Abstract
We address existing controversies regarding neuroanatomical substrates of reading‐aloud processes according to the dual‐route processing models, in this particular instance in a series of 49 individuals with brain tumors who performed several reading tasks of real‐time neuropsychological testing during surgery (low‐ to high‐grade cerebral neoplasms involving the left hemisphere). We explored how reading abilities in individuals with brain tumors evolve during and after surgery for a brain tumor, and we studied the reading performance in a sample of 33 individuals in a 4‐month follow‐up after surgery. Impaired reading performance was seen pre‐surgery in 7 individuals with brain tumors, intra‐surgery in 18 individuals, at immediate post‐surgery testing in 26 individuals, and at follow‐up in 5 individuals. We classified their reading disorders according to operational criteria for either phonological or surface dyslexia. Neuroimaging results are discussed within the theoretical framework of the dual‐route model of reading. Lesion‐mask subtraction analyses revealed that areas selectively related with phonological dyslexia were located—along with the left hemisphere dorsal stream—in the Rolandic operculum, the inferior frontal gyrus, the precentral gyrus, the supramarginal gyrus, the insula (and/or the underlying external capsule), and parts of the superior longitudinal fasciculus, whereas lesions related to surface dyslexia involved the ventral stream, that is, the left middle and inferior temporal gyrus and parts of the left inferior longitudinal fasciculus.
Keywords: functional recovery, individuals with brain tumors, MRI, phonological dyslexia, plasticity, real‐time neuropsychological testing (RTNT), surface dyslexia
We explored how reading abilities in individuals with neurosurgical lesions evolve during and after surgery for a brain tumor, and we studied the reading performance in a sample of 33 individuals in a 4‐month follow‐up after surgery.
Abbreviations
- AF
arcuate fasciculus
- EOR
extent of resection
- HGG
high‐grade glioma
- IC
internal capsule
- IFOF
inferior fronto‐occipital fasciculus
- ILF
inferior longitudinal fasciculus
- LGG
low‐grade glioma
- RTNT
real‐time neuropsychological testing
- SLF
superior longitudinal fasciculus
- VWFA
visual word form area
1. INTRODUCTION
The neuroanatomical correlates of reading‐aloud processes are a crucial issue in cognitive neurolinguistic research and they are still not fully elucidated. The main objective of this study is to examine the neural correlates of the two main routes of reading aloud namely the lexical‐semantic route mediating direct access from word shape to meaning (often reported as “ventral route”) (Taylor, 2013) and the sublexical phonological route mediating grapheme‐to‐phoneme mapping (often reported as “dorsal route”).
Italian is a shallow orthography language. The sublexical phonological routine is thus sufficient for reading the great majority of words. There are, however, exceptions in reading, mainly concerning word stress, which in Italian generally falls on the penultimate syllable, but in three‐syllable or longer words, stress may fall on the ante‐penultimate syllable. This information is neither phonologically predictable nor diacritically marked and must therefore be processed along the lexical route (i.e., it has to be stored in the phonological output lexicon).
Most data about the neuroanatomical correlates of reading aloud processes come from functional neuroimaging studies of healthy participants (Price, 2012; Taylor, 2013), while data from individuals with brain damage mainly involve single case studies, and only a few studies involved large samples of individuals with left‐hemisphere damage suffering from acquired reading disorders (Cloutman, Newhart, Davis, Heidler‐Gary, & Hillis, 2011; Long, Sebastian, Faria, & Hillis, 2018; Purcell et al., 2017; Ripamonti et al., 2014; Sebastian et al., 2014). Overall, at the cortical level, the functional magnetic imaging literature designates the left occipito‐temporal region and the left inferior temporal cortex (Cohen et al., 2000), the posterior portion of the left middle temporal gyrus (Simos et al., 2002), and the pars triangularis of the left inferior frontal gyrus (Jobard, Crivello, & Tzourio‐Mazoyer, 2003) as the neuroanatomical correlates of the lexical‐semantic route of reading. In contrast, the sublexical route has mainly been found associated with the left temporo‐parietal junction, the opercular part of the left inferior frontal gyrus (Burton, Small, & Blumstein, 2000; Jobard et al., 2003; Pugh et al., 2000; Simos et al., 2002) or a variety of perisylvian cortical regions (Rapcsak et al., 2009). At the subcortical level, diffusion tensor imaging (DTI) studies designate the inferior fronto‐occipital fasciculus (IFOF), and the inferior longitudinal fasciculus (ILF) (Catani & Thiebaut de Schotten, 2008; Fernandez‐Miranda et al., 2008; Martino, Brogna, Robles, Vergani, & Duffau, 2010) as the subcortical correlates of the lexical‐semantic route of reading (Vandermosten, Boets, Poelmans, et al., 2012; Vandermosten, Boets, Wouters, & Ghesquiere, 2012), while the left arcuate fasciculus (AF) (Vandermosten, Boets, Poelmans, et al., 2012; Vandermosten, Boets, Wouters, & Ghesquiere, 2012) has mainly been found associated with the sublexical route. These functional neuroimaging data are only partially consistent with data arising from anatomo‐clinical correlation studies in individuals with brain damage suffering from either surface or phonological dyslexia. 1 For example, a study involving individuals with focal brain damage (Ripamonti et al., 2014) did not find any evidence for an involvement of the pars triangularis of the left inferior frontal gyrus, contrary to frequent reports in the neuroimaging literature of it being part of the circuitry related to the ventral lexical‐semantic route of reading (Fiebach, Friederici, Müller, & von Cramon, 2002; Jobard et al., 2003). By contrast, surface dyslexia was found to correlate with the postero‐inferior part of the left insula (and/or the underlying external capsule) together with the left postero‐inferior and anterior temporal areas (Ripamonti et al., 2014). In addition, it is unclear whether surface dyslexia occurs after damage to the left occipito‐temporal region, to the left inferior temporal cortex (Cohen et al., 2000), or to the posterior portion of the left middle temporal gyrus (Simos et al., 2002). Similarly, at the subcortical level Ripamonti et al. (2014) did not find any evidence for an involvement of the left inferior frontal gyrus, which structural neuroimaging literature considers to be among the subcortical tracts related to surface dyslexia (Vandermosten, Boets, Poelmans, et al., 2012; Vandermosten, Boets, Wouters, & Ghesquiere, 2012). In Ripamonti et al.'s (2014) study, only the left IFOF was found to be associated with this type of reading impairment. As to the correlates of phonological dyslexia, few studies reported evidence for an involvement of the opercular part of the left inferior frontal gyrus(e.g., Ripamonti et al., 2014), which is considered part of the circuitry related to the sublexical route of reading in the neuroimaging literature (e.g., Jobard et al., 2003; Pugh et al., 2000; Simos et al., 2002), while other correlative neuropsychological studies reported an involvement of frontoparietal (e.g., Lesch & Martin, 1998), fronto‐temporo‐parietal (e.g., Hamilton & Coslett, 2008), frontal (e.g., Ferreres, López, & China, 2003), temporal (e.g., Sasanuma, 1996), frontotemporal (e.g., Adair, Schwartz, Williamson, Raymer, & Heilman, 1999), or temporoparietal (e.g., Sato, Patterson, Fushimi, Maxim, & Bryan, 2008) areas. At subcortical level, the AF, which is considered by some authors of the structural neuroimaging literature to be the salient white‐matter tract related to the dorsal sublexical reading route (Vandermosten, Boets, Poelmans, et al., 2012; Vandermosten, Boets, Wouters, & Ghesquiere, 2012), was not found among the correlates of phonological dyslexia in Ripamonti et al.'s (2014) study.
In most individuals with vascular etiology, cerebral lesions are relatively large and are not limited to a single lobe but encompass several brain structures, often perisylvian areas. Neurosurgical lesions offer the possibility to verify previous lesional data on the neuroanatomy of reading taken from individuals with vascular damage. Given that neurosurgical lesions tend to be more circumscribed, we expected more selective neuroanatomical patterns allowing for the investigation of controversies (Taylor, 2013; Price, 2015; Ripamonti et al., 2014; Danelli et al., 2015) about the anatomical substrates of lexical and sublexical reading. One of the very few studies addressing post‐surgery reading abilities in seven individuals with left hemisphere glioma showed that resection of the posterior portion of the ILF connecting the visual cortex to the VWFA induced long‐term, global reading impairment, while resection of the terminations of the left posterior segment of the AF and of the posterior part of the inferior temporal cortex induced irregular word and pseudoword reading impairment with no damage to regular words (mixed dyslexia), while resection of the anterior portion of the ILF did not induce any reading impairment (Zemmoura, Herbet, Moritz‐Gasser, & Duffau, 2015). In a previous study carried out by our group (Tomasino et al., 2015), we described patient CF, an Italian native speaker who developed an extremely selective surface reading and spelling deficit following a tumor in the left temporal lobe. The majority of CF's reading errors were lexical stress errors, like [ˈgondola] → *[gonˈdola] 2 (the only possible type of surface reading error in Italian, owing to the extreme regularity of print‐to‐sound correspondence). CF's deficit was associated with anatomical damage to the left ventral/lexical reading route (as evidenced by fractional anisotropy decrease along the left IFOF) and relatively preserved dorsal/sublexical route (with rather full integrity of the left superior longitudinal fasciculus [SLF]). Taken together, these results suggest that reading impairments may appear after resection in very different areas.
In the present study, we further addressed the neuroanatomical correlates of the lexical and sublexical reading routes in a large sample of Italian individuals with neurosurgical lesions. To do so, we asked them to read aloud a list of words and phonologically plausible nonwords. The aim of such analysis was to identify individuals with brain tumors suffering from surface dyslexia and phonological dyslexia, that is, damage to either the ventral lexical or the dorsal sublexical route of reading. In order to assess the integrity of the lexical and sublexical reading routes, we identified two lists of words and pseudowords, which were matched on several dimensions, such as syllable length and CV structure (presence/absence of consonant clusters, presence/absence of double letters). The operational criterion indicating over‐reliance on the sublexical grapheme‐to‐phoneme conversion route is a poor stress assignment of three‐syllable words and relatively preserved reading of regular words and pseudowords, a pattern of damage that is usually called surface dyslexia (see above). Given the virtual absence of irregular words in the Italian reading system, surface dyslexia is usually identified by the rate of stress errors in three‐syllable (or longer) words. By contrast, the critical pattern indicating over‐reliance on the lexical route is poor reading of pseudowords, and more accurate reading of words, a pattern of damage that is usually called phonological dyslexia. Brain‐damaged individuals with phonological dyslexia may occasionally also show imageability and grammatical class effects, that is, better performance on concrete nouns than on abstract nouns or function words (see Coltheart, 1996; Marchand & Friedman, 2005): therefore, such effects may determine low performance along the lexical route, although not all individuals with brain damage suffering from phonological dyslexia show lexical effects in word reading (Friedman & Kohn, 1990; Funnell, 1983). For this reason we avoided items that might induce confounding lexical effects (abstract nouns, function words). Finally, undifferentiated reading deficit was defined for brain‐damaged individuals that did not meet the criteria for either dyslexia types.
