Abstract
Many studies suggested shared psychological and neural representations for first‐hand physical pain and empathy for others' pain, both of which depend strongly upon top–down controlled mechanisms such as attention. This study aimed to assess the interindividual variation in first‐hand physical pain and empathy for pain, and whether their relationship is dependent upon attention. We recruited participants exhibiting high and low sensitivity to first‐hand pain (HPS and LPS), and adopted pain empathy paradigms involving attention directed toward or withdrawn from pain of another. Relative to the LPS group, participants in the HPS group estimated greater pain intensity experienced by others, felt greater unpleasantness when viewing others in pain, and exhibited greater sensitivity in discriminating others' pain. Electroencephalographic data showed that when attention was directed toward others' pain, only participants in the HPS group exhibited significant pain empathic effects on the N1 component of event‐related potentials and on the α‐oscillation response. These empathic neural responses mediated the linkage between first‐hand pain sensitivity and empathic behavioral responses. Nevertheless, empathic responses were comparable between two groups when attention was withdrawn from others' pain. These results demonstrate a shared sensitivity to first‐hand pain and empathy for pain provided that attention is directed toward pain.
Keywords: attention, empathy for pain, event‐related potentials (ERPs), first‐hand pain, α‐oscillation
This study compared the empathic responses between individuals with high and low sensitivity to first‐hand pain, during empathy for pain tasks in which attention was either directed toward or withdrawn from pain cues in the visual displays. Results showed that individuals with high sensitivity to first‐hand painexhibited greater empathic responses to others' pain, provided that attention was directed toward the pain being experienced by another. It suggests that top‐down control of attention matters in the link between first‐hand pain sensitivity and empathy for others' pain, thus supporting a qualified version of the “shared representations” theory of empathy.

1. INTRODUCTION
Empathy is the ability to share and understand others' emotional experiences (de Vignemont & Singer, 2006) and plays an important role in social interactions (Decety, Bartal, Uzefovsky, & Knafo‐Noam, 2016). According to the “shared representations” theory (Jackson, Meltzoff, & Decety, 2005; Preston & de Waal, 2002), empathy for others' pain relies on the same psychological and neural representations that underlie first‐hand experience of physical pain. Consistent with this theory, many neuroimaging studies have shown that vicariously witnessing pain in others activates brain areas such as the bilateral anterior insula and anterior midcingulate cortex, which partially overlap with those associated with first‐hand experience of pain (Jackson et al., 2005; Lamm, Decety, & Singer, 2011; Singer et al., 2004). Beyond the evidence for the overlapping brain activations, experimentally manipulating the first‐hand experience of pain can affect empathic responses to others' pain (Mischkowski, Crocker, & Way, 2016; Rütgen, Seidel, Silani, et al., 2015), thus providing more causal evidence for the “shared representations” theory. For example, the common prescription painkiller acetaminophen can reduce empathy for others' pain (Mischkowski et al., 2016), suggesting that pharmacologically inhibiting the neural circuits necessary for experiencing one's own pain also inhibits the perception/experience of others' pain. Further, electroencephalographic (EEG) studies have shown similar modulations of self and other‐related pain processing (Meng et al., 2013; Peng, Huang, Liu, & Cui, 2019; Rütgen, Seidel, Riečanský, & Lamm, 2015), for example, placebo analgesia induction procedure equivalently reduces behavioral responses and the affective‐motivational P2 component on event‐related potentials (ERPs) to self and other‐related pain (Rütgen, Seidel, Riečanský, et al., 2015). The evidence for the association between the perception of first‐hand pain and others' pain predicts shared sensitivity to first‐hand physical pain and empathy for others' pain.
Nevertheless, other evidence suggests that specialized neural circuits are required for pain empathy (Krishnan et al., 2016), because empathy for others' pain primarily reflects cognitive experiences whose experiential qualities are hard to mimic or simulate directly (Hooker, Verosky, Germine, Knight, & D'Esposito, 2008). By combining functional neuroimaging with multivariate pattern analyses, Krishnan et al. (2016) showed that somatic pain (heat‐related pain) and vicarious pain (viewing pictures of others' being injured) were represented by dissociable multivariate brain patterns, respectively localized within somatosensory and mentalizing‐related circuits. Beyond these dissociable brain patterns, dissociable sensitivity to first‐hand and empathic pain has been observed among individuals with autism spectrum conditions (Chen et al., 2017) and patients with somatoform pain disorder (Peng, Meng, et al., 2019) who exhibited hypersensitivity to first‐hand pain but hyposensitivity to vicarious pain. The evidence for the disassociation between perceptions of first‐hand pain and others' pain predicts asynchronous sensitivity to first‐hand physical pain and empathy for others' pain, under certain conditions.
In line with first‐hand experiences of physical pain that are modulated by attention (Legrain, Guérit, Bruyer, & Plaghki, 2002; Tracey et al., 2002), empathic responses to others' pain also depend strongly upon this top–down controlled mechanism (Y. Fan & Han, 2008; Gu & Han, 2007; Meng, Shen, Li, & Peng, 2019). Previous studies manipulated top–down attention to others' pain by asking participants either to rate pain intensity felt by the model (attention directed toward painful aspects of the stimuli) or to count the number of hands in the stimulus displays (attention withdrawn from the painful aspects of the stimuli), while perceptual features of the stimuli were controlled (Y. Fan & Han, 2008; Gu & Han, 2007). Brain regions associated with empathy for pain (e.g., anterior cingulate cortex and insula) became active when paying attention to the painful aspect of the stimuli, but not when directing attention away from the painful aspect (Gu & Han, 2007). The attentional constraint on empathic pain processing was further confirmed by the temporal dynamics of ERP responses (Y. Fan & Han, 2008; Meng et al., 2019). Given that neural correlates of empathic pain processing are sensitive to top–down attentional modulation, the linkage between sensitivity to first‐hand pain and empathy for pain is likely dependent upon top–down modulations in attention.
Therefore, this study aimed to clarify the interindividual linkage between sensitivity to first‐hand pain and empathy for others' pain among healthy individuals, and whether this linkage can be influenced by manipulating top–down attention. Given that EEG/ERP measurement provides a window for investigating the temporal dynamics of neural responses to perceiving others' pain, we recruited participants exhibiting high and low sensitivity to first‐hand pain (HPS and LPS), and compared their behavioral and EEG/ERP responses during pain‐empathy tasks in which they directed their attention toward or away from pain cues. Previous ERP studies of empathy for pain (Decety, Yang, & Cheng, 2010; Y. Fan & Han, 2008; Meng et al., 2013) have shown early effects of pain in the fronto‐central N1 and N2 components, as well as latter effects of pain in the centro‐parietal P3 and late positive potential (LPP) components. Pain effects on these ERP components have been associated with early automatic and late controlled processes of others' pain (Cheng, Chen, & Decety, 2014; Fabi & Leuthold, 2017; Y. Fan & Han, 2008). In addition, observation of others' painful situation induced a greater suppression of brain oscillations within the alpha frequency band (α band: 8–14 Hz) as compared to the observation of nonpainful situation (Fabi & Leuthold, 2017; Li, Meng, Li, Yang, & Yuan, 2017; Motoyama, Ogata, Hoka, & Tobimatsu, 2017; Perry, Bentin, Bartal, Lamm, & Decety, 2010), manifested as greater alpha event‐related desynchronization (α‐ERD) reflecting cortical activations associated with stimulus significance (De Cesarei & Codispoti, 2011; Schubring & Schupp, 2019; Simons, Detenber, Cuthbert, Schwartz, & Reiss, 2003). These empathy‐related neural responses (ERPs and α‐ERD) during the tasks were compared between participants in the HPS and LPS groups, for identifying the linkage between first‐hand pain sensitivity and empathy for others' pain.
2. MATERIALS AND METHODS
2.1. Participants
A total of 1,318 healthy college students from Shenzhen University (Guangdong, China) filled out the Chinese version of the 17‐item Pain Sensitivity Questionnaire (PSQ)—a validated self‐rating measure of pain perception that covers painful situations in daily life (Quan et al., 2018; Ruscheweyh, Marziniak, Stumpenhorst, Reinholz, & Knecht, 2009). According to the distribution of PSQ scores (4.82 ± 0.04, Figure S1 of Supplementary Materials), we assigned participants to HPS or LPS groups if their PSQ scores landed in the upper (PSQ ≥5.86) or lower (PSQ ≤3.79) quartiles, respectively. Then, a subset of participants from the HPS and LPS groups were randomly selected and contacted by the experimenters to inform about the EEG experiment procedure. As a result, a total of 62 participants were recruited to participate in the EEG experiment, composed of the HPS group (n = 29; 13 females, aged 20.79 ± 0.41 years) and LPS group (n = 33; 19 females, aged 20.21 ± 0.37 years). Groups did not differ in age (t 60 = −1.06, p = .29, independent‐sample t‐test) or sex (χ 2 = 1.00, p = .32, chi‐square test). All participants were right‐handed and had normal or corrected‐to‐normal vision. No participants reported medical conditions associated with acute or chronic pain, cardiovascular or neurological diseases, psychiatric disorders, or current use of any medication. All participants gave their written informed consent before the experiments according to Declaration of Helsinki and all experimental procedures were approved by the Ethics Committee of Shenzhen University.
