli, an indicator of fecal contamination, can help interpret outcomes of water, sanitation, and hygiene trials. While E. coli is not a perfect proxy for bacterial pathogens, much less viruses and protozoa, the presence of E. coli in drinking water and on hands has been associated with diarrhea in children.18−20 Two studies have compared two or more pathways using exposure assessment to identify which pathway primarily contributes to children’s overall intake of E. coli.21,22 In a rural Tanzania study that modeled the hand mouthing and drinking water pathways, 99.7% of children’s E. coli exposure was due to contacts between children’s hands and mouths while only 0.3% of E. coli exposure was from drinking water.22 In urban Ghana, ingestion of water, food, and soil, hand-to-mouth contact, and flies were investigated as potential pathways of fecal exposure. The authors identified food as the pathway contributing 99% of children’s E. coli ingestion.21 However, due to a lack of data on hand-to-mouth and object-to-mouth exposures among children in low-income communities at the time these studies were published, both used hand- and object-mouthing frequencies and other data from children in the U.S. The impact of these nonlocal exposure measures on the study results is unclear as children’s interactions with their environment are likely context-specific.17,23 In this study, we used location-specific measurements of environmental fecal contamination and location-specific data on children’s environmental exposures to estimate children’s intake of the fecal indicator bacteria E. coli.
Figure 1.
Pathways of exposure to fecal contamination (adapted with permission from ref (24)).
Methods
Modeled Pathways
Our model drew on unpublished and published data from studies of children’s exposure to multiple pathways of fecal transmission in rural Bangladesh, and the levels of fecal contamination documented along these pathways. The seven pathways of fecal ingestion included in our model are (1) hand-to-mouth contact with the child’s own hands, (2) hand-to-mouth contact with caregivers’ hands, (3) object-to-mouth contacts, (4) ingestion of food, (5) ingestion of water, (6) direct soil ingestion, and (7) direct feces ingestion. Since we did not observe flies on or in children’s mouths, we assumed that all transmission from flies was captured in the food pathway and therefore did not include a separate fly pathway. We modeled E. coli ingested by children in the control and combined water, sanitation, and hygiene (WSH) arms of the WASH Benefits trial in Bangladesh.25 Interventions in the WSH arm included dual-pit latrines provided for every household in the study household’s compound. The compounds also received a potty for young children and a sani-scoop for removal and safe disposal of child and animal feces. In addition, the study household received a water storage vessel with a cover and tap, point-of-use water chlorination tablets, two handwashing stations with soapy water bottles. Hardware was delivered within a behavior change program that encouraged regular use of these hardware components through periodic household visits from local community health promoters. Promoters visited intervention households six times per month on average; they did not visit households in the control arm.
Model Overview
We used a probabilistic Monte Carlo simulation to model the amount of human and animal fecal matter, proxied by E. coli, ingested by rural Bangladeshi children <36 months old. We compared the relative contribution of each pathway to the total quantity of E. coli ingested for children in different age groups. Monte Carlo simulations run multiple iterations of a model, each time randomly selecting a value from each parameter’s distribution to use in the current iteration. We accounted for ingestion through placing contaminated hands and objects in the mouth (eqs 1–3), consumption of food (eq 4) and water (eq 5), direct soil ingestion (eq 6), and direct feces ingestion (eq 7). We summed the values to calculate total ingestion (eq 8). Briefly, for hands and objects, we multiplied the load of E. coli on the hand or object by the surface area of the hand or object mouthed, transfer efficiency, frequency of mouthing of hands or objects per hour, and number of hours a child is awake during the day. For food, water, soil, and feces, we multiplied the concentration of E. coli in food, water, soil, and feces by the quantity of the food, water, soil, and feces ingested per day. For pathways with estimated per-hour frequencies, we selected from an age-relevant distribution the number of hours in a day the child was awake, calculated a value for each hour or partial hour, and summed these values to calculate a daily estimate. We have previously used a similar model to estimate children’s ingestion of soil.26 For the model presented here, we ran 10000 simulations for each age group (3–5 months, 6–11 months, 12–17 months, 18–23 months, and 24–35 months old). Age groups were selected based on guidance from the U.S. Environmental Protection Agency on selecting age groups for assessing exposure to environmental contaminants.27 Parameter abbreviations, descriptions, and distributions are given in Table S1; parameter distributions were drawn from the referenced source, except for those marked “This study”, which are described in this paper and SI.
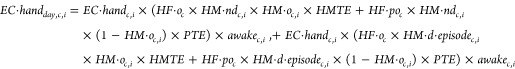 |
1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
We assumed that E. coli concentrations expressed as colony-forming units (CFU) were equivalent to those expressed as most probable number (MPN) and conducted all analysis using concentrations in MPN. We used the Kruskal–Wallis and Dunn tests to compare the median E. coli intake across pathways and age groups. Modeling and statistical analysis were conducted using R version 3.3.3.
Parameters
Frequency of Hand-to-mouth (HM.x) and Object-to-Mouth Contacts (OMc,i)
We collected data on the frequency that rural Bangladesh children put hands and objects into their mouths during 234 in-person structured observations and video observations of children 3–45 months old.17,23 A subset of children participating in the WASH Benefits trial in Bangladesh25 were observed at their homes for 5–6 h during daylight hours and each of their hand-to-mouth, object-to-mouth, and hand-to-object contacts were recorded using a computer-based software, Virtual Timing Device.28 Structured observations were conducted on 149 children 3–18 months old.17 A subset of 30 of these children participated in video observations conducted longitudinally at four time points from 2014 to 2016, when children ranged from 3 to 47 months old.23 We calculated the hourly frequency of hand-to-mouth contacts separately for contacts with the child’s own hand (HM.ndc,i) or caregiver’s hand (HM.ndm,i) while not eating. We also enumerated the number of contacts per hour when a child fed herself (HM.d.episodec,i) or was fed by a caregiver (HM.d.episodem,i). The detailed methods are reported elsewhere.17,23
Frequency and Quantity of Direct Soil (SMc,i, SWDI) and Fecal Matter (FMc,i, FWDI) Ingestion
The structured and video observations were also analyzed for the prevalence and frequency of direct soil and fecal matter ingestion (Table S1). Our observations, which took place both during the morning and afternoon, covered approximately half of children’s waking hours,29 so we assumed that the prevalence of soil and fecal matter ingestion per day was double what we observed.
We used these exposure data, combined with information on the load of soil on child and caregiver hands and objects, to model the quantity of soil directly and indirectly ingested each day.26 On the basis of our observations of direct soil and fecal ingestion and lacking additional data on the mass of feces consumed per ingestion event, we assumed that the dry mass of fecal matter consumed in one ingestion event (FWDI) was equivalent to the dry mass of soil ingested in one ingestion event (SWDI).
Food Consumption (Fc,i)
Among Bengali children, 3–5 months old, approximately 56% exclusively breastfeed, 26% consume breastmilk and liquids, and 18% breastmilk and solid foods.30,31 Data on mass of nonbreastmilk food consumed by children <6 months old were unavailable; we assumed that children who consume liquid food in addition to breastmilk consume 10% of the dry grams ingested by children 6–11 months old and children who eat solid food to complement breastmilk consume 20% of the dry grams ingested by children 6–11 months old. On the basis of published and unpublished data regarding the amount of food consumed by children, and the total amounts and ratios of fat, protein, carbohydrates, and total calories consumed by children 24–35 months old,32,33 we estimated the amount of food consumed by children 6–11 months old using published values for the total number of calories and grams of protein they consume.34 Lacking data more recent than 1985 on the quantity of food consumed by children 12–23 months old, we estimated their food consumption by averaging the values for children 6–11 and 24–35 months old.
