Abstract
Tertiary lymphoid structures (TLS) are ectopic lymphoid aggregates that phenotypically resemble conventional secondary lymphoid organs (SLO) and are commonly found at sites of chronic inflammation. They are also found in a wide variety of primary and metastatic human tumors. The presence of tumor-associated TLS (TA-TLS) is associated with prolonged patient survival, higher rates of disease-free survival, and a favorable response to current cancer therapies. However, the immune responses that occur in these structures, and how they contribute to improved clinical outcomes, remain incompletely understood. Additionally, it is unknown how heterogeneity in TA-TLS cellular composition, structural organization, and anatomical location influences their functionality and prognostic significance. Understanding more about TA-TLS development, formation, and function may offer new therapeutic options to modulate antitumor immunity.
Introduction:
It is well appreciated that tumor immune infiltrates are prognostic indicators for patient survival and response to immunotherapies. Infiltrates enriched for CD8+ T-cells have a positive prognostic value, whereas those enriched for myeloid cells have a negative prognostic value (1,2). Given the general view that endogenous antibody responses contribute little to antitumor immunity, it is surprising that B-lymphocytes also have a positive prognostic value (3). Also, CD8+ T-cells disseminated throughout the tumor parenchyma have a stronger prognostic value than those confined to the perivascular space surrounding intratumoral blood vessels (4) or those aggregated immediately outside of the tumor mass (5). On the other hand, immune infiltrates organized into TA-TLS also have significant positive prognostic value. These structures have been extensively reviewed previously (6,7) and we have incorporated the details of these reviews by reference. In this Cancer Immunology at a Crossroads, we summarize what is known about the composition, organization, function, and mechanisms underlying TA-TLS formation, and highlight issues that remain to be understood in order to harness these structures for therapeutic purposes.
Composition, organization, and heterogeneity of TA-TLS:
TA-TLS were initially described in melanoma and in non-small cell lung cancer (NSCLC), and are documented in a variety of primary and metastatic tumor types (6,7). Histological elements most frequently used to identify human TA-TLS include one or more of the following: tumor vessels expressing peripheral node addressin (PNAd), mature dendritic cells (DC) expressing lysosome-associated membrane glycoprotein (DC-LAMP), dense aggregates of T- and/or B-cells, follicular helper T-cells (TFH), and cells resembling follicular dendritic cells (FDC) (6,7) (Table 1). Most TA-TLS are organized “classically”, with distinct T-cell/DC and B-cell/FDC compartments (Table 1), and one or more of the homeostatic chemokines CCL19, CCL21, CXCL12, and CXCL13, which organize the SLO microarchitecture, are documented in TA-TLS by immunohistochemistry (6,7) (Fig. 1). Composite gene signatures are used for the detection of TA-TLS (Table 1). Expression of the plasma cell specific marker B-cell maturation antigen (BCMA) is associated with the presence of TA-TLS in ovarian cancer (8). A more comprehensive 19-gene signature identifying B-cells and TH1 T-cells is associated with the presence of TA-TLS in gastric cancer (9), and an 8-gene signature identifying TFH cells is predictive of the presence of TA-TLS in breast cancer (10). A 12-chemokine gene signature is also predictive of the presence of TA-TLS in colorectal (11), melanoma (11), breast (12), and hepatocellular carcinoma (13). Finally, a 9-gene signature has been identified by comparing CD8+/CD20+ and CD8+/CD20neg melanomas (14). Collectively, these components provide a baseline for identifying TA-TLS. However, most human studies have relied on only one or a small number of these markers (6). Additionally, the criteria typically used are largely oriented towards elements that support antitumor immunity, although regulatory T-cells (Treg) have occasionally been reported (15,16). A particularly interesting study demonstrates distinct Treg, TH1, and TH17 biased profiles in TA-TLS associated with response or lack of response to a mesothelin vaccine (17). However, it is still unknown whether other immunosuppressive cells (myeloid-derived suppressor cells, some populations of innate lymphocytes or natural killer T-cell cells) can be present in TA-TLS. Thus, there is likely to be significant unappreciated heterogeneity in TA-TLS cellular composition. Also, some reports have identified loose aggregates of lymphocytes as TA-TLS (13,18), although they are typically tightly aggregated structures. Whether these are nascent or senescent TA-TLS, or something altogether different, remains unclear. We believe that the field as a whole should move to utilize a comprehensive set of markers to identify these structures to ensure that their heterogeneity, function, and prognostic value can be more thoroughly evaluated.