The Fear of Pain Questionnaire (FPQ; McNeil & Rainwater, 1998) and the Pain Catastrophizing Scale (PCS; Sullivan, Bishop, & Pivik, 1995) were administered to participants in both groups to assess their cognitive attitudes toward pain (e.g., beliefs and thoughts about pain). In addition, their empathy trait was assessed using the Interpersonal Reactivity Index (IRI; Davis, 1983), which consists of four subscales: perspective taking, empathic fantasy, empathic concern, and personal distress. Following this, participants in both groups completed the first‐hand pain and empathy for pain experiments, which were conducted on two separate days. The order of experiments was counterbalanced across the participants.
2.2. Assessment of first‐hand pain sensitivity
To validate that the two groups differed in their sensitivity to first‐hand physical pain, all participants were asked to complete an experimental pain‐sensitivity assessment. We used a constant current stimulator (type: SXC‐4A, Sanxia Technique, Inc., China) to deliver electrical stimulation through ring electrodes placed on the fourth finger of the left hand. A series of single pulse electrical stimulations (pulse duration: 50 ms; intensity range: 300to 2,500 μA; in ascending steps of 100 μA) was delivered with an intertrial interval varying randomly between 4,000 and 6,000 ms. After each stimulation, participants were instructed to rate the perceived pain intensity using an 11‐point numerical rating scale (NRS) ranging from 0 (no pain) to 10 (unbearable pain). Subjective ratings to electrical stimulations at varying intensities were defined as the individual stimulus–response function that depicted the relationship between objective stimulation intensity and subjective pain intensity.
2.3. Assessment of empathy for others' pain
A classical pain empathy experiment was adopted by exposing participants to pictures depicting others in painful or nonpainful situations (Fabi & Leuthold, 2017; Y. Fan & Han, 2008; Gonzalez‐Liencres, Brown, Tas, Breidenstein, & Brüne, 2016; Gu & Han, 2007). The empathic stimuli used in the experiment were 60 color pictures of hands in painful or nonpainful situations (30 pictures per type; Meng et al., 2013). The same 60 pictures contained either one or two hands (30 pictures per type). All the situations in the pictures depicted ordinary events that occasionally happen in daily life (Figure S2 of Supplementary Materials). All the events shown in the nonpainful pictures corresponded to those in the painful pictures, but without the nociceptive component. The luminance, contrast, and color were well matched between each pair of painful and nonpainful pictures. During the experiment, all digital pictures were presented at the center of a black background, with a visual angle of 12.8° × 7.7° at a viewing distance of 100 cm. Stimulus presentation was controlled using the E‐prime 3.0 software (Psychology Software Tools, Inc., Pittsburgh, PA).
2.3.1. Pain judgment and hands counting tasks
As illustrated in Figure 1a, each participant was first instructed to complete the Pain Judgment Task and the Hands Counting Task, the order of which was counterbalanced across the participants. Each experimental trial started with a 500‐ms fixation on a black screen. After a blank interval that randomly lasted 1,000–2,000 ms, a painful or nonpainful picture was presented. In the Pain Judgment Task, participants were required to respond as quickly and as accurately as possible by pressing a key on the keyboard (either “F” or “J”) to indicate whether the situation depicted in the picture was painful or not. In the Hands Counting Task, participants were required to respond as quickly and as accurately as possible by pressing a key (either “F” or “J”) to indicate how many hands were in the picture (one or two). Each experimental task comprised 60 trials, 30 of which contained painful pictures. The intertrial interval varied randomly between 2,000 and 4,000 ms. Key‐assignment for both tasks was counterbalanced across participants. Behavioral data including reaction times (RTs) and accuracies (ACCs), as well as EEG data were continuously recorded throughout the tasks.
FIGURE 1.
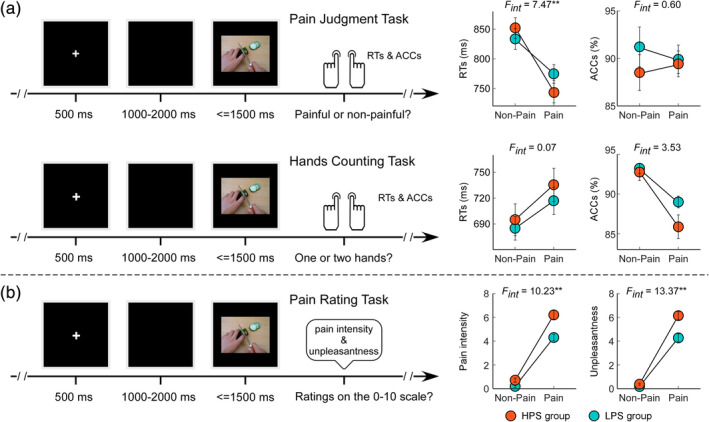
Experimental design and behavioral results. The empathy for pain experiment used the Pain Judgment and Hands Counting Tasks (a), as well as the Pain Rating Task (b). For each task, either painful or nonpainful pictorial stimulation was presented. The stimulations were identical for the three tasks. Participants were required to judge whether the pictorial stimulation was painful or nonpainful during the Pain Judgment Task, whether it contained one or two hands during the Hands Counting Task, and report the intensity of the depicted pain intensity and their own feeling of unpleasantness on a 0–10 numerical rating scale (NRS) during the Pain Rating Task. RTs and ACCs obtained during the Pain Judgment and Hands Counting Tasks, as well as subjective ratings of imaged pain intensity and self‐experienced unpleasantness obtained during the Pain Rating Task, were compared between HPS (orange) and LPS (blue) groups. Faster responses were made to painful stimulations than to nonpainful stimulations during the Pain Judgment Task, and slower responses were made to the painful stimulations than to the nonpainful stimulations during the Hands Counting Task. Compared with nonpainful stimulations, painful stimulations were judged as more painful for others and more unpleasant for themselves. Data are expressed as mean ± SEM. **p < .01
2.3.2. Pain rating task
In addition to these two EEG tasks, participants also completed the Pain Rating Task (Figure 1b), in which they reported the pain intensity being experienced by the model in the picture and the degree of unpleasantness they felt upon viewing it. Ratings were made on the predefined 0–10 NRS, with 0 for no pain/unpleasantness, and 10 for unbearable pain/unpleasantness. Note that the pictures used in the three different tasks (Pain Judgment Task, Hands Counting Task, and Pain Rating Task) were identical.
2.4. EEG recordings
EEG data were collected during the Pain Judgment and Hands Counting Tasks. Participants sat on a comfortable chair in a silent and temperature‐controlled room. They were instructed to focus on the stimuli, keep their eyes open, and gaze at a fixation point on the screen. EEG data were recorded using 64 Ag‐AgCl scalp electrodes placed according to the International 10–20 system (Brain Products GmbH; bandpass filter: 0.01–100 Hz; sampling rate: 1000 Hz). The electrode‐to‐skin impedances were kept below 10 kΩ for all electrodes. Electro‐oculographic (EOG) signals were simultaneously recorded using surface electrodes to monitor ocular movements and eye blinks.
2.5. EEG data processing
EEG data were processed using EEGLAB (Delorme & Makeig, 2004), an open source toolbox for the MATLAB environment (The MathWorks, Inc., Natick, MA). Continuous EEG data were bandpass filtered between 0.1 and 30 Hz. For each experimental task, EEG epochs that were time locked to the onset of the pictorial stimulation were extracted using a 1,500‐ms time window (500 ms prestimulus and 1,000 ms poststimulus) and baseline corrected using the prestimulus interval. EEG epochs were visually inspected, and trials contaminated by eye blinks and movements were corrected using an independent component analysis (ICA) algorithm (Delorme & Makeig, 2004). In all datasets, these independent components had a large EOG channel contribution and a frontal scalp distribution. After ICA and an additional baseline correction, EEG trials were re‐referenced to the bilateral mastoid electrodes.