Water Consumption (Wc,i)
Since there does not exist a published study on the volume of drinking water consumed by children in Bangladesh, we used drinking water consumption data from children in West Bengal and the body mass data from children in the WASH Benefits study to estimate drinking water ingestion. A study of 117 rural West Bengali children 7 months to 15 years old reported that children ingest as drinking water an arithmetic mean of 87 mL water/kg body weight/day.35 We estimated amount of water consumed per day by multiplying the 87 mL water/kg body weight by the empirical distribution of body weights of children in the specified age group measured in the WASH Benefits study. For exclusively breastfed children <6 months old, consumption of drinking water was set to 0 mL/day.
E. coli Contamination of Water (ECwater,c,i), Hands (EChand,c,i, EChand,m,i), Food (ECfood,c,i), Objects (ECobj), and Soil (ECsoil)
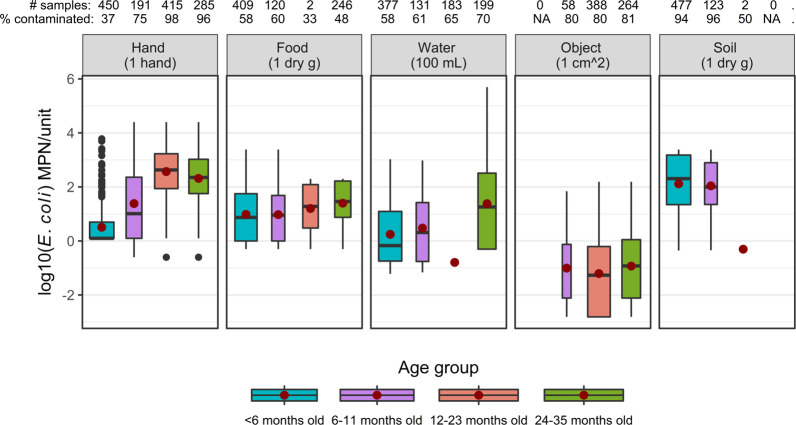
We utilized environmental samples collected as part of WASH Benefits in the trial’s control and combined water, sanitation, and hygiene arms for data of fecal contamination along these pathways.8,9 The WASH Benefits team sampled stored drinking water, children’s hands, food that would have been served to young children (predominantly samples of rice), sentinel toys (nonporous plastic balls, radius = 5 cm), and soil from where young children last played in the courtyard. Samples were collected approximately four months, one year, and two years after the initiation of the WASH Benefits interventions.8,9 We used age group-specific data for E. coli contamination of hands, food, water, and objects as environmental media demonstrated that E. coli concentrations varied by age group (Kruskal–Wallis test for difference among age groups: p < 0.005 for hands, food, and water; p = 0.07 for objects; and p = 0.09 for soil after removing the 24–35-month-old age group, which was represented by only two samples) (Figure 2). In this study, we used the empirical E. coli concentrations from all sampling periods. Given that prior studies did not find a difference between the levels of contamination on the hands of children and their mothers,36 we assigned mothers’ hands the same empirical distribution of E. coli as children’s hands.
Figure 2.
E. coli contamination of various environmental media, by age group.
E. coli Per Gram Feces (ECfeces) and Feces Moisture Content (FMC)
We calculated the E. coli consumed from direct feces ingestion by multiplying the quantity of feces ingested by the concentration of E. coli in fecal matter (wet weight). A study of animal feces in urban Bangladesh found the mean concentration of E. coli in chicken feces was 8.5 log10MPN/g wet-weight feces, whereas for ducks it was 7.6 log10MPN/wet g; goat feces had 7.8 log10E. coli MPN/wet g feces and cows 6.8 log10MPN/wet g.37 Children were observed consuming the feces of poultry, cows/buffalos, and goat/sheep, so we used the geometric mean concentration of E. coli in these types of feces (mean 7.7 log10MPN/wet g, standard deviation [sd] = 0.89 log10MPN/wet g) to convert mass of ingested feces into the quantity of ingested E. coli.
To calculate the wet weight of feces ingested, we multiplied the dry weight of feces consumed by the moisture content of ingested animal feces, which are common in Bangladeshi courtyards.38 We estimated the moisture content of ingested feces by taking the mean feces moisture content for chickens (74%39), cows (85%40,41), and goats (22%42) as the mode of a triangular distribution with an assumed minimum of 0.20 and a maximum of 0.90. We represented these values with the distribution beta(6.31, 4.82).
Other Parameters
Additional details on model parameters, including the transfer efficiency, the peri-oral transfer efficiency, the surface area children’s and mothers’ hands, the fraction of the hand that touches the mouth during an oral or peri-oral hand-to-mouth contact, and the fraction of oral and peri-oral contacts have been previously described.26
Relative Contribution and Sensitivity Analyses
We calculated the relative contribution of each pathway to the total quantity of E. coli ingested by finding the percent each pathway contributed to the total in each simulation. To assess if E. coli ingestion across seasons reflected trends in diarrhea rates across seasons, we also conducted separate analyses for the rainy (June-October) and dry (November-May) seasons. We compared results modeled based on data from the control and WSH arms of the WASH Benefits Trial.
We did not estimate fecal matter ingested from all pathways because E. coli can grow in food and soil and thus the amount of E. coli ingested may not represent the amount of fecal matter that was originally deposited. However, we did model fecal matter ingestion through the pathways of drinking water and children mouthing their own hands (whether eating or not) in order to compare our results to those of a study of rural Tanzanian children.22 For this comparison, we used the same conversion factor of E. coli to human feces that was used in the Tanzania study.22
We performed a sensitivity analysis to assess whether variability in the concentration of fecal contamination in the environment or the degree of children’s interaction with the environment more strongly influences the amount of E. coli they ingest. For this analysis, we set all parameters to their median values except the parameter of interest, which was set to its 25th percentile for the first model run and 75th percentile for the second model run. We compared the ratio of outcomes resulting from the 75th and 25th percentile runs.22 To assess extreme differences in child mouthing—frequencies outside the range of those observed in Bangladesh,—we doubled and tripled the 75th percentiles of these distributions, correspondingly set the 25th percentile to one-half and one-third of their original values, and reran the sensitivity analysis to determine how the influence of the hand- and object-mouthing parameters changed.
Results
Our model estimated that in rural Bangladesh, children in the control and WSH arms of the WASH Benefits trial ingested similar amounts of E. coli (Table S2, Figure S1). Children <6 months old in the control arm ingested a median of 3.6 log10MPN E. coli/day, whereas E. coli ingestion among children 6–35 months old was approximately one log higher: 4.7 log10MPN E. coli/day among children 6–11 months old, 4.9 log10MPN E. coli/day among children 12–23 months old, and 4.6 log10MPN E. coli/day among children 24–35 months old. Among children in the WSH arm in the same age group, E. coli ingestion was within 0.1 log10MPN E. coli/day of the estimates among controls. With respect to seasonal variation, median ingestion of E. coli was 4.4 log10MPN/day in the dry season and 4.7 log10MPN/day in the rainy season among children of all ages in the control arm; median ingestion of E. coli was 4.3 log10MPN/day in the dry season and 4.8 log10MPN/day in the rainy season among children of all ages in the WSH arm.
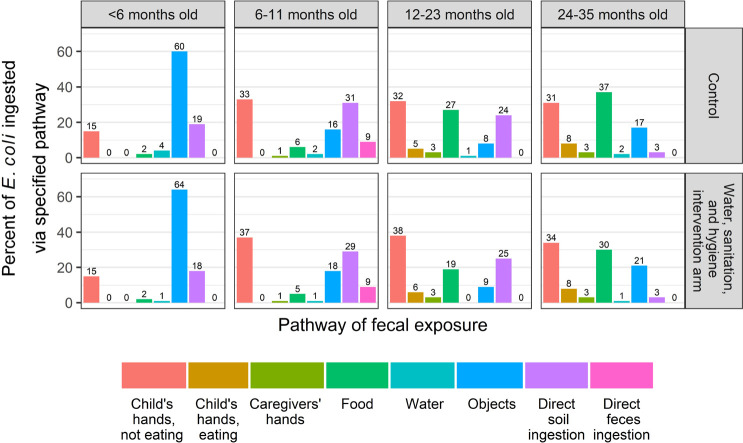
The primary pathways of E. coli ingestion varied by age group but were similar across arms (Figure 3). Among all children in age groups in both arms and combining both seasons, the percentage of total E. coli ingestion resulting from children mouthing their own hands while not eating ranged from 15 to 38%. Direct soil ingestion was the secondary pathway for children 0–23 months old, with a contribution that ranged from 18 to 31% of total E. coli ingestion, depending on age group and study arm. For children <6 months old, mouthing objects was the primary pathway of E. coli ingestion, contributing 60% of total E. coli ingestion among children in the control arm and 64% among children in the WSH arm. The contribution of food to total ingested E. coli increased as children aged, with children <12 months ingesting 2–6% of total E. coli from food and children 12–35 months old ingesting 19–37% of total E. coli from food, depending on age group and study arm.