Table 1:
Characteristics of TA-TLS in Human Tumors and their Prognostic Value
| Tumor Type | TA-TLS markers by IHCa | TA-TLS gene signatures | TA-TLS location | TA-TLS organization | # patients | TA-TLS association | Ref. |
|---|---|---|---|---|---|---|---|
| Bladder, primary | T- and B-cells, CD21 (FDC), PNAd, DC-LAMP | - | Peritumoral | Classical | 28 | Higher tumor grade | (61) |
| Breast, primary | PNAd, DC-LAMP | - | Peritumoral and intratumoral | Classical | 146 | Favorable overall survival | (62,63) |
| T- and B-cells, TFH, GC B-cells | TFH | Peritumoral and intratumoral | Classical | 794 | Favorable overall survival and response to chemotherapy | (10) | |
| T- and B-cells, GC B-cells, CD21 (FDC), PNAd, DC-LAMP | - | Peritumoral | Classical | 290 | Higher tumor grade | (40) | |
| Mononuclear aggregates | - | Peritumoral | ND | 796 | Favorable overall survival | (64) | |
| Mononuclear aggregates | - | Peritumoral | ND | 447 | Favorable overall survival and response to adjuvant Trastuzumab in patients with HER2+ tumors | (46) | |
| T- and B-cells, CD21 (FDC) | - | Peritumoral | Classical | 248 | Favorable overall survival in patients with HER2+ tumors | (65) | |
| Mononuclear aggregates, T- and B-cells, PNAd | - | Peritumoral | Non-classical | 108 | Favorable response to neoadjuvant chemotherapy | (45) | |
| Mononuclear aggregates | - | Peritumoral | ND | 769 | Favorable overall survival | (30) | |
| Cervical, primary | Mononuclear aggregates, T- and B-cells, Ki67, PNAd | - | Peritumoral | Non-classical | 12 | Found in only vaccinated patients | (50) |
| Colorectal, primary | T- and B-cells, GC B-cells, CD21 (FDC) | 12-chemokine | Peritumoral and intratumoral | Classical | 21 | Favorable overall survival | (11) |
| T- and B-cells, CD21 (FDC), PNAd, CCL21, CXCL13 | - | Peritumoral | Classical | 351 | Favorable overall survival | (41) | |
| Mononuclear aggregates, T- and B-cells | 12-chemokine | Peritumoral | Classical | 39 | Favorable overall survival | (12) | |
| Colorectal, lung metastatic | T- and B-cells, PNAd, DC-LAMP | - | Intratumoral | Classical | 192 | Favorable overall survival | (24) |
| Mononuclear aggregates, T-cells, CD45RO+ T-cells, Foxp3+ cells | - | Peritumoral and intratumoral | ND | 57 | No evaluation | (16) | |
| Colorectal, liver metastatic | Mononuclear aggregates, B-cells, GC B-cells, macrophages | - | Peritumoral (Non-tumor liver tissue) | ND | 65 | Favorable overall survival | (66) |
| Gastric, metastatic | Mononuclear aggregates, T- and B-cells, DC-LAMP, PNAd | TH1 and B-cell | Intratumoral | Classical | 365 | Favorable overall survival | (9) |
| Mononuclear aggregates, T- and B-cells, GC B-cells, CD21 (FDC), DC-LAMP, PNAd, CCL21 and CXCL13 | - | Intratumoral | Classical | 176 | Advanced clinical disease; no impact on overall survival | (67) | |
| Liver, primary | Mononuclear aggregates | - | Intratumoral | ND | 273 | Favorable overall survival | (68) |
| Mononuclear aggregates | 12-chemokine | Intratumoral | ND | 221 | Favorable overall survival | (68) | |
| Mononuclear aggregates | - | Peritumoral (Non-tumor liver tissue) | ND | 217 | No impact on overall survival | (68) | |
| Mononuclear aggregates, T- and B-cells, CD68 (macrophages), Ly6G (neutrophils), Foxp3, CD21 (FDC) | 12-chemokine | Peritumoral (Non-tumor liver tissue) | Non-classical | 82 | Unfavorable overall survival | (13) | |
| Mononuclear aggregates, T- and B-cells, macrophages, Foxp3, CD21 (FDC) | - | Intratumoral | Non-classical | 462 | Favorable overall survival | (43) | |
| Lung, primary | Mononuclear aggregates, T- and B-cells, CD68 (macrophages), CD21 (FDC) | - | Peritumoral | Classical | 74 | Favorable overall survival | (32) |