2.5.1. Time‐domain analysis
Single‐trial ERP waveforms elicited by painful and nonpainful stimulations during the Pain Judgment and Hands Counting Tasks were averaged for each participant, thus yielding two average waveforms for each participant that were time locked to the onset of pictorial stimulation for each task. Subsequently, single‐participant averaged ERP waveforms were averaged to obtain group‐level waveforms, and scalp topographies were computed by spline interpolation. Dominant ERP components, including N1, N2, P3 and LPP, were identified according to the scalp topographies of grand average ERP activity and previous pain‐empathy studies (Decety et al., 2010; Y. Fan & Han, 2008; Meng et al., 2013). Specifically, N1 and N2 amplitudes were measured at fronto‐central electrodes (F1, Fz, F2, FC1, FCz, FC2) between 125–155 ms and 230–260 ms after pictorial stimulation onset, respectively. P3 amplitudes were measured at parietal electrodes (P1, Pz, P2, PO3, POz, PO4) between 300–400 ms after pictorial stimulation onset; LPP amplitudes were measured at centro‐parietal electrodes (CP1, CPz, CP2, P1, Pz, P2) between 400–800 ms after pictorial stimulation onset. Electrodes and time windows for assessing amplitudes of these ERP components were chosen according to the visual inspection of the grand average ERP waveforms and scalp topographies, as well as previous ERP studies using the same set of pictorial stimuli (Cui, Ma, & Luo, 2016; Meng et al., 2013; Zheng, Lyu, & Jackson, 2018).
2.5.2. Time‐frequency analysis
EEG time course in response to painful and nonpainful stimulations was transformed to the time‐frequency domain to identify EEG oscillatory responses related to empathy for pain. A time‐frequency distribution (TFD) of the EEG time course was obtained using a windowed Fourier transform with a fixed 250‐ms Hanning window (Zhang, Hu, Hung, Mouraux, & Iannetti, 2012). For each time course, the windowed Fourier transform yielded a complex time‐frequency estimate at each point on the time‐frequency plane, extending from −500 to 1,000 ms (in 2‐ms intervals) in the time domain, and from 1 to 30 Hz (in 1‐Hz intervals) in the frequency domain. The resulting spectrogram, P(t, f) = |F(t, f)|2, represents the signal power as a joint function of time and frequency at each time‐frequency point. The spectrograms were baseline corrected (reference interval: −400 to −100 ms relative to pictorial stimulation onset) at each frequency f using the normalization approach. This reference interval was chosen to reduce the adverse influence of spectral estimates biased by windowing poststimulus activity and padding values. Previous studies have shown that (a) greater α‐ERD response occurs over the centro‐parietal regions after exposure to others' painful situations than to neutral situations (Fabi & Leuthold, 2017; Motoyama et al., 2017; Perry et al., 2010), and that (b) α‐ERD response was more pronounced in response to more arousing stimuli compared to less arousing and neutral contents (De Cesarei & Codispoti, 2011; Schubring & Schupp, 2019; Simons et al., 2003). Thus, for each participant and stimulation condition, we measured α‐ERD magnitudes at centro‐parietal electrodes (CP1, CPz, CP2, P1, Pz, P2) by averaging the oscillation magnitudes in the 8–14 Hz frequency range and within 300–800 ms after pictorial stimulation onset. Scalp topographies of α‐ERD magnitudes were computed by spline interpolation.
2.6. Statistical analysis
All statistical analyses were carried out using the IBM SPSS statistical analysis package (version 22; IBM Corp., Armonk, NY). To validate the differences in first‐hand pain sensitivity between participants in the HPS and LPS groups, subjective ratings to electrical stimulations (300–2,500 μA, in steps of 100 μA) were compared between the two groups using independent‐sample t‐tests. The false discovery rate (FDR) method was used to correct the significance level for multiple comparisons (Benjamini & Hochberg, 1995). Psychometric scores, including the PSQ, FPQ, PCS, and the four subscales of the IRI, were also compared between HPS and LPS groups using independent‐sample t‐tests.
Behavioral responses (including RTs and ACCs) and neural responses (including ERP amplitudes and α‐ERD magnitudes) to observing painful and nonpainful situations during the Pain Judgment and Hands Counting Tasks were obtained for each participant. These responses were compared using a mixed‐design three‐way analysis of variance (ANOVA) with a between‐participant factor of Group (HPS vs. LPS group) and two within‐participant factors of Stimulation (painful vs. nonpainful stimulation) and Task (Pain Judgment vs. Hands Counting Task). When we found a significant three‐way interaction, we performed a post hoc two‐way ANOVA with factors of Stimulation and Group, separately for the Pain Judgment Task and Hands Counting Task. Subjective ratings to nonpainful and painful stimulations in the Pain Rating Task, including imagined pain intensity experienced by others and self‐experienced unpleasantness, were obtained for each participant. These ratings were compared using a two‐way ANOVA with a between‐participant factor of Group (HPS vs. LPS group) and a within‐participant factor of Stimulation (painful vs. nonpainful stimulation). When the two‐way interaction was of significance, post hoc comparisons were performed.
To test whether the effects of first‐hand pain sensitivity on pain empathic behavioral responses were driven by neural processing of others' pain, a participant‐level mediation analysis was performed using the SPSS version of the PROCESS macro (Hayes, 2017). In the mediation models, independent variable (X) was first‐hand pain sensitivity (1 for HPS group; −1 for LPS group); pain empathic effects on behavioral responses were the dependent variable (Y); pain empathic effects on neural responses were the mediator (M). For each mediation model, five path coefficients were calculated in a regression‐based approach, which quantified the relationship of X to M (path a), the relationship of M to Y controlling for X (path b), the relationship of X to Y (path c, the total effect of X on Y), the relationship of X to Y controlling for M (path c′, the direct effect of X on Y), and the mediation effect (path a*b, the indirect effect of X on Y through M). We performed a percentile bootstrap estimation analysis with 5,000 bootstrapped samples to calculate the mediation effect (a*b). It determined the indirect effects of first‐hand pain sensitivity on empathic behavioral responses through neural responses. Analysis yielded the 95% confidence intervals (CIs) of the indirect effects. These effects were considered statistically significant at p < .05 when the 95% CIs did not include zero. The effect size for the mediation analysis was quantified as the relative mediation effect (P M), referring to the proportion of the total effect that is mediated (Preacher & Kelley, 2011). It is calculated as P M = 1 – c′/c, in which c is the regression coefficient for the relationship of X to Y, c′ is the regression coefficient for the relationship of X to Y controlling for M.
3. RESULTS
3.1. First‐hand pain sensitivity
As shown in Figure 2, pain‐intensity ratings did not differ between groups when electrical stimulation intensity was lower than 700 μA (subthreshold stimulation, p fdr > .05). However, when the electrical stimulation intensity was higher than 700 μA (suprathreshold stimulation), the HPS group reported higher pain intensity than did the LPS group (pfdr < .05), despite receiving identical electrical stimulation. Thus, the HPS group was more sensitive to physical pain than the LPS group, which validated the group categorization based on the PSQ scores. Independent‐sample t‐tests further revealed that total scores on the PSQ, FPQ, and PCS were significantly greater for the HPS group than for the LPS group (p < .001 for PSQ and FPQ, p = .01 for PCS, Table 1), suggesting a more negative attitude toward pain (including more negative thoughts and emotions) for participants in the HPS group.
FIGURE 2.
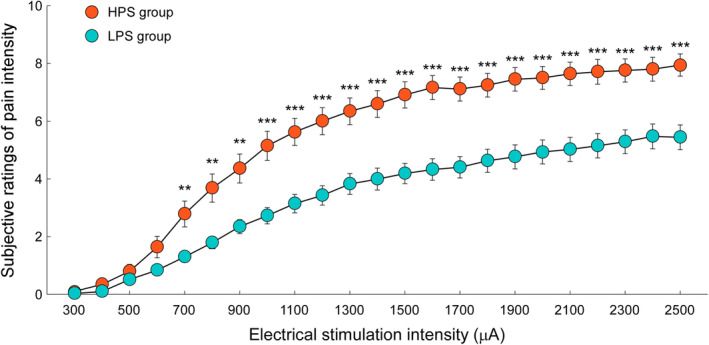
Subjective ratings of pain intensity to electrical stimulations. Subjective ratings of pain intensity elicited by electrical stimulations (ranging from 300 to 2,500 μA, in steps of 100 μA) were compared between HPS (orange) and LPS (blue) groups. Data are expressed as mean ± SEM. In contrast to the LPS group, participants in the HPS group reported greater ratings of pain intensity in response to identical suprathreshold electrical stimulations (>700 μA). **p fdr < .01; ***: p fdr < .001
TABLE 1.
Psychometric variables for HPS and LPS groups
| Grand average | Statistics | |||
|---|---|---|---|---|
| HPS group | LPS group | t 60 | p | |
| PSQ | 6.89 ± 0.13 | 2.78 ± 0.12 | 22.72 | <.001 |
| FPQ | 100.34 ± 2.74 | 81.39 ± 1.99 | 5.69 | <.001 |
| PCS | 22.45 ± 1.93 | 16.27 ± 1.26 | 2.68 | .01 |
| IRI | ||||
| Perspective taking | 15.59 ± 0.76 | 14.91 ± 0.53 | 0.70 | .48 |
| Fantasy | 17.55 ± 0.73 | 15.39 ± 0.62 | 2.27 | .027 |
| Empathic concerns | 16.00 ± 0.58 | 16.39 ± 0.54 | 0.49 | .62 |
| Personal distress | 14.55 ± 1.01 | 10.24 ± 0.70 | 3.58 | .001 |
Notes: Data are expressed as mean ± SEM. Statistical comparisons between HPS and LPS groups were conducted using independent‐sample t‐tests.