An analysis of reading errors produced by individuals with brain tumors enabled us to identify cases with surface dyslexia and with phonological dyslexia. We then addressed the localization of their lesions by creating volumes of interest drawn on the lesion boundaries. Furthermore, we created lesions overlays of brain tumor for individuals with phonological dyslexia and with surface dyslexia, and applied the subtraction procedure in order to isolate cortical and subcortical areas selectively damaged in individuals suffering from phonological or surface dyslexia.
Besides the investigation of neuroanatomical correlates of the lexical and sublexical routes, the second goal of the study was to monitor how reading performance evolves as an effect of lesion/surgery and possible post‐surgery changes. In their study, Zemmoura et al. (2015) reported the effects of resection on reading: only two out of seven individuals with brain tumors fully recovered at a 3‐month follow‐up. In Tomasino et al.'s (2015) study no data were available about patient CF's follow‐up examination. Thus, it is unknown whether there was a recovery in his reading ability. There are some single‐case reports indicating post‐surgery reorganization and recovery of reading performance. Kamada et al. (2004) described a gradual reading improvement in a patient after resection of a mesial temporal glioma involving the left fusiform gyrus: reading improvement was detected at 3 and 8 months after surgery. Other authors (Hayashi, Okita, Kinoshita, Miyashita, & Nakada, 2014) reported a complete recovery about 6 months after second surgery for a left posterior basal temporal glioma. In the present study, we addressed the evolution of reading impairments in a large sample of Italian individuals with neurosurgical lesions. We investigated their reading ability before, during and immediately after surgery, as well as after a 4‐month follow‐up. Intra‐surgery testing refers to a recently developed method called “real‐time neuropsychological testing” (RTNT) (Skrap, Marin, Ius, Fabbro, & Tomasino, 2016), RTNT consists in continuous testing of the cognitive abilities of individuals with brain tumors throughout resection. RTNT allows for collection of both neuroanatomical and functional information during surgery. As far as neuroanatomical information is concerned, RTNT is a brain mapping technique with a clinical aim. The advantage of such procedure is very much similar to the localization scope of direct electrical stimulation (DES). DES (e.g., Berger & Ojemann, 1992; Ojemann, Ojemann, Lettich, & Berger, 1989) allows one to generate a map of points defined as “positive” because electrical stimulation in a given site, both at cortical and subcortical level, causes interference with a certain function (e.g., anomia or speech arrest) or elicits a motor response (e.g., a hand or lip movement).
RTNT enables one to collect real‐time information about spatial localization of the deficit and the concomitantly observed performance decrease. During RTNT, the coordinates and position of the site where resection causes a decrease in the patient's performance are recorded on the patient's MRI images displayed by the neuronavigation system.
As far as functional information is concerned, RTNT is an intraoperative treatment verification procedure. RTNT is continuously performed during all resection phases. This neuropsychological monitoring allows one to obtain a real‐time analysis of the resection effect on cognitive functioning. Besides being a clinical tool during awake surgery, RTNT offers the possibility to collect in vivo data while resection occurs, which can help to understand the mechanisms involved in a certain cognitive activity. Compared to DES, which is performed between resection phases, RTNT is performed throughout the resection.
In the present study, RTNT during awake surgery—including the assessment of reading—was carried out for each patient to map and preserve the cognitive functions while maximizing the extent of resection (EoR).
Two previous studies addressed reading abilities during awake surgery: a single case study (Roux et al., 2004), and Zemmoura et al.'s (2015) study including seven individuals with brain tumors. In both studies, reading was assessed intra‐surgery by means of DES. In Zemmoura et al.'s (2015) study, despite the use of DES, all individuals with brain tumors experienced immediate post‐surgery deficits, which were permanent in five out of seven individuals . Because of this possible dissonance between intra‐operative mapping and postoperative outcome, we used this novel RTNT approach in order to obtain a real‐time view of what happens to cognitive functioning during resection. RTNT allows in vivo reading performance data collection while resection occurs in cortical and subcortical areas, which allows to understand the mechanisms involved in reading.
2. MATERIALS AND METHODS
2.1. Participants
The main criterion for inclusion in the present study was performance on a reading task by means of the RTNT procedure during awake surgery (Skrap et al., 2016). All participants underwent neurosurgical resections involving different types of tumors in the left hemisphere areas (see Supplementary Table S1). The sample included 49 individuals with brain tumors who were tested and underwent neurosurgical treatment at the Neurosurgery Department (Azienda Sanitaria Universitaria Integrata, Udine). Other inclusion criteria were (a) being native Italian speakers, (b) having normal or corrected‐to‐normal vision, and (c) having no history of previous cerebral vascular damage, psychiatric disease, or drug abuse. We also excluded individuals with brain tumors who reported learning disabilities or history of developmental language problems and those who scored pathologically on a general nonverbal intelligence test (Raven Colored Progressive Matrices (Basso, Capitani, & Laiacona, 1987)). Participants had a mean age of 42.24 ± 14.6 and mean education of 12.87 ± 3.39 years, and included 24 males and 25 females, 44 right‐handers, 3 left‐handers, and 2 ambidextrous (Oldfield, 1971) (see Supplementary Table S1). On neuropsychological tasks, they all performed successfully on a temporal orientation task (Spinnler & Tognoni, 1987) and tests tapping oral (De Renzi, Piezcuro, & Vignolo, 1966) and ideomotor apraxia (De Renzi, Motti, & Nichelli, 1980), 2/49 participants scored below the normal range on a verbal short‐term memory task (WAIS Digit Span Forward) (Orsini et al., 1987), 6/49 on a verbal working memory task (WAIS Digit Span Backward) (Orsini et al., 1987), 2/49 on the Token Test (De Renzi & Faglioni, 1978), 8/49 on a picture naming task of objects and 12/49 of actions (Miceli, Laudanna, Burani, & Capasso, 1994), and 7/49 participants on a verbal fluency task (Novelli et al., 1996) (see Supplementary Table S2).
Conventional T2‐weighted MR imaging revealed 28 low‐grade gliomas (LGG) and 14 high‐grade gliomas (HGG), 3 metastases, 4 cavernomas, 1 pilocytic astrocytoma, and 1 dysembryoplastic neuroepithelial tumor (DNET) (see Supplementary Table S1). The overlay plot of all the participants' lesions shows the most frequently damaged voxels in the sample (see bar code in Supplementary Figure S1). An analysis of the lesion distribution shows maximum overlap in the left fusiform gyrus, hippocampus, inferior, middle and superior temporal gyri, inferior parietal lobule, insula (and/or the underlying external capsule), Rolandic operculum and inferior frontal gyrus. The mean lesion volume was 53.78 ± 56.45 cm3 (see Supplementary Table S1). The mean (%) EoR, calculated on postoperatory T2‐weighted MR images (Ius et al., 2012) was 93.16%.
The study was approved by the Ethics Committee of our Institution and carried out in accordance with the 2013 Fortaleza version of the Helsinki Declaration and subsequent amendments. All participants gave their written consent for inclusion in the study.
2.2. Word and pseudoword reading tasks before, immediately after surgery, and after a 4‐month follow‐up
The participants' ability to read words (N = 92) and pseudowords (N = 45) was assessed by the word and pseudoword reading‐aloud tasks taken from the Italian BADA (Batteria per l'analisi dei deficit afasici, Battery for the analysis of aphasic disorders) (Miceli et al., 1994). Stimuli were presented individually on an A4 sheet with no time limit. Answers were recorded and transcribed by the experimenter for later analysis. The examiner took note of long latencies, participants' self corrections, “staccato” productions, and letter‐by‐letter reading attempts. For three‐syllable or longer words, the examiner recorded the position of the principal word stress, when a stress‐position error was produced.
2.3. Intra‐surgery reading assessment
All individuals with brain tumors underwent awake surgery (Ius et al., 2012). Surgery was performed under cortical and subcortical white matter Direct Electrical Stimulation, according to Berger and Ojemann's intraoperative technique (Berger & Ojemann, 1992; Ojemann et al., 1989). To obtain best feedback on the status of the cognitive functions during surgery, we also performed RTNT (Skrap et al., 2016).
2.3.1. Monitoring reading performance
RTNT is a sequence of several neuropsychological tasks (e.g., lexical comprehension, picture naming, repetition, limb apraxia, short‐term memory) that are continuously alternated. In RTNT, as previously described (Skrap et al., 2016), we selected a series of cognitive tasks based on the functional role of the area involved in the resection and on the individuals' preoperative neuropsychological profile. The task sequence followed a fixed order (e.g., for the language skills: 15 items of object naming, 10 items of phonemic discrimination, 10 items of word reading, 10 items of word repetition, 10 items of pseudoword reading, 10 items of pseudoword repetition, 16 items of phonological discrimination, digit‐span, 10 items of lexical decision, 16 items of action naming, and 4 items of picture description). The sequence of tasks was repeated (presenting a different stimulus sublist for each sequence) until resection ended. As soon as individuals with neurosurgical lesions started suffering from a decrement with respect to the 70% threshold, the neurosurgeon was informed.
While for pre‐surgery, immediately post‐surgery, and follow‐up evaluations individuals with brain tumors are classified as pathological or unimpaired according to the criteria described by Miceli et al. (1994), this was not feasible for intra‐surgery evaluations, where the task is administered in sub‐lists of items. In the clinical routine we used the 70% accuracy threshold established in a previous study (Skrap et al., 2016), in which the threshold was found to correlate with the follow‐up performance. In Skrap et al., 2016 we reported that the threshold “[…] was established by examining a preliminary group of patients in whom we tested the feasibility of our technique […]. Thus this threshold was correlated to follow‐up performance. For example, for the object naming task, 1) we identified patients with pathology at the follow‐up examination and calculated their mean performance, which was around 75%; and 2) we identified their RTNT level of performance on the same task and calculated their mean accuracy, which was around 70%. Consequently, we considered this level of performance as a warning to stop surgery.” RTNT started at the beginning of resection and ended at the beginning of hemostasis, that is, the surgical procedure stopping bleeding caused by the tumoral tissue excision.
