Abstract
Objective.
To characterize risk factors of venous thromboembolism (VTE) and to examine effects of VTE on survival of women with cervical cancer.
Methods.
This is a retrospective study examining consecutive stage I–IV cervical cancer cases diagnosed between 2000 and 2014. Cumulative risk of VTE after cervical cancer diagnosis was evaluated by a time-dependent analysis, expressing adjusted-hazard ratio [HR] and 95% confidence interval [CI]. Survival analysis was performed to determine independent risk factors for progression-free survival (PFS) and disease-specific overall survival (OS).
Results.
VTE was recorded in 98 (12.3%, 95%CI 11.6–22.8) out of 798 cases with 1-, 2-, and 5-year cumulative incidences after cervical cancer diagnosis being 8.4%, 11.3%, and 18.7%, respectively. On multivariable analysis, advanced-stage disease (2-year cumulative risk, distant metastatic disease 44.8% [HR 4.13, 95%CI 1.06–10.7, P = 0.003], and locally-advanced disease 13.4% [HR 2.46, 95%CI 1.17–4.43, P = 0.004]) were independently associated with increased risk of VTE compared to early-stage disease (stage IA1–IB1 4.1%). In addition, low albumin level (HR per unit change, 0.59, 95%CI 0.40–0.85, P = 0.005) and chemotherapy treatment (HR 2.46, 95%CI 1.30–4.66, P = 0.006) remained independent risk factors associated with increased risk of VTE. On univariate analysis, VTE was significantly associated with decreased PFS (5-year rates, 22.3% versus 68.7%, P < 0.001) and OS (5-year rates, 55.1% versus 90.0%, P < 0.001). On multivariable analysis, VTE remained an independent prognostic factor associated with decreased PFS (HR 1.95, 95%CI 1.43–2.67, P < 0.001) and OS (HR 3.54, 95%CI 2.04–6.13, P < 0.001).
Conclusion.
VTE represents aggressive tumor behavior and poor patient condition, and is an independent prognostic factor for decreased survival in women with cervical cancer.
Keywords: Cervical cancer, Venous thromboembolism, Risk factor, Chemotherapy, Albumin, Survival
1. Introduction
Cervical cancer is the most common gynecologic malignancy worldwide [1]. While cure is highly achievable in early-stage disease, metastatic or recurrent disease is challenging to cure in cervical cancer. Given the effectiveness of treatment modalities in cervical cancer, it would be useful to identify and predict risk factors that may compromise survival outcomes.
Malignancy is a known risk factor for developing venous thromboembolism (VTE). VTE was first described by Armand Trousseau in the 19th century, and has been historically theorized to occur in the setting of endothelial damage, venous stasis, and hypercoagulability [2]. In general, the risk of VTE increases approximately 7-fold in the presence of malignancy and approximately 20-fold in the presence of distant metastases [3]. This known association between VTE and malignancy has been described in various types of gynecologic malignancies including ovarian and endometrial cancers [4,5].
For cervical cancer, previous studies have not completely outlined the characteristics and outcome of VTE [6]. Reported incidence of VTE in cervical cancer varies due to heterogeneous study populations (0–34%), and identification of additional risk factors for VTE development in women with cervical cancer is relatively understudied [6]. Because cervical cancer with VTE was associated with the poorest survival among gynecologic malignancies [7], it is paramount to examine if VTE impacts survival in women with cervical cancer. The aim of this study was to (i) identify independent risk factors for developing VTE in women with cervical cancer, and (ii) to evaluate survival outcomes related to VTE in cervical cancer.
2. Patients and methods
2.1. Eligibility criteria
After Institutional Review Board approval was obtained, consecutive cases of stage I-IV invasive cervical cancer diagnosed and managed at LAC + USC Medical Center between January 1, 2000 and December 31, 2014 were examined. An institutional pathology database was used to identify those eligible cases by searching the keyword “cervical cancer”. Cases with pre-invasive cervical dysplasia, sarcoma, and metastatic tumors to the uterine cervix were excluded from the search. Patients with past history of VTE were also excluded. Among eligible cases, patient demographics, laboratory test results, tumor characteristics, treatment pattern, information for VTE, and survival outcome were collected from medical records. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines were consulted to outline the study description for this retrospective cohort study [8].
2.2. Clinical information
Patient demographics at cervical cancer diagnosis included age, ethnicity, body mass index (BMI, kg/m2), medical comorbidities (hypertension, diabetes mellitus, and hypercholesterolemia), and cigarette use. Laboratory test results at cervical cancer diagnosis included white blood cell counts (WBC, 109/L), hemoglobin levels (g/dL), platelet counts (109/L), blood urea nitrogen levels (BUN, mg/dL), creatinine levels (mg/dL), bicarbonate levels (mEq/L), and albumin levels (g/dL). Tumor characteristics included histologic subtypes and cancer stage. Patterns for the initial treatment after cervical cancer diagnosis included primary hysterectomy, systemic chemotherapy type, and whole pelvic radiotherapy (WPRT). Radiation sensitizing chemotherapy was not considered systemic chemotherapy. Among recurrent cases, use of salvage chemotherapy was also recorded. For survival outcomes, progression-free survival (PFS) and disease-specific overall survival (OS) were examined.
VTE observed between the initial cervical cancer diagnosis and the last follow-up date was recorded. The diagnosis of VTE was determined by imaging including Doppler study of the extremities, computed tomography (CT) pulmonary artery angiogram, or ventilation-perfusion lung scan. In our institution, systemic imaging with CT scan is not routinely performed during the post-treatment follow-up course other than at 3 months post-radiation. Otherwise, CT scanning is performed when recurrence/progression is clinically suspected. Type of VTE was examined as follows: deep venous thrombosis (DVT) alone, pulmonary embolism (PE) alone, and DVT/PE combined. Detail of treatment for VTE was recorded as follows: heparin, low-molecular weight heparin (LMWH), warfarin, and inferior vena cava (IVC) filter.