Abbreviations: FPQ, Fear of Pain Questionnaire; HPS, high pain sensitivity; IRI, Interpersonal Reactivity Index; LPS, low pain sensitivity; PCS, Pain Catastrophizing Scale; PSQ, Pain Sensitivity Questionnaire.
3.2. Empathic behavioral responses
Scores on the personal distress and fantasy subscales of the IRI were higher for the HPS group than the LPS group (p = .001 and p = .027, respectively, Table 1). However, scores on other two subscales did not significantly differ between groups. Behavioral responses (including RTs and ACCs) during the Pain Judgment and Hands Counting Tasks were compared using a mixed‐design three‐way ANOVA with factors of Group, Stimulation, and Task. Relevant statistics are summarized in Table 2. Subjective ratings to painful and nonpainful stimulations (including estimated pain intensity in others and self‐experienced unpleasantness) during the Pain Rating Task were compared using two‐way ANOVA with factors of Group and Stimulation.
TABLE 2.
Statistical comparisons of neural responses during Pain Judgment and Hands Counting Tasks for HPS and LPS groups
| RTs | ACCs | N1 amp. | N2 amp. | P3 amp. | LPP amp. | α‐ERD mag. | ||
|---|---|---|---|---|---|---|---|---|
| Group | F = 0.05 | F = 1.75 | F = 0.78 | F = 1.24 | F = 0.23 | F = 0.01 | F = 0.54 | |
| Stimulation | F = 20.07*** | F = 14.51*** | F = 3.11 | F = 1.26 | F = 1.44 | F = 63.19*** | F = 1.35 | |
| Task | F = 71.24*** | F = 0.18 | F = 2.87 | F = 1.45 | F = 24.47*** | F = 14.13*** | F = 0.98 | |
| Group × Stimulation | F = 3.60 | F = 0.02 | F = 4.96* | F = 0.01 | F = 2.63 | F = 0.56 | F = 3.11 | |
| Group × Task | F = 0.81 | F = 0.01 | F = 0.001 | F = 0.09 | F = 0.15 | F = 1.77 | F = 0.03 | |
| Stimulation × Task | F = 139.17*** | F = 10.20** | F = 0.88 | F = 0.004 | F = 8.99** | F = 63.77*** | F = 0.38 | |
| Group × Stimulation × Task | F = 7.93** | F = 2.11 | F = 4.51* | F = 0.18 | F = 1.67 | F = 0.03 | F = 4.35* | |
| Post hoc two‐way ANOVA | ||||||||
| Pain Judgment Task | Stimulation | F = 88.33*** | F = 3.43 | F = 1.30 | ||||
| Group | F = 0.06 | F = 0.76 | F = 0.33 | |||||
| Stimulation × Group | F = 7.47** | F = 9.25** | F = 6.23* | |||||
| Hands Counting Task | Stimulation | F = 47.24*** | F = 0.22 | F = 0.15 | ||||
| Group | F = 0.39 | F = 0.66 | F = 0.70 | |||||
| Stimulation × Group | F = 0.67 | F = 0.01 | F = 0.12 | |||||
Notes: Statistics were obtained by applying mixed‐design three‐way ANOVA with one between‐participant factor of Group (HPS or LPS group) and two within‐participant factors of Stimulation (painful vs. nonpainful stimulation) and Task (Pain Judgment vs. Hands Counting Task) on behavioral and neural responses during the empathy for pain tasks.
***p < .001; **p < .01; *p < .05.
3.2.1. Pain judgment and hands counting tasks
Grand average behavioral responses during the Pain Judgment and Hands Counting Tasks are shown in Figure 1a. As revealed by the mixed‐design three‐way ANOVA, ACCs were significantly modulated by the main effect of Stimulation (F 1,60 = 14.51, p < .001, η p 2 = 0.20) such that ACCs for painful stimulations were significantly lower than those for nonpainful stimulations. They were also significantly modulated by the interaction between Stimulation and Task (F 1,60 = 10.20, p = .002, η p 2 = 0.15): although ACCs for painful stimulations were lower than those for nonpainful stimulations during the Hands Counting Task (p < .001), no significant difference was observed during the Pain Judgment Task (p = .89). Analysis of RTs showed main effects of Stimulation (F 1,60 = 20.07, p < .001, η p 2 = 0.25) and Task (F 1,60 = 71.24, p < .001, η p 2 = 0.54), such that faster responses were made to painful stimulations than to nonpainful stimulations, and to stimulations in the Hands Counting Task than in the Pain Judgment Task. Analysis also revealed a significant three‐way interaction of Group, Stimulation, and Task (F 1,60 = 7.93, p = .007, η p 2 = 0.12). Post hoc two‐way ANOVA on the RTs during the Pain Judgment Task showed a significant main effect of Stimulation (F 1,60 = 88.33, p < .001, η p 2 = 0.60) as well as a significant interaction between Stimulation and Group (F 1,60 = 7.47, p = .008, η p 2 = 0.11). While faster responses were made to painful stimulations than to nonpainful stimulations, the differential RTs to painful and nonpainful stimulations were significantly greater in the HPS group than in the LPS group (−107.25 ± 14.62 ms vs. −58.93 ± 10.48 ms, p = .008). In contrast, post hoc two‐way ANOVA on the RTs during the Hands Counting Task only showed a significant main effect of Stimulation (F 1,60 = 47.24, p < .001, η p 2 = 0.44) such that responses to painful stimulations were slower than those to nonpainful stimulations. These results suggested that when attention was directed toward painful aspects of pictorial stimulations, pain cues in the pictures facilitated behavioral reactions overall, but this facilitation effect was greater for the HPS group. In contrast, when attention was directed away from the painful aspects of pictorial stimulations, pain cues in the pictures interfered with the ability to discriminate the number of hands similarly for both groups.
3.2.2. Pain rating task
Grand average intensity ratings of the pain depicted in the pictures, as well as the degree of unpleasantness felt by the participants when viewing them, are shown in Figure 1b. Both ratings were significantly modulated by the main effect of Stimulation (pain intensity: F 1,60 = 494.97, p < .001, η p 2 = 0.89; unpleasantness: F 1,60 = 466.74, p < .001, η p 2 = 0.89), such that pain intensity and unpleasantness ratings were higher for painful stimulations than for nonpainful stimulations. These ratings were also significantly modulated by the main effect of Group (pain intensity: F 1,60 = 19.45, p < .001, η p 2 = 0.25; unpleasantness: F 1,60 = 15.65, p < .001, η p 2 = 0.21), such that ratings given by the HPS group were higher than those provided by the LPS group. Importantly, we found a significant interaction between Stimulation and Group for both pain intensity (F 1,60 = 10.23, p = .002, η p 2 = 0.15) and unpleasantness ratings (F 1,60 = 13.37, p = .001, η p 2 = 0.18). Post hoc comparisons revealed that the differences of ratings to painful and nonpainful stimulations were significantly greater for the HPS group than for the LPS group (pain intensity: 5.48 ± 0.33 vs. 4.11 ± 0.29, p = .002; unpleasantness: 5.76 ± 0.31 vs. 4.09 ± 0.33, p = .001). These results indicated that while both groups exhibited significantly empathic ratings toward others in pain, these empathic ratings (i.e., greater ratings for painful stimulations than for nonpainful ones) were significantly larger for the HPS group.
3.3. Empathic brain responses
Grand average ERP activity during the Pain Judgment and Hands Counting Tasks, measured at fronto‐central electrodes and parietal electrodes, are shown in Figure 3 (for the Pain Judgment Task) and in Figure S3 (for the Hands Counting Task, see Supplementary Materials). Additionally, ERP activities measured at bilateral occipital electrodes (PO3, PO4, PO7, PO8, O1, O2) were shown in Figure S4 (Supplementary Materials). Consistent with previous studies (Cui et al., 2016; Decety et al., 2010; Meng et al., 2013), painful and nonpainful stimulations elicited N1 and N2 responses with maximal distribution over fronto‐central electrodes, followed by P3 and LPP responses with maximal distribution over parietal and centro‐parietal electrodes, respectively. Amplitudes of these ERP components were compared using three‐way ANOVA with factors of Group, Stimulation, and Task. Relevant statistics are summarized in Table 2.
FIGURE 3.