Reading is one of the tasks included in the RTNT. The word stimuli (N = 92) taken from the BADA (Miceli et al., 1994) were divided into 5 sublists of 20 items each (with 8 repeated stimuli), while pseudoword stimuli (N = 45) are divided into 5 sublists of 10 items each (with 5 repeated stimuli). Each sublist is shown at different stages of surgery until the end of resection. When a first reading sublist was achieved, testing continued with the remaining cognitive tasks of the RTNT sequence, after which the next reading sublist was administered, and so on. During RTNT, reading stimuli were presented (“Presentation,” Neurobehavioral Systems Inc., CA) via a monitor at a distance of about 30 cm. The verbal output of the participants as well as of the neuropsychologist was audio‐recorded and transcribed.
2.3.2. Localizing sites for which resection caused a decrease in reading performance
The aim of RTNT is the early detection of any sign of reading impairment in order to avoid postoperative deficits (irrespective of the surface or phonological dyslexia type). During RTNT, cortical and subcortical sites for which resection caused a decrease in the patient's reading performance (and the respective x, y, and z coordinates in the patient's MRI space) were collected and recorded as 3D data points on the patient's MRI images displayed by the neuronavigation system. We then normalized the ROIs to the Montreal Neurological Institute (MNI) space using the Normalize MRI routine of the “Clinical Toolbox” software (https://www.nitrc.org/projects/clinicaltbx/) for SPM8 (https://www.fil.ion.ucl.ac.uk/spm/software/spm8/).
2.4. MRI structural data
We used structural imaging data routinely acquired during pre‐surgery investigations. A 3‐T Philips Achieva whole‐body scanner was used to acquire structural data using a SENSE‐Head‐8 channel head coil and a custom‐built head restrainer to minimize head movements. High‐resolution T2‐weighted and post‐gadolinium contrast T1‐weighted anatomical MR images were acquired by using a T1‐weighted 3D magnetization‐prepared rapid acquisition gradient fast field echo (T1W_3D_TFE SENSE) pulse sequence (TR = 8.2 s, TE = 3.7 s, FOV = 24 cm, 190 sagittal slices of 1 mm thickness, flip angle = 8°, voxel size: 1 × 1 × 1 mm) and a T2‐weighted 3D magnetization‐prepared rapid acquisition gradient fast field echo (T2W_3D_TFE SENSE) pulse sequence (TR = 2,500 ms, TE = 35 ms, FOV = 24 cm, 190 sagittal slices of 1 mm thickness, flip angle = 90°, voxel size: 1 × 1 × 1 mm).
2.5. Data analysis
2.5.1. Behavioral measures
Assessment pre‐surgery and immediately post‐surgery and at follow‐up
According to Miceli et al.'s (1994) criteria, the cut‐off accuracy is 90/92 for the word reading task and 43/45 for the pseudoword reading task. Cut‐offs refer to the performance of 20 healthy adults (Miceli et al., 1994) with at least 8 years of education. On the basis of cut‐off scores, participants were classified as impaired or unimpaired for either words or pseudowords. Healthy controls show ceiling levels of performance at reading both words and pseudowords. Thus, even a few errors made by a patient indicate pathological performance.
Cut‐off scores allowed for the diagnosis of a reading deficit. To address the type of reading deficit, from the original list of 92 words and 45 pseudowords, we identified three lists of items that are critical for the diagnosis of either surface dyslexia or phonological dyslexia.
From the 92‐word list we identified a first set of 25 three‐syllable items that are critical for the diagnosis of surface dyslexia, that is, testing stress assignment in three‐syllable or longer words (see Introduction). In addition, from the 92‐word list we identified a critical list of 19 three‐ and two‐syllable words (critical words). Finally, from the 45 pseudowords, we identified a third list of 19 critical items (critical pseudowords). Critical words and critical pseudowords were matched on several dimensions, such as syllable length and CV structure (presence/absence of consonant clusters, presence/absence of double letters).
Following the principles reported in Ripamonti et al. (2014), the major operational criterion for surface dyslexia was a predominant occurrence (>10%) of stress errors (regardless of other types of errors), that is, ≥3 out of 25 items. Considering that reading three‐syllable words along the GPC routine allow for a chance accuracy of 50%, 10% of word‐stress errors is a reasonable error rate, since we aimed at detecting mild surface dyslexia cases, too. In addition, no—or only mild—impairment shall emerge when reading pseudowords. In contrast, to meet the operational criterion for phonological dyslexia in our assessment, performance on critical words had to be significantly better than that on critical pseudowords. Undifferentiated reading deficit was defined for participants that did not meet the criteria for either dyslexia types.
Fisher exact tests were performed using SPSS software (Statistics for Windows, version 25.0, Armonk, NY) to compare reading accuracy for critical words versus reading accuracy for critical pseudowords in both pre‐surgery, immediate post‐surgery, and follow‐up data.
Intra‐surgery reading assessment
Decreases in reading performance were estimated based on significant Fisher exact test between pre‐ and intra‐surgery accuracy (correct vs. error and pre‐surgery vs. intra‐surgery).
Finally, to assess the predictive value of intra‐surgery assessment, we compared the last intra‐surgery RTNT reading performance with the immediate post‐surgery performance by means of a Pearson correlation analysis. The analysis was carried out on the items that were tested during the last RTNT reading task, which is representative of each patient's reading ability during the last resection phase.
We used IBM SPSS Statistics for Windows, version 25.0, Armonk, to perform the correlation analysis.
2.5.2. MRI structural data
Structural MRI preprocessing was performed on a UNIX workstation (Ubuntu 8.04 LTS. i386. http://www.ubuntu.com/) using MATLAB r2007b (The MathWorks Inc., Natick, MA) and SPM8 (Statistical Parametric Mapping software; Wellcome Department of Imaging Neuroscience, London, UK). Volumes of interest of the participants' lesions were drawn on their T2 MRI scans using the MRIcron software (https://www.nitrc.org/projects/mricron). We then normalized the ROIs to the MNI space using the Normalize MRI routine of the “Clinical Toolbox” software (https://www.nitrc.org/projects/clinicaltbx/) for SPM8 (https://www.fil.ion.ucl.ac.uk/spm/software/spm8/).
Overlays and subtractions
Overlaps were created by using the lesion maps of the participants with dyslexia detected at immediate post‐surgery time. We first overlapped the volumes of interest of participants with surface dyslexia, with phonological dyslexia, and with undifferentiated dyslexia (i.e., patients that did not meet the criteria for either dyslexia types), by using the MRIcron procedure (https://www.nitrc.org/projects/mricron). The outputs are three percentage overlay maps portraying the % of overlapping lesions, for each type of dyslexia, for each voxel on a color scale.
We then applied the subtraction procedure in order to identify the specific lesions causing selective damage to either the lexical (surface dyslexia) or the sublexical route of reading (phonological dyslexia) by subtracting the percentage overlay map of participants with phonological dyslexia from the percentage overlay map of participants with surface dyslexia (and vice versa). The outputs are two percentage overlay maps showing the percentage of overlapping lesions exclusively related to either phonological or surface dyslexia, for each voxel on a color scale (Karnath, Berger, Küker, & Rorden, 2004; Ripamonti et al., 2014).
3. RESULTS
3.1. Pre‐surgery: Effect of tumor
Reading performance was impaired in 7/49 participants (4 HGG, 2 metastases and 1 LGG, see Table 1 and Figure 1). An analysis of participants' performance pre‐surgery indicates that the reading impairment in 2/7 individuals could be classified as phonological dyslexia, since reading of critical pseudowords was significantly more impaired than that of critical words (Case 12, Case 20; see Table 2), the reading impairment of the remaining three cases was classified as undifferentiated dyslexia.
TABLE 1.