2.3. Definition
Obesity was defined as BMI ≥ 30 kg/m2. Cancer stage was based on the International Federation of Gynecology and Obstetrics (FIGO) classification [9]. In this study, cancer stage was further grouped into the following: early-stage (stage IA1–IB1), locally-advanced stage (stage IB2-IVA), and distant metastasis (stage IVB). Various cutoff levels for serum albumin were tested for cumulative risk of VTE based on previous studies (4.0 versus 3.5 g/dL), and the cutoff level of 4.0 g/dL was chosen as hypoalbuminemia due to larger statistical value in log-rank compared to the cutoff of 3.5 g/dL (27 versus 11) for this study [10,11]. PFS was defined as the time interval between the initial cervical cancer diagnosis and the date of the first disease recurrence/progression or the last date of follow-up if censored. OS was defined as time interval between the initial cervical cancer diagnosis and the date of death due to cervical cancer or the last date of follow-up if patient was alive or died of other causes. Patients who were lost to follow-up were censored at the date of the last visit.
2.4. Statistical analysis
Continuous variables were assessed for normality (Kolmogorov-Smirnov test) and expressed as appropriate (mean with SD or median with range). Student’s t-test or Mann-Whitney U test was used to assess statistical significance for continuous variables as appropriate. Categorical variables were evaluated with the Fisher’s exact test or chi-square test as appropriate. Because VTE is a time-dependent event after cervical cancer diagnosis, survival analyses with a log-rank test for univariate analysis and Cox proportional hazard regression models for multivariable analysis were used to assess the cumulative incidence and risk of VTE after cervical cancer diagnosis. Significant covariates with P < 0.05 on univariate analysis were initially entered into the multivariable model; then, least significant covariates were removed from the model until the final model retained significant covariates (conditional backward method). Age, BMI, and laboratory test results were entered as continuous variables in the model. Other categorical variables were grouped in a priori manner. Significances of PFS and OS were also examined in a similar fashion with survival analysis. Magnitude of statistical significance for survival analysis was expressed with hazard ratio (HR) and 95% confidence interval (CI). The Kaplan-Meier method was used to construct cumulative incidence and survival curves. A P < 0.05 was considered statistically significant (all, 2-tailed). The Statistical Package for Social Science software (SPSS, version 22.0, IL) was used for all analyses.
3. Results
There were 815 cases of cervical cancer identified during the study period. Of those, there were 13 cases with no medical record. Among 802 cases of cervical cancer with available medical records, 4 (0.5%) cases were excluded due to a past history of VTE. The remaining 798 cases of cervical cancer without past history of VTE represented the study population. The patient characteristics are shown in Table 1. In the entire cohort, the median age was 48.9 years old and the majority were Hispanic (72.1%). Obesity was seen in 39.2% of the study population. Medical comorbidities were relatively not prevalent in this study population (hypertension 25.1%, diabetes mellitus 14.1%, and hypercholesterolemia 8.1%). Statin use was seen in 53.9% of the dyslipidemic patients. Cigarette use was seen in approximately one seventh (14.7%). Hypoalbuminemia was seen in 38.3% of the cases. The most common histology was squamous cell carcinoma (75.8%), and the majority of our study patients had locally-advanced disease (56.1%). The most common primary treatment modality was WPRT (60.4%). Among 482 patients who received WPRT, 426 (88.4%) patients received concurrent chemo-radiotherapy with cisplatin being the most common agent (94.1%). Among chemotherapy regimens used for the primary treatment, cisplatin/gemcitabine was the most common regimen given in 42 (52.5%) patients (Table S1).
Table 1.
Patient demographics.
| All |
VTE (+) |
VTE (−) |
P-Value |
|
|---|---|---|---|---|
| N = 798 (100%) | n = 98 (12.3%) | n = 700 (87.7%) | ||
| Age (years) | 48.9 (15.6–88.7) | 51.3 (21.8–83.5) | 48.8 (15.6–88.7) | 0.06 |
| Ethnicity | 0.11 | |||
| Caucasian | 65 (8.1%) | 5 (5.1%) | 60 (8.6%) | |
| Black | 50 (6.3%) | 11 (11.2%) | 39 (5.6%) | |
| Hispanic | 575 (72.1%) | 73 (74.5%) | 502 (71.7%) | |
| Asian | 104 (13.0%) | 9 (9.2%) | 95 (13.6%) | |
| Others | 4 (0.5%) | 0 | 4 (0.6%) | |
| BMI (kg/m2) | 28.0 (16.5–58.3) | 27.5 (18.4–53.1) | 28.1 (16.5–58.3) | 0.41 |
| Hypertension | 0.11 | |||
| No | 586 (74.9%) | 67 (68.4%) | 519 (75.9%) | |
| Yes | 196 (25.1%) | 31 (31.6%) | 165 (24.1%) | |
| Diabetes mellitus | 0.49 | |||
| No | 672 (85.9%) | 82 (83.7%) | 590 (86.3%) | |
| Yes | 110 (14.1%) | 16 (16.3%) | 94 (13.7%) | |
| Hypercholesterolemia | 0.45 | |||
| No | 719 (91.9%) | 92 (93.9%) | 627 (91.7%) | |
| Yes | 63 (8.1%) | 6 (6.1%) | 57 (8.3%) | |
| Cigarette use | 0.63 | |||
| No | 661 (85.3%) | 82 (83.7%) | 579 (85.5%) | |
| Yes | 114 (14.7%) | 16 (16.3%) | 98 (14.5%) | |
| Laboratory results | ||||
| WBC (×109/L) | 8.3 (2.6–30.0) | 8.8 (2.7–22.0) | 8.2 (2.6–30.0) | 0.018 |
| Platelet (×109/L) | 301 (23–964) | 319 (119–782) | 296 (23–964) | 0.015 |
| Hemoglobin (g/dL) | 12.2 (3.7–17.0) | 11.9 (3.7–17.0) | 12.3 (3.8–16.0) | 0.031 |
| BUN (mg/dL) | 12.0 (2–173) | 12.0 (4.0–80.0) | 12.0 (2.0–173.0) | 0.53 |
| Creatinine (mg/dL) | 0.6 (0.3–16.7) | 0.6 (0.3–7.4) | 0.6 (0.3–16.7) | 0.60 |
| HCO3 (mEq/L) | 25.0 (10.0–33.0) | 25.0 (16.0–31.0) | 25.0 (10.0–33.0) | 0.36 |
| Albumin (g/dL) | 4.1 (2.0–5.3) | 3.8 (2.1–4.9) | 4.1 (2.0–5.3) | <0.001 |
| Histology | 0.34 | |||
| Squamous cell | 605 (75.8%) | 77 (78.6%) | 528 (75.4%) | |
| Adenocarcinoma | 138 (17.3%) | 17 (17.3%) | 121 (17.3%) | |
| Adenosquamous | 32 (4.0%) | 4 (4.1%) | 28 (4.0%) | |
| Others | 23 (2.9%) | 0 | 23 (3.3%) | |
| Stage | <0.001 | |||
| Early stage† | 290 (36.6%) | 16 (16.7%) | 274 (39.4%) | |
| Locally advanced stage | 444 (56.1%) | 63 (65.6%) | 381 (54.7%) | |
| Distant metastasis | 58 (7.3%) | 17 (17.7%) | 41 (5.9%) | |
| Primary hysterectomy | <0.001 | |||
| No | 576 (72.2%) | 86 (87.8%) | 490 (70.0%) | |
| Yes | 222 (27.8%) | 12 (12.2%) | 210 (30.0%) | |
| WPRT* | <0.001 | |||
| No | 316 (39.6%) | 23 (23.5%) | 293 (41.9%) | |
| Yes | 482 (60.4%) | 75 (76.5%) | 407 (58.1%) | |
| Systemic chemotherapy | <0.001 | |||
| No | 718 (90.0%) | 71 (72.4%) | 647 (92.4%) | |
| Yes | 80 (10.0%) | 27 (27.6%) | 53 (7.6%) |
Bold numbers indicate significance at P < 0.05.