Figure 3.
Relative contribution of multiple pathways to total fecal bacteria ingestion among young children in rural Bangladesh.
When we considered only the pathways of drinking water and children mouthing their own hands unrelated to eating, and assumed that all of the E. coli ingested from these pathways was derived from human feces, we calculated that Bangladeshi children <6 months old ingested a median of 0.01 mg wet-weight feces/day, children 6–11 months old 0.37 mg/day, children 12–23 months old 0.92 mg/day and children 24–35 months old 0.54 mg/day; mouthing of children’s hands was responsible for 80–97% of fecal matter ingestion. When we assumed that the E. coli ingested from mouthing hands and drinking water was from a mixture of poultry, cow, and goat feces instead of human feces,38 the estimated ingestion of feces dropped by half, with a low of 0.0044 mg wet-weight feces/day for children <6 months old to a high of 0.47 mg wet-weight feces/day for children 12–23 months old.
The parameters whose variation most influenced the estimate of fecal bacteria ingestion were those associated with the primary pathway of ingestion in each age group. As direct soil ingestion was the primary contributor to E. coli ingestion among children 6–23 months old, the frequency of direct soil ingestion, the concentration of E. coli in the soil, and the mass of soil ingested per direct ingestion event had the strongest influence on the model for this age range. For children 6–11 months old, the 75th percentile of the frequency of direct soil ingestion was 7.8 times/day compared to the 25th percentile of 0 times/day (many children did not ingest any soil). The estimate of total E. coli ingestion resulting from using the 75th percentile of direct soil ingestion frequency was 154 times higher than the estimate resulting from the 25th percentile. Similarly, total E. coli ingestion estimates were 28 times higher for children <6 months old when the 75th vs 25th percentile values were used for the concentration of E. coli on objects and 7 times higher when the 75th vs 25th percentile values were used for the frequency of mouthing objects (Table S3). The model-estimated E. coli ingestion was not sensitive to the frequency of feeding events or the frequency of children’s hand-to-mouth contacts during feeding, the concentration of E. coli on caregiver’s hands or in water or food, the quantity of water consumed, the frequency of direct feces ingestion events, or mass of feces ingested per direct fecal matter ingestion event.
Discussion
A substantial fraction of the E. coli ingested by children is from pathways that are not directly addressed by household-level water, sanitation, and hygiene interventions: directly ingesting soil that is contaminated by animal feces and mouthing hands and objects contaminated by this soil. This could explain why low-cost, household-level interventions that have focused on water treatment and storage, latrine construction and usage, and caregivers washing their own hands often have not reduced children’s exposure to fecal contamination enough to observe large reductions in child diarrhea6,7,43 and/or linear growth.6,7,25
Our model shows that the WSH intervention implemented by WASH Benefits in Bangladesh did not impact the quantity of E. coli ingested. The WSH interventions sought to reduce contamination of the environment by better containing human feces, storing treated drinking water, and increasing handwashing with soap. There was high uptake of all interventions, except for child potties and sani-scoops for removal of animal feces: 15 months after the intervention was implemented in the WSH arm, 50% of stored water had a chlorine residual, 55% of water was stored fully covered, hands were washed with soap after 74% of defecation events, and adults used a hygienic latrine for defecation in 97% of defecation events, while a potty was used in 37% of child defecation events, a sani-scoop used in 25% human feces disposal events and 21% of animal feces disposal events, and animal feces were observed in 85% of compounds.44 The prevalence and concentration of E. coli in stored water was lower in the WSH arm than the control arm when measured four months, one year and two years after intervention initiation, but the prevalence and concentration of E. coli on objects (sentinel balls) was higher at year one.8 The prevalence and concentration of E. coli on hands, in food, and in soil was not significantly different between the WSH and control arms at any of the sampling time points.8,9 As the primary pathways of E. coli ingestion were mouthing hands, soil, food, and objects, the conclusion that the intervention did not decrease the prevalence or concentration of E. coli in these environmental reservoir explains the model finding that total ingestion of E. coli was not lower in the WSH arm.
Despite finding no reduction in the prevalence or concentration of E. coli in hand, soil, food, and objects,8,9 the prevalence of reported diarrhea in the WSH arm was 38% lower in the WSH arm compared with the control arm.25 One explanation for this apparent discrepancy between environmental, modeling, and observed child health results is that the reduction in diarrhea was primarily driven by a reduction in diarrhea caused by viral or protozoan infections, and E. coli are a poor proxy for viruses and protozoa in the environment.45 The sanitation and control arms did not have significantly different concentrations of pathogenic E. coli genes in hand rinse, stored water, or soil samples, nor were there significantly different concentrations of rotavirus in soil, water, and hand rinse samples38 or norovirus in hand rinse samples.46 However, diarrhea could have been due to viruses that were not tested for, including sapovirus, adenovirus, and astrovirus47 and/or the water and handwashing interventions may have been associated with larger reductions of viruses in the environment than seen in the sanitation arm. Indeed, analysis of stool from children in the WSH arm has a significantly lower prevalence of adenovirus, norovirus, sapovirus and Giardia as compared to stool from children in the control arm.48,49 This evidence is consistent with our model’s conclusion that across the WSH and control arms there was no significant difference in children’s ingestion of the fecal indicator bacteria, E. coli this suggests that there may have been no difference in exposure to bacterial pathogens but cannot be used to infer whether or not there were differences in exposure to viral or protozoan pathogens.
The relative contribution of each pathway to total E. coli ingestion differed by age group. This finding suggests that both during planning and evaluation, interventions to reduce exposure to fecal matter should consider child age. While our results suggest that children in all age groups would have substantially reduced exposure to fecal matter if they had less contaminated hands, preventing direct soil ingestion would provide a greater benefit to children <24 months old and food hygiene interventions would provide a greater benefit to children >24 months old. As different interventions will likely not have the same effect on children in different age groups, the age of children in a study should be considered when estimating and evaluating an intervention’s effect size.