| Mononuclear aggregates, T- and B-cells, CD21 (FDC), PNAd | - | Peritumoral | Classical | 151 | Favorable overall survival only after neoadjuvant chemotherapy | (69) | |
| Mononuclear aggregates, T- and B-cells, CD21 (FDC), DC-LAMP | - | Intratumoral | Classical | 74 | Favorable overall survival | (19) | |
| Mononuclear aggregates, T- and B-cells, CD21 (FDC), DC-LAMP | TH1/cytotoxic | Peritumoral and intratumoral | Classical | 362 | Favorable overall survival | (20) | |
| Mononuclear aggregates, T- and B-cells, GC B-cells, CD21 (FDC), DC-LAMP, PNAd, CCL21, CXCL13 | - | Peritumoral | Classical and non-classical | 138 | Favorable overall survival | (36) | |
| Renal clear-cell, lung metastatic | T- and B-cells, PNAd, DC-LAMP | - | Peritumoral | Classical | 57 | Unfavorable overall survival | (24) |
| Melanoma, primary | Mononuclear aggregates, T-cells, DC-LAMP | - | Peritumoral | ND | 82 | Favorable overall survival | (70) |
| CD45RO+ T-cells, B-cells, CD21 (FDC), PNAd | - | Peritumoral | ND | 39 | No impact on overall survival | (71) | |
| T- and B-cells, PNAd | - | Peritumoral | Non-classical | 225 | No impact on overall survival | (72) | |
| Melanoma, metastatic | T- and B-cells, GC B-cells, CD21 (FDC), DC-LAMP, PNAd | FDC | Peritumoral | Classical | 29 | No impact on overall survival | (26) |
| Mononuclear aggregates, T- and B-cells, macrophages, Foxp3+ cells | 12-chemokine | Peritumoral | Classical | 10 | Favorable overall survival | (73) | |
| T- and B-cells, GC B-cells | CD20+ and CD8+ associated | Peritumoral | Classical | 177 | Favorable overall survival and response to checkpoint immunotherapy | (14) | |
| Mononuclear aggregates, T- and B-cells, CD21 (FDC), Foxp3+ cells | CD20+ B-cell | Peritumoral | Non-classical | 127 | Favorable overall survival and response to checkpoint immunotherapy | (47) | |
| Mononuclear aggregates, T- and B-cells, naïve & activated B-cells, Foxp3+ cells | B-cell plasmablast-like | Peritumoral | Non-classical | 10 | Favorable response to checkpoint immunotherapy | (48) | |
| Oral, primary | Mononuclear aggregates, T- and B-cells, macrophages, DC-LAMP, PNAd | 12-chemokine | Peritumoral | Classical | 80 | Favorable overall survival | (74) |
| Ovarian, metastatic | Mononuclear aggregates, T- and B-cells, GC B-cells, CD21 (FDC), DC-LAMP, PNAd | BCMA | Peritumoral and intratumoral | Non-classical | 172 | Favorable overall survival | (8) |
| Mononuclear aggregates, DC-LAMP | - | Peritumoral and intratumoral | ND | 147 | Favorable overall survival | (75) | |
| Pancreatic, primary | Mononuclear aggregates | - | Intratumoral | ND | 308 | Favorable overall survival | (42) |
| Mononuclear aggregates, T- and B-cells, DC-LAMP, PNAd, CCL21, CXCL13 | - | Intratumoral | Classical | 104 | Favorable overall survival | (76) | |
| Mononuclear aggregates, T- and B-cells, CD45RO+ T-cells, DC-LAMP, CD21 (FDC), Ki67, CD68 (macrophages), Foxp3+ cells, Tbet+ cells, PD-L1, CCL21 | - | Intratumoral | Classical | 93 | Found in only vaccinated patients | (17) | |
| Soft-tissue, primary | T- and B-cells, CD21 (FDC), DC-LAMP, PNAd | 12-chemokine | Intratumoral | Classical and non-classical | 47 | Favorable response to checkpoint immunotherapy | (49) |
| Prostate, primary | Mononuclear aggregates, T- and B-cells, CD21 (FDC), DC-LAMP, PNAd, CD68 (macrophages) | - | Peritumoral | Non-classical | 17 | No impact on overall survival | (15) |
GC, germinal center; ND, not determined. Other abbreviations are defined in the text.
Figure 1: Cellular, organization, and location heterogeneity associated with tumor-associated tertiary lymphoid structures.
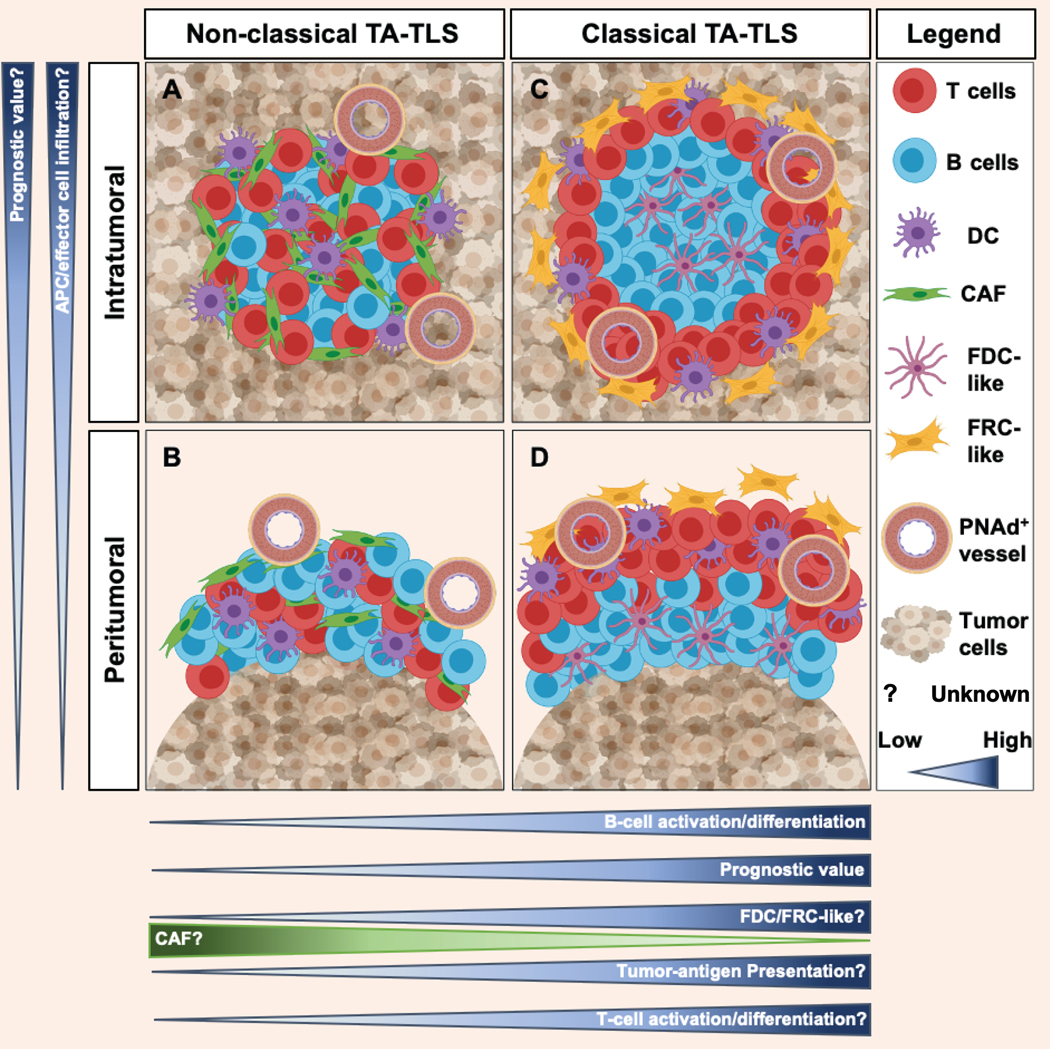