Number (%) or mean (±SD) is shown. Mann-Whitney U test, Fisher’s exact test, or chi-square test for P-values (comparison between VTE cases versus non-VTE cases). Significant P-values are emboldened. 16 missing data for hypertension, diabetes mellitus, and hypercholesterolemia; 23 missing data for smoker; 11 missing data for WBC, Platelet and Hemoglobin; 29 missing data for albumin, BUN, creatinine and HCO3; and 6 missing data for stage.
treatment patterns included: primary hysterectomy n = 210, excision alone n = 27, radiotherapy n = 27, lost to follow-up n = 15, declined treatment n = 7, no record n = 3, and aborted hysterectomy n = 1.
426 (88.4%) patients received concurrent chemo-radiotherapy.
Abbreviations: WBC, white blood cell; BUN, Blood urea nitrogen; HCO3, bicarbonate; WPRT, whole pelvic radiation therapy; and VTE, venous thromboembolism.
VTE was recorded in 98 (12.3%, 95%CI 11.6–22.8) cases with 1-, 2-, and 5-year cumulative incidences after cervical cancer diagnosis being 8.4%, 11.3%, and 18.7%, respectively (Fig. 1A). VTE cases were more likely to have higher WBC counts and platelet counts while having lower hemoglobin and albumin levels (all, P < 0.05; Table 1). Patients who developed VTE were more likely to have locally-advanced stage and have distant metastasis compared to non-VTE patients (P < 0.001). Characteristics of VTE were shown in Table S2. DVT alone was the most common type of VTE (85.4%) followed by DVT/PE (8.3%) and PE alone (6.3%). Median time to develop VTE was 9.2 months, and the most common time frame of VTE development after cervical cancer diagnosis was VTE after 6 months from cervical cancer diagnosis (59.3%) and approximately one fifth of patients developed VTE within 1 month from cervical cancer diagnosis (18.8%). There were 7 cases (0.9%) of VTE diagnosed at the time of cervical cancer diagnosis. There was no occurrences of postoperative VTE within 30 days after hysterectomy for stage IA1–IB1 cervical cancer cases. Among 57 women who developed VTE 6 months after the initial cervical cancer diagnosis, there were 45 cases (78.9%) with active/ongoing cancer. Similarly, there were 25 cases of VTE diagnosed 2 years or later from the initial cervical cancer diagnosis: of those, 20 cases (80%) were associated with recurrent/progressed disease. LMHW alone was the most common treatment approach (70.5%), and there were 15 (15.3%) patients who received an IVC filter.
Fig. 1.
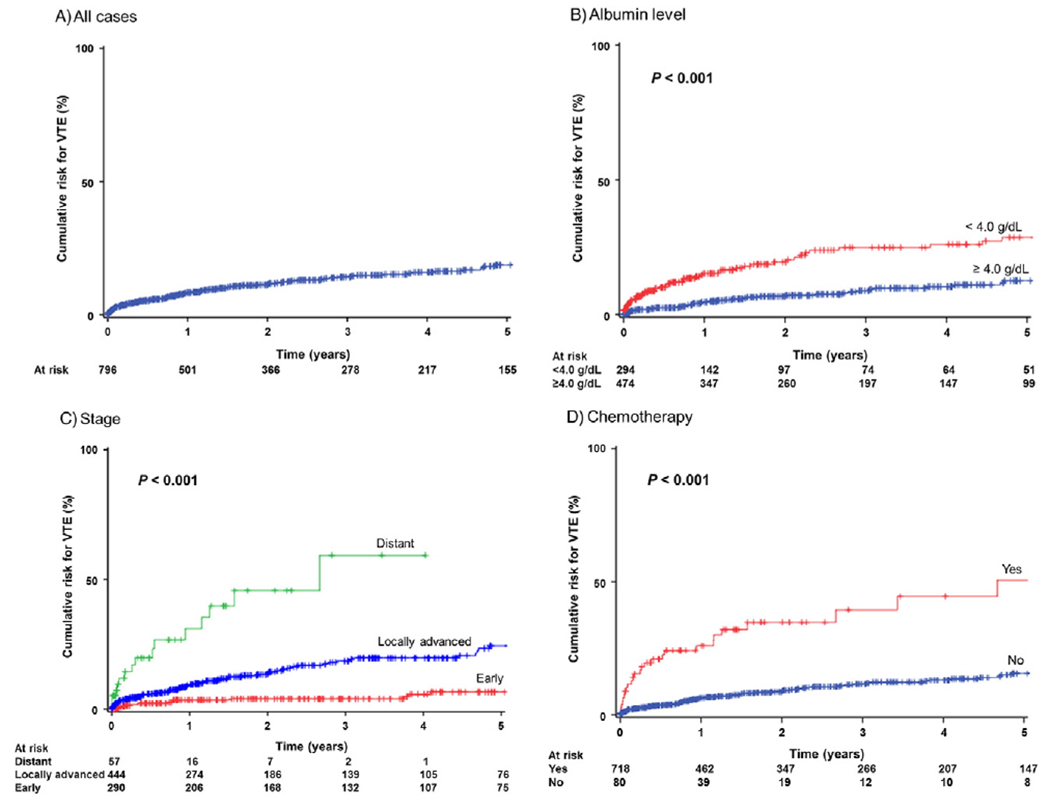
Cumulative incidence curves for venous thromboembolism. Log-rank test for P-value. Cumulative risks for VTE are shown for all cases (panel A), albumin level at cervical cancer diagnosis (panel B), cancer stage (panel C), and systemic chemotherapy for the initial treatment (panel D). Number at risk indicates patients who had VTE and who were censored in each time point period. Events for VTE are shown as proportional upward changes in the Kaplan-Meier curves. Censored cases (active surveillance or lost to follow-up) are shown as vertical bars. Abbreviation: VTE, venous thromboembolism.