Reducing direct soil ingestion may be crucial for reducing children’s exposure to fecal contamination. Direct soil ingestion, a pathway that has historically received little attention, is a primary pathway of E. coli ingestion among children 6–23 months old in rural Bangladesh. Exposure to E. coli, and by proxy to fecal-oral pathogens, in soil may explain why direct soil ingestion has been associated with diarrhea and markers of enteric enteropathy.13,50 Manipulating items allows infants to practice fine motor skills and helps them gain information about the properties of the item, which contributes to their conceptual and language development. As such, generally restricting children’s grasping of items to reduce their exposure to soil may not be preferred. Instead, strategies to limit children’s exposure to and direct ingestion of soil include improved flooring and hygienic play pens. Improving indoor flooring in urban Mexico was associated with reduced parasite loads, diarrheal episodes, and incidence of anemia among children <5 years old,51 while play pens with plastic mats for children in rural Ethiopia were unsuccessful at reducing diarrhea.52
Among children ≥12 months old, consumption of contaminated food contributed 27–37% of total E. coli ingested, indicating that improved food hygiene intervention could substantially reduce exposure to fecal pathogens for children in this age group. Recent studies have explored the degree of contamination in foods consumed by young children.8,9,53−55 Potential mechanisms of food contamination include contamination by dust, flies, and contaminated hands.56−59 Food hygiene interventions that focus on handwashing before food preparation and covering cooked food during storage could reduce food contamination and diarrhea.57,58
The sensitivity analysis suggests that our E. coli ingestion results for children in rural Bangladesh would be generalizable to contexts with similar levels of environmental contamination, prevalence of direct soil ingestion, object mouthing frequencies, and drinking water quality. However, the relative contribution of each pathway may differ across settings. For example, for children living in locations with lower levels of soil contamination13,60 or less opportunity to put soil into their mouths because the ground is covered with concrete, vinyl, or other flooring materials,50 the contribution of soil ingested E. coli may be less than that in our study. The sensitivity analysis also indicates that for children >6 months old, differences in hand- and object-mouthing frequencies between children in different countries has a relatively small impact on their estimated ingestion of E. coli. However, exposure factors for U.S. children alone cannot be used to model fecal intake of children in other countries because they do not include frequencies of soil mouthing or relevant quantities of food consumption. The model’s extreme sensitivity to particular variables indicates the need for more data to make assessments about fecal ingestion pathways in contexts that differ from rural Bangladesh.
Comparison with previous studies supports our model outputs. Considering only the pathways of drinking contaminated water and hand mouthing and assuming that all ingested E. coli represents freshly deposited human fecal matter, our study and other previous studies have similar estimates of fecal matter ingestion (0.01–0.92 mg wet-weight feces/day vs 0.098 mg or 0.93 mg wet-weight feces/day, depending on fecal indicator used for the estimate) and the fraction of ingestion from mouthing contaminated hands (80–97% vs 97%).22 Our estimates for the amount of E. coli ingested by children in rural Bangladesh (3.6–4.9 log10 MPN E. coli/day) was less than the estimate for children <5 years old in urban Ghana (8–16 log10 CFU E. coli/day), likely due to assumptions regarding Ghanaian children’s consumption of highly contaminated raw produce.21
This analysis uses E. coli as an indicator of fecal contamination, but E. coli is an imperfect proxy for fecal contamination and for enteric pathogen exposure. Naturally occurring E. coli can persist and multiply in tropical soils and surface waters, so some of the E. coli we detected in environmental samples measured was potentially not derived from feces.61 As a result, we may have overestimated the concentration of fecal matter in environmental media. Even if E. coli was a perfect proxy for the presence of fecal contamination, not all feces are contaminated with pathogens, so the presence of E. coli would not indicate the presence of pathogens. While the quantity of E. coli has been associated with the quantity of pathogenic E. coli genes,62 the presence of E. coli and fecal contamination does not always indicate the presence of pathogens. While the presence of the fecal indicator bacteria E. coli in household drinking water is associated with an increased risk of diarrhea,18 there is no simple dose–response relationship as there is an imperfect correlation between E. coli and enteric pathogens and acquired immunity influences the relationship between pathogen ingestion and the presence of symptomatic or asymptomatic infection.63 Our model demonstrates that consumption of drinking water contributes a minor portion of total ingested E. coli among children in rural Bangladesh, so lack of a dose–response or threshold association between E. coil and drinking water does not necessarily indicate a lack of association in the total quantity of E. coli ingested and diarrhea or other gastrointestinal disorders.19 Even if our model estimates of absolute quantity of E. coli ingested are inaccurate, our primary conclusions, which are based on the relative ingestion of E. coli from each pathway, may still be helpful in targeting pathways to reduce the probability or severity of illness.
The amount of E. coli ingested from the environmental reservoirs that we have considered (hands, water, food, objects, soil, and feces) may not be directly comparable because E. coli grows and dies off at different rates in these media.64−66 As such, the amount of E. coli in one environmental medium may reflect a different amount of deposited fecal matter (and associated pathogens) than the same amount of E. coli in a different environmental medium. The type of environmental reservoir may also influence the way fecal contamination interacts with the gut microbiome to generate immature or otherwise unhealthy microbial ecologies.67 Additionally, environmental reservoir type and characteristics such as moisture, temperature, pH, and surface area may influence pathogen growth, survival, bioavailability, infectious dose, and/or virulence. For example, waterborne typhoid outbreaks due to Salmonella typhi were associated with longer incubation periods and lower attack rates than foodborne outbreaks.68
Limitations to the data used for modeling and the modeling approach, including a small sample of children observed and not modeling rare events that may remove soil from hands, are detailed elsewhere.26 Uncertainties in transfer efficiencies and uncertainty in quantity of soil directly consumed during each direct soil consumption event are due to limited empirical data on these influential parameters and could substantially influence the estimate of total E. coli ingested by changing the estimated amount of soil ingested.26 However, these limitations are not expected to change the comparison between arms or the relative contribution of each pathway to children’s ingestion of E. coli because all pathways other than ingestion of food and water would be affected similarly by altering these transfer efficiency and direct soil ingestion parameters. The amount of water consumed and the concentration of E. coli in water are both low, so even if the transfer efficiencies and soil consumption were overestimated, rural Bangladeshi children’s ingestion of E. coli through drinking water is expected to remain negligible. Given the larger amount of food consumed, the relative contribution of food to total E. coli ingestion could increase. An additional limitation is that we use environmental contamination data specific to the control and WSH arms but the behavioral data were derived only from observations of children in the control arm. However, given children’s young ages, we do not expect that children were aware of the intervention or that the intervention impacted their hand-to-mouth and object-to-mouth contact frequencies.
Low-cost, household-level water, sanitation, and hygiene interventions that focus on water treatment, latrine usage, and caregiver’s hand cleanliness may be insufficient to substantially reduce fecal exposure among young children who live in settings where soil, children’s hands, food, and mouthed objects are highly contaminated with feces and there are ample opportunities for direct soil ingestion. Animal husbandry interventions, such as confining animals to areas of the home property where children would be discouraged from playing, may more effectively reduce fecal contamination of the soil than addressing human sanitation.38,69 More research into the prevalence, frequency, and quantity of soil that children of different ages ingest in different cultural and physical settings could improve our understanding of exposure to pathogens, as well as the accuracy of risk assessments for other environmental contaminants, such as lead. To prevent environmental enteropathy and diarrheal disease in young children, transformative WASH should include the examination of interventions to reduce direct soil ingestion and fecal contamination of children’s hands, food, mouthed objects, and household soil.
Acknowledgments
We gratefully acknowledge the WASH Benefits – Bangladesh study families who participated in the exposure assessment and provided environmental samples. This material is based upon work supported by the Bill and Melinda Gates Foundation (OPPGD759), World Bank, Stanford Wood’s Institute for the Environment Goldman Graduate Fellowship and the National Science Foundation Graduate Research Fellowship under Grant No. DGE-114747. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation. The authors declare that they have no conflicts of interest.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.0c02606.