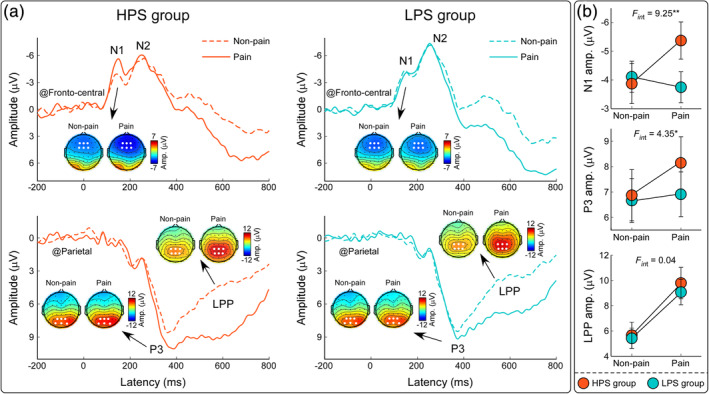
ERP responses during the Pain Judgment Task. Grand average ERP waveforms and scalp topographies for HPS (orange lines) and LPS (blue lines) groups were elicited by painful (solid lines) and nonpainful (dashed lines) pictorial stimulations during the Pain Judgment Task. Displayed waveforms were measured at fronto‐central (F1, Fz, F2, FC1, FCz, FC2) and parietal (P1, Pz, P2, PO3, POz, PO4) electrodes. Amplitudes of dominant components (N1, P3, and LPP) elicited by painful and nonpainful stimulations were compared between HPS and LPS groups. Electrodes used to evaluate the ERP amplitudes were marked using enlarged white dots on the corresponding scalp topographies. Data are expressed as mean ± SEM. *p < .05, **p < .01
The three‐way ANOVA applied to the fronto‐central N1 amplitudes revealed a significant interaction between Stimulation, Task, and Group (F 1,60 = 4.51, p = .038, η p 2 = 0.07). Post hoc two‐way ANOVA on the N1 amplitudes during the Pain Judgment Task showed a significant interaction between Stimulation and Group (F 1,60 = 9.25, p = .003, η p 2 = 0.13). While participants in the HPS group had significantly greater N1 amplitudes in response to painful stimulations than to nonpainful stimulations (−5.37 ± 0.61 μV vs. −3.87 ± 0.63 μV, p = .001), this difference was nonsignificant in the LPS group (−3.75 ± 0.54 μV vs. −4.11 ± 0.54 μV, p = .39). Post hoc two‐way ANOVA on the N1 amplitudes during the Hands Counting Task did not reveal any significant main effect or interaction (p > .05 for all comparisons). Both parietal P3 amplitudes and centro‐parietal LPP amplitudes were significantly modulated by the main effect of Task (P3: F 1,60 = 24.47, p < .001, η p 2 = 0.29; LPP: F 1,60 = 14.13, p < .001, η p 2 = 0.19), such that amplitudes were larger during the Pain Judgment Task than during the Hands Counting Task. They were also significantly modulated by the interaction between Stimulation and Task (P3: F 1,60 = 8.99, p = .004, η p 2 = 0.13; LPP: F 1,60 = 63.77, p < .001, η p 2 = 0.52). P3 and LPP amplitudes were significantly greater for painful stimulations than for nonpainful stimulations during the Pain Judgment Task (P3: p = .005; LPP: p < .001), but not during the Hands Counting Task (p > .05 for both comparisons). In addition, centro‐parietal LPP amplitudes were also modulated by the main effect of Stimulation (F 1,60 = 63.19, p < .001, η p 2 = 0.51) such that amplitudes evoked by painful stimulations were greater than those evoked by nonpainful stimulations. Nevertheless, we did not find any main effect or interaction that affected fronto‐central N2 amplitudes (p > .05 for all comparisons).
Grand average TFDs during the Pain Judgment and Hands Counting Tasks, measured at centro‐parietal electrodes, are shown in Figure 4 (for the Pain Judgment Task) and in Figure S5 (for the Hands Counting Task, see Supplementary Materials). Nonpainful and painful pictorial stimulations elicited a long‐lasting decrease of EEG oscillatory power within the alpha frequency band (α‐ERD) in both tasks. The scalp topographies of the α‐ERD response (300–800 ms and 8–14 Hz) induced by pictorial stimulations differed between groups; they were centrally and occipitally distributed for the HPS group, but occipitally distributed for the LPS group. The mixed‐design ANOVA applied on centro‐parietal α‐ERD magnitudes revealed a significant three‐way interaction among Group, Stimulation, and Task (F 1,60 = 4.35, p = .041, η p 2 = 0.07). Post hoc two‐way ANOVA on the α‐ERD magnitudes during the Pain Judgment Task showed a significant interaction between Group and Stimulation (F 1,60 = 6.23, p = .015, η p 2 = 0.09). While painful stimulations induced greater α‐ERD magnitudes than nonpainful stimulations for participants in the HPS group (−5.24 ± 0.81 vs. −3.83 ± 0.68, p = .016), no significant difference was observed in the LPS group (−3.73 ± 0.76 vs. −4.25 ± 0.63, p = .33). Two‐way ANOVA on the α‐ERD magnitudes during the Hands Counting Task did not reveal any significant main effect or interaction (p > .05 for all comparisons).
FIGURE 4.
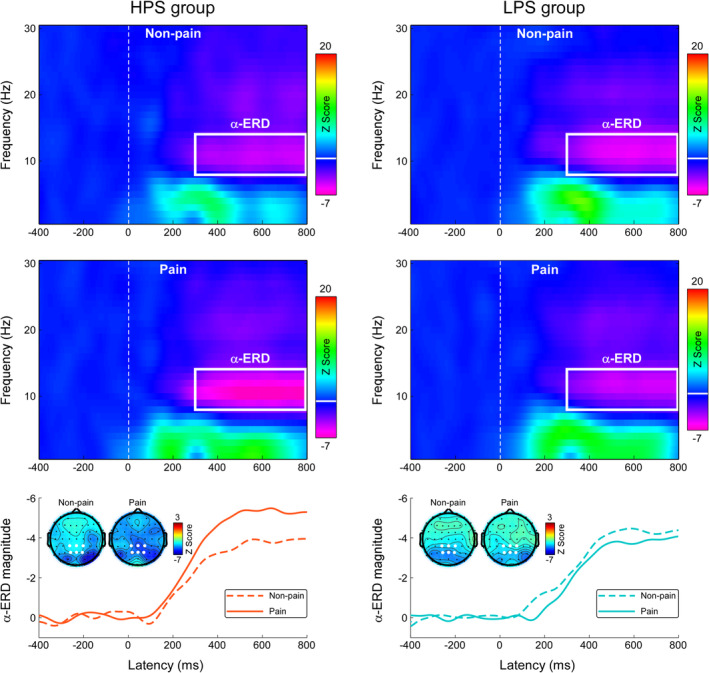
Time‐frequency responses during the Pain Judgment Task. Time‐frequency distributions of neural responses for HPS and LPS groups were elicited by nonpainful and painful stimulations during the Pain Judgment Task. The color scale represents the increase or decrease of the oscillatory magnitude relative to a prestimulus interval (−400 to −100 ms). Displayed signals were measured at centro‐parietal electrodes (CP1, CPz, CP2, P1, Pz, and P2). Both nonpainful and painful stimulations elicited a long‐lasting α‐ERD response (8–14 Hz in frequency and 300–800 ms in latency, marked using white rectangles). The time‐course of α‐ERD magnitudes in response to painful (solid line) and nonpainful (dashed line) stimulations was obtained by averaging across 8–14 Hz. Electrodes used to evaluate the α‐ERD magnitude are marked using enlarged white dots on the scalp topographies
These EEG/ERP results indicated that when attention was directed toward the pain depicted in pictorial stimulations, participants in the HPS group exhibited significant pain empathic effects on N1 responses and α‐ERD magnitudes. In contrast, those in the LPS group did not.
3.4. Mediation model
In light of the association between first‐hand pain sensitivity and pain empathic responses during the Pain Judgment Task, we next tested whether empathic brain responses mediated this relationship. In the mediation model, first‐hand pain sensitivity (HPS vs. LPS group) was the predictor (X), pain empathic behavioral response was the outcome (Y), and pain empathic brain response was the mediator (M). The bootstrap CIs revealed that the indirect effect of first‐hand pain sensitivity on the empathic behavioral response (facilitated RTs to others' pain) via the empathic N1 response differed from zero with 95% confidence (a*b = −6.80, SE = 4.02, CI = [−15.89, −0.16], Figure 5a), in which pain empathic effects on RTs and N1 amplitudes were derived from their differences between painful and nonpainful conditions during the Pain Judgment Task. Overall, the mediating effect of empathic N1 amplitudes accounted for 28.17% (P M = 1–17.36/24.16) of the linkage between first‐hand pain sensitivity and facilitated RTs in response to others' pain.
FIGURE 5.