Because VTE is a time-dependent event after cervical cancer diagnosis, survival analysis was performed to determine independent predictors for developing VTE (Table 2). On multivariable analysis, albumin level at cervical cancer diagnosis (Fig. 1B), stage (Fig. 1C), and systemic chemotherapy (Fig. 1D) remained independent risk factors associated with developing VTE. Specifically, distant metastatic disease (2-year cumulative incidence of VTE 44.8%, HR 4.13, 95%CI 1.60–10.7, P = 0.003) and locally-advanced disease (13.4%, HR 2.46, 95%CI 1.17–4.43, P = 0.004) were independently associated with increased risk of VTE compared to early-stage disease (4.1%). In addition, low albumin level (HR per unit change, 0.59,95%CI 0.40–0.85, P = 0.005) and systemic chemotherapy treatment (HR2.46, 95%CI 1.30–4.66, P = 0.006) remained independent risk factors associated with increased risk of VTE. When chemotherapy regimens were compared, the cisplatin and gemcitabine combination regimen had a higher cumulative incidence of VTE compared to other regimens but it did not reach statistical significance (2-year cumulative incidence rate, 45.8% versus 26.7%, HR 2.07, 95%CI 0.92–4.66, P = 0.08). Similarly, among cases who received concurrent chemoradiotherapy, use of cisplatin radiosensitization was associated with higher cumulative risk of VTE compared to non-cisplatin counterparts although it did not reach statistical significance (2-year cumulative incidence rate, 12.4% versus 0%, P = 0.14).
Table 2.
Independent risk factors of venous thromboembolism.
| Univariate |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| No. | 2-yr (%) | HR (95%CI) | P-Value | HR (95%CI) | P-Value | |
| Age (per unit) | 798 | 1.02 (1.00–1.04) | 0.035 | |||
| Stage | <0.001 | |||||
| Early stage | 290 | 4.1% | 1 | 1 | ||
| Local advanced | 444 | 13.4% | 3.11 (1.80–5.39) | 2.46 (1.37–4.43) | 0.004 | |
| Distant metastasis | 58 | 44.8% | 12.8 (6.29–26.0) | 4.13 (1.60–10.7) | 0.003 | |
| Laboratory results | ||||||
| WBC (per unit) | 798 | 1.09 (1.04–1.15) | 0.001 | |||
| Platelet (per unit) | 798 | 1.00 (1.00–1.01) | <0.001 | |||
| Hemoglobin (per unit) | 798 | 0.87 (0.80–0.94) | 0.022 | |||
| Albumin (per unit) | 798 | 0.41 (0.28–0.58) | <0.001 | 0.59 (0.40–0.85) | 0.005 | |
| Primary hysterectomy | <0.001 | |||||
| No | 576 | 14.6% | 1 | |||
| Yes | 222 | 4.0% | 0.28 (0.15–0.50) | |||
| WPRT | 0.015 | |||||
| No | 316 | 8.2% | 1 | |||
| Yes | 482 | 12.7% | 1.79 (1.11–2.88) | |||
| Systemic chemotherapy | <0.001 | |||||
| No | 718 | 8.5% | 1 | 1 | ||
| Yes | 80 | 37.0% | 4.74 (3.00–7.47) | 2.46 (1.30–4.66) | 0.006 | |
Log-rank test for univariable analysis (among all covariates tested in Table 1, only significant covariates are listed). A Cox proportional hazard regression model for multivariable analysis (conditional backward method). Significant P-values are emboldened. Abbreviations: HR, Hazard ratio; 95%CI, 95% confidence interval; 2-yr (%), 2-year cumulative proportion; and WPRT, whole pelvic radiation therapy.
Among 80 women who received systemic chemotherapy for the initial treatment, there were 11 (13.8%, 95%CI 6.2–21.3) cases who developed VTE during or within a month after completion of chemotherapy (n = 7, 63.6% during chemotherapy; and n = 3, 27.3%, 2 weeks after completion of chemotherapy; and n = 1, 9.1%, 1 month after completion of chemotherapy). There were 3 (27.3%) women who developed DVT/PE. The risk of developing VTE during and within 1 month after chemotherapy was significantly higher in the combination regimen with cisplatin and gemcitabine (11 out of 42 cases, 26.2%) compared to other regimens (0 out of 38 cases, P < 0.001). Similarly, VTE risk during systemic chemotherapy was significantly higher in the cisplatin/gemcitabine regimen compared to others (18.4% versus 0%, P = 0.013).
Among 114 recurrent cases, there were 30 cases (26.3%) in whom VTE was diagnosed after recurrence of cervical cancer with 1-, 2-, and 5-year cumulative risks being were 3.8%, 7.3%, and 34.5%, respectively. There were 7 women with recurrent disease in whom VTE was diagnosed during salvage chemotherapy (5 cases during the first line regimen [cisplatin doublet 4 cases, and carboplatin doublet 1 case], 1 case during the 3rd line regimen with pemetrexed, and 1 case during the 4th line regimen with cisplatin).