Participants' word and pseudoword % reading accuracy during RTNT (intra‐surgery accuracies reflect the average of the RTNT runs) and % accuracy pre‐surgery, immediately post‐surgery and at F‐up. Decreases in the reading performance with respect to pre‐surgery (according to Fisher exact test) and pathological performance pre‐surgery, post‐surgery and at F‐up (according to BADA cut‐offs which are 90/92 for the word reading task and 43/45 for the pseudoword reading task) are highlighted in gray
| Case No. | Etiology | RTNT: intra‐surgery | Pre‐surgery (PRE), immediately post‐surgery (1W) and at F‐up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W mean% | Fisher exact test | PW mean% | Fisher exact test | PRE W% | 1W W% | F‐up W% | PRE PW% | 1W PW% | F‐up PW% | ||
| 1 | LGG | 48 | p < .05 | 74 | p < .05 | 99 | 25 | 89 | 93 | 33 | 76 |
| 2 | LGG | 99 | p > .05 | 97 | p > .05 | 100 | 90 | 100 | 100 | 69 | 98 |
| 3 | HGG | 100 | p > .05 | 100 | 93 | 100 | 100 | 100 | 100 | ||
| 4 | LGG | 93 | p < .05 | 80 | p < .05 | 100 | 97 | 100 | 100 | 87 | 100 |
| 5 | LGG | 81 | p < .05 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 6 | HGG | 70 | p < .05 | 95 | p < .05 | 93 | 78 | n.a. | 84 | 71 | n.a. |
| 7 | LGG | 80 | p < .05 | 46 | p < .05 | 100 | 62 | 100 | 98 | 29 | 100 |
| 8 | LGG | 100 | p > .05 | 100 | p < .05 | 100 | 100 | n.a. | 93 | 87 | 100 |
| 9 | HGG | 67 | p < .05 | 69 | p < .05 | 100 | 65 | n.a. | 96 | 94 | 100 |
| 10 | LGG | 88 | p < .05 | 56 | p < .05 | 99 | 91 | n.a. | 100 | 89 | n.a. |
| 11 | LGG | 46 | p < .05 | 100 | p > .05 | 100 | 89 | 97 | 98 | 89 | 91 |
| 12 | Metastasis | 37 | p < .05 | 100 | 92 | n.a. | 84 | 62 | n.a. | ||
| 13 | LGG | 89 | p < .05 | 100 | p > .05 | 100 | 89 | 99 | 98 | 82 | 100 |
| 14 | LGG | 57 | p < .05 | 97 | p > .05 | 100 | 100 | 99 | 98 | 100 | 100 |
| 15 | HGG | 93 | p > .05 | 95 | p > .05 | 92 | 73 | n.a. | 89 | 84 | n.a. |
| 16 | HGG | 27 | p < .05 | 78 | 79 | n.a. | 69 | 80 | n.a. | ||
| 17 | Metastasis | 90 | p < .05 | 98 | 93 | n.a. | 84 | 80 | n.a. | ||
| 18 | HGG | 84 | p < .05 | 98 | 100 | 97 | 98 | 100 | 93 | ||
| 19 | HGG | 95 | p > .05 | 100 | p > .05 | 98 | 100 | 100 | 98 | 98 | n.a. |
| 20 | HGG | 93 | p < .05 | 85 | p > .05 | 100 | 98 | n.a. | 89 | 58 | n.a. |
| 21 | Cavernoma | 100 | p > .05 | 100 | 100 | n.a. | 100 | 93 | n.a. | ||
| 22 | LGG | 100 | p > .05 | 100 | 100 | 100 | 100 | 87 | 100 | ||
| 23 | DNET | 93 | p > .05 | 100 | p > .05 | 98 | 45 | 99 | 100 | 44 | 93 |
| 24 | LGG | 80 | p < .05 | 90 | p < .05 | 100 | 92 | n.a. | 100 | 24 | n.a. |
| 25 | LGG | 100 | p > .05 | 96 | 98 | 100 | 100 | 91 | 100 | ||
| 26 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 90 | 100 | 100 | 98 | 98 |
| 27 | Metastasis | 100 | p > .05 | 100 | p > .05 | 100 | 100 | n.a. | 98 | 87 | n.a. |
| 28 | LGG | 90 | p < .05 | 100 | 93 | n.a. | 96 | 100 | n.a. | ||
| 29 | HGG | 72 | p < .05 | 94 | p < .05 | 99 | 86 | 100 | 100 | 89 | 96 |
| 30 | HGG | 100 | p > .05 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 31 | Pilocytic astrocytoma | 100 | p > .05 | 100 | 100 | n.a. | 100 | 100 | n.a. | ||
| 32 | LGG | 100 | p > .05 | 100 | p > .05 | 99 | 100 | 100 | 100 | 100 | 100 |
| 33 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 99 | 100 | 100 | 100 | 100 |
| 34 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 100 | 100 | 100 | 100 | 100 |
| 35 | HGG | 100 | p > .05 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 36 | LGG | 100 | p > .05 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 37 | Cavernoma | 100 | p > .05 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 38 | HGG | 90 | p < .05 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 39 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 99 | 100 | 98 | 100 | 100 |
| 40 | LGG | 100 | 100 | 100 | 100 | 100 | 100 | ||||
| 41 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 4.4 | 100 | 96 | 2.22 | 100 |
| 42 | HGG | 100 | — | 100 | — | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 43 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 100 | n.a. | 100 | 100 | n.a. |
| 44 | Cavernoma | 100 | 100 | 100 | 100 | 98 | 100 | ||||
| 45 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 100 | 100 | 100 | 100 | 100 |
| 46 | LGG | 100 | p > .05 | 100 | p > .05 | 100 | 99 | 100 | 100 | 93 | 100 |
| 47 | HGG | 100 | p > .05 | 100 | 100 | 100 | 100 | 98 | 100 | ||
| 48 | LGG | 98 | p > .05 | 100 | p > .05 | 100 | 100 | 99 | 100 | 100 | 98 |
| 49 | Cavernoma | 100 | p > .05 | 96 | p > .05 | 100 | 100 | n.a. | 100 | 100 | n.a. |
Note: n.a. = non administered.
Abbreviations: BADA, Batteria per l'analisi dei deficit afasici, Battery for the analysis of aphasic disorders; DNET, dysembryoplastic neuroepithelial tumor; F‐up, follow‐up; HGG, high‐grade glioma; LGG, low‐grade glioma; RTNT, real‐time neuropsychological testing.
FIGURE 1.
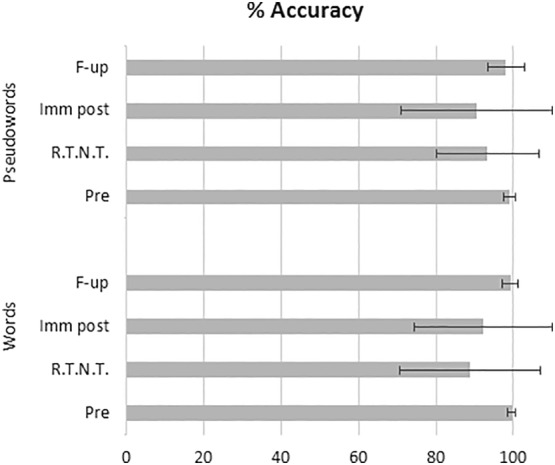
Patients' mean % of word and pseudoword reading accuracy pre‐surgery (pre), immediately post‐surgery (imm post) and at follow‐up (F‐up) testing and at different runs during real‐time neuropsychological testing (RTNT)
TABLE 2.
The distribution of the x, y, and z coordinates of the LH resection sites causing a significant decrease in the participants' reading performance
| Case No. | MNI | Area | ||
|---|---|---|---|---|
| x | y | z | ||
| 1 | −44 | −49 | −29 | Inferior parietal cortex |
| 4 | −49 | −2 | 29 | Precentral gyrus |
| 5 | −35 | −10 | 1 | Insula |
| 6 | −57 | −31 | 35 | Inferior parietal cortex |
| 7 | −35 | −4 | 30 | Inferior frontal gyrus |
| 9 | −38 | −51 | 21 | Superior longitudinal fasciculus |
| 10 | −33 | −29 | 23 | Parietal operculum |
| 11 | −53 | −47 | 11 | Middle temporal gyrus |
| 12 | −42 | −8 | 51 | Precentral gyrus |
| 13 | −41 | −44 | 10 | Middle temporal gyrus |
| 14 | −42 | −33 | 27 | Parietal operculum |
| 16 | −39 | −61 | 18 | Middle temporal gyrus |
| 17 | −33 | −45 | 28 | Superior longitudinal fasciculus |
| 18 | −53 | −50 | 13 | Superior temporal gyrus |
| 20 | −49 | −35 | 49 | Intraparietal area |
| 24 | −57 | −45 | −2 | Middle temporal gyrus |
| 28 | −36 | −4 | 31 | Precentral gyrus |
| 29 | −56 | −41 | 30 | Inferior parietal cortex |
Abbreviations: MNI, Montreal Neurological Institute.
3.2. Intra‐surgery reading assessment
RTNT is performed to detect in real‐time a decrease in performance and stop resection, if needed, in order to avoid cognitive deficits due to surgery. A decrease in performance (vs. pre‐surgery data) for either words or pseudowords (see Table 1 and Figure 1) was found in 19/49 participants (both with HGG and LGG). Performance remained above 70% accuracy in 11/19 participants. In eight cases, while reading accuracy was normal at the completion of the previous RTNT run, it suddenly decreased below 70% (in the clinical routine we use the 70% accuracy threshold (Skrap et al., 2016), when the next reading sublist was administered.
3.3. Localizing sites for which resection caused a decrease in reading performance
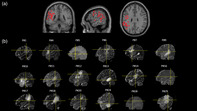
Figure 2 shows the normalized cortical and subcortical 3D data points corresponding to the resection areas that caused a significant decrease in the participants' reading performance: normalized cortical and subcortical 3D data points were superimposed on coronal, sagittal, and axial sections of the single subject's brain template provided by SPM8 (A) and on each patient's T1 (Case P#5 and P#28) or T2 MRI images (B). The distribution of the x, y, and z site coordinates (see Table 2) ranged from the posterior inferior and middle temporal gyrus to the temporo‐parietal areas and the precentral gyrus.
FIGURE 2.
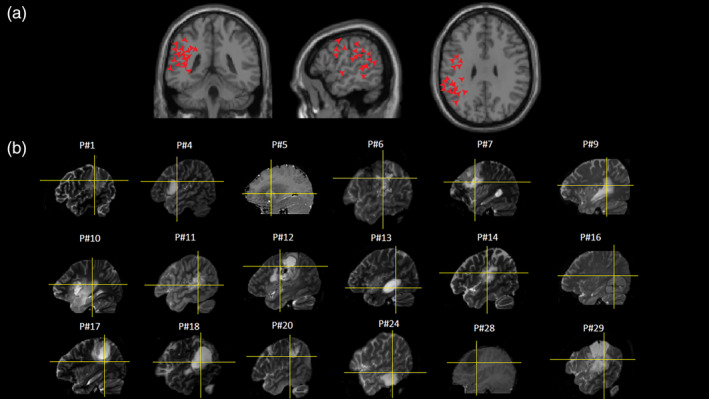
(a) 3D data points corresponding to the resection areas causing significant decrease in the participants' reading performance superimposed on coronal, sagittal, and axial sections of the single subject's brain template provided by SPM8. (b) Sagittal sections of single cases' T1 (Cases P#5 and P#28) or T2 MRI images: the cross line indicates the resection areas causing significant decrease in the participants' real‐time neuropsychological testing (RTNT) reading performance
3.4. Correlation between RTNT and immediate post‐surgery performance
The predictive value of the intra‐surgery assessment was confirmed by a significant correlation between accuracy during the last RTNT reading task and that obtained for the corresponding items during the immediate post‐surgery assessment stage, both for word (r[47] = .340, p < .05) and pseudoword (r[32] = .484, p < .01) lists.
3.5. Immediate post‐surgery outcome: Effect of resection
Reading performance was impaired in 26/49 participants (14 LGG; 7 HGG, 3 metastases, 1 cavernoma, 1 DNET) (see Table 1 and Figure 1). Only one of the seven participants who performed below the normal range pre‐surgery recovered to normal levels after surgery (Case 25). The post‐surgery reading impairment fitted the operational criteria for surface dyslexia in four participants (Cases 2, 3, 11, and 13; see Table 3), and was consistent with the operational criteria for phonological dyslexia in 11 participants (Cases 2, 6, 7, 8, 12, 20, 23, 24, 27, 28, and 41; see Table 3 for Wilcoxon Z values); the remaining 11 cases were classified as undifferentiated. In one patient (Case 2), the post‐surgery reading impairment fitted the operational criteria for both surface and phonological dyslexia.
TABLE 3.