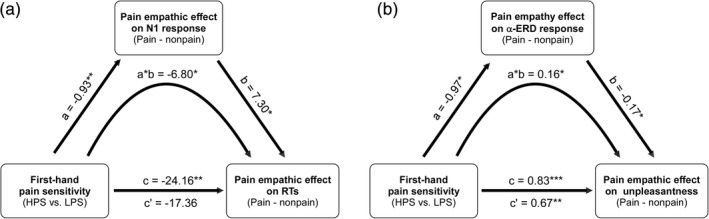
Empathic brain responses mediate the link between first‐hand pain sensitivity and empathic behavioral responses. The mediation model included first‐hand pain sensitivity as the independent variable (1 for HPS group, −1 for LPS group), pain empathic effects on behavioral responses (RTs in the Pain Judgment Task, and self‐experienced unpleasantness ratings in the Pain Rating Task) as the dependent variables, and pain empathic effects on the N1 and α‐ERD responses as the mediators. Pain empathic effects on brain and behavioral responses were derived from the differences between painful and nonpainful conditions. Pain empathic N1 responses mediated the link between first‐hand pain sensitivity and facilitated RTs to others' pain (a). The pain empathic α‐ERD response mediated the link between first‐hand pain sensitivity and affective responses to others' pain (b). *p < .05, **p < .01, ***p < .001
The indirect effect of first‐hand pain sensitivity on the empathic unpleasantness ratings via the centro‐parietal α‐ERD responses was different from zero with 95% confidence (a*b = 0.16, SE = 0.08, CI = [0.03, 0.35], Figure 5b), in which pain empathic effects on unpleasantness ratings and α‐ERD responses were derived from their differences between painful and nonpainful conditions during the Pain Rating Task (for unpleasantness ratings) and the Pain Judgment Task (for α‐ERD responses). Overall, the mediating effect of α‐ERD responses accounted for 19.28% (P M = 1–0.67/0.83) of the linkage between first‐hand pain sensitivity and self‐experienced unpleasantness in response to observing others' pain.
4. DISCUSSION
The present study investigated the interindividual relationship between sensitivity to first‐hand pain and empathy for others' pain and examined whether this relationship is modulated by attention. Behaviorally, compared with the LPS group, participants in the HPS group reported greater intensity of pain thought to be experienced by others, as well as greater personal unpleasantness felt when viewing others in pain. When attention was directed toward pain of another, only participants in the HPS group exhibited significant pain empathic effects on fronto‐central N1 responses and on centro‐parietal α‐ERD responses, which was accompanied by their greater sensitivity in discriminating others' pain. Importantly, pain empathic effects on N1 responses mediated the link between first‐hand pain sensitivity and facilitated RTs to others' pain. Similarly, pain empathic effects on the α‐ERD response mediated the link between first‐hand pain sensitivity and self‐experienced unpleasantness to others' pain. However, when participants' attention was directed away from the pain being experienced by another, empathic behavioral and neural responses were comparable between these two groups. These experiments demonstrate a shared sensitivity to first‐hand pain and empathy for pain, provided that attention is directed toward the pain being experienced by another.
Researchers assume that empathy involves at least two psychological mechanisms (Shamay‐Tsoory, Aharon‐Peretz, & Perry, 2009; Singer & Lamm, 2009): (a) cognitive processes of empathy that capture the capacity to infer others' mental states and (b) affective processes that allow emotional reactions to others' emotional states. Accurately understanding other's pain is fundamental to effectively caring for people in pain, and observer's affective responses to seeing others in pain might trigger an altruistic motivation to help them (de Waal, 2008; Goubert et al., 2005). To disentangle these two aspects of empathy, we instructed participants to estimate the intensity of pain experienced by the model in the picture and to report self‐experienced unpleasantness in response to the pictorial stimulations (Pain Rating Task). Although both groups provided higher pain‐intensity ratings as well as higher feelings of unpleasantness for painful stimulations than for nonpainful stimulations, the differential ratings (empathic ratings) were greater for the HPS group. In addition to being more sensitive to first‐hand pain (higher scores on the PSQ and greater pain perception in response to identical electrical stimulation), participants in the HPS group tended to exhibit amplified empathy for others' pain in both their cognition and affect. This finding was further supported by the reports of greater fantasy and personal distress in the HPS group on the empathy trait questionnaire: people with great sensitivity to first‐hand pain tend to transpose themselves imaginatively into others' emotions more easily and become more anxious when witnessing the suffering of others.
Two‐choice stimulus‐categorization tasks, including the Pain Judgment and Hands Counting Tasks, were employed to assess top–down attentional modulation of empathy for pain. Given that the pictorial stimulations used in these tasks were identical, the contrast between them should remove any effects related to stimulus properties and mainly reflect the contribution of attention (Fabi & Leuthold, 2017; Y. Fan & Han, 2008; Gonzalez‐Liencres et al., 2016). During the Pain Judgment Task with attention directed toward the painful aspects of the pictorial stimulations, responses to the painful stimulations were faster than those to the nonpainful stimulations, and this pain‐facilitation effect was greater for the HPS group than for the LPS group. During the Hands Counting Task with attention directed away from the painful aspects of the pictorial stimulations, responses to the painful stimulations were slower than those to the nonpainful stimulations, and this pain‐interference effect was comparable between the HPS and LPS groups. Attention was likely attracted by task‐irrelevant pain‐related features in the pictures, thereby interfering with the ability to quickly determine the number of hands (Fabi & Leuthold, 2017). Between‐group differences in the pain‐facilitation effect during the Pain Judgment Task indicated that participants in the HPS group had a greater efficacy in discriminating and categorizing others' pain when attention was directed toward painful aspects of pictorial stimulations, thus providing the evidence for the link between first‐hand pain sensitivity and pain empathic effects on behavioral reactions.
Time‐domain ERP results revealed a between‐group difference in the pain empathic effect on the fronto‐central N1 response during the Pain Judgment Task. The N1 response to painful and nonpainful stimulations differed significantly for the HPS group, but not for the LPS group. Pain effects on the fronto‐central N1 response have been reported in other ERP studies investigating empathy for pain (Cui, Zhu, & Luo, 2017; Decety et al., 2010; Gonzalez‐Liencres et al., 2016), and have been associated with the automatic activation of affective arousal or emotional sharing of empathy. Our results suggested that automatic, stimulus‐driven empathic processing could be influenced by the physical pain sensitivity of the observers when attention is directed toward pain cues. Importantly, the degree of the pain empathic effect on N1 amplitude explained the link between first‐hand pain sensitivity and facilitated RTs to others' pain. Studies investigating affective processing have also shown the different fronto‐central N1 responses between negative and neutral stimuli, which was suggested to reflect the preferential processing of stimulation with negative valence (Hilimire, Mienaltowski, Blanchard‐Fields, & Corballis, 2014; Luo, Feng, He, Wang, & Luo, 2010). Painful situations could be perceived as more salient and more arousing by participants in the HPS group (manifested as an earlier differentiation between painful and nonpainful stimulations) and thus preferentially attract attention early in the information processing stream, thus facilitating the subsequent discrimination process. At a later stage of information processing, we observed pain empathic effects on P3 and LPP responses during the Pain Judgment Task. These responses are often interpreted in terms of sustained attentional processing and cognitive evaluation of motivationally relevant stimuli (Polich, 2007; Schupp, Flaisch, Stockburger, & Junghöfer, 2006), which, in the context of empathy, might contribute to the late controlled process of social understanding and emotional regulation (Cheng, Hung, & Decety, 2012; Decety et al., 2010; Y.‐T. Fan, Chen, Chen, Decety, & Cheng, 2013). The late empathic processing of others' pain does not depend on sensitivity to first‐hand pain as indicated by the comparable empathic neural responses at later stages (e.g., pain empathic effects on LPP responses) between participants in the HPS and LPS groups. However, because participants were required to categorize the type of stimulations by pressing buttons, we cannot completely exclude the possibility that these late‐stage ERP components were contaminated by the preparation or execution of the motor response.
As a compliment to the time‐domain findings, we also identified a between‐group difference in pain empathic effects on centro‐parietal α‐ERD responses during the Pain Judgment Task. The α‐ERD responses to painful and nonpainful stimulations differed significantly for the HPS group, but not for the LPS group. Importantly, pain empathic modulation of the α‐ERD responses mediated the influence of first‐hand pain sensitivity on self‐experienced unpleasantness in response to others' pain, suggesting the contribution of α‐ERD response in shared sensitivity to first‐hand pain and others' pain. The modulation of α‐oscillations during the observation of others' pain has been reported in previous studies (Fabi & Leuthold, 2017; Li et al., 2017; Motoyama et al., 2017; Perry et al., 2010). The degree of modulation on α‐oscillation desynchronization has been associated with subjective feelings of pain in others and self‐unpleasantness (Li et al., 2017; Mu, Fan, Mao, & Han, 2008) as well as with emotional arousal during affective picture processing (De Cesarei & Codispoti, 2011; Schubring & Schupp, 2019; Simons et al., 2003). Functionally, Klimesch, Sauseng, and Hanslmayr (2007) proposed that an increase in α‐oscillation reflects top–down, inhibitory control processes, while a decrease in α‐oscillation is associated with gradual release of inhibition that is associated with the emergence of complex spreading of activation processes. Therefore, the greater pain empathic effect on the α‐ERD response that we observed in the HPS group indicates enhanced cortical activation and affective processing of pain in others, provided that pain of another was the focus of attention.