Survival analysis was performed. Median follow-up times of patients who died of cervical cancer and patients without evidence of disease were 21.7 and 40.7 months, respectively (entire cohort, 22.8 months). There were 239 (29.0%) patients who developed recurrence/progression of cervical cancer, and there were 74 (9.3%) patients who died of disease. No patient died of VTE. There were 31 cases who were lost to follow-up within 1 month after cervical cancer diagnosis. On univariate analysis, VTE was significantly associated with decreased PFS compared to non-VTE (5-year rates, 22.3% versus 68.7%, P < 0.001; Fig. 2A). After controlling for other significant covariates, VTE remained an independent prognostic factor associated with decreased PFS on multivariable analysis (HR 1.95, 95%CI 1.43–2.67, P < 0.001; Table 3). The magnitude of this statistical significance was the third highest in the model. Other independent prognostic factors for PFS included BUN and albumin levels, stage, primary hysterectomy, and WPRT (all, P < 0.01). Among those who received systemic chemotherapy, 2-year PFS rates were similar between cisplatin/gemcitabine combination versus others (30.0% versus 23.4%, P = 0.70).
Fig. 2.
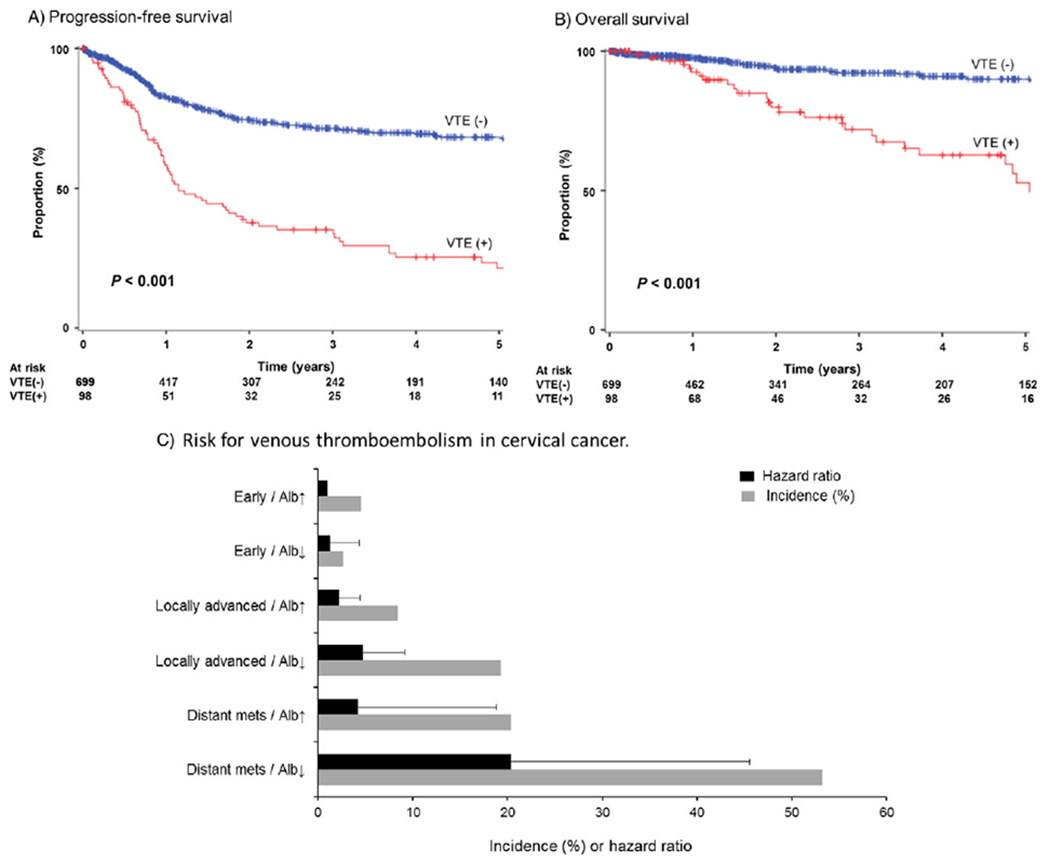
Survival curves and cumulative risk of venous thromboembolism. Log-rank test for P-value. (A) Progression-free survival stratified by VTE. (B) Disease-specific overall survival stratified by VTE. (C) 2-year cumulative incidence and risk for VTE based on patient factor (albumin level) and tumor factor (cancer stage). Number at risk indicates patients who had recurrence, progression, or cervical cancer death in each time point period. Events for survival are shown as proportional downward changes in the Kaplan-Meier curves. Censored cases (active surveillance or lost to follow-up) are shown as vertical bars. Abbreviations: VTE, venous thromboembolism; Alb↓, albumin level < 4.0 g/dL at cervical cancer diagnosis; and Alb↑, albumin level ≥4.0 g/dL at cervical cancer diagnosis.
Table 3.
Multivariable analysis for progression-free survival.
| Univariate |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| No. | 5-yr (%) | HR (95%CI) | P-Value | HR (95%CI) | P-Value | |
| Age (per unit) | 798 | 1.02 (1.01–1.03) | <0.001 | |||
| Ethnicity | 0.001 | |||||
| Caucasian | 65 | 64.6% | 1 | |||
| Black | 50 | 39.8% | 2.10 (1.13–3.91) | |||
| Hispanic | 575 | 63.8% | 0.91 (0.55–1.49) | |||
| Asian | 104 | 54.9% | 1.36 (0.76–2.44) | |||
| Laboratory results | ||||||
| WBC (per unit) | 798 | 1.07 (1.03–1.10) | <0.001 | |||
| Platelet (per unit) | 798 | 1.00 (1.00–1.01) | <0.001 | |||
| Hemoglobin (per unit) | 798 | 0.80 (0.77–0.84) | <0.001 | |||
| BUN (per unit) | 798 | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.00–1.02) | 0.002 | |
| Creatinine (per unit) | 798 | 1.18 (1.12–1.25) | <0.001 | |||
| HCO3 (per unit) | 798 | 0.92 (0.88–0.96) | <0.001 | |||
| Albumin (per unit) | 798 | 0.35 (0.28–0.43) | <0.001 | 0.66 (0.51–0.86) | 0.002 | |
| Histology | 0.047 | |||||
| Squamous cell | 605 | 60.6% | 1 | |||
| Adenocarcinoma | 138 | 66.9% | 0.83 (0.58–1.18) | |||
| Adenosquamous | 32 | 61.6% | 0.92 (0.47–1.80) | |||
| Other | 23 | 47.4% | 2.07 (1.15–3.71) | |||
| Stage | <0.001 | |||||
| Early stage | 290 | 90.5% | 1 | 1 | ||
| Locally-advanced stage | 444 | 51.1% | 6.87 (4.36–10.8) | 6.62 (3.36–13.0) | <0.001 | |
| Distant metastasis | 58 | 42.5% | 39.4 (23.5–66.2) | 22.0 (10.9–44.4) | <0.001 | |
| Primary hysterectomy | <0.001 | |||||
| No | 576 | 49.7% | 1 | 1 | ||
| Yes | 222 | 89.6% | 0.15 (0.09–0.24) | 0.36 (0.19–0.68) | 0.002 | |
| WPRT | 0.002 | |||||
| No | 316 | 75.5% | 1 | 1 | ||
| Yes | 482 | 54.8% | 1.57 (1.17–2.10) | 0.26 (0.17–0.39) | <0.001 | |
| Systemic chemotherapy | <0.001 | |||||
| No | 718 | 67.4% | 1 | |||
| Yes | 80 | 18.0% | 4.70 (3.51–6.28) | |||
| VTE | <0.001 | |||||
| No | 700 | 68.7% | 1 | 1 | ||
| Yes | 98 | 22.3% | 3.24 (2.45–4.29) | 1.95 (1.43–2.67) | <0.001 | |
Log-rank test for univariable analysis (among all covariates tested in Table 1, only significant covariates are listed). A Cox proportional hazard regression model for multivariable analysis (conditional backward method). Significant P-values are emboldened. Abbreviations: HR, Hazard ratio; 95%CI, 95% confidence interval; 5-yr (%), 5-year proportion; WBC, white blood cell; BUN, Blood urea nitrogen; HCO3, bicarbonate; VTE, venous thromboembolism; and WPRT, whole pelvic radiation therapy.