Modeled log10 E. coli MPN ingestion/day among children in the WASH Benefits Bangladesh control and WSH arms (Table S1). Modeled fecal bacteria ingestion by young children in rural Bangladesh (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Korpe P. S.; Petri W. A. Environmental Enteropathy: Critical Implications of a Poorly Understood Condition. Trends Mol. Med. 2012, 18 (6), 328–336. 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger C.; Forouzanfar M.; Rao P. C; Khalil I.; Brown A.; Reiner R. C; Fullman N.; Thompson R. L; Abajobir A.; Ahmed M.; Alemayohu M. A.; Alvis-Guzman N.; Amare A. T; Antonio C. A.; Asayesh H.; Avokpaho E.; Awasthi A.; Bacha U.; Barac A.; Betsue B. D.; Beyene A. S.; Boneya D. J.; Malta D. C.; Dandona L.; Dandona R.; Dubey M.; Eshrati B.; Fitchett J. R A; Gebrehiwot T. T.; Hailu G. B.; Horino M.; Hotez P. J; Jibat T.; Jonas J. B; Kasaeian A.; Kissoon N.; Kotloff K.; Koyanagi A.; Kumar G A.; Rai R. K.; Lal A.; El Razek H. M. A.; Mengistie M. A.; Moe C.; Patton G.; Platts-Mills J. A; Qorbani M.; Ram U.; Roba H. S.; Sanabria J.; Sartorius B.; Sawhney M.; Shigematsu M.; Sreeramareddy C.; Swaminathan S.; Tedla B. A.; Jagiellonian R. T.-M.; Ukwaja K.; Werdecker A.; Widdowson M.-A.; Yonemoto N.; El Sayed Zaki M.; Lim S. S; Naghavi M.; Vos T.; Hay S. I; Murray C. J L; Mokdad A. H Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17 (9), 909–948. 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman H.; Hoddinott J.; Kinsey B. Long Term Consequences of Early Childhood Malnutrition. Oxf. Econ. Pap. 2006, 58 (3), 450–474. 10.1093/oep/gpl008. [DOI] [Google Scholar]
- Berkman D. S.; Lescano A. G.; Gillman R. H.; Lopez S. L.; Black M. M. Effects of Stunting, Diarrhoeal Disease, and Parasitic Infection. Lancet 2002, 359 (9306), 564–571. 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- Cairncross S.; Hunt C.; Boisson S.; Bostoen K.; Curtis V.; Fung I. C. H.; Schmidt W. P. Water, Sanitation and Hygiene for the Prevention of Diarrhoea. Int. J. Epidemiol. 2010, 39, i193. 10.1093/ije/dyq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Null C.; Stewart C. P.; Pickering A. J.; Dentz H. N.; Arnold B. F.; Arnold C. D.; Benjamin-Chung J.; Clasen T.; Dewey K. G.; Fernald L. C. H.; Hubbard A. E.; Kariger P.; Lin A.; Luby S. P.; Mertens A.; Njenga S. M.; Nyambane G.; Ram P. K.; Colford J. M. Effects of Water Quality, Sanitation, Handwashing, and Nutritional Interventions on Diarrhoea and Child Growth in Rural Kenya: A Cluster-Randomised Controlled Trial. Lancet Glob. Health 2018, 6 (3), e316-e329 10.1016/S2214-109X(18)30005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen T.; Boisson S.; Routray P.; Torondel B.; Bell M.; Cumming O.; Ensink J.; Freeman M.; Jenkins M.; Odagiri M.; Ray S.; Sinha A.; Suar M.; Schmidt W.-P. Effectiveness of a Rural Sanitation Programme on Diarrhoea, Soil-Transmitted Helminth Infection, and Child Malnutrition in Odisha, India: A Cluster-Randomised Trial. Lancet Glob. Health 2014, 2 (11), e645 10.1016/S2214-109X(14)70307-9. [DOI] [PubMed] [Google Scholar]
- Ercumen A.; Mertens A.; Arnold B. F.; Benjamin-Chung J.; Hubbard A. E.; Ahmed M. A.; Kabir M. H.; Rahman Khalil Md. M.; Kumar A.; Rahman Md. S.; Parvez S. M.; Unicomb L.; Rahman M.; Ram P. K.; Clasen T.; Luby S. P.; Colford J. M. Effects of Single and Combined Water, Sanitation and Handwashing Interventions on Fecal Contamination in the Domestic Environment: A Cluster-Randomized Controlled Trial in Rural Bangladesh. Environ. Sci. Technol. 2018, 52 (21), 12078–12088. 10.1021/acs.est.8b05153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercumen A.; Pickering A. J.; Kwong L. H.; Mertens A.; Arnold B. F.; Benjamin-Chung J.; Hubbard A. E.; Alam M.; Sen D.; Islam S.; Rahman Md. Z.; Kullmann C.; Chase C.; Ahmed R.; Parvez S. M.; Unicomb L.; Rahman M.; Ram P. K.; Clasen T.; Luby S. P.; Colford J. M. Do Sanitation Improvements Reduce Fecal Contamination of Water, Hands, Food, Soil, and Flies? Evidence from a Cluster-Randomized Controlled Trial in Rural Bangladesh. Environ. Sci. Technol. 2018, 52 (21), 12089–12097. 10.1021/acs.est.8b02988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclar G. D.; Penakalapati G.; Amato H. K.; Garn J. V.; Alexander K.; Freeman M. C.; Boisson S.; Medlicott K. O.; Clasen T. Assessing the Impact of Sanitation on Indicators of Fecal Exposure along Principal Transmission Pathways: A Systematic Review. Int. J. Hyg. Environ. Health 2016, 219 (8), 709–723. 10.1016/j.ijheh.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Bauza V.; Guest J. S. The Effect of Young Children’s Faeces Disposal Practices on Child Growth: Evidence from 34 Countries. Trop. Med. Int. Health 2017, 22 (10), 1233–1248. 10.1111/tmi.12930. [DOI] [PubMed] [Google Scholar]
- Bauza V.; Byrne D. M.; Trimmer J. T.; Lardizabal A.; Atiim P.; Asigbee M. A. K.; Guest J. S. Child Soil Ingestion in Rural Ghana: Frequency, Caregiver Perceptions, Relationship with Household Floor Material, and Associations with Child Diarrhoea. Trop. Med. Int. Health 2018, 23, 558. 10.1111/tmi.13050. [DOI] [PubMed] [Google Scholar]
- George C. M.; Oldja L.; Lee G. O.; Biswas S.; Perin J.; Lee G. O.; Kosek M.; Sack R. B.; Ahmed S.; Haque R.; Parvin T.; Azmi I. J.; Bhuyian S. I.; Talukder K. A.; Mohammad S.; Faruque A. G. Geophagy Is Associated with Environmental Enteropathy and Stunting in Children in Rural Bangladesh. Am. J. Trop. Med. Hyg. 2015, 92 (6), 1117–1124. 10.4269/ajtmh.14-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin J.; Thomas A.; Oldja L.; Ahmed S.; Parvin T.; Bhuyian S. I.; Sarker B.; Biswas S. K.; Faruque A. S. G.; Sack R. B.; George C. M. Geophagy Is Associated with Growth Faltering in Children in Rural Bangladesh. J. Pediatr. 2016, 178, 34–39. 10.1016/j.jpeds.2016.06.077. [DOI] [PubMed] [Google Scholar]
- Zambrano L. D.; Levy K.; Menezes N. P.; Freeman M. C. Human Diarrhea Infections Associated with Domestic Animal Husbandry: A Systematic Review and Meta-Analysis. Trans. R. Soc. Trop. Med. Hyg. 2014, 108 (6), 313–325. 10.1093/trstmh/tru056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujcic J.; Ram P. K.; Hussain F.; Unicomb L.; Gope P. S.; Abedin J.; Mahmud Z. H.; Sirajul Islam M.; Luby S. P. Toys and Toilets: Cross-Sectional Study Using Children’s Toys to Evaluate Environmental Faecal Contamination in Rural Bangladeshi Households with Different Sanitation Facilities and Practices. Trop. Med. Int. Health 2014, 19 (5), 528–536. 10.1111/tmi.12292. [DOI] [PubMed] [Google Scholar]