Participants diagnosed with phonological or surface dyslexia. Number of errors out of the critical lists for 19 words (W err) and 19 pseudowords (pW err) and number of stress errors (Stress err) out of the critical lists for 25 words. Significant differences (Fisher exact test) between W and pW accuracy are shown in dark gray (phonological dyslexics) as are data for patients with a number of stress errors >10% of critical items (surface dyslexics) in light gray
| Pre | Immediate post | Follow‐up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | W err | pW err | Fisher exact test | Stress err | Classified as | W err | pW err | Fisher exact test | Stress err | Classified as | W err | pW err | Fisher exact test | Stress err | Classified as |
| 1 | 0 | 2 | p > .05 | 12 | 17 | p > .05 | Undiff | 1 | 6 | p > .05 | Undiff | ||||
| 2 | 0 | 0 | 0 | 2 | 10 | p < .05 | 3 | Surf/phon | — | — | — | ||||
| 3 | 0 | 0 | 0 | 1 | 0 | p > .05 | 3 | Surf | 0 | 0 | 0 | ||||
| 4 | 0 | 0 | 0 | 1 | 4 | p > .05 | Undiff | 0 | 0 | 0 | |||||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 6 | 1 | 3 | p > .05 | 2 | Undiff | 5 | 12 | p < .05 | 2 | Phon | — | — | — | ||
| 7 | 0 | 0 | 0 | 5 | 15 | p < .05 | Phon | 0 | 0 | 0 | |||||
| 8 | 0 | 3 | p > .05 | 0 | 5 | p < .05 | Phon | — | — | — | |||||
| 9 | 0 | 1 | p > .05 | 4 | 7 | p > .05 | 1 | Undiff | — | — | — | ||||
| 10 | 0 | 0 | 0 | 2 | 5 | p > .05 | 2 | Undiff | — | — | — | ||||
| 11 | 0 | 1 | p > .051 | 3 | 3 | 0 | 6 | Surf | 0 | 2 | p > .05 | — | Undiff | ||
| 12 | 0 | 4 | p > .05 | Undiff | 0 | 11 | p < .05 | Phon | — | — | — | ||||
| 13 | 0 | 0 | 0 | 2 | 6 | p > .05 | 5 | Surf | 1 | 0 | p > .05 | Undiff | |||
| 14 | 0 | 1 | p > .05 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 15 | 4 | 2 | p > .05 | 2 | Undiff | 5 | 4 | p > .05 | 2 | Undiff | — | — | — | ||
| 16 | 2 | 10 | p < .05 | 1 | Phon | 1 | 5 | p > .05 | 1 | Undiff | — | — | — | ||
| 17 | 0 | 6 | p < .05 | Phon | 1 | 4 | p > .05 | Undiff | — | — | — | ||||
| 18 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | p > .05 | Undiff | |||||
| 19 | 0 | 0 | 0 | 1 | 0 | 1 | −1 | 0 | 0 | 0 | |||||
| 20 | 0 | 4 | p > .05 | Undiff | 1 | 10 | p < .05 | Phon | — | — | — | ||||
| 21 | 0 | 0 | 0 | 0 | 3 | p > .05 | — | — | — | ||||||
| 22 | 0 | 0 | 0 | 0 | 3 | p > .05 | 0 | 0 | 0 | ||||||
| 23 | 0 | 0 | 0 | 7 | 15 | p < .05 | 1 | Phon | 0 | 3 | p > .05 | Undiff | |||
| 24 | 0 | 0 | 0 | 0 | 18 | p < .05 | Phon | — | — | — | |||||
| 25 | 0 | 0 | 0 | Undiff | 0 | 2 | p > .05 | Undiff | 0 | 0 | 0 | ||||
| 26 | 0 | 0 | 0 | 1 | 0 | p > .05 | 1 | Undiff | 0 | 1 | p > .05 | Undiff | |||
| 27 | 0 | 1 | p > .05 | 0 | 5 | p < .05 | Phon | — | — | — | |||||
| 28 | 0 | 1 | p > .05 | 0 | 7 | p < .05 | Phon | — | — | — | |||||
| 29 | 0 | 0 | 0 | 2 | 3 | p > .05 | Undiff | 0 | 1 | p > .05 | Undiff | ||||
| 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 31 | 0 | 0 | 0 | 0 | 0 | — | — | — | |||||||
| 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 34 | 0 | 0 | 0 | 0 | 0 | — | — | — | |||||||
| 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 41 | 0 | 2 | p > .05 | 11 | 19 | p < .05 | Phon | 0 | 0 | 0 | |||||
| 42 | 0 | 0 | 0 | 0 | 1 | p > .05 | — | — | — | ||||||
| 43 | 0 | 1 | p > .05 | 0 | 0 | 0 | 0 | 0 | |||||||
| 44 | 0 | 0 | 0 | 0 | 1 | p > .05 | 0 | 0 | 0 | ||||||
| 45 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 46 | 0 | 0 | 0 | 1 | 1 | p > .05 | 0 | 0 | 0 | ||||||
| 47 | 0 | 0 | 0 | 0 | 1 | p > .05 | 0 | 0 | 0 | ||||||
| 48 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | p > .05 | |||||||
| 49 | 0 | 0 | 0 | 0 | 0 | — | — | — | |||||||
3.6. Follow‐up outcome: Effect of spontaneous recovery
Four months after surgery, 33/49 participants (67%: 22 LGG, 9 HGG, 2 cavernomas and 1 DNET) were reassessed with the same reading task employed before and immediately after tumor resection. Reading performance was still impaired in only 5/33 participants (2 LGG, 2 HGG, 1 DNET) (performance was >70% accuracy for all patients). Of these five participants, none corresponded to the original seven participants who performed below normal range before surgery. In particular, only 2/11 participants with LGG with impaired reading performance at 1 week post‐surgery were still below the normal range at follow‐up assessment. This shows a remarkable recovery rate in the participants' reading ability. Almost all individuals whose word reading performance worsened on immediate post‐surgery testing (mean % accuracy = 92.09 ± 17.85) recovered significantly at follow‐up (mean % accuracy = 99.54 ± 1.0, t(28) = −2.34, p = .026) and returned to their pre‐surgery accuracy rate (t(28) = .64, p = .526, n.s.; see Table 1 and Figure 1). Similarly, participants whose performance on pseudoword reading significantly worsened on immediate post‐surgery testing (mean % accuracy = 89.84 ± 19.96) recovered at follow‐up (mean % accuracy = 99.04 ± 1.64, t(28) = −2.43, p = .021) and returned to their pre‐surgery accuracy rate (t(28) = 1.309, p = .201, n.s.; see Table 1 and Figure 1).
More in detail, the lexical reading damage vanished in all four participants meeting the operational criteria for surface dyslexia on immediate post‐surgery testing (Cases 2, 3, 11, and 13) (see Table 3). By contrast, only 3 of the 11 participants meeting the operational criteria for phonological dyslexia post‐surgery could be reassessed and showed full resolution of their reading impairment (see Table 3).
3.7. MRI structural data (subtraction procedure)
The structural neuroimaging analysis allowed for direct subtraction of neural pathways underlying the sublexical route from pathways underlying the lexical route (and vice versa). The overlay for phonological dyslexia (see Figure 3) was created by overlapping the lesion maps of the 10 participants diagnosed with such reading impairment detected at immediate post‐surgery stage (Cases 6, 7, 8, 12, 20, 23, 24, 27, 28, and 41). The overlay for surface dyslexia was created using the lesion maps of the three participants with such type of dyslexia detected at immediate post‐surgery stage. Case 2 (whose reading profile corresponded to the operational criteria for both surface and phonological dyslexia) was excluded.
FIGURE 3.
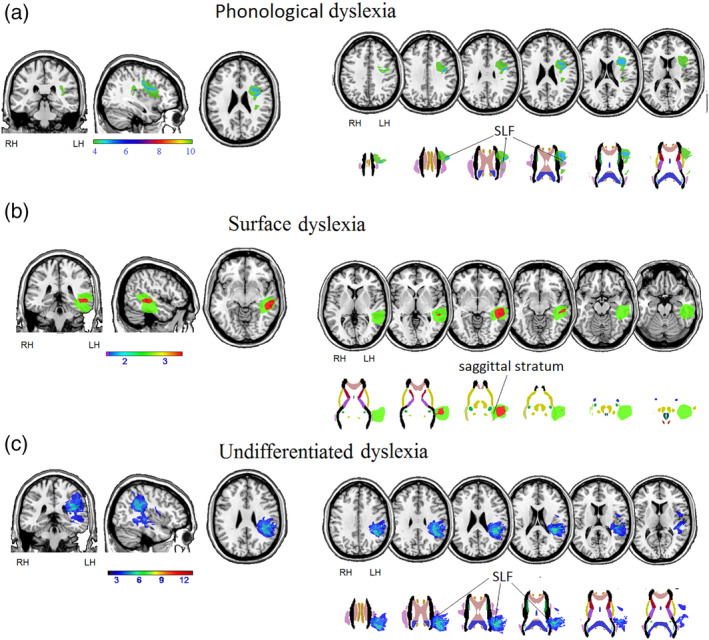
Overlays of lesion masks for participants with (a) phonological dyslexia, (b) surface dyslexia, and (c) undifferentiated dyslexia on coronal, sagittal, and axial sections of the T1 MRIcro template and on axial sections at cortical (first row) and subcortical level (second row); images taken from the John Hopkins University (JHU) diffusion tensor imaging (DTI)‐based white‐matter atlases (Hua et al., 2008). The color bar represents the maximum density of the participants' lesion overlays. MR images are displayed in radiological convention
3.7.1. Overlays
The maximum overlap of lesion masks for participants with phonological dyslexia occurred in the Rolandic operculum, inferior frontal gyrus and, at subcortical level, in the anterior part of the SLF. The maximum overlap of lesion masks for participants with surface dyslexia occurred in the middle temporal gyrus and, at the subcortical level, in part of the sagittal stratum (including the ILF and the IFOF). The maximum overlap of lesion masks for participants with undifferentiated dyslexia occurred in the precentral gyrus, insula, and superior temporal gyrus, and, at the subcortical level, in part of the external capsule.
3.7.2. Subtractions
Direct subtraction of the phonological dyslexia plot from the surface dyslexia plot (% surface > % phonological dyslexia) evidenced a specific involvement of the left middle and inferior temporal gyrus, and, at the subcortical level, of the posterior part of the sagittal stratum (IFOF + ILF) and in the retrolenticular part of the internal capsule (IC) (Table 4 and Figure 4a).
TABLE 4.