For OS, patients who developed VTE had a significantly lower 5-year rate compared to patients without VTE (55.1% versus 90.0%, P < 0.001; Fig. 2B). On multivariable analysis, VTE remained an independent prognostic factor associated with decreased OS (HR 3.54, 95%CI 2.04–6.13, P < 0.001). Other independent prognostic factors for OS included platelet counts, albumin levels, histology, stage, primary hysterectomy, and WPRT (all, P < 0.01; Table 4). Among those with significant covariates in multivariable analysis, VTE had the second largest magnitude of statistical significance following behind distant metastatic disease (HR 5.12, 95%CI 1.60–16.4, P = 0.006).
Table 4.
Multivariable analysis for disease-specific overall survival.
| Univariate |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| No. | 5-yr (%) | HR (95%CI) | P-Value | HR (95%CI) | P-Value | |
| Ethnicity | 0.006 | |||||
| Caucasian | 65 | 92.4% | 1 | |||
| Black | 50 | 56.8% | 3.00 (1.02–8.78) | |||
| Hispanic | 575 | 86.8% | 0.88 (0.35–2.20) | |||
| Asian | 104 | 82.6% | 1.15 (0.39–3.45) | |||
| Cigarette use | 0.009 | |||||
| No | 661 | 87.4% | 1 | |||
| Yes | 114 | 69.2% | 2.07 (1.19–3.61) | |||
| Laboratory results | ||||||
| WBC (per unit) | 798 | 1.10 (1.04–1.17) | 0.001 | |||
| Platelet (per unit) | 798 | 1.00 (1.00–1.01) | <0.001 | 1.00 (1.00–1.01) | 0.039 | |
| Hemoglobin (per unit) | 798 | 0.83 (0.75–0.91) | 0.001 | |||
| BUN (per unit) | 798 | 1.02 (1.01–1.03) | 0.008 | |||
| Creatinine (per unit) | 798 | 1.16 (1.03–1.30) | 0.014 | |||
| Albumin (per unit) | 798 | 0.32 (0.21–0.47) | <0.001 | 0.46 (0.29–0.72) | 0.001 | |
| Histology | 0.001 | |||||
| Squamous cell | 605 | 83.6% | 1 | 1 | ||
| Adenocarcinoma | 138 | 93.6% | 0.39 (0.17–0.91) | 0.37 (0.16–0.87) | 0.023 | |
| Adenosquamous | 32 | 86.9% | 0.63 (0.16–2.59) | 0.87 (0.21–3.62) | 0.85 | |
| Other | 23 | 67.3% | 3.14 (1.35–7.27) | 3.37 (1.37–8.27) | 0.008 | |
| Stage | <0.001 | |||||
| Early stage | 290 | 96.8% | 1 | 1 | ||
| Locally-advanced stage | 444 | 80.8% | 5.09 (2.41–10.8) | 1.25 (0.46–3.44) | 0.66 | |
| Distant metastasis | 58 | 25.2% | 37.1 (15.6–88.3) | 5.12 (1.60–16.4) | 0.006 | |
| Primary hysterectomy | <0.001 | |||||
| No | 576 | 78.7% | 1 | 1 | ||
| Yes | 222 | 98.0% | 0.13 (0.05–0.33) | 0.22 (0.06–0.77) | 0.018 | |
| Systemic chemotherapy | <0.001 | |||||
| No | 718 | 89.1% | 1 | |||
| Yes | 80 | 46.3% | 6.90 (4.23–11.3) | |||
| VTE | <0.001 | |||||
| No | 700 | 90.0% | 1 | 1 | ||
| Yes | 98 | 55.1% | 4.65 (2.90–7.45) | 3.54 (2.04–6.13) | <0.001 | |
Log-rank test for univariable analysis (among all covariates tested in Table 1, only significant covariates are listed). A Cox proportional hazard regression model for multivariable analysis (conditional backward method). Significant P-values are emboldened. Abbreviations: HR, Hazard ratio; 95%CI, 95% confidence interval; 5-yr (%), 5-year proportion WBC, white blood cell; BUN, Blood urea nitrogen; and VTE, venous thromboembolism.
Lastly, 2-year cumulative incidence of VTE was stratified by the combination patterns of stage and albumin levels at cervical cancer diagnosis (Fig. 2C). Risks of VTE were similar between distant metastatic disease without hypoalbuminemia and locally-advanced disease with hypoalbuminemia (2-year cumulative incidence, 20.4% versus 19.3%, P = 0.77). However, risk of VTE was significantly elevated when women had hypoalbuminemia in the setting of distant metastatic disease (yes versus no, 53.2% versus 20.4%, P = 0.023).
4. Discussion
The main finding of our study was that VTE occurrence was associated with decreased survival outcomes in women with cervical cancer. Furthermore, this study identified independent risk factors for VTE development in cervical cancer including advanced-stage disease, low serum albumin level, and receiving systemic chemotherapy.