- Kwong L. H.; Ercumen A.; Pickering A. J.; Unicomb L.; Davis J.; Luby S. P. Hand- and Object-Mouthing of Rural Bangladeshi Children 3–18 Months Old. Int. J. Environ. Res. Public Health 2016, 13 (6), 563. 10.3390/ijerph13060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J. S.; Ercumen A.; Colford J. M. Coliform Bacteria as Indicators of Diarrheal Risk in Household Drinking Water: Systematic Review and Meta-Analysis. PLoS One 2014, 9 (9), e107429 10.1371/journal.pone.0107429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A. J.; Ercumen A.; Arnold B. F.; Kwong L. H.; Parvez S. M.; Alam M.; Sen D.; Islam S.; Kullmann C.; Chase C.; Ahmed R.; Unicomb L.; Colford J. M.; Luby S. P. Fecal Indicator Bacteria along Multiple Environmental Transmission Pathways (Water, Hands, Food, Soil, Flies) and Subsequent Child Diarrhea in Rural Bangladesh. Environ. Sci. Technol. 2018, 52 (14), 7928–7936. 10.1021/acs.est.8b00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard F.; Pickering A. J.; Ercumen A.; Brown J.; Chang H.; Clasen T. Fecal Contamination of the Environment and Child Health: A Systematic Review and Meta-Analysis Using Individual Participant Data. Lancet Planet. Health 2020, 4, e405. 10.1016/S2542-5196(20)30195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Moe C. L.; Null C.; Raj S. J.; Baker K. K.; Robb K. A.; Yakubu H.; Ampofo J. A.; Wellington N.; Freeman M. C.; Armah G.; Reese H. E.; Peprah D.; Teunis P. F. M. Multipathway Quantitative Assessment of Exposure to Fecal Contamination for Young Children in Low-Income Urban Environments in Accra, Ghana: The Sanipath Analytical Approach. Am. J. Trop. Med. Hyg. 2017, 97 (4), 1009–1019. 10.4269/ajtmh.16-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli M. C. M.; Davis J.; Boehm A. B. Hand-to-Mouth Contacts Result in Greater Ingestion of Feces than Dietary Water Consumption in Tanzania: A Quantitative Fecal Exposure Assessment Model. Environ. Sci. Technol. 2015, 49 (3), 1912–1920. 10.1021/es505555f. [DOI] [PubMed] [Google Scholar]
- Kwong L. H.; Ercumen A.; Pickering A. J.; Unicomb L.; Davis J.; Luby S. P. Age-Related Changes to Environmental Exposure: Variation in the Frequency That Young Children Place Hands and Objects in Their Mouths. J. Exposure Sci. Environ. Epidemiol. 2020, 30, 205–216. 10.1038/s41370-019-0115-8. [DOI] [PubMed] [Google Scholar]
- Wagner E. G.; Lanoix J. N.. Excreta Disposal for Rural Areas and Small Communities; Monograph Series 39; World Health Organization: Geneva, Switzerland, 1958. [PubMed] [Google Scholar]
- Luby S. P.; Rahman M.; Arnold B. F.; Unicomb L.; Ashraf S.; Winch P. J.; Stewart C. P.; Begum F.; Ercumen A.; Ram P. K.; Das K. K.; Abedin J.; Clasen T. F.; Dewey K. G.; Fernald L. C.; Null C.; Ahmed T.; Bill F.; Foundation M. G.. Effects of Water Quality, Sanitation, Handwashing, and Nutritional Interventions on Diarrhoea and Child Growth in Rural Bangladesh: A Cluster Randomised Controlled Trial. Lancet Glob. Health 2018, No. 6 (17), . e302. 10.1016/S2214-109X(17)30490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong L. H.; Ercumen A.; Pickering A. J.; Unicomb L.; Davis J.; Leckie J. O.; Luby S. P. Soil Ingestion among Young Children in Rural Bangladesh. J. Exposure Sci. Environ. Epidemiol. 2019, xx (xx), xx 10.1038/s41370-019-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants; EPA/630/P-03/003F; U.S. Environmental Protection Agency: Washington D.C., 2005. [Google Scholar]
- Julian T. R.; Bustos C.; Kwong L. H.; Badilla A. D.; Lee J.; Bischel H. N.; Canales R. A. Quantifying Human-Environment Interactions Using Videography in the Context of Infectious Disease Transmission. Geospatial Health 2018, 631. 10.4081/gh.2018.631. [DOI] [PubMed] [Google Scholar]
- Galland B. C.; Taylor B. J.; Elder D. E.; Herbison P. Normal Sleep Patterns in Infants and Children: A Systematic Review of Observational Studies. Sleep Med. Rev. 2012, 16 (3), 213–222. 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Bangladesh Bureau of Statistics and UNICEF Bangladesh. Bangladesh Multiple Indicator Cluster Survey 2012–2013, Progotir Pathey: Final Report; Dhaka, Bangladesh, 2014.
- Saha K. K.; Frongillo E.; Alam D. S.; Arifeen S. E.; Persson L. A.; Rasmussen K. M. Household Food Security Is Associated with Infant Feeding Practices in Rural Bangladesh. J. Nutr. 2008, 138, 1383–1390. 10.1093/jn/138.7.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte N. F.; Wong W. W.; Hopkinson J. M.; Heinz C. J.; Mehta N. R.; Smith E. O. Energy Requirements Derived from Total Energy Expenditure and Energy Deposition during the First 2 y of Life. Am. J. Clin. Nutr. 2000, 72 (72), 1558–1569. 10.1093/ajcn/72.6.1558. [DOI] [PubMed] [Google Scholar]
- Arsenault J. E.; Yakes E. A.; Hossain M. B.; Islam M. M.; Ahmed T.; Hotz C.; Lewis B.; Rahman A. S.; Jamil K. M.; Brown K. H. The Current High Prevalence of Dietary Zinc Inadequacy among Children and Women in Rural Bangladesh Could Be Substantially Ameliorated by Zinc Biofortification of Rice. J. Nutr. 2010, 140 (9), 1683–1690. 10.3945/jn.110.123059. [DOI] [PubMed] [Google Scholar]
- Kimmons J. E.; Dewey K. G.; Haque E.; Chakraborty J.; Osendarp S. J. M.; Brown K. H. Low Nutrient Intakes among Infants in Rural Bangladesh Are Attributable to Low Intake and Micronutrient Density of Complementary Foods. J. Nutr. 2005, 135 (3), 444–451. 10.1093/jn/135.3.444. [DOI] [PubMed] [Google Scholar]
- Hossain M. A.; Rahman M. M.; Murrill M.; Das B.; Roy B.; Dey S.; Maity D.; Chakraborti D. Water Consumption Patterns and Factors Contributing to Water Consumption in Arsenic Affected Population of Rural West Bengal, India. Sci. Total Environ. 2013, 0, 1217–1224. 10.1016/j.scitotenv.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriewer A.; Odagiri M.; Wuertz S.; Misra P. R.; Panigrahi P.; Clasen T.; Jenkins M. W. Human and Animal Fecal Contamination of Community Water Sources, Stored Drinking Water and Hands in Rural India Measured with Validated Microbial Source Tracking Assays. Am. J. Trop. Med. Hyg. 2015, 93 (3), 509–516. 10.4269/ajtmh.14-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. R.; Pickering A. J.; Harris M.; Doza S.; Islam M. S.; Unicomb L.; Luby S.; Davis J.; Boehm A. B. Ruminants Contribute Fecal Contamination to the Urban Household Environment in Dhaka, Bangladesh. Environ. Sci. Technol. 2016, 50 (9), 4642–4649. 10.1021/acs.est.5b06282. [DOI] [PubMed] [Google Scholar]