TA-TLS are aggregates of T-cells, B-cells, dendritic cells (DC), and fibroblastic reticular cells (FRC)-/follicular dendritic cells (FDC)-like that are in juxtaposition to a tumor vessel expressing peripheral node addressin (PNAd). They can be found either intratumorally (A) or peritumorally (B). Limited evidence suggests that intratumoral structures may have greater prognostic significance, but this has not been widely established. It is also unknown whether the peritumoral location limits infiltration of APC and/or effector cells. TA-TLS can exhibit either a classical organization that contains discrete T-cell/DC and B-cell/FDC compartments (C) or a non-classical organization (D). Non-classical TA-TLS usually contain B-cells that are less activated than those in classical structures. Evidence suggests that classical TA-TLS have greater prognostic value than non-classical structures, and classical structures containing germinal centers may have the greatest value. While CAF support TA-TLS formation in murine melanoma, the role of CAF and of associated FRC-/FDC-like cells in supporting formation of non-classical and classical TA-TLS in other murine and human tumors remains to be determined. Similarly, it is unknown whether the lack of compartmentalization in non-classical TA-TLS limits tumor-antigen presentation and T-cell activation relative to classical structures, and whether any of these variables alter effector T-cell differentiation. Addressing these unknowns will determine how heterogeneity in TA-TLS cellular composition, structural organization, and anatomical location influences their functionality and prognostic impact.
Despite the limited characterization in many studies, TA-TLS from different tumor types vary in cellular composition and organization. B-cells with immature, naïve, activated, memory, and plasma cell phenotypes are evident to varying extents in different TA-TLS (6). In NSCLC, TA-TLS contain large numbers of mature DC-LAMP+ DC (19,20), but these are absent in those associated with lung metastatic renal cell carcinoma (21). TFH cells are common features of breast cancer TA-TLS (22), whereas those associated with prostate (15) and lung metastatic colorectal cancer (16) contain large numbers of Tregs. While this could indicate that TA-TLS in different tumor types or anatomical locations contain different T-cell subpopulations, none of these studies evaluated both. The majority of studies have identified human TA-TLS as having a peritumoral location, whereas a smaller number have identified TA-TLS as intratumoral, usually in addition to peritumoral structures (6) (Table 1, Fig. 1). However, in germ cell tumors (23), hepatocellular carcinoma (13), and lung metastatic renal cell carcinoma (24), TA-TLS are largely intratumoral, and exhibit a non-classical organization lacking discrete T- and B-cell compartments. However, peritumoral TA-TLS are sometimes inside the tumor albeit near the tumor-invasive margin and sometimes fully outside the margin (25). This distinction may have important consequences for TA-TLS function. In addition, there is concern that the peritumoral TA-TLS identified in lymph node metastases may represent residual lymphoid follicles (14). In human melanoma, TLS frequently develop in metastatic lesions, but are largely absent from primary tumors, despite the presence of a PNAd+ vasculature (26). Similarly, TA-TLS are found in intraperitoneal, but not in subcutaneous murine tumors (27–29), and are observed frequently in primary breast tumors but largely absent in metastatic brain lesions (30). Thus, TA-TLS presence and structural organization are associated with tumor microenvironment and anatomical location, although the factors responsible, and the overall impact on TA-TLS functionality remains to be determined.