To date, there are limited data regarding incidence and risk factors associated with VTE in cervical cancer [6]. In our study, overall 2-year cumulative incidence of VTE was 11.3%, but there was a disproportionally elevated risk of developing VTE in distant metastatic disease in cervical cancer with 2-year cumulative incidence being 44.8%. When hypoalbuminemia is present in the setting of distant metastatic disease, the risk of developing VTE exceeds > 50% (2-year cumulative incidence, 53.2%). This risk of VTE in metastatic cervical cancer is significantly higher than what previous studies reported for VTE incidence in stage IV cervical cancer (27.8%) [12] and is comparable to other known thrombogenic gynecologic malignancies such as advanced-stage ovarian clear cell cancer (43.1%) and advanced-stage high-risk endometrial cancer with risk factors (42.9–46.2%) [4,13]. While the exact mechanism of increased VTE risk in distant metastatic cervical cancer is not known, a possible role of interleukin 6 (IL-6) may be of interest. That is, cervical cancer is associated with an increased expression of IL-6 that is associated with tumor growth via the vascular endothelial growth factor pathway [14,15]. This IL-6 mediated tumor progression and VTE development may partly suggest an association of distant metastatic cervical cancer and an increased risk of VTE found in our study [13].
In this study, patients receiving chemotherapy were more likely to develop VTE. It is now well recognized that chemotherapy increases the risk of VTE in cancer patients. A recent study showed that chemotherapy can increase the risk of VTE in cancer patients from 4.1–6.5 folds [16]. The most common chemotherapeutic agent in our study was cisplatin followed by gemcitabine. In a large number of studies, increased risk of VTE with cisplatin has been shown in a panel of solid tumors [17]. The salient mechanism of cisplatin-associated thrombosis may be due to endovascular toxicity increasing von Willebrand factor and to endothelial cell damage by procoagulant microparticles [17,18]. Gemcitabine is known to increase the risk of VTE [19], however, a recent meta-analysis of 19 clinical trials did not show a statistical difference in VTE risk between gemcitabine and non-gemcitabine regimens [20]. The role of gemcitabine in the coagulation cascade and hemostasis is largely unknown with explanations including increased platelet aggregation and activation of the coagulation cascade.
In our study, all the VTE events during systemic chemotherapy were seen in the combination regimen with cisplatin and gemcitabine. Nevertheless, it will be premature to conclude that this regimen is associated with increased VTE risk in cervical cancer for several reasons. First, cisplatin was used in the majority of cases that made statistical comparison to non-cisplatin counterparts difficult. Second, other factors such as prolonged immobilization and preexisting coagulation abnormalities likely put the patients who are receiving chemotherapy at higher risk for thrombosis, and these were not controlled for in this analysis.
The high risk of VTE in the setting of metastatic or recurrent cervical cancer in women receiving combination cisplatin based chemotherapy raises the question as to whether VTE prophylaxis should be considered in this population especially given the possible role of LMWH in improving outcome [22,23]. However, LMWH is both expensive and might be problematic in a population prone to bleeding because of previous irradiation and the common presence of a pelvic tumor. These considerations, in combination with the fact that no woman died of VTE in this study, do not allow us to make a recommendation of LMWH use in all women being treated with chemotherapy for metastatic or recurrent cervical cancer. The decision as to whether to consider LMWH prophylaxis should be individualized based on each patient’s particular characteristics.
The last independent risk factor for VTE formation was low albumin level. Low albumin level is also associated with poor prognosis in our study. In a recent study, low albumin level in cancer patients was associated with increased risk of VTE and mortality [24]. The mechanism for this association is not well understood. However, it has been suggested that lower albumin levels may be associated with higher fibrinogen and factor VIII levels, which can reflect a hypercoagulable tendency [25]. It has also has been proposed that low albumin levels may lead due to increased liver protein synthesis, resulting in high concentrations of procoagulant factors [26]. Last, low albumin levels are considered indicative of poor general health and low daily activity, which can predispose patients to a higher risk of VTE related to venous stasis. In the present study, univariate analysis showed that WBC, platelet, and hemoglobin levels were also associated with increased risk of VTE. Many of these risk factors are consistent with earlier studies showing that elevated platelet counts, increased WBC counts and low hemoglobin are associated with associated VTE [27,28]. However, these studies did not control for albumin level. In our study, after multivariable analysis controlled for albumin level, these hematologic parameters were no longer significant for progression-free survival.
A strength of this study is that comprehensive analyses were performed to examine the significance of VTE in cervical cancer with a relatively large patient population. A weakness is that this was a retrospective study that may have missed possible confounding factors. For instance, this study did not examine biologic factors such as plasma level of coagulation factors. The relatively short follow-up time may lead to us missing possible VTE and survival events due to lead-time bias; however, this study chose a time-dependent analysis in consideration of this weakness. Among censored cases, this study was unable to distinguish women lost to follow-up from women undergoing active surveillance due to its retrospective nature. In addition, our study population was predominantly Hispanic, thus our findings might not be generalizable to other populations. A limitation is that systemic chemotherapy was only examined for the initial treatment but not for recurrent/progressive disease. Finally, universal laboratory testing and imaging for VTE was not performed in this study which may underestimate the true number of VTE incidents including subclinical thrombosis.
In summary, VTE is a marker for aggressive tumor behavior and poor patient condition, and is associated with poor survival in women with cervical cancer. The role of IL-6 in VTE development and tumor progression in cervical cancer is an important research question. If IL-6 is found to be a mechanism linking VTE formation and cancer progression, targeting the IL-6 pathway may have a possible therapeutic role in advanced cervical cancer. Since statins target the IL-6 pathway and reduce by IL-6 levels, women with advanced cervical cancer might benefit from statin use [29]. Statins are also reported to reduce the risk of overall cancer-related mortality demonstrated in a population-based study [30]. To our knowledge, there is no prior study which examined the association between statin use and risk of VTE in cancer patients, and the possible dual effect of statins merits future investigation.
Supplementary Material
HIGHLIGHTS.
Metastatic cervical cancer has a significantly high risk of developing venous thromboembolism (VTE).
VTE is a surrogate marker for aggressive tumor behavior and poor patient condition in cervical cancer.
Systemic chemotherapy was associated with increased risk of VTE.