- Boehm A. B.; Wang D.; Ercumen A.; Shea M.; Harris A. R.; Shanks O. C.; Kelty C.; Ahmed A.; Mahmud Z. H.; Arnold B. F.; Chase C.; Kullmann C.; Colford J. M.; Luby S. P.; Pickering A. J. Occurrence of Host-Associated Fecal Markers on Child Hands, Household Soil, and Drinking Water in Rural Bangladeshi Households. Environ. Sci. Technol. Lett. 2016, 3 (11), 393–398. 10.1021/acs.estlett.6b00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven-Hangoor E.; Paton N.; Van de Linde I.; Verstegen M.; Hendriks W. Moisture Content in Broiler Excreta Is Influenced by Excreta Nutrient Contents. J. Anim. Sci. 2013, 91 (12), 5705–5713. 10.2527/jas.2013-6573. [DOI] [PubMed] [Google Scholar]
- Geldreich E. E.; Bordner R. H.; Huff C. B.; Clark H. F.; Kabler P. W. Type Distribution of Coliform Bacteria in the Feces of Warm-Blooded Animals. Water Pollut. Control Fed. 1962, 34 (3), 295–301. [Google Scholar]
- Himathongkham S.; Bahari S.; Riemann H.; Cliver D. Survival of Escherichia Coli O157:H7 and Salmonella Typhimurium in Cow Manure and Cow Manure Slurry. FEMS Microbiol. Lett. 1999, 178 (2), 251–257. 10.1111/j.1574-6968.1999.tb08684.x. [DOI] [PubMed] [Google Scholar]
- Garg V.; Yadav Y.; Sheoran A.; Chand S.; Kaushik P. Livestock Excreta Management through Vermicomposting Using an Epigeic Earthworm Eisenia Foetida. Environmentalist 2006, 26 (4), 269–276. 10.1007/s10669-006-8641-z. [DOI] [Google Scholar]
- Wolf J.; Hunter P. R.; Freeman M. C.; Cumming O.; Clasen T.; Bartram J.; Higgins J. P. T.; Johnston R.; Medlicott K.; Boisson S.; Prüss-Ustün A. Impact of Drinking Water, Sanitation and Handwashing with Soap on Childhood Diarrhoeal Disease: Updated Meta-Analysis and Meta-Regression. Trop. Med. Int. Health 2018, 23 (5), 508–525. 10.1111/tmi.13051. [DOI] [PubMed] [Google Scholar]
- Parvez S. M.; Azad R.; Rahman M.; Unicomb L.; Ram P. K.; Naser A. M.; Stewart C. P.; Jannat K.; Rahman M. J.; Leontsini E.; Winch P. J.; Luby S. P. Achieving Optimal Technology and Behavioral Uptake of Single and Combined Interventions of Water, Sanitation Hygiene and Nutrition, in an Efficacy Trial (WASH Benefits) in Rural Bangladesh. Trials 2018, 19, 2710. 10.1186/s13063-018-2710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschke J. S.; Sobsey M. D. Comparative Reduction of Norwalk Virus, Poliovirus Type 1, F+ RNA Coliphage MS2 and Escherichia Coli in Miniature Soil Columns. Water Sci. Technol. 2003, 47 (3), 85–90. 10.2166/wst.2003.0168. [DOI] [PubMed] [Google Scholar]
- Fuhrmeister E. R.; Ercumen A.; Pickering A. J.; Jeanis K. M.; Crider Y.; Ahmed M.; Brown S.; Alam M.; Sen D.; Islam S.; Kabir M. H.; Islam M.; Rahman M.; Kwong L. H.; Arnold B. F.; Luby S. P.; Colford J. M.; Nelson K. L. Effect of Sanitation Improvements on Pathogens and Microbial Source Tracking Markers in the Rural Bangladeshi Household Environment. Environ. Sci. Technol. 2020, 54, 4316. 10.1021/acs.est.9b04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills J. A.; Liu J.; Rogawski E. T.; Kabir F.; Lertsethtakarn P.; Siguas M.; Khan S. S.; Praharaj I.; Murei A.; Nshama R.; Mujaga B.; Havt A.; Maciel I. A.; McMurry T. L.; Operario D. J.; Taniuchi M.; Gratz J.; Stroup S. E.; Roberts J. H.; Kalam A.; Aziz F.; Qureshi S.; Islam M. O.; Sakpaisal P.; Silapong S.; Yori P. P.; Rajendiran R.; Benny B.; McGrath M.; McCormick B. J. J.; Seidman J. C.; Lang D.; Gottlieb M.; Guerrant R. L.; Lima A. A. M.; Leite J. P.; Samie A.; Bessong P. O.; Page N.; Bodhidatta L.; Mason C.; Shrestha S.; Kiwelu I.; Mduma E. R.; Iqbal N. T.; Bhutta Z. A.; Ahmed T.; Haque R.; Kang G.; Kosek M. N.; Houpt E. R.; Acosta A. M.; Rios de Burga R.; Chavez C. B.; Flores J. T.; Olotegui M. P.; Pinedo S. R.; Trigoso D. R.; Vasquez A. O.; Ahmed I.; Alam D.; Ali A.; Rasheed M.; Soofi S.; Turab A.; Yousafzai A.; Zaidi A. K.; Shrestha B.; Rayamajhi B. B.; Strand T.; Ammu G.; Babji S.; Bose A.; George A. T.; Hariraju D.; Jennifer M. S.; John S.; Kaki S.; Karunakaran P.; Koshy B.; Lazarus R. P.; Muliyil J.; Ragasudha P.; Raghava M. V.; Raju S.; Ramachandran A.; Ramadas R.; Ramanujam K.; Rose A.; Roshan R.; Sharma S. L.; Sundaram S.; Thomas R. J.; Pan W. K.; Ambikapathi R.; Carreon J. D.; Doan V.; Hoest C.; Knobler S.; Miller M. A.; Psaki S.; Rasmussen Z.; Richard S. A.; Tountas K. H.; Svensen E.; Amour C.; Bayyo E.; Mvungi R.; Pascal J.; Yarrot L.; Barrett L.; Dillingham R.; Petri W. A.; Scharf R.; Ahmed A. S.; Alam M. A.; Haque U.; Hossain M. I.; Islam M.; Mahfuz M.; Mondal D.; Nahar B.; Tofail F.; Chandyo R. K.; Shrestha P. S.; Shrestha R.; Ulak M.; Bauck A.; Black R.; Caulfield L.; Checkley W.; Lee G.; Schulze K.; Scott S.; Murray-Kolb L. E.; Ross A. C.; Schaefer B.; Simons S.; Pendergast L.; Abreu C. B.; Costa H.; Di Moura A.; Filho J. Q.; Leite Á. M.; Lima N. L.; Lima I. F.; Maciel B. L.; Medeiros P. H.; Moraes M.; Mota F. S.; Oriá R. B.; Quetz J.; Soares A. M.; Mota R. M.; Patil C. L.; Mahopo C.; Maphula A.; Nyathi E. Use of Quantitative Molecular Diagnostic Methods to Assess the Aetiology, Burden, and Clinical Characteristics of Diarrhoea in Children in Low-Resource Settings: A Reanalysis of the MAL-ED Cohort Study. Lancet Glob. Health 2018, 6 (12), e1309 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grembi J. A; Lin A.; Karim M. A.; Islam M. O.; Miah R.; Arnold B. F; McQuade E. T R.; Ali S.; Rahman M. Z.; Hussain Z.; Shoab A. K; Famida S. L; Hossen M. S.; Mutsuddi P.; Rahman M.; Unicomb L.; Haque R.; Taniuchi M.; Liu J.; Platts-Mills J. A; Holmes S. P; Stewart C. P; Benjamin-Chung J.; Colford J. M; Houpt E. R; Luby S. P Effect of Water, Sanitation, Handwashing and Nutrition Interventions on Enteropathogens in Children 14 Months Old: A Cluster-Randomized Controlled Trial in Rural Bangladesh. J. Infect. Dis. 2020, 549. 10.1093/infdis/jiaa549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A.; Ercumen A.; Benjamin-Chung J.; Arnold B. F.; Das S.; Haque R.; Ashraf S.; Parvez S. M.; Unicomb L.; Rahman M.; Hubbard A. E.; Stewart C. P.; Colford J. M.; Luby S. P. Effects of Water, Sanitation, Handwashing, and Nutritional Interventions on Child Enteric Protozoan Infections in Rural Bangladesh: A Cluster-Randomized Controlled Trial. Clin. Infect. Dis. 2018, 320. 10.1093/cid/ciy320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauza V.; Ocharo R. M.; Nguyen T. H.; Guest J. S. Soil Ingestion Is Associated with Child Diarrhea in an Urban Slum of Nairobi, Kenya. Am. J. Trop. Med. Hyg. 2017, 96 (3), 569–575. 10.4269/ajtmh.16-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M. D.; Galiani S.; Gertler P. J.; Martinez S.; Titiunik R. Housing, Health, and Happiness. Am. Econ. J. Econ. Policy 2009, 1 (1), 75–105. 10.1257/pol.1.1.75. [DOI] [Google Scholar]