During the Hands Counting Task when attention was directed away from the painful aspects of the pictorial stimulations, the pain empathic effects on the neural responses (e.g., N1 and α‐ERD) were inhibited or eliminated in both HPS and LPS groups. Regardless of the physical pain sensitivity in the observers, EEG/ERP activity involved in empathic processing was inhibited by manipulating top‐down attention. In line with this observation, the engagement of brain regions associated with empathic pain processing, including the anterior cingulate cortex, the insula, and the frontal cortex, took place only when participants paid attention to the pain of another, but was eliminated when they counted the number of hands in the painful stimuli (Gu & Han, 2007). Similar top‐down attention modulation on ERP responses during empathy for pain tasks was also reported by previous studies (Fabi & Leuthold, 2017; Y. Fan & Han, 2008; Meng et al., 2019). Empathic neural response to others' in potentially painful situations is not likely a purely automatic and effortless process (Y. Fan & Han, 2008; Gu & Han, 2007). Rather, it requires attention to be focused on the pain being experienced by another, not simply focused on any aspect of the other person. Therefore, due to the top‐down attention modulation of empathic processing, individual variations in first‐hand physical pain and empathy for others' pain could be influenced by attention manipulation. This understanding might help explain the asynchronous sensitivity to first‐hand pain and others' pain in some clinical samples (Chen et al., 2017; Peng, Meng, et al., 2019). For example, atypical linkage might arise from abnormal attentional control such as paying less attention to pain of another.
Among healthy individuals, we have shown that individuals with greater sensitivity to first‐hand pain tend to have greater empathic behavioral and EEG/ERP responses to others' pain during a task when attention was directed toward others' pain. The results suggest an overlap in sensitivity to first‐hand pain and empathy for others' pain at a between‐participant level provided that attention remains focused on the pain being experienced by another. This supports a qualified version of the “shared representations” theory of empathy—empathy for others' pain partially relies upon processes functionally equivalent to those engaged by first‐hand pain experiences (Jackson et al., 2005; Preston & de Waal, 2002). This finding parallels the behavior seen in patients with fibromyalgia who have increased sensitivity to first‐hand physical pain and provide greater empathic ratings of others' pain (Fallon, Li, Chiu, Nurmikko, & Stancak, 2015). Similar to first‐hand pain that signals potential or actual injury/ illness in the body, the perception of pain in others, acting as an empathic signal, alerts individuals that a conspecific is at risk, attracts their attention, and motivates social behaviors (Craig, 2009). Either perceiving pain in ourselves or seeing pain in others should activate the threat‐detection system and protect the individual from a present or potential danger (Ibáñez et al., 2011; Yamada & Decety, 2009). The similar evolutional significance might explain the observed shared sensitivity between first‐hand pain and empathy for others' pain among healthy individuals. This understanding might help explain the similar modulation of self‐ and other‐related pain processing by factors such as placebo analgesia and predictability (Peng, Meng, et al., 2019; Rütgen, Seidel, Riečanský, et al., 2015), as well as the altered responsiveness to others' pain among individuals with altered first‐hand pain experiences (Danziger, Prkachin, & Willer, 2006). Despite these implications, the relationship between behavioral and neural responses during the empathy for pain tasks (e.g., neural correlates of pain‐facilitation effect on RTs during the Pain Judgment Task) deserved to be further disentangled in the future studies along with a randomly recruited and larger sample size.
5. CONCLUSIONS
This study revealed a shared sensitivity between first‐hand physical pain and empathy for others' pain when attention was directed toward the pain being experienced by another. When judging or rating the pain observed in others, people with higher sensitivity to first‐hand pain tended to exhibit greater empathy for pain than those with lower sensitivity. However, when attention was withdrawn from pain of the other person, individual pain sensitivity did not influence empathic responses. Our findings suggest that top‐down control of attention matters in the link between first‐hand pain sensitivity and empathy for others' pain, thus supporting a qualified version of the “shared representations” theory of empathy.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 31871127), the Features Innovative Projects of Guangdong Province Ordinary University (2019KTSCX149), and the Shenzhen Basic Research Project (JCYJ20190808154413592).
Li X, Liu Y, Ye Q, Lu X, Peng W. The linkage between first‐hand pain sensitivity and empathy for others' pain: Attention matters. Hum Brain Mapp. 2020;41:4815–4828. 10.1002/hbm.25160
Xiaoyun Li and Yang Liu contributed equally to this study.
Funding information National Natural Science Foundation of China, Grant/Award Number: 31871127; Features Innovative Projects of Guangdong Province Ordinary University, Grant/Award Number: 2019KTSCX149; Shenzhen Basic Research Project, Grant/Award Number: JCYJ20190808154413592
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 57(1), 289–300. [Google Scholar]
- Chen, C. , Hung, A. Y. , Fan, Y. T. , Tan, S. , Hong, H. , & Cheng, Y. (2017). Linkage between pain sensitivity and empathic response in adolescents with autism spectrum conditions and conduct disorder symptoms. Autism Research, 10(2), 267–275. [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Chen, C. , & Decety, J. (2014). An EEG/ERP investigation of the development of empathy in early and middle childhood. Developmental Cognitive Neuroscience, 10, 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. , Hung, A.‐Y. , & Decety, J. (2012). Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Development and Psychopathology, 24(2), 623–636. [DOI] [PubMed] [Google Scholar]
- Craig, K. D. (2009). The social communication model of pain. Canadian Psychology/Psychologie Canadienne, 50(1), 22–32. [Google Scholar]
- Cui, F. , Ma, N. , & Luo, Y. J. (2016). Moral judgment modulates neural responses to the perception of other's pain: An ERP study. Scientific Reports, 6, 20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, F. , Zhu, X. , & Luo, Y. (2017). Social contexts modulate neural responses in the processing of others' pain: An event‐related potential study. Cognitive, Affective, & Behavioral Neuroscience, 17(4), 850–857. [DOI] [PubMed] [Google Scholar]
- Danziger, N. , Prkachin, K. M. , & Willer, J.‐C. (2006). Is pain the price of empathy? The perception of others' pain in patients with congenital insensitivity to pain. Brain, 129(9), 2494–2507. [DOI] [PubMed] [Google Scholar]
- Davis, M. H. (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44(1), 113–126. [Google Scholar]
- De Cesarei, A. , & Codispoti, M. (2011). Affective modulation of the LPP and α‐ERD during picture viewing. Psychophysiology, 48(10), 1397–1404. [DOI] [PubMed] [Google Scholar]
- de Vignemont, F. , & Singer, T. (2006). The empathic brain: How, when and why? Trends in Cognitive Sciences, 10(10), 435–441. [DOI] [PubMed] [Google Scholar]
- de Waal, F. B. (2008). Putting the altruism back into altruism: The evolution of empathy. Annual Review of Psychology, 59, 279–300. [DOI] [PubMed] [Google Scholar]
- Decety, J. , Bartal, I. B.‐A. , Uzefovsky, F. , & Knafo‐Noam, A. (2016). Empathy as a driver of prosocial behaviour: Highly conserved neurobehavioural mechanisms across species. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371(1686), 20150077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety, J. , Yang, C.‐Y. , & Cheng, Y. (2010). Physicians down‐regulate their pain empathy response: An event‐related brain potential study. NeuroImage, 50(4), 1676–1682. [DOI] [PubMed] [Google Scholar]
- Delorme, A. , & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Fabi, S. , & Leuthold, H. (2017). Empathy for pain influences perceptual and motor processing: Evidence from response force, ERPs, and EEG oscillations. Social Neuroscience, 12(6), 701–716. [DOI] [PubMed] [Google Scholar]
- Fallon, N. , Li, X. , Chiu, Y. , Nurmikko, T. , & Stancak, A. (2015). Altered cortical processing of observed pain in patients with fibromyalgia syndrome. The Journal of Pain, 16(8), 717–726. [DOI] [PubMed] [Google Scholar]
- Fan, Y. , & Han, S. (2008). Temporal dynamic of neural mechanisms involved in empathy for pain: An event‐related brain potential study. Neuropsychologia, 46(1), 160–173. [DOI] [PubMed] [Google Scholar]