Subtractions of lesion masks of participants with phonological dyslexia > surface dyslexia and vice versa (the percentage of participants with a lesion involving the area is reported). The neuroanatomical labels are derived from the automated anatomical labeling (Tzourio‐Mazoyer et al., 2002) for the cortical, and from the JHU atlas (Hua et al., 2008) for the subcortical part of the lesion overlay
| Subtraction | Subtraction | |||||||
|---|---|---|---|---|---|---|---|---|
| Phonological > surface | Surface > phonological | |||||||
| x | y | z | % overlap a | x | y | z | % overlap a | |
| Cortical | ||||||||
| Middle temporal gyrus | — | — | — | — | −55 | −30 | −2 | 90% |
| Superior temporal gyrus | — | — | — | — | −46 | −26 | −2 | 80% |
| Inferior temporal gyrus | — | — | — | — | −55 | −32 | −24 | 57% |
| Supramarginal gyrus | −49 | −38 | 26 | 30% | — | — | — | — |
| Rolandic operculum | −42 | −32 | 24 | 30% | — | — | — | — |
| Insula | −40 | 10 | 8 | 40% | — | — | — | — |
| Inferior frontal gyrus (pars triangularis) | −41 | 22 | 26 | 40% | — | — | — | — |
| Superior frontal gyrus | −23 | 9 | 55 | 30% | — | — | — | — |
| Subcortical | ||||||||
| Sagittal stratum (IFOF and ILF) | — | — | — | — | −41 | −24 | −15 | 57% |
| Retrolenticular part of the posterior corona radiata | — | — | — | — | −40 | 38 | 3 | 40% |
| Superior corona radiata | −24 | 2 | 33 | 40% | — | — | — | — |
| Superior longitudinal fasciculus | −3 | 8 | 23 | 50% | — | — | — | — |
Abbreviations: IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; JHU, John Hopkins University.
Percent of phonological dyslexic participants who had lesions of voxels in which none of the surface dyslexics had a lesion, and vice versa. The % overlap is reported as provided by the MRIcron software. Under the Draw MRIcron menu, we selected “Statistics” and then the “Batch Descriptives” function. After selecting the lesion subtraction map, the MRIcron function returned the percentage of overlap between lesions and specific structures.
FIGURE 4.
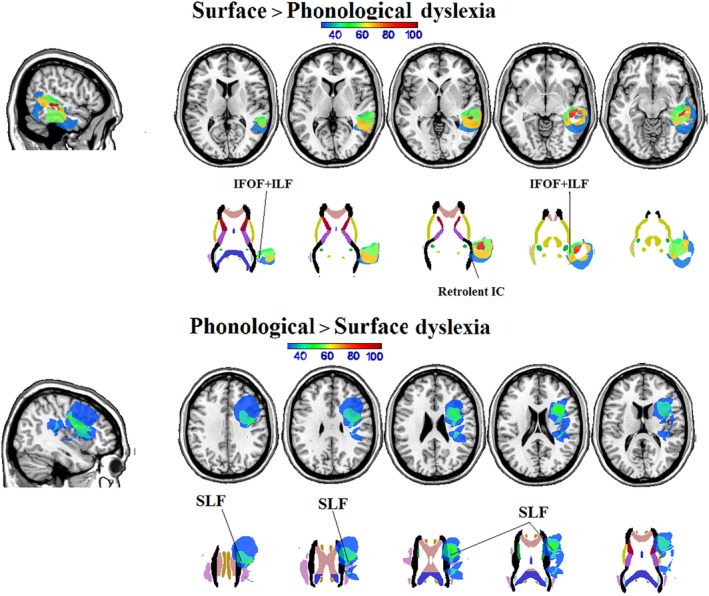
Subtraction analyses (minimum 30% overlap) of lesion masks on coronal, sagittal, and axial sections of the T1 MRIcro template and on axial sections at cortical (first row) and subcortical level (second row; images taken from the John Hopkins University (JHU) diffusion tensor imaging (DTI)‐based white‐matter atlases (Hua et al., 2008) for participants with (a) surface dyslexia > participants with phonological dyslexia, and (b) phonological dyslexia > participants with surface dyslexia. The color bar represents the maximum density of the individuals' lesion overlays. MR images are displayed in radiological convention (left is right and vice versa)
The reverse subtraction (% phonological > % surface dyslexia) evidenced a lesional pattern that includes the left Rolandic operculum, left inferior frontal gyrus (pars triangularis), left supramaginal gyrus, left anterior insula (and/or the underlying external capsule) and, at the subcortical level, the left SLF, and superior corona radiata (Table 4 and Figure 4b).
4. DISCUSSION
Combining neuropsychological data concerning neurosurgical symptoms with results of lesion reconstruction analysis is a promising approach to assess neuropsychological effects before, during, and after brain tumor excision. Neurosurgical lesions are generally much more circumscribed than vascular lesions. The aim of the present study was reporting pre‐surgery, immediately post‐surgery, and follow‐up performance of those individuals with brain tumors for whom we assessed reading skills while resection occurred. We addressed the neuroanatomical correlates of the lexical semantic and the sublexical routes and studied how reading abilities evolve in individuals with neurosurgical lesions. We found that only few individuals suffered from dyslexia at pre‐surgery testing, that decreases in the reading performance could be detected in real time during surgery, that these impairments worsened in the immediate post‐surgery stage, and that they mostly recovered during 4‐month follow‐up.
4.1. Neuroanatomical correlates
The area arising from our overlay study and the following subtraction analyses shall be considered as the anatomical location of the sub lexical and lexical‐semantic functional pathways, rather than the specific location of the single units underlying the reading processes.
4.1.1. Surface dyslexia following left brain damage to the ventral stream
Previous studies found that the anatomical localization of surface dyslexia ranges from the left superior, middle, and inferior temporal gyri, the left temporo‐parietal, and parieto‐occipital areas, to the left anterior temporal regions (see Section 1). Given the circumscribed aspect of neurosurgical lesions, we expected more selective neuroanatomical patterns. We found that the maximum lesion overlap for surface dyslexia (and, consequently, for the ventral lexical reading processes) involved a circumscribed area located between the left posterior portion of the middle (90% of overlap), superior (80% of overlap), and inferior (57% of overlap) temporal gyri. Despite the limited number of cases included in the study, this indicates a much more selective anatomical correlate than that described in previous studies, following which surface dyslexia resulted from lesions involving either the left temporoparietal (Ferreres, Cuiyino, & Olmedo, 2005; Marshall & Newcombe, 1973) or parieto‐occipital structures (Raman & Weekes, 2005). Our results are consistent with the assumption that brain tumor individuals with surface dyslexia have lesions involving the posterior and the inferior part of the left middle and inferior temporal gyri (e.g., see Ripamonti et al., 2014 and Binder et al., 2016 for surface dyslexia in individuals with stroke, Brambati et al., 2009 and Henry, Beeson, Alexander, & Rapcsak, 2012 for surface dyslexia in semantic dementia and/or primary progressive aphasia).
At the subcortical level, the maximal overlap of lesion volumes of interest occurred in the posterior part of the sagittal stratum (IFOF + ILF) and in the retrolenticular part of IC. According to several studies, the IFOF and the ILF (Catani & Thiebaut de Schotten, 2008; Fernandez‐Miranda et al., 2008; Martino et al., 2010) are part of the neuroanatomical correlates of the ventral reading route (Epelbaum et al., 2008; Schlaggar & McCandliss, 2007). Consistent with the association of these regions with surface dyslexia, our results complement data that stress the importance of the left IFOF and ILF when reading along the lexical route. Data confirm the view that the left ILF may be considered (Vandermosten, Boets, Poelmans, et al., 2012; Vandermosten, Boets, Wouters, & Ghesquiere, 2012) the subcortical tract that is related to surface dyslexia, although other authors found only an involvement of the IFOF (Ripamonti et al., 2014).
These results complement, on a larger sample of participants, findings from a previous study (Tomasino et al., 2015) carried out on patient CF, which we mentioned in the Introduction, who developed surface dyslexia and dysgraphia due to a tumor in the left temporal lobe. The majority of CF's reading mistakes were lexical stress errors. CF's neural damage involved the ventral lexical route (as evidenced by a fractional anisotropy decrease along the IFOF) with a relatively preserved dorsal phonological route (as evidenced by a rather full integrity of the SLF). Thus, our results seem to further confirm the involvement of the IFOF and ILF in reading along the ventral route.
4.1.2. Phonological dyslexia following left brain damage to the dorsal stream
The subtraction procedure (% phonological > % surface dyslexia) revealed an association of phonological dyslexia with circumscribed damage to the left insula (and/or the underlying external capsule), the left inferior frontal gyrus, and the left Rolandic operculum. These findings complement previous results suggesting that phonological dyslexia would depend on large left frontoparietal (Denes, Cipolotti, & Semenza, 1987; Lesch & Martin, 1998) or fronto‐temporo‐parietal lesions (e.g., Hamilton & Coslett, 2008; Marchand & Friedman, 2005; Rapcsak et al., 2009). These areas are part of the dorsal reading route, which allows sublexical grapheme‐to‐phoneme mapping.
In addition, data confirm the role of the left SLF, which is part of the AF, in the sublexical grapheme‐to‐phoneme conversion reading route (Vandermosten, Boets, Poelmans, et al., 2012; Vandermosten, Boets, Wouters, & Ghesquiere, 2012). Previous studies on Italian individuals with vascular lesions (Ripamonti et al., 2014) did not find specific contribution of the left SLF.
4.2. Evolution of reading abilities in individuals with neurosurgical lesions
Tumors, in particular low‐grade ones, grow very slowly so it is possible that, before surgery and thanks to neuroplasticity, left‐lateralized language and associated cognitive processes may shift to either ipsilateral perilesional structures or controlateral structures. In our sample of individuals with neurosurgical lesions, reading abilities were rather resistant to tumor growth. Only 7/49 participants were below the normal range pre‐surgery. In four out of seven participants, the reading impairment was consistent with a phonological dyslexia pattern of damage. At immediate post‐surgery evaluation, the reading disorder was still consistent with phonological dyslexia in two of these four cases, while it evolved to undifferentiated dyslexia in two cases.
During surgery, and while performing RTNT, 18 participants showed a decrease in performance, which helped in limiting functional consequences (indeed most cases recovered at follow‐up assessment, see below). One of the most debated issues in awake surgery is the reliability of awake mapping (e.g., Borchers, Himmelbach, Logothetis, & Karnath, 2011). RTNT allows to detect the first signs of functional consequences of resection as soon as they take place. In a previous study we reported (Skrap et al., 2016) that decreases in individuals' performance detected during RTNT most often disappear post‐surgery. We argued (Skrap et al., 2016) that the mechanical manipulation of the tissue can transiently condition the implicated function. Other examples of such effect occur during intramedullary surgery (Sala, Bricolo, Faccioli, Lanteri, & Gerosa, 2007) and in resections close to the cortico‐spinal tract (Skrap et al., 2018). In 10/18 cases, reading accuracy remained >70%, similarly to data reported in Skrap et al. (2016). In eight cases, while reading accuracy was in the normal range at the completion of the last‐but‐one RTNT run, it suddenly decreased when the last reading sublist was administered.