- Reid B.; Orgle J.; Roy K.; Pongolani C.; Chileshe M.; Stoltzfus R. Characterizing Potential Risks of Fecal-Oral Microbial Transmission for Infants and Young Children in Rural Zambia. Am. J. Trop. Med. Hyg. 2018, 98 (3), 816–823. 10.4269/ajtmh.17-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. A.; Ahmed T.; Faruque A. S. G.; Rahman S.; Das S. K.; Ahmed D.; Fattori V.; Clarke R.; Endtz H. P.; Cravioto A. Microbiological Quality of Complementary Foods and Its Association with Diarrhoeal Morbidity and Nutritional Status of Bangladeshi Children. Eur. J. Clin. Nutr. 2012, 66 (11), 1242–1246. 10.1038/ejcn.2012.94. [DOI] [PubMed] [Google Scholar]
- Islam M. S.; Mahmud Z. H.; Gope P. S.; Zaman R. U.; Hossain Z.; Islam M. S.; Mondal D.; Sharker M. A. Y.; Islam K.; Jahan H.; Bhuiya A.; Endtz H. P.; Cravioto A.; Curtis V.; Toure O.; Cairncross S. Hygiene Intervention Reduces Contamination of Weaning Food in Bangladesh. Trop. Med. Int. Health 2012, 18 (3), 250–258. 10.1111/tmi.12051. [DOI] [PubMed] [Google Scholar]
- Taulo S.; Wetlesen A.; Abrahamsen R. K.; Narvhus J. A.; Mkakosya R. Quantification and Variability of Escherichia Coli and Staphylococcus Aureus Cross-Contamination during Serving and Consumption of Cooked Thick Porridge in Lungwena Rural Households, Malawi. Food Control 2009, 20 (12), 1158–1166. 10.1016/j.foodcont.2009.03.009. [DOI] [Google Scholar]
- Lindeberg Y. L.; Egedal K.; Hossain Z. Z.; Phelps M.; Tulsiani S.; Farhana I.; Begum A.; Jensen P. K. M. Can Escherichia Coli Fly? The Role of Flies as Transmitters of E. coli to Food in an Urban Slum in Bangladesh. Trop. Med. Int. Health 2018, 23 (1), 2–9. 10.1111/tmi.13003. [DOI] [PubMed] [Google Scholar]
- Parvez S. M.; Kwong L.; Rahman M. J.; Ercumen A.; Pickering A. J.; Ghosh P. K; Rahman M. Z.; Das K. K.; Luby S. P.; Unicomb L. E. coli Contamination of Complementary Foods and Associations with Domestic Hygiene in Rural Bangladesh. Trop. Med. Int. Health 2017, 22 (5), 547–557. 10.1111/tmi.12849. [DOI] [PubMed] [Google Scholar]
- Luby S. P.; Halder A. K.; Huda T.; Unicomb L.; Johnston R. B. The Effect of Handwashing at Recommended Times with Water Alone and with Soap on Child Diarrhea in Rural Bangladesh: An Observational Study. PLoS Med. 2011, 8 (6), e1001052 10.1371/journal.pmed.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doza S.; Jabeen Rahman M.; Islam M. A.; Kwong L. H.; Unicomb L.; Ercumen A.; Pickering A. J.; Parvez S. M.; Naser A. M.; Ashraf S.; Das K. K.; Luby S. P. Prevalence and Association of Escherichia Coli and Diarrheagenic Escherichia Coli in Stored Foods for Young Children and Flies Caught in the Same Households in Rural Bangladesh. Am. J. Trop. Med. Hyg. 2018, 98 (4), 1031–1038. 10.4269/ajtmh.17-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A. J.; Julian T. R.; Marks S. J.; Mattioli M. C.; Boehm A. B.; Schwab K. J.; Davis J. Fecal Contamination and Diarrheal Pathogens on Surfaces and in Soils among Tanzanian Households with and without Improved Sanitation. Environ. Sci. Technol. 2012, 46 (11), 5736–5743. 10.1021/es300022c. [DOI] [PubMed] [Google Scholar]
- Byappanahalli M.; Fujioka R. Evidence That Tropical Soil Environment Can Support the Growth of Escherichia Coli. Water Sci. Technol. 1998, 38 (12), 171–174. 10.2166/wst.1998.0533. [DOI] [Google Scholar]
- Fuhrmeister E. R.; Ercumen A.; Pickering A. J.; Jeanis K. M.; Ahmed M.; Brown S.; Arnold B. F.; Hubbard A. E.; Alam M.; Sen D.; Islam S.; Kabir M. H.; Kwong L. H.; Islam M.; Unicomb L.; Rahman M.; Boehm A. B.; Luby S. P.; Colford J. M.; Nelson K. L. Predictors of Enteric Pathogens in the Domestic Environment from Human and Animal Sources in Rural Bangladesh. Environ. Sci. Technol. 2019, 53 (17), 10023–10033. 10.1021/acs.est.8b07192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M.; Sobuz S. U.; Begum S.; Platts-Mills J. A.; Liu J.; Yang Z.; Wang X.-Q.; Petri W. A.; Haque R.; Houpt E. R. Etiology of Diarrhea in Bangladeshi Infants in the First Year of Life Analyzed Using Molecular Methods. J. Infect. Dis. 2013, 208 (11), 1794–1802. 10.1093/infdis/jit507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellefont L. A.; McMeekin T. A.; Ross T. Performance Evaluation of a Model Describing the Effects of Temperature, Water Activity, PH and Lactic Acid Concentration on the Growth of Escherichia Coli. Int. J. Food Microbiol. 2003, 82 (1), 45–58. 10.1016/S0168-1605(02)00253-2. [DOI] [PubMed] [Google Scholar]
- Topp E.; Welsh M.; Tien Y.-C.; Dang A.; Lazarovits G.; Conn K.; Zhu H. Strain-Dependent Variability in Growth and Survival of Escherichia Coli in Agricultural Soil. FEMS Microbiol. Ecol. 2003, 44 (3), 303–308. 10.1016/S0168-6496(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Medema G. J.; Bahar M.; Schets F. M. Survival of Cryptosporidium Parvum, Escherichia Coli, Faecal Enterococci, and Clostridium Perfringens in River Water: Influence of Temperature and Autochthonous Microorganisms. Water Sci. Technol. 1997, 35 (11–12), 249–252. 10.2166/wst.1997.0742. [DOI] [Google Scholar]
- Raman A. S.; Gehrig J. L.; Venkatesh S.; Chang H.-W.; Hibberd M. C.; Subramanian S.; Kang G.; Bessong P. O.; Lima A. A. M.; Kosek M. N.; Petri W. A.; Rodionov D. A.; Arzamasov A. A.; Leyn S. A.; Osterman A. L.; Huq S.; Mostafa I.; Islam M.; Mahfuz M.; Haque R.; Ahmed T.; Barratt M. J.; Gordon J. I. A Sparse Covarying Unit That Describes Healthy and Impaired Human Gut Microbiota Development. Science 2019, 365 (6449), eaau4735 10.1126/science.aau4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J.; Bradley D. The Relationship between Infecting Dose and Severity of Disease in Reported Outbreaks of Salmonella Infections. Epidemiol. Infect. 1992, 109, 371–388. 10.1017/S0950268800050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercumen A.; Pickering A. J.; Kwong L. H.; Arnold B. F.; Parvez S. M.; Alam M.; Sen D.; Islam S.; Kullmann C.; Chase C.; Ahmed R.; Unicomb L.; Luby S. P.; Colford J. M. Animal Feces Contribute to Domestic Fecal Contamination: Evidence from E. coli Measured in Water, Hands, Food, Flies, and Soil in Bangladesh. Environ. Sci. Technol. 2017, 51 (15), 8725–8734. 10.1021/acs.est.7b01710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.