Functional characteristics of TA-TLS:
It has been suggested that TA-TLS serve as sites for sustained generation of in situ immune responses that are focused towards tumor antigens (6,7), but evidence for this remains somewhat limited. We demonstrated that tumor vessels expressing PNAd, a hallmark of TA-TLS, supported infiltration of naïve T- and B-cells (27,29), and TA-TLS in NSCLC contain large accumulations of naïve T- and B-cells (20,31,32). Thus, TA-TLS could promote a continual influx of naïve cells for sustaining immunity. Whereas TA-TLS vary in the number of mature DC they contain, larger numbers of mature DC are associated with larger numbers of T-cells with a TH1/cytotoxic immune profile (19,20), suggesting active antigen presentation to T-cells in TA-TLS. A linear relationship between the density of TA-TLS and the levels of intratumoral activated T- and/or B-cells has also been described (6,7). While this observation may suggest that TA-TLS support TIL development, our own work (29), described below, demonstrates that TIL support TA-TLS development.
Aggregation of tumor-associated B-cells into a follicle-like structure is one of the most dramatic and defining features of TA-TLS. Although B-cells can be positive or negative mediators of antitumor immunity (33), B-cells in classically organized TA-TLS often express markers associated with germinal center activity (34,32,35,36), and show higher degrees of clonal amplification, rearranged immunoglobulins, somatic hypermutations, and isotype switching than those in tumor parenchyma (26,37), suggesting active antitumor humoral responses in these structures (Fig. 1). TFH cells are also commonly found in the B-cell compartment of TA-TLS (38). These observations suggest that TA-TLS support the activation and differentiation of B-cells into antibody producing cells, and they are consistent with the idea that TA-TLS promote the in situ generation of tumor-specific antibody that augments antitumor immunity.
Despite these intriguing observations, there are a number of unaddressed issues that may limit the contribution of TA-TLS to producing effective antitumor immunity. Lymphatic vessels are rarely reported in TA-TLS, leading to uncertainties about tumor-antigen and cellular transport to these structures. Given the relatively small size of TA-TLS, it is unclear what fraction of the resident naïve T- and B-cells are tumor-antigen specific, and how frequently they turn over. Also, it is unclear whether DC in TA-TLS come from tumor parenchyma, adjacent tissue, or blood-derived inflammatory monocytes. Similarly, it is unclear whether and how these cells acquire tumor antigen, and if DC in TA-TLS are more mature than those in tumor parenchyma or tumor-draining SLO. We do not know how the effector and exhaustion marker profiles of T-cells in TA-TLS compare to those in the surrounding tumor parenchyma and tumor-draining SLO. We also do not know how immunosuppressive elements in the tumor microenvironment, such as hypoxia, indoleamine 2, 3-dioxygenase, nitric oxide, arginase, TGF-β, and immune checkpoint ligands, impact immune responses that occur in TA-TLS. Finally, it is unknown whether B-cells in TA-TLS support antitumor immunity through tumor-antigen presentation to T-cells, secretion of pro-inflammatory cytokines (33), or the direct action of in situ produced antibody, or whether regulatory B-cells, which can enhance tumor development by suppressing antitumor immunity (33), exist in TA-TLS. Since B-cells are a major component of TA-TLS, it is particularly important to have a more comprehensive understanding of the function(s) of these cells in these structures.
Prognostic significance and immunological impact of TA-TLS:
The prognostic impact of TA-TLS has been extensively evaluated. Several studies have pointed to a significant relationship between the densities of TA-TLS and overall patient survival (6,7), although there are exceptions (13,39,40) (Table 1). Because TA-TLS are associated with higher densities of CD8+ TIL, it remains possible that TA-TLS are simply proxies for more robust intratumoral T-cell effector activity. However, multivariate studies in NSCLC (20) and colorectal cancer (41) have established that the prognostic value of TA-TLS independent of TIL density. Intratumoral TA-TLS are more significantly associated with enhanced patient survival than peritumoral TA-TLS in pancreatic cancer (42) and early-stage hepatocellular carcinoma (43). Also, oral squamous cell carcinoma patients whose tumors contained higher proportions of classically organized TA-TLS tended to survive longer, although this was not statistically significant (44). Two studies show that germinal centers within TA-TLS determine their prognostic value in colorectal (35) and lung squamous cell carcinoma (36). However, it is important to establish more generally whether peritumoral and intratumoral, or classically and non-classically organized TA-TLS differ in their association with patient survival (Fig. 1).