RTNT is complementary—and not alternative—to DES. The two methodological approaches differ in several aspects. In DES, the risk of negative mapping (i.e., DES has no effect, Sanai, Mirzadeh, & Berger, 2008) is relatively high. Negative mapping may be due to several factors, such as an inappropriate task for the target area, or a network of areas subserving a certain cognitive function that cannot be conditioned by the small area where DES exerts its electrical interference (Nathan, Sinha, Gordon, Lesser, & Thakor, 1993). Negative mapping does not necessarily imply lack of functional role of the stimulated area and there is a risk of resecting tissue that is crucial for a certain function. Often, post‐surgery assessment can show impairments in performance for the crucial function. By using RTNT we try to reduce such risk. The RTNT procedure is performed continuously during resection, while DES is performed discontinuously. RTNT implies the use of different tasks and allows for continuous feedback on the individuals' cognitive status. In addition, DES allows only for short testing periods, while RTNT does not impose time limitations. In the absence of DES responses (negative mapping) the surgeon cannot rely on proper feedback during the procedure and may be less prone to perform an extensive resection. DES is suitable and reliable for simple functions such as when testing the motor system, or areas producing speech arrest under stimulation. By contrast, DES becomes less conclusive when complex functions are tested (also within neural areas related to the language domain). The DES feedback type is “all‐or‐nothing”: it can evoke an effect, or it may produce a negative mapping. RTNT adds information on a continuum that can be expressed in percentage of accuracy.
A decrease in reading performance persisted in the immediate post‐surgery condition, suggesting that compensatory or reorganization mechanisms were not active yet. A critical issue concerns the role of cerebral edema immediately post‐surgery. Tumors and surgical resection normally cause edema of the perilesional tissue and it becomes highly probable that individuals will have some decrease in language skills (and other cognitive functions) after surgery. Very often, the improvement starts a few days after surgical treatment.
The follow‐up evaluation revealed the effect of spontaneous recovery on the post‐surgery reading impairment. In particular, only 2 out of the 11 individuals with LGG who at 1 week post‐surgery had an impaired performance and were examined at follow‐up were still below the normal range. It is not possible to draw conclusions on whether the improvement was due to either the disappearance of perilesional cerebral edema, which may have occurred at some point between immediate post‐surgery testing and testing that was performed 4 months later, or to neuroplasticity, or both.
There are sparse single case reports in the literature indicating post‐surgery reorganization and recovery of the reading performance. Severe dyslexia after radical resection of a mesial temporal glioma involving the left fusiform gyrus has been reported (Kamada et al., 2004): pre‐surgery magnetoencephalography (MEG), performed in this case during reading tasks, revealed a functional activation in the anterior portion of the left superior temporal gyrus and the left fusiform gyrus. The authors reported a gradual improvement in the patient's reading abilities, as detected at 3 and 8 months after surgery, accompanied by an overshot recovery of MEG responses recorded in the spared left superior temporal gyrus. In another example of post‐surgery reorganization (Hayashi et al., 2014), after each ablation of a two‐stage excision of a left posterior basal temporal glioma, the patient developed transient pure alexia, yet recovered completely about 6 months after second surgery. Interestingly, DTI‐tractography obtained 6 months after first‐stage surgery showed that the IFOF had shifted caudally and the ILF medially and that the two fiber tracts partially overlapped. However, the recovery could also be partially due to a decreased edema occurring early post‐surgery.
Our findings complement the above‐mentioned studies by reporting data on a large sample of individuals with neurosurgical lesions. The ability of brain structures—even adult brain structures—to reorganize is well known (Hübener & Bonhoeffer, 2014; Merzenich, 2013). This is likely due to the slow growth of neoplasms, which may induce early compensatory mechanisms as early as pre‐surgery so that an effective functional compensation is rapidly achieved after surgery. In addition, the majority of these studies only considered changes related to tumor growth pre‐surgery, whereas little is known about post‐surgery compensation/restoration. Surgeons need to remove the tumor mass to the greatest possible extent while avoiding—as far as possible—postoperative deficits. Resections of tumors invading cognitive areas are at risk of producing cognitive deficits, especially in individuals with LGG, given that the neoplastic tissue may still be functional. In a previous study on individuals with neurosurgical lesions we showed that RTNT is a reliable testing method for the surgeon's need to preserve the functions subserved by the affected brain areas (Skrap et al., 2016) during resection. With respect to reading abilities, the present study also shows that the RTNT procedure is highly predictive of post‐surgery reading performance. In addition, surgeons can be more prone to perform gross total resections when they know that reading abilities may be easily recovered after brain surgery.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors wish to thank Dario Marin for his help with the neuropsychological data collection. The authors also acknowledge the support by the staff of the Medical Imaging Centre.
Tomasino B, Ius T, Skrap M, Luzzatti C. Phonological and surface dyslexia in individuals with brain tumors: Performance pre‐, intra‐, immediately post‐surgery and at follow‐up. Hum Brain Mapp. 2020;41:5015–5031. 10.1002/hbm.25176
Endnotes
In a cognitive neuropsychological frame (Binder et al., 2016; Brambati, Ogar, Neuhaus, Miller, & Gorno‐Tempini, 2009; Newcombe & Marshall, 1987; Schubert & McCloskey, 2015), it is customary to use the term “dyslexia” to designate both vascular and degenerative (PPA) reading impairments. By contrast, “alexia” is preferentially employed in a clinical neurological context: since in the present study, we analyze and discuss data within a cognitive frame, we adhere to such tradition by using the term “dyslexia.”
In linguistics, an * usually marks a wrong or ungrammatical expression.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adair, J. C. , Schwartz, R. L. , Williamson, D. J. G. , Raymer, A. M. , & Heilman, K. M. (1999). Articulatory processes and phonologic dyslexia. Neuropsychiatry, Neuropsychology and Behavioural Neurology, 12, 121–127. [PubMed] [Google Scholar]
- Basso, A. , Capitani, E. , & Laiacona, M. (1987). Raven's coloured progressive matrices: Normative values on 305 adult normal controls. Functional Neurology, 2, 189–194. [PubMed] [Google Scholar]
- Berger, M. S. , & Ojemann, G. A. (1992). Intraoperative brain mapping techniques in neuro‐oncology. Stereotactic Functional Neurosurgery, 58, 153–161. [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Pillay, S. B. , Humphries, C. J. , Gross, W. L. , Graves, W. W. , & Book, D. S. (2016). Surface errors without semantic impairment in acquired dyslexia: A voxel‐based lesion‐symptom mapping study. Brain, 139, 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers, S. , Himmelbach, M. , Logothetis, N. , & Karnath, H. O. (2011). Direct electrical stimulation of human cortex—The gold standard for mapping brain functions? Nature Review Neuroscience, 13, 63–70. [DOI] [PubMed] [Google Scholar]
- Brambati, S. M. , Ogar, J. , Neuhaus, J. , Miller, B. L. , & Gorno‐Tempini, M. L. (2009). Reading disorders in primary progressive aphasia: A behavioral and neuroimaging study. Neuropsychologia, 47, 1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, M. W. , Small, S. L. , & Blumstein, S. E. (2000). The role of segmentation in phonological processing: An fMRI investigation. Journal of Cognitive Neuroscience, 12, 679–690. [DOI] [PubMed] [Google Scholar]
- Catani, M. , & Thiebaut de Schotten, M. (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex, 44, 1105–1132. [DOI] [PubMed] [Google Scholar]
- Cloutman, L. L. , Newhart, M. , Davis, C. L. , Heidler‐Gary, J. , & Hillis, A. E. (2011). Neuroanatomical correlates of oral reading in acute left hemispheric stroke. Brain and Language, 116, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, L. , Dehaene, S. , Naccache, L. , Lehéricy, S. , Dehaene‐Lambertz, G. , Hénaff, M. A. , & Michel, F. (2000). The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain, 123, 291–307. [DOI] [PubMed] [Google Scholar]
- Coltheart M. (Ed.). (1996). Phonological dyslexia. Hove, England: Psychology Press. [Google Scholar]
- Danelli, L. , Marelli, M. , Berlingeri, M. , Tettamanti, M. , Sberna, M. , Paulesu, E. , & Luzzatti, C. (2015). Framing effects reveal discrete lexical‐semantic and sublexical procedures in reading: An fMRI study. Frontiers in Psychology, 6, 1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Renzi, E. , & Faglioni, P. (1978). Normative data and screening power of a shortened version of the token test. Cortex, 14, 41–49. [DOI] [PubMed] [Google Scholar]
- de Renzi, E. , Motti, F. , & Nichelli, P. (1980). Imitating gestures. A quantitative approach to ideomotor apraxia. Archives of Neurology, 37, 6–10. [DOI] [PubMed] [Google Scholar]
- de Renzi, E. , Piezcuro, A. , & Vignolo, L. A. (1966). Oral apraxia and aphasia. Cortex, 2, 50–73. [Google Scholar]
- Denes, G. , Cipolotti, L. , & Semenza, C. (1987). How does a phonologycal dyslexic read words she has never seen? Cognitive Neuropsychology, 4, 11–31. [Google Scholar]
- Epelbaum, S., Pinel, P., Gaillard, R., Delmaire, C., Perrin, M., Dupont, S., … Cohen, L. (2008). Pure alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex, 44, 962–974. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Miranda, J. C. , Rhoton, A. L., Jr. , varez‐Linera, J. , Kakizawa, Y. , Choi, C. , & de Oliveira, E. P. (2008). Three‐dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery, 62, 989–1026. [DOI] [PubMed] [Google Scholar]
- Ferreres, A. R. , Cuiyino, M. M. , & Olmedo, A. (2005). Acquired surface dyslexia in Spanish. Behavioural Neurology, 16, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreres, A. R. , López, C. V. , & China, N. (2003). Phonological alexia with vowel–consonant dissociation in non‐word reading. Brain and Language, 84, 399–413. [DOI] [PubMed] [Google Scholar]