Acknowledgments
Financial support: Ensign Endowment for Gynecologic Cancer Research (K.M.)
Footnotes
Disclosure statement
There is no conflict of interest in all authors.
Appendix A.: Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2016.06.012.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. , Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012, Int. J. Cancer 136 (2015) E359–E386. [DOI] [PubMed] [Google Scholar]
- [2].Wang X, Fu S, Freedman RS, Kavanagh JJ, Venous thromboembolism syndrome in gynecological cancer, Int. J. Gynecol. Cancer 16 (Suppl. 1) (2006) 458–471. [DOI] [PubMed] [Google Scholar]
- [3].Blom JW, Doggen CJ, Osanto S, Rosendaal FR, Malignancies, prothrombotic mutations, and the risk of venous thrombosis, J. Am. Med. Assoc 293 (2005) 715–722. [DOI] [PubMed] [Google Scholar]
- [4].Matsuo K, Yessaian AA, Lin YG, Pham HQ, Muderspach LI, Liebman HA, et al. , Predictive model of venous thromboembolism in endometrial cancer, Gynecol. Oncol 128 (2013) 544–551. [DOI] [PubMed] [Google Scholar]
- [5].Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. , Paraneoplastic thrombocytosis in ovarian cancer, N. Engl. J. Med 366 (2012) 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barbera L, Thomas G, Venous thromboembolism in cervical cancer, Lancet Oncol. 9 (2008) 54–60. [DOI] [PubMed] [Google Scholar]
- [7].Morgan MA, Iyengar TD, Napiorkowski BE, Rubin SC, Mikuta JJ, The clinical course of deep vein thrombosis in patients with gynecologic cancer, Gynecol. Oncol 84 (2002) 67–71. [DOI] [PubMed] [Google Scholar]
- [8].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].FIGO, Staging for carcinoma of the vulva, cervix, and corpus uteri, Int. J. Gynaecol. Obstet 125 (2014) 97–98. [DOI] [PubMed] [Google Scholar]
- [10].Sejima T, Iwamoto H, Masago T, Morizane S, Yao A, Isoyama T, et al. , Low preoperative levels of serum albumin predict lymph node metastases and ultimately correlate with a biochemical recurrence of prostate cancer in radical prostatectomy patients, Cent. Eur.J. Urol 66 (2013) 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Uppal S, Al-Niaimi A, Rice LW, Rose SL, Kushner DM, Spencer RJ, et al. , Preoperative hypoalbuminemia is an independent predictor of poor perioperative outcomes in women undergoing open surgery for gynecologic malignancies, Gynecol. Oncol 131 (2013)416–422. [DOI] [PubMed] [Google Scholar]
- [12].Satoh T, Matsumoto K, Tanaka YO, Akiyama A, Nakao S, Sakurai M, et al. , Incidence of venous thromboembolism before treatment in cervical cancer and the impact of management on venous thromboembolism after commencement of treatment, Thromb. Res 131 (2013) e127–e132. [DOI] [PubMed] [Google Scholar]
- [13].Matsuo K, Hasegawa K, Yoshino K, Murakami R, Hisamatsu T, Stone RL, et al. , Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma, Eur. J. Cancer 51 (2015) 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. , Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway, Oncogene 22 (2003) 1517–1527. [DOI] [PubMed] [Google Scholar]
- [15].Wei LH, Kuo ML, Chen CA, Cheng WF, Cheng SP, Hsieh FJ, et al. , Interleukin-6 in cervical cancer: the relationship with vascular endothelial growth factor, Gynecol. Oncol 82 (2001) 49–56. [DOI] [PubMed] [Google Scholar]
- [16].Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd., Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study, Arch. Intern. Med 160 (2000) 809–815. [DOI] [PubMed] [Google Scholar]
- [17].Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, et al. , High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis, J. Clin. Oncol 29 (2011) 3466–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anders JC, Grigsby PW, Singh AK, Cisplatin chemotherapy (without erythropoietin) and risk of life-threatening thromboembolic events in carcinoma of the uterine cervix: the tip of the iceberg? A review of the literature, Radiat. Oncol 1 (2006) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dasanu CA, Gemcitabine: vascular toxicity and prothrombotic potential, Expert Opin. Drug Saf 7 (2008) 703–716. [DOI] [PubMed] [Google Scholar]
- [20].Qi WX, Lin F, Sun YJ, Tang LN, Shen Z, Yao Y, Risk of venous and arterial thromboembolic events in cancer patients treated with gemcitabine: a systematic review and meta-analysis, Br. J. Clin. Pharmacol 76 (2013) 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Icli F, Akbulut H, Utkan G, Yalcin B, Dincol D, Isikdogan A, et al. , Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer, J. Surg. Oncol 95 (2007) 507–512. [DOI] [PubMed] [Google Scholar]
- [23].Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, et al. , Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer, Eur.J. Cancer 48 (2012) 1283–1292. [DOI] [PubMed] [Google Scholar]
- [24].Konigsbrugge O, Posch F, Riedl J, Reitter EM, Zielinski C, Pabinger I, et al. , Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients, Oncologist 21 (2016) 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Folsom AR, Lutsey PL, Heckbert SR, Cushman M, Serum albumin and risk of venous thromboembolism, Thromb. Haemost 104 (2010) 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pincus KJ, Hynicka LM, Prophylaxis of thromboembolic events in patients with nephrotic syndrome, Ann. Pharmacother 47 (2013) 725–734. [DOI] [PubMed] [Google Scholar]
- [27].Lyman GH, Khorana AA, Falanga A, Thrombosis and cancer: emerging data for the practicing oncologist, American Society of Clinical Oncology Educational Book/ASCO American Society of Clinical Oncology Meeting, 2013. [DOI] [PubMed] [Google Scholar]
- [28].Kyrle PA, Predicting recurrent venous thromboembolism in cancer: is it possible? Thromb. Res 133 (Suppl. 2) (2014) S17–S22. [DOI] [PubMed] [Google Scholar]
- [29].Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD Jr., Wakefield TW, Diaz JA, Statins, inflammation and deep vein thrombosis: a systematic review, J. Thromb. Thrombolysis 33 (2012) 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nielsen SF, Nordestgaard BG, Bojesen SE, Statin use and reduced cancer-related mortality, N. Engl. J. Med 367 (2012) 1792–1802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