An area of immense interest is whether TA-TLS are associated with patient responsiveness to cancer therapies (Table 1). Interestingly, the presence of TA-TLS was initially associated with a favorable response to neoadjuvant chemotherapy in breast cancer (10,45). Similarly, densities of TA-TLS in HER2+ breast cancer strongly correlate with disease-free survival and responsiveness to adjuvant Trastuzumab (46). The presence of B-cells and/or TA-TLS prior to treatment is associated with favorable responses to checkpoint blockade immunotherapies in melanoma (14,47,48) and soft-tissue sarcoma (49), and one of these studies presents evidence suggesting that immunotherapy might increase TLS density (47). Immune checkpoint treatment of murine tumors increases the number and size of TA-TLS, and promotes a classical organization, in association with diminished tumor outgrowth (29). These observations establish that TA-TLS may be important predictors of patient response to chemotherapy and immunotherapy, along with overall intratumoral CD8+ TIL, mutational burden, and PD-L1 expression. At the same time, there is a suggestion that TA-TLS may be the site at which these therapies act. However, the range of tumors in which TA-TLS are identified is larger than the range that responds to immune checkpoint blockade. Whether this is a consequence of additional regulatory mechanisms, and whether these operate within the TA-TLS, remains to be determined. As above, it is important to establish more generally whether peritumoral and intratumoral, or classically and non-classically organized TA-TLS, differ in their association with treatment responses.
Cellular and molecular mechanisms regulating the development of TA-TLS:
Given the considerations above, it is highly attractive to develop immunotherapeutic approaches that induce or augment TA-TLS formation. Interestingly, vaccination induce TLS formation in association with pancreatic tumors (17) and human papilloma virus-driven cervical intraepithelial neoplasia (50). Transgenic overexpression of lymphotoxin-β receptor ligands (51), injection of recombinant LIGHT (CD258) (52), intratumoral administration of CCL21 (53), and intratumoral injection of DCs engineered to overexpress T-bet (54), IL-36 (55), or CCL21 (56,57) all induce TA-TLS in murine tumors. However, there are limited reports of spontaneous TA-TLS development in murine tumors (27,29,41,58), and the mechanisms driving their formation have mostly remained unknown. In murine melanoma, we demonstrate that spontaneous development of intratumoral PNAd+ CCL21+ vasculature is controlled by effector CD8+ T-cells and natural killer cells secreting lymphotoxin-α3 and IFNγ (27). We show that these cells, together with B-cells expressing lymphotoxin-α1β2, act coordinately as surrogate lymphoid tissue inducer cells to drive TA-TLS development (29). We also demonstrate that a population of cancer-associated fibroblasts (CAF) form a reticular network co-extensive with the immune cells in TA-TLS, and also express high levels of the B-cell attracting chemokine CXCL13, and the B-cell survival factors BAFF and APRIL (27–29). The CXCL13 receptor CXCR5 is critical for intratumoral B-cell accumulation and TA-TLS formation (29). These findings demonstrate a novel and previously undescribed role of CAF as surrogate lymphoid tissue organizer cells that orchestrate TA-TLS development.
CAF populations in TA-TLS are also associated with human melanoma (29). However, in breast cancer, TFH cells are reported as a CXCL13 source (10), raising the possibility that these cells also aid in TA-TLS development or long-term maintenance. TA-TLS in late-stage NSCLC are associated with a distinct population of CXCL13 producing CD8+ T-cells (59). Conversely, TA-TLS associated with early-stage NSCLC contain a population of type 3 innate lymphocytes (60). This suggests that the cells responsible for initiating and maintaining TA-TLS in NSCLC may evolve over time, although the direct activity of these cell populations has not yet been demonstrated. However, it is unknown whether these cells exist in TA-TLS from other tumor types, and whether different anatomical compartments contain tissue-resident fibroblasts that are more prone to become organizer-like cells. In addition, it is unknown how CAFs or other cell populations may resemble follicular dendritic cells and fibroblastic reticular cells, which respectively promote the formation of distinct T- and B-cell compartments in SLO and are likely to be necessary for TA-TLS with a classical organization (Fig. 1). It is entirely unknown what promotes the development of TA-TLS in peritumoral and intratumoral locations. To the extent that CAFs promote TA-TLS development, it is unknown how this may be related structurally to the formation of desmoplastic stroma by other CAF populations. Overall, while suggesting some general molecular mechanisms may operate to promote TA-TLS development and maintenance, these results also suggest that the cellular sources of these molecules may vary based on tumor type, anatomical location, and/or tumor evolution.
Conclusions:
Based on the preponderance of evidence, it seems highly desirable to induce and/or augment TA-TLS development as a new aspect of cancer immunotherapy, either alone or in combination with immunotherapy or chemotherapy. However, given the significant number of studies that show negative prognostic associations, and the heterogeneity of TA-TLS organization, consideration should also be given to optimizing their functionality. Cellular and molecular mechanisms responsible for spontaneous TA-TLS formation, and strategies to induce these structures, are described in murine models. However, much remains to be done to understand the overall heterogeneity and functionality of these structures in human tumors, and the tumor, tissue, and temporal elements that may control these properties. These in turn will enable more refined understanding of the properties of TA-TLS that are of greatest value in determining patient survival, and applicability to the widest array of different cancer types.
Acknowledgments:
This work was supported by the United States Public Health Service Grants CA78400 and CA181794 (to V.H.E.). A.B.R. was supported by USPHS Training Grant T32AI007496, and was a recipient of the University of Virginia School of Medicine Wagner Fellowship.
Footnotes
Disclosure of potential conflicts of interest:
The author declares no competing financial interests. Correspondence should be addressed to V.H.E.