- Fiebach, C. J. , Friederici, A. D. , Müller, K. , & von Cramon, D. Y. (2002). FMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience, 14, 11–23. [DOI] [PubMed] [Google Scholar]
- Friedman, R. B. , & Kohn, S. E. (1990). Impaired activation of the phonological lexicon: Effects upon oral reading. Brain and Language, 38, 278–297. [DOI] [PubMed] [Google Scholar]
- Funnell, E. (1983). Phonological processes in reading. New evidence from acquired dyslexia. British Journal of Psychology, 74, 159–180. [DOI] [PubMed] [Google Scholar]
- Hamilton, A. C. , & Coslett, H. B. (2008). Role of inflectional regularity and semantic transparency in reading morphologically complex words: Evidence from acquired dyslexia. Neurocase, 14, 347–368. [DOI] [PubMed] [Google Scholar]
- Hayashi, Y. , Okita, H. , Kinoshita, M. , Miyashita, K. , & Nakada, M. (2014). Functional recovery from pure dyslexia with preservation of subcortical association fiber networks. Interdisciplinary Neurosurgery, 1, 59–62. [Google Scholar]
- Henry, M. L. , Beeson, P. M. , Alexander, G. E. , & Rapcsak, S. Z. (2012). Written language impairments in primary progressive aphasia: A reflection of damage to central semantic and phonological processes. Journal of Cognitive Neuroscience, 24, 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., Calabresi, … Mori, S. (2008). Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract‐specific quantification. NeuroImage, 39, 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübener, M. , & Bonhoeffer, T. (2014). Neuronal plasticity: Beyond the critical period. Cell, 159, 727–737. [DOI] [PubMed] [Google Scholar]
- Ius, T. , Isola, M. , Budai, R. , Pauletto, G. , Tomasino, B. , Fadiga, L. , & Skrap, M. (2012). Low‐grade glioma surgery in eloquent areas: Volumetric analysis of extent of resection and its impact on overall survival. A single‐institution experience in 190 patients: Clinical article. Journal of Neurosurgery, 117, 1039–1052. [DOI] [PubMed] [Google Scholar]
- Jobard, G. , Crivello, F. , & Tzourio‐Mazoyer, N. (2003). Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. NeuroImage, 20, 693–712. [DOI] [PubMed] [Google Scholar]
- Kamada, K. , Sawamura, Y. , Takeuchi, F. , Houkin, K. , Kawaguchi, H. , Iwasaki, Y. , & Kuriki, S. (2004). Gradual recovery from dyslexia and related serial magnetoencephalographic changes in the lexicosemantic centers after resection of a mesial temporal astrocytoma. Journal of Neurosurgery, 100, 1101–1106. [DOI] [PubMed] [Google Scholar]
- Karnath, H.‐O. , Berger, M. F. , Küker, W. , & Rorden, C. (2004). The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cerebral Cortex, 14, 1164–1172. [DOI] [PubMed] [Google Scholar]
- Lesch, M. F. , & Martin, R. C. (1998). The representation of sublexical orthographic‐phonologic correspondences: Evidence from phonological dyslexia. The Quarterly Journal of Experimental Psychology: Section A, 51, 905–938. [DOI] [PubMed] [Google Scholar]
- Long, C. , Sebastian, R. , Faria, A. V. , & Hillis, A. E. (2018). Longitudinal imaging of reading and naming recovery after stroke. Aphasiology, 32, 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand, Y. , & Friedman, R. B. (2005). Impaired oral reading in two atypical dyslexics: A comparison with a computational lexical‐analogy model. Brain and Language, 93, 255–266. [DOI] [PubMed] [Google Scholar]
- Marshall, J. C. , & Newcombe, F. (1973). Patterns of paralexia: A psycholinguistic approach. Journal of Psycholinguistic Research, 2, 175–199. [DOI] [PubMed] [Google Scholar]
- Martino, J. , Brogna, C. , Robles, S. G. , Vergani, F. , & Duffau, H. (2010). Anatomic dissection of the inferior fronto‐occipital fasciculus revisited in the lights of brain stimulation data. Cortex, 46, 691–699. [DOI] [PubMed] [Google Scholar]
- Merzenich, M. M. (2013). Soft‐wired: How the new science of brain plasticity can change your life. San Francisco, CA: Lightning Source. [Google Scholar]
- Miceli, G. , Laudanna, A. , Burani, C. , & Capasso, R. (1994). Batteria per l'analisi dei deficit afasici: BADA [BADA: A battery for the assessment of aphasic disorders.]. Roma: CEPSAG. [Google Scholar]
- Nathan, S. S. , Sinha, S. R. , Gordon, B. , Lesser, R. P. , & Thakor, N. V. (1993). Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalography and Clinical Neurophysiology, 86, 183–192. [DOI] [PubMed] [Google Scholar]
- Newcombe, F. , & Marshall, J. C. (1987). Transcoding and lexical stabilization in deep dyslexia In Coltheart M., Patterson K., & Marshall J. C. (Eds.), Deep dyslexia (2nd ed., pp. 176–188). New York, NY: Routledge. [Google Scholar]
- Novelli, G. , Papagno, C. , Capitani, E. , Laiacona, M. , Vallar, G. , & Cappa, S. F. (1996). Tre test clinici di ricerca e produzione lessicale.Taratura su soggetti normali. Archivio di Psicologia, Neurologia e Psichiatria, 47, 477–505. [Google Scholar]
- Ojemann, G. , Ojemann, J. , Lettich, E. , & Berger, M. (1989). Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery, 71, 316–326. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Orsini, A. , Grossi, D. , Capitani, E. , Laiacona, M. , Papagno, C. , & Vallar, G. (1987). Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Italian Journal of Neurological Science, 8, 539–548. [DOI] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62, 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, A.R. , Bonner, M.F. , Peelle, J.E. , & Grossman, M. (2015). Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. The Journal of Neuroscience, 35, 3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, A. R., Bonner, M. F., Peelle, J. E., & Grossman, M. (2015). The angular gyrus in developmental dyslexia: Task‐specific differences in functional connectivity within posterior cortex. Psychological Science, 11, 51–56. [DOI] [PubMed] [Google Scholar]
- Purcell, J. , Sebastian, R. , Leigh, R. , Jarso, S. , Davis, C. , Posner, J. , … Hillis, A. E. (2017). Recovery of orthographic processing after stroke: A longitudinal fMRI study. Cortex, 92, 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, I. , & Weekes, B. S. (2005). Acquired dyslexia in a Turkish‐English speaker. Annals of Dyslexia, 55, 79–104. [DOI] [PubMed] [Google Scholar]
- Rapcsak, S. Z. , Beeson, P. M. , Henry, M. L. , Leyden, A. , Kim, E. , Rising, K. , … Cho, H. S. (2009). Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex, 45, 575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti, E. , Aggujaro, S. , Molteni, F. , Zonca, G. , Frustaci, M. , & Luzzatti, C. (2014). The anatomical foundations of acquired reading disorders: A neuropsychological verification of the dual‐route model of reading. Brain and Language, 134, 44–67. [DOI] [PubMed] [Google Scholar]
- Roux, F. E. , Lubrano, V. , Lauwers‐Cances, V. , Trémoulet, M. , Mascott, C. R. , & Démonet, J. F. (2004). Intra‐operative mapping of cortical areas involved in reading in mono‐ and bilingual patients. Brain, 127, 1796–1810. [DOI] [PubMed] [Google Scholar]
- Sala, F. , Bricolo, A. , Faccioli, F. , Lanteri, P. , & Gerosa, M. (2007). Surgery for intramedullary spinal cord tumors: The role of intraoperative (neurophysiological) monitoring. European Spine Journal, 16(Suppl 2), S130–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai, N. , Mirzadeh, Z. , & Berger, M. S. (2008). Functional outcome after language mapping for glioma resection. New England Journal of Medicine, 358, 18–27. [DOI] [PubMed] [Google Scholar]
- Sasanuma, S. (1996). Phonological alexia in Japanese: A case study. Cognitive Neuropsychology, 13, 823–848. [Google Scholar]
- Sato, H. , Patterson, K. , Fushimi, T. , Maxim, J. , & Bryan, K. (2008). Deep dyslexia for kanji and phonological dyslexia for kana: Different manifestations from a common source. Neurocase, 14, 508–524. [DOI] [PubMed] [Google Scholar]
- Schlaggar, B. L. , & McCandliss, B. D. (2007). Development of neural systems for reading. Annual Review Neurosciences, 30, 475–503. [DOI] [PubMed] [Google Scholar]
- Schubert, T. , & McCloskey, M. (2015). Recognition of oral spelling is diagnostic of the central reading processes. Cognitive Neuropsychology, 32, 80–88. [DOI] [PubMed] [Google Scholar]
- Sebastian, R. , Gomez, Y. , Leigh, R. , Davis, C. , Newhart, M. , & Hillis, A. E. (2014). The roles of occipitotemporal cortex in reading, spelling, and naming. Cognitive Neuropsychology, 31, 511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos, P. G. , Breier, J. I. , Fletcher, J. M. , Foorman, B. R. , Castillo, E. M. , & Papanicolaou, A. C. (2002). Brain mechanisms for reading words and pseudowords: An integrated approach. Cerebral Cortex, 12, 297–305. [DOI] [PubMed] [Google Scholar]
- Skrap, M. , Marin, D. , Ius, T. , Fabbro, F. , & Tomasino, B. (2016). Brain mapping: A novel intraoperative neuropsychological approach. Journal of Neurosurgery, 5, 1–11. [DOI] [PubMed] [Google Scholar]
- Skrap, M. , Vescovi, M. C. , Pauletto, G. , Maieron, M. , Tomasino, B. , Bagatto, D. , & Tuniz, F. (2018). Supratentorial cavernous malformations involving the corticospinal tract and sensory motor cortex: Treatment strategies, surgical considerations, and outcomes. Operative Neurosurgery, 15(5), 483–497. [DOI] [PubMed] [Google Scholar]
- Spinnler, M. , & Tognoni, G. (1987). Standardizzazione e taratura italiana di test neuropsicologici. The Italian Journal of Neurological Science, 6(Suppl 8), 1–120. [PubMed] [Google Scholar]
- Taylor, J. S. (2013). Can cognitive models explain brain activation during word and pseudoword reading? A meta‐analysis of 36 neuroimaging studies. Psychological Bulletin, 139, 766–791. [DOI] [PubMed] [Google Scholar]
- Tomasino, B., Marin, D., Maieron, M., D'Agostini, S., Fabbro, F., Skrap, M., & Luzzatti, C. (2015). Double‐letter processing in surface dyslexia and dysgraphia following a left temporal lesion: A multimodal neuroimaging study. Cortex, 73, 112–130. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Vandermosten, M. , Boets, B. , Poelmans, H. , Sunaert, S. , Wouters, J. , & Ghesquiere, P. (2012). A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain, 135, 935–948. [DOI] [PubMed] [Google Scholar]
- Vandermosten, M. , Boets, B. , Wouters, J. , & Ghesquiere, P. (2012). A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Review, 36, 1532–1552. [DOI] [PubMed] [Google Scholar]
- Zemmoura, I. , Herbet, G. , Moritz‐Gasser, S. , & Duffau, H. (2015). New insights into the neural network mediating reading processes provided by cortico‐subcortical electrical mapping. Human Brain Mapping, 36, 2215–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