- Fan, Y.‐T. , Chen, C. , Chen, S.‐C. , Decety, J. , & Cheng, Y. (2013). Empathic arousal and social understanding in individuals with autism: Evidence from fMRI and ERP measurements. Social Cognitive and Affective Neuroscience, 9(8), 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Liencres, C. , Brown, E. C. , Tas, C. , Breidenstein, A. , & Brüne, M. (2016). Alterations in event‐related potential responses to empathy for pain in schizophrenia. Psychiatry Research, 241, 14–21. [DOI] [PubMed] [Google Scholar]
- Goubert, L. , Craig, K. D. , Vervoort, T. , Morley, S. , Sullivan, M. J. , de, C. W. A. C. , … Crombez, G. (2005). Facing others in pain: The effects of empathy. Pain, 118(3), 285–288. [DOI] [PubMed] [Google Scholar]
- Gu, X. , & Han, S. (2007). Attention and reality constraints on the neural processes of empathy for pain. NeuroImage, 36(1), 256–267. [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2017). Introduction to mediation, moderation, and conditional process analysis: A regression‐based approach. New York, NY: The Guilford Press. [Google Scholar]
- Hilimire, M. R. , Mienaltowski, A. , Blanchard‐Fields, F. , & Corballis, P. M. (2014). Age‐related differences in event‐related potentials for early visual processing of emotional faces. Social Cognitive and Affective Neuroscience, 9(7), 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker, C. I. , Verosky, S. C. , Germine, L. T. , Knight, R. T. , & D'Esposito, M. (2008). Mentalizing about emotion and its relationship to empathy. Social Cognitive and Affective Neuroscience, 3(3), 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez, A. , Hurtado, E. , Lobos, A. , Escobar, J. , Trujillo, N. , Baez, S. , … Decety, J. (2011). Subliminal presentation of other faces (but not own face) primes behavioral and evoked cortical processing of empathy for pain. Brain Research, 1398, 72–85. [DOI] [PubMed] [Google Scholar]
- Jackson, P. L. , Meltzoff, A. , & Decety, J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24(3), 771–779. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. , Sauseng, P. , & Hanslmayr, S. (2007). EEG alpha oscillations: The inhibition–timing hypothesis. Brain Research Reviews, 53(1), 63–88. [DOI] [PubMed] [Google Scholar]
- Krishnan, A. , Woo, C.‐W. , Chang, L. J. , Ruzic, L. , Gu, X. , Lopez‐Sola, M. , … Wager, T. D. (2016). Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife, 5, e15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm, C. , Decety, J. , & Singer, T. (2011). Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. [DOI] [PubMed] [Google Scholar]
- Legrain, V. , Guérit, J. M. , Bruyer, R. , & Plaghki, L. (2002). Attentional modulation of the nociceptive processing into the human brain: Selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain, 99(1–2), 21–39. [DOI] [PubMed] [Google Scholar]
- Li, X. , Meng, X. , Li, H. , Yang, J. , & Yuan, J. (2017). The impact of mood on empathy for pain: Evidence from an EEG study. Psychophysiology, 54(9), 1311–1322. [DOI] [PubMed] [Google Scholar]
- Luo, W. , Feng, W. , He, W. , Wang, N. Y. , & Luo, Y. J. (2010). Three stages of facial expression processing: ERP study with rapid serial visual presentation. NeuroImage, 49(2), 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, D. W. , & Rainwater, A. J. (1998). Development of the fear of pain questionnaire‐III. Journal of Behavioral Medicine, 21(4), 389–410. [DOI] [PubMed] [Google Scholar]
- Meng, J. , Jackson, T. , Chen, H. , Hu, L. , Yang, Z. , Su, Y. , & Huang, X. (2013). Pain perception in the self and observation of others: An ERP investigation. NeuroImage, 72, 164–173. [DOI] [PubMed] [Google Scholar]
- Meng, J. , Shen, L. , Li, Z. , & Peng, W. (2019). Top‐down attention modulation on the perception of others' vocal pain: An event‐related potential study. Neuropsychologia, 133, 107177. [DOI] [PubMed] [Google Scholar]
- Mischkowski, D. , Crocker, J. , & Way, B. M. (2016). From painkiller to empathy killer: Acetaminophen (paracetamol) reduces empathy for pain. Social Cognitive and Affective Neuroscience, 11(9), 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama, Y. , Ogata, K. , Hoka, S. , & Tobimatsu, S. (2017). Frequency‐dependent changes in sensorimotor and pain affective systems induced by empathy for pain. Journal of Pain Research, 10, 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, Y. , Fan, Y. , Mao, L. , & Han, S. (2008). Event‐related theta and alpha oscillations mediate empathy for pain. Brain Research, 1234, 128–136. [DOI] [PubMed] [Google Scholar]
- Peng, W. , Huang, X. , Liu, Y. , & Cui, F. (2019). Predictability modulates the anticipation and perception of pain in both self and others. Social Cognitive and Affective Neuroscience, 14(7), 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, W. , Meng, J. , Lou, Y. , Li, X. , Lei, Y. , & Yan, D. (2019). Reduced empathic pain processing in patients with somatoform pain disorder: Evidence from behavioral and neurophysiological measures. International Journal of Psychophysiology, 139, 40–47. [DOI] [PubMed] [Google Scholar]
- Perry, A. , Bentin, S. , Bartal, I. B. , Lamm, C. , & Decety, J. (2010). “Feeling” the pain of those who are different from us: Modulation of EEG in the mu/alpha range. Cognitive, Affective, & Behavioral Neuroscience, 10(4), 493–504. [DOI] [PubMed] [Google Scholar]
- Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher, K. J. , & Kelley, K. (2011). Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods, 16(2), 93–115. [DOI] [PubMed] [Google Scholar]
- Preston, S. D. , & de Waal, F. B. (2002). Empathy: Its ultimate and proximate bases. The Behavioral and Brain Sciences, 25(1), 20–71. [DOI] [PubMed] [Google Scholar]
- Quan, X. , Fong, D. Y. T. , Leung, A. Y. M. , Liao, Q. , Ruscheweyh, R. , & Chau, P. H. (2018). Validation of the mandarin Chinese version of the pain sensitivity questionnaire. Pain Practice, 18(2), 180–193. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh, R. , Marziniak, M. , Stumpenhorst, F. , Reinholz, J. , & Knecht, S. (2009). Pain sensitivity can be assessed by self‐rating: Development and validation of the pain sensitivity questionnaire. Pain, 146(1–2), 65–74. [DOI] [PubMed] [Google Scholar]
- Rütgen, M. , Seidel, E. M. , Riečanský, I. , & Lamm, C. (2015). Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first‐hand emotion experience. The Journal of Neuroscience, 35(23), 8938–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgen, M. , Seidel, E.‐M. , Silani, G. , Riečanský, I. , Hummer, A. , Windischberger, C. , … Lamm, C. (2015). Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proceedings of the National Academy of Sciences of the United States of America, 112(41), E5638–E5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubring, D. , & Schupp, H. T. (2019). Affective picture processing: Alpha‐ and lower beta‐band desynchronization reflects emotional arousal. Psychophysiology, 56(8), e13386. [DOI] [PubMed] [Google Scholar]
- Schupp, H. T. , Flaisch, T. , Stockburger, J. , & Junghöfer, M. (2006). Emotion and attention: Event‐related brain potential studies. Progress in Brain Research, 156, 31–51. [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory, S. G. , Aharon‐Peretz, J. , & Perry, D. (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–627. [DOI] [PubMed] [Google Scholar]
- Simons, R. F. , Detenber, B. H. , Cuthbert, B. N. , Schwartz, D. D. , & Reiss, J. E. (2003). Attention to television: Alpha power and its relationship to image motion and emotional content. Media Psychology, 5(3), 283–301. [Google Scholar]
- Singer, T. , & Lamm, C. (2009). The social neuroscience of empathy. Annals of the New York Academy of Sciences, 1156, 81–96. [DOI] [PubMed] [Google Scholar]
- Singer, T. , Seymour, B. , O'doherty, J. , Kaube, H. , Dolan, R. J. , & Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–1162. [DOI] [PubMed] [Google Scholar]
- Sullivan, M. J. , Bishop, S. R. , & Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7(4), 524–532. [Google Scholar]
- Tracey, I. , Ploghaus, A. , Gati, J. S. , Clare, S. , Smith, S. , Menon, R. S. , & Matthews, P. M. (2002). Imaging attentional modulation of pain in the periaqueductal gray in humans. The Journal of Neuroscience, 22(7), 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, M. , & Decety, J. (2009). Unconscious affective processing and empathy: An investigation of subliminal priming on the detection of painful facial expressions. Pain, 143(1–2), 71–75. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Hu, L. , Hung, Y. S. , Mouraux, A. , & Iannetti, G. (2012). Gamma‐band oscillations in the primary somatosensory cortex—A direct and obligatory correlate of subjective pain intensity. The Journal of Neuroscience, 32(22), 7429–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, P. , Lyu, Z. , & Jackson, T. (2018). Fear of pain and event‐related potentials during exposure to image‐cued somatosensory stimulation. Brain Research, 1695, 91–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