References:
- 1.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang T-H, et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wouters MCA, Nelson BH. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin Cancer Res. 2018;24:6125–35. [DOI] [PubMed] [Google Scholar]
- 4.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–25. [DOI] [PubMed] [Google Scholar]
- 7.Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J Immunol. 2018;200:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res. 2016;22:3005–15. [DOI] [PubMed] [Google Scholar]
- 9.Hennequin A, Derangère V, Boidot R, Apetoh L, Vincent J, Orry D, et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2016;5:e1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011;179:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakaran S, Rizk VT, Ma Z, Cheng C-H, Berglund AE, Coppola D, et al. Evaluation of invasive breast cancer samples using a 12-chemokine gene expression score: correlation with clinical outcomes. Breast Cancer Res. 2017;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16:1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrita R, Lauss M, Sanna A, Donia M, Larsen MS, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–5. [DOI] [PubMed] [Google Scholar]
- 15.de la L García-Hernández M, Uribe-Uribe NO, Espinosa-González R, Kast WM, Khader SA, Rangel-Moreno J. A Unique Cellular and Molecular Microenvironment Is Present in Tertiary Lymphoid Organs of Patients with Spontaneous Prostate Cancer Regression. Front Immunol. 2017;8:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweiger T, Berghoff AS, Glogner C, Glueck O, Rajky O, Traxler D, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin Exp Metastasis. 2016;33:727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowman AM, Hickman AW, Mauldin IS, Mahmutovic A, Gru AA, Slingluff CL. Lymphoid aggregates in desmoplastic melanoma have features of tertiary lymphoid structures. Melanoma Res. 2018;28:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–7. [DOI] [PubMed] [Google Scholar]
- 20.Goc J, Germain C, Vo-Bourgais TKD, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–15. [DOI] [PubMed] [Google Scholar]
- 21.Giraldo NA, Becht E, Pagès F, Skliris G, Verkarre V, Vano Y, et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin Cancer Res. 2015;21:3031–40. [DOI] [PubMed] [Google Scholar]
- 22.Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2:e91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis SN, Mallozzi SS, Rodig SJ, Cronk KM, McArdel SL, Caron T, et al. The microenvironment of germ cell tumors harbors a prominent antigen-driven humoral response. J Immunol. 2009;182:3310–7. [DOI] [PubMed] [Google Scholar]
- 24.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M-C, Riquet M, et al. Characteristics and Clinical Impacts of the Immune Environments in Colorectal and Renal Cell Carcinoma Lung Metastases: Influence of Tumor Origin. Clin Cancer Res. 2013;19:4079–91. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Erazo L, Rhodes JL, Marion VC, Kemp RA. Tertiary lymphoid structures in cancer – considerations for patient prognosis. Cell Mol Immunol. 2020;17:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipponi A, Mercier M, Seremet T, Baurain J-F, Théate I, van den Oord J, et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. [DOI] [PubMed] [Google Scholar]
- 27.Peske JD, Thompson ED, Gemta L, Baylis RA, Fu Y-X, Engelhard VH. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun. 2015;6:7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez AB, Peske JD, Engelhard VH. Identification and characterization of tertiary lymphoid structures in murine melanoma In: Dieu-Nosjean M-C., editor. Tert. Lymphoid Struct. Methods Protoc. New York, NY: Humana Press; 2018. page 241–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez AB, Peske JD, Woods AN, Leick KM, Mauldin IS, Young SJ, et al. Immune Mechanisms Orchestrate Tertiary Lymphoid Structures in Tumors Via Cancer-Associated Fibroblasts [Internet]. 2020. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3575119 [DOI] [PMC free article] [PubMed]
- 30.Lee M, Heo S-H, Song IH, Rajayi H, Park HS, Park IA, et al. Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod Pathol. 2019;32:70–80. [DOI] [PubMed] [Google Scholar]
- 31.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–9. [DOI] [PubMed] [Google Scholar]
- 32.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–44. [DOI] [PubMed] [Google Scholar]
- 33.Guo FF, Cui JW. The Role of Tumor-Infiltrating B Cells in Tumor Immunity. J Oncol. 2019;2019:2592419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–92. [DOI] [PubMed] [Google Scholar]
- 35.Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebenhüner A, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7:e1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res. 2018;78:1308–20. [DOI] [PubMed] [Google Scholar]
- 37.Selitsky SR, Mose LE, Smith CC, Chai S, Hoadley KA, Dittmer DP, et al. Prognostic value of B cells in cutaneous melanoma. Genome Med. 2019;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu-Trantien C, Willard-Gallo K. Tumor-infiltrating follicular helper T cells. Oncoimmunology. 2013;2:e26066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bento DC, Jones E, Junaid S, Tull J, Williams GT, Godkin A, et al. High endothelial venules are rare in colorectal cancers but accumulate in extra-tumoral areas with disease progression. OncoImmunology. 2015;4:e974374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Figenschau SL, Fismen S, Fenton KA, Fenton C, Mortensen ES. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer. 2015;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20:2147–58. [DOI] [PubMed] [Google Scholar]
- 42.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112:1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Wang J, Liu H, Lan T, Xu L, Wang G, et al. Existence of intratumoral tertiary lymphoid structures is associated with immune cells infiltration and predicts better prognosis in early-stage hepatocellular carcinoma. Aging. 2020;12:3451–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E. Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC Clin Pathol. 2014;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song IH, Heo S-H, Bang WS, Park HS, Park IA, Kim Y-A, et al. Predictive Value of Tertiary Lymphoid Structures Assessed by High Endothelial Venule Counts in the Neoadjuvant Setting of Triple-Negative Breast Cancer. Cancer Res Treat. 2017;49:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn J-H, et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes and the Tertiary Lymphoid Structures in HER2-Positive Breast Cancer Treated With Adjuvant Trastuzumab. Am J Clin Pathol. 2015;144:278–88. [DOI] [PubMed] [Google Scholar]
- 47.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griss J, Bauer W, Wagner C, Simon M, Chen M, Grabmeier-Pfistershammer K, et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun. 2019;10:4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petitprez F, de Reyniès A, Keung EZ, Chen TW-W, Sun C-M, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–60. [DOI] [PubMed] [Google Scholar]
- 50.Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular Therapeutic Vaccination Targeting HPV16 Induces T Cell Responses That Localize in Mucosal Lesions. Sci Transl Med. 2014;6:221ra13–221ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang H, Zhu M, Qiao J, Fu Y-X. Lymphotoxin signaling in tertiary lymphoid structures and immunotherapy. Cell Mol Immunol. 2017;14:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansson-Percival A, He B, Li Z-J, Kjellén A, Russell K, Li J, et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol. 2017;18:1207–17. [DOI] [PubMed] [Google Scholar]
- 53.Turnquist HR, Lin X, Ashour AE, Hollingsworth MA, Singh RK, Talmadge JE, et al. CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int J Oncol. 2007;30:631–9. [PubMed] [Google Scholar]
- 54.Chen L, Taylor JL, Sabins NC, Lowe DB, Qu Y, You Z, et al. Extranodal induction of therapeutic immunity in the tumor microenvironment after intratumoral delivery of Tbet gene-modified dendritic cells. Cancer Gene Ther. 2013;20:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstein AM, Chen L, Brzana EA, Patil PR, Taylor JL, Fabian KL, et al. Tbet and IL-36γ cooperate in therapeutic DC-mediated promotion of ectopic lymphoid organogenesis in the tumor microenvironment. Oncoimmunology. 2017;6:e1322238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirk CJ, Hartigan-O’Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–802. [PubMed] [Google Scholar]
- 57.Yang S-C, Batra RK, Hillinger S, Reckamp KL, Strieter RM, Dubinett SM, et al. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2006;66:3205–13. [DOI] [PubMed] [Google Scholar]
- 58.Joshi NS, Akama-Garren EH, Lu Y, Lee D-Y, Chang GP, Li A, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrega P, Loiacono F, Di Carlo E, Scaramuccia A, Mora M, Conte R, et al. NCR+ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun. 2015;6:8280. [DOI] [PubMed] [Google Scholar]
- 61.Koti M, Xu AS, Ren KYM, Visram K, Ren R, Berman DM, et al. Tertiary Lymphoid Structures Associate with Tumour Stage in Urothelial Bladder Cancer. Bladder Cancer Amst Neth. 2017;3:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie J-J, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–87. [DOI] [PubMed] [Google Scholar]
- 63.Martinet L, Filleron T, Guellec SL, Rochaix P, Garrido I, Girard J-P. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with Lymphotoxin β–producing dendritic cells in human breast cancer. J Immunol. 2013;191:2001–8. [DOI] [PubMed] [Google Scholar]
- 64.Lee HJ, Park IA, Song IH, Shin S-J, Kim JY, Yu JH, et al. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2015;69:422–30. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Tsang JYS, Hlaing T, Hu J, Ni Y-B, Chan SK, et al. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. The Oncologist. 2017;22:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meshcheryakova A, Tamandl D, Bajna E, Stift J, Mittlboeck M, Svoboda M, et al. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PloS One. 2014;9:e99008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hill DG, Yu L, Gao H, Balic JJ, West A, Oshima H, et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int J Cancer. 2018;143:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70:58–65. [DOI] [PubMed] [Google Scholar]
- 69.Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, et al. Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology. 2016;5:e1255394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ladanyi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ladányi A, Sebestyén T, Mohos A, Liszkay G, Somlai B, Tóth E, et al. Ectopic lymphoid structures in primary cutaneous melanoma. Pathol Oncol Res. 2014;20:981–5. [DOI] [PubMed] [Google Scholar]
- 72.Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, et al. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012;1:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012;2:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wirsing AM, Ervik IK, Seppola M, Uhlin-Hansen L, Steigen SE, Hadler-Olsen E. Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma. Mod Pathol. 2018;31:910–22. [DOI] [PubMed] [Google Scholar]
- 75.Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer. 2018;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castino GF, Cortese N, Capretti G, Serio S, Di Caro G, Mineri R, et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology. 2015;5:e1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]